Introduction
Cerebrovascular disease is one of the main diseases
that threaten human health, and it ranks the top among the three
major causes of mortality in China, with ischemic cerebrovascular
disease, a common and frequently occurring disease that causes
mortality and disability, accounting for 60–80% (1). At present, early thrombolytic
therapy is applied for ischemic cerebrovascular disease so as to
restore the local blood supply; however, the resulting reperfusion
will aggravate the further injury of the ischemic tissue (2,3).
As a result, there is an urgent requirement to search for an
effective drug for treating ischemia- and reperfusion-induced brain
injury.
Brain anoxia and ischemia subsequent to cerebral
infarction will result in a series of pathophysiological processes
(4). The neurons, glial cells and
vascular endothelial cells will develop edema, necrosis and
apoptosis as a result of the lack of nutrient and energy supply, as
well as the inability to discharge metabolic waste, accompanied
with inflammatory factor release, cascade amplification, and free
radical and calcium ion overload (5). During this process, a series of
molecules, including nucleic acids and proteins, will acquire
profound changes, which will aggravate or improve the pathological
process of cerebral infarction, while their mutual equilibrium and
development will influence the outcome and prognosis for cerebral
infarction (6).
The cerebral infarction is a complicated
pathophysiological process: Firstly, the growth factor receptor,
which can promote angiogenesis on the vascular endothelial cell, is
activated together with the relevant enzymes in the body; secondly,
the activated vascular endothelial cell begins to release
metalloprotease so as to degrade the basilar membrane; thirdly, the
vascular endothelial cell migrates to the corresponding position,
secretes and forms the surrounding matrix, and proliferates into
the budding structure, which continues to enlarge and form the
annular tube cavity; and finally, the perivascular cell, smooth
muscle cell and astrocyte proliferate, migrate, differentiate, and
occlude to form the mature vascular cavity (7,8).
MicroRNAs (miRNAs/miRs), a type of endogenous
non-coding RNA discovered in eukaryotes, has regulatory functions,
and the mature miRNAs have 20–25 nucleotides. miRNA binds with the
3′-terminal non-coding domain of a specific target gene and forms
the silencing complex, which will inhibit the translation of the
target mRNA or promote the degradation of the mRNA, thus exerting
its biological functions (9). As
has been previously demonstrated, miRNAs regulate angiogenesis
under physiological and pathological conditions through regulation
of the key molecules of angiogenesis (10).
Toll-like receptor (TLR) was first identified in
fruit flies, and it was named since its extracellular fragment was
homologous to a type of fruit fly protein Toll (11). The TLR family is a signal energy
transformation molecular family, which serves a key role in the
immune response and inflammatory response (11). TLR is a type of transmembrane
receptor that is mainly distributed in immune cells and on the
cavitary epithelial cell surface, and can identify various
pathogen-associated molecular patterns, induce the inherent immune
response of the body, induce a series of gene activations through
various signal transduction pathways, promote increased
inflammatory cytokine secretion and cause a systemic inflammatory
response (12).
Attention has been paid to the roles of TLR4 ligands
myeloid differentiation primary response protein MyD88 (MyD88) and
Toll/interleukin-1 receptor domain-containing adapter protein
(TIRAP), as well as the negative feedback protein that gradually
influences the TLR signal transduction pathway, Toll-interacting
protein, in cerebral infarction. Recently, there has been
increasing evidence of the TLR4-TIRAP-MyD88 signal pathway
(13,14).
The active cyclic AMP response element binding
protein (CREB) participates in multiple processes, including
neurodevelopment, synaptic plasticity, memory function,
regeneration and cell survival, so as to cope with all manner of
stimulations, such as ischemia (15). Since 1990, phosphorylated (p-)CREB
has been extensively researched in multiple ischemic models, and
its downstream neuroprotection targets include apoptosis regulator
Bcl-2 (Bcl-2) and brain-derived neurotrophic factor (BDNF), and
thus promote the survival of the ischemic neurons (16). The present study investigated the
role and mechanism of miR-497 to regulate cerebral infarction.
Materials and methods
Clinical specimens
Male patients with cerebral infarction (n=6; 55–62
years old) and healthy volunteers (n=4; 57–65 years old) from
Tangshan Worker Hospital (Tangshan, Hebei, China) were enrolled in
the present study between October 2015 and March 2016. All patients
provided written informed consent and the study was approved by the
Medical Ethics Committee of Tangshan Worker Hospital. Whole blood
was collected and centrifuged at 1,000 x g for 10 min at room
temperature. Serum was stored at −80°C for further experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analyses
The total RNA of every serum sample was isolated
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer’s protocols. cDNA
was reverse transcribed from 20–50 ng total RNA using the miRCURY
LNA™ Universal RT microRNA PCR (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA (1 μl) was used for RT-qPCR (CFX-96
PCR machine; Bio-Rad Laboratories, Inc., Hercules, CA, USA) with
the SYBR-Green Supermix (Invitrogen; Thermo Fisher Scientific,
Inc.). RT-qPCR was performed at 95°C for 5 min, followed by 40
cycles of 95°C for 30 sec, 60°C for 30 sec and 70°C for 20 sec.
miR-497 expression was quantificated using 2−∆∆Ct
(17).
Cell culture, transfection and ischemic
injury of N2A cells
Mouse N2A cells were obtained from the Cell Culture
Center of Chinese Peking Union Medical College (Beijing, China) and
cultivated in Dulbecco’s modified Eagle’s medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum,
in a 5% CO2/95% O2 atmosphere at 37°C. N2A
cells were transfected with miR-497 (100 ng;
5′-CCACCCCGGTCCTGCTCCCG-3′ and 5′-GGTGGGGGAGGCACCGCCGAGG-3′) and
negative vector (Sangon Biotech Shanghai Co., Ltd., Shanghai,
China) using Lipofectamine 2000® (Invitrogen; Thermo
Fisher Scientific, Inc.). Following transfection for 24 h, N2A
cells were replaced by glucose-free DMEM (Gibco; Thermo Fisher
Scientific, Inc.) and 50 ng/ml LPS in a hypoxic chamber (5%
CO2, 1% O2 and 94% N2) for 4 h,
and then under normoxic conditions for 24 h. TLR4 inhibitor
(TAK-242, 2–5 μM) was added to the N2A cells for 4 h. CREB
inhibitor (666-15, 5 μM) was added to the N2A cells for 12
h.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
N2A cells were seeded in 96-well plates at a density
of 2×104 cells/well and incubated with MTT (0.5 mg/ml)
for 3 h. The medium was removed from each well and dimethyl
sulfoxide (100 μl) was added. Absorbance was measured using
an Infinite M200 PRO Multimode Microplate (Tecan Group, Ltd.,
Mannedorf, Switzerland) at 492 nm.
Apoptosis assay
N2A cells were seeded in 6-well plates at a density
of 2×106 cells/well and incubated with Annexin
V-fluorescein isothiocyanate and propidium iodide (Bio-Rad
Laboratories, Inc.) at 25°C for 5-15 min (in the dark). The total
number of apoptotic cells was quantified by a flow cytometer (BRYTE
HS; Bio-Rad Laboratories, Inc.) and FlowJo 7.6 (Tree Star, Inc.,
Ashland, OR, USA).
Measurement of inflammation factors
Total protein was isolated from the N2A cells with
an ice-cold cell lysis reagent (FNN0091; Thermo Fisher Scientific,
Inc., Rockford, IL, USA) according to the manufacturer’s protocols.
The protein concentration was measured using the Pierce
bicinchoninic acid assay (BCA) reagent (Thermo Fisher Scientific,
Inc.). Proteins (5 μg) were incubated with enzyme-linked
immunosorbent assay (ELISA) kits [interleukin (IL)-1β (H002), IL-6
(H007) and IL-8 (H008); Nanjing Jiangcheng Bioengineering
Institute, Nanjing, China]. Absorbance was measured using Infinite
M200 PRO Multimode Microplate (Tecan Group, Ltd.) at 450 nm.
Caspase-3/9 activity assay
Total protein was isolated from N2A cells with an
ice-cold cell lysis reagent (FNN0091; Thermo Fisher Scientific,
Inc.) according to the manufacturer’s protocols. The protein
concentration was measured using the Pierce BCA reagent (Thermo
Fisher Scientific, Inc.). Proteins (5 μg) were incubated
with caspase-3/9 activity kits (Bio-Rad Laboratories, Inc.).
Absorbance was measured using Infinite M200 PRO Multimode
Microplate (Tecan Group, Ltd.) at 405 nm.
Western blot analysis
Total protein was isolated from N2A cells with an
ice-cold cell lysis reagent (FNN0091; Thermo Fisher Scientific,
Inc.) according to the manufacturer’s protocols. The protein
concentration was measured using the Pierce BCA reagent (Thermo
Fisher Scientific, Inc.). Proteins (50 μg) were separated
using 8–12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto polyvinylidene difluoride
membranes (Thermo Fisher Scientific, Inc.). Membranes were blocked
with 5% bovine serum albumin for 1 h at 37°C and incubated with
anti-TLR4 (catalog no. sc-10741; dilution, 1:500), anti-MyD88
(catalog no. sc-11356; dilution, 1:500), anti-nuclear factor-κB
(NF-κB; catalog no. sc-109; dilution, 1:500), anti-IL-1 receptor
associated kinase (IRAK1; catalog no. sc-7883; dilution, 1:500),
anti-p-CREB (catalog no. sc-101663; catalog no. 1:500) and
anti-glyceraldehyde 3-phosphate dehydrogenase (GADPH; catalog no.
sc-25778; dilution 1:500) (all Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) at 4°C overnight. A goat anti-rabbit IgG
horseradish peroxidase-conjugated secondary antibody (sc-2004;
1:5,000; Santa Cruz Biotechnology, Inc.) was used to incubate the
membranes for 1 h at 37°C, and a Novex ECL Chemiluminescent
Substrate reagent kit (WP20005; Thermo Fisher Scientific, Inc.) was
used to visualize the signal bands. Signal bands were acquired
using the ChemiDoc XRS+ imaging system (Bio-Rad
Laboratories, Inc.).
