Introduction
Human telomerase reverse transcriptase (hTERT) is
highly expressed in almost all types of cancer and maintains the
integrity of telomere sequences required for an immortal phenotype
(1,2). Due to this association between the
level of hTERT and cancer, hTERT has been suggested as an effective
target for anticancer vaccines (3). Phase I/II clinical trials using the
hTERT peptide fragment, GV1001, led to immune activation when
cluster of differentiation 4+ (CD4+) T cells
were surveyed (4). A
dose-escalating phase I/II study in patients with pancreatic cancer
revealed prolonged survival rates in patients receiving the GV1001
peptide with an immunologic response, compared with those without
an immune response (5). Another
study showed the activation of cytotoxic T cells and an
anti-leukemic response in patients with B-cell chronic lymphocytic
leukemia when administered with GV1001 (6). Therefore, earlier clinical studies
suggested potential anticancer effects of GV1001, primarily through
immunologic activation.
GV1001 is a 16-amino acid peptide corresponding to
hTERT 616–626 sequences (EARPALLTSRLRFIPK), which lies within the
reverse transcriptase functional domain (5,7).
Previous studies have demonstrated direct cellular effects of
GV1001, beyond its immunologic functions, as an anticancer vaccine,
including anti-inflammatory effects (8–10),
tumor suppressive effects (11,12) and antiviral activities (13,14). GV1001 has also been found to
protect against β-amyloid-induced neurotoxicity in neural stem
cells (NSCs), suggesting its potential therapeutic effects for
neurodegenerative diseases (15);
NSCs showed enhanced cell proliferation and survival when treated
with β-amyloid in the presence of GV1001. Furthermore, GV1001
administration in tumor xenografts was shown to suppress fibrotic
tissue surrounding the tumor nodules, allowing for increased
tumoral susceptibility to chemotherapeutic agents and cell death
(12). These studies demonstrated
the versatility of GV1001 with multiple biological effects,
independent of the immune activation originally intended as an
anticancer vaccine.
Radiation induces normal tissue injuries, which are
characterized at the histologic level by loss of parenchymal cells,
excessive fibrosis and tissue atrophy. Fibrotic changes occur as
result of the loss of epithelial identity and function. There is a
long list of systemic diseases eventually resulting in organ
failure due to fibrotic changes with significant mortality rates;
it has been estimated that organ fibrosis is responsible for ~45%
of all chronic systemic disease-associated mortality (16). The primary driver of tissue
fibrosis is transforming growth factor-β (TGF-β) signaling, as
evidenced in radiation-induced pulmonary scarring, through the
induction of myofibroblast differentiation (17,18). Following exposure to ionizing
radiation (IR), the expression of TGF-β1 has been shown to increase
in a dose-dependent manner in the rat liver (19). Similarly, in a mouse model of
radiation-induced lung injury, fibrosis development was accompanied
by an increase in the expression of TGF-β1 and activation of the
TGF-β1 signal transduction pathway (20). As there is substantial evidence
supporting the importance of TGF-β1 in the development of
radiation-induced tissue damage, TGF-β1 and its signaling molecules
have become logical targets for molecular therapies designed to
prevent or reduce normal tissue injury following irradiation
exposure. In the present study, the effects of GV1001 on
radioprotection and antifibrosis were examined in primary normal
human oral keratinocytes (NHOKs) and fibroblasts (NHOFs). The data
indicated that GV1001 protected the NHOKs from IR-induced damage
and suppressed epithelial-mesenchymal transition (EMT) in cells
exposed to IR, allowing the cells continued proliferation with
maintenance of the epithelial phenotype. In addition, GV1001
directly suppressed TGF-β-induced EMT in epithelial cells and
myofibroblast differentiation. When GV1001 was administered by
subcutaneous injection in mice exposed to bleomycin (BLM), the
level of dermal fibrosis and the synthesis of collagen type III α1
chain (Col3a1) were significantly reduced in the dermal layer of
the skin. Collectively, the data indicated that GV1001 may be of
therapeutic benefit against tissue injury and fibrosis resulting
from IR exposure through direct suppression of the TGF-β signaling
pathway.
Materials and methods
Cells and cell culture
Primary NHOK and NHOF cultures were established from
discarded oral mucosa without patient identification or medical
information, under exemption from the Institutional Review Board of
UCLA (Los Angeles, CA, USA) as described previously (21). Briefly, the discarded oral mucosal
tissues of approximately 25 mm2 were collected from
healthy patients who were undergoing routine dental procedures at
the UCLA Dental Center between 2010 and 2016. The oral mucosal
tissues were minced into 2 mm2 × 0.5 mm sections and
incubated in 2.5 mg/ml dispase solution (cat. no. 17105; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) in 37°C for 1 h,
then the epithelial layers and connective layers were separated.
The separated epithelial layers were minced and subjected to
trypsin digestion (cat. no. 25200056; Gibco; Thermo Fisher
Scientific, Inc.) in 37°C for 5 min to harvest the individual
epithelial cells to establish NHOK. The separated connective tissue
layers were minced and digested with collagenase (cat. no.
17100017; Gibco; Thermo Fisher Scientific, Inc.) in 37°C for 1 h to
harvest the individual mesenchymal cells to establish NHOF. NHOKs
were cultured in EpiLife supplemented with human keratinocyte
growth supplement (HKGS; Cascade Biologics; Thermo Fisher
Scientific, Inc.), and NHOFs were cultured in DMEM supplemented
with 10% FBS (Invitrogen; Thermo Fisher Scientific, Inc.). For
radiation exposure, NHOKs were exposed to varying doses of IR using
a Mark I-30 Cesium-137 irradiator (JL Shepherd & Associates,
San Fernando, CA, USA) with the delivery rate of 4.86 Gy
min−1. IR exposure led to the induction of a senescent
phenotype, described as stress-induced premature senescence (SIPS)
(22). To confirm SIPS, NHOKs
were stained for senescence-associated β-galactosidase (SA β-Gal)
activity, which was viewed/imaged under an Olympus phase-contrast
microscope (Olympus Corporation, Tokyo, Japan) (21). Squamous cell carcinomas (SCC) 4
cell line was obtained from the American Type Culture Collection
(ATCC, Manassas, VA, USA) and cultured in DMEM/F12 (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Invitrogen;
Thermo Fisher Scientific, Inc.) and 0.4 µg/ml hydrocortisol
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Reagents
The following primary antibodies were used in the
present study: GAPDH (cat. no. sc-47724), zinc finger E-box binding
homeobox 1 (ZEB1; cat. no. sc-25388), E-Cadherin (E-Cad; cat. no.
sc-21791), P21/WAF1 (cat. no. sc-397), 53BP1 (cat. no. sc-22760),
P16INK4A (cat. no. sc-468), phosphorylated (p-)Akt
(Ser473; cat. no. sc-7985), collagen type I α1 chain (Col1a1; cat.
no. sc-8784) and Col3a1 (cat. no. sc-28888) from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA); GRHL2 (cat. no.
