Introduction
Low back pain (LBP), a serious social health problem
in modern society, has imposed a huge burden on the health care
system (1,2). Related studies have proved
intervertebral disc (IVD) degeneration to play the most important
role in pathology of LBP and other spine degenerative diseases
(3,4). Nowadays, however, the IVD
degeneration mechanism has not be completely elucidated. There is
evidence that nucleus pulposus (NP) cell apoptosis, which is
triggered by biomechanical and biochemical stimulus in degenerative
progression (5,6), plays an important role in IVD
degeneration (7,8).
Previous studies demonstrate that excessive reactive
oxygen species (ROS) may impair the mitochondrial function,
resulting in apoptosis (9). In
IVD, ROS can be produced by NP cells in response to many kinds of
biomechanical and biochemical stimulus, such as compression
loading, high glucose and hydrogen peroxide (10–13). Meanwhile, nitric oxide (NO) is
also proved to induce IVD cell apoptosis during degeneration
(14). NO can suppress activity
of cytochrome oxidase, leading to the reduction of the electron
transport chain and the production of superoxide anions (15). NO production of NP cells may be
induced by stimulation of interleukin (IL)-1, -10 and interferon
(INF)-γ, which are involved in IVD degeneration (16,17). Sodium nitroprusside (SNP), a
widely used donor of NO, is often adopted to investigate the
mechanism of chondrocyte apoptosis induced by NO (18–21). Treatment of SNP can also cause
mitochondrial dysfunction in chondrocyte, which is characterized by
decline of mitochondrial membrane potential (ΔΨm) and release of
cytochrome c (21–23). Similarly, as to IVD, SNP has been
also used as an apoptosis inducer in annulus fibrosus cells, which
are chondrocyte-like, suggesting that SNP can induce both
endoplasmic reticulum and mitochondrial stress in annulus fibrosus
cells (24).
Resveratrol (RV;
3,5,4′-trihydroxy-trans-stilbene), a natural polyphenol
compound which could be extracted from grapes, has previously been
proved to possess anti-inflammatory, antioxidant, and anticancer
bioactivities in different kinds of cells and tissues (25–29). RV can protect IVD from
degeneration by reducing levels of proinflammatory cytokines and
activating silent mating type information regulator 2 homolog 1
(SIRT1) (30–34). However, antioxidant properties of
RV in IVD have not been elucidated yet.
The present study aimed to explore the effects of RV
on SNP induced NP cell apoptosis and investigate related mechanism.
For the first time, our results indicate that RV can protect NP
cells from SNP induced apoptosis, through scavenging excessive ROS
rather than NO provided by SNP.
Materials and methods
Materials and reagents
SNP, RV, dimethyl sulfoxide (DMSO), N-acetyl
cysteine (NAC), carboxy-PTIO (PTIO), type II collagenase,
L-ascorbic acid, Hoechst 33258, 4′,6-diamidino-2-phenylindole
(DAPI) and insulin-transferrin-selenium were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Dulbecco's modified Eagle's
medium/nutrient mixture F-12 (DMEM/F12), trypsin,
penicillin/streptomycin and fetal bovine serum (FBS) was purchased
from Gibco (Carlsbad, CA, USA). Cell Counting Kit-8 (CCK-8) was
purchased from Dojindo (Kumamoto, Japan). Caspase-3, -8, and -9
activity assay kits, 2′,7′-dichlorofluorescin diacetate (DCFH-DA),
3-amino,4-aminomethyl-2′,7′-difluorescein, diacetate (DAF-FM DA),
Actin-Tracker Green and Tubulin-Trakcer Red were from purchased
from Beyotime Institute of Biotechnology (Jiangsu, China). Annexin
V-FITC/propidium iodide (PI) apoptosis detection kit and
tetramethylrhodamine methyl ester (TMRM) was purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA). In situ
cell death detection kit was purchased from Roche Diagnostics
(Basel, Switzerland).
SNP was dissolved with phosphate-buffered saline
(PBS) and NAC was dissolved with ultrapure water just before
experiment. RV and PTIO were dissolved with DMSO. It is ensured
that the working concentration of DMSO in medium was <1%
throughout all experiments. The treatment time and concentration of
RV and SNP were determined by our experiments. The pretreatment
time of PTIO and NAC is 4 h, while their concentration is
respectively 100 μM and 2 mM.
NP cell isolation and culture
This present study was carried out in accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. Our
experiment protocol was approved by the Animal Care and Experiment
Committee of Shanghai Jiao Tong University School of Medicine. Ten
3-month-old Sprague-Dawley rats were provided by Experimental
Animal Center of Shanghai Ninth People's Hospital for in
vitro experiments. NP cell isolation and culture were carried
out as we previously described (35). The second-generation NP cells were
adopted in the following experiments. NP cells were seeded into
96-well plates (5×103/well for cell viability assay) or
6-well plates (1×105/well for apoptosis assay, caspases
activity assay, intracellular ROS and NO measurement and
mitochondrial membrane potential assay) for at least 24 h before
treatment of any reagent.
Cell viability, apoptosis and caspase
activity assay
According to manufacturer's instructions, cell
viability was measured with CCK-8 (Dojindo) using a microplate
reader (Omega Bio-Tek, Inc., Norcross, GA, USA). All cell viability
experiments were performed 6 times. Flow cytometry (FCM) was
carried out for analysis of apoptosis rates with Annexin V-FITC/PI
apoptosis detection kit following instructions, while 10,000 NP
cells were collected for each FCM analysis. Apoptosis rates were
calculated as Q2 (Annexin V-FITC-positive and PI-positive) + Q3
(Annexin V-FITC-positive and PI-negative). NP cells were stained
with 0.5 μg/ml Hoechst 33258 for 20 min in dark and then
imaged by a fluorescent microscope (IX71; Olmypus, Tokyo, Japan).
Caspase-3, -8 and -9 activities were detected with caspase-3, -8,
and -9 activity assay kits following manufacturer's instructions
using a microplate reader (Omega Bio-Tek, Inc.). The caspase
activity levels were expressed as relative activity with control as
standard.
Mitochondrial membrane potential
assay
Mitochondrial membrane potential was measured with
TMRM staining. NP cells were incubated with 100 nM TMRM at 37°C in
dark for 30 min, washed with PBS 3 times and covered with fresh
medium. NP cells were subsequently imaged using microscope (IX71,
Olmypus). The excitation wavelength for TMRM was 549 nm. Three
images (×200) of each kind of treatment were obtained for
quantitative analysis of fluorescence intensity of the TMRM using
IPP version 6.0 software (Media Cybernetics, Bethesda, MD, USA).
