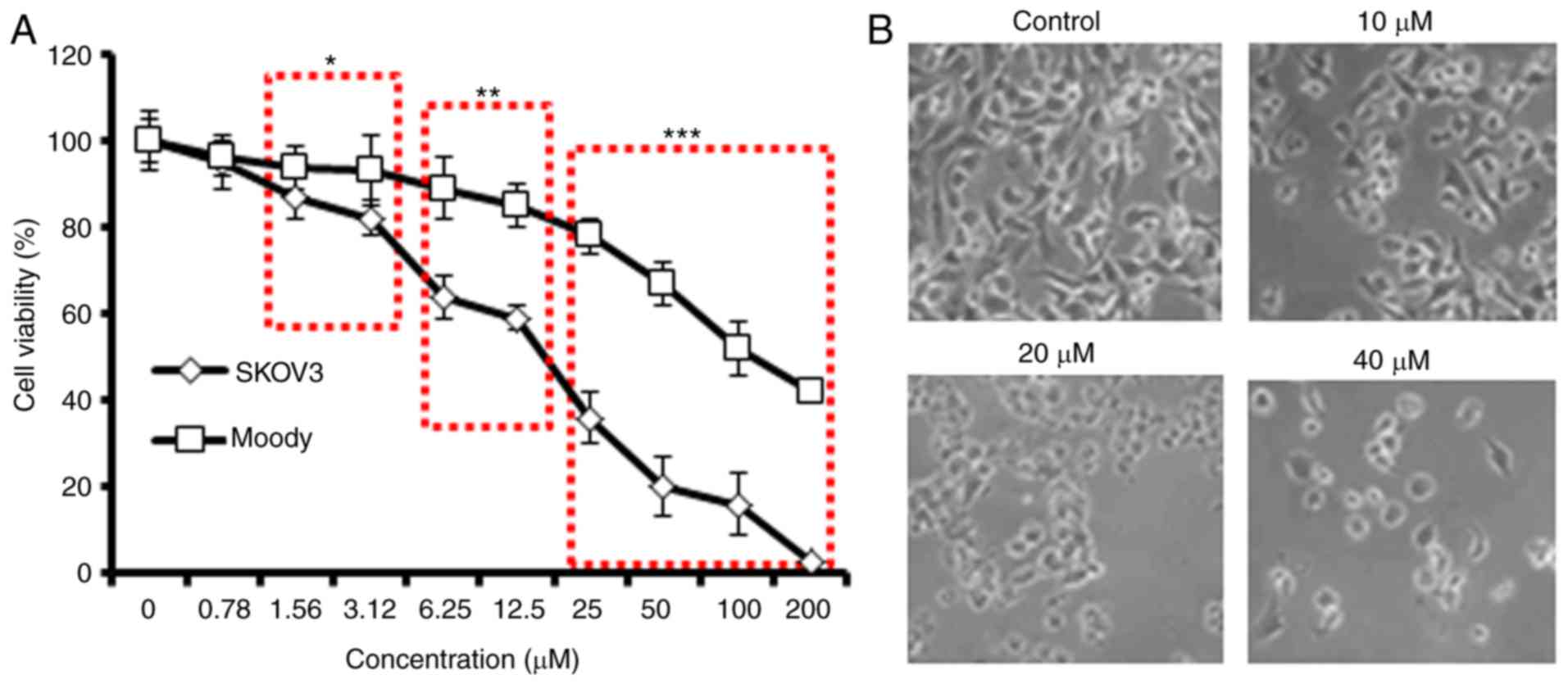
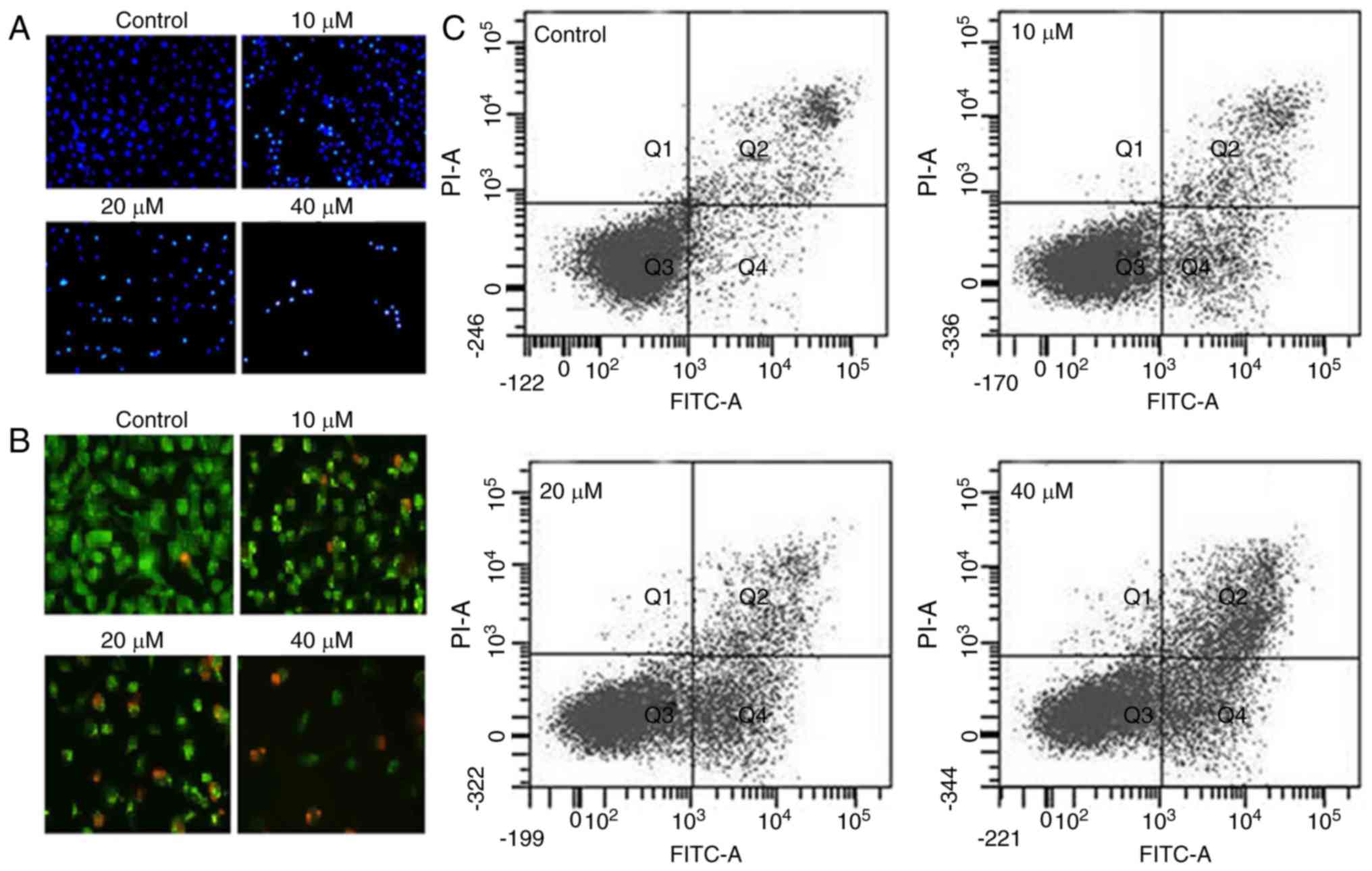
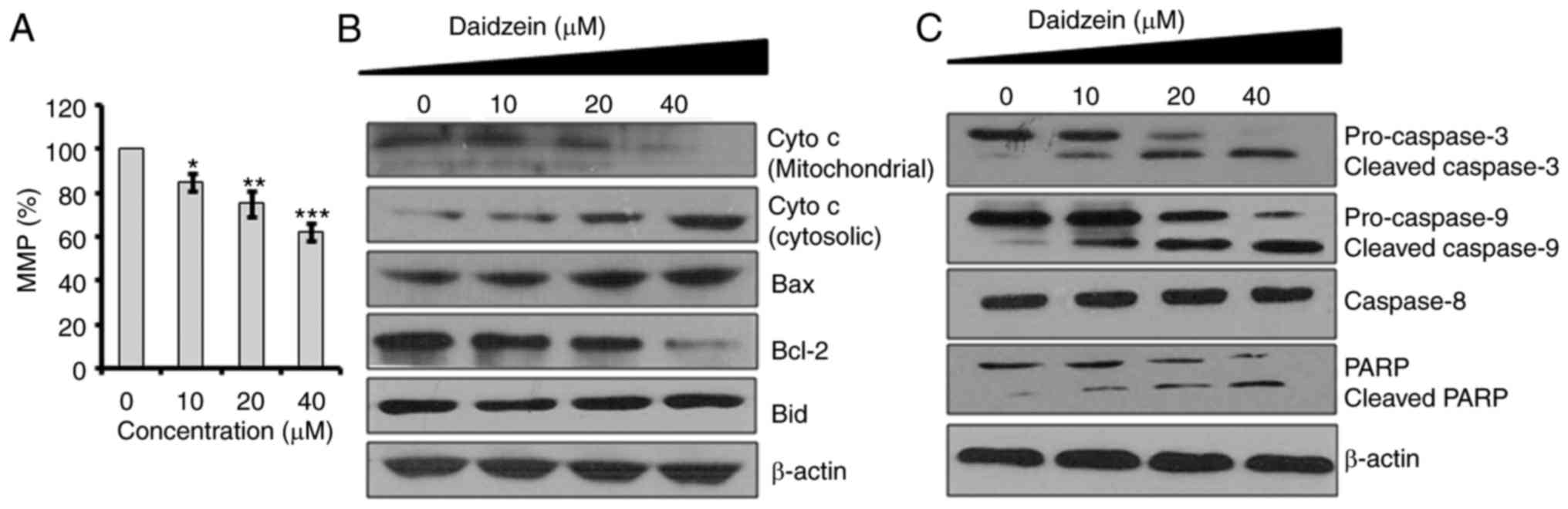
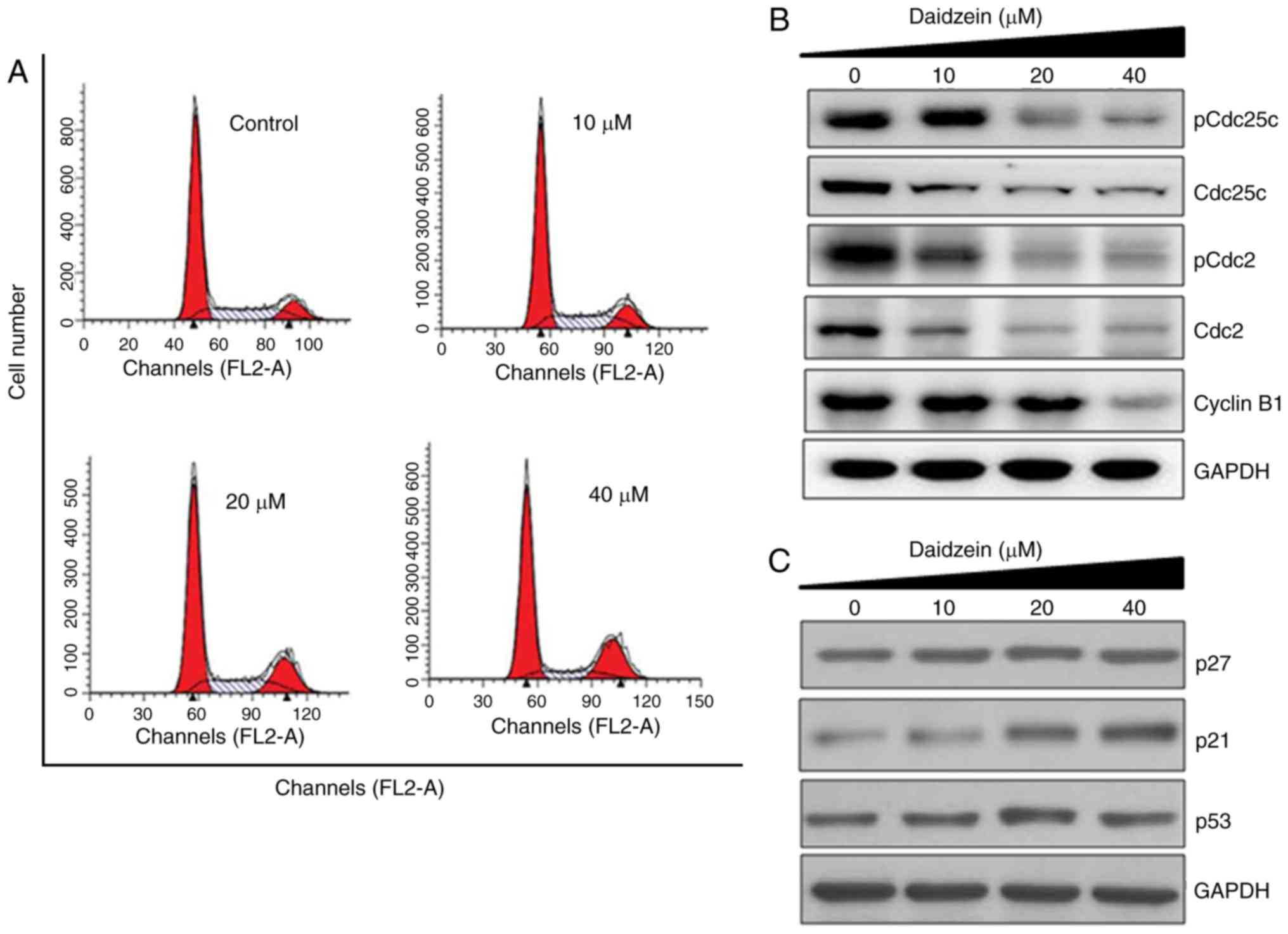
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Can J Clin. 64:9–29. 2014. View Article : Google Scholar
|
|
2
|
Bast RC Jr, Urban N, Shridhar V, Smith D,
Zhang Z, Skates S, Lu K, Liu J, Fishman D and Mills G: Early
detection of ovarian cancer: Promise and reality. Cancer Treat Res.
107:61–97. 2002.PubMed/NCBI
|
