Introduction
Esophageal cancer is a lethal malignancy with
>440,000 new cases arising around the world every year (1). In Asia, squamous cell carcinoma is
the primary type among a variety of esophageal cancers (2). Curative surgery for esophageal
squamous cell carcinoma (ESCC) was believed to be promising and may
provide an increased chance of survival for patients with ESCC.
However, with a 5-year survival rate of 20-50%, the prognosis of
these patients is unsatisfactory (3,4).
Distant metastases following surgery is a major cause of mortality
for these patients. Although early studies indicated that
neoadjuvant chemoradiotherapy (NCRT) may effectively reduce lymph
node metastasis and offer a good opportunity for margin-negative
resection, it remains controversial whether NCRT improves treatment
outcomes in patients with resectable ESCC (5,6).
The nuclear factor (NF)-κB pathway contains a number
of transcription factors (RelA/p65, c-Rel, RelB, p50 and p52),
which may form ≥12 kinds of homodimers or heterodimers. NF-κB p65
is the most well-studied transcription factor of the NF-κB
signaling pathway. Activation of the NF-κB pathway releases p65
from its inhibitor, and promotes the translocation of p65 into the
nucleus to drive the transcription of various key genes (7). The phosphorylation of p65 induces a
conformational change to enhance its binding to DNA (8). Incorrect regulation of NF-κB has
been implicated in a number of types of disease, including cancer
(9). Research in ESCC cell lines
and ESCC tissues has indicated that the NF-κB pathway is
constitutively activated in ESCC, and targeting NF-κB may
effectively block fast cell growth and inhibit the strong
metastatic ability of ESCC cells (10). In patients with ESCC, activation
of the NF-κB pathway was closely associated with a poor prognostic
outcome, and it was deemed likely that patients with a complete
pathological response may benefit from NCRT (11).
The hedgehog signaling pathway is one of the key
mediators of development in humans. It is involved in embryonic
formation, tissue homoeostasis, tumor initiation and tumor
development (12,13). Due to its central role in stem
cell regeneration, the hedgehog pathway is crucial for the
maintenance of cancer cell stemness and thus contributes to cancer
cell metastasis (14). The
glioma-associated oncogene homolog 1 (Gli1), a zinc finger
transcription factor, is the key mediator of the hedgehog pathway
that regulates a number of genes important for tumor occurrence and
progression (15).
Hyper-activation of Gli1 has been implicated in a number of cancer
types. In ESCC, the hedgehog pathway was activated upon epidermal
growth factor stimulation and cooperated with the
phosphatidylinositol 3-kinase/RAC-α serine/threonine-protein kinase
pathway and mitogen-activated protein kinase pathway to promote
cancer cell survival and growth (16). Overexpression of Gli1 was observed
in ESCC tissues, particularly in ESCC cells with strong invasive
and metastatic capabilities (17). Gli1 was positively regulated by
the NF-κB pathway in claudin-low breast cancer (18). However, the association between
the hedgehog pathway and the NF-κB pathway, and their status in
response to NCRT, were largely unknown.
In the present study, it was demonstrated that the
NF-κB pathway and the hedgehog pathway were hyperactivated in
patients with ESCC following NCRT. Low expression of either NF-κB
or Gli1 was associated with better overall survival (OS). In
addition, there was a strong association between NF-κB p65 and Gli1
in ESCC patient samples. In the ESCC cell line TE-8, there was a
decrease in cell proliferation and cellular metastasis following
inhibition of the NF-κB pathway or hedgehog pathway by small
molecules. Notably, inhibition of the NF-κB pathway induced a sharp
decrease in Gli1, whereas inhibition of the hedgehog pathway
inactivated the NF-κB pathway. The data suggested that
overactivation of and interplay between the NF-κB pathway and the
hedgehog pathway were involved in poor prognosis in patients with
ESCC who underwent NCRT.
Materials and methods
Patients
Between July 2006 and September 2010, tumor samples
from 54 patients with ESCC who underwent NCRT prior to surgery at
the Nanjing General Hospital of Nanjing Command (Nanjing, China)
were collected following surgical resection. Tissue samples were
immediately stored at −80°C until use. The tumors of patients were
staged according to the American Joint Committee on Cancer (Edition
7) (19). The patients included
41 men and 13 women, aged between 40 and 78 years. A total of 11
patients were classified as stage II, 30 patients were classified
as stage III, and 13 patients were classified as stage IV. The
study was approved by the Ethics Committee of Nanjing General
Hospital of Nanjing Command and written consent from each patient
was obtained.
Neoadjuvant chemoradiotherapy and
surgery
All 54 patients received NCRT prior to surgery.
Chemotherapy included 5-flurouracil (5-FU) in combination with
cisplatin (CDDP). 5-FU was administrated at 500 mg/m2
per day by a 5 h continuous intravenous (i.v.) infusion starting on
day 1, and CDDP was administered at 25 mg/m2 at a 2-h
i.v. infusion on days 1-5. During radiation therapy, patients
received five fractions of 2 Gy radiation per week over 4 weeks, at
a total dose of 30-40 Gy. In the first week, radiation therapy was
conducted in combination with chemotherapy, whereas radiation
therapy alone was performed for the next 3 weeks. After 4 weeks of
NCRT, total thoracic esophagectomy was performed and tumor tissues
were used for following experiments.
Immunohistochemistry (IHC) staining
Primary antibodies against NF-κB p65 (cat. no.
8242S) and Gli1 (cat. no. 3538S) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Horseradish peroxidase
(HRP)-conjugated goat anti-rabbit secondary antibody (cat. no.
SA00001-2) was purchased from ProteinTech Group, Inc. (Chicago, IL,
USA). ESCC tumor samples were fixed with 4% formalin for 2 h at
room temperature and then processed using the Max Vision™ kit
(Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China) by following the
manufacturer's protocol. Briefly, all samples were subjected to
antigen retrieval by heating at a high temperature of 95°C in 0.01
M sodium citrate buffer (pH 6.0) for 20 min. Tissues were embedded
in paraffin. Subsequently, 3% H2O2 was added
to the slices to block the activity of endogenous peroxidase at
room temperature for 15 min. The sections were then incubated with
anti-NF-κB (1:500) or anti-Gli1 (1:300) antibodies at 37°C for 1 h.
