Introduction
Periodontitis is accompanied by inflammation and
alveolar bone loss, and the progression of periodontitis can lead
to tooth loss (1). To restore
lost periodontal structures, a number of treatments, including
guided tissue regeneration, bone grafting and enamel matrix
derivatives, are available in animal models and in clinical
settings. However, complete regeneration is hardly observed.
Low-intensity pulsed ultrasound (LIPUS) is a non-invasive acoustic
radiation at intensities ranging 30-100 mW/cm2. LIPUS
has been demonstrated to accelerate fracture healing in both
experimental and clinical studies (2–5).
Since LIPUS displayed beneficial effects in accelerating the
fracture healing process, an increasing number of studies have been
conducted to treat periodontal disease (6–11).
A previous study from our group has demonstrated that LIPUS can
increase the number, volume, and area of new alveolar bone
trabeculae, which display potential uses in periodontal
regeneration (10). From a
biological perspective, it has been reported that mechanical stress
induces a variety of cellular events including proliferation
(12,13), differentiation (14) and migration (15,16). As a biophysical stimulus, LIPUS
may have a similar function since it transmits mechanical energy as
acoustical pressure waves into cells and tissues (3). LIPUS triggers a series of
biochemical reactions at the cellular level and can stimulate cell
proliferation, osteogenic differentiation and the production of
extracellular matrix (17).
However, the detailed mechanism of LIPUS-promoted fracture healing
and periodontal regeneration is not fully elucidated yet.
Reparative cell migration is a crucial cellular
event during the healing of wounds in the periodontal ligament
(18). Stromal cell-derived
factor 1 (SDF-1), also known as C-X-C motif chemokine ligand 12, is
a type of chemokine expressed by a variety of tissues. The
significant role of SDF-1 in stem cell homing and tissue
regeneration has been well-demonstrated in the literature (19–21). A prior study has demonstrated that
LIPUS induces the homing of circulating osteogenic progenitors to
the fracture site (22).
Subsequently, researchers demonstrated that using LIPUS to
stimulate rat bone marrow-derived stem cells (BMSCs) resulted in
enhanced SDF-1 expression and recruitment of BMSCs (23). Kimura et al (24) reported that SDF-1 expression was
increased around periodontal tissue defects and that endogenous
stem cells were recruited to the wound site. Therefore, recruitment
of stem cells may be a novel target for periodontal treatment
(24). In addition, researchers
found that SDF-1 could induce collagen I expression, proliferation
and migration of human periodontal ligament cells (PDLSCs)
(25), which may help periodontal
ligament repair and regeneration. Although these studies concluded
that LIPUS could stimulate SDF-1 expression, how LIPUS promotes
periodontal regeneration remains unknown.
Twist family bHLH transcription factor 1 (TWIST1)
encodes a basic helix-loop-helix transcription factor, known to
contribute to mesodermal and skeletal tissue development (26) and to cell migration in the
epithelial-mesenchymal transition (EMT) (27,28). Mahmoud et al (29) demonstrated that TWIST1 can be
regulated by low shear stress in endothelial cells (ECs), which
further enhances EC proliferation and migration. Desprat et
al (30) reported that TWIST1
can be regulated by mechanical force during Drosophila development.
In addition, a previous study suggested that occlusal force might
regulate TWIST1 gene expression in the periodontal ligament
(31). Furthermore, TWIST1 was
demonstrated to directly activate SDF-1 expression, which promotes
cell migration (26). These
results suggested that TWIST1 may be involved in the signal
transduction of LIPUS.
Therefore, the present study hypothesized that LIPUS
may regulate the production of SDF-1 through TWIST1 in PDLSCs, an
effect that may benefit periodontal tissue regeneration.
Materials and methods
Cell culture
All experiments in the present study were approved
by the Committee of Ethics of Chongqing Medical University
(Chongqing, China) and written informed consent was acquired from
each patient.
Healthy premolars were extracted from patients
(between April and July 2017; n=5, 12–18 years of age) for
orthodontic reasons at the College of Stomatology, Chongqing
Medical University (Chongqing, China). PDLSCs were isolated and
cultured as described previously (32), with minor modifications. Briefly,
the periodontal ligament was scraped from the middle third of the
root surface, minced using sterile scissors, and digested in a
solution of 3 mg/ml collagenase type I (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 1 h at 37°C. The suspension was
centrifuged, seeded into T25 flasks and cultured in α minimum
essential medium (α-MEM; Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA), supplemented with 10% fetal bovine serum (FBS;
HyClone; GE Healthcare Life Sciences) and 1%
penicillin/streptomycin solution (Solarbio Co., Ltd., Beijing,
China) in a humidified atmosphere of 5% CO2 at 37°C. The
medium was refreshed every three days. Passage 3–4 PDLSCs were used
in the following experiments.
Flow cytometry
Briefly, PDLSCs were trypsinized and washed with
PBS. Then, the cells were stained with primary antibodies including
phycoerythrin (PE)-conjugated mouse anti-human CD34 (BD
Biosciences, San Jose, CA, USA, catalog number: 555822),
PE-conjugated mouse anti-human CD73 (BD Biosciences, catalog
number: 550257), PE-conjugated mouse anti-human CD146 (BD
Biosciences, catalog number: 550315) and fluorescein
isothiocyanate-conjugated mouse anti-human STRO-1 (BioLegend, San
Diego, CA, USA, catalog number: 340106) according to the
manufacturer's protocols. Flow cytometry was performed on a BD
Influx flow cytometer (BD Biosciences) and analyzed using a BD
FACS™ Software version 1.0 (BD Biosciences).
Differentiation assays
The differentiation assay was performed according to
published methods (33). Briefly,
PDLSCs were induced with osteogenic medium containing 10% FBS, 5 mM
L-glycerophosphate (Solarbio Co., Ltd.,), 100 nM dexamethasone
(Solarbio Co., Ltd.) and 50 mg/ml ascorbic acid (Solarbio Co.,
Ltd.) for 21 days. Then, the cells were fixed in 4%
paraformaldehyde and stained with 0.2% alizarin red solution
(Solarbio Co., Ltd.). For adipogenic differentiation, PDLSCs were
cultured in α-MEM supplemented with 10% FBS, 2 mM insulin (Solarbio
Co., Ltd.), 0.5 mM isobutylmethylxanthine (Solarbio Co., Ltd.), and
10 nM dexamethasone (Solarbio Co., Ltd.) for 14 days. The cells
were fixed and stained with Oil Red O solution (Solarbio Co.,
Ltd.). The control cells were cultured in α-MEM with 10% FBS.
Images were acquired under a phase-contrast inverted microscope
(Nikon Corporation, Tokyo, Japan).
