Introduction
Cytomegalovirus (CMV) is the most significant cause
of developmental disorders associated with intrauterine infection
in humans, potentially resulting in hearing loss (1–3).
Only 10–15% of children with congenital CMV infection exhibit
clinical signs at birth, although even children who appear
asymptomatic at birth are at risk of neurodevelopmental sequelae
(2,4). Sensorineural hearing loss (SNHL)
occurs in symptomatic and asymptomatic CMV infections (5,6).
It was reported that the progression of CMV brain infection in
neonatal mice pivots on innate and adaptive immune responses
(7). A previous study by our
group indicated that cleaved caspase-1 and downstream inflammatory
factors, including interleukin (IL)-1β and IL-18, were activated in
CMV-infected cochleae (8).
However, little is known regarding the factors that initiate the
inflammatory process in SNHL induced by CMV.
In the inner ear, the spiral ganglion neurons serve
the important function of conveying electric signals to the brain
(9). Inflammation may also damage
the spiral ganglion neurons (SGN) of the inner ear through the
round window membrane and cause SNHL (10). In addition, Schachtele et
al (1) have reported that
cochlear SGN apoptosis occurred in neonatal mice with murine (m)
CMV infection, leading to SNHL. However, the mechanisms underlying
the induction of this apoptotic process of SGN have remained
elusive.
Studies on viral brain infection in adult and
neonatal mice suggest that recruitment of neutrophils and
macrophages is the primary innate defense mechanism against CMV
(11). However, these immune
cells may also be harmful to the inner ear by production of
reactive oxygen species (ROS) (1). Substantial evidence has suggested
that ROS has an important role in the pathogenesis of inflammation
and tissue injury (12,13).
Intracellular ROS generation may induce the
activation of the nucleotide-binding oligomerization domain-like
receptor protein 3 (NLRP3) inflammasome in response to a variety of
cellular stressors (14). The
NLRP3 inflammasome acts as a molecular platform through inducing
the maturation of pro-inflammatory cytokines, including IL-1β and
IL-18 (15,16). A number of studies have indicated
that the NLRP3 inflammasome, which consists of NLRP3,
apoptosis-associated speck-like protein containing a
carboxy-terminal caspase recruitment domain (ASC) and caspase-1, is
associated with cell dysfunction. It has been demonstrated that
NLRP3 inflammasome activation is critical for the cell damage under
various circumstances (17).
However, it has remained elusive whether CMV activates the NLRP3
inflammasome through ROS in the whole inner ear and SGN.
In the present study, a model of hearing loss was
established in mice inoculated with murine (M)CMV early in the
postnatal period to observe whether the MCMV-induced hearing loss
is based on the generation of ROS to induce inflammation in
vivo and in cultured SGN. The results indicated that CMV
induces SNHL, at least in part, via this mechanism.
Materials and methods
Reagents
Anti-NLRP3 (1:1,000 dilution; cat. no. 19771-1-AP)
antibody was purchased from ProteinTech Group, Inc. (Chicago, IL,
USA). Anti-ASC antibody (1:1,000 dilution; cat. no. ST1121) and
anti-caspase-1 (p20) antibody (1:1,000 dilution; cat. no. AP1043)
were purchased from Chemicon (EMD Millipore, Billerica, MA, USA).
Anti-β-actin antibody (1:10,000 dilution; cat. no. 13E5) was
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA),
and pro-IL-18 and IL-18 (1:1,000 dilution; cat. no. BAF-1520)
pro-IL-1β and IL-1β (1:1,000 dilution; cat. no. AF-401-SP)
antibodies were purchased from R&D Systems (Minneapolis, MN,
USA). Anti-NeuN (1:500 dilution; cat. no. ab177487) and ROS
inhibitor N-acetyl-L-cysteine (NAC; 1:1,000 dilution; cat. no.
ab143032) were purchased from Abcam (Cambridge, UK). ELISA kits for
IL-1β/18 were purchased from USCN Life Sciences, Inc. (Wuhan,
China).
Virus and animals
MCMV (Smith strain) was provided by Dr Meng Hong
(Medical College of Shandong University, Jinan, China). Specific
identification of the virus was described previously (18). MCMV was replicated in NIH3T3 cells
(American Type Culture Collection, Manassas, VA, USA) cultured with
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), streptomycin
(300 g/ml) and penicillin (300 U/ml). The supernatant of the
MCMV-infected NIH3T3 cells was centrifuged at 1,600 × g for 10 min
at 4°C. Viral stock titers were determined using 3T3 cells as 50%
tissue culture infective doses (2,000 pfu) per milliliter. MCMV was
stored as aliquots at −80°C until use.
Murine mouse model
Newborn BALB/c mice (within 24 h of birth, weight
1.5–2 g, n=80) were divided into two groups (each group had 40
mice, 1:1 male to female ratio). MCMV suspension (50% tissue
culture infective dose=2,000 pfu/ml, 15 ml) was slowly injected
into the cerebral hemisphere of mice using a micro syringe along
the sagittal suture, with a depth of 2–3 mm (19). Following injection, the injected
group and the control group mice were separated into two cages and
fed by hand to mimic the maternal feeding, and were maintained at
room temperature under a 12 h light/dark cycle and a humidity of
50–60%. Mice were observed for their growth and developmental
status daily (19). Mice were
supplied by the Animal Center at Xuzhou Medical University (Xuzhou,
China). Animal experiments were performed in accordance with a
protocol approved by the Ethics Committee of the Experimental
Animal Center at Xuzhou Medical University (Xuzhou, China).
Evaluation of hearing loss
Hearing loss was evaluated at 3 weeks of age by
measuring the auditory brainstem responses (ABRs). Mice were
anesthetized with 100 mg/kg ketamine hydrochloride (INN ketamine)
and 10 mg/kg xylazine, and intubated with a 22-gauge catheter, and
artificially ventilated (Hallowell EMC, Pittsfield, MA, USA)
through an intraperitoneal injection. Click stimuli were generated
and ABR waveforms were recorded in decreasing 1 dB intervals from a
90 dB sound pressure level until a threshold was reached (no
waveforms were visualized).
Primary culture and identification of
SGN
Ten neonatal BALB/c mice were immersed in 75%
ethanol after receiving anesthesia by inhalation of ethyl ether.
The bilateral temporal bones were removed, and the spiral ganglion
tissue was dissected from the stria vascularis and basilar
membrane. The tissue was then digested with 0.25% trypsin (without
EDTA) (cat. no. A610609; Sangon Biotech Co., Ltd., Shanghai, China)
for 30 min at 37°C. After the samples were centrifuged for 8 min at
1,600 × g, the cells were resuspended and placed in 35-mm cell
culture plates (2×104 cells) coated with poly-L-lysine.
Cells were maintained in DMEM/F12 (GE Healthcare, Little Chalfont,
UK), supplemented with 20% FBS, 10% neurotrophic factor B27 and 1%
penicillin-streptomycin, and placed in an incubator with 5%
CO2 and 95% air at 37°C. Cytarabine (5 µmol/ml)
was used to purify the SGN for 72 h. NF-200 (1:500 dilution; cat.
no. ab177487; Abcam) was the primary antibody added at 4°C for 24
h, followed by the Alexa Fluor 488 goat anti-mouse IgG (H+L)
secondary antibody at 37°C for 30 min (1:500 dilution; cat. no.
