Introduction
Spinal cord injury (SCI) is a serious trauma of the
nervous system (1). It may cause
paralysis and sphincter disturbance, resulting in patients becoming
bedridden long-term, and may even be life-threatening, which is a
heavy burden to society and the patient's family. The morbidity of
SCI is ~20-40 per million population, and there are predicted to be
~2,500,000 individuals affected by SCI, with an annual growth in
incidence of >100,000 (2). The
expense of nursing and treatment is very high due to the lack of
effective treatments and rehabilitation measures (3). The morbidity of SCI in Beijing in
2002 was reported to be 50 per million population, which is much
higher than the mean morbidity worldwide (4). The frequency of earthquakes, mining,
construction and traffic accidents in China is high, which is
likely to result in a higher morbidity compared with that in other
countries. Due to the consistent and rapid development of the
economy in China, SCI has become a clear threat to health and
quality of life.
The phosphatidylinositol 3-kinase (PI3K)/Akt signal
transduction pathway affects cell proliferation, differentiation,
migration and apoptosis (5).
Recent studies have demonstrated that this pathway is associated
with a variety of cerebral injuries (5,6).
The PI3K/Akt pathway has been observed to have a regulatory effect
of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)
receptor in the central nervous system (6). In the optic nerve cells and
cerebellar tracts of mice, it has been observed that
oligodendrocytes are dynamically regulated through the PI3K/Akt
pathway and AMPA receptor-mediated Ca2+-signaling
(7).
Fibroblast growth factor 2 (FGF-2) is a
multi-functional growth factor that is extensively distributed in
various tissues of the body and promotes cell proliferation,
differentiation, migration and angiogenesis (8). FGF-2 has been demonstrated to be an
important endogenous cardioprotective protein (9). Previous studies have indicated that
the protective effects of exogenous FGF-2 are associated with a
signal transduction pathway comprising fibroblast growth factor
receptor 1 (FGFR1), activated protein kinase C (PKC) and
mitogen-activated protein kinase (MAPK) (10). Activation of the FGFR1 tyrosine
kinase induces activation of the phospholipase C-PKC, Ras-MAPK and
PI3K/Akt signaling pathways; the PI3K/Akt pathway is independent of
PKC, PKA and MAPK (11). It
participates in the regulation of the physiological or pathological
processes of nerve cells (12).
In addition, it serves a crucial role in cell proliferation,
differentiation and apoptosis (12). Studies have shown that in nerve
cells, retinal photoreceptor cells and human umbilical vein
endothelial cells, FGF-2 antagonizes the cell apoptosis induced by
oxidative stress through activation of the PI3K/Akt signaling
pathway. Forkhead box O3 (FOXO3a) has been demonstrated to be an
essential transcription factor downstream of the PI3K/Akt signal
pathway (11,12). It is vital in the antagonism of
oxidative stress-induced apoptosis (13). The aim of the present study was to
investigate the expression and role of PI3K/Akt/FOXO3a in the
regeneration of the spinal cord following SCI in adult rats, and
explore the underlying mechanism.
Materials and methods
SCI surgery
All surgical interventions were carried out in
accordance with the Guide for the Care and Use of Laboratory
Animals of PLA General Hospital and were approved by the ethics
committee of Chinese PLA General Hospital (Beijing, China). Male
Sprague-Dawley rats (n=24; age, 6-7 weeks, weight, 200-230 g) were
purchased from Beijing Vital River Laboratory Animal Technology
Co., Ltd. and housed at 22-23°C, 55-60% humidity, under a
light/dark cycle (8:00-20:00), with free access to food and water.
The rats were deeply anesthetized with 350 mg/kg chloral hydrate
intraperitoneally. All rats were randomly assigned to control,
SCI-1 day, SCI-2 day and SCI-3 day (n=6 rat/group) and 18 rats
underwent SCI surgery. Dorsal laminectomy at the level of the ninth
thoracic vertebra was conducted and the spinal cord was contused by
dropping a rod 2.0 mm in diameter. Following this, the overlying
muscles and skin were closed in layers with 4-0 silk sutures and
staples.
Hematoxylin and eosin (H&E)
staining
The experimental rats (n=6) were sacrificed on days
1, 2 and 3 following SCI, and spinal cord tissue was extracted and
fixed in 10% neutral formalin for 3 days. The spinal cord tissue
was sliced into 1-cm sections, which were dehydrated using a graded
alcohol series. The tissue samples were then sliced into
20-μm longitudinal sections. The sections were stained with
hematoxylin for 5 min, then washed with water for 10 min, treated
with ethanol for 10 sec to remove excess stain, and washed with
water for 10 min. Finally, they were stained with eosin for 5 min
and washed with water for 10 min. The stained sections were
dehydrated through a graded alcohol series, permeabilized with
xylene, and mounted with neutral resin. Imaging was performed using
a confocal microscope (LSM 700: Zeiss AG, Oberkochen, Germany) with
ImageJ 3.0 software (National Institutes of Health, Bethesda, MD,
USA).
Western blotting
Spinal cord tissue samples and cultured cells were
each homogenized in RIPA assay (Beyotime Institute of
Biotechnology, Jiangsu, China) and then centrifuged at 10,000 × g
for 20 min at 4°C. The protein content of the supernatant was
measured using a bicinchoninic acid assay (Beyotime Institute of
Biotechnology). Samples containing 100 μg protein were
subjected to 10-12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and then transferred to a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA). The membrane was
blocked in TBS with Tween 20 with 5% non-fat milk for 1 h at 37°C
prior to incubation with the primary antibodies anti-PI3K (sc-7174;
1:500), anti-p-Akt (sc-7985-R; 1:2,000), anti-FOXO3a (12829;
1:3,000), anti-cyclin-dependent kinase inhibitor 1B
(p27kip1; sc-756; 1:2,000) and anti-β-actin (sc-10731;
1:5,000) (all from Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
at 37°C for 1 h. The membrane was then incubated with the
appropriate horseradish peroxidase-conjugated secondary antibody
(sc-2004; 1:5,000; Santa Cruz Biotechnology, Inc.) at 37°C for 1 h
and visualized using an enhanced chemiluminescence system (Pierce;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and quantified
using ImageJ 6.0 software.
Enzyme-linked immunosorbent assay
Tumor necrosis factor (TNF)-α was quantified in
Sprague-Dawley rat cell cultures using a mouse TNF-α Enzyme-Linked
Immunosorbent assay kit (RTA00; R&D Systems, Inc., Minneapolis,
MN, USA) according to the manufacturer's protocol. The lower limit
of detection of the kit was <20 pg/ml.
Spinal cord cell culture and
downregulation of PI3K
Primary dissociated cell cultures were prepared from
the dorsal spinal cords of Sprague-Dawley rats. Collagenase 0.25%
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used to digest
the spinal cords for 10-20 min, and the obtained cells were then
cultured with Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich; Merck KGaA) containing 5% fetal bovine serum for 1
week. The spinal cord cells were stimulated with lipopolysaccharide
(LPS; 4 μg/ml; Beyotime Institute of Biotechnology) for 2 h
without PI3K inhibitor (with negative plasmid), and then treated
with 10 μmol/l PI3K inhibitor (LY294002; Beyotime Institute
of Biotechnology) for 1, 2 and 3 days.
