Introduction
Chronic kidney disease (CKD) has become a major
public health concern worldwide with higher morbidity and mortality
(1). Renal fibrosis is generally
recognized as the most prominent feature of CKD and the final
common pathway leading to end-stage renal failure (2). The pathological changes associated
with renal fibrosis mainly involve the deposition of extracellular
matrix (ECM) which is thought to be produced by myofibroblasts
(3). Epithelial-mesenchymal
transition (EMT) is a complex process in which differentiated
epithelial cells undergo a phenotypic conversion that gives rise to
the matrix-producing fibroblasts and myofibroblasts (4). In the process of EMT, epithelial
cells degrade the basement membrane to become migratory, with
several epithelial markers such as E-cadherin, cytokeratin 18
(CK18) loss and mesenchymal markers such as N-cadherin, fibronectin
and α-smooth muscle actin (α-SMA) acquired (5,6).
EMT has been proved to be a critical step in the pathogenesis and
progression of tubuloint-erstitial fibrosis, whereby renal tubular
epithelial cells change phenotypically and functionally into
myofibroblasts (7). Therefore, it
is important to prevent tubular epithelial cells from undergoing
EMT to prevent renal fibrosis.
As is known, chronic inflammation is acknowledged to
be pivotal in the development and progression of CKD (8). When kidneys are exposed to
inflammatory stimuli, the evaluated release of transforming growth
factor-β1 (TGF-β1) eventually activates EMT of renal tubular cells
(9). TGF-β1, a well-known master
cytokine/growth factor, is considered as an important
well-established regulator of EMT. It could effectively regulate
the transdifferentiation of tubular epithelial cells into
myofibroblasts in renal fibrosis primarily via Smad-dependent
pathway (10,11). Upon TGF-β1 binding to its
receptors, Serine/Threonine kinases are activated and induce
phosphorylation of Smad2/Smad3, then phosphorylated Smad2/3 partner
with Smad4 translocate into the nucleus where they regulate the
transcription of the target genes responsible for EMT (10). Integrin linked kinase (ILK) is an
intracellular serine/threonine kinase involved in cell-matrix
interactions. It is shown to be a key intracellular mediator that
controls TGF-β1-induced-EMT in renal tubular epithelial cells
(11,12). Although the involvement of ILK in
tubular EMT has been established based on several lines of
evidence, intriguingly, many components of ILK signaling, including
ILK and β1-integrin are induced simultaneously by TGF-β1 in a
Smad-dependent manner (4,12). Therefore, it is widely accepted
that TGF-β1 plays an important role in promoting renal tubular
EMT.
P311, as a binding protein of the TGF-β1 latency
associated protein, is an 8-kDa, 68-amino acid, intracellular
polypeptide that is highly conserved across species and expressed
in brain, smooth muscle, regenerating tissues, and malignant
glioblastomas (13-19). It has been shown to be of
importance in the process of myofibroblast differentiation and
fibrosis with the functions of promoting embryonic development,
wound healing, as well as nerve and lung regeneration. Some
researchers reported that P311 transfection into fibroblast cells
induced phenotypic changes consistent with myofibroblast
transformation, decreased TGF-β1 signaling and caused an inhibition
in collagen expression (15).
Their findings suggested that P311 may be involved in facilitating
wound healing and/or minimizing scarring during wound repair via
preventing fibrosis.
Although previous study has suggested P311 may be an
important factor in myofibroblast transformation and in the
progression of fibrosis, the related studies on P311 on renal
fibrosis are limited and the mechanisms of P311 in the progression
of renal tubulointerstitial fibrosis remain largely unknown. In our
previous studies, we found that P311 may be involved in the
pathogenesis of renal fibrosis by inhibiting EMT process via
TGF-β1-Smad-ILK pathway in NRK-52E cells (20). In the present study, we further
examined the effect of P311 on TGF-β1-mediated EMT in a rat model
of unilateral ureteral occlusion (UUO) renal fibrosis. After
construction the recombinant adenovirus p311 (also called Ad-P311)
was transferred it into UUO rats, the preventing effect and
possible mechanism of P311 on TGF-β1-mediated EMT were
explored.
Materials and methods
Animals
Forty male Sprague-Dawley rats (8-week-old, 200±10
g) were purchased from Shandong Experimental Animal Center (Jinan,
China), and were given free access to water and food throughout the
experiments. The rats were acclimatized for at least 1 week prior
to the experiments. All of the animal experimental protocols were
handled in accordance with the Code of Ethics of the World Medical
Association, and all research procedures were permitted by Medical
Ethics Committee of Provincial Hospital Affiliated to Shandong
University.
Establishment of the UUO model
UUO was performed as described previously (21). Briefly, after induction of general
anesthesia by intraperitoneal injection of 3% pentobarbital (Sigma,
St. Louis, MO, USA) (1 ml/kg body wt), the abdominal cavity was
exposed via midline incision and the left ureter was ligated at 2
points with 4-0 silk (Niccho Kogyo Co., Ltd., Tokyo, Japan).
Ureters of the sham-operated rats were manipulated, but not
ligated, and were used as controls.
Experimental protocol
The rats were randomly divided into four groups and
given different treatment, each group consisted of ten animals as
follows: control group (or sham surgery group), UUO group, control
+ Ad-P311 group, and UUO + Ad-P311 group. Ad-P311 was constructed
as described previously by the current authors and stored at −80°C
for use (20). After
establishment of the UUO model successfully, the rats in control
group and UUO group were injected with 0.5 ml normal saline through
the tail vein each week for 4 weeks, while the rats in control +
Ad-P311 group and UUO + Ad-P311 group were injected with 0.5 ml
P311 adenovirus by tail vein per week for 4 weeks. Then, rats were
sacrificed and their kidneys were taken and blood analysis was
performed. Serum creatinine (Cr), blood urea nitrogen (BUN) and
albumin (ALB) (BioVision, Inc., Milpitas, CA, USA) levels in the
blood were tested according to the introduction of detection kits.
Part of the kidneys was fixed in 10% formalin solution and embedded
in paraffin as 3 μm sections for hematoxylin and eosin
(H&E), Masson's trichrome and immunohistochemical staining. The
other part of the kidneys was stored at −80°C for western blot
analysis, which was performed as previously described to detect the
protein expressions of E-cadherin, α-SMA, TGF-β1, phosphorylated
Smad2/3 (pSmad2/3), Smad7 and ILK (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA).
H&E staining and Masson staining
To observe the histological morphology changes of
kidney tissue, H&E staining and Masson staining were performed.
First, kidney tissues were fixed with 10% neutral formaldehyde and
dehydrated in graded ethanol. After permeation in xylene, they were
embedded in paraffin. The paraffin blocks were cut into 2 μm
slices, mounted onto glass slides and stained by standard
techniques of H&E staining and Masson staining according to
previous studies (10,22). Renal tubular injury index
including inflammation, cell infiltration, interstitial fibrosis,
interstitial edema, cell vacuolar degeneration, tubular atrophy,
and tubular expansion were measured to assess the renal
interstitial lesions. Ten different fields were selected to
estimate the level of renal injury index with H&E staining
using bio-image analysis system (Bio-Profile). Each parameter was
evaluated and given a score from 0 to 4+, (0, no
changes; 1+, changes affecting 5-25% of the sample;
2+, changes affecting 25-50%; 3+, changes
affecting 50-75%; 4+, changes affecting 75-100%)
(23). The severity of
interstitial fibrosis was estimated by scanning 10 no-repeated
fields in each sample with Masson staining. Blue-stained fibrotic
areas were quantified by the Image-Pro plus 6.0 software (Media
Cybernetics, Rockville, MD, USA) (23). The results were expressed by the
proportion of the relative volume of the scanned interstitium. The
cases with H&E and Masson staining were evaluated by two
investigators independently and any discrepancy was resolved by a
group discussion.