The fluorescence intensity of the TMRM was expressed as mean
density in IPP version 6.0 software.
Measurement of intracellular ROS and
NO
To measure intracellular ROS or NO level, NP cells
was incubated with DCFH-DA (10 μM) for 30 min or with DAF-FM
DA (5 μM) for 20 min in dark at 37°C. After washed with PBS
3 times, NP cells were collected and suspended in fresh medium. The
fluorescence intensity was detected using a microplate reader
(Omega Bio-Tek, Inc.). The excitation wavelengths for DCFH-DA and
DAF-FM DA are 488 and 495 nm respectively. The experiments were
repeated 3 times.
Imaging of cytoskeletal and morphological
structure
Imaging of cytoskeletal structure was carried out as
previously described (29). After
staining with Actin-Tracker Green and Tubulin-Trakcer Red,
cytoskeletons (×400) were imaged using a fluorescent microscope
(LEICA DM4000B; Leica Microsystems GmbH, Wetzlar, Germany). The
excitation wavelengths for Actin-Tracker Green and Tubulin-Trakcer
Red are 488 and 543 nm respectively. After cell treatment, NP cells
(×200) were imaged under transmitted light illumination using
microscope (IX71; Olmypus).
Organ culture and TUNEL assay
Rat disc organ culture was carried out as we
previously described (35). Ex
vivo, discs were pretreated with 100 μM RV for 24 h or 2
mM NAC for 12 h or 100 μM PTIO for 12 h, then treated with
or without 1 mM SNP for 18 h. Then the harvested discs were fixed
in 4% paraformaldehyde, and then decalcified with EDTA for 2 weeks.
After embedding with paraffin, 5-μm thick serial
mid-sagittal sections of discs were made for slides. Mid-sagittal
sections of discs were analyzed for apoptosis using in situ
cell death detection kit according to manufacturer's instructions.
DAPI staining was conducted for indication of total cells.
TUNEL-positive apoptotic cells and DAPI-positive total cells of NP
area on mid-sagittal sections of discs were identified by
fluorescent microscope (IX71; Olmypus), and apoptosis rate was
calculated as the percentage of numbers of TUNEL-positive cells to
the numbers of total cells using IPP version 6.0 software (Media
Cybernetics). The quantitative analysis was performed on three ×200
fields/section (three sections/disc and three discs for each kinds
of treatment).
Statistical analysis
All statistical data were expressed as mean ±
standard deviation. Results were statistically analyzed by a
one-way analysis of variance (ANOVA) with multiple comparisons
using SPSS 19.0 (IBM, Inc., New York, NY, USA). P-values <0.05
were considered to indicate statistically significant
difference.
Results
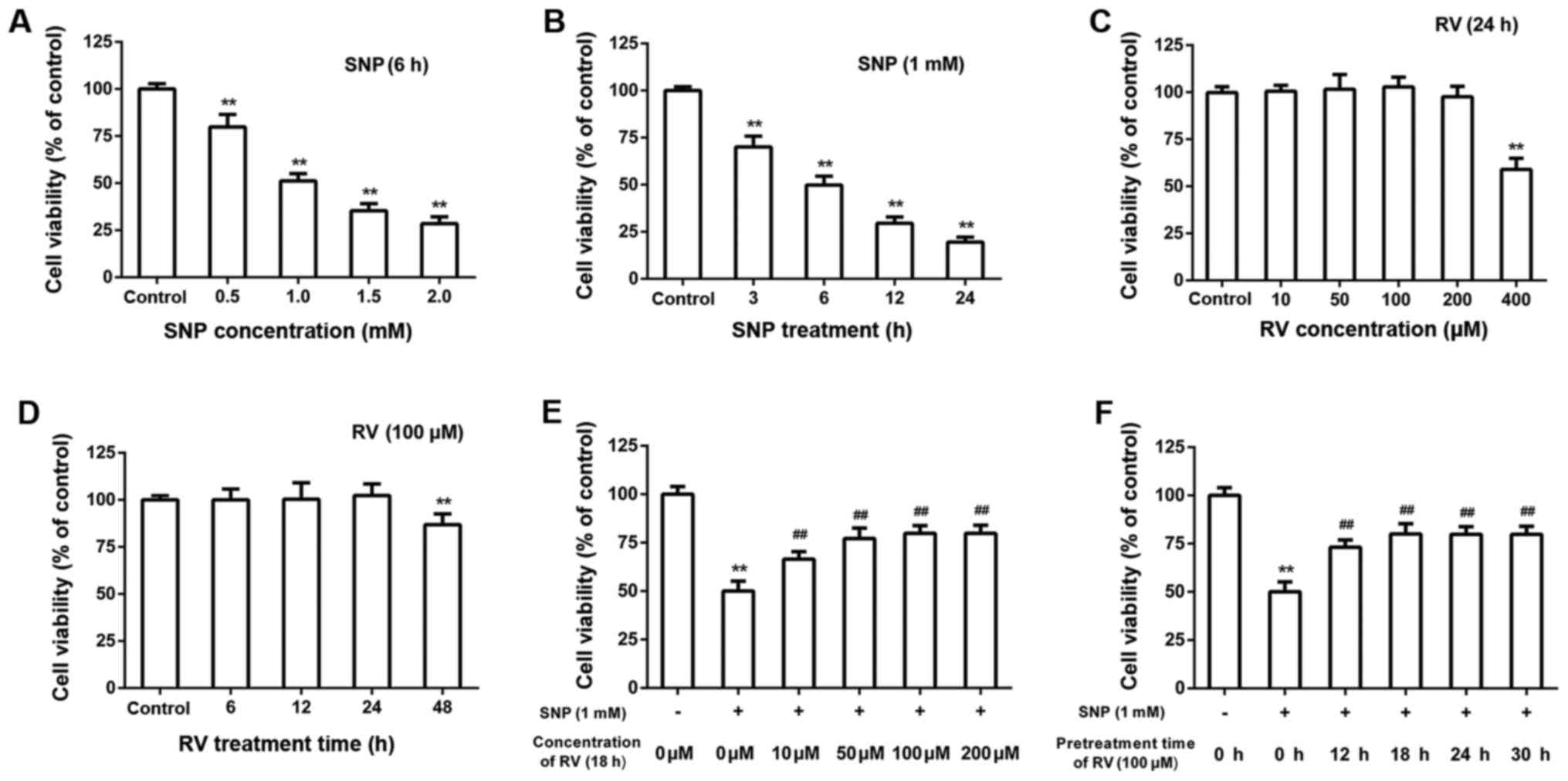
Dose and time-dependent effects of SNP
and RV on NP cell viability
As shown by CCK-8 assays, SNP induced NP cells
cytotoxicity in a dose and time-dependent manner (Fig. 1A and B). Treatment concentration
of SNP was set as 1 mM and treatment time of SNP was set as 6 h
throughout the following experiments without indication. Treatment
of RV for 24 h did not exert obvious cytotoxicity on NP cells when
RV concentration was no higher than 200 μM (Fig. 1C). Treatment of 100 μM RV
did not exert significant cytotoxicity on NP cells when treatment
time was no longer than 24 h (Fig.