Measurement of the regeneration of spinal
cord cells
In the LPS-challenged cells following 1, 2 and 3
days of treatment with LY294002, the effect of PI3K inhibition on
the viability of the spinal cord cells was determined using a
colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay. In this assay, 50 μl MTT solution (1
mg/ml final concentration) was added to the cells in each well of a
96-well plate and the plate was incubated for 4 h at 37°C. The
formazan was removed by the addition of 100 μl
dimethylsulfoxide and the optical density was measured with a
microplate reader (Multiskan; Labsystems Diagnostics Oy, Helsinki,
Finland) at 540 nm.
Overexpression of FOXO3a using an
adenoviral vectors
A FOXO3a adenoviral vector was designed by and
purchased from Takara Biotechnology Co., Ltd. (Dalian, China). The
primers of the FOXO3a adenoviral vector were: Sense,
5′-CCCGGTGCGTGCCTATCAGGGGC-3′ and antisense,
5′-CCGACTTCTCGTCCCCTCG-3′. Spinal cord cells induced by LPS (4
μg/ml) for 2 h were infected with FOXO3a adenoviral vectors
(100 nM) in DMEM/F12 for 8 h. The medium was replaced with fresh
complete culture medium prior to subsequent analysis.
Statistical analysis
Results are presented as the mean ± standard error
of the mean using SPSS 17.0 (experiments were repeated in
triplicate). One-way analysis of variance followed by Turkey's post
hoc multiple comparison tests were used for statistical analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Histological examination of the spinal
cord and the expression of PI3K and Akt proteins in a rat model of
SCI
H&E staining of the spinal cord tissue (Fig. 1A) revealed a complete and clear
structure in the control group, with no cavities and densely
arranged nerve fibers. However, the nerve fibers were loosely
arranged and appeared shorter and less numerous at 1, 2 and 3 days
following SCI compared with those in the control group.
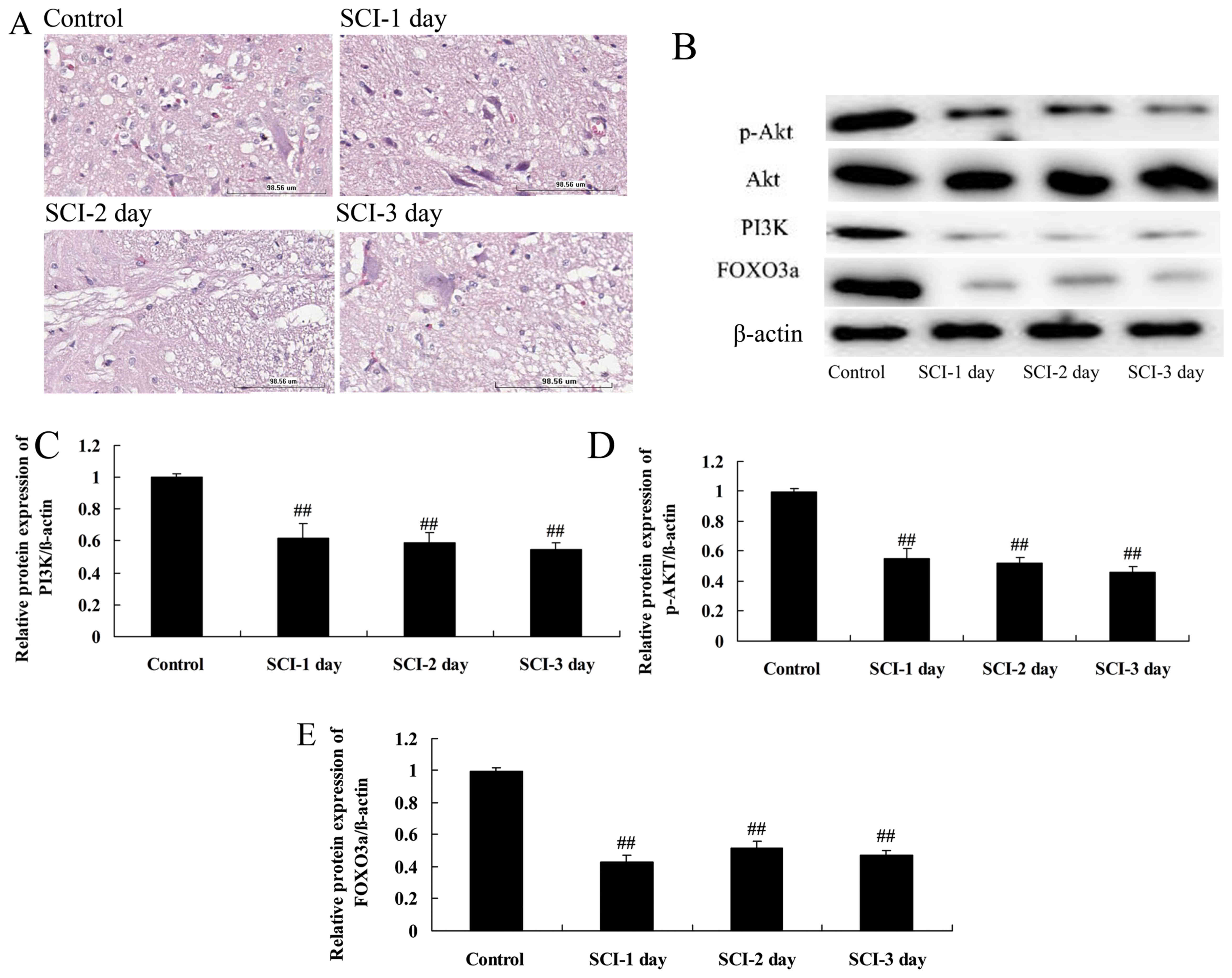 | Figure 1Spinal cord histology and the
expression of PI3K, Akt, p-Akt and FOXO3a proteins in a rat model
of SCI. (A) Hematoxylin and eosin-stained spinal cord tissue 1, 2
and 3 days following SCI compared with the control. Scale bar,
98.56 μm. (B) Western blots of PI3K, Akt, p-Akt and FOXO3a
and quantitative analysis of (C) PI3K, (D) p-Akt and (E) FOXO3a
protein expression levels in the SCI model rats.
##P<0.01 vs. the control group. PI3K,
phosphatidylinositol 3-kinase; p, phosphorylated; FOXO3a, forkhead
box O3; SCI, spinal cord injury. |
To investigate the involvement of the PI3K/Akt
signaling pathway in the tissue damage associated with SCI, the
PI3K and p-Akt protein levels in the spinal cord tissue were
determined in the rats following SCI using western blotting. The
protein levels of PI3K and p-Akt were significantly suppressed in
the rats 1, 2 and 3 days following SCI compared with the respective
expression levels in the control group (Fig. 1B–D). However, Akt protein
expression was not changes in the rats 1, 2 and 3 days following
SCI compared with the respective expression levels in the control
group (Fig. 1B–D). This suggests
that the PI3K/Akt signaling pathway is inhibited following SCI.
