Introduction
Ischemic brain injury, or ischemic stroke, is a
major cause of mortality and disability worldwide (1). Rapid revascularization of the
occluded vessels, followed by timely reperfusion, is considered one
of the most effective approaches to cerebral ischemic injury
treatment. However, this therapy method may evoke subsequent
ischemia-reperfusion (I/R) injury. I/R damage is an important event
in each case and includes the restoration of blood flow following a
critical duration of ischemia, which is the primary cause of
post-ischemic tissue injury (2,3).
I/R injury also induces cognitive deficit, which significantly
affects the quality of life of patients who have had a stroke.
However, the therapeutic efficacy remains unsatisfactory.
A previous study revealed that cerebral I/R injury
differentially changed the expression of numerous noncoding RNAs,
termed micro RNAs (miRNAs), upregulating the expression of specific
genes and downregulating the level of others (4). Long non-coding RNAs (lncRNAs) are
not responsible for protein coding or translation. As a class of
key regulatory RNAs, lncRNAs have important roles in various
biological processes in mammals, including genomic imprinting, dose
compensation, gene regulation, chromatin organization and
alternative splicing (5).
Furthermore, lncRNAs have been previously identified to be involved
in various human diseases. A pervious study revealed that a large
number of genomes produced lncRNAs of in humans or mice; however,
their effect and function remain to be elucidated (6). LncRNAs are a demonstrated class of
novel key regulators in cerebrovascular endothelial pathologies
that arise following ischemic injury, such as stroke (7). According to a previous study,
ischemic stroke induces expressional changes in both lncRNA genes
and protein-coding genes, which suggests that the so-called
'stroke-responsive' lncRNAs may have roles in plasticity post
ischemic injury (8).
It has been previously reported that a
nuclear-residing lncRNA, metastasis-associated lung adenocarcinoma
transcript 1 (MALAT1), is involved in the proliferation and
survival of cancer cells, as well as in tumor metastasis (9). MALAT1 is also enriched in
endothelial cells, serving a crucial role in maintaining
endothelial cell functions. The abnormal expression of MALAT1 is
associated with endothelial cell dysfunction, including
inflammatory processes induced by hyperglycemia, angiogenesis and
proliferation (9–11). However, there are limited reports
regarding the potential role of MALAT1 in I/R injury.
Evidence obtained from both human and animal studies
demonstrates that voluntary exercise may improve cognitive function
and facilitate neuronal plasticity in I/R-models (4,12).
As a rehabilitation strategy, voluntary wheel-running is a
demonstrated effective intervention for facilitating motor recovery
(13). However, the underlying
molecular and epidemic mechanisms remain to be elucidated. The
present study investigated the possible effects of MALAT1 on
voluntary running-wheel (RW) exercise-induced cognitive improvement
following I/R injury.
Materials and methods
Animal I/R models and spontaneous
exercise
A total of 56 male C57BL/6J mice with an average
weight of 28.31±2.19 g, aged 4 to 4.5 months, were purchased from
the Changzhi Medical College. Animal use and care were in
accordance with the animal care guidelines, which conformed to the
Guide for the Care and Use of Laboratory Animals, published by the
US National Institutes of Health (NIH Publication No. 85–23,
revised 1996). All protocols performed in the current study were
approved by the Changzhi Medical College Administrative Committee
of Experimental Animal Care and Use (license no. cz08329).
Prior to the experiments, the mice were allowed to
acclimate to their environment for 1week. A total of 14 mice
underwent a sham-operation and were used as the sham group. For the
I/R injury model, 28 mice were subjected to 45 min of ischemia
followed by reperfusion, as previously described (4). Overall, 7 sham-operated mice and 35
I/R-injured mice were separately housed, each in cages equipped
with a RW (sham-runners, n=7; I/R-runners, n=35). The remaining
untreated mice served as the sedentary control group (I/R
non-runners, n=7; sham non-runners, n=7), and were housed in cages
with an immobilized RW, as previously described (14,15). All mice used in this work were
bred at Changzhi Medical College in a restricted access,
temperature-controlled (at 22°C) animal central on a 12-h
light/dark cycle, food and water ad libitum. To investigate
the potential roles of MALAT1 involved in spontaneous-RW-induced
neuroplasticity, the I/R-runners were sub-divided into five groups
(I/R-runners, n=7; I/R-runners + MALAT1 siRNA, n=7; I/R-runners +
MALAT1 scrambled siRNA, n=7; I/R-runners + MALAT1, n=7; I/R-runners
+ blank control, n=7). RWs with a diameter of 12 cm and a width of
5 cm (Nalge Nunc International, Rochester, NY, USA) were used to
equip the cages for I/R-runners. The number of revolutions was
monitored and recorded using Vital Viewer Data Acquisition System
version V-4000 (Mini Mitter, Sunriver, OR, USA). Given that 7 pm to
7 am is the most active period for rodents, the average number of
revolutions was calculated for each night during this period. The
mice were allowed unlimited access to the RWs in their individual
cages.
Focal cerebral I/R operation
During surgery, 5% isoflurane was used to induce
anesthesia, and 1–2% isoflurane was used for maintenance. Focal
ischemic injury was generated as described previously (16,17). After 45 min of ischemia,
reperfusion was achieved by permanently withdrawing the suture, as
previously described (4).
Neurological deficits evaluation
The neurological deficits of mice were assessed at
1, 2 and 3 days post-operation (dpo). Focal neurological scores
(FNS), using a 28-point scale, were used to assess neurological
deficits, as previously described (18). The total score for each animal
ranged from 0–28, the higher scores indicate a greater impairment
(18).
MALAT1/MALAT1 siRNA treatment
By transducing with synthetic MALAT1 or
siRNA-targeted MALAT1, MALAT1 expression levels were upregulated or
downregulated, respectively. Mus musculus (Mmu)-MALAT1,
MALAT1 siRNA and scrambled siRNA were designed and purchased from
Applied Biological Materials Inc. (Richmond, BC, Canada).
I/R-runners received various treatments via intraperitoneal
injection with mmu-MALAT1 (10 nmol/20 g body weight, I/R-runners +
MALAT1, n=7), MALAT1 blank control (I/R-runners + MALAT1 blank,
n=7), mmu-MALAT1 siRNA (5 nmol/20 g body weight, I/R-runners +
MALAT1 siRNA, n=7), or scrambled siRNA (I/R-runners + MALAT1
scrambled siRNA, n=7), 5 h after I/R injury and twice per week
prior to sacrifice (15).
Morris water maze (MWM) test
At 25–30 days after I/R injury, induced immediately
followed running, the MWM test was used to evaluate spatial
learning and memory, as described previously (15,19). Each mouse underwent the trial
thrice daily for six consecutive days. The percentages of time, the
path taken in the target quadrant, and the number of target
platform crossings were recorded and analyzed using TOPSCAN™
version 2.0 (Clever Sys, Inc., Reston, VA, USA).
