Introduction
Breast cancer is the most prevalent female malignant
neoplasm worldwide, the frequency of which has increased in recent
years (1). Breast cancer
management includes systemic treatments, such as hormone therapy,
which suppresses estrogen synthesis or uptake, and
chemotherapeutics; as well as topical treatments, including
radiotherapy and surgery. Traditional therapeutics that induce
inhibition of cell proliferation and DNA replication not only do
not possess tumor cell selectivity, but also exert several adverse
effects and are associated with inadequate response rates (2,3).
Contemporary therapies intend to selectively target cancer cells,
in order to improve cancer cure rates and decrease cytotoxicity to
normal cells. In breast cancer, these efforts have resulted in the
generation of therapies that target aromatase (the P450 enzyme that
transforms androgens to estrogens) or estrogen receptors. Recently
developed drugs, including letrozole and tamoxifen, exhibit
specificity against aromatase and estrogen receptors, respectively;
these drugs have produced promising results. However, these drugs
are reliant on tumors exhibiting hormone dependency, mainly in
estrogen receptor-positive breast cancers (4–6).
One of the main medical developments in the last few
decades is cancer chemotherapy. However, these drugs often exhibit
limited therapeutic index, and the obtained responses are often
unpredictable as well as palliative. Conversely, targeted therapy
is focused on cancer-specific signaling pathways and molecules;
therefore, it induces less nonspecific toxicities. Tyrosine kinases
are important targets, since they have a significant role in the
modulation of tumorigenesis and signaling (7–9).
Consequently, novel targeted therapies, which
modulate the activity of numerous tyrosine kinase receptors were
involved into the therapeutic outlines. Therefore, research has
focused on developing novel inhibitory drugs associated with
tyrosine kinase receptor pathways. Imatinib mesylate (also known as
STI571 or Gleevec) is a targeted, synthetic, oral anti-cancer drug,
with a low-molecular weight. Imatinib mesylate is a
2-phenylaminopyridine derivative, which was developed as a
selective inhibitor of the BCR-ABL fusion gene product; BCR-ABL is
a tyrosine kinase that is contained in a translocation
[t(9;22)(q34;q11)] between chromosomes 9 and 22, which is
cytogenetically evident as the Philadelphia chromosome (10,11). Imatinib usage in cancers other
than those typified by BCR-ABL expression is relevant, due to its
biological activity on other type III receptor tyrosine kinases,
particularly platelet-derived growth factor (PDGF) and c-Kit
[cluster of differentiation (CD)117] receptors, which are
functionally and structurally closely connected (12,13). On binding their corresponding
ligands, PDGF and stem cell factor (SCF), these receptors serve a
significant role in signal transduction that leads to cell
survival. Imatinib inhibits signal transduction in a similar manner
to its effects on the BCR-ABL transcribed protein, resulting in
either selective proliferation inhibition or apoptosis induction.
Notably, imatinib has no effect on any other closely-connected
non-mutant protein kinase. (14,15).
CD117 is encoded by c-Kit, and is a transmembrane
tyrosine kinase receptor that has a significant role in the
development of small-cell lung cancer, gastrointestinal stromal
tumors, breast cancer and melanoma (16,17).
The expression of PDGFR has been established in
malignant breast tissue and adjacent stromal cells, including the
pericytes that support blood vessels. Preclinical studies have
demonstrated that imatinib exerts antitumor activity in a model of
breast carcinoma, particularly in osteolytic bone metastases
(18).
Imatinib was initially used to treat chronic myeloid
leukemia, resulting in full remission in most patients (19). Furthermore, in solid tumors,
imatinib has been reported to exert antiproliferative activity by
suppressing tyrosine kinase receptor signaling, including PDGFRs
and c-Kit. It is possible to detect receptor expression in numerous
types of cancer, including melanoma, meningioma, prostate, ovary,
breast and pancreatic cancer (11,20,21).
Blocking the site of intracellular ATP binding can
inhibit stimulation of these kinases and the resulting signal
transduction. Imatinib can reduce paracrine and autocrine receptor
stimulation, as well as the irregular activation of mutated kinases
that develops in certain carcinoma cells (22,23). Consequently, imatinib has been
used as a chemotherapeutic agent in gastrointestinal stromal tumor
therapy, where it deactivates the pathognomonic c-Kit kinase
(24). In addition, imatinib
inhibits dermatofibrosarcoma protuberans progression, and promotes
apoptosis in leukemia and neuroblastoma. In some types of cancer
and metastases, incubation with imatinib has been reported to
inhibit the aforementioned receptors and suppress cell
proliferation (22,25,26). Furthermore, imatinib has been
revealed to inhibit the invasiveness of a panel of human epithelial
breast cancer cells with various invasive capacities (13).
To evaluate the antiproliferative effects of
imatinib mesylate through the gene and protein expression of
PDGFR-β and c-Kit, and their corresponding ligands PDGF-BB and SCF,
ZR-75-1 and MDA-MB-231 breast cancer cell lines were used. ZR-75-1
and MDA-MB-231 cells comprise human tumorigenic epithelial mammary
gland cells; ZR-75-1 cells were derived from ascites of the
metastatic site of ductal carcinoma in a 63-year-old female
patient, whereas MDA-MB-231 cells were derived from pleural
effusion of the metastatic site of adenocarcinoma in a 51-year-old
female patient.
The present study aimed to evaluate the in
vitro antiproliferative effects of imatinib mesylate on breast
carcinoma cell lines, and to investigate whether these effects were
accompanied by apoptotic induction or cell cytostatic activity.
Furthermore, the gene and protein expression levels of PDGFR-β and
c-Kit, and their corresponding ligands PDGF-BB and SCF, were
detected in breast cancer cells following treatment with imatinib,
in order to investigate its potential targets in breast cancer
therapy.
Materials and methods
Materials
Imatinib mesylate was provided by OsvahPharma Co.
(Tehran, Iran). RPMI-1640 cell culture medium was supplied by
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Fetal bovine serum
(FBS) was obtained from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). All other reagents were of analytical grade and
deionized water was obtained by reverse osmosis.
Cell culture
The estrogen receptor-positive ZR-75-1 breast
carcinoma cell line [American Type Culture Collection
(ATCC®) CRL-1500™] and the triple-negative breast
carcinoma cell line MDA-MB-231 (ATCC® HTB-26™) were used
in the present study. Cell lines were purchased from ATCC
(Manassas, VA, USA). Cell lines were cultured at 37°C in an
atmosphere containing 5% CO2 in RPMI-1640 medium
supplemented with 5% FBS.
Cell treatment
Cells cultured in the aforementioned conditions were
harvested from 75 cm2 flasks, rinsed with PBS, and
seeded in 96-well plates containing complete cell culture medium at
a seeding density of 2,500 cells/well (ZR-75-1) and 1,500
cells/well (MDA-MB-231).
Each treatment group was cultured in triplicate.
Following 24 h of culture, adherent cells were incubated in normal
medium containing 2, 3, 4, 5, 6, 7, 8, 9 or 10 μM imatinib
mesylate for 24, 48, 96, 120 and 144 h at 37°C. Every 48 h, the
medium was completely replaced.
Cells were also exposed to 1 mM hydrogen peroxide to
ensure minimal viability (positive control), which caused full cell
death, as verified by the trypan blue viability staining. For
maximal viability, untreated cells which incubated with complete
medium without any drug (negative control).
In vitro proliferation assay
Cell lines were cultured and treated as
aforementioned, after which the Cell Titer Aqueous One Solution
assay (MTS; Promega Corporation, Madison, WI, USA) was conducted
according to the manufacturer's protocol, to investigate whether
cell proliferation was inhibited.
The half maximal inhibitory concentration
(IC50) value of imatinib, which resulted in 50%
mortality of viable cells, was measured using the following
equation: IC50 = [(50 − A)/(B − A)] x (D − C) + C, where
A is the concentration which resulted in <50% mortality; B is
the concentration which resulted in ≥50% mortality; C is the
concentration of imatinib mesylate that gives A% inhibition, and D
is the concentration of imatinib mesylate that gives B% inhibition
(27). Subsequently, the
IC50 value was used in further experiments. Student's
t-test was conducted for statistical analysis and P<0.05 was
considered to indicate a statistically significant difference.
Terminal
deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)
assay
Fragmentation of oligonucleosomal DNA (a hallmark of
apoptosis) was analyzed using the cell death detection ELISA plus
kit (Roche Diagnostics GmbH, Mannheim, Germany), to determine
whether apoptosis was induced by imatinib mesylate. Briefly,
24-well plates were seeded with 10,000 ZR-75-1 cells/well and 6,000
MDA-MB-231 cells/well.