Immunocytofluorescence
N2A cells (1–2×104 cells/ml) were seeded
at cell chamber slides and fixed with 4% formaldehyde at 4°C for 15
min. Next, the cells were blocked with 5% BSA assay in
phosphate-buffered saline (PBS) for 1 h at 37°C and stained with
anti-TLR4 antibody (dilution, 1:100; catalog no. sc-10741; Santa
Cruz Biotechnology, Inc.) at 4°C overnight. The cells were
incubated with appropriate fluorescent secondary antibodies
(dilution, 1:500; catalog no. sc-362272; Santa Cruz Biotechnology,
Inc.) at room temperature for 1 h and then stained by DAPI assay at
room temperature for 15 min. Cell images were obtained using an
FV300 confocal microscope (Olympus Corporation, Tokyo, Japan).
Statistical analysis
All data are expressed as the mean ± standard error
of mean. SPSS 17.0 software was used (IBM, Armonk, NY, USA). Two
groups were compared using Student’s t-test. Differences among
groups were compared using one-way analysis of variance followed by
Tukey’s post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
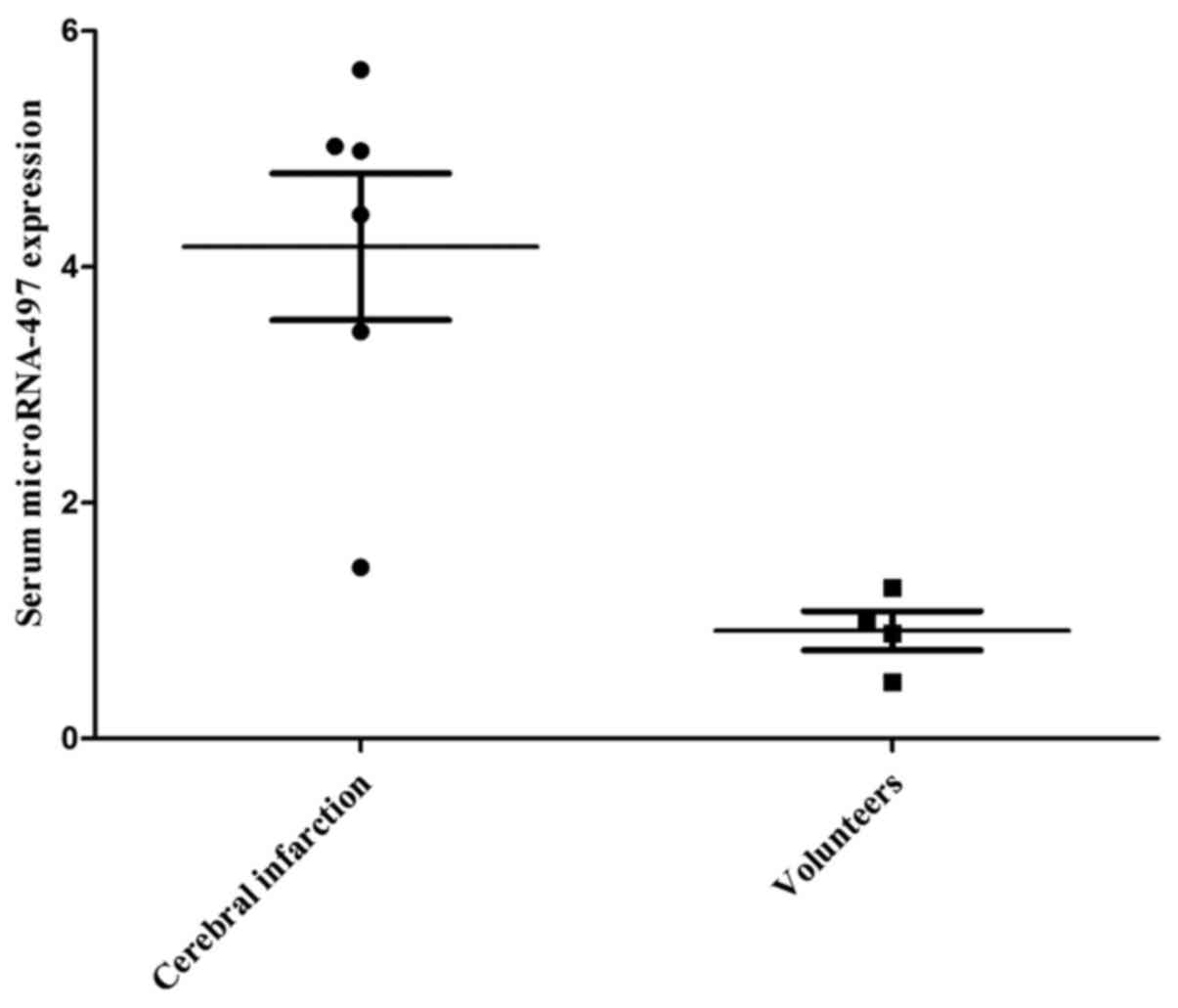
Serum miR-497 expression in patients with
cerebral infarction
The serum microRNA-497 expression of patients with
cerebral infarction was first determined in the present study.
miR-497 expression was upregulated in patients with cerebral
infarction compared with that in the healthy volunteers (Fig. 1). This result showed that miR-497
may affect cerebral infarction.
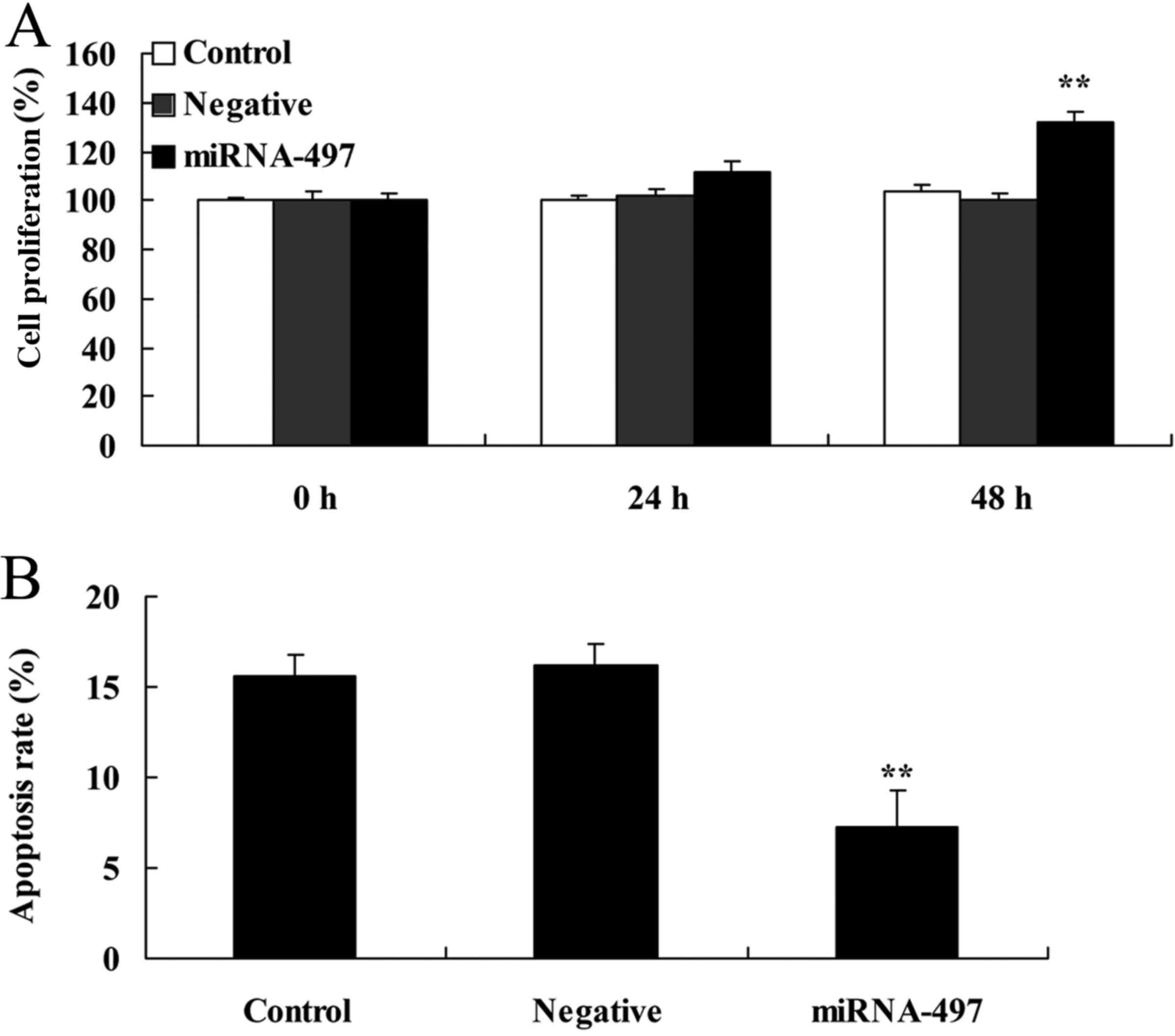
Overexpression of miR-497 induces cell
proliferation and decreases apoptosis in N2A cells
Overexpression of miR-497 significantly induced cell
proliferation and decreased apoptosis in the cerebral infarction
model in vitro compared with the negative group (Fig. 2).