Immunohistochemical staining
To analyze the protein expression of α-SMA, pSmad2/3
and Smad7, immunohistochemical staining was performed as described
previously (22). First, paraffin
sections were de-paraffinized, hydrated, and immersed in 0.3%
hydrogen peroxide in methanol for 30 min to block the endogenous
peroxidase activity. Second, the sections were incubated in primary
antibodies (α-SMA, Smad2/3 and Smad7) overnight at 4°C, followed by
incubation in anti-mouse secondary antibody for 1 h at room
temperature. Third, visualization was carried out using the DAB
horseradish peroxidase color development kit (Beyotime Institute of
Biotechnology, Shanghai, China), and slides were counter-stained in
hematoxylin-1. Finally, ten random fields were examined per slice
for expression of α-SMA, Smad2/3, and Smad7 using a Leica DM2500
optical microscope at a magnification of ×200. The mean optical
density (MOD) was measured by Image-Pro Plus 6.0 image analysis
software. All cases in immunohistochemical staining were evaluated
by two investigators independently and any discrepancy was resolved
by a group discussion.
Western blot analysis
Thirty micrograms of total cellular proteins were
resolved by sodium dodecyl sulfate-poly-acrylamide gel
electrophoresis (SDS-PAGE) and transferred onto polyvinylidene
fluoride (PVDF) transfer membranes by western blotting (24). The results were quantified using
ImageJ (National Institutes of Health, Bethesda, MD, USA). The
following antibodies were used: E-cadherin, α-SMA, TGF-β1,
pSmad2/3, Smad7 and ILK (Santa Cruz Biotechnology, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). All
experiments were performed in triplicate and the data are expressed
as the mean ± standard deviation (SD). The statistical significance
of differences was calculated using the t-test and one-way analysis
of variance (ANOVA), and p<0.05 was considered statistically
significant.
Results
The general condition of rats
After treatment for 4 weeks, the rats in control
group and control + Ad-P311 group were still in good shape, glossy
coat color and obesity, while the rats in UUO model group had
different degrees of anorexia, low spirits, matt coat color, and
kidney enlargement, and even individual rats were dead from kidney
failure (there were 5 deaths including 3 in UUO group and 2 in UUO
+ Ad-P311 group). However, the above symptoms were improved by the
administration of Ad-P311 in UUO rats. In addition, there was a
great change in body weight of rats after establishment of the UUO
model and treatment with Ad-P311 (data not shown). After 4 weeks of
treatment, body weights in UUO group (p<0.01) and UUO + Ad-P311
group (p<0.05) had different degrees of weight loss compared to
the control group and control + Ad-P311 group, while there was no
significant difference in control group and control + Ad-P311 group
(p>0.05). Moreover, the weight loss was reversed significantly
by the administration of Ad-P311 in UUO + Ad-P311 group compared to
that in UUO group (p<0.05).
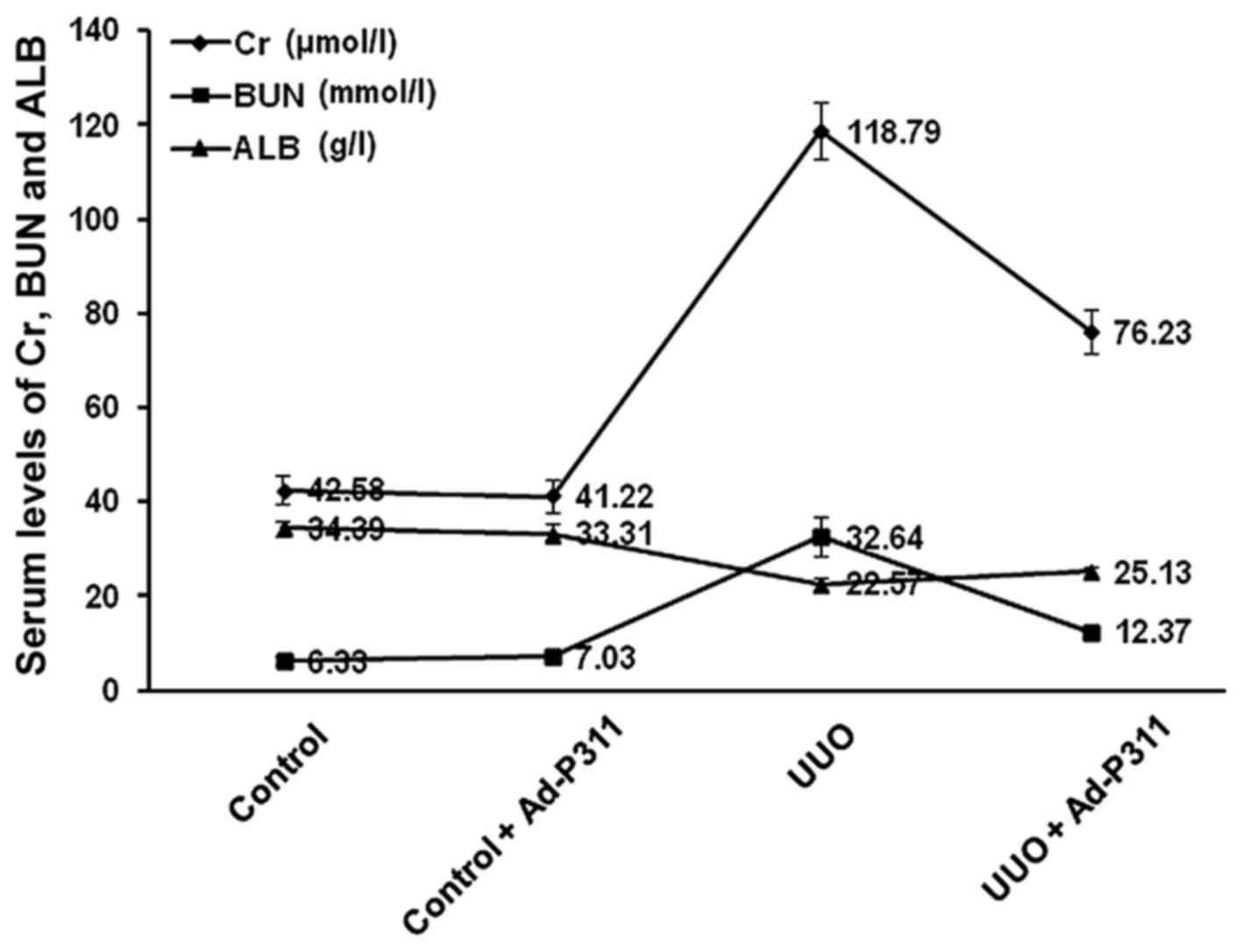
The serum levels of Cr, BUN and ALB
As shown in Fig.