The slices were washed with PBS and incubated with secondary
antibody (1:2,000) for 30 min at room temperature. The signal was
developed with DAB solution for 5 min at room temperature.
Hematoxylin was used for nuclei visualization for 30 sec at room
temperature. For semi-quantification of NF-κB and Gli1 expression,
images from NF-κB- or Gli1-stained slides were captured at ×40
magnification under a standard light microscope (five fields per
slide). The threshold values used for NF-κB and Gli1 were positive
if ≥10% cells exhibited clear positive staining with the
antibodies.
Cell culture and reagents
The ESCC cell line, TE-8, was purchased from RIKEN
BioResource Center (Tsukuba, Japan). The cells were cultured in
RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (HyClone; GE Healthcare,
Chicago, IL, USA) and 1% penicillin-streptomycin (Thermo Fisher
Scientific, Inc.), in a 37°C incubator supplemented with 5%
CO2. GANT61 and Bay 11-7082 were purchased from Selleck
Chemicals (Houston, TX, USA).
Western blotting
The 5-μm frozen tissue samples at −80°C were
thawed on ice, and lysates were prepared with
radio-immunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). The antibody against phospho-NF-κB
p65 (Ser536) (cat. no. 3033S; 1:1,000) was purchased from Cell
Signaling Technology, Inc. and the anti-GAPDH antibody (cat. no.
G8795; 1:8,000) was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). The HRP-conjugated goat anti-mouse antibody
(cat. no. SA00001-1; 1:10,000) was purchased from ProteinTech
Group, Inc.
The concentration of protein lysates was determined
using a Bicinchoninic Acid kit for Protein Determination
(Sigma-Aldrich; Merck KGaA). Protein lysates (30 μg each)
were loaded onto an 8% SDS-PAGE gel, and subsequently transferred
to a polyvinylidene difluoride membrane. The membrane was blocked
with 5% non-fat milk at room temperature for 1 h, and incubated
with the indicated primary antibodies at room temperature for 1 h,
and followed by incubation with secondary antibodies at room
temperature for a further 1 h. Blots were developed with
SuperSignal West Femto Maximum Sensitivity substrate (Pierce;
Thermo Fisher Scientific, Inc.) and the images were obtained by
using ImageQuant LAS 4000 (GE Healthcare) using ImageQuant TL
8.0.
Reverse
transcription-quantitative-polymerase chain reaction (RT-qPCR)
For RT-qPCR, total RNA from tumor samples was
prepared using an RNeasy kit (Qiagen GmbH, Hilden, Germany),
according to the manufacturer's protocol. Reverse transcription of
RNA was performed with a PrimeScript RT reagent kit (Takara
Biotechnology, Co., Ltd., Dalian, China). PCR was performed using
SYBR Premix Ex Taq kit (Takara Biotechnology Co., Ltd.) on a
Bio-Rad CFX96 Real-Time PCR system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and normalized to the internal control GAPDH.
The qPCR program was as follows: Stage 1, 95°C for 30 sec; stage 2,
95°C for 5 sec and 60°C for 30 sec (35 repeats). The relative
expression of genes was calculated using the 2−∆∆Cq
method (20). The primer
sequences were as follows: GAPDH forward, AATCCCATCACCATCTTCCA;
GAPDH reverse, TGGACTCCACGACGTACTCA; NF-κB p65 forward,
ATGGCAGACGATGATCCCTAC; NF-κB p65 reverse, CGGAATCGAAATCCCCTCTGTT;
Gli1 forward, GTGCAAGTCAAGCCAGAACA; and Gli1 reverse,
ATAGGGGCCTGACTGGAGAT.
Cell invasion assay
Cell invasion assays were performed using Transwell
permeable supports (Corning Incorporated, Corning, NY, USA),
according to manufacturer's protocol. Cells of 90% confluence were
treated and incubated with dimethyl sulfoxide (DMSO; 0.02%), GANT61
(2 μM) or Bay 11-7082 (1 μM) in a 37°C incubator for
24 h, then plated onto a Matrigel-coated membrane in the upper
chamber of a 24-well insert containing serum-free RPMI-1640 medium.
The bottom chamber contained RPMI-1640 medium supplemented with 10%
FBS. The cells were subsequently cultured with DMSO (0.02%), GANT61
(2 μM) or Bay 11-7082 (1 μM) in a 37°C incubator for
48 h. Subsequently, the bottom of the chamber insert was collected
and fixed with 10% methanol for 5 min, and stained with crystal
violet for 5 min at room temperature. Cells that remained in the
upper chamber were removed with a cotton swab. Images of cells that
invaded into the bottom area were captured with an inverted
microscope (five fields per well) and the cell number was
calculated with ImageJ software (version 1.50) (National Institutes
of Health, Bethesda, MD, USA). Each experiment was performed in
triplicate.
Cell proliferation assay
The cell proliferation assay was conducted with a
Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan). Briefly, cells were seeded onto a 96 well
plate at 50% confluence, and the next day, the medium was replaced
with medium containing DMSO (0.02%), GANT61 (2 μM) or Bay
11-7082 (1 μM) and incubated at 37°C for 72 h. A total of 10
μl CCK-8 solution was added into each well and incubated at
37°C for 2 h, and absorbance was measured at a wavelength of 450
nm. The cell proliferation curves were generated using GraphPad
Prism version 6.0 software (GraphPad Software Inc., La Jolla, CA,
USA).
Statistical analysis
All data were analyzed using GraphPad Prism version
6.0 software (GraphPad Software Inc.). The values were expressed as
the mean ± standard deviation. Student's t-test was applied to
compare continuous variables and Pearson's Chi-squared test was
employed to compare dichotomous variables. P<0.05 was considered
to indicate a statistically significant difference.
OS was defined as the length of time from the date
of ESCC diagnosis to either mortality of the patient or the date of
the last available information on vital status. Distant
metastasis-free survival (DMFS) was defined as the period from the
date of ESCC diagnosis to the date of metastasis detection.
