Introduction
Cardiovascular diseases are the leading causes of
morbidity and mortality around the world, according to the World
Health Organization, and with the increasingly aging population,
almost 23,600,000 individuals are predicted to succumb to mortality
from cardiovascular diseases by 2030 (1). However, the majority of cases of
patients with cardiovascular diseases succumbing to mortality are
due to myocardial infarction (MI), which is most frequently caused
by insufficient blood supply to the heart (2). Currently, increased attention is
being paid to the development of timely and effective interventions
to recover the blood flow in the ischemic myocardium, including
coronary angioplasty, coronary artery bypass surgery and
thrombolytic treatments (3,4).
Although these therapeutic approaches significantly decrease the
risk of post-ischemic complications, including heart failure and
arrhythmia, the prognosis in terms of post-ischemic 30-day
mortality and morbidity rates, remains relatively poor (5,6).
In addition, several studies have shown that reperfusion strategies
are usually accompanied by marked changes in mitochondrial
permeability transition pore opening; generation of reactive oxygen
species and reactive nitrogen species; activation of neutrophils,
platelets and complements; inflammatory reactions; intracellular
distribution of Ca2+; and irreversible cell death. These
events may ultimately induce more serious cardiomyocyte dysfunction
and worsen tissue damage (5,7).
Therefore, to maintain the blood flow to myocardial
ischemia-reperfusion (I/R)-injured tissues, prevent cardiomyocyte
damage and re-establish the function of the heart, physicians and
researchers are focusing investigations on the mechanisms
underlying the development and progression of myocardial I/R
injury.
MicroRNAs (miRNAs) constitute a large class of
phylogenetically conserved, small, single-stranded, non-coding RNA
molecules of 19–25 nucleotides, which downregulate gene expression,
possibly regulating up to 90% of human genes, in a
sequence-dependent manner via the degradation or translational
inhibition of their target mRNAs (8,9).
In previous years, numerous studies have documented that miRNAs are
key transcriptional regulators that are involved in several
clinical scenarios of cardiovascular diseases, including cardiac
arrhythmia, cardiac hypertrophy, heart failure, cardiac fibrosis,
cardiac ischemia and vascular atherosclerosis (10,11). Furthermore, the upregulation or
downregulation of the expression of an individual miRNA is capable
of facilitating or alleviating the pathological alterations in
cardiovascular diseases, suggesting that miRNAs may be innovative
therapeutic targets in cardiovascular diseases (12). For example, miRNA (miR)-22 can
suppress the apoptosis of cardiomyocytes by targeting the cAMP
response element binding-binding protein; therefore, miR-22 has
been considered a novel therapeutic target for preventing
myocardial I/R injury (13).
Additionally, miRNAs are used as non-invasive, sensitive diagnostic
biomarkers in cardiovascular diseases (10,14) as heart tissue miRNAs can be
released into the circulating blood; for example, miR-1, miR-126,
miR-208 and miR-499 are utilized in the diagnosis of acute MI
(15). Therefore, the accurate
modulation of miRNAs or circulating miRNAs offers a promising
therapeutic strategy or diagnostic index in cardiovascular
diseases, respectively (11,16).
Previous studies have demonstrated that circulating
miR-221-3p is a novel marker for the early prediction of acute MI
(17). In addition, plasma
miR-221-3p was shown to be decreased in patients with early-stage
arteriosclerosis obliterans; therefore, it was applied as a
biomarker for arteriosclerosis obliterans (18). However, the specific role of
miR-221-3p in myocardial I/R injury remains to be elucidated.
Therefore, in the present study, a myocardial damage model and a
rat model of myocardial I/R were established to examine the effects
of miR-221-3p on the occurrence and development of myocardial
damage.
Materials and methods
Cell culture and treatments
The H9c2 rat cardiomyocytes line, originally derived
from embryonic rat heart tissue, was purchased from American Type
Culture Collection (Manassas, VA, USA) and grown in Dulbecco’s
modified Eagle’s medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with the addition of 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 2 mmol/l glutamine, 100
U/ml penicillin and 0.1 mg/ml streptomycin at 37°C in a humidified
atmosphere of 5% CO2. For the passages, the H9c2 cells
were washed with phosphate-buffered saline (PBS) twice and then
trypsinized with 0.25% trypsin once they reached 80–90%
confluence.
The H9c2 cells were seeded in 6-well plates at a
density of 1×105 cells/well and treated with 0, 150 and
700 μM hydrogen peroxide (H2O2),
respectively, for 0, 6, 12 and 24 h. The concentration of 700
μM H2O2 was selected for subsequent
experiments. The H9c2 cells cultured in 6-well plates were randomly
divided into six groups (n=3 per group): Control group,
H2O2 group, miR-control group, miR-221-3p
group, H2O2+miR-221-3p group and
H2O2+p57 group. The miR-control plasmid,
miR-221-3p mimics and p57 mimics, synthesized by Sangon Biotech
Co., Ltd. (Shanghai, China) were transfected into the H9c2 cells
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer’s protocol.
Animal experiments
Male Sprague Dawley rats, aged 8-10 weeks, weighted
~220 g, were obtained from Weitong Lihua Company (Beijing, China)
and housed under a standard specific pathogen-free environment with
a 12-h light and 12-h dark cycle at 25±2°C. The rats received free
access to autoclaved chow and water. All procedures and protocols
for the animal experiments were performed in accordance with the
Guidelines for the Care and Use of Laboratory Animals, with prior
approval by the Animal Ethical Experimentation Committee of
Shandong University (Shandong, China; approval no. KYLL-2017029).