FOXO3a protein levels in a rat model of
SCI
Whether FOXO3a participates in the PI3K/Akt
signaling pathway in the rat model of SCI was investigated by
measuring the protein levels of FOXO3a using western blotting. The
FOXO3a protein levels at 1, 2 and 3 days following SCI were
significantly lower compared with those in the control group
(Fig. 1B and E). This suggests
that the PI3K/Akt/FOXO3a signaling pathway was inhibited following
SCI in the rat model.
PI3K inhibition reduces PI3K and p-Akt
protein levels in spinal cord cells in vitro
The effects of a PI3K inhibitor on the levels of
various proteins in LPS-induced spinal cord cells were investigated
using western blotting (Fig. 2).
The results demonstrated that the protein levels of PI3K and p-Akt
were significantly suppressed by treatment with a PI3K inhibitor
for 1, 2 or 3 days compared with those in the control group
(Fig. 2A–C), confirming that the
PI3K/Akt pathway was downregulated.
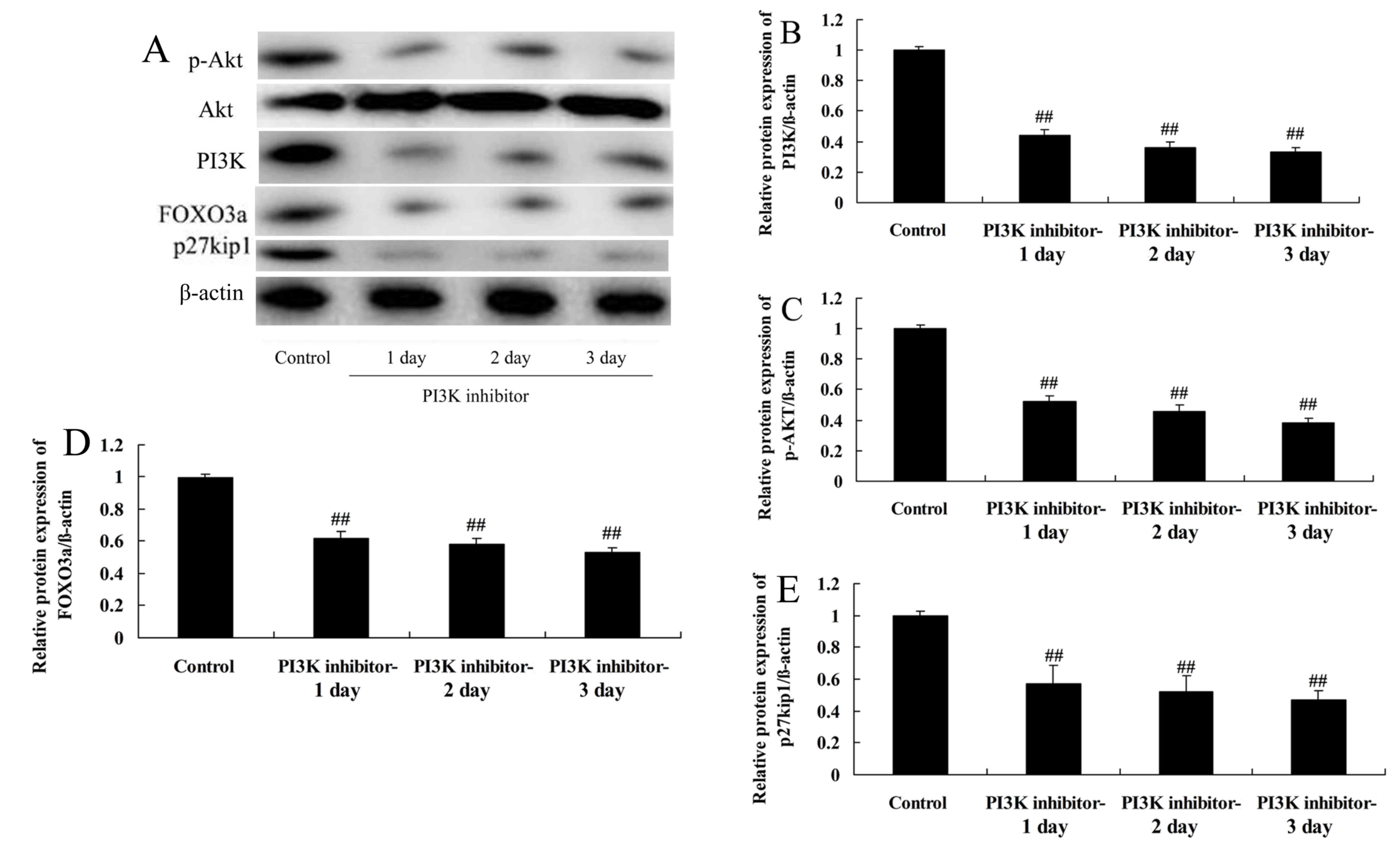 | Figure 2Effect of PI3K inhibition on PI3K,
Akt, p-Akt, FOXO3a and p27kip1 protein levels in spinal
cord cells. Control cells were treated with LPS (4 μg/ml)
for 2 h, and cells in the PI3K inhibitor-1, 2 and 3 day groups were
treated with LPS (4 μg/ml) for 2 h and then with 10
μmol/l LY294002 for 1, 2 and 3 days. (A) Western blotting of
PI3K, Akt, p-Akt, FOXO3a and p27kip1, and quantitative
analysis of (B) PI3K, (C) p-Akt, (D) FOXO3a and (E)
p27kip1 protein levels. ##P<0.01 vs. the
control group. PI3K, phosphatidylinositol 3-kinase; p,
phosphorylated; FOXO3a, forkhead box O3; p27kip1,
anti-cyclin-dependent kinase inhibitor 1B; LPS,