Triphenyl tetrazolium chloride (TTC)
staining
TTC staining was performed for infarct volume
assessments, as previously described (20). Briefly, coronal sections of the
brain, 2-mm thickness, were incubated with 2% TTC at 37°C for 30
min with gentle agitation. The sections were then fixed at 22–25°C
in 10% formalin with PBS for 15 min, and subjected to serial
staining steps. Finally, the stained slices were collected for
imaging. The infarct volume was quantified and analyzed by Leica
image software DMI6000B (Leica Microsystems, Wetzlar, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The level of MALAT1 in the cerebral cortex and the
hippocampus from sham and I/R injured mice was determined using
RT-qPCR as previously described (7). Total RNA was isolated using
TRIzol® (Thermo Fisher Scientific, Inc., Waltham, MA,
USA), according to the manufacturer's protocol. cDNA was
synthesized by using Oligo(dT)18 and MMLV reverse
transcriptase (Promega Corporation, Madison, WI) at 22–25°C. The iQ
SYBR® Green supermix (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used for qPCR detection, according to the
manufacturer's protocol. GAPDH served as the endogenous control.
LncRNAs expression levels were normalized by calculating the
lncRNAs to GAPDH expression ratio (2−ΔΔCq) (21). qPCR amplification was performed at
95°C for 3 min, followed by 40 cycles at 95°C for 15 sec and 62°C
for 60 sec. The primer sequences were as follows: GAPDH, forward:
5′-CGA GAT CCC TCC AAA ATC AA-3′ and reverse: 5′-TTC ACA CCC ATG
ACG AAC AT-3′; MALAT1, forward 5′-GTG ATG CGA GTT GTT CTC CG-3′ and
reverse 5′-CTG GCT GCC TCA ATG CCT AC-3′ (7,22).
Cell apoptosis assay
Hippocampal tissues were fixed at 22–25°C in 4%
formalin with PBS for 30 min, and subjected to serial staining
steps. Apoptosis within the hippocampal tissues was evaluated using
a TUNEL-POD In Situ apoptosis detection kit (Roche Molecular
Systems, Inc., Pleasanton, CA, USA), according to the
manufacturer's protocol. The TUNEL-positive/apoptotic cells, as
detected by DAB staining at 22–25°C for 5–10 min (Beyotime
Institute of Biotechnology, Shanghai, China), were presented as a
percentage of the total cells. A phase contrast microscope (Leica)
was used for imaging at ×40 magnification and 5 fields of view were
used. Three independent experiments were performed.
Western blot analysis
Western blotting was used to determine the
expression of cytokines associated with apoptosis, which may have
been altered by spontaneous RW in I/R injured mice. Antibodies
against B cell leukemia/lymphoma 2 (Bcl-2), BCL2-associated X
(Bax), BCL2 associated agonist of cell death (Bad), B-cell
lymphoma-extra large (Bcl-xL), caspase-3/-8, and GAPDH were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Tissues were homogenized on ice in a RIPA lysis buffer (P0013C;
Beyotime Institute of Biotechnology) and centrifuged at 4,500 × g
for 25 min. Protein concentration was assayed with BCA reagent
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). Total 50 mg
cell lysate protein per lane extracts from each cell lines were
separated using 15% SDS-PAGE and transferred to nitrocellulose
membranes. The membranes were then blocked with phosphate-buffered
saline containing 0.05% Tween-20 (PBST) with 10% non-fat dry milk
(Sigma-Aldrich; Merck Millipore) overnight at 4°C for 12 h, then
the membrane was washed three times for 10 min each time. This was
followed by incubation with anti-Bad (1:800; cat. no. AB008;
Beyotime Institute of Biotechnology), anti-Bcl-2 (1:500; cat. no.
AB112; Beyotime Institute of Biotechnology), anti-Bax (1:500; cat.
no. ab32503; Abcam, Cambridge, UK), anti-Bcl-xL (1:1,000; cat. no.
ab32370; Abcam), and anti-caspase-3 (1:1,000; cat. no. ab13847;
Abcam) and anti-caspase-8 (1:500; cat. no. ab25901; Abcam) primary
antibodies at 4°C overnight. After washing 3 times with PBST for 10
min each, the membrane was incubated with a horseradish
peroxidase-conjugated goat anti-rabbit IgG (1:2,500; cat. no.
A0208; Beyotime Institute of Biotechnology) for 2 h at room
temperature. The blots were then subjected to enhanced
chemiluminesence-based detection (Beyotime Institute of
Biotechnology) as previously described (23,24). The semi-quantitated target
proteins were analyzed for each group by using Bio-Rad Gel
Imagining system (ChemiDoc™ XRS+; Bio-Rad Laboratories, Hercules,
CA, USA) (23,24).
Statistical analysis
Values are presented as the mean ± standard
deviation. FNS were analyzed using the Kruskal-Wallis test,
followed by the Mann-Whitney U test with Bonferroni's correction.
Significant differences between the groups were determined using
Student's t-test. Two-way repeated-measures ANOVA, followed by
Tukey's test, was used for the MWM test analysis. SPSS version 19.0
(IMB Corporation, Armonk, NY, USA) was used for statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Neurological scores
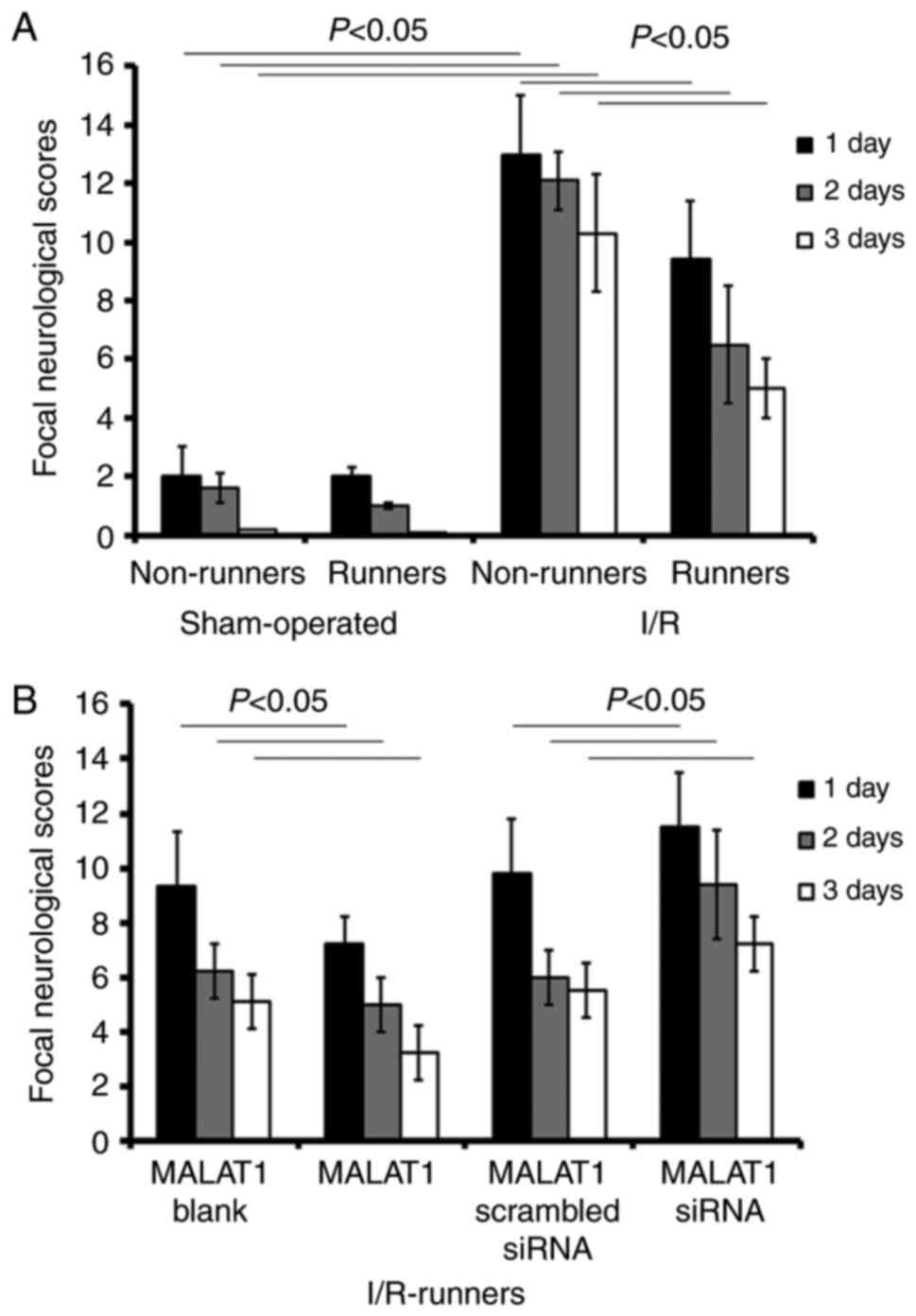
The data from the present study revealed that
voluntary RW partially rescued neurological impairment, whereas
siRNA targeting MALAT1 neutralized that neuroprotection. I/R
surgery lead to increased scores relative to the sham operation
(I/R Non-runners vs. sham non-runners; P<0.05; Fig. 1). RW exercise significantly
reduced neurological scores from 1–3 dpo (I/R Non-runners vs.
