Introduction
Germ cell can generate sperm and oocyte which are
the basis for sexual reproduction (1). To produce a functional sperm, an
unformed male germ cell undergoes a coordinated set of events that
are collectively called spermatogenesis. In many animals, sperm
cells are derived from a first germline cell population of
primordial germ cells (PGCs) that are set aside early in
embryogenesis (2,3). In the male gonads, PGCs initiate
differentiation toward spermatogenesis.
For more than a century, researchers have been
focusing on spermatogenesis in vitro (4). Previous studies have indicated that
retinoic acid (RA) can trigger differentiation of GS cells
(5,6). RA, the active derivative of vitamin
A, controls the entry of germ cells into meiosis in avian, mice and
humans (1,7,8).
It has been demonstrated that RA and stem cell factor (SCF) are
crucial for the maintenance of normal spermatogenesis in rodents
and human spermatogonial stem cells (SSCs) (6,9).
SCF has been evidenced to induce human and mouse SSCs to
differentiate into haploid spermatids in vitro (6,10)
and the SCF/KIT system plays key role in SSC proliferation and
entrance meiosis (11). Moreover,
RA has been indicated to trigger germ cell meiotic origination in
the chicken (12). Unfortunately,
there is no evidence showing the functions of SCF in induction of
PGCs spermatogenesis. Therefore, RA and SCF were chosen in this
study to induce the differentiation of chicken PGCs.
In this study, we successfully generated haploid
spermatids by RA and SCF inducing chicken PGCs directly in
vitro. We have indicated that RA leads to PGC differentiation,
and SCF can improve the efficiency of induction. In addition to
providing an in vitro model for chicken PGC spermatogenesis
through RA and SCF stimulation. Our results support the recent
protocol that RA can trigger germ cell differentiation without
somatic testicular cells (13).
Our results demonstrated that chicken PGCs could be induced into
male gametes. It may be a great animal model for study of
spermatogenesis. We have built an efficient proposal to induce
haploid spermatids in vitro, it could provide abundant
operating materials for spermatozoal function research promoting
the use of stem cell transplanting engineering in clinical
infertility.
Materials and methods
Experimental animals
All the animal procedures were approved by the
Institutional Animal Care and Use Committee of Chinese Academy of
Agricultural Sciences. Chicken embryos were provided by the
Experimental Animal Base Institute of Animal Sciences, Chinese
Academy of Agricultural Sciences, Beijing, China. The use of
animals in research and all experimental procedures involving
chicken embryos were conducted in accordance with the guidelines
established by the Institutional Animal Care and Use Committee at
the University of Medicine and Dentistry of New Jersey-Robert Wood
Johnson Medical School.
Isolation and in vitro culture of chicken
PGCs
Fertile eggs were incubated at 38°C and 50% humidity
for 5.5 day. Gonads of the chicken embryos developed at stage 28
(14), were isolated using
stereoscopic microscope, then embryonic gonadal tissues were
collected and dissociated with 0.125% trypsin-0.02%
ethylenediaminetetraacetic acid (EDTA). After neutralized with
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum (FBS), the gonadal cells were collected by
centrifugation.
The precipitated cells were re-suspended in PGC cell
culture medium, which consisted of DMEM medium replenished with 10%
FBS (Gibco, Grand Island, NY, USA), 2% chicken serum and
supplemented with 10 ng/ml of human SCF (hSCF), 10 U/ml of leukemia
inhibitory factor (LIF), 20 ng/ml of human basic fibroblast growth
factor (bFGF) (all from PeproTech, London, UK), 1X
penicillin/streptomycin (Life Technologies, Grand Island, NY, USA).
Cell suspensions were seeded into 6-well culture plates with
chicken embryonic fibroblasts (CEFs) as feeding layer (15) at a density of
1×104/well, and cultured at 37.5°C in 5% CO2.
The PGC colonies were dissociated and moved into fresh plates with
CEFs using trypsin-EDTA treatment for subculture (16).
Embryonic tissue collection
Following separation of the embryonic gonadal
tissues respectively, ~10 mg of embryonic tissue was collected and
placed in 10 ml of digestion buffer (10 mM Tris, 1 mM EDTA, 1% SDS,
pH 8.0 containing 10 mg/ml Proteinase-K) and incubated overnight at
45°C. Samples were cooled to 4°C and centrifuged at 12,000 rpm for
10 min. The supernatant was transferred to a fresh micro-centrifuge
tube, diluted to 200 µl with water and used in PCR
reactions.
PAS staining and alkaline phosphatase
activity assay
For PAS staining, the cell colonies of the cultured
chicken PGCs were fixed with 4% paraformaldehyde for 20 min and
washed 3×5 min with phosphate-buffered saline (PBS). The cell
colonies were then submerged to periodic acid solution
(Sigma-Aldrich, St. Louis, MO, USA) for 5 min at room temperature.
After washing twice with PBS, the cell colonies were immersed in
Schiff's solution (Sigma-Aldrich) for 15 min at room temperature.
Then PAS-stained PGC colonies were observed under an inverted
microscope after washing twice with PBS. Alkaline phosphatase (AKP)
activity was detected by AKP substrate kit (Sigma-Aldrich)
according to the manufacturer's instruction. Images were captured
with a computer-assisted video camera (IX-71 inverted research
microscope; Olympus, Tokyo, Japan).
Karyotyping
The chicken PGCs were incubated in 0.5 µg/ml
colcemid (Karyomax; Invitrogen, Carlsbad, CA, USA) at 37.5°C in 5%
CO2 for 5 h, then the chicken PGCs were dissociated with
0.125% trypsin-0.02% EDTA. The cells were then centrifuged and
resuspended in 0.075 M KCl solution at 37°C. After 30 min, the
cells were centrifuged at 200 × g for 8 min and the pellet was
fixed in 3:1 methanol: glacial acetic acid. For metaphase analysis
the cell suspension was dropped on the frozen glass slides, stained
with Giemsa (Amresco, Solon, OH, USA) and analysed for the 6 pairs
of macro-chromosomes and the sex chromosomes. At least 20 metaphase
spreads were counted for every chicken PGC passage.
Immunocytochemistry staining
For immunocytochemical analysis, the chicken PGCs or
differentiated cells from PGCs were fixed in 4% paraformaldehyde
(PFA) in PBS and permeabilized with 0.4% Triton X-100. Blocking
with 10% goat serum was performed for 1 h prior to the incubation
with primary antibodies. The cells were incubated with primary
antibodies anti-SSEA-1, BLIMP1, Oct4, Sox2 or ACR at 4°C overnight,
followed by goat anti-rabbit FITC or anti-mouse Alexa Fluor 594
(red)-labeled secondary antibody for 1 h. DAPI was used to label
the cell nuclei, and images were captured with a fluorescence
microscope (Nikon TE-2000-E inverted microscope; Nikon, Tokyo,
Japan).
