Introduction
Ischemic heart disease (IHD) is among the leading
causes of disease-associated mortality worldwide (1). Cardiac oxidative injury during IHD,
including myocardial infarction (MI) and myocardial
ischemia/reperfusion (I/R), leads to cardiomyocyte apoptosis and
results in physiopathological processes, such as fibrosis,
hypertrophy and ventricular remodeling, ultimately leading to heart
failure (2). Excessive oxidative
stress may inhibit pro-survival signaling, reduce cell viability
and trigger cell apoptosis (3).
Activating pro-survival signaling pathways and rescuing
cardiomyocytes from apoptosis has been demonstrated to be an
effective IHD treatment strategy (4).
Flaxseed (Linum usitatissimum L.) is a
functional food and has potential health benefits in a variety of
diseases, including MI, atherosclerosis, diabetes and
hyperlipidaemia (5-7). Secoisolariciresinol diglucoside
(SDG) is a plant lignan extracted from flaxseed that belongs to the
polyphenolic class of chemical compounds. It has been previously
demonstrated that SDG is a potential agent for cardioprotection
(8). SDG has been demonstrated
previously to reduce infarct size and to recover aortic flow and
function in an I/R model (8,9).
It was also determined that SDG improved left ventricular function,
inhibited ventricular remodeling, and promoted angiogenesis in an
MI model via upregulation of vascular endothelial growth factor
protein expression levels (9). In
addition, a previous study demonstrated that SDG prevented
oxidative stress-induced inflammation and apoptosis in cultured
cardiomyocytes (10). These
findings suggested that SDG is a potential agent for
cardioprotection. However, the underlying mechanisms remain to be
elucidated.
Janus kinase 2 (JAK2)/signal transducer and
activator of transcription 3 (STAT3) has been demonstrated to be a
key signaling pathway in cardioprotection (11,12). Activation of JAK2 and STAT3 is
enhanced by ischemic or hypoxia preconditioning in I/R or
hypoxia/reoxygenation cell models (13,14). Previous studies revealed that
suppression of JAK2 by the JAK inhibitor AG490 eliminated the
cardioprotective effects of ischemic preconditioning in myocardial
I/R (13,14). In addition, deficiency of STAT3
has been demonstrated to increase apoptosis in an
inflammation-induced heart damage and myocardial I/R model
(15,16). However, whether SDG may protect
cardiomyocytes against oxidative stress-induced apoptosis through
the JAK2/STAT3 signaling pathway remains to be determined. The
present study used an H2O2-induced in
vitro cardiomyocyte apoptosis model to investigate whether the
JAK2/STAT3 pathway was involved in the anti-apoptotic effect of
SDG.
Materials and methods
Cells and reagents
The H9C2 cell line, which derives from embryonic rat
heart cells, was purchased from the Cell Bank of Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China). SDG
was purchased from Sigma-Aldrich (Merck Millipore; Darmstadt,
Germany; purity >97% as determined by HPLC). JAK2/STAT3
inhibitor WP1066 was purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA; purity >97% as determined by HPLC). Antibodies
against poly-(ADP-ribose) polymerase (PARP, cat. no. 9532), p-JAK2
(Tyr-1007/Tyr-1008, cat. no. 3771), JAK2 (cat. no. 3230), p-STAT3
(Tyr-705, cat. no. 9145), p-STAT3 (Ser-727, cat. no. 9134), STAT3
(cat. no. 12640), Src (cat. no. 2191), B-cell lymphoma-extra-large
(Bcl-xL, cat. no. 2762) and induced myeloid leukemia cell
differentiation protein (Mcl-1, cat. no. 94296) were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). P-Src (Tyr-418,
cat. no. ab40660) was purchased from Abcam (Cambridge, MA, USA).
Annexin V/propidium iodide (PI) Apoptosis Detection kit was
purchased from BD Biosciences (San Jose, CA, USA). Remaining
reagents used in the study were obtained from commercial
sources.
MTT cell viability assay
H9C2 cells were plated at a density of
5.×103 cells per well in 96-well plates. Cells were
treated with 50, 100 and 150 μM SDG with or without 5
μM JAK2/STAT3 inhibitor for 24 h at 37°C and were treated
with 400 μM H2O2 for 2 h at 37°C. Cell
viability was detected using an MTT assay as previously described
(17). Briefly, following
treatment, the culture medium was removed from each well and
replaced with 0.5 mg/ml MTT solution for 4 h at 37°C. Following
incubation, culture medium was removed and 150 μl dimethyl
sulfoxide (Sigma-Aldrich; Merck Millipore) per well was added for
formazan solubilization. The formazan was quantified by determining
the absorbance at 490 nm, using an microplate reader (Thermo Fisher
Scientific, Inc., Walham, MA, USA). The viability of the
cardiomyocytes (%) was calculated as follows: Viability (% of
control)=optical density (OD) mean test group/OD mean control group
×100.
Measurement of lactate dehydrogenase
(LDH) levels
H9C2 cells were plated at a density of
5.0×103 cells/well in 96-well plates. H9C2 cells were
pretreated with 50, 100 and 150 μM SDG for 24 h at 37°C, and
were stimulated with 400 μM H2O2 for 2
h at 37°C. Subsequently, 96-well plates were centrifuged at 400 × g
for 5 min at 4°C to obtain the cellular supernatant and the LDH
release was quantified using LDH Release assay kit (cat. no. C0016;
Beyotime Institute of Biotechnology, Haimen, China) following the
manufacturer's protocol.
Flow cytometric quantification of
apoptosis
Flow cytometry (BD Biosciences) was performed to
quanitfy cell apoptosis. H9C2 cells were treated with indicated
doses of SDG with/without the JAK2/STAT3 inhibitor for 24 h and
then treated with H2O2 for 2 h. The apoptosis
index of H9C2 cells was detected using the Annexin V/PI Apoptosis
Detection kit following the manufacturer's protocol. The data was
analyzed using FlowJo version 6.0 (Tree Star, Inc., Ashland, OR,
USA).
