Introduction
Osteoblasts serve an important role in bone
formation and remodeling, which is active throughout life (1). It has previously been demonstrated
that the differentiation of osteoblasts is likely to affect bone
formation (2). Deficiencies in
osteoblast differentiation and function have been reported to be
involved in various pathologies, including osteoporosis, delayed
bone fracture healing and osteonecrosis (3,4).
Mineralization of the extracellular matrix represents the terminal
stage of osteoblast differentiation and is thought to be a sign of
osteoblast maturation (5).
Adenosine monophosphate (AMP)-activated protein
kinase (AMPK) is a heterotrimeric complex that is comprised of a
catabolic α subunit, and regulatory β and γ subunits; the isoforms
of these subunits are widely expressed in various tissues,
including bone (6). The
activation of AMPK turns on catabolism and turns off anabolism, and
it a key sensor of energy homeostasis (7). The activation of AMPK can be
mediated by AMP, or its upstream kinases, including the tumor
suppressor liver kinase B1 and calmodulin kinase kinase (8). Physiological stressors, including
low nutrient levels, prolonged exercise or pharmacological inducers
[5-aminoimdazole-4-car boxamide-1-β-D-ribofuranoside (AICAR) and
metformin] can lead to the activation of AMPK. The AMPK pathway
coordinates cell growth, autophagy and metabolism via transcription
and direct effects on metabolic enzymes (9). Previous studies have confirmed that
activation of AMPK positively regulates osteoblast differentiation
and mineralization via numerous pathways, for example, by
inhibition of the mevalonate pathway and stimulation of endothelial
nitric oxide synthase (eNOS) and bone morphogenetic protein (BMP)-2
expression, and by induction of Dlx5-dependent runt-related
transcription factor 2 (Runx2) expression (10,11).
Autophagy refers to a cell degradation process
whereby cytoplasmic materials, including aggregates, long-lived
proteins and damaged organelles, are transported to lysosomes for
degradation and the content is recycled (12). Autophagy has a critical role in
physiological conditions, and autophagic deficiency is associated
with certain diseases, including infectious diseases, cancer and
neurodegeneration (13).
Autophagy in osteoblasts is involved in mineralization and bone
homeostasis; notably, autophagic vacuoles have been reported to act
as vehicles for secretion of mineralization matrix (1). The UNC-51-like autophagy activating
kinase (ULK) complex, which consists of ULK-1/2, autophagy-related
protein (ATG)-13, ATG-101 and FIP200, initiates the autophagy
process (14,15), and the mammalian target of
rapamycin (mTOR) has been recognized as a major pathway that
regulates autophagy (16).
Furthermore, it has been suggested that AMPK can induce autophagy
by directly activating ULK1 or via the inhibition of mTOR (9). However, the association between
AMPK, autophagy, and osteoblast differentiation and mineralization
remains to be fully elucidated.
The present study aimed to explore whether
activation of AMPK could enhance osteoblast differentiation and
mineralization via the induction of autophagy.
Materials and methods
Reagents and antibodies
AICAR (cat. no. HY-13417), compound C (cat. no.
HY-13418A), 3-methyladenine (3-MA; cat. no. HY-19312) and
chloroquine diphosphatase (CQ; cat. no. HY-17589) were purchased
from MCE China (Shanghai, China). Ascorbic acid,
β-glycerophosphatase, and reagents used in Masson staining,
Alizarin red staining and electron microscopy sample processing
were obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Anti-AMPKα (rabbit anti-mouse, monoclonal, cat. no. ab32047) and
phosphorylated (p)-AMPKα (rabbit anti-mouse, monoclonal, cat. no.
ab133448 for western blotting, and rabbit anti-mouse, polyclonal,
cat. no. ab194920 for immunohistochemistry), microtubule-associated
proteins 1A/1B light chain 3B (LC3B; rabbit anti-mouse, polyclonal,
cat. no. ab48394), p62 (mouse anti-mouse, monoclonal, cat. no.
ab56416) and β-actin (mouse anti-mouse, monoclonal, cat. no.
ab8226) were purchased from Abcam (Shanghai, China).
Rabbit model of radius nonunion
A total of 24 male New Zealand white rabbits (age,
12 weeks; weight, 2.0-2.5 kg; obtained from Wuhan Wanqianjiahe
Experimental Animals Co Ltd., Wuhan, China; cat. no.
42010000001221) were used in the present study. The animals were
housed individually in rabbit cages under a 12-h light/dark cycle
(on between 7:00 a.m. and 7:00 p.m.) with unrestricted access to
food and water, and under specific pathogen-free (SPF) conditions
at a constant room temperature of 22-24°C. The animal experiments
were conducted according to the National Institutes of Health Guide
for the Care and Use of Laboratory Animals (17), and the present study was approved
by the Wuhan University Zhongnan Hospital Research Committee
(Wuhan, China). Rabbits were randomly assigned into two groups: The
nonunion group and the healing group (n=12 rabbits/group). The
rabbits were anesthetized with an intravenous injection of 30 mg/kg
pentobarbital sodium solution. In the nonunion group, bilateral 20
mm bone defects were created in the middle portion of the radiuses.
For the healing group, 10 mm bone defects were made in the middle
portion of both radiuses (18,19). The normal bone was treated as the
control group. The bone defects in both groups were not filled with
any material (20) and the
incisions were closed using 5-0 sutures. Calluses in the fracture
ends were obtained from three rabbits (sacrificed prior to callus
collection) every 4 weeks following surgery (4, 8, 12 and 16
weeks). After decalcification by 10% EDTA (pH 7.2-7.3) at 37°C for
14-60 days (when the calluses became soft and could be cut using a
knife), the calluses were fixed in 4% paraformaldehyde at room
temperature for 24 h, embedded in paraffin, sectioned (4-mm thick
sections) and examined by Masson staining and immunohistochemical
staining (primary antibodies against AMPK, p-AMPK, LC3B and p62
were employed). Radiographs were captured of each forelimb 16 weeks
post-operation.
Masson staining
The paraffin-embedded sections were deparaffinized
and rehydrated through graded alcohol (100% for 5 min, 95% for 3
min,and 70% for 2 min), and then washed in distilled water for 2
min. Then the sections were stained in Weigert's iron hematoxylin
working solution for 10 min and rinsed under running warm tap water
for 5 min. The sections were then differentiated in 1% hydrochloric
acid (dissolved in alcohol) for 5 sec and rinsed under running warm
tap water for 5 min. The sections were then stained by xylidine
ponceau and acid fuchsin solution for 4 min and rinsed under tap
water for 1 min. Afterwards, the sections were differentiated in 1%
phosphomolybdic acid for 5 min and spin-dried and then stained in
aniline blue solution for 5 min. The sections were rinsed in 1%
glacial acetic acid for 1 min and washed with distilled water. The
sections were then quickly dehydrated through 95% alcohol, absolute
ethyl alcohol and cleared in xylene. Finally, they were mounted
with Permount™ mounting medium and observed under a Nikon E100
microscope (Nikon, Tokyo, Japan).
Immunohistochemistry
Briefly, the sections were cleared in xylene and
dehydrated in ethanol. Antigen retrieval was then performed using
10 mmol/l sodium citrate buffer solution. Afterwards, endogenous
peroxidase was blocked by 3% H2O2 for 15 min.
The sections were then incubated in 10% normal goat serum followed
by incubation with rabbit monoclonal (or polyclonal) primary
antibody overnight at 4°C, and incubated with
horseradish-peroxidase conjugated secondary antibody (goat
anti-rabbit) and washed 3 times with PBS buffer for 5 min each. DAB
was added to the stain and then the slides were immersed and washed
by distilled water. Finally, the sections were dehydrated in 95%
ethanol, 100% ethanol, and xylene and mounted and observed under a
Nikon E100 microscope (Nikon).