lipopolysaccharide. |
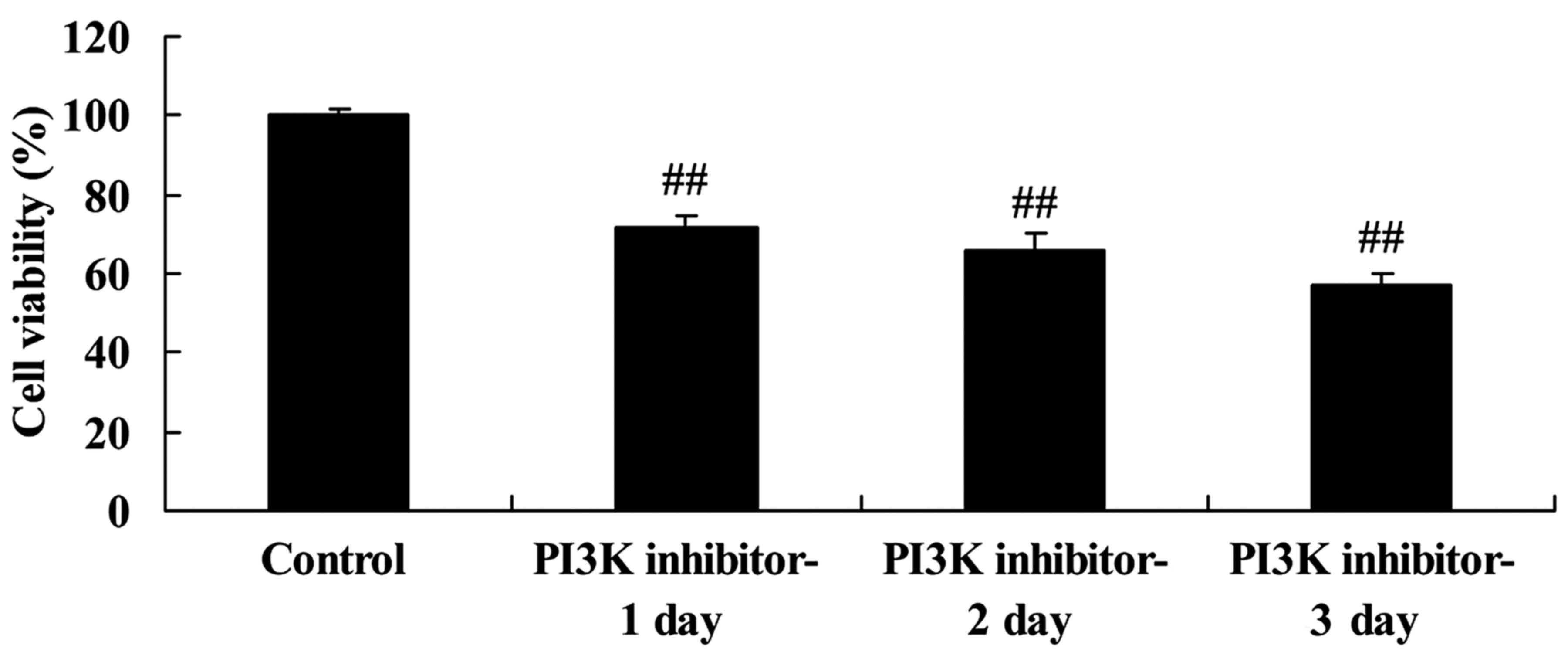
Downregulation of PI3K inhibits the
regeneration of spinal cord cells
The effect of PI3K/Akt pathway inhibition on the
viability of the LPS-induced spinal cord cells was investigated
using an MTT assay. The PI3K inhibitor significantly inhibited the
viability of the LPS-induced spinal cord cells compared with that
of the control group following 1, 2 and 3 days of treatment
(Fig. 3). This suggests that the
downregulation of PI3K reduced cell viability in this in
vitro SCI model.
Downregulation of PI3K reduces FOXO3a
protein levels in spinal cord cells
To further investigate the role of inhibition of the
PI3K/Akt pathway in LPS-induced spinal cord cell damage, FOXO3a
protein levels were determined in the LPS-induced spinal cord cells
using western blotting. The inhibition of PI3K significantly
reduced FOXO3a protein levels in the LPS-induced spinal cord cells,
compared with those in the control group (Fig. 2A and D). This suggests that FOXO3a
may participate in the PI3K/Akt signaling pathway in SCI.
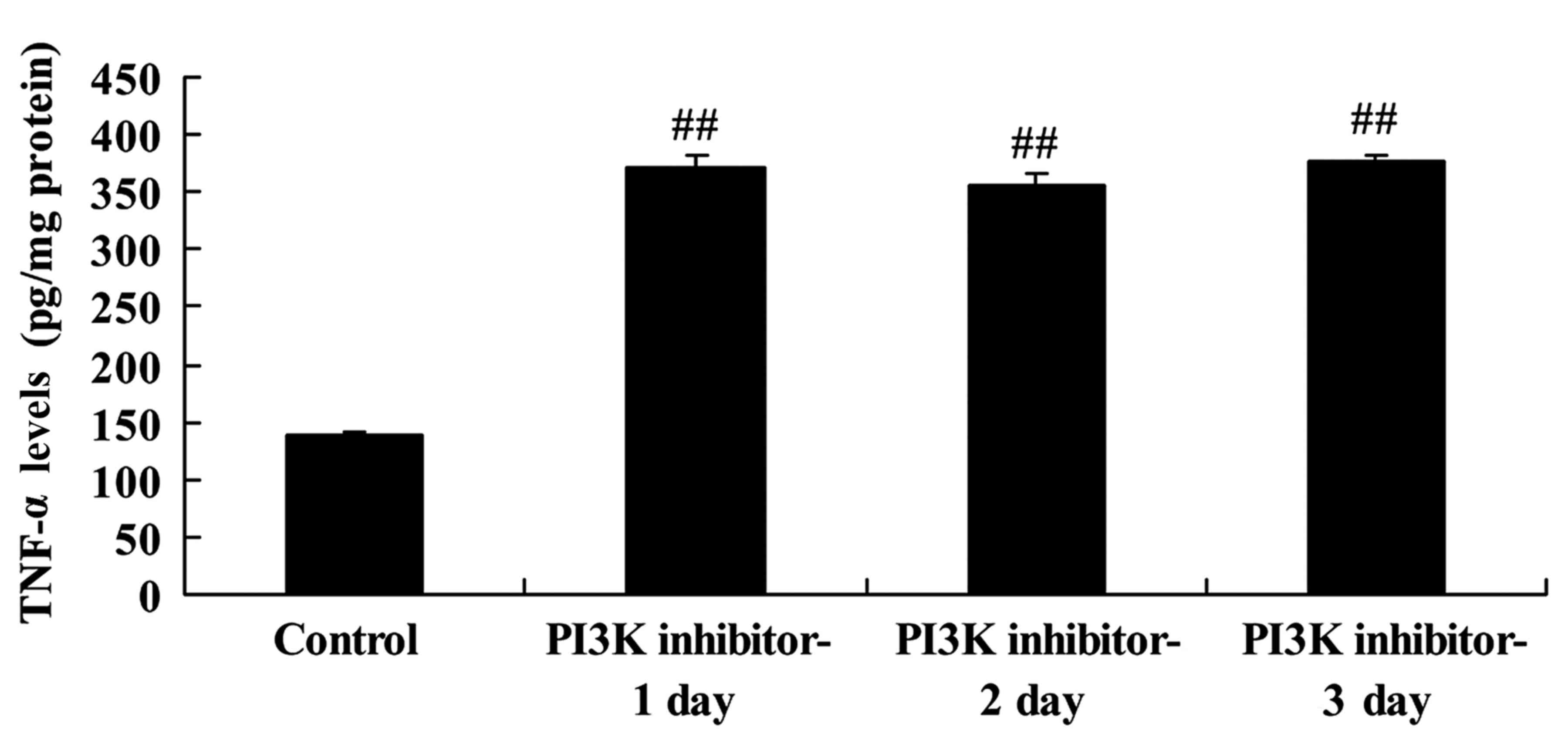
Downregulation of PI3K increases tumor
necrosis factor (TNF)-α levels in spinal cord cells
The effect of PI3K inhibition on TNF-α activity was
evaluated to assess its involvement in the pathogenic mechanism of
SCI. TNF-α levels in the LPS-induced spinal cord cells were
significantly increased in the PI3K inhibitor group on days 1, 2
and 3 compared with those in the control group (Fig. 4). This indicates that the PI3K/Akt
signaling pathway affected TNF-α levels in this in vitro SCI
model, and may have inflammatory effects.