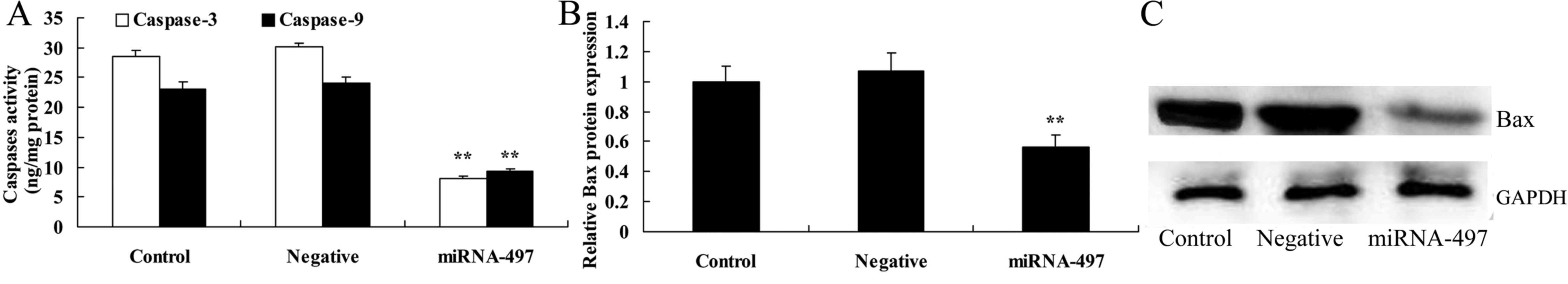
Overexpression of microRNA-497 decreases
caspase-3 and caspase-9 activities, and suppresses Bax protein
expression in N2A cells
To define the functional role of miR-497 in cerebral
infarction, caspase-3 and caspase-9 activities were measured using
ELISA kits. The overexpression of miR-497 significantly decreased
the caspase-3 and caspase-9 activities, and suppressed Bax protein
expression in the cerebral infarction model in vitro
compared with that in the negative group (Fig. 3).
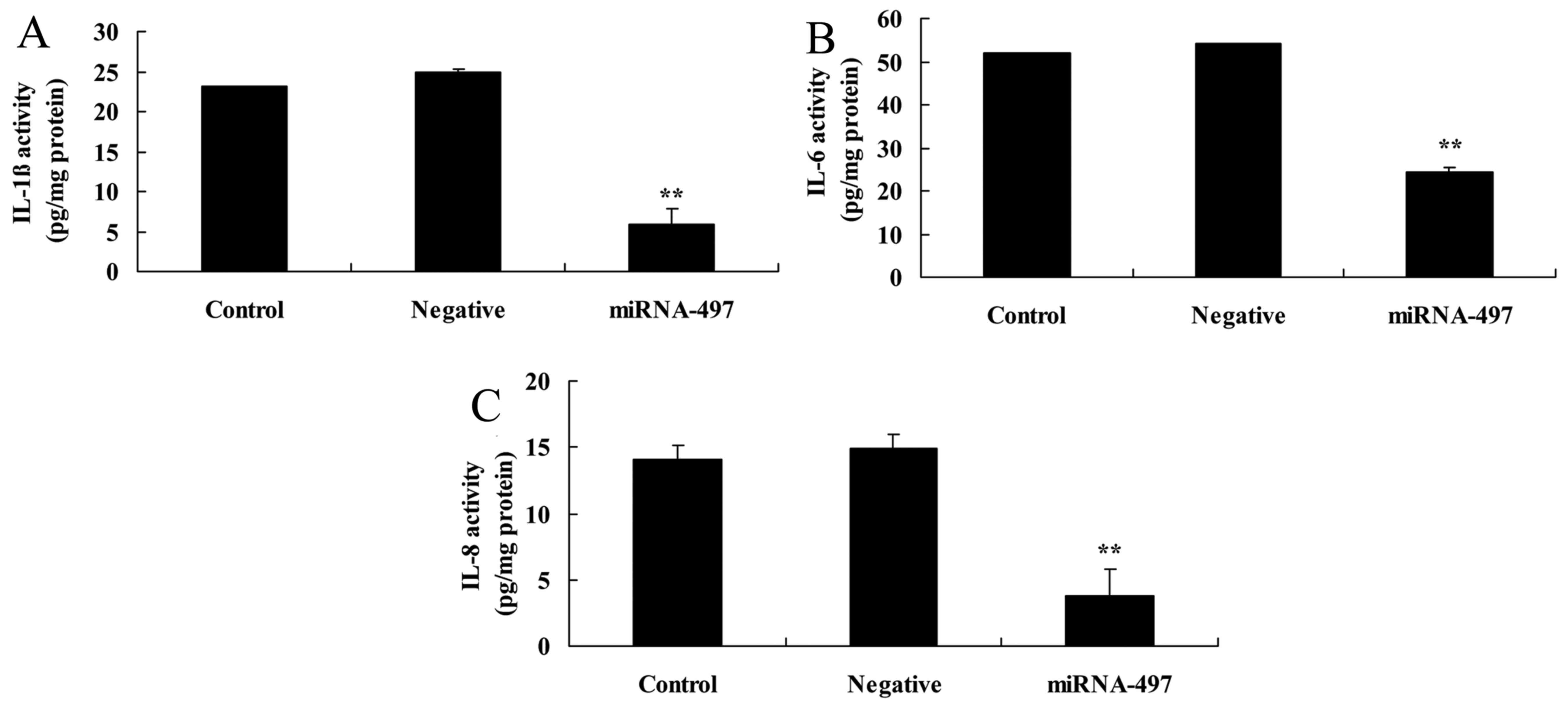
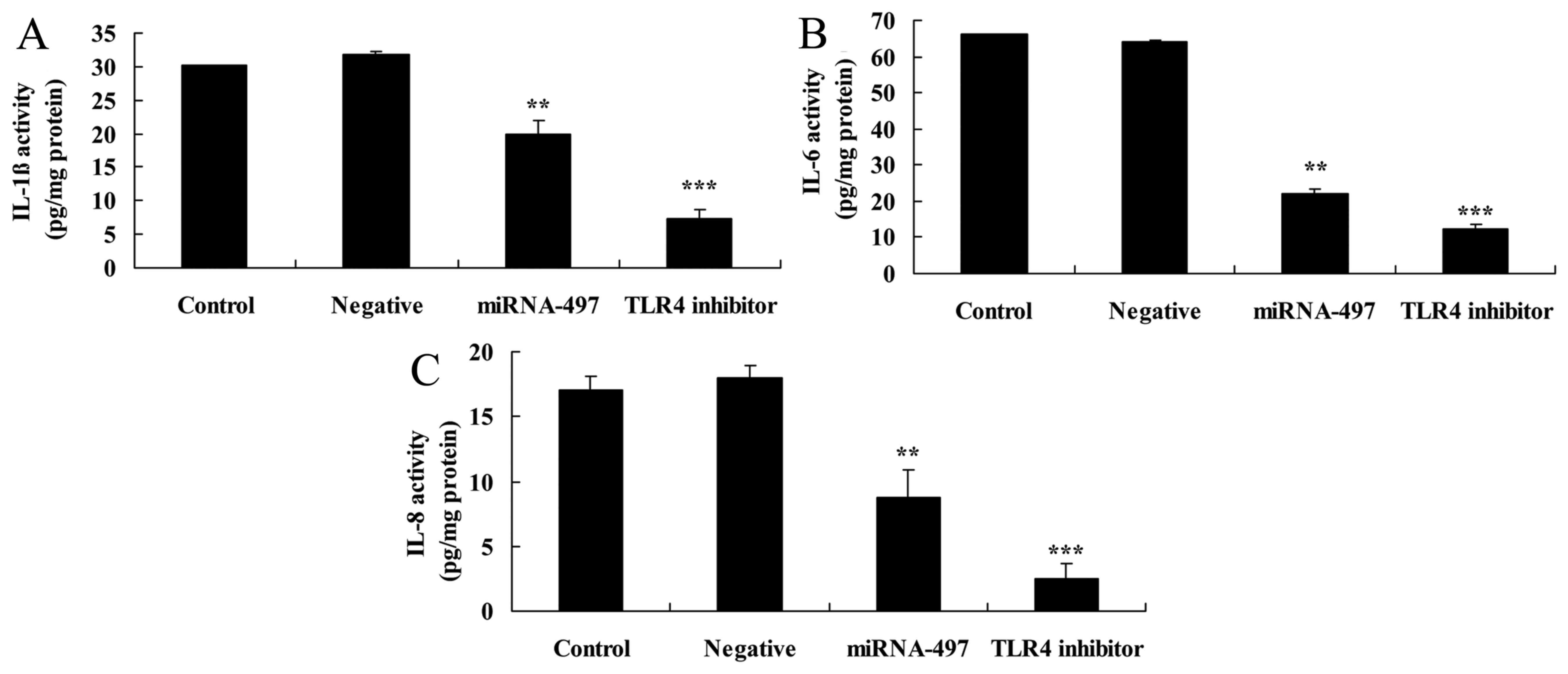
Overexpression of miR-497 reduces
inflammation factors in N2A cells
Next, the effects of miR-497 overexpression on
inflammation factors in cerebral infarction were investigated.
Compared with the negative group, the miR-497 overexpression group
exhibited significantly reduced IL-1β, IL-6 and IL-8 levels in the
cerebral infarction model in vitro (Fig. 4).