Western blot assay
Western blot assay was performed as previously
described (18). Briefly, protein
extracts of cultured cells were prepared in RIPA lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China). Protein
concentration of each sample was detected using BCA protein assay
kit (Thermo Fisher Scientific, Inc.). Protein samples (15
μg) were separated by 10% sodium dodecyl sulfate gel
electrophoresis, and were transferred onto polyvinylidene fluoride
membranes (Merck Millipore), subsequently blocked in Tris-buffered
saline including 5% non-fat milk. Membranes were incubated at 4°C
overnight with the corresponding primary antibodies [PARP 1:1,000,
p-STAT3 (Tyr-705) 1:1,000, p-STAT3 (Ser-727), STAT3 1:1,000, p-JAK2
(Tyr-1007/ Tyr-1008) 1:1,000, JAK2 1:1,000, p-Src (Tyr-418)
1:1,000, Src 1:1,000, Mcl-1 1:1,000, Bcl-xL 1:1,000, GAPDH
1:5,000]. Then membranes were incubated with horseradish
peroxidase-conjugated anti-rabbit secondary antibody (cat no. 7074;
1:1,000; Cell Signaling Technology, Inc.) for 1 h at 37°C. Each
experiment was repeated three times. Band intensities were
quantified using ImageJ version 1.47 (National Institutes of
Helath, Bethesda, MD, USA) and normalized to the intensities of
GAPDH loading control bands.
Molecular simulation study
Molecular docking and dynamics analyses (MD) were
performed to investigate the mechanism of interaction between SDG
and JAK2. X-ray crystallographic structures of JAK2 (PDB ID: 4C61)
(19) were used as starting
structures for prediction of the binding site on SDG (CID:
9917980). The crystallographic structures of the JAK2 protein were
prepared for molecular docking by removing water and
co-crystallized ligands. Energy minimization of ligands was
performed using YASARA version 16.9.23 (www.yasara.org). The protein kinase domain of JAK2 was
defined in the present study as the protein-ligand binding site of
JAK2. Molecular docking simulation was performed using Autodock
Vina version 1.1.2 (vina.scripps.edu; The Scripps Research Institute, La
Jolla, CA, USA) (20). PyMol
version 1.3 and Ligplot+ version 1.4 were used to
visualize the JAK2-SDG complex (21,22). The conformation with the lowest
binding energy (lower is better) was used as star conformation for
MD simulation.
MD simulation was performed using YASARA (23). All simulations were run with the
AMBER ff99sb force field option selected. The JAK2-SDG complex was
solvated in a cube box of 0.9% NaCl, and the distance between
solute and the box was 5 Å. Simulations were run at 298 K followed
by simulated annealing minimizations which start at 298 K and
velocities were scaled down with 0.9 every ten steps for 500 steps.
Subsequently, 70 ns MD simulation was performed with a time step of
2 fsec and the simulation snapshots were saved every 100 psec.
Statistical analysis
Data are presented as the mean ± standard deviation.
Calculations were performed using SPSS version 13.0 (SPSS, Inc.,
Chicago, IL, USA). One-way analysis of variance was performed to
determine the significance of multiple comparisons. Least
Significant Difference was used for statistical comparisons between
treatment groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
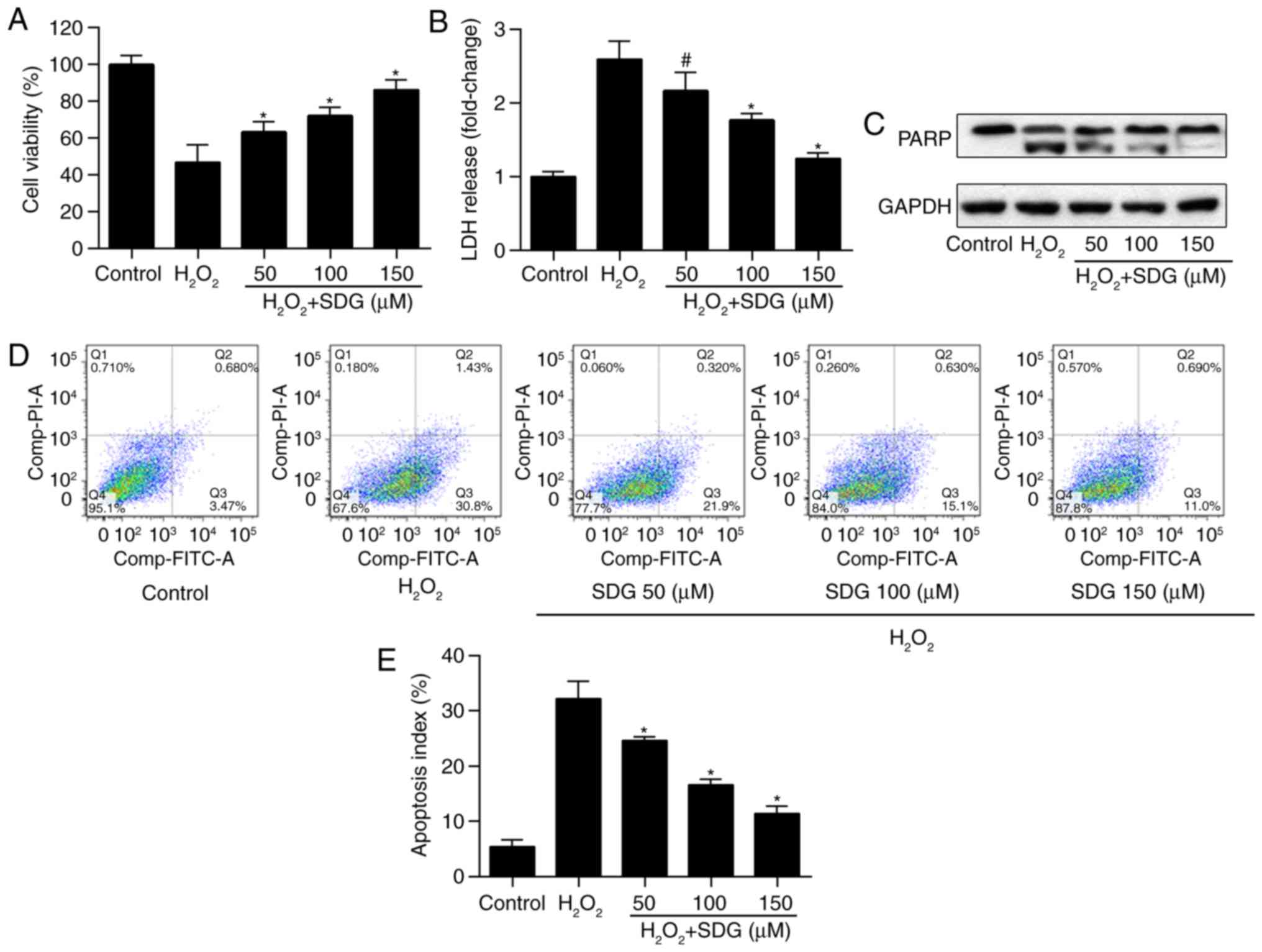
Pretreatment with SDG attenuates
oxidative stress-mediated apoptosis in H9C2 cells
To investigate whether SDG prevents H9C2 from
oxidative stress-mediated apoptosis, cells were pretreated with
various doses of SDG for 24 h and subsequently stimulated with
H2O2 for 2 h. Cell viability of H9C2 was
reduced by H2O2 treatment, but was increased
in a dose-dependent manner in SDG treatment groups (Fig. 1A). LDH release assay indicated
that treatment with H2O2 significantly
increased LDH release, whereas SDG treatment reduced it, in a
dose-dependent manner (Fig. 1B).