H00079977) from Abnova (Taipei City, Taiwan); N-Cadherin (N-Cad;
cat. no. 610920) from BD Biosciences (San Jose, CA, USA);
fibronectin (FN; cat. no. F3648), α-smooth muscle actin (α-SMA;
cat. no. A5228) and Snail (cat. no. SAB1406456) from Sigma-Aldrich;
Merck KGaA; p-small mothers against decapentaplegic (Smad)3
(ser423/425; cat. no. 9520), p-Smad2 (ser465/467; cat. no. 3108),
Smad4 (cat. no. 38454) and p-p53 (Ser15; cat. no. 9284) from Cell
Signaling Technology, Inc. (Danvers, MA, USA); and γ-H2AX (phospho
S139; cat. no. ab2893) from Abcam (Cambridge, MA, USA). Alexa
Fluor® anti-rabbit or anti-mouse secondary antibodies
were from Thermo Fisher Scientific, Inc. TGF-β1 (cat. no. 100-21)
was purchased from PeproTech, Inc. (Grand Island, NY, USA); TGF-β
receptor I kinase inhibitor (TRI; cat. no. 616451) was obtained
from Calbiochem; EMD Millipore (Billerica, MA, USA). BLM was
purchased from Sigma-Aldrich; Merck KGaA; GV1001 was provided by
Gemvax & Kael Co. Ltd. (Seoul, Korea).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated using TRIzol reagent (Thermo
Fisher Scientific, Inc.). DNA-free total RNA (5 µg) was used
for the RT reaction, followed by qPCR for each 1 µl cDNA
sample with 1 µl primer pair mix (5 pmol/µl each
primer) and LC480 SYBR Green I master using universal cycling
conditions in a LightCycler® 480 (Roche Diagnostics,
South San Francisco, CA, USA). The primer sequences were obtained
from the Universal Probe Library (Roche Diagnostics). The PCR
cycling conditions were as follows: 45 cycles of 10 sec at 95°C, 45
sec at 55°C, and 20 sec at 72°C. The second derivative Cq value
determination method was used to compare the fold-differences
(23). Cq was the cycle at which
the threshold was crossed. The experiments were performed in
triplicate. The primer sequences used for the PCR to determine gene
expression were as follows: Col1a1, forward
5′-GGGATTCCCTGGACCTAAAG-3′ and reverse 5′-GGAACACCTCGCTCTCCA-3′;
Col3a1, forward 5′-CTGGACCCCAGGGTCTTC-3′ and reverse
5′-CATCTGATCCAGGGTTTCCA-3′; FN, forward
5′-GGGAGAATAAGCTGTACCATCG-3′ and reverse
5′-TCCATTACCAAGACACACACACT-3′; N-Cad, forward
5′-CTCCATGTGCCGGATAGC-3′ and reverse 5′-CGATTTCACCAGAAGCCTCTAC-3′;
ZEB1, forward 5′-AACTGCTGGGAGGATGACAC-3′ and reverse
5′-TCCTGCTTCATCTGCCTGA-3′; ZEB2, forward 5′-CCAGACCGCAATTAACAATG-3′
and reverse 5′-ATGCTGACTGCATGACCATC-3′; glyceraldehyde 3-phosphate
dehy drogenase (GAPDH), forward 5′-AGCCACATCGCTCAGACAC-3′ and
reverse 5′-GCCCAATACGACCAAATCC-3′.
Western blot analysis
Whole cell extracts (WCEs) from the cultured cells
were isolated using lysis buffer [1% Triton X-100, 20 mM Tris-HCl
(pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium
pyrophosphate, 1 µM β-glycerophosphate, 1 mM sodium
orthovanadate and 1 mg/ml PMSF]. The protein concentration of the
lysates was measured using a Bio-Rad protein assay kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Equal amounts of protein
(20 µg/lane) were separated by 8 or 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto an immobilon membrane (EMD Millipore), which was
incubated in blocking solution containing 5% bovine serum albumin
(BSA; Sigma-Aldrich; Merck KGaA) in Tris-buffered saline (TBS) for
30 min at room temperature. The membrane was then incubated with
primary antibodies (e.g., GRHL2 and N-Cad) diluted in blocking
solution (1:1,000) at 4°C overnight. Following washing with TBST
three times, the membrane was incubated with anti-mouse or rabbit
secondary antibodies (1:2,000) for 1 h at room temperature and then
exposed to the chemiluminescence reagent (GE Healthcare Life
Sciences, Piscataway, NJ, USA) for signal detection.
Immunofluorescence staining (IFS) of
cells and tissue specimens
The cells were cultured in an Nunc™ Lab-Tek™ II
Chamber Slide™ system (Thermo Fisher Scientific, Inc.) to reach
70–80% confluence, and then fixed in 2% paraformaldehyde for 20
min. The cells were permeabilized with 0.2% Triton X-100 in
phosphate-buffered saline (PBS) for 10 min, and then blocked for 1
h in PBS containing 2% BSA (Sigma-Aldrich; Merck KGaA) at room
temperature and incubated overnight at 4°C with primary antibodies
(ZEB1 and α-SMA; 1:100). Following three washes with PBS, the cells
were incubated with the secondary antibodies, Alexa
Fluor® 594 goat anti-rabbit IgG or Alexa
Fluor® 488 goat anti-mouse IgG (1:400; Thermo Fisher
Scientific, Inc.) for 1 h at room temperature. Slides were mounted
in Prolong Gold w/DAPI (Invitrogen; Thermo Fisher Scientific,
Inc.). Images were captured with an Olympus epifluorescence
inverted microscope (Olympus Corporation).
Paraffin-embedded histological sections were stained
with hematoxylin and eosin (H&E) for determination of the
histological changes in the dermis. The mouse skin specimens were
fixed in 4% (wt/vol) paraformaldehyde at 4°C for 24 h. The samples
were embedded in paraffin, sectioned at 4 µm thickness, and
stained as described previously (24). Briefly, the tissue slides were
dewaxed, and antigen retrieval was performed using an unmasking
solution (Vector Laboratories, Inc., Burlingame, CA, USA) boiled in
a microwave for 20 min. The slides were then incubated in 3%
hydrogen peroxide in PBS for 10 min to quench the ZEB endogenous
peroxidase. The slides were sequentially washed with distilled
water and PBS, and incubated in blocking solution (2.5% BSA in PBS)
for 30 min at room temperature. The slides were incubated with
Col3a1 primary antibody diluted in blocking solution (1:100) at 4°C
overnight. Following washing with PBS, the slides were incubated
with the secondary antibody, Alexa Fluor® 594 goat
anti-rabbit IgG (1:400; Thermo Fisher Scientific, Inc.) for 1 h at
room temperature. The slides were mounted in Prolong Gold w/DAPI
(Invitrogen; Thermo Fisher Scientific, Inc.).
DNA foci detection
Following irradiation, the cells were fixed in 2%
paraformaldehyde for 20 min at room temperature. The cells were
permeabilized with 0.5% TritonX-100 and blocked with 5% FBS in PBS.
The cells were then incubated with 53BP1 or γ-H2A.X primary
antibody overnight at 4°C in 1% BSA. The coverslips were mounted
with ProLong® Gold antifade reagent with DAPI to
counterstain the nuclei. The foci were quantified using Cell
Profiler to count cells with >3 foci per nucleus in ≥10
different fields from each experiment under confocal microscopy
(Olympus Corporation). Experimental data were determined as the
average of three independent experiments.
Chromatin immunoprecipitation (ChIP)
assay
A ChIP assay was performed based on the protocols
described in our previous study (22). The sequences for the PCR primers
of gene promoters were as follows, Col1a1 (-1,750 bp), forward
5′-TCTCCCAGGTGTCTGTCTCC-3′ and reverse 5′-AGGAGAGGGGTCTGCTGAG-3′;
Col1a1 (-1,000 bp), forward 5′-AGGGGGAAAAACTGCTTTGT-3′ and reverse
5′-TCCGACCTCTCTCCTCTGAA-3′; Col1a1 (−200 bp), forward
5′-CCACTTGGGTGTTTGAGCA-3′ and reverse 5′-CCTCCCCTCCACTCCTTC-3′;
Col3a1 promoter, forward 5′-TCTCCATTGCCAGATATAGCC-3′ and reverse
5′-TCCAGATCCTCATCCACAAA-3′. The promoter regions analyzed in the
present study for p-Smad2 enrichment included the Smad-binding
element (SBE), AGACA sequence.
Wound healing assay
SCC4 cells (2×105 cells per well) were
seeded in 6-well plate and cultured for 48 h in culture medium. A
scratch (wound) was then introduced in the confluent cell layer
using a P200 yellow tip. The cells were washed three times with
medium to remove detached cells. The cells were then incubated with
or without TGF-β (10 ng/ml) or GV1001 (1 µM) for 48 h and
images of a defined wound spot were captured with a computer-aided
phase contrast microscope (Olympus). Experiments were performed in
triplicate.
BLM-induced dermal fibrosis model
Pathogen-free, female C57BL/6 mice (6 week-old;
Jackson Laboratory, Ben Harbor, ME, USA) were used for BLM-induced
dermal fibrosis by subcutaneous injection of BLM to the shaved
upper back skin for 4 weeks. The animals were housed in a
pathogen-free environment with a 12 h light/dark cycle in the
Division of Laboratory and Animal Medicine at UCLA (Los Angeles,
CA, USA). The experiments were performed based on the protocol
approved by the Institutional Animal Care and Use Committee. A
total of 20 mice were divided into four groups: Control group,
injected subcutaneously (s.q.) with 100 µl vehicle (PBS);
BLM group, injected with 100 µl BLM solution (100
µg/ml); BLM and Low GV1001 group, injected with 1 mg/kg
GV1001 and 100 µg/ml BLM; BLM and high GV1001 group,
injected with 5 mg/kg GV1001 and 100 µg/ml BLM. Each group
included five mice. The s.q. injection continued daily for 4 weeks
and the shaved upper back skin tissues were harvested for
histological assessment of the lesions.