Comparison between OS and DMFS between patients with negative and
positive IHC staining was achieved using the Kaplan-Meier
method.
Results
Hyperactivation of the NF-κB and hedgehog
signaling pathways in tumors from patients with ESCC following
NCRT
To investigate the status of the NF-κB and hedgehog
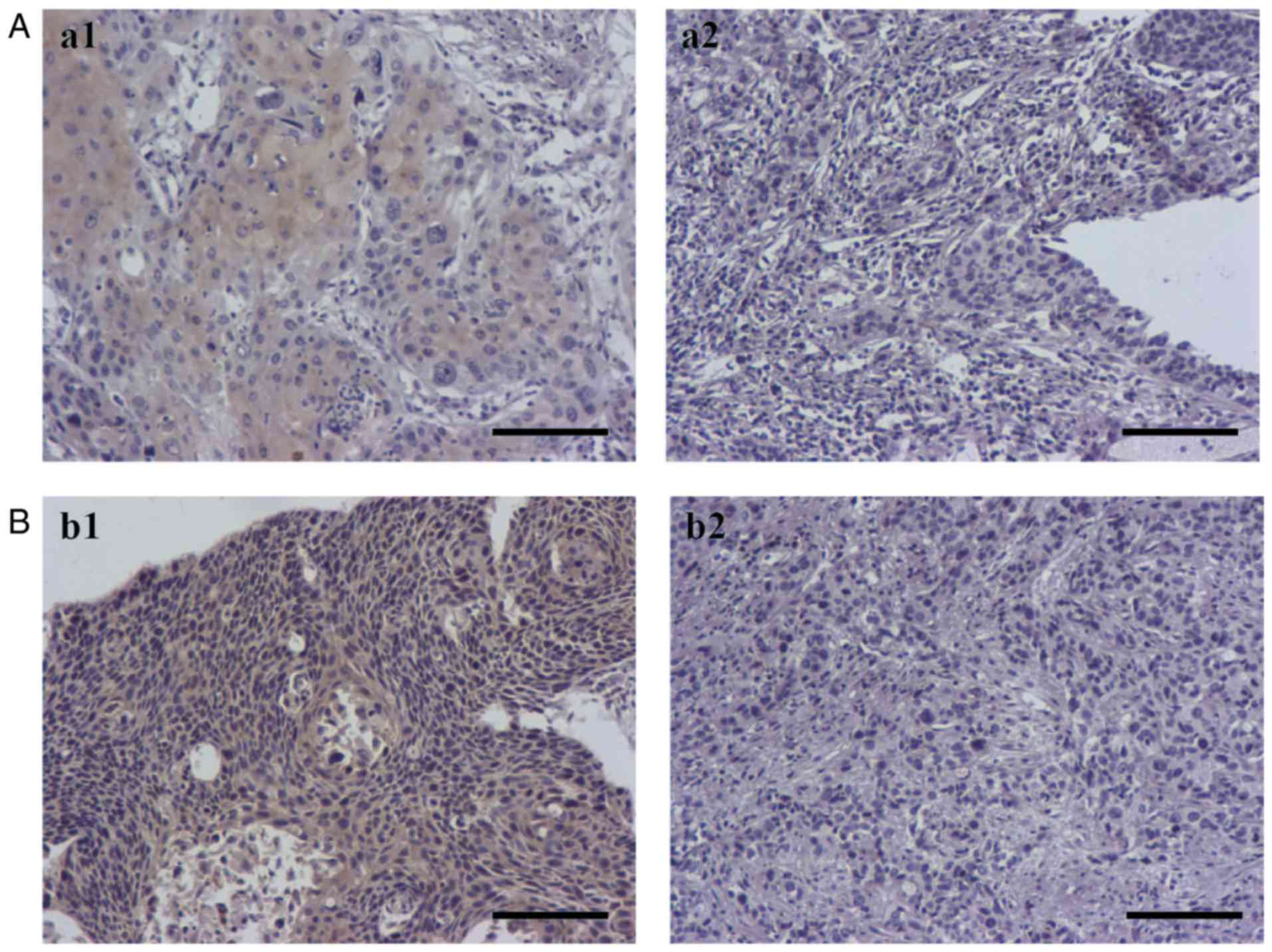
signaling pathways following NCRT, IHC was performed to evaluate
the expression levels of NF-κB p65 and Gli1 in tissue samples from
patients who underwent NCRT prior to surgery. A total of 32 out of
54 cases were positive for NF-κB p65, 28 out of 54 cases were
positive for Gli1 and 20 cases were positive for NF-κB p65 and Gli1
(Table I). Representative
expression for positive and negative staining of NF-κB p65 and Gli1
in ESCC tissue samples was demonstrated in Fig. 1.
 | Table ICharacteristics of patients. |
Table I
Characteristics of patients.