Subsequently, the protective effect of SDG against
H2O2-induced apoptosis was examined, by
detecting PARP expression and performing Annexin/PI assay. It was
determined that SDG treatment led to a dose-dependent reduction of
cleaved PARP expression levels (Fig.
1C). Accordingly, SDG treatment significantly reduced the
apoptosis index of H9C2 cells (Fig.
1D and E). This suggested that SDG protected cardiomyocytes
against oxidative stress-mediated cell apoptosis.
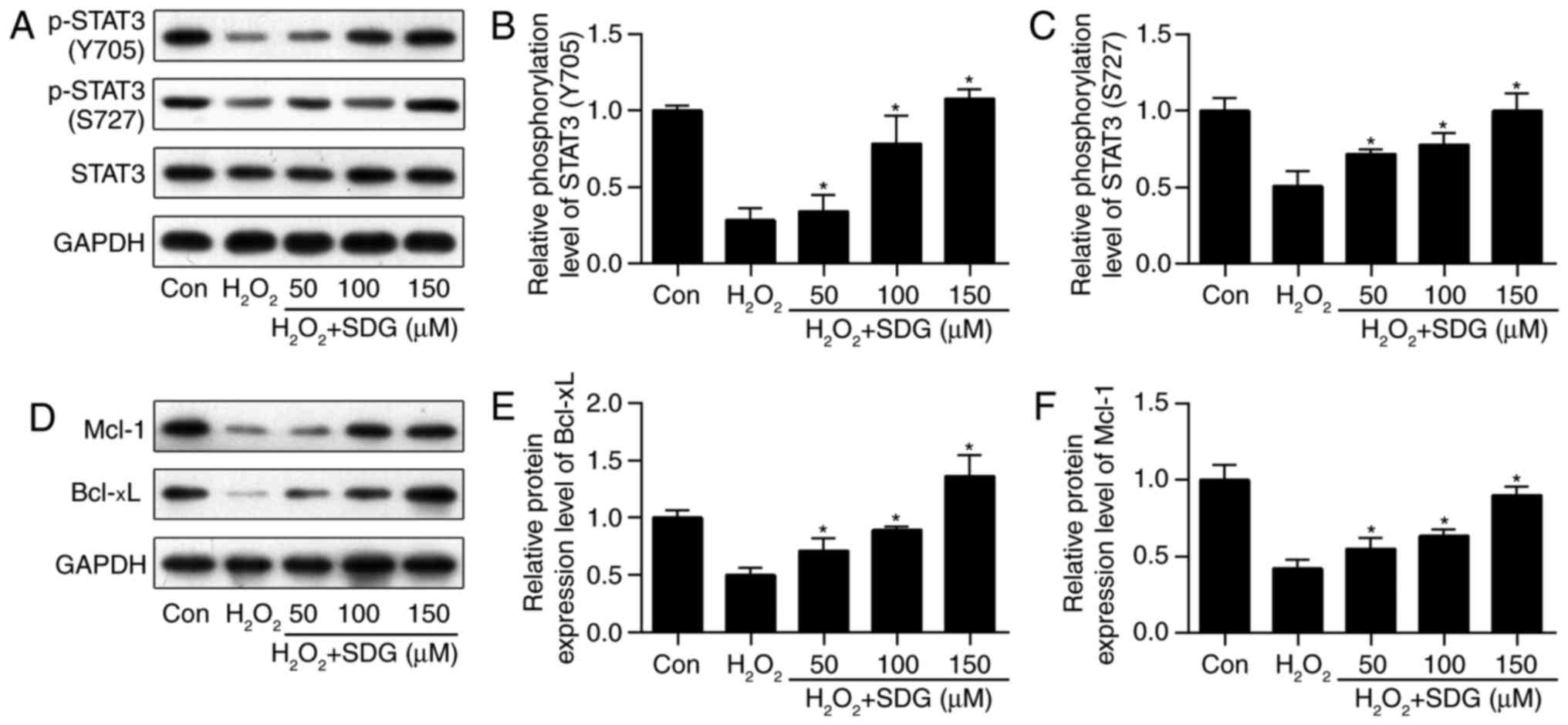
SDG pretreatment rescued STAT3 signaling
activation in H2O2-treated H9C2
It has been previously established that STAT3
activation prevents cardiomyocyte apoptosis (15,16). Therefore, whether SDG increased
the activity of STAT3 in H2O2-treated H9C2
cells was investigated. It was demonstrated that SDG caused a
dose-dependent increase in phosphorylated STAT3 (p-STAT3, at
Tyr-705 and Ser-727) expression levels (Fig. 2A-C). It was further examined
whether SDG affected STAT3-target genes in H9C2 cells. Mcl-1 and
Bcl-xL are target genes of STAT3 involved in cell apoptosis
(24). It was observed that Mcl-1
and Bcl-xL protein levels were significantly increased in
H2O2-treated H9C2 cells following SDG
treatment (Fig. 2D-F). These
findings indicated that SDG rescued STAT3 signaling activation in
H2O2-treated H9C2 cardiomyocytes.