1D). Pretreatment of RV for 18 h showed dose-dependent
protective effects on SNP induced cytotoxicity (Fig. 1E). Pretreatment of 100 μM
RV protected NP cells from SNP induced cytotoxicity in a
time-dependent manner (Fig. 1F).
Treatment concentration of RV was set as 100 μM and
treatment time of RV was set as 18 h throughout the following
experiments without indication.
RV protects against SNP induced NP cell
apoptosis by scavenging ROS instead of NO in vitro
In order to investigate the mechanism of protective
effects of RV, NAC as an established ROS scanvenger and PTIO as an
established NO scanvenger were adopted in the following
experiments. FCM assay with Annexin V-FITC/PI staining showed that
SNP induced significant NP cell apoptosis, which was significantly
rescued by RV and NAC instead of PTIO (Fig. 2A and B). CCK-8 assays demonstrated
that RV and NAC instead of PTIO significantly suppressed SNP
induced cytotoxicity, while NAC or PTIO alone did not show obvious
cytotoxicity (Fig. 2C).
Meanwhile, RV and NAC instead of PTIO markedly inhibited activation
of caspase-3 and -9 induced by SNP (Fig. 2D). However, SNP did not
significantly activate caspase-8 (Fig. 2D). Hoechst 33258 staining images
showed that RV and NAC instead of PTIO effectively decreased
apoptotic nuclei containing condensed or fragmented chromatin
induced by SNP, which is consistent with FCM assay with Annexin
V-FITC/PI staining (Fig. 2E). As
to mitochondrial membrane potential, TMRM staining and quantitative
analysis demonstrated that significant loss of ΔΨm was caused by
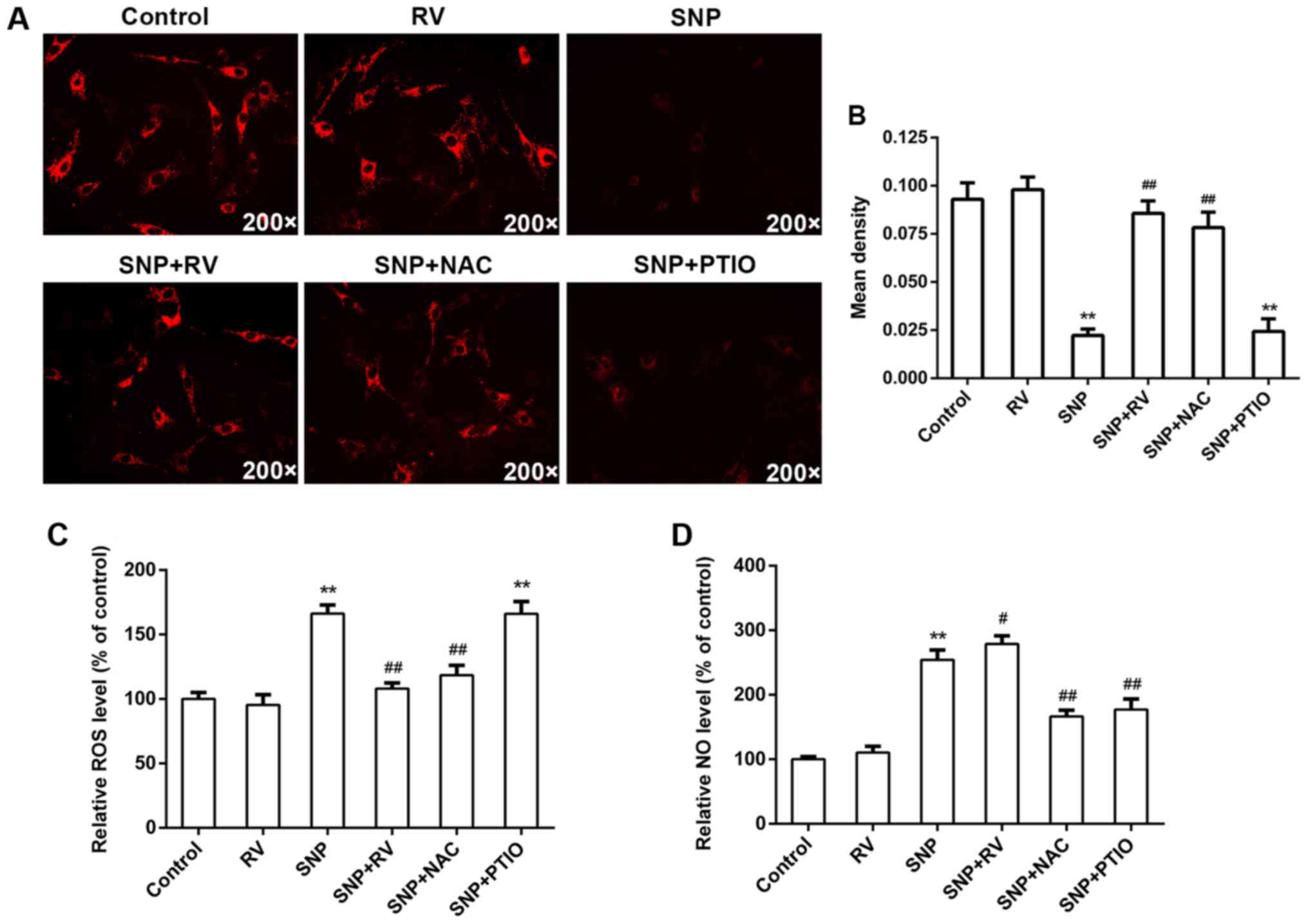
SNP and effectively rescued by RV and NAC but not PTIO (Fig. 3A and B). As shown by intracellular
ROS and NO analysis, SNP significantly induced production of
intracellular ROS (Fig. 3C) and
NO (Fig. 3D). Pretreatment with
RV obviously suppressed SNP induced excessive intracellular ROS
production, but did not suppress SNP induced NO production
(Fig. 3C and D). As expected, NAC
significantly decreased the SNP induced high intracellular ROS and
NO production, while PTIO significantly decreased the SNP induced
high intracellular NO production but not ROS production.