I/R-runners; P<0.05; Fig. 1).
Compared with the I/R-runners + MALAT1 blank controls, MALAT1
treatment significantly decreased neurological scores from 1-3dpo
(I/R-runners + MALAT1 vs. I/R-runners + MALAT1 blank; P<0.05;
respectively). Conversely, scores were significantly reduced from
1–3dpo in I/R-runners receiving MALAT1 siRNA treatment compared
with the MALAT1 scrambled siRNA group (I/R runners + MALAT1 siRNA
vs. I/R runners + MALAT1 scrambled siRNA, P<0.05; Fig. 1).
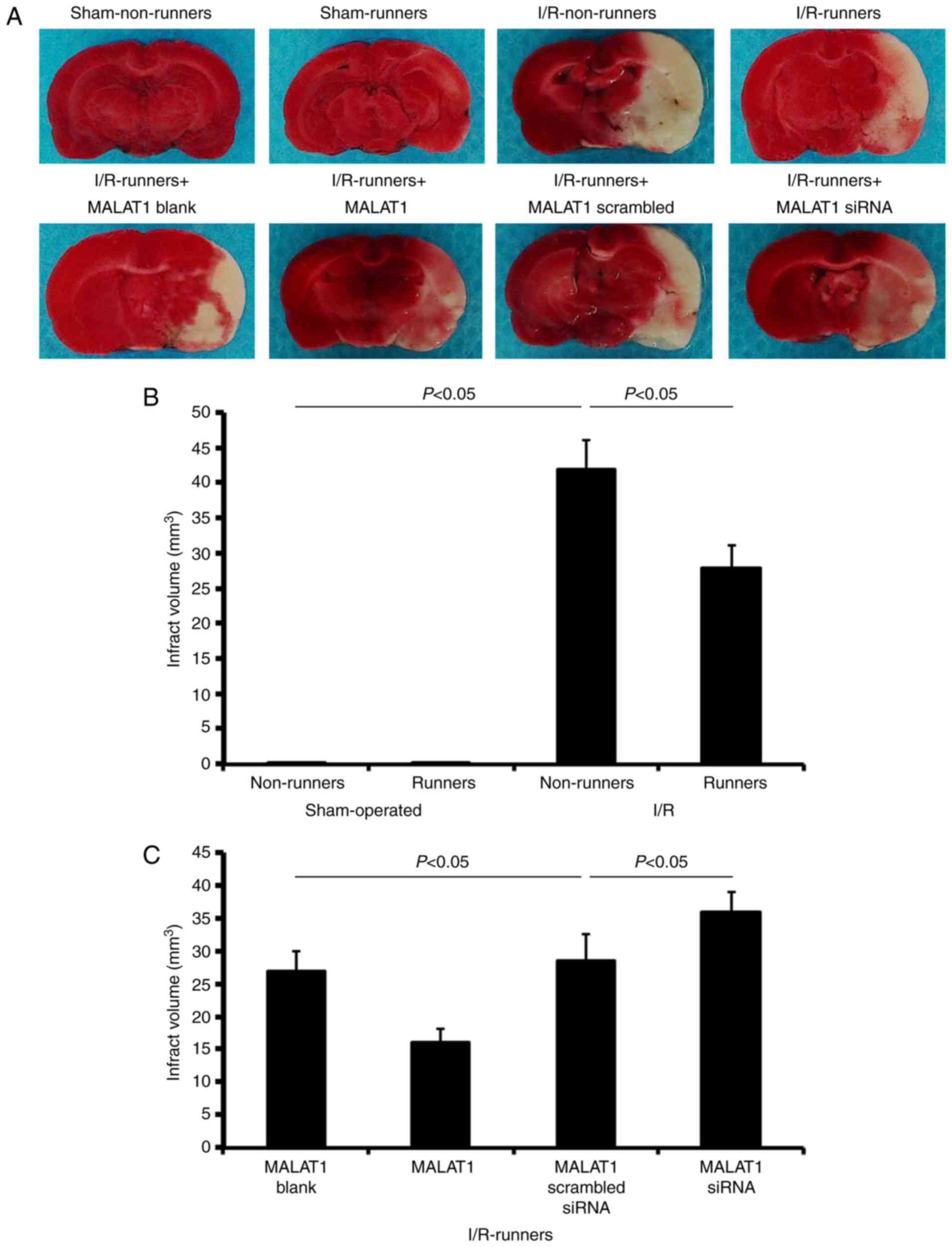
Infarct volume evaluation
TTC staining revealed no observable damage in the
cerebrums of mice subjected to a sham operation (Fig. 2). I/R injuries led to a large
infarction, which was attenuated by voluntary RW following the
injury (I/R non-runners vs. I/R runners; P<0.05; Fig. 2). Treatment with MALAT1 increased
the neuroprotective effects induced by RW following I/R injury (I/R
runners + MALAT1 vs. I/R runners + MALAT1 blank control; P<0.05;
Fig. 2), where MALAT1 siRNA
administration partially neutralized these effects (I/R runners +
MALAT1 siRNA vs. I/R runners + MALAT1 scrambled siRNA; P<0.05;
Fig. 2).
