Introduction
Intestinal ischemia/reperfusion (I/R) injury is a
common clinical event that occurs during surgery for abdominal
aortic aneurysm, mesenteric artery occlusion, small bowel
transplantation, cardiopulmonary bypass, strangulated hernias,
trauma, and shock (1). Acute
intestinal ischemia is a life-threatening emergency that is
associated with substantial morbidity and mortality (2). However, the exact mechanism involved
in the pathogenesis of acute intestinal ischemia has not yet been
clearly defined. The intestinal mucosal barrier is a complex entity
comprising different structural, mechanical, physical, immune,
biological and chemical components, among others. Due to the
presence of intestinal epithelial cells (IECs) and intestinal
intraepithelial lymphocytes (IELs) in the intestinal mucosa, the
epithelial layer is a key factor that determines the barrier
function of the intestinal mucosa (3,4).
IELs mainly consist of T cells, which are interspersed among the
IECs in the small intestine; there are ~20 IELs for every 100 IECs
in the small intestine (5). IELs
play an important role in the maintenance of mucosal homeostasis,
as they form the first line of immune defense against invasion,
while they also preserve the integrity of the mucosal barrier
(6). I/R-induced acute intestinal
mucosal damage significantly promotes apoptosis of IELs and affects
the phenotype and function of IELs (7). The immune function of IELs may be
regulated by IECs. In order for the intestinal barrier to function
properly, there must be communication between the IELs and the
IECs, which is initiated through the activation of IECs by direct
contact or through the effects of secreted factors on IELs,
maintaining their growth and metabolism. However, the mechanism
underlying this dialogue remains unclear (8).
Bone morphogenetic proteins (BMPs) play a pivotal
role in the patterning and cellular fate of numerous tissues and
organs, including the intestinal mucosal epithelium (9). Over 30 BMPs have been reported, and
they all belong to the larger transforming growth factor (TGF)-β
family. BMP signaling includes the canonical Smad signaling pathway
and the non-canonical mitogen-activated protein kinase and nuclear
factor (NF)-κB signaling pathways (10). BMPs are primarily found in
epithelial cells (ECs) in the intestinal mucosal barrier. It was
previously reported that the abundance of BMP2, BMP4 and BMP
receptors at the protein level was greater in the total parenteral
nutrition (TPN) group compared with that in the control (11). In addition, hypoxia and I/R were
found to increase the expression of BMP2/4 and upregulated the
expression of the BMP receptors BMPRIA and BMPRII in an in
vivo mouse intestinal I/R model. I/R also activated the NF-κB
signaling pathway, which led to increased levels of inflammatory
factors, such as tumor necrosis factor (TNF)-α and interleukin
(IL)-6; this in turn aggravated the damage to the intestinal
mucosal barrier (12). Therefore,
the expression of BMP4 was increased in the intestinal mucosal
epithelial cells under simulated stress conditions. Moreover,
BMP2/4 signaling is involved in thymocyte development (13), and it has been reported that it
directly regulates fetal T-cell development through BMPRIA.
Additionally, use of the specific BMP antagonist NOGGIN revealed
that BMP2/4 signaling is a negative regulator of fetal T-cell
development (14). BMP4 treatment
of human-mouse fetal thymus organ culture resulted in a reduction
in the recovery of human cells and the inhibition of
CD34+ progenitor cell proliferation and differentiation
(15). NF-κB signaling proteins
comprise a family of pleiotropic transcription factors that are
involved in the embryonic development of various organs,
inflammation, the immune response, cell survival, proliferation and
differentiation (16).
Additionally, it was determined that NF-κB is activated within a
few hours after I/R, leading to upregulation of inflammatory
proteins (17).
IL-7 is produced by stromal cell components in the
bone marrow, thymus and in peripheral lymphoid compartments, and is
a master regulator of T-cell development and homeostasis (18,19). IL-7 binds to the IL-7R
heterodimer, which is composed of the IL-7-specific IL-7R α-chain
(CD127) and the γc-chain (CD132). IL-7 induces CD127, which is
linked to the activation of the janus kinase/signal transducer and
activator of transcription 5 (STAT5) signaling pathway. This
pathway is crucial for cell survival and the upregulation of
anti-apoptotic Bcl-2 proteins, which maintain T-cell homeostasis
and prevent the progression of inflammation (20,21). IL-7 signaling and the CD127
receptor were found to maintain the balance of regulatory T cells
in vivo (22). In the
intestine, IL-7 is produced by IECs and, in turn, IL-7 receptors
(CD127 and CD132) have been detected on IELs (23). The IL-7/IL-7R signaling pathway
plays a key role in the regulation of IELs (24), as IL-7 has been demonstrated to
increase the numbers and functional capacity of IELs. It was
previously demonstrated that TPN results in loss of IEC-derived
IL-7, which leads to a marked weakening in the function and
phenotype of IELs (25,26). IL-7 produced in the thymus is also
essential for the development of IELs (27). Therefore, IL-7/IL-7R signaling
appears to be crucial for the maintenance of the function and
growth of IELs.
It was reported that the interplay of BMP4 and IL-7
is key to the maintenance of the human thymic progenitor
population. BMP4 downregulates the expression of CD127 in the
precursor cell population, which leads to a reduction in the
proliferation and differentiation of CD34+ progenitor
cells. In addition, IL-7 stimulates the expansion and
differentiation of intrathymic precursors (13,15). Therefore, it was hypothesized that
the interplay between BMP4 and IL-7 regulates the number and
function of IELs following I/R. Our data indicated that IELs
express functional BMP receptors. BMP proteins activate the NF-κB
signaling pathway, which increases apoptosis, but they also inhibit
apoptosis induced by IL-7/IL-7R signaling and maintain the number
of IELs, indicating that the interplay between the BMP4 and IL-7
signaling pathways is important in the dialogue between IECs and
IELs.
Materials and methods
Preparation of test substances
Anti-p-NF-κB-PE (cat. no. 9401S; 1:50) was purchased
from Cell Signaling Technology, Inc. (Boston, MA, USA).
Anti-CD45-APC (cat. no. MHCD4527; 1:200), anti-BMPRIA-FITC (cat.
no. 11-7301-34; 1:150), anti-BMPRIB-FITC (cat. no. 11-6587-72;
1:150) and anti-CD127-Alexa Fluor 488 (cat. no. 53-1271-80; 1:100)
were purchased from eBioscience (San Diego, CA, USA). The
anti-GAPDH antibody (cat. no. 10494-1-AP; 1:5,000) was purchased
from the ProteinTech group, Inc. (Chicago, IL, USA), whereas
anti-BMP4 (cat. no. sc-6267; 1:500), anti-IL7 (cat. no. sc-365306;
1:500) and anti-BMPRIA (cat. no. sc-293175; 1:200) were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Recombinant BMP4 protein (cat. no. 315-27; 30 ng/ml) and
recombinant murine IL-7 protein (cat. no. 217-17; 2 ng/ml) were
both purchased from ProteinTech Group, Inc. Pyrrolidine
dithiocarbamate (PDTC; cat. no. S1808; 30 ng/ml), an inhibitor of
NF-κB, was purchased from Beyotime Institute of Biotechnology
(Wuhan, China).
Cell culture
IEC-6 IECs were purchased from the American Type
Culture Collection (Manassas, VA, USA) and were grown in Dulbecco's
modified Eagle's medium (Hyclone, Logan, UT, USA) supplemented with
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Carlsbad, CA, USA), 100 IU/ml penicillin, and 100 mg/ml
streptomycin. IEC-6 cells were cultured at 37°C under normoxic (5%
Co2 and 20% O2) conditions (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). For the western blot analysis,
IL-7 was added to the medium for 6 h.
Western blot analysis
The cells were washed twice with phosphate-buffered
saline (PBS) prior to lysis in cold RIPA buffer [50 mM Tris, 150 mM
NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl
sulfate (SDS) and 2 mM sodium pyrophosphate]. The samples were then
mixed with loading buffer and boiled for 5 min prior to
electrophoresis. The proteins were loaded onto 8–10% SDS-PAGE gels
and were subjected to electrophoresis at 100 V for 2 h. After
electrophoresis, the proteins were electroblotted onto
nitrocellulose membranes at 200 mA for 2 h. Non-specific binding
was blocked by incubation in Tris-buffered saline (TBS) with 0.1%
Tween-20 (TBS-T) and 5% skimmed milk. The transferred membranes
were incubated overnight at 4°C with the following primary
antibodies: Anti-BMP4 (1:500), anti-BMPRIA (1:200) and anti-GAPDH
(1:5,000). After washing three times in TBS-T, the membranes were
incubated with anti-rabbit IgG (cat. no. BA1001; Zhongshan Bio-Tech
Co., Ltd., Beijing, China) conjugated to horseradish peroxidase at
a dilution of 1:3,000 in TBS-T containing 5% skimmed milk for 1 h
at 37°C. After three additional washes in TBS-T, the signals were
visualized with the SuperSignal West Pico Trial kit (Pierce
Biotechnology, Inc., Rockford, IL, USA) and detected with Image
Station 4000R (Kodak, Rochester, NY, USA).
