Introduction
Ovarian cancer is one of the most life-threatening
gynecological malignancies. It accounts for ~3% of all cancer cases
in women, with 240,000 new cases diagnosed annually and an annual
mortality rate of 150,000 from ovarian cancer across the globe
(1). In the US alone, ~22,000
ovarian cancer cases are diagnosed and ~14,000 individuals succumb
to mortality annually (2).
Ovarian cancer is often referred to as a 'silent killer' as 80% of
patients show no symptoms of the disease until an advanced stage if
the cancer develops within the ovaries (3). It has been reported in the last few
decades that 20% of patients diagnosed with ovarian cancer at an
early stage have >90% survival rate (3). Although efficient surgical
interventions and the use of different combinations of anticancer
drugs have shown promising results, the overall cure rate remains
at only 30% (4). Due to the
distinct biology of ovarian cancer, the selection of treatment
options and effective drug combinations remain limited (4). Therefore, there is an urgent
requirement to examine novel and more effective drugs for the
treatment of ovarian cancer. Consistently, natural products have
shown a diverse range of human health-promoting properties since
times immemorial (5). Natural
products are mainly secondary metabolites synthesized by living
organisms to adapt to and survive under extreme environmental
conditions. Due to the tendency of these secondary metabolites to
interact with enzymes, receptors and other cellular entities, they
exhibit appropriate drug properties (5). Statistical data between 1981 and
2010 have revealed that a number of natural products have been
established as sources of novel drugs (5). Furthermore, the diverse molecular
scaffolds of natural products often exhibit drug properties
(6), and are considered essential
for combinatorial (7,8) and diversity-oriented synthesis
(9) for the development of
effective bioactive molecules.
It has been reported that, of the 155 small-molecule
anticancer drugs, the number of drugs that are natural products
and/or synthesized from natural products accounts for 72.9%
(10). Among these, natural
sources from plants represent an important source of anticancer
drugs; and several plant-derived compounds are currently used as
anticancer drugs or are being assessed in clinical trials (11–13). Isoflavones are plant secondary
metabolites with substantial pharmacological potential (14). They are common food ingredients
and, therefore, are considered safe (14,15). Several studies have reported that
the consumption of isoflavonoids is inversely proportional to the
risk of cancer (14,15). Daidzein (7,4-dihydroxyisoflavone),
a flavone of plant origin, has been reported to exhibit anticancer
activity against several types of cancer, including breast and
ovarian cancer. However, the anticancer effect of daidzein has not
been thoroughly investigated and the detailed mechanisms remain to
be elucidated. In addition, the RAF/mitogen-activated protein
kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)
signaling pathways have been reported to be crucial in the
tumorigenesis and progression of several types of cancer, including
ovarian cancer (16). Therefore,
the present study was designed to examine the anticancer activity
of daidzein in vitro and in vivo, and to attempt to
investigate the underlying mechanisms.
Materials and methods
Chemicals, reagents and cell
cultures
Daidzein (98% pure by HPLC) and other chemicals were
procured from Sigma-Aldrich; Merck Millipore (Darmstadt, Germany)
unless otherwise mentioned. The normal human ovarian epithelial
cell line (Moody), and the Caov-3, OVACAR-3, SKOV3 and A2780 human
ovarian tumor cell lines were obtained from the Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China). For
culturing of the cells, RPMI-1640 media was used, which also
contained fetal bovine serum (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) (10%) and appropriate antibiotics (streptomycin
100 μg/ml and penicillin G 100 U/ml). The cultures were
maintained in an atmosphere containing 5% CO2 at
37°C.
CCK-8 assay for the assessment of cell
viability
The cell viability of the non-cancerous ovarian
cells (Moody) and the ovarian cancer cells (SKOV3) was determined
using a CCK-8 assay. In brief, 5×103 cells were seeded
in a 96-wel lplate and maintained at 37°C in a humidified 5%
CO2 atmosphere. Following incubation overnight, the
cells were treated with varied doses of daidzein (0, 0.78, 1.56,
3.12, 6.25, 12.5, 25, 25, 50, 100 and 200 μM) for 24 h.
Subsequently, 10 μl of CCK-8 was added into each well and
incubated again at 37°C for 1 h. The optical density (OD) at
OD450 nm was determined using a microplate
spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
and reported as a percentage of the control.
AO/EB, DAPI and Annexin V/propidium
iodide (PI) staining
The SKOV3 cells were cultured at a density of
2×105 cells/well in six-well plates, subjected to varied
doses of daidzein (0, 10, 20 and 40 μM) and incubated for 24
h at 37°C. The cells were then stained with a mixture of AO and EB.
The stained cells were examined under the fluorescent microscope.
DAPI staining was performed by incubating the cells in 6-well
plates and treated with different concentrations of daidzein (0,
10, 20 and 40 μM). The cells were then washed with PBS,
fixed in formaldehyde (10%) and the washed with PBS. The cells were
subjected to DAPI staining and then examined using fluorescence
microscopy. For the estimation of apoptosis, the cells were
subjected to Annexin V/PI double staining, using a similar
procedure to that used for DAPI staining, and was investigated
using flow cytometry.
Determination of mitochondrial membrane
potential (MMP)
The SKOV3 cells were cultured at 2×105
cells/well in a six-well plate and incubated for 24 h with 0, 10,
20 and 40 μM daidzein at 37°C in an atmosphere consisting of
5% CO2 and 95% air. The cells from all samples were then
harvested and subjected to washing with PBS, and mixed with 500
μl of DiOC6 (1 μmol/l) for the estimation
of MMP at 37°C for 30 min. Subsequently, flow cytometry was used to
examine the cell samples.
Cell cycle analysis
Following staining with PI, the cells were subjected
to flow cytometry for the evaluation of cell cycle phase
distribution. Briefly, the SKOV3 cells were seeded at a density of
2×105 and treated with varied doses of daidzein (0, 10,
20 and 40 μM) for 24 h, following which the cells were
harvested and placed in ethanol (70%) at 4°C. Following overnight
incubation, the cells were harvested and centrifuged for 10 min at
800 x g, washed in PBS and finally suspended in PBS (250 ml). This
was followed by RNase treatment for ~20 min. The cell cycle phase
was then determined using flow cytometry following PI staining.
Cell migration assay
The migration of SKOV3 cells was determined using a
wound healing assay. Briefly, the cells were cultured until
confluence. The SKOV3 cells were then treated with different
concentrations of daidzein (0, 5, 10 and 20 μM) and a
scratch was introduced to the cell culture using a sterile pipette
tip, followed by incubation for 24 h. The wound healing capacity of
the daidzein-treated cells was determined by comparing the wound
length with that of the untreated control cells.