A11032; Thermo Fisher Scientific, Inc.) in order to identify
neurons.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA of mouse cochlea was extracted using
TRIzol reagent according to the manufacturer's protocol (Tiangen
Biotech, Beijing, China). Complementary DNA (cDNA) was synthesized
from 1 mg total RNA using the ImProm-II™ Reverse Transcription
System (Promega Corp., Madison, WI, USA). PCR amplification of the
cDNA and quantification was then performed using SYBR Green
(Stratagene; Agilent Technologies, Inc., Santa Clara, CA, USA). The
PCR conditions for the Mx3000P QPCR System (Stratagene) were as
follows: 40 denaturation cycles at 95°C for 10 sec, annealing at
60°C for 10 sec and elongation at 72°C for 10 sec. The relative
product levels were quantified using the 2(-Delta Delta C(T))
method (20). Data presented are
representative of three independent experiments. β-actin as
internal standard was arbitrarily assigned a value of 1.0. The
sequence-specific primers used for qPCR were as follows: IL-18
sense, 5′-CCAAGGAAATCGGCCTCTAT-3′ and antisense,
5′-TTGTTCTCACAGGAGAGAGTTGA-3′; IL-1β sense,
5′-AAGAAGAACCCGTCCTCTGCAACA-3′ and antisense,
5′-TCAGCTCATACGTGCCAGACAACA-3′; β-actin (control) sense,
5′-GGGTCAGAAGGATTCCTATG-3′ and antisense,
5′-GGTCTCAAACATGATCTGGG-3′.
Western blot analysis
Western blot analysis was performed as described
previously (21). The cells were
solubilized in lysis buffer (100 mmol/l Tris-HCl, 2% SDS, 10%
glycerin, 100 mmol/l DL-dithiothreitol and protease inhibitors; pH
6.8). Protein extraction of the cytosolic and mitochondrial
fractions was performed using a multiple centrifugation method as
described previously (8,21). The sample protein concentrations
were determined using a BCA protein assay kit (Thermo Fisher
Scientific, Inc.). Protein (30 ng) were separated using SDS-PAGE
(10% gel) and then electrotransferred onto a nitrocellulose
membrane (EMD Millipore). After blocking for 3 h with 3% bovine
serum albumin (BSA) (E661003; Sangon Biotech Co., Ltd., Shanghai,
China) in Tris-buffered saline with 0.1% Tween-20 (TBST), membranes
were incubated overnight at 4°C with primary antibodies in TBST
containing 3% BSA and then fluorescently labeled secondary antibody
(1:10,000; cat. no. A7650; Sigma-Aldrich; Merck KGaA) for 1 h at
room temperature. After washing, the protein bands were scanned
with the Odyssey Infrared Imaging System (Li-Cor Biosciences,
Waltham, MA, USA) (without ECL/staining).
Immunofluorescence
Cells were grown in 48-well plates. After the
respective treatments, cells were washed twice with PBS and fixed
with freshly prepared 4% paraformaldehyde at room temperature for
15 min. For inner ear tissue staining, inner ear tissue was
horizontally sliced into 5-µm sections, which were mounted
on glass slides. Antigen accessibility was increased by treatment
with 2% Triton X-100 for 10 min at 37°C. The samples were then
blocked with 3% BSA for 30 min. The samples and cells were
incubated with the primary antibodies overnight at 4°C. After
washing three times with PBS, samples and cells were stained with a
secondary antibody Alexa Fluor 488 goat anti-mouse IgG (H+L) (1:500
dilution; cat. no. A11032; Thermo Fisher Scientific, Inc.) for 1 h
at 37°C. The nuclei were counterstained with DAPI for 15 min. After
each incubation, the samples and cells were washed thrice with PBS
for 5 min each. The morphology of SGN cells and the inner ear
tissues were observed and images were captured with an Olympus
DSU-IX81 confocal microscope (Olympus, Tokyo, Japan).
Detection of intracellular ROS
The generation of intracellular ROS was measured by
monitoring the increasing fluorescence of 2070-dichlorofluorescein
(DCF). 2070-dichlo-rodihydorofluorescein diacetate (DCFH-DA;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) is cell-permeant and
enters the cells where intracellular esterases cleave off the
diacetated group. The resulting DCFH was retained in the cytoplasm
and oxidized to DCF by ROS. SGN were seeded into each well of a
48-well plate. After 24 h of infection with 10 µl MCMV/1 ml
DMEM at 2,000 pfu/ml, cells were then washed once with phenol
red-free medium and incubated in 200 µl DCFH-DA working
solution (20 mM) at 37°C for 30 min. The cells were observed under
a fluorescence microscope (Olympus). Fluorescence (488 nm
excitation and 530 nm emission) was measured after 30 min using a
microplate fluorometer (Thermo Fisher Scientific, Inc.). In order
to inhibit the generation of ROS, cells were pretreated with 5 mM
NAC (ROS inhibitor) for 2 h (37°C) prior to MCMV injection.
Measurement of total superoxide dismutase
(T-SOD) and malondialdehyde (MDA)
T-SOD and MDA activities were measured using
detection kits (KGT00150 and KGT003; Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China) according to the manufacturers' protocols.
T-SOD activities were examined via the xanthine oxidase method. MDA
levels were detected using the 2-thiobarbituric acid method.
Colorimetric measurements were preformed using a multi-mode
microplate reader (Synergy 2; Bio-Tek, Winooski, VT, USA).
ELISA
The ELISA kits of IL-1β included the pro-forms and
IL-18 included the pro-forms were purchased from USCN Life Sciences
(cat. nos. SEA563Mu and SEA064Mu). Cell supernatants were
collected. For all ELISAs, the substrate solution was
tetramethylbenzidine, the reaction was blocked using
2NH2SO4, and absorbance was read at 450 nm.
The assays were carried out according to the manufacturer's
protocol. For all assays, Nunc MaxiSorp 96 well ELISA plates were
used (cat. no. 439454; Thermo Fisher Scientific, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). Values are expressed as the mean ±
standard error of the mean. The data of Fig. 1B were analyzed with the Mann
Whitney U test and the data of Figs.
2 and 3 were analyzed by
Student's t-test. The data of Fig.