|
3
|
Berek JS: Ovarian cancer. Practical
Gynecologic Oncology. Berek JS and Hacker NF: Lippincott Williams
& Wilkins; Philadelphia, PA: pp. 443epp. 5112005
|
|
4
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: New opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Over B, Wetzel S, Grütter C, Nakai Y,
Renner S, Rauh D and Waldmann H: Natural-product-derived fragments
for fragment-based ligand discovery. Nat Chem. 5:21–28. 2013.
View Article : Google Scholar
|
|
7
|
Clough J, Chen S, Gordon EM, Hackbarth C,
Lam S, Trias J, White RJ, Candiani G, Donadio S, Romanò G, et al:
Combinatorial modification of natural products: Synthesis and in
vitro analysis of derivatives of thiazole peptide antibiotic GE2270
A: A-ring modifications. Biorg Med Chem Lett. 13:3409–3414. 2003.
View Article : Google Scholar
|
|
8
|
Cordier C, Morton D, Murrison S, Nelson A
and O'Leary-Steele C: Natural products as an inspiration in the
diversity-oriented synthesis of bioactive compound libraries. Nat
Prod Rep. 25:719–737. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the last 25 years. J Nat Prod.
70:461–477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cragg GM, Grothaus PG and Newman DJ:
Impact of natural products on developing new anti-cancer agents.
Chem Rev. 109:3012–3043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shah U, Shah R, Acharya S and Acharya N:
Novel anticancer agents from plant sources. Chin J Nat Med.