After 24 h, the cell culture medium was replaced
with complete medium containing the corresponding IC50
concentrations of imatinib mesylate. The non-treated control group
was cultured in normal medium, whereas the positive control was
provided with the kit. Every 48 h, the medium was changed (18). After 48, 72, 96, 120 and 144 h,
the assay was conducted according to the manufacturer's protocol,
and an ELISA reader was used to obtain spectrophotometric data at
405 nm.
Cell cycle analysis
ZR-75-1 and MDA-MB-231 cell lines were treated with
imatinib mesylate in the exponential growth phase for 72 or 144 h.
After the incubation, cells were harvested, washed twice with
ice-cold PBS and were fixed in 700 μl cold 80% ethanol at
4°C overnight. Subsequently, the cells were washed with cold PBS
and suspended in 500 μl ice-cold PBS. Cells were then
stained with 200 μl propidium iodide (PI; 50 μg/ml)
in the presence of 50 μl RNase A (100 μg/ml) for 40
min at room temperature. RNase A was used to degrade the remaining
RNA content, in order to ensure PI bound to the DNA of experimental
cell lines only. Cell cycle distribution was analyzed using a flow
cytometer (BD FACSCanto II; BD Biosciences, San Jose, CA, USA) and
ModFit LT v3.0 software (Verity Software House, Inc., Topsham, ME,
USA).
Gene expression analysis
To evaluate the potential alterations in PDGFR-β and
c-Kit expression, as well as the expression of their relevant
ligands PDGF-B and SCF, in cells treated with imatinib, cell lines
were cultured in 6-well plates with RPMI-1640 containing 5% FBS.
After 24 h of cell adhesion, the cells were cultured in normal
medium containing the corresponding IC50 concentrations
of imatinib mesylate for 144 h. The medium was replaced every 48 h;
subsequently, total RNA was extracted and gene expression was
analyzed.
Receptor expression
In each individual cell line, the expression levels
of the tyrosine kinase receptors PDGFR-β and c-Kit, and their
relevant ligands PDGF-B and SCF, were detected at the
transcriptional level. RNeasy mini kit was used to extract total
RNA and RNase-Free DNase (both Qiagen, Inc., Valencia, CA, USA) was
used to eliminate genomic DNA. Extracted RNA was stored at
−70°C.
Total RNA underwent reverse transcription-polymerase
chain reaction (RT-PCR) amplification of nDNA-encoded PDGFR-β,
c-Kit, PDGF-B and SCF genes. β-actin was employed as a reference
gene for gene expression analysis.
Relative quantification of the transcriptional
levels of the genes encoding PDGFR-β, c-Kit, PDGF-B and SCF was
conducted using QuantiTect Primer assay kit (Qiagen, Inc.,
Singapore, Singapore) and RT-PCR SYBR-Green detection according to
the One-Step RT-PCR standard protocol. Total RNA (0.1 μg)
extracted from treated and untreated breast cancer cell lines
underwent One-Step RT-PCR amplifications in 50 μl reactions
containing QuantiTect SYBR-Green RT-PCR master mix, QuantiTect
Primer assay (Fig. 1), QuantiTect
RT Mix and RNase-free water.
Applied Biosystems StepOne Plus Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was used for
amplification according to the following protocol: Transcriptase
was activated for 30 min at 50°C for RT, after which temperature
was increased to 95°C for 15 min to activate HotStar Taq DNA
Polymerase, inactivate transcriptase and denature the resultant
cDNA template. Subsequently, 35 cycles of three-step cycling were
conducted, including denaturation at 94°C for 15 sec, annealing for
30 sec at 55°C and extension for 30 sec at 72°C. Finally,
amplification was completed with a final extension step at 10 min
for 72°C.
Each test was repeated three times in duplicate
tubes. To monitor the reagents used in the assay for probable
contamination, control tubes without template RNA were incorporated
into duplicates, together with the appropriate primer sets in each
test. In addition, PCR amplification was conducted on negative
control tubes without template RNA. The efficiency of the four
primer pairs was evaluated. Amplification cycle was determined
using StepOne Software v2.3 (Applied Biosystems; Thermo Fisher
Scientific, Inc.), in which product buildup was beyond the
threshold (Cq). RT-qPCR Cq values were analyzed using standard REST
2009 software, which is a standalone software tool developed by
Qiagen and M. Pfaffl (Technical University Munich, Munich, Germany)
for gene expression data analysis (28). In order to confirm the reliability
of the results in each experiment, the mean Cq of each sample for a
given gene in each experiment (repeated four times) was normalized
to the mean Cq value of the reference gene in untreated and treated
cell lines, which was employed as control (β-actin).
Relative gene expression in untreated groups was
calculated according to the following equation : Relative gene
expression = 1/(Cq / Cq Hk), where Cq is the average Cq value of
the genes of interest in untreated cell lines and Cq Hk is the
average Cq value of β-actin (29).
Western blot analysis
To determine the effects of imatinib mesylate on the
protein expression levels of PDGFR-β, c-Kit, and their
corresponding ligands PDGF-BB and SCF, cells were seeded in 12-well
plates and were treated with the corresponding IC50
concentrations of imatinib mesylate for 144 h. Cell lysis buffer
[120 mM NaCl, 50 mM Tris-HCl (pH 8.0), 1 mM phenylmethylsulfonyl
fluoride and 0.5% NP-40) was used to extract total cell proteins.
Subsequently, 40 μg proteins which were quantified by
nano-drop instrument, were separated by 10% SDS-PAGE, and proteins
were then transferred to a polyvinylidene difluoride (PVDF)
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
membranes were blocked with 5% nonfat milk in TBS-Tween-20 buffer 7
(1.5 M NaCl, 0.12 M Tris-base, 0.1% Tween-20) at room temperature
for 1 h, after which they were incubated with the relevant primary
antibodies at 4°C overnight. Subsequently, the membranes were
incubated with alkaline phosphatase-conjugated secondary antibody
at room temperature for 45 min. The following primary antibodies:
Anti-PDGFR-β (P-20) (sc-339), anti-c-Kit (Ab 81) (sc-13508),
anti-β-actin (C4) (sc-47778), anti-PDGF-BB (H-55) (sc-7878) and
anti-SCF (G-19) (sc-1303), were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) The PVDF membranes were
incubated for 45 min with alkaline phosphatase-conjugated
anti-mouse/anti-rabbit or anti-goat (1:5,000) secondary antibodies
and were then washed twice with TBST. The blots were developed
using BCIP/NBT colorimetric detection (Santa Cruz Biotechnology,
Inc.), in order to semi-quantify target protein bands using ImageJ
1.47v software.
Statistical analysis
To determine the significance between treated and
untreated control groups, ANOVA was performed. Statistical analysis
was conducted using the REST 2009 software, which is a standalone
software tool, established by M. Pfaffl (Technical University
Munich) and Qiagen, Inc. (28),
for analysis of comparative-quantitative gene expression data, and
Microsoft Office Excel 2013 (Microsoft Corporation, Redmond, WA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Imatinib suppresses late, but not early,
proliferation of breast cancer cells
To evaluate the antiproliferative and cytotoxic
effects of imatinib mesylate on breast cancer cell lines, an MTS
cell proliferation assay was conducted. Both cell lines were
treated for 96, 120 and 144 h with 2–10 μM clinically
relevant imatinib mesylate concentrations (30) in the presence of FBS-containing
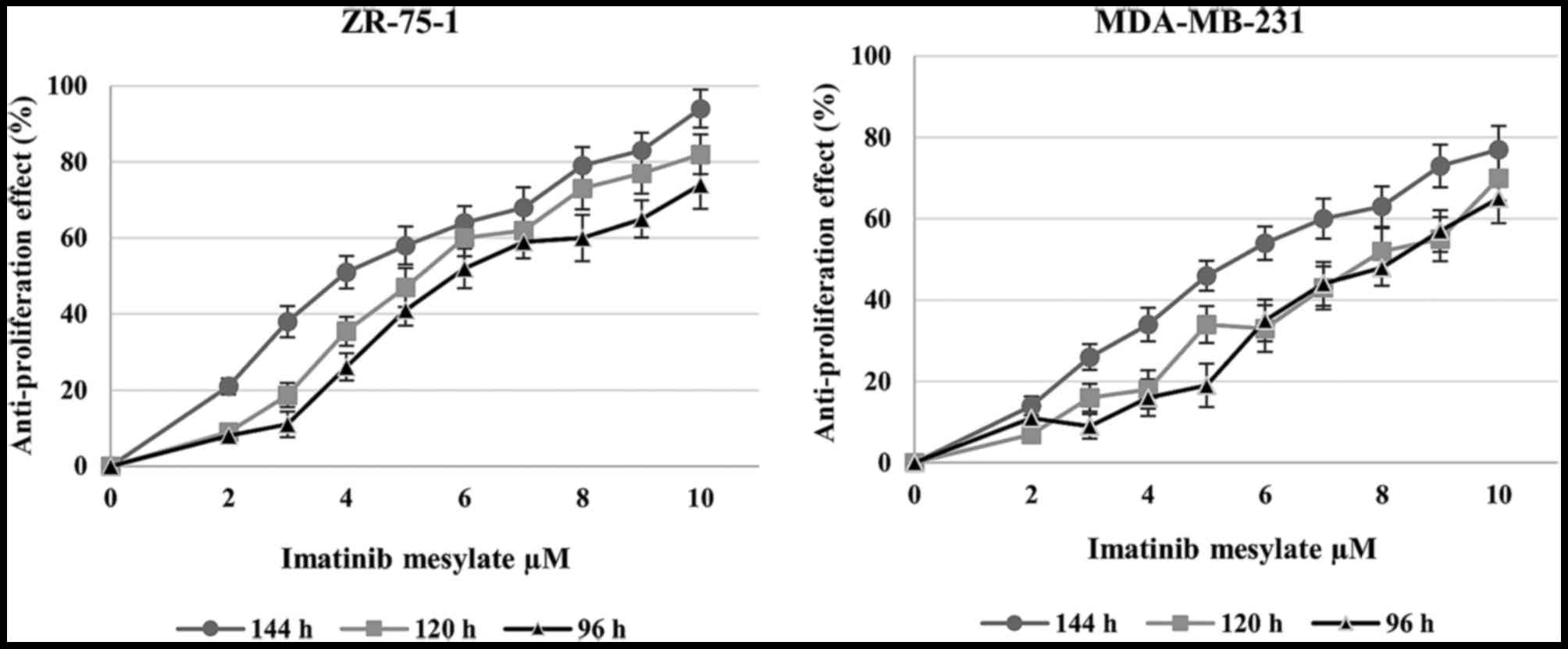
medium. As shown in Fig. 3,
imatinib mesylate revealed a significant dose-dependent effect on
MDA-MB-231 and ZR-75-1 breast carcinoma cancer cell lines. No
significant effect was detected in cells treated with imatinib for
48 and 96 h; however, in ZR-75-1 cells, 144 h treatment with <6
μM imatinib mesylate resulted in a significant increase in
proliferation inhibition. However, 96 and 120 h treatment with
higher concentrations of imatinib mesylate (>6 μM) did
not exert time-dependent antiproliferative effects.