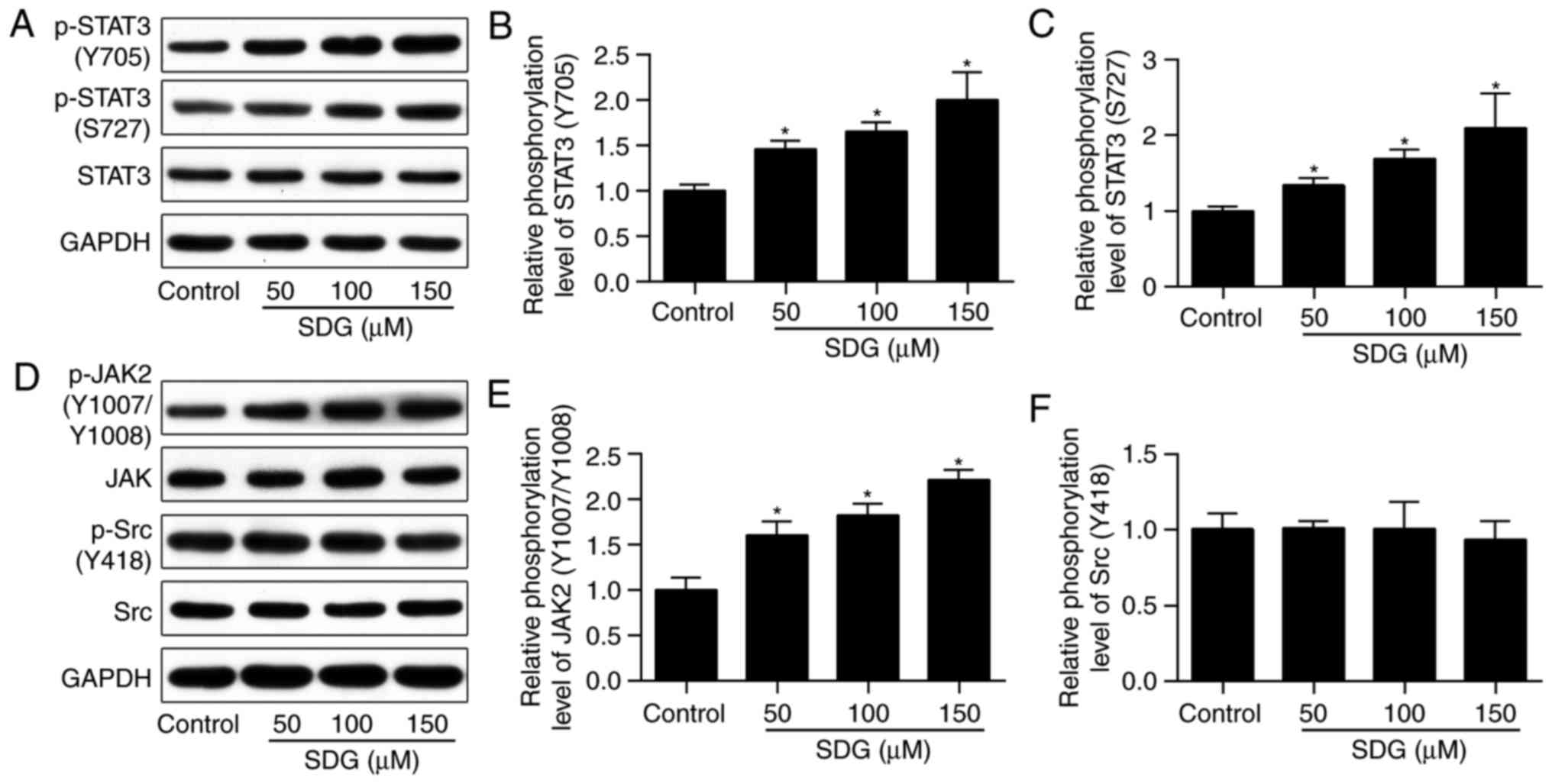
Pretreatment with SDG alone enhances
activation of STAT3 and JAK2, but not Src
To determine whether SDG activated the
STAT3-associated signaling pathway directly or through inhibition
of oxidative stress, phosphorylation levels of STAT3 following
pretreatment with indicated concentrations of SDG for 24 h were
investigated. As illustrated in Fig.
3A-C, pretreatment with SDG led to a dose-dependent increase of
expression levels of p-STAT3 at the Tyr-705 and Ser-727 sites. It
has been previously established that STAT3 may be activated by JAK2
and non-receptor tyrosine kinase Src (25,26). Therefore, the present study
determined if activation of JAK2 and/or Src occurred in SDG-treated
H9C2 cells. As presented in Fig.
3D-F, phosphorylation of Src did not change following SDG
treatment, and expression of p-JAK2 was increased in SDG-treated
groups. These findings suggested that SDG may directly activate the
JAK2/STAT3 signaling pathway.
Inhibition of JAK2/STAT3 attenuates the
anti-apoptotic effects of SDG
In order to determine if the anti-apoptotic effect
of SDG in H9C2 cells was due to the activation of the JAK2/STAT3
signaling pathway, WP1066 which is a JAK2/STAT3 inhibitor (27) was used. As illustrated in Fig. 4A-D, phosphorylation of JAK2 and
STAT3 was increased by SDG treatment; however, it was reduced in
H9C2 cells treated with with WP1066 and with/without SDG. These
results indicated that co-treatment with WP1066 inhibited the
activation effect of SDG on the JAK2/STAT3 signaling pathway.
Following treatment with SDG and/or WP1066 for 24 h, H9C2 cells
were treated with H2O2 for 2 h, and
phosphorylation of JAK2 and STAT3 was measured by western blot
analysis. As demonstrated in Fig.