Analysis of protein expression by western
blot analysis
Total proteins from the untreated and
daidzein-treated cells were isolated in RIPA lysis buffer. The
concentrations of the proteins in each assay were determined by BCA
assay. Equal quantities of protein extracts from each group were
then run on SDS-PAGE gels (10%) and then transferred onto a
polyvinylidene fluoride membrane. This was followed by blocking
with 5% non-fat milk and incubation at room temperature for 1 h.
The membranes were then incubated with a specific primary antibody
(MEK, sc-6250; pMEk, sc-136542; ERK, sc-93; pERK, sc-7383; MMP-9,
sc-176046; MMP-2 sc-176407; pCdc25c, sc-327; pCd25c, sc-56296;
pCdc2, sc-12340; Cdc2, sc-54; Cyclin B1, sc-70898; p27, sc-1641;
p21, sc-122305; p53, sc-47698; Cyto C, sc-65396; Bax, 623621; BCl2,
sc-509; pro-caspase-3, sc-113427; cleaved caspase-3, sc-176260;
pro-caspase-9, sc-81650; cleaved caspase-9, sc-81663; PARP,
sc-8007; Cleaved PARP, sc-23461; Actin, sc-58673; GAPDH, sc-47724)
overnight at 4°C (all 1:1,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), followed by incubation with horse radish
peroxidase-conjugated anti-rabbit secondary antibody (1:1,000;
sc-2372; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. The protein bands of interest were visualized using an
ECL Advanced Western Blot Detection kit (GE Healthcare Life
Sciences, Uppsala, Sweden).
In vivo xenograft experiment
For the xenograft study, the whole procedure was
performed as per the animal ethics guidelines and was approved by
the animal ethics committee of Huai'an First People's hospital,
Nanjing University (Huai'an, China; approval no. NUX278/2016; 12
May 2016). The six-week old, immunodeficient nude mice (14 male and
14 female) weighing 25–30 g were procured from the Animal Centre of
Nanjing University and housed in sterile stainless-steel cages in a
12-h light:dark cycle at 22°C and with ~50% relative humidity. The
mice received a subcutaneous injection in the left flank ofSKVO3
cells (5×106). As the tumors became visible, the mice
were administrated i.p. with DMSO (0.1%), dissolved daidzein and
diluted with normal saline at concentrations of 10, 20 and 40
μg/kg body weight. The doses were administrated three times
each week (Monday, Thursday and Saturday) and the control mice
received 0.1% DMSO in normal saline only. The tumor volume and size
were estimated every week using standard procedures. During and at
the end of the 4-week period, the mice were sacrificed by inhaling
of deep anesthesia with isoflurane (2.5% of the oxygen supplied for
2 h) and organs were collected for further experimentation. The
study was terminated at the 4-week end point as a marked difference
was observed in the tumor volume and weight at this point.
Statistical analysis
The data are presented as the mean ± standard
deviation. Statistical significance and IC50 values were
analyzed using GraphPad Prism Demo, Version 5 (GraphPad Software,
Inc., San Diego, CA, USA). Student's t-test was used for comparison
between two samples, and one-way analysis of variance followed by
Tukey's test was used for comparisons between more than two samples
for statistical analysis. P<0.01 was considered to indicate a
statistically significant difference.
Results
Daidzein exerts antiproliferative effects
on SKOV3 cells
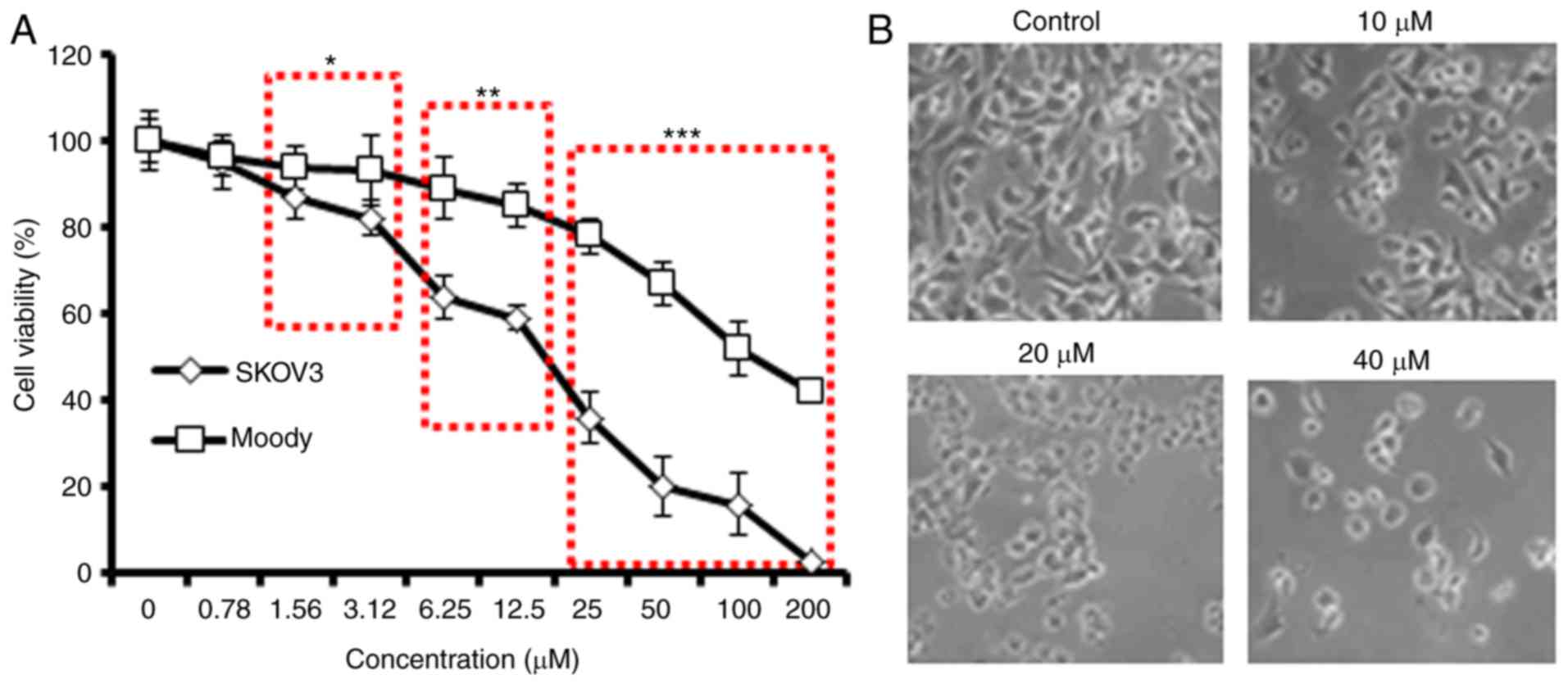
The antiproliferative effect of daidzein against a
panel of human ovarian cancer cells and normal (Moody) ovarian
cells was evaluated using a CCK-8 assay (Table I). The results indicated that, of
all ovarian cancer cell lines, daidzein exerted the most marked
dose-dependent antiproliferative effects on SKVO3 cells. However,
daidzein was found to be less cytotoxic against the normal cells
(Fig. 1A). The IC50 of
daidzein against the SKOV3 cells was 20 μM, compared with
the IC50 of 100 μM for the normal ovarian cells.