5 were analyzed by one-way analysis of variance followed by
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
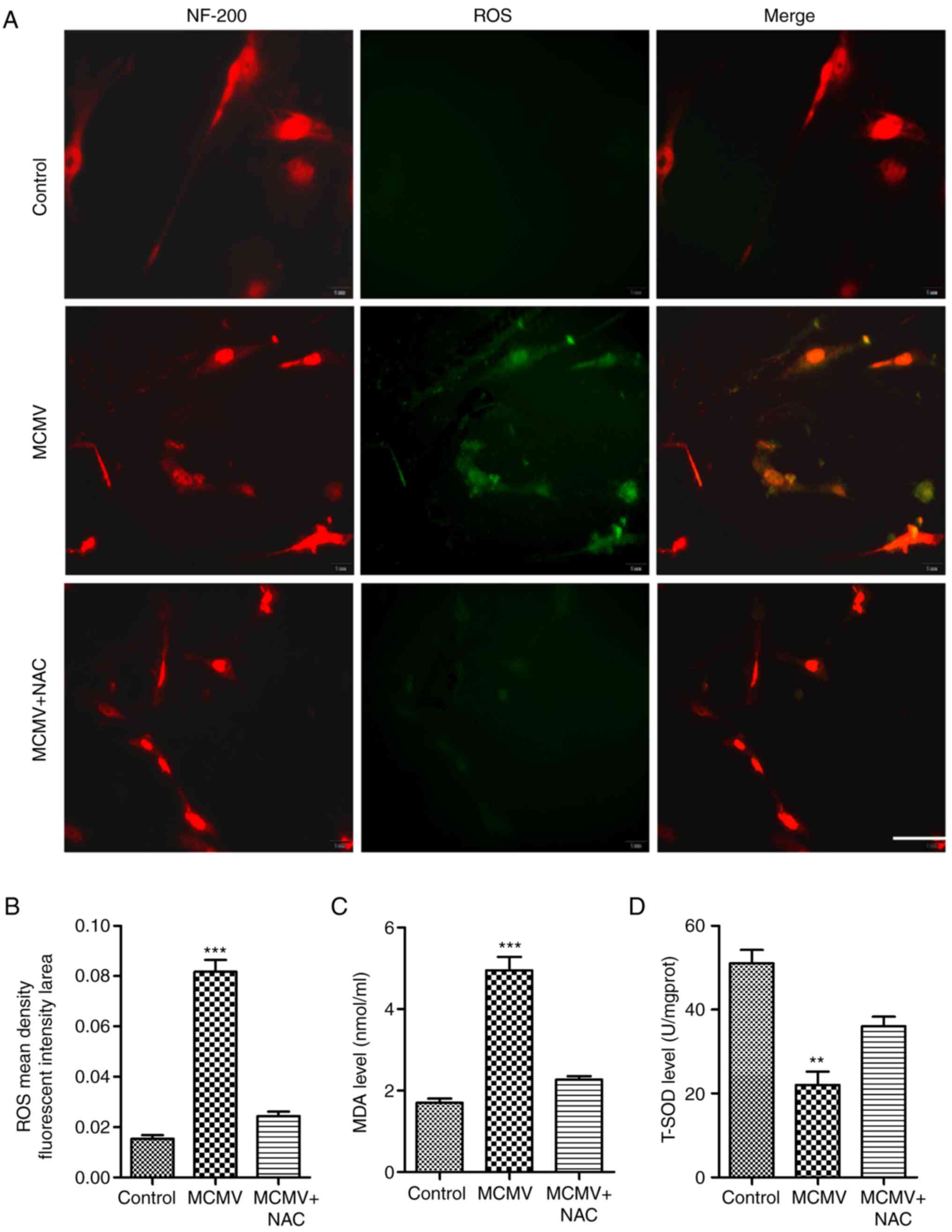 | Figure 5CMV-induced ROS expression in
cultured SGN. (A) ROS levels were measured with the
dichlorodihydorofluorescein diacetate fluorescence probe (green).
Immunohistochemical staining for NF-200 (red), a marker for SGN,
was also performed. ROS inhibitor NAC decreased the ROS levels
(scale bar, 50 µm). (B) Mean fluorescent intensity of ROS
was determined from A. (C) MDA levels were examined with the
2-thiobarbituric acid method. (D) T-SOD activities were examined
via the xanthine oxidase method. **P<0.01;
***P<0.001 vs. the control and MCMV+NAC groups.
Values are expressed as the mean ± standard deviation. NF,
neurofilament; MCMV, murine congenital cytomegalovirus; SGN, spiral
ganglion neurons; ROS, reactive oxygen species; MDA,
malondialdehyde; NAC, N-acetyl-L-cysteine; T-SOD, total superoxide
dismutase. |
Results
MCMV causes hearing loss in neonatal
mouse model
To establish the model of CMV-induced SNHL, neonatal
BALB/c mice were injected into the right cerebral lobe with the
strain of MCMV (2,000 pfu/ml, 15 ml) no later than 24 h after
birth. The results of ABR parameters at 21 days post MCMV infection
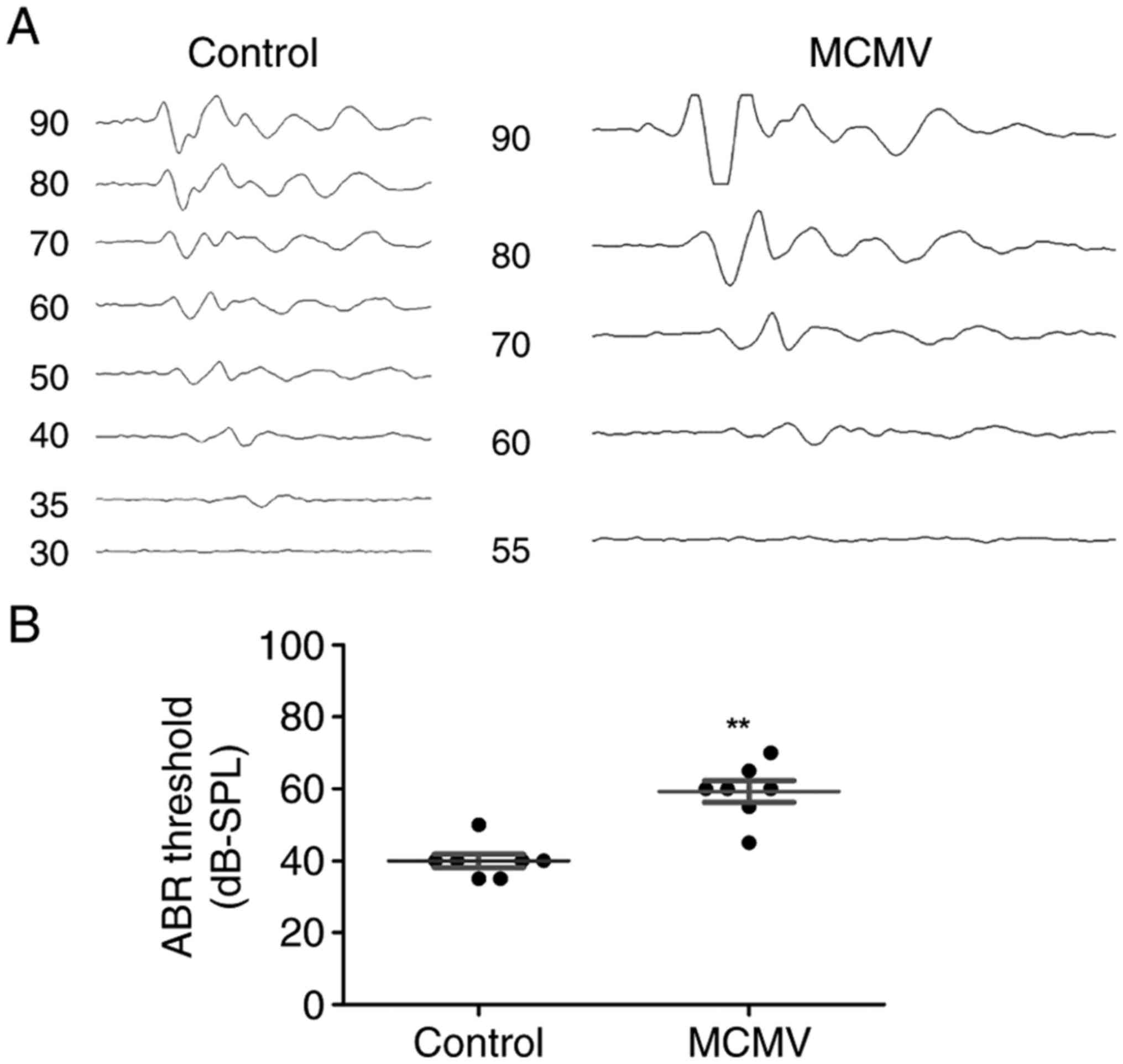
were identified. As presented in Fig.