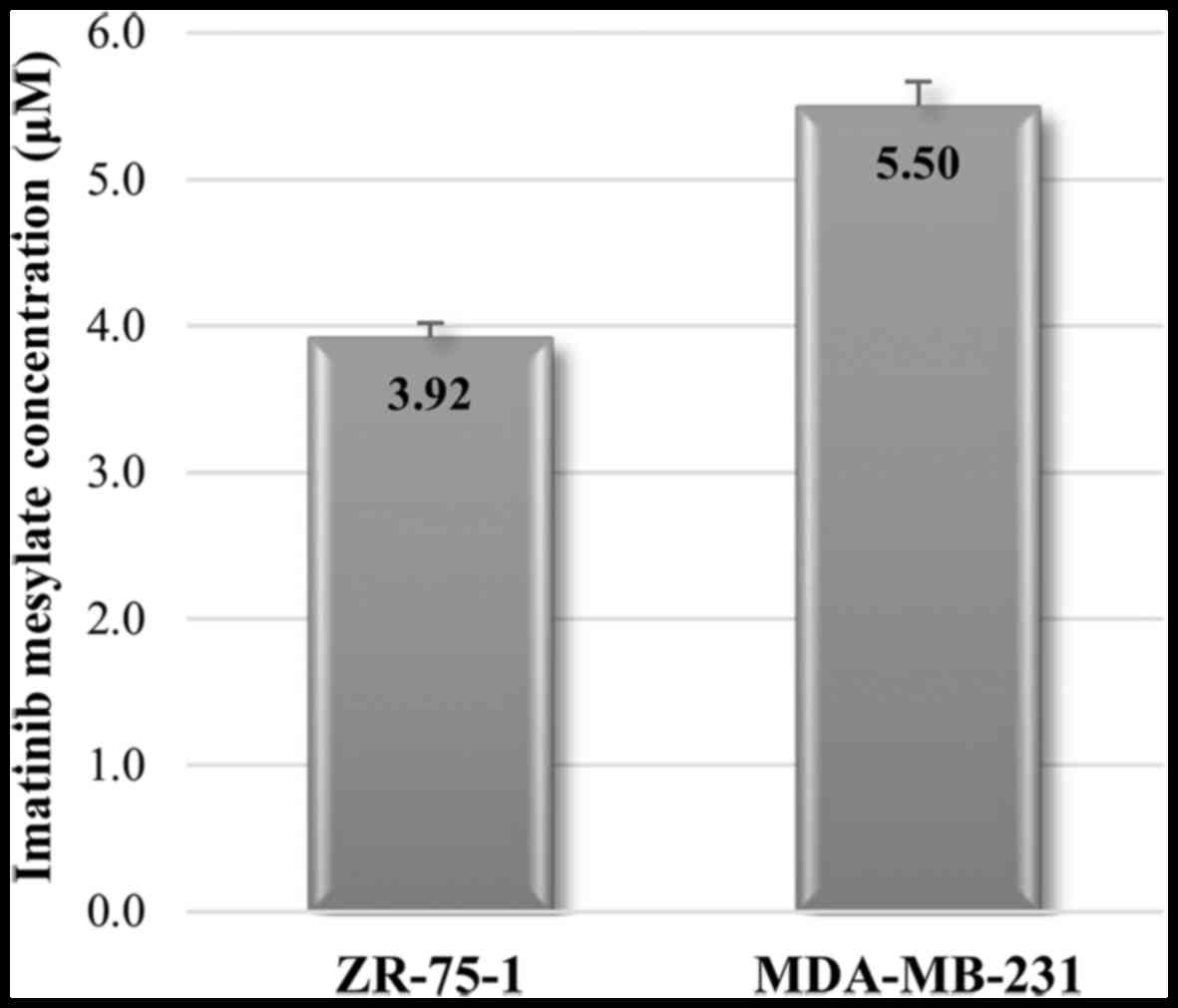
The average IC50 values of imatinib
mesylate were determined 144 h after treatment, as follows: 5.5
μM for MDA-MB-231 and 3.92 μM for ZR-75-1 (Fig. 2).
The results of the present study revealed that 144 h
treatment had the most significant toxic effect on both breast
cancer cell lines. Therefore, 144 h exposure time was selected for
further experiments.
Apoptosis is induced with long-term
imatinib treatment
The apoptotic effects of imatinib mesylate on breast
cancer cell lines were determined using the quantitative TUNEL
assay, which detects DNA breakage as a hallmark of late apoptosis.
After 96 h, treatment of each cell line with the relevant
IC50 value of imatinib mesylate resulted in a
significant increase in the number of apoptotic cells compared with
in the untreated group. In the present study, 144 h of exposure was
selected for further study in order to determine the inhibitory
effects of imatinib on the two cell lines.