In addition, daidzein affected the morphology of the SKOV3 cells
(Fig. 1B). As daidzein exhibited
the lowest IC50 against SKOV3 cells, subsequent
experiments were performed using only this cell line. As the
concentration of daidzein was increased, the SKOV3 cancer cells
became rounder, shrunken and detached from the substratum (Fig. 1B), which are important
morphological changes associated with apoptosis.
 | Table IIC50 values of daidzein
against different ovarian cancer cell lines. Experiments were
performed in three biological replicates. |
Table I
IC50 values of daidzein
against different ovarian cancer cell lines. Experiments were
performed in three biological replicates.
| Cell line | IC50
(μM) |
|---|
| Caov-3 | 25 |
| OVACAR-3 | 25 |
| SKOV3 | 20 |
| A2780 | 40 |
| Moody (normal cell
line) | 100 |
Daidzein triggers mitochondrial
apoptosis
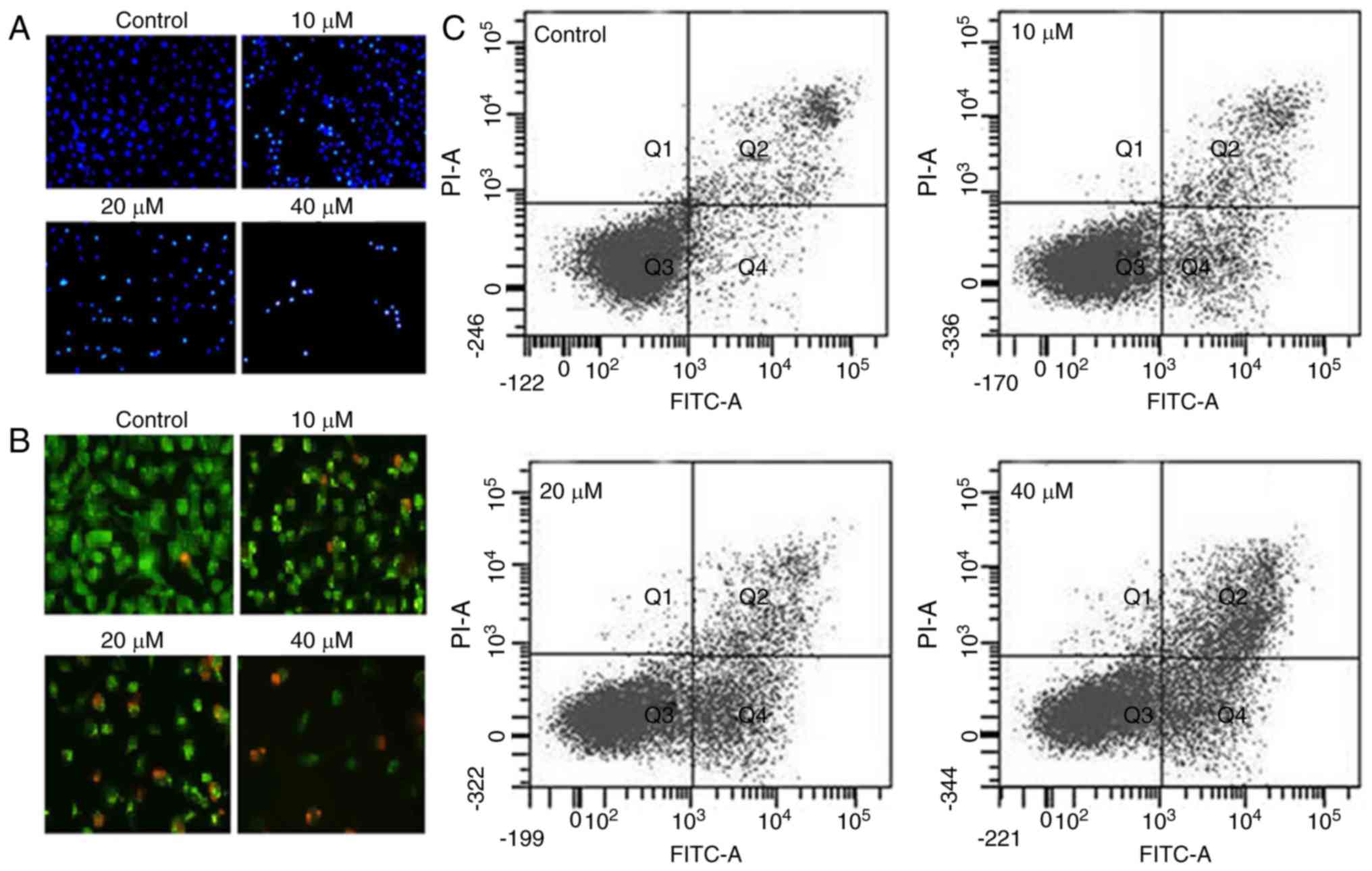
As daidzein induced morphological changes in the
SKOV3 cells characteristic of apoptosis, DAPI and AO/EB staining
were performed. The results indicated that daidzein induced
apoptosis of the SKOV3 cells, as evident from the increasing number
of nuclei stained white in the case of DAPI staining (Fig. 2A) and showing orange fluorescence
in the case of AO/EB staining (Fig.
2B). To estimate the apoptotic cell populations, Annexin V/PI
double staining was performed, and the results indicated that the
proportion of apoptotic cells increased with the increase in the
concentration of daidzein (Fig.
2C). The apoptotic cell populations were 5.2, 11.3, 35.7 and
48.8% at the concentrations of 0, 10, 20 and 40 μM daidzein,
respectively. Subsequently, the effect of different concentrations
of daidzein on MMP was determined. The results indicated that
daidzein caused a reduction in the MMP of SKOV3 cells in a
dose-dependent manner (Fig. 3A).