Inhibition of PI3K reduces
p27kip1 protein levels in spinal cord cells
The effect of PI3K inhibition on the
p27kip1 protein levels of LPS-induced spinal cord cells
was explored using western blotting. In the cells, a significant
reduction in p27kip1 protein levels was observed on days
1, 2 and 3 in the cells treated with PI3K inhibitor compared with
the control group (Fig. 2A and
E). This demonstrates that the PI3K/Akt signaling pathway
affected p27kip1 expression levels in this in
vitro SCI model.
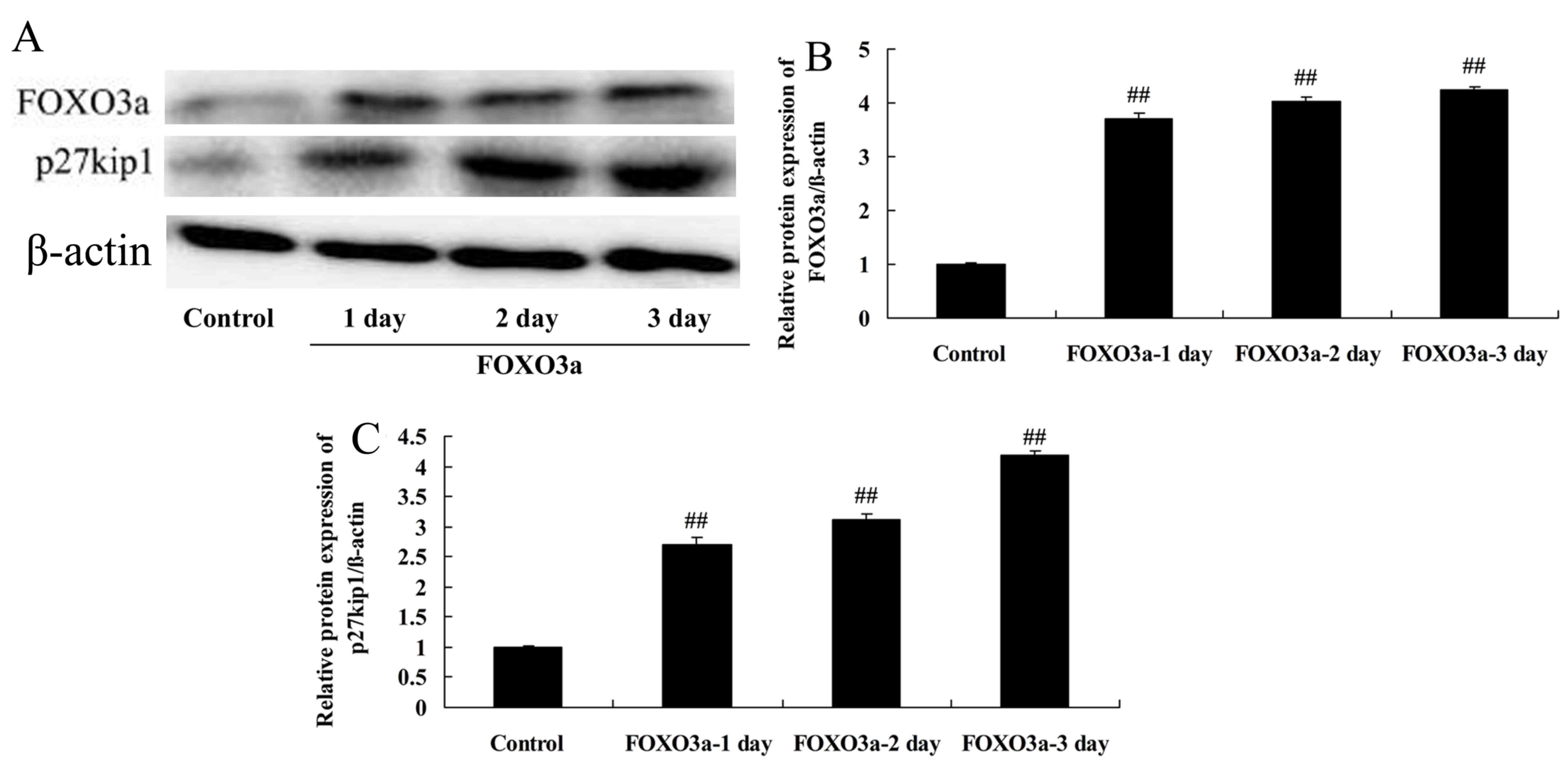
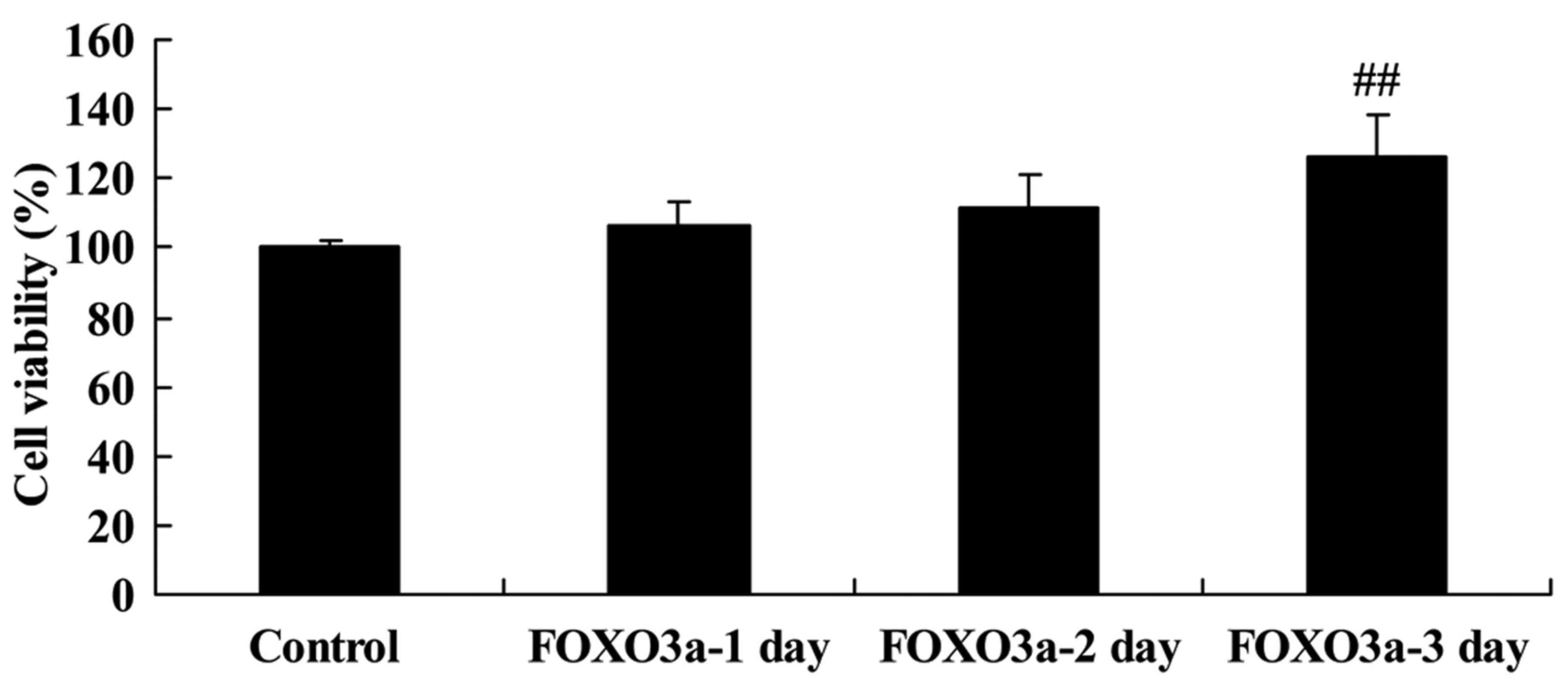
Overexpression of FOXO3a improves the
regeneration of spinal cord cells
The mechanism of PI3K/Akt signaling in the
progression of SCI was further examined via the overexpression of
FOXO3a. The effects of FOXO3a overexpression on the levels of
various proteins in LPS-induced spinal cells were evaluated using
western blotting (Fig. 5). The
results confirmed that transfection with a FOXO3a vector
significantly increased the FOXO3a protein levels in the
LPS-induced spinal cord cells compared with those in the control
group (Fig. 5A and B).