Following acclimatization for at least 1 week, the rats were
randomly divided into seven groups (n=3 per group): Sham group,
myocardial I/R-0 min group, myocardial I/R-60 min group, myocardial
I/R-120 min group, myocardial I/R+β-galactosidase (β-gal) group,
myocardial I/R+miR-221-3p group and myocardial I/R+p57 group.
For I/R surgery, the rats were initially
anesthetized with 2% isoflurane gas inhalation. Subsequently, the
left coronary artery (LCA) was exposed using a left thoracotomy at
the fifth intercostal space. Following LCA ligation with 7-0 silk
sutures, a smooth catheter was applied on the artery to achieve
ischemia for 0, 60 and 120 min, respectively, followed by
reperfusion, which was induced by releasing the ligature and
removing the catheter. The myocardial ischemia and reperfusion were
visually confirmed by changes in myocardial color. During the
surgical procedure, the body temperature of the rats was maintained
at 37°C using a thermo-heating pad. The rats in the sham group
underwent surgery in a similar manner, but without the LCA I/R, and
were treated with saline. However, the rats used in the β-gal,
miR-221-3p mimetic and p57 mimetic groups received intravenous
injections (50 mg/kg/day) of β-gal, miR-221-3p and p57 for 5
consecutive days prior to surgery. The rats were sacrificed 3 days
following the induction of I/R to measure the expression of
miR-221-3p, expression of p57, myosin+ cell ratio and
fractional shortening of the left ventricular diameter (FSLVD)
ratio.
Cell death analysis
Fluorescein Annexin V-FITC/propidium iodide (PI)
double labeling was performed using the Annexin V-FITC apoptosis
detection kit (Beyotime Institute of Biotechnology, Shanghai,
China), following the manufacturer’s protocol to detect the
apoptosis of H9c2 cells subjected to the different concentrations
of H2O2. The harvested cells, washed with
cold PBS, were treated with 195 μl of Annexin V-FITC binding
buffer. Following thorough mixing, the cells were incubated with 5
μl of Annexin V-FITC and 10 μl of PI per sample at
room temperature (20–25°C) for 20 min in the dark. The cells were
analyzed on a FACScan flow cytometer within 1 h. Annexin
V-FITC+/PI− cells represented apoptotic
cells, and Annexin V-FITC−/PI+ cells
represented necrotic cells. Additionally, the cells treated with
H2O2+miR-221-3p and
H2O2+p57, which showed only PI staining and
necrotic cells, were counterstained with DAPI (Beyotime Institute
of Biotechnology) and counted under a fluorescence microscope
(Leica Microsystems GmbH, Solms, Germany).
Lactate dehydrogenase (LDH) activity
measurement
LDH, an enzyme that catalyzes the conversion between
lactate and pyruvic acid, is a diagnostic criterion for MI
(19). LDH activity was measured
for each group using an LDH detection kit (Beyotime Institute of
Biotechnology) according to the manufacturer’s protocol. Briefly,
100 μl of supernatant (1,200 × g for 10 min at 4°C) for each
group was prepared in a non-sterile, clear 96-well plate and mixed
with 100 μl of LDH reaction. Following incubation at room
temperature for 3 min in the dark, 30 μl of HCl was added to
each sample. Ultimately, the optical density of each well was
examined at a wavelength of 490 nm in a multimode plate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Reverse
transcription-quantitative-polymerase chain reaction (RT-qPCR)
detection
Total RNA from three replicates per sample was
isolated using TRIzol reagent (Tiangen Biotech Co., Ltd., Beijing,
China) extraction method and subsequently cleaned using RNeasy Mini
Columns (Qiagen GmbH, Dusseldorf, Germany). The concentration and
purity of the extracted RNA were assessed by measuring the optical
density at 260 and 280 nm, respectively, using a NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). For the
analysis of miR-221-3p, equal quantities of RNA were then
polyadenylated and reverse transcribed into complementary DNA
(cDNA) using an mir-X miRNA First-Strand Synthesis kit (Takara Bio,
Inc., Tokyo, Japan), according to the manufacturer’s protocol. The
cDNA (1 μg) of each sample was amplified with SYBR Advantage
qPCR Premix (Takara Bio, Inc.) on a 7500 Fast Real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) using the
following procedure in a 96-well optical plate: 95°C for 10 sec; 40
cycles of 95°C for 10 sec and 60°C for 40 sec; and dissociation at
95°C for 60 sec, 55°C for 30 sec and 95°C for 30 sec. For p57
evaluation, RT-qPCR analysis was performed on a 7500 Fast Real-Time
PCR system using a One Step SYBR® PrimeScript™ RT-PCR
kit (Takara Bio, Inc.) in accordance with the manufacturer’s
protocol. The amplification cycles consisted of one cycle at 42°C
for 5 min and one cycle of initial denaturation at 95°C for 10 sec,
followed by 40 cycles of a two-step procedure consisting of
denaturation at 95°C for 5 sec and annealing at 60°C for 34 sec.
