Introduction
Viral hepatitis affects millions of people
worldwide, and is now the leading cause of cirrhosis in China.
Cirrhosis is a pathological manifestation of end-stage liver
disease that contributes significantly to the high mortality of
liver diseases. The most important part in the formation of hepatic
fibrosis is extracellular matrix (ECM) deposition from hepatic
stellate cells (HSCs) (1). HSCs
are the final target cells in all types of liver fibrosis. HSCs are
activated and transformed into muscle fibroblast-like cells, which
causes increased synthesis and degradation of collagen (2).
At present, orthotropic liver transplantation is the
last resort for the treatment of cirrhosis. However, due to the
shortage of donor organs, orthotropic liver transplantation is
restricted clinically (3), and
alternative treatment strategies are urgently needed.
Stem cell therapy as a potential therapeutic method
has attracted increased attention. Mesenchymal stem cells (MSCs)
are important members of the stem cell family. They have the
characteristics of multi-differentiation potential, hematopoietic
support and promotion of stem cell transplantation, immune
regulation and self replication (4). Human umbilical cord MSCs (hUC-MSCs)
are derived from umbilical cord tissue. Aspiration of MSCs does not
require invasive procedures, which differs from bone marrow MSCs
(BM-MSCs). hUC-MSCs are routinely discarded after delivery, without
ethical consideration. There have been few studies on the treatment
of liver fibrosis with tissue-derived MSCs (umbilical cord,
placenta, or adipose tissue), and their role in the treatment of
liver fibrosis has rarely been compared with that of hUC-MSCs and
BM-MSCs. MSCs can secrete various cytokines in a paracrine manner,
which can promote liver repair, such as hepatocyte growth factor
(5), while others can inhibit the
occurrence of liver cirrhosis (6).
A recent investigation found that hUC-MSCs can
accelerate the resolution of acute liver injury without any
differentiation and manipulation (7). Additionally, it has been confirmed
histologically that transplantation of hUC-MSCs into rats with
CCl4-induced liver fibrosis results in significant
reduction of liver fibrosis (8).
However, to the best of our knowledge, there are few studies
concerning the mechanism of action of hUC-MSCs on HSCs, and what
type of signal transduction pathways are used in HSCs.
Transforming growth factor-β1 (TGF-β1) is a key
member of the TGF-β superfamily and plays a critical role in the
development of hepatic fibrosis. The Smads protein family is
located on HSCs and is divided into receptor activation and
inhibitory Smads, such as Smad3 and Smad7. TGF-β1/Smads is an
important signaling pathway in hepatic fibrosis (9). In this study, we confirmed that
hUC-MSCs could inhibit proliferation of HSCs, and clarified the
effect on the TGF-β1/Smads pathway when hUC-MSCs were co-cultured
with HSCs. This will provide a theoretical basis for the
therapeutic use of MSCs in the treatment of liver fibrosis.
Materials and methods
Materials
Human HSC cell line LX2 was kindly gifted by Scott
L. Friedman, Mount Sinai School of Medicine, New York, NY, USA. We
used Dulbecco's modified Eagle's medium (DMEM)-low glucose (LG)
culture medium and fetal bovine serum (FBS; HyClone, Logan, UT,
USA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) reagent cartridge (Sigma, St. Louis, MO, USA), TGF-β1
enzyme-linked immunosorbent assay (ELISA) reagent cartridge
(R&D Systems, Minneapolis, MN, USA), human TGF-β1 and β-actin
antibody (Bioworld, Minneapolis, MN, USA), human Smad7 antibody
(Abcam, Hong Kong, China), horseradish-peroxidase-labeled goat
anti-rabbit IgG (Bioworld), RNA extraction reagent RNAiao Plus,
reverse transcription kit (both from Takara Biotechnology, Dalian,
Japan), ECL light kit (Thermo Fisher Scientific, Inc., Waltham,
USA), polyvinylidene fluoride (PVDF) membrane (Millipore,
Billerica, MA, USA), semipermeable Transwell insert film (Corning
Costar, Acton, MA, USA), Hoechst 33324 dye (Sigma), BCA protein
concentration assay kit (Beijing Soledad Bao Biological Technology
Co., Ltd., Beijing, China), and protein lysate (Biotechnology
Research Institute, Haimen, China) in our experiment.
Culture of hUC-MSCs
hUC-MSC cultures were established from the umbilical
cords of healthy donors using the direct plastic adherence method
after informed consent had been obtained. The study was approved by
the Ethics Committee of the School of Life Science and
Biopharmaceutics of Lanzhou University (Lanzhou, China) and
performed in accordance with the Helsinki Declaration. The cord
tissue pieces were minced into 3-5-mm long fragments, plated
separately in 100-mm diameter polystyrene tissue culture dishes and
maintained in Dulbecco's modified Eagle's medium-low glucose
(DMEM-LG) medium with 10% FBS at 37°C in a humidified atmosphere
with 5% CO2. The culture medium was changed on day 7 and
then every 3-4 days. Approximately 3 weeks later, when
well-developed colonies of fibroblast-like cells were 80-90%
confluent, the cord tissue pieces were removed and the cultures
were washed and harvested with 0.25% trypsin. The cells were then
seeded in new 100-mm diameter flasks for further expansion
(6).
Culture of HSCs
LX2 cells were grown in DMEM-LG medium with 10% FBS
in 5% CO2 at 37°C. For all of the experiments,
subconfluent cells (80%) were incubated in 25-cm2
culture bottles for different time periods (24 and 48 h).
Establishment of co-culture system
For indirect co-culture, hUC-MSCs and LX2 cells were
seeded at a 1:1 ratio in each well of a 6-well plate, using
Transwell membranes (24 mm diameter, 0.4 μm pore size;
Corning Costar). Approximately 105 LX2 cells were placed
in the lower chamber with 105 hUC-MSCs placed on the
membrane insert. Co-cultures were maintained in DMED-LG with 10%
FBS for 24 or 48 h.
The upper and lower double-cell co-culture system
was established between hUC-MSCs and HSCs as the experimental
group. HSCs were cultured alone as a negative control group.
Growth curve
hU-MSC and LX2 cell suspensions were inoculated in
96-well plates for 9 days. Living cells from three wells were
harvested and counted serially at 24-h intervals. Then a growth
curve was plotted.
MTT assay
Cell proliferation inhibition rate of each group was
evaluated by MTT assay. Cells were seeded into the aforementioned
co-culture system in 2 ml of medium in each well and cultured for
24 or 48 h. The Transwell membranes were removed and MTT solution
[5 mg/ml in phosphate-buffered saline (PBS)] was added to each well
and plates were incubated for an additional 4 h at 37°C. Dimethyl
sulfoxide (DMSO) was added to each well, followed by incubation on
a shaker at 10 min at 37°C. The liquid was transferred into 96-well
plates, 150 μl/hole, 10 wells/group. Absorbance was measured
on a microplate reader (Bio-Rad, Hercules, CA, USA) at 490 nm. Cell
growth inhibition rate = (1-A value of experimental group/control
group A value) ×100% were then calculated.
