Introduction
Neuropathic pain syndromes, i.e., pain after a
lesion or disease of the peripheral or central nervous system
(CNS), are clinically characterised by spontaneous pain (ongoing
pain, paroxysms) and evoked types of pain (hyperalgesia, allodynia)
(1). Previous studies have
focused on the relationship between the neurotransmitters involved
in neuropathic pain and modulators such as serotonin, opioids and
adenosine (2–4). Recently, increasing evidence
indicates that spinal microglia contribute to the establishment and
maintenance of central sensitisation in chronic neuropathic pain
(5). The activation of microglia
stimulates the synthesis and release of brain-derived neurotrophic
factor (BDNF) (6), which
modulates neuronal excitability within the spinal cord and plays an
indispensable role in central sensitisation (7). BDNF mediates its action through
various intracellular signalling pathways triggered by the
activation of tyrosine kinase receptor B (TrkB) (8). Electro-acupuncture (EA) is widely
accepted as a viable therapeutic intervention for chronic pain
treatment (9,10); however, the biological basis and
the precise mechanism that underlies the analgesic effect of EA
remain unknown. In traditional Chinese medicine, the Zusanli
(ST-36) acupoint of the 'Stomach Meridian of Foot-Yangming' and the
Yanglingquan (GB-34) acupoint of the 'Gall Bladder Meridian of
Foot-Shaoyang' are commonly used to treat neuropathic pain
(11,12). The aim of this study was to
investigate whether the analgesic effect of EA is associated with
the following mechanisms: i) the inhibition of the activation of
spinal microglia; ii) the disruption of the BDNF-TrkB signalling
pathway.
Materials and methods
Animals
The Institutional Animal Care and Use Committee of
Wenzhou Medical University approved all experiments, which were
performed according to the guidelines of the National Institutes of
Health Guide for the Care and Use of Laboratory Animals (NIH
Publications no. 80-23, revised 1978). All efforts were made to
minimise the number of animals used and their suffering. Male
Sprague Dawley rats, which weighed 200–250 g, were used in this
study. All animals were housed in plastic boxes at a temperature of
22–24°C and were provided ad libitum food and water under a
12 h reversed light-dark cycle.
Chronic constriction injury model
As the most commonly used animal model of
neuropathic pain, the chronic constriction injury (CCI) model,
which was generated based on previously described methods, was
implemented to investigate the questions asked in this study
(13). Briefly, male rats were
anaesthetised with 4% chloral hydrate (10 ml/kg, i.p.); the
adequacy of the anaesthesia was verified based on a lack of
response to a nociceptive stimulus. The right sciatic nerve was
exposed at the mid-thigh level and approached to the sciatic
trifurcation; four ligature knots (4-0 chromic gut) were loosely
tied, with ~1 mm between the knots. The knots slightly constricted
the nerve; however, they did not interrupt the circulation through
the epineural vasculature. For the rats in the sham CCI group, the
sciatic nerve was exposed for 2–3 min without ligatures. The rats
were randomly divided into 4 groups, including the normal group,
sham CCI group, CCI group and CCI plus EA group. There were 15
rats/group. The mechanical withdrawal threshold (MWT) and the
thermal withdrawal latency (TWL) of every rat were measured as an
assessment of nociception prior to the CCI operation and at days 3,
5, 7, 10, 12 and 14 after the CCI operation. The behavioural
testing was conducted between 14:00 and 18:00.
MWT
The MWT was measured using a 2392 Electronic von
Frey Anesthesiometer (IITC Life Science, Woodland Hills, CA, USA)
to evaluate mechanical allodynia. The animals were individually
placed inside wire-mesh-bottom cages (20×14×16 cm) and allowed to
adapt for 30 min prior to testing. The plantar surface of the paw
was stimulated with a series of ascending von Frey filaments that
ranged from 0.1 to 70 g until the rat twitched its paw. The maximum
force was recorded at which the animal briskly lifted its hind paw.
Each rat was tested 6 times at intervals of 5 min, and the average
value was used as the MWT.
TWL
A 37370 plantar test apparatus (Ugo-Basile, Milan,
Italy) was used to measure the TWL to estimate thermal
hyperalgesia. The rats were placed in a transparent, square,
bottomless acrylic box (17×11.5×14 cm). After 15 min, the radiant
heat was set to 60°C and was applied via a direct beam of light to
the foot pad of each hind paw through a glass plate. The light beam
turned off automatically when the rat lifted its paw. The cut-off
time for the heat stimulation was 40 sec. Each hind paw was
alternately tested at 10 min intervals. Each rat was tested 5
times, and the average value for the withdrawal time was used as
the TWL.
EA stimulation
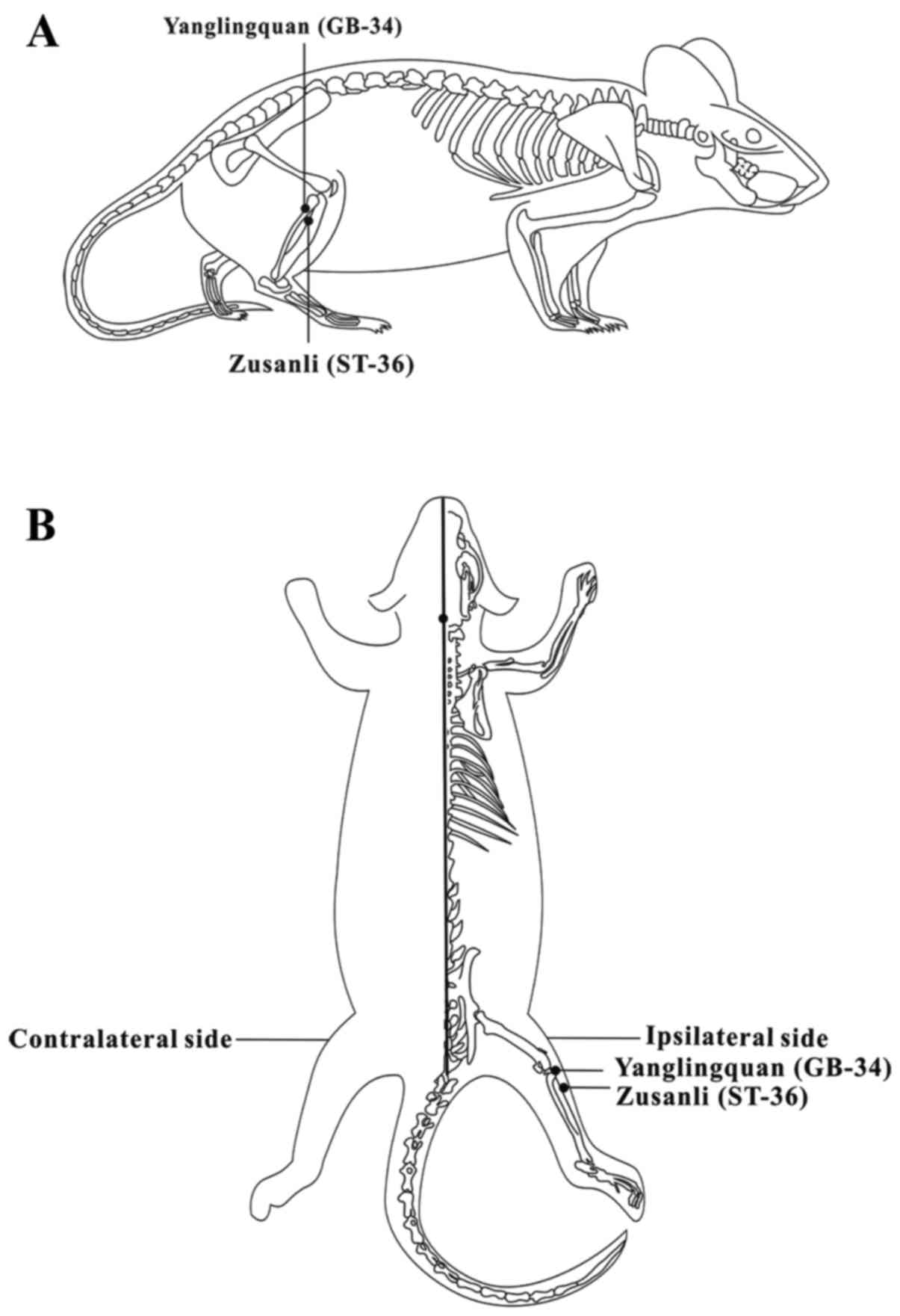
One needle was placed at the Zusanli acupoint
(ST-36), which was located 5 mm beneath the capitulum fibulae and
lateral posterior to the knee-joint, and another needle was placed
at the Yanglingquan acupoint (GB-34), which was located on the gall
bladder meridian and was ~5 mm superior-lateral to ST-36 (14,15) (Fig.