The relative change in miRNA and mRNA expression was determined
using the 2−∆∆Cq method (20) with normalization to U6 snRNA and
18S rRNA, respectively. The sequences of primers used in the
present study are listed in Table
I.
 | Table ISequences of primers used for qRT-PCR
assays. |
Table I
Sequences of primers used for qRT-PCR
assays.
| miRNA or gene | Primer
sequences |
|---|
| miR-221-3p | F:
5′-AGCTACATTGTCTGCTGGGTTTC-3′ |
| p57 | F:
5′-CCGTTCATGTAGCAGCAACCG-3′ |
| R:
5′-ACCAGTGTACCTTCTCGTGCAG-3′ |
| 18S rRNA | F:
5′-CCTGGATACCGCAGCTAGGA-3′ |
| R:
5′-GCGGCGCAATACGAATGCCCC-3′ |
Cardiac function assessment
Transthoracic echocardiography, as a noninvasive
technology, is usually used to efficiently evaluate cardiac
function, including the FSLVD (21). Transthoracic echocardiographic
examination was performed on the rats in each group, which were
anesthetized but remained conscious. When FSLVD was measured in
vivo, the rats were sacrificed by CO2 inhalation to
ameliorate animal suffering, and the hearts were removed to examine
myosin+ cells and the gene and protein expression levels
of p57. The myosin in myocardial cells provides energy to the
beating heart via the transduction of ATP to mechanical activity
(22). Therefore, myosin-antibody
injection is a common method for detecting myosin+ cells
in rat hearts.
Western blot analysis
The H9c2 cell samples from three independent
experiments and whole-heart tissue samples from three rats were
rinsed with pre-cooled PBS three times and stored at −80°C until
evaluation using western blot assays. The proteins from each sample
were obtained by treatment with ice-cold radioimmunoprecipitation
assay lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl,
0.1% Nonidet P-40, and a mixture of protease inhibitors for 20 min
on ice. The total protein concentration was measured using a BCA
protein assay kit (Beyotime Institute of Biotechnology). The
protein samples (100 μg) were mixed with 2X loading buffer
(Takara Bio, Inc.), boiled for 8 min at boiling water and
separated, based on the molecular weight, by 8–10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. When the bromophenol
blue reached the bottom of the gel, the proteins were transferred
onto polyvinylidene fluoride (PVDF) membranes at a constant current
of 200 mA for 60 min with a semi-dry instrument (Bio-Rad
Laboratories, Inc.), followed by sealing with 5% skim milk at room
temperature for 2 h. Subsequently, the primary antibodies against
p57 (1:1,000 dilution in 5% skim milk; ab33169; Abcam, Cambridge,
USA) and GAPDH (as an internal reference, 1:10,000 dilution in 5%
skim milk; ab8245; Abcam) were allowed to react with the PVDF
membranes overnight at 4°C. Following extensive washing with
Tris-buffered saline containing 0.1% Tween-20 (TBST) three times,
the membranes were incubated with secondary antibodies, including
goat anti-mouse IgG and goat anti-rabbit IgG (1:12,000 diluted in
5% skim milk; ab191866 and ab193651; Abcam) at room temperature
with gentle agitation for 2 h at room temperature. The PVDF
membranes were washed three times with TBST; the proteins were
detected by enhanced chemiluminescence reagents (Beyotime Institute
of Biotechnology) and visualized on an X-ray film (Kodak,
Rochester, USA). Finally, the relative protein expression levels
were quantified by densitometry using ImageJ (version 2) analysis
software (National Institutes of Health, Bethesda, MD, USA).
Dual-luciferase reporter assay
To construct a luciferase reporter vector, the p57
3’untranslated region (UTR) fragment containing putative binding
sites and mutant sites for miR-221-3p was amplified using PCR and
cloned in the psiCHECK-2 vector (Promega Corporation, Madison, WI,
USA). For the analysis of luciferase activity, the 293T cells were
cultured in 24-well plates at ~80% confluence and co-transfected
with wild-type (WT)-p57 or Mutant-p57 vectors in addition to the
commercially available control psiCHECK-2 plasmid (blank group),
miR-221-3p mimics, miR-221-3p inhibitor, negative control (NC)
plasmid or NC inhibitor from Sangon Biotech Co., Ltd. for 48 h
using Lipofectamine 2000, according to the manufacturer’s protocol.
Subsequently, a dual-luciferase reporter assay was performed using
the Dual-Luciferase Reporter Assay kit (Promega Corporation),
following the manufacturer’s protocol, and the relative luciferase
activity was calculated by obtaining the ratio of firefly
fluorescence and Renilla fluorescence.
Statistical analysis
All data were analyzed with SPSS 18.0 software
(SPSS, Inc., Chicago, IL, USA) and are presented as the mean ±
standard deviation of at least three independent experiments. The
differences were evaluated using Student’s t-test (two-tailed) for
two experimental groups and one-way analysis of variance for more
than two experimental groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
Myocardial damage model establishment in
H9c2 cells
The H9c2 cells were incubated with different
concentrations of H2O2 (0, 150 and 700
μM) for myocardial damage model establishment. The results
showed that H9c2 cell death, including apoptosis and necrosis,
increased markedly for the cells treated with 150 and 700 μM
H2O2, compared with that observed for 0
μM H2O2 (Fig. 1A). Subsequently, the activity of
LDH was measured in the H9c2 cells treated with 150 and 700
μM H2O2. As shown in Fig. 1B, LDH activity was elevated in a
time-dependent manner following challenge with 700 μM
H2O2, however, no significant changes were
observed in the 150 μM H2O2 group.
Based on these results, challenge with 700 μM
H2O2 for 24 h was selected as a myocardial
damage model for subsequent experiments.