1A, a significantly increased ABR threshold was detected in a
representative CMV-infected mouse compared with those of a
representative control mouse. The ABR thresholds for individual
ears in control and MCMV-injected mice are displayed in Fig. 1B. These results revealed that the
model of CMV-induced SNHL was successfully established.
MCMV infection increases cochlear
pro-inflammatory mediators
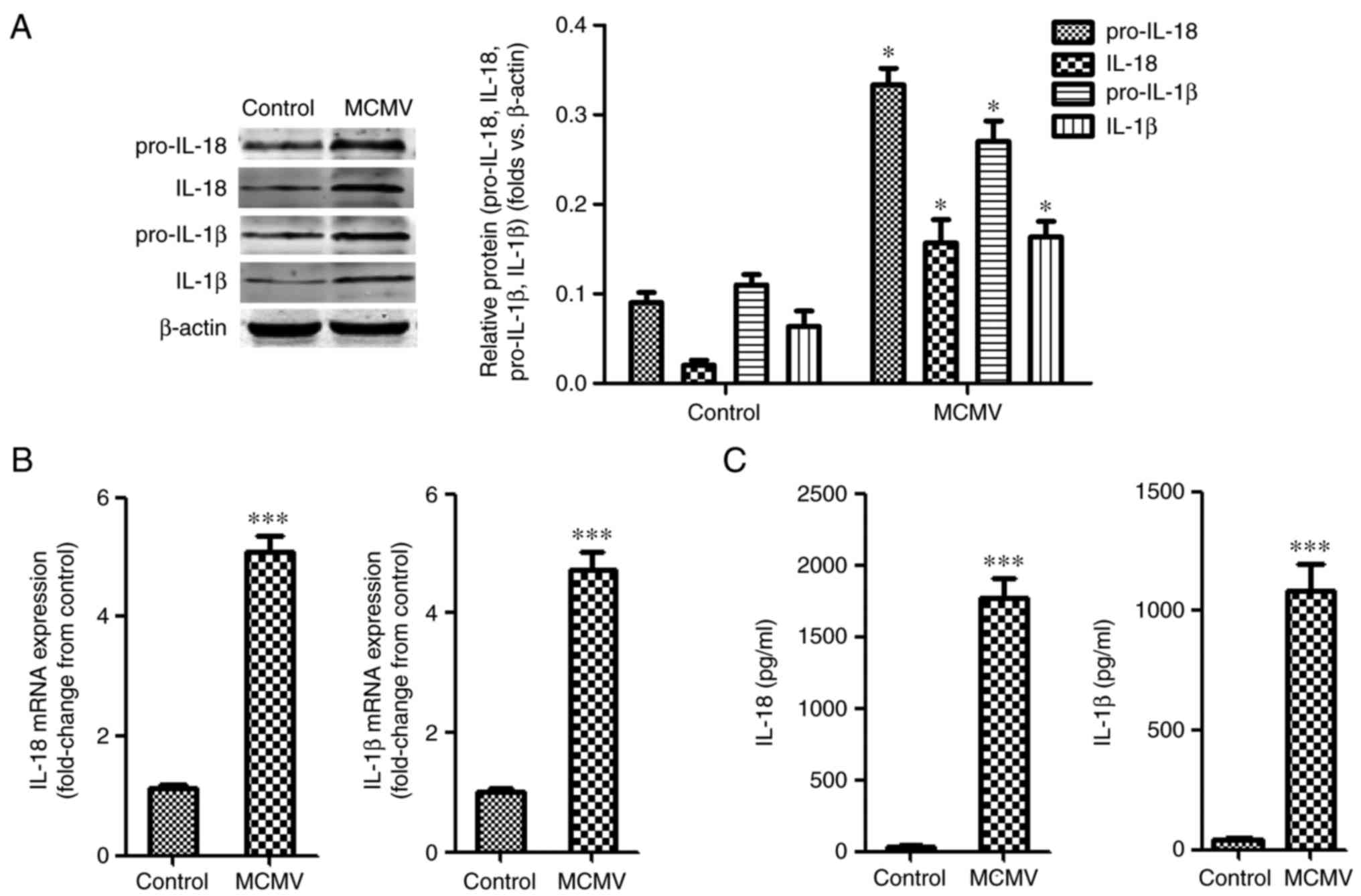
To assess the expression of pro-inflammatory
mediators during MCMV infection, the protein levels of pro-IL-18/1β
and IL-18/1β after MCMV infection were evaluated in mouse inner
ears tissues. As presented in Fig.
2A, the protein levels of pro-IL-18/1β were increased in
MCMV-infected mice, and the protein levels of IL-18/1β displayed
the same trends. Regarding the temporal expression of IL-18/1β
mRNA, the mRNA levels of IL18 and IL-1β in mouse inner ear tissues
were raised after infection with MCMV (Fig. 2B). The secreted levels of IL-1β
and IL-18 in inner ear tissues were also increased (Fig. 2C).