4E-J, co-treatment of WP1066 significantly reduced the
phosphorylation levels of JAK2 and STAT3, as well as the expression
levels of Mcl-1 and Bcl-xL, were increased by SDG in
H2O2-treated H9C2 cells. These results
confirmed that WP1066 abolished the activation effect of SDG on the
JAK2/STAT3 signaling pathway. Finally, MTT and Annexin V/PI
staining was performed to determine the role of JAK2/STAT3
signaling in the anti-apoptotic effect of SDG. As demonstrated in
Fig. 4K, viablity of H9C2 cells
was significantly reduced by treatment with
H2O2 or WP1066 alone. SDG treatment rescued
the viability of H2O2-stimulated H9C2 cells,
whereas inhibition of JAK2/STAT3 impeded the protective effects of
SDG on cell viability, reducing it from 82.5 to 50.3%. Accordingly,
inhibition of JAK2/STAT3 partly abolished the anti-apoptotic
effects of SDG on apoptotic rate from 10.8 to 25.6% (Fig. 4L). These results implied that the
anti-apoptotic effect of SDG was, partially at least, due to the
activation of the JAK2/STAT3 signaling pathway.
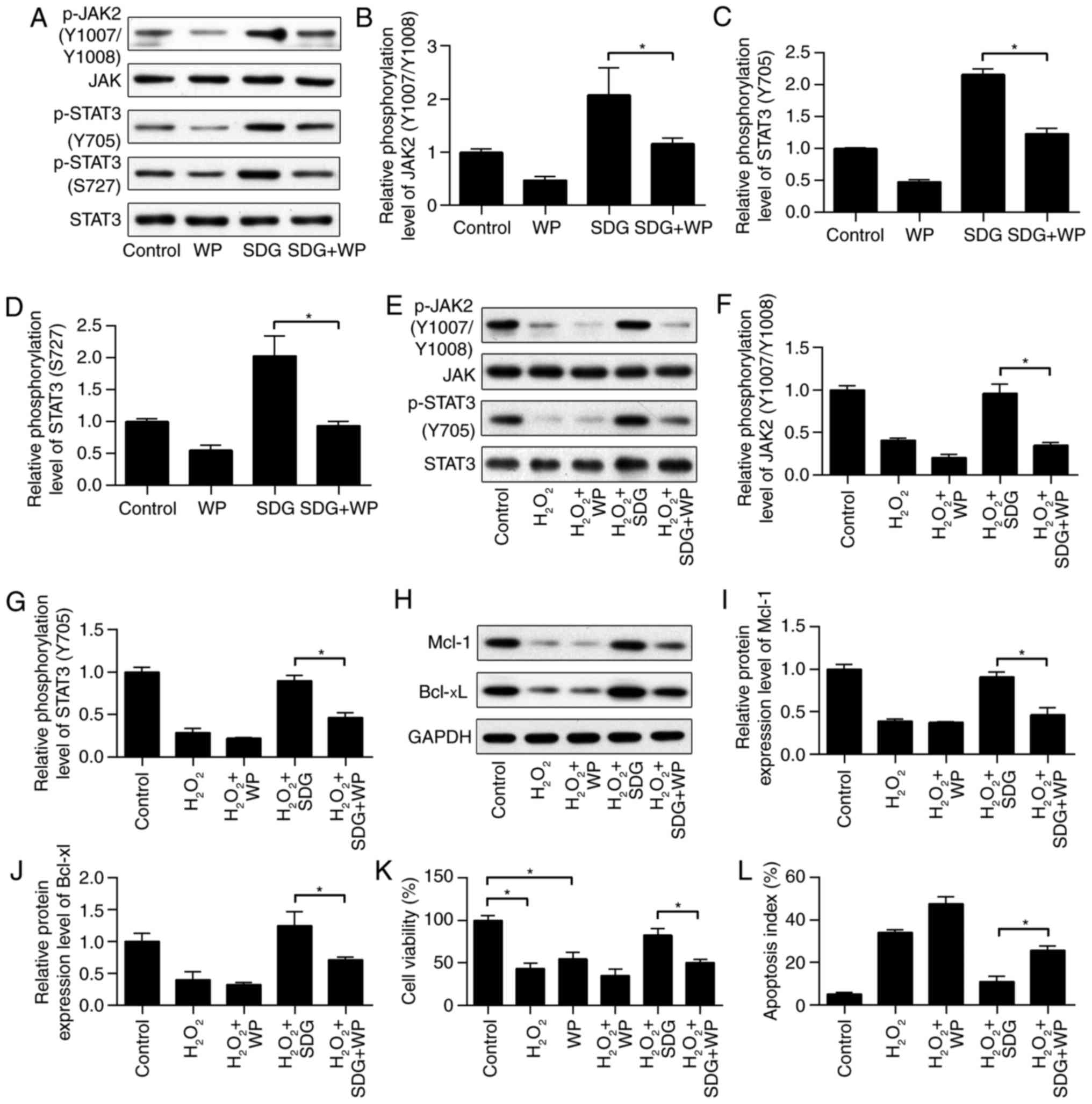 | Figure 4Inhibition of JAK2/STAT3 signaling
attenuated the anti-apoptotic effects of SDG. H9C2 cells were
pretreated with SDG (150 μM) with or without the JAK2/STAT3
inhibitor WP1066 (5 μM). Expression levels of (A) p-JAK2,
JAK2, p-STAT3 (Tyr-705), STAT3 (Ser-727) and STAT3 and (B-D)
quantification of their relative phosphorylation levels, as
measured by western blot analysis (n=3). H9C2 cells were pretreated
with SDG (150 μM) with or without the JAK2/STAT3 inhibitor
WP1066 (5 μM), following stimulation with
H2O2 (400 μM) for 2 h. Expression
levels of (E) p-JAK2, JAK2, p-STAT3 (Tyr-705), and STAT3 and (F and
G) quantification of their relative phosphorylation levels, as
measured by western blot analysis (n=3). Expression levels of (H)
Mcl-1 and Bcl-xL and (I and J) quantification of their relative
protein levels, as measured by western blot analysis (n=3). (K)
Viability of H9C2 cells (n=6) and (L) apoptosis ratio (n=3), as
measured by MTT and Annexin V/PI staining, respectively. Data are
presented as the mean ± standard deviation. *P<0.01.