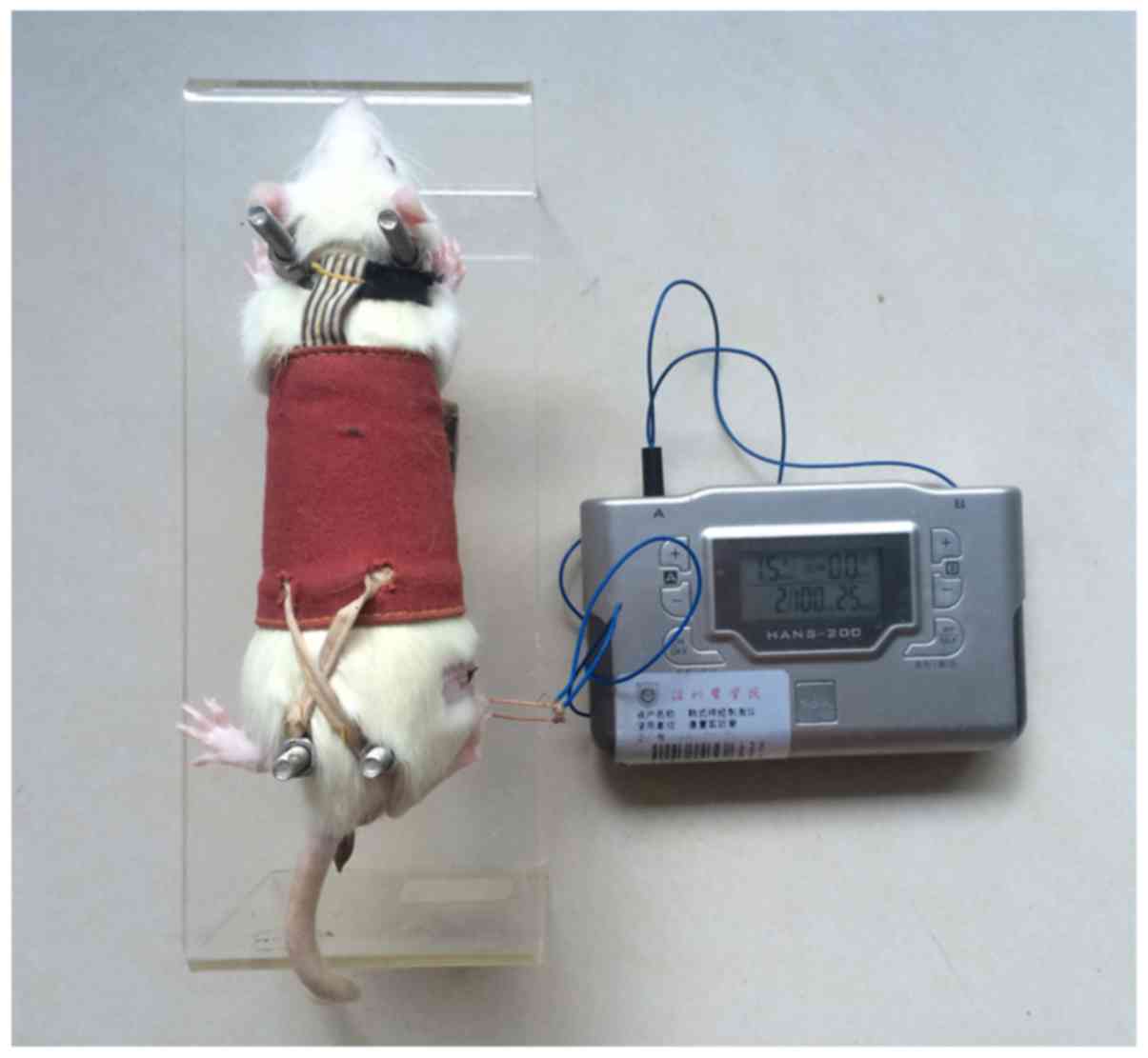
1). The rats were allowed to recover for 7 days; EA started on
the eighth day after the CCI surgery and lasted for 7 days. EA was
performed between 09:00 and 11:00 every day. The rats were
restrained in an immobilisation apparatus designed by our
laboratory (patent application no. 201110021482.5; State
Intellectual Property Office) without anaesthesia; the apparatus is
convenient for acupuncture research and comfortable for the
experimental rats, thereby reducing their stress (16,17) (Fig.
2). Stainless steel needles were inserted at ST-36 and GB-34 at
a depth of 2-3 mm. A recent study indicated that alternating
stimulation at low (2 Hz) and high (100 Hz) frequencies (referred
to as 2/100 EA) elicits a synergistic analgesic effect (18). The intensity of EA was determined
by observing slight shrinkage in the muscle, which was ~1.5 mA.
Therefore, stimulation (current of 2/100 Hz, 1.5 mA) was delivered
using an electrical stimulation device (HANS-200E; Jisheng Medical
Instruments, Nanjing, China) for 30 min daily.