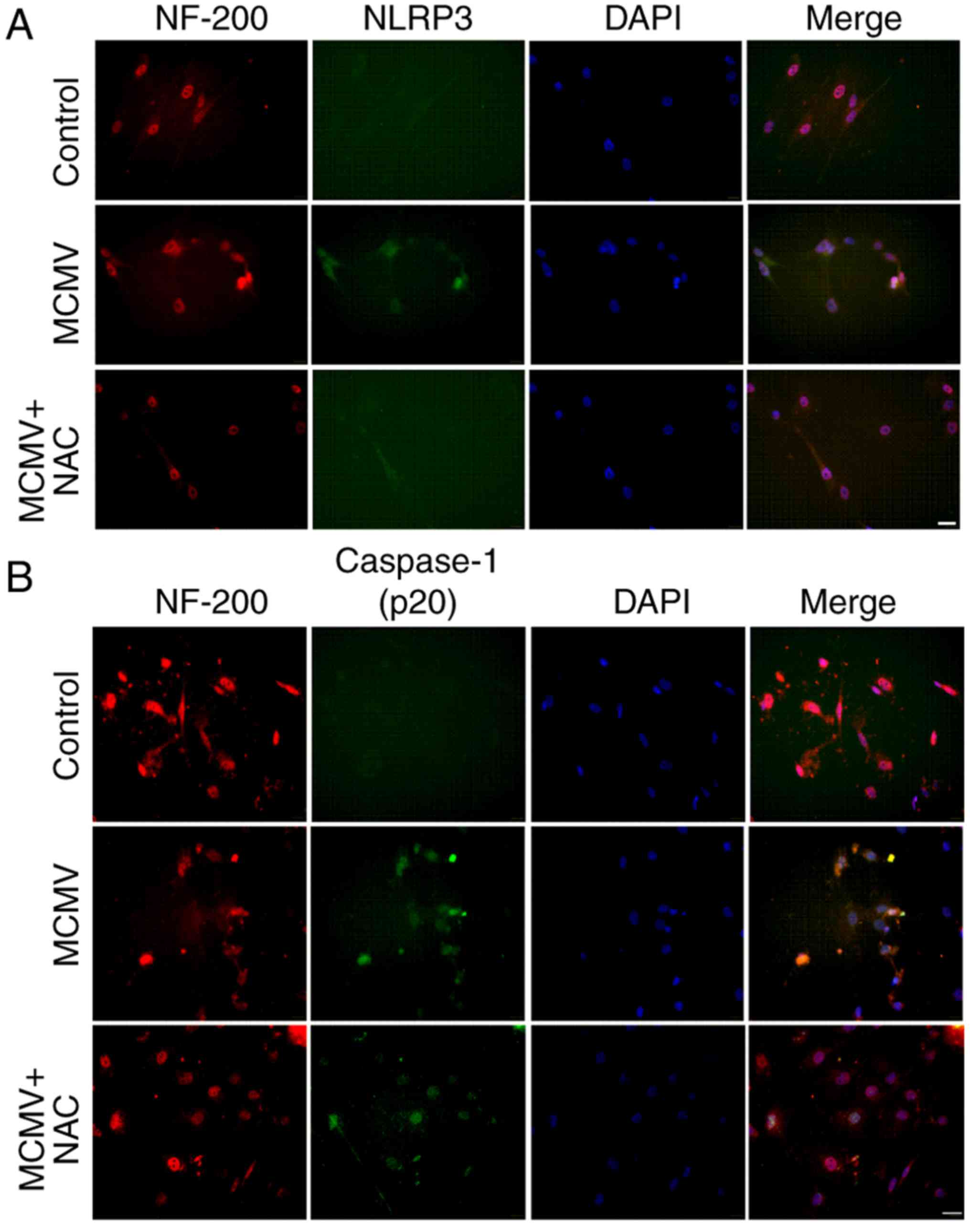
MCMV induces NLRP3 inflammasome
activation in cochlea
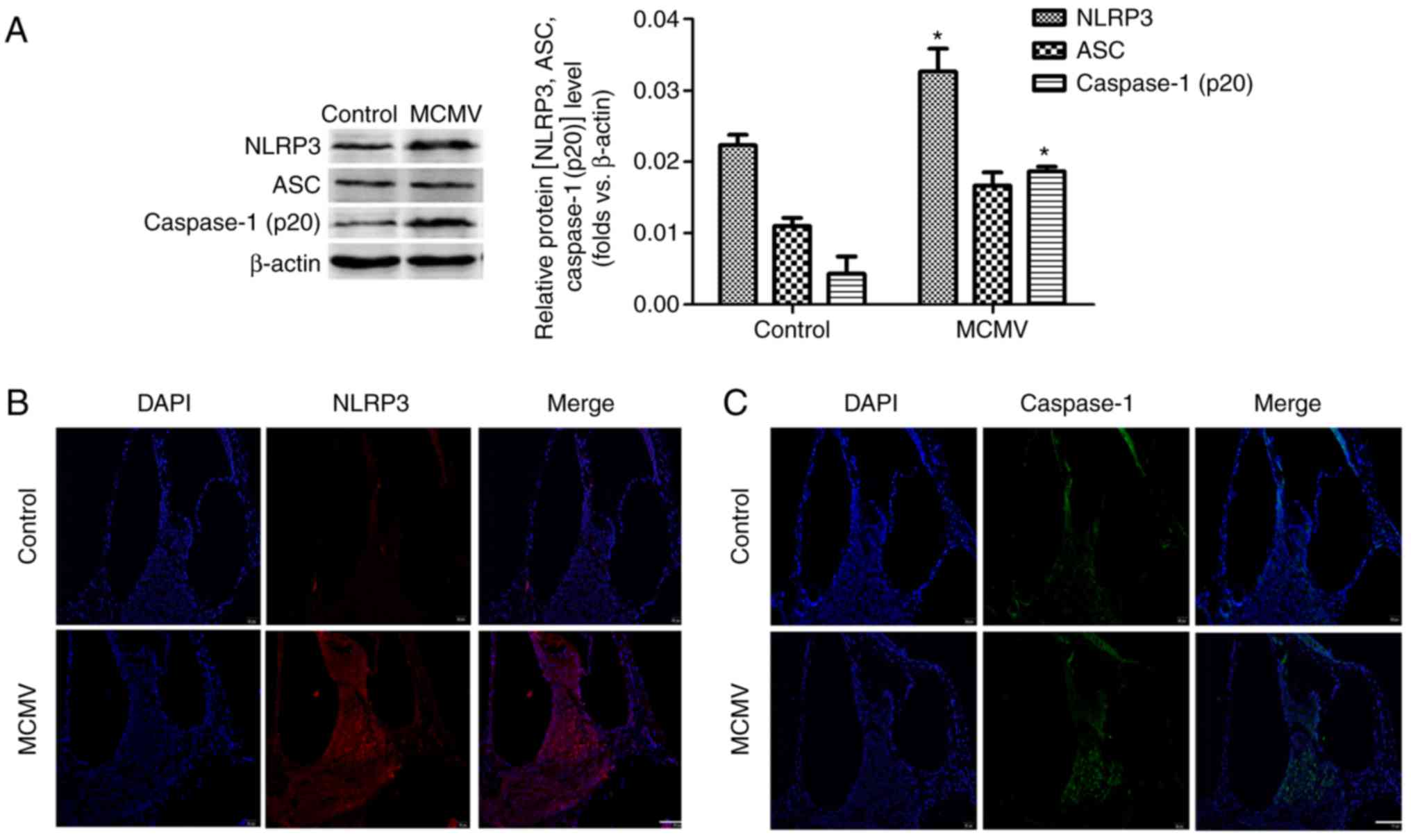
To assess whether MCMV induces NLRP3 inflammasome
activation in the cochlea, the neonatal BALB/c mice were infected
with MCMV and after 3 weeks, western blot analysis was performed to
assess the protein levels of NLRP3, ASC and caspase-1 (P20). As
indicated in Fig. 3A, NLRP3 and
caspase-1 (p20) were increased in cochlea after MCMV infection.
However, MCMV had no impact on the level of ASC protein.
Immunofluorescence analysis of NLRP3 and caspase-1 (p20) confirmed
these outcomes (Fig. 3B and C).