Statistical analysis
Statistical evaluations were performed using SPSS
version 11.0 (SPSS, Inc., Chicago, IL, USA). All numerical data
were expressed as the mean ± standard deviation. Statistical
analysis was performed using Student's t-test (two-tailed) for
quantitative experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
GV1001 suppresses IR-induced premature
senescence by reducing the genotoxic effect
Our previous study showed that exposure of primary
human keratinocytes to IR triggered a senescence response (22). To examine the effect of GV1001 on
the cellular response to IR, NHOKs were exposed to 6 Gy IR and the
cells were cultured in the presence or absence of GV1001. The
rapidly proliferating NHOKs underwent cell proliferation arrest
upon irradiation and exhibited cellular morphology consistent with
senescence, including a flattened cytosolic region and perinuclear
vacuolization (21), whereas
these senescing effects of IR were reduced in the cells treated
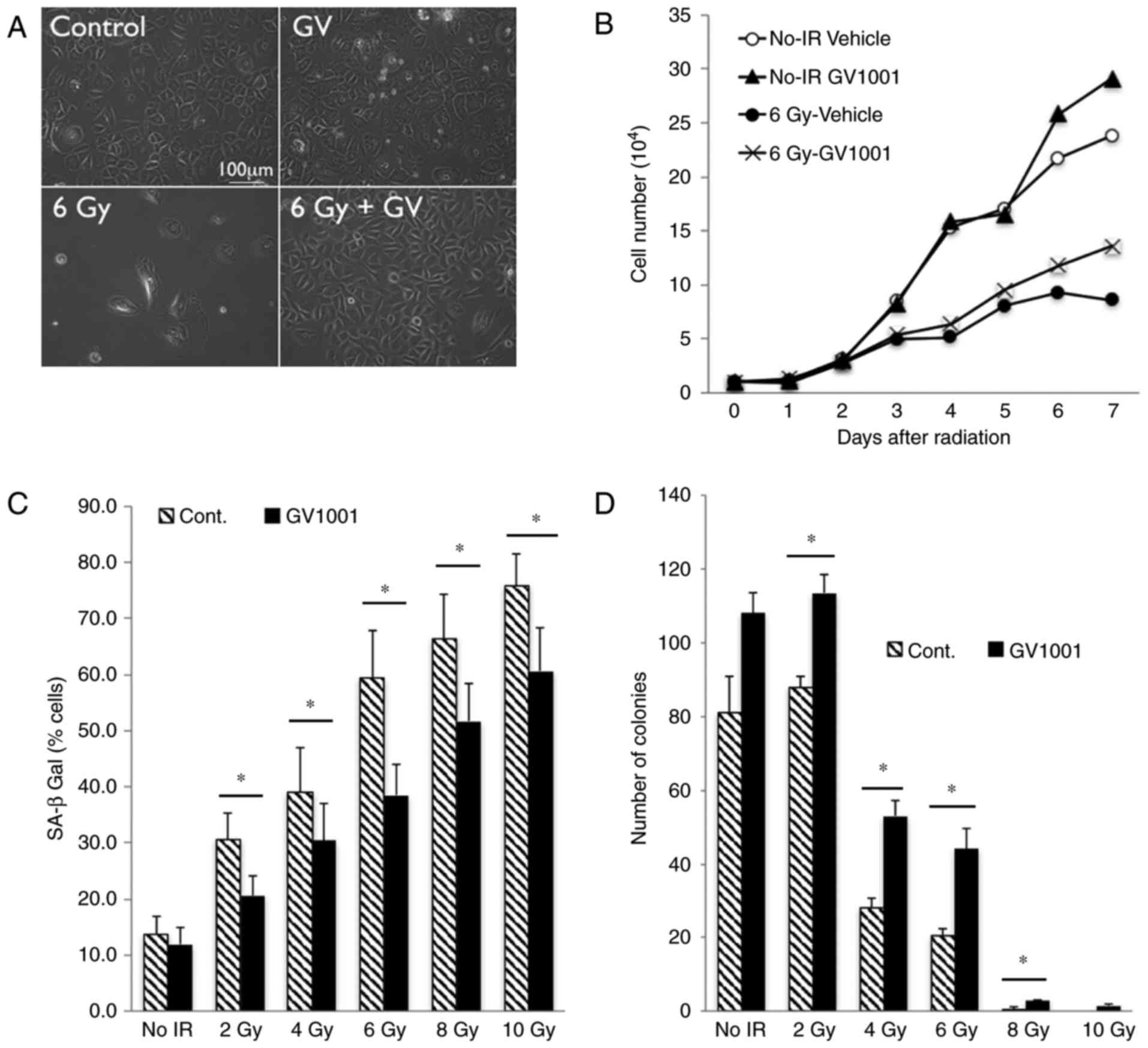
with GV1001 (Fig. 1A). While
irradiation inhibited cell proliferation, treatment of the NHOKs
with GV1001 mitigated the growth suppressive effects of IR
(Fig. 1B). With increased dose of
IR, the percentage of cells expressing SA β-Gal was increased in
the NHOKs; however, GV1001 treatment significantly reduced the
percentage of cells stained positively for SA β-Gal (Fig. 1C). Similarly, the colony forming
ability of the cultured NHOKs was suppressed by exposure to IR,
whereas this effect was mitigated by GV1001 treatment (Fig. 1D). These results demonstrated that
GV1001 treatment ameliorated IR-induced premature senescence in
cultured NHOKs.
Keratinocyte proliferation is regulated in part
through Grainyhead-like 2 (GRHL2), which is a novel transregulator
of the hTERT gene and determines epithelial phenotype through the
suppression of EMT regulators (25–27). The western blot analysis showed
that the endogenous level of GRHL2 was induced by GV1001, even
following exposure to 6 Gy IR (Fig.
2A). GV1001 treatment led to reduction in the level of N-Cad,
encoded by the CDH2 gene, which is elevated during EMT (28), consistent with the increased
expression of GRHL2. As EMT is induced by TGF-β signaling, these
data indicated the possible activation of the TGF-β pathway in
NHOKs following IR exposure. IR also led to p53 phosphorylation
(Ser15) and an increased level of p21/WAF1 cyclin-dependent kinase
inhibitor, however, these protein levels were reduced in the cells
treated with GV1001. p-Akt (Ser473), which is necessary for
radioresistance in cells by enhancing DNA double strand break (DSB)
repair (29), was elevated in the
NHOKs treated with GV1001. The level of DNA DSB in the irradiated
cells was measured directly by staining for 53BP1 and γ-H2AX, which
form fluorescent foci at the sites of DSBs (30,31), as shown in Fig. 2B. The NHOKs were irradiated with 6
Gy IR in the presence or absence of GV1001, and the percentages of
cells with intranuclear 53BP1 and γ-H2AX foci were determined at 10
days post-IR by IFS using confocal microscopy. There was elevation
of DSBs in the irradiated cells, compared with the control cells
without IR exposure, and the level of DSBs was decreased in cells
cultured with GV1001, indicating that GV1001 enhanced the removal
of DSBs in the cells (Fig. 2C).
Taken together, these data indicated that GV1001 suppressed
IR-induced premature senescence, in part via mitigating the
genotoxic effects.
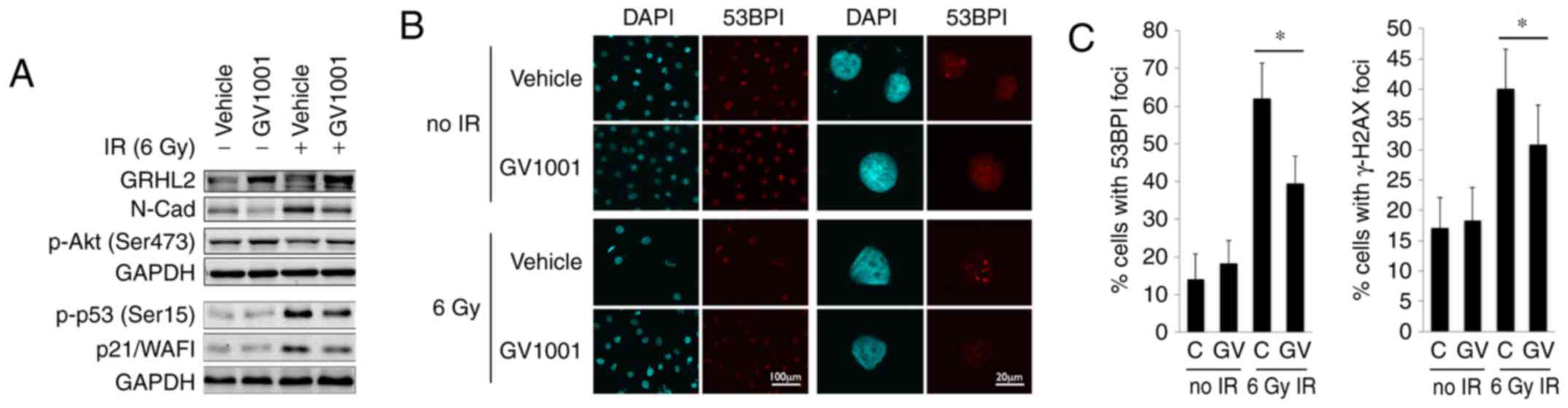 | Figure 2GV1001 treatment reduces the level of
DNA double strand breaks in NHOKs exposed to IR. (A) Western blot
analysis was performed with NHOKs 10 days following exposure to 6
Gy IR with or without GV1001 (1 µM) for GRHL2, N-Cad, p-Akt
(Ser473), p-p53 (Ser15) and p21/WAF1. GAPDH was used as a loading
control. (B) NHOKs were exposed to 6 Gy IR in the presence or
absence of GV1001 (1 µM), were stained for 53BP1 and were
viewed under confocal microscopy to detect 53BP1 intranuclear foci.