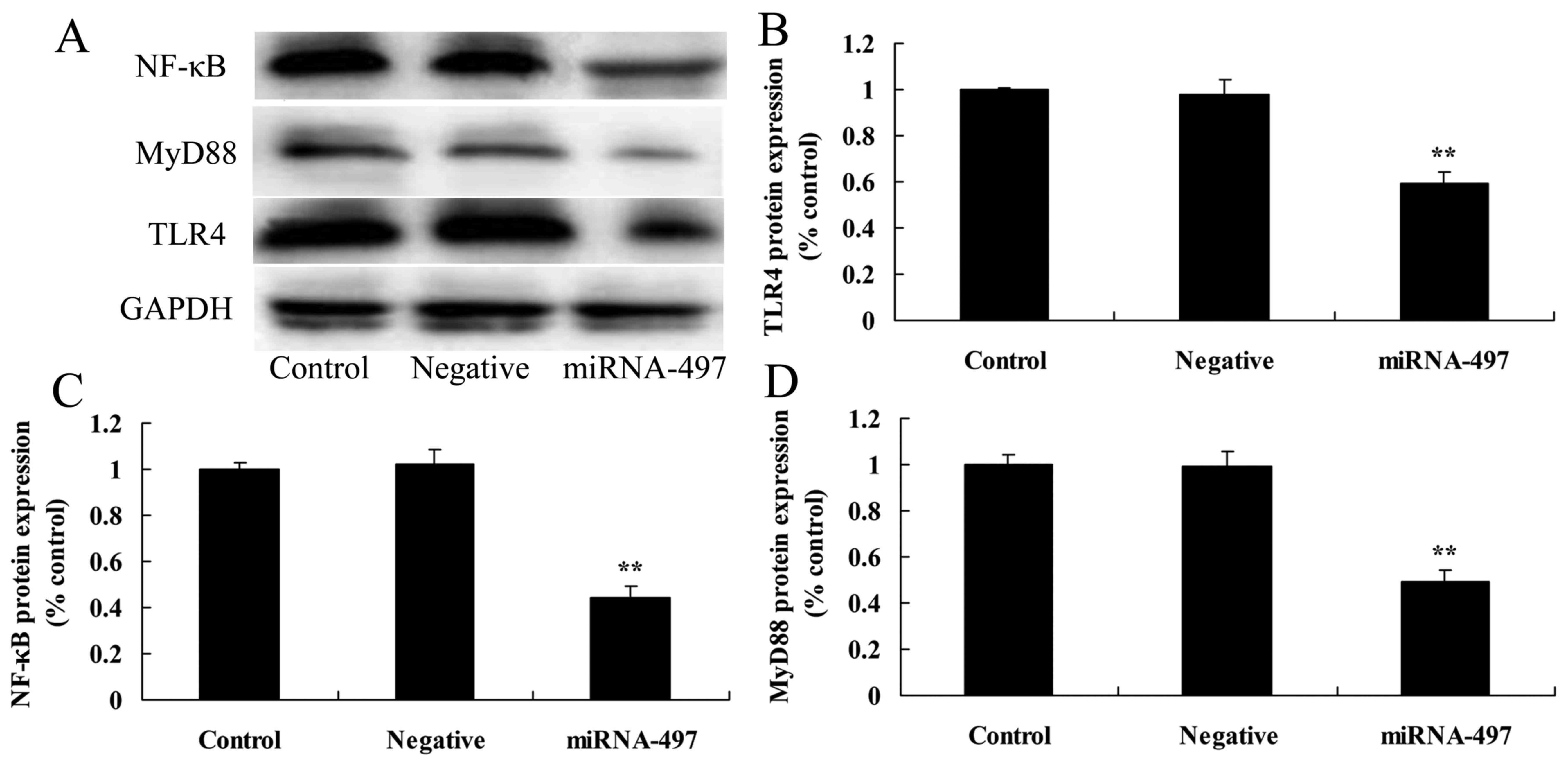
Overexpression of miR-497 suppresses
TLR4, MyD88 and NF-κB protein expression in N2A cells
To investigate the anti-inflammatory functional role
of miR-497 in cerebral infarction, TLR4, MyD88 and NF-κB protein
expression was measured using western blot analysis. The results of
western blot analysis showed that the overexpression of miR-497
significantly suppressed TLR4, MyD88 and NF-κB protein expression
in cerebral infarction compared with that in the negative group
(Fig. 5).
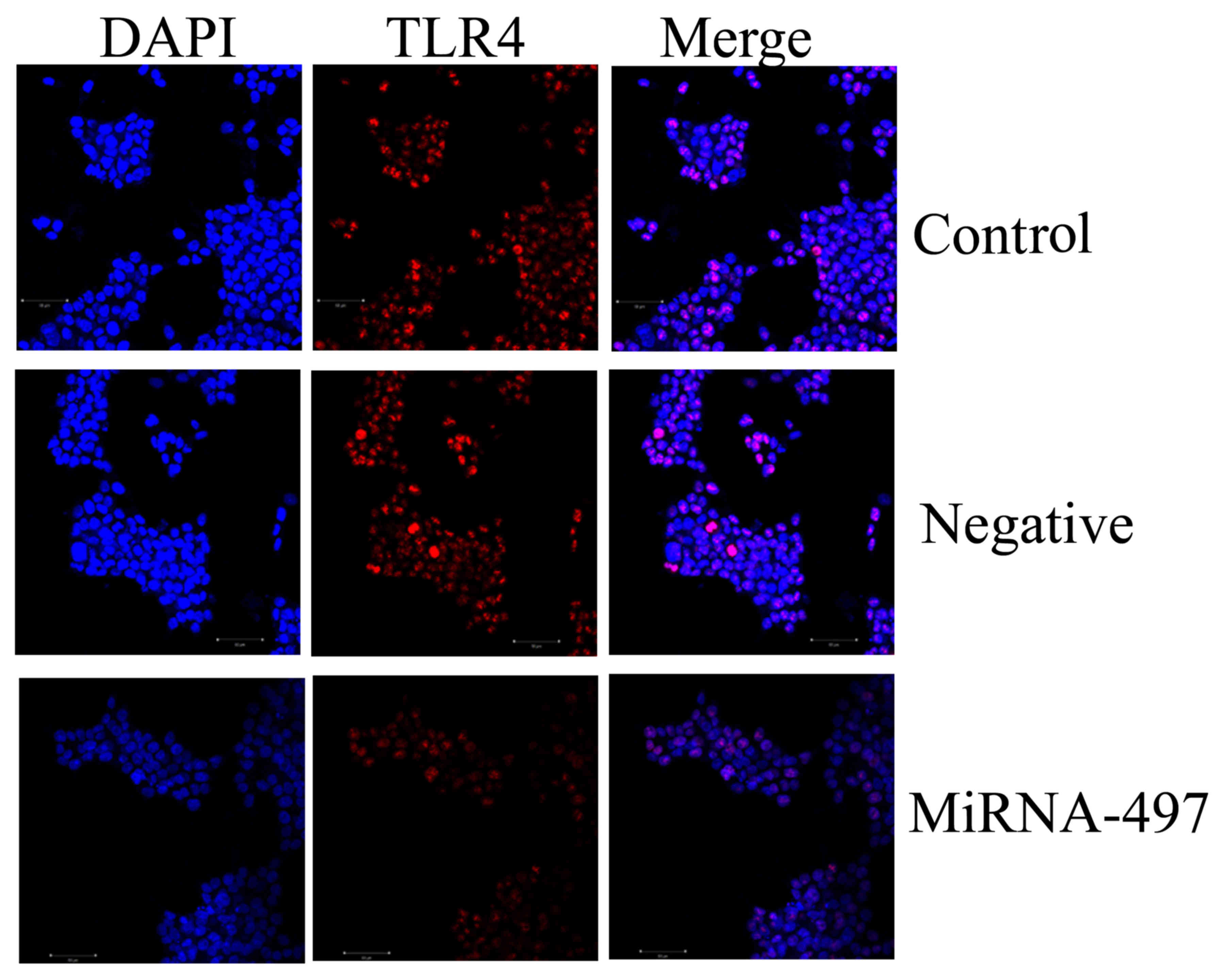
TLR4 protein expression in N2A cells
using immunocytofluorescence
Immunocytofluorescence was used to determine TLR4
protein expression in the N2A cells. As shown in Fig. 6, TLR4 protein expression was
significantly suppressed in the N2A cells by miR-497 overexpression
compared with that in the negative group.