1, the serum levels of Cr and BUN in UUO group (Cr 118.79±6.14
μmol/l; BUN 32.64±4.02 mmol/l) were higher than those in
control group (Cr 42.58±2.95 μmol/l; BUN 6.33±1.1 mmol/l)
and control + Ad-P311 group (Cr 41.22±3.36 μmol/l; BUN
7.03±0.90 mmol/l) (p<0.01), while there was no significant
difference in control group and control + Ad-P311 group
(p>0.05). However, Ad-P311 administration substantially
decreased the serum levels of Cr and BUN. The serum levels of Cr
and BUN in UUO + Ad-P311 group (Cr 76.23±4.45 μmol/l; BUN
12.37±1.33 mmol/l) were lower than those in UUO group (p<0.01).
The serum levels of ALB in UUO group (ALB 22.57±1.43 g/l) were
higher than those in control group (ALB 34.39±1.66 g/l) and control
+ Ad-P311 group (ALB 33.31±1.95 g/l) (p<0.01), while there was
no significant difference in control group and control + Ad-P311
group (p>0.05). However, Ad-P311 administration substantially
increased the serum levels of ALB. The serum levels of ALB in UUO +
Ad-P311 group (ALB 25.13±0.97 g/l) were higher than those in UUO
group (p<0.01).
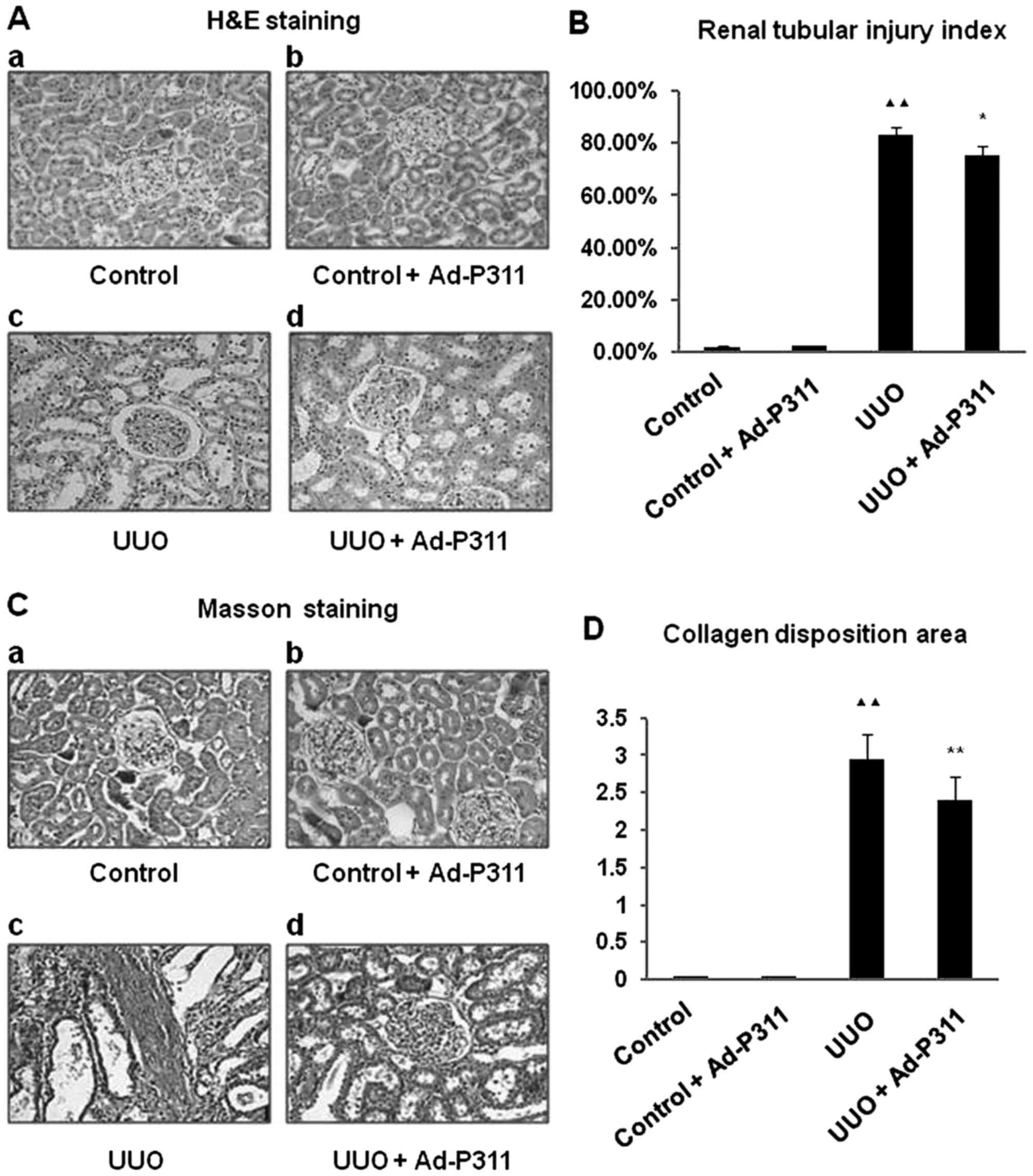
Ad-P311 attenuated UUO-induced
fibrosis
H&E staining demonstrated that there were no
histological changes in the kidneys of sham rats as shown in
control group (Fig. 2A–a) and
control + Ad-P311 group (Fig.
2A–b). By contrast, the kidneys developed remarkable
pathological changes such as interstitial fibrosis, tubular
expansion, and atrophy and inflammatory cell invasion in UUO rats
as shown in UUO group (Fig.
2A–c). However, these histological lesions in the kidneys of
UUO rats were attenuated by the administration of Ad-P311 as shown
in UUO + Ad-P311 group (Fig.
2A–d). Consistent with the pathological changes in the
experimental kidneys, UUO surgery resulted in ~8-fold increase of
the renal tubular injury index, but the extent of damage was
remarkably decreased from 83.20±2.80% (UUO group) to 75.60±3.40%
(UUO + Ad-P311 group) by the administration of Ad-P311 (Fig. 2B). Masson staining showed that
collagen deposition and fibrosis area were significantly decreased
in UUO + Ad-P311 group compared to UUO group (Fig. 2C). The collagen accumulation had
prominently increased ~3-fold in the renal interstitium of UUO
group, while administration of Ad-P311 decreased the amount of
collagen deposition from 2.94±0.34 (UUO group) to 2.39±0.33 (UUO +
Ad-P311 group), compared to UUO group (p<0.01) (Fig. 2D).