Bcl-xL, B-cell lymphoma-extra-large; Mcl-1, induced myeloid
leukemia cell differentiation protein; JAK2, Janus kinase 2; p,
phosphorylated; SDG, secoisolariciresinol diglucoside; STAT3,
signal transducer and activator of transcription 3; WP, WP1066
JAK2/STAT3 inhibitor. |
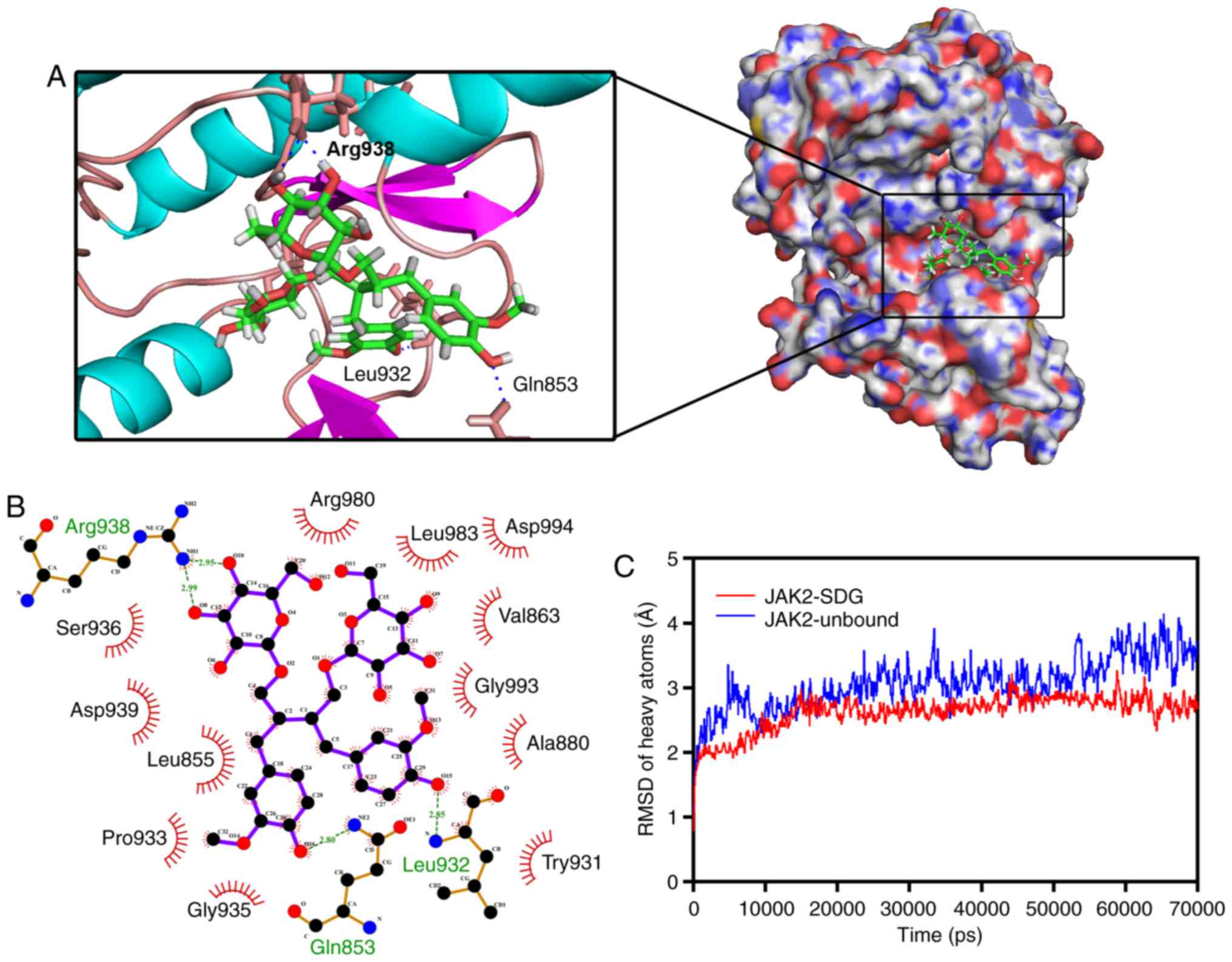
SDG directly targets the kinase domain of
JAK2
The present findings demonstrated that SDG
significantly enhanced the phosphorylation levels of JAK2.
Therefore, it is possible that SDG directly activates JAK2.
Subsequently, the interaction between the protein kinase domain of
JAK2 and SDG was investigated, by molecular docking and MD
simulation. SDG molecule was docked into the protein kinase domain
of the JAK2 protein (PDB: 4C61). The binding energy of the JAK2-SDG
complex was −8.258 kcal/mol. Three- and two-dimensional structures
of the JAK2-SDG complex are presented in Fig. 5A and B. Two hydrogen bonds were
formed between SDG and JAK2 Leu-932 and Gln-853. The distance of
hydrogen bonds between SDG and JAK2 Leu-932 and Gln-853 were Å,
2.85 and 2.80 Å, respectively. Two hydrogen bonds were formed
between SDG and Arg-938 of JAK2, and the distance of hydrogen bonds
between them was 2.99 and 2.95 Å, respectively. The best
conformation of JAK2-SDG was used as the star conformation for the
MD simulation by YASARA. Fig. 5C
demonstrates the evolution of backbone atoms and the
root-mean-square deviation (RMSD) of the complex with respect to
the minimized structure. The RMSD track in the last 50 nsec
fluctuated from 2.52 to 4.14 Å in the unbound state of the JAK2
protein, and from 2.32 to 3.26 Å in the JAK2-SDG complex. These
results suggested strong binding between the kinase domain of JAK2
and SDG, which indicated that SDG may directly target JAK2.
Discussion
SDG is isolated from flaxseed and has a number of
health benefits, such as cardioprotective activity (8). In the present study, the
anti-apoptotic effect of SDG was investigated in
H2O2-treated H9C2 cells and it was
demonstrated that SDG pretreatment significantly reduced apoptosis
of cells in the presence of H2O2, which is in
agreement with a previous study (10).