Cell culture
Mouse MC3T3-E1 osteoblasts (cat. no. GNM15) were
purchased from Cell Bank of Chinese Academy of Sciences (Shanghai,
China) and were cultured in α-minimum essential medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (Gibco) and 1% penicillin-streptomycin. For
osteogenic differentiation and mineralization, cells were
maintained in medium supplemented with ascorbic acid (50
μg/ml) and β-glycerol phosphatase (10 mM). The medium was
changed every 3 days. Cells were treated with AICAR (10 μm)
in the presence or absence of 3-MA (5 mm) or CQ (10 μm) for
24 h at 37°C. Cells in the control group were treated with PBS.
Transmission electron microscopy
After the indicated time and treatment, MC3T3-E1
cells were fixed with 2.5% glutaraldehyde in 0.1 M sodium
dihydrogen phosphatase (pH 7.4) for 4 h at 4°C and were then fixed
with 1% OsO4 for 1 h at room temperature, followed by
dehydration using graded ethanol solutions and gradual infiltration
with epoxy resin. Ultra-thin sections (60–80 nm) were stained with
uranyl acetate and lead citrate, and were observed under a
transmission electron microscope (Hitachi H-7500; Hitachi, Ltd.,
Tokyo, Japan). In addition, the density of autophagosomes was
calculated using the following formula: Number of autophagic
vacuoles/number of osteoblasts, as previously described (21); the values are presented relative
to the control group.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The mRNA expression levels of alkaline phosphatase
(ALP), osteocalcin (OCN), Runx2 and β-actin were determined by
RT-qPCR using SYBR-Green. Total RNA was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Subsequently, ~1
μg RNA underwent RT, in order to generate single-stranded
cDNA. Total RNA (500 ng) was reverse-transcribed using RevertAid
M-MuLV RT, oligo(dT)18 (cat. no. 639505; Takara, Dalian, China) in
a 20-μl system according to the manufacturer's instructions.
Reactions were incubated at 42°C for 60 min, then 70°C for 5 min
and 16°C hold. The thermal conditions for qPCR (Kapa Biosystems,
Boston, MA, USA) were as follows: 95°C for 5 min, followed by 40
cycles at 94°C for 15 sec, 60°C for 1 min and 70°C 30 sec. The
final extension step was 70°C for 5 min and hold at 4°C. β-actin
was used as the internal control gene. The primer sequences were as
follows: ALP, forward 5′-AACCCAGACACAAGCATTCC-3′, reverse
5′-GAGAGCGAAGGGTCAGTCAG-3′; OCN, forward
5′-TGCTTGTGACGAGCTATCAG-3′, reverse 5′-GAGGACAGGGAGGATCAAGT-3′;
Runx2, forward 5′-AAGTGCGGTGCAAACTTTCT-3′, reverse
5′-TCTCGGTGGCTGGTAGTGA-3′; Beclin 1 (BCN1), forward
5′-GTTGCCGTTATACTGTTCT-3′ and reverse 5′-TTTCCACCTCTTCTTTGA-3′ and
β-actin forward, 5′-CCCATCTACGAGGGCTAT-3′ and reverse,
5′-TGTCACGCACGATTTCC-3′. All reactions were performed in triplicate
and the 2−∆∆Cq method was used to quantify the results
(22).
ALP activity assay
Before the cells were used for ALP activity assay
and mineralization assay, they were cultured for a few days and the
6-well plates had a cell density of about 2.5×106/well.
ALP activity was measured by 5-bromo-4-chloro-3-indolyl phosphate
(BCIP)/nitro blue tetrazolium (NBT) staining. Cells
(2×105/well) cultured in 6-well plates (culture medium,
2 ml/well) were fixed with 4% paraformaldehyde and washed three
times with double-distilled water, after which they were treated
with BCIP/NBT solution (cat. no. C3206; Beyotime Institute of
Biotechnology, Shanghai, China) for 30 min at 37°C; staining images
were then captured using an Olympus IX50 microscope (Olympus,
Tokyo, Japan).
Mineralization assay
Cells (2×105/well) were rinsed three
times with PBS and were fixed with 4% paraformaldehyde for 1 h at
4°C, after which they were washed three times with double-distilled
water and stained with 1% Alizarin red solution (pH 4.3) for 30 min
at room temperature; the dye was removed with water. Images of the
stained cells were then captured using an Olympus IX50 microscope
(Olympus). For quantitative analysis, the stained cultures were
destained with 100 mmol/l cetylpyridinium chloride for 1 h at room
temperature. The absorbance of the released stain was measured at
550 nm and is presented relative to the control group.
Western blot analysis
Cells were rinsed three times with ice-cold PBS and
were lysed in lysis buffer [30 mM Tris-HCl (pH 8.0), 150 mM NaCl,
1% NP-40; Bio-Swamp, Shanghai, China] containing 1 mM
phenylmethylsulfonyl fluoride. Protein concentration was determined
using a bicinchoninic acid assay (cat. no. P0009; Beyotime
Institute of Biotechnology) assay. Protein samples (10 μg)
were separated by 12% SDS-PAGE and electrotransferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% non-fat milk/Tris-buffered
saline containing 0.05% Tween followed by incubation overnight at
4°C with antibodies against total-AMPKα, p-AMPKα, p62 and LC3B
(1:1,000 dilution). Appropriate peroxidase-conjugated secondary
antibodies (goat anti-rabbit IgG; Bio-Swamp) were used to detect
specific antibody binding. Protein bands were visualized using a
chemiluminescence kit (cat. no. 32106; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. ImageJ software
version 1.48 (National Institutes of Health, Bethesda, MD, USA) was
used to semi-quantify band intensity.
Silencing of BCN1
Small interfering (si)RNA targeting mouse BCN1
(siBCN1) and scrambled control siRNA (siControl) were chemically
synthesized by Wuhan Myhalic Biotechnology Co., Ltd. (Wuhan,
China). Cells were plated in 6-well plates at the density of
2×105/well and cultured for 24 h in growth medium
without antibiotics, after which they were transfected with siRNAs
(30 nM) using Lipo fectamine® 2000 reagent (cat. no.
11668027; Invitrogen; Thermo Fisher Scientific, Inc.) and cultured
for 6 h at 37°C. Subsequently, the medium was changed and RT-qPCR
was performed 24, 48 and 72 h post-transfection, in order to assess
the silencing effect; the most efficient siRNA was thus selected. A
total of 24 h post-transfection using the selected siRNA,
differentiation medium was added and the cells were treated with or
without AICAR (10 μm) for 8 days at 37°C. After 8 days,
total RNA was collected from each group and RT-qPCR was performed
to relatively quantify the expression levels of ALP, OCN and Runx2.
In addition, ALP activity was assessed 8 days post-transfection.
The siRNA sequences were as follows: siBCN1-I, sense
5′-GAUGGUGUCUCUCGAAGAUTT-3′, antisense 5′-AUCUUCGAGAGACACCAUCTT-3′;
siBCN1-II, sense 5′-GGCACAAUCAAUAAUUUCATT-3′, antisense
5′-UGAAAUUAUUGAUUGUGCCTT-3′; siBCN1-III, sense
5′-GGAGUGGAAUGAAAUCAAUTT, antisense 5′-AUUGAUUUCAUUCCACUCCTT-3′;
and siControl, sense 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′. The silencing effect of the selected
siRNA was verified by RT-qPCR and western blot analysis at 24, 48
and 72 h post-transfection.