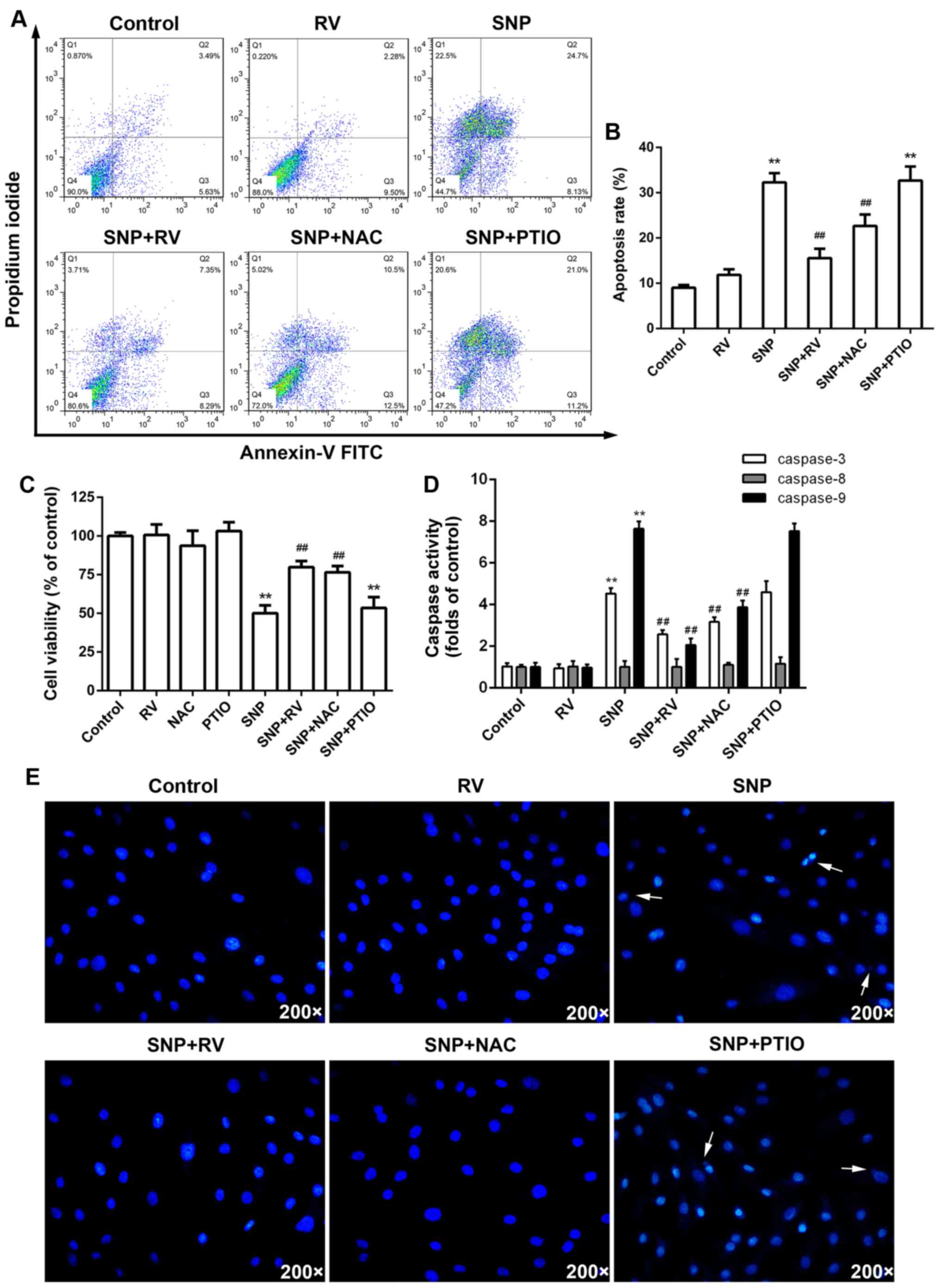 | Figure 2Effects of resveratrol (RV), N-acetyl
cysteine (NAC) and PTIO on sodium nitroprusside (SNP) induced
nucleus pulposus (NP) cell apoptosis. (A) NP cell apoptosis
analyzed by flow cytometry (FCM) with Annexin V/propidium iodide
(PI) staining. (B) Quantitative analysis of apoptosis rates.
**p<0.01, compared to control;
##p<0.01, compared to SNP alone group. (C) Effects of
RV, NAC and PTIO on SNP induced NP cell cytotoxiity analyzed by
Cell Counting Kit-8 (CCK-8) assay. **p<0.01, compared
to control; ##p<0.01, compared to SNP alone group.
(D) Effects of RV, NAC, PTIO and SNP on caspases activity.
**p<0.01, compared to control;
##p<0.01, compared to SNP alone group. (E) Hoechst
33258 staining images of NP cells. Arrows show the apoptotic nuclei
containing condensed or fragmented chromatin. |
Overall, ROS, not NO, production is probably the key
mechanism of SNP induced NP cell apoptosis. RV can effectively
scavenge intracellular ROS instead of NO, subsequently protecting
NP cells from SNP induced apoptosis.