RNA isolation and PCR analysis
Total RNA was extracted from the PGC colonies at
third passage or induced cells after 48 h using TRIzol
(Invitrogen). After DNase I treatment to remove the potential
contamination of genomic DNA, 2.0 µg of total RNA were
reverse transcribed into cDNA using an RNA PCR kit (AMV) Ver.3.0
(Takara Bio Co., Dalian, China). The gene expression analysis was
detected by ABI StepOnePlus Real-time PCR thermal cycling
instrument (Applied Biosystems, Foster City, CA, USA). The stage
specific genes CVH, BLIMP1 and the stem cell specific genes Pouv
and Nanog (the primer information is shown in Table I), the Xhol repeat sequence
and 18S ribosomal gene (the primer information is shown in Table II) and the hallmarks of meiotic
germ cells and haploid germ cell gene acrosin (ACR), DNA meiotic
recombinase 1 (DMC1), BOULE, Dazl, protamine 1 (PRM1) and
synaptonemal complex protein 1 (SYCP1) were detected (the primer
information is shown in Table
III), the PCR reactions were performed by the PCR Master Mix
kit (Promega, Madison, WI, USA), and the PCR products were
visualized by electrophoresis on 2.5% agarose gels. The qPCR was
carried out on an ABI 7300 HT real-time PCR machine (Applied
Biosystems) with the reaction volume of 30 µl consisting of
complementary DNA from 15 ng of the original RNA template, 400 nM
of each of the gene-specific forward and reverse primers, and 15
µl SYBR Premix Ex Taq (Takara Bio Co.). Then, qRT-PCR was
carried out in triplicate with a SYBR Premix Ex Taq in an ABI 7500
Real-time PCR detection system (Applied Biosystems). After
normalization with glyceraldehyde 3-phosphate dehydrogenase
(GAPDH), relative RNA levels in the samples were calculated by the
comparative threshold cycle method. The sequences of primers for
PCR are listed in Table III.
The ΔΔCt method was used to calculate relative fold-change values
between samples.
 | Table IThe primer information for PGC
identification. |
Table I
The primer information for PGC
identification.
| Gene name | Primer
sequences | Circles | Product length
(bp) | Tm (°C) |
|---|
| CVH | F:
5′-TGGTACTAGATGAAGCAGAC-3′ | 35 | 373 | 56 |
| R:
5′-GATGGTAGGTTCTCTTGACA-3′ | | | |
| BLIMP1 | F:
5′-AACCGAATCAACGAGGAG-3′ | 35 | 249 | 55 |
| R:
5′-GTGACTGTGAGGCAACTT-3′ | | | |
| POUV | F:
5′-TTCAGCCAGACCACCATC-3′ | 35 | 100 | 57 |
| R:
5′-CTGCCTCATTGAGCCAAC-3′ | | | |
| NANOG | F:
5′-ATGGCTGTGGAGGATGAG-3′ | 35 | 209 | 56 |
| R:
5′-TGATGCCGTACAGGAGAG-3′ | | | |
| GAPDH | F:
5′-AGGTGCTGAGTATGTTGTG-3′ | 35 | 269 | 55 |
| R:
5′-CATGGACAGTGGTCATAAGA-3′ | | | |
 | Table IIThe primer information for male and
female identification. |
Table II
The primer information for male and
female identification.
| Gene name | Primer
sequences | Circles | Product length
(bp) | Tm (°C) |
|---|
| Xhol | F:
5′-CCCAAATATAACACGCTTCACT-3′ | 35 | 417 | 57 |
| R:
5′-GAAATGAATTATTTTCTGGCGAC-3′ | | | |
| 18S ribosomal
gene | F:
5′-AGCTCTTTCTCGATTCCGTG-3′ | 35 | 256 | 60 |
| R:
5′-GGGTAGACACAAGCTGAGCC-3′ | | | |
 | Table IIIThe primer information for meiotic
germ cell identification. |
Table III
The primer information for meiotic
germ cell identification.
| Gene name | Primer
sequences | Circles | Product length
(bp) | Tm (°C) |
|---|
| SYCP1 | F:
5′-GCGTTGTCTGTGGTGATA-3′ | 35 | 89 | 55 |
| R:
5′-GGTTATGCTGCGTCTGAA-3′ | | | |
| BOULE | F:
5′-GCACAGAAGATATTACAAGAGG-3′ | 35 | 108 | 55 |
| R:
5′-TACATTAGAACGAGGCATCC-3′ | | | |
| DAZL | F:
5′-CAATATGGTACTGTGAAGGAG-3′ | 35 | 126 | 54 |
| R:
5′-GACACTGATCTGTGATTCTAC-3′ | | | |
| ACR | F:
5′-TTCTGGCTCATTGGTGTG-3′ | 35 | 150 | 54 |
| R:
5′-TGGATATGGCGTTGTAGTAG-3′ | | | |
| DMC1 | F:
5′-TCTTCGTGACATTGCTGAT-3′ | 35 | 125 | 55 |
| R:
5′-GGAACTTGGCTGCTACATA-3′ | | | |
| STRA8 | F:
5′-CAGTGGAGGTAACAGTGAG-3′ | 35 | 92 | 55 |
| R:
5′-AACCAGCAGCAACATCAA-3′ | | | |
| GAPDH | F:
5′-AACCAGCCAAGTATGATGAT-3′ | 35 | 113 | 55 |
| R:
5′-ACCATTGAAGTCACAGGAG-3′ | | | |
Induction of sperm
The induction solution which consisted of DMEM
medium replenished with 10% FBS, 2% chicken serum and supplemented
with RA and SCF were prepared. The PGCs at passage 3 were chosen
and reseeded with induction solution into 24-well plates at a
density of 5×104/ml in an incubator in 5% CO2
at 37°C.
Flow cytometry
Flow cytometry was performed to quantify DNA content
of chicken embryonal PGCs with different density of RA and SCF
treatment. In brief, the samples were washed twice in PBS and fixed
in cold 70% ethanol, then incubated with a solution containing 25
µg/ml propidium iodide (PI) (Sigma-Aldrich), 40 µg/ml
RNase (Invitrogen), and 0.3% Tween-20 in PBS at room temperature
for 20 min, cells were analyzed with a FACSCalibur system (Beckman
Coulter, Brea, CA, USA).
Statistical analysis
Statistical analyses of the data were performed with
a one-way ANOVA, followed by the Tukey-Kramer honestly significant
difference (HSD) test for the three sets of results. A P-value of
<0.05 was considered statistically significant. Statistical
analyses were done with a JMP Statistical Discovery software (SAS
Institute, Cary, NC, USA).