Mitochondrial injury causes the release of cytochrome c (cyt
c) into the cytoplasm and promotes apoptotic factors, which
activate the caspase cascade and mitochondria-triggered apoptosis
(14). To investigate whether
daidzein induces apoptosis via this mechanism in the SKOV3 cells,
the expression levels of cyt c, B-cell lymphoma 2-associated
X protein (Bax), BH3 interacting-domain death agonist (Bid),
caspase-3, -9 and -8, and poly (ADP-ribose) polymerase (PARP) were
determined using western blot analysis (Fig. 3B). The results showed that
daidzein increased the cytosolic levels of cyt c, Bax,
cleaved caspase-3 and -9, and cleaved PARP, compared with levels in
the control (Fig. 3C).
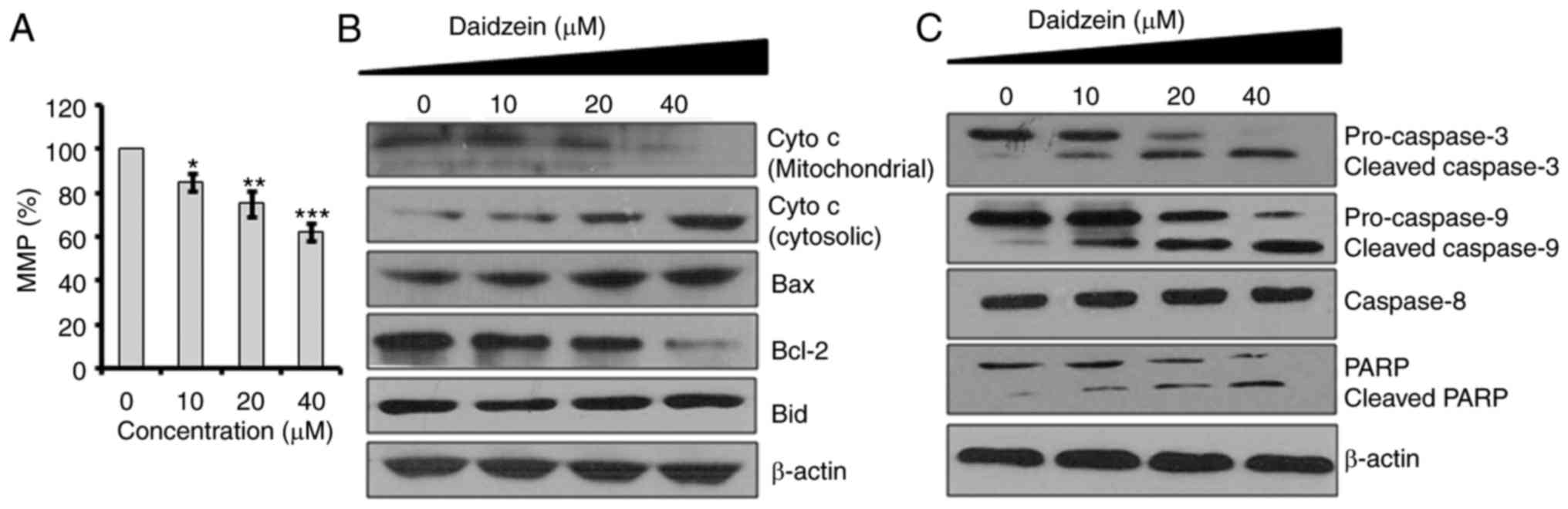 | Figure 3Effects of daidzein on MMP. Effects
of the indicated doses of daidzein on (A) MMP, and the expression
of (B) cyto c, Bax, Bcl-2 and Bid, (C) caspase-3, caspase-9,
caspase-8 and PARP in SKOV3 cells. The experiments were performed
in triplicate and presented as the mean ± standard deviation
(*P<0.01, **P<0.001 and
***P<0.0001, vs. control). MMP, mitochondrial
membrane potential; cyto c, cytochrome c; Bcl-2,
B-cell lymphoma 2; Bax, Bcl-2-associated X protein; Bid, BH3
interacting-domaindeath agonist; PARP, poly (ADP-ribose)
polymerase. |
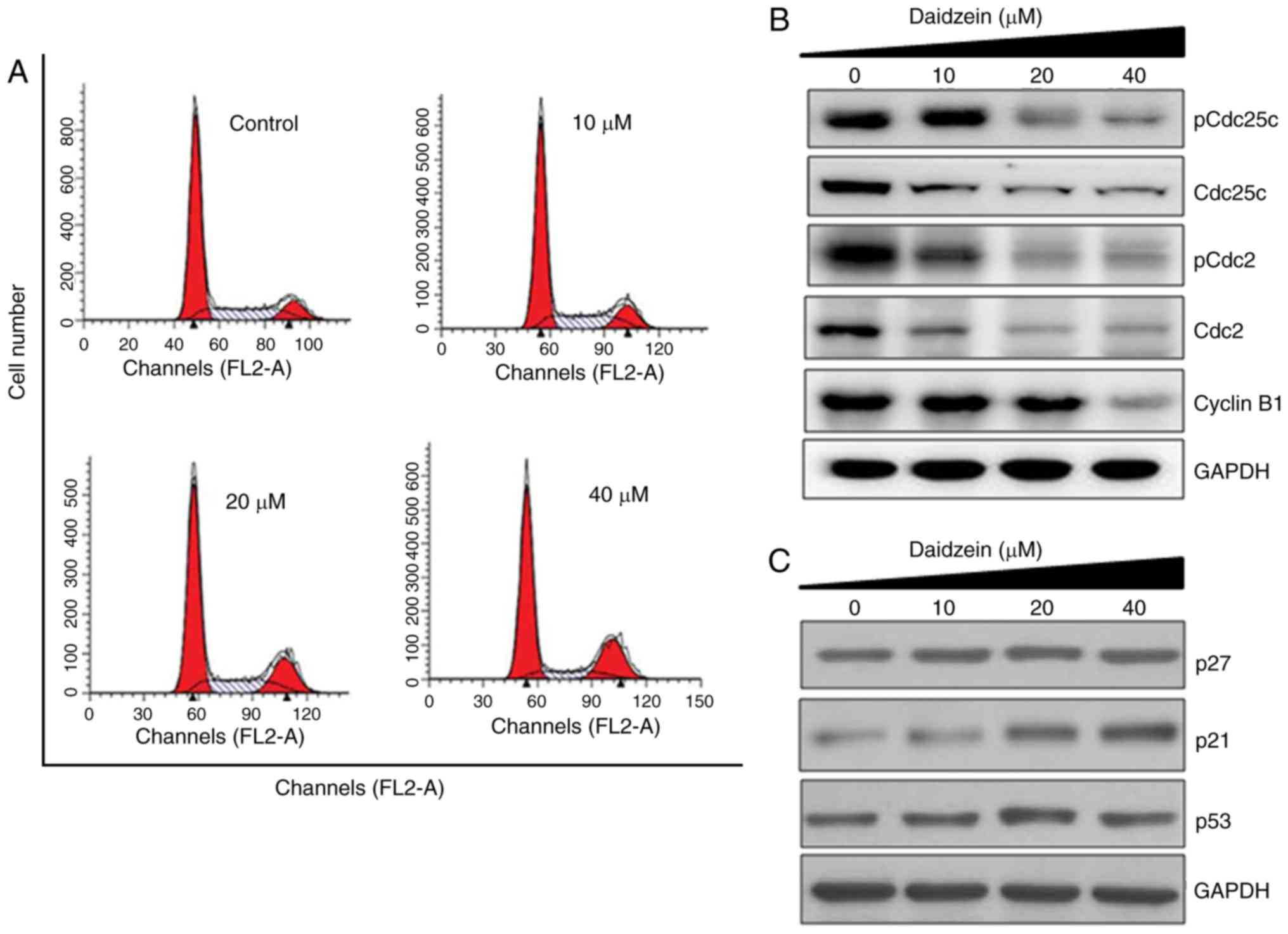
Daidzein induces G2/M cell cycle
arrest
Cell cycle arrest is one of the important mechanisms
by which anticancer agents exert their inhibitory effects.