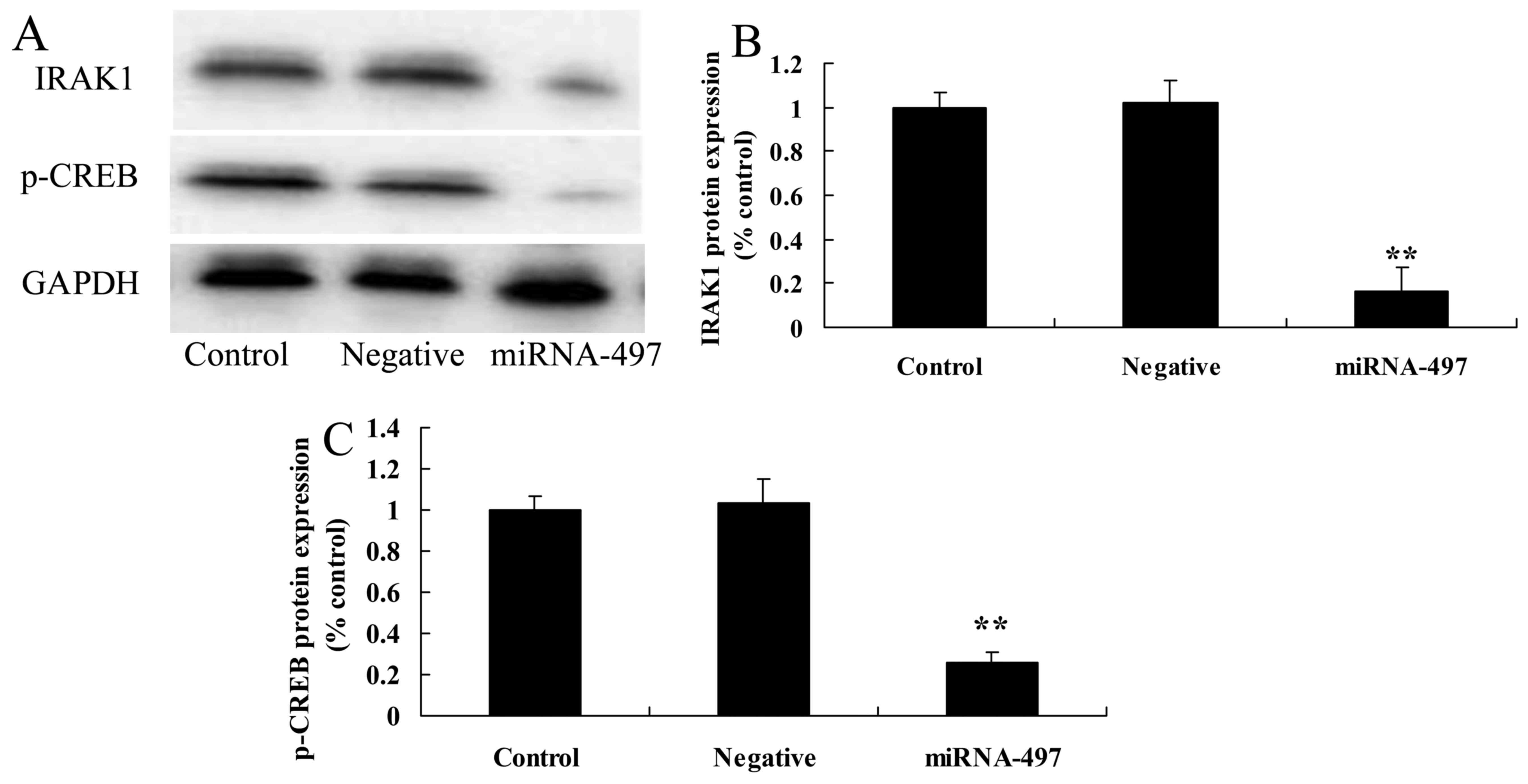
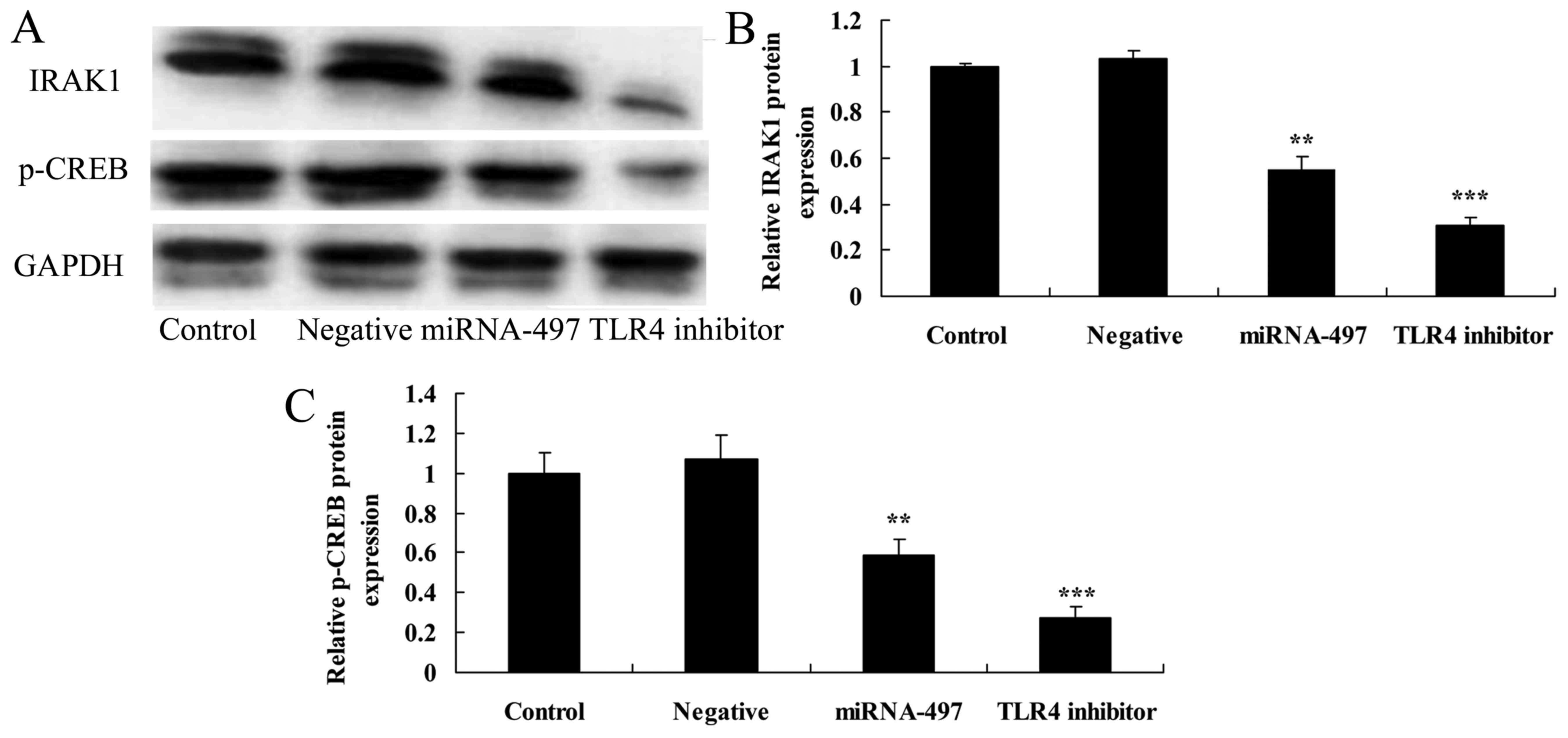
Overexpression of miR-497 suppresses
IRAK1 and p-CREB protein expression in N2A cells
To determine the anti-inflammatory functional role
of miR-497 in cerebral infarction, IRAK1 and p-CREB protein
expression was measured using western blot analysis. It was found
that miR-497 overexpression significantly suppressed IRAK1 and
p-CREB protein expression in cerebral infarction compared with that
in the negative group (Fig.
7).
Following miR-497 overexpression, TLR4
inhibitor suppresses TLR4, MyD88 and NF-κB protein expression in
N2A cells
The TLR4 inhibitor was used to inhibit TLR4 protein
expression in cerebral infarction. As shown in Fig. 7, TLR4 inhibitor significantly
suppressed TLR4, MyD88 and NF-κB protein expression in the N2A
cells following miR-497 overexpression compared with that in the
miR-497 overexpression group (Fig.
8).
Following miR-497 overexpression, TLR4
inhibitor suppresses inflammation factors in N2A cells
Next, it was determined whether TLR4 inhibitor
influences inflammation factors in N2A cells following miR-497
overexpression. Compared with miR-497 overexpression alone, the
TLR4 inhibitor significantly suppressed IL-1β, IL-6 and IL-8 levels
in the N2A cells following miR-497 overexpression (Fig. 9).
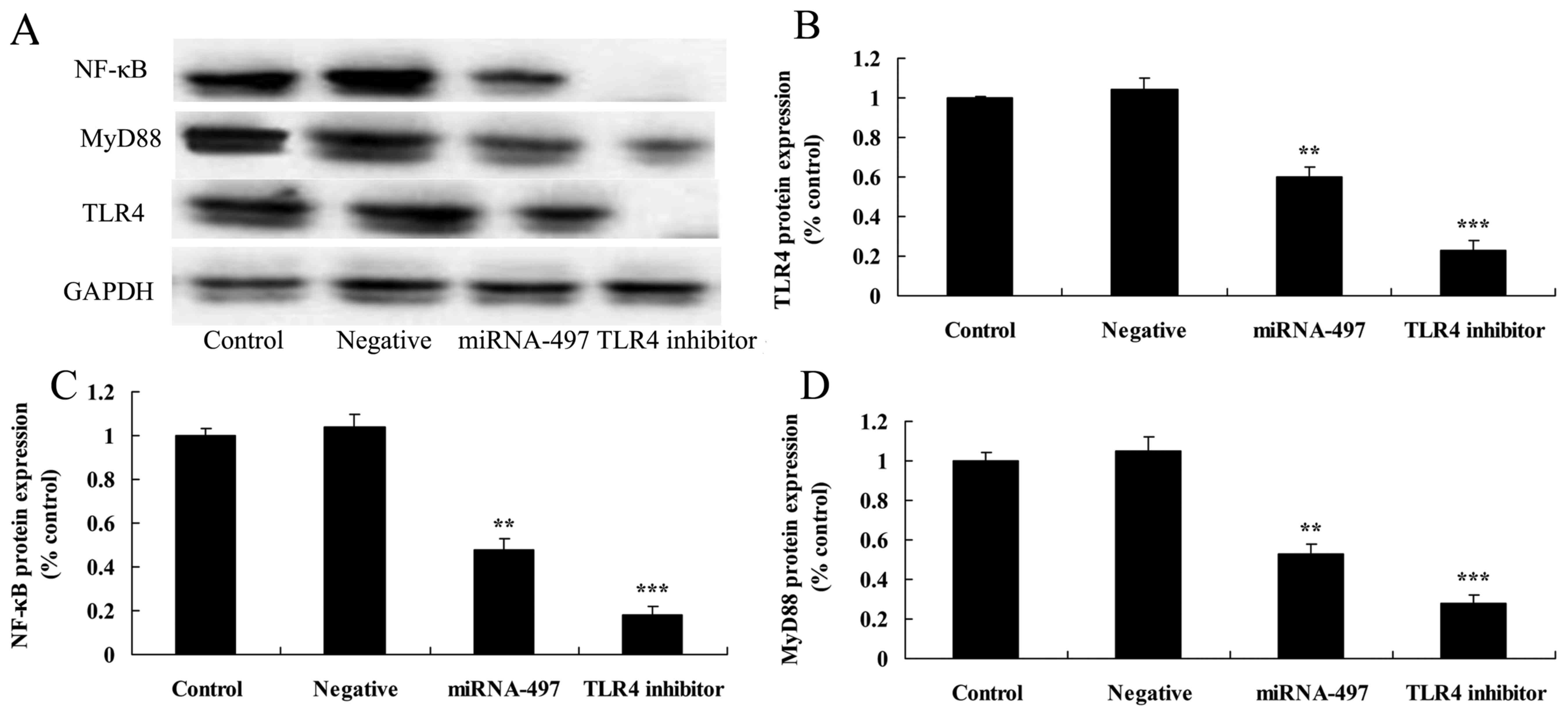 | Figure 9Following miR-497 overexpression,
TLR4 inhibitor suppresses TLR4, MyD88 and NF-κB protein expression
in N2A cells. Following miR-497 overexpression, TLR4 inhibitor
suppressed TLR4, MyD88 and NF-κB protein expression, as determined
using (A) western blot assays and (B-D) statistical analysis in N2A
cells. **P<0.01 compared with negative group;