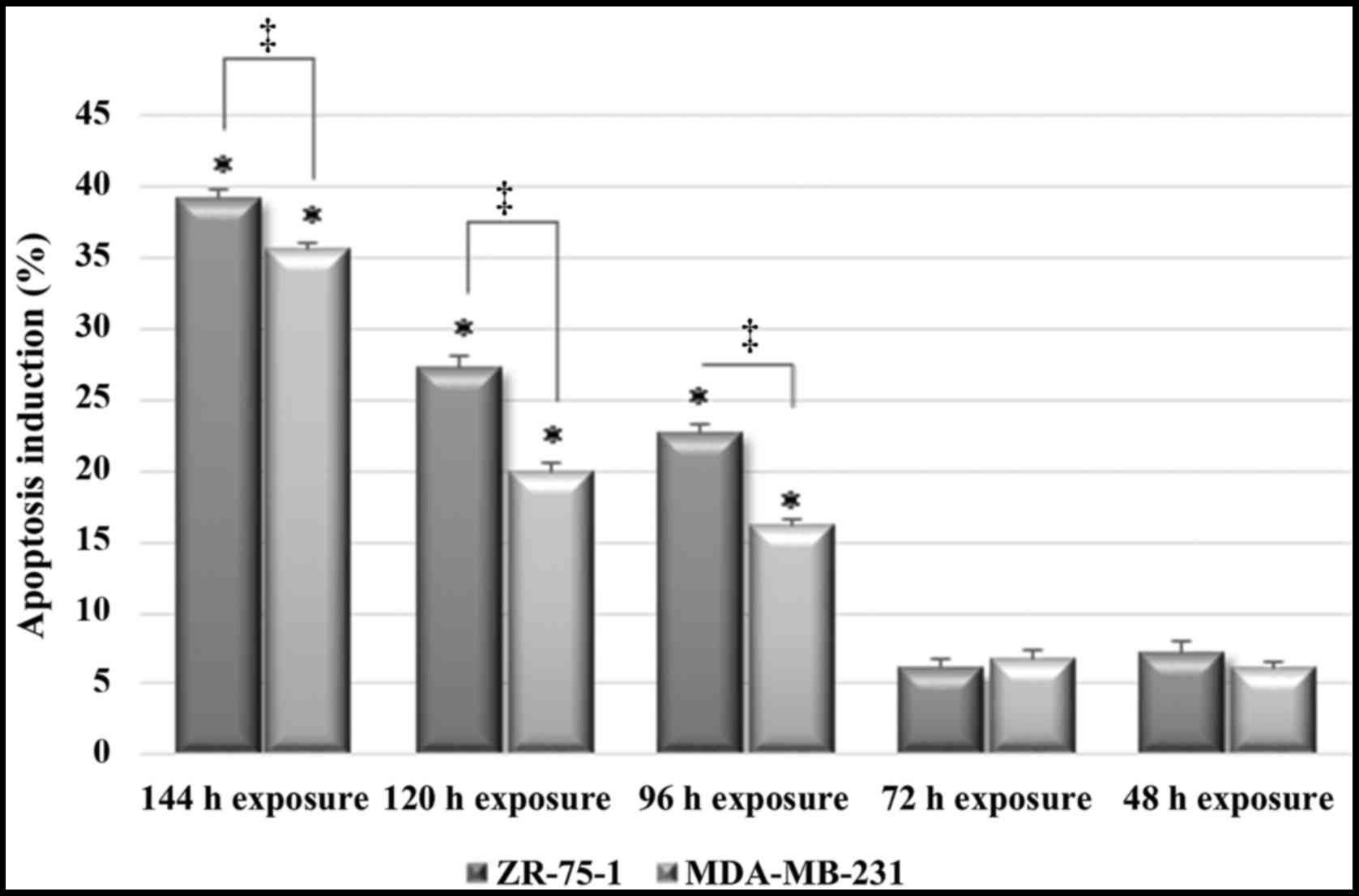
As demonstrated in Fig. 4, the highest percentage of
apoptotic cells (39.2%) was detected in the ZR-75-1 group after 144
h, whereas the percentage of apoptotic MDA-MB-231 cells was 35.6%
at 144 h, which was slightly lower than the percentage of apoptotic
ZR-75-1 cells. However, treatment with imatinib for 120 and 96 h
resulted in a significant difference in apoptotic index between
MDA-MB-231 and ZR-75-1 cells, this may be due to the inhibitory
mechanism of action of imatinib mesylate. Therefore, the results of
a TUNEL assay indicated that the ZR-75-1 cell line was more
sensitive to imatinib mesylate-induced apoptosis compared with the
MDA-MB-231 cell line. Apoptotic induction was confirmed in the
positive control group, which was provided by the Cell Death
Detection ELISA plus kit (Roche Diagnostics; data not shown).
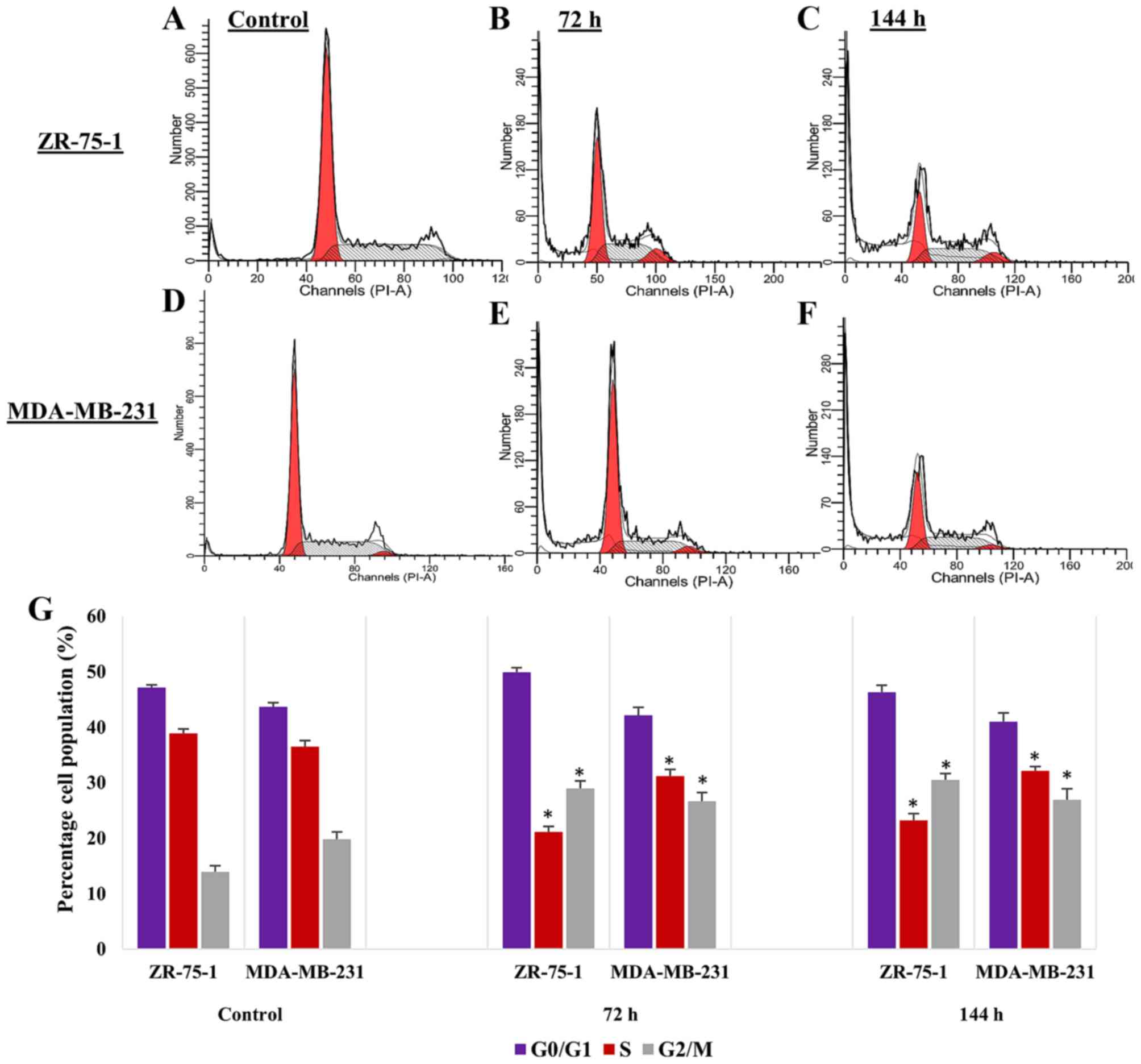
Imatinib induces cell cycle arrest at
G2/M phase
The present study indicated that, in breast
carcinoma cell lines, imatinib mesylate treatment significantly
suppressed cell proliferation after 96 h; however, the
antiproliferative effects of imatinib mesylate treatment after 72 h
were previously confirmed by Weigel et al (18) in a panel of breast cancer cell
lines. Furthermore, in the present study, after 72 h of treatment,
apoptosis was not significantly induced by imatinib. Conversely,
after 96 h of treatment, apoptosis was significantly induced in the
breast cancer cell lines. Subsequently, a cell cycle analysis was
conducted to determine the effects of imatinib mesylate on cell
cycle progression in ZR-75-1 and MDA-MB-231 cell lines (Fig. 5). Similar to the results of the
proliferation assay, the cell cycle analysis in breast carcinoma
cell lines revealed that imatinib suppressed carcinoma cell
proliferation, as indicated by a significant increase in cells in
G2/M phase and a significant decrease in cells in S
phase. Furthermore, the results indicated that suppression of cell
proliferation after 72 h of treatment was due only to cell
cytostatic activity; however, after 144 h, induction of apoptosis,
as well as cell cytostatic activity, was responsible for the
antiproliferative effects of imatinib mesylate on ZR-75-1 and
MDA-MB-231 breast carcinoma cell lines.