Hoechst
The co-culture and control groups were cultured for
24 or 48 h. Cells were fixed with 3.7% paraformaldehyde for 30 min
at room temperature, washed with PBS, and stained with Hoechst
33324 at a final concentration of 5 μg/ml at 37°C for 6-9
min. Cells were observed under a fluorescence microscope equipped
with a UV filter. The images were recorded on a computer with a
digital camera (Olympus, Takachiho, Japan) attached to the
microscope, and the images were processed by computer. The Hoechst
reagent was taken up by the nuclei of the cells, and apoptotic
cells exhibited a bright blue fluorescence.
ELISA
TGF-β1 protein was measured in the LX2-conditioned
medium after co-culture with the hUC-MSCs for 24 or 48 h.
Serum-deprived LX2 cells served as a positive control. We used a
commercial ELISA kit (R&D Systems). Reverse transcription
polymerase chain reaction (RT-PCR). Total RNA was extracted from
the hUC-MSCs and LX2 cells using the RNA Plus kit. TGF-β1, primers
forward, 5′-CCACAACGAAATCTATGAC-3′ and reverse,
5′-GTATTTCTGGTACAGCTCCA-3; Smad3, primers forward,
5′-CTGGCTACCTGAGTGAAGATG-3′ and reverse,
5′-TGTGAAGCGTGGAATGTCTC-3′; Smad7, primers forward,
5′-TCTGCGAACTAGAGTCTCCC-3′ and reverse, 5′-ACGCACCAGTGTGACCGATC-3′
were used for PCR. Reverse transcription was carried out using the
Takara PrimeScript reagent kit with 1 μg total RNA as a
template and oligo(dT) as a primer. All semi-quantitative PCR
experiments were performed using the same serially diluted cDNA
batches as templates. Amplification was performed at 95°C for 30
sec and 95°C for 5 sec followed by 40 cycles of 60°C for 30 sec and
analyzed by fluorescence quantitative thermal cycling PCR
(Bio-Rad). The PCR of human β-actin was performed as a control.
TGF-β1, Smad7 mRNA expression levels were detected. The data were
calculated by the 2−ΔΔCT method.
Apoptosis analysis
To detect early apoptotic changes, LX2 cells were
cultured alone or co-cultured with hUC-MSCs for 24 or 48 h, as
described previously. Apoptotic cell death was detected by Annexin
V/propidium iodide (PI) staining using the MEBCYTO apoptosis kit
(MBL, Nagoya, Japan). LX2 cells from the various cultures were
digested with 2.5 g/l trypsin, harvested, washed and resuspended in
300 μl binding buffer, followed by incubation with 5
μl Annexin V-FITC and 5 μl PI at room temperature for
15 min in the dark. Following incubation, 200 μl binding
buffer was added and the cell samples were measured using flow
cytometry (BD Biosciences, New Jersey, NY, USA).
Western blotting
Cells were harvested in 0.15 ml
radio-immunoprecipitation assay (RIPA) lysis buffer with protease
inhibitors and centrifuged at 12,000 rpm for 5 min. The
supernatants were assayed for protein concentration. Protein
content was measured by BCA protein concentration determination
kit. The sample size was 30 μg. Protein samples were heated
at 100°C for 10 min before loading and the samples were subjected
to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred to PVDF membranes. Membranes were
blocked with 5% skimmed milk powder in TBST buffer (20 mmol/l Tris,
500 mmol/l NaCl, and 0.1% Tween-20) for 2 h at 37°C with gentle
shaking. Membranes were incubated overnight at 4°C with various
primary antibodies. The following primary antibodies were used:
1:1,000 rabbit polyclonal anti-TGF-β1, 1:1,000 rabbit polyclonal
anti-Smad7, and 1:1,000 rabbit polyclonal anti-Smad3. The membranes
were washed with TBST buffer and incubated in the appropriate
peroxidase-conjugated secondary antibody solution at a 1:5,000
dilution before they were finally developed with enhanced
chemiluminescence. The density of the individual bands was
quantified using a densitometric scanner with Gel-pro Analyzer
(Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
All statistical calculations were performed using
GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). The
data are presented as the mean ± SD. When applicable, Student's
unpaired t-test, one-way ANOVA and Holm-Sidak test were used to
determine significance. p<0.05 was considered statistically
significant.
Results
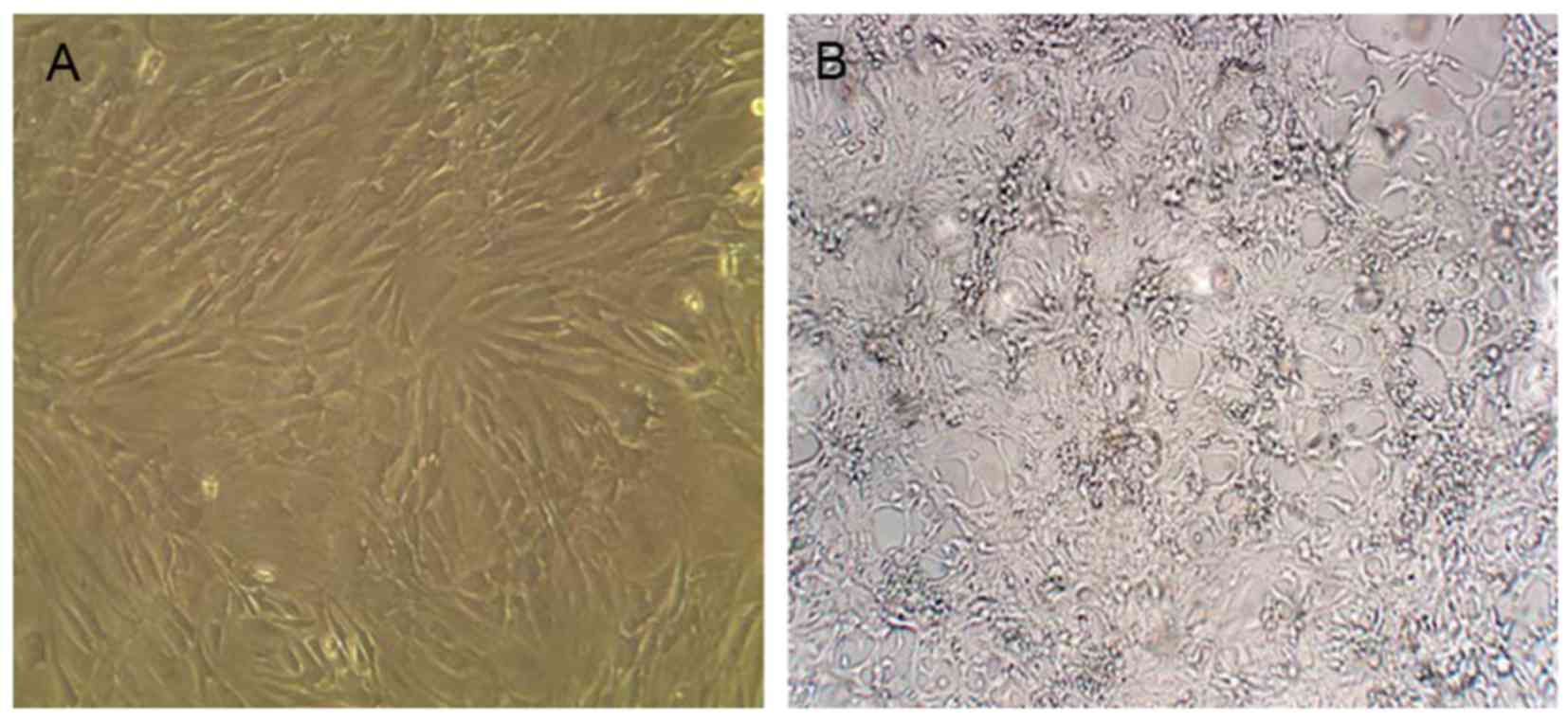
Morphological changes and growth of
cells
Umbilical cords tissue block attaching to the wall
was observed after 1-2 days, and some cells crawled out of the
tissue block. The cultured hUC-MSCs became spindle-shaped on day 6.