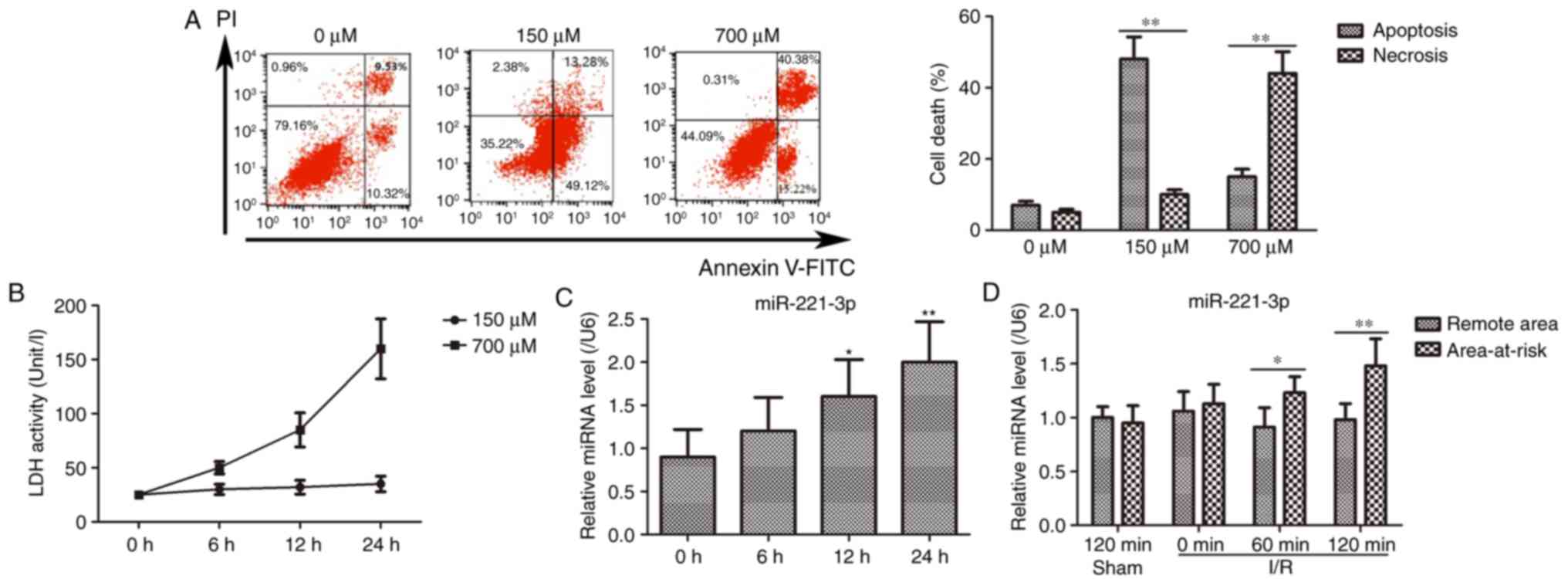 | Figure 1Myocardial damage model establishment
and expression of miR-221-3p. (A) Comparisons of cell death in H9c2
cells treated with various concentrations of
H2O2. The representative images are presented
on the left panel, and the statistical histogram is shown on the
right panel. **P<0.01. (B) LDH activity measurement
in the H9c2 cells treated with different concentrations of
H2O2 for 0, 6, 12 and 24 h. (C) Expression of
miR-221-3p was increased in the H9c2 cells treated with 700
μM H2O2 for 0, 6, 12 and 24 h.
*P<0.05 and **P<0.01, vs. 0 h. (D)
Expression of miR-221-3p in the area-at-risk was higher than that
in remote areas in the I/R rats. *P<0.05,
**P<0.01. miR, microRNA; LDH, lactate dehydrogenase;
I/R, ischemia-reperfusion; H2O2, hydrogen
peroxide; PI, propidium iodide. |
miR-221-3p is upregulated in H9c2 cells
treated with H2O2 and rats following I/R
surgery
In the H9c2 cells subjected to
H2O2 treatment, the expression level of
miR-221-3p was gradually augmented with time (Fig. 1C). Furthermore, in the I/R rat
model, the expression level of miR-221-3p in the area-at-risk was
gradually upregulated with increasing ischemia duration, compared
with that in the sham group, whereas no notable change in
expression was observed in remote areas with different ischemia
durations (Fig. 1D). Therefore,
these findings indicated that miR-221-3p was induced in myocardial
damage and may be important in the progress of myocardial
damage.
