Introduction
In recent years, drowning accidents have received
growing attention. Approximately 50,000 people succumb to seawater
(SW) drowning annually, and it has become the third leading cause
of accidental death (1,2). After SW drowning, victims are
hypoxemic, due to severely damaged gas exchange; as a result,
alveolar epithelial cells and pulmonary capillary epithelial cells
are damaged, causing alveolar hemorrhage and exudate, pulmonary
interstitial edema, and ventilation-perfusion mismatch, which
ultimately lead to acute lung injury (ALI) and can further develop
into acute respiratory distress syndrome (ARDS) (3).
Previous studies have suggested that apoptosis may
be involved in the ALI/ARDS pathological process (4,5).
Our previous study confirmed that SW inhalation may lead to
apoptosis of rat alveolar epithelial cells (6). However, there are few studies
regarding the mechanism by which apoptosis contributes to
SW-induced acute lung injury (SW-ALI) or acute respiratory distress
syndrome (SW-ARDS).
Reactive oxygen species (ROS), including oxygen ions
and peroxides, serve an important role in regulating cell growth,
survival and death (7). Increased
cellular ROS levels can activate various signaling pathways,
resulting in DNA damage and apoptosis (8). Endoplasmic reticulum (ER) stress is
the stress response of the body to external stimuli. When
stimulated by external stimuli, cells activate ER stress and
further activate signaling pathways, which induce cell death,
inflammation and apoptosis (9).
Numerous studies have suggested that increased ROS generation and
ER stress interact with each other (10,11).
The present study aimed to investigate whether ROS
and ER stress pathways are involved in SW-induced apoptosis, which
is in turn involved in the pathological process of SW-ALI and
SW-ARDS.
Materials and methods
Reagents
Antibodies against phosphorylated (p)-protein kinase
R-like ER kinase (PERK; ab192591), PERK (ab79483),
inositol-requiring kinase 1α (IRE1α; ab37073), p-IRE1α (ab48187),
activating transcription factor 6α (ATF6α), p-50ATF6α (ab37149),
glucose-regulated protein 78 (GRP78; ab21685), CCAAT/enhancer
binding protein homologous protein (CHOP; ab11419), p-c-Jun
N-terminal kinase (JNK; ab124956), JNK (ab179461) and caspase-3
(ab13847) were purchased from Abcam (Cambridge, UK). N-acetyl
L-cysteine (NAC), 4-phenylbutyric acid (4-PBA) and thap-sigargin
(Thap) were also purchased from Abcam. The Annexin V-fluorescein
isothiocyanate (FITC) apoptosis detection kit was purchased from
Roche Diagnostics (Indianapolis, IN, USA). Cell Counting kit-8
(CCK-8) and 2′,7′-dichlorofluorescein diacetate (DCFH-DA) were
purchased from Beyotime Institute of Biotechnology (Shanghai,
China). SW (osmolality, 1,300 mmol/l; pH 8.2; relative density
1.05; salt content, 34.421%; NaCl, 26.518 g/l; MgSO4,
3.305 g/l; MgCl2, 2.447 g/l; CaCl2, 1.141
g/l; KCl, 0.725 g/l; NaHCO3, 0.202 g/l; and NaBr, 0.083
g/l) was prepared based on the overall composition of the East
China Sea, which was provided by the Chinese Ocean Bureau (Beijing,
China).
Animal preparation
Male Sprague-Dawley rats (age, 5-7 weeks; weight,
200±20 g) were obtained from the Animal Center of the Fourth
Military Medical University (Xi'an, China). The rats were
maintained in a temperature-controlled room (20-22°C) with 40-50%
humidity, under a 12-h light/dark cycle. All rats were given ad
libitum access to standard laboratory chow and water. Prior to
experimentation, rats were fasted for 12 h, but were allowed free
access to water. The present study was approved by the Animal Care
and Use Committee of the Fourth Military Medical University, and
experiments were conducted in accordance with the National
Institutes of Health guidelines regulating the Care and Use of
Laboratory Animals (12).
Firstly, 25 Sprague-Dawley rats were randomly
divided into the following five groups: Normal control group (were
treated as SW groups, but no liquid was injected into the trachea),
2-h SW group (2 h), 4-h SW group (4 h), 6-h SW group (6 h) and 8-h
SW group (8 h). The rats were anesthetized with 20% urethane (1.0
g/kg), administered intraperitoneally, prior to treatment with SW.
SW (4 ml/kg body weight) was injected into both lungs though the
trachea at a constant speed over 4 min. The rats were kept in a
supine and 30-degree head-up position during the experiment.
Secondly, 30 Sprague-Dawley rats (independent of the initial 25
rats) were randomly divided into the following six groups: Control
group, SW group, NAC control group (150 mg/kg), NAC + SW group,
4-PBA control group (30 mg/kg) and 4-PBA + SW group. The rats were
pretreated with NAC or 4-PBA, which were administered
intraperitoneally, 2 h prior to SW administration. Rats were
sacrificed 4 h after SW administration. Rats in the control, NAC
and 4-PBA groups were treated with saline instead of SW. Finally,
the rats were sacrificed by an overdose of anesthesia at the
indicated time-points; subsequently, lung tissues were harvested
and processed.
A549 cell culture and treatment
Human lung alveolar epithelial A549 cells were
purchased from American Type Culture Collection (Manassas, VA, USA)
and were cultured in RPMI-1640 medium (Hyclone, Logan, UT, USA)
supplemented with 100 µg/ml streptomycin, 100 U/ml penicillin and
10% fetal bovine serum (Sijiqing, Hangzhou, China) in a humidified
atmosphere containing 95% air and 5% CO2 at 37°C. For
the subsequent experiments, cells were collected during the
logarithmic growth phase under the same conditions. A549 cells
(1×104/ml) were inoculated into 6-well plates in culture
medium overnight, after which, SW was added at final concentrations
of 10, 20, 40 and 60% for 4 h, or 25% for 2, 4, 6 or 8 h. NAC (5
mM), 4-PBA (2 mM) and Thap (150 nM) were prepared in advance and
added to cells 2 h prior to SW administration. Mean fluorescence
intensity was analyzed using ImageJ software (version 1.51j8;
National Institutes of Health, Bethesda, MD, USA).
ROS assay
The DCFH-DA probe was used to detect ROS generation.
Briefly, A549 cells were inoculated into plates and were treated
when they reached 75% confluence. Subsequently, cells were
incubated with DCFH-DA (10 µM) at 37°C for 45 min. Finally, cells
were washed three times with PBS and imaged under a fluorescence
microscope.
Lung wet-to-dry weight (W/D) ratio
The lung W/D ratio is considered an index of
pulmonary edema. The left lungs (n=5) were weighed immediately
after the rats were sacrificed, and were then subjected to
desiccation at 55°C for 72 h to determine the dry weight. The lung
W/D ratio was calculated by dividing the wet weight by the dry
weight.
Histopathological examination
To visually evaluate the severity of lung injury,
the right lower lungs of the rats were separated and fixed with 10%
formalin at the indicated time-points. Subsequently, the tissues
were embedded in paraffin and cut into 5 µm sections, which were
mounted on silanized slides and stained with hematoxylin and eosin
(H&E). The sections were observed using an Olympus microscope
(Olympus, Tokyo, Japan).
Immunohistochemical analysis of GRP78
expression in rat lungs
Rat lung sections were deparaffinized and
rehydrated, after which they were incubated in 3% hydrogen peroxide
for 10 min to quench endogenous peroxidase activity. After boiling
the sections in 0.01 mol/l citrate buffer (microwave oven heating,
at high fire for 2 min and medium fire for 8 min) for antigen
recovery, slides were incubated with anti-GRP78 antibody (1:200) at
4°C for 1 night. Subsequently, the sections were incubated with
horseradish peroxidase-conjugated secondary antibody at room
temperature for 1 h, as described in the instructions provided in
the immunohistochemistry kit (1:400; #13079; Cell Signaling
Technology, Inc., Danvers, MA, USA). The sections were observed
using an Olympus microscope (Olympus, Tokyo, Japan).
Flow cytometry
A549 cells undergoing apoptosis were digested with
parenzyme at the indicated time-points, and were then washed twice
with PBS prior to being resuspended in Annexin binding buffer. A549
cell apoptosis was investigated following the addition of propidium
iodide and FITC-conjugated Annexin V, according to the
manufacturer's protocol. The results were analyzed by flow
cytometry (EXP032; Beckman Coulter, Brea, CA, USA).