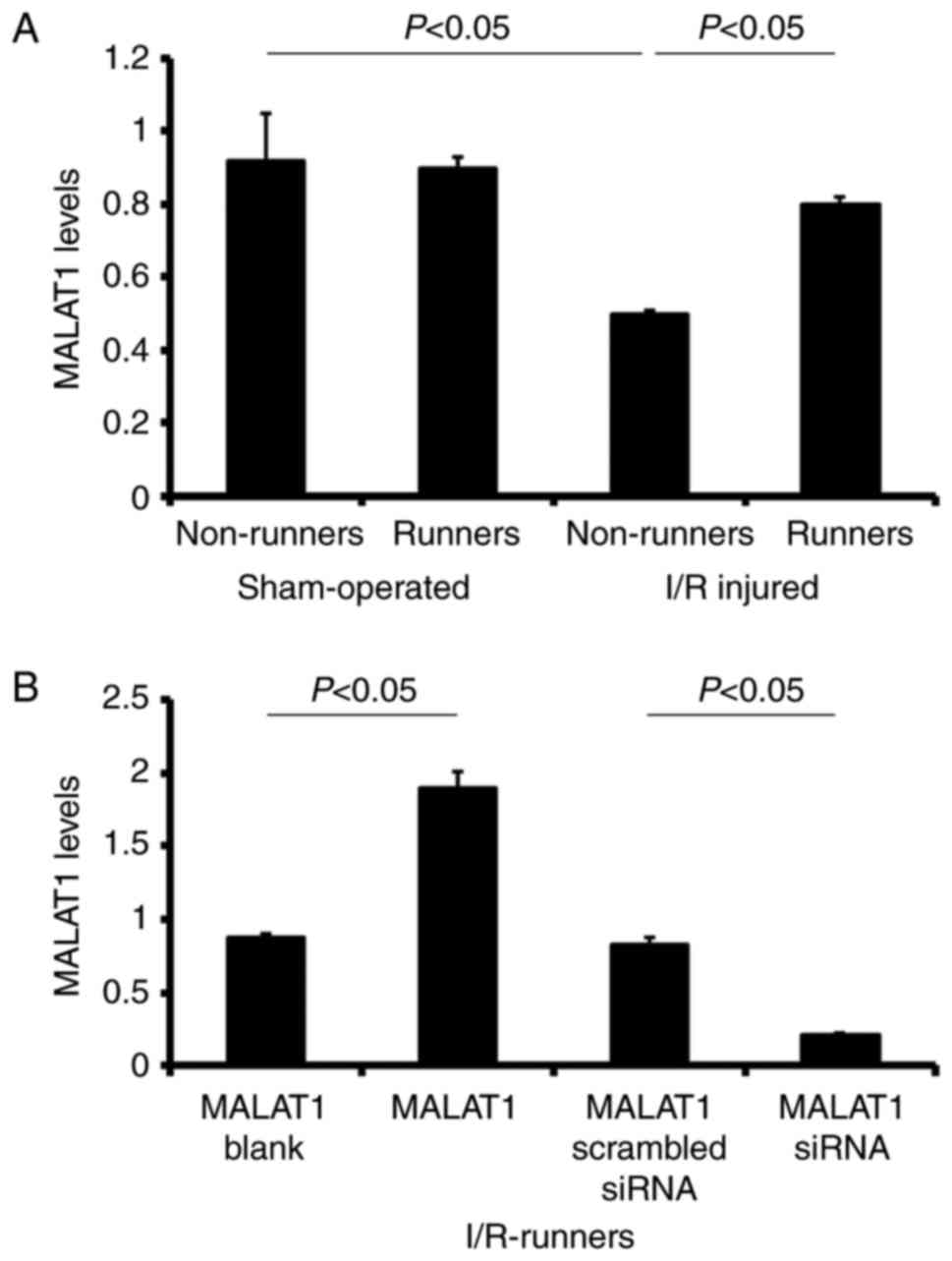
Changes to MALAT1 levels in different
groups
Following I/R injury, MALAT1 expression levels were
significantly reduced in the I/R non-runners, compared with in the
sham non-runners (P<0.05). There were no significant differences
between sham-operated runners and the non-runners (sham non-runners
vs. sham-runners; P>0.05). Spontaneous RW increased the
expression levels of MALAT1 in I/R injured mice. The expression
levels of MALAT1 increased following voluntary RW training in
I/R-runners (I/R non-runners vs. I/R runners, P<0.05, Fig. 3).
MALAT1-treatment of I/R-runners induced significant
upregulation of MALAT1 expression in the I/R-runners + MALAT1 group
vs. I/R-runners + MALAT1 blank (P<0.05). siRNA targeting MALAT1
effectively reduced MALAT1 expression levels in I/R-runners, and in
MALAT1 siRNA-treated mice vs. I/R-runners + MALAT1 scrambled siRNA
(P<0.05). The MALAT1 levels showed no significant difference
between the two matched control groups (I/R-runners + MALAT1 blank
vs. I/R-runners + MALAT1 scrambled siRNA, P>0.05, Fig. 3).
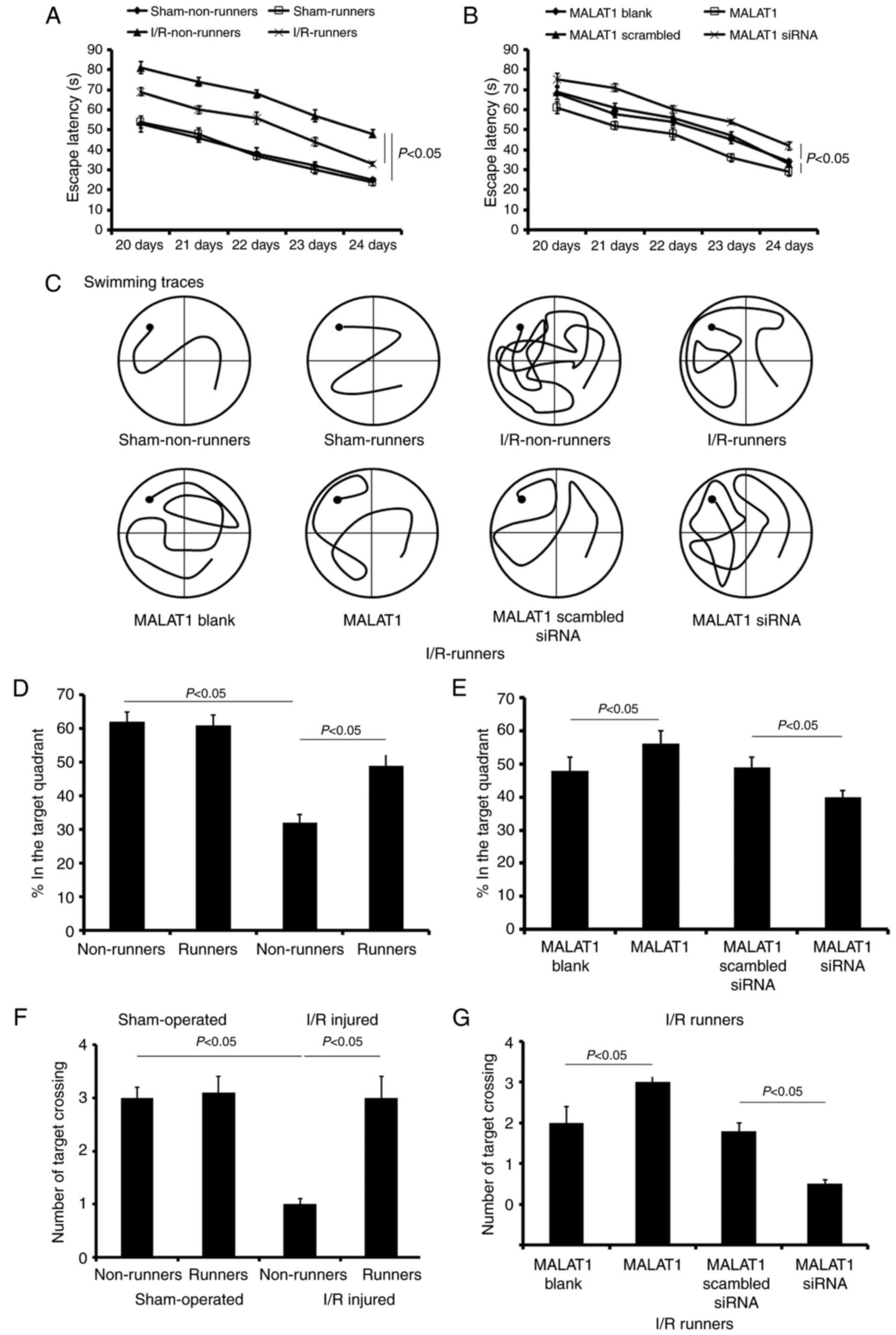
Spatial learning and memory
alterations
The present study used the MWM test to assess the
spatial learning of mice subjected to various treatments. During
the initial 5 training days, spatial learning loss was assessed by
a hidden platform test. The mice in sham-operated groups showed no
significant cognitive deficiency. Voluntary RW had no effect on the
cognitive changes in sham-operated mice, but induced obvious
improvements in spatial learning and memory in I/R injured mice.