Animal experiments
Male C57BL/6 pathogen-free mice, aged 6–8 weeks
(weight, 20–30 g), were purchased from the Laboratory Animal Center
of the Third Military Medical University (Chongqing, China), and
maintained in a temperature-, humidity- and light-controlled
environment. The mice were randomly assigned to one of two groups
(n=12/group), the first of which was the I/R group. Following
intraperitoneal anesthesia with 40 mg/kg pentobarbital, the abdomen
was opened at the midline, and the superior mesenteric artery was
occluded for 30 min using non-traumatic vascular clamps, followed
by predefined times of reperfusion. The second group was the sham
group, which included animals that were subjected to anesthesia and
laparotomy without ischemia. All mice were sacrificed after 6 h by
CO2 asphyxiation. The jejunal part of the small
intestine was cut along the longitudinal axis, washed in
physiological saline, and immediately frozen in liquid nitrogen and
stored at −70°C until use. The present study was approved by the
Ethics Committee of Xinqiao Hospital, the Third Military Medical
University. All animals were handled according to the guidelines
for the Care and Use of Laboratory Animals (NIH publication no.
85–23, revised 1996).
Immunofluorescence analysis
In the present study, 10-µm frozen sections
were cut from the jejunum and were fixed onto slides. After
fixation in 4% formaldehyde for 20 min, the sections were incubated
in 3% H2O2 for 30 min to quench endogenous
peroxidase activity. Non-specific binding was blocked with 5%
bovine serum albumin (BSA) in PBS for 30 min at room temperature.
The sections were then incubated overnight at 4°C in 3% BSA in PBS
with the following primary antibodies: Anti-BMP4 and anti-IL-7, at
a dilution of 1:50. The sections were washed three times with BSA
in PBS and were incubated with Alexa 488-conjugated goat
anti-rabbit antibody for 1 h at RT. After the nuclei were stained
with DAPI, images were captured and analyzed with a Leica TCSSP
confocal imaging system (Leica, Heidelberg, Germany).
Isolation of IELs from the small
intestine
The isolation of IELs was performed according to the
protocol of Mosley and Klein (28). Briefly, the small bowel was placed
in RPMI-1640 tissue culture media supplemented with 10% FBS (both
from Gibco; Thermo Fisher Scientific). The sections were cut into
5-mm pieces, washed in an extraction buffer, and incubated in the
same buffer with continuous brisk stirring at 37°C for 25 min. The
supernatant was filtered rapidly through a glass wool column. After
centrifugation, the pellets were purified in 40% isotonic Percoll
(GE Healthcare Biosciences, Pittsburgh, PA, USA) and reconstituted
in RPMI tissue culture media. The purity of the IELs was >95%,
as assessed by trypan blue exclusion staining. Isolated IELs were
counted with a Neubauer hemocytometer (Reichert Technologies,
Buffalo, NY, USA).
IEL cell culture
IELs were isolated from the small intestine and
grown in RPMI-1640 supplemented with 10% FBS, 100 IU/ml penicillin
and 100 mg/ml streptomycin. The IELs were cultured at 37°C under
normoxic (5% Co2 and 20% O2) conditions
(Thermo Fisher Scientific, Inc.), and the viability of the IELs
automatically decreased under culture without interference. For
flow cytometry, BMP4, NOGGIN, PDTC and IL-7 were added to the
medium for 6 h.
Flow cytometric analysis
The IEL phenotype was assessed with fluorescent
antibody staining detected by flow cytometry. The following
anti-mouse monoclonal antibodies were used: CD45-APC, BMPRIA-FITC,
BMPRIB-FITC and CD127-Alexa Fluor 488. Isotype-matched, irrelevant
antibodies were used as negative controls. The apoptotic ratios for
the IELs in the different groups were measured by flow cytometry
according to the manufacturer's instructions. Double staining for
FITC-Annexin V binding and cellular DNA using propidium iodide were
performed as described in the manufacturer's protocol. The
acquisition and analysis were performed with MoFlo (Beckman
Coulter, Brea, CA, USA).
Reverse trancsription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was isolated with TRIzol reagent (Takara
Biotechnology Co., Ltd., Dalian, China). Single-stranded cDNA was
synthesized with Moloney murine leukemia virus reverse
transcriptase (Takara Biotechnology Co., Ltd.) and then used for
PCR analysis. RT-PCR was performed with a SYBR PrimeScript RT kit
according to the manufacturer's instructions (Takara Biotechnology
Co., Ltd.). Amplifications were performed under the following
conditions: 94°C for 5 min, followed by 35 cycles at 94°C for 30
sec, 59°C for 30 sec, and 72°C for 30 sec, followed by 72°C for 10
min in a PCR System 7500 (Applied Biosystems, Carlsbad, CA, USA).
The following PCR primers were used: BMPRIA forward,
5′-CTTATTCTGCTGCTTGTGGTC-3′ and reverse, 5′-AGCGGTTAGACACGATTGG-3′;
BMPRIB forward, 5′-AGGGTCAGATTTTCAATGTCG-3′ and reverse,
5′-GAGGTCGGGCTTCTTGTCT-3′; BMPRII forward,
5′-GATACAGAATGTTGACAGGAGACAGG-3′ and reverse,
5′-GGAAATTGCAGGTTTGTAATGATCTC-3′; ActRIA forward,
5′-GGTTCCCAATGACCCAAGTTT-3′ and reverse,
5′-CGAGCGAGGTTAGGGTGGTT-3′; p-NF-κB forward,
5′-CCTCGGGACAAACAGCCTC-3′ and reverse, 5′-CACGGCGCGCTAAAGTAAAG-3′;
IKb forward, 5′-CCTCACCCTTCCCCAATAAT-3′ and reverse,
5′-GTGTGAATGGTGCCTGTGAC-3′; β-actin forward,
5′-ACCGTGAAAAGATGACCCAGATC-3′ and reverse,
5′-GCCACAGGATTCCATACCCAG-3′; RT-qPCR products were directly loaded
onto non-denaturing 2% agarose gels, stained with SYBR Safe
(Invitrogen Life Technologies; Thermo Fisher Scientific, Carlsbad,
CA, USA), and visualized under UV transillumination. The
specificity of the primers was determined by sequencing the
amplification products.
Statistical analysis
Statistical analyses were performed with SPSS 13.0
statistical software (SPSS Inc., Chicago, IL, USA). All data are
expressed as the means ± standard deviation. The results were
analyzed using analysis of variance. Statistical significance was
defined as P<0.05.