The STAT3 signaling pathway is important for cell
resistance to apoptosis. Inhibition of STAT3 has been previously
reported in heart failure in human patients and animal models
(28,29). The present study revealed that
H2O2 significantly reduced the activation of
STAT3. A previous study revealed that treatment with
H2O2 increased STAT3 activation in H9C2 cells
(30); however, the duration of
exposure to H2O2 (30 min) was shorter than
that in the present study. SDG pretreatment significantly rescued
phosphorylation levels of STAT3 in
H2O2-treated cardiomyocytes. Following
phosphorylation at Tyr-705 and Ser-727, STAT3 translocates into the
nucleus and binds to specific DNA response elements in the promoter
region of STAT3-target genes (31). Expression levels of Bcl-xL and
Mcl-1, which are STAT3-target genes, were reduced following
H2O2 exposure, and increased in SDG
pretreatment groups. These results indicated that treatment with
SDG rescued the activation of the STAT3 pathway in
H2O2-treated cardiomyocytes.
In the present study, H2O2
significantly reduced the activation of STAT3. Whether SDG directly
activated the STAT3-associated signaling pathway or inhibited
oxidative stress was also investigated. Cardiomyocytes were treated
with SDG alone and the phosphorylation levels of STAT3 were
quantified which demonstrated that SDG significantly activated
STAT3, indicating that SDG directly activated STAT3-related
signaling pathway. However, the possibility that SDG inhibited
oxidative stress level by activating oxidative stress-associated
signaling pathway cannot be ruled out. It has been previously
demonstrated that STAT3 may be activated by upstream tyrosine
kinases including Src and JAK2 (31,32). To determine whether SDG activated
STAT3 or its upstream signaling molecules, phosphorylation levels
of JAK2 and Src were investigated. In the present study, SDG
increased JAK2 phosphorylation in a dose-dependent manner, whereas
phosphorylation of Src was not altered. These results indicated
that SDG may activate STAT3 signaling through activation of
JAK2.
Subsequently, the function of JAK2/STAT3 in the
anti-apoptotic action of SDG was investigated using WP1066, a JAK2
and STAT3 inhibitor (27). The
anti-apoptotic effects of SDG were counteracted by WP1006,
suggesting that JAK2/STAT3 is the pharmacological target of SDG and
activation of JAK2/STAT3 by SDG may be a novel treatment strategy
for cardiac protection. It has been previously demonstrated that
SDG induced angiogenesis in human coronary arteriolar endothelial
cells (8). It was also
demonstrated that flaxseed extract, which contains SDG, induced
proliferation and migration of human dermal fibroblasts and may be
a novel therapy for infected wounds (33).
The present findings suggested that SDG may target
JAK2. Therefore, molecular docking and MD simulation was performed
to determine whether SDG may bind the protein kinase domain of
JAK2. The binding energy of the JAK2-SDG complex indicated a strong
binding ability. Furthermore, MD simulation results demonstrated
that JAK2-SDG binding conformation was stable. These results
indicated that SDG directly targets JAK2, acting as a potential
JAK2 agonist.
In conclusion, SDG was demonstrated to exert
anti-apoptotic activities. These effects were partially mediated
via activation of the JAK2/STAT3 signaling pathway and it was
demonstrated that SDG may be a potential JAK2 agonist. As
JAK2/STAT3 activation has an essential role in anti-apoptosis
(28,29), the present findings may provide a
novel perspective for investigating the mechanism by which SDG or
flaxseed may aid in treating IHD.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81673805, 81373575,
81673949, and 81601779), Guangdong Natural Science Foundation
(grant nos. 2014A030313495 and 2014A030310210), the Science and
Technology Planning Project of Guangdong Province (grant nos.
2014A020221013 and 2014A020221059), the Science Technology and
Innovation Committee of Shenzhen (grant no. JCYJ20150630164505508)
and the Traditional Chinese Medicine Bureau of Guangdong Province
(grant no. 20161260).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman
M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C,
et al: Heart disease and stroke statistics-2017 update: A report
from the American heart association. Circulation. 135:e146–e603.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh SS and Kang PM: Mechanisms and
inhibitors of apoptosis in cardiovascular diseases. Curr Pharm Des.
17:1783–1793. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao ZQ: Oxidative stress-elicited
myocardial apoptosis during reperfusion. Curr Opin Pharmacol.
4:159–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hamacher-Brady A, Brady NR and Gottlieb
RA: The interplay between pro-death and pro-survival signaling
pathways in myocardial ischemia/reperfusion injury: Apoptosis meets
autophagy. Cardiovasc Drugs Ther. 20:445–462. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Slavova-Kazakova A, Karamać M, Kancheva V
and Amarowicz R: Antioxidant activity of flaxseed extracts in lipid
systems. Molecules. 21:E172015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prasad K and Dhar A: Flaxseed and
diabetes. Curr Pharm Des. 22:141–144. 2016. View Article : Google Scholar
|
|
7
|
Prasad K: Flaxseed and cardiovascular
health. J Cardiovasc Pharmacol. 54:369–377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Penumathsa SV, Koneru S, Thirunavukkarasu
M, Zhan L, Prasad K and Maulik N: Secoisolariciresinol diglucoside:
Relevance to angiogenesis and cardioprotection against
ischemia-reperfusion injury. J Pharmacol Exp Ther. 320:951–959.
2007. View Article : Google Scholar
|
|
9
|
Penumathsa SV, Koneru S, Zhan L, John S,
Menon VP, Prasad K and Maulik N: Secoisolariciresinol diglucoside
induces neovascularization-mediated cardioprotection against
ischemia-reperfusion injury in hypercholesterolemic myocardium. J
Mol Cell Cardiol. 44:170–179. 2008. View Article : Google Scholar
|
|
10
|
Puukila S, Bryan S, Laakso A, Abdel-Malak
J, Gurney C, Agostino A, Belló-Klein A, Prasad K and Khaper N:
Secoisolariciresinol diglucoside abrogates oxidative stress-induced
damage in cardiac iron overload condition. PLoS One.