Statistical analysis
All experiments were repeated at least three times
and the results are expressed as the means ± standard error of the
mean. The statistical analysis was performed using SPSS 19.0
software. The statistical analysis of differences between groups
was evaluated by Student's t-test, or one-way analysis of variance
followed by Fisher's least significant difference test. P<0.05
was considered to indicate a statistically significant
difference.
Results
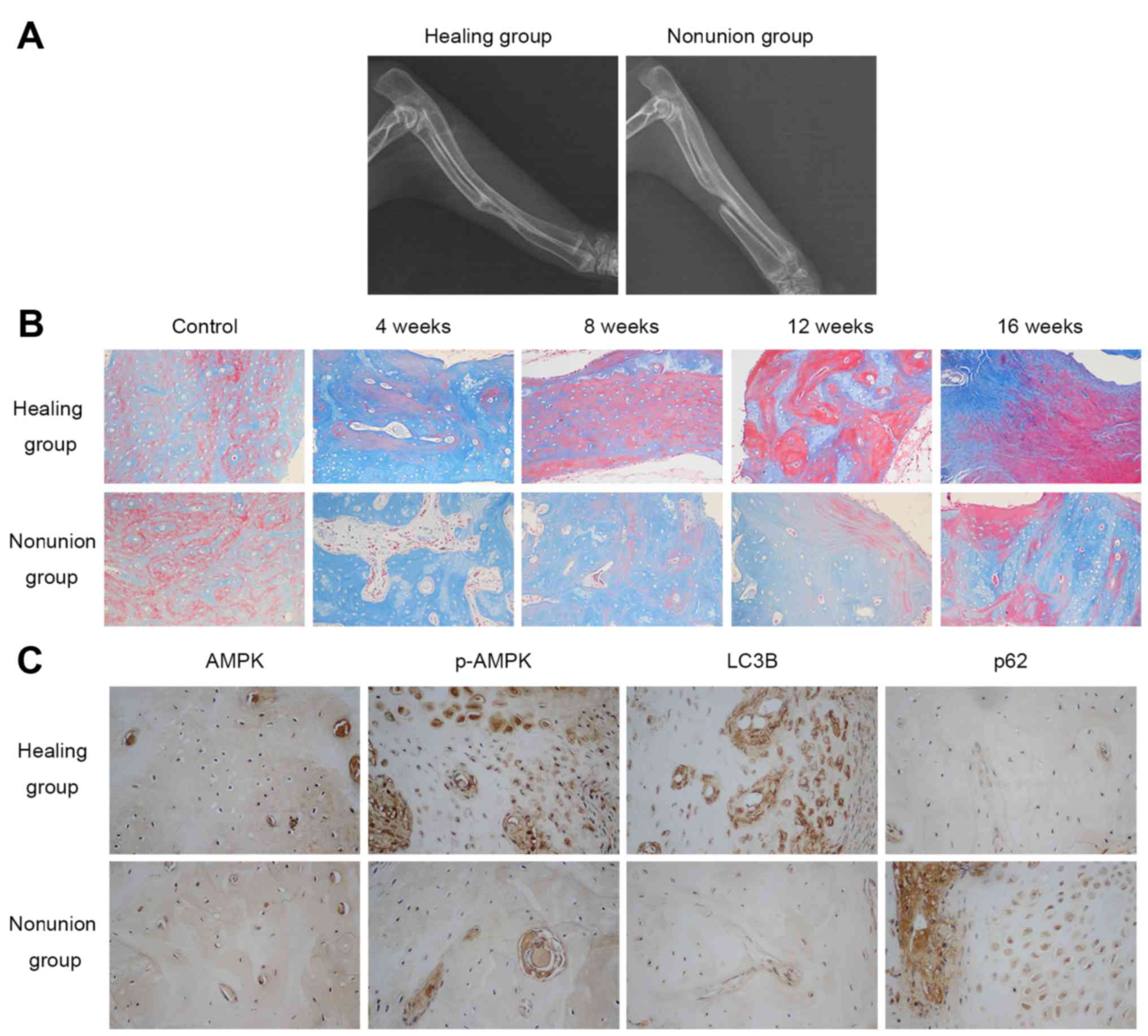
Radiographic and histological analysis of
bone healing status in rabbits
A total of 16 weeks following surgery, the bilateral
forelimbs of the rabbits in both groups were examined by
radiography. As shown in Fig. 1A,
rabbits in the healing group all achieved bony union in the
radiuses, whereas nonunion was observed in the group that underwent
20 mm radial bone osteotomy, thus confirming the nonunion model had
been successfully generated. Masson staining detected the
maturation process of newly formed bone by staining osteoid and
connective tissues blue, whereas mineralized bone was dyed red. The
results indicated that new bone formation took place in both
groups; however, the mineralization process of the healing group
was more prominent and faster compared with in the nonunion group,
as illustrated by a higher percentage of red staining at the same
timepoints (4, 8, 12, 16 weeks post-operation) in the healing group
(Fig. 1B). Collectively, these
results suggested that new bone mineralization and maturation is
restrained in the nonunion group.
Expression levels of AMPK and
autophagy-associated markers in calluses
The present study examined the protein expression
levels of AMPK, p-AMPK and autophagy-associated markers (LC3B and
p62) in the calluses at various timepoints (4, 8, 12 and 16 weeks
post-operation) by immunohistochemistry. As shown in Fig. 1C, there was no obvious difference
in the expression of AMPK between the two groups 4 weeks after
surgery, whereas p-AMPK and LC3B expression was higher, and p62
expression was lower in the healing group. With regards to other
time-points, no marked differences were detected between the
groups. These results indicated that AMPK activation and autophagic
activity were reduced in the nonunion group 4 weeks
post-operation.
AMPK activation stimulates the osteogenic
differentiation of MC3T3-E1 cells
ALP, OCN and Runx2 have been identified as typical
markers of osteogenic differentiation (23). To investigate whether AMPK
activation may stimulate the differentiation and mineralization of
MC3T3-E1 cells, AICAR was added to the osteogenic differentiation
medium in the presence or absence of compound C for 8 days, and the
mRNA expression levels of ALP, OCN and Runx2 were assessed by
RT-qPCR. In addition, ALP activity was detected by BCIP/NBT
staining. Alizarin red staining was also applied to evaluate
mineralization after 21 days of culture with the indicated
treatment. AICAR significantly promoted the mRNA expression levels
of ALP, OCN and Runx2 in a dose-dependent manner, whereas compound
C, the AMPK inhibitor, reversed the effects of AICAR (Fig. 2A). In addition, BCIP/NBT staining
indicated that AICAR enhanced ALP activity, whereas compound C
suppressed the effects of AICAR in a dose-dependent manner
(Fig. 2B). Furthermore, AICAR
enhanced the mineralization of MC3T3-E1 cells, whereas compound C
inhibited the effects of AICAR (Fig.
2C and D). Taken together, these results indicated that AMPK
activation may promote the differentiation and mineralization of
MC3T3-E1 osteoblasts.