Results
Characterization of PGCs
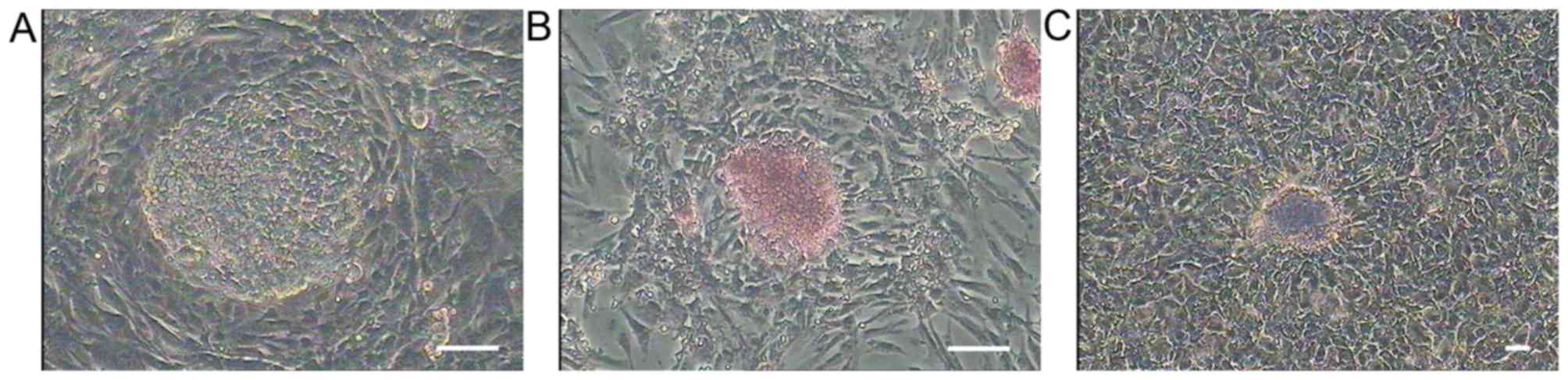
In this study, we established a stable PGC cell line
(Fig. 1A) which could culture for
a long time and subculture on an average of 3 days, and it
supported the later sperm induction usage. The PGCs expressed the
stage specific genes CVH and Blimp1, the stem cell specific genes
Pouv and Nanog (Fig. 2), were
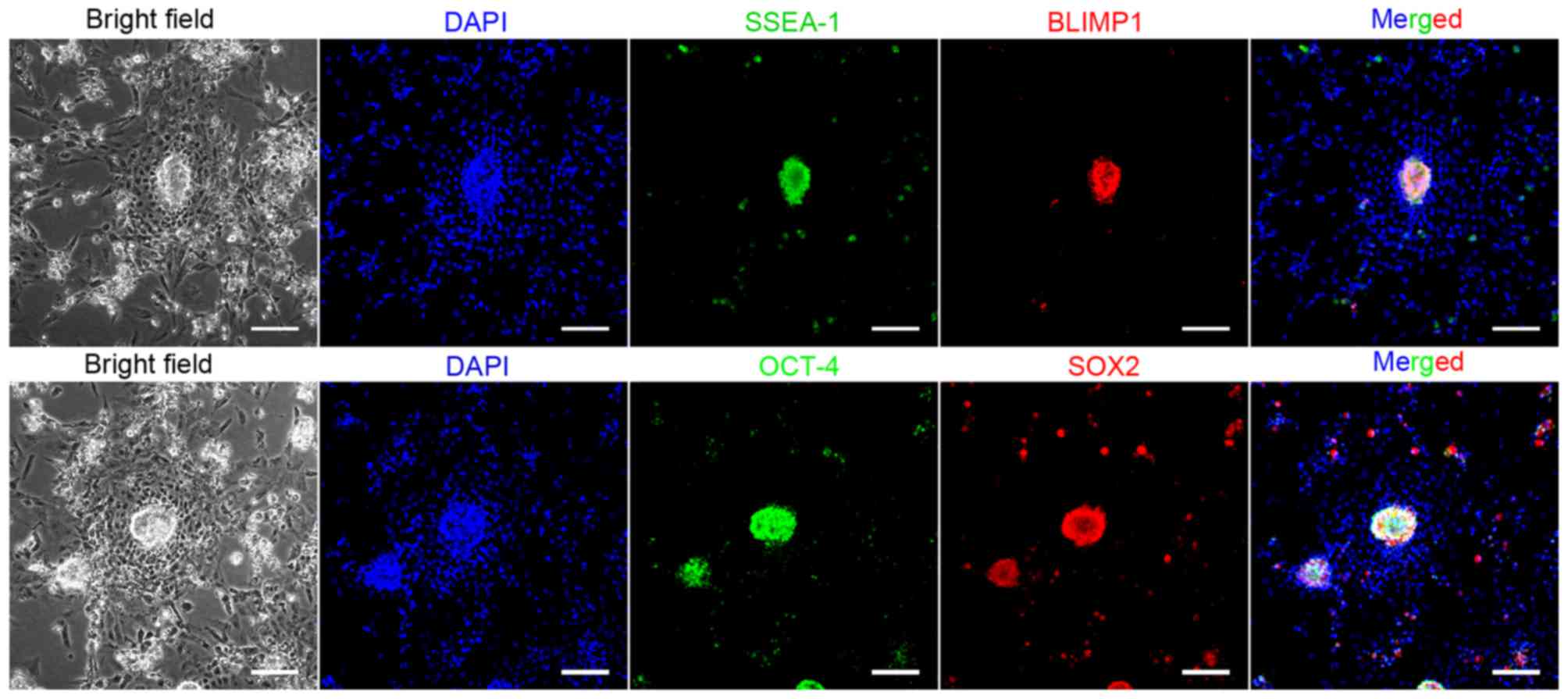
strongly positive for AKP and PAS staining (Fig. 1B and C). We examined the three
passages of PGCs which expressed stage specific surface makers
SSEA-1, BLIMP1 and stem cell makers Oct4 and Sox2 by
immunocytochemistry staining (Fig.
3), these results suggested unique characteristics for the PGCs
cell line.