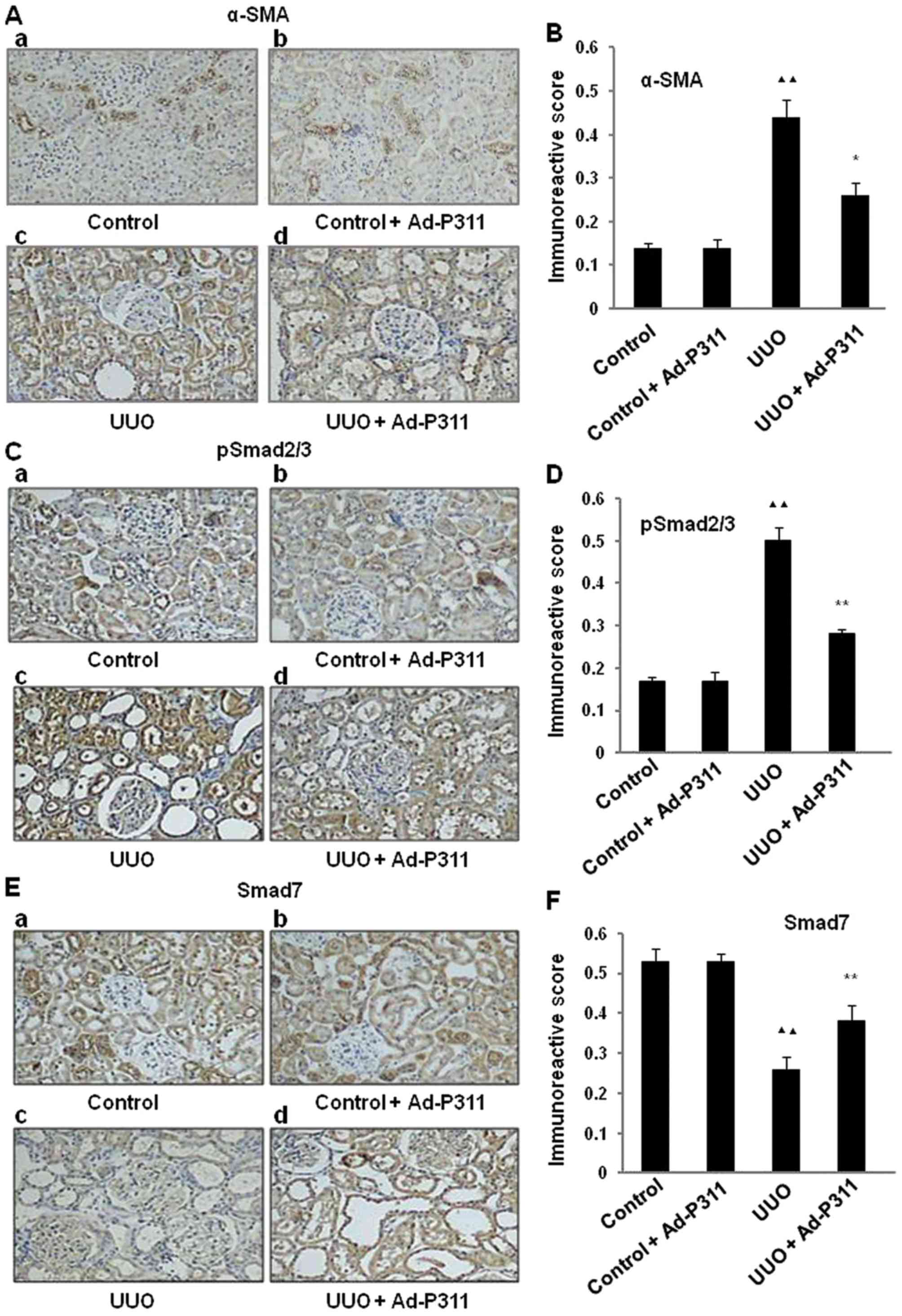
Ad-P311 reverses the expression α-SMA,
pSmad2/3 and Smad7 detected by immunohistochemical staining in UUO
kidneys
To explore the effect of Ad-P311 on EMT-related
markers in UUO kidneys, the expression of the mesenchymal marker
α-SMA in UUO kidneys was investigated by immunohistochemical
staining. As shown in Fig. 3A and
B, the protein expression of α-SMA was increased significantly
in UUO group compared to that in the sham operated control kidneys
(control group and control + Ad-P311 group). In contrast, the
increased expression of α-SMA in UUO group was reversed by the
administration of Ad-P311 as shown in UUO + Ad-P311 group.
Furthermore, to explore the possible mechanism of
Ad-P311 on EMT of UUO kidneys, the protein expression of Smad2/3
and Smad7 was investigated by immunohistochemical staining. As
shown in Fig. 3C and D, the
protein expression of pSmad2/3 was dramatically elevated in UUO
group compared with the sham groups (control group and control +
Ad-P311 group), but Ad-P311 administration (UUO + Ad-P311 group)
substantially ameliorated this elevation induced by UUO surgery
(UUO group). As shown in Fig. 3E and
F, the protein expression of Smad7 was decreased significantly
in UUO group compared to that in the sham groups (control group and
control + Ad-P311 group), while the administration of Ad-P311
alleviated its decrease as shown in UUO + Ad-P311 group.
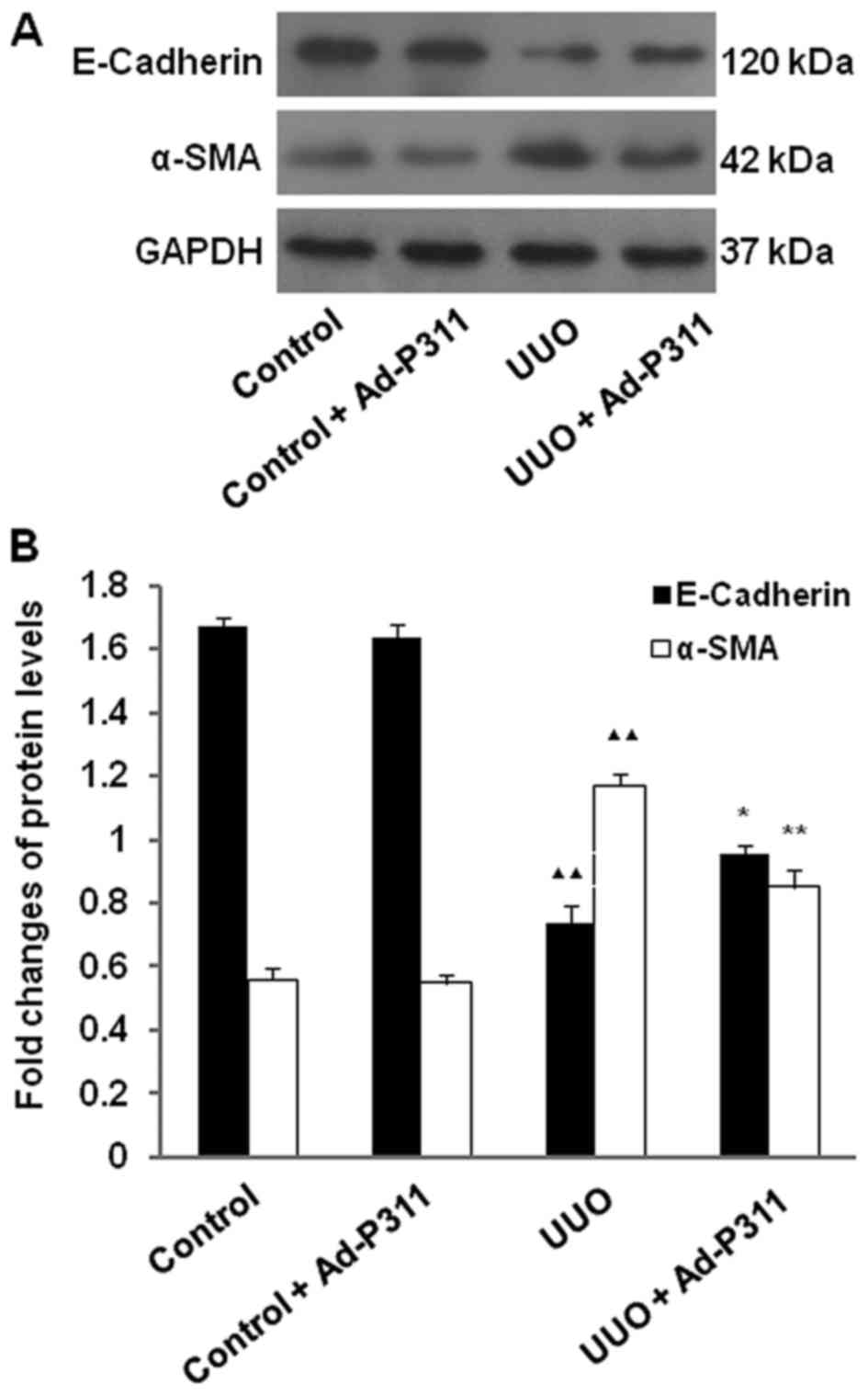
Ad-P311 reversed the expression of EMT
related proteins detected by western blot analysis in UUO
kidneys
To further explore the effect of Ad-P311 on EMT
related markers in UUO kidneys, the expression of the epithelial
marker E-cadherin, and the mesenchymal marker α-SMA in UUO kidneys
was examined by western blot analysis. As shown in Fig. 4, the protein level of E-cadherin
was decreased significantly in UUO group compared with the sham
groups (control group and control + Ad-P311 group), but
administration of Ad-P311 alleviated its decrease as shown in UUO +
Ad-P311 group. In contrast, the protein expression of α-SMA was
increased significantly in UUO group compared to that in the sham
operated control kidneys (control group and control + Ad-P311
group), while the increased expression of α-SMA in UUO group was
reversed by the administration of Ad-P311 as shown in UUO + Ad-P311
group (Fig. 4).