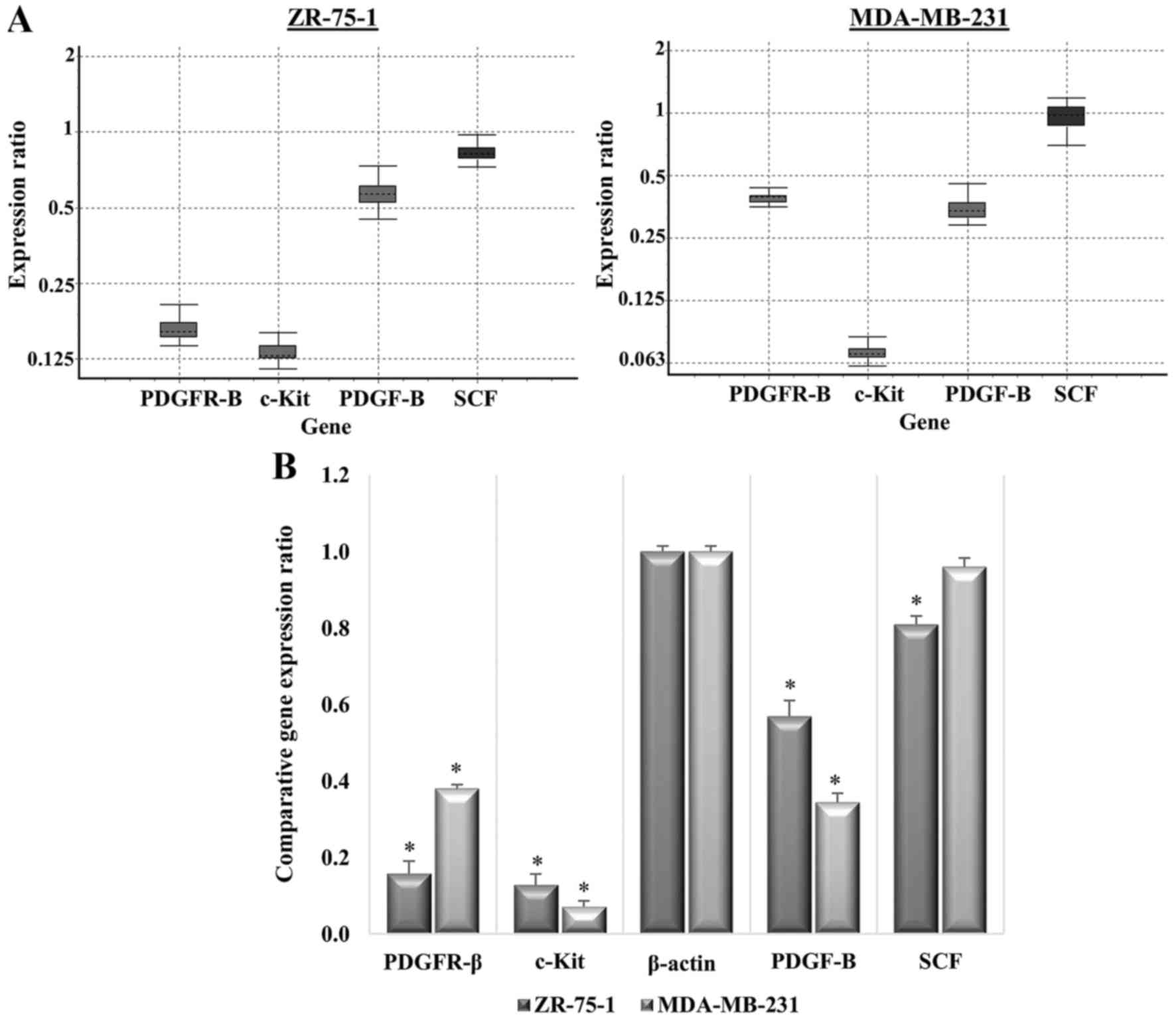
PDGFR-β, c-Kit, PDGF-BB and SCF gene
expression in breast cancer cell lines
The expression levels of genes encoding PDGFR-β,
c-Kit, and their corresponding ligands PDGF-B and SCF, in breast
carcinoma cell lines were investigated using RT-qPCR. The β-actin
housekeeping gene was used to normalize the relative expression
levels of genes of interest.
The results of RT-qPCR are presented in Fig. 6 and revealed that the four genes
of interest were expressed in both carcinoma cell lines with
variable expression levels. The mRNA expression levels of PDGFR-β
were expressed in both carcinoma cell lines at similar levels.
Similar to the levels of its corresponding receptor, PDGF-B mRNA
exhibited high expression in both carcinoma cell lines; however,
higher expression was detected in the ZR-75-1 cell line. c-Kit
expression was significantly less in MDA-MB-231 cells compared to
ZR-75-1, whereas the expression of its complementary ligand, SCF,
was equally expressed in both cancer cell lines. These findings
indicated that the breast carcinoma cell lines express at least two
potential targets for imatinib. Furthermore, protein expression
levels were confirmed by western blotting.
Imatinib affects PDGFR-β/PDGF-B and
c-Kit/SCF gene expression
The relative quantification method was used for gene
expression analysis. Gene expression of treated samples was
compared with the untreated control group, and was normalized to
the endogenous control (housekeeping gene) using REST 2009
software. Following treatment of each cell line with the specific
IC50 value of imatinib, the expression levels of
PDGFR-β, PDGF-B and c-Kit were significantly downregulated compared
with in the untreated group (P<0.05), whereas SCF was
significantly but slightly downregulated in the ZR-75-1 cell line,
but was not significantly altered in the MDA-MB-231 cell line.
According to relative gene expression analysis, as
presented in Fig. 7 and Table I, the ZR-75-1 cell line exhibited
reduced suppression with regards to expression levels compared with
the MDA-MB-231 cell line. Results of the PDGF-β gene expression
analysis revealed that, similar to its corresponding receptor, its
expression was relatively downregulated in the imatinib-treated
group compared with in the untreated control group; relative
expression levels were lower in the MDA-MB-231 cell line (mean mRNA
expression level, 0.34) compared with in the ZR-75-1 cell line
(mean mRNA expression level, 0.57). In addition, c-Kit was markedly
downregulated in MDA-MB-231 and ZR-75-1 cell lines (mean mRNA
expression levels, 0.07 and 0.13, respectively), whereas the
complementary ligand SCF was not significantly altered in the
MDA-MB-231 cell line, and was insignificantly downregulated in
ZR-75-1 cells (mean mRNA expression level, 0.82).
 | Table IRelative gene expression profile
report. |
Table I
Relative gene expression profile
report.
| Gene | Type | Expression | Std. Error | P(H1) | Result |
|---|
| ZR-75-1 | | | | | |
| β-actin | REF | 1.000 | | | |
| PDGFR-β | TRG | 0.164 | 0.149–0.181 | 0.033 | Down |
| c-Kit | TRG | 0.131 | 0.119–0.142 | 0.029 | Down |
| PDGF-B | TRG | 0.570 | 0.502–0.650 | 0.028 | Down |
| SCF | TRG | 0.824 | 0.766–0.888 | 0.026 | Down |
| MDA-MB-231 | | | | | |
| β-actin | REF | 1.000 | | | |
| PDGFR-β | TRG | 0.389 | 0.363–0.408 | 0.020 | Down |
| c-Kit | TRG | 0.070 | 0.064–0.076 | 0.012 | Down |
| PDGF-B | TRG | 0.344 | 0.302–0.384 | 0.020 | Down |
| SCF | TRG | 0.958 | 0.829–1.144 | 0.668 | |
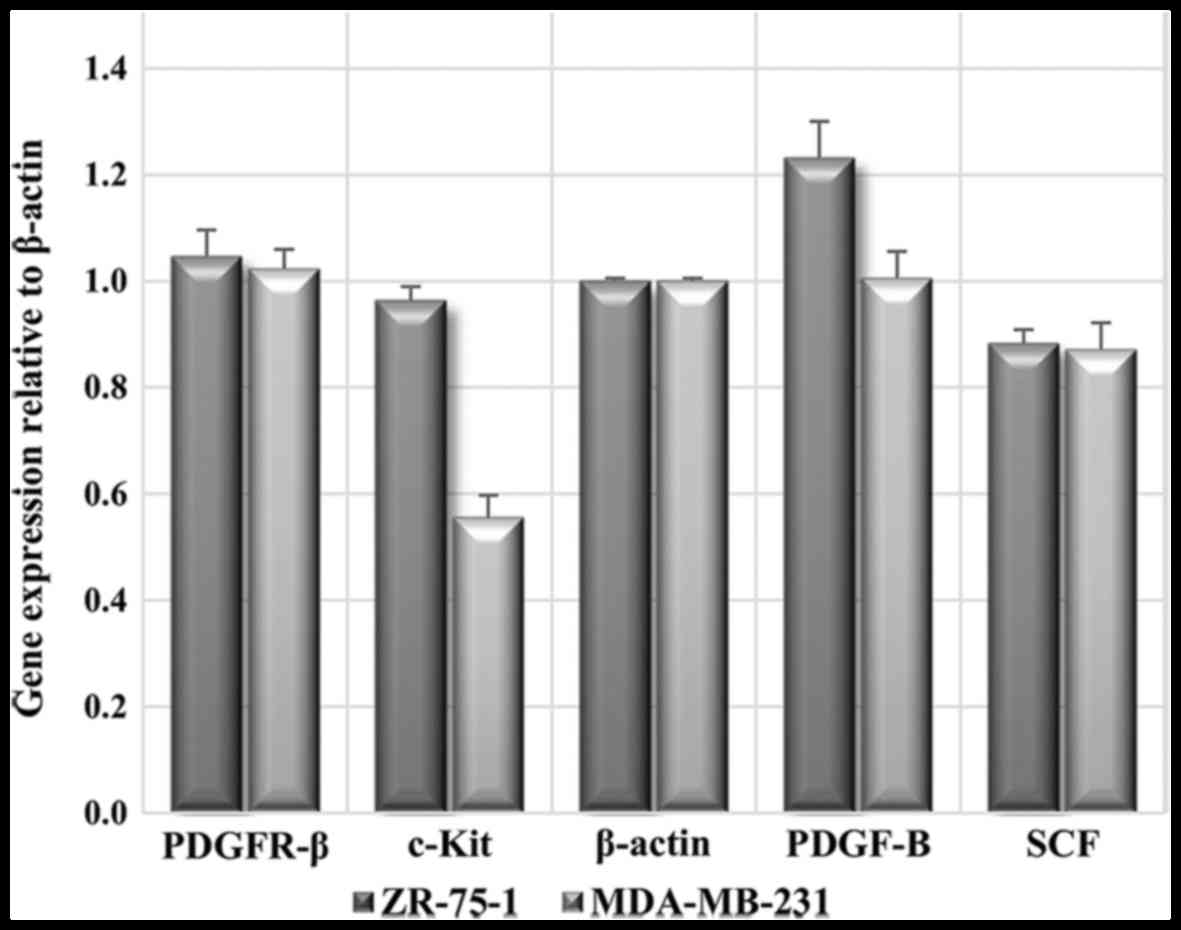
PDGFR-β/PDGF-BB and c-Kit/SCF protein
expression is also affected by imatinib
To evaluate the expression and regulation of genes
and proteins of interest under imatinib mesylate treatment, RT-qPCR
and western blotting were conducted. In order to confirm the
results of the gene expression study, post-transcriptional
regulation was analyzed using western blotting.