Results
The BMP signaling pathway is functionally
active in IELs
It has been previously reported that IEC dysfunction
induced by BMP2/4 activates NF-κB signaling following I/R (12). In a similar manner, the inhibition
of CD34+ progenitor cell differentiation under
stimulation of the BMP2/4 signaling pathway has also been
demonstrated (15). To determine
whether BMP4 signaling also activates NF-κB signaling in IELs, it
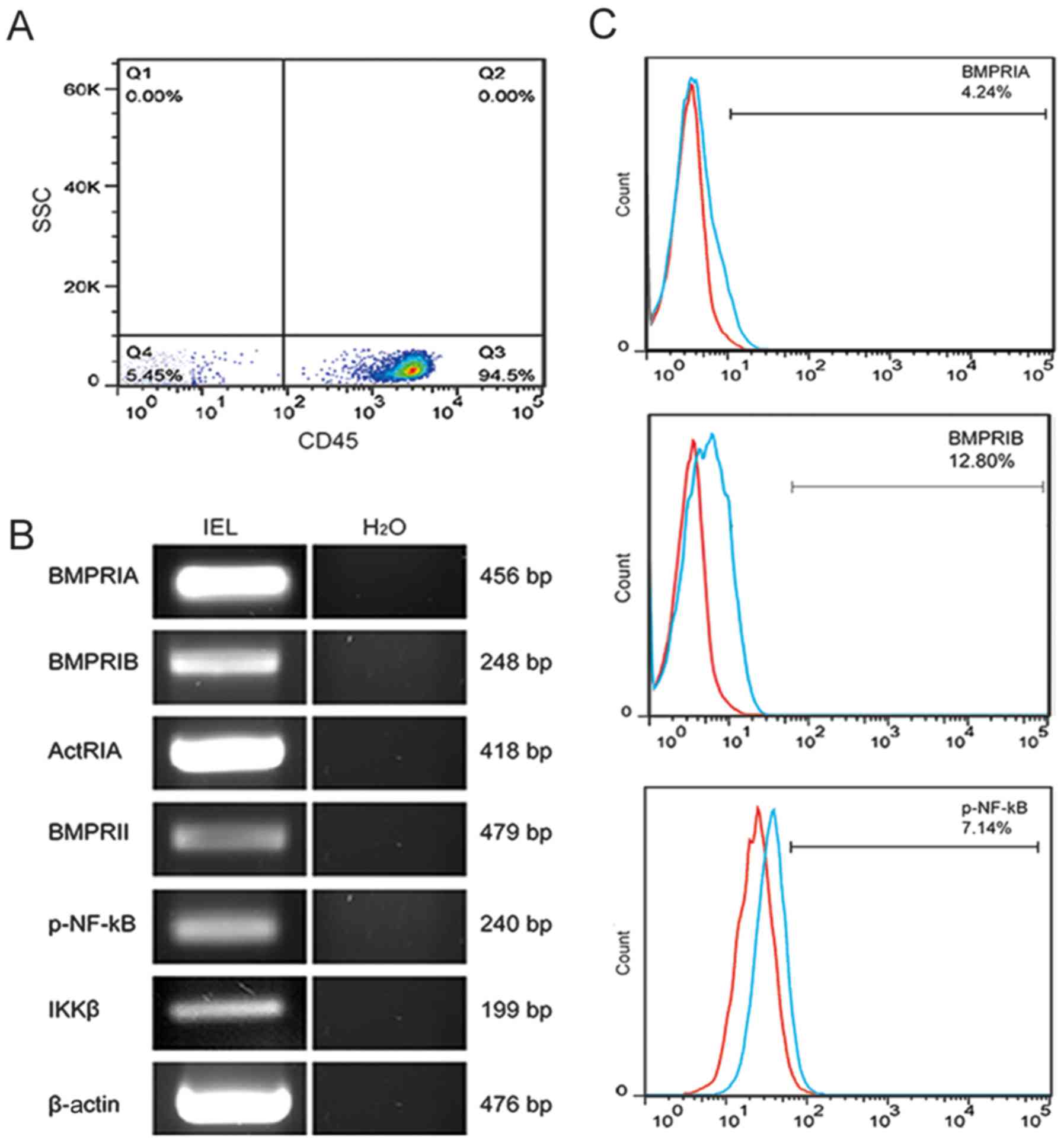
was first demonstrated by flow cytometry that the purity of IELs
isolated from the small intestine of mice reached 94.5%, as shown
in Fig. 1A. RT-PCR analysis
demonstrated that IELs expressed three type I BMP receptors
(BMPRIA, BaMPRIB and ActRIA), as well as one type II BMP receptor
(BMPRII). Similarly, IELs expressed specific RNA for p-NF-κB
(IKKβ), as shown in Fig. 1B. The
flow cytometric analysis revealed that the IELs expressed BMPRIA
(4.24±0.37%), BMPRIB (12.8±0.67%), and phosphorylated NF-κB (P65)
(7.14±1.36%), as shown in Fig.
1C. Therefore, it may be hypothesized that BMP binds to BMP
receptors and activates NF-κB signaling in IELs of the small
intestine.
I/R induces an increase the expression of
BMP4 in IECs and upregulates BMP receptors and the phosphorylation
of NF-κB in IELs
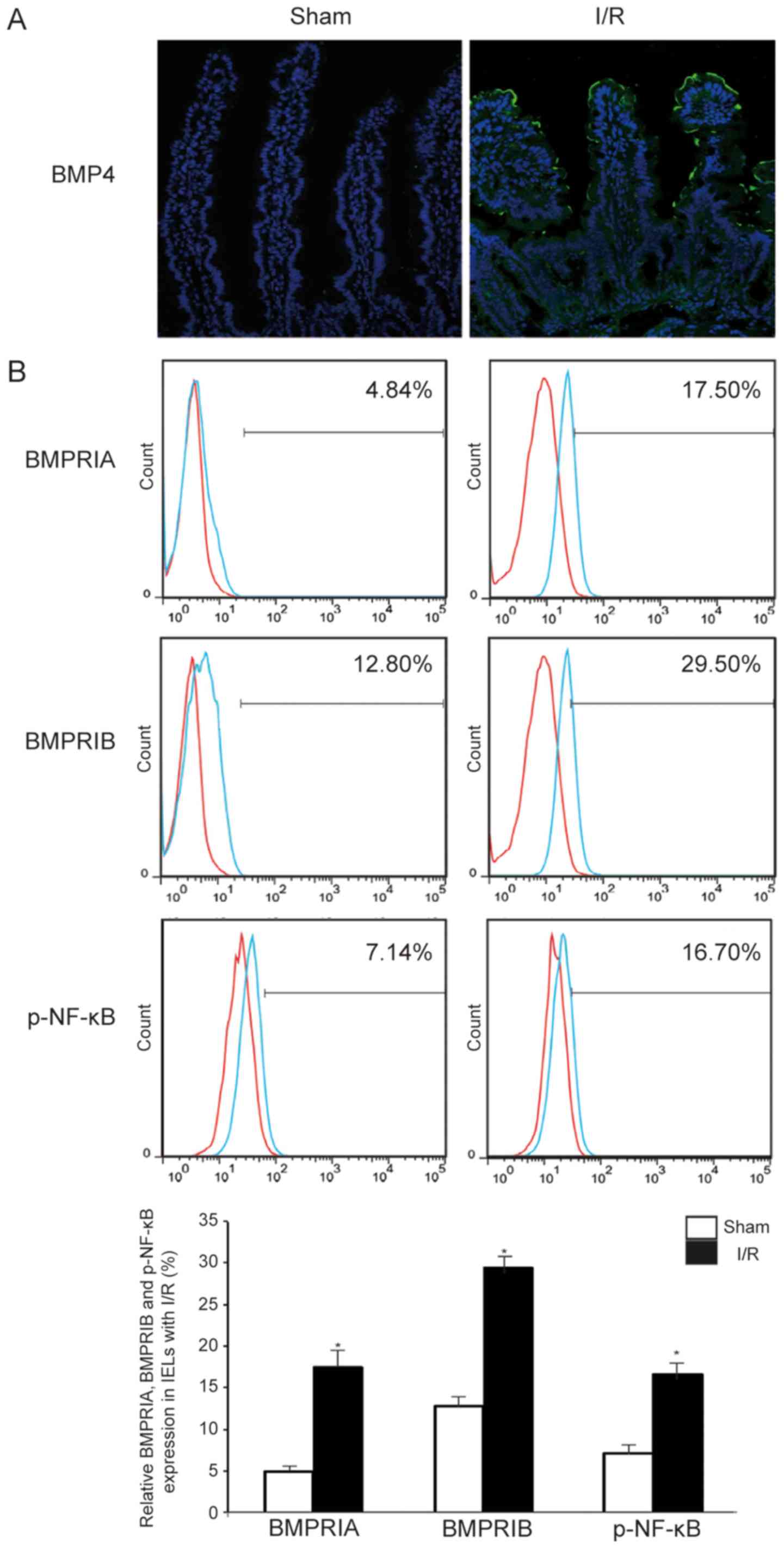
A previous study revealed more severe destruction of
the intestinal morphology and some changes in protein secretion 6 h
after I/R (29); moreover, the
expression of the BMP4 protein was determined in IECs. The protein
level of BMP4 in IECs was analyzed by immunofluorescence in the I/R
group compared with the sham group, and the expression level of
BMP4 was found to be markedly increased in IECs, as shown in
Fig. 2A. Moreover, it was
recently reported that I/R significantly altered IEL-derived
cytokine expression in IELs (7).
To better understand the changes in BMP receptors and NF-κB
signaling in IELs following I/R, flow cytometry was performed and
it demonstrated that the expression of BMPRIA, BMPRIB and
phosphorylated NF-κB increased following I/R (BMPRIA, 12.66±0.91%;
BMPRIB, 16.70±2.22%; and p-NF-κB, 8.56±2.87%), as shown in Fig. 2B. These data suggest that I/R
induces expression of IEC-derived BMP4 and activation of NF-κB
signaling in IELs.