Western blot analysis
Right upper lung tissues and A549 cells were lysed
with protein extraction reagent (P0013; Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. Protein
concentration was determined using BCA protein quantification kits
(ab102536; Abcam). Proteins (30 µg proteins in each group) were
separated by 12% gradient SDS-PAGE and were transferred to
polyvinylidene fluoride membranes. Nonspecific binding was blocked
with 10% non-fat dry milk in Tris-buffered saline (TBS) at room
temperature for 1 h. The membranes were then incubated overnight at
4°C with anti-PERK (1:1,000 dilution), anti-p-PERK (1:1,000
dilution), anti-GRP78 (1:1,000 dilution), anti-JNK (1:1,000
dilution), anti-p-JNK (1:1,000 dilution), anti-CHOP (1:1,000
dilution), anti-IRE1α (1:1,000 dilution), anti-p-IRE1α (1:1,000
dilution), anti-p-50ATF6α (1:1,000 dilution), anti-cleaved
caspase-3 (1:1,000 dilution) and anti-GAPDH (1:1,000 dilution;
ab8245; Abcam). After washing with TBS containing 20% Tween-20 (20%
Tween-20: TBS =2.6:1,000), the membranes were incubated with an
HRP-labeled goat anti-rabbit IgG secondary antibody (1:7,500
dilution; A0208; Beyotime Institute of Biotechnology) at room
temperature for 1.5 h. The blots were visualized using an enhanced
chemiluminescent detection system (Bio-Rad, Hercules, CA, USA).
TUNEL detection of lung cell
apoptosis
TUNEL assay was used to detect apoptotic cells in
rat lung tissues via an In Situ Cell Death Detection kit
(MK500; Takara, Otsu, Japan) according to the manufacturer's
protocol. Briefly, sections were incubated with proteinase K for 30
min after dewaxing and rehydration. TUNEL staining results were
analyzed by imageJ software. The sections were then incubated with
TUNEL solution for 60 min and were incubated with alkaline
phosphatase conversion solution for 30 min. Apoptotic cells were
detected by incubation with 3,3′-diaminobenzidine
tetrahydrochloride chromogen for 20 min, and the results were
analyzed using a digital imaging system.
Statistical analysis
All data are presented as the means ± standard error
of the mean, and each experiment was performed at least three
times. Multiple groups were compared using one-way analysis of
variance followed by a Tukey's test. Statistical analyses were
conducted using GraphPad Prism software version 5.01 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
SW exposure induces pulmonary edema,
histological alterations and apoptosis in rat lungs
To evaluate the degree of pulmonary edema induced by
SW aspiration, lung W/D ratios were calculated following various
durations of SW exposure. The W/D ratio was higher in the SW group
compared with in the normal group. This difference was significant
at 2 h and then gradually decreased (Fig. 1A). To investigate alterations in
the structure of pulmonary tissue following SW aspiration,
histological analysis was performed. The results indicated that
disruption of the lung alveolar architecture occurred in response
to SW aspiration; this disruption peaked at 4 h and then gradually
decreased (Fig. 1B).
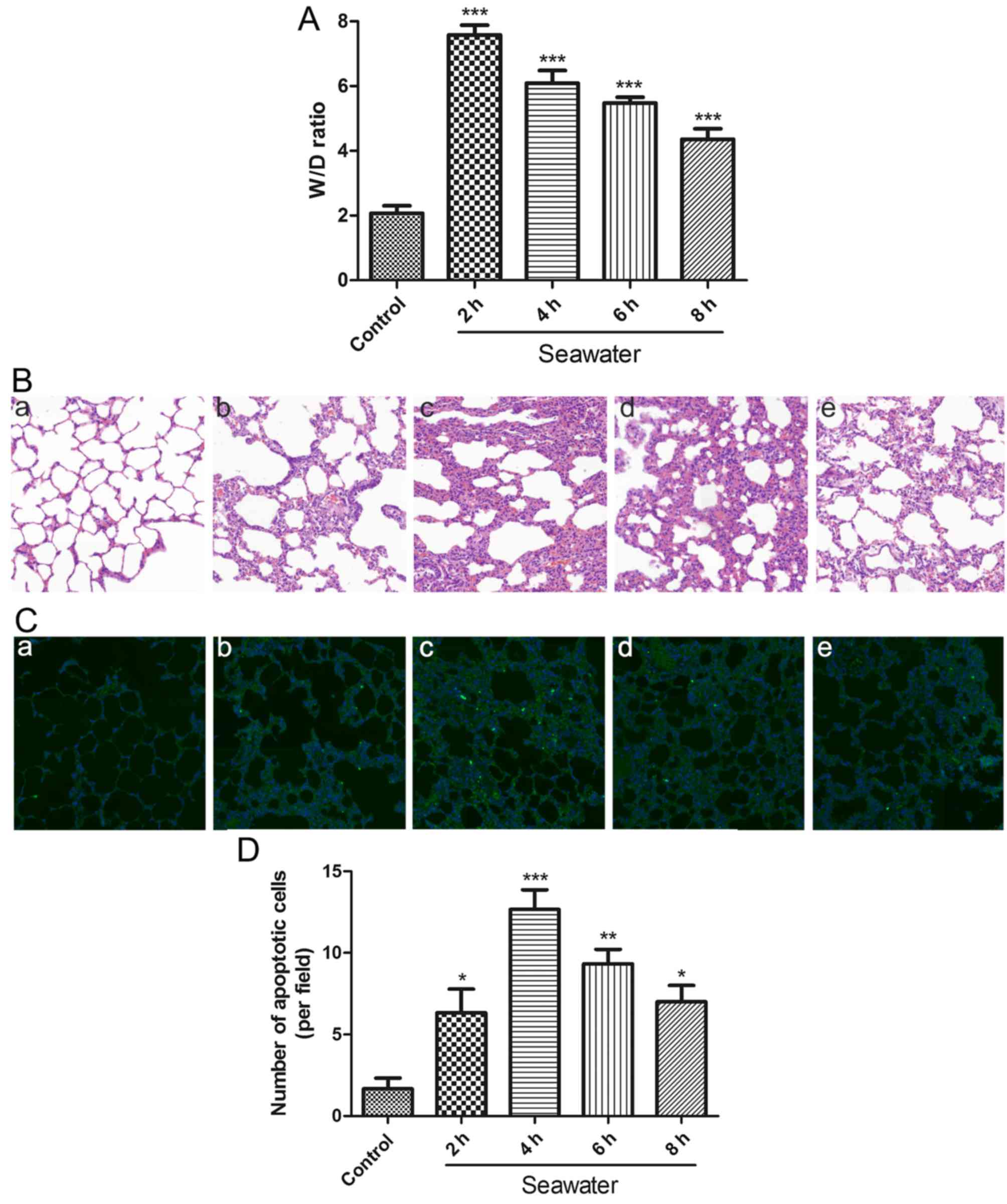 | Figure 1SW induces pulmonary edema,
histopathological alterations and apoptosis in rat lungs. (A)
Time-dependent effects of SW on lung W/D ratio (n=5). (B)
Time-dependent effects of SW on lung histopathological alterations
in rats (hematoxylin and eosin staining; original magnification,
×20). (a) Control, and (b) 2 h, (c) 4 h, (d) 6 h and (e) 8 h SW
groups. (C) SW aspiration-induced apoptosis in rat lungs [TUNEL
assay staining; original magnification, ×20). (a) Control, and (b)
2 h, (c) 4 h, (d) 6 h and (e) 8 h SW groups. (D) Number of
apoptotic cells per field in (C). Data are presented as the means ±
standard error of the mean, n=5. *P<0.05,
**P<0.01 and ***P<0.001 vs. the control
group. W/D, wet/dry; SW, seawater. |
In order to assess whether SW aspiration induced
apoptosis in rat lungs, lung tissue sections were stained according
to the TUNEL assay. The degree of apoptosis clearly paralleled the
histological alterations (Fig. 1C and
D). These results suggested that SW aspiration may induce edema
and ALI in rat lungs, which may be associated with apoptosis.
SW inhibits cell growth and induces
apoptosis of A549 cells
To investigate the cytotoxic effects of SW on A549
cells, the CCK-8 assay was used to evaluate the extent of cell
growth inhibition induced by SW. Notably, following treatment with
20, 40, 60 or 80% SW for 4 h, cell viability decreased in a
dose-dependent manner (Fig. 2A).
Subsequently, A549 cells were treated with 25% SW for 2, 4, 6 or 8
h, and a time-dependent decrease in cell viability was detected
(Fig. 2B). These findings
suggested that SW may induce cell growth inhibition, and that the
cytotoxic effects of SW on A549 cells may be associated with SW
dose and treatment time.