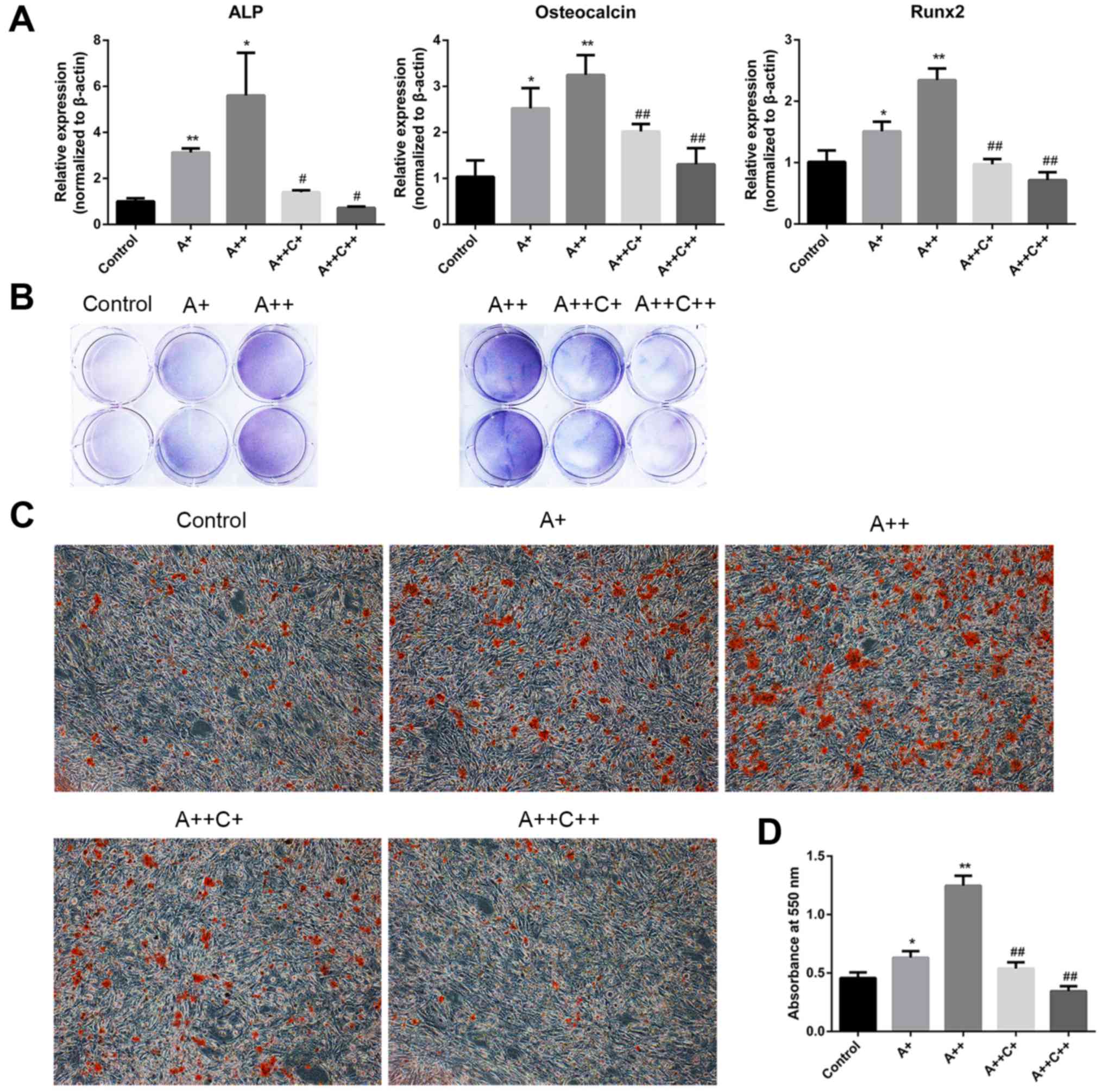 | Figure 2AMPK activation promotes the
differentiation and mineralization of MC3T3-E1 cells. (A and B)
Cells were treated with AICAR (A+, 1 μM; ++, 10 μM)
and compound C (C+, 0.1 μM; ++, 1 μM) in
differentiation medium for 8 days. (A) ALP, osteocalcin and Runx2
mRNA expression were detected by reverse transcription-quantitative
polymerase chain reaction. (B) 5-Bromo-4-chloro-3-indolyl
phosphate/nitro blue tetrazolium staining was used to determine ALP
activity. (C) Cells were cultured for 21 days; representative
images (magnification, ×100) of mineralized nodules stained by
Alizarin red staining are presented. (D) Quantification of Alizarin
red staining with cetylpyridinium chloride. Absorbance was measured
at 550 nm. *P<0.05, **P<0.01 compared
with the control group; #P<0.05,
##P<0.01 compared with the A++ group. AICAR,
5-aminoimdazole-4-carboxamide-1-β-D-ribofuranoside; ALP, alkaline
phosphatase; AMPK, adenosine monophosphate-activated protein
kinase; Rnx2, runt-related transcription factor 2. |
AMPK activation induces autophagy in
MC3T3-E1 cells
To determine whether AMPK activation induces
autophagy, MC3T3-E1 cells were treated with AICAR for 24 h in the
presence or absence of compound C; the protein expression levels of
AMPK and p-AMPK, as well as the autophagy markers LC3B-I, LC3B-II
and p62, were assessed by western blot analysis (Fig. 3A–D). Furthermore, autophagosomes
were detected by transmission electron microscopy (Fig. 3E and F). Treatment with AICAR
significantly stimulated activation of AMPK after 30 min of
treatment; p-AMPK expression reached its peak after 24 h of AICAR
treatment. In addition, LC3B-II expression was increased after 30
min of AICAR treatment, whereas p62 expression was downregulated
after 4 h; the lowest expression levels were detected at 24 h
(Fig. 3A). Relative autophagosome
density was higher following 24 h of AICAR treatment compared with
in the control group (Fig. 3E and
F). These results suggested that AICAR may stimulate AMPK
activation and autophagy. To further confirm that AMPK activation
by AICAR could induce autophagy, cells were incubated with AICAR
for 24 h in combination with compound C, which is a specific AMPK
inhibitor. As demonstrated in Fig.
3B, compound C markedly reduced LC3B-II expression, whereas p62
expression was increased. In addition, relative autophagosome
density was markedly reduced by compound C compared with in the
AICAR group. Taken together, these results indicated that AMPK
activation may induce autophagy.