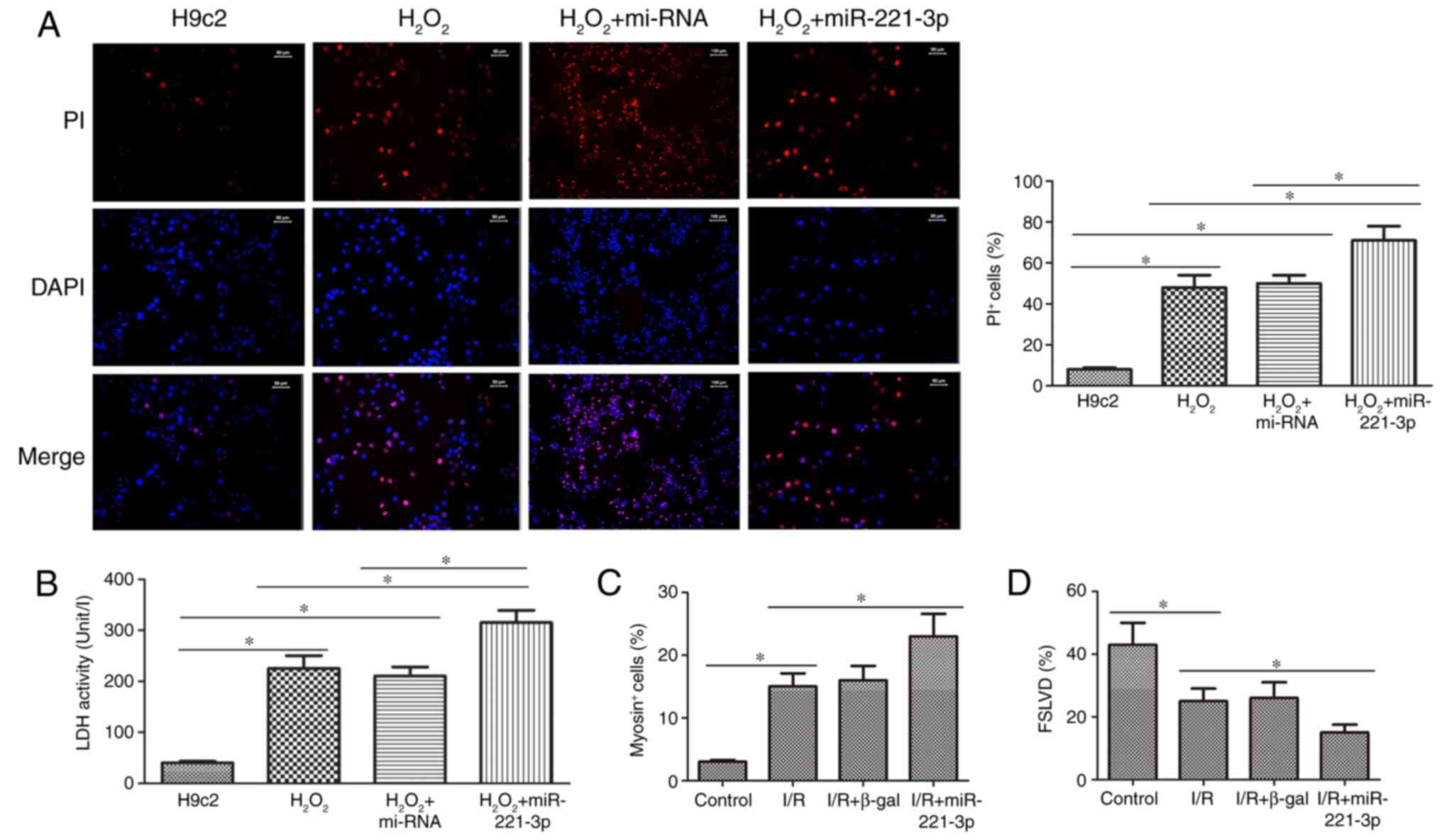
miR-221-3p exacerbates myocardial injury
in H9c2 cells treated with H2O2 and rats
following I/R surgery
As shown in Fig.
2A, the number of PI+ cells in the
H2O2 and
H2O2+miR-221-3p groups increased markedly,
compared with that in the H9c2 group. The number of PI+
cells in the H2O2+miR-221-3p group was higher
than that in the H2O2 group. In addition, the
change in LDH activity in the H9c2, H2O2 and
H2O2+miR-221-3p groups correlated with the
alteration in PI+ cells in these groups (Fig. 2B). In the I/R rat model, the
number of myosin+ cells was significantly elevated,
compared with that in the control group, however, the highest
increase in myosin+ cells was observed in the
I/R+miR-221-3p group (Fig. 2C).
In addition, FSLVD% was markedly decreased in the I/R rat model,
compared with that in the control group, and I/R+miR-221-3p
treatment led to the lowest FSLVD% in the rats (Fig. 2D). Therefore, these data suggested
that miR-221-3p promoted the development of myocardial injury in
the H9c2 cells treated with H2O2 and the rats
subjected to I/R surgery.