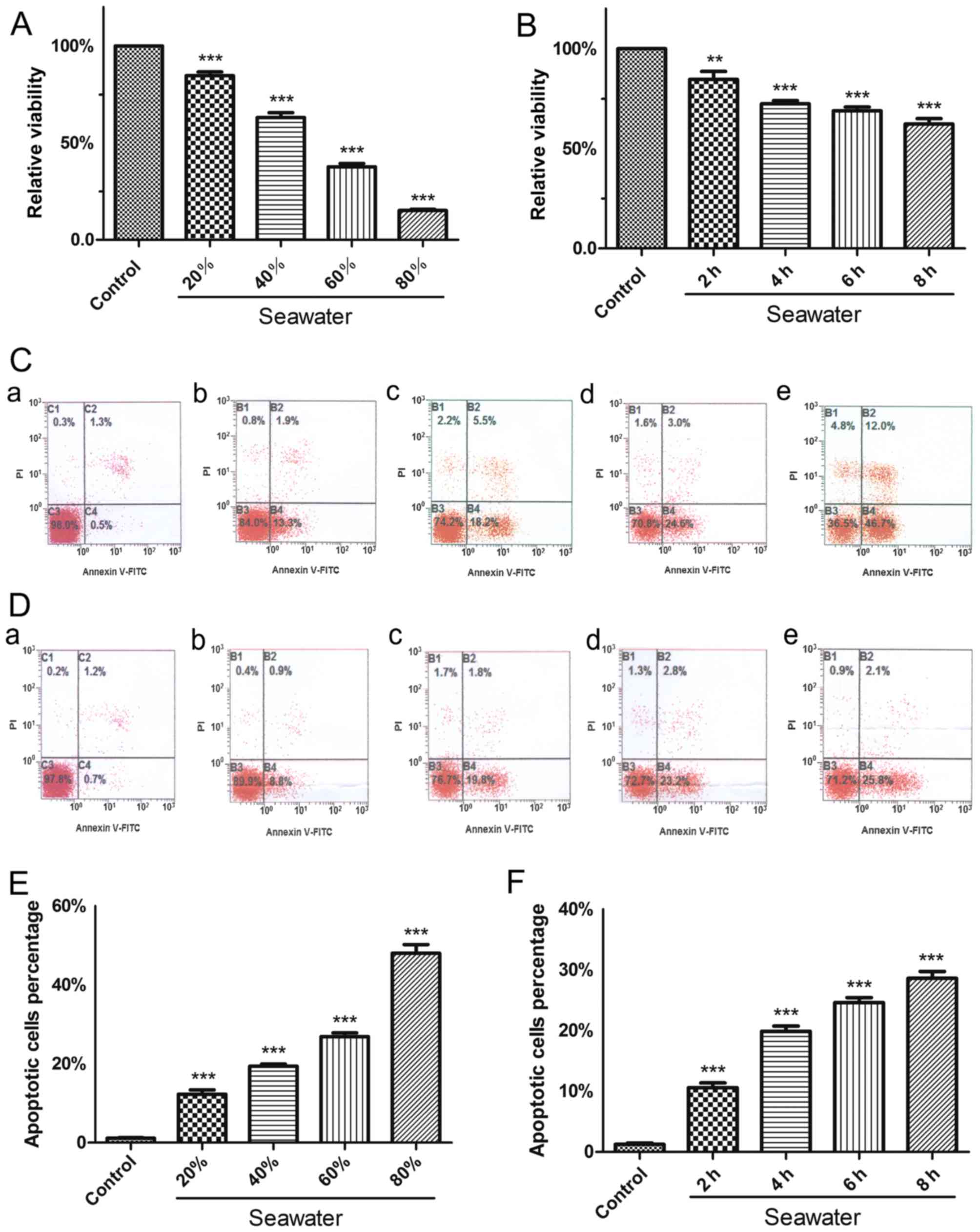 | Figure 2SW inhibits growth and induces
apoptosis of A549 cells. (A) Dose-dependent effects of SW on cell
viability. A549 cells were treated with various concentrations of
SW (20, 40, 60 and 80%) for 4 h, and Cell Counting kit-8 assay was
used to measure cell viability. (B) Time-dependent effects of SW on
cell viability. The cells were treated with 25% SW for various
durations (2, 4, 6 and 8 h), and cell viability was measured as in
(A). (C) Dose-dependent effects of SW on cell apoptosis. A549 cells
were treated as in (A); subsequently, cell apoptosis was assessed
by Annexin V-FITC/PI staining. (a) Control, and (b) 20%, (c) 40%,
(d) 60% and (e) 80% SW groups. (D) Time-dependent effects of SW on
cell apoptosis. A549 cells were treated as in (B), and cell
apoptosis was measured as in (C). (a) Control, and (b) 2 h, (c) 4
h, (d) 6 h and (e) 8 h SW groups. (E) Percentage of apoptotic cells
in (C). (F) Percentage of apoptotic cells in (D). Data are
presented as the means ± standard error of the mean, n=5.
***P<0.001 vs. the control group. FITC, fluorescein
isothiocayanate; PI, propidium iodide; SW, seawater. |
The present study also assessed whether SW had a
proapoptotic effect in vitro (Fig. 2C–F). Following treatment with 20,
40, 60 or 80% SW for 4 h, apoptosis of A549 cells was measured by
flow cytometry. The results indicated that the percentage of
apoptotic cells was increased as the concentration of SW increased
(Fig. 2C and E). Furthermore, a
time-dependent increase in cell apoptosis was detected when cells
were treated with 25% SW for various durations (Fig. 2D and F). The results of the cell
apoptosis and growth inhibition experiments were similar, thus
suggesting that SW induces cell growth inhibition, which may be
associated with apoptosis.
SW induces ROS generation in A549
cells
Previous studies have indicated that numerous
undesirable external stimuli can trigger ROS generation, and that
ROS serve an important role in cell injury and the development of
several diseases (13,14). Therefore, it was predicted that SW
may induce the generation of ROS in A549 cells. To verify this
hypothesis, the present study assessed the levels of ROS using the
DCFH-DA probe following treatment of A549 cells with various
concentrations of SW (20, 40 or 60%) for 4 h, or with 25% SW for
various durations (2, 4, 6 Or 8 H). Subsequently, images were
captured using fluorescence microscopy and were evaluated (Fig. 3). SW significantly increased ROS
levels in a dose-dependent (Fig. 3A
and D) and time-dependent manner (Fig. 3B and E). Notably, SW-induced ROS
generation was markedly impaired when cells were pretreated with
the ROS inhibitor NAC (5 mM) for 2 h (Fig. 3C and F). These results suggested
that SW stimulation can trigger ROS generation, which may affect
cell survival.
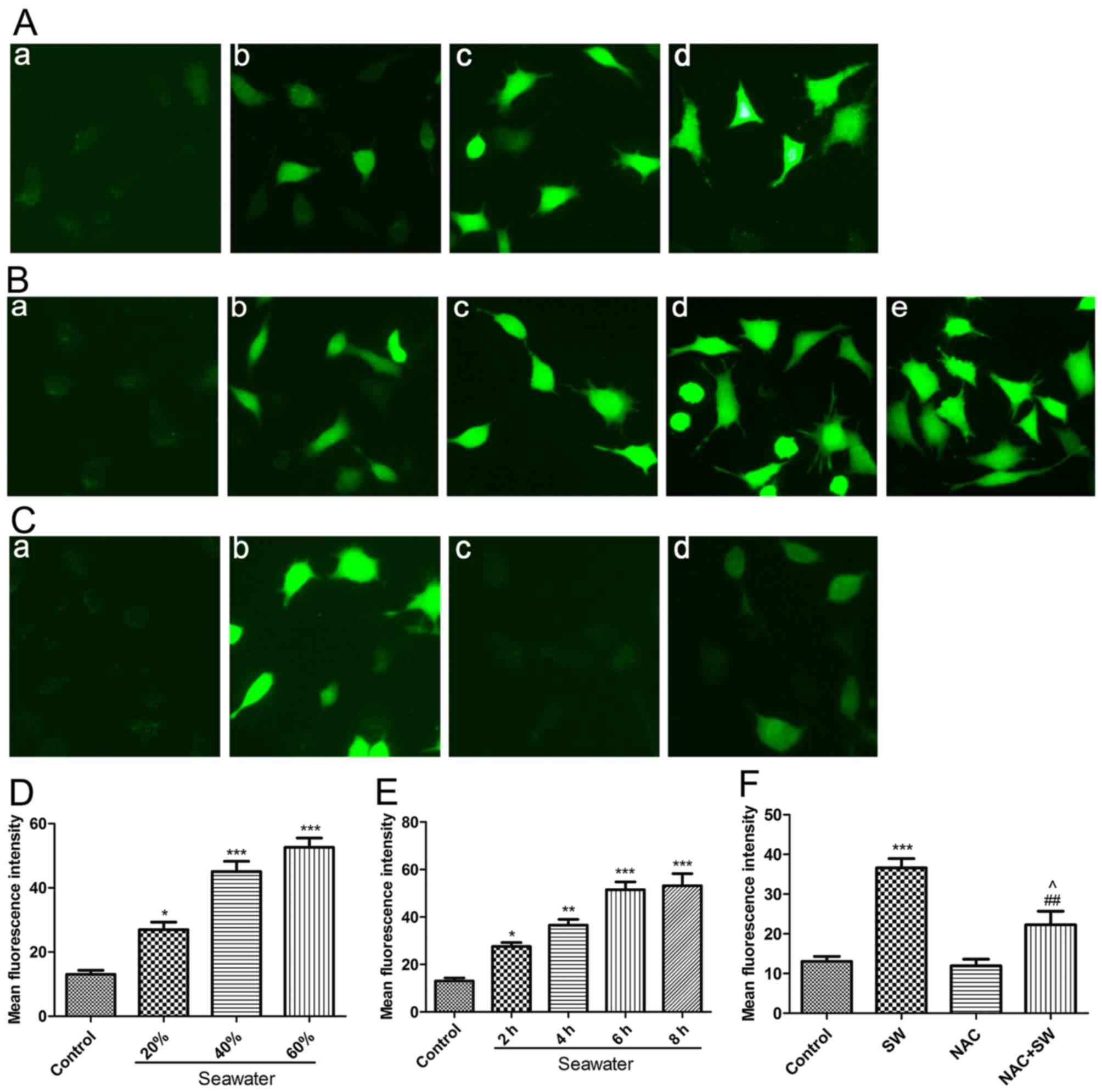 | Figure 3SW triggers ROS generation. (A)
Dose-dependent effects of SW on cellular ROS levels. A549 cells
were treated with the indicated concentrations of SW for 4 h, and
cellular ROS levels were assessed using 2′,7′-dichlorofluorescein
diacetate and were visualized with a fluorescence microscope. (a)
Control, and (b) 20%, (c) 40%, (d) 60% and (e) 80% SW groups
(magnification, ×100). (B) Time-dependent effects of SW on cellular
ROS levels. A549 cells were treated with 25% SW for various
durations, and cellular ROS levels were assessed as described in
(A). (a) Control, and (b) 2 h, (c) 4 h, (d) 6 h and (e) 8 h SW
groups (magnification, ×100). (C) Effects of NAC on SW-induced ROS
generation. Following pretreatment with 5 mM NAC for 2 h, cells
were treated with 25% SW for 4 h and cellular ROS levels were
assessed. (a) Control, (b) SW, (c) NAC and (d) NAC + SW groups
(magnification, ×100). (D) Mean fluorescence intensity of (A) was
analyzed using ImageJ software. (E) Mean fluorescence intensity of
(B) was analyzed using ImageJ software. (F) Mean fluorescence
intensity of (C) was analyzed using ImageJ software. Data are
presented as the means ± standard error of the mean, n=5.