The cells had a long fusiform or flat shape after ~10 days. The
fused cells were elongated and similar to fibroblasts that were in
parallel or spiral-like growth after 21 days and cell fusion was
80% (Fig. 1A). The third passage
cells were for transplant spare.
LX2 cells reached >80% cell fusion after 5-8
days. The growth of cells retained its original status without
change of culture medium for 1-2 days. After 3 days, cell growth
entered the logarithmic phase, and reached a plateau after 5 days
(Fig. 1B). LX2 showed obvious
growth and proliferation when spared 3-4 generations were used in
the experiment.
The upper and lower double-cell co-culture system
was established between hUC-MSCs and HSCs as the experimental
group. HSCs were cultured alone as a negative control group.
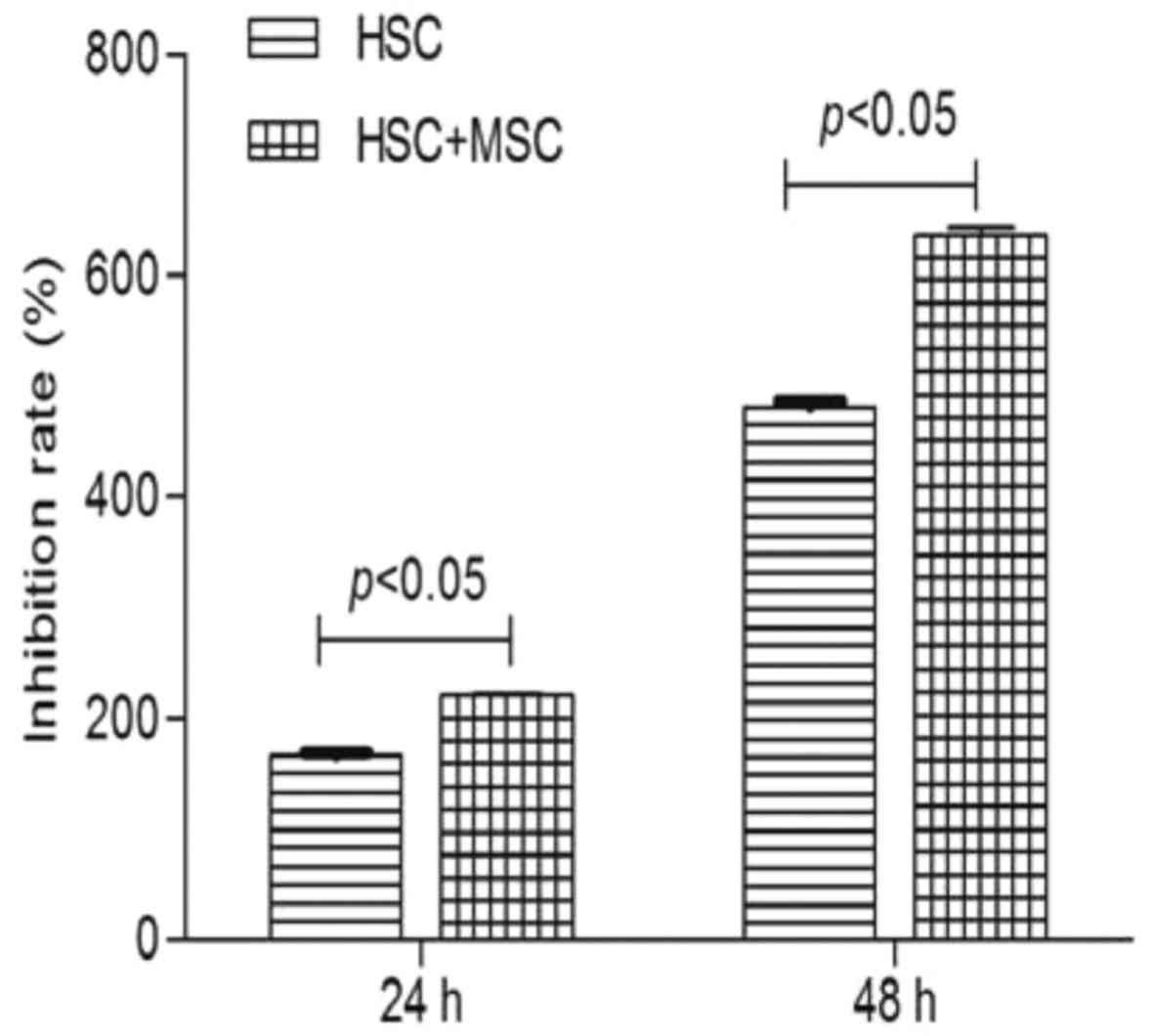
Inhibition of HSC proliferation by
hUC-MSCs
We detected the absorbance of hUC-MSCs on HSCs by
MTT assay after being cultured for 24 and 48 h. Cell growth
inhibition rates (average absorbance of each inhibited
group/non-inhibited group) were then calculated. The inhibition
rate of hUC-MSCs on HSCs at 24 and 48 h was 2.21±0.02 and
6.37±0.06%, respectively. A significant difference was observed
between the co-culture experimental group (2.21±0.02 and
6.37±0.06%) and negative control group (1.66±0.02 and 4.82±0.05%)
at 24 h (p<0.05) and 48 h (p<0.05) (Fig. 2). hUC-MSCs significantly inhibited
the proliferation of HSCs in a time-dependent manner at 24 and 48
h.