11:16–23. 2013. View Article : Google Scholar
|
|
12
|
Zhang X, Chen LX, Ouyang L, Cheng Y and
Liu B: Plant natural compounds: Targeting pathways of autophagy as
anti-cancer therapeutic agents. Cell Prolif. 45:466–476. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liggins J, Bluck LJ, Runswick S, Atkinson
C, Coward WA and Bingham SA: Daidzein and genistein contents of
vegetables. British J Nutr. 84:717–725. 2000.
|
|
14
|
Liggins J, Bluck LJ, Runswick S, Atkinson
C, Coward WA and Bingham SA: Bingham Daidzein and genistein content
of fruits and nuts. J Nutr Biochem. 11:326–331. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cancer Genome Atlas Research Network:
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ullah MF, Ahmad A, Bhat SH, Khan HY,
Zubair H, Sarkar FH and Hadi SM: Simulating hypoxia-induced acidic
environment in cancer cells facilitates mobilization and
redox-cycling of genomic copper by daidzein leading to pro-oxidant
cell death: Implications for the sensitization of resistant hypoxic
cancer cells to therapeutic challenges. Biometals. 29:299–310.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi EJ and Kim GH: Daidzein causes cell
cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7
and MDA-MB-453 cells. Phytomedicine. 15:683–690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin S, Zhang QY, Kang XM, Wang JX and Zhao
WH: Daidzein induces MCF-7 breast cancer cell apoptosis via the
mitochondrial pathway. Ann Oncol. 21:263–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang M, Liu Y, Gao Y and Li S:
Silibinin-induced glioma cell apoptosis by PI3K-mediated but
Akt-independent down-regulation of FoxM1 expression. Euro J
Pharmacol. 765:346–354. 2015. View Article : Google Scholar
|
|
21
|
Lee HG, Park WJ, Shin SJ, Kwon SH, Cha SD,
Seo YH, Jeong JH, Lee JY and Cho CH: Hsp90 inhibitor SY-016 induces
G2/M arrest and apoptosis in paclitaxel-resistant human ovarian
cancer cells. Oncol Lett. 13:2817–2822. 2017. View Article : Google Scholar
|
|
22
|
Gautier J, Solomon MJ, Booher RN, Bazan JF
and Kirschner MW: cdc25 is a specific tyrosine phosphatase that
directly activates p34cdc2. Cell. 67:197–211. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K
and Linn S: Molecular mechanisms of mammalian DNA repair and the
DNA damage checkpoints. Ann Rev Biochem. 73:39–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baus F, Gire V, Fisher D, Piette J and
Dulić V: Permanent cell cycle exit in G2 phase after DNA
damage in normal human fibro-blasts. EMBO J. 22:3992–4002. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsu YL, Kuo PL, Lin LT and Lin CC: Asiatic
acid, a triterpene, induces apoptosis and cell cycle arrest through
activation of extracellular signal-regulated kinase and p38
mitogen-activated protein kinase pathways in human breast cancer
cells. J Pharmacol Exp Ther. 313:333–344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun SY, Hail N Jr and Lotan R: Apoptosis
as a novel target for cancer chemoprevention. J Natl Can Inst.
96:662–672. 2004. View Article : Google Scholar
|
|
28
|
Singer G, Oldt R III, Cohen Y, Wang BG,
Sidransky D and Kurman RJ; Shih IeM: Mutations in BRAF and KRAS
characterize the development of low-grade ovarian serous carcinoma.
J Natl Cancer Inst. 95:484–486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Auner V, Kriegshäuser G, Tong D, Horvat R,
Reinthaller A, Mustea A and Zeillinger R: KRAS mutation analysis in
ovarian samples using a high sensitivity biochip assay. BMC Can.
9:1112009. View Article : Google Scholar
|
|
30
|
Scholzen T and Gerdes J: The Ki-67
protein: From the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Noble P, Vyas M, Al-Attar A, Durrant S,
Scholefield J and Durrant L: High levels of cleaved caspase-3 in
colorectal tumour stroma predict good survival. Br J Cancer.
108:2097–2105. 2013. View Article : Google Scholar : PubMed/NCBI
|