*P<0.05, **P<0.01 and
***P<0.001 vs. the control group; ##P<0.01 vs. the
SW group; and ^P<0.05 vs. the NAC group. NAC,
N-acetyl-L-cysteine; ROS, reactive oxygen species; SW,
seawater. |
ROS are implicated in SW-induced cell
apoptosis and growth inhibition in vivo and in vitro
Previous studies have reported that ROS generation
is closely associated with apoptosis (15,16). Therefore, the present study
investigated whether ROS are involved in SW-induced cell growth
inhibition and apoptosis (Fig.
4). Rats were administered a ROS scavenger, NAC (150 mg/kg),
via intraperitoneal injection 2 h prior to SW inhalation;
subsequently, the levels of cell apoptosis were evaluated using a
TUNEL assay. The results revealed that pretreatment with NAC
attenuated SW-induced apoptosis (Fig.
4A and C). Similarly, using flow cytometry, the results
indicated that pretreatment with NAC (5 mM) for 2 h significantly
alleviated SW-induced apoptosis of A549 cells (Fig. 4B and D). Subsequently, the present
study detected the expression of apoptosis-associated proteins
(caspase-3 and p-JNK) in A549 cells. SW administration
significantly increased the protein expression levels of caspase-3
and p-JNK; however, NAC pretreatment reversed these effects
(Fig. 4E–G). Furthermore, similar
results were detected with regards to SW-induced inhibition of A549
cell growth (Fig. 4H). These
results indicated that ROS generation may be involved in SW-induced
cell apoptosis and growth inhibition.
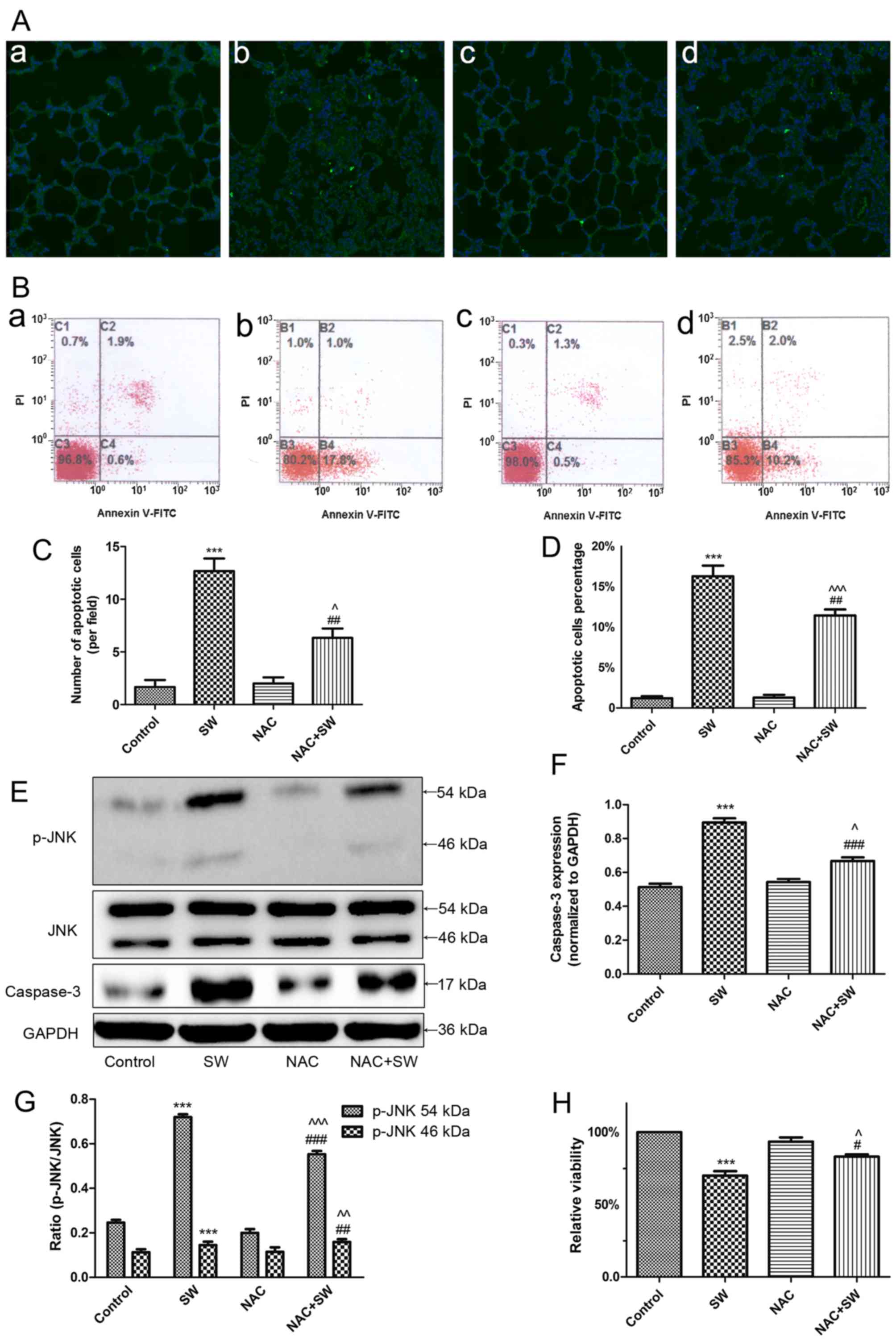 | Figure 4SW-induced growth inhibition and
apoptosis depend on ROS. (A) Effects of NAC (150 mg/kg)
pretreatment on SW-induced apoptosis in rats (TUNEL staining;
magnification, ×10). (B) Effects of NAC (5 mM) on SW-induced
apoptosis of A549 cells (Annexin V-FITC/PI staining). (a) Control,
(b) SW, (c) NAC and (d) NAC + SW groups. (C) Number of apoptotic
cells per field in (A). (D) Percentage of apoptotic cells in (B).
(E-G) Effects of NAC (5 mM) on the expression of the
apoptosis-associated proteins caspase-3 and p-JNK in A549 cells.