LIPUS treatment
Then, 24 h following cell seeding, LIPUS at various
intensities (30, 60 and 90 mW/cm2) was applied to
stimulate the PDLSCs for 0.5 h in a water bath at 37°C, according
to our previous study using a LIPUS device (National Engineering
Research Center of Ultrasound Medicine, Chongqing, China) (34). The LIPUS conditions were at a
frequency of 1.5 MHz, a pulse duty cycle of 1:4, and a pulse
repetition frequency of 1.0 kHz. The concentrations of recombinant
human SDF-1α (PeproTech, Inc., Rocky Hill, NJ, USA) and AMD3100
(Sigma-Aldrich; Merck KGaA) used in the present study were 100
ng/ml and 5 μg/ml, respectively, according to the literature
(35).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cells from LIPUS-treated and non-treated groups were
collected for total RNA isolation using RNAiso Plus (Takara
Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's protocols. Then, the RNA was reverse transcribed
into cDNA using PrimeScript RT master mix (Takara Biotechnology
Co., Ltd.). qPCR was conducted with SYBR Premix Ex Taq II (Tli
RNaseH Plus; Takara Biotechnology Co., Ltd) on a CFX96 TouchTM
Real-Time PCR detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Thermocycling conditions were: 1 cycle at 95°C
for 30 sec, following 40 cycles of 95°C for 5 sec and 60°C for 31
sec, then 1 cycle of 95°C for 15 sec, 60°C for 1 min, and 95°C for
15 sec. Relative mRNA expression was calculated by the
2−ΔΔCq method (36).
Primer sequences were as follows: SDF-1, forward
5′-TGTGCATTGACCCGAAGCTA-3′ and reverse 5′-CACACCTGGTCCTCATGGTT-3′;
TWIST1, forward 5′-TCCAAATTCAAAGAAACAGGGCG-3′ and reverse
5′-CAGAATGCAGAGGTGTGAGGA-3′; and GAPDH, forward
5′-CTTTGGTATCGTGGAAGGACTC-3′and reverse
5′-GTAGAGGCAGGGATGATGTTCT-3′.
ELISA
The supernatants were collected and tested with the
SDF-1 ELISA kit (catalog number: SEA122Hu; Cloud-Clone Corp.,
Houston, TX, USA), according to the manufacturer's protocols. The
optical absorbance was measured at 450 nm with an EnSpire Multimode
plate reader (PerkinElmer, Inc., Waltham, MA, USA). The
concentration of SDF-1 was determined by comparing the optical
density of the samples to the standard curve.
Wound healing assay
PDLSCs were seeded at a density of 10,000
cells/cm2 in 6-well plates. The culture medium was
removed after 24 h, and a wound was made in the center of each well
by scratching with a 200 μl pipette tip. Then, the cells
were washed twice with PBS and cultured with serum-free media prior
to exposure to the LIPUS method described above. Scratch wounds
were imaged using an inverted microscope (Nikon Corporation) at 0,
6, 12, and 24 h post-wounding, and areas were measured using
Image-Pro-Plus software (Media Cybernetics, Inc., Rockville, MD,
USA) according to the published protocol (37).
Transwell migration assay
Migration assays were assessed in 6-well transwell
inserts with 8 μm pore membrane filters (Corning
Incorporated, Corning, NY, USA), as described previously (38). PDLSCs were grown to subconfluence
(70%) prior to harvesting by trypsinization and seeded into the
upper chamber (1×105 cells per chamber). Serum-free
media were used at the upper and lower chamber. Following overnight
incubation under 5% CO2 at 37°C, the cells remaining on
the top of the membrane were removed, and cells that had migrated
to the bottom side were fixed with 95% ethanol for 10 min and
stained with 0.1% crystal violet. Images were captured and counted
under a light microscope.
Small interfering (si) RNA
transfection
Three different siRNAs specific against TWIST1, and
one scrambled sequence serving as control, were obtained from
Sangon Biotech Co., Ltd. (Shanghai, China). The sequences were:
TWIST1-812, sense 5'-GGUACAUCGACUUCCUCUATT-3′ and antisense
5′-UAGAGGAAGUCGAUGUACCTT-3′; TWIST1-991, sense
5′-CCGGAGACCUAGAUGUCAUTT-3′ and antisense
5′-AUGACAUCUAGGUCUCCGGTT-3′; TWIST1-1577, sense
5′-GGUGUCUAAAUGCAUUCAUTT-3′ and antisense
5′-AUGAAUGCAUUUAGACACCTT-3′); and scramble-siRNA, sense
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′. PDLSCs were seeded in a 6-well plate.
On the following day, overnight transfection was performed with
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) using 2 μg of siRNA, according to
the manufacturer's protocols. After 24 h, RT-qPCR and ELISA assays
were performed to evaluate the efficiency of siRNA transfection as
described above.
LIPUS treatment of the TWIST1-knockdown
PDLSCs
To evaluate the biological effect of TWIST1 on
PDLSCs, six treatment groups were examined: Group 1, control; group
2, scramble-siRNA; group 3, TWIST1-siRNA; group 4, control+LIPUS;
group 5, Scramble-siRNA+LIPUS; and group 6, TWIST1-siRNA+LIPUS.
After 24 h, wound healing assay and transwell migration assay were
performed as described above.