Immunofluorescence
On day 14, the rats were deeply anaesthetised as
previously described and perfused via the aorta with 200 ml of
normal saline, followed by 250 ml of 4% paraformaldehyde in 0.1 M
phosphate-buffered saline (PBS, pH 7.4). The L4-L6 segments of the
spinal cord were excised and fixed. Paraffin sections (5 µm
thick) were mounted on poly-L-lysine-coated slides for
immunofluorescence. They were deparaffinised with xylene and
rehydrated through a graded ethanol series. After washing, the
sections were blocked with 3% H2O2 for 10 min
and treated with a sodium citrate buffer at 95°C for antigen
retrieval for 20 min. After blocking in 10% normal goat serum in
0.01 M PBS with 0.3% Triton X-100 for 1 h at room temperature, the
sections were incubated overnight at 4°C with rabbit anti-ionised
calcium binding adaptor molecule 1 (Iba1) (a microglia marker,
1:200, 019-19741; Wako, Osaka, Japan) and sheep anti-BDNF (1:100,
ab75040; Abcam, Cambridge, MA, USA). Following the primary antibody
incubation, the sections were incubated for 30 min at 37°C with the
following secondary antibodies: fluorescein (FITC)-conjugated
AffiniPure goat anti-rabbit IgG (1:200, BS10950; Bioworld,
Minneapolis, MN, USA) and DyLight 594-labelled donkey anti-sheep
IgG (1:200, ab96941; Abcam). The sections were subsequently stained
with diamidino-2-phenylindole dihydrochloride (DAPI) (1:1,000;
Beyotime Corp., Shanghai, China). Finally, the sections were washed
with PBS, and coverslips were mounted onto the slides using
antifade mounting medium (Beyotime Corp.). The specific
distributions of BDNF and Iba1 expression within the spinal cord
sections were determined with a fluorescence microscope (Olympus,
Tokyo, Japan).
Quantitative real-time PCR
At the end of EA treatment, 5 randomly-selected rats
from each group were deeply anesthetised with 4% chloral hydrate
and perfused via the aorta with 400 ml of normal saline. The lower
lumbar enlargement (L4-6) region of the spinal cord was separated.
Total RNA (1 µg) was extracted from the tissue using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). First-Strand cDNA was
synthesised by a reverse transcriptase kit (A3500; Promega,
Madison, WI, USA) according to the manufacturer's instructions.
Real-time amplification, using SYBR-Green Supermix (QPK-212; Toyobo
Corporation, Osaka, Japan) and a LightCycler 480 system (Roche,
Indianapolis, IN, USA), was performed using the following
sequences: Iba1, sense, GGATGGGATCAACAAG CACT and antisense,
TCCATTGCCATTCAGATCAA; BDNF, sense, CTTGGAGAAGGAAACCGCCT and
antisense, GTCC ACACAAAGCTCTCGGA; TrkB, sense, GCTTCTGGAGGG
CTTCTCTT and antisense, TGTTCTCTGGGTCAATGCTG; and RPS16, sense,
AAGTCTTCGGACGCAAGAAA and anti-sense, TTGCCCAGAAGCAGAACAG. The qPCR
conditions were as follows: 95°C for 5 min, followed by 40 cycles
of 95°C for 10 sec, 60°C for 10 sec, and 72°C for 10 sec. All
samples were performed in triplicate. RPS16 was used as an internal
control. The relative expression levels were analysed using the
2−ΔΔCt method with the relative expression software tool
(19).
Western blotting
The rats were deeply anesthetised as previously
described on day 14 after the CCI operation. A laminectomy was
rapidly performed to expose the lumbar spinal cord, and the L4-L6
segment was excised. The segments were immediately stored at −80°C
for further analysis. The cell lysates were lysed in fresh RIPA
protein lysis buffer that contained (RIPA: PMSF=100:1) on ice. The
samples were subsequently incubated for 30 min and centrifuged at
15,294 × g for 5 min at 4°C, and the supernatant was collected. A
BCA protein assay kit (Beyotime Corp.) was used to determine the
protein concentration of each sample. The homogenate was heated to
100°C for 10 min and centrifuged again at 15,294 × g for 1 min.
Equal amounts (80 µg) of protein were subjected to 8 and 12%
Tris-HCl sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) gel (Bio-Rad Laboratories, Hercules, CA, USA) separation