(H) Effects of NAC on SW-induced cell growth inhibition (Cell
Counting kit-8 assay). Data are presented as the means ± standard
error of the mean, n=5. ***P<0.001 vs. the control
group; #P<0.05 and ##P<0.01 vs. the SW
group; ^P<0.05, ^^P<0.01 and
^^^P<0.001 vs. the NAC group. FITC, fluorescein
isothiocyanate; JNK, c-Jun N-terminal kinae; NAC,
N-acetyl-L-cysteine; p-JNK, phosphorylated-JNK; PI, propidium
iodide; ROS, reactive oxygen species; SW, seawater. |
SW administration activates ER stress in
vitro and in vivo
To determine whether SW administration can activate
ER stress, the present study detected the expression levels of
GRP78 and CHOP, which are considered essential proteins in the ER
stress response (17-19). The results suggested that as the
length of SW exposure increased, GRP78 and CHOP expression
increased and peaked at 4 h before gradually decreasing in
vivo (Fig. 5A). In
vitro, SW exposure time-dependently increased the expression of
GRP78 and CHOP (Fig. 5B). In
addition, A549 cells were pretreated with an ER stress inhibitor,
4-PBA (2 mM), 2 h prior to SW exposure. Treatment with an ER stress
inducer, Thap (150 nM), for 4 h was used as a positive control,
after which the expression levels of GRP78 and CHOP were detected.
The results revealed that 4-PBA (30 mg/kg) pretreatment clearly
reduced SW-induced expression of GRP78 and CHOP; however, Thap
treatment increased GRP78 and CHOP expression (Fig. 5C). In addition, rats were
pretreated with 4-PBA 2 h prior to SW inhalation and
immunohistochemistry performed to detect GRP78 in rat lungs. 4-PBA
pretreatment reduced SW-induced expression of GRP78 in rat lungs
(Fig. 5D). These results
suggested that SW administration may activate ER stress.
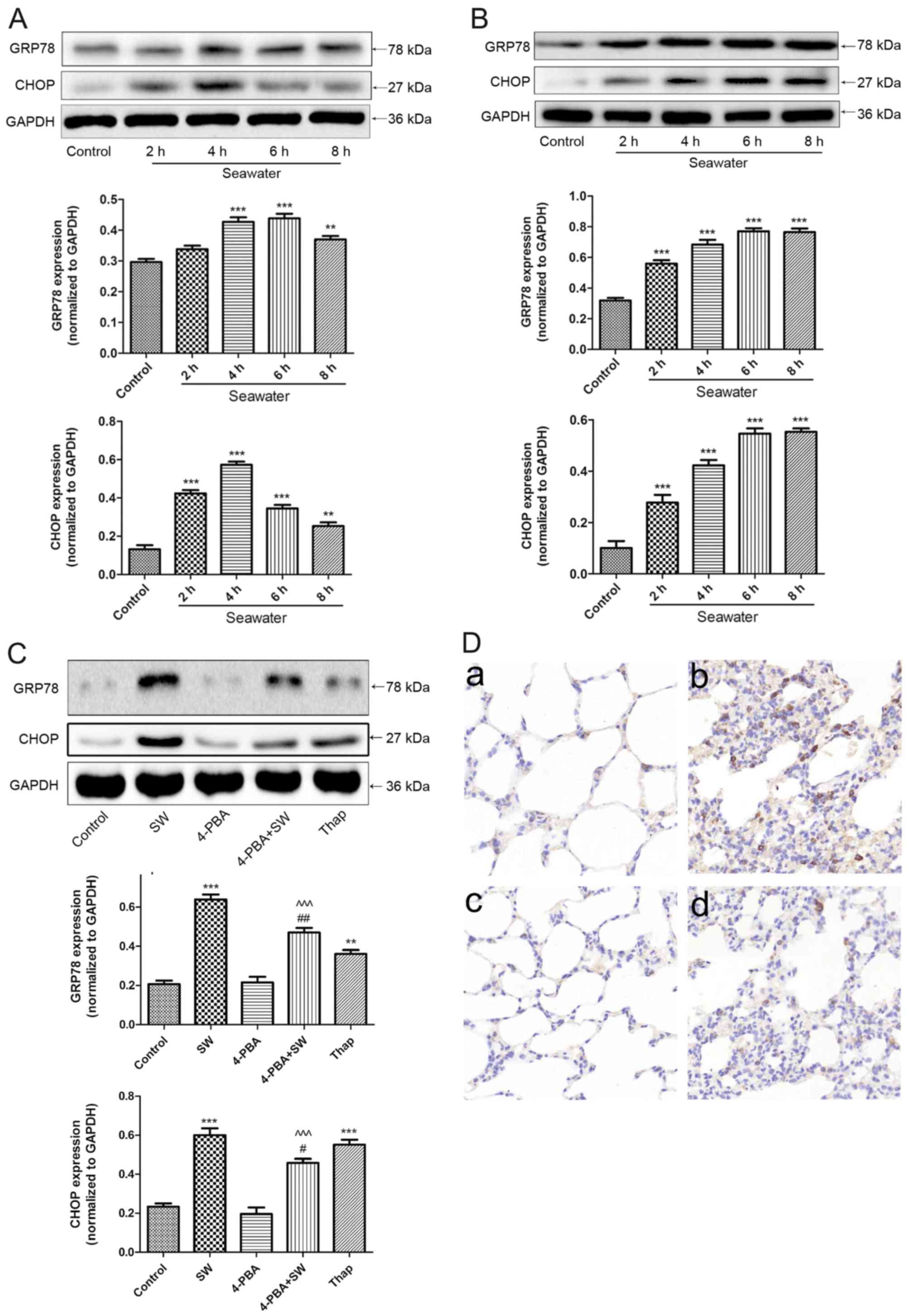 | Figure 5ER stress is activated by SW.
Time-dependent effects of SW on the ER stress-associated proteins
GRP78 and CHOP in (A) rat lungs and (B) A549 cells. A549 cells or
rats were treated with SW for the indicated ti mes, and the
expression levels of GRP78 and CHOP were assessed by western blot
analysis. (C) Effects of ER stress inhibitors or activators on
SW-induced ER stress in A549 cells. Following pretreatment with 2
mM 4-PBA or 150 nM Thap alone as the positive control of SW for 2
h, A549 cells were treated with 25% SW for 4 h, and the expression
of ER stress-associated proteins was assessed. (D) Effects of ER
stress inhibitors on SW-induced ER stress in rat lungs. Following
pretreatment with 4-PBA (30 mg/kg), the rats were treated with SW
for 4 h, and immunohistochemistry was used to detect GRP78
expression in rat lungs. (a) Control, (b) SW, (c) 4-PBA and (d)
4-PBA + SW groups (magnification, ×10). Data are presented as the
means ± standard error of the mean, n=5. **P<0.01 and
***P<0.001 vs. the control group;
#P<0.05 and ##P<0.01 vs. the SW group;
^^^P<0.001 vs. 4-PBA group. 4-PBA, 4-phenylbutyric
acid; CHOP, CCAAT/enhancer binding protein homologous protein; ER,
endoplasmic reticulum; SW, seawater; Thap, thapsigargin. |
ER stress is implicated in SW-induced
cell apoptosis and growth inhibition in vivo and in vitro
Numerous studies have suggested that ER stress is
involved in several cellular activities, particularly apoptosis
(20-22). Therefore, the present study
investigated whether ER stress is involved in SW-induced cell
injury (Fig. 6). The rats were
intraperitoneally injected with 4-PBA 2 h prior to SW inhalation,
after which cell apoptosis was evaluated using a TUNEL assay. The
results indicated that pretreatment with 4-PBA attenuated
SW-induced apoptosis (Fig. 6A and
C). Similarly, using flow cytometry, pretreatment with 4-PBA (2
mM) for 2 h significantly alleviated SW-induced apoptosis of A549
cells; conversely, Thap, an ER inducer, increased apoptosis
(Fig. 6B and D). Subsequently,
the present study detected the expression of apoptosis-associated
proteins, caspase-3 and p-JNK. Notably, 4-PBA significantly reduced
the expression of apoptosis-associated proteins, whereas Thap
increased their expression (Fig.
6E–G). Furthermore, similar effects were detected on SW-induced
growth inhibition (Fig. 6H).
Collectively, these results revealed that SW-induced cell growth
inhibition and apoptosis in rat lung tissues and A549 cells are at
least somewhat dependent on ER stress signaling pathways.