Therefore, the present study also determined the effect of daidzein
treatment on cell cycle phase dissemination of the SKOV3 cells
(Fig. 4A). The results indicated
that the number of SKOV3 cells was significantly enhanced in the G2
phase at doses of 0-40 μM daidzein, leading to G2/M cell
cycle phase arrest (Fig. 4A).
Additionally, the populations of SKOV3 cells in the G2 phase were
marginally enhanced at the dosage of 10 μM, moderately
enhanced at 20 μM and markedly increased at 40 μM,
indicating the dose-dependent effect of daidzein. Western blot
analysis was then performed to investigate the effect of daidzein
on the expression of G2/M cell cycle controlling proteins,
including cyclin B1, Cdc25c and Cdc2, in SKOV3 cells. Treatment of
the SKOV3 cells with daidzein led to a concentration-dependent
reduction in the protein levels of pCdc25c (Ser216), Cdc25c, pCdc2
(Tyr15), Cdc2 and cyclin B1 in the SKOV3 cells (Fig. 4B). Daidzein also caused an
increase in the expression of p21, whereas no significant effect
was reported on the expression levels of p27 or p53 (Fig. 4C).
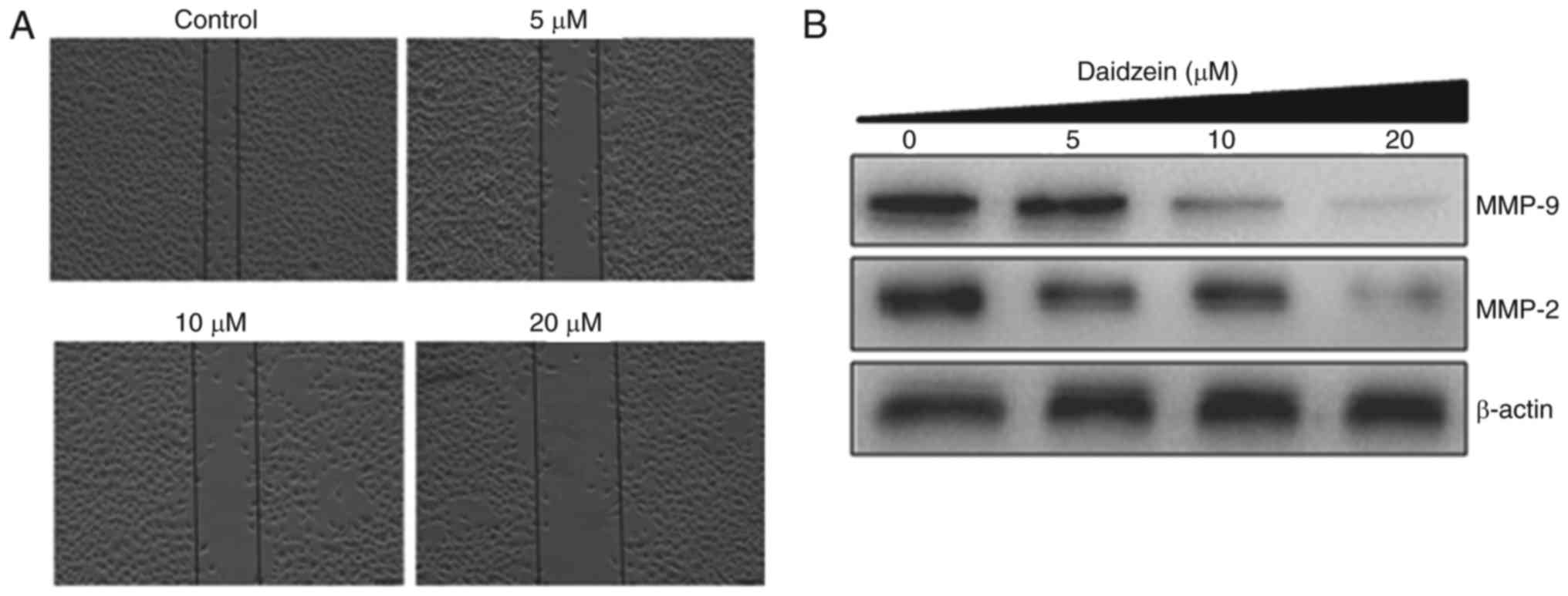
Daidzein inhibits cell migration
The present study also examined whether daidzein can
inhibit the migration of SKOV3 cancer cells at the different
concentrations using a wound-healing assay. The results of the
wound-healing assay showed that daidzein reduced the migratory
capability of the SKOV3 cells in a dose-dependent manner. In the
control group, the cells exhibited the capacity to migrate, whereas
treatment led to cells showing reduced potential to migrate, as
shown in Fig. 5A. Additionally,
daidzein caused a reduction in the expression levels of MMP-9 and
MMP-2 in a dose-dependent manner (Fig. 5B).