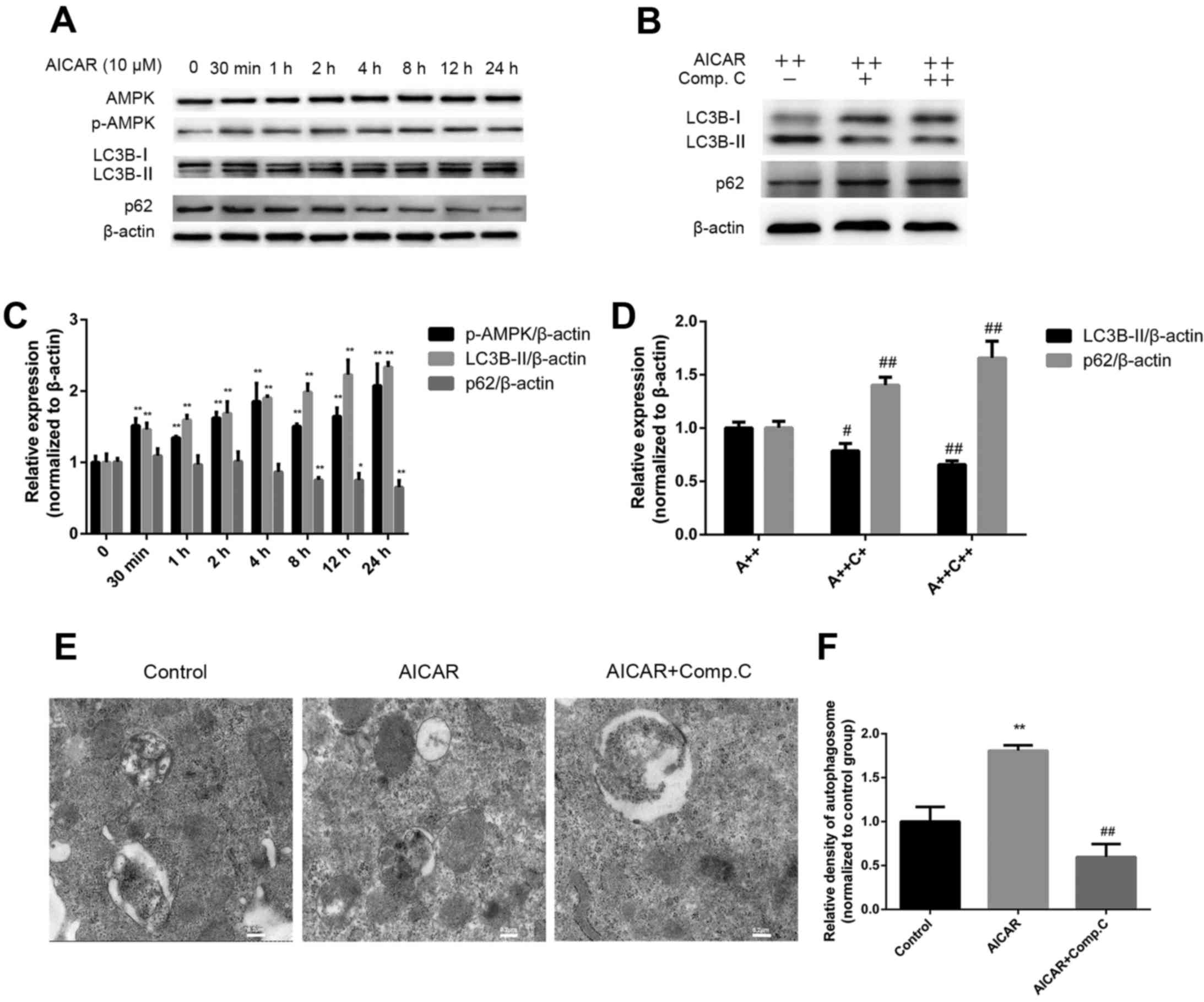 | Figure 3AMPK activation induces autophagy.
(A) Cells were treated with AICAR and (B) AICAR (A++, 10 μM)
+ compound C (C++, 1 μM) for 24 h, after which western blot
analysis was performed. (C and D) Semi-quantitative analysis of
western blotting was conducted using ImageJ software and density
values were normalized to β-actin, with the control group set as 1.
(E) Cells were treated with AICAR (10 μM) or AICAR (10
μM) + compound C (1 μM) for 24 h. Representative
images of autophagosomes were captured by transmission electron
microscopy (scale bar, 0.2 μm). (F) Relative quantification
of autophagosome density; values of the control group were set as
1. *P<0.05, **P<0.01 compared with the
control group; #P<0.05, ##P<0.01
compared with the AICAR(A++) group. AICAR,
5-aminoimdazole-4-carboxamide-1-β-D-ribofuranoside; AMPK, adenosine
monophosphate-activated protein kinase; LC3B,
microtubule-associated proteins 1A/1B light chain 3B; p-AMPK,
phosphorylated-AMPK. |
AMPK activation enhances differentiation
and mineralization in MC3T3-E1 cells via the induction of
autophagy
The aforementioned results demonstrated that AMPK
activation could stimulate osteoblast differentiation and
mineralization, during which autophagy was also induced. The
present study subsequently examined whether the effects of AMPK
activation on osteoblast differentiation and mineralization were
mediated by autophagy induction. MC3T3-E1 cells were cotreated with
AICAR and the following autophagy inhibitors: 3-MA, which
suppresses initiation of autophagosome formation, and CQ, which
inhibits the degradation of cargoes in the autophagosome, thus
suppressing the final steps of autophagy. Western blot analysis
verified the inhibition of autophagy by 3-MA and CQ (Fig. 4A and B). The results revealed that
3-MA and CQ markedly reduced ALP activity (Fig. 4C), and the mRNA expression levels
of ALP, OCN and Runx2 in AICAR-treated cells (Fig. 4D). In addition, mineralization was
suppressed by 3-MA and CQ compared with in cells treated with AICAR
alone (Fig. 4E). To further
confirm that the stimulatory effects of AMPK activation on
osteoblast differentiation were due to autophagy, siRNA against
BCN1 was used to more specifically inhibit autophagy. siBCN1-II was
selected as the most efficient siRNA (Fig. 5A) and the silencing effect was
confirmed by RT-qPCR and western blot analysis (Fig. 5B and C). As shown in Fig. 5D and E, ALP activity, and ALP, OCN
and Runx2 mRNA expression were markedly decreased by siBCN1-II
compared with in the AICAR group. Collectively, these results
provide evidence to suggest that autophagy is involved in
osteoblast differentiation and mineralization induced by AMPK
activation.
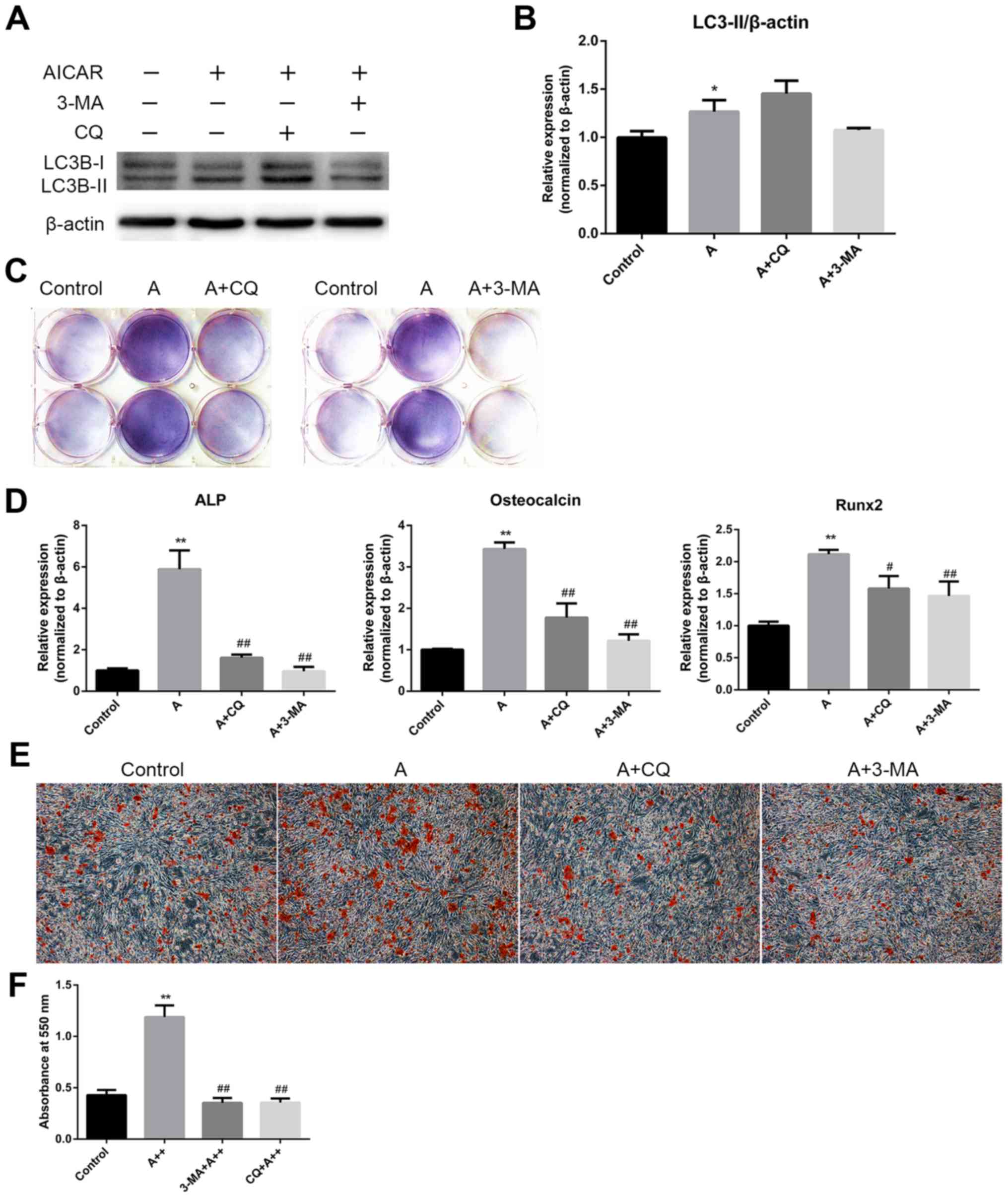 | Figure 4AMPK activation enhances
differentiation and mineralization of MC3T3-E1 cells via the
induction of autophagy. (A) Cells were treated with AICAR (10
μM) in the presence or absence of 3-MA (5 mM) or CQ (10
μM) for 24 h, after which western blot analysis was
conducted. (B) Semi-quantitative results of western blot analysis.