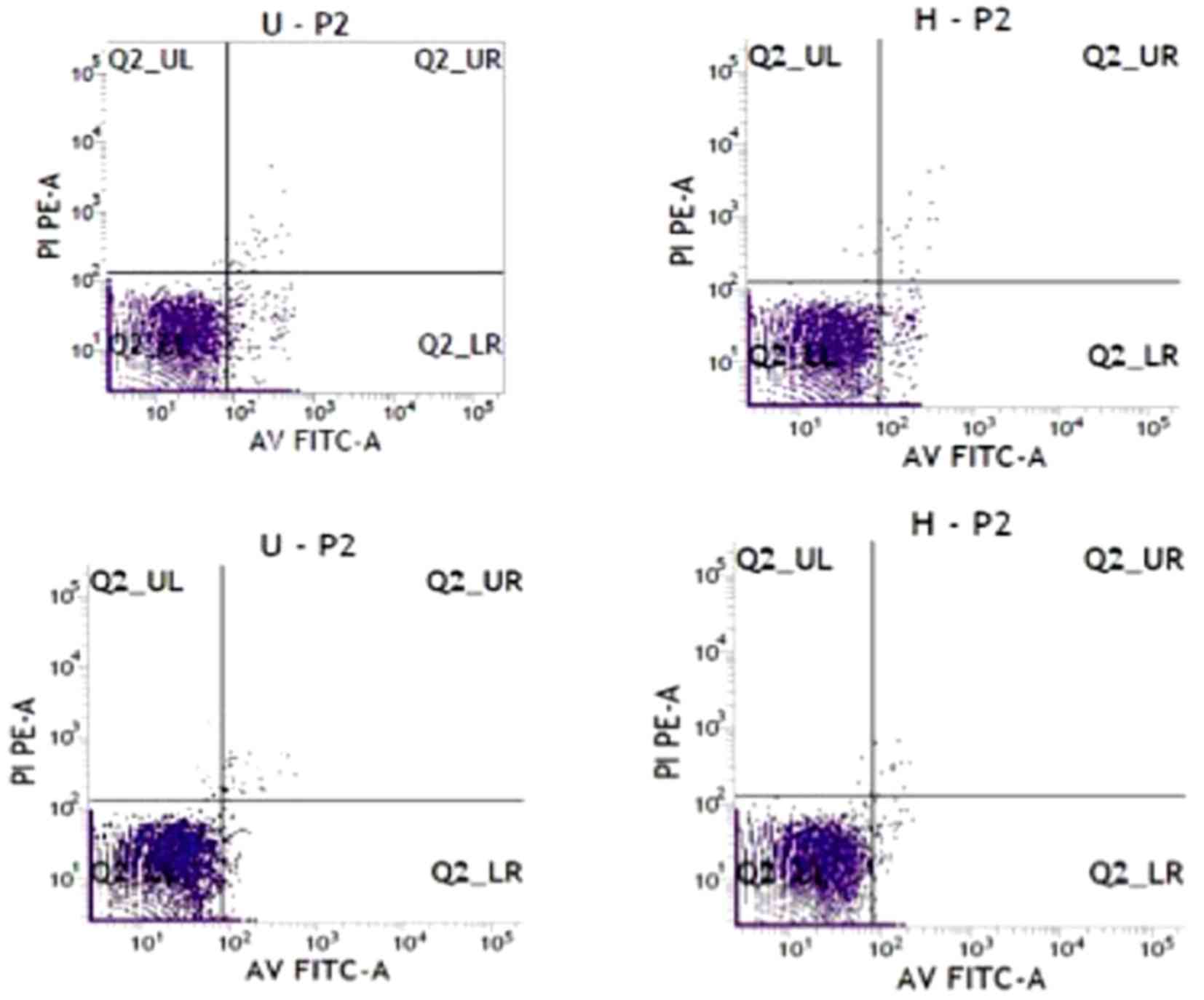
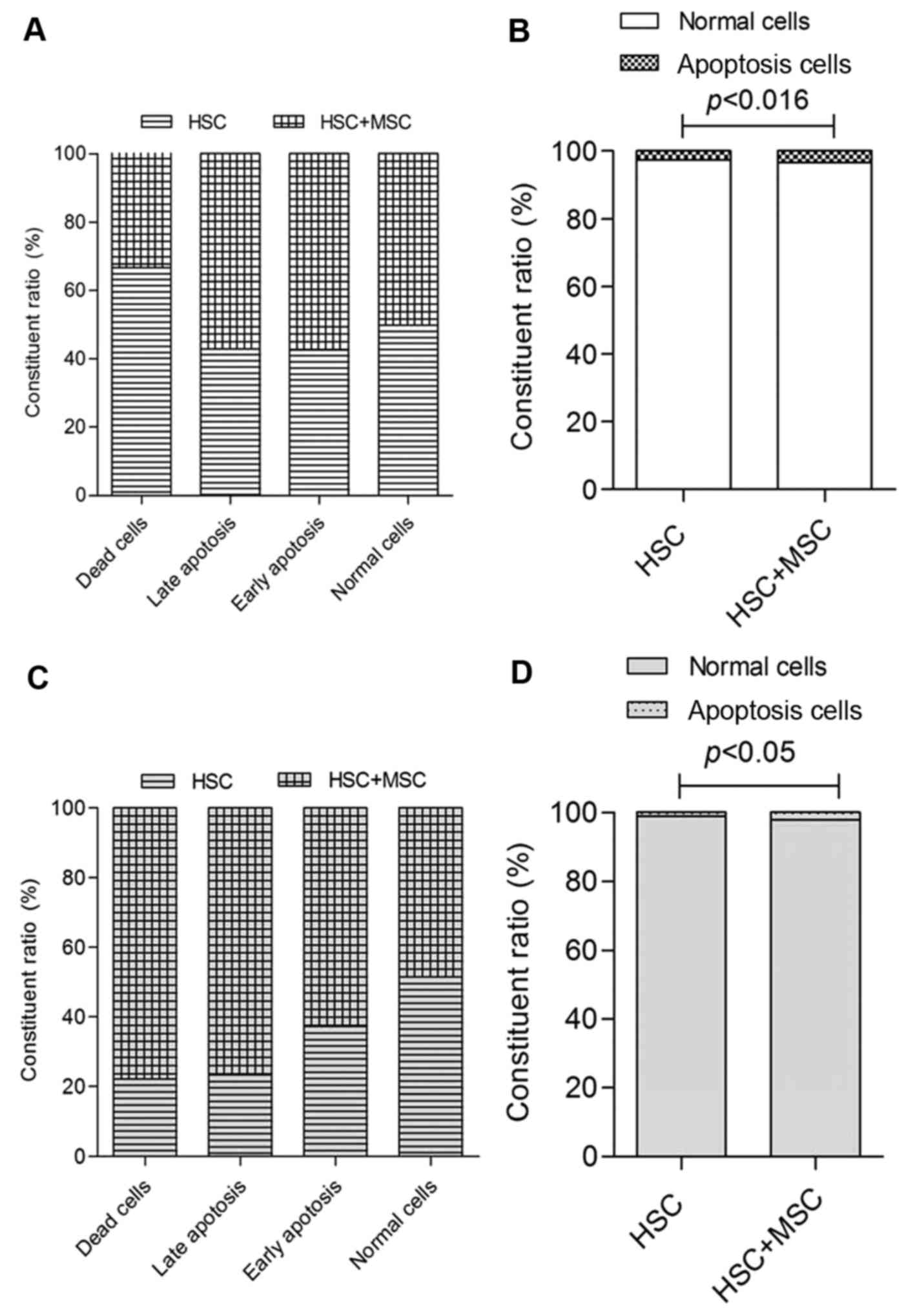
Apoptosis of HSCs induced by
hUC-MSCs
After hUC-MSCs co-cultured with LX2 for 24 and 48 h,
the cells were double stained with Annexin V-FITC and PI to detect
apoptosis rate of LX2 using flow cytometry. Apoptosis of LX2 cells
in the co-culture system was significantly increased compared with
the control group (Figs. 3 and
4). The effect of hU-MSCs on LX2
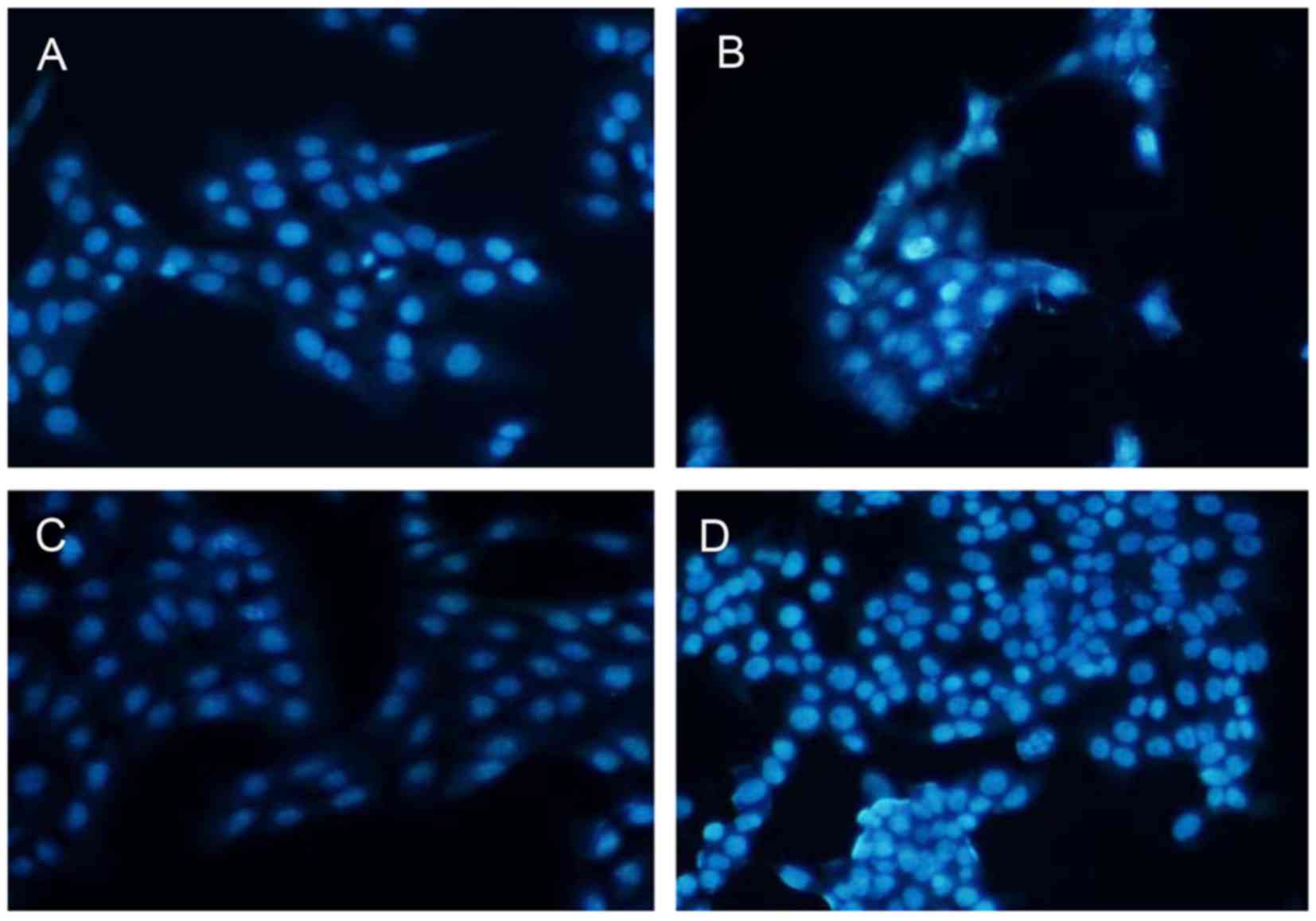
cell apoptosis was evaluated by Hoechst 33342 staining. Apoptotic
cells demonstrating nuclear condensation were detected by Hoechst
33342 staining and fluorescence microscopy. As illustrated in