Furthermore, the overexpression of FOXO3a significantly increased
the viability of the LPS-induced spinal cord cells compared with
that of the control group at 3 days after transfection (Fig. 6). This indicates that FOXO3a has a
potential role in the regulation of SCI progression.
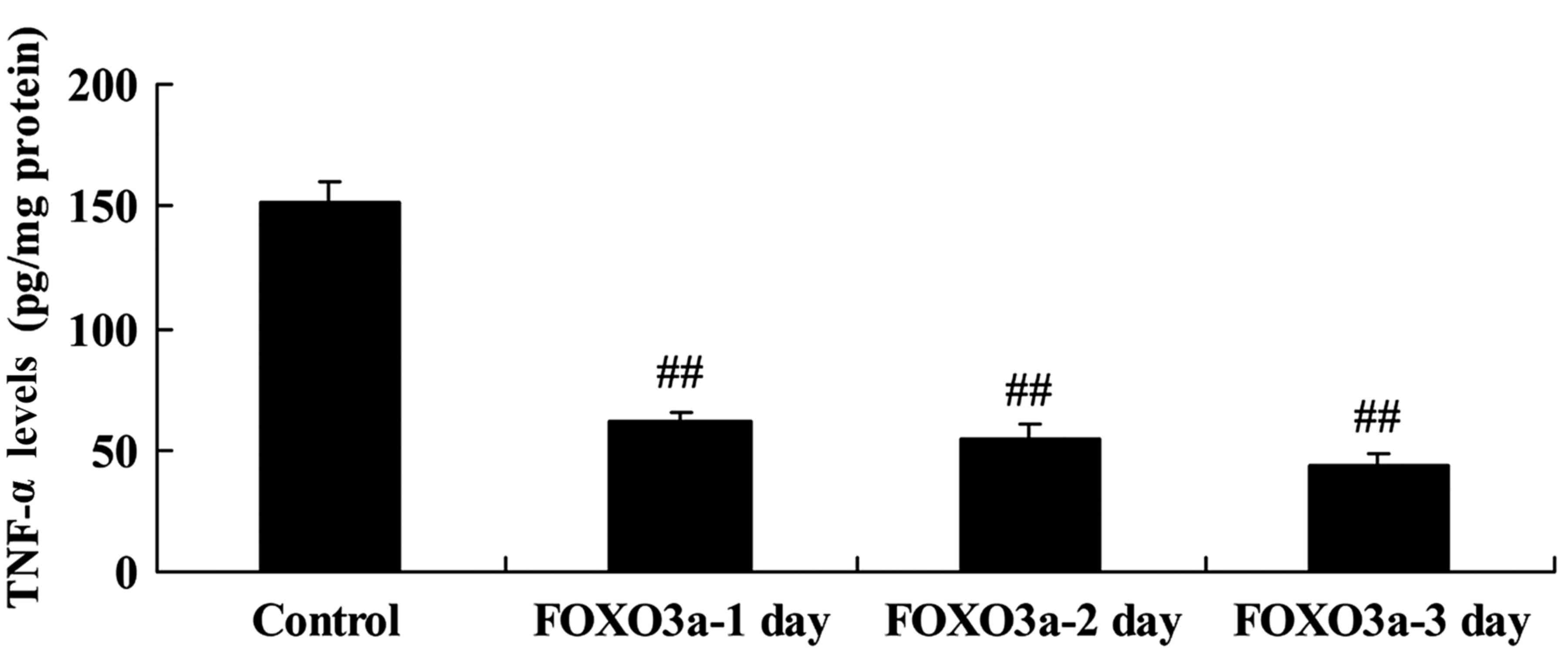
Overexpression of FOXO3a reduces TNF-α
levels in spinal cord cells
The effect of FOXO3a overexpression on TNF-α levels
in LPS-induced spinal cord cells was evaluated. The overexpression
of FOXO3a significantly reduced TNF-α levels in the LPS-induced
spinal cord cells on days 1, 2 and 3 compared with those in the
control group (Fig. 7). These
results suggest that the PI3K/Akt/FOXO3a signaling pathway affects
TNF-α levels in SCI.
Overexpression of FOXO3a increases the
p27kip1 protein levels of spinal cord cells
The effect of the overexpression of FOXO3a on
p27kip1 protein levels in LPS-induced spinal cord cells
was investigated. The overexpression of FOXO3a significantly
increased the p27kip1 protein levels of the LPS-induced
spinal cord cells compared with those in the control group
(Fig. 5A and C). This indicates
that the PI3K/Akt/FOXO3a signaling pathway affected
p27kip1 in this SCI model.
Discussion
The PI3K/Akt/FOXO3a pathway has been demonstrated to
serve an important role in the apoptosis of rat neurons induced by
hypoxia and ischemia (7). The
present study verified that FOXO3a is regulated via PI3K/Akt. In a
previous study involving a mouse model of cardiac
ischemia/reperfusion, the PI3K/Akt/FOXO3a signaling pathway was
demonstrated to mediate the cardioprotective effects of bromelain
and sodium sulfonate (14). It
has been speculated that FGF-2 promotes the phosphorylation of
FOXO3a, inhibits its nuclear translocation and reduces the
expression of pro-apoptotic proteins, thereby inhibiting the
oxidative stress-induced apoptosis of nerve cells (11). The present study provides novel
evidence that the protein levels of PI3K, p-Akt and FOXO3a were
significantly suppressed in an adult rat model of SCI.
SCI involves nervous system damage, and generally
leads to severe motor and sensory dysfunction (4). SCI includes primary damage and
sequential damage caused by primary factors. The primary injury is
an instantaneous and irreversible event, while the sequential
injury arises on the basis of the primary injury and may
potentially be attenuated (15).