Statistical analysis
All data are presented as the mean ± standard
deviation from at least three replicates. The results were analyzed
with one- or two-way analysis of variance or Student's t-test with
Holm-Sidak test as a post hoc test, as appropriate, using GraphPad
Prism version 6.01 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
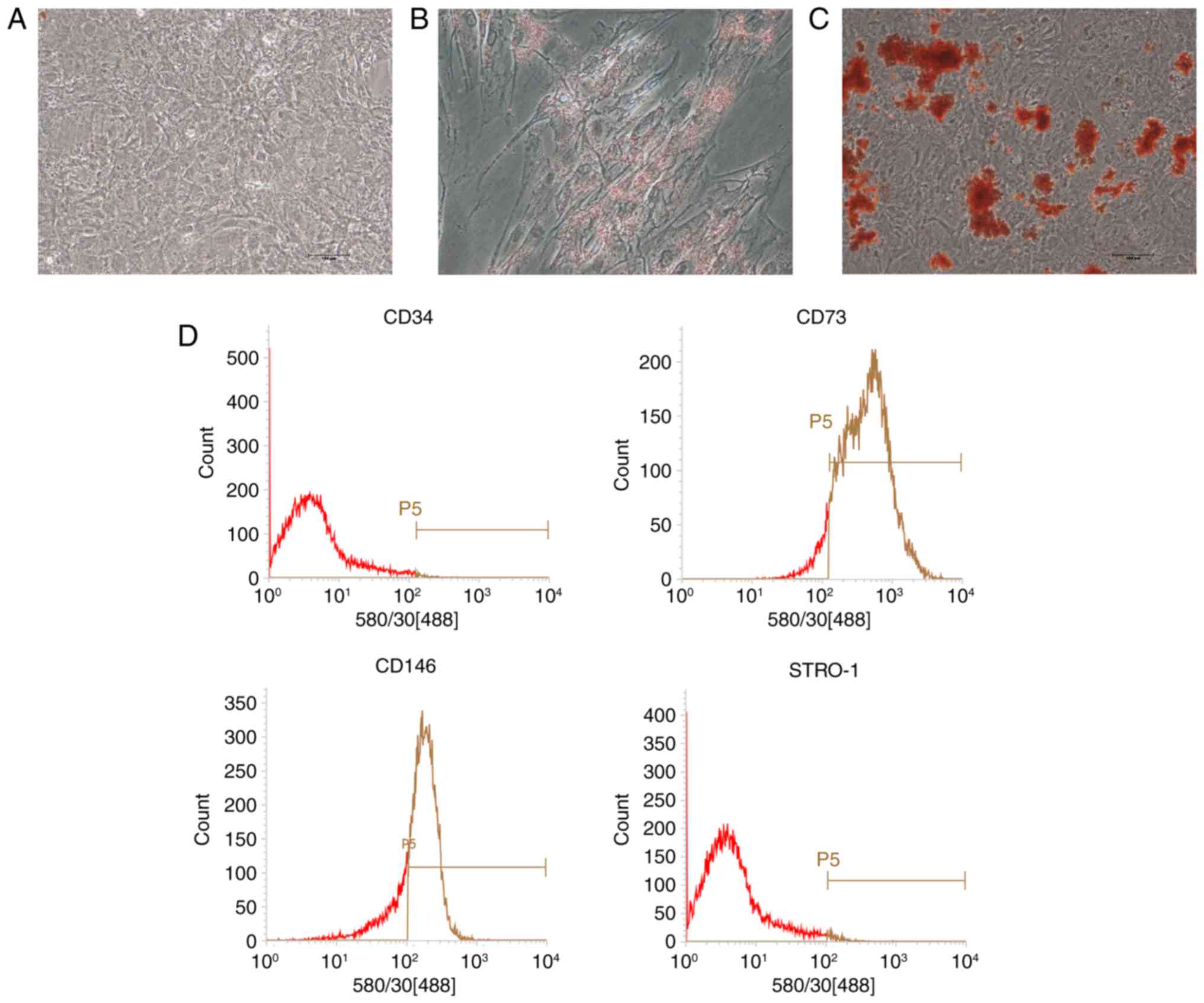
Characterization of PDLSCs
After cell passage, PDLSCs displayed a
spindle-shaped morphology similar to mesenchymal stem cells (MSCs;
Fig. 1A). Under adipogenic
conditions, lipid droplets were observed in the cytoplasm as
indicated by Oil Red O staining (Fig.
1B). Mineralized nodules were detected via Alizarin red
staining in osteogenic-induced cultures (Fig. 1C). No direct evidence of positive
staining was observed in the control group (Fig. 1A). Flow cytometry analysis
revealed that PDLSCs were positive for the MSC markers CD73 and
CD146, but negative for the hematopoi-etic lineage marker CD34 and
the early progenitor marker STRO-1 (Fig. 1D).
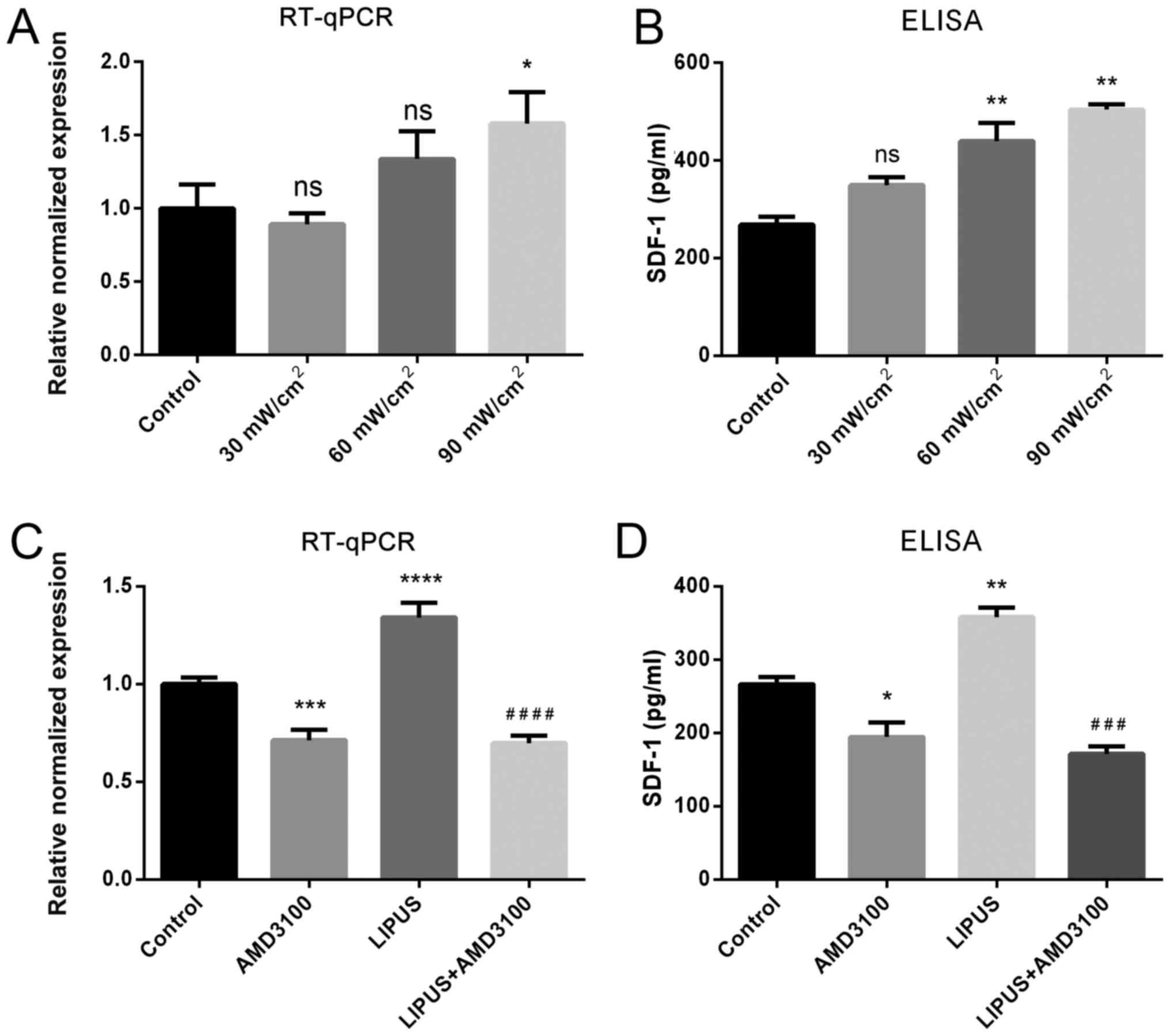
LIPUS enhances the expression of SDF-1 in
PDLSCs
To evaluate the effect of LIPUS intensity on SDF-1
expression, PDLSCs were treated with different LIPUS intensities.