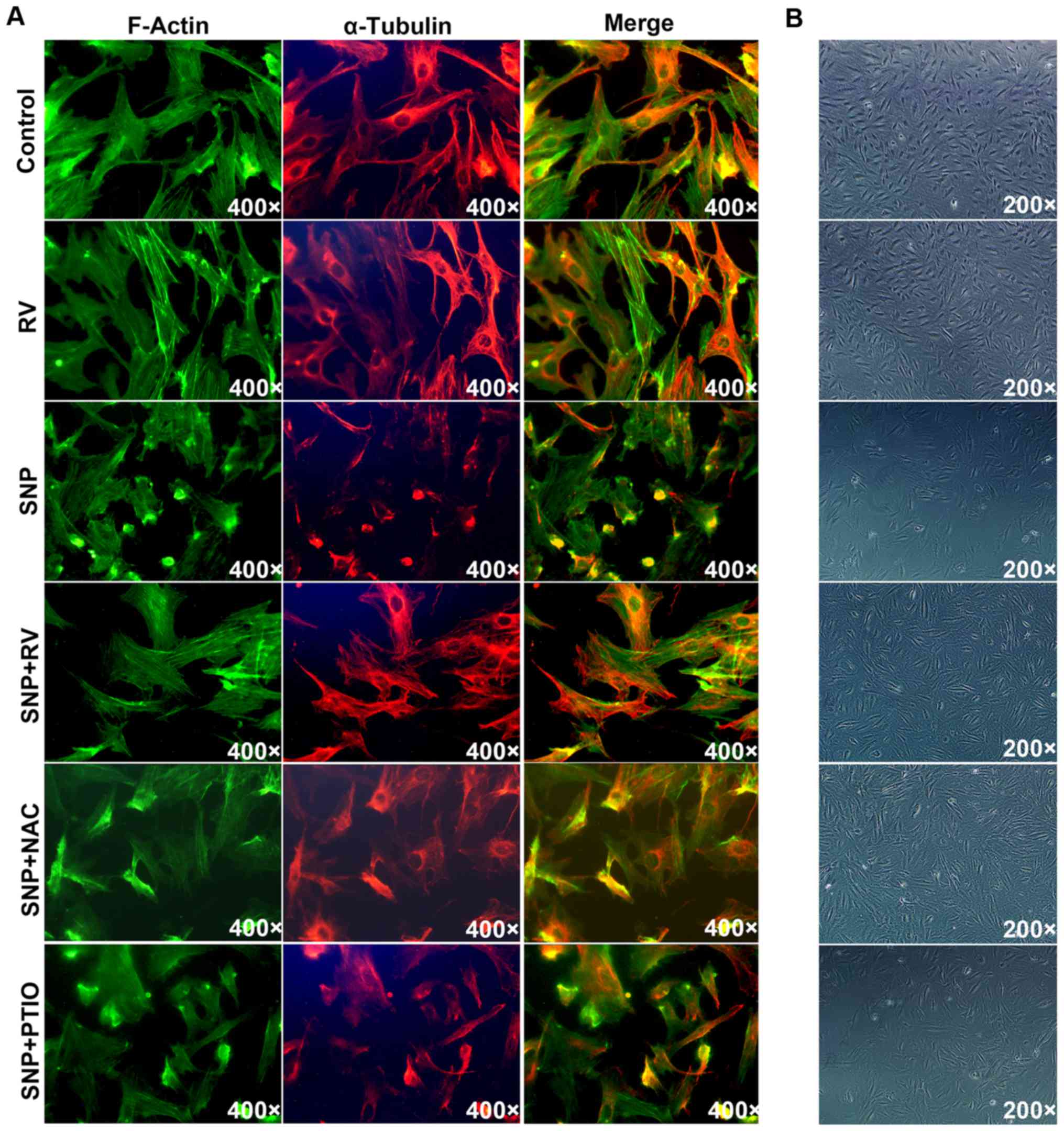
RV protects NP cells from SNP induced
disruption of cytoskeletal and morphological structure
Actin-Tracker Green and Tubulin-Trakcer Red were
used to mark F-actin and α-tubulin respectively under fluorescent
microscope. In control and RV treated NP cells, normal cytoskeleton
was observed with regularly distributed F-actin filament and
microtubule (Fig. 4A). SNP
treatment caused cytoskeleton shrinkage, by curling up F-actin
filaments and disrupting microtubule structure (Fig. 4A). The SNP induced cytoskeleton
shrinkage was significantly rescued by RV and NAC instead of PTIO
(Fig. 4A). Under microscope with
transmitted light illumination, SNP induced obvious cell shrinkage
compared to control, which was significantly prevented by RV and
NAC but not PTIO (Fig. 4B).
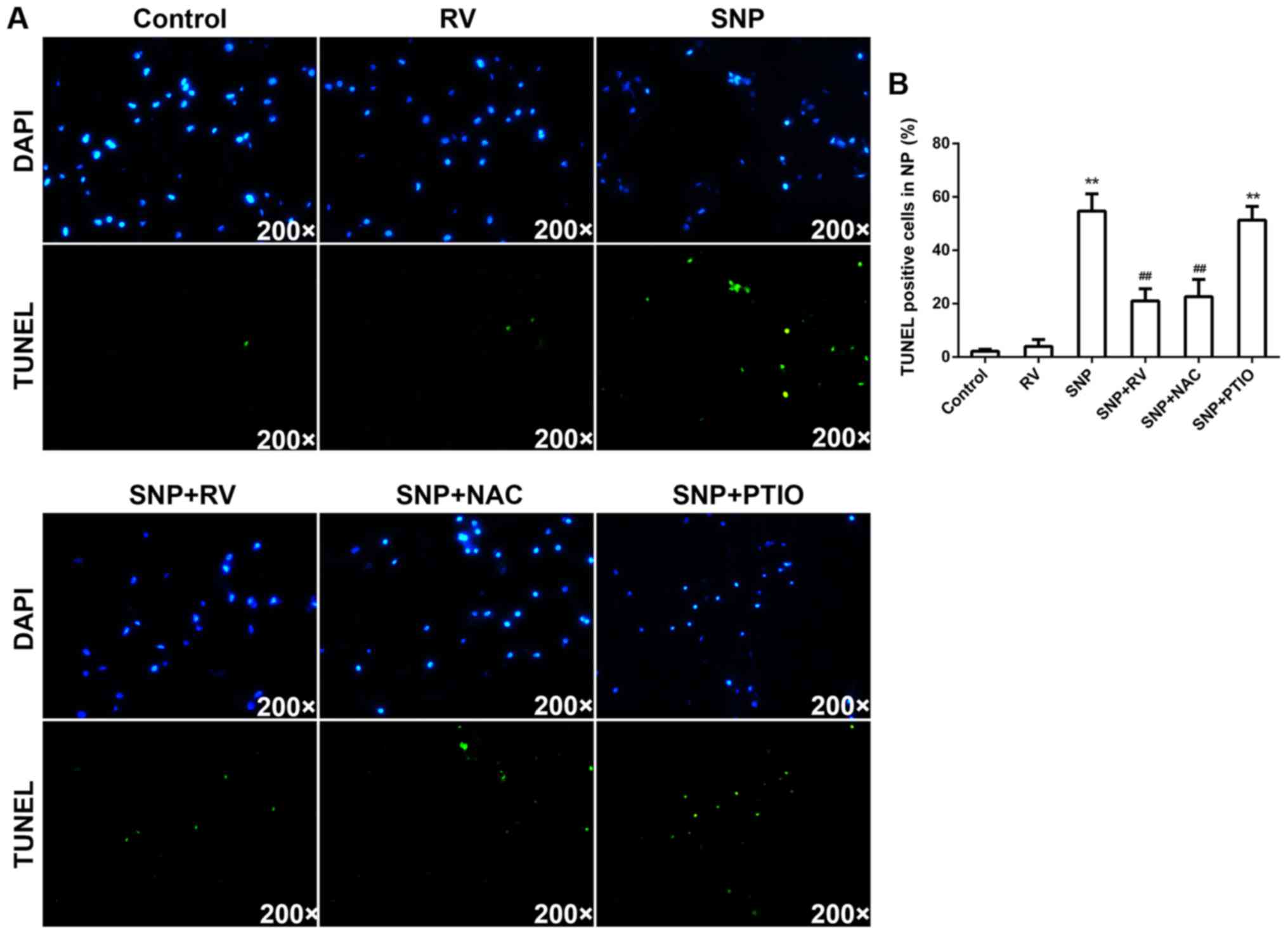
RV protects against SNP induced NP cell
apoptosis by scavenging ROS instead of NO ex vivo
Organ culture was also carried out to analyze
effects of RV on NP cells ex vivo. Treatment of SNP ex
vivo significantly increased the TUNEL-positive cells
percentage of NP area on mid-sagittal sections of discs compared to
control (Fig. 5A and B).
Consistent with in vitro results, RV and NAC significantly
decreased TUNEL-positive cells percentage on mid-sagittal sections
of discs compared to SNP treated group, while PTIO exerted no
significant effects (Fig. 5A and
B).
Discussion
Our results demonstrate that SNP can potently induce
NP cell apoptosis in a ROS dependent rather than NO dependent
manner. For the first time, RV is found to effectively scavenge ROS
instead of NO in NP cells, through which RV significantly inhibits
SNP induced NP cell apoptosis.
In previous cartilaginous studies, SNP is usually
adopted as a NO donor to investigate NO related apoptosis and NO is
considered to be the key mediator of apoptosis (18,36,37). As to IVD, Zhao et al proved
that both endoplasmic reticulum and mitochondria play a role in SNP
induced annulus fibrosus cell apoptosis and speculated that high
concentration of NO provided by SNP acted as the main upstream
mediator (24). However, no study
focused on effects of SNP on NP cells. Moreover, a recent study
show that ROS rather than NO plays the key role in SNP induced in
rabbit articular chondrocytes apoptosis, suggesting a new mechanism
of SNP induced apoptosis (29).
In our study, both intracellular NO and ROS level was raised by SNP
treatment. Nevertheless, PTIO, which significantly scavenged
intracellular NO, could not retard SNP induced NP cell cytotoxicity
and apoptosis, which indicated that SNP induces NP cell apoptosis
in a NO independent manner.
It has been reported that SNP can hinder electron
transfer process and raise the level of reduced cytochrome
c, resulting in production of ROS (38). Meanwhile, mitochondrial membrane