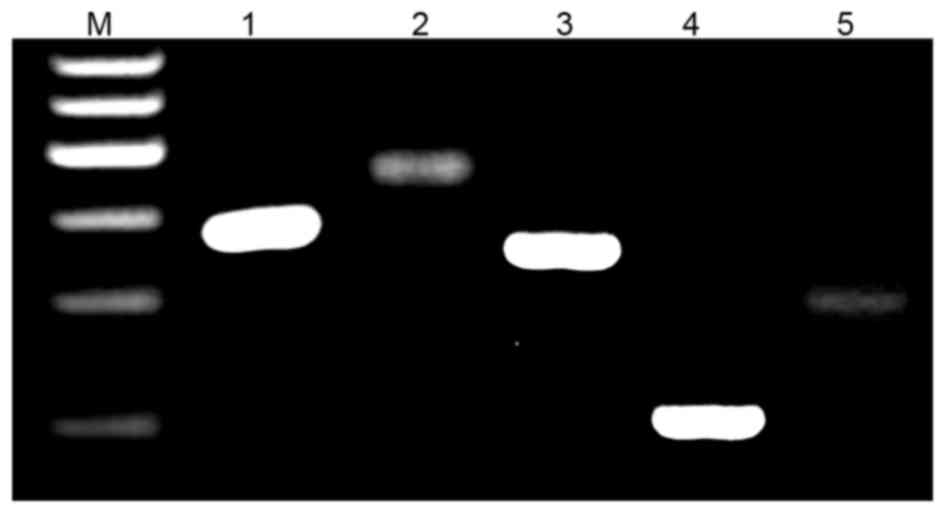
To distinguish whether PGC is a female or a male the
W chromosome-specific Xhol family and ribosomal repeat
primers previously established (17) were used. Both pairs of primers
perform as expected on female and male DNA in separate reactions.
The W-repeat primers produce a product only with female DNA, while
the ribosomal gene primers produce a product with both male and
female DNA (Fig. 4A).
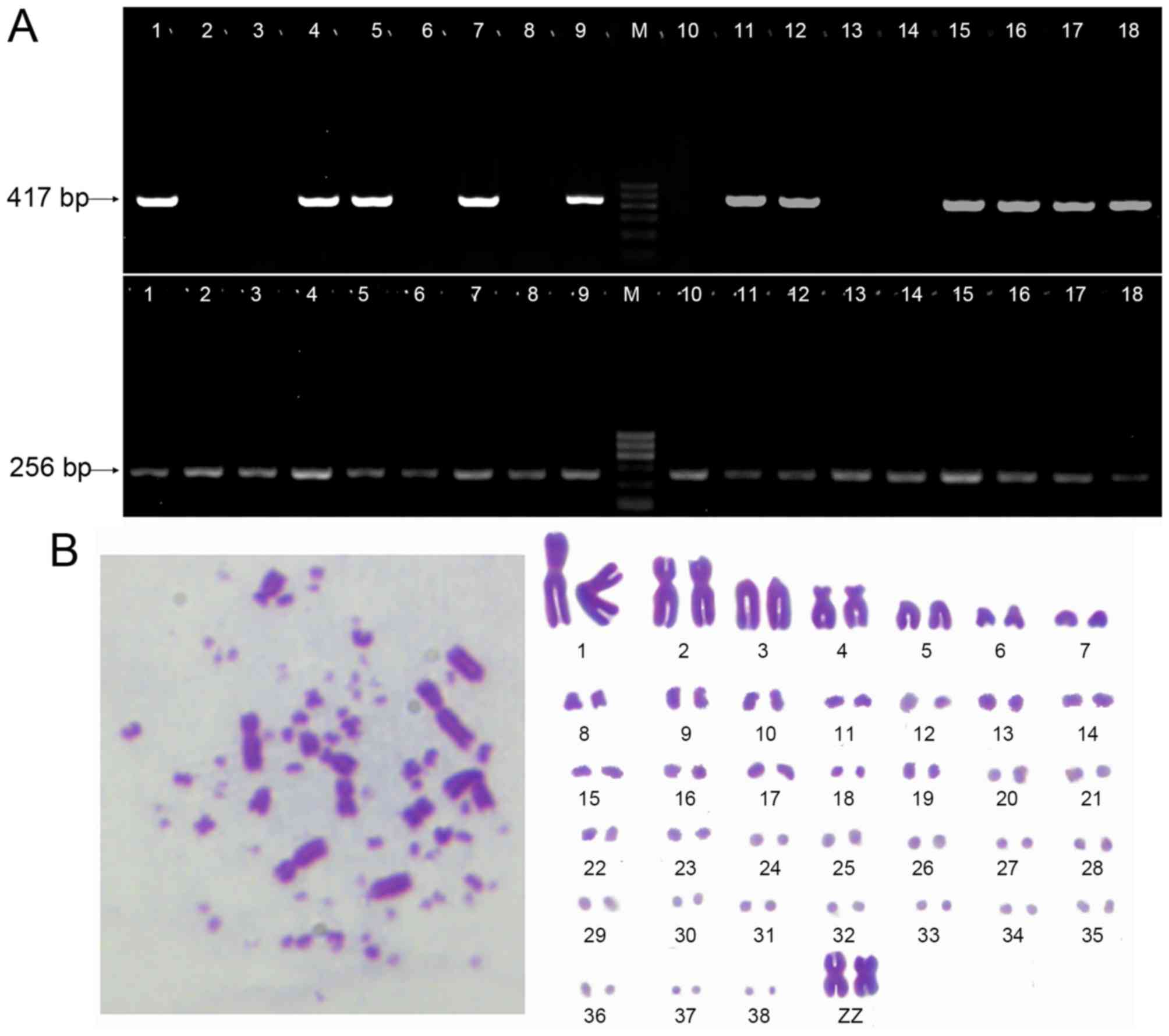
Furthermore, we checked the chromosome karyotype of the fourth
passage. Karyotype analysis revealed that the PGCs were diploid
(2n=78) with 9 pairs of macrochromosomes and 30 pairs of
microchromosomes. The sex chromosome type was ZZ (Fig. 4B). PGCs karyotypes had no
variation after subculture.
Effect of RA on PGCs meiosis
RA has been demonstrated to play essential roles in
promoting germ cell spermatogenesis in chicken. Thus various
density of RA were chosen to induce PGC cells spermatogenesis. The
PGCs were treated without or with RA for differentiation. Different
concentrations of RA ranging from 0.5 to 2 nmol/ml, were used to
optimize the condition for inducing spermatogenesis of chicken
PGCs. qRT-PCR assays showed that the expression of ACR was the
highest in PGCs treated with 1 nmol/ml RA compared to other
concentrations of RA; the expression of SYCP1 was higher in PGCs
treated with 0.5 and 1 nmol/ml RA than other concentrations of RA;
the expression of BOULE was higher in PGCs treated with 0.5, 1 and
2 nmol/ml RA than control, and thus 1 nmol/ml RA was used to induce
the differentiation of chicken PGCs (Fig. 5).