| Characteristic | NF-κB p65
| χ2 test
P-value | Gli1
| χ2 test
P-value | NF-κB p65 and Gli1
| χ2 test
P-value |
|---|
| Positive cases
(%) | Negative cases
(%) | Positive cases
(%) | Negative cases
(%) | Positive cases
(%) | Negative cases
(%) |
|---|
| Sex | | | | | | | | | |
| Male | 24 (44) | 17 (31) | 1.00 | 23 (43) | 18 (33) | 0.35 | 16 (30) | 25 (46) | 0.75 |
| Female | 8 (15) | 5 (9) | | 5 (9) | 8 (15) | | 4 (7) | 9 (17) | |
| Age (years) | | | | | | | | | |
| ≤50 | 15 (28) | 10 (19) | 1.00 | 11 (20) | 14 (26) | 0.41 | 10 (19) | 15 (28) | 0.78 |
| >50 | 17 (31) | 12 (22) | | 17 (31) | 12 (22) | | 10 (19) | 19 (35) | |
| Clinical stage | | | | | | | | | |
| II | 3 (6) | 8 (15) | 0.04 | 2 (4) | 9 (17) | 0.01 | 2 (4) | 9 (17) | 0.01 |
| III | 19 (35) | 11 (20) | | 16 (30) | 14 (26) | | 9 (17) | 21 (39) | |
| IV | 10 (19) | 3 (6) | | 10 (19) | 3 (6) | | 9 (17) | 4 (7) | |
| Total | 32 (59) | 22 (41) | | 28 (52) | 26 (48) | | 20 (37) | 34 (63) | |
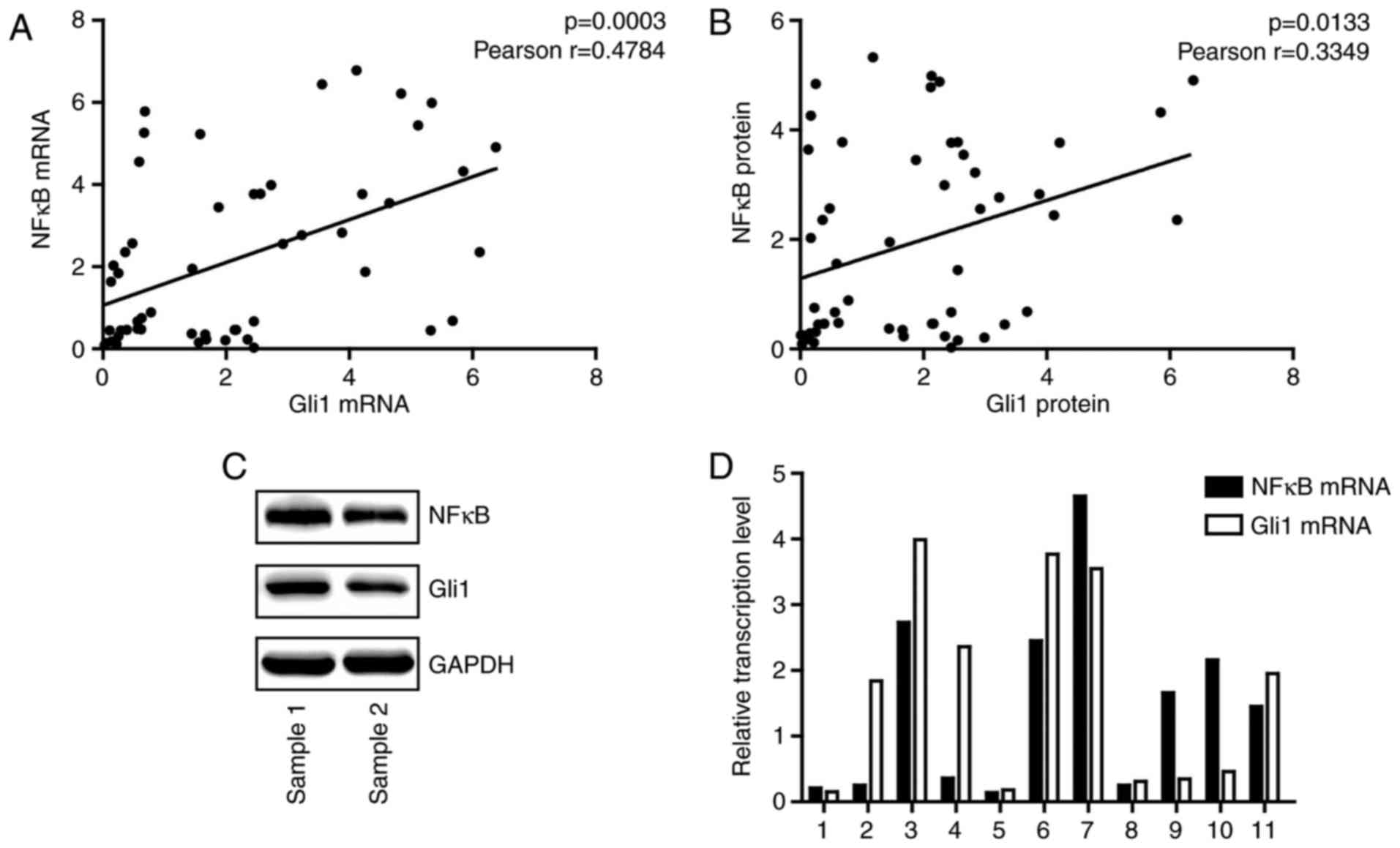
To further examine the association between the NF-κB
pathway and the hedgehog pathway, RT-qPCR analysis and western
blotting were performed to detect the mRNA and protein expression
levels of NF-κB p65 and Gli1, respectively. At the transcriptional
and translational level, NF-κB p65 was positively associated with
Gli1 (Fig. 2A and B).
Representative examples of western blotting and RT-qPCR are
presented in Fig. 2C and D,
respectively.