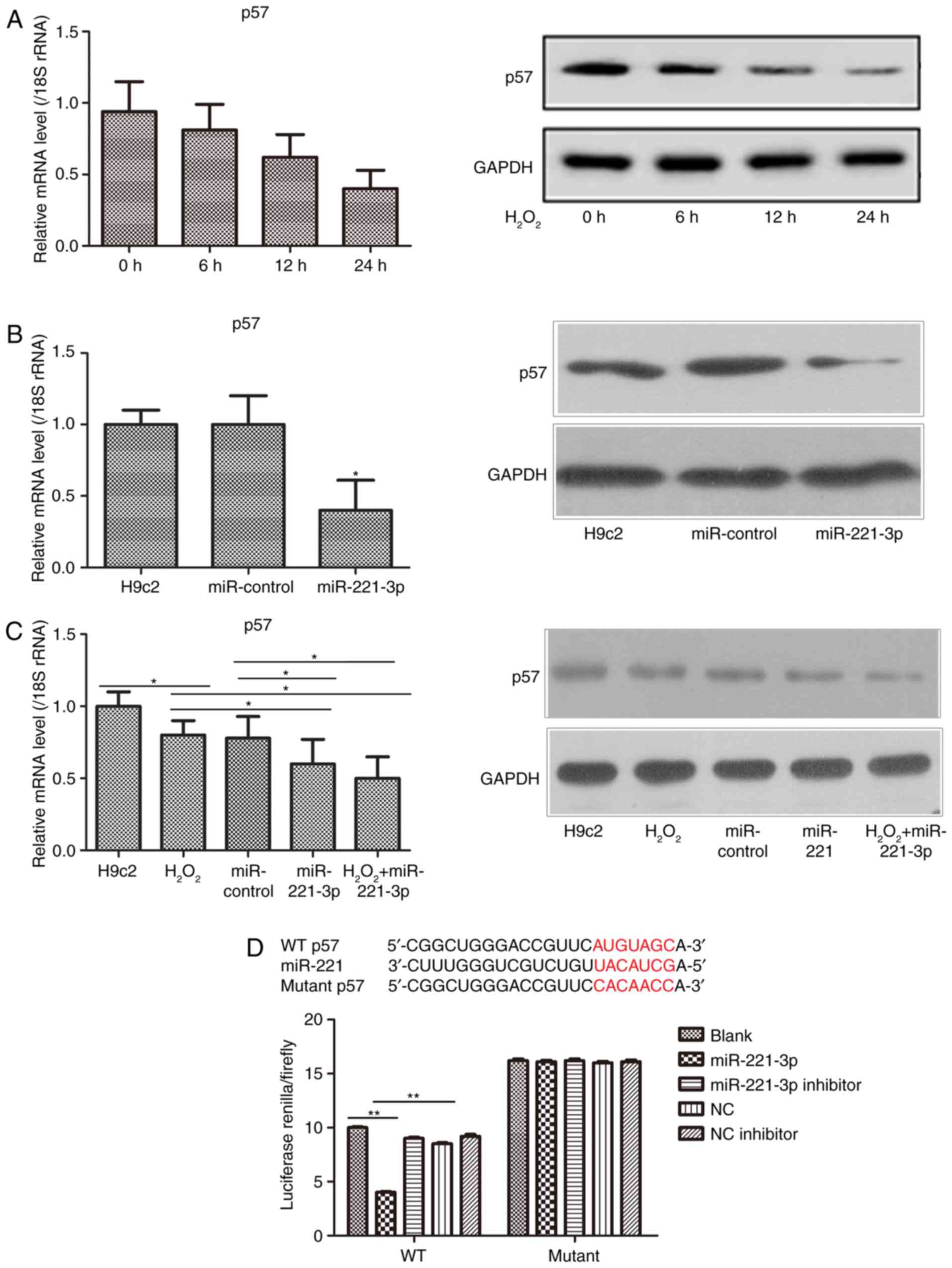
p57 is a direct target of miR-221-3p
In the H9c2 cell myocardial damage model, it was
found that the gene and protein expression levels of p57 were
reduced in a time-dependent manner (Fig. 3A). Furthermore, following the
overexpression of miR-221-3p, the gene and protein expression
levels of p57 in the H9c2 cells were markedly decreased (Fig. 3B). The gene and protein expression
levels of p57 were also examined in the H9c2 cells treated with
H2O2+miR-221-3p, and it was found that the
downregulation of p57 was lower, compared with that in the
H2O2 group and miR-221-3p group (Fig. 3C). However, based on the
increasing expression of miR-221-3p in the
H2O2 treatment groups, it was hypothesized
that p57 may be a potential target of miR-221-3p. A dual-luciferase
reporter assay was then used to confirm the above hypothesis. The
result showed that the activity of luciferase was markedly
decreased following co-transfection with the WT-p57 3′UTR vector
and miR-221-3p mimic plasmid. By contrast, the luciferase activity
was notably increased following co-transfection with the WT-p57
3′UTR vector and miR-221-3p inhibitor (Fig. 3D). However, no changes were
observed in cells co-transfected with the Mutant-p57 3′UTR vector
and miR-221-3p mimic/inhibitor or NC/NC inhibitor. Taken together,
it was confirmed that p57 may be the direct target of
miR-221-3p.
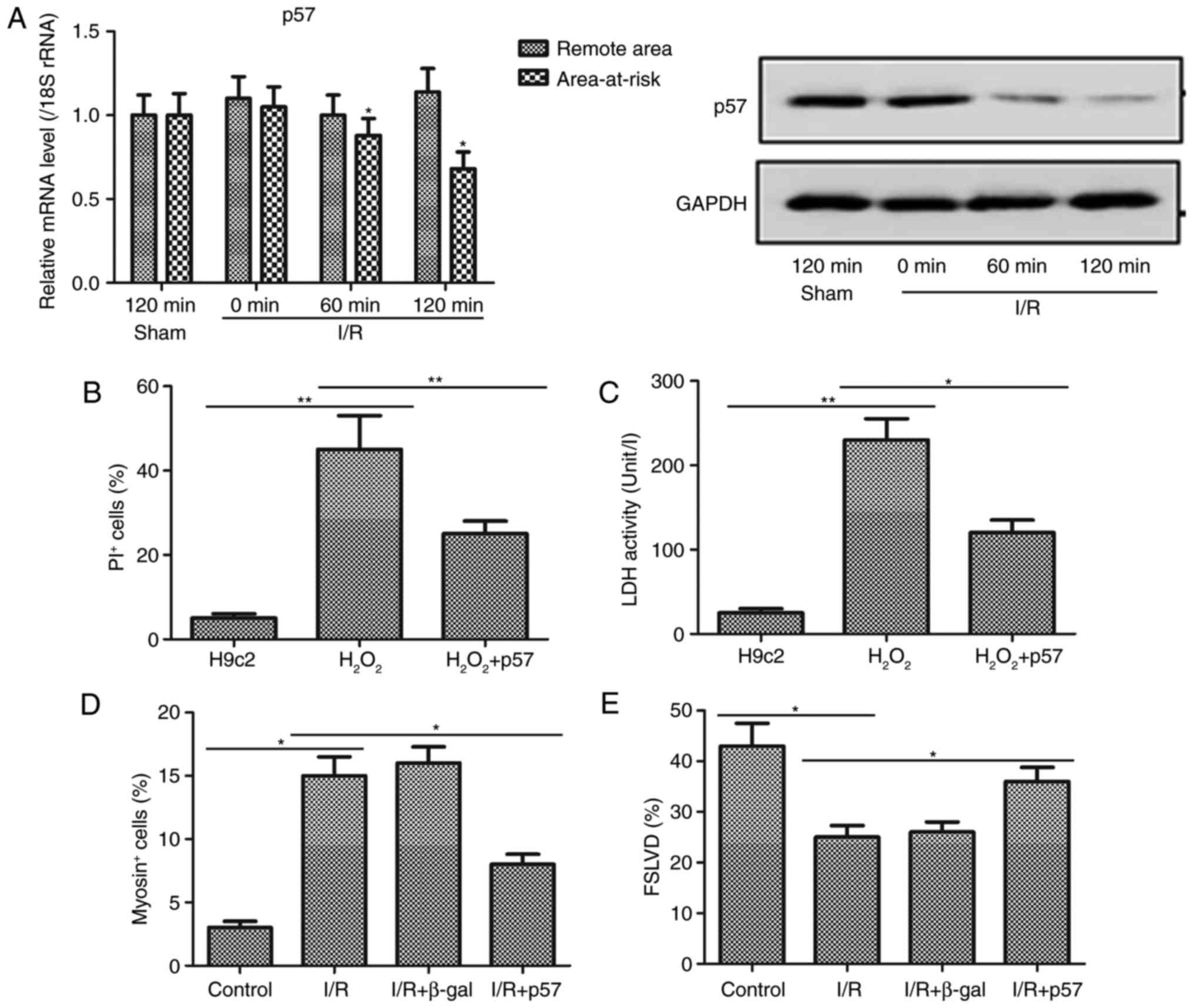
p57 attenuates myocardial injury in H9c2
cells treated with H2O2 and rats following
I/R surgery
Further investigation of the effect of p57 on
myocardial injury was performed in the I/R rats. p57 was
transfected into the H9c2 cells and administered to the rats prior
to H2O2 treatment and I/R surgery,
respectively. It was observed that the gene and protein expression
levels of p57 were downregulated in the area-at-risk of the I/R
rats, compared with levels in the sham group, and no differences in
the expression of p57 were observed in the remote area between the
sham and I/R groups (Fig. 4A).
This result, combined with the results obtained for the expression
of miR-221-3p in I/R rats, further indicated that p57 may be the
direct target of miR-221-3p. In the myocardial damage cell model,
the number of PI+ cells and the activity of LDH were
markedly increased (Fig. 4B and
C). However, it was observed that these changes were less
marked in the H2O2+p57 group than in the
H2O2 group. Furthermore, I/R surgery in the
rats caused marked upregulation and down-regulation in the number
of myosin+ cells and FSLVD%, respectively (Fig. 4D and E). The overexpression of p57