Fig. 5, co-culture of LX2 cells
and hU-MSCs for 24 h showed more cells with condensed and
fragmented nuclei than in the negative control group. Similar
results were obtained at 48 h. The number of apoptotic bodies in
the experimental group was significantly increased compared with
the control group.
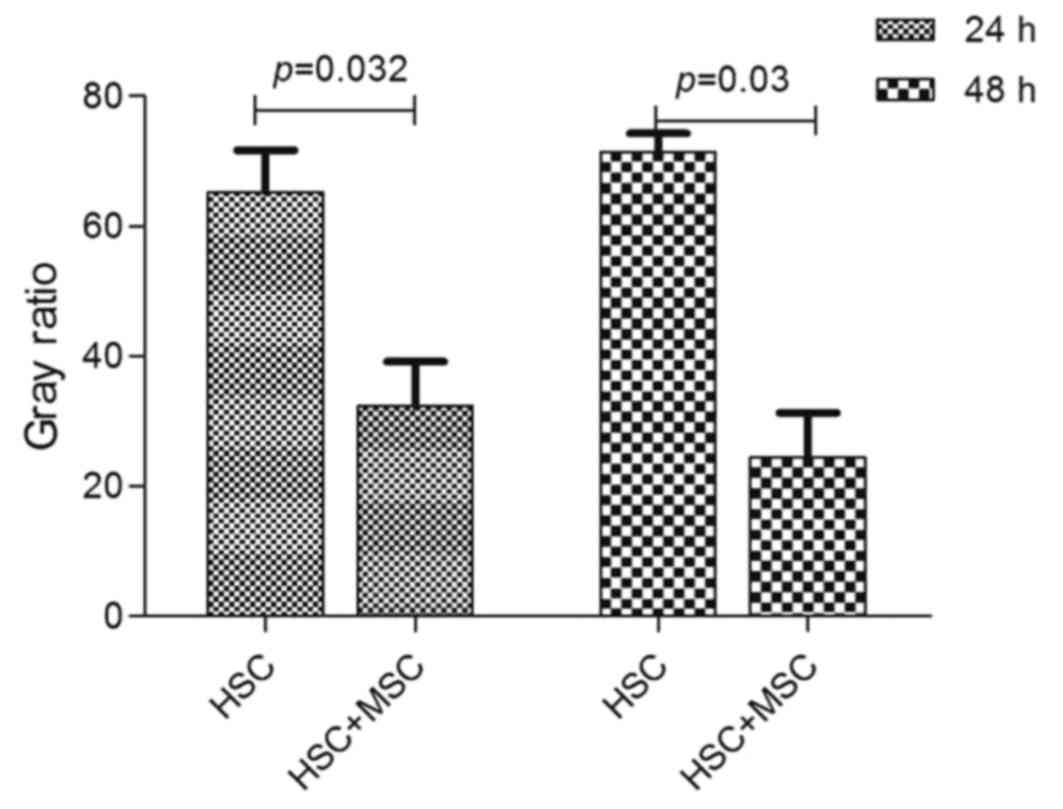
Decreased level of TGF-β1 caused by
hUC-MSCs
TGF-β1 is a cytokine that plays a central role in
fibrosis. To investigate whether hU-MSCs affected TGF-β1 production
in the co-culture system, the cells were subjected to ELISA to
measure production of the profibrotic cytokine TGF-β1 by LX2.