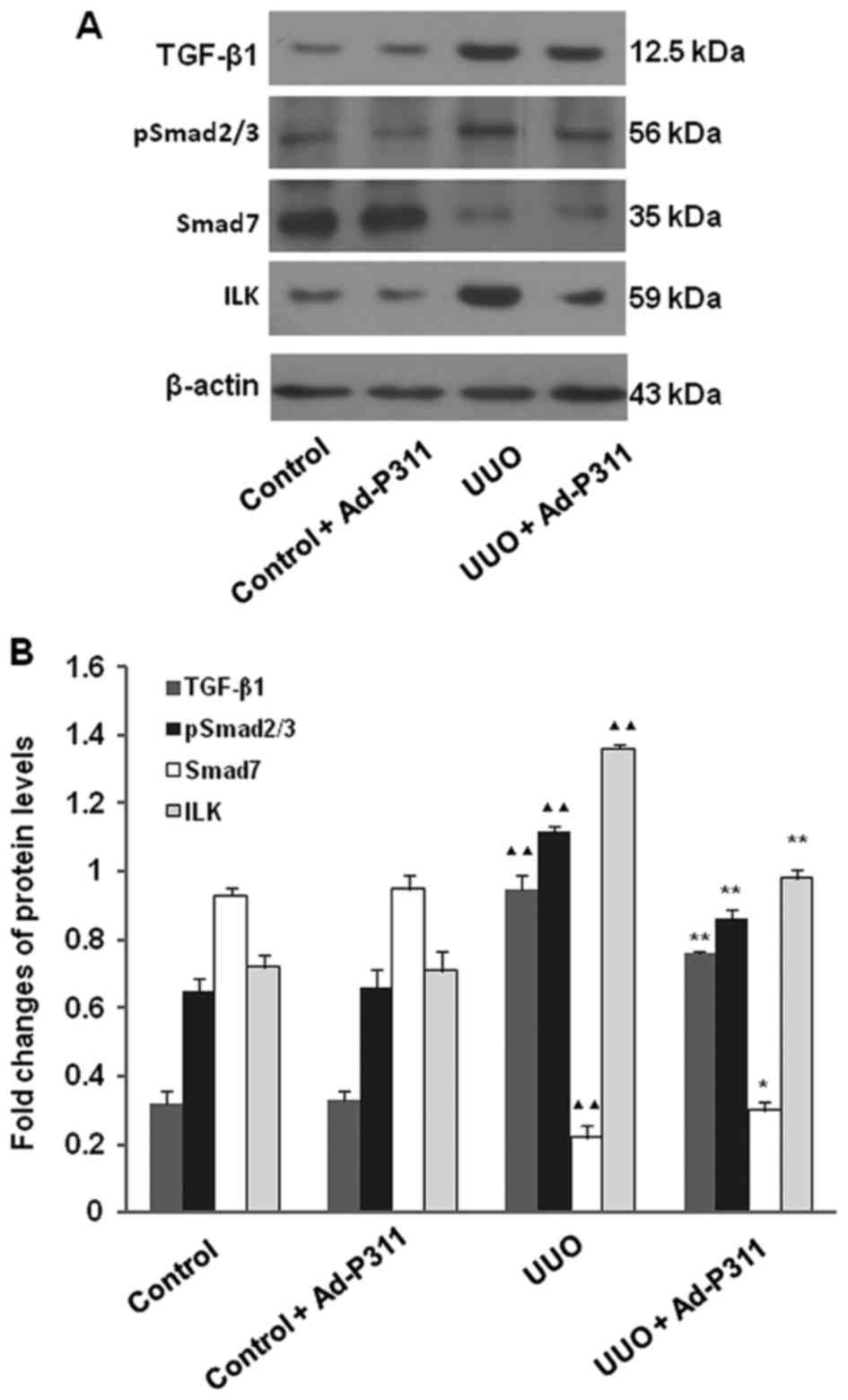
To explore the possible mechanism of Ad-P311 on EMT
of UUO kidneys, the protein expression of EMT-related proteins
TGF-β1, pSmad2/3, Smad7 and ILK were measured by western blot
analysis. As shown in Fig. 5, the
protein expression of TGF-β1, pSmad2/3 and ILK were increased
significantly in UUO group compared to that in the sham operated
control kidneys (control group and control + Ad-P311 group), but
administration of Ad-P311 alleviated all of above changes
significantly as shown in UUO + Ad-P311 group. In contrast, the
protein expression of Smad7 was decreased significantly in UUO
group compared to that in the sham operated control kidneys
(control group and control + Ad-P311 group), while the decreased
expression of Smad7 in UUO group was reversed by the administration
of Ad-P311 as shown in UUO + Ad-P311 group (Fig. 5).
Discussion
P311 is a conserved 8-kDa intracellular protein
expressed in vascular and visceral smooth muscle beds, the nervous
system, regenerating tissues as well as malignant glioblastomas
(16,17,19,25). Previous studies have suggested
P311 may be an important factor in myofibroblast transformation and
in the progression of fibrosis (13-20). However, the related studies on
P311 on renal fibrosis are limited and the mechanisms of P311 in
the progression of CKD remain largely unknown. In our previous
studies, we found that P311 may be involved in the pathogenesis of
renal fibrosis by inhibiting EMT process via TGF-β1-Smad-ILK
pathway in NRK-52E cells (20).