for 30 min at 70 V and 60 min at 120 V. After electrophoresis, the
proteins were transferred onto PVDF membranes (Millipore Corp.,
Billerica, MA, USA) at 300 mA for immunoblotting. After blocking
with 5% skim milk for 2 h at room temperature, the membranes were
incubated overnight at 4°C with the following primary antibodies:
rabbit anti-BDNF (1:1,000, ab108319; Abcam); rabbit anti-TrkB
(phospho-Tyr705) (1:1,000, orb106262; Biorbyt, Cambridge, UK);
rabbit anti-TrkB (1:700, ab33655; Abcam); and rabbit
anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:5,000,
AP0063; Bioworld). After three washes in TBST, the membranes were
subsequently incubated with a horseradish peroxidase-conjugated
goat anti-rabbit IgG (1:5,000, BL003A; Biosharp, St. Louis, MO,
USA) for 2 h at room temperature. Protein bands were visualised
using an enhanced chemiluminescence (ECL) kit (Beyotime Corp.). The
band density was quantified via detection with a DNR
micro-chemiluminescence gel imaging system (DNR Bio-Imaging
Systems, Jerusalem, Israel). Moreover, each band density was
normalised to the density of GAPDH.
Statistical analysis
SPSS 16.0 software was used for the statistical
analysis. A statistical evaluation of the data was performed using
a one-way analysis of variance (ANOVA) (P<0.05, P<0.01 and
P<0.001, followed by post hoc comparisons using the LSD or
Kruskal-Wallis method. Data from the mechanical allodynia and
thermal hyperalgesia tests were analysed via repeated-measure
tests. All experimental data are expressed as the mean ± standard
deviation (SD), and P<0.05 indicates a statistically significant
difference.
Results
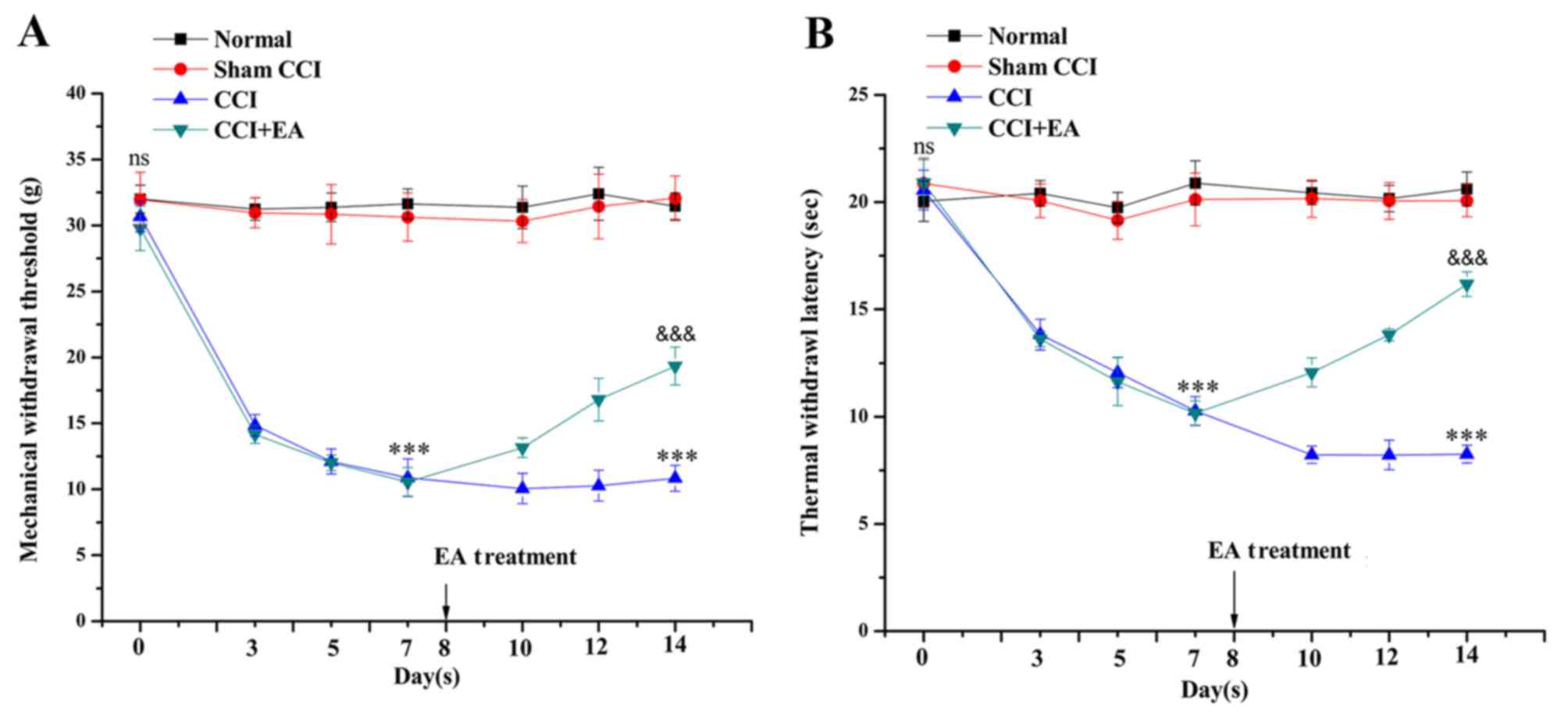
EA treatment reduces mechanical allodynia
and thermal hyperalgesia in CCI rats
In this study, we investigated the potential
efficacy of EA treatments in a rat model of CCI with regard to
neuropathic pain. To determine the time-course of the changes in
hyperalgesia and allodynia, baseline measurements were obtained
prior to surgery. The baseline measures of the MWT and TWL on both
hind paws did not differ among the four groups. In the CCI group,
the MWT was markedly reduced after surgery, from 30.6±1.15 to
10.9±1.42 g, compared with the values in the normal and sham groups
(P<0.001). The TWL was reduced from 21.9±0.87 to 10.2±0.67 sec
on day 7 (P<0.001). On day 14, the MWT and TWL values of the
CCI+EA group were significantly increased compared with the CCI
group (P<0.001). These findings imply that EA treatment
increases the mechanical and thermal pain thresholds in rats
suffering from neuropathic pain following CCI surgery (Fig. 3).
Inhibitory effect of EA on the activation
of microglia and BDNF expression in the spinal cord of CCI rats
demonstrated by immunofluorescence
The activation of microglia and BDNF expression in
the L4-L6 segment of the spinal dorsal horn was assessed via
immunofluorescence. An increased number of activated microglia was
identified accompanied by an upregulated expression of BDNF, which
was mainly expressed in microglia. In addition, we also identified
an increased level of BDNF in the neurons of the CCI group, which
was released by activated microglia. However, CCI-induced
activation of microglia and a high expression of BDNF were
significantly inhibited by EA treatment (Fig. 4).
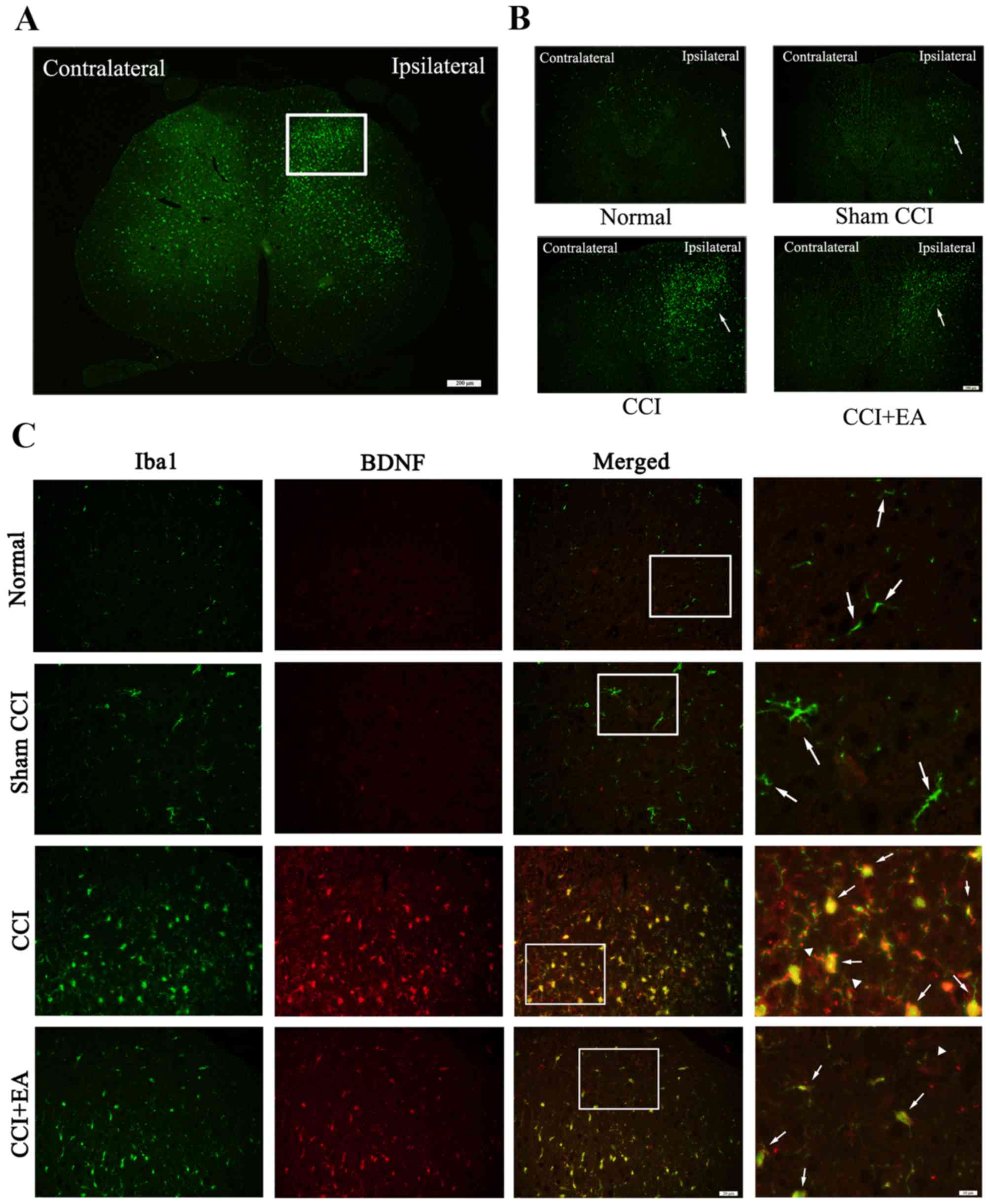 | Figure 4Chronic constrictive injury (CCI)
induces microglia activation subsequent to the release of microglia
brain-derived neurotrophic factor (BDNF). (A) White rectangle was
the dorsal horn of the injured side of the spinal cord,
immunofluorescence showed the amount of ionised calcium binding
adaptor molecule 1 ionised calcium binding adaptor molecule 1
(Iba1) in the injured ipsilateral side relative to the
contralateral, Iba1 was the marker for microglia. Scale bar, 200
µm. (B) Compare the expression of Iba1 in the injury
ipsilateral side among the four groups, Scale bar, 100 µm.