The results demonstrated that an intensity of 30 mW/cm2
for 30 min/day did not affect SDF-1 mRNA or protein expression,
while both 60 and 90 mW/cm2 for 30 min/day resulted in
positive effects on SDF-1 expression, and 90 mW/m2 for
30 min/day had a significant promoting effect on SDF-1 expression,
as indicated by RT-qPCR and ELISA (Fig. 2A). Thus, the LIPUS treatment
conditions of 90 mW/cm2 for 30 min/day were adopted in
subsequent experiments. RT-qPCR and ELISA results revealed that
LIPUS treatment significantly enhanced SDF-1 mRNA transcription and
protein secretion compared to untreated PDLSCs (Fig. 2B). By contrast, the CXCR4 specific
antagonist AMD3100 inhibited SDF-1 mRNA expression and protein
secretion compared with untreated PDLSCs (Fig. 2B). In addition, the CXCR4 specific
antagonist AMD3100 significantly blocked the LIPUS-promoted SDF-1
upregulation (Fig. 2B). These
results suggested that LIPUS significantly upregulated SDF-1 both
at the mRNA and protein level.
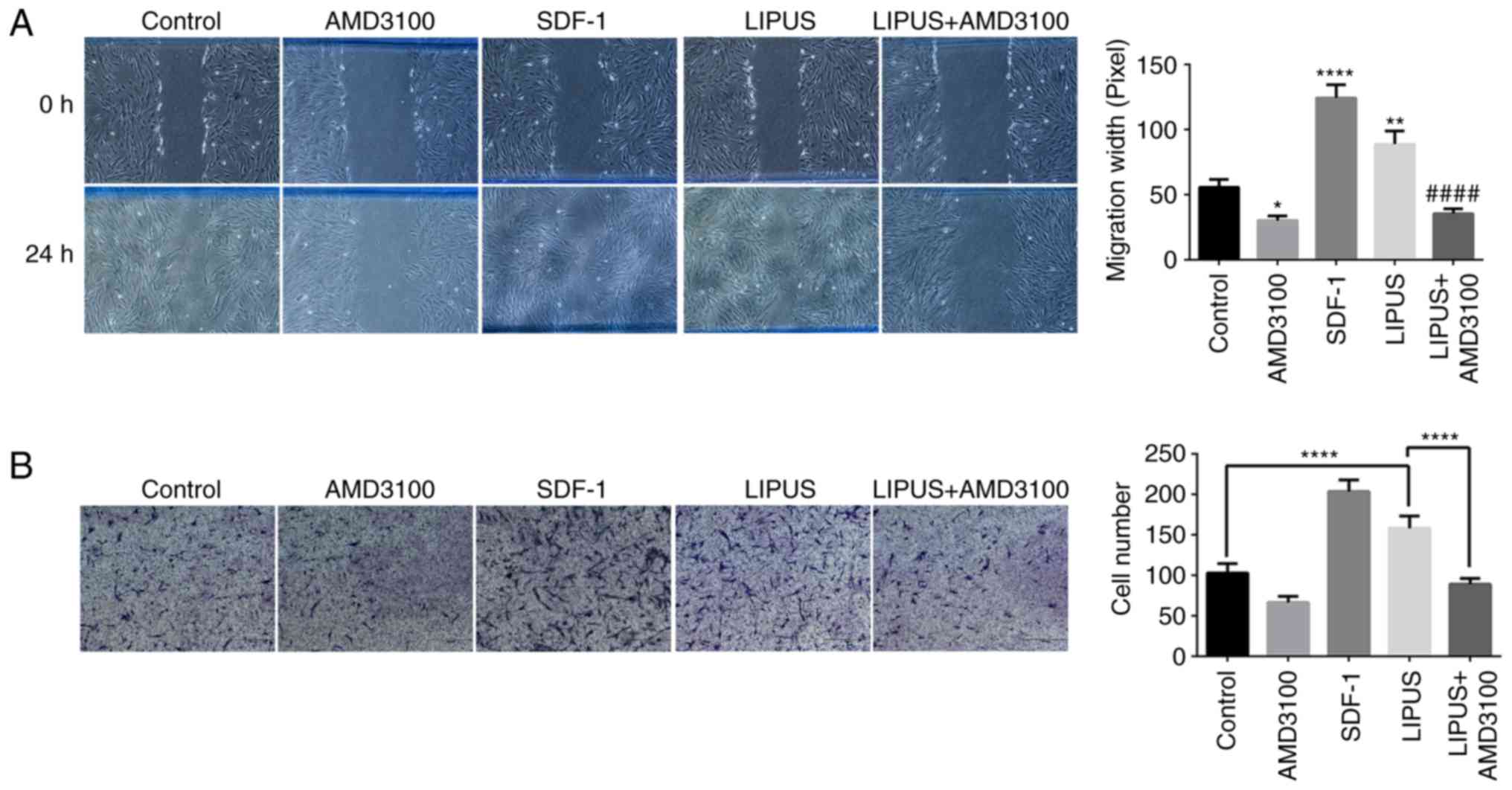
LIPUS promotes migration of PDLSCs
To examine whether LIPUS exhibited biological
effects relevant to the migration of PDLSCs, wound healing assays
were performed. As illustrated in Fig. 3A, PDLSC migration was determined
by measuring the diameters of wounded spaces on 6-well plates. Both
SDF-1 addition and LIPUS treatment enhanced the migration of PDLSCs
compared with the control group after 24 h of incubation (Fig. 3A); however, AMD3100 significantly
inhibited the LIPUS-induced migration of PDLSCs (Fig. 3A). To further confirm the
promoting effect of LIPUS on PDLSC migration, transwell assay was
performed. As presented in Fig.
3B, significantly higher numbers of crystal violet-stained
transmigrated cells were counted in the lower membrane side of the
LIPUS-treated groups compared with the control group (Fig. 3B). Similar to the wound healing
assay, addition of AMD3100 significantly inhibited LIPUS-induced
migration compared with LIPUS treatment alone (Fig. 3B).