Western blot images were semi-quantified three times
using ImageJ 1.47v software (National Institutes of Health,
Bethesda, MD, USA), and mean results were considered relative
protein expression levels. The relative expression levels of
examined proteins in untreated carcinoma cell lines are presented
in Fig. 8. The results revealed
that PDGFR-β protein was highly expressed in both examined breast
carcinoma cell lines. Similar to its corresponding receptor, the
protein expression levels of PDGF-BB were expressed in both cell
lines, with markedly higher expression in ZR-75-1 cell line. c-Kit
protein expression, as an imatinib mesylate target, was markedly
lower than PDGFR-β, with the lowest expression in the MDA-MB-231
cell line; however, SCF protein was moderately expressed in both
carcinoma cell lines.
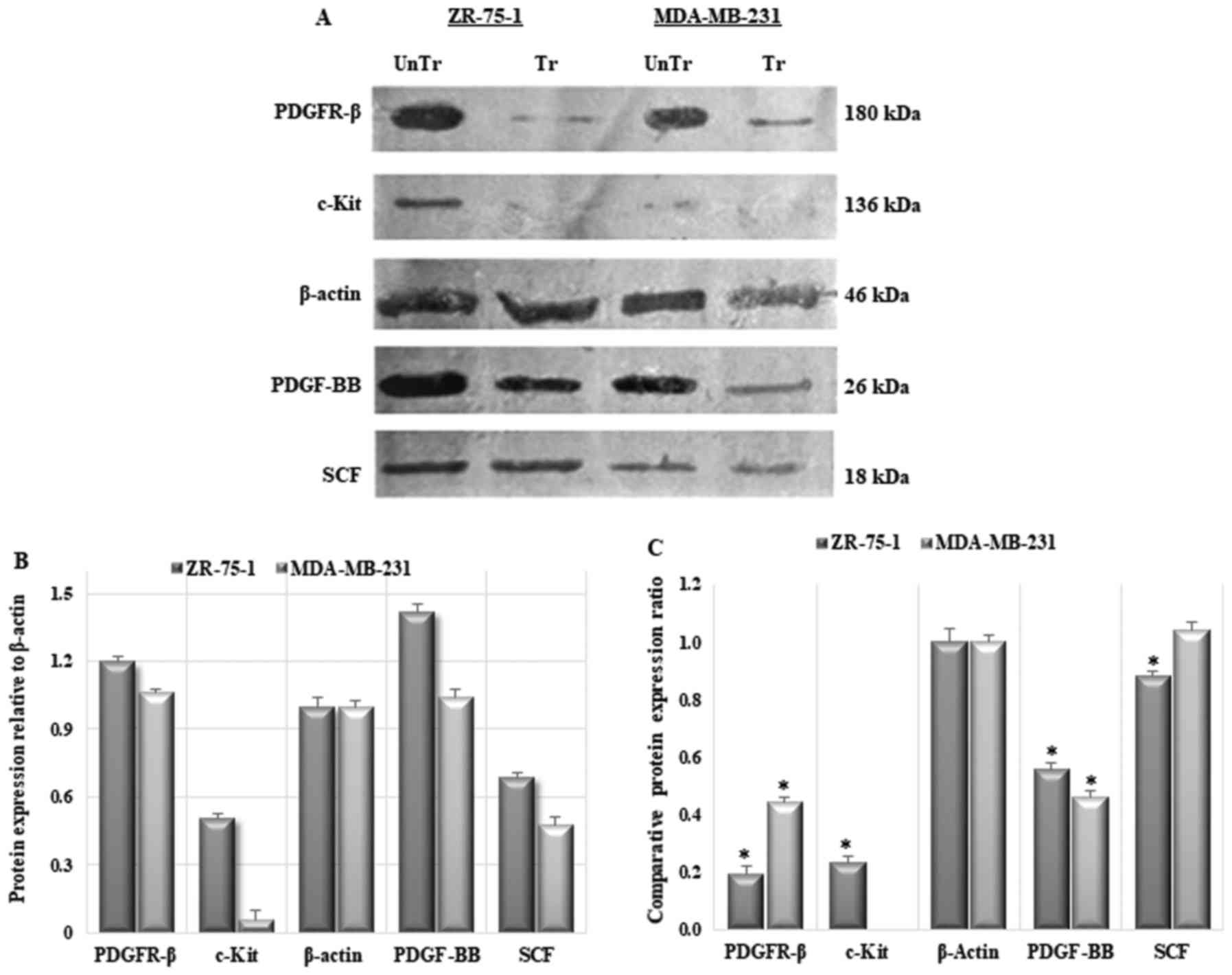 | Figure 8Protein expression profiling of
cancer cell lines and effects of imatinib mesylate on PDGFR-β,
c-Kit, PDGF-BB and SCF protein expression compared with untreated
cell lines. (A) Western blot analysis. (B) Comparative expression
of PDGFR-β, c-Kit, PDGF-BB and SCF proteins compared to the
housekeeping protein (β-actin; control) in untreated breast
carcinoma cell lines. PDGF, platelet-derived growth factor; PDGFR,
PDGF receptor; SCF, stem cell factor. (C) Protein expression
pattern profile. Data are presented as the mean ± standard
deviation (n=3); data were normalized to 1. *P<0.05
compared with the untreated control cells. PDGF, platelet-derived
growth factor; PDGFR, PDGF receptor; SCF, stem cell factor. |
Western blot analysis demonstrated that PDGFR-β,
c-Kit and PDGF-BB protein expression was significantly
downregulated in cells treated with the corresponding
IC50 values of imatinib mesylate compared with in the
untreated control cells, with β-actin as internal control.
As shown in Fig.
8, the protein expression levels of PDGFR-β were significantly
suppressed (P<0.05) in both treated cell lines compared with in
the untreated control cells; expression was strongly suppressed in
the ZR-75-1 cell line (mean protein expression level, 0.19) and was
also suppressed in MDA-MB-231 cells (protein expression, 0.44).
Relative expression levels of PDGF-BB were significantly suppressed
in ZR-75-1 and MDA-MB-231 cell lines (mean protein expression
levels, 0.56 and 0.46, respectively). The protein expression levels
of c-Kit were markedly reduced in ZR-75-1 cells (mean protein
expression level, 0.24), whereas c-Kit protein expression was very
low in untreated cells, and was completely suppressed and
undetectable in treated MDA-MB-231 cells. Furthermore, the
complementary ligand SCF did not exhibit any significant
alterations in protein expression in imatinib mesylate-treated
MDA-MB-231 cells, whereas SCF protein expression was downregulated
in the ZR-75-1 cell line (mean protein expression level, 0.88).