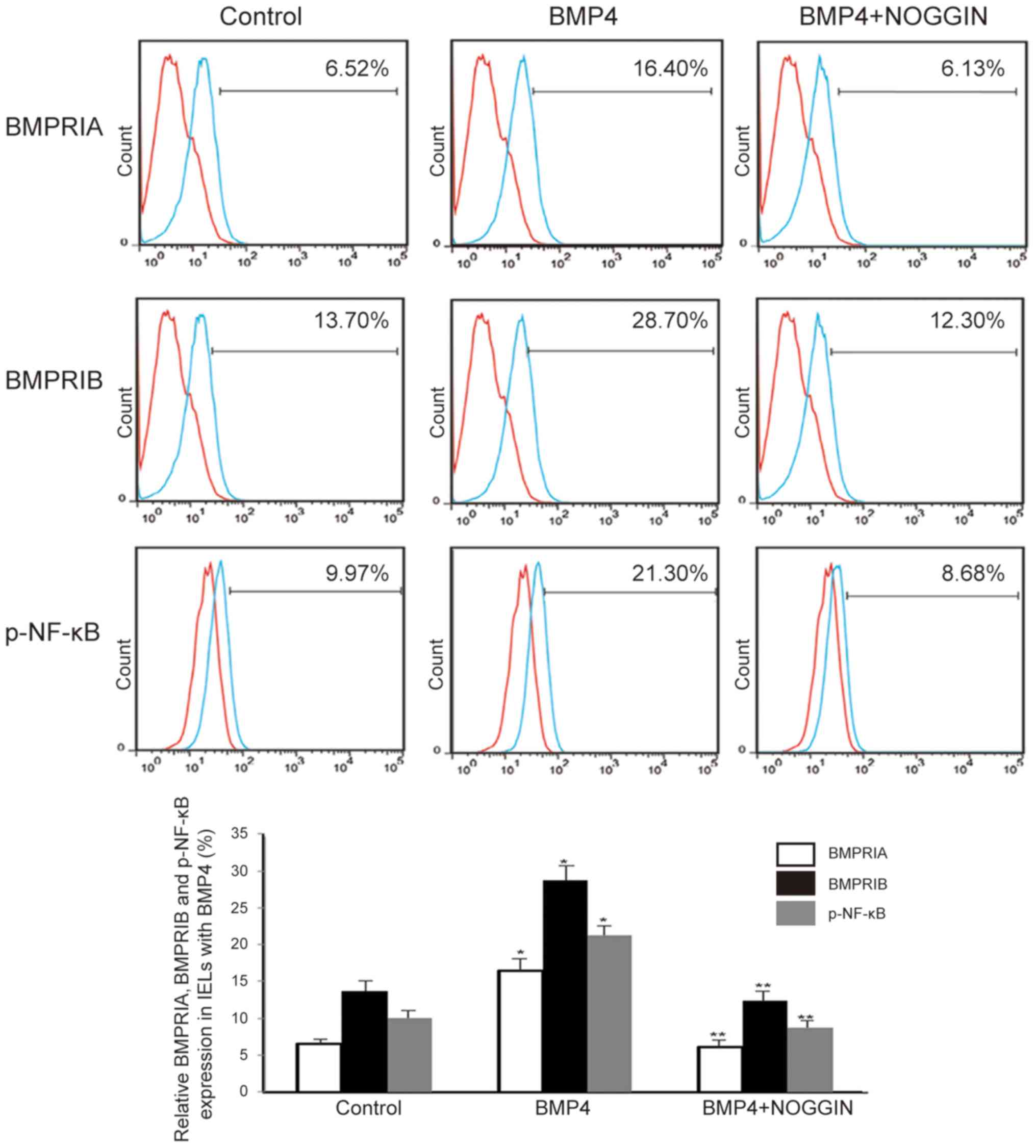
Exogenous BMP4 regulates the NF-κB
signaling pathway in IELs
The primary BMP receptors include two type I
receptors (BMPRIA and BMPRIB) and one type II receptor (BMPRII).
Typically, BMP4 binds to BMPRIA and BMPRIB, both of which have a
high-affinity binding site for BMP4 (9). To determine whether the activation
of NF-κB signaling in IELs involves IEC-derived BMP4, IELs from the
small intestines of mice were isolated and cultured. It was
observed that the protein levels of BMPRIA, BMPRIB and
phosphorylated NF-κB were highly upregulated in IELs that were
cultured in the presence of BMP4 (30 ng/ml), as assessed by flow
cytometry (BMPRIA, 9.88±0.56%; BMPRIB, 15.00±1.42%; and p-NF-κB,
11.33±1.45%). However, this effect was abolished by the
BMP-specific antagonist NOGGIN (30 ng/ml), as shown in Fig. 3. Therefore, it is hypothesized
that I/R induces the activation of NF-κB signaling in IELs,
possibly through IEC-derived BMP4.
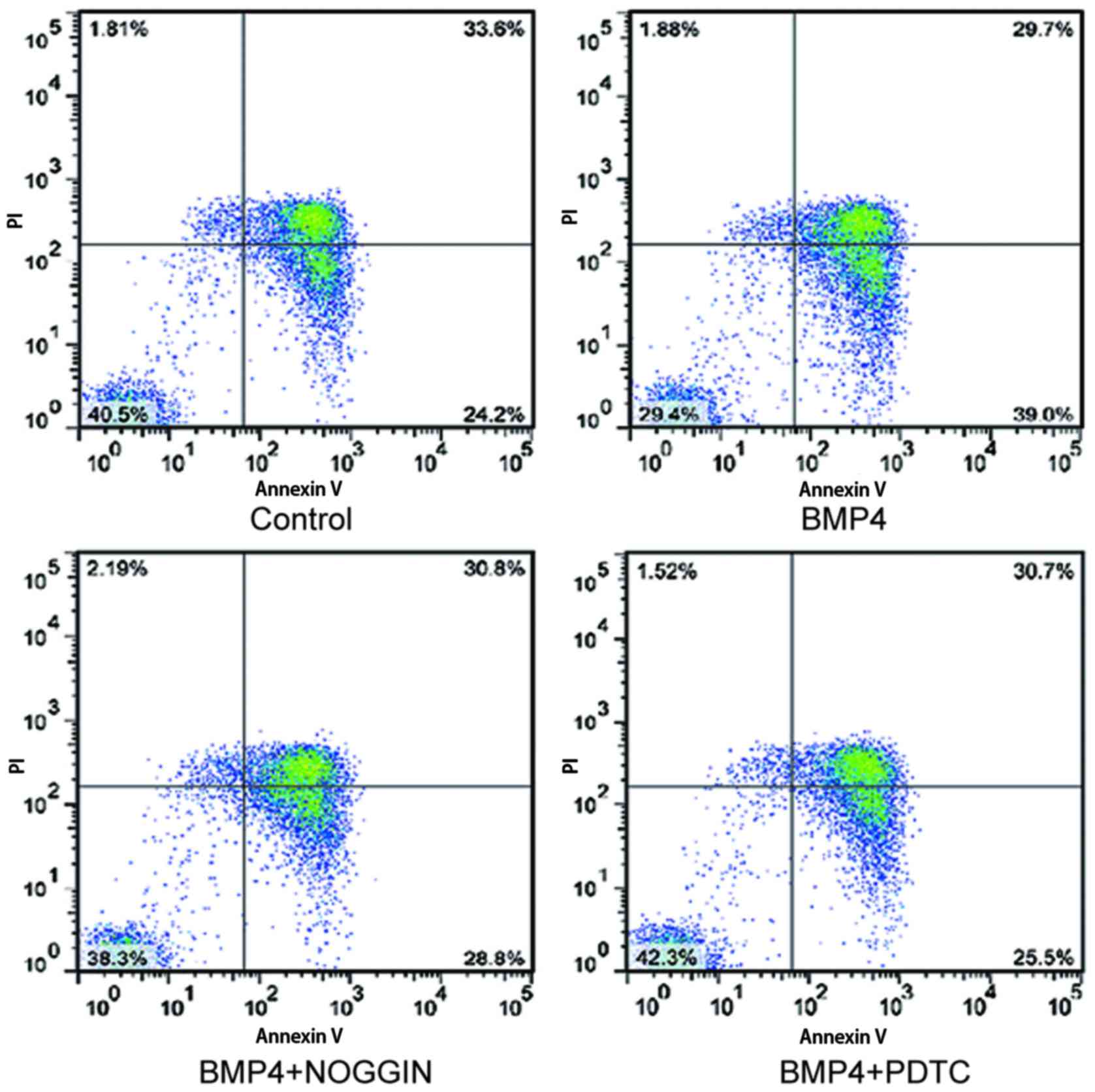
BMP4/NF-κB signaling enhances the
apoptosis of IELs cells
The effects of BMP4 on apoptosis of IELs were next
evaluated following addition of exogenous recombinant BMP4 to the
IEL cultures. Although the viability of the IELs decreased from
94.5 to 45% under without interference cultured, it was also
observed that BMP4 induces IELs to undergo apoptosis (13.20±2.25%).
Activation of NF-κB in IELs by IEC-derived BMP4 (30 ng/ml) was
observed. The blockade of BMP activity by the BMP antagonist NOGGIN
(30 ng/ml), or by PDTC (30 ng/ml), resulted in a marked decrease in
IEL apoptosis, as shown in Fig.
4. Therefore, these results strongly suggest that IEC-derived
BMP4 activates NF-κB signaling in IELs, which leads to enhanced IEL
apoptosis.