DAPI staining revealed the nuclei (original magnification of left 2
panels, ×100; scale bar, 100 µm; n=3; original magnification
of right panels, ×500; scale bar, 20 µm; n=3). (C)
Percentages of cells with 53BP1 or γ-H2AX intranuclear foci (>3
foci per nucleus) were counted in ≥10 various fields from each
experiment and plotted. Error bars represent the mean ± standard
deviation. *P<0.05. NHOKs, normal human oral
keratinocytes; IR, ionizing radiation; GRHL2, Grainyhead-like 2;
N-Cad, N-Cadherin; p-, phosphorylated. |
GV1001 inhibits TGF-β signaling
The senescent phenotype in cells exposed to IR is
regulated through the auto/paracrine activity of TGF-β (17,32). The irradiation of NHOKs also led
to the activation of TGF-β signaling molecules, inducing the
phosphorylation of Smad2/3 complex and the level of Smad4 (Fig. 3), suggesting their role in
premature senescence. In addition, the irradiated cells exhibited a
mesenchymal phenotype and expressed higher levels of TGF-β target
genes, including N-Cad, ZEB1, FN and Snail. Treatment of the cells
with GV1001 decreased the expression of these TGF-β target
mesenchymal markers and TGF-β signaling molecules, including
p-Smad2/3 and Smad4, compared with the control cells. The
expression of target proteins and signaling induced by TGF-β were
also eliminated by exposure to 1 µM TRI, which was used as
the positive control for GV1001 treatment. These data indicated
that the mesenchymal phenotype induced by IR was prevented by
GV1001 in the NHOKs, possibly through suppressing TGF-β
signaling.
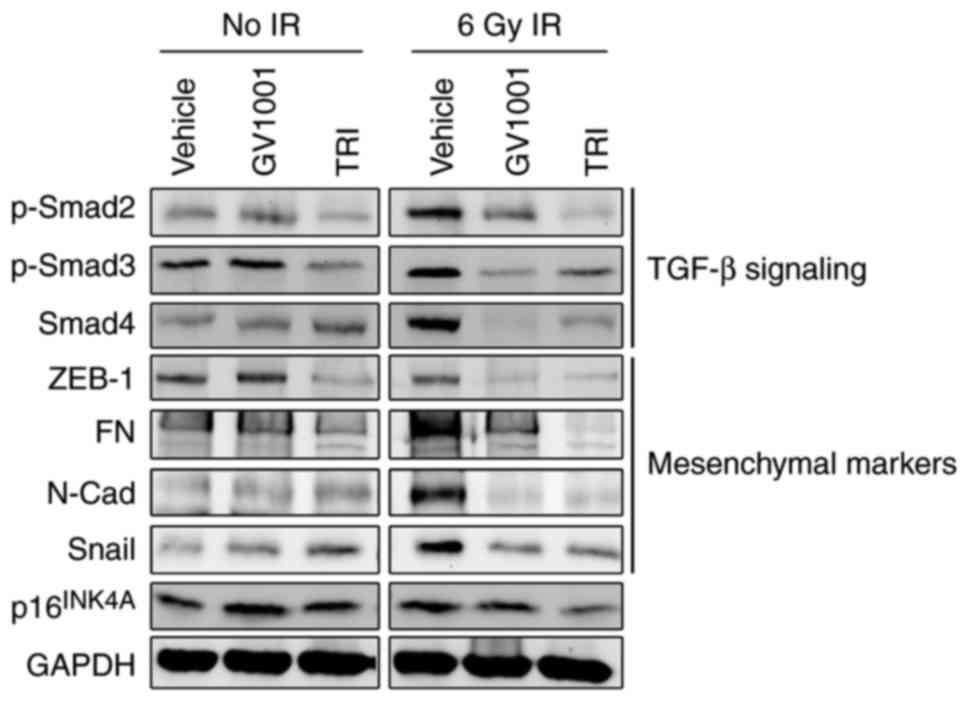 | Figure 3GV1001 suppresses TGF-β signaling and
EMT in NHOKs exposed to IR. NHOKs were exposed to 6 Gy IR and
maintained in culture for 10 days in the presence of GV1001 (1
µM) or TRI (1 µM). Western blot analysis was
performed for TGF-β signaling molecules, p-Smad2/3 and Smad4, and
TGF-β target mesenchymal markers, ZEB1, FN, N-Cad and Snail. GAPDH
was used as a loading control. NHOKs, normal human oral
keratinocytes; IR, ionizing radiation; TGF-β, transforming growth
factor-β; TRI, TGF-β receptor inhibitor; Smad, small mothers
against decapentaplegic; p-, phosphorylated; ZEB1, zinc finger
E-box binding homeobox 1; FN, fibronectin; N-Cad, N-Cadherin. |
To examine the effects of GV1001 on TGF-β signaling,
rapidly proliferating NHOKs were exposed to TGF-β in the presence
or absence of GV1001. Exposure to TGF-β for up to 10 days led to
induction of a mesenchymal phenotype through EMT (Fig. 4A). It was also found that GV1001
treatment reduced the expression of Snail and α-SMA in the cells
exposed to TGF-β, in addition to TGF-β signaling molecules,
including p-Smad2/3 and Smad 4 (Fig.
4B). TGF-β treatment also led to enhanced cell migration of the
SCC4 cell line, reminiscent of the mesenchymal phenotype, and
GV1001 almost completely prevented the enhanced migratory effects
of TGF-β (Fig. 4C). In the NHOFs,
the expression of TGF-β target molecules and mesenchymal markers,
including Col1a1, Col3a1, FN, N-Cad, ZEB1 and ZEB2, was suppressed
in the cells treated with GV1001 (Fig. 5A). The effect of GV1001 on binding
of p-Smad to the SBE was also confirmed by a ChIP assay of NHOFs
exposed to TGF-β (Fig. 5B and C).
Treatment of the cells with GV1001 markedly suppressed binding of
Smad2 on the Col1a1 and Col3a1 gene promoter regions, indicating
that GV1001 interfered with TGF-β-mediated target gene
transcription in NHOFs.