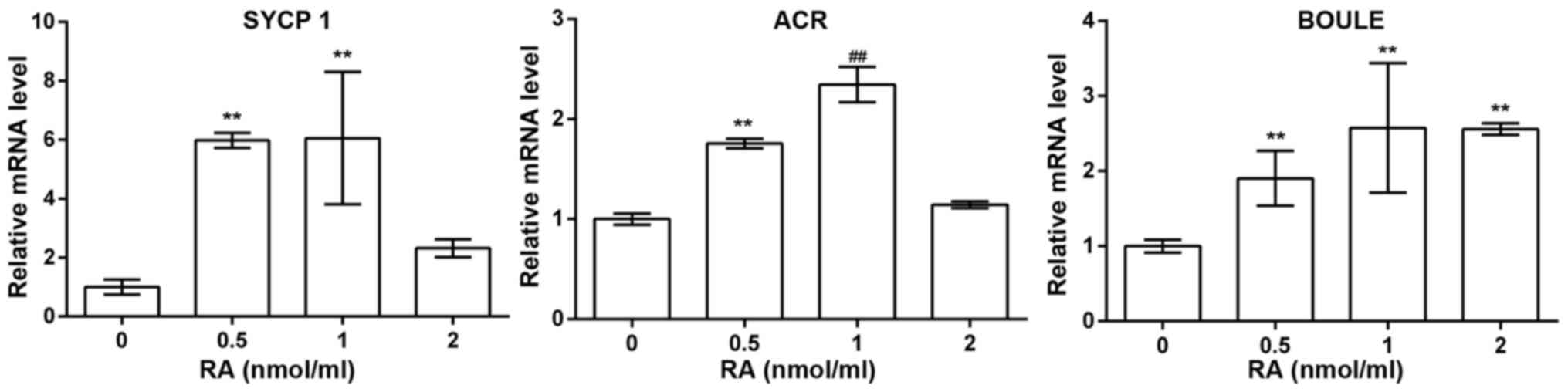 | Figure 5Real-time RT-PCR revealed mRNA
expression of synaptonemal complex protein 1 (SYCP1) (1.00±0.25,
5.98±0.26, 6.06±2.25, 2.32±0.30, n=3), acrosin (ACR) (1.00±0.06,
1.76±0.05, 2.34±0.18, 1.14±0.03, n=3), and BOULE (1.00±0.09,
1.90±0.36, 2.58±0.86, 2.56±0.08, n=3) in primordial germ cells
(PGCs) treated with 0, 0.5, 1 and 2 nmol/ml of retinoic acid (RA),
respectively. **P<0.01 compared with 0 nmol/ml of RA;
##P<0.01 compared with 0.5 nmol/ml of RA. |
RA and SCF promote PGC meiotic
efficiency
Previous studies evidenced that SCF was essential
for the maintenance of normal spermatogenesis (6,9).
SCF is a cytokine that binds to the c-kit receptor which is
expressed in PGCs and spermatogonia (18). SCF/c-kit plays an essential role
in the regulation of spermatogenesis. Studies with type A
spermatogonia have shown that SCF induces DNA synthesis and
proliferation (10). Thus we
added the SCF into induction solution with RA for inducement.
Morphological analysis displayed a different shape of PGCs with or
without RA and SCF treatment. Excitingly, more male germ cells
became enlarged and elongated in PGCs treated with RA and SCF
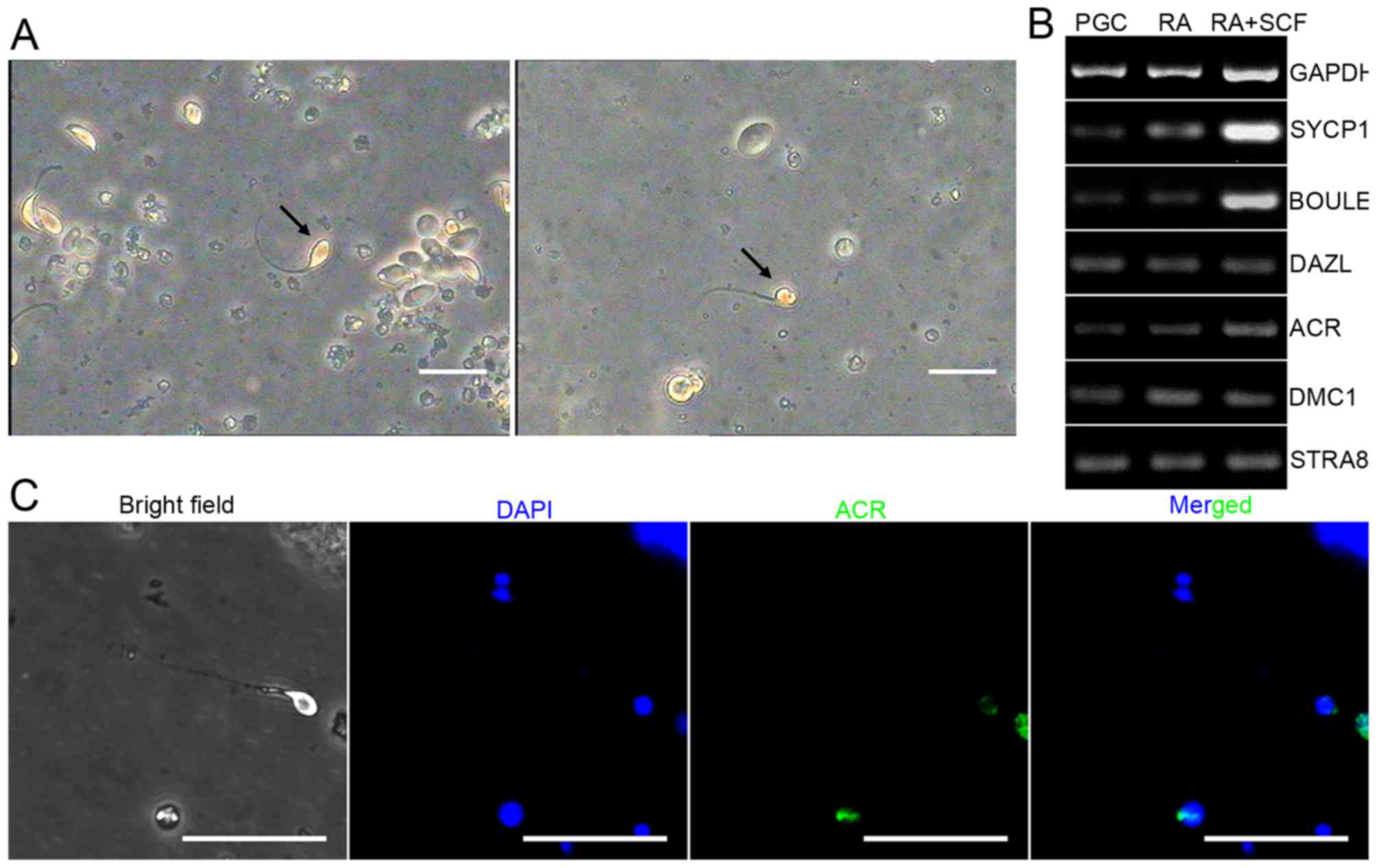
compared to the group without RA or SCF (Fig. 6A). Finally, we examined the
differentiated cells which expressed sperm specific surface marker
ACR by immunocytochemistry staining (Fig. 6C).