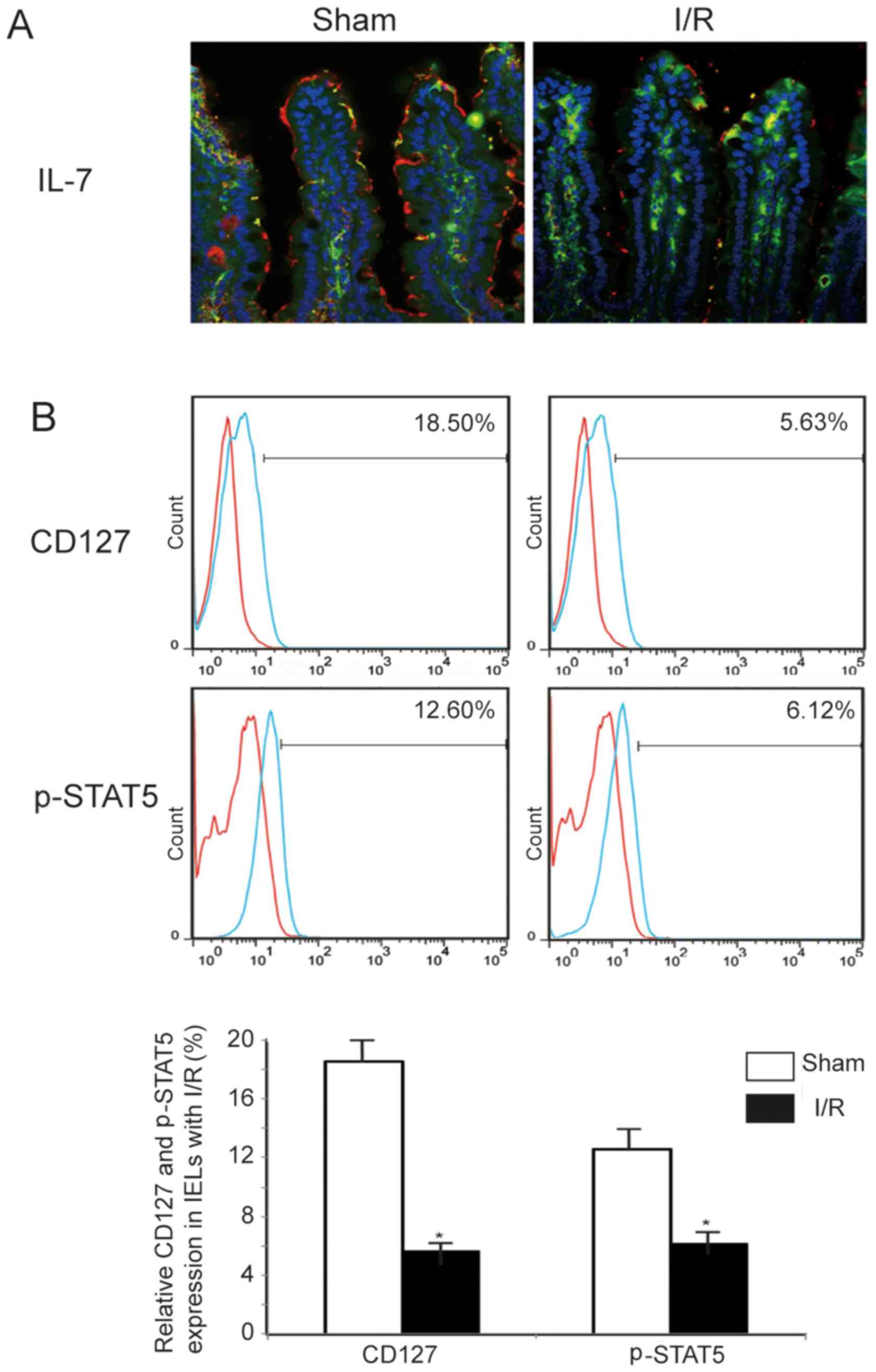
I/R induces a decrease the expression of
IL-7 in IECs and downregulates the expression of IL-7Rα/CD127 and
phosphorylated STAT5 in IELs
IEC-derived IL-7 plays a key role in the regulation
of the development and homeostasis of neighboring IELs. IL-7 was
also shown to significantly affect the phenotype and function of
IELs, and caused an increase in the number of IELs (30). In a previous study, an decrease in
IL-7 expression was confirmed in an intestinal I/R mouse model
(31). In addition, by
immunofluorescence analysis, a decreased expression of IL-7 in the
cytoplasm of IECs after I/R was also confirmed compared with sham
animals. Moreover, it has been reported that IL-7Rα/CD127 may
induce the phosphorylation of STAT5, which acts to maintain T-cell
homeostasis and prevents the progression of inflammation. To better
understand CD127 and the changes in phosphorylation of STAT5 in
IELs after I/R, flow cytometric analysis was performed and revealed
that CD127 expression and the phosphorylation of STAT5 decreased
after I/R (CD127, 12.87±1.32%; and p-STAT5, 6.48±1.23%), as shown
in Fig. 5. These data suggest
that the IL-7/CD127 signaling pathway is inhibited in IECs after
I/R, further aggravating the deterioration of intestinal barrier
function.
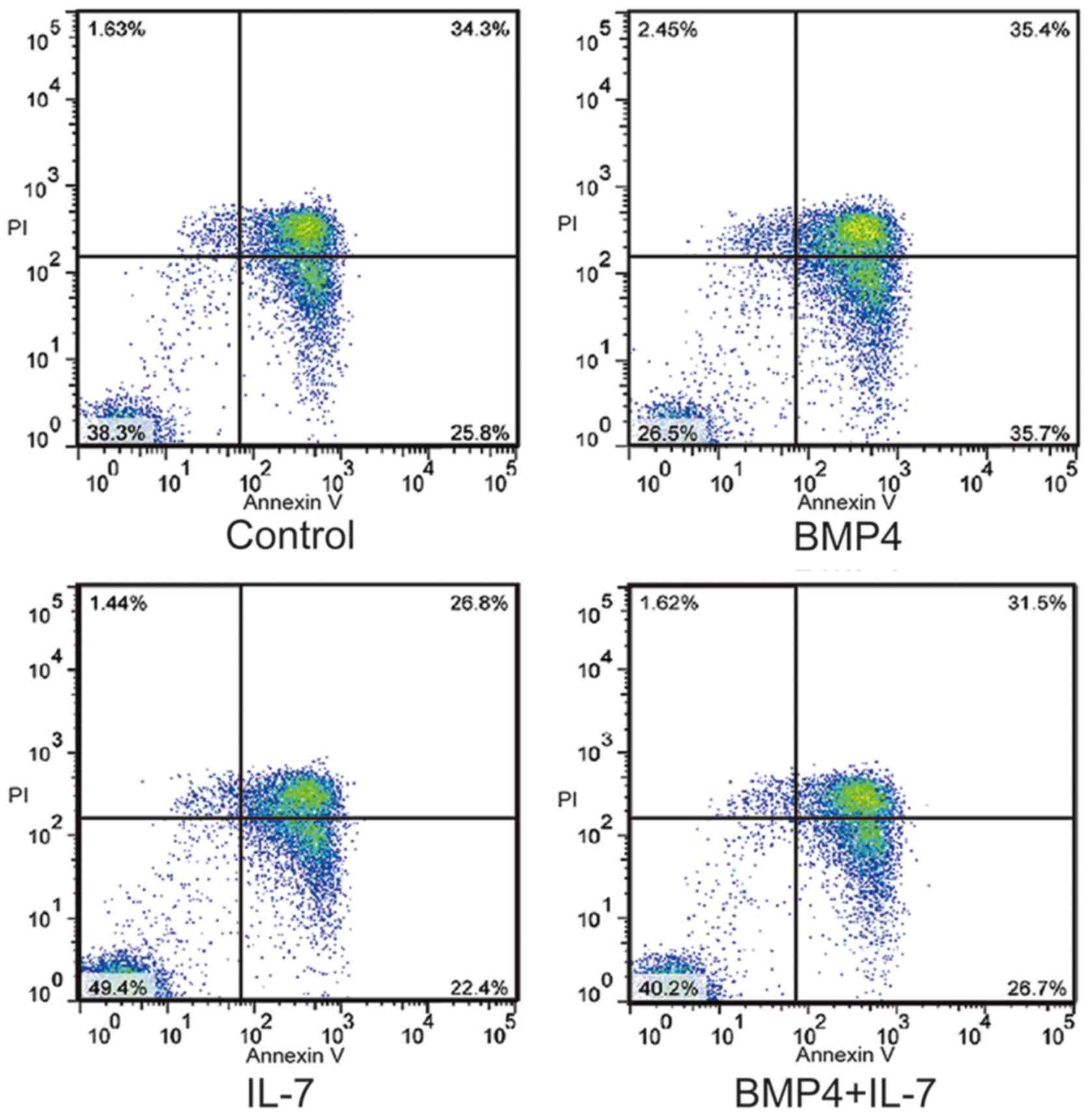
IL-7 inhibits BMP4, which induces the
apoptosis of IELs
It is a known fact that the interaction between BMP4
and IL-7 maintains the balance of proliferation and differentiation
in CD34+ cells. To establish whether the interaction
between BMP4 and IL-7 regulates the number of IELs, exogenous BMP4
(30 ng/ml) and IL-7 (2 ng/ml) proteins were added to cultured IELs
for 6 h. Flow cytometry demonstrated that treatment with IL-7
reduced the extent of apoptosis of IELs (11.1±0.93%), and enhanced
the apoptosis of IELs in the presence of BMP4 (11.8±0.47%). Of
note, no difference in the apoptosis of IELs in the presence of
BMP4 combined with IL-7 stimulation was observed compared with the
control group, as shown in Fig.