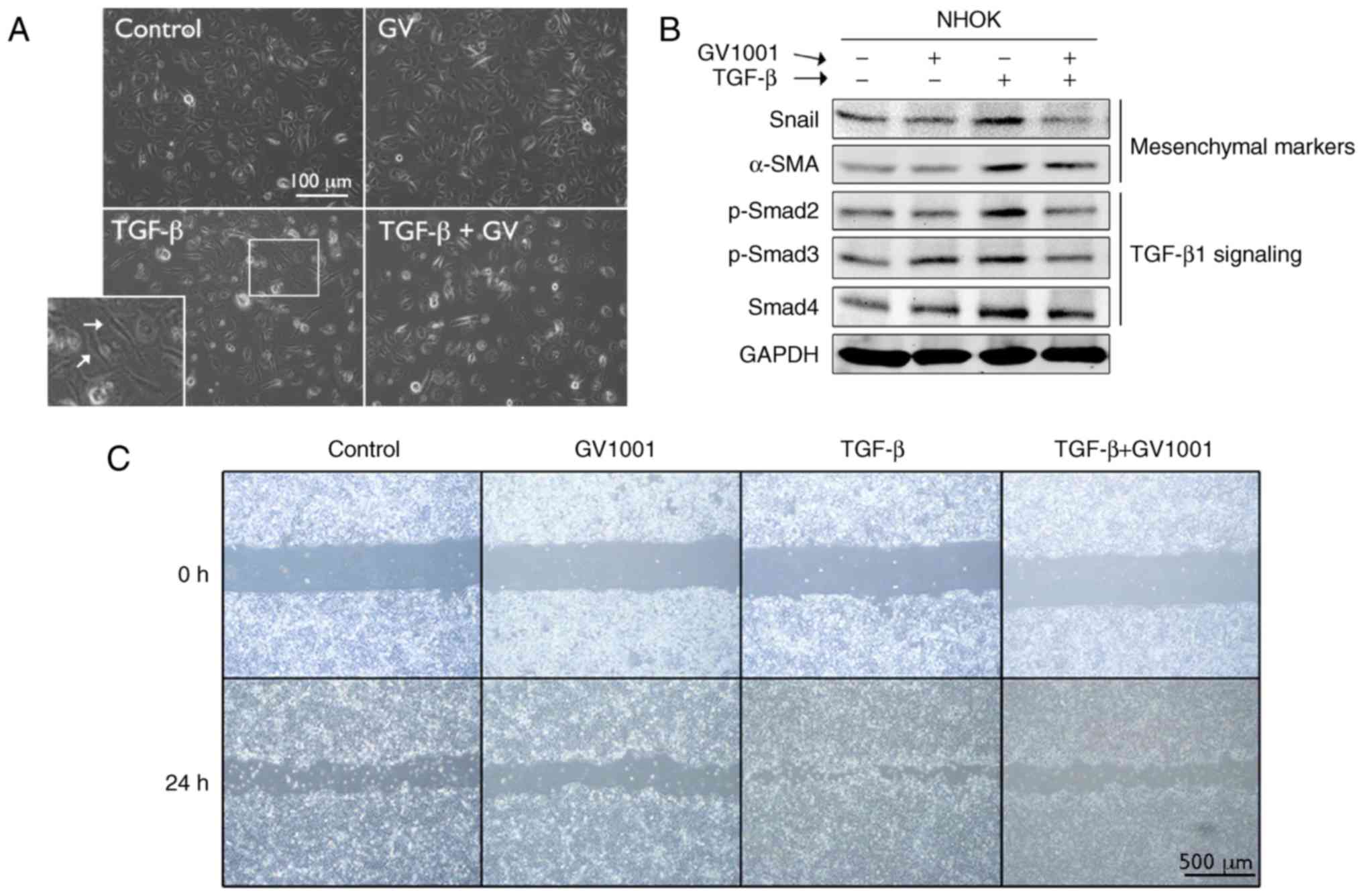 | Figure 4GV1001 suppresses TGF-β-induced EMT
in NHOKs. (A) Rapidly proliferating NHOKs were exposed to TGF-β (10
ng/ml) for 10 days to induce EMT (shown in islet, arrows). The same
cultures were also maintained in the presence or absence of GV1001
(1 µM) (original magnification, ×100; scale bar, 100
µm; n=3). (B) Western blot analysis was performed with NHOKs
exposed to TGF-β (10 ng/ml) with GV1001 (1 µM) or the
control for Snail, α-SMA, p-Smad2/3 and Smad4. GAPDH was used as a
loading control. (C) Cell migration of SCC4 cells exposed to TGF-β
(10 ng/ml) with or without GV1001 (1 µM) for 24 h (original
magnification, ×40; scale bar, 500 µm; n=3). NHOKs, normal
human oral keratinocytes; EMT, epithelial-mesencyhmal transition;
TGF-β, transforming growth factor-β; α-SMA, α-smooth muscle actin;
Smad, small mothers against decapentaplegic; p-, phosphorylated;
GV, GV1001. |
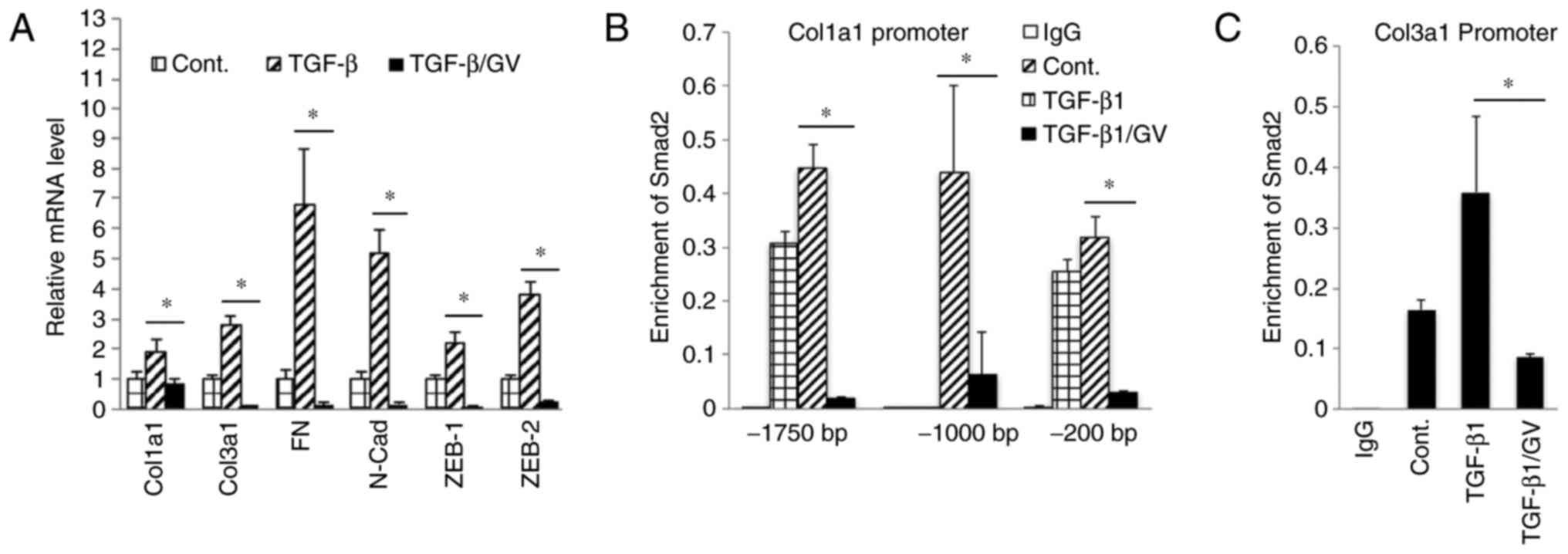 | Figure 5GV1001 inhibits Smad2 binding to
target gene promoters. (A) Reverse transcription-quantitative
polymerase chain reaction analysis was performed on NHOFs exposed
to TGF-β (10 ng/ml) with GV1001 (1 µM) or control for 10
days, for the gene expression of Col1a1, Col3a1, FN, N-Cad, Zeb1
and Zeb2. (B and C) NHOFs were cultured with 10 ng/ml TGF-β and
GV1001 (1 µM) for 10 days. Chromatin immunoprecipitation was
performed for Smad2 enrichment on Col1a1 and Col3a1 promoter
regions. Error bars indicate the mean ± standard deviation.
*P<0.05. NHOKs, normal human oral keratinocytes;
TGF-β, transforming growth factor-β; Col1a1, collagen type I α1
chain; Col3a1, collagen type III α1 chain; ZEB1, zinc finger E-box
binding homeobox 1; FN, fibronectin; N-Cad, N-Cadherin; Smad, small
mothers against decapentaplegic; Cont, control; GV, GV1001. |
GV1001 inhibits BLM-induced dermal
fibrosis
In the subsequent experiments, the effects of GV1001
in fibrotic tissue formation were determined. Whether GV1001
treatment inhibited TGF-β signaling in myofibroblast
differentiation was assessed. Rapidly proliferating NHOFs were
exposed to TGF-β, which triggered the induction of α-SMA,
specialized actin molecules found in myofibroblasts, in addition to
Col3a1, Col1a1, Snail, FN and N-Cad (Fig. 6A). These changes occurred with
marked induction of p-Smad2. However, when the cells were
co-treated with GV1001, there were marked reductions in the levels
of α-SMA, N-Cad, FN and p-Smad in the cells. Similarly, IFS
revealed induced α-SMA in the NHOFs by TGF-β treatment, in addition
to ZEB1, a mesenchymal transcription factor directly regulating the
expression of α-SMA (33),
whereas the levels of α-SMA and ZEB1 were suppressed by GV1001
treatment (Fig. 6B). These data
indicated that GV1001 inhibited TGF-β-induced myofibroblast
differentiation through the suppression of ZEB.