There was no significant difference in the escape latencies between
sham non-runners and sham-runners (P>0.05; Fig. 4A). I/R non-runners appeared to
take longer to find the hidden platform compared with I/R-runners
(F1,7=16.35; P<0.01; Fig. 4A).
Following MALAT1 treatment, I/R-runners had
significantly longer escape latencies compared with mice from the
I/R-runners + MALAT1 group vs. I/R-runners + MALAT1 blank
(F1,7=7.62; P<0.05; Fig. 4B). The latency differences were
appeared significantly at the third training day (Tukey's test;
P<0.05; Fig. 4B). Conversely,
MALAT1 siRNA partially neutralized the cognitive improvement
induced by spontaneous RW exercise. MALAT1 siRNA-treated I/R mice
took longer to find the hidden platform, compared with MALAT1
scrambled siRNA treated ones (F1,7=13.28; P<0.01;
Fig. 4B). However, no significant
difference was identified in the escape latencies between the two
control groups (I/R-runners + MALAT1 blank vs. I/R-runners + MALAT1
scrambled siRNA; P>0.05; Fig.
4B). The swimming traces at the fifth training day for mice in
different groups are presented in Fig. 4C.
Following the hidden platform test, a probe test was
performed to evaluate spatial retention on the sixth day.
Sham-runners exhibited no obvious differences when compared with
sham-Non-runners for any of the tested parameters (P>0.05;
Fig. 4). However, sham
non-runners spent longer in the target quadrant compared with the
I/R non-runners (P<0.05, Fig.
4D). Voluntary RW induced notable improvement in memory
retention. For I/R-runners, the time spent and number of paths
travelled in the target quadrant, as well as the number of target
crossings, was increased, compared with I/R non-runners (P<0.05,
Fig. 4). Synthetic MALAT1
treatment enhanced the cognitive improvement induced by voluntary
RW in I/R mice, whereas MALAT1 siRNA administration partially
attenuated these effects. The I/R runners + MALAT1 group showed
increase in the test duration, number of paths travelled and number
of target crossings in the target quadrant when compared with the
I/runners + MALAT1 blank group (P<0.05, Fig. 4). However, mice in I/R runners +
MALAT1 siRNA groups revealed a reduction in the time spent, the
number of paths travelled and the number of target crossings (vs.
I/R runners + MALAT1 scrambled siRNA, P<0.05, Fig. 4). The two control groups,
I/R-runners + MALAT1 blank and I/R-runners + MALAT1 scrambled siRNA
ones, showed no statistical difference (P>0.05).
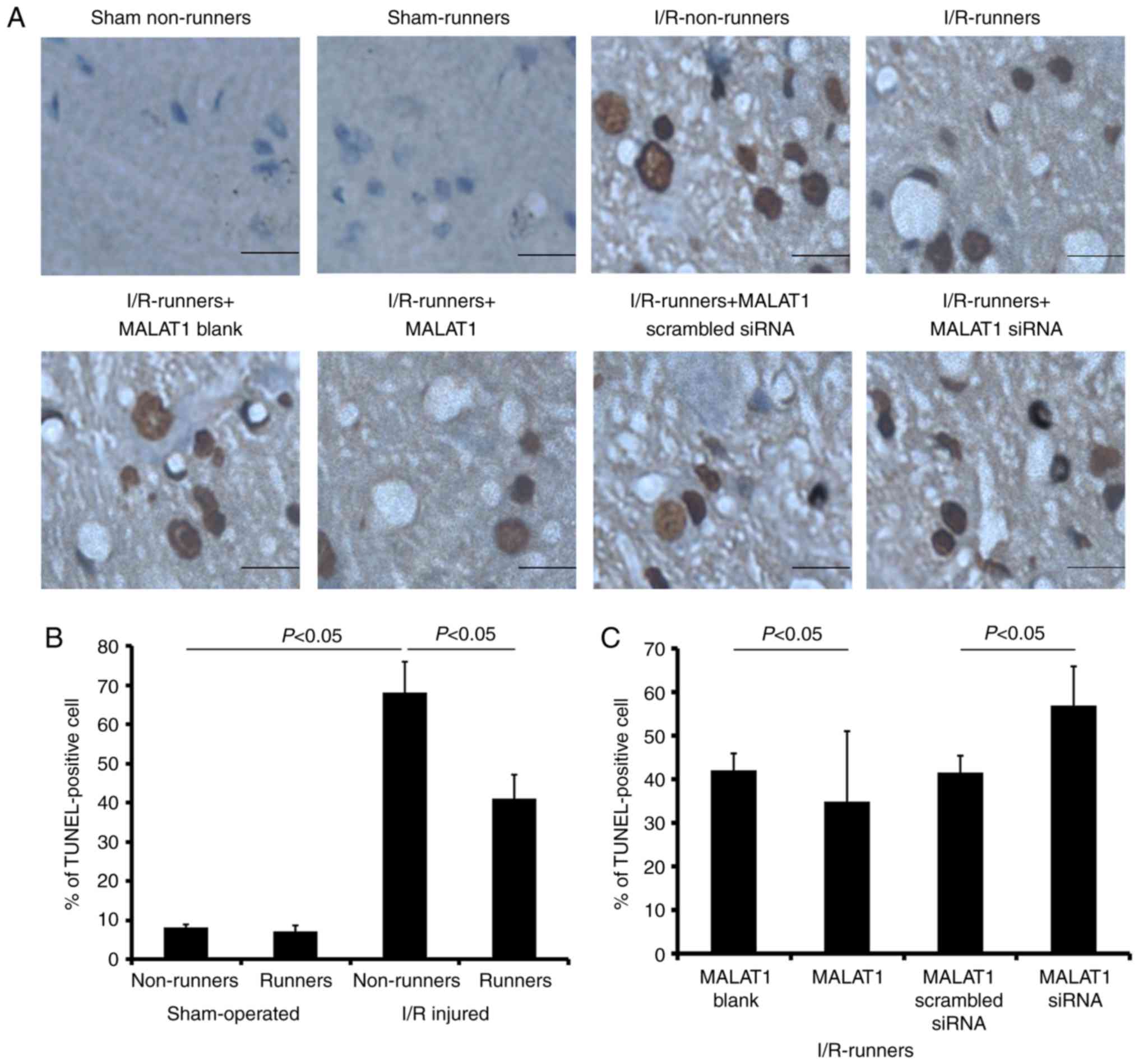
Apoptosis induced by I/R injury was
reversed by RW
Voluntary RW had no effect on hippocampal cell
apoptosis under sham operated conditions. The apoptotic cell
proportion was increased in the hippocampus following I/R injury
(I/R non-runners vs. sham non-runners, P<0.05); however,
significantly reduced in mice subjected to voluntary RW
(I/R-runners vs. I/R non-runners, P<0.05; Fig. 5). Administration of MALAT1 further
reduced the number of apoptotic hippocampal cells in the
I/R-runners (I/R-runners + MALAT1 vs. I/R-runners + MALAT1 blank,
P<0.05). Conversely, MALAT1 siRNA treatment neutralized the
effects of RW and increased the proportion of apoptotic cells
(I/R-runners + MALAT1 siRNA vs. I/R-runners + MALAT1 scrambled
siRNA, P<0.05).