***P<0.01 compared with miR-497 overexpression group.
TLR4, Toll-like receptor 4; NF-κB, nuclear factor-κB; miR/miRNA,
microRNA; MyD88, myeloid differentiation primary response protein
MyD88; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
Following miR-497 overexpression, TLR4
inhibitor suppresses IRAK1 and p-CREB protein expression in N2A
cells
The effects of TLR4 inhibitor on IRAK1 and p-CREB
protein expression in N2A cells were assessed. Results indicated
that TLR4 inhibitor significantly suppressed IRAK1 and p-CREB
protein expression in the N2A cells following miR-497
overexpression compared with that in the miR-497 overexpression
group (Fig. 10).
Following miR-497 overexpression, CREB
inhibitor suppresses IRAK1 and p-CREB protein expression in N2A
cells
For western blot analysis, CREB inhibitor was used
to inhibit CREB protein expression in cerebral infarction. As shown
in Fig. 11, CREB inhibitor was
able to inhibit Bax, IRAK1 and p-CREB protein expression in N2A
cells following miR-497 overexpression compared with that in the
miR-497 overexpression group (Fig.
11).
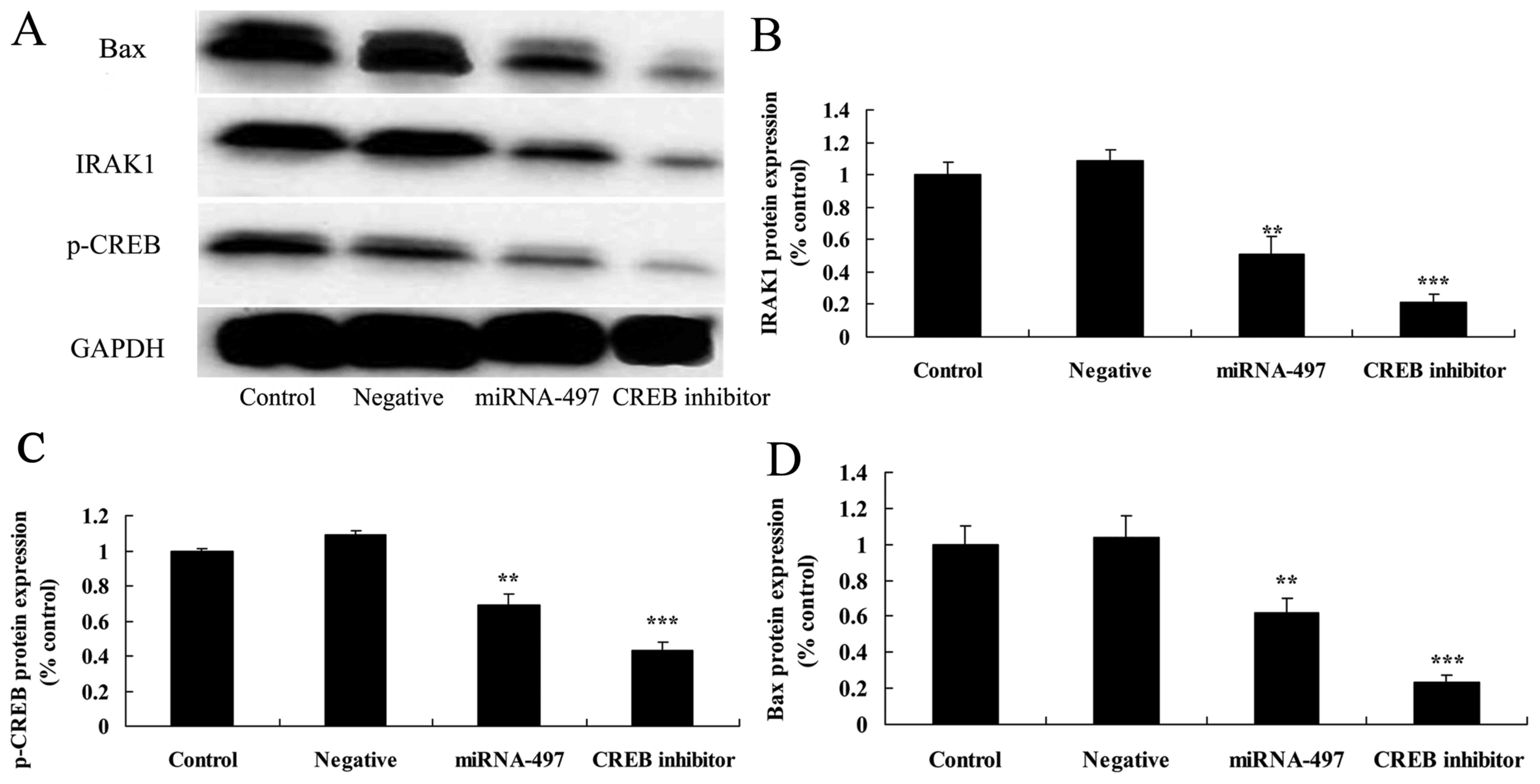 | Figure 11Following miR-497 overexpression,
CREB inhibitor suppresses IRAK1, p-CREB and Bax protein expression
in N2A cells. Following miR-497 overexpression, CREB inhibitor
suppressed IRAK1, p-CREB and Bax protein expression, as determined
using (A) western blot assays and (B-D) statistical analysis in the
N2A cells. **P<0.01 compared with negative group;
***P<0.01 compared with miR-497 overexpression group.
IRAK1, IL-1 receptor-associated kinase; CREB, cyclic AMP response
element binding protein; p-, phosphorylated; GAPDH, glyceraldehyde
3-phosphate dehydrogenase; miR/miRNA, microRNA. |
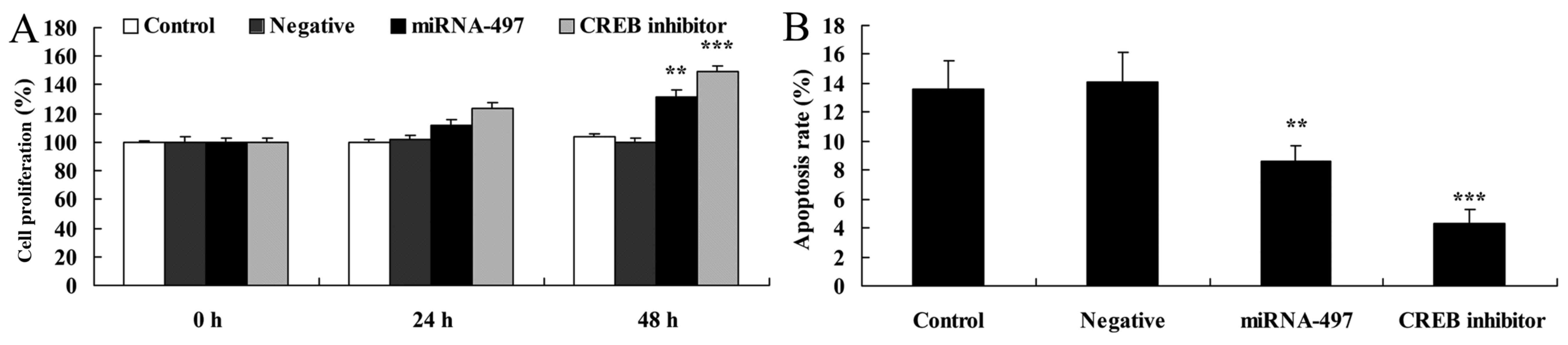
Following miR-497 overexpression, CREB
inhibitor induces cell proliferation and decreases apoptosis in N2A
cells
Next, the role of CREB in the effects of miR-497 on
cell proliferation and apoptosis in N2A cells following miR-497
overexpression was determined. CREB inhibitor induced cell
proliferation and decreased apoptosis in the N2A cells following
miR-497 overexpression compared with that in the miR-497
overexpression group (Fig.