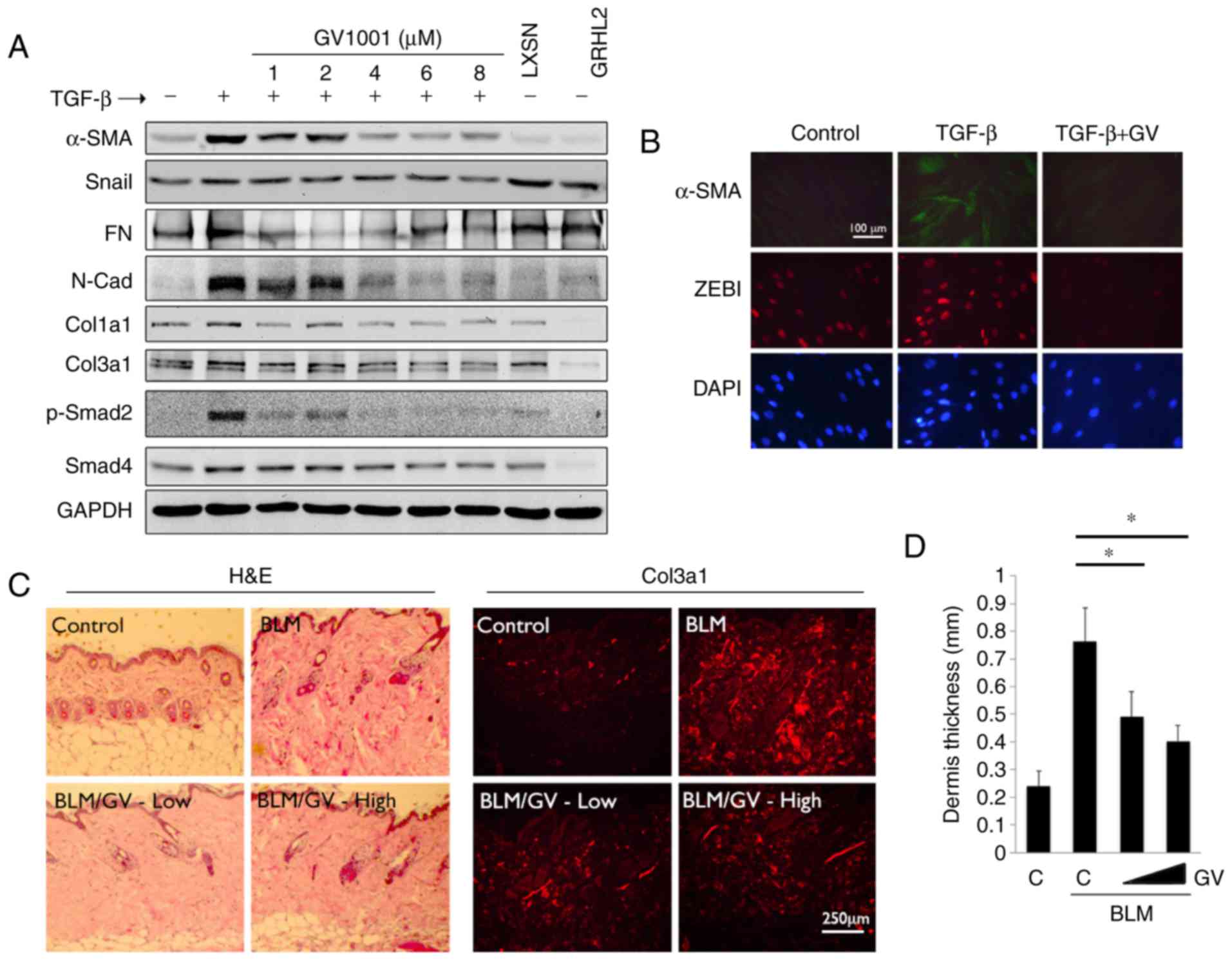 | Figure 6GV1001 attenuates the dermal fibrotic
lesions induced by BLM. (A) NHOF cultures were treated with 10
ng/ml TGF-β for 10 days and GV1001 was added at varying
concentrations between 1 and 8 µM. Western blot analysis was
performed with whole cell extracts for α-SMA, Snail, FN, N-Cad,
Col1a1 and Col3a1, and p-Smad2 and Smad4. GAPDH was used as a
loading control. NHOFs with ectopic overexpression of GRHL2 were
also included as a negative control, as GRHL2 inhibited fibrogenic
differentiation and TGF-β signaling. (B) IFS was performed for
α-SMA and ZEB1 in NHOFs treated with 10 ng/ml TGF-β and GV1001 (1
µM) for 10 days (original magnification, ×100; scale bar,
100 µm; n=3). (C) C57BL/6 mice were exposed to BLM (100
µg/ml) with or without GV1001, administered at low (1 mg/kg)
or high (5 mg/kg) doses, by daily subcutaneous injection into the
dorsal flank. Following 4 weeks of BLM injection, mice were
sacrificed for histological examination by H&E staining. IFS
was also performed with the skin biopsy samples for Col3a1
deposition in mice exposed to BLM with or without GV1001 (original
magnification, ×100; scale bar, 250 µm; n=5). (D)
Quantitation of dermal thickness was plotted in the groups with or
without BLM and GV1001 administration. Error bars indicate the mean
± standard deviation. *P<0.05. NHOKs, normal human
oral keratinocytes; BLM, bleomycin; IFS, immunofluorescence
staining; TGF-β, transforming growth factor-β; α-SMA, α-smooth
muscle actin; Col1a1, collagen type I α1 chain; Col3a1, collagen
type III α1 chain; ZEB1, zinc finger E-box binding homeobox 1; FN,
fibronectin; N-Cad, N-Cadherin; Smad, small mothers against
decapentaplegic; p-, phosphorylated; GRHL2, Grainyhead-like 2;
H&E, hematoxylin and eosin; GV, GV1001; C, control. |
To confirm the antifibrogenic property of GV1001
in vivo, dermal fibrosis was induced in C57BL/6 mice by s.q.
injection of BLM with or without co-administration of GV1001. BLM
treatment for 4 weeks led to marked dermal sclerosis in the mice,
which was characterized histologically by a thickened dermis with
increased deposition of collagen bundles (Fig. 6C). When GV1001 was administered to
these mice, there was significant reduction in the thickness of the
fibrotic lesions, and a reduction in dermal levels of Col3a1, as
determined by IF staining (Fig. 6C
and D). Therefore, GV1001 exerted antifibrotic effects in
vivo and may directly impair the fibrogenic differentiation of
dermal fibroblasts.
Discussion
The present study demonstrated a novel role of
GV1001, a 16-amino acid peptide sequence of hTERT protein,
originally developed as an immunogenic anticancer vaccine (5), in modulating the cellular responses
to IR. The irradiation of NHOKs led to suppressed cell
proliferation and EMT, possibly through the activation of TGF-β
signaling, and these phenotypic changes induced by IR were notably
reduced following GV1001 treatment. These data suggested that
GV1001 may exert its protective and anti-EMT effects through
suppressing TGF-β signaling, however, the detailed mechanism
requires further investigation. In addition, the radioprotective
effects of GV1001 reported in the present study were based on in
vitro experiments and require validation by further experiments
in vivo. The radioprotective effects of GV1001 were also
linked to elevated DNA repair activities. This was evidenced as the
increased level of p-Akt (Ser473), which has been shown to regulate
cellular radioprotection against IR (29,34), and reduced level of active
p53/p21WAF1 proteins in the NHOKs treated with GV1001. This was in
accordance with an earlier report showing the protective effects of
GV1001 against oxidative stress in neural stem cells, allowing for
continued cell proliferation and reduced cell death following
exposure to H2O2 (35). Further investigations aim to
elucidate the detailed mechanism by which GV1001 modulates the
genotoxic effects of IR.
Of note, GV1001 treatment markedly elevated the
level of GRHL2, a transcription factor with diverse biological
functions and a multitude of target genes, including hTERT,
proliferating cell nuclear antigen, p63, microRNA-200 family genes
and epidermal cell differentiation complex genes (24–26,36). GRHL2 promotes epithelial cell
proliferation in part through the transcription regulation of
hTERT; the ectopic overexpression of GRHL2 in primary NHOKs led to
marked extension of cell life span with continued expression of
hTERT and telomerase activity (25). As GV1001 is derived from the hTERT
protein sequence, the fact that GV1001 enhanced GRHL2 may indicate
a feed-forward mechanism by which it further elevates the level of
hTERT through the expression of GRHL2.