10:e1228522015. View Article : Google Scholar
|
|
11
|
Boengler K, Hilfiker-Kleiner D, Drexler H,
Heusch G and Schulz R: The myocardial JAK/STAT pathway: From
protection to failure. Pharmacol Ther. 120:172–185. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bolli R, Stein AB, Guo Y, Wang OL, Rokosh
G, Dawn B, Molkentin JD, Sanganalmath SK, Zhu Y and Xuan YT: A
murine model of inducible, cardiac-specific deletion of STAT3: Its
use to determine the role of STAT3 in the upregulation of
cardioprotective proteins by ischemic preconditioning. J Mol Cell
Cardiol. 50:589–597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xuan YT, Guo Y, Han H, Zhu Y and Bolli R:
An essential role of the JAK-STAT pathway in ischemic
preconditioning. Proc Natl Acad Sci USA. 98:9050–9055. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suleman N, Somers S, Smith R, Opie LH and
Lecour SC: Dual activation of STAT-3 and Akt is required during the
trigger phase of ischaemic preconditioning. Cardiovasc Res.
79:127–133. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hilfiker-Kleiner D, Hilfiker A, Fuchs M,
Kaminski K, Schaefer A, Schieffer B, Hillmer A, Schmiedl A, Ding Z,
Podewski E, et al: Signal transducer and activator of transcription
3 is required for myocardial capillary growth, control of
interstitial matrix deposition, and heart protection from ischemic
injury. Circ Res. 95:187–195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jacoby JJ, Kalinowski A, Liu MG, Zhang SS,
Gao Q, Chai GX, Ji L, Iwamoto Y, Li E, Schneider M, et al:
Cardiomyocyte-restricted knockout of STAT3 results in higher
sensitivity to inflammation, cardiac fibrosis, and heart failure
with advanced age. Proc Natl Acad Sci USA. 100:12929–12934. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu B, Zhang J, Liu W, Liu N, Fu X, Kwan H
and Liu S, Liu B, Zhang S, Yu Z and Liu S: Calycosin inhibits
oxidative stress-induced cardiomyocyte apoptosis via activating
estrogen receptor-α/β. Bioorg Med Chem Lett. 26:181–185. 2016.
View Article : Google Scholar
|
|
18
|
Liu B, Liu NN, Liu WH, Zhang SW, Zhang JZ,
Li AQ and Liu SM: Inhibition of lectin-like oxidized low-density
lipoprotein receptor-1 reduces cardiac fibroblast proliferation by
suppressing GATA binding protein 4. Biochem Biophys Res Commun.
475:329–334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Su Q, Ioannidis S, Chuaqui C, Almeida L,
Alimzhanov M, Bebernitz G, Bell K, Block M, Howard T, Huang S, et
al: Discovery of 1-methyl-1H-imidazole derivatives as potent Jak2
inhibitors. J Med Chem. 57:144–158. 2014. View Article : Google Scholar
|
|
20
|
Trott O and Olson AJ: AutoDock Vina:
Improving the speed and accuracy of docking with a new scoring
function, efficient optimization, and multithreading. J Comput
Chem. 31:455–461. 2010.
|
|
21
|
Yang Y, Hu B and Lill MA: WATsite2.0 with
PyMOL Plugin: Hydration site prediction and visualization. Methods
Mol Biol. 1611:123–134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Laskowski RA and Swindells MB:
LigPlot+: Multiple ligand-protein interaction diagrams
for drug discovery. J Chem Inf Model. 51:2778–2786. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krieger E, Koraimann G and Vriend G:
Increasing the precision of comparative models with YASARA NOVA-a
self-parameterizing force field. Proteins. 47:393–402. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Teng Y, Ross JL and Cowell JK: The
involvement of JAK-STAT3 in cell motility, invasion, and
metastasis. JAKSTAT. 3:e280862014.PubMed/NCBI
|
|
25
|
Nam S, Xie J, Perkins A, Ma Y, Yang F, Wu
J, Wang Y, Xu RZ, Huang W, Horne DA and Jove R: Novel synthetic
derivatives of the natural product berbamine inhibit Jak2/Stat3
signaling and induce apoptosis of human melanoma cells. Mol Oncol.
6:484–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niu G, Bowman T, Huang M, Shivers S,
Reintgen D, Daud A, Chang A, Kraker A, Jove R and Yu H: Roles of
activated Src and Stat3 signaling in melanoma tumor cell growth.
Oncogene. 21:7001–7010. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song B, Jin H, Yu X, Zhang Z, Yu H, Ye J,
Xu Y, Zhou T, Oudit GY, Ye JY, et al: Angiotensin-converting enzyme
2 attenuates oxidative stress and VSMC proliferation via the
JAK2/STAT3/SOCS3 and profilin-1/MAPK signaling pathways. Regul
Pept. 185:44–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Podewski EK, Hilfiker-Kleiner D, Hilfiker
A, Morawietz H, Lichtenberg A, Wollert KC and Drexler H:
Alterations in Janus kinase (JAK)-signal transducers and activators
of transcription (STAT) signaling in patients with end-stage
dilated cardiomyopathy. Circulation. 107:798–802. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang W, Qu X, Chen B, Snyder M, Wang M,
Li B, Tang Y, Chen H, Zhu W, Zhan L, et al: Critical roles of STAT3
in β-adrenergic functions in the heart. Circulation. 133:48–61.
2016. View Article : Google Scholar
|
|
30
|
Reed DK and Arany I: Sex hormones
differentially modulate STAT3-dependent antioxidant responses
during oxidative stress in renal proximal tubule cells. In Vivo.
28:1097–1100. 2014.PubMed/NCBI
|
|
31
|
Carpenter RL and Lo HW: STAT3 target genes
relevant to human cancers. Cancers (Basel). 6:897–925. 2014.
View Article : Google Scholar
|
|
32
|
Chen-Scarabelli C, Saravolatz IL, McCaukey
R, Scarabelli G, Di Rezze J, Mohanty B, Barry S, Latchman D,
Georgiadis V, McCormick J, et al: The cardioprotective effects of
urocortin are mediated via activation of the Src tyrosine
kinase-STAT3 pathway. JAKSTAT. 2:e248122013.
|
|
33
|
Czemplik M, Kulma A, Bazela K and Szopa J:
The biomedical potential of genetically modified flax seeds
overexpressing the glucosyltransferase gene. BMC Complement Altern
Med. 12:2512012. View Article : Google Scholar : PubMed/NCBI
|