(C) Representative photomicrographs of immunofluorescence showed
colocalization of Iba1 (green, the arrows stand for
Iba1+ cells, represent activated microglia) and BDNF
(red, the arrows represent BDNF+ cells released from
activated microglia, the white triangle represent the BDNF
expressed in neurons in the spinal dorsal horns). Scale bar, 20
µm. The right side of the column; scale bar, 10
µm. |
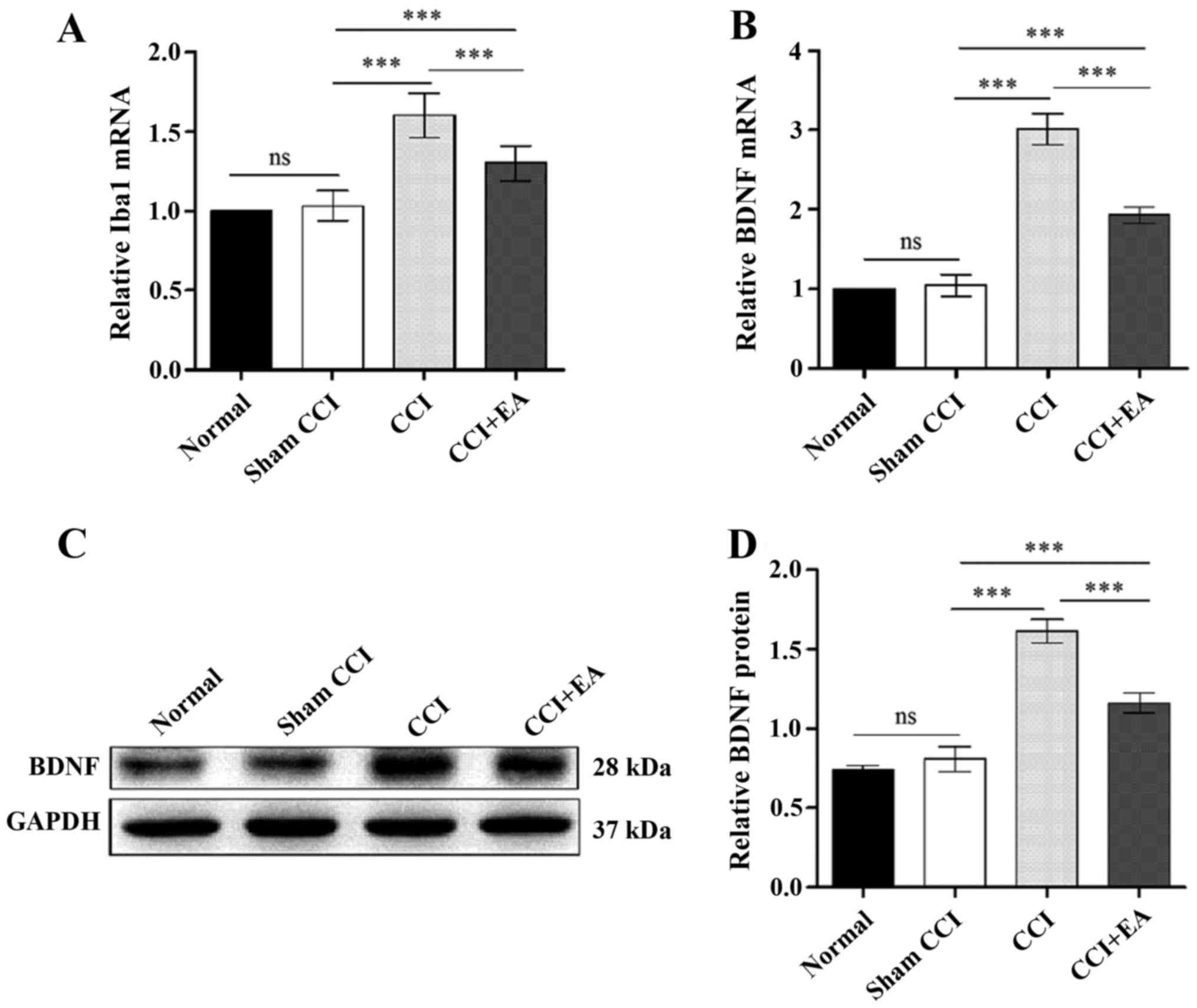
EA treatment downregulates spinal
microglia activation and BDNF expression at the mRNA and protein
levels
To assess the role of microglia and BDNF in the
spinal cord, qPCR analyses of microglia and BDNF were performed in
each group. The qPCR analysis of the rats with CCI-induced
neuropathic pain indicated significant decrease in the levels of
microglia and BDNF in the CCI+EA rats compared with the CCI rats
(P<0.001) (Fig. 5A and B). The
BDNF expression at the protein level was analysed via western
blotting, which indicated a strong increase in BDNF after the CCI
operation. The relative optical density (ROD) value for the BDNF
protein expression in the CCI group was significantly increased
compared with in the normal, sham CCI and EA groups (P<0.001)
(Fig. 5C and D), and EA treatment
decreased the BDNF expression in the EA group. Taken together,
these findings suggest a direct link between the effects of EA
treatment and microglia activation and BDNF expression in CCI
rats.
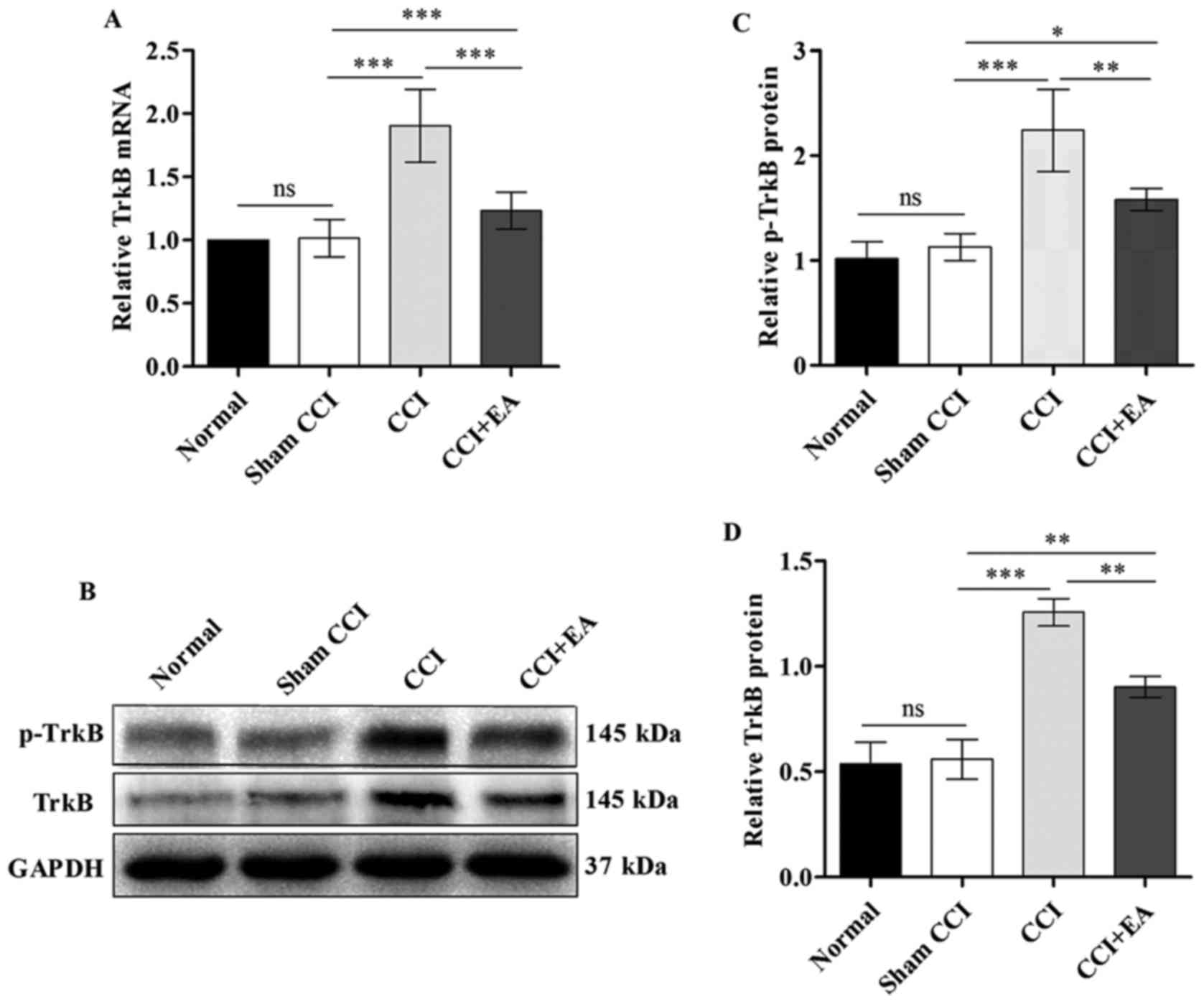
EA attenuates the overexpression of TrkB
at both the mRNA and protein levels
A substantial decrease in TrkB mRNA expression was
identified in the spinal cord after EA relative to the expression
in the CCI rats (P<0.001) (Fig.