In the present study, GV1001 treatment suppressed
TGF-β signaling, including the expression of p-Smad2/3 and Smad4,
and reduced the expression of mesenchymal markers, including ZEB1,
FN, N-Cad and Snail, in NHOKs exposed to IR. These findings may be
linked to the effect that GV1001 enhanced the level of GRHL2, which
is also known to be a master regulator of epithelial phenotype,
inhibiting TGF-β-induced EMT (26,37). The present study also provided
evidence that GV1001 suppressed the fibrogenic differentiation of
NHOFs treated with TGF-β; GV1001 suppressed the phosphorylation of
Smad2 and decreased the level of α-SMA, a marker of myofibroblasts
involved in wound contraction (38). GV1001 markedly inhibited p-Smad2
binding to the Col1a1 and Col3a1 promoter regions, which may be
associated with a reduced level of tissue fibrosis. As GRHL2 is an
epithelial-specific protein, the suppressive effects of GV1001
against TGF-β signaling in NHOFs were not GRHL2-dependent, however,
the mechanisms remain to be elucidated.
Tissue fibrosis commonly occurs with terminal stages
of organ failure, which results from chronic inflammatory
reactions, including chronic wound and radiation tissue damage, and
fibrotic changes can be observed in several organ systems. One of
the core cellular constituents of fibrosis is myofibroblasts,
marked by α-SMA, which migrate into the wound area and cause wound
contraction and the secretion of extracellular matrix proteins.
Myofibroblasts were originally considered to arise from the
resident fibroblasts in local tissues upon activation (39), however, several studies have
indicated the role of adjacent epithelial and endothelial cells as
the source of myofibroblasts for tissue fibrosis through EMT
(40,41). EMT may also account for
radiation-induced tissue fibrosis in patients exposed to
therapeutic radiation. Pulmonary fibrosis in patients with lung
cancer occurs with increased α-SMA-positive cells in the lung
interstitium and EMT in alveolar epithelial cells, driven by
Forkhead Box M1 transcription factor (17,42). In addition, head/neck irradiation
in patients leads to salivary gland atrophy, termed xerostomia,
resulting in salivary gland dysfunction, primarily through fibrotic
changes (43). The involvement of
EMT for xerostomia is not documented, however, similar glandular
fibrotic tissues of the lacrimal gland have been reported to show
increased markers of EMT, including Snail, α-SMA, and reduced
E-cadherin, in patients with Mikulicz's syndrome, which causes
abnormal enlargement of glands in the head/neck area (44). Therefore, by suppressing
IR-induced EMT, GV1001 may have therapeutic benefit against the
tissue fibrosis that results from IR exposure.
Acknowledgments
The authors are thankful to the Gemvax & Kael
Co. Ltd. (Seoul, Korea) for providing the GV1001 peptide.
Abbreviations:
|
TGF-β
|
transforming growth factor-β
|
|
GRHL2
|
Grainyhead-like 2
|
|
FN
|
fibronectin
|
|
hTERT
|
human telomerase reverse
transcriptase
|
|
E-Cad
|
E-cadherin
|
|
N-Cad
|
N-cadherin
|
|
NHOF
|
normal human oral fibroblast
|
|
NHOK
|
normal human oral keratinocyte
|
|
Col1a1
|
collagen type I α1 chain
|
|
Col3a1
|
collagen type III α1 chain
|
|
IR
|
ionizing radiation
|
|
NSC
|
neural stem cell
|
|
IPF
|
idiopathic pulmonary fibrosis
|
|
EMT
|
epithelial-mesenchymal transition
|
|
SA β-Gal
|
senescence-associated
β-galactosidase
|
|
WCE
|
whole cell extract
|
|
BLM
|
bleomycin
|
|
RT
|
reverse transcription
|
Notes
[1]
Funding
This study was supported in part by grants from the
NIDCR/NIH (grant nos. 1R56DE024593 and R03DE024259), the University
of California Cancer Research Coordinating Committee (grant no.
20152529), and Gemvax & Kael Co. Ltd. (Seoul, Korea). MKK was
also supported by the Jack A. Weichman Endowed Fund.
[2] Availability
of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
WC contributed to conception, design, data
acquisition, analysis and interpretation, and critically revised
the manuscript; KHS analyzed and interpreted the in vivo
data about GV1001 inhibiting dermal fibrosis, and critically
revised the manuscript; SK contributed to conception of research
question and data interpretation; WJS interpreted data and
critically revised the manuscript; RHK contributed to conception,
data interpretation and critically revised the manuscript; NHP
interpreted data and critically revised the manuscript; MKK
contributed to conception, design, data analysis, interpretation,
drafted and critically revised the manuscript. All authors read and
approved the final manuscript.
[4] Ethics
approval and consent to participate
The animal experiments were performed based on the
protocol approved by the Institutional Animal Care and Use
Committee of UCLA. The discarded human tissues were obtained
without patient identification or medical information, under
exemption from the Institutional Review Board of UCLA.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
SK is employed at Gemvax & Kael Co. Ltd. (Seoul,
Korea) and TELOID Inc (Los Angeles, CA, USA). Other authors declare
that there are no competing interests.
References
|
1
|
Kim HR, Christensen R and Park NH, Sapp P,
Kang MK and Park NH: Elevated expression of hTERT is associated
with dysplastic cell transformation during human oral
carcinogenesis in situ. Clin Cancer Res. 7:3079–3086.
2001.PubMed/NCBI
|
|
2
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Greener M: Telomerase: The search for a
universal cancer vaccine. Mol Med Today. 6:2572000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brunsvig PF, Aamdal S, Gjertsen MK,
Kvalheim G, Markowski-Grimsrud CJ, Sve I, Dyrhaug M, Trachsel S,
Møller M, Eriksen JA and Gaudernack G: Telomerase peptide
vaccination: A phase I/II study in patients with non-small cell
lung cancer. Cancer Immunol Immunother. 55:1553–1564. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bernhardt SL, Gjertsen MK, Trachsel S,
Møller M, Eriksen JA, Meo M, Buanes T and Gaudernack G: Telomerase
peptide vaccination of patients with non-resectable pancreatic
cancer: A dose escalating phase I/II study. Br J Cancer.
95:1474–1482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kokhaei P, Palma M, Hansson L, Osterborg
A, Mellstedt H and Choudhury A: Telomerase (hTERT 611-626) serves
as a tumor antigen in B-cell chronic lymphocytic leukemia and
generates spontaneously antileukemic, cytotoxic T cells. Exp
Hematol. 35:297–304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nicholls C, Li H, Wang JQ and Liu JP:
Molecular regulation of telomerase activity in aging. Protein Cell.
2:726–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang JE, Kim HJ, Yi E, Jheon S and Kim K:
Reduction of ischaemia-reperfusion injury in a rat lung
transplantation model by low-concentration GV1001. Eur J
Cardiothorac Surg. 50:972–979. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi J, Kim H, Kim Y, Jang M, Jeon J,
Hwang YI, Shon WJ, Song YW, Kang JS and Lee WJ: The
anti-inflammatory effect of GV1001 mediated by the downregulation
of ENO1-induced pro-inflammatory cytokine production. Immune Netw.
15:291–303. 2015. View Article : Google Scholar
|
|
10
|
Ko YJ, Kwon KY, Kum KY, Lee WC, Baek SH,
Kang MK and Shon WJ: The anti-inflammatory effect of human
telomerase-derived peptide on P. gingivalis
lipopolysaccharide-induced inflammatory cytokine production and its
mechanism in human dental pulp cells. Mediators Inflamm.
2015:3851272015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim BK, Kim BR, Lee HJ, Lee SA and Kim BJ,
Kim H, Won YS, Shon WJ, Lee NR, Inn KS and Kim BJ:
Tumor-suppressive effect of a telomerase-derived peptide by
inhibiting hypoxia-induced HIF-1α-VEGF signaling axis.