Expression of apoptosis-associated
proteins
Quantitative analysis was performed using western
blotting. Following I/R injury, the expression levels of Bax, Bad,
and caspase-3/-8 were increased, whereas those of Bcl-2 and Bcl-xL
were reduced (I/R non-runners vs. sham non-runners; P<0.05;
Fig. 6). RW exercise protected
hippocampal cells from apoptosis. The present findings demonstrated
that spontaneous RW downregulated the expression of Bax, Bad and
caspase-3/-8, whereas Bcl-2 and Bcl-xL expression levels were
upregulated in the hippocampus following I/R injury (I/R-runners
vs. I/R-Non-runners; P<0.05; Fig.
6). Consistent with the TUNNEL staining results, MALAT1
treatment increased the inhibition of apoptosis induced by RW in
I/R mice, whereas MALAT1 siRNA administration attenuated this
effect. Specifically, the Bax, Bad, and caspase-3/-8 levels were
reduced, whereas the Bcl-2 and Bcl-xL levels increased following
MALAT1 treatment (I/R-runners + MALAT1 vs. I/R-runners + MALAT1
blank; P<0.05; Fig. 6). The
expression levels of Bax, Bad, and caspase-3/-8 were partially
upregulated and Bcl-2 and Bcl-xL expression levels were reduced, by
MALAT1 siRNA administration in I/R-runners (I/R-runners + MALAT1
siRNA vs. I/R-runners + MALAT1 scrambled siRNA; P<0.05). The
expression of apoptosis-associated proteins tested in sham operated
groups showed no significant difference following RW (sham
non-runners vs. sham-runners, P<0.05). There were also no
significant differences identified between the MALAT1 treatment
control groups.
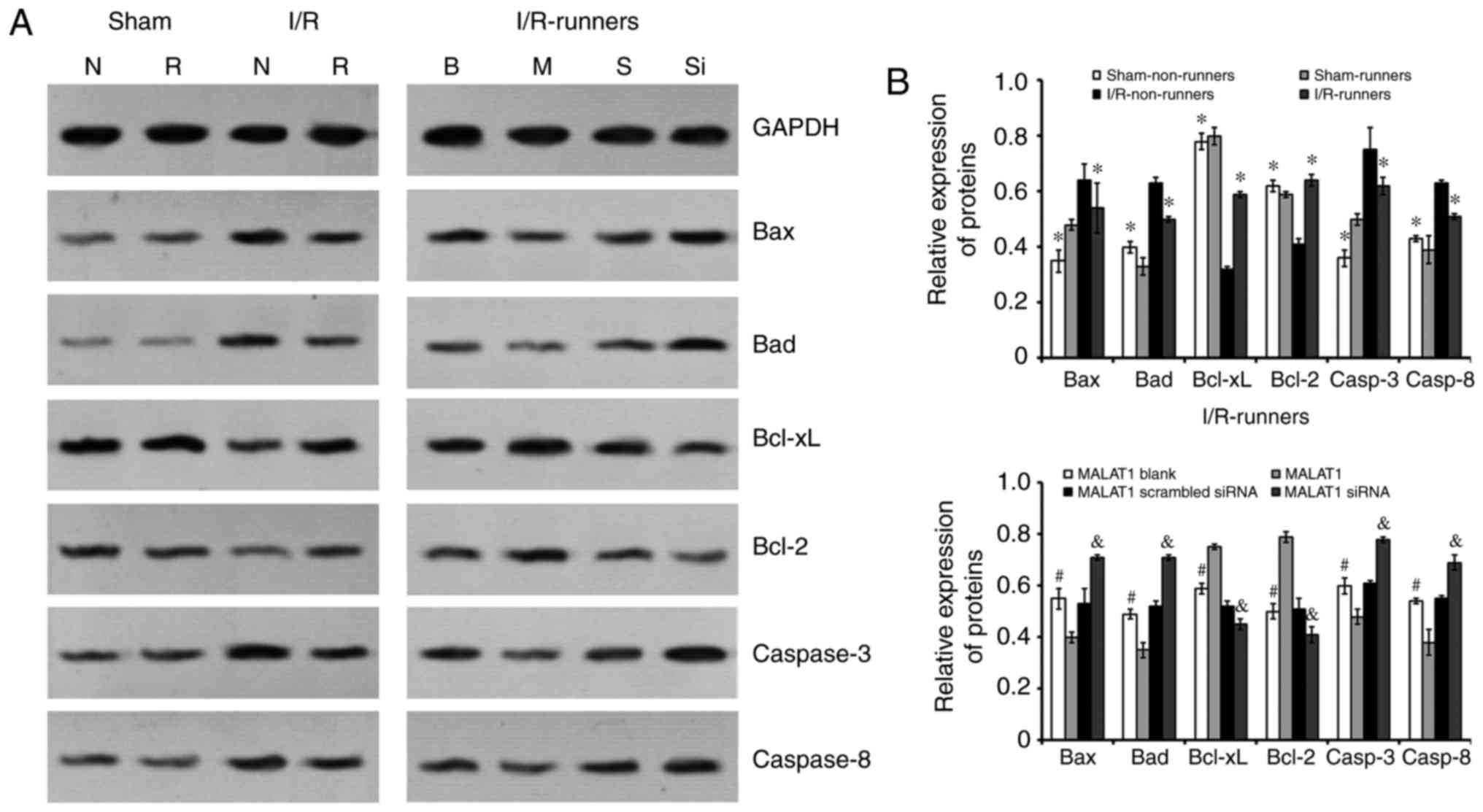 | Figure 6Expression of apoptosis-associated
proteins in the hippocampus of mice from different groups. (A)
Representative bands of the different proteins detected by western
blotting. (B) Quantitative analysis of the apoptosis-associated
proteins. *P<0.05 vs. I/R-Non-runners;
#P<0.05 vs. MALAT1-treated; &P<0.05
vs. MALAT1 scrambled siRNA group. Data are presented as the mean ±
standard deviation. siRNA, small interfering RNA; MALAT1,
metastasis-associated lung adenocarcinoma transcript 1; I/R,
ischemia/reperfusion; B, MALAT1 Blank; M, MALAT1; S, MALAT1
scrambled siRNA; Si, MALAT1 siRNA; N, Non-runners; R, runners;
Bcl-2, B cell leukemia/lymphoma 2; Bax, BCL2-associated X; Bad,
BCL2 associated agonist of cell death; Bcl-xL, B-cell
lymphoma-extra large. |
Discussion
The present study aimed to investigate whether
increased hippocampal MALAT1 expression induced spontaneous RW
following I/R injury in mice, which may be associated with
cognitive recovery. The current findings revealed that I/R injury
induced significant neurological and cognitive function deficits,
as well as increased neuronal apoptosis in mice. Spontaneous RW
partially restored this neurological and cognitive impairment and
reduced hippocampal apoptosis. Simultaneously, the expression of
MALAT1, an lncRNA demonstrated to be involved in cancer cell
proliferation and metastasis was significantly restored following
RW exercise. Specific siRNA targeting MALAT1 neutralized the
neuroprotective effects induced by voluntary RW exercise, whereas
MALAT1 administration enhanced these effects.