12).
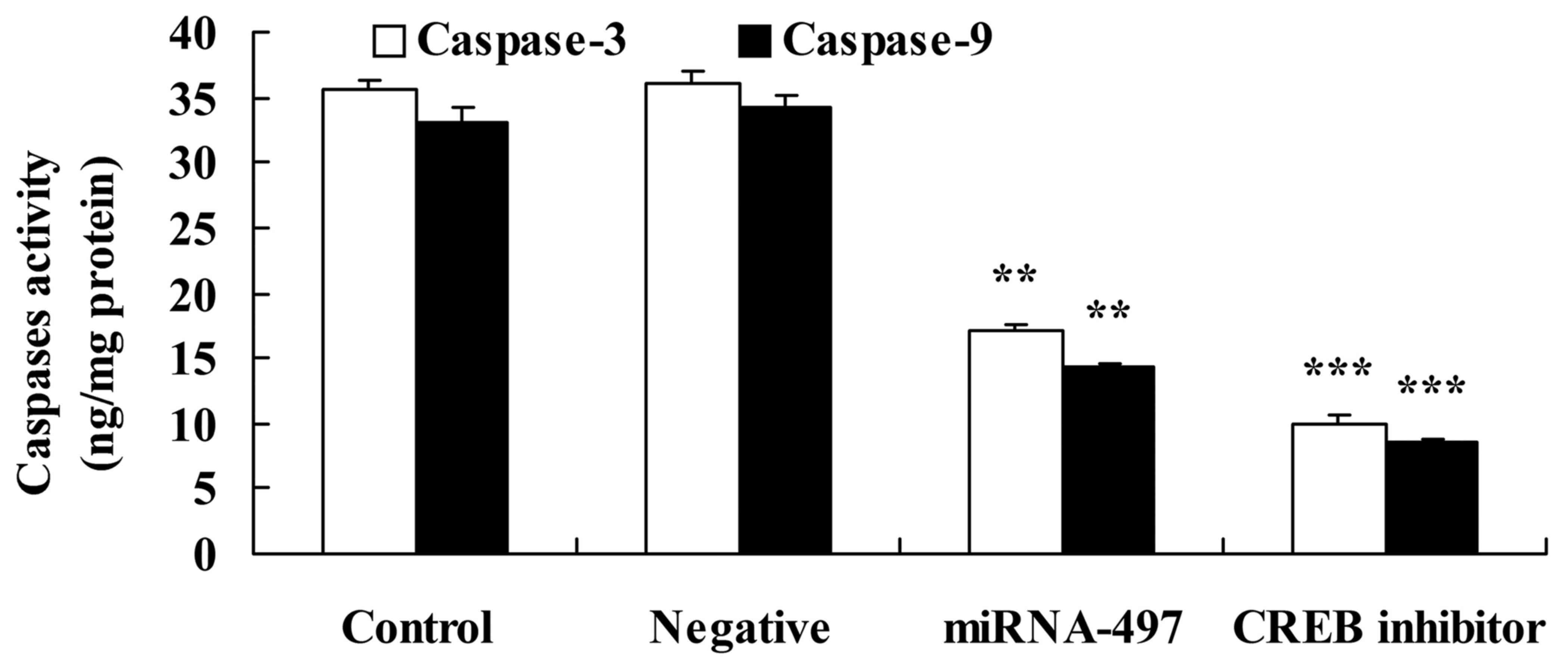
Following microRNA-497 overexpression,
CREB inhibitor decreases caspase-3 and caspase-9 activities, and
suppresses Bax protein expression in N2A cells
Fig. 13 showed
that following miR-497 overexpression, CREB inhibitor decreased
caspase-3 and caspase-9 activities in the N2A cells compared with
that in the miR-497 overexpression group.
Discussion
A large amount of the experimental research data
from the middle cerebral arterial embolism models verify that
inflammatory cell adhesion molecules, chemokines and cytokines
serve key roles in cell apoptosis following focal cerebral ischemia
reperfusion injury (18).
Investigating the mechanism of the focal cerebral ischemia
reperfusion injury can directly instruct some drugs, with
anti-inflammation and anti-apoptosis being the targets used in the
clinic to treat and intervene in strokes (19).
The present results indicated that serum miR-497
expression in patients with cerebral infarction was upregulated
compared with that in healthy volunteers. Furthermore, it was also
demonstrated that overexpression of miR-497 significantly induced
cell proliferation, decreased apoptosis, and reduced IL-1β, IL-6
and IL-8 levels in the cerebral infarction model in vitro.
Li et al (20)
demonstrated that inhibition of miR-497 ameliorates
anoxia/reoxygenation injury in cardiomyocytes by suppressing cell
apoptosis.
TLR is also extensively expressed in the central
nervous system, and is mainly distributed in the neuroglial cells,
including microglia, astrocytes and oligodendrocytes (21). The excessive inflammatory response
in the brain tissue following focal cerebral ischemia reperfusion
is one of the important mechanisms leading to reperfusion injury,
which requires the infiltration of inflammatory cells and the
involvement of certain cytokines, intercellular adhesion molecules
and chemokines (22). TLR has
been found to serve an important role in the inflammatory response
following focal cerebral ischemia reperfusion. In the cerebral
ischemic response, TLR may induce the occurrence of the
inflammatory response through identifying the endogenous ligands
released in the cerebral ischemic injury (23). The mechanism of action of TLR in
cerebral ischemia has become a research focus in recent years, and
currently, TLR2, TLR, TLR9 and particularly TLR4 have a relatively
well-established association with cerebral ischemia (22). The present study next examined the
significant suppression of TLR4, MyD88 and NF-κB protein expression
in cerebral infarction following the overexpression of miR-497. Xu
et al (24) demonstrated
that miR-497 could inhibit the inflammation and apoptosis of spinal
cord ischemia-reperfusion injury through IRAK1 of TLR4 and the CREB
signaling pathway.
Once the TLR4 system is activated, it can activate
the expression of NF-κB through the MyD88-dependent and
MyD88-independent signal pathways, and thus mediate the
upregulation of certain inflammatory response factors, including
tumor necrosis factor-α (TNF-α) and IL-1 (13,14). It has been demonstrated by
research that the innate immune response and the inflammatory
response serve a fairly important role in focal cerebral ischemia
reperfusion injury (14). The
present results indicated that TLR4 inhibitor suppressed
inflammation factors in N2A cells following miR-497 overexpression.
Kong et al (25) reported
that miR-497 may exhibit an anticancer function by regulating the
NF-κB signaling pathway in human prostate cancer cells.
The transient ischemia of brain tissue will lead to
cerebral ischemic injury, while the inflammatory response following
the restoration of the brain blood supply will cause a second brain
injury, known as an ischemia reperfusion injury (26). The latest research suggests that
the innate immune response and the inflammatory response serve
extremely critical roles in focal cerebral ischemia reperfusion
injury (27). As an important
nuclear transcription factor, NF-κB mediates the synthesis of
certain genes, such as TNF-α, and it is closely associated with the
immuno-inflammatory response following focal cerebral ischemia
reperfusion injury (28).
Numerous studies have confirmed that puerarin can protect against
the inflammatory response injury following focal cerebral ischemia
reperfusion injury through the inhibition of NF-κB activity and the
reduction in TNF-α expression (14,29). These present results indicated
that TLR4 inhibitor suppressed TLR4, MyD88 and NF-κB protein
expression, and reduced IRAK1 and p-CREB protein expression in the
N2A cells following miR-497 overexpression.
The TLR4-TIRAP-MyD88 signal pathway can take part in
the phagocytosis and degradation of amyloid β in the early stage of
Alzheimer’s disease (AD), and can reduce its deposition, but the
release of its subsequent activated inflammatory factors will cause
neuronal apoptosis and accelerate the course of AD (30). In addition, research on mouse and
human atherosclerosis has shown that TLR4 is closely associated
with the development of atherosclerosis (26). The intense activation of the
TLR4-TIRAP-MyD88 signaling pathway will result in the secretion of
a high level of inflammatory cytokines, and will accelerate plaque
formation and development (12).
The present results showed that miR-497 overexpression
significantly suppressed IRAK1 and p-CREB protein expression in
cerebral infarction in N2A cells following miR-497
overexpression.
Extracellular signal-regulated kinase (ERK) is a
member of the MAPK sub-family, which will be phosphorylated when
upstream mitogen-activated protein kinase kinase is intensively
stimulated by the calcium influx of the N-methyl-D-aspartate
receptor calcium channel within the synapse (31). p-ERK has an essential role in
regulating neuronal survival during the pathological stage of
stroke, and inhibits the activation of the associated pro-apoptotic
molecular mechanisms (32). The
activated ERK develops nuclear translocation and phosphorylates the
downstream CREB through the p90 ribosomal S6 kinase (31). p-CREB regulates the expression of
the Bcl-2, C-fos and BDNF genes, promotes the expression of the
antioxidant enzyme and anti-apoptotic protein, enhances ischemia
tolerance and reduces delayed neuronal death (29,32). The present results demonstrated
that CREB inhibitor suppressed p-CREB protein expression, induced
cell proliferation, decreased apoptosis and caspase-3 and caspase-9
activities, and suppressed Bax protein expression in N2A cells
following miR-497 overexpression. Xu et al (24) demonstrated that miR-497 could
inhibit the inflammation and apoptosis of spinal cord
ischemia-reperfusion injury through IRAK1 of the TLR4 and CREB
signaling pathways.
In conclusion, the present study data demonstrated
that miR-497 attenuated cerebral infarction in patients through
anti-inflammation and anti-apoptosis mechanisms by regulating the
TLR4 and CREB signaling pathways. Furthermore, miR-497/TLR4 and
CREB signaling pathways may have an impact on the molecular target
spot of cerebral infarction patients in the future.