potential can be reduced by SNP in many kinds of tissues (39–42). Loss of mitochondrial membrane
potential means disruption of mitochondrial membrane function,
which leads to the transfer of ROS into cytoplasm subsequently
inducing cell death (43). As
shown by our results, SNP induced an excessive production of ROS,
loss of mitochondrial membrane potential and apoptosis. According
to our results, we conclude that ROS instead of NO is the key
mediator of SNP induced NP cell apoptosis. Interestingly, SNP
seemed to exert no obvious effects on caspase-8 activity when it
significantly activated caspase-3 and -9. It is probably due to
that SNP induces NP cell apoptosis mainly via an intrinsic
apoptotic pathway (44). In the
present study, RV showed potent effects of scavenging ROS to
inhibit SNP induced apoptosis, which is comparable to NAC. However,
no obvious effects of RV were observed on SNP induced NO production
unlike NAC and PTIO.
To further prove the effects of RV, an ex
vivo study was also carried out. Among studies adopting disc
organ culture system, few of them focus on NP cells apoptosis
induced by oxidative stress (45–47). Considering the rapid effects of
SNP in vitro, we modified the organ culture system with
relatively short culture time. For the first time, it is found that
ex vivo SNP treatment on IVD caused significant NP cell
apoptosis under the established disc organ culture condition.
Consistent with in vitro results, RV and NAC but not PTIO
partly recued the NP cell apoptosis ex vivo.
RV has been reported to exert antioxidative effects
in many kinds of tissues, while the antioxidative potential of RV
in NP cells is proved by us for the first time (48–50). As oxidative stress plays an
important role in degeneration related disc cell apoptosis,
antioxidative treatment is a promising therapeutic strategy
(10–13). There have been studies aiming to
retard IVD degeneration with antioxidative drugs and biomaterials
(13,51,52). The antioxidative potential makes
RV a good choice for treatment of IVD degeneration in future
targeting at degeneration related disc cell apoptosis.
In general, the present study demonstrated that ROS
is the key mediator in SNP induced NP cell apoptosis. RV
significantly inhibit SNP induced NP cell apoptosis by scavenging
ROS but not NO in vitro and ex vivo. With potent
antioxidative activity, RV would be a favorable candidate for
protection against oxidative stress related disc cell
apoptosis.
Acknowledgments
The technical support from Chuan Jiang and Lei Wang
is appreciated by the authors.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Hart LG, Deyo RA and Cherkin DC: Physician
office visits for low back pain. Frequency, clinical evaluation,
and treatment patterns from a U.S. national survey. Spine.