We next assessed the differentiation potential of
PGCs. RT-PCR showed that the transcripts of SYCP1, BOULE, DAZL,
STRA8, DMC1 and ACR, hallmarks of meiotic germ cells and haploid
germ cells, respectively (1,19),
were enhanced in PGCs with RA and SCF treatment compared to the
control without SCF and RA (Fig.
6B), and real-time PCR revealed that the mRNA levels of SYCP1,
BOULE, DMC1 and ACR were significantly upregulated in PGCs with RA
and SCF treatment compared to the control without SCF or RA
(Fig. 7). Considered the above,
these results evidence that RA and SCF promote the differentiating
efficiency of chicken PGCs into meiotic male germ cells and haploid
cells at transcriptional level.
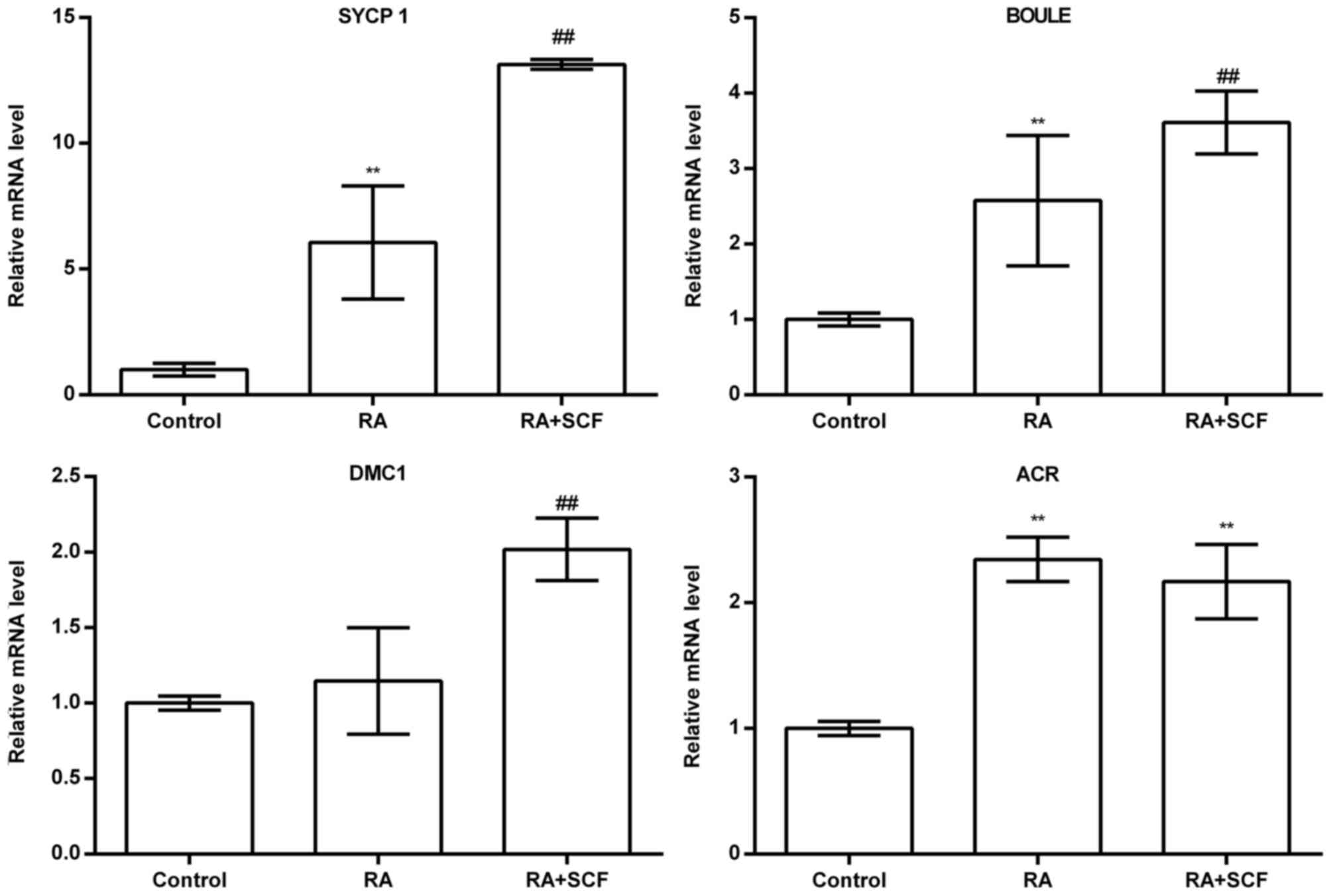 | Figure 7Real-time RT-PCR revealed mRNA
expression of synaptonemal complex protein 1 (SYCP1) (1.00±0.25,
6.06±2.25, 13.14±0.19, n=3), BOULE (1.00±0.09, 2.58±0.86,
3.61±0.42, n=3), DNA meiotic recombinase 1 (DMC1) (1.00±0.04,
1.14±0.35, 2.02±0.21, n=3), and acrosin (ACR) (1.00±0.06,
2.34±0.18, 2.16±0.30, n=3) in primordial germ cells (PGCs) treated
without retinoic acid (RA) and stem cell factor (SCF), with RA, and
with RA and SCF, respectively. **P<0.01 compared with
control; ##P<0.01 compared with RA. |
RA and SCF induce increase of haploid
cell population
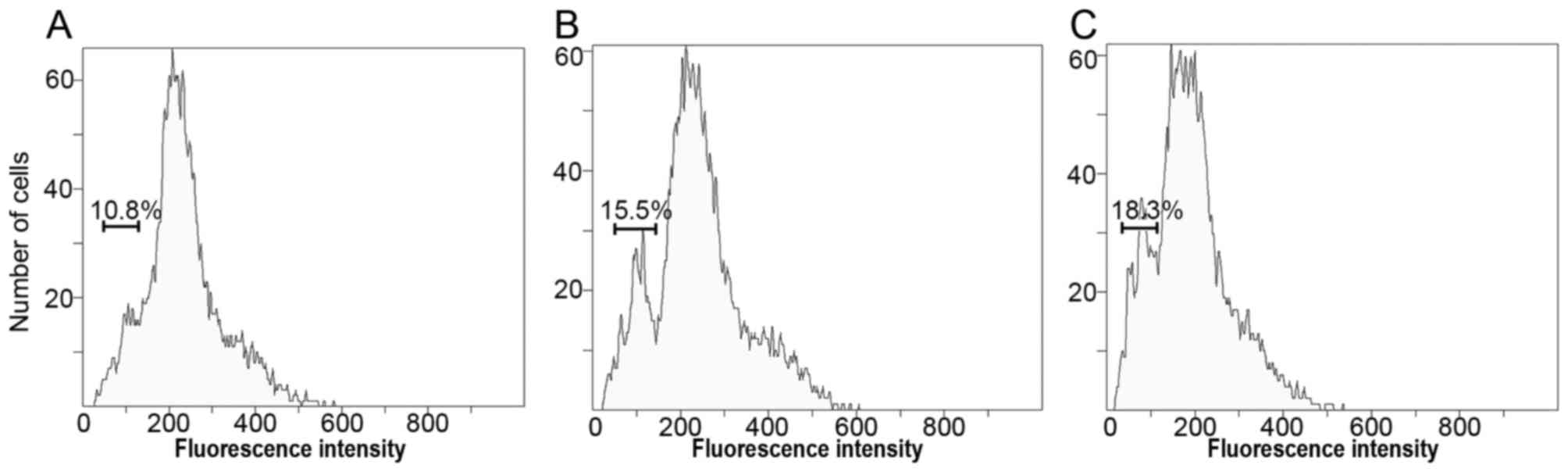
To evaluate the ploidy levels of chicken PGCs
without or with RA and SCF treatment, flow cytometry were used to
analyze DNA contents. Notably, the haploid cells originated from
10.8 to 15.5% in PGCs with RA treatment, 18.3% in PGCs with RA and
SCF treatment compared to the control (Fig. 8). These data further implicate
that RA and SCF improve the differentiating efficiency of PGCs into
haploid spermatids.