TGF-β1 protein in LX2 was significantly decreased in the co-culture
group compared with the negative control group at 24 h, with
similar results at 48 h (p<0.05) (Fig. 6).
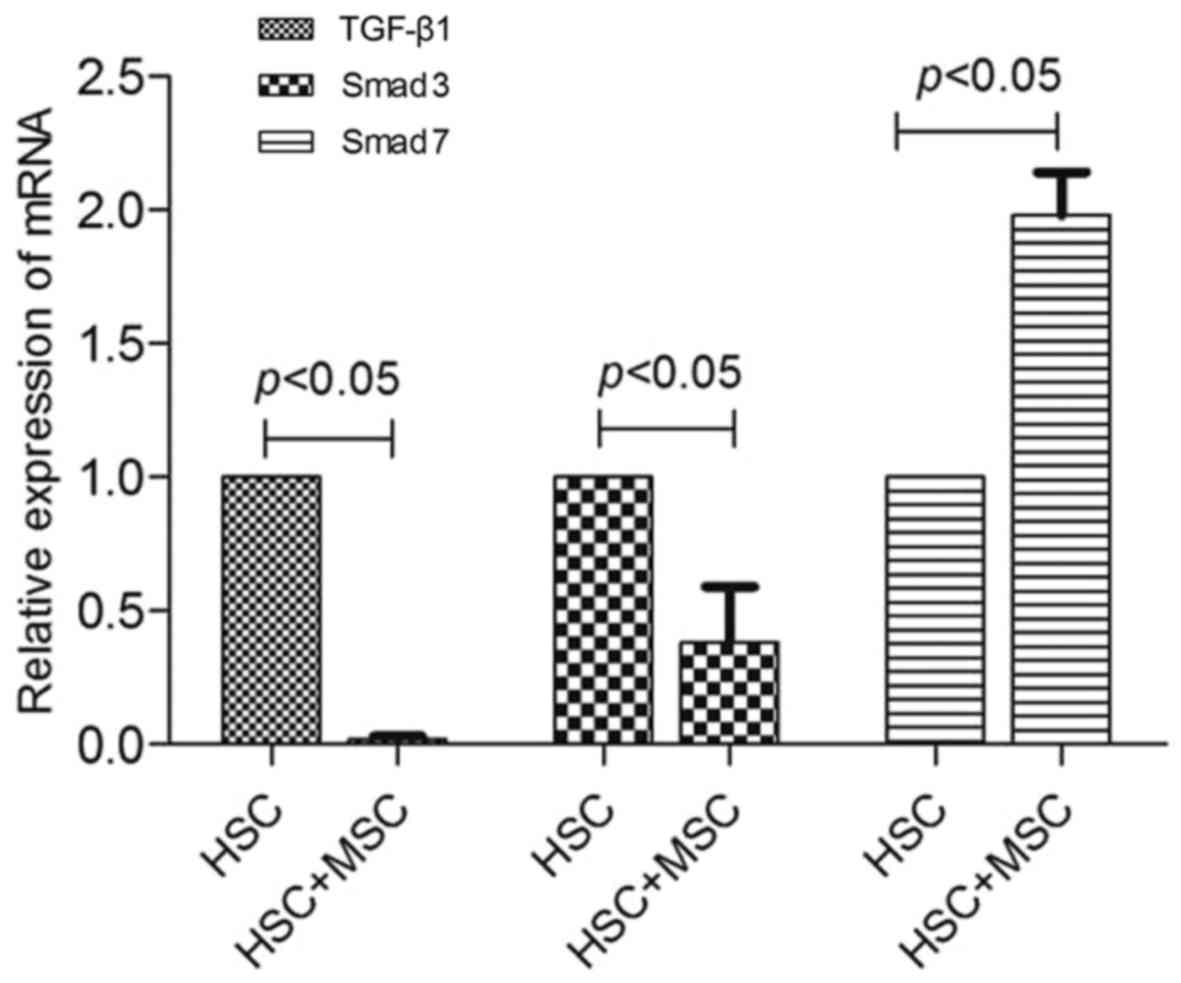
The expression of TGF-β1, Smad3 and Smad7
mRNA
TGF-β1, Smad3 and Smad7 mRNA expression in LX2 cells
was determined by RT-PCR. After 24 h co-culture, TGF-β1 and Smad3
mRNA expression in the experimental group was significantly lower
than that in the negative control group (p<0.05), but Smad7 mRNA
expression increased compared with that in the negative control
group (p<0.05) (Fig. 7). LX2
cells synthesized and secreted less TGF-β1 and Smad3 and more Smad7
after being co-cultured with hUC-MSCs.
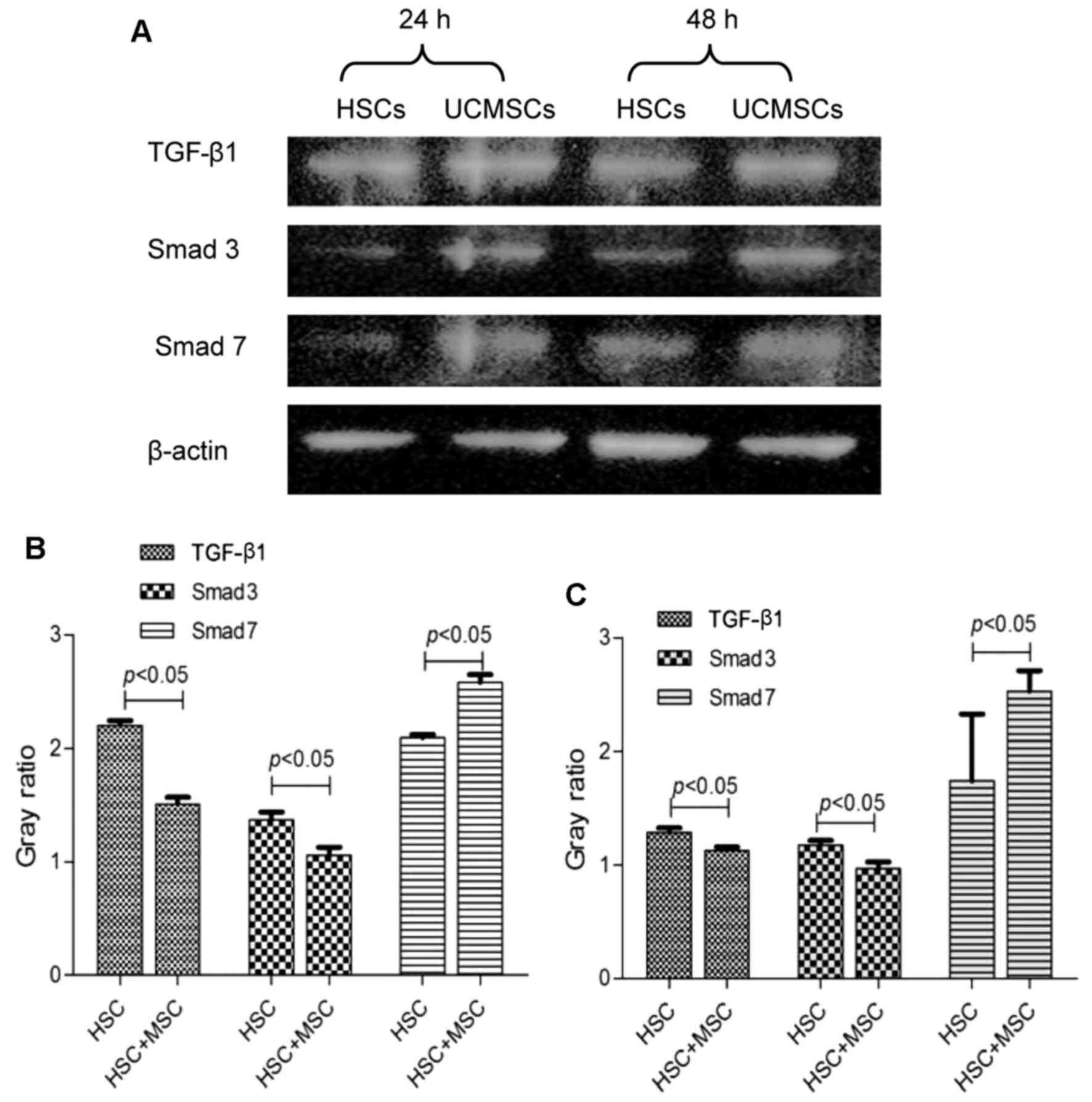
Protein expression of TGF-β1, Smad3 and
Smad7 by hUC-MSCs
After the hUC-MSCs were co-cultured with LX2 cells
for 24 h, TGF-β1 and Smad3 protein began to decrease, and their
expression (1.51±0.06 and 1.06±0.07) was significantly lower than
that in the control group (2.20±0.04 and 1.37±0.07) (p<0.05).