6A). The TrkB expression at the protein level in the spinal
cord was analysed via western blotting. The ROD for the p-TrkB and
TrkB protein expression in the CCI group were significantly
increased compared with the normal and sham CCI groups
(P<0.001). Similar to the results of qPCR analysis, the level of
p-TrkB and TrkB protein was significantly decreased after EA
treatment (P<0.01) (Fig.
6B–D). These findings indicate that EA significantly inhibits
the expression of TrkB at both the mRNA and protein levels.
Discussion
Acupuncture has been used for more than 3,000 years
in China and is generally regarded as a safe and effective method
to alleviate pain in humans and experimental animals (20–23). EA treatments apply various levels
of stimulating currents to acupoints through acupuncture needles.
EA is widely practiced worldwide; however, the biological basis of
the analgesic effects of EA on neuropathic pain remains unclear.
Various neurotransmitters and neuromodulators, including primarily
opioid peptides, glutamate, norepinephrine, serotonin,
g-amino-butyric acid and adenosine have been suggested to be
responsible for the beneficial effects of EA on neuropathic pain
(23,24).
CCI rats have been demonstrated to exhibit
behavioural signs of spontaneous pain and hyperalgesia as a result
of noxious thermal and mechanical stimuli (23). In this study, mechanical allodynia
and thermal hyperalgesia persisted in the CCI group; however, all
rats in the group began to exhibit signs of pain relief after 7
days of EA treatment at the ST-36 and GB-34 acupoints. This finding
indicates acupuncture may provide an effective treatment for pain
and has potential in clinical applications.
One of the most significant advances in pain
research was the finding that neurons are not the only cell type
involved in the aetiology of chronic pain. This discovery caused a
radical shift from the previous dogma that neuronal dysfunction
alone accounted for pain pathologies to a novel type of thinking,
in which all cell types within the CNS should be considered with
respect to their involvement in neuropathic pain (25). In recent years, additional studies
focused on this topic have suggested a significant role of
microglia in the maintenance of normal neuronal physiology in the
CNS. The activation of microglia has been associated with symptoms
of neuropathic pain such as allodynia or hyperalgesia (26). Accumulated evidence suggests that
spinal microglia are involved in the modulation of chronic pain and
EA analgesia (27,28). Choi et al demonstrated that
acupuncture not only relieves mechanical allodynia and thermal
hyperalgesia but also decreases the proportion of activated
microglia at the L4-5 when applied at the Shuigou (GV26) and
Yanglingquan (GB-34) acupoints following SCI-induced neuropathic
pain (29). Shan et al
provided the first indication that repeated EA significantly blocks
the activation of spinal microglia and reduces the release of
proinflammatory cytokines and other pain-enhancing substances in
the spinal cord, which suggests that the anti-allodynic and
anti-hyperalgesic effects of EA may be associated with its capacity
to inhibit spinal microglia activation (30).
Ionised calcium binding adaptor molecule 1 (Iba1)
comprises a sensitive marker associated with activated microglia
(31). In this study, we
demonstrated that EA treatment increased the MWT and TWL values.
Furthermore, our immunofluorescence-based analysis indicated that
the CCI-induced activation of microglia was significantly inhibited
by EA treatment.
Activated microglia secrete various biologically
active signalling molecules, including BDNF, which is a crucial
molecule for signalling between microglia and neurons. The
inhibition of this microglia-neuron signalling pathway may prevent
tactile allodynia (32). Many
studies have indicated that microglia are closely associated with
the actions of BDNF; thus, the stimulation of BDNF release appears
to be caused by activated microglia. These actions contributed to
the establishment and maintenance of central sensitisation in
chronic neuropathic pain. The present data illustrate that the
proportion of activated microglia and the BDNF protein level were
both increased in the spinal dorsal horn in association with
pathological pain. However, EA significantly reversed this
phenomenon by decreasing the activation of microglia and BDNF
expression, thereby attenuating the hyperalgesia and allodynia
associated with neuropathic pain.