20:11–19. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katz JN: Lumbar disc disorders and
low-back pain: socioeconomic factors and consequences. J Bone Joint
Surg Am. 88(Suppl 2): 21–24. 2006.PubMed/NCBI
|
|
3
|
Schwarzer AC, Aprill CN, Derby R, Fortin
J, Kine G and Bogduk N: The relative contributions of the disc and
zygapophyseal joint in chronic low back pain. Spine. 19:801–806.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dario AB, Ferreira ML, Refshauge KM, Lima
TS, Ordoñana JR and Ferreira PH: The relationship between obesity,
low back pain, and lumbar disc degeneration when genetics and the
environment are considered: a systematic review of twin studies.
Spine J. 15:1106–1117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walsh AJ and Lotz JC: Biological response
of the intervertebral disc to dynamic loading. J Biomech.
37:329–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahsan R, Tajima N, Chosa E, Sugamata M,
Sumida M and Hamada M: Biochemical and morphological changes in
herniated human intervertebral disc. J Orthop Sci. 6:510–518. 2001.
View Article : Google Scholar
|
|
7
|
Ding F, Shao ZW and Xiong LM: Cell death
in intervertebral disc degeneration. Apoptosis. 18:777–785. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kepler CK, Ponnappan RK, Tannoury CA,
Risbud MV and Anderson DG: The molecular basis of intervertebral
disc degeneration. Spine J. 13:318–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kowaltowski AJ and Vercesi AE:
Mitochondrial damage induced by conditions of oxidative stress.
Free Radic Biol Med. 26:463–471. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding F, Shao ZW, Yang SH, Wu Q, Gao F and
Xiong LM: Role of mitochondrial pathway in compression-induced
apoptosis of nucleus pulposus cells. Apoptosis. 17:579–590. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma KG, Shao ZW, Yang SH, Wang J, Wang BC,
Xiong LM, Wu Q and Chen SF: Autophagy is activated in
compression-induced cell degeneration and is mediated by reactive
oxygen species in nucleus pulposus cells exposed to compression.
Osteoarthritis Cartilage. 21:2030–2038. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park EY and Park JB: High glucose-induced
oxidative stress promotes autophagy through mitochondrial damage in
rat notochordal cells. Int Orthop. 37:2507–2514. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang L, Rong Z, Zeng M, Cao Y, Gong X, Lin
L, Chen Y, Cao W, Zhu L and Dong W: Pyrroloquinoline quinone
protects nucleus pulposus cells from hydrogen peroxide-induced
apoptosis by inhibiting the mitochondria-mediated pathway. Eur
Spine J. 24:1702–1710. 2015. View Article : Google Scholar
|
|
14
|
Kohyama K, Saura R, Doita M and Mizuno K:
Intervertebral disc cell apoptosis by nitric oxide: biological
understanding of intervertebral disc degeneration. Kobe J Med Sci.
46:283–295. 2000.
|
|
15
|
Fermor B, Christensen SE, Youn I, Cernanec
JM, Davies CM and Weinberg JB: Oxygen, nitric oxide and articular
cartilage. Eur Cell Mater. 13:56–65; discussion 65. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Studer RK, Gilbertson LG, Georgescu H,
Sowa G, Vo N and Kang JD: p38 MAPK inhibition modulates rabbit
nucleus pulposus cell response to IL-1. J Orthop Res. 26:991–998.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Katsuno R, Hasegawa T, Iwashina T, Sakai
D, Mikawa Y and Mochida J: Age-related effects of cocultured rat
nucleus pulposus cells and macrophages on nitric oxide production
and cytokine imbalance. Spine. 33:845–849. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Blanco FJ, Ochs RL, Schwarz H and Lotz M:
Chondrocyte apoptosis induced by nitric oxide. Am J Pathol.
146:75–85. 1995.PubMed/NCBI
|
|
19
|
Yoon JB, Kim SJ, Hwang SG, Chang S, Kang
SS and Chun JS: Non-steroidal anti-inflammatory drugs inhibit
nitric oxide-induced apoptosis and dedifferentiation of articular
chondrocytes independent of cyclooxygenase activity. J Biol Chem.
278:15319–15325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JS, Park ZY, Yoo YJ, Yu SS and Chun
JS: p38 kinase mediates nitric oxide-induced apoptosis of
chondrocytes through the inhibition of protein kinase C zeta by
blocking autophosphorylation. Cell Death Differ. 12:201–212. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu GJ, Chen TG, Chang HC, Chiu WT, Chang
CC and Chen RM: Nitric oxide from both exogenous and endogenous
sources activates mitochondria-dependent events and induces insults
to human chondrocytes. J Cell Biochem. 101:1520–1531. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maneiro E, López-Armada MJ, de Andres MC,
Caramés B, Martín MA, Bonilla A, Del Hoyo P, Galdo F, Arenas J and
Blanco FJ: Effect of nitric oxide on mitochondrial respiratory
activity of human articular chondrocytes. Ann Rheum Dis.
64:388–395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SW, Song YS, Shin SH, Kim KT, Park YC,
Park BS, Yun I, Kim K, Lee SY, Chung WT, et al: Cilostazol protects
rat chondrocytes against nitric oxide-induced apoptosis in vitro
and prevents cartilage destruction in a rat model of
osteoarthritis. Arthritis Rheum. 58:790–800. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao CQ, Zhang YH, Jiang SD, Jiang LS and
Dai LY: Both endoplasmic reticulum and mitochondria are involved in
disc cell apoptosis and intervertebral disc degeneration in rats.
Age (Dordr). 32:161–177. 2010. View Article : Google Scholar
|
|
25
|
Haider UG, Sorescu D, Griendling KK,
Vollmar AM and Dirsch VM: Resveratrol suppresses angiotensin
II-induced Akt/protein kinase B and p70 S6 kinase phosphorylation
and subsequent hypertrophy in rat aortic smooth muscle cells. Mol
Pharmacol. 62:772–777. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhat KPL, Kosmeder JW 2nd and Pezzuto JM:
Biological effects of resveratrol. Antioxid Redox Signal.
3:1041–1064. 2001. View Article : Google Scholar
|
|
27
|
Bertelli AA, Ferrara F, Diana G, Fulgenzi
A, Corsi M, Ponti W, Ferrero ME and Bertelli A: Resveratrol, a
natural stilbene in grapes and wine, enhances intraphagocytosis in
human promonocytes: a co-factor in antiinflammatory and anticancer
chemopreventive activity. Int J Tissue React. 21:93–104. 1999.
|
|
28
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liang Q, Wang XP and Chen TS: Resveratrol
protects rabbit articular chondrocyte against sodium
nitroprusside-induced apoptosis via scavenging ROS. Apoptosis.