In our previous studies, we also determined whether p311 expressed
in the kidney of rats transfected with Ad-p311 by quantitative
real-time RT-PCR assay. P311 was expressed in the kidneys of UUO
rats but not in the kidneys of sham surgery rats. Furthermore, P311
gene could be highly and stably transfected into UUO rats with
adenovirus mediation (data not shown). Thus, in the present study,
the effect and possible mechanism of adenovirus-mediated P311 on
TGF-β1-mediated EMT in UUO rats were further investigated. We found
that adeno-virus-mediated P311 was capable of improving fibrosis by
regulating expressions of EMT related markers in vivo and
the underlying mechanisms may involve the TGF-β1-Smad-ILK signaling
pathway.
CKD is characterized by an irreversible decrease in
kidney function and renal fibrosis is considered as a common
pathological hallmark of progressive CKD (26). UUO is recognized as an ideal
experimental model of renal interstitial fibrosis. Ureteral
obstruction leads to a significant inflammatory and fibrotic
response, followed by infiltration of inflammatory cells, renal
interstitial myofibroblast proliferation, and ECM accumulation in
the renal interstitium (27). We
established the UUO model successfully and administered Ad-P311
into UUO rats by the tail vein. Then the general condition of rats
and the serum levels of Cr, BUN and ALB were evaluated. The rats in
UUO model group had different degrees of anorexia, low spirits,
matt coat color, kidney enlargement, weight loss, and even
individual rats dead from kidney failure, but these symptoms were
improved by the administration of Ad-P311 in UUO rats. Moreover,
the serum levels of Cr and BUN were increased significantly and the
serum levels of ALB were decreased significantly in the UUO rats.
However, the increased serum levels of Cr and BUN and the decreased
serum levels of ALB were reversed by the administration of Ad-P311
in UUO rats. In addition, H&E staining and Masson staining
indicated that the kidneys developed remarkable pathological
changes such as interstitial fibrosis, tubular expansion, and
atrophy and inflammatory cell invasion in UUO rats. However, these
histological lesions in the kidneys of UUO rats were attenuated
after administration of Ad-P311 with the renal tubular injury index
and collagen disposition area reversed. These findings suggested
Ad-P311 alleviated kidney function and renal fibrosis in UUO
rats.
Renal fibrosis is generally considered a failure of
tissue injury/repair response, which is closely associated with
chronic interstitial inflammation (11). The progression of renal fibrosis
primarily involves transdifferentiation of renal fibroblasts into
myofibroblasts and infiltration of inflammatory cells, with
production and release of profibrotic cytokines and growth factors,
such as TGF-β1, TNF-α, IL-1β, IL-6, and PDGF (28). TGF-β1 as a key paracrine/autocrine
growth factor and profibrotic cytokine plays a key role in renal
fibrosis, which promotes tubular epithelial myofibroblast
transdifferentiation and increases the synthesis and accumulation
of ECM proteins (29,30). P311 is an intracytoplasmic protein
that can bind to TGF-β-latent associated protein (LAP) and
downregulates the expression of TGF-β1 and TGF-β2 (14). Moreover, Pan et al have
reported that P311 in myofibroblast transformation could decrease
TGF-β1 signaling and cause inhibition in collagen expression,
suggesting that P311 may be involved in preventing fibrosis during
wound repair (15).
In the present study, the protein expression of
TGF-β1 was increased significantly in UUO kidneys, but
administration of Ad-P311 alleviated its increase. These findings
indicated that adenovirus-mediated P311 could ameliorate renal
fibrosis through regulating TGF-β1 in UUO rats. However, Yao et
al used P311−/− and P311+/+ C57BL/6 mice
to establish UUO model and found that P311 could promote renal
fibrosis via TGFβ1/Smad signaling (31). This finding differs from our data.
Although the previous study suggested P311 may be an important
factor in myofibroblast transformation and in the progression of
fibrosis, the related studies of P311 on renal fibrosis are limited
and the mechanisms of P311 in the progression of renal fibrosis
remains largely unknown. Thus, it is difficult to explain this
discrepancy with our results. The authors consider the following
reasons may be responsible for the discrepancy. Since P311
inhibited profibrotic cytokine TGF-β1 accompanied with the
resultant decrease in collagen expression, P311 levels may be one
of the factors contributing to determine whether a lesion will heal
faster or with less fibrosis (15). It should be stressed, however,
that although P311 inhibits TGF-β1 synthesis, myofibroblasts remain
responsive to exogenous TGF-β1, as the inhibition effect of P311 on
collagen was overcome by exogenous TGF-β1. Moreover, noteworthy
that myofibroblasts are not the only source of TGF-β1, which is
mainly produced by inflammatory cells normally present at the wound
site (32-34). Therefore, depending on the
magnitude and duration of the inflammatory response, the autocrine
anti-fibrogenic effect of P311 may be partially counterbalanced or
completely offset by the paracrine production of TGF-β1 (15). In a noncomplicated wound, however,
inflammatory cells are present at early stages of repair, and then
gradually disappear from the site. Thus, the antifibrogenic effect
of P311 may increase over time and become maximal toward the end of
the reparative process (15).
This seems interesting as the relationship between P311 and TGF-β1
is like two competitors sitting on a seesaw. During renal fibrosis,
at early stages of repair with abundant inflammatory cells, the
antifibrogenic effect of P311 may be offset by TGF-β1 and exhibit
pro-fibrogenic effect, while at advanced stages of repair with
inflammatory cells gradually disappeared, the antifibrogenic effect
of P311 may increase over time and become maximal toward the end of
the reparative process.
Tubular EMT has been widely accepted as the
underlying mechanisms of renal fibrosis (26). Damaged tubular epithelial cells
undergo EMT and then transfer crucial signals towards renal
interstitial to activate phenotypic transition of fibroblasts and
sustain inflammation, resulting in progressive renal fibrosis
(35). In CKD, TGF-β1 is
considered a crucial regulator of EMT which mediates the initiation
and progression of interstitial fibrosis (31). It can induce the activation of
fibroblasts to undergo a phenotypic transition to myofibroblasts
with tubular epithelial cells losing their adhesion molecules (such
as E-cadherin), and gaining the mesenchymal cell markers (such as
α-SMA) (4). In the present study,
the protein expression of α-SMA was increased significantly and the
protein expression of E-cadherin was decreased significantly in UUO
kidneys compared to that in the sham operated control kidneys,
while the changed expressions of α-SMA and E-cadherin in UUO
kidneys was reversed by the administration of Ad-P311. These
results indicated that P311 could prevent TGF-β1-mediated EMT in
UUO kidneys.
The TGF-β1/Smad signaling pathway has been reported
to play a key role in initiating and completing the entire EMT
course in fibrotic disease pathogenesis (36). During this signaling pathway, both
Smad2 and Smad3 proteins are phosphorylated in response to TGF-β
receptor activation and in subsequent events become downstream
mediators of TGF-β signaling. Phosphorylated Smad2 and Smad3 then
bind to the common Smad4 and form the Smad complex, which
translocates into the nucleus to regulate the target gene
transcription, including Smad7 (32). Here we found that pSmad2/3
expression was increased and Smad7 expression was decreased
significantly in UUO kidneys compared to that in the sham operated
control kidneys, but administration of Ad-P311 alleviated all of
above changes significantly. These results indicated that P311
regulates not only TGF-β1 but also the Smad signaling pathway,
which may be involved in the regulation of renal fibrosis by P311.