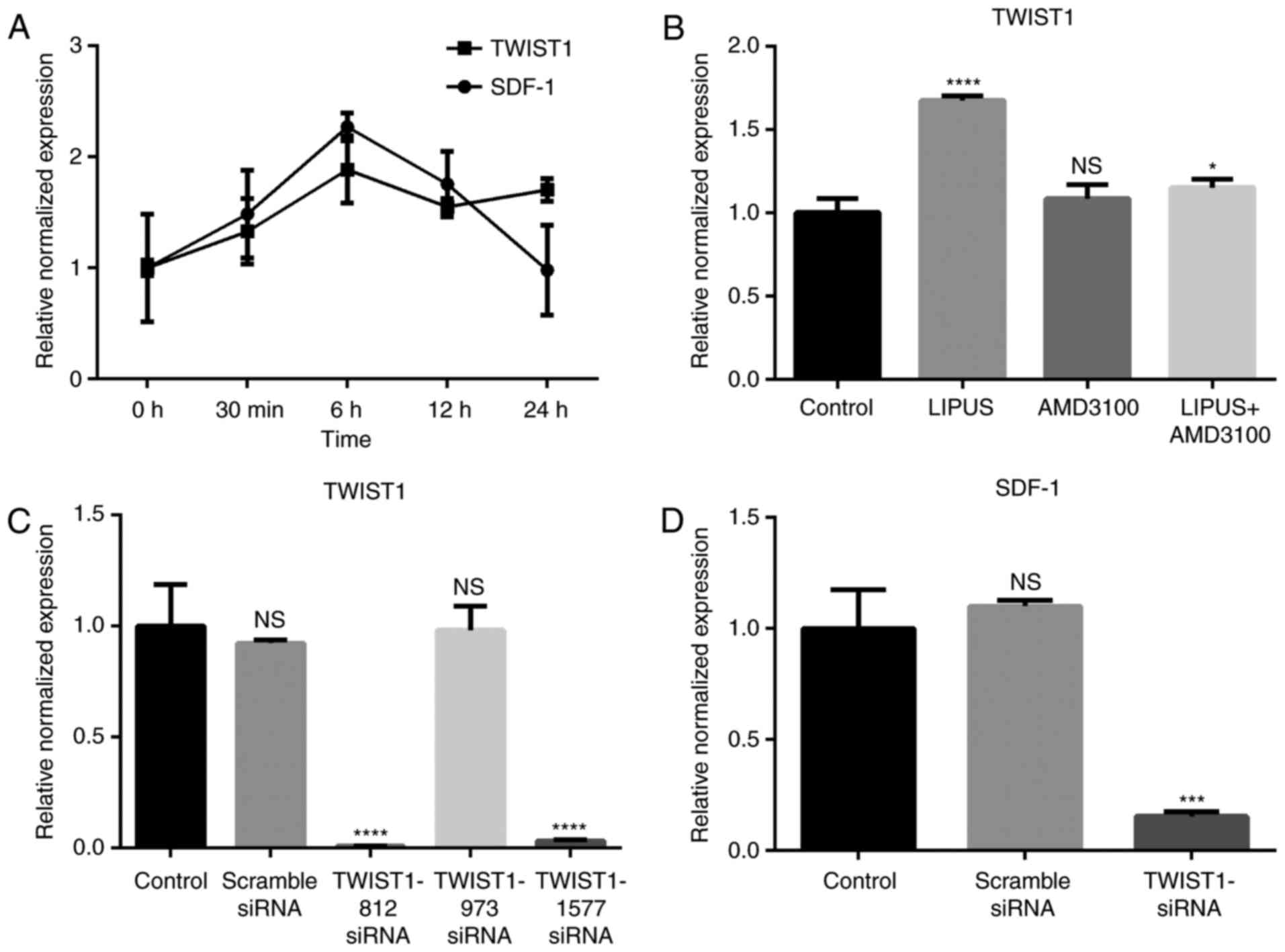
SDF-1 expression is associated with
TWIST1 expression in PDLSCs
SDF-1 mRNA expression started to increase
immediately following LIPUS treatment (90 mW/cm2, 30
min/day; Fig. 4A). SDF-1 reached
maximal expression at 6 h post-treatment and then decreased with
time (Fig. 4A). TWIST1 mRNA
expression displayed an increasing trend from 0–6 h post-treatment
and then maintained a high expression level compared with the
untreated group (Fig. 4A).
Blocking the SDF-1/CXCR4 signaling pathway with AMD3100 did not
affect the expression of TWIST1 (Fig.
4B), however LIPUS treatment with addition of AMD3100 inhibited
TWIST1 expression (Fig. 4B).
Knockdown of TWIST1 in PDLSCs decreases
expression of SDF-1
To investigate if LIPUS promoted SDF-1 expression
through TWIST1, three siRNA sequences targeting TWIST1 were
synthesized. TWIST1 mRNA expression was efficiently decreased by
TWIST1-812 and TWIST1-1577 siRNA transfection, as evidenced by
RT-qPCR results (Fig. 4C).
TWIST1-1577 siRNA was then used in subsequent experiments. Compared
with the other groups, SDF-1 expression levels were significantly
decreased by TWIST1 siRNA transfection following LIPUS treatment
(Fig. 4D). These results indicate
that TWIST1 may be an upstream regulator of SDF-1.