19:1354–1363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Phillips FM, An HS, Ellman M, Thonar
EJ, Wu W, Park D and Im HJ: The action of resveratrol, a
phytoestrogen found in grapes, on the intervertebral disc. Spine.
33:2586–2595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wuertz K, Quero L, Sekiguchi M, Klawitter
M, Nerlich A, Konno S, Kikuchi S and Boos N: The red wine
polyphenol resveratrol shows promising potential for the treatment
of nucleus pulposus-mediated pain in vitro and in vivo. Spine.
36:E1373–E1384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang D, Hu Z, Hao J, He B, Gan Q, Zhong X,
Zhang X, Shen J, Fang J and Jiang W: SIRT1 inhibits apoptosis of
degenerative human disc nucleus pulposus cells through activation
of Akt pathway. Age (Dordr). 35:1741–1753. 2013. View Article : Google Scholar
|
|
33
|
Jiang W, Zhang X, Hao J, Shen J, Fang J,
Dong W, Wang D, Zhang X, Shui W, Luo Y, et al: SIRT1 protects
against apoptosis by promoting autophagy in degenerative human disc
nucleus pulposus cells. Sci Rep. 4:74562014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xia X, Guo J, Lu F and Jiang J: SIRT1
plays a protective role in intervertebral disc degeneration in a
puncture-induced Rodent model. Spine. 40:E515–E524. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li K, Li Y, Ma Z and Zhao J: Crocin exerts
anti-inflammatory and anti-catabolic effects on rat intervertebral
discs by suppressing the activation of JNK. Int J Mol Med.
36:1291–1299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Del Carlo M Jr and Loeser RF: Nitric
oxide-mediated chondrocyte cell death requires the generation of
additional reactive oxygen species. Arthritis Rheum. 46:394–403.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eo SH, Cho H and Kim SJ: Resveratrol
inhibits nitric oxide-induced apoptosis via the NF-kappa B pathway
in rabbit articular chondrocytes. Biomol Ther (Seoul). 21:364–370.
2013. View Article : Google Scholar
|
|
38
|
Giorgio M, Migliaccio E, Orsini F,
Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S,
Marcaccio M, et al: Electron transfer between cytochrome c and
p66Shc generates reactive oxygen species that trigger
mitochondrial apoptosis. Cell. 122:221–233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang CN, Duan GL, Liu YJ, Yu Q, Tang XL,
Zhao W, Li XH, Zhu XY and Ni X: Overproduction of nitric oxide by
endothelial cells and macrophages contributes to mitochondrial
oxidative stress in adrenocortical cells and adrenal insufficiency
during endotoxemia. Free Radic Biol Med. 83:31–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Baek MW, Seong KJ, Jeong YJ, Kim GM, Park
HJ, Kim SH, Chung HJ, Kim WJ and Jung JY: Nitric oxide induces
apoptosis in human gingival fibroblast through
mitochondria-dependent pathway and JNK activation. Int Endod J.
48:287–297. 2015. View Article : Google Scholar
|
|
41
|
de Andrés MC, Maneiro E, Martín MA, Arenas
J and Blanco FJ: Nitric oxide compounds have different effects
profiles on human articular chondrocyte metabolism. Arthritis Res
Ther. 15:R1152013. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mahesh R, Jung HW, Kim GW, Kim YS and Park
YK: Cryptotanshinone from Salviae miltiorrhizae radix inhibits
sodium-nitroprusside-induced apoptosis in neuro-2a cells. Phytother
Res. 26:1211–1219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fleury C, Mignotte B and Vayssière JL:
Mitochondrial reactive oxygen species in cell death signaling.
Biochimie. 84:131–141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ryter SW, Kim HP, Hoetzel A, Park JW,
Nakahira K, Wang X and Choi AM: Mechanisms of cell death in
oxidative stress. Antioxid Redox Signal. 9:49–89. 2007. View Article : Google Scholar
|
|
45
|
Markova DZ, Kepler CK, Addya S, Murray HB,
Vaccaro AR, Shapiro IM, Anderson DG, Albert TJ and Risbud MV: An
organ culture system to model early degenerative changes of the
intervertebral disc II: profiling global gene expression changes.
Arthritis Res Ther. 15:R1212013. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim JS, Ellman MB, Yan D, An HS, Kc R, Li
X, Chen D, Xiao G, Cs-Szabo G, Hoskin DW, et al: Lactoferricin
mediates anti-inflammatory and anti-catabolic effects via
inhibition of IL-1 and LPS activity in the intervertebral disc. J
Cell Physiol. 228:1884–1896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Risbud MV, Di Martino A, Guttapalli A,
Seghatoleslami R, Denaro V, Vaccaro AR, Albert TJ and Shapiro IM:
Toward an optimum system for intervertebral disc organ culture:
TGF-beta 3 enhances nucleus pulposus and anulus fibrosus survival
and function through modulation of TGF-beta-R expression and ERK
signaling. Spine. 31:884–890. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu H, Li GN, Xie J, Li R, Chen QH, Chen
JZ, Wei ZH, Kang LN and Xu B: Resveratrol ameliorates myocardial
fibrosis by inhibiting ROS/ERK/TGF-β/periostin pathway in
STZ-induced diabetic mice. BMC Cardiovasc Disord. 16:52016.
View Article : Google Scholar
|
|
49
|
Abengózar-Vela A, Calonge M, Stern ME,
González-García MJ and Enríquez-De-Salamanca A: Quercetin and
resveratrol decrease the inflammatory and oxidative responses in
human ocular surface epithelial cells. Invest Ophthalmol Vis Sci.
56:2709–2719. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang R, Liu YY, Liu XY, Jia SW, Zhao J,
Cui D and Wang L: Resveratrol protects neurons and the myocardium
by reducing oxidative stress and ameliorating mitochondria damage
in a cerebral ischemia rat model. Cell Physiol Biochem. 34:854–864.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang X, Jin L, Yao L, Shen FH, Shimer AL
and Li X: Antioxidative nanofullerol prevents intervertebral disk
degeneration. Int J Nanomedicine. 9:2419–2430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cheng YH, Yang SH and Lin FH:
Thermosensitive chitosan-gelatin-glycerol phosphate hydrogel as a
controlled release system of ferulic acid for nucleus pulposus
regeneration. Biomaterials. 32:6953–6961. 2011. View Article : Google Scholar : PubMed/NCBI
|