Discussion
We examined the efficiency of in vitro
expansion of chicken PGCs which were cultured for 6 weeks.
Establishment of the long-term in vitro culture system for
PGCs could provide numerous cells for research (20). In our culture system the PGCs were
strongly positive for AKP and PAS staining (21,22) and its karyotype kept no variation
after subculture. The size of PGCs, large amounts of glycogen
granules in the cytoplasm and large nuclei, are bigger than somatic
cells. This result implicated that the PGC wants normal growth and
development.
To obtain conclusive evidence that the PGCs were
derived from male chicken embryos, we investigated whether these
cells presented female or male phenotypes. The female and male
gonads of the chicken embryos developed at stage 28 are
morphologically similar known as the indifferent gonad. Gonads of
female and male, respectively, develop into ovarian and testicular
characteristics after the indifferent stage. Previous studies
demonstrated a kind of repetitive DNA sequences from W chromosome
present specifically in the female chicken (17,23). Thus, we evidenced that a PCR
reaction, containing primers specific to both the W chromosome
sequence and the 18S ribosomal gene, can readily make a clear
distinction between female and male DNA. The karyotype analysis is
also an important research means of cytogenetics. It can not only
quickly identify mutated chromosomes, but also distinguish male and
female phenotypes (6,24). Combining these two methods, we
were able to separate the male PGCs from chicken embryos.
RA has been shown to be a meiosis-inducing
substance. Several studies have suggested the importance of RA in
germ cell meiotic initiation (6,9,25).
Our results suggested, in agreement with previous studies, that 1
nmol/ml RA induced PGC meiosis efficiently. SSCs originate from
PGCs. During avian and mammalian development, PGCs migrate along
the dorsal mesentery into the genital ridges, and their
differentiation lasts several weeks (26,27), while PGC differentiation into
spermatigonia and spermatigonia to reach the haploid sperm needs a
long time-period (28). SCF has
been reported to be crucial in spermatogonial differentiation as
well as meiotic initiation (10).
Our results indicated that RA and SCF promote the greater
differentiating efficiency of chicken PGCs into meiotic male germ
cells and haploid cells then RA was induced. Crosstalk between RA
and the SCF pathway stimulated differentiation of male germ cells
toward the meiotic stages (29).
Various types of detection methods, including
quantitative PCR, RT-PCR and FACS assays were used to assess the
differentiation potential of chicken PGCs. The expression of
several genes for meiotic and haploid cells, including SYCP1,
BOULE, DMC1 and ACR (1,6), in chicken PGCs were evidently
upregulated after treatment with RA and SCF. SYCP1, major component
of the transverse filaments of synaptonemal complexes (SCS), can be
used to measure the synaptonemal complex, which formed between
homologous chromosomes during meiotic prophase (30). BOULE, with a key role in germ cell
development, is a member of the DAZ gene family. Loss of this gene
function results in azoospermia. Previous studies found that they
originally appeared in the meiotic G2/M transition (31). DMC1 encodes the DNA
strand-exchange protein, which is essential for repairing
double-strand DNA breaks during mitosis and meiosis and meiotic
homologous recombination (32).
ACR, a typical serine proteinase with trypsin-like specificity, is
the key proteinase present in the acrosome of mature spermatozoa.
Research demonstrated that the mRNA for proacrosin is expressed
only in the postmeiotic stages of spermatogenesis (33). The DNA content of chicken PGCs
with RA and SCF treatment was detected by FACS. Although the DNA
ploidy levels show that PGCs have the ability of spontaneous
differentiation into haploid cells, the number of haploid cells was
obviously increased in chicken PGCs by RA and SCF treatment. These
markers for meiosis and postmeiosis obviously show that RA and SCF
promote the differentiation of chicken PGCs into the post-meiotic
stage and finally differentiate into haploid spermatids.
In this study, we evidenced that RA and SCF are
effective in promoting the differentiation of chicken PGCs from
gonadal ridges into cells with phenotypic features, and DNA content
of haploid spermatids. This study thus offers an approach to
generate functional haploid spermatids from PGCs, which could
provide male gametes for clinical treatment and transgenic animal
research.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 31472099).
References
|
1
|
Mi Y, He B, Li J and Zhang C: Progesterone
regulates chicken embryonic germ cell meiotic initiation
independent of retinoic acid signaling. Theriogenology. 82:195–203.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng LX, Chen Y, Dettin L, Pera RA, Herr
JC, Goldberg E and Dym M: Generation and in vitro differentiation
of a spermatogonial cell line. Science. 297:392–395. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saitou M and Yamaji M: Germ cell
specification in mice: Signaling, transcription regulation, and
epigenetic consequences. Reproduction. 139:931–942. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martinovitch PN: Development in vitro of
the mammalian gonad. Nature. 139:4131937. View Article : Google Scholar
|
|
5
|
Dann CT, Alvarado AL, Molyneux LA, Denard
BS, Garbers DL and Porteus MH: Spermatogonial stem cell
self-renewal requires OCT4, a factor downregulated during retinoic
acid-induced differentiation. Stem Cells. 26:2928–2937. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang S, Ping P, Ma M, Li P, Tian R, Yang
H, Liu Y, Gong Y, Zhang Z, Li Z, et al: Generation of haploid
spermatids with fertilization and development capacity from human
spermatogonial stem cells of cryptorchid patients. Stem Cell Rep.