(C and D) Cells were cotreated with AICAR (10 μM) and 3-MA
(5 mM) or CQ (10 μM) for 8 days. (C)
5-Bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium
staining was used to detect ALP activity. (D) ALP, OCN and Runx2
mRNA expression was detected using reverse transcription-polymerase
chain reaction. (E) Cells were cultured for 21 days; representative
images (magnification, ×100) of Alizarin red staining are
presented. (F) Quantification of Alizarin red staining.
*P<0.05, **P<0.01 compared with the
control group; #P<0.05, ##P<0.01
compared with the AICAR(A++) group. 3-MA, 3-methyladenine; AICAR,
5-aminoimdazole-4-carboxamide-1-β-D-ribofuranoside; ALP, alkaline
phosphatase; AMPK, adenosine monophosphate-activated protein
kinase; CQ, chloroquine diphosphatase; OCN, osteocalcin; Runx2,
runt-related transcription factor 2. |
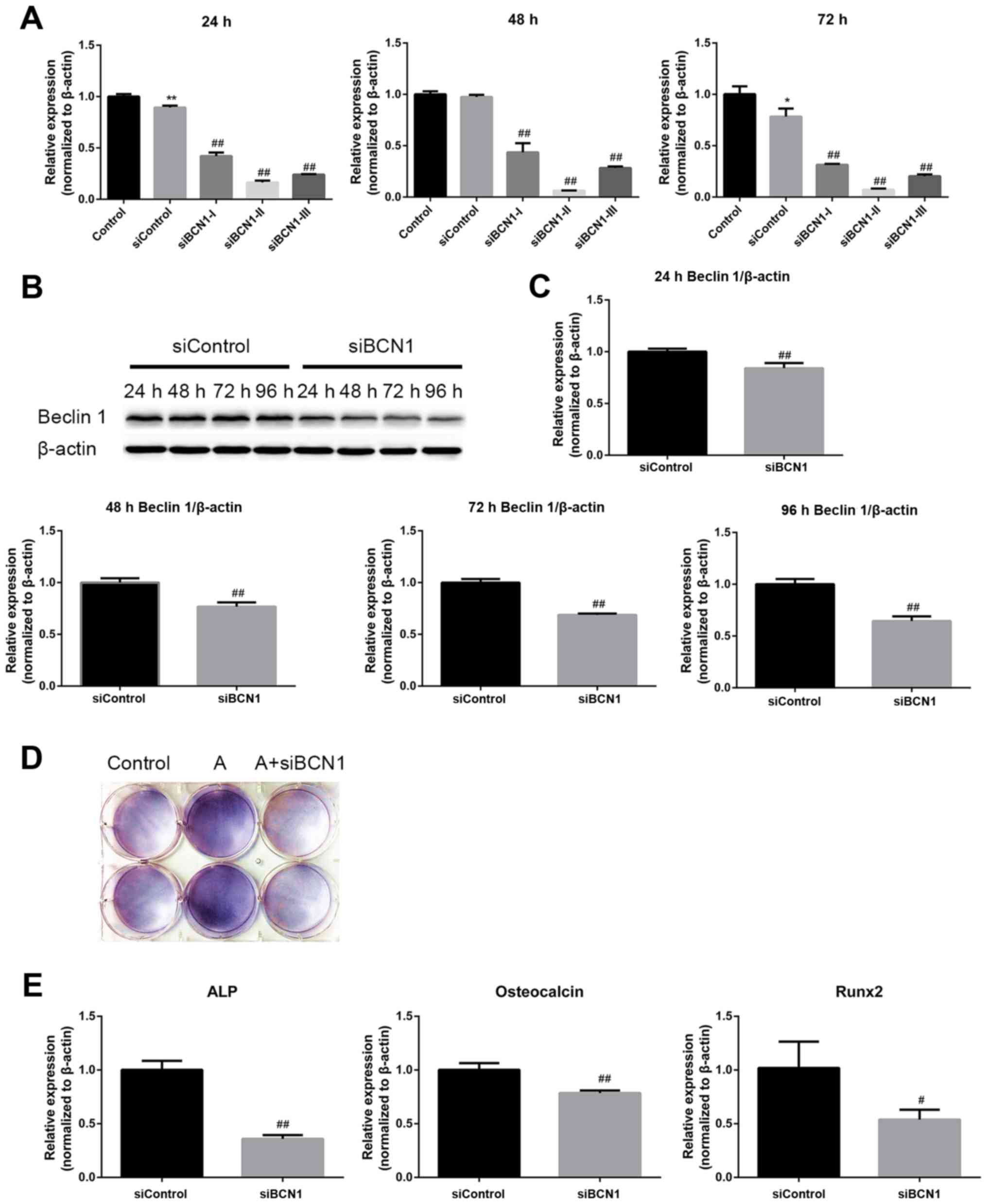 | Figure 5AMPK activation enhances
differentiation of MC3T3-E1 cells via the induction of autophagy.
(A) Cells were transfected with the indicated siRNAs, and the mRNA
expression levels of BCN1 were measured by RT-qPCR 24, 48 and 72 h
post-transfection to assess the silencing effect; siBCN1-II was the
most efficient siRNA and was used for further study. (B) Cells were
transfected with siBCN1-II and siControl, western blot analyis was
performed using an antibody against BCN1 24, 48, 72 and 96 h
post-transfection, in order to verify the silencing effect. (C)
Semi-quantitative results of western blot analysis. (D and E) A
total of 24 h post-transfection with siBCN1-II or siControl,
differentiation medium was added and cells were treated with or
without AICAR (10 μM) for 8 days. (D)
5-Bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium
staining was used to detect ALP activity. (E) ALP, OCN and Runx2
mRNA expression was detected by RT-PCR. siControl cells were
treated with AICAR. *P<0.05, **P<0.01
compared with the control group; #P<0.05,
##P<0.01 compared with the siControl group. AICAR,
5-aminoimdazole-4-carboxamide-1-β-D-ribofuranoside; ALP, alkaline
phosphatase; AMPK, adenosine monophosphate-activated protein
kinase; BCN1, Beclin 1; OCN, osteocalcin; Runx2, runt-related
transcription factor 2; si/siRNA, small interfering RNA. |
Discussion
The results of the present study suggested that bone
maturation is impaired in a rabbit model of nonunion, which was
accompanied by decreased AMPK activation and autophagic activity in
the early phase of fracture healing (4 weeks post-surgery).