6. These results indicate that IEC-derived IL-7 signaling
promotes the survival of IELs and neutralizes IEC-derived BMP4,
which promotes the apoptosis of IELs following I/R.
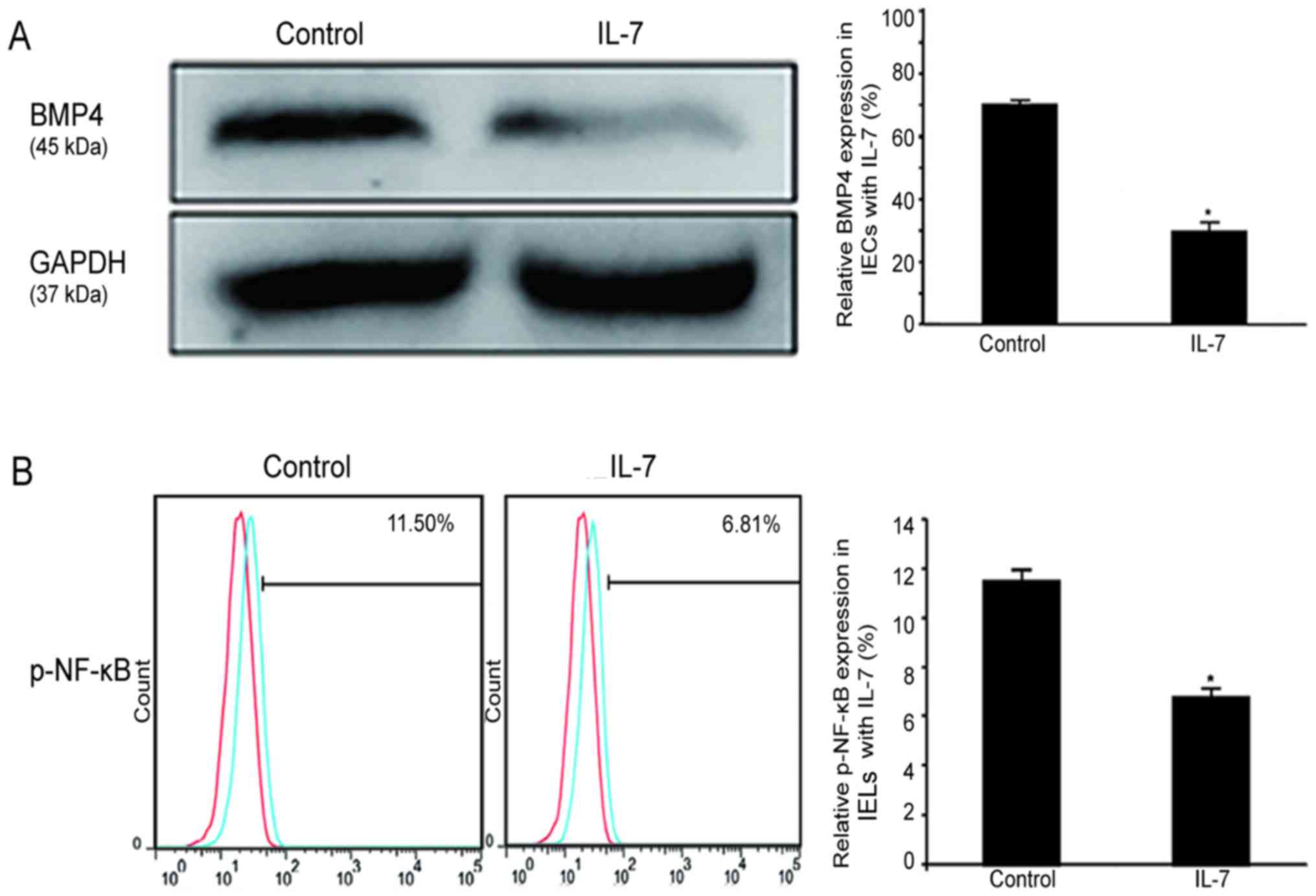
IL-7 downregulates the BMP/NF-κB
signaling pathway in IECs and in IELs
To determine how to delay the induction of IEL
apoptosis by BMP4 in the presence of IL-7, the expression of BMP4
in IECs under IL-7 protein (2 ng/ml) stimulation was evaluated by
western blot analysis. The expression level of BMP4 was found to be
significantly decreased, as shown in Fig. 7A. Moreover, flow cytometry
revealed that the expression of phosphorylated NF-κB protein was
downregulated as a result of IL-7 (2 ng/ml) stimulation in IELs in
culture (5.18±1.12%), as shown in Fig. 7B. NF-κB signaling is known to
increase the levels of inflammatory factors. It was hypothesized
that IL-7 downregulates BMP4 and the phosphorylation of NF-κB,
thereby decreasing the expression of inflammatory factors to
prevent the apoptosis of IELs.
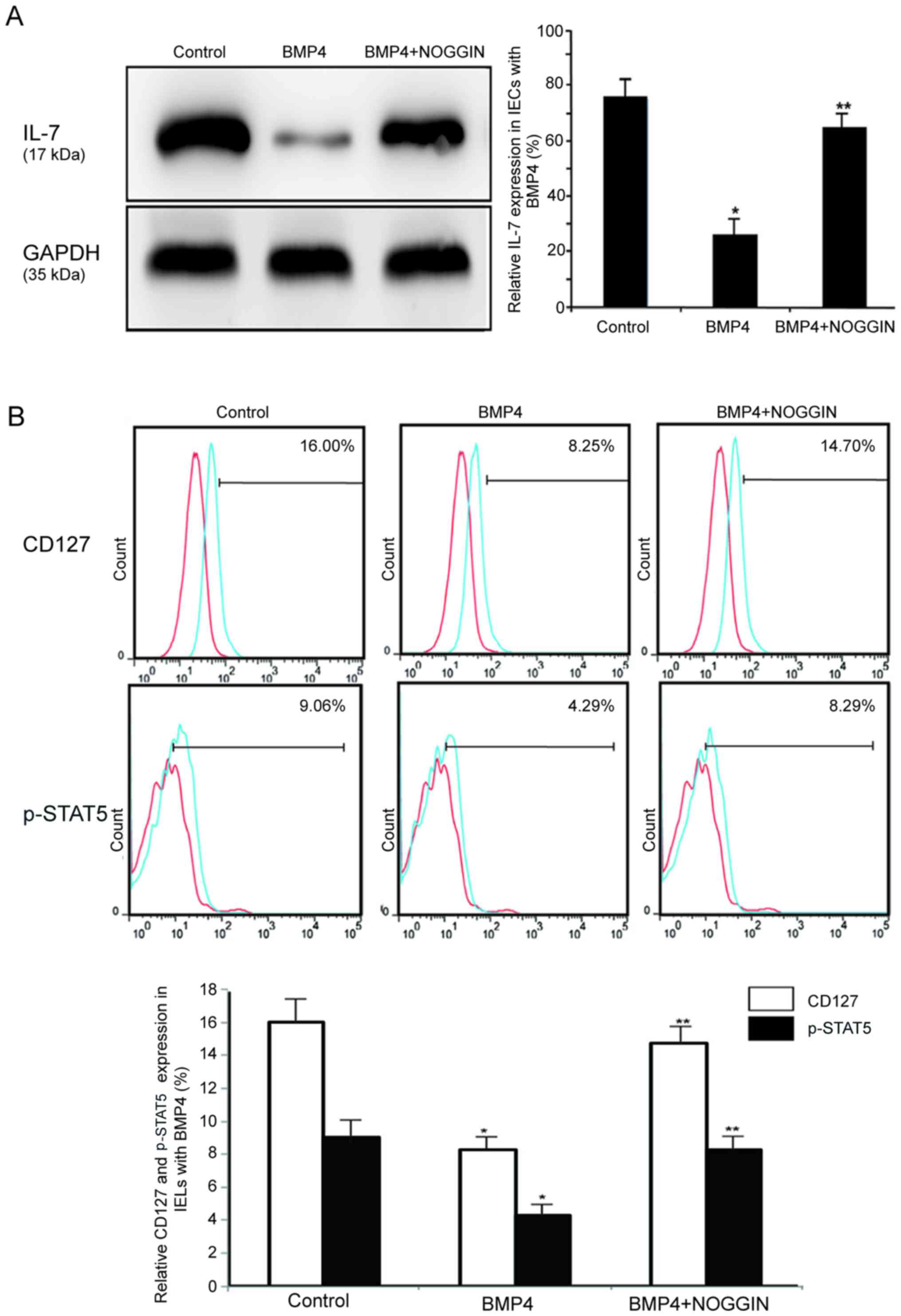
BMP4 regulates the IL-7/CD127 signaling
pathway in IECs and IELs
The majority of studies have demonstrated the role
of IL-7 in T-cell homeostasis, in that CD127 induces STAT5
activation. The expression levels of CD127 were found to be highly
downregulated in BMP4-treated CD34+ cells (15). Therefore, it was hypothesized that
BMP4 promotes the apoptosis of IELs by downregulating CD127 in
intestinal mucosal epithelial cells. Western blot analysis revealed
that BMP4 (30 ng/ml) decreased the expression of the IL-7 protein
in IECs, as shown in Fig. 8A. The
expression level of CD127 dictates the levels of IL-7, which affect
T-cell sensitivity (22).