ILK is a serine/threonine protein kinase and a major regulator of
integrin signaling, interacting with the cytoplasmic domains of
integrin β1 and β3 subunits. It has a fundamental role in the
regulation of cell survival, proliferation, and migration (37). TGF-β1-mediated EMT has been
suggested to be dependent on ILK function during renal fibrosis
depending on intracellular Smad signaling (11). Therefore, ILK is shown to be a key
intracellular mediator that controls TGF-β1-induced-EMT in renal
tubular epithelial cells. We studied the effects of P311 on ILK
expression, which was the important downstream mediator of
TGF-β1/Smads signaling pathway. Here we found that ILK expression
was increased significantly in UUO kidneys compared to that in the
sham operated control kidneys, however, administration of Ad-P311
significantly reversed of above changes. Taken together, these
findings indicated that P311 attenuated TGF-β1-mediated EMT via
regulating the protein expression of pSmad2/3, Smad7 and ILK in UUO
kidneys.
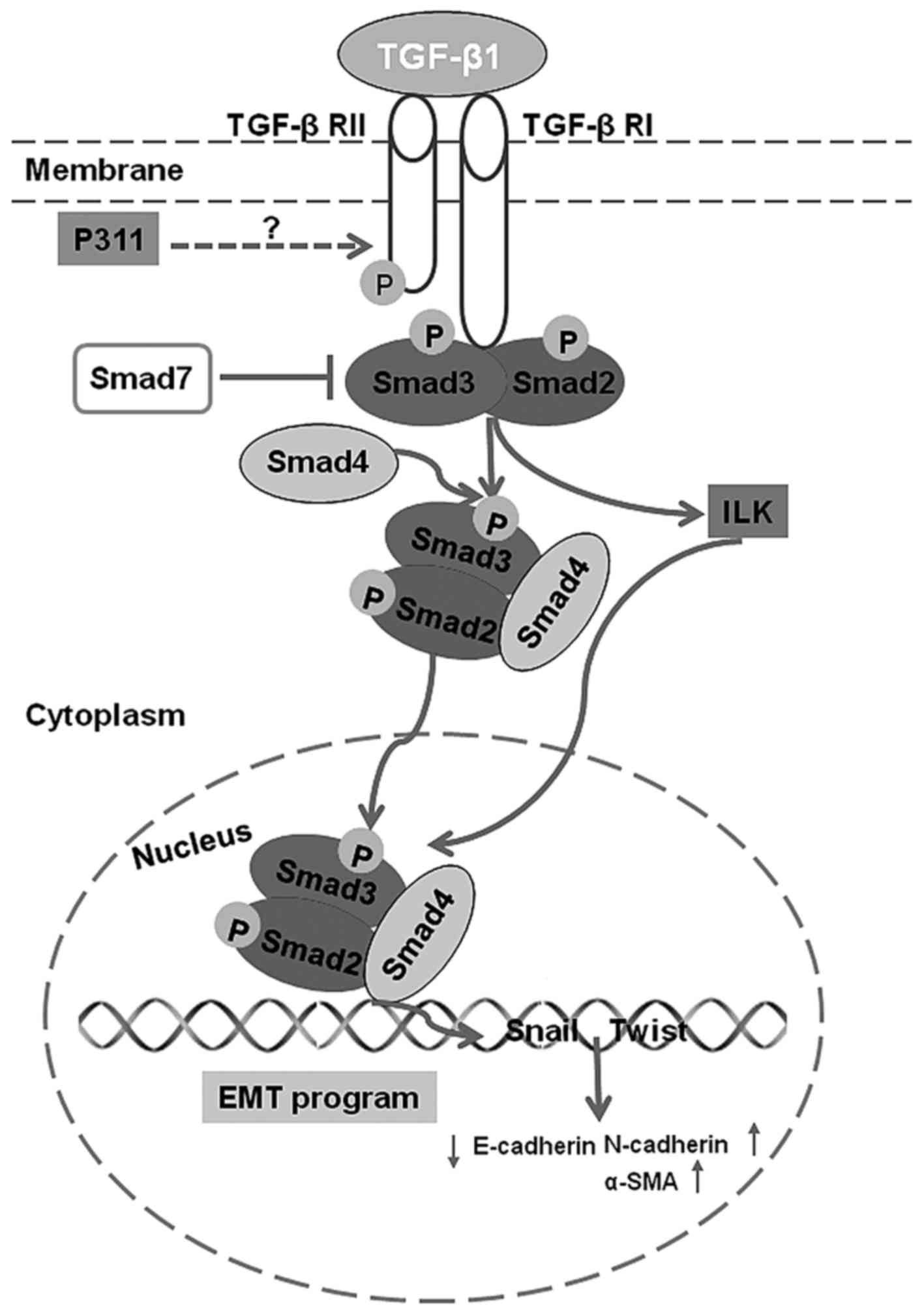
In conclusion, our data suggest that P311 may be
involved in the pathogenesis of renal fibrosis by blocking
TGF-β1-mediated EMT via TGF-β1-Smad-ILK pathway (Fig. 6) in UUO kidneys. P311 may be a
novel target for the control of renal fibrosis and the progression
of CKD.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
CKD
|
chronic kidney disease
|
|
UUO
|
unilateral ureteral occlusion
|
|
α-SMA
|
α-smooth muscle actin
|
|
ILK
|
integrin linked kinase
|
|
TGF-β1
|
transforming growth factor-β1
|
Acknowledgments
Not applicable.
Notes
[1]
Funding
The present study was supported by the National
Natural Science Foundation of China (no. 81273682) and the Science
and Technology Development Program of Shandong Province (no.
2010G0020220).
[2] Availability
of data and material
All data generated or analyzed during this study are
included in this published article.
[3] Authors'
contributions
GMS was responsible for the concept and design of
the study. FHQ, PPC, and XL completed the experiments and performed
the data analysis. FHQ was a major contributor in writing the
manuscript. All authors read and approved the final manuscript.
[4] Ethics
approval and consent to participate
All the experimental procedures were approved by the
Institutional Animal Care and Use Committee of Shandong Provincial
Hospital Affiliated to Shandong University (no. 2017-208).
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Jha V, Garcia-Garcia G, Iseki K, Li Z,
Naicker S, Plattner B, Saran R, Wang AY and Yang CW: Chronic kidney
disease: Global dimension and perspectives. Lancet. 382:260–272.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tonelli M, Wiebe N, Culleton B, House A,
Rabbat C, Fok M, McAlister F and Garg AX: Chronic kidney disease
and mortality risk: A systematic review. J Am Soc Nephrol.
17:2034–2047. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boor P, Ostendorf T and Floege J: Renal
fibrosis: Novel insights into mechanisms and therapeutic targets.
Nat Rev Nephrol. 6:643–656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y: New insights into
epithelial-mesenchymal transition in kidney fibrosis. J Am Soc
Nephrol. 21:212–222. 2010. View Article : Google Scholar
|
|
5
|
Kriz W, Kaissling B and Le Hir M:
Epithelial-mesenchymal transition (EMT) in kidney fibrosis: Fact or
fantasy? J Clin Invest. 121:468–474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo Y, Li Z, Ding R, Li H, Zhang L, Yuan W
and Wang Y: Parathyroid hormone induces epithelial-to-mesenchymal
transition via the Wnt/β-catenin signaling pathway in human renal
proximal tubular cells. Int J Clin Exp Pathol. 7:5978–5987.