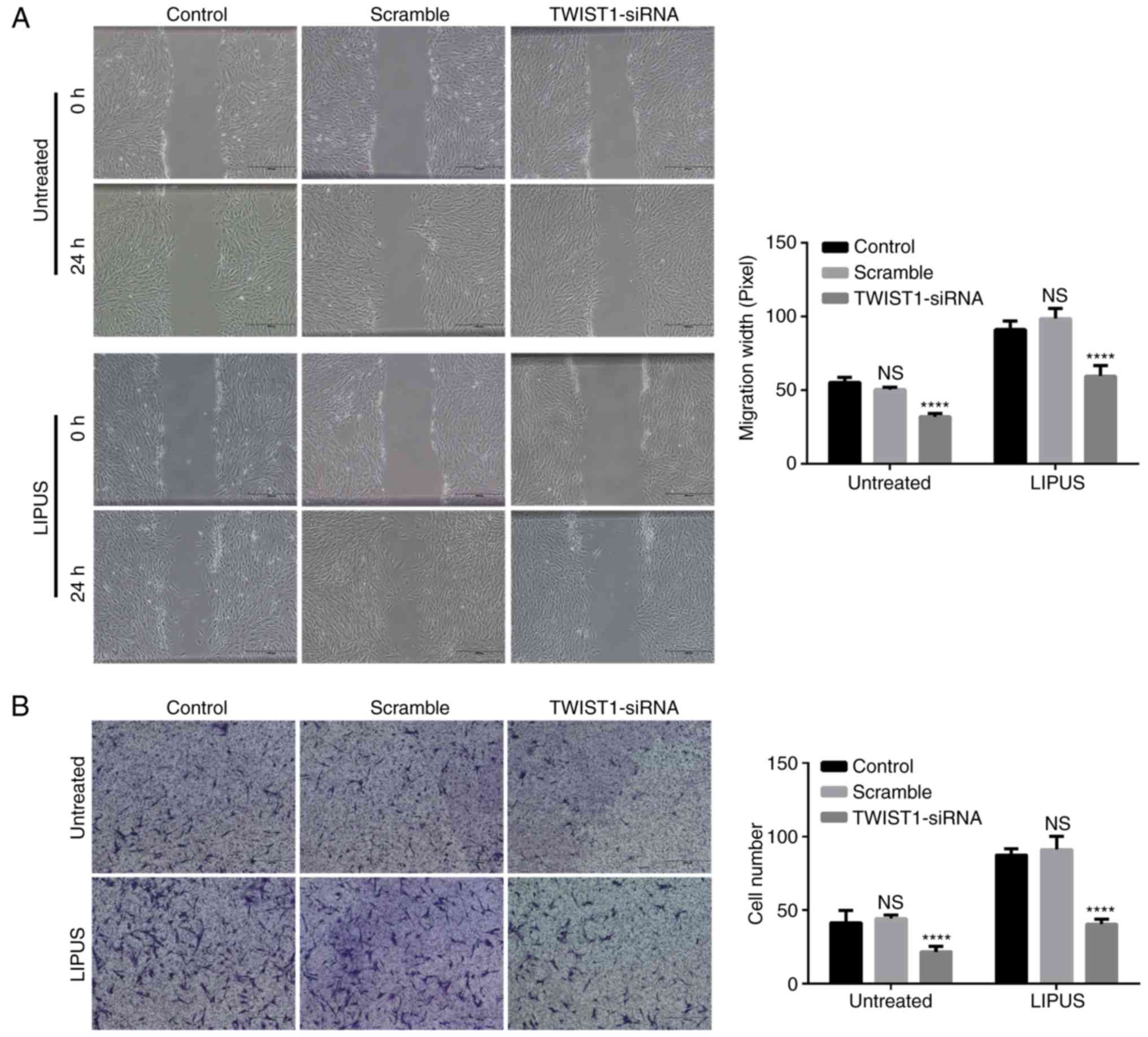
Knockdown of TWIST1 in PDLSCs blocks the
LIPUS-promoted PDLSC migration
Migration assay was performed in PDLSCs following
knockdown of TWIST1. TWIST1 siRNA silencing significantly inhibited
not only natural migration but also LIPUS-promoted migration of
PDLSCs, as presented in Fig. 5A.
By contrast, the scramble-siRNA control did not block the migration
of PDLSCs (Fig. 5B). Similarly,
the results from the transwell migration assay also demonstrated
that knockdown of TWIST1 significantly blocked LIPUS-promoted PDLSC
migration (Fig. 5B).
Discussion
Several possible cellular and molecular mechanisms
are responsible for periodontal repair. First, LIPUS promotes the
osteogenic differentiation of PDLSCs via bone morphogenetic
protein-Smad (39) and p38
mitogen-activated protein kinase (40) signaling pathways. Second, LIPUS
can regulate the inflammation status of periodontitis by
suppressing the toll-like receptor 4-nuclear factor κB signaling
pathway (34). Extracellular
signal-regulated kinase and receptor activator of nuclear factor
kappa-B ligand signaling may also be involved in the
immunomodulation by LIPUS treatment (8). Third, as indicated by the current
study, LIPUS promotes PDLSCs migration via the TWIST1/SDF-1
signaling pathway.
Endogenous MSCs have been reported to promote repair
of injured tissue by homing to injured sites (41). MSCs are an important cellular
constituent of the periodontal ligament, which is responsible for
the repair and turnover of the periodontium (42). Seo et al (43) isolated MSCs and used them to
generate cementum and periodontal ligament in vivo. The
present findings suggest that the PDLSCs that were isolated possess
MSC properties, such as multipotency, and express MSC markers,
which is consistent with previous studies (44). MSC mobilization has been reported
to participate in periodontal tissue homeostasis (24,45). SDF-1 has a significant role in the
recruitment and engraftment of stem cells in wound sites (20,21,46). Numerous studies have evaluated
cell homing effects in periodontal defects. In a murine study,
SDF-1 expression was demonstrated to increase around periodontal
defects and in periodontal ligaments (24). Another study suggested that LIPUS
accelerates fracture healing by promoting the homing of circulating
osteogenic progenitors to the fracture site (22). The current study demonstrated that
LIPUS enhanced the migration of PDLSCs, which indicates that LIPUS
has the potential to accelerate endogenous periodontal MSC
recruitment.
Previous literature has reported the SDF-1
expression-promoting effects of LIPUS. Immunofluorescence staining
has demonstrated that LIPUS treatment increases SDF-1 expression at
the fracture site (22). Further
exploration demonstrated that LIPUS increased SDF-1 transcription
and translation by in vitro experiments (23). When ultrasound was combined with
microbubbles to treat MSCs, the expression of SDF-1/CXCR4 and the
migration ability were significantly improved (47). Consistent with a fracture healing
study (23), the present study
demonstrated that LIPUS treatment promoted gene and protein
expression of SDF-1 in PDLSCs. Blocking SDF-1/CXCR4 with the
specific AMD3100 antagonist suppressed the promoting effect of
SDF-1 secretion and cell migration of LIPUS. These results
suggested that the SDF-1/CXCR4 pathway is a crucial molecular
mechanism underlying LIPUS-promoted cell migration.
Mechanical stimuli, such as strain and shear stress,
have been recognized to have a profound impact on stem cell
behavior (48,49). The mechanism by which mechanical
forces are transduced into biochemical signals is complicated and
not yet fully clarified (49).
Recently, TWIST1 was suggested to have a potential role in alveolar
bone-periodontal ligament interface remodeling (50). An earlier study reported that
occlusal forces might have putative roles in TWIST gene expression
in the periodontal ligament (31), whereas TWIST1 was documented to
increase SDF-1 promoter activity in a dose dependent manner in
BMSCs (26). These studies
suggested that mechanical stress might regulate SDF-1 expression
through TWIST1. To validate the hypothesis, the role of TWIST1 in
regulating SDF-1 expression was explored in the present study. The
current findings suggested that TWIST1 expression was strongly
correlated with SDF-1 expression. Knockdown of TWIST1 by siRNA
abolished the LIPUS-induced SDF-1 expression, which indicated that
TWIST1 may be a sensor for pressure waves. In addition, knockdown
of TWIST1 could not only inhibit the migration of PDLSCs but also
blocked the LIPUS-induced cell migration. These findings indicated
that TWIST1 may be an upstream regulator of SDF-1, which has an
important role in cell migration. Likewise, a TWIST1-G3BP2
mechanotransduction pathway was revealed to drive EMT, invasion and
metastasis in response to biomechanical signals from the tumor
microenvironment (51). However,
knockdown of TWIST1 did not completely block migration of PDLCs,
which suggested that compensation mechanisms might exist. For
instance, an earlier study has reported that the expression of
other chemokine receptors, like CCR1, CCR4, and CCR7, but not
CXCR4, drive hMSC migration (52). Thus, the mechanisms by which MSCs
are recruited to periodontal tissues are yet to be fully explored.