3:663–675. 2014. View Article : Google Scholar
|
|
7
|
Childs AJ, Cowan G, Kinnell HL and
Anderson RA: Saunders PT. Retinoic Acid signalling and the control
of meiotic entry in the human fetal gonad. PLoS One. 6:e202492011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohta K, Lin Y, Hogg N, Yamamoto M and
Yamazaki Y: Direct effects of retinoic acid on entry of fetal male
germ cells into meiosis in mice. Biol Reprod. 83:1056–1063. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bowles J, Knight D, Smith C, Wilhelm D,
Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ,
Rossant J, et al: Retinoid signaling determines germ cell fate in
mice. Science. 312:596–600. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng LX, Ravindranath N and Dym M: Stem
cell factor/c-kit upregulates cyclin D3 and promotes cell cycle
progression via the phosphoinositide 3-kinase/70 S6 kinase pathway
in spermatogonia. J Biol Chem. 275:25572–25576. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mithraprabhu S and Loveland KL: Control of
KIT signalling in male germ cells: What can we learn from other
systems. Reproduction. 138:743–757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smith CA, Roeszler KN, Bowles J, Koopman P
and Sinclair AH: Onset of meiosis in the chicken embryo; evidence
of a role for retinoic acid. BMC Dev Biol. 8:852008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou Q, Li Y, Nie R, Friel P, Mitchell D,
Evanoff RM, Pouchnik D, Banasik B, McCarrey JR, Small C, et al:
Expression of stimulated by retinoic acid gene 8 (Stra8) and
maturation of murine gonocytes and spermatogonia induced by
retinoic acid in vitro. Biol Reprod. 78:537–545. 2008. View Article : Google Scholar
|
|
14
|
Hamburger V and Hamilton HL: A series of
normal stages in the development of the chick embryo. J Morphol.
88:49–92. 1951. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi JW, Kim S, Kim TM, Kim YM, Seo HW,
Park TS, Jeong JW, Song G and Han JY: Basic fibroblast growth
factor activates MEK/ERK cell signaling pathway and stimulates the
proliferation of chicken primordial germ cells. PLoS One.
5:e129682010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu CX, Wang WL, Zhao RY, Wang HT, Liu YY,
Wang SY and Zhou HM: Isolation, culture, and characterization of
primordial germ cells in Mongolian sheep. In Vitro Cell Dev Biol
Anim. 50:207–213. 2014. View Article : Google Scholar
|
|
17
|
Clinton M, Haines L, Belloir B and McBride
D: Sexing chick embryos: A rapid and simple protocol. Br Poult Sci.
42:134–138. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rossi P, Sette C, Dolci S and Geremia R:
Role of c-kit in mammalian spermatogenesis. J Endocrinol Invest.
23:609–615. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
West JA, Park IH, Daley GQ and Geijsen N:
In vitro generation of germ cells from murine embryonic stem cells.
Nat Protoc. 1:2026–2036. 2006. View Article : Google Scholar
|
|
20
|
Shiue YL, Tailiu JJ, Liou JF, Lu HT, Tai
C, Shiau JW and Chen LR: Establishment of the long-term in vitro
culture system for chicken primordial germ cells. Reprod Domest
Anim. 44:55–61. 2009. View Article : Google Scholar
|
|
21
|
Jung JG, Kim DK, Park TS, Lee SD, Lim JM
and Han JY: Development of novel markers for the characterization
of chicken primordial germ cells. Stem Cells. 23:689–698. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Hou L, Li C, Guan W, Chen L, Li X,
Yue W and Ma Y: Isolation, culture and biological characteristics
of primordial germ cells from Beijing fatty chicken. J Reprod Dev.
56:303–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tone M, Nakano N, Takao E, Narisawa S and
Mizuno S: Demonstration of W chromosome-specific repetitive DNA
sequences in the domestic fowl Gallus g domesticus. Chromosoma.
86:551–569. 1982. View Article : Google Scholar
|
|
24
|
Nishida C, Ishijima J, Ishishita S, Yamada
K, Griffin DK, Yamazaki T and Matsuda Y: Karyotype reorganization
with conserved genomic compartmentalization in dot-shaped
microchromosomes in the Japanese mountain hawk-eagle (Nisaetus
nipalensis orientalis, Accipitridae). Cytogenet Genome Res.
141:284–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eguizabal C, Montserrat N, Vassena R,
Barragan M, Garreta E, Garcia-Quevedo L, Vidal F, Giorgetti A,
Veiga A and Izpisua Belmonte JC: Complete meiosis from human
induced pluripotent stem cells. Stem Cells. 29:1186–1195. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Childs AJ and Anderson RA: Experimental
approaches to the study of human primordial germ cells. Methods Mol
Biol. 825:199–210. 2012. View Article : Google Scholar
|
|
27
|
Macdonald J, Glover JD, Taylor L, Sang HM
and McGrew MJ: Characterisation and germline transmission of
cultured avian primordial germ cells. PLoS One. 5:e155182010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amann RP: The cycle of the seminiferous
epithelium in humans: A need to revisit. J Androl. 29:469–487.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pellegrini M, Filipponi D, Gori M, Barrios
F, Lolicato F, Grimaldi P, Rossi P, Jannini EA, Geremia R and Dolci
S: ATRA and KL promote differentiation toward the meiotic program
of male germ cells. Cell Cycle. 7:3878–3888. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Türeci O, Sahin U, Zwick C, Koslowski M,
Seitz G and Pfreundschuh M: Identification of a meiosis-specific
protein as a member of the class of cancer/testis antigens. Proc
Natl Acad Sci USA. 95:5211–5216. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kee K, Angeles VT, Flores M, Nguyen HN and
Reijo Pera RA: Human DAZL, DAZ and BOULE genes modulate primordial
germ-cell and haploid gamete formation. Nature. 462:222–225. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Habu T, Taki T, West A, Nishimune Y and
Morita T: The mouse and human homologs of DMC1, the yeast
meiosis-specific homologous recombination gene, have a common
unique form of exon-skipped transcript in meiosis. Nucleic Acids
Res. 24:470–477. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zahn A, Furlong LI, Biancotti JC,
Ghiringhelli PD, Marijn-Briggiler CI and Vazquez-Levin MH:
Evaluation of the proacrosin/acrosin system and its mechanism of
activation in human sperm extracts. J Reprod Immunol. 54:43–63.
2002. View Article : Google Scholar : PubMed/NCBI
|