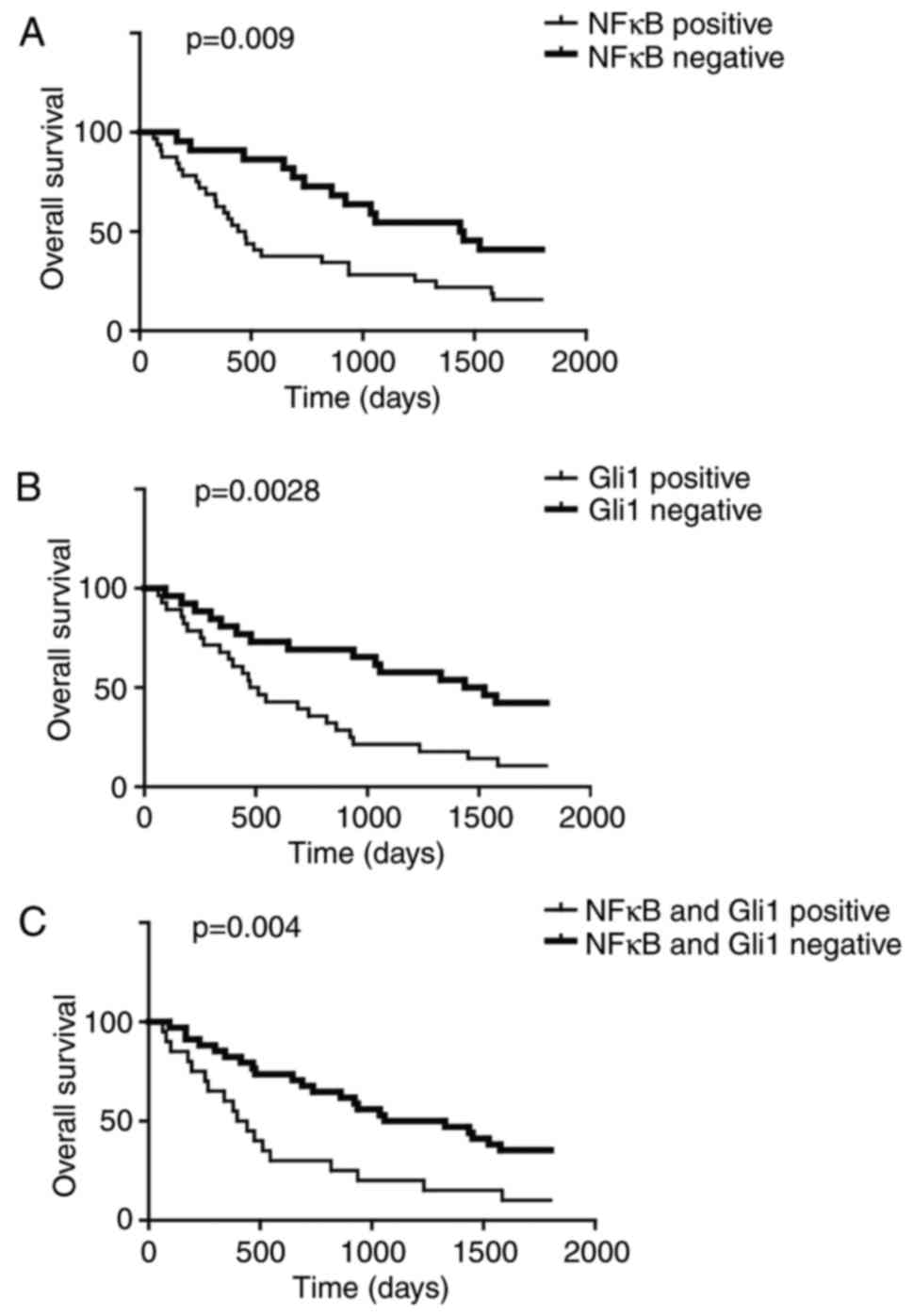
High NF-κB p65 and Gli1 expression is
associated with poor prognosis
The NF-κB pathway and hedgehog pathway are involved
in cancer initiation and development, and therefore, the present
study analyzed OS between patients that exhibited positive and
negative NF-κB p65 expression levels. The OS of NF-κB p65-positive
patients was significantly lower compared with that of the NF-κB
p65-negative patients (Fig. 3A).
In addition, comparison of OS between Gli1-positive and -negative
patients indicated that Gli1 positivity was associated with a
poorer survival rate (Fig. 3B).
This association was additionally observed when NF-κB p65 and Gli1
were positive (Fig. 3C). These
data implied that NF-κB p65 and Gli1 were important for predicting
patient survival, and that NCRT may improve patient treatment
outcomes via inhibition of these two proteins.
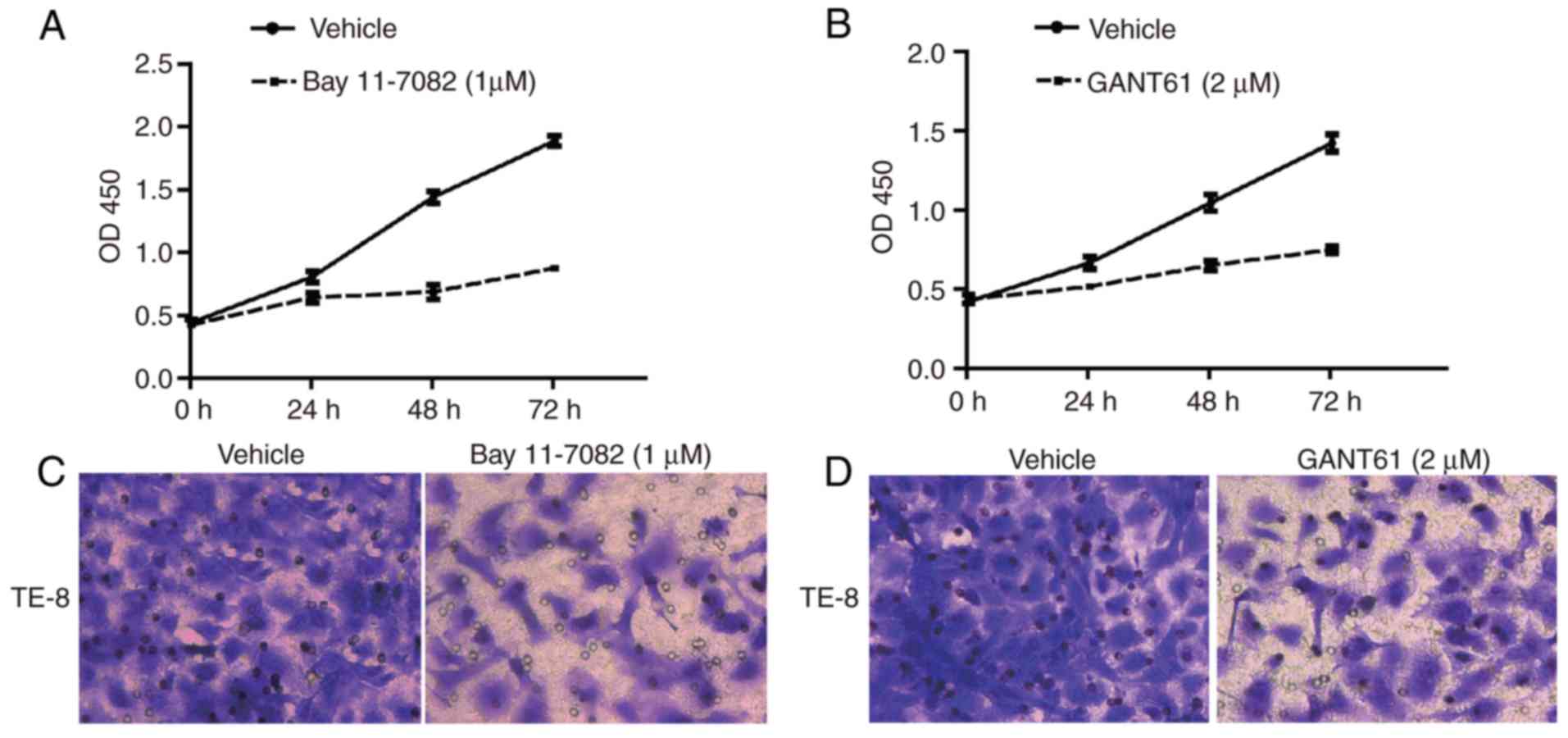
NF-κB and hedgehog signaling pathways are
crucial for ESCC cell survival and invasion
To examine the role of the NF-κB pathway and
hedgehog pathway in ESCC cell behavior, cell proliferation was
determined following inhibition of the NF-κB or hedgehog pathways
with inhibitors. Inhibition of NF-κB with Bay 11-7082 resulted in a
decrease in cell growth in TE-8 cells, an ESCC cell line (Fig. 4A). In addition, inhibition of Gli1
with GANT61 resulted in a decrease in proliferation rate in TE-8
cells (Fig. 4B). Blocking either
the NF-κB pathway or hedgehog pathway reduced the number of cells
that invaded through the Matrigel membrane in the chambers,
suggesting their importance in promoting ESCC cell metastasis
(Fig. 4C and D).