Furthermore, in vitro experiments indicated that AMPK
activation induces autophagy, as well as osteoblast differentiation
and mineralization, which can be reversed by the AMPK inhibitor
compound C, by inhibition of autophagy with pharmacological
inhibitors, or by BCN1 gene silencing. These results suggested that
AMPK activation may promote osteoblast differentiation and
mineralization via the induction of autophagy.
Osteoblasts, which are derived from mesenchymal
precursor cells, are responsible for bone formation, and have a
vital role in bone development and fracture repair (24). As osteoblasts differentiate, they
secrete collagen and eventually form a mineralized extracellular
matrix. Due to their increased expression during the
differentiation process, ALP, OCN and Runx2 are widely used as
markers to assess osteoblast differentiation (23). Osteoblast differentiation can be
regulated by diverse molecules and signaling pathways, including
Runx2, osterix, BMPs and Wnts (25,26). Agents capable of stimulating
osteoblast differentiation have been reported to be beneficial for
bone formation; for example, metformin and statins can stimulate
bone formation by inducing BMP-2 and eNOS expression (27,28). Furthermore, drugs that target
osteoblast differentiation to enhance bone formation have been
applied in some clinical situations; for example, recombinant
human-BMP-2 has been used to strengthen spinal fusion and BMP-7 has
been used for the treatment of long-bone nonunion (25).
Nonunion refers to a situation where a bone fracture
fails to heal over a prolonged period of time; it is not rare, with
an overall incidence of 5-10% among patients with fractures
(29). Nonunion can be caused by
numerous factors, including a large bone defect, infection,
mechanical instability, soft tissue interposition, loss of
vascularization and severe soft tissue damage (30-33). However, the underlying molecular
mechanism remains elusive. Fracture healing is a complex
physiological process in which several factors and cells interact
in a coordinated manner (34).
Osteoblasts have an essential role in new bone formation and bone
remodeling by producing osteoid, which then becomes mineralized
during the differentiation process (35). In the present study, radiographs
captured 16 weeks post-operation detected nonunion in the nonunion
group. Masson staining revealed that bone mineralization and
maturation was markedly delayed in the nonunion group, which may be
attributed to the prolonged existence of osteoid and impairment of
the mineralization process. Based on these findings, it may be
hypothesized that osteoblast differentiation is impaired in the
development of nonunion.
AMPK has been recognized as a key energy sensor and
metabolic modulator in regulating cell proliferation,
differentiation, apoptosis, autophagy and mitochondrial function
via various signaling pathways (36-38). It has been reported that
activation of AMPK is able to stimulate osteoblast differentiation
via eNOS and BMP-2 expression, as well as through the SMAD family
member 1/5/8-Dlx5-Runx2 signaling pathway (10,28). Furthermore, AMPK activity has been
reported to serve a crucial role in in vitro bone nodule
formation and in vivo bone mass maintenance; knockout of
AMPK gene expression may result in reduced cortical and trabecular
bone parameters (8). The present
results were in accordance with those of previous studies and
indicated that activation of AMPK could enhance the differentiation
and mineralization of MC3T3-E1 cells.
It has previously been demonstrated that autophagy
is induced during the differentiation process of osteoblasts, and
deficiencies in the expression of autophagy-essential genes may
result in decreased mineralization capacity; furthermore,
autophagic vacuoles may act as vehicles for the secretion of
mineralization matrix (1).
Notably, mechanical stress, which is an important stimulator for
bone cells and can improve bone strength, has been revealed to be
able to induce autophagy in osteoblasts; however, the process is
transient (39,40). In line with these results, the
present study indicated that autophagy was induced during
AMPK-promoted MC3T3-E1 osteogenic differentiation, and inhibition
of auto phagy prevented the differentiation process.
A previous in vitro study demonstrated that a
significant increase in osteoblast mineralization could be observed
after 3-4 weeks cultivation (5).
In addition, in vivo experiments revealed that hard callus
and new bone formation could be detected in the margin of bone ends
in rabbits 4 weeks after radius defect creation (41). The present results from
immunohistochemical staining of AMPK, p-AMPK, LC3B and p62 in the
calluses of fracture ends 4 weeks after surgery indicated that AMPK
activity, as well as autophagy, was increased in the healing group
compared with in the nonunion group. These events occurred at
almost the same time period; therefore, considering the important
role osteoblasts, AMPK and autophagy have in bone formation and
remodeling, it may be hypothesized that AMPK and autophagy are
involved in osteoblast differentiation, mineralization and the
fracture healing process in vivo.
Inoki et al reported that AMPK may promote
the initiation of autophagy via inhibition of mTOR (42). Furthermore, it has been revealed
that AMPK may induce autophagy via direct phosphorylation of ULK1,
which is essential in the initiation of autophagy (43). AMPK could also induce autophagy by
directly phosphorylating the phosphatidylinositol 3-kinase
catalytic subunit type 3 complex and BCN1, which are essential for
autophagosome formation (44).
Consistent with these results, the present study suggested that
AMPK activation could induce autophagy in MC3T3-E1 cells.
The present study has some limitations. Firstly, the
in vivo experiment was based on observation; agonists or
inhibitors of AMPK and autophagy were not used to treat the
animals, as there is insufficient evidence supporting the
efficiency of these agents when applied in vivo. In
addition, the signaling pathway by which AMPK activation induces
autophagy and how autophagy stimulates osteoblast differentiation
were not investigated; therefore, further research is required to
elucidate these issues.
In conclusion, the results of the present study
demonstrated that AMPK activation may stimulate osteoblast
differentiation and mineralization through the induction of
autophagy. In addition, AMPK activation and autophagy may be
involved in the fracture healing process in vivo. The
present study provides evidence to suggest that enhancing AMPK
activation and autophagic activity may be a potential novel
approach to promote fracture healing.
Acknowledgments
Not applicable.
Notes
[1]
Funding
The present study was supported by the Hubei
Provincial Natural Scie nce Foundation of China (grant nos.
2015CFB209); the Fundamental Research Funds for the Central
Universities (grant no. 2042015kf0070); and the Zhongnan Hospital
of Wuhan University Science, Technology and Innovation Seed Fund,
Project znpy2016004.
[2] Availability
of data and materials
Materials described in the manuscript, including all
relevant raw data, will be freely available to any researcher
wishing to use them for non-commercial purposes, without breaching
participant confidentiality.
[3] Authors'
contributions
YL wrote the manuscript. YL, JS and WS performed the
experiments. LC and ZD designed the study and reviewed the
manuscript. All authors read and approved the manuscript.
[4] Ethics
approval and consent to participate
The present study was approved by the Wuhan
University Zhongnan Hospital Research Committee (Wuhan, China).