Similar results were found at 48 h, their expression (1.13±0.03 and
0.97±0.06) was significantly lower than that in the control group
(1.29±0.04 and 1.18±0.04) (p<0.05). After 24 and 48 h
co-culture, Smad7 protein expression in the experimental group
(2.58±0.07 and 2.35±0.18) increased significantly compared with
that in the control group (2.09±0.13 and 1.74±0.59) (p<0.05)
(Fig. 8).
Discussion
Our study is believed to be the first to show that
hUC-MSCs inhibit the proliferation of HSCs by affecting the
TGF-β/Smads pathway, and the formation of liver fibrosis. It is
well known that HSCs are the main source of ECM, and the
proliferation and activation of HSCs may promote the occurrence of
hepatic fibrosis. It has been shown previously that the MSCs may be
affected by the signal transduction pathway during activation of
HSCs (10), but the effect of
hUC-MSCs on HSCs is not clear. This study confirmed that using an
in vitro model, hUC-MSCs could inhibit proliferation of HSCs
by inhibiting the TGF-β/Smads pathway.
Prior to this study, we used BM-MSCs to inhibit
liver fibrosis (11). However,
compared with BM-MSCs, hUC-MSCs are more primitive, have greater
differentiation ability and low immunogenicity, are not subject to
ethical constraints, and are easy to culture through a noninvasive
procedure. Røsland et al (12) reported a 45.8% rate of spontaneous
malignant transformation during culture of BM-MSCs. It has been
suggested that spontaneous malignant transformation represents a
biohazard in long-term ex vivo expansion of BM-MSCs, but
hUC-MSCs propagating in continuous culture ultimately enter
senescence and are not susceptible to spontaneous malignant
transformation (13). Therefore,
we chose hUC-MSCs for this study. We separated the hUC-MSCs from
the umbilical cord for culture, and verified their ability to
differentiate into fat cells in vitro.
The proliferation and activation of HSCs is an
important step in the development of hepatic fibrosis (14). We would like to understand further
whether hUC-MSCs can inhibit the proliferation of HSCs by
regulating their proliferation. We used Transwell migration assay
to co-culture hUC-MSCs and HSCs, and confirmed that hUC-MSCs
inhibited the proliferation of HSCs. We then used the the flow
cytometry technique to verify that the increase in HSC inhibition
was the result of apoptosis of hUC-MSCs and not the result of death
of HSCs. Finally, we directly observed apoptosis of HSCs in
co-culture with hUC-MSCs by Hoechst staining. At the same time, we
concluded that these effects were not through direct contact among
cells, but rather by cytokines secreted into the culture medium.
ELISA showed that hUC-MSCs secreted low levels of TGF-β1, while
HSCs secreted a large amount, and the TGF-β1 levels of co-cultured
HSCs were significantly decreased. These results are similar to
those reported previously in a study of low levels of TGF-β1 in
liver cirrhosis (15).
Previous studies have indicated that hUC-MSC therapy
results in significant improvement of liver function and hepatic
fibrosis, but the specific mechanism is still unclear.
Liver fibrosis is related to gene expression of many
cytokines, such as TGF-β, platelet-derived growth factor,
endothelin, fibroblast growth factor, connective tissue growth
factor and leptin, and there are multiple signaling pathways
involved in the formation of liver fibrosis (16-19). TGF-β is a cytokine that causes
hepatic fibrosis and plays an important role in the activation of
muscle fibroblasts. It has been shown that, in the 6 weeks after
CCl4-induced liver fibrosis, TGF-β1 levels in the serum
and liver increase (9). The
TGF-β/Smads signal transduction pathway is the most important in
liver fibrosis, therefore, we are also interested in the effects of
hUC-MSCs on the pathway. The Smads protein family is located on
HSCs and is divided into receptor activation and inhibitory Smads.
The former includes Smad3, which can transfer the signal from the
cytoplasm to the nucleus, and promote formation of liver fibrosis.
Inhibitory Smads include Smad7, which can inhibit the formation of
Smads complexes and the signal transduction process, thereby
inhibiting the formation of fibrosis (20). Many studies have shown that
activation of the TGF-β/Smads signaling pathway can induce collagen
deposition (21). Our experiments
found that the levels of TGF-β1 and Smad2 secreted by HSCs
decreased after co-culture, while the concentration of Smad7
increased. These data confirmed that hUC-MSCs inhibit the
TGF-β1/Smads pathway to inhibit proliferation and promote apoptosis
of HSCs, thereby inhibiting the formation of liver fibrosis.
There are still a few issues to resolve. For
example, will similar results be obtained in vivo. Previous
studies have shown that inhibition of the TGF-β1/Smad pathway may
lead to tumor occurrence (22).
This needs further research.
In conclusion, this study is believed to be the
first to demonstrate hUC-MSCs inhibit proliferation and induce
apoptosis of HSCs by paracrine inhibition of the TGF-β1/Smads
pathway. Our results indicate the potential of hUC-MSCs as a method
for the treatment of liver fibrosis. However, the complexity of the
mechanism requires further study.