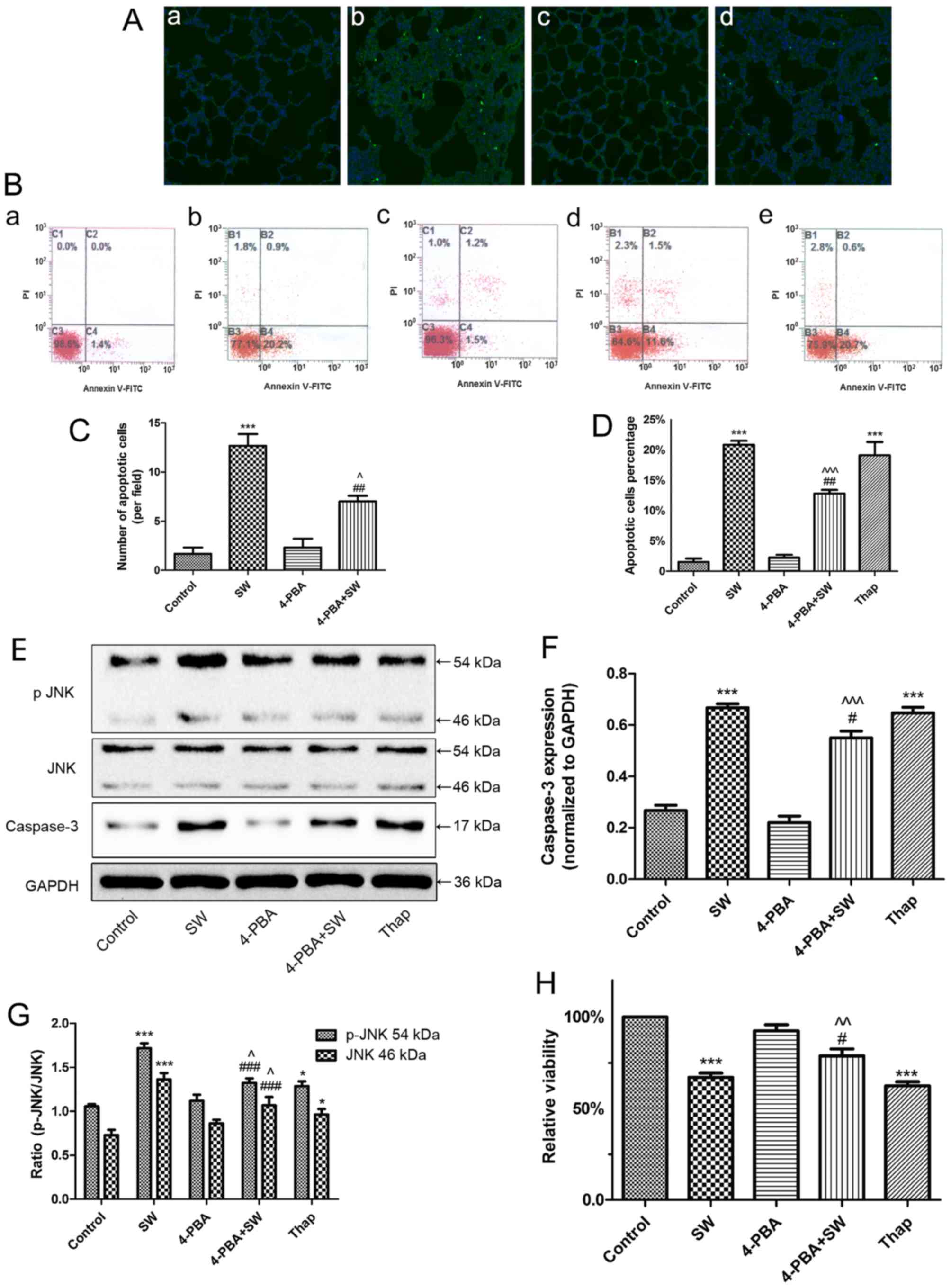 | Figure 6SW-induced cell apoptosis and growth
inhibition are dependent on endoplasmic reticulum stress. (A)
Effects of 4-PBA (30 mg/kg) on SW-induced apoptosis in rats (TUNEL
staining). (a) Control, (b) SW, (c) 4-PBA and (d) 4-PBA + SW groups
(magnification, ×10). (B) Effects of 4-PBA (2 mM) or Thap (150 nM)
on SW-induced apoptosis of A549 cells (Annexin V-FITC/PI staining).
(a) Control, (b) SW, (c) 4-PBA, (d) 4-PBA + SW and (e) Thap groups.
(C) Number of apoptotic cells per field in (A). (D) Percentage of
apoptotic cells in (B). (E-G) Effects of 4-PBA or Thap on the
expression of apoptosis-associated proteins caspase-3 and p-JNK.
(H) Effects of 4-PBA or Thap on SW-induced cell growth inhibition
(Cell Counting kit-8 assay). Data are presented as the means ±
standard error of the mean, n=5. *P<0.05 and
***P<0.001 vs. the control group;
#P<0.05, ##P<0.01 and
###P<0.001 vs. the SW group; ^P<0.05,
^^P<0.01 and ^^^P<0.001 vs. the 4-PBA
group. 4-PBA, 4-phenylbutyric acid; FITC, fluorescein
isothiocyanate; JNK, c-Jun N-terminal kinae; p-JNK,
phosphorylated-JNK; PI, propidium iodide; SW, seawater; Thap,
thapsigargin. |
SW administration activates ROS and ER
stress, which interact to induce cell damage
To investigate the association between ER stress and
SW-induced ROS generation, A549 cells were pretreated with a ROS
scavenger, NAC (5 mM), 2 h prior to SW exposure and the expression
levels of ER stress-associated proteins, GRP78 (Fig. 7A), p-50ATF-6α (Fig. 7B), p-IRE1α (Fig. 7C), p-PERK (Fig. 7D) and CHOP (Fig. 7E), were detected. The SW-induced
expression of these proteins was attenuated following NAC
pretreatment, thus suggesting that SW-induced ROS generation may
activate ER stress.
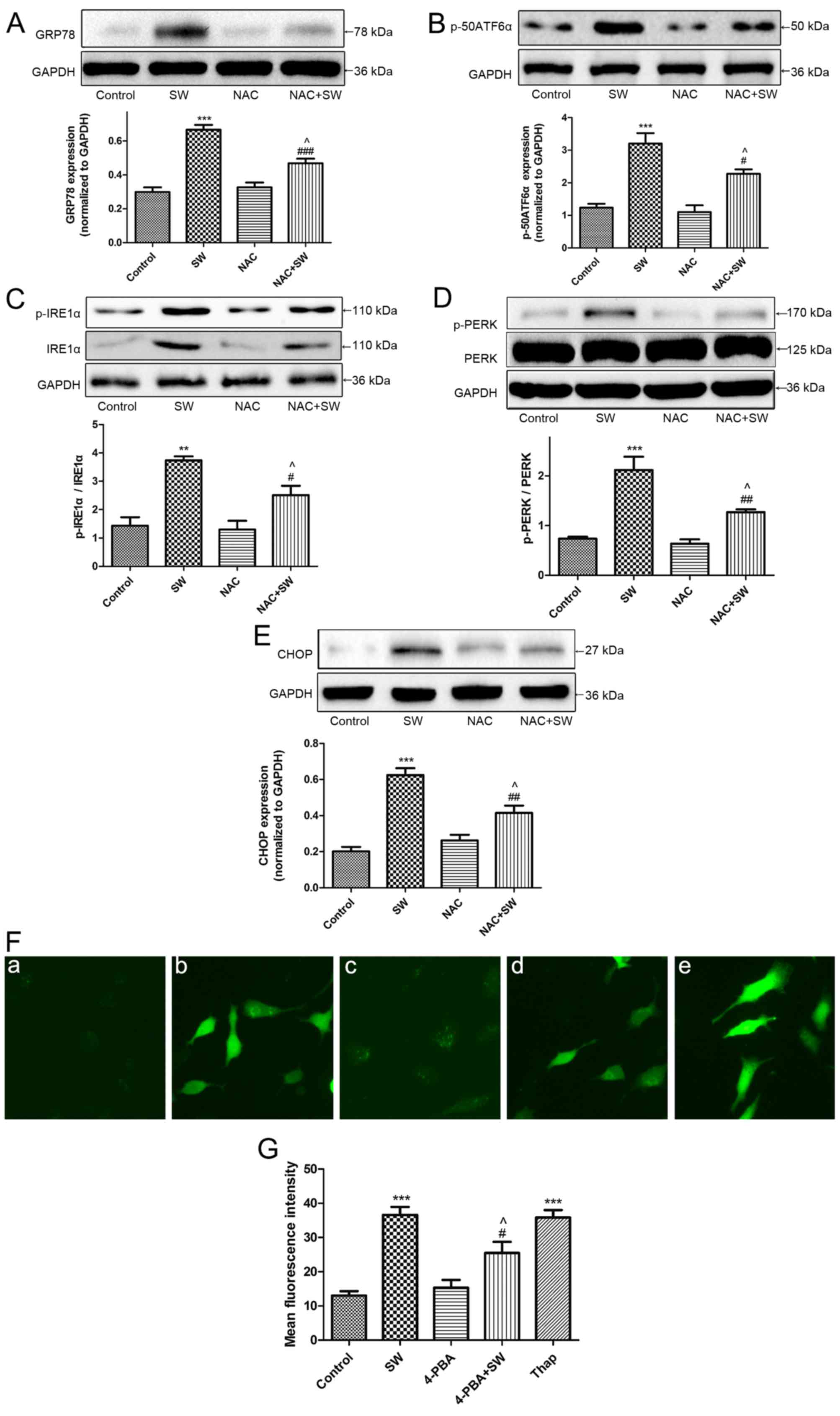 | Figure 7SW induces ROS and ER stress, which
interact with each other. (A-E) Effects of NAC on SW-induced ER
stress. Following pretreatment with 5 mM NAC for 2 h, cells were
treated with 25% SW for 4 h; then, the expression of the ER
stress-associated proteins GRP78, p-50ATF6α, p-IRE1α, p-PERK and
CHOP was evaluated by western blot analysis. (F) Effects of 4-PBA
or Thap on SW-induced ROS generation. Following pretreatment with 2
mM 4-PBA or 150 nM Thap for 2 h, cells were treated with 25% SW for
4 h, and cellular ROS levels were assessed using
2′,7′-dichlorofluorescein diacetate and visualized with a
fluorescence microscope (magnification, ×100). (G) Mean
fluorescence intensity of (F) was analyzed using ImageJ software.