The pathological condition of cerebral ischemia is
responsible for learning and memory impairments, and adversely
affects the everyday and social lives of survivors (25). The present study revealed that I/R
injury may lead to significant neurological loss, learning and
memory deficits, which is consistent with previous findings
(4,26).
Previous studies have demonstrated that exercise has
beneficial effects and promotes functional recovery in animal
models of stroke (27,28). Voluntary RW is reportedly the most
valid treatment for promoting the recovery of motor function. Rats
that underwent voluntary exercise also had a lower corticosterone
stress response, compared with other trialed exercise paradigms
(13). However, the underlying
molecular mechanisms reported have been inconsistent and remain to
be fully elucidated. The present study revealed that voluntary RW
exercise partially reverses hippocampal MALAT1 expression, reduces
infarction volume, decreases the hippocampal TUNEL-positive cell
proportion and alters the expression of apoptosis-associated
proteins. MALAT1 siRNA treatment neutralized the neuroprotective
effects induced by voluntary RW exercise, whereas exogenous MALAT1
administration increased these protective effects. Therefore, the
present study hypothesized that the voluntary RW exercise-induced
recovery of cognitive functions may be associated with hippocampal
apoptosis inhibition mediated by MALAT1.
Cerebral miRNA and piwi-interacting RNA profiles
change extensively, these may be involved in neuroplasticity and
the modulation of brain damage following ischemic injury. Miao
et al (4) revealed that
cortical and hippocampal miRNA expression alterations in I/R mice
were responsible for ischemic postconditioning-induced
neuroprotection. Previous studies reported that ischemia also
influences the lncRNA profile bioinformatics analysis identified a
>90% sequence homology between several stroke-responsive lncRNAs
genes and protein-coding genes located on different chromosomes,
indicating that these lncRNAs may be pseudogenes (8,29,30). Many of the 'stroke-responsive'
lncRNAs are homologous to protein-coding genes that are associated
with ribosomal complex formation, splicing, translation initiation
and the nuclear import of mRNAs. Therefore, they may stabilize
those mRNAs in order to partially recover the protein generation
inhibited by the stroke, during the acute phase following injury
(8). Previous studies have
determined that these 'stroke-responsive' lncRNAs may be involved
in chromatin modifications, transcription factor activity and
apoptosis regulation (31–33).
The present study revealed that the lncRNA MALAT1
level was significantly reduced following I/R injury and was
partially restored by spontaneous RW exercise. MALAT1 siRNA
treatment neutralized the neuroprotection induced by voluntary RW
exercise, including cognitive recovery and apoptosis inhibition,
whereas treatment with MALAT1 enhanced these effects.
MALAT1 is involved with the survival of various
tumor types and may act as a valid prognostic biomarker for cancer
(34). A previous study revealed
its role in extensive biological processes. For example, MALAT1
lncRNA was demonstrated to protect human brain vascular endothelial
cells from oxygen-glucose deprivation and re-oxygenation-induced
apoptosis in vitro (22).
The current study revealed that voluntary RW exercise setting
protected I/R injured mice from the cognitive decline induced by
MALAT1-regulated apoptosis inhibition.
The present study used TUNEL staining and protein
level detection to identify hippocampal apoptosis following the
application of different treatments. The apoptosis
prevention-induced by voluntary RW following I/R injury might
depend on endogenic MALAT1 regulation. Spontaneous RW downregulated
Bax, Bad and caspase-3/-8, whereas Bcl-2 and Bcl-xL were
upregulated in the hippocampus of mice post I/R injury. Those
expressional alterations were partially reversed by MALAT1 siRNA
treatment and enhanced by the administration of exogenous MALAT1.
Apoptosis, a complex biochemical process, is influenced by a
variety of factors (35,36). It has been previously established
that the Bcl-2 family functions as inhibitors (such as Bcl-2 and
Bcl-xL) or promotors (such as Bax, Bcl-xS, Bad, and Bak) during
cell apoptosis. Caspase-3 activation is considered a key mechanism
of apoptosis (37,38). Therefore, the current findings
indicate that voluntary RW may inhibit the damage caused by
apoptosis with MALAT1 regulation, in I/R mice.
In conclusion, the present study demonstrated that
I/R injury induced significant neurological deficits and cognitive
impairments, as well as increased neuronal apoptosis in mice.
Spontaneous RW exercise may partially restore these impairments,
reduce hippocampal apoptosis and alter the expression of
apoptosis-associated proteins. siRNA targeting MALAT1 neutralized
the neuroprotective effects induced by voluntary RW exercise,
whereas exogenous MALAT1 enhanced these effects. The present
findings suggested that spontaneous RW exercise had a
neuroprotective role, via MALAT1 regulation, following I/R injury
in mice.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Durukan A and Tatlisumak T:
Preconditioning-induced ischemic tolerance: A window into
endogenous gearing for cerebroprotection. Exp Transl Stroke Med.
2:22010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao H, Sapolsky RM and Steinberg GK:
Interrupting reperfusion as a stroke therapy: Ischemic
postconditioning reduces infarct size after focal ischemia in rats.
J Cereb Blood Flow Metab. 26:1114–1121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin XM, Zhang ZY, Wang LF and Zhang L, Liu
Y, Liu XL, Yang XC, Cui L and Zhang L: Attenuation of tumor
necrosis factor-alpha elevation and improved heart function by
postconditioning for 60 sec in patients with acute myocardial
infarction. Chin Med. 123:1833–1839. 2010.
|
|
4
|
Miao W, Bao TH, Han JH, Yin M, Zhang J,
Yan Y and Zhu YH: Neuroprotection induced by post-conditioning
following ischemia/reperfusion in mice is associated with altered
microRNA expression. Mol Med Rep. 14:2582–2588. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niland CN, Merry CR and Khalil AM:
Emerging roles for long non-coding RNAs in cancer and neurological
disorders. Front Genet. 3:252012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huttenhofer A, Schattner P and Polacek N:
Non-coding RNAs: Hope or hype. Trends Genet. 21:289–297. 2005.