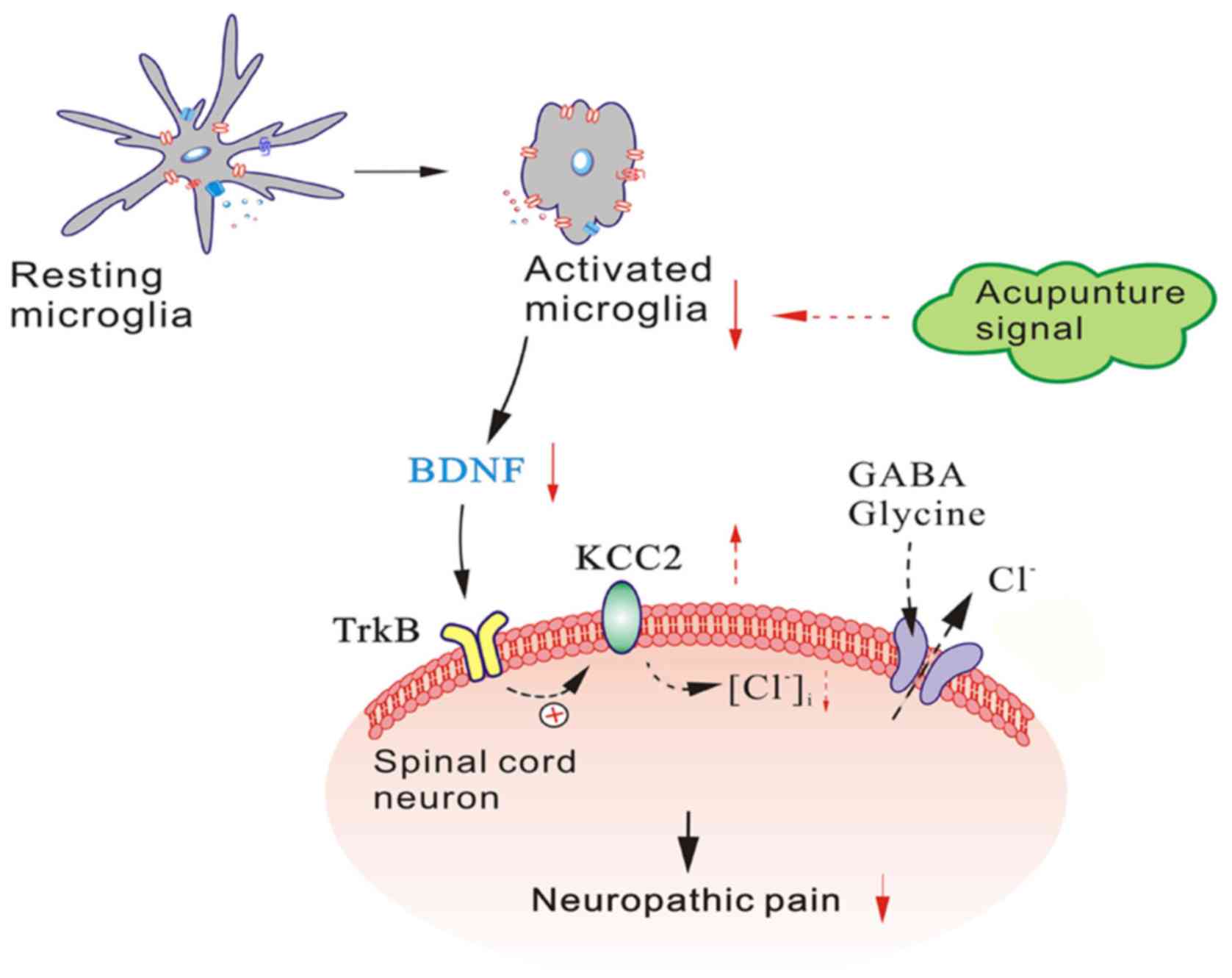
Furthermore, recent studies have indicated that BDNF
exerts its effect by binding to its high affinity receptor TrkB,
and that the BDNF-TrkB pathway may be an important early step in
the transition from normal to pathophysiological processing in the
spinal dorsal horn (33). Both
in vivo and in vitro studies, have demonstrated that
the activation of the BDNF-TrkB pathway leads to a downregulation
of potassium-chloride co-transporter (KCC2) expression in neurons
(32,34–36). The expression of KCC2, the main
Cl− transporter in spinal lamina I neurons, is
downregulated by BDNF, which causes an increase in intracellular
[Cl−] within these cells (35). As a result, the opening of γ-amino
butyric acid (GABA)A or glycine channels becomes less effective in
producing inhibition, and in approximately one-third of lamina I
neurons, GABA-evoked responses are converted from hyperpolarising
to depolarising responses (32,37,38).
In this study, the expression of TrkB in the L4-L6
segment was examined via western blotting and qPCR analysis, which
demonstrated that EA treatment decreased expression of p-TrkB and
TrkB receptors. These data further support the notion that
BDNF-TrkB signalling pathway interactions mediated by spinal
microglia play a crucial role in the development of the
hyperalgesia and allodynia associated with chronic pain. Based on
these findings, we suspect that EA may relieve neuropathic pain via
the regulation of this signalling pathway (Fig. 7). In future studies, we intend to
apply pharmacological, gene knock-out and other methods to further
test and verify this hypothesis.
In conclusion, we demonstrated that mechanical
allodynia and thermal hyperalgesia appear to be attenuated by EA
via its analgesia effects. Excessive spinal microglia activation
induced by nerve injury, which activates the BDNF-TrkB signalling
pathway and maintains the pathophysiological processes that lead to
neuropathic pain, is substantially inhibited by EA. However,
further verification of the overall effects and the underlying
molecular mechanism of EA in animals with peripheral nerve injuries
is necessary.
Acknowledgments
The experiments were carried out with the help of
the Scientific Research Center, The Second Affiliated Hospital of
Wenzhou Medical University.
Notes
[1]
Funding
This study was supported by a grant from the Natural
Science Foundation of Zhejiang Province (LY16H270016) and the
Foundation of Wenzhou Scientific and Technological Bureau Project
(Y20140221).
[2] Availability
of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
WZT, SHJ and conceived and designed the experiments.
SSL and XJ performed the experiments. XRQ, GHY and PPG analyzed the
data and created the images. WZT and SSL wrote the paper. BL and
SHJ contributed to the modification of the manuscript. All authors
read and approved the final manuscript.
[4] Ethics
approval and consent to participate
The Institutional Animal Care and Use Committee of
Wenzhou Medical University approved all experiments, which were
performed according to the guidelines of the National Institutes of
Health Guide for the Care and Use of Laboratory Animals (NIH
Publications no. 80-23, revised 1978).
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Baron R: Neuropathic pain: A clinical
perspective. Handb Exp Pharmacol. 194:3–30. 2009. View Article : Google Scholar
|
|
2
|
Goettl VM, Huang Y, Hackshaw KV and
Stephens RL Jr: Reduced basal release of serotonin from the
ventrobasal thalamus of the rat in a model of neuropathic pain.
Pain. 99:359–366. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith HS: Opioids and neuropathic pain.
Pain Physician. 15(Suppl 3): ES93–ES110. 2012.PubMed/NCBI
|
|
4
|
Ren W, Tu W, Jiang S, Cheng R and Du Y:
Electroacupuncture improves neuropathic pain: Adenosine, adenosine
5′-triphosphate disodium and their receptors perhaps change
simultaneously. Neural Regen Res. 7:2618–2623. 2012.PubMed/NCBI
|
|
5
|
Tu W, Wang W, Xi H, He R, Gao L and Jiang
S: Regulation of neurotrophin-3 and interleukin-1beta and
inhibition of spinal glial activation contribute to the analgesic
effect of electroacu-puncture in chronic neuropathic pain states of
rats. Evid Based Complement Alternat Med. 2015:6420812015.
View Article : Google Scholar
|
|
6
|
Tsuda M, Beggs S, Salter MW and Inoue K:
Microglia and intractable chronic pain. Glia. 61:55–61. 2013.