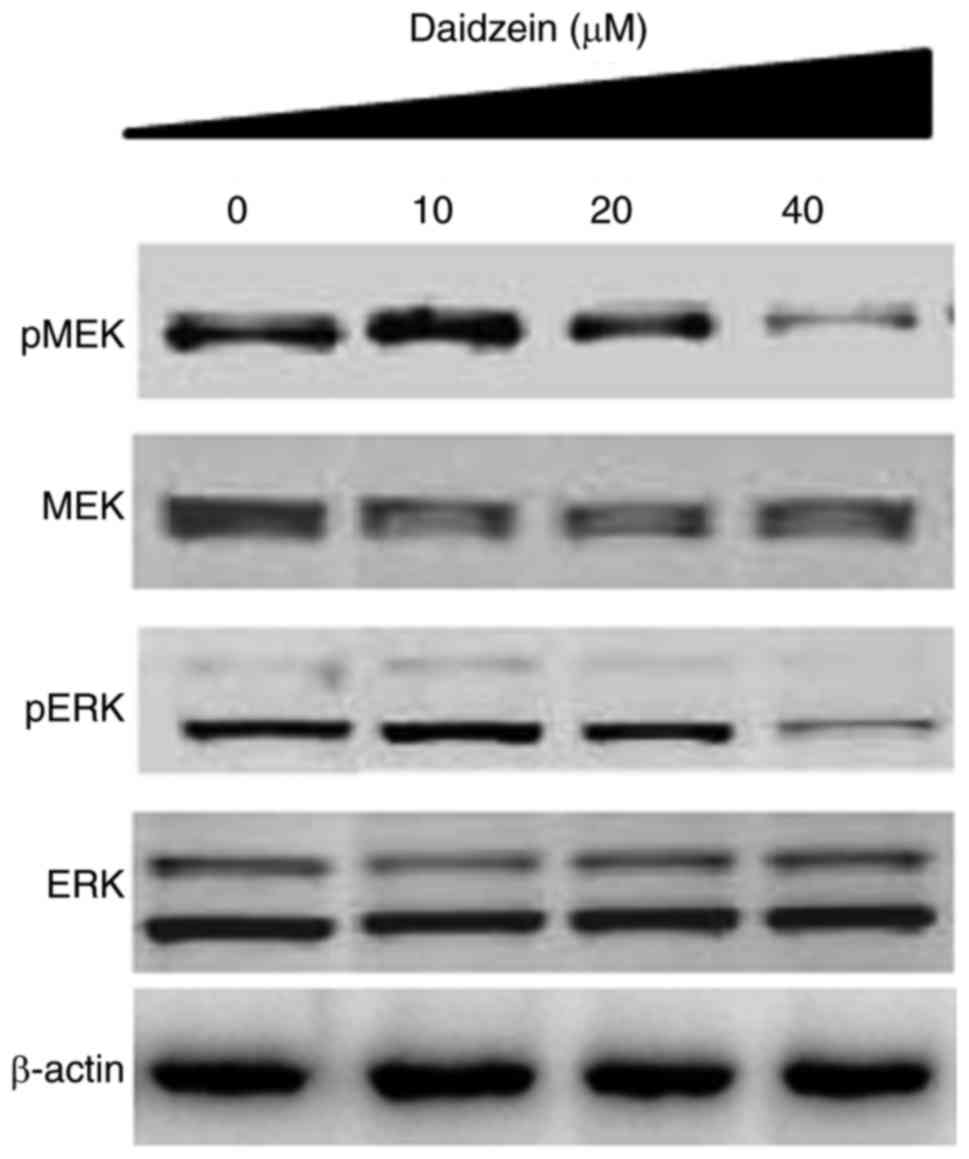
Daidzein inhibits the RAF/MEK/ERK
signaling pathway
The RAF/MEK/ERK signaling pathway has been shown to
be important in the tumorigenesis and progression of several types
of cancer, including ovarian cancer. Therefore, the present study
evaluated the effect of various concentrations of daidzein on the
RAF/MEK/ERK cascade. The results indicated that daidzein suppressed
the phosphorylation of MEK and ERK in a dose-dependent manner
(Fig. 6).
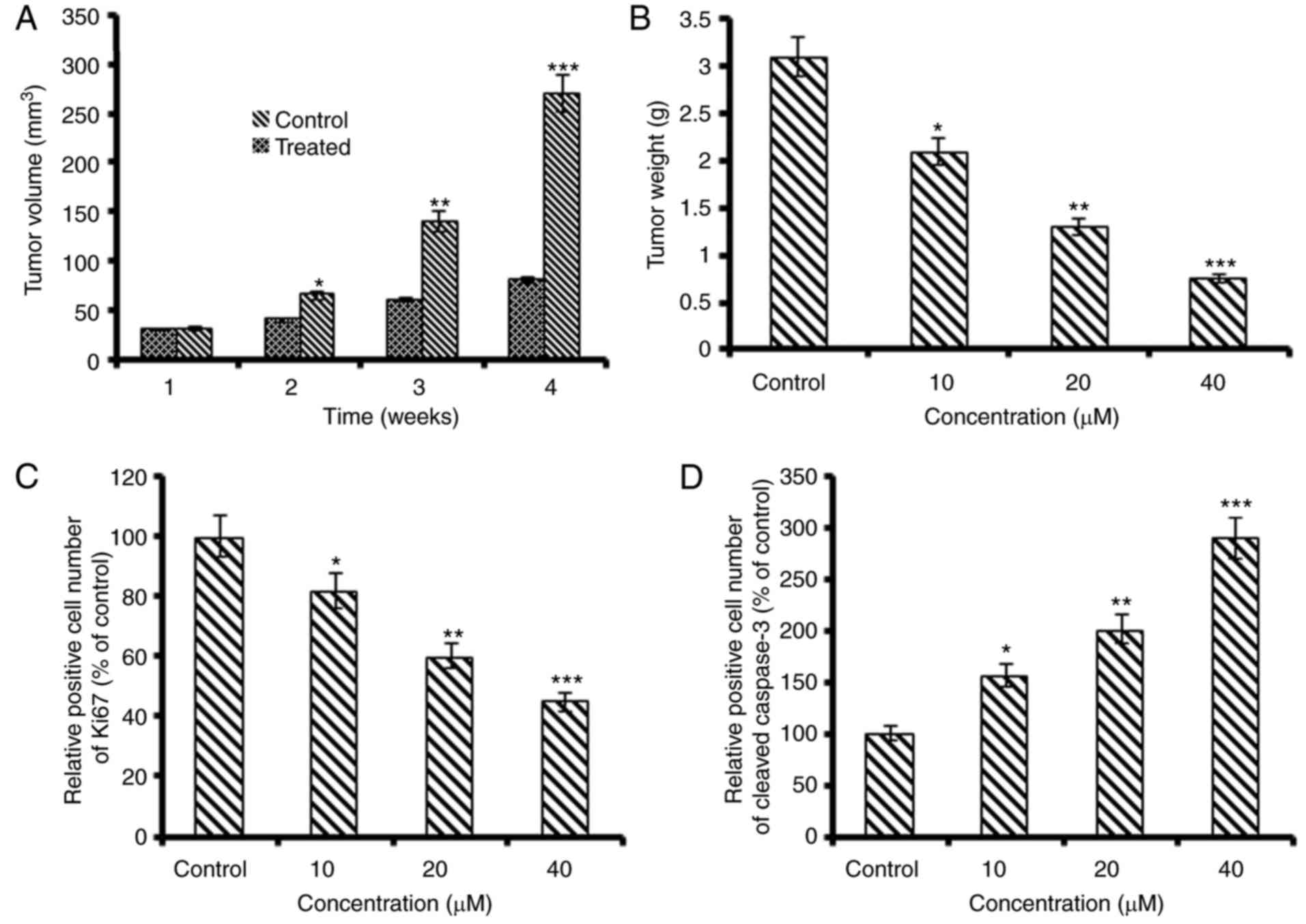
Daidzein inhibits tumor growth in
vivo
To examine the anti-cancer effects of daidzein in
vivo, it was assessed in nude mice xenograft models. The
results revealed that SKOV3 tumor growth was significantly
suppressed by daidzein administration, compared with that in the
control group. At the end of the 4-week period of daidzein
treatment, the average tumor growth and volume in the untreated
control group were considerably higher than those in the treated
groups (Fig. 7A and B). In
addition, the in vivo growth inhibitory potential was
concentration- and time-dependent. The protein expression level of
Ki-67 in the xenografted tumors was downregulated and that of
cleaved caspase-3 was enhanced following daidzein treatment
(Fig. 7C and D).