(a) Control, (b) SW, (c) 4-PBA and (d) 4-PBA + SW groups. Data are
presented as the means ± standard error of the mean, n=5.
**P<0.01 and ***P<0.001 vs. the control
group; #P<0.05, ##P<0.01 and
###P<0.001 vs. the SW group; ^P<0.05
vs. the NAC or 4-PBA group. 4-PBA, 4-phenylbutyric acid; ATF6α,
activating transcription factor 6α; CHOP, CCAAT/enhancer binding
protein homologous protein; ER, endoplasmic reticulum; GRP78,
glucose-regulated protien 78; IRE1α, inositol-requiring kinase 1α;
NAC, N-acetyl-L-cysteine; p-, phosphorylated; PERK, protien kinase
R-like ER kinase; SW, seawater; Thap, thapsigargin. |
The present study also pretreated A549 cells with an
ER stress inhibitor, 4-PBA (2 mM), prior to SW exposure, and with
an ER stress inducer Thap (150 nM) as a positive control;
subsequently, the levels of cellular ROS were detected. Notably,
ROS generation was attenuated by 4-PBA pretreatment, whereas it was
enhanced by Thap treatment (Fig. 7F
and G) revealing that ER stress is involved in ROS generation
in response to SW administration. These results indicated that
SW-induced ER stress and ROS generation may interact with each
other to induce cell injury.
Discussion
Drowning has gradually become one of the most common
causes of accidental death worldwide (1). Drowning victims experience hypoxia,
because SW inhalation obstructs the airway. In addition, SW with
high osmolality can directly damage alveolar epithelial cells.
These factors induce ALI or ARDS. The present study demonstrated
that SW inhalation could induce apoptosis in vivo and in
vitro. The results indicated that SW exposure induced cell
apoptosis and growth inhibition, which are key factors in SW-ALI
and SW-ARDS, via ROS generation and ER stress pathways. These
findings were supported by the following: i) SW dose- and
time-dependently inhibited A549 cell growth and induced cell
apoptosis; furthermore, SW inhalation increased the W/D ratio of
rat lungs and induced histopathological alterations; ii) SW
exposure stimulated ROS production, and inhibiting ROS generation
with NAC significantly improved SW-induced apoptosis and growth
inhibition; and iii) SW administration induced ER stress, whereas
suppressing ER stress with 4-PBA alleviated SW-induced apoptosis
and cell growth inhibition.
ALI is a syndrome that results from acute pulmonary
inflammation, which is accompanied by epithelial cell damage and
increased pulmonary effusion, thus resulting in pulmonary edema and
respiratory failure (1,23,24). The present study demonstrated that
SW inhalation was able to induce marked histopathological
alterations in rat lungs, which peaked at 4 h. Furthermore, SW
administration induced pulmonary edema, which was evidenced by
measurement of the lung W/D ratio. SW exposure also inhibited A549
cell growth in vitro. Numerous studies have suggested that
alveolar epithelial cell apoptosis serves an important role in the
pathological process of ALI/ARDS (25-27). In the present in vitro
study, the number of apoptotic cells exhibited a time-dependent
increase when A549 cells were treated with SW. In addition, the
number of apoptotic cells was increased in SW-treated rats compared
with in control rats, and reached a peak at 4 h. In addition, the
degree of alveolar epithelial cell apoptosis was consistent with
the trend in pathological alterations determined by H&E
staining. These results suggested that SW may induce cell
apoptosis, growth inhibition and histopathological lesions, which
may contribute to the pathological process of SW-ALI.
The term ROS refers to cellular oxygen free
radicals, including superoxide (O2•−),
hydrogen peroxide (H2O2) and hydroxyl radical
(•OH), which can be generated as a result of exposure to
toxic agents and as oxygen byproducts (28,29). As a second messenger, ROS can
regulate cell proliferation, apoptosis and transformation (30,31). Therefore, the present study
assessed whether ROS generation is involved in SW-induced cell
growth inhibition and apoptosis. The present study indicated that
SW dose- and time-dependently increased ROS generation in A549
cells. In addition, suppressing ROS generation by pretreating A549
cells with the ROS scavenger NAC significantly decreased the
expression of p-JNK and caspase-3, which are both important in
triggering apoptosis. Furthermore, NAC pretreatment ameliorated
SW-induced apoptosis and histopathological alterations in rat
lungs. These results suggested that SW inhalation-induced ALI/ARDS
may partially depend on ROS generation.
The ER is an important regulator of protein
synthesis and folding; when stimulated by external stimuli,
misfolded and unfolded proteins accumulate in the ER cavity to
activate the unfolded protein response (UPR), which is an ER stress
response (32,33). There are three ER-resident
transmembrane proteins, namely PERK, ATF6α and IRE1α, which can
initiate the UPR to activate ER stress, and which may be involved
in apoptosis (19,33,34). Previous studies have suggested
that CHOP can be activated by the UPR signaling pathways to induce
apoptosis (35,36). The present study revealed that SW
exposure time-dependently upregulated the expression of the ER
stress marker protein GRP78 in vitro. In addition, the
expression levels of GRP78 were increased in rats with SW
inhalation, and peaked at 4 h. Notably, the alterations in CHOP
expression were consistent with those in GRP78 expression in
vitro and in vivo. Blocking ER stress with an ER stress
inhibitor, 4-PBA, not only decreased the expression of SW-induced
GRP78 and CHOP, but also decreased p-JNK and caspase-3 expression.
Furthermore, 4-PBA pretreatment significantly improved rat lung
cell apoptosis and histopathological alteration (Fig. 5D). These results suggested that
SW-induced apoptosis and cell injury partially depend on ER stress,
which may trigger CHOP and p-JNK and then activate the caspase-3
apoptosis signaling pathway.
Previous studies have suggested that ROS generation
and ER stress are closely related; in particular, excessive
accumulation of intracellular ROS can induce ER stress (35,37). In addition, ER stress can increase
ROS generation (11,37). In the present study, blocking ROS
generation with NAC decreased the expression of GRP78, p-PERK,
p-IRE1α, p-50ATF6α and CHOP, thus suggesting that ROS generation
was an upstream factor in the induction of SW-induced ER stress.
Conversely, blocking ER stress with 4-PBA significantly decreased
intracellular ROS generation. These results indicated that ROS
generation and ER stress interact with each other to induce cell
damage in response to SW exposure; however, the exact underlying
mechanism requires further investigation.
In conclusion, the present study demonstrated that
SW administration can trigger alveolar epithelial cell apoptosis
and growth inhibition to induce ALI/ARDS, which may partially
depend on ROS generation and the ER stress pathway. The involvement
of ROS and the ER stress pathway in SW-ALI provides a potential
clinical treatment strategy. However, the pathogenesis of SW-ALI is
complex, and there are other signaling mechanisms involved in the
regulation of alveolar epithelial cell apoptosis. In addition, the
exact mechanism underlying the interaction between ROS generation
and ER stress requires further investigation.
Acknowledgments
Not applicable.
Notes
[1]
Funding
The present study was supported by research grants
from the National Natural Science Foundation of China (grant no.
81570067).
[2] Availability
of data and materials
We declared that materials described in the
manuscript, including all relevant raw data, will be freely
available to any scientist wishing to use them for non-commercial
purposes, without breaching participant confidentiality.
[3] Authors'
contributions
PCL, YJL, DGM and FGJ conceived and designed the
study. PCL, BRW, CCL, XL, YJL and WSQ performed the experiments.
PCL, BRW and YJL wrote the paper. DGM and FGJ reviewed and edited
the manuscript. All authors read and approved the manuscript.
[4] Ethics
approval and consent to participate
The present study was approved by the Animal Care
and Use Committee of the Fourth Military Medical University (Xi'an,
China).
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
financial interests.