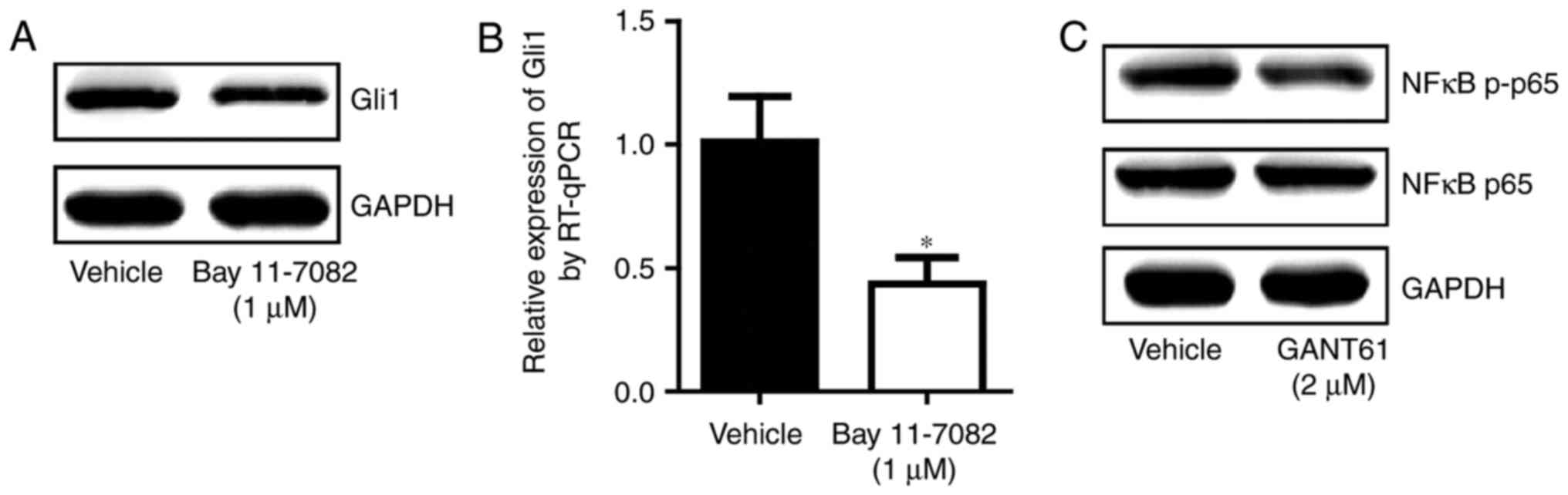
NF-κB pathway and hedgehog pathway form a
positive loop in ESCC cells
As the NF-κB and hedgehog signaling pathways are
crucial for ESCC development, the present study determined their
association in ESCC. Inhibition of the NF-κB pathway resulted in a
decrease in Gli1 at the mRNA and protein levels (Fig. 5A and B). Treatment with the
hedgehog pathway inhibitor reduced the phosphorylation of NF-κB p65
(Fig. 5C). This suggested that
interplay between the NF-κB pathway and the hedgehog pathway may
exist and that these two pathways may cooperate to promote ESCC
development.
Discussion
Although substantial advantages have been achieved
in the screening, diagnosis and treatment of ESCC, the prognosis
for patients with ESCC remains poor. Surgical resection was
considered to provide a better survival for ESCC patients; however,
numerous patients continued to succumb as a result of recurrence or
distant metastasis (21). In a
phase III randomized trial, compared with the surgical treatment
alone group of patients, NCRT prior to surgery did not improve OS,
which was 47.5% with trimodal therapy vs. 53% with surgery at 3
years, with a P-value of 0.94 (22). In a large randomized trial that
included 366 patients, the OS of trimodal therapy was significantly
improved compared with surgery alone, with a median OS of 49.4
months in the NCRT group and 24 months for the surgery group
(23). The function of NCRT in
ESCC has been debated for a number of years. The dysregulation of
numerous proteins has been proven to predict recurrence and
prognosis in patients with ESCC following NCRT (24-26). In this respect, inhibition of a
number of key molecules was demonstrated to be effective in
enhancing the sensitivity of ESCC cells towards chemotherapy or
chemoradiotherapy (10,27,28). The present study demonstrated that
overactivation of the NF-κB and hedgehog signaling pathways, and
their interplay, was associated with poor prognosis post-NCRT.