View Article : Google Scholar
|
|
7
|
Zhang Q, Matsuura K, Kleiner DE, Zamboni
F, Alter HJ and Farci P: Analysis of long noncoding RNA expression
in hepatocellular carcinoma of different viral etiology. J Transl
Med. 14:3282016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dharap A, Nakka VP and Vemuganti R: Effect
of focal ischemia on long noncoding RNAs. Stroke. 43:2800–2802.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Puthanveetil P, Chen S, Feng B, Gautam A
and Chakrabarti S: Long non-coding RNA MALAT1 regulates
hyperglycaemia induced inflammatory process in the endothelial
cells. J Cell Mol Med. 19:1418–1425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Michalik KM, You X, Manavski Y,
Doddaballapur A, Zörnig M, Braun T, John D, Ponomareva Y, Chen W,
Uchida S, et al: Long noncoding RNA MALAT1 regulates endothelial
cell function and vessel growth. Circ Res. 114:1389–1397. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thum T and Fiedler J: LINCing MALAT1 and
angiogenesis. Circ Res. 114:1366–1368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu T, Zhou FJ, Chang YF, Li YS, Liu GC,
Hong Y, Chen HL, Xiyang YB and Bao TH: miR21 is associated with the
cognitive improvement following voluntary running wheel exercise in
TBI mice. J Mol Neurosci. 57:114–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ke Z, Yip SP, Li L, Zheng XX and Tong KY:
The effects of voluntary, involuntary, and forced exercises on
brain-derived neurotrophic factor and motor function recovery: A
rat brain ischemia model. PLoS One. 6:e166432011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bao TH, Miao W, Han JH, Yin M, Yan Y, Wang
WW and Zhu YH: Spontaneous running wheel improves cognitive
functions of mouse associated with miRNA expressional alteration in
hippocampus following traumatic brain injury. J Mol Neurosci.
54:622–629. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
XiYang YB, Wang YC, Zhao Y, Ru J, Lu BT,
Zhang YN, Wang NC, Hu WY, Liu J, Yang JW, et al: Sodium channel
voltage-gated beta 2 plays a vital role in brain aging associated
with synaptic plasticity and expression of COX5A and FGF-2. Mol
Neurobiol. 53:955–967. 2016. View Article : Google Scholar
|
|
16
|
Xiong X, Gu L, Zhang H, Xu B, Zhu S and
Zhao H: The protective effects of T cell deficiency against brain
injury are ischemic model-dependent in rats. Neurochem Int.
62:265–270. 2013. View Article : Google Scholar :
|
|
17
|
Joo SP, Xie W, Xiong X, Xu B and Zhao H:
Ischemic postconditioning protects against focal cerebral ischemia
by inhibiting brain inflammation while attenuating peripheral
lymphopenia in mice. Neuroscience. 23:149–157. 2013. View Article : Google Scholar
|
|
18
|
Hill JK, Gunion-Rinker L, Kulhanek D,
Lessov N, Kim S, Clark WM, Dixon MP, Nishi R, Stenzel-Poore MP and
Eckenstein FP: Temporal modulation of cytokine expression following
focal cerebral ischemia in mice. Brain Res. 820:45–54. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Loane DJ, Pocivavsek A, Moussa CE,
Thompson R, Matsuoka Y, Faden AI, Rebeck GW and Burns MP: Amyloid
precursor protein secretases as therapeutic targets for traumatic
brain injury. Nat Med. 15:377–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong G, Xiang L, Yuan L, Hu L, Wu W, Cai
L, Yin L and Dong H: Protective effect of glycyrrhizin, a direct
HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced
inflammation, oxidative stress, and apoptosis in rats. PLoS One.
9:e894502014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar
|
|
22
|
Xin JW and Jiang YG: Long noncoding RNA
MALAT1 inhibits apoptosis induced by oxygen-glucose deprivation and
reoxygenation in human brain microvascular endothelial cells. Exp
Ther Med. 13:1225–1234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuete V, Sandjo LP, Mbaveng AT, Seukep JA,
Ngadjui BT and Efferth T: Cytotoxicity of selected Cameroonian
medicinal plants and Nauclea pobeguinii towards multi-factorial
drug-resistant cancer cells. BMC Complement Altern Med. 15:3092015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Skup M, Dwornik A, Macias M, Suleczak D,
Wiater M and Czarkoeska-Bauch J: Long-term locomotor training
upregulates TrkB(FL) receptor-like proteins, brain-derived
neurotrophic factor, and neurotrophin 4 with different topographies
of expression in oligodendroglia and neurons in the spinal cord.
Exp Neurol. 176:289–307. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vaughan L, Bushnell C, Bell CL and
Espeland MA: Global cognitive function before, surrounding,
andafter ischemic stroke: The role of risk and protective factors
varies with time among ischemic stroke survivors. Neuropsychol Dev
Cogn B Aging Neuropsychol Cogn. 23:117–131. 2016. View Article : Google Scholar
|
|
26
|
Moghimi M, Parvardeh S, Zanjani TM and
Ghafghazi S: Protective effect of α-terpineol against impairment of
hippocampal synaptic plasticity and spatial memory following
transient cerebral ischemia in rats. Iran J Basic Med Sci.
19:960–969. 2016.PubMed/NCBI
|
|
27
|
DeBow SB, Davies MA, Clarke HL and
Colbourne F: Constraint-induced movement therapy and rehabilitation
exercises lessen motor deficits and volume of brain injury after
striatal hemorrhagic stroke in rats. Stroke. 34:1021–1026. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burnett MG, Shimazu T, Szabados T,
Muramatsu H, Detre JA and Greenberg JH: Electrical forepaw
stimulation during reversible forebrain ischemia decreases infarct
volume. Stroke. 37:1327–1331. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dharap A, Nakka VP and Vemuganti R:
Altered expression of PIWI RNA in the rat brain after transient
focal ischemia. Stroke. 42:1105–1109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hirotsune S, Yoshida N, Chen A, Garrett L,
Sugiyama F, Takahashi S, Yagami K, Wynshaw-Boris A and Yoshiki A:
An expressed pseudogene regulates the messenger-RNA stability of
its homologous coding gene. Nature. 423:91–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huarte M, Guttman M, Feldser D, Garber M,
Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M,
et al: A largeintergenic noncoding RNA induced by p53 mediates
global gene repression in the p53 response. Cell. 142:409–419.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang J, Yuan L, Zhang X, Hamblin MH, Zhu
T, Meng F, Li Y, Chen YE and Yin KJ: Altered long non-coding RNA
transcriptomic profiles in brain microvascular endothelium after
cerebral ischemia. Exp Neurol. 277:162–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan J, Zhou X, Dang Y, Yin C and Zhang G:
Prognostic role of the long non-coding RNA metastasis-associated
lung adenocarcinoma transcript 1 in various cancers: A
meta-analysis. Mol Clin Oncol. 4:100–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vaux D and Korsmeyer S: Cell death in
development. Cell. 96:245–254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Korsmeyer SJ: BCL-2 gene family and the
regulation of programmed cell death. Cancer Res. 59(7 Suppl):
S1693–S1700. 1999.
|
|
38
|
Cohen GM: Caspases: The executioners of
apoptosis. Biochem J. 326:1–16. 1997. View Article : Google Scholar : PubMed/NCBI
|