Acknowledgments
Not applicable.
References
|
1
|
Thangarajh M, Yang G, Fuchs D, Ponisio MR,
McKinstry RC, Jaju A, Noetzel MJ, Casella JF, Barron-Casella E,
Hooper WC, et al: Magnetic resonance angiography-defined
intracranial vasculopathy is associated with silent cerebral
infarcts and glucose-6-phosphate dehydrogenase mutation in children
with sickle cell anaemia. Br J Haematol. 159:352–359. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Su Y, Fan L, Zhang Y, Zhang Y, Ye H, Gao
D, Chen W and Liu G: Improved Neurological Outcome With Mild
Hypothermia in Surviving Patients With Massive Cerebral Hemispheric
Infarction. Stroke. 47:457–463. 2016. View Article : Google Scholar
|
|
3
|
Taomoto K, Ohnishi H, Kuga Y, Nakashima K,
Ichioka T, Kodama Y, Kubota H, Tominaga T, Hirose T, Hayashi M, et
al: Platelet function and spontaneous thrombolytic activity of
patients with cerebral infarction assessed by the global thrombosis
test. Pathophysiol Haemost Thromb. 37:43–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miwa K, Tanaka M, Okazaki S, Furukado S,
Sakaguchi M and Kitagawa K: Relations of blood inflammatory marker
levels with cerebral microbleeds. Stroke. 42:3202–3206. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhan J, Qin W, Zhang Y, Jiang J, Ma H, Li
Q and Luo Y: Upregulation of neuronal zinc finger protein A20
expression is required for electroacupuncture to attenuate the
cerebral inflammatory injury mediated by the nuclear factor-kB
signaling pathway in cerebral ischemia/reperfusion rats. J
Neuroinflammation. 13:2582016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sarajärvi T, Lipsanen A, Mäkinen P,
Peräniemi S, Soininen H, Haapasalo A, Jolkkonen J and Hiltunen M:
Bepridil decreases Aβ and calcium levels in the thalamus after
middle cerebral artery occlusion in rats. J Cell Mol Med.
16:2754–2767. 2012. View Article : Google Scholar
|
|
7
|
Du R, Teng JF, Wang Y, Zhao XY and Shi ZB:
Clinical study of Butylphthalide combined with Xue Shuan Tong on
serum inflammatory factors and prognosis effect of patients with
cerebral infarction. Pak J Pharm Sci. 28(Suppl 5): 1823–1827.
2015.PubMed/NCBI
|
|
8
|
Kim SJ, Shin HY, Ha YS, Kim JW, Kang KW,
Na DL and Bang OY: Paradoxical embolism as a cause of silent brain
infarctions in healthy subjects: The ICONS study (Identification of
the Cause of Silent Cerebral Infarction in Healthy Subjects). Eur J
Neurol. 20:353–360. 2013. View Article : Google Scholar
|
|
9
|
Zhang J, Yuan L, Zhang X, Hamblin MH, Zhu
T, Meng F, Li Y, Chen YE and Yin KJ: Altered long non-coding RNA
transcriptomic profiles in brain microvascular endothelium after
cerebral ischemia. Exp Neurol. 277:162–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu XS, Chopp M, Zhang RL and Zhang ZG:
MicroRNAs in cerebral ischemia-induced neurogenesis. J Neuropathol
Exp Neurol. 72:718–722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eklind S, Mallard C, Leverin AL, Gilland
E, Blomgren K, Mattsby-Baltzer I and Hagberg H: Bacterial endotoxin
sensitizes the immature brain to hypoxic–ischaemic injury. Eur J
Neurosci. 13:1101–1106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan H, Li L, Zhang X, Liu Y, Yang C, Yang
Y and Yin J: Oxymatrine downregulates TLR4, TLR2, MyD88, and
NF-kappaB and protects rat brains against focal ischemia. Mediators
Inflamm. 2009:7047062009. View Article : Google Scholar
|
|
13
|
Li Y, Xu XL, Zhao D, Pan LN, Huang CW, Guo
LJ, Lu Q and Wang J: TLR3 ligand poly IC attenuates reactive
astrogliosis and improves recovery of rats after focal cerebral
ischemia. CNS Neurosci Ther. 21:905–913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Belinga VF, Wu GJ, Yan FL and Limbenga EA:
Splenectomy following MCAO inhibits the TLR4-NF-κB signaling
pathway and protects the brain from neurodegeneration in rats. J
Neuroimmunol. 293:105–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin R, Lin Y, Tao J, Chen B, Yu K, Chen J,
Li X and Chen LD: Electroacupuncture ameliorates learning and
memory in rats with cerebral ischemia-reperfusion injury by
inhibiting oxidative stress and promoting p-CREB expression in the
hippocampus. Mol Med Rep. 12:6807–6814. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bell KF, Bent RJ, Meese-Tamuri S, Ali A,
Forder JP and Aarts MM: Calmodulin kinase IV-dependent CREB
activation is required for neuroprotection via NMDA receptor-PSD95
disruption. J Neurochem. 126:274–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Ferguson S and Macdonald RL: Predictors of
cerebral infarction in patients with aneurysmal subarachnoid
hemorrhage. Neurosurgery. 60:658–667. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ueno H, Koyama H, Mima Y, Fukumoto S,
Tanaka S, Shoji T, Emoto M, Shoji T, Nishizawa Y and Inaba M:
Comparison of the effect of cilostazol with aspirin on circulating
endothelial progenitor cells and small-dense LDL cholesterol in
diabetic patients with cerebral ischemia: A randomized controlled
pilot trial. J Atheroscler Thromb. 18:883–890. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Zeng Z, Li Q, Xu Q, Xie J, Hao H,
Luo G, Liao W, Bin J, Huang X, et al: Inhibition of microRNA-497
ameliorates anoxia/reoxygenation injury in cardiomyocytes by
suppressing cell apoptosis and enhancing autophagy. Oncotarget.
6:18829–18844. 2015.PubMed/NCBI
|
|
21
|
Caso JR, Pradillo JM, Hurtado O, Lorenzo
P, Moro MA and Lizasoain I: Toll-like receptor 4 is involved in
brain damage and inflammation after experimental stroke.
Circulation. 115:1599–1608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao CX, Yang QW, Lv FL, Cui J, Fu HB and
Wang JZ: Reduced cerebral ischemia-reperfusion injury in Toll-like
receptor 4 deficient mice. Biochem Biophys Res Commun. 353:509–514.
2007. View Article : Google Scholar
|
|
23
|
Wang Y, Ge P and Zhu Y: TLR2 and TLR4 in
the brain injury caused by cerebral ischemia and reperfusion.
Mediators Inflamm. 2013:1246142013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu M, Wang HF, Zhang YY and Zhuang HW:
Protection of rats spinal cord ischemia-reperfusion injury by
inhibition of miR-497 on inflammation and apoptosis: Possible role
in pediatrics. Biomed Pharmacother. 81:337–344. 2016. View Article : Google Scholar
|
|
25
|
Kong XJ, Duan LJ, Qian XQ, Xu D, Liu HL,
Zhu YJ and Qi J: Tumor-suppressive microRNA-497 targets IKKβ to
regulate NF-κB signaling pathway in human prostate cancer cells. Am
J Cancer Res. 5:1795–1804. 2015.
|
|
26
|
Kunz A, Abe T, Hochrainer K, Shimamura M,
Anrather J, Racchumi G, Zhou P and Iadecola C: Nuclear
factor-kappaB activation and postischemic inflammation are
suppressed in CD36-null mice after middle cerebral artery
occlusion. J Neurosci. 28:1649–1658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie W, Fang L, Gan S and Xuan H:
Interleukin-19 alleviates brain injury by anti-inflammatory effects
in a mice model of focal cerebral ischemia. Brain Res.
1650:172–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu XC, Jiang T, Zhang QQ, Cao L, Tan MS,
Wang HF, Ding ZZ, Tan L and Yu JT: Chronic Metformin
Preconditioning provides neuroprotection via suppression of
NF-κB-mediated inflammatory pathway in rats with permanent cerebral
ischemia. Mol Neurobiol. 52:375–385. 2015. View Article : Google Scholar
|
|
29
|
Zhang X, Zhang X, Wang C, Li Y, Dong L,
Cui L, Wang L, Liu Z, Qiao H, Zhu C, et al: Neuroprotection of
early and short-time applying berberine in the acute phase of
cerebral ischemia: Upregulated pAkt, pGSK and pCREB, downregulated
NF-κB expression, ameliorated BBB permeability. Brain Res.
1459:61–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qiao H, Zhang X, Zhu C, Dong L, Wang L,
Zhang X, Xing Y, Wang C, Ji Y and Cao X: Luteolin downregulates
TLR4, TLR5, NF-κB and p-p38MAPK expression, upregulates the p-ERK
expression, and protects rat brains against focal ischemia. Brain
Res. 1448:71–81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choi IY, Ju C, Anthony Jalin AM, Lee DI,
Prather PL and Kim WK: Activation of cannabinoid CB2
receptor-mediated AMPK/CREB pathway reduces cerebral ischemic
injury. Am J Pathol. 182:928–939. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng CY, Tang NY, Kao ST and Hsieh CL:
Ferulic acid administered at various time points protects against
cerebral infarction by activating p38 MAPK/p90RSK/CREB/Bcl-2
anti-apoptotic signaling in the subacute phase of cerebral
ischemia-reperfusion injury in rats. PLoS One. 11:e01557482016.
View Article : Google Scholar : PubMed/NCBI
|