The extensive infiltration of inflammatory cells and generation of
inflammatory cytokines following SCI are the principal factors
leading to sequential spinal cord injury. Since sequential injury
of the spinal cord can be inhibited, the treatment of SCI is
focused on relieving or preventing the sequential injury, and is an
essential objective of the early treatment of SCI (16).
TNF-α is an inflammatory cytokine that serves major
roles in inflammatory reactions and immune regulation (17). Studies have shown that following
SCI, TNF-α rapidly and consistently participates in the sequential
spinal cord injury process as an injury factor (16,17). It is a cytokine that increases
following SCI, upregulates the generation of other cytokines, and
induces the generation of arachidonic acid, lipid peroxides and
oxygen free radicals that may damage cytomembranes and increase the
vascular permeability of blood (18). Such characteristics may be
associated with the mechanism of formation of edema following SCI
(18). In the present study, it
was observed that the inhibition of PI3K significantly increased
the TNF-α levels in the LPS-induced spinal cord cells, which may be
the cause of the reduction in cell viability that was observed.
Cell apoptosis has multiple upstream pathways,
including the death receptor-mediated, mitochondrial and
endoplasmic reticulum pathways (19). The PI3K/Akt pathway is important
for controlling cell apoptosis and promoting proliferation through
affecting the activation state of various downstream effector
molecules (20). In the present
study, the inhibition of PI3K significantly reduced the protein
levels of FOXO3a, PI3K, p-Akt and p27kip1 in the
LPS-induced spinal cord cells. The PI3K/Akt/FOXO3a pathway has been
demonstrated to be an essential pathway in the control of cell
apoptosis (21). In this pathway,
when PI3K/Akt is activated, the phosphorylation of FOXO3a is
stimulated and FOXO3a transfers from the cell nucleus to the
cytoplasm, which reduces the expression of Bcl-2-like protein 11
(Bim) and thus inhibits cell apoptosis; however, if the PI3K/Akt
signaling pathway is inhibited, FOXO3a is dephosphorylated and
transfers to the nucleus from the cytoplasm, thereby increasing Bim
expression and initiating cell apoptosis (22). Studies have indicated that in
Alzheimer's disease, β amyloid protein inhibits the PI3K/Akt
signaling pathway which increases the nuclear transfer of FOXO3a
protein, thereby increasing the production of the pro-apoptotic
protein Bim and inducing nerve cell apoptosis (22,23). In the present study, the
overexpression of FOXO3a significantly increased the cell viability
and reduced the TNF-α activity of LPS-induced spinal cord cells.
This is consistent with a previous study in which Pun et al
(24) indicated that the sirtuin
1-FOXO3a axis regulates LPS-induced TNF-α expression and serves a
crucial role in globular adiponectin-induced autophagy in
macrophages.
The FOXO3a protein, as a transcription factor
associated with cell cycle regulation and apoptosis induction,
serves a vital role in cell proliferation, cell apoptosis, cell
cycle arrest, cell senescence, cell differentiation, tumor
angiogenesis, DNA rehabilitation and oxidative stress, mainly
through regulating the expression of downstream genes (25). FOXO3a downstream proteins include
apoptosis-mediating proteins such as Bim, TNF-related
apoptosis-inducing ligand, death receptor (DR)4 and DR5; cell cycle
regulators including cyclin-dependent kinase inhibitor 1 and
p27kip1; ataxia-telangiectasia mutated, which controls
rehabilitation from DNA injury; and manganese superoxide dismutase,
which protects against anti-oxidative stress (25). p27kip1 is a negative
regulator of the cell cycle, the expression level of which changes
during different processes of the cell cycle (22). It serves a vital role in the
regulation of cell cycle arrest and thus inhibits cell
proliferation. It has been reported that in addition to regulating
the cell cycle and cell proliferation, p27kip1 also
regulates cell migration, apoptosis and autophagy (22). Furthermore, p27kip1 has
been demonstrated to regulate the cell cycle, proliferation and
migration of vascular smooth muscle cells, atherosclerosis and the
formation of neointima (23). The
overexpression of FOXO3a significantly increased the protein levels
of p27kip1 in the LPS-induced spinal cord cells in the
present study, indicating that FOXO3a affects p27kip1 in
SCI. This is consistent with a study by Pramod and Shivakumar
(26), which reported that
p27kip1 was activated via a FOXO3a-dependent mechanism
during cardiac fibroblast growth.
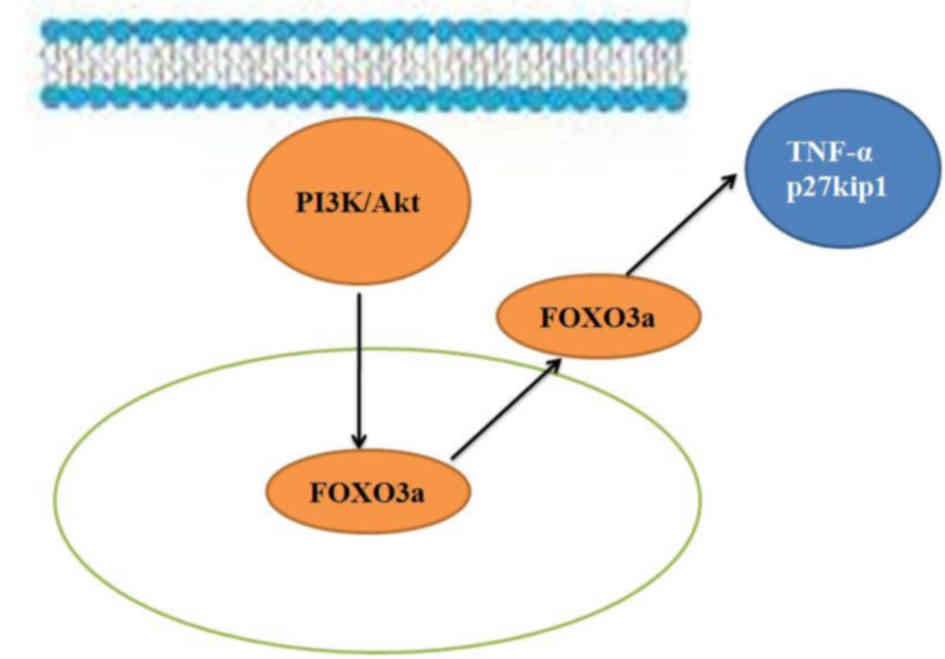
In conclusion, the results of the present study
suggest that the PI3K/Akt/FOXO3a signaling pathway regulates
regeneration of the spinal cord following SCI in adult rats through
its effects on TNF-α and p27kip1 expression (Fig. 8). Furthermore, regulation of the
PI3K/Akt/FOXO3a pathway is potentially an important mechanism that
could be targeted in the treatment of SCI and may provide a novel
therapeutic strategy for clinical application.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Pramodhyakul W, Wattanapan P,
Siritaratiwat W, Eungpinichpong W and Amatachaya S: Immediate
effects of obstacle crossing training in independent ambulatory
patients with spinal cord injury. Spinal Cord. 51:379–383. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Theiss RD, Hornby TG, Rymer WZ and Schmit
BD: Riluzole decreases flexion withdrawal reflex but not voluntary
ankle torque in human chronic spinal cord injury. J Neurophysiol.