In esophageal cancer, NF-κB activation prior to
therapy was associated with chemotherapy resistance and contributed
to metastasis, and eventually led to patient mortality (29). Another study on localized
esophageal cancer demonstrated that activated NF-κB was associated
with chemoradiation resistance, and inversely associated with
metastatic potential and OS (30). However, the above studies were
based on a cohort of patients including esophageal adenocarcinoma
and squamous cell carcinoma. In ESCC, the present study
demonstrated that the NF-κB signaling pathway was active in a
majority of patients (32 of 54 cases) that received NCRT, and
patients with NF-κB p65 positive IHC staining exhibited
significantly shorter OS compared with patients with negative
staining of NF-κB p65. This suggested that NF-κB was a predictor
for poor prognosis in patients undergoing NCRT.
The hedgehog signaling pathway was essential for
esophageal tumor formation, and associated with invasion and a poor
prognosis. In patients with ESCC undergoing NCRT, Gli1 expression
was observed to be a predictor for patients with poor treatment
outcomes (31). Inhibitor of
NF-κB, an inhibitor of NF-κB signaling, was discovered to serve a
role in the phosphorylation of Gli1, and thus regulated its
transcriptional activity in diffuse large B cell lymphoma (32). In pancreatic cancer, NF-κB
activation induced the activation of the hedgehog pathway by
targeting sonic hedgehog protein (33). In the present study, it was
confirmed that Gli1 was activated in patients with ESCC following
NCRT and was associated with clinical outcome. A total of 20 out of
54 patients exhibited overexpression of NF-κB p65 and Gli1.
Additionally, the present data revealed that the expression of
NF-κB p65 was associated with Gli1 in the samples analyzed. In the
ESCC cell line TE-8, inhibition of either NF-κB or Gli1 resulted in
decreased cell proliferation and cell invasion ability, and
treatment with an NF-κB inhibitor reduced the mRNA and protein
expression levels of Gli1 in TE-8. A number of oncogenes, including
epidermal growth factor receptors (ErbB), have been reported to
contribute to carcinogenesis by activating the hedgehog pathway and
the NF-κB pathway (34,35). As overexpression of ErbB was
frequently observed in ESCC, ErbB may activate the hedgehog and
NF-κB pathways to promote ESCC progression. A study in refractory
acute myeloid leukemia cells reported that inhibition of smoothened
homolog, a transducer of the hedgehog pathway, was accompanied by a
decrease in the expression of nuclear NF-κB p65 (36). Inhibition of Gli1 by an inhibitor
additionally resulted in inactivation of the NF-κB pathway by
reducing the phosphorylation levels of NF-κB p65 in TE-8 cells.
Therefore, interplay between the NF-κB pathway and the hedgehog
pathway may exist in patients with ESCC undergoing NCRT.
In conclusion, the present study suggested that
crosstalk between the NF-κB pathway and hedgehog pathway may be a
predictor for prognosis in patients with ESCC following NCRT and,
thus, may be a putative therapeutic target.
Acknowledgments
The present study was supported by the Medical
Innovation Program of Nanjing Military Region (grant no.
12Z22).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Global Burden of Disease Cancer
Collaboration; Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The Global Burden of Cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu N, Chen Z, Pang L, Ma Q and Chen G:
Prognostic significance of lymph node characteristics on survival
in esophageal squamous cell carcinomas. Wiener Klin Wochenschr.
125:26–33. 2013. View Article : Google Scholar
|
|
4
|
Miyasaka D, Okushiba S, Sasaki T, Ebihara
Y, Kawada M, Kawarada Y, Kitashiro S, Katoh H, Miyamoto M,
Shichinohe T and Hirano S: Clinical evaluation of the feasibility
of minimally invasive surgery in esophageal cancer. Asian J Endosc
Surg. 6:26–32. 2013. View Article : Google Scholar
|
|
5
|
Buderi SI, Shackcloth M and Page RD: Does
neoadjuvant chemoradiotherapy increase survival in patients with
resectable oesophageal cancer? Interact Cardiovasc Thorac Surg.
24:115–120. 2017. View Article : Google Scholar
|
|
6
|
Duan XF, Tang P and Yu ZT: Neoadjuvant
chemoradiotherapy for resectable esophageal cancer: An in-depth
study of randomized controlled trials and literature review. Cancer
Biol Med. 11:191–201. 2014.PubMed/NCBI
|
|
7
|
Hoffmann A, Natoli G and Ghosh G:
Transcriptional regulation via the NF-kappaB signaling module.
Oncogene. 25:6706–6716. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Milanovic M, Kracht M and Schmitz ML: The
cytokine-induced conformational switch of nuclear factor κB p65 is
mediated by p65 phosphorylation. Biochem J. 457:401–413. 2014.
View Article : Google Scholar
|
|
9
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar
|
|
10
|
Li B, Li YY, Tsao SW and Cheung AL:
Targeting NF-kappaB signaling pathway suppresses tumor growth,
angiogenesis, and metastasis of human esophageal cancer. Mol Cancer
Ther. 8:2635–2644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim HJ, Hawke N and Baldwin AS: NF-kappaB
and IKK as therapeutic targets in cancer. Cell Death Differ.
13:738–747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramsbottom SA and Pownall ME: Regulation
of Hedgehog signalling inside and outside the cell. J Dev Biol.
4:232016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pak E and Segal RA: Hedgehog signal
transduction: Key players, oncogenic drivers, and cancer therapy.
Dev Cell. 38:333–344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agliano A, Calvo A and Box C: The
challenge of targeting cancer stem cells to halt metastasis. Semin
Cancer Biol. 44:25–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruiz i Altaba A: Gli proteins encode
context-dependent positive and negative functions: Implications for
development and disease. Development. 126:3205–3216.
1999.PubMed/NCBI
|
|
16
|
Wei L and Xu Z: Cross-signaling among
phosphinositide-3 kinase, mitogen-activated protein kinase and
sonic hedgehog pathways exists in esophageal cancer. Int J Cancer.