These results revealed that the activity of NLRP3 inflammasome in
cochlea was induced after infection with MCMV. MCMV also damaged
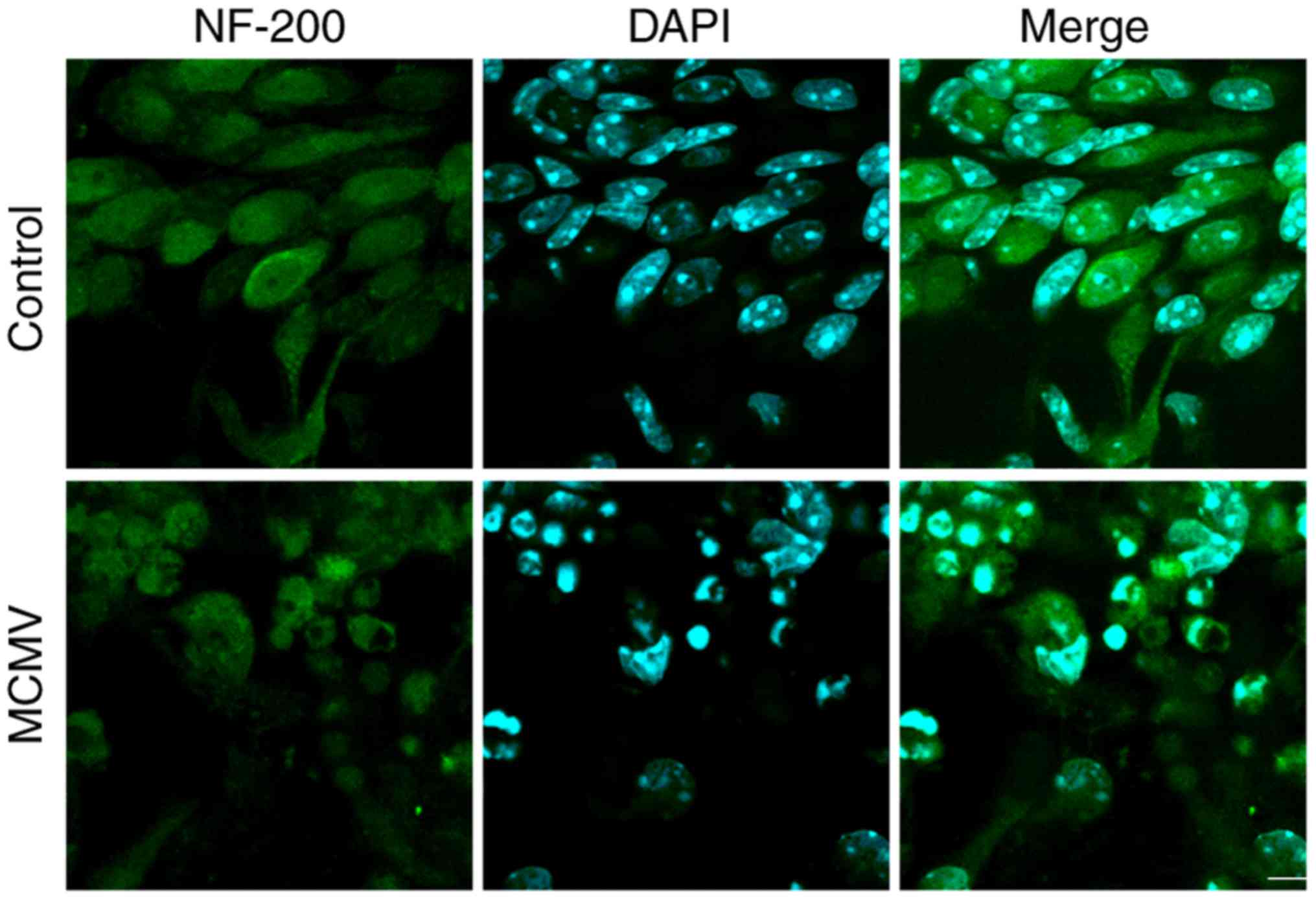
cultured SGN in vitro. SGN cells have important roles in
processes associated with neurotransmitters. To investigate whether
MCMV damages SGN cells, these cells were cultured in vitro
and infected with MCMV for 24 h, following which SGN was marked by
NF-200 antibody and cells were counterstained with DAPI. As
presented in Fig. 4, MCMV induced
and shrinkage of cells in SGN cells in contrast to the control
group. These results suggested that MCMV damages the cultured SGN
in vitro.
CMV induces the generation of ROS in
cultured SGN in vitro
Prolonged exposure to excessive ROS has been
implicated in neurotoxicity during viral brain infection (22). To investigate whether MCMV induced
ROS production by SGN in vitro, the DCFH-DA assay was
employed to determine the level of ROS. As presented in Fig. 5A and B, the cellular fluorescence
intensity of the metabolite DCF was increased to multiple folds of
the level in the control group, which was inhibited in the presence
of the ROS inhibitor NAC. MDA was also changed in a similar manner
to ROS (Fig. 5C). As expected,
T-SOD activity displayed an opposite trend (Fig. 5D). These results suggested that
CMV increased the level of ROS and MDA in the cultured SGN.
MCMV induces NLRP3 inflammasome
activation in cultured SGN
The involvement of the NLRP3 inflammasome pathway in
cultured SGN was then verified. The expression of NLRP3 and
caspase-1 (P20) was detected by immunofluorescence.
Immunofluorescence staining for the NLRP3 inflammasome was apparent
in the cultured SGN infected with MCMV, while virtually no staining
was observed in the control group, and the inflammatory effect of
MCMV was reduced when the ROS inhibitor NAC was applied (Fig. 6A). The expression of caspase-1
(P20) displayed the same tendency (Fig. 6B). These results clearly indicated
that MCMV markedly increases the expression of NLRP3 and caspase-1
(p20) in the cultured SGN.