Biomaterials. 35:2924–2933. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park JK, Kim Y, Kim H, Jeon J, Kim TW,
Park JH, Hwnag YI, Lee WJ and Kang JS: The anti-fibrotic effect of
GV1001 combined with gemcitabine on treatment of pancreatic ductal
adenocarcinoma. Oncotarget. 7:75081–75093. 2016.PubMed/NCBI
|
|
13
|
Kim H, Choi MS, Inn KS and Kim BJ:
Inhibition of HIV-1 reactivation by a telomerase-derived peptide in
a HSP90-dependent manner. Sci Rep. 6:288962016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee SA, Kim J, Sim J, Kim SG, Kook YH,
Park CG, Kim HR and Kim BJ: A telomerase-derived peptide regulates
reactive oxygen species and hepatitis C virus RNA replication in
HCV-infected cells via heat shock protein 90. Biochem Biophys Res
Commun. 471:156–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park HH, Lee KY, Kim S, Lee JW, Choi NY,
Lee EH, Lee YJ, Lee SH and Koh SH: Novel vaccine peptide GV1001
effectively blocks β-amyloid toxicity by mimicking the
extra-telomeric functions of human telomerase reverse
transcriptase. Neurobiol Aging. 35:1255–1274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bitterman PB and Henke CA:
Fibroproliferative disorders. Chest. 99(3 Suppl): 81S–84S. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Judge JL, Owens KM, Pollock SJ, Woeller
CF, Thatcher TH, Williams JP, Phipps RP, Sime PJ and Kottmann RM:
Ionizing radiation induces myofibroblast differentiation via
lactate dehydrogenase. Am J Physiol Lung Cell Mol Physiol.
309:L879–L887. 2015.PubMed/NCBI
|
|
18
|
Decologne N, Kolb M, Margetts PJ,
Menetrier F, Artur Y, Garrido C, Gauldie J, Camus P and Bonniaud P:
TGF-beta1 induces progressive pleural scarring and subpleural
fibrosis. J Immunol. 179:6043–6051. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anscher MS, Crocker IR and Jirtle RL:
Jirtle, transforming growth factor-beta 1 expression in irradiated
liver. Radiat Res. 122:77–85. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Franko AJ, Sharplin J, Ghahary A and
Barcellos-Hoff MH: Immunohistochemical localization of transforming
growth factor beta and tumor necrosis factor alpha in the lungs of
fibrosis-prone and 'non-fibrosing' mice during the latent period
and early phase after irradiation. Radiat Res. 147:245–256. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang MK, Bibb C, Baluda MA, Rey O and Park
NH: In vitro replication and differentiation of normal human oral
keratinocytes. Exp Cell Res. 258:288–297. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong Q, Oh JE, Chen W, Kim R, Kim RH, Shin
KH, McBride WH, Park NH and Kang MK: Radioprotective effects of
Bmi-1 involve epigenetic silencing of oxidase genes and enhanced
DNA repair in normal human keratinocytes. J Invest Dermatol.
131:1216–1225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Larionov A, Krause A and Miller W: A
standard curve based method for relative real time PCR data
processing. BMC Bioinformatics. 6:622005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen W, Xiao Liu Z, Oh JE, Shin KH, Kim
RH, Jiang M, Park NH and Kang MK: Grainyhead-like 2 (GRHL2)
inhibits keratinocyte differentiation through epigenetic mechanism.
Cell Death Dis. 3:e4502012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen W, Dong Q, Shin KH, Kim RH, Oh JE,
Park NH and Kang MK: Grainyhead-like 2 enhances the human
telomerase reverse transcriptase gene expression by inhibiting DNA
methylation at the 5′-CpG island in normal human keratinocytes. J
Biol Chem. 285:40852–40863. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen W, Yi JK, Shimane T, Mehrazarin S,
Lin YL, Shin KH, Kim RH, Park NH and Kang MK: Grainyhead-like 2
regulates epithelial plasticity and stemness in oral cancer cells.
Carcinogenesis. 37:500–510. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cieply B, Farris J, Denvir J, Ford HL and
Frisch SM: Epithelial-mesenchymal transition and tumor suppression
are controlled by a reciprocal feedback loop between ZEB1 and
Grainyhead-like-2. Cancer Res. 73:6299–6309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma T, Zhao Y, Wei K, Yao G, Pan C, Liu B,
Xia Y, He Z, Qi X, Li Z, et al: MicroRNA-124 functions as a tumor
suppressor by regulating CDH2 and epithelial-mesenchymal transition
in non-small cell lung cancer. Cell Physiol Biochem. 38:1563–1574.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holler M, Grottke A, Mueck K, Manes J,
Jücker M, Rodemann HP and Toulany M: Dual Targeting of Akt and
mTORC1 impairs repair of DNA double-strand breaks and increases
radiation sensitivity of human tumor cells. PLoS One.
11:e01547452016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Borodkina A, Shatrova A, Abushik P,
Nikolsky N and Burova E: Interaction between ROS dependent DNA
damage, mitochondria and p38 MAPK underlies senescence of human
adult stem cells. Aging (Albany NY). 6:481–495. 2014. View Article : Google Scholar
|
|
31
|
Vandevoorde C, Vral A, Vandekerckhove B,
Philippé J and Thierens H: Radiation sensitivity of human CD34(+)
cells versus peripheral blood T lymphocytes of newborns and adults:
DNA Repair and Mutagenic Effects. Radiat Res. 185:580–590. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liakou E, Mavrogonatou E, Pratsinis H,
Rizou S, Evangelou K, Panagiotou PN, Karamanos NK, Gorgoulis VG and
Kletsas D: Ionizing radiation-mediated premature senescence and
paracrine interactions with cancer cells enhance the expression of
syndecan 1 in human breast stromal fibroblasts: The role of TGF-β.
Aging (Albany NY). 8:1650–1669. 2016. View Article : Google Scholar
|
|
33
|
Chang YC, Tsai CH, Lai YL, Yu CC, Chi WY,
Li JJ and Chang WW: Arecoline-induced myofibroblast
transdifferentiation from human buccal mucosal fibroblasts is
mediated by ZEB1. J Cell Mol Med. 18:698–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park HS, You GE, Yang KH, Kim JY, An S,
Song JY, Lee SJ, Lim YK and Nam SY: Role of AKT and ERK pathways in
controlling sensitivity to ionizing radiation and adaptive response
induced by low-dose radiation in human immune cells. Eur J Cell
Biol. 94:653–660. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park HH, Yu HJ, Kim S, Kim G, Choi NY, Lee
EH, Lee YJ, Yoon MY, Lee KY and Koh SH: Neural stem cells injured
by oxidative stress can be rejuvenated by GV1001, a novel peptide,
through scavenging free radicals and enhancing survival signals.
Neurotoxicology. 55:131–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mehrazarin S, Chen W, Oh JE, Liu ZX, Kang
KL, Yi JK, Kim RH, Shin KH, Park NH and Kang MK: The p63 gene is
regulated by grainyhead-like 2 (GRHL2) through reciprocal feedback
and determines the epithelial phenotype in human keratinocytes. J
Biol Chem. 290:19999–20008. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cieply B, Riley P IV, Pifer PM, Widmeyer
J, Addison JB, Ivanov AV, Denvir J and Frisch SM: Suppression of
the epithelial-mesenchymal transition by Grainyhead-like-2. Cancer
Res. 72:2440–2453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Penke LR, Huang SK, White ES and
Peters-Golden M: Prostaglandin E2 inhibits α-smooth muscle actin
transcription during myofibroblast differentiation via distinct
mechanisms of modulation of serum response factor and
myocardin-related transcription factor-A. J Biol Chem.
289:17151–17162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hinz B: The role of myofibroblasts in
wound healing. Curr Res Transl Med. 64:171–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Willis BC, duBois RM and Borok Z:
Epithelial origin of myofibroblasts during fibrosis in the lung.
Proc Am Thorac Soc. 3:377–382. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zeisberg EM, Tarnavski O, Zeisberg M,
Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT,
Roberts Ab, et al: Endothelial-to-mesenchymal transition
contributes to cardiac fibrosis. Nat Med. 13:952–961. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Balli D, Ustiyan V, Zhang Y, Wang IC,
Masino AJ, Ren X, Whitsett JA, Kalinichenko VV and Kalin TV: Foxm1
transcription factor is required for lung fibrosis and
epithelial-to-mesenchymal transition. EMBO J. 32:231–244. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saleh J, Figueiredo MA, Cherubini K and
Salum FG: Salivary hypofunction: An update on aetiology, diagnosis
and therapeutics. Arch Oral Biol. 60:242–255. 2015. View Article : Google Scholar
|
|
44
|
Fukui M, Ogawa Y, Shimmura S, Hatou S,
Ichihashi Y, Yaguchi S, Hirayama M, Kawakita T and Tsubota K:
Possible involvement of epithelial-mesenchymal transition in
fibrosis associated with IgG4-related Mikulicz's disease. Mod
Rheumatol. 25:737–743. 2015. View Article : Google Scholar : PubMed/NCBI
|