Combined with previous studies, the present findings suggest that
TWIST1 might be a mechanical stress sensor during
mechanotransduction.
In conclusion, the current study demonstrated that
LIPUS treatment promoted SDF-1 expression and enhanced PDLSC
migration. TWIST1 may be a potential sensor in LIPUS-mediated
mechanical signal transduction. However, how LIPUS transduces
signals from TWIST1 to SDF-1 needs to be clarified in future
studies. Nevertheless, these results provide a new molecular and
cellular basis for LIPUS-mediated periodontal disease
treatment.
Acknowledgments
Not applicable.
References
|
1
|
Pihlstrom BL, Michalowicz BS and Johnson
NW: Periodontal diseases. Lancet. 366:1809–1820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pounder NM and Harrison AJ: Low intensity
pulsed ultrasound for fracture healing: A review of the clinical
evidence and the associated biological mechanism of action.
Ultrasonics. 48:330–338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malizos KN, Hantes ME, Protopappas V and
Papachristos A: Low-intensity pulsed ultrasound for bone healing:
An overview. Injury. 37(Suppl 1): S56–S62. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pilla AA, Mont MA, Nasser PR, Khan SA,
Figueiredo M, Kaufman JJ and Siffert RS: Non-invasive low-intensity
pulsed ultrasound accelerates bone healing in the rabbit. J Orthop
Trauma. 4:246–253. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheung WH, Chin WC, Wei FY, Li G and Leung
KS: Applications of exogenous mesenchymal stem cells and low
intensity pulsed ultrasound enhance fracture healing in rat model.
Ultrasound Med Biol. 39:117–125. 2013. View Article : Google Scholar
|
|
6
|
Ikai H, Tamura T, Watanabe T, Itou M,
Sugaya A, Iwabuchi S, Mikuni-Takagaki Y and Deguchi S:
Low-intensity pulsed ultrasound accelerates periodontal wound
healing after flap surgery. J Periodontal Res. 43:212–216. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El-Bialy T, Alhadlaq A and Lam B: Effect
of therapeutic ultrasound on human periodontal ligament cells for
dental and periodontal tissue engineering. Open Dent J. 6:235–239.
2012. View Article : Google Scholar
|
|
8
|
Kusuyama J, Nakamura T, Ohnishi T, Eiraku
N, Noguchi K and Matsuguchi T: Low-intensity pulsed ultrasound
(LIPUS) promotes BMP9-induced osteogenesis and suppresses
inflammatory responses in human periodontal ligament-derived stem
cells. J Orthop Trauma. 31:S42017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhuang D, Ji Z, Bi L, Wang X, Zhou Q and
Cao W: Low-intensity ultrasound combined with hematoporphyrin
monomethyl ether in the treatment of experimental periodontitis in
rats. Biomed Res Int. 2016:71567162016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Chai Z, Zhang Y, Deng F, Wang Z
and Song J: Influence of low-intensity pulsed ultrasound on
osteogenic tissue regeneration in a periodontal injury model: X-ray
image alterations assessed by micro-computed tomography.
Ultrasonics. 54:1581–1584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu XQ, Li YM, Guo J, Zhang LH, Li D and
Gai XD: Effect of low intensity pulsed ultrasound on repairing the
periodontal bone of Beagle canines. Asian Pac J Trop Med.
7:325–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng G, Tse J, Jain RK and Munn LL:
Micro-environmental mechanical stress controls tumor spheroid size
and morphology by suppressing proliferation and inducing apoptosis
in cancer cells. PloS One. 4:e46322009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salgarella AR, Cafarelli A, Ricotti L,
Capineri L, Dario P and Menciassi A: Optimal ultrasound exposure
conditions for maximizing C2C12 muscle cell proliferation and
differentiation. Ultrasound Med Biol. 43:1452–1465. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Altman GH, Horan RL, Martin I, Farhadi J,
Stark PR, Volloch V, Richmond JC, Vunjak-Novakovic G and Kaplan DL:
Cell differentiation by mechanical stress. FASEB J. 16:270–272.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li C, Wernig F, Leitges M, Hu Y and Xu Q:
Mechanical stress-activated PKCdelta regulates smooth muscle cell
migration. FASEB J. 17:2106–2108. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jang KW, Ding L, Seol D, Lim TH,
Buckwalter JA and Martin JA: Low-intensity pulsed ultrasound
promotes chondrogenic progenitor cell migration via focal adhesion
kinase pathway. Ultrasound Med Biol. 40:1177–1186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Padilla F, Puts R, Vico L and Raum K:
Stimulation of bone repair with ultrasound: A review of the
possible mechanic effects. Ultrasonics. 54:1125–1145. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gould TR, Melcher AH and Brunette DM:
Migration and division of progenitor cell populations in
periodontal ligament after wounding. J Periodontal Res. 15:20–42.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peled A, Petit I, Kollet O, Magid M,
Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, et al:
Dependence of human stem cell engraftment and repopulation of
NOD/SCID mice on CXCR4. Science. 283:845–848. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Askari AT, Unzek S, Popovic ZB, Goldman
CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD,
DiCorleto PE, et al: Effect of stromal-cell-derived factor 1 on
stem-cell homing and tissue regeneration in ischaemic
cardiomyopathy. Lancet. 362:697–703. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng Y, Fu X, Lou X and Fu B: Stromal
cell-derived factor 1 protects human periodontal ligament stem
cells against hydrogen peroxide-induced apoptosis. Mol Med Rep.
16:5001–5006. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumagai K, Takeuchi R, Ishikawa H,
Yamaguchi Y, Fujisawa T, Kuniya T, Takagawa S, Muschler GF and
Saito T: Low-intensity pulsed ultrasound accelerates fracture
healing by stimulation of recruitment of both local and circulating
osteogenic progenitors. J Orthop Res. 30:1516–1521. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wei FY, Leung KS, Li G, Qin J, Chow SK,
Huang S, Sun MH, Qin L and Cheung WH: Low intensity pulsed
ultrasound enhanced mesenchymal stem cell recruitment through
stromal derived factor-1 signaling in fracture healing. PloS One.
9:e1067222014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kimura Y, Komaki M, Iwasaki K, Sata M,
Izumi Y and Morita I: Recruitment of bone marrow-derived cells to
periodontal tissue defects. Front Cell Dev Biol. 2:192014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du L, Yang P and Ge S: Stromal
cell-derived factor-1 significantly induces proliferation,
migration, and collagen type I expression in a human periodontal
ligament stem cell subpopulation. J Periodontol. 83:379–388. 2012.