Discussion
Of all forms of gynecological cancer, ovarian cancer
is responsible for the highest mortality rate, mainly due to
diagnosis being at advanced stages (17). Although the majority of patients
react to debulking surgery and combinatorial therapy using taxane
and platinum, there is frequent relapse of the disease due to
intrinsic and acquired resistance. Therefore, novel options are
required for the improved management and treatment of this disease
at diagnosis and/or to offer an effective second line treatment. In
the present study, the anti-cancer effects of daidzein against
ovarian cancer cells were investigated. The results indicated that
daidzein significantly suppressed the growth (IC5020
μM) of SKOV3 cells. However, daidzein showed comparatively
less cytotoxicity (IC50100 μM) towards the normal
ovarian cells, indicating that daidzein selectively targeted cancer
cells (Fig. 1). These results are
in accordance with those of previous studies, in which daidzein has
been shown to suppress the proliferation of several types of cancer
(14,18-20). Daidzein treatment also triggered
several morphological changes in SKOV3 cells, which are
characteristic of apoptosis. Following this observation, DAPI and
AO/EB staining were performed to examine whether daidzein induces
the apoptosis of SKOV3 cells. It was observed that daidzein
increased the DNA fragmentation, observed using DAPI, and led to an
increase in orange fluorescence following AO/EB staining, which are
changes indicative of apoptosis. These results were substantiated
by the results of Annexin V/IP staining, which showed that daidzein
treatment led to a significant increase in the apoptotic cell
populations of SKOV3 cells (Fig.
2). These results are supported by a previous report that
daidzein induces apoptosis in cancer cells (20). To determine whether the apoptosis
follows the mitochondrial route, the present study determined the
effect of daidzein on MMP, and it was observed that daidzein
significantly decreased MMP, which was associated with upregulation
in the expression levels of cyt c, Bax, cleaved caspase-3
and -9, and cleaved PARP, compared with levels in the control SKOV3
cells (Fig. 3). It has been
reported that drugs with apoptosis-inducing properties exhibit the
potential to minimize potential drug resistance (21) and the results of the present study
clearly indicated that daidzein had apoptosis-inducting properties,
suggesting that daidzein may be an important lead molecule for the
treatment of ovarian cancer. In addition to apoptosis, cell cycle
arrest is another mechanism by which anticancer agents inhibit
cancer cell proliferation. The results of the present study
revealed that daidzein caused G2/M cell cycle arrest. Apoptotic
cell death is triggered when explicit checkpoints are arrested
during cell cycle. Consistent with this, several anticancer agents
cause cell cycle arrest and have been found to be clinically
effective for cancer treatment (22). The increase of cells in the G2/M
phase prevents cells undergoing mitosis (23,24). Treatment of the SKOV3 cells with
daidzein led to incomplete cell division, revealing that cells at
the G2/M phase were unsuccessful in entering and undergoing mitosis
due to the suppression of G2/M regulatory proteins, including
cyclin B1, Cdc25C and Cdc2 (Fig.
4). p21WAF1 is an important inhibitor in modulating cell cycle
progression (25). There is
evidence indicating that p21WAF1 is linked to suppression of the
expression of the Cdc2/cyclin B1 complex (26,27). The results of the present study
revealed that daidzein suppressed the expression of cyclin B1,
Cdc25C and Cdc2, and increased the protein expression levels of
p21/WAF1 (Fig. 5B). However,
daidzein had no significant effect on the expression of either p27
or p53. These results indicated that daidzein stimulated the
expression of p21WAF1, which in turn triggered G2/M phase arrest of
the p53-independent pathway. Cell migration is one of the important
characteristics of cancer cell progression and metastasis (28), and the inhibition of cell
migration may be beneficial in suppressing metastasis under in
vivo conditions. In the present study, it was observed that
daidzein inhibited the migration of SKOV3 cells, which was
associated with a concomitant decrease in the expression of MMP-2/9
(Fig. 5). Dysregulation of the
RAS/ERK pathway is common in all histotypes of ovarian cancer, and
targeting this pathway may offer a novel alternative (29,30). Therefore, the present study
determined the effect of daidzein on the RAF/MEK/ERK pathway, which
revealed that daidzein inhibited the phosphorylation of MEK and
ERK, indicating daidzein may be a potential candidate for targeting
this pathway (Fig. 6). However,
daidzein-induced G2/M cell cycle arrest may not be linked to
inhibition of the RAF/MEK/ERK pathway. Due to the complexity and
cross talk between the pathways, daidzein may induce G2/M2 cell
cycle arrest by a mechanism other than inhibition of RAF/MEK/ERK
pathway and further investigations are require to elucidate
this.