129:275–284. 2011. View Article : Google Scholar
|
|
17
|
Min S, Xiaoyan X, Fanghui P, Yamei W,
Xiaoli Y and Feng W: The glioma-associated oncogene homolog 1
promotes epithelial-mesenchymal transition in human esophageal
squamous cell cancer by inhibiting E-cadherin via Snail. Cancer
Gene Therapy. 20:379–385. 2013. View Article : Google Scholar
|
|
18
|
Colavito SA, Zou MR, Yan Q, Nguyen DX and
Stern DF: Significance of glioma-associated oncogene homolog 1
(GLI1) expression in claudin-low breast cancer and crosstalk with
the nuclear factor kappa-light-chain-enhancer of activated B cells
(NFkappaB) pathway. Breast Cancer Res. 16:4442014. View Article : Google Scholar
|
|
19
|
Rice TW, Blackstone EH and Rusch VW: 7th
edition of the AJCC cancer staging manual: Esophagus and
esophagogastric junction. Ann Surg Oncol. 17:1721–1724. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Mariette C, Piessen G and Triboulet JP:
Therapeutic strategies in oesophageal carcinoma: Role of surgery
and other modalities. Lancet Oncol. 8:545–553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mariette C, Dahan L, Mornex F, Maillard E,
Thomas PA, Meunier B, Boige V, Pezet D, Robb WB, Le Brun-Ly V, et
al: Surgery alone versus chemoradiotherapy followed by surgery for
stage I and II esophageal cancer: Final analysis of randomized
controlled phase III trial FFCD 9901. J Clin Oncol. 32:2416–2422.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Hagen P, Hulshof MC, van Lanschot JJ,
Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ,
Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al: Preoperative
chemoradiotherapy for esophageal or junctional cancer. N Engl J
Med. 366:2074–2084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koishi K, Yoshikawa R, Tsujimura T,
Hashimoto-Tamaoki T, Kojima S, Yanagi H, Yamamura T and Fujiwara Y:
Persistent CXCR4 expression after preoperative chemoradiotherapy
predicts early recurrence and poor prognosis in esophageal cancer.
World J Gastroenterol. 12:7585–7590. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshikawa R, Fujiwara Y, Koishi K, Kojima
S, Matsumoto T, Yanagi H, Yamamura T, Hashimoto-Tamaoki T,
Nishigami T and Tsujimura T: Cyclooxygenase-2 expression after
preoperative chemoradiotherapy correlates with more frequent
esophageal cancer recurrence. World J Gastroenterol. 13:2283–2288.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Olphen SH, Biermann K, Shapiro J,
Wijnhoven BP, Toxopeus EL, van der Gaast A, Stoop HA, van Lanschot
JJ, Spaander MC, Bruno MJ and Looijenga LH: P53 and SOX2 protein
expression predicts esophageal adenocarcinoma in response to
neoadjuvant chemoradiotherapy. Ann Surg. 265:347–355. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leszczynska KB, Dobrynin G, Leslie RE,
Ient J, Boumelha AJ, Senra JM, Hawkins MA, Maughan T, Mukherjee S
and Hammond EM: Preclinical testing of an Atr inhibitor
demonstrates improved response to standard therapies for esophageal
cancer. Radiother Oncol. 121:232–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li DJ, Shi M and Wang Z: RUNX3 reverses
cisplatin resistance in esophageal squamous cell carcinoma via
suppression of the protein kinase B pathway. Thorac Cancer.
7:570–580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Izzo JG, Correa AM, Wu TT, Malhotra U,
Chao CK, Luthra R, Ensor J, Dekovich A, Liao Z, Hittelman WN, et
al: Pretherapy nuclear factor-kappaB status, chemoradiation
resistance, and metastatic progression in esophageal carcinoma. Mol
Cancer Ther. 5:2844–2850. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Izzo JG, Malhotra U, Wu TT, Ensor J,
Luthra R, Lee JH, Swisher SG, Liao Z, Chao KS, Hittelman WN, et al:
Association of activated transcription factor nuclear factor kappab
with chemoradiation resistance and poor outcome in esophageal
carcinoma. J Clin Oncol. 24:748–754. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoshikawa R, Nakano Y, Tao L, Koishi K,
Matsumoto T, Sasako M, Tsujimura T, Hashimoto-Tamaoki T and
Fujiwara Y: Hedgehog signal activation in oesophageal cancer
patients undergoing neoadjuvant chemoradiotherapy. Br J Cancer.
98:1670–1674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Agarwal NK, Kim CH, Kunkalla K, Konno H,
Tjendra Y, Kwon D, Blonska M, Kozloski GA, Moy VT, Verdun RE, et
al: Active IKKβ promotes the stability of GLI1 oncogene in diffuse
large B-cell lymphoma. Blood. 127:605–615. 2016. View Article : Google Scholar :
|
|
33
|
Nakashima H, Nakamura M, Yamaguchi H,
Yamanaka N, Akiyoshi T, Koga K, Yamaguchi K, Tsuneyoshi M, Tanaka M
and Katano M: Nuclear factor-kappaB contributes to hedgehog
signaling pathway activation through sonic hedgehog induction in
pancreatic cancer. Cancer Res. 66:7041–7049. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Benvenuto M, Fantini M, Masuelli L, De
Smaele E, Zazzeroni F, Tresoldi I, Calabrese G, Galvano F, Modesti
A and Bei R: Inhibition of ErbB receptors, Hedgehog and NF-kappaB
signaling by polyphenols in cancer. Front Biosci. 18:1290–1310.
2013. View Article : Google Scholar
|
|
35
|
Fitzgerald TL, Lertpiriyapong K, Cocco L,
Martelli AM, Libra M, Candido S, Montalto G, Cervello M, Steelman
L, Abrams SL and McCubrey JA: Roles of EGFR and KRAS and their
downstream signaling pathways in pancreatic cancer and pancreatic
cancer stem cells. Adv Biol Regul. 59:65–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li X, Chen F, Zhu Q, Ding B, Zhong Q,
Huang K, Jiang X, Wang Z, Yin C, Zhu Y, et al: Gli-1/PI3K/AKT/NF-kB
pathway mediates resistance to radiation and is a target for
reversion of responses in refractory acute myeloid leukemia cells.
Oncotarget. 7:33004–33015. 2016.PubMed/NCBI
|