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Nollet M, Santucci-Darmanin S, Breuil V,
Al-Sahlanee R, Cros C, Topi M, Momier D, Samson M, Pagnotta S,
Cailleteau L, et al: Autophagy in osteoblasts is involved in
mineralization and bone homeostasis. Autophagy. 10:1965–1977. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan GK and Duque G: Age-related bone
loss: old bone, new facts. Gerontology. 48:62–71. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Homma Y, Zimmermann G and Hernigou P:
Cellular therapies for the treatment of non-union: the past,
present and future. Injury. 44(Suppl 1): S46–S49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu Y, Zhou J, Ao R and Yu B: A-769662
protects osteoblasts from hydrogen dioxide-induced apoptosis
through activating of AMP-activated protein kinase (AMPK). Int J
Mol Sci. 15:11190–11203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quarles LD, Yohay DA, Lever LW, Caton R
and Wenstrup RJ: Distinct proliferative and differentiated stages
of murine MC3T3-E1 cells in culture: an in vitro model of
osteoblast development. J Bone Miner Res. 7:683–692. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhong X, Xiu LL, Wei GH, Liu YY, Su L, Cao
XP, Li YB and Xiao HP: Bezafibrate enhances proliferation and
differentiation of osteoblastic MC3T3-E1 cells via AMPK and eNOS
activation. Acta Pharmacol Sin. 32:591–600. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hardie DG, Hawley SA and Scott JW:
AMP-activated protein kinase - development of the energy sensor
concept. J Physiol. 574:7–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shah M, Kola B, Bataveljic A, Arnett TR,
Viollet B, Saxon L, Korbonits M and Chenu C: AMP-activated protein
kinase (AMPK) activation regulates in vitro bone formation and bone
mass. Bone. 47:309–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jang WG, Kim EJ, Lee KN, Son HJ and Koh
JT: AMP-activated protein kinase (AMPK) positively regulates
osteoblast differentiation via induction of Dlx5-dependent Runx2
expression in MC3T3E1 cells. Biochem Biophys Res Commun.
404:1004–1009. 2011. View Article : Google Scholar
|
|
11
|
Kanazawa I, Yamaguchi T, Yano S, Yamauchi
M and Sugimoto T: Activation of AMP kinase and inhibition of Rho
kinase induce the mineralization of osteoblastic MC3T3-E1 cells
through endo-thelial NOS and BMP-2 expression. Am J Physiol
Endocrinol Metab. 296:E139–E146. 2009. View Article : Google Scholar
|
|
12
|
Pierrefite-Carle V, Santucci-Darmanin S,
Breuil V, Camuzard O and Carle GF: Autophagy in bone: self-eating
to stay in balance. Ageing Res Rev. 24(Pt B): 206–217. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Auto-phagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hosokawa N, Sasaki T, Iemura S, Natsume T,
Hara T and Mizushima N: Atg101, a novel mammalian autophagy protein
interacting with Atg13. Autophagy. 5:973–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mercer CA, Kaliappan A and Dennis PB: A
novel, human Atg13 binding protein, Atg101, interacts with ULK1 and
is essential for macroautophagy. Autophagy. 5:649–662. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Glick D, Barth S and Macleod KF:
Autophagy: cellular and molecular mechanisms. J Pathol. 221:3–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the care and use of laboratory animals. 8th
edition. Washington (DC): National Academies Press (US); 2011
|
|
18
|
Farso Nielsen F, Karring T and Gogolewski
S: Biodegradable guide for bone regeneration. Polyurethane
membranes tested in rabbit radius defects. Acta Orthop Scand.
63:66–69. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shafiei Z, Bigham AS, Dehghani SN and
Nezhad ST: Fresh cortical autograft versus fresh cortical allograft
effects on experimental bone healing in rabbits: radiological,
histopathological and biomechanical evaluation. Cell Tissue Bank.
10:19–26. 2009. View Article : Google Scholar
|
|
20
|
Oryan A, Alidadi S and Moshiri A: Current
concerns regarding healing of bone defects. Hard Tissue. 2:132013.
View Article : Google Scholar
|
|
21
|
Saito T, Asai K, Sato S, Hayashi M, Adachi
A, Sasaki Y, Takano H, Mizuno K and Shimizu W: Autophagic vacuoles
in cardiomyocytes of dilated cardiomyopathy with initially
decompensated heart failure predict improved prognosis. Autophagy.
12:579–587. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Aubin JE, Liu F, Malaval L and Gupta AK:
Osteoblast and chondroblast differentiation. Bone. 17(Suppl 2):
77S–83S. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shapiro F: Bone development and its
relation to fracture repair. The role of mesenchymal osteoblasts
and surface osteoblasts. Eur Cell Mater. 15:53–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khosla S, Westendorf JJ and Oursler MJ:
Building bone to reverse osteoporosis and repair fractures. J Clin
Invest. 118:421–428. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakashima K and de Crombrugghe B:
Transcriptional mechanisms in osteoblast differentiation and bone
formation. Trends Genet. 19:458–466. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garrett IR, Gutierrez G and Mundy GR:
Statins and bone formation. Curr Pharm Des. 7:715–736. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanazawa I, Yamaguchi T, Yano S, Yamauchi
M and Sugimoto T: Metformin enhances the differentiation and
mineralization of osteoblastic MC3T3-E1 cells via AMP kinase
activation as well as eNOS and BMP-2 expression. Biochem Biophys
Res Commun. 375:414–419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gómez-Barrena E, Rosset P, Lozano D,
Stanovici J, Ermthaller C and Gerbhard F: Bone fracture healing:
cell therapy in delayed unions and nonunions. Bone. 70:93–101.
2015. View Article : Google Scholar
|
|
30
|
Altner PC, Grana L and Gordon M: An
experimental study on the significance of muscle tissue
interposition on fracture healing. Clin Orthop Relat Res.
111:269–273. 1975. View Article : Google Scholar
|
|
31
|
Heiple KG and Herndon CH: The pathologic
physiology of nonunion. Clin Orthop Relat Res. 43:11–21. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hietaniemi K, Peltonen J and Paavolainen
P: An experimental model for non-union in rats. Injury. 26:681–686.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sarahrudi K, Mousavi M, Grossschmidt K,
Sela N, König F, Vécsei V and Aharinejad S: Combination of
anorganic bovine-derived hydroxyapatite with binding peptide does
not enhance bone healing in a critical-size defect in a rabbit
model. J Orthop Res. 26:759–763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsiridis E, Upadhyay N and Giannoudis P:
Molecular aspects of fracture healing: which are the important
molecules? Injury. 38(Suppl 1): S11–S25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harada S and Rodan GA: Control of
osteoblast function and regulation of bone mass. Nature.
423:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Steinberg GR and Kemp BE: AMPK in health
and disease. Physiol Rev. 89:1025–1078. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Greer EL, Oskoui PR, Banko MR, Maniar JM,
Gygi MP, Gygi SP and Brunet A: The energy sensor AMP-activated
protein kinase directly regulates the mammalian FOXO3 transcription
factor. J Biol Chem. 282:30107–30119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang S, Song P and Zou MH: AMP-activated
protein kinase, stress responses and cardiovascular diseases. Clin
Sci (Lond). 122:555–573. 2012. View Article : Google Scholar
|
|
39
|
King JS, Veltman DM and Insall RH: The
induction of autophagy by mechanical stress. Autophagy.
7:1490–1499. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Klein-Nulend J, Bacabac RG and Bakker AD:
Mechanical loading and how it affects bone cells: the role of the
osteocyte cytoskeleton in maintaining our skeleton. Eur Cell Mater.
24:278–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao L, Zhao J, Wang S, Wang J and Liu J:
Comparative study between tissue-engineered periosteum and
structural allograft in rabbit critical-sized radial defect model.
J Biomed Mater Res B Appl Biomater. 97:1–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Inoki K, Zhu T and Guan KL: TSC2 mediates
cellular energy response to control cell growth and survival. Cell.
115:577–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Egan DF, Shackelford DB, Mihaylova MM,
Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor
R, et al: Phosphorylation of ULK1 (hATG1) by AMP-activated protein
kinase connects energy sensing to mitophagy. Science. 331:456–461.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim J, Kim YC, Fang C, Russell RC, Kim JH,
Fan W, Liu R, Zhong Q and Guan KL: Differential regulation of
distinct Vps34 complexes by AMPK in nutrient stress and autophagy.
Cell. 152:290–303. 2013. View Article : Google Scholar : PubMed/NCBI
|