may reverse the injury caused by I/R, as the myosin+cells and FSLVD
values in I/R+p57 group were less markedly different to the control
compared with in the I/R+β-gal and I/R group. Therefore, these data
suggested that p57 may be vital in reducing myocardial injury.
Discussion
Myocardial I/R injury has been widely accepted as
one of the most common and detrimental causes of cardiovascular
diseases (23,24). To date, the treatment approaches
for myocardial I/R injury remain unsatisfactory (5). Therefore, the early and precise
diagnosis of myocardial I/R may prevent the development and
progression of myocardial impairments. Studies have increasingly
revealed the potential roles of miRNAs as diagnostic biomarkers or
therapeutic targets in the treatment of myocardial I/R (11,14). For example, miR-29a and let7
elicited cardioprotective effects by regulating insulin-like growth
factor-1, which inhibits the cell apoptotic signaling pathway
following myocardial I/R, thereby offering theoretical support for
future therapeutic investigations on the modulation of miR-29a and
let7 (25). However, in the
present study, it was found that miR-221-3p was significantly
upregulated in H2O2-treated H9c2 cells and
that it promoted H2O2-induced H9c2 cell
death, including apoptosis and necrosis, in vitro. It is
well known that the apoptosis of myocardiocytes is a critical
pathologic basis of myocardial I/R injury, and that it can lead to
myocardial cell loss, accelerated cardiac dysfunction and even
heart failure (26,27). Several studies have focused on
methods of alleviating the cell death of myocardiocytes to
attenuate myocardial I/R injury. For example, transforming growth
factor β1 (TGFβ1) was shown to protect the myocardium from
apoptosis following myocardial I/R injury; therefore, TGFβ1 may
serve as a novel therapeutic target (28). The data in the present study
showed that miR-221-3p elevated LDH activity in H9c2 cells
incubated with H2O2. LDH is known to be
released from cells when the cell membranes are disrupted (19); therefore, in the present study,
the increased LDH activity of H9c2 cells represented increased
damage to cell membranes. Additionally, in the rat myocardial I/R
injury model, the results indicated that miR-221-3p was markedly
increased in the area-at-risk and that, following the forced
expression of miR-221-3p, the number of myosin+ cells
and FSLVD% were upregulated and downregulated, respectively. These
results indicated that miR-221-3p aggravated the myocardial injury
in vivo. Therefore, inhibiting miR-221-3p may prevent
myocardial I/R injury, which may be a novel therapeutic
strategy.