2014.
|
|
7
|
Wang Q, Wang Y, Huang X, Liang W and Xiong
Z and Xiong Z: Integrin β4 in EMT: An implication of renal
diseases. Int J Clin Exp Med. 8:6967–6976. 2015.
|
|
8
|
Wynn TA: Cellular and molecular mechanisms
of fibrosis. J Pathol. 214:199–210. 2008. View Article : Google Scholar
|
|
9
|
Impellizzeri D, Esposito E, Attley J and
Cuzzocrea S: Targeting inflammation: New therapeutic approaches in
chronic kidney disease (CKD). Pharmacol Res. 81:91–102. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia L, Ma X, Gui B, Ge H, Wang L, Ou Y,
Tian L, Chen Z, Duan Z, Han J, et al: Sorafenib ameliorates renal
fibrosis through inhibition of TGF-β-induced epithelial-mesenchymal
transition. PLoS One. 10:e01177572015. View Article : Google Scholar
|
|
11
|
Kim MK, Maeng YI, Sung WJ, Oh HK, Park JB,
Yoon GS, Cho CH and Park KK: The differential expression of TGF-β1,
ILK and wnt signaling inducing epithelial to mesenchymal transition
in human renal fibrogenesis: An immunohistochemical study. Int J
Clin Exp Pathol. 6:1747–1758. 2013.
|
|
12
|
Li Y, Yang J, Dai C, Wu C and Liu Y: Role
for integrin-linked kinase in mediating tubular epithelial to
mesenchymal transition and renal interstitial fibrogenesis. J Clin
Invest. 112:503–516. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Penn JW, Grobbelaar AO and Rolfe KJ: The
role of the TGF-β family in wound healing, burns and scarring: A
review. Int J Burns Trauma. 2:18–28. 2012.
|
|
14
|
Paliwal S, Shi J, Dhru U, Zhou Y and
Schuger L: P311 binds to the latency associated protein and
downregulates the expression of TGF-beta1 and TGF-beta2. Biochem
Biophys Res Commun. 315:1104–1109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan D, Zhe X, Jakkaraju S, Taylor GA and
Schuger L: P311 induces a TGF-beta1-independent, nonfibrogenic
myofibroblast phenotype. J Clin Invest. 110:1349–1358. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mariani L, McDonough WS, Hoelzinger DB,
Beaudry C, Kaczmarek E, Coons SW, Giese A, Moghaddam M, Seiler RW
and Berens ME: Identification and validation of P311 as a
glioblastoma invasion gene using laser capture microdissection.
Cancer Res. 61:4190–4196. 2001.PubMed/NCBI
|
|
17
|
Fujitani M, Yamagishi S, Che YH, Hata K,
Kubo T, Ino H, Tohyama M and Yamashita T: P311 accelerates nerve
regeneration of the axotomized facial nerve. J Neurochem.
91:737–744. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao L, Leung JK, Yamamoto H, Goswami S,
Kheradmand F and Vu TH: Identification of P311 as a potential gene
regulating alveolar generation. Am J Respir Cell Mol Biol.
35:48–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan J, Peng X, Luo G, Ma B, Cao C, He W,
Yuan S, Li S, Wilkins JA and Wu J: Investigating the role of P311
in the hyper-trophic scar. PLoS One. 5:e99952010. View Article : Google Scholar
|
|
20
|
Qi F, Cai P, Liu X, Peng M and Si G:
Adenovirus-mediated P311 inhibits TGF-β1-induced
epithelial-mesenchymal transition in NRK-52E cells via
TGF-β1-Smad-ILK pathway. Biosci Trends. 9:299–306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baba I, Egi Y, Utsumi H, Kakimoto T and
Suzuki K: Inhibitory effects of fasudil on renal interstitial
fibrosis induced by unilateral ureteral obstruction. Mol Med Rep.
12:8010–8020. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin Y, Qi F, Song Z, Zhang B and Teng J:
Ferulic acid combined with astragaloside IV protects against
vascular endothelial dysfunction in diabetic rats. Biosci Trends.
8:217–226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu QF, Ye JM, Deng ZY, Yu LX, Sun Q and
Li SS: Ameliorating effect of Klotho on endoplasmic reticulum
stress and renal fibrosis induced by unilateral ureteral
obstruction. Iran J Kidney Dis. 9:291–297. 2015.PubMed/NCBI
|
|
24
|
Lu H, Dong J, Zhang Y, Li C, Yu Q and Tang
W: Pathological changes in primary cilia: A novel mechanism of
graft cholangiopathy caused by prolonged cold preservation in a rat
model of orthotopic liver transplantation. Biosci Trends.
8:206–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Badri KR, Yue M, Carretero OA, Aramgam SL,
Cao J, Sharkady S, Kim GH, Taylor GA, Byron KL and Schuger L: Blood
pressure homeostasis is maintained by a P311-TGF-β axis. J Clin
Invest. 123:4502–4512. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshinaga T, Uwabe K, Naito S, Higashino
K, Nakano T, Numata Y and Kihara A: AM251 suppresses
epithelial-mesenchymal transition of renal tubular epithelial
cells. PLoS One. 11:e01678482016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Ma J, Guo C, Chen C, Yin Z, Zhang
X and Chen X: Danggui buxue tang attenuates tubulointerstitial
fibrosis via suppressing NLRP3 inflammasome in a rat model of
unilateral ureteral obstruction. Biomed Res Int. 2016:93684832016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Y: Cellular and molecular mechanisms
of renal fibrosis. Nat Rev Nephrol. 7:684–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fan JM, Ng YY, Hill PA, Nikolic-Paterson
DJ, Mu W, Atkins RC and Lan HY: Transforming growth factor-beta
regulates tubular epithelial-myofibroblast transdifferentiation in
vitro. Kidney Int. 56:1455–1467. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang F, Xie X, Fan J, Wang L, Guo D, Yang
L, Ma X, Zhang L and Li Z: Expression of P311, a transforming
growth factor beta latency-associated protein-binding protein, in
human kidneys with IgA nephropathy. Int Urol Nephrol. 42:811–819.
2010. View Article : Google Scholar :
|
|
31
|
Loboda A, Sobczak M, Jozkowicz A and Dulak
J: TGF-β1/Smads and miR-21 in renal fibrosis and inflammation.
Mediators Inflamm. 2016:83192832016. View Article : Google Scholar
|
|
32
|
Yao Z, Yang S, He W, Li L, Xu R, Zhang X,
Li H, Zhan R, Sun W, Tan J, et al: P311 promotes renal fibrosis via
TGFβ1/Smad signaling. Sci Rep. 5:170322015. View Article : Google Scholar
|
|
33
|
Martin P: Wound healing - aiming for
perfect skin regeneration. Science. 276:75–81. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Allison SJ: Fibrosis: Targeting EMT to
reverse renal fibrosis. Nat Rev Nephrol. 11:5652015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
O'Connor JW and Gomez EW: Biomechanics of
TGFβ-induced epithelial-mesenchymal transition: Implications for
fibrosis and cancer. Clin Transl Med. 3:232014. View Article : Google Scholar
|
|
37
|
Wei MG, Sun W, He WM, Ni L and Yang YY:
Ferulic acid attenuates TGF-β1-induced renal cellular fibrosis in
NRK-52E cells by inhibiting Smad/ILK/Snail pathway. Evid Based
Complement Alternat Med. 2015:6197202015. View Article : Google Scholar
|