References
|
1
|
Han F, Luo Y, Li Y, Liu Z, Xu D, Jin F and
Li Z: Seawater induces apoptosis in alveolar epithelial cells via
the Fas/FasL-mediated pathway. Respir Physiol Neurobiol. 182:71–80.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soar J, Deakin CD, Nolan JP, Abbas G,
Alfonzo A, Handley AJ, Lockey D, Perkins GD and Thies K; European
Resuscitation Council: European Resuscitation Council guidelines
for resuscitation 2005. Section 7. Cardiac arrest in special
circumstances. Resuscitation. 67(Suppl 1): S135–S170. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ibsen LM and Koch T: Submersion and
asphyxial injury. Crit Care Med. 30(Suppl 11): S402–S408. 2002.
View Article : Google Scholar
|
|
4
|
Matsuda N, Yamamoto S, Takano K, Kageyama
S, Kurobe Y, Yoshihara Y, Takano Y and Hattori Y: Silencing of
fas-associated death domain protects mice from septic lung
inflammation and apoptosis. Am J Respir Crit Care Med. 179:806–815.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kitamura Y, Hashimoto S, Mizuta N,
Kobayashi A, Kooguchi K, Fujiwara I and Nakajima H:
Fas/FasL-dependent apoptosis of alveolar cells after
lipopolysaccharide-induced lung injury in mice. Am J Respir Crit
Care Med. 163:762–769. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li JH, Xu M, Xie XY, Fan QX, Mu DG, Zhang
Y, Cao FL, Wang YX, Zhao PT, Zhang B, et al: Tanshinone IIA
suppresses lung injury and apoptosis, and modulates protein kinase
B and extracellular signal-regulated protein kinase pathways in
rats challenged with seawater exposure. Clin Exp Pharmacol Physiol.
38:269–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li ZY, Yang Y, Ming M and Liu B:
Mitochondrial ROS generation for regulation of autophagic pathways
in cancer. Biochem Biophys Res Commun. 414:5–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mazure NM and Pouysségur J:
Hypoxia-induced autophagy: Cell death or cell survival. Curr Opin
Cell Biol. 22:177–180. 2010. View Article : Google Scholar
|
|
9
|
Joyce MA, Walters KA, Lamb SE, Yeh MM, Zhu
LF, Kneteman N, Doyle JS, Katze MG and Tyrrell DL: HCV induces
oxidative and ER stress, and sensitizes infected cells to apoptosis
in SCID/Alb-uPA mice. PLoS Pathog. 5:e10002912009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park IJ, Yang WK, Nam SH, Hong J, Yang KR,
Kim J, Kim SS, Choe W, Kang I and Ha J: Cryptotanshinone induces G1
cell cycle arrest and autophagic cell death by activating the
AMP-activated protein kinase signal pathway in HepG2 hepatoma.
Apoptosis. 19:615–628. 2014. View Article : Google Scholar
|
|
11
|
Malhotra JD and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress: A vicious cycle or a
double-edged sword. Antioxid Redox Signal. 9:2277–2293. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Institutes of Health (NIH)
publication No 85-23, revised in 1985
|
|
13
|
Lu TH, Su CC, Tang FC, Chen CH, Yen CC,
Fang KM, Lee I, Hung DZ and Chen YW: Chloroacetic acid triggers
apoptosis in neuronal cells via a reactive oxygen species-induced
endoplasmic reticulum stress signaling pathway. Chem Biol Interact.
225:1–12. 2015. View Article : Google Scholar
|
|
14
|
Ott M, Gogvadze V, Orrenius S and
Zhivotovsky B: Mitochondria, oxidative stress and cell death.
Apoptosis. 12:913–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang WY, Albert CJ and Ford DA:
Alpha-chlorofatty acid accumulates in activated monocytes and
causes apoptosis through reactive oxygen species production and
endoplasmic reticulum stress. Arterioscler Thromb Vasc Biol.
34:526–532. 2014. View Article : Google Scholar
|
|
16
|
Moungjaroen J, Nimmannit U, Callery PS,
Wang L, Azad N, Lipipun V, Chanvorachote P and Rojanasakul Y:
Reactive oxygen species mediate caspase activation and apoptosis
induced by lipoic acid in human lung epithelial cancer cells
through Bcl-2 downregulation. J Pharmacol Exp Ther. 319:1062–1069.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou Y, Shu F, Liang X, Chang H, Shi L,
Peng X, Zhu J and Mi M: Ampelopsin induces cell growth inhibition
and apoptosis in breast cancer cells through ROS generation and
endoplasmic reticulum stress pathway. PLoS One. 9:e890212014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Quick QA and Faison MO: CHOP and caspase 3
induction underlie glioblastoma cell death in response to
endoplasmic reticulum stress. Exp Ther Med. 3:487–492. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han J, Back SH, Hur J, Lin YH,
Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M,
et al: ER-stress-induced transcriptional regulation increases
protein synthesis leading to cell death. Nat Cell Biol. 15:481–490.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Syed DN, Lall RK, Chamcheu JC, Haidar O
and Mukhtar H: Involvement of ER stress and activation of apoptotic
pathways in fisetin induced cytotoxicity in human melanoma. Arch
Biochem Biophys. 563:108–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou CH, Lin FL, Hou SM and Liu JF:
Hyperthermia induces apoptosis through endoplasmic reticulum and
reactive oxygen species in human osteosarcoma cells. Int J Mol Sci.
15:17380–17395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gropper MA and Wiener-Kronish J: The
epithelium in acute lung injury/acute respiratory distress
syndrome. Curr Opin Crit Care. 14:11–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Flecknoe S, Harding R, Maritz G and Hooper
SB: Increased lung expansion alters the proportions of type I and
type II alveolar epithelial cells in fetal sheep. Am J Physiol Lung
Cell Mol Physiol. 278:L1180–L1185. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perl M, Lomas-Neira J, Chung CS and Ayala
A: Epithelial cell apoptosis and neutrophil recruitment in acute
lung injury-a unifying hypothesis? What we have learned from small
interfering RNAs. Mol Med. 14:465–475. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Imazu Y, Yanagi S, Miyoshi K, Tsubouchi H,
Yamashita S, Matsumoto N, Ashitani J, Kangawa K and Nakazato M:
Ghrelin ameliorates bleomycin-induced acute lung injury by
protecting alveolar epithelial cells and suppressing lung
inflammation. Eur J Pharmacol. 672:153–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyake Y, Kaise H, Isono K, Koseki H,
Kohno K and Tanaka M: Protective role of macrophages in
noninflammatory lung injury caused by selective ablation of
alveolar epithelial type II cells. J Immunol. 178:5001–5009. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sen CK and Packer L: Antioxidant and redox
regulation of gene transcription. FASEB J. 10:709–720. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Al-Mehdi AB, Pastukh VM, Swiger BM, Reed
DJ, Patel MR, Bardwell GC, Pastukh VV, Alexeyev MF and Gillespie
MN: Perinuclear mitochondrial clustering creates an oxidant-rich
nuclear domain required for hypoxia-induced transcription. Sci
Signal. 5:ra472012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dalton TP, Shertzer HG and Puga A:
Regulation of gene expression by reactive oxygen. Annu Rev
Pharmacol Toxicol. 39:67–101. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Azad MB, Chen Y and Gibson SB: Regulation
of autophagy by reactive oxygen species (ROS): Implications for
cancer progression and treatment. Antioxid Redox Signal.
11:777–790. 2009. View Article : Google Scholar
|
|
32
|
Feldman DE, Chauhan V and Koong AC: The
unfolded protein response: A novel component of the hypoxic stress
response in tumors. Mol Cancer Res. 3:597–605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mandl J, Mészáros T, Bánhegyi G and Csala
M: Minireview: Endoplasmic reticulum stress: Control in protein,
lipid, and signal homeostasis. Mol Endocrinol. 27:384–393. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Szegezdi E, Fitzgerald U and Samali A:
Caspase-12 and ER-stress-mediated apoptosis: The story so far. Ann
NY Acad Sci. 1010:186–194. 2003. View Article : Google Scholar
|
|
35
|
McCullough KD, Martindale JL, Klotz LO, Aw
TY and Holbrook NJ: Gadd153 sensitizes cells to endoplasmic
reticulum stress by downregulating Bcl2 and perturbing the cellular
redox state. Mol Cell Biol. 21:1249–1259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Woo KJ, Lee TJ, Lee SH, Lee JM, Seo JH,
Jeong YJ, Park JW and Kwon TK: Elevated gadd153/chop expression
during resveratrol-induced apoptosis in human colon cancer cells.
Biochem Pharmacol. 73:68–76. 2007. View Article : Google Scholar
|
|
37
|
Pierre AS, Minville-Walz M, Fèvre C,
Hichami A, Gresti J, Pichon L, Bellenger S, Bellenger J,
Ghiringhelli F, Narce M, et al: Trans-10, cis-12 conjugated
linoleic acid induced cell death in human colon cancer cells
through reactive oxygen species-mediated ER stress. Biochim Biophys
Acta. 1831:759–768. 2013. View Article : Google Scholar : PubMed/NCBI
|





