Discussion
Imatinib mesylate is a molecularly-specific
anticancer agent, which targets the tyrosine kinase receptors
PDGFRs and c-Kit (22). According
to the findings of the present study, imatinib mesylate treatment
induced apoptosis of breast carcinoma cells, particularly after 96
h, which may be attributed to the regulation of PDGFR and c-Kit
pathways. The incubation time required to induce apoptosis of other
cell types varies. For example, neuroblastoma cells exhibit signs
of apoptosis after 48 h of incubation, whereas osteosarcoma cells
undergo apoptosis after 120 h (31,32). The proapoptotic effects of
imatinib have been reported in breast cancer cells in the present
study, as well as in other types of cancer cells in previous
studies; however, imatinib does not induce apoptosis at all time
points. For example, in a previous study, in ovarian carcinoma
cells, imatinib exerted G1/G0 cytostatic
activity (21). In addition,
apoptosis among vascular endothelial cells is not directly related
to imatinib treatment and might be the consequence of other factors
(21). Furthermore, Uziel et
al (33) reported the
cytostatic activity of imatinib in Ewing sarcoma cells in
G2/M phase. The results of the present study detected a
significant G2/M phase cell cycle arrest, and a
significant reduction in the number of cells at S phase, in breast
carcinoma cell lines under imatinib mesylate treatment, and the
antiproliferative effects of imatinib were detected, which was
concordant with the findings of Malavaki et al (13). Furthermore, the IC50
values of imatinib can reach between 4 and 6 μM in human
blood serum in vivo (34).
Therefore, the in-vitro IC50 of imatinib mesylate
against MDA-MB-231 and ZR-75-1 which was calculated in this study
can be availed in blood.
Numerous diseases, including cancer, cardiovascular
diseases and developmental defects, have been reported to be
associated with the abnormal regulation of cytokines or growth
factors, and corresponding receptors. It is well known that breast
carcinoma tissues express PDGF and some types of carcinoma express
c-Kit. c-Kit and PDGFR availability in target cells is important
for cellular reactions dependent on cytokines/PDGF, and is reliant
on their regulation (35–37). The present study examined the
expression of the mature forms of PDGFR-β and c-Kit, as well as
their corresponding ligands PDGF-BB and SCF, in breast cancer cell
lines.
The PDGF-BB complementary ligand of PDGFR-β was
markedly expressed in both studied cell lines. Furthermore, the
receptor tyrosine kinase c-Kit, and its complementary ligand SCF,
were expressed by both cell lines, and therefore may be considered
additional targets for imatinib mesylate treatment. These findings
indicated that at least two potential targets for imatinib were
expressed by the studied breast carcinoma cell lines.
PDGF protein expression, as a ligand for PDGFRs, was
detected in both studied breast carcinoma cell lines. It has been
suggested that the paracrine and autocrine effects of PDGF-BB on
stimulation of the PDGFR-β signaling pathway are essential for
tumor angiogenesis and cell proliferation of breast cancer
(38); this has also been
observed in other malignancies, including glioblastoma and ovarian
cancer (39,40). The present study suggested that
imatinib may inhibit proliferation of breast cancer cell lines by
exerting effects on this pathway.
Treatment with imatinib led to reduced gene and
protein expression of PDGFR-β in both examined breast cancer cell
lines, as determined by RT-qPCR. This outcome has also been
reported in neuroblastoma cells, osteosarcoma, ovarian cancer and
bone endothelial cells. In addition, it has been reported that
autocrine PDGFR signaling may stimulate metastasis of breast
cancer. In a mouse model, this could be prevented by imatinib
treatment and overexpression of the dominant-negative form of PDGFR
(41–43).
Notably, in both cell lines used in the present
study, treatment with imatinib mesylate resulted in transcriptional
and post-transcriptional downregulation of PDGFR-β and c-Kit
receptors. Alterations in ligand expression were also analyzed. It
is usually presumed that ligand expression increases due to
receptor inhibition. However, receptor inhibition may have an
effect on the downstream kinase cascade pathway in paracrine
signaling, and particularly in autocrine signaling, thus modulating
ligand expression. Results of the gene and protein expression
analyses established PDGF-BB as an autocrine/paracrine ligand that
was downregulated in response to imatinib in both cell lines.
However, imatinib treatment did not significantly downregulate SCF
expression, which may be attributed to endocrine signaling of this
cytokine.
The present results confirmed that imatinib mesylate
not only suppressed tyrosine kinase receptor expression, but also
affected the expression of the receptor tyrosine kinase ligand,
PDGF, which may lead to tumor suppression. However, imatinib could
not control the expression of SCF.
Comparison between the sensitivities of ZR-75-1
cells, which expressed PDGFR-β and c-Kit, and MDA-MB-231 cells,
which possessed low c-Kit expression, to imatinib mesylate
treatment verified that PDGFR-β and c-Kit are targets of imatinib
mesylate inhibition. In addition, suppression of PDGFR-β and c-Kit
expression via imatinib mesylate may lead to increased inhibition
and anticancer effects.
In conclusion, the present study revealed that
imatinib mesylate-induced modulation of PDGFR-β, c-Kit and PDGF-BB
may result in cell death and growth suppression of breast cancer
cell lines via apoptosis or cell cycle arrest. Furthermore,
imatinib mesylate targets the PDGFR-β and c-Kit signaling pathways
via its effects on downstream kinase cascade regulation,
consequently resulting in expression modulation whenever the
receptor inhibition result in the gene or protein expression, it
confirm the downstream regulation. Therefore, the present study
suggested that PDGFR-β and c-Kit expression may be able to predict
the effectiveness of imatinib and monitor its potential therapeutic
effects in patients with breast cancer. Consequently, imatinib
mesylate may be considered a promising medication for the treatment
of breast cancer.
Abbreviations:
|
PDGF
|
platelet-derived growth factor
|
|
SCF
|
stem cell factor
|
|
FBS
|
fetal bovine serum
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
Acknowledgments
The present study was supported by the University of
Malaya IPPP research grant (grant no. PV047/2011A). The funders had
no role in study design, data collection and analysis, decision to
publish, or preparation of the manuscript. The authors of the
present study would like to thank OsvahPharma Co. (Tehran, Iran)
for their gift of imatinib mesylate, and the Department of
Pharmaceutics, Faculty of Pharmacy, Tehran University of Medical
Sciences and the Professor Alborzi Clinical Microbiology Research
Center (PACMRC) for providing and supporting the use of technical
equipment.
References
|
1
|
Stewart B and Wild CP: World Cancer Report
2014. WORLD. 2015.
|
|
2
|
Coates AS, Winer EP, Goldhirsch A, Gelber
RD, Gnant M, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel
Members: Tailoring therapies--improving the management of early
breast cancer: St Gallen International Expert Consensus on the
Primary Therapy of Early Breast Cancer 2015. Ann Oncol.
26:1533–1546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lopez L, Carvajal C, Gallardo M and Russo
N: Evolution in breast cancer suspicion and extent of surgery at a
radio-oncology center. Rev Chil Cir. 66:241–244. 2014.
|
|
4
|
Ross JS, Fletcher JA, Bloom KJ, Linette
GP, Stec J, Symmans WF, Pusztai L and Hortobagyi GN: Targeted
therapy in breast cancer: The HER-2/neu gene and protein. Mol Cell
Proteomics. 3:379–398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ellis MJ, Coop A, Singh B, Mauriac L,
Llombert-Cussac A, Jänicke F, Miller WR, Evans DB, Dugan M, Brady
C, et al: Letrozole is more effective neoadjuvant endocrine therapy
than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen
receptor-positive primary breast cancer: Evidence from a phase III
randomized trial. J Clin Oncol. 19:3808–3816. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jordan VC: Tamoxifen (ICI46,474) as a
targeted therapy to treat and prevent breast cancer. Br J
Pharmacol. 147(Suppl 1): S269–S276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gschwind A, Fischer OM and Ullrich A: The
discovery of receptor tyrosine kinases: Targets for cancer therapy.
Nat Rev Cancer. 4:361–370. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
LaVallee TM, Alvarado D, Garton AJ,
Trombetta ES, Gedrich R and McMahon G: Emerging receptor tyrosine
kinase drug targets: Implications for antibody-based therapies for
oncology and immunologic disorders. Crit Rev Oncog. 20:485–508.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kadivar A, Kamalidehghan B, Javar HA,
Davoudi ET, Zaharuddin ND, Sabeti B, Chung LY and Noordin MI:
Formulation and in vitro, in vivo evaluation of effervescent
floating sustained-release imatinib mesylate tablet. PLoS One.
10:e01268742015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manley PW, Cowan-Jacob SW, Buchdunger E,
Fabbro D, Fendrich G, Furet P, Meyer T and Zimmermann J: Imatinib:
A selective tyrosine kinase inhibitor. Eur J Cancer. 38(Suppl 5):
S19–S27. 2002. View Article : Google Scholar
|
|
11
|
Radford IR: Imatinib. Novartis Curr Opin
Investig Drugs. 3:492–499. 2002.
|
|
12
|
Buchdunger E, Cioffi CL, Law N, Stover D,
Ohno-Jones S, Druker BJ and Lydon NB: Abl protein-tyrosine kinase
inhibitor STI571 inhibits in vitro signal transduction mediated by
c-kit and platelet-derived growth factor receptors. J Pharmacol Exp
Ther. 295:139–145. 2000.PubMed/NCBI
|
|
13
|
Malavaki CJ, Roussidis AE, Gialeli C,
Kletsas D, Tsegenidis T, Theocharis AD, Tzanakakis GN and Karamanos
NK: Imatinib as a key inhibitor of the platelet-derived growth
factor receptor mediated expression of cell surface heparan sulfate
proteoglycans and functional properties of breast cancer cells.