However, the expression of CD127 and the phosphorylation of STAT5
were downregulated in IELs that were cultured in the presence of
BMP4 (30 ng/ml) (CD127, 7.30±0.48%; and p-STAT5, 4.77±1.77%), but
these effects were abolished with the application of NOGGIN (30
ng/ml), as shown in Fig. 8B.
Therefore, it was hypothesized that BMP4 reduces IL-7 and IEL
sensitivity through the downregulation of CD127 and the expression
of phosphorylated STAT5, which would in turn negatively affect the
number and function of IELs.
Discussion
The present study demonstrated that IEC-derived BMP4
activates the NF-κB signaling pathway to promote apoptosis of IELs
following I/R. IL-7 exerts these inhibitory effects by
counteracting the actions of BMP4. Previous studies have
investigated the roles of the BMP proteins in early intestinal
development and in the proliferation and differentiation of IECs
(32,33). We observed that the expression of
BMP4 increases and that the expression of BMP receptors is also
upregulated in IECs after hypoxia and I/R. BMP4 then activates
NF-κB signaling, which leads to increased levels of inflammatory
factors, such as TNF-α and IL-6. These factors can destroy the
intestinal mucosal barrier, weakening its function (12). IEC-derived IL-7 plays a crucial
role in the control of the development and homeostasis of
neighboring IELs (23). Previous
studies investigated the regulation of the population of progenitor
cells in the human thymus and indicated that the balance between
signals occurs as a result of the interplay between the BMP and
IL-7 signaling pathways (15).
However, the association between the interaction of the BMP and
IL-7 signaling pathways and the regulation of IEL apoptosis
following I/R has rarely been reported.
The BMP signaling pathway plays a critical role in
inflammatory reactions (34,36). It was therefore hypothesized that
the BMP signaling pathway contributes to the mechanisms that are
involved in the promotion of apoptosis of IELs. The present study
investigated the expression of BMP receptors (BMPRIA, BMPRIB,
ActRIA and BMPRII) and phosphorylated NF-κB protein in IELs by
RT-PCR and flow cytometry. Following I/R, IECs produce BMP4
abundantly, and IELs express increased levels of BMPRIA, BMPRIB and
phosphorylated NF-κB proteins. Thus, the evidence suggests that BMP
may activate NF-κB signaling in IELs after I/R. The addition of
exogenous recombinant BMP4 to isolated and cultured IELs was found
to directly activate NF-κB signaling, and this effect was weakened
by the addition of the BMP-specific antagonist NOGGIN. It is known
that an increase in the inflammatory cytokines IL-6 and TNF-α is
mediated via the NF-κB signaling pathway (12) and, thus, flow cytometry was used
and revealed that the addition of exogenous recombinant BMP4
promoted IEL apoptosis. This effect was attenuated in part by
either the BMP-specific antagonist NOGGIN or the NF-κB inhibitor
PDTC. The present study provides new evidence that, following I/R,
BMP4 derived from IECs may bind to the BMP receptors on IELs. The
phosphorylation of NF-κB allows for its translocation to the
nucleus, so that it can trigger the transcription of genes that are
involved in inflammatory cellular responses and other types of
signaling (35). This, in turn,
promotes the apoptosis of IELs. It was also recently confirmed that
activating the canonical Smad signaling pathway promotes the
apoptosis of IELs after I/R. However, the underlying mechanism
requires further elucidation.
The administration of IEC-derived IL-7 protein
significantly affects the function and phenotype of IELs. The
IL-7-induced increase in the number of IELs may be associated with
the increased rate of IEL proliferation observed by
immunofluorescence; however, the expression level of the IL-7
protein was found to be decreased in IECs following I/R for 6 h.
IL-7 affects T-cell sensitivity through the expression of CD127,
and it is possible that the availability of IL-7 is limited in
vivo (36,37). Moreover, IL-7 induces the
phosphorylation of STAT5, which then translocates into the nucleus
where it controls the expression of target genes that are involved
in the survival and proliferation of T cells (38). It has been reported that BMP4
exerts an inhibitory effect on proliferation and differentiation
and, thus counteracts the functions of IL-7 in human intrathymic
CD34+ precursor cells (15). These effects are mediated by
BMP4-induced downregulation of CD127 expression in CD34+
precursor cells. CD127 deletion caused a 30% reduction in the
numbers of CD4+ and CD8+ T cells, which
confirms a key role for IL-7R in the maintenance of peripheral
T-cell survival (39). More
importantly, other data directly indicate that BMP4 downregulates
the level of CD127 and p-STAT5 expression in IELs in culture, which
leads to a reduction in the sensitivity of IELs to IL-7. Therefore,
BMP4 induces a decrease in the number of IELs and weakens IEL
function. Based on these data, it may be inferred that IEC-derived
BMP4 not only activates NF-κB signaling to induce IEL apoptosis,
but also downregulates CD127 and p-STAT5 protein expression to
shorten the survival of IELs. IEC-derived IL-7 directly affects the
number and function of IELs; furthermore, it was observed that IL-7
protein expression decreases following BMP4 stimulation. It may
thus be hypothesized that the decreasing expression of IL-7 was
associated with the increasing expression of BMP4 in IECs following
I/R. The reasons for this finding are not clear and require
confirmation in future experiments.
The protein level of BMP4 in IECs, as well as the
phosphorylation of NF-κB in IELs, were found to be significantly
downregulated in the presence of IL-7. The addition of exogenous
IL-7 markedly reversed BMP4-induced apoptosis of IELs. As a result,
it may be inferred that IL-7 exerts protective effects in terms of
the number and function of IELs through the downregulation of
deleterious factors that are triggered by the BMP/NF-κB signaling
pathways. This protective effect decreases IEL apoptosis after I/R,
effectively preventing further deterioration of intestinal barrier
function.
The regulation of NF-κB expression in developing T
cells and the role of this pathway in selection and lineage
determination is complex (16).
Recent studies reported that basal nuclear NF-κB activity plays an
important role in the transcription of CD127, which facilitates the
responsiveness of naïve T cells to the prosurvival effects of IL-7
and allows for T-cell persistence in vivo (40). Additionally, a developmental
function has been reported for NF-κB signaling in the homeostatic
maturation of new T cells via the regulation of IL-7Rα expression
(41). Our data revealed an
increase the phosphorylation of NF-κB and decrease in IL-7/CD127
protein expression in IELs after I/R. However, whether there is an
association between NF-κB and IL-7/CD127 remains unclear. Hence, it
is recommended that the association between NF-κB and IL-7/CD127 is
further investigated in a mouse model of I/R.
To the best of our knowledge, the present study is
the first to report that BMP4 expression was increased in IECs and
that IEC-derived BMP4 can directly activate NF-κB signaling and
induce apoptosis of IELs following I/R. Additionally, IEC-derived
IL-7 was shown to downregulate BMP4 signaling after I/R, which
limits the extent of IEL apoptosis. This may result in the
maintenance of the balance of the number of IELs and their function
following I/R. All these effects indicate the importance of the
interplay of BMP4 and IL-7 and the dialogue between IECs and IELs
in the intergrity of the intestinal mucosal barrier.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. NSFC 81071532 and
81370479 to C.J.Z.).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Collard CD and Gelman S: Pathophysiology,
clinical manifestations, and prevention of ischemia-reperfusion
injury. Anesthesiology. 94:1133–1138. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grootjans J, Lenaerts K, Derikx JP,
Matthijsen RA, de Bruïne AP, van Bijnen AA, van Dam RM, Dejong CH
and Buurman WA: Human intestinal ischemia-reperfusion-induced
inflammation characterized: experiences from a new translational
model. Am J Pathol. 176:2283–2291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Andrade ME, Araújo RS, de Barros PA,
Soares AD, Abrantes FA, Generoso SV, Fernandes SO and Cardoso VN:
The role of immunomodulators on intestinal barrier homeostasis in
experimental models. Clin Nutr. 34:1080–1087. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andres SF, Simmons JG, Mah AT, Santoro MA,
Van Landeghem L and Lund PK: Insulin receptor isoform switching in
intestinal stem cells, progenitors, differentiated lineages and
tumors: evidence that IR-B limits proliferation. J Cell Sci.