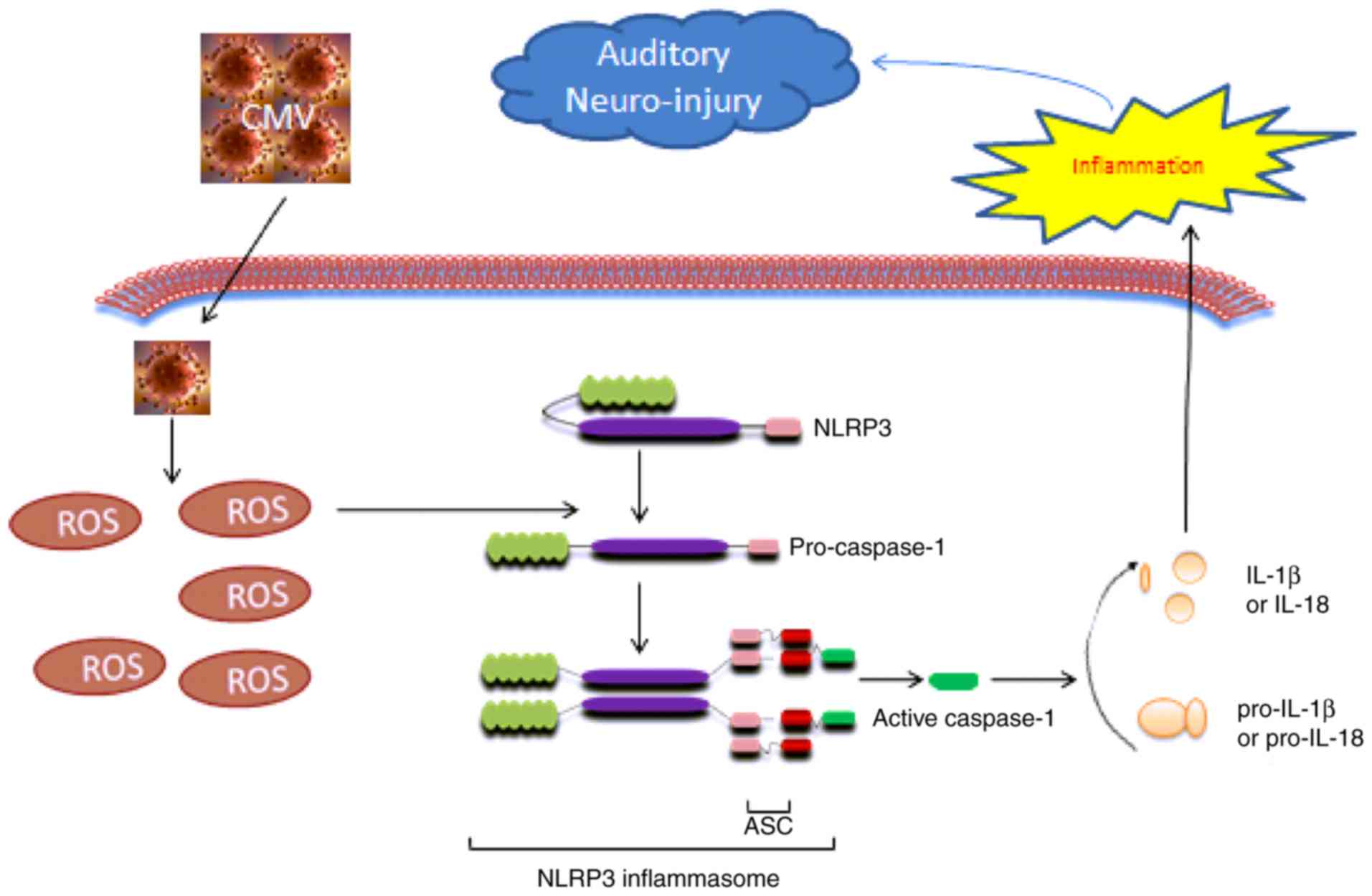
Discussion
To the best of our knowledge, the present study
demonstrated that MCMV actives inflammation in vivo and in
cultured SGN cells. The possible effects of MCMV may be due to the
activation of ROS (Fig. 7) as
MCMV increased the level of ROS and activated NLRP3 inflammasome in
the cochlea and cultured spiral ganglion neurons, resulting in
caspase-1 activation and an increase in IL-1β and IL-18 maturation
and release. These results suggested that ROS-induced inflammation
via the NLRP3 inflammasome is a mechanism of MCMV-associated
SNHL.
The inflammasome is multiprotein complex localized
within the cytoplasm of the cell and is responsible for the
maturation of pro-inflammatory cytokines, including IL-1β and
IL-18, as well as the mediation of a highly inflammatory form of
cell death (23). The canonical
inflammasome includes NLR (including NLRP1, NLRP3 and NLRC4) and
non-NLR (e.g. AIM2) types, which is mediated by activation of
caspase-1 in response to pathogen-associated molecular patterns and
damage-associated molecular pattern molecules (24,25). Sester et al (26) reported that AIM2 antagonist
protein p202 had a high expression and low IL-1β output in response
to transfected DNA and mouse CMV infection in an autoimmune
hemolytic anemia model. Rathinam et al (27) reported that AIM2 binds to
cytosolic DNA and initiates inflammasome responses in response to
viruses including MCMV and vaccinia, as well as the cytosolic
bacterium Francisella tularensis. The results of a previous
study by our group are consistent with these previous studies
(8). The NLRP3 inflammasome has
been implicated in the pathogenesis of a wide variety of diseases,
including genetically inherited autoinflammatory conditions, as
well as chronic diseases, in which NLRP3 is abnormally activated
(28). However, it has remained
elusive whether MCMV exerts its effects by affecting the NLRP3
inflammasome in mouse cochlea. The present study demonstrated that
MCMV stimulated NLRP3 activity in mouse cochlea. In combination
with a previous study by our group, the conclusion was drawn that
the effects of MCMV are not only dependent on AIM2, but also
NLRP3.
As is well known, the overproduction of ROS causes
severe damage to cellular macromolecules and ROS production is an
important intracellular inducer of autophagy (9). An accumulating body of evidence has
also indicated that ROS has an important role in CMV infection.
Xiao et al (29) described
that patients infected with human CMV experience an imbalance of
redox homeostasis that causes accumulation of ROS at the cellular
level. The present study proved that MCMV stimulated ROS activity
in mouse cochlea. In addition, MCMV promoted the production of ROS
through decreasing SOD activities. This is the underlying mechanism
for MCMV-induced SNHL.
Furthermore, the present study proved for the first
time, to the best of our knowledge, that MCMV infection increases
the level of ROS and activates the NLRP3 inflammasome in cultured
SGN in vitro. During the hearing process, SGN receive an
electrical signal input from cochlear hair cells and project from
the cochlea to the cochlear nucleus; subsequently, the electrical
signals are transmitted to the auditory cortex (30). Therefore, the SGN are referred to
as the first level of neurons of the auditory system. The
dysfunction of SGN often leads to SNHL and causes implantable
hearing device failure (9).
Esperanza et al (31)
suggested that the expression of pro-inflammatory cytokines
promotes intra-cochlear fibrosis, as well as loss of the auditory
hair cells and SGN. The results of the present study indicated that
MCMV triggers ROS-induced inflammation in cultured SGN. This may be
the mechanism to explain the SGN loss in SNHL caused by MCMV. The
underlying mechanisms require to be further assessed in future
studies.
In conclusion, the present study indicated that MCMV
activates the NLRP3 inflammasome via production of ROS in mouse
cochleae and in cultured SGN. Therefore, ROS-induced NLRP3
inflammasome activation is potentially a novel target for the
prevention and treatment of CMV-associated SNHL.
Acknowledgments
The authors would like to express their sincere
gratitude to Dr Meng Hong (Medical College of Shandong University,
Jinan, China) for providing MCMV (Smith strain).
Notes
[1]
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81250042, 81470684 and
81270173) and the Postdoctoral Science Foundation of China (grant
no. 2015M571818).
[2] Availability
of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
All the authors conceived and designed the study
protocol. HD, YQ, WZ, CW and XS conceived and designed the
experiments. SQ, SZ, MC, WJ and BX performed the experiments. WJ,
BX and MC analyzed the data. SQ, SZ, MC, HD and YQ provided
reagents/materials/analysis tools. WZ, XS, HD, WJ and YQ wrote the
paper. All the authors gave final approval of the version to be
submitted.
[4] Ethics
approval and consent to participate
Animal experiments were performed in accordance with
a protocol approved by the Ethics Committee of the Experimental
Animal Center at Xuzhou Medical University (Xuzhou, China).