The in vivo evaluation of daidzein revealed
that it markedly inhibitedSKOV3 ovarian tumor growth, compared with
that in the untreated control group and induced with no apparent
toxicity. The Ki67 and cleaved caspase-3 proteins are considered
important cellular markers of proliferation and apoptosis,
respectively (31). The decrease
in Ki67-positive cells and significant increase in the expression
of cleaved caspase-3 suggested that daidzein effectively inhibited
ovarian cancer cell growth in vivo and maybe an important
lead molecule.
Taken together, the results of the present study
indicated that daidzein exerted significant anticancer effects
towards SKOV3 cancer cells by inducing mitochondrial apoptosis and
cell cycle arrest. Daidzein also inhibited tumor growth in
vivo, indicating that it may offer potential as a lead molecule
in the management of ovarian cancer and warrants further
investigation.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Can J Clin. 64:9–29. 2014. View Article : Google Scholar
|
|
2
|
Bast RC Jr, Urban N, Shridhar V, Smith D,
Zhang Z, Skates S, Lu K, Liu J, Fishman D and Mills G: Early
detection of ovarian cancer: Promise and reality. Cancer Treat Res.
107:61–97. 2002.PubMed/NCBI
|
|
3
|
Berek JS: Ovarian cancer. Practical
Gynecologic Oncology. Berek JS and Hacker NF: Lippincott Williams
& Wilkins; Philadelphia, PA: pp. 443epp. 5112005
|
|
4
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: New opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Over B, Wetzel S, Grütter C, Nakai Y,
Renner S, Rauh D and Waldmann H: Natural-product-derived fragments
for fragment-based ligand discovery. Nat Chem. 5:21–28. 2013.
View Article : Google Scholar
|
|
7
|
Clough J, Chen S, Gordon EM, Hackbarth C,
Lam S, Trias J, White RJ, Candiani G, Donadio S, Romanò G, et al:
Combinatorial modification of natural products: Synthesis and in
vitro analysis of derivatives of thiazole peptide antibiotic GE2270
A: A-ring modifications. Biorg Med Chem Lett. 13:3409–3414. 2003.
View Article : Google Scholar
|
|
8
|
Cordier C, Morton D, Murrison S, Nelson A
and O'Leary-Steele C: Natural products as an inspiration in the
diversity-oriented synthesis of bioactive compound libraries. Nat
Prod Rep. 25:719–737. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the last 25 years. J Nat Prod.
70:461–477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cragg GM, Grothaus PG and Newman DJ:
Impact of natural products on developing new anti-cancer agents.
Chem Rev. 109:3012–3043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shah U, Shah R, Acharya S and Acharya N:
Novel anticancer agents from plant sources. Chin J Nat Med.
11:16–23. 2013. View Article : Google Scholar
|
|
12
|
Zhang X, Chen LX, Ouyang L, Cheng Y and
Liu B: Plant natural compounds: Targeting pathways of autophagy as
anti-cancer therapeutic agents. Cell Prolif. 45:466–476. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liggins J, Bluck LJ, Runswick S, Atkinson
C, Coward WA and Bingham SA: Daidzein and genistein contents of
vegetables. British J Nutr. 84:717–725. 2000.
|
|
14
|
Liggins J, Bluck LJ, Runswick S, Atkinson
C, Coward WA and Bingham SA: Bingham Daidzein and genistein content
of fruits and nuts. J Nutr Biochem. 11:326–331. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cancer Genome Atlas Research Network:
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ullah MF, Ahmad A, Bhat SH, Khan HY,
Zubair H, Sarkar FH and Hadi SM: Simulating hypoxia-induced acidic
environment in cancer cells facilitates mobilization and
redox-cycling of genomic copper by daidzein leading to pro-oxidant
cell death: Implications for the sensitization of resistant hypoxic
cancer cells to therapeutic challenges. Biometals. 29:299–310.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi EJ and Kim GH: Daidzein causes cell
cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7
and MDA-MB-453 cells. Phytomedicine. 15:683–690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin S, Zhang QY, Kang XM, Wang JX and Zhao
WH: Daidzein induces MCF-7 breast cancer cell apoptosis via the
mitochondrial pathway. Ann Oncol. 21:263–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang M, Liu Y, Gao Y and Li S:
Silibinin-induced glioma cell apoptosis by PI3K-mediated but
Akt-independent down-regulation of FoxM1 expression. Euro J
Pharmacol. 765:346–354. 2015. View Article : Google Scholar
|
|
21
|
Lee HG, Park WJ, Shin SJ, Kwon SH, Cha SD,
Seo YH, Jeong JH, Lee JY and Cho CH: Hsp90 inhibitor SY-016 induces
G2/M arrest and apoptosis in paclitaxel-resistant human ovarian
cancer cells. Oncol Lett. 13:2817–2822. 2017. View Article : Google Scholar
|
|
22
|
Gautier J, Solomon MJ, Booher RN, Bazan JF
and Kirschner MW: cdc25 is a specific tyrosine phosphatase that
directly activates p34cdc2. Cell. 67:197–211. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K
and Linn S: Molecular mechanisms of mammalian DNA repair and the
DNA damage checkpoints. Ann Rev Biochem. 73:39–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baus F, Gire V, Fisher D, Piette J and
Dulić V: Permanent cell cycle exit in G2 phase after DNA
damage in normal human fibro-blasts. EMBO J. 22:3992–4002. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsu YL, Kuo PL, Lin LT and Lin CC: Asiatic
acid, a triterpene, induces apoptosis and cell cycle arrest through
activation of extracellular signal-regulated kinase and p38
mitogen-activated protein kinase pathways in human breast cancer
cells. J Pharmacol Exp Ther. 313:333–344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun SY, Hail N Jr and Lotan R: Apoptosis
as a novel target for cancer chemoprevention. J Natl Can Inst.
96:662–672. 2004. View Article : Google Scholar
|
|
28
|
Singer G, Oldt R III, Cohen Y, Wang BG,
Sidransky D and Kurman RJ; Shih IeM: Mutations in BRAF and KRAS
characterize the development of low-grade ovarian serous carcinoma.
J Natl Cancer Inst. 95:484–486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Auner V, Kriegshäuser G, Tong D, Horvat R,
Reinthaller A, Mustea A and Zeillinger R: KRAS mutation analysis in
ovarian samples using a high sensitivity biochip assay. BMC Can.
9:1112009. View Article : Google Scholar
|
|
30
|
Scholzen T and Gerdes J: The Ki-67
protein: From the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Noble P, Vyas M, Al-Attar A, Durrant S,
Scholefield J and Durrant L: High levels of cleaved caspase-3 in
colorectal tumour stroma predict good survival. Br J Cancer.
108:2097–2105. 2013. View Article : Google Scholar : PubMed/NCBI
|