126:5645–5656. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beagley KW, Fujihashi K, Lagoo AS,
Lagoo-Deenadaylan S, Black CA, Murray AM, Sharmanov AT, Yamamoto M,
McGhee JR, Elson CO, et al: Differences in intraepithelial
lymphocyte T cell subsets isolated from murine small versus large
intestine. J Immunol. 154:5611–5619. 1995.PubMed/NCBI
|
|
6
|
Cheroutre H, Lambolez F and Mucida D: The
light and dark sides of intestinal intraepithelial lymphocytes. Nat
Rev Immunol. 11:445–456. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qiu Y, Yu M, Yang Y, Sheng H, Wang W, Sun
L, Chen G, Liu Y, Xiao W and Yang H: Disturbance of intraepithelial
lymphocytes in a murine model of acute intestinal
ischemia̸reperfusion. J Mol Histol. 45:217–227. 2014. View Article : Google Scholar
|
|
8
|
Qiu Y and Yang H: Effects of
intraepithelial lymphocyte-derived cytokines on intestinal mucosal
barrier function. J Interferon Cytokine Res. 33:551–562. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyazono K, Kamiya Y and Morikawa M: Bone
morphogenetic protein receptors and signal transduction. J Biochem.
147:35–51. 2010. View Article : Google Scholar
|
|
10
|
Core AB, Canali S and Babitt JL:
Hemojuvelin and bone morphogenetic protein (BMP) signaling in iron
homeostasis. Front Pharmacol. 5:1042014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang C, Feng Y, Yang H, Koga H and
Teitelbaum DH: The bone morphogenetic protein signaling pathway is
upregulated in a mouse model of total parenteral nutrition. J Nutr.
139:1315–1321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen K, Xie W, Luo B, Xiao W, Teitelbaum
DH, Yang H, Zhang K and Zhang C: Intestinal mucosal barrier is
injured by BMP2/4 via activation of NF-κB signals after ischemic
reperfusion. Mediators Inflamm. 2014:9015302014. View Article : Google Scholar
|
|
13
|
Yoshioka Y, Ono M, Osaki M, Konishi I and
Sakaguchi S: Differential effects of inhibition of bone morphogenic
protein (BMP) signalling on T-cell activation and differentiation.
Eur J Immunol. 42:749–759. 2012. View Article : Google Scholar
|
|
14
|
Hager-Theodorides AL, Ross SE, Sahni H,
Mishina Y, Furmanski AL and Crompton T: Direct BMP2/4 signaling
through BMP receptor IA regulates fetal thymocyte progenitor
homeostasis and differentiation to CD4+CD8+
double-positive cell. Cell Cycle. 13:324–333. 2014. View Article : Google Scholar
|
|
15
|
Varas A, Sacedón R, Hidalgo L, Martínez
VG, Valencia J, Cejalvo T, Zapata A, Hernández-López C and Vicente
A: Interplay between BMP4 and IL-7 in human intrathymic precursor
cells. Cell Cycle. 8:4119–4126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gerondakis S, Fulford TS, Messina L and
Grumont RJ: NF-κB control of T cell development. Nat Immunol.
15:15–25. 2014. View
Article : Google Scholar
|
|
17
|
Fan Z, Jing H, Yao J, Li Y, Hu X, Shao H,
Shen G, Pan J, Luo F and Tian X: The protective effects of curcumin
on experimental acute liver lesion induced by intestinal
ischemia-reperfusion through inhibiting the pathway of NF-κB in a
rat model. Oxid Med Cell Longev. 2014:1916242014. View Article : Google Scholar
|
|
18
|
Kim GY, Hong C and Park JH: Seeing is
believing: illuminating the source of in vivo interleukin-7. Immune
Netw. 11:1–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sprent J and Surh CD: Normal T cell
homeostasis: the conversion of naive cells into memory-phenotype
cells. Nat Immunol. 12:478–484. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chetoui N, Boisvert M, Gendron S and
Aoudjit F: Interleukin-7 promotes the survival of human
CD4+ effector/memory T cells by up-regulating Bcl-2
proteins and activating the JAK/STAT signalling pathway.
Immunology. 130:418–426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tal N, Shochat C, Geron I, Bercovich D and
Izraeli S: Interleukin 7 and thymic stromal lymphopoietin: from
immunity to leukemia. Cell Mol Life Sci. 71:365–378. 2014.
View Article : Google Scholar
|
|
22
|
Carrette F and Surh CD: IL-7 signaling and
CD127 receptor regulation in the control of T cell homeostasis.
Semin Immunol. 24:209–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Monneret G, Villars-Méchin A, Demaret J,
Foray AP and Venet F: Interleukin-7, a new immunoadjuvant for the
treatment of septic shock. Med Sci (Paris). 30:160–165. 2014.In
French. View Article : Google Scholar
|
|
24
|
Geiselhart LA, Humphries CA, Gregorio TA,
Mou S, Subleski J and Komschlies KL: IL-7 administration alters the
CD4:CD8 ratio, increases T cell numbers, and increases T cell
function in the absence of activation. J Immunol. 166:3019–3027.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cai YJ, Wang WS, Yang Y, Sun LH,
Teitelbaum DH and Yang H: Up-regulation of intestinal epithelial
cell derived IL-7 expression by keratinocyte growth factor through
STAT1/IRF-1, IRF-2 pathway. PLoS one. 8:e586472013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang H, Gumucio DL and Teitelbaum DH:
Intestinal specific overexpression of interleukin-7 attenuates the
alternation of intestinal intraepithelial lymphocytes after total
parenteral nutrition administration. Ann Surg. 248:849–856. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shitara S, Hara T, Liang B, Wagatsuma K,
Zuklys S, Holländer GA, Nakase H, Chiba T, Tani-ichi S and Ikuta K:
IL-7 produced by thymic epithelial cells plays a major role in the
development of thymocytes and TCRγδ+ intraepithelial
lymphocytes. J Immunol. 190:6173–6179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mosley RL and Klein JR: A rapid method for
isolating murine intestine intraepithelial lymphocytes with high
yield and purity. J Immunol Methods. 156:19–26. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vollmar B and Menger MD: Intestinal
ischemia/reperfusion: microcirculatory pathology and functional
consequences. Langenbecks Arch Surg. 396:13–29. 2011. View Article : Google Scholar
|
|
30
|
Ji T, Xu C, Sun L, Yu M, Peng K, Qiu Y,
Xiao W and Yang H: Aryl hydrocarbon receptor activation
down-regulates IL-7 and reduces inflammation in a mouse model of
DSS-induced colitis. Dig Dis Sci. 60:1958–1966. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cai YJ, Wang WS, Liang HY, Sun LH,
Teitelbaum DH and Yang H: Keratinocyte growth factor up-regulates
interleukin-7 expression following intestinal ischemia/reperfusion
in vitro and in vivo. Int J Clin Exp Pathol. 5:569–580.
2012.PubMed/NCBI
|
|
32
|
Jain AP, Pundir S and Sharma A: Bone
morphogenetic proteins: the anomalous molecules. J Indian Soc
Periodontol. 17:583–586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bandyopadhyay A, Yadav PS and Prashar P:
BMP signaling in development and diseases: a pharmacological
perspective. Biochem Pharmacol. 85:857–864. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bruun C, Christensen GL, Jacobsen ML,
Kanstrup MB, Jensen PR, Fjordvang H, Mandrup-Poulsen T and
Billestrup N: Inhibition of beta cell growth and function by bone
morphogenetic proteins. Diabetologia. 57:2546–2554. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan R, Li Y, Zhang L, Xia N, Liu Q, Sun H
and Guo H: Augmenter of liver regeneration attenuates inflammation
of renal ischemia/reperfusion injury through the NF-kappa B pathway
in rats. Int Urol Nephrol. 47:861–868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park JH, Yu Q, Erman B, Appelbaum JS,
Montoya-Durango D, Grimes HL and Singer A: Suppression of IL7Ralpha
transcription by IL-7 and other prosurvival cytokines: a novel
mechanism for maximizing IL-7-dependent T cell survival. Immunity.
21:289–302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Henriques CM, Rino J, Nibbs RJ, Graham GJ
and Barata JT: IL-7 induces rapid clathrin-mediated internalization
and JAK3-dependent degradation of IL-7Ralpha in T cells. Blood.
115:3269–3277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang Q, Li WQ, Aiello FB, Mazzucchelli R,
Asefa B, Khaled AR and Durum SK: Cell biology of IL-7, a key
lymphotrophin. Cytokine Growth Factor Rev. 16:513–533. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jacobs SR, Michalek RD and Rathmell JC:
IL-7 is essential for homeostatic control of T cell metabolism in
vivo. J Immunol. 184:3461–3469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Miller ML, Mashayekhi M, Chen L, Zhou P,
Liu X, Michelotti M, Tramontini Gunn N, Powers S, Zhu X, Evaristo
C, et al: Basal NF-κB controls IL-7 responsiveness of quiescent
naïve T cells. Proc Natl Acad Sci USA. 111:7397–7402. 2014.
View Article : Google Scholar
|
|
41
|
Silva A, Cornish G, Ley SC and Seddon B:
NF-κB signaling mediates homeostatic maturation of new T cells.
Proc Natl Acad Sci USA. 111:E846–E855. 2014. View Article : Google Scholar
|