FEBS J. 280:2477–2489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong Y, Han Q, Zou Y, Deng Z, Lu X, Wang
X, Zhang W, Jin H, Su J, Jiang T, et al: Long-term exposure to
imatinib reduced cancer stem cell ability through induction of cell
differentiation via activation of MAPK signaling in glioblastoma
cells. Mol Cell Biochem. 370:89–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Verweij J, van Oosterom A, Blay J-Y,
Judson I, Rodenhuis S, van der Graaf W, Radford J, Le Cesne A,
Hogendoorn PC, di Paola ED, et al: Imatinib mesylate (STI-571
Glivec, Gleevec) is an active agent for gastrointestinal stromal
tumours, but does not yield responses in other soft-tissue sarcomas
that are unselected for a molecular target. Results from an EORTC
Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer.
39:2006–2011. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paschka P, Marcucci G, Ruppert AS, Mrózek
K, Chen H, Kittles RA, Vukosavljevic T, Perrotti D, Vardiman JW,
Carroll AJ, et al Cancer and Leukemia Group B: Adverse prognostic
significance of KIT mutations in adult acute myeloid leukemia with
inv(16) and t(8;21): A Cancer and Leukemia Group B Study. J Clin
Oncol. 24:3904–3911. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heinrich MC, Griffith DJ, Druker BJ, Wait
CL, Ott KA and Zigler AJ: Inhibition of c-kit receptor tyrosine
kinase activity by STI 571, a selective tyrosine kinase inhibitor.
Blood. 96:925–932. 2000.PubMed/NCBI
|
|
18
|
Weigel MT, Meinhold-Heerlein I,
Bauerschlag DO, Schem C, Bauer M, Jonat W, Maass N and Mundhenke C:
Combination of imatinib and vinorelbine enhances cell growth
inhibition in breast cancer cells via PDGFR β signalling. Cancer
Lett. 273:70–79. 2009. View Article : Google Scholar
|
|
19
|
Demetri GD, von Mehren M, Blanke CD, Van
den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA,
Singer S, Janicek M, et al: Efficacy and safety of imatinib
mesylate in advanced gastrointestinal stromal tumors. N Engl J Med.
347:472–480. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bond M, Bernstein ML, Pappo A, Schultz KR,
Krailo M, Blaney SM and Adamson PC: A phase II study of imatinib
mesylate in children with refractory or relapsed solid tumors: A
Children's Oncology Group study. Pediatr Blood Cancer. 50:254–258.
2008. View Article : Google Scholar
|
|
21
|
Matei D, Chang DD and Jeng M-H: Imatinib
mesylate (Gleevec) inhibits ovarian cancer cell growth through a
mechanism dependent on platelet-derived growth factor receptor α
and Akt inactivation. Clin Cancer Res. 10:681–690. 2004. View Article : Google Scholar
|
|
22
|
Gross DJ, Munter G, Bitan M, Siegal T,
Gabizon A, Weitzen R, Merimsky O, Ackerstein A, Salmon A, Sella A,
et al Israel Glivec in Solid Tumors Study Group: The role of
imatinib mesylate (Glivec) for treatment of patients with malignant
endocrine tumors positive for c-kit or PDGF-R. Endocr Relat Cancer.
13:535–540. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Jong JS, van Diest PJ, van der Valk P
and Baak JP: Expression of growth factors, growth inhibiting
factors, and their receptors in invasive breast cancer. I: An
inventory in search of autocrine and paracrine loops. J Pathol.
184:44–52. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lopes LF and Bacchi CE: Imatinib treatment
for gastrointestinal stromal tumour (GIST). J Cell Mol Med.
14:42–50. 2010. View Article : Google Scholar
|
|
25
|
Kim KB, Eton O, Davis DW, Frazier ML,
McConkey DJ, Diwan AH, Papadopoulos NE, Bedikian AY, Camacho LH,
Ross MI, et al: Phase II trial of imatinib mesylate in patients
with metastatic melanoma. Br J Cancer. 99:734–740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rutkowski P, Van Glabbeke M, Rankin CJ,
Ruka W, Rubin BP, Debiec-Rychter M, Lazar A, Gelderblom H, Sciot R,
Lopez-Terrada D, et al European Organisation for Research and
Treatment of Cancer Soft Tissue/Bone Sarcoma Group; Southwest
Oncology Group: Imatinib mesylate in advanced dermatofibrosarcoma
protuberans: Pooled analysis of two phase II clinical trials. J
Clin Oncol. 28:1772–1779. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mahata S, Maru S, Shukla S, Pandey A,
Mugesh G, Das BC and Bharti AC: Anticancer property of Bryophyllum
pinnata (Lam.) Oken leaf on human cervical cancer cells. BMC
Complement Altern Med. 12:152012. View Article : Google Scholar
|
|
28
|
Pfaffl MW, Horgan GW and Dempfle L:
Relative expression software tool (REST) for group-wise comparison
and statistical analysis of relative expression results in
real-time PCR. Nucleic Acids Res. 30:e362002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
30
|
Hu S, Franke RM, Filipski KK, Hu C, Orwick
SJ, de Bruijn EA, Burger H, Baker SD and Sparreboom A: Interaction
of imatinib with human organic ion carriers. Clin Cancer Res.
14:3141–3148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beppu K, Jaboine J, Merchant MS, Mackall
CL and Thiele CJ: Effect of imatinib mesylate on neuroblastoma
tumorigenesis and vascular endothelial growth factor expression. J
Natl Cancer Inst. 96:46–55. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin S, Shen JN, Wang J, Huang G and Zhou
JG: Oridonin induced apoptosis through Akt and MAPKs signaling
pathways in human osteosarcoma cells. Cancer Biol Ther. 6:261–268.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Uziel O, Fenig E, Nordenberg J, Beery E,
Reshef H, Sandbank J, Birenbaum M, Bakhanashvili M, Yerushalmi R,
Luria D, et al: Imatinib mesylate (Gleevec) downregulates
telomerase activity and inhibits proliferation in
telomerase-expressing cell lines. Br J Cancer. 92:1881–1891. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peng B, Lloyd P and Schran H: Clinical
pharmacokinetics of imatinib. Clin Pharmacokinet. 44:879–894. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Heldin CH: Targeting the PDGF signaling
pathway in tumor treatment. Cell Commun Signal. 11:972013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsimberidou AM, Kurzrock R and Anderson
KC: The role of angiogenesis in cancer. Target Ther Transl Cancer
Res. 5:642015.
|
|
37
|
Kofler S, Nickel T and Weis M: Role of
cytokines in cardiovascular diseases: A focus on endothelial
responses to inflammation. Clin Sci (Lond). 108:205–213. 2005.
View Article : Google Scholar
|
|
38
|
Vrekoussis T, Stathopoulos EN, Kafousi M,
Navrozoglou I and Zoras O: Expression of endothelial PDGF receptors
α and β in breast cancer: Up-regulation of endothelial PDGF
receptor β. Oncol Rep. 17:1115–1119. 2007.PubMed/NCBI
|
|
39
|
Nazarenko I, Hede S-M, He X, Hedrén A,
Thompson J, Lindström MS and Nistér M: PDGF and PDGF receptors in
glioma. Ups J Med Sci. 117:99–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wilczynski SP, Chen Y-Y, Chen W, Howell
SB, Shively JE and Alberts DS: Expression and mutational analysis
of tyrosine kinase receptors c-kit, PDGFRalpha, and PDGFRbeta in
ovarian cancers. Hum Pathol. 36:242–249. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jechlinger M, Sommer A, Moriggl R, Seither
P, Kraut N, Capodiecci P, Donovan M, Cordon-Cardo C, Beug H and
Grünert S: Autocrine PDGFR signaling promotes mammary cancer
metastasis. J Clin Invest. 116:1561–1570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Geng L, Shinohara ET, Kim D, Tan J, Osusky
K, Shyr Y and Hallahan DE: STI571 (Gleevec) improves tumor growth
delay and survival in irradiated mouse models of glioblastoma. Int
J Radiat Oncol Biol Phys. 64:263–271. 2006. View Article : Google Scholar
|
|
43
|
Board R and Jayson GC: Platelet-derived
growth factor receptor (PDGFR): A target for anticancer
therapeutics. Drug Resist Updat. 8:75–83. 2005. View Article : Google Scholar : PubMed/NCBI
|