View Article : Google Scholar
|
|
7
|
Zhang X, Xu Y, Wang J, Zhou Q, Pu S, Jiang
W and Du D: The effect of intrathecal administration of glial
activation inhibitors on dorsal horn BDNF overexpression and hind
paw mechanical allodynia in spinal nerve ligated rats. J Neural
Transm Vienna. 119:329–336. 2012. View Article : Google Scholar
|
|
8
|
Pandya CD, Kutiyanawalla A and Pillai A:
BDNF-TrkB signaling and neuroprotection in schizophrenia. Asian J
Psychiatr. 6:22–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xing GG, Liu FY, Qu XX, Han JS and Wan Y:
Long-term synaptic plasticity in the spinal dorsal horn and its
modulation by electroacupuncture in rats with neuropathic pain. Exp
Neurol. 208:323–332. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SK, Park JH, Bae SJ, Kim JH, Hwang BG,
Min BI, Park DS and Na HS: Effects of electroacupuncture on cold
allodynia in a rat model of neuropathic pain: Mediation by spinal
adrenergic and serotonergic receptors. Exp Neurol. 195:430–436.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lau WK, Lau YM, Zhang HQ, Wong SC and Bian
ZX: Electroacupuncture versus celecoxib for neuropathic pain in rat
SNL model. Neuroscience. 170:655–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun S, Cao H, Han M, Li TT, Zhao ZQ and
Zhang YQ: Evidence for suppression of electroacupuncture on spinal
glial activation and behavioral hypersensitivity in a rat model of
monoarthritis. Brain Res Bull. 75:83–93. 2008. View Article : Google Scholar
|
|
13
|
Fox A, Kesingland A, Gentry C, McNair K,
Patel S, Urban L and James I: The role of central and peripheral
Cannabinoid1 receptors in the antihyperalgesic activity of
cannabinoids in a model of neuropathic pain. Pain. 92:91–100. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang C, Li HT, Shi YS, Han JS and Wan Y:
Ketamine potentiates the effect of electroacupuncture on mechanical
allodynia in a rat model of neuropathic pain. Neurosci Lett.
368:327–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao YH, Wang JY, Qiao LN, Chen SP, Tan LH,
Xu QL and Liu JL: NK cells mediate the cumulative analgesic effect
of electroacupuncture in a rat model of neuropathic pain. BMC
Complement Altern Med. 14:3162014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tu WZ, Cheng RD, Cheng B, Lu J, Cao F, Lin
HY, Jiang YX, Wang JZ, Chen H and Jiang SH: Analgesic effect of
electroacupuncture on chronic neuropathic pain mediated by P2X3
receptors in rat dorsal root ganglion neurons. Neurochem Int.
60:379–386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang WS, Tu WZ, Cheng RD, He R, Ruan LH,
Zhang L, Gong YS, Fan XF, Hu J, Cheng B, et al: Electroacupuncture
and A-317491 depress the transmission of pain on primary afferent
mediated by the P2X3 receptor in rats with chronic neuropathic pain
states. J Neurosci Res. 92:1703–1713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Silva JR, Silva ML and Prado WA: Analgesia
induced by 2- or 100-Hz electroacupuncture in the rat tail-flick
test depends on the activation of different descending pain
inhibitory mechanisms. J Pain. 12:51–60. 2011. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Filshie J: The non-drug treatment of
neuralgic and neuropathic pain of malignancy. Cancer Surv.
7:161–193. 1988.PubMed/NCBI
|
|
21
|
Wong JY and Rapson LM: Acupuncture in the
management of pain of musculoskeletal and neurologic origin. Phys
Med Rehabil Clin N Am. 10:531–545. vii–viii. 1999.PubMed/NCBI
|
|
22
|
Qin Z, Liu X, Yao Q, Zhai Y and Liu Z:
Acupuncture for treating sciatica: A systematic review protocol.
BMJ Open. 5:e0074982015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao ZQ: Neural mechanism underlying
acupuncture analgesia. Prog Neurobiol. 85:355–375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen S, Wang S, Rong P, Wang J, Qiao L,
Feng X, Liu J and Zhang J: Acupuncture for visceral pain: neural
substrates and potential mechanisms. Evid Based Complement Alternat
Med. 2014:6095942014. View Article : Google Scholar
|
|
25
|
Trang T, Beggs S and Salter MW:
Brain-derived neurotrophic factor from microglia: A molecular
substrate for neuropathic pain. Neuron Glia Biol. 7:99–108. 2011.
View Article : Google Scholar
|
|
26
|
Mika J, Zychowska M, Popiolek-Barczyk K,
Rojewska E and Przewlocka B: Importance of glial activation in
neuropathic pain. Eur J Pharmacol. 716:106–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Inoue K, Tsuda M and Tozaki-Saitoh H: Role
of the glia in neuropathic pain caused by peripheral nerve injury.
Brain Nerve. 64:1233–1239. 2012.In Japanese. PubMed/NCBI
|
|
28
|
Liang LL, Yang JL, Lü N, Gu XY, Zhang YQ
and Zhao ZQ: Synergetic analgesia of propentofylline and
electroacupuncture by interrupting spinal glial function in rats.
Neurochem Res. 35:1780–1786. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi DC, Lee JY, Lim EJ, Baik HH, Oh TH
and Yune TY: Inhibition of ROS-induced P38MAPK and ERK activation
in microglia by acupuncture relieves neuropathic pain after spinal
cord injury in rats. Exp Neurol. 236:268–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shan S, Qi-Liang MY, Hong C, Tingting L,
Mei H, Haili P, Yan-Qing W, Zhi-Qi Z and Yu-Qiu Z: Is functional
state of spinal microglia involved in the anti-allodynic and
anti-hyperalgesic effects of electroacupuncture in rat model of
monoarthritis? Neurobiol Dis. 26:558–568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Narita M, Yoshida T, Nakajima M, Narita M,
Miyatake M, Takagi T, Yajima Y and Suzuki T: Direct evidence for
spinal cord microglia in the development of a neuropathic pain-like
state in mice. J Neurochem. 97:1337–1348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Coull JA, Beggs S, Boudreau D, Boivin D,
Tsuda M, Inoue K, Gravel C, Salter MW and De Koninck Y: BDNF from
microglia causes the shift in neuronal anion gradient underlying
neuropathic pain. Nature. 438:1017–1021. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang W, Liu LY and Xu TL: Reduced
potassium-chloride co-transporter expression in spinal cord dorsal
horn neurons contributes to inflammatory pain hypersensitivity in
rats. Neuroscience. 152:502–510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rivera C, Voipio J, Thomas-Crusells J, Li
H, Emri Z, Sipilä S, Payne JA, Minichiello L, Saarma M and Kaila K:
Mechanism of activity-dependent downregulation of the
neuron-specific K-Cl cotransporter KCC2. J Neurosci. 24:4683–4691.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coull JA, Boudreau D, Bachand K, Prescott
SA, Nault F, Sík A, De Koninck P and De Koninck Y: Trans-synaptic
shift in anion gradient in spinal lamina I neurons as a mechanism
of neuropathic pain. Nature. 424:938–942. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Biggs JE, Lu VB, Stebbing MJ,
Balasubramanyan S and Smith PA: Is BDNF sufficient for information
transfer between microglia and dorsal horn neurons during the onset
of central sensitization? Mol Pain. 6:442010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kahle KT, Staley KJ, Nahed BV, Gamba G,
Hebert SC, Lifton RP and Mount DB: Roles of the cation-chloride
cotransporters in neurological disease. Nat Clin Pract Neurol.
4:490–503. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cordero-Erausquin M, Coull JA, Boudreau D,
Rolland M and De Koninck Y: Differential maturation of GABA action
and anion reversal potential in spinal lamina I neurons: Impact of
chloride extrusion capacity. J Neurosci. 25:9613–9623. 2005.
View Article : Google Scholar : PubMed/NCBI
|