View Article : Google Scholar
|
|
26
|
Arthur A, Cakouros D, Cooper L, Nguyen T,
Isenmann S, Zannettino AC, Glackin CA and Gronthos S: Twist-1
enhances bone marrow mesenchymal stromal cell support of
hematopoiesis by modulating CXCL12 expression. Stem Cells.
34:504–509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weiss MB, Abel EV, Dadpey N and Aplin AE:
FOXD3 modulates migration through direct transcriptional repression
of TWIST1 in melanoma. Mol Cancer Res. 12:1314–1323. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duan Y, He Q, Yue K, Si H, Wang J, Zhou X
and Wang X: Hypoxia induced Bcl-2/Twist1 complex promotes tumor
cell invasion in oral squamous cell carcinoma. Oncotarget.
8:7729–7739. 2017. View Article : Google Scholar :
|
|
29
|
Mahmoud MM, Kim HR, Xing R, Hsiao S,
Mammoto A, Chen J, Serbanovic-Canic J, Feng S, Bowden NP, Maguire
R, et al: TWIST1 integrates endothelial responses to flow in
vascular dysfunction and atherosclerosis. Circ Res. 119:450–462.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Desprat N, Supatto W, Pouille PA,
Beaurepaire E and Farge E: Tissue deformation modulates twist
expression to determine anterior midgut differentiation in
Drosophila embryos. Dev Cell. 15:470–477. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Afanador E, Yokozeki M, Oba Y, Kitase Y,
Takahashi T, Kudo A and Moriyama K: Messenger RNA expression of
periostin and Twist transiently decrease by occlusal hypofunction
in mouse periodontal ligament. Arch Oral Biol. 50:1023–1031. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Li H, Tian Y, Yang Y, Chen G, Guo W
and Tian W: Cytoskeletal binding proteins distinguish cultured
dental follicle cells and periodontal ligament cells. Exp Cell Res.
345:6–16. 2016. View Article : Google Scholar
|
|
33
|
Tian Y, Bai D, Guo W, Li J, Zeng J, Yang
L, Jiang Z, Feng L, Yu M and Tian W: Comparison of human dental
follicle cells and human periodontal ligament cells for dentin
tissue regeneration. Regen Med. 10:461–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X, Hu B, Sun J, Li J, Liu S and Song
J: Inhibitory effect of low-intensity pulsed ultrasound on the
expression of lipopolysaccharide-induced inflammatory factors in
U937 cells. J Ultrasound Med. 36:2419–2429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu S, Li L, Wang G, Shen W, Xu Y, Liu Z,
Zhuo Z, Xia H, Gao Y and Tan K: Ultrasound-targeted stromal
cell-derived factor-1-loaded microbubble destruction promotes
mesenchymal stem cell homing to kidneys in diabetic nephropathy
rats. Int J Nanomedicine. 9:5639–5651. 2014.PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔC T method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
37
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao H, Priebe W, Glod J and Banerjee D:
Activation of signal transducers and activators of transcription 3
and focal adhesion kinase by stromal cell-derived factor 1 is
required for migration of human mesenchymal stem cells in response
to tumor cell-conditioned medium. Stem Cells. 27:857–865. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Z, Ren L, Deng F, Wang Z and Song J:
Low-intensity pulsed ultrasound induces osteogenic differentiation
of human periodontal ligament cells through activation of bone
morphogenetic protein-smad signaling. J Ultrasound Med. 33:865–873.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ren L, Yang Z, Song J, Wang Z, Deng F and
Li W: Involvement of p38 MAPK pathway in low intensity pulsed
ultrasound induced osteogenic differentiation of human periodontal
ligament cells. Ultrasonics. 53:686–690. 2013. View Article : Google Scholar
|
|
41
|
Karp JM and Leng Teo GS: Mesenchymal stem
cell homing: The devil is in the details. Cell Stem Cell.
4:206–216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nanci A and Bosshardt DD: Structure of
periodontal tissues in health and disease. Periodontol 2000.
40:11–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multi-potent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Choi JK, Hwang HI and Jang YJ: The
efficiency of the in vitro osteo/dentinogenic differentiation of
human dental pulp cells, periodontal ligament cells and gingival
fibroblasts. Int J Mol Med. 35:161–168. 2015. View Article : Google Scholar
|
|
45
|
Zhou J, Shi S, Shi Y, Xie H, Chen L, He Y,
Guo W, Wen L and Jin Y: Role of bone marrow-derived progenitor
cells in the maintenance and regeneration of dental mesenchymal
tissues. J Cell Physiol. 226:2081–2090. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lapidot T and Kollet O: The essential
roles of the chemokine SDF-1 and its receptor CXCR4 in human stem
cell homing and repopulation of transplanted immune-deficient
NOD/SCID and NOD/SCID/B2mnull mice. Leukemia.
16:1992–2003. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li L, Wu S, Li P, Zhuo L, Gao Y and Xu Y:
Hypoxic preconditioning combined with microbubble-mediated
ultrasound effect on MSCs promote SDF-1/CXCR4 expression and its
migration ability: An in vitro study. Cell Biochem Biophys.
73:749–757. 2015. View Article : Google Scholar
|
|
48
|
Kshitiz, Park J, Kim P, Helen W, Engler
AJ, Levchenko A and Kim DH: Control of stem cell fate and function
by engineering physical microenvironments. Integr Biol.
4:1008–1018. 2012. View Article : Google Scholar
|
|
49
|
Guilak F, Cohen DM, Estes BT, Gimble JM,
Liedtke W and Chen CS: Control of stem cell fate by physical
interactions with the extracellular matrix. Cell Stem Cell.
5:17–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yan Y, Tian Z, Guan Q, Bai D, Zhang J and
Han X: The role of Twist1 in stem cell differentiation through
mechanical cues: A review and hypothesis. Br J Med Med Res. 17:1–9.
2016. View Article : Google Scholar
|
|
51
|
Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH,
Majeski HE, Chen AC, Sah RL, Taylor SS, Engler AJ and Yang J:
Matrix stiffness drives epithelial-mesenchymal transition and
tumour metastasis through a TWIST1-G3BP2 mechanotransduction
pathway. Nat Cell Biol. 17:678–688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Von Lüttichau I, Notohamiprodjo M,
Wechselberger A, Peters C, Henger A, Seliger C, Djafarzadeh R, Huss
R and Nelson PJ: Human adult CD34− progenitor cells
functionally express the chemokine receptors CCR1, CCR4, CCR7,
CXCR5, and CCR10 but not CXCR4. Stem Cells Dev. 14:329–336. 2005.
View Article : Google Scholar
|