105:2781–2790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Totosy de Zepetnek JO, Pelletier CA, Hicks
AL and MacDonald MJ: Following the physical activity guidelines for
adults with spinal cord injury for 16 weeks does not improve
vascular health: A randomized controlled trial. Arch Phys Med
Rehabil. 96:1566–1575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saberi H, Derakhshanrad N and Yekaninejad
MS: Comparison of neurological and functional outcomes after
administration of granulocyte-colony-stimulating factor in
motor-complete versus motor-incomplete postrehabilitated, chronic
spinal cord injuries: A phase I/II study. Cell Transplant. 23(Suppl
1): S19–S23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jung SY, Kim DY, Yune TY, Shin DH, Baek SB
and Kim CJ: Treadmill exercise reduces spinal cord injury-induced
apoptosis by activating the PI3K/Akt pathway in rats. Exp Ther Med.
7:587–593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng B, Ye L, Zhou Y, Zhu S, Wang Q, Shi
H, Chen D, Wei X, Wang Z, Li X, et al: Epidermal growth factor
attenuates blood-spinal cord barrier disruption via PI3K/Akt/Rac1
pathway after acute spinal cord injury. J Cell Mol Med.
20:1062–1075. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Felix MS, Bauer S, Darlot F, Muscatelli F,
Kastner A, Gauthier P and Matarazzo V: Activation of Akt/FKHR in
the medulla oblongata contributes to spontaneous respiratory
recovery after incomplete spinal cord injury in adult rats.
Neurobiol Dis. 69:93–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guzen FP, de Araújo DP, Lucena EE, de
Morais HH, Cavalcanti JR, do Nascimento ES Jr, Costa MS and
Cavalcante JS: Effect of FGF-2 and sciatic nerve grafting on ChAT
expression in dorsal root ganglia neurons of spinal cord transected
rats. Neurosci Lett. 616:43–48. 2016. View Article : Google Scholar
|
|
9
|
Allodi I, Mecollari V, González-Pérez F,
Eggers R, Hoyng S, Verhaagen J, Navarro X and Udina E: Schwann
cells transduced with a lentiviral vector encoding Fgf-2 promote
motor neuron regeneration following sciatic nerve injury. Glia.
62:1736–1746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adeeb N and Mortazavi MM: The role of FGF2
in spinal cord trauma and regeneration research. Brain Behav.
4:105–107. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang S, Huan W, Wei H, Shi J, Fan J, Zhao
J, Shen A and Teng H: FOXO3a/p27kip1 expression and
essential role after acute spinal cord injury in adult rat. J Cell
Biochem. 114:354–365. 2013. View Article : Google Scholar
|
|
12
|
Qin W, Pan J, Wu Y, Bauman WA and Cardozo
C: Protection against dexamethasone-induced muscle atrophy is
related to modulation by testosterone of FOXO1 and PGC-1α. Biochem
Biophys Res Commun. 403:473–478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Léger B, Senese R, Al-Khodairy AW, Dériaz
O, Gobelet C, Giacobino JP and Russell AP: Atrogin-1, MuRF1, and
FoXO, as well as phosphorylated GSK-3beta and 4E-BP1 are reduced in
skeletal muscle of chronic spinal cord-injured patients. Muscle
Nerve. 40:69–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia M and Zhu Y: FOXO3a involvement in the
release of TNF-α stimulated by ATP in spinal cord astrocytes. J Mol
Neurosci. 51:792–804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Machova Urdzikova L, Karova K, Ruzicka J,
Kloudova A, Shannon C, Dubisova J, Murali R, Kubinova S, Sykova E,
Jhanwar-Uniyal M, et al: The anti-inflammatory compound curcumin
enhances locomotor and sensory recovery after spinal cord injury in
rats by immunomodulation. Int J Mol Sci. 17:E492015. View Article : Google Scholar
|
|
16
|
Zhou Y, Zhang H, Zheng B, Ye L, Zhu S,
Johnson NR, Wang Z, Wei X, Chen D, Cao G, et al: Retinoic acid
induced-autophagic flux inhibits ER-stress dependent apoptosis and
prevents disruption of blood-spinal cord barrier after spinal cord
injury. Int J Biol Sci. 12:87–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldshmit Y, Frisca F, Pinto AR, Pébay A,
Tang JK, Siegel AL, Kaslin J and Currie PD: Fgf2 improves
functional recovery-decreasing gliosis and increasing radial glia
and neural progenitor cells after spinal cord injury. Brain Behav.
4:187–200. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kurtoglu T, Basoglu H, Ozkisacik EA, Cetin
NK, Tataroglu C, Yenisey C and Discigil B: Effects of cilostazol on
oxidative stress, systemic cytokine release, and spinal cord injury
in a rat model of transient aortic occlusion. Ann Vasc Surg.
28:479–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang HY, Wang ZG, Wu FZ, Kong XX, Yang J,
Lin BB, Zhu SP, Lin L, Gan CS, Fu XB, et al: Regulation of
autophagy and ubiquitinated protein accumulation by bFGF promotes
functional recovery and neural protection in a rat model of spinal
cord injury. Mol Neurobiol. 48:452–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong M, Yang G, Liu H, Liu X, Lin S, Sun D
and Wang Y: Aged black garlic extract inhibits HT29 colon cancer
cell growth via the PI3K/Akt signaling pathway. Biomed Rep.
2:250–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Wang H, Tao Y, Zhang S, Wang J and
Feng X: Necroptosis inhibitor necrostatin-1 promotes cell
protection and physiological function in traumatic spinal cord
injury. Neuroscience. 266:91–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li A, Wang J, Wu M, Zhang X and Zhang H:
The inhibition of activated hepatic stellate cells proliferation by
arctigenin through G0/G1 phase cell cycle arrest: Persistent
p27(Kip1) induction by interfering with PI3K/Akt/FOXO3a signaling
pathway. Eur J Pharmacol. 747:71–87. 2015. View Article : Google Scholar
|
|
23
|
Li CJ, Chang JK, Chou CH, Wang GJ and Ho
ML: The PI3K/Akt/FOXO3a/27Kip1 signaling contributes to
anti-inflammatory drug-suppressed proliferation of human
osteoblasts. Biochem Pharmacol. 79:926–937. 2010. View Article : Google Scholar
|
|
24
|
Pun NT, Subedi A, Kim MJ and Park PH:
Globular adiponectin causes tolerance to LPS-induced TNF-α
expression via autophagy induction in RAW 264.7 macrophages:
Involvement of SIRT1/FoxO3A axis. PLoS One. 10:e01246362015.
View Article : Google Scholar
|
|
25
|
Kelley K and Berberich SJ: FHIT gene
expression is repressed by mitogenic signaling through the
PI3K/AKT/FOXO pathway. Am J Cancer Res. 1:62–70. 2011.PubMed/NCBI
|
|
26
|
Pramod S and Shivakumar K: Mechanisms in
cardiac fibroblast growth: An obligate role for Skp2 and FOXO3a in
ERK1/2 MAPK-dependent regulation of p27kip1. Am J
Physiol Heart Circ Physiol. 306:H844–H855. 2014. View Article : Google Scholar : PubMed/NCBI
|