Abbreviations:
|
MSC
|
mesenchymal stem cells
|
|
hUC-MSCs
|
human umbilical cord mesenchymal stem
cells
|
|
BMSCs
|
bone marrow mesenchymal stem cells
|
|
ECM
|
extracellular matrix
|
|
TGF-β
|
transforming growth factor-β
|
|
MTT
|
3-(4,5-dimethylthiazol-2-2yl)-2,5-diphenyltetrazolium bromide
|
|
ELISA
|
enzyme linked immunosorbent assay
|
|
PBS
|
phosphate-buffered saline
|
|
FBS
|
fetal bovine serum
|
|
DMEM-LG
|
Dulbecco's modified Eagle's medium-low
glucose
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
RIPA
|
radio-immunoprecipitation assay
|
|
SDS-PAGE
|
sodium dodecyl sulfate-polyacrylamide
gel electrophoresis
|
|
PVDF
|
polyvinylidene fluoride
|
|
TBST
|
Tris-buffered saline Tween-20
|
Acknowledgments
Not applicable.
Notes
[1]
Funding
This study was supported by the Natural Science
Foundation of Gansu Province, China (grant no. 1506RJZA263) and the
Major Projects of Science and Technology of Gansu Province, China
(grant no. 1302FKDA029).
[2] Availability
of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
LTZ and JFL conceived and designed the experiments.
XBP and XQF performed the experiments. XBP, HC and XRM analyzed the
data. XBP wrote the paper.
[4] Ethics
approval and consent to participate
The study protocol was conducted in accordance with
the provisions of the Declaration of Helsinki, 1975 and approved by
the Institutional Review Board of the First Hospital of Lanzhou
University.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Volarevic V, Nurkovic J, Arsenijevic N and
Stojkovic M: Concise review: Therapeutic potential of mesenchymal
stem cells for the treatment of acute liver failure and cirrhosis.
Stem Cells. 32:2818–2823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berardis S, Dwisthi Sattwika P, Najimi M
and Sokal EM: Use of mesenchymal stem cells to treat liver
fibrosis: Current situation and future prospects. World J
Gastroenterol. 21:742–758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang L, Ye JS, Decot V, Stoltz JF and de
Isla N: Research on stem cells as candidates to be differentiated
into hepatocytes. Biomed Mater Eng. 22:105–111. 2012.PubMed/NCBI
|
|
4
|
Secunda R, Vennila R, Mohanashankar AM,
Rajasundari M, Jeswanth S and Surendran R: Isolation, expansion and
characterisation of mesenchymal stem cells from human bone marrow,
adipose tissue, umbilical cord blood and matrix: A comparative
study. Cytotechnology. 67:793–807. 2015. View Article : Google Scholar :
|
|
5
|
Berardis S, Lombard C, Evraerts J, El
Taghdouini A, Rosseels V, Sancho-Bru P, Lozano JJ, van Grunsven L,
Sokal E and Najimi M: Gene expression profiling and secretome
analysis differentiate adult-derived human liver stem/progenitor
cells and human hepatic stellate cells. PLoS One. 9:e861372014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pan RL, Wang P, Xiang LX and Shao JZ:
Delta-like 1 serves as a new target and contributor to liver
fibrosis down-regulated by mesenchymal stem cell transplantation. J
Biol Chem. 286:12340–12348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burra P, Arcidiacono D, Bizzaro D, Chioato
T, Di Liddo R, Banerjee A, Cappon A, Bo P, Conconi MT, Parnigotto
PP, et al: Systemic administration of a novel human umbilical cord
mesenchymal stem cells population accelerates the resolution of
acute liver injury. BMC Gastroenterol. 12:882012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakaida I, Terai S, Yamamoto N, Aoyama K,
Ishikawa T, Nishina H and Okita K: Transplantation of bone marrow
cells reduces CCl4-induced liver fibrosis in mice.
Hepatology. 40:1304–1311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li T, Yan Y, Wang B, Qian H, Zhang X, Shen
L, Wang M, Zhou Y, Zhu W, Li W, et al: Exosomes derived from human
umbilical cord mesenchymal stem cells alleviate liver fibrosis.
Stem Cells Dev. 22:845–854. 2013. View Article : Google Scholar :
|
|
10
|
Eom YW, Shim KY and Baik SK: Mesenchymal
stem cell therapy for liver fibrosis. Korean J Intern Med.
30:580–589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang LT, Fang XQ, Chen QF, Chen H, Xiao
P, Peng XB, Zhang SX, Li JF and Mao XR: Bone marrow-derived
mesenchymal stem cells inhibit the proliferation of hepatic
stellate cells by inhibiting the transforming growth factor β
pathway. Mol Med Rep. 12:7227–7232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Røsland GV, Svendsen A, Torsvik A, Sobala
E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R,
Lønning PE, et al: Long-term cultures of bone marrow-derived human
mesenchymal stem cells frequently undergo spontaneous malignant
transformation. Cancer Res. 69:5331–5339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang Q, Chen Q, Lai X, Liu S, Chen Y,
Zheng Z, Xie Q, Maldonado M, Cai Z, Qin S, et al: Malignant
transformation potentials of human umbilical cord mesenchymal stem
cells both spontaneously and via 3-methycholanthrene induction.
PLoS One. 8:e818442013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Yu X, Chen E and Li L:
Liver-derived human mesenchymal stem cells: A novel therapeutic
source for liver diseases. Stem Cell Res Ther. 7:712016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim WH, Matsumoto K, Bessho K and Nakamura
T: Growth inhibition and apoptosis in liver myofibroblasts promoted
by hepatocyte growth factor leads to resolution from liver
cirrhosis. Am J Pathol. 166:1017–1028. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao YL, Zhu RT and Sun YL:
Epithelial-mesenchymal transition in liver fibrosis. Biomed Rep.
4:269–274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Borkham-Kamphorst E, Herrmann J, Stoll D,
Treptau J, Gressner AM and Weiskirchen R: Dominant-negative soluble
PDGF-beta receptor inhibits hepatic stellate cell activation and
attenuates liver fibrosis. Lab Invest. 84:766–777. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saxena NK, Titus MA, Ding X, Floyd J,
Srinivasan S, Sitaraman SV and Anania FA: Leptin as a novel
profibrogenic cytokine in hepatic stellate cells: Mitogenesis and
inhibition of apoptosis mediated by extracellular regulated kinase
(Erk) and Akt phosphorylation. FASEB J. 18:1612–1614. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lang T, Ikejima K, Yoshikawa M, Enomoto N,
Iijima K, Kitamura T, Takei Y and Sato N: Leptin facilitates
proliferation of hepatic stellate cells through up-regulation of
platelet-derived growth factor receptor. Biochem Biophys Res
Commun. 323:1091–1095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hata A and Chen YG: TGF-β signaling from
receptors to Smads. Cold Spring Harb Perspect Biol. 8:a0220612016.
View Article : Google Scholar
|
|
21
|
Argentou N, Germanidis G, Hytiroglou P,
Apostolou E, Vassiliadis T, Patsiaoura K, Sideras P, Germenis AE
and Speletas M: TGF-β signaling is activated in patients with
chronic HBV infection and repressed by SMAD7 overexpression after
successful antiviral treatment. Inflamm Res. 65:355–365. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu B, Zhou YN, Li Q, Wu ZQ, Zhang ZY, Ji
R, Guo QH and Liu W: Correlations of TGF-betaRII, Smad4 and Smad7
expression to clinicopathologic characteristics and prognosis of
gastric cancer. Ai Zheng. 28:538–542. 2009.In Chinese. PubMed/NCBI
|