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Schachtele SJ, Mutnal MB, Schleiss MR and
Lokensgard JR: Cytomegalovirus-induced sensorineural hearing loss
with persistent cochlear inflammation in neonatal mice. J
Neurovirol. 17:201–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheeran MC, Lokensgard JR and Schleiss MR:
Neuropathogenesis of congenital cytomegalovirus infection: Disease
mechanisms and prospects for intervention. Clin Microbiol Rev.
22:99–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li L, Kosugi I, Han GP, Kawasaki H, Arai
Y, Takeshita T and Tsutsui Y: Induction of cytomegalovirus-infected
labyrinthitis in newborn mice by lipopolysaccharide: A model for
hearing loss in congenital CMV infection. Lab Invest. 88:722–730.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grosse SD, Ross DS and Dollard SC:
Congenital cytomegalovirus (CMV) infection as a cause of permanent
bilateral hearing loss: A quantitative assessment. J Clin Virol.
41:57–62. 2008. View Article : Google Scholar
|
|
5
|
Ikuta K, Ogawa H, Hashimoto H, Okano W,
Tani A, Sato E, Kosugi I, Kobayashi T, Omori K and Suzutani T:
Restricted infection of murine cytomegalovirus (MCMV) in neonatal
mice with MCMV-induced sensorineural hearing loss. J Clin Virol.
69:138–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Patel R, Ren C, Taggart MG, Firpo
MA, Schleiss MR and Park AH: A comparison of different murine
models for cytomegalovirus-induced sensorineural hearing loss.
Laryngoscope. 123:2801–2806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bantug GR, Cekinovic D, Bradford R, Koontz
T, Jonjic S and Britt WJ: CD8+ T lymphocytes control
murine cytomegalovirus replication in the central nervous system of
newborn animals. J Immunol. 181:2111–2123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xi S, Yanfen D, Ya L, Zenlu Z, Huan L,
Shiwei Q, Yaohan L, Weiwei G and Yuehua Q: Inflammasome activation
in mouse inner ear in response to MCMV induced hearing loss. J
Otol. 10:143–149. 2015. View Article : Google Scholar
|
|
9
|
Zuo WQ, Hu YJ, Yang Y, Zhao XY, Zhang YY,
Kong W and Kong WJ: Sensitivity of spiral ganglion neurons to
damage caused by mobile phone electromagnetic radiation will
increase in lipopolysaccharide-induced inflammation in vitro model.
J Neuroinflammation. 12:1052015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Juhn SK, Jung MK, Hoffman MD, Drew BR,
Preciado DA, Sausen NJ, Jung TT, Kim BH, Park SY, Lin J, et al: The
role of inflammatory mediators in the pathogenesis of otitis media
and sequelae. Clin Exp Otorhinolaryngol. 1:117–138. 2008.
View Article : Google Scholar
|
|
11
|
Nesin M and Cunningham-Rundles S:
Cytokines and neonates. Am J Perinatol. 17:393–404. 2000.
View Article : Google Scholar
|
|
12
|
Riedl MA and Nel AE: Importance of
oxidative stress in the pathogenesis and treatment of asthma. Curr
Opin Allergy Clin Immunol. 8:49–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ciencewicki J, Trivedi S and Kleeberger
SR: Oxidants and the pathogenesis of lung diseases. J Allergy Clin
Immunol. 122:456–470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou R, Yazdi AS, Menu P and Tschopp J: A
role for mitochondria in NLRP3 inflammasome activation. Nature.
469:221–225. 2011. View Article : Google Scholar
|
|
15
|
Schroder K, Zhou R and Tschopp J: The
NLRP3 inflammasome: A sensor for metabolic danger? Science.
327:296–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martinon F, Pétrilli V, Mayor A, Tardivel
A and Tschopp J: Gout-associated uric acid crystals activate the
NALP3 inflammasome. Nature. 440:237–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stinski MF: Human cytomegalovirus:
Glycoproteins associated with virions and dense bodies. J Virol.
19:594–609. 1976.PubMed/NCBI
|
|
19
|
Li X, Shi X, Wang C, Niu H, Zeng L and
Qiao Y: Cochlear Spiral ganglion neuron apoptosis in neonatal mice
with murine cytomegalovirus-induced sensorineural hearing loss. J
Am Acad Audiol. 27:345–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔC T method. Methods. 25:402–408.
2001. View Article : Google Scholar
|
|
21
|
Shi X, Tian B, Ma C, Liu L, Zhang N, Na Y,
Li J, Lu J and Qiao Y: GSK3β activity is essential for
senescence-associated heterochromatin foci (SAHF) formation induced
by HMGA2 in WI38 cells. Am J Transl Res. 9:167–174. 2017.
|
|
22
|
Schachtele SJ, Hu S, Little MR and
Lokensgard JR: Herpes simplex virus induces neural oxidative damage
via microglial cell Toll-like receptor-2. J Neuroinflammation.
7:352010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo B, Li B, Wang W, Liu X, Xia Y, Zhang
C, Zhang M, Zhang Y and An F: NLRP3 gene silencing ameliorates
diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One.
9:e1047712014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie M, Yu Y, Kang R, Zhu S, Yang L, Zeng
L, Sun X, Yang M, Billiar TR, Wang H, et al: PKM2-dependent
glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat
Commun. 7:132802016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zorman J, Susjan P and Hafner-Bratkovic I:
Shikonin suppresses NLRP3 and AIM2 inflammasomes by direct
inhibition of caspase-1. PLoS One. 11:e01598262016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sester DP, Sagulenko V, Thygesen SJ,
Cridland JA, Loi YS, Cridland SO, Masters SL, Genske U, Hornung V,
Andoniou CE, et al: Deficient NLRP3 and AIM2 inflammasome function
in autoimmune NZB mice. J Immunol. 195:1233–1241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rathinam VA, Jiang ZZ, Waggoner SN, Sharma
S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, et
al: The AIM2 inflammasome is essential for host defense against
cytosolic bacteria and DNA viruses. Nat Immunol. 11:395–403. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abderrazak A, Syrovets T, Couchie D, El
Hadri K, Friguet B, Simmet T and Rouis M: NLRP3 inflammasome: From
a danger signal sensor to a regulatory node of oxidative stress and
inflammatory diseases. Redox Biol. 4:296–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiao J, Deng J, Lv L, Kang Q, Ma P, Yan F,
Song X, Gao B, Zhang Y and Xu J: Hydrogen peroxide induce human
cytomegalovirus replication through the activation of p38-MAPK
signaling pathway. Viruses. 7:2816–2833. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bailey EM and Green SH: Postnatal
expression of neurotrophic factors accessible to spiral ganglion
neurons in the auditory system of adult hearing and deafened rats.
J Neurosci. 34:13110–13126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bas E, Goncalves S, Adams M, Dinh CT, Bas
JM, Van De Water TR and Eshraghi AA: Spiral ganglion cells and
macrophages initiate neuro-inflammation and scarring following
cochlear implantation. Front Cell Neurosci. 9:3032015. View Article : Google Scholar : PubMed/NCBI
|