To further determine the mechanism of miR-221-3p
function, its potential targets were examined using TargetScan
software (http://www.targetscan.org/vert_71/). In the present
study, the data demonstrated that the mRNA and protein levels of
p57 were markedly reduced in the H2O2-treated
H9c2 cells and I/R-treated rats, which exhibited an opposite trend
to that of miR-221-3p. In addition, a significant inverse
correlation between miR-221 and p57 was reported in a previous
study of human hepatocellular carcinoma (29), which offered a reasonable basis to
support the hypothesis that miR-221-3p may directly regulate p57.
Additionally, the dual-luciferase reporter assay confirmed that p57
was the direct target of miR-221-3p in the present study.
Furthermore, according to previous studies, p57 exerts a
cardioprotective role in cardiovascular diseases. For example, the
overexpression of p57 has a protective effect against
atherosclerosis and MI (30), and
the forced cardiac expression of p57 has been shown to attenuate
injury due to I/R in the adult mouse heart (31). Therefore, these previous findings
and the results of the present study showed that the decreased
expression of p57 may serve as a potential risk factor for
myocardial damage in vitro and in vivo. In the
p57-overexpression experiments in vitro and in vivo,
it was observed that the enhanced expression of p57 notably reduced
cell death and LDH activity in the
H2O2-treated H9c2 cells, whereas the
upregulated expression of p57 apparently suppressed the number of
myosin+ cells and improved FSLVD% in the I/R-induced
rats. These findings indicated that treatment with p57 may promote
heart function following myocardial I/R injury.
In conclusion, the data obtained in the present
study provided the first evidence that miR-221-3p contributed to
cardiomyocyte injury in H2O2-treated H9c2
cells and a rat myocardial I/R model by targeting p57. Furthermore,
following elevation of the expression of p57, the myocardial
impairment and function improved in the in vitro and in
vivo experiments. Taken together, these results collectively
indicated that miR-221-3p may serve as a biomarker for the
development of myocardial I/R injury and that p57 may be a
promising novel agent for cardioprotection against myocardial I/R
injury.
Acknowledgments
Not applicable.
References
|
1
|
Dongó E, Hornyák I, Benko Z and Kiss L:
The cardioprotective potential of hydrogen sulfide in myocardial
ischemia/reperfusion injury (Review). Acta Physiol Hung.
98:369–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neri M, Riezzo I, Pascale N, Pomara C and
Turillazzi E: Ischemia/reperfusion injury following acute
myocardial infarction: A critical issue for clinicians and forensic
pathologists. Mediators Inflamm. 2017:70183932017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wernly JA: Ischemia, reperfusion, and the
role of surgery in the treatment of cardiogenic shock secondary to
acute myocardial infarction: An interpretative review. J Surg Res.
117:6–21. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bagheri F, Khori V, Alizadeh AM,
Khalighfard S, Khodayari S and Khodayari H: Reactive oxygen
species-mediated cardiac-reperfusion injury: Mechanisms and
therapies. Life Sci. 165:43–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoffman JW Jr, Gilbert TB, Poston RS and
Silldorff EP: Myocardial reperfusion injury: Etiology, mechanisms,
and therapies. J Extra Corpor Technol. 36:391–411. 2014.
|
|
6
|
Johnson AP, Parlow JL, Whitehead M, Xu J,
Rohland S and Milne B: Body mass index, outcomes, and mortality
following cardiac surgery in Ontario, Canada. J Am Heart Assoc.
4:e0021402015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Braunersreuther V and Jaquet V: Reactive
oxygen species in myocardial reperfusion injury: From
physiopathology to therapeutic approaches. Curr Pharm Biotechnol.
13:97–114. 2012. View Article : Google Scholar
|
|
8
|
Davis-Dusenbery BN and Hata A: Mechanisms
of control of microRNA biogenesis. J Biochem. 148:381–392.
2010.PubMed/NCBI
|
|
9
|
Liu X, Fortin K and Mourelatos Z:
MicroRNAs: Biogenesis and molecular functions. Brain Pathol.
18:113–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Devaux Y, Creemers EE, Boon RA, Werfel S,
Thum T, Engelhardt S, Dimmeler S and Squire I: Circular RNAs in
heart failure. Eur J Heart Fail. 27:2017.
|
|
11
|
Pan ZW, Lu YJ and Yang BF: MicroRNAs: A
novel class of potential therapeutic targets for cardiovascular
diseases. Acta Pharmacol Sin. 31:1–9. 2010. View Article : Google Scholar
|
|
12
|
Frost RJ and van Rooij E: MiRNAs as
therapeutic targets in ischemic heart disease. J Cardiovasc Transl
Res. 3:280–289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang J, Chen L, Yang J, Ding J, Li S, Wu
H, Zhang J, Fan Z, Dong W and Li X: MicroRNA-22 targeting CBP
protects against myocardial ischemia-reperfusion injury through
anti-apoptosis in mice. Mol Biol Rep. 41:555–561. 2014. View Article : Google Scholar
|
|
14
|
Lima J Jr, Batty JA, Sinclair H and
Kunadian V: MicroRNAs in ischemic heart disease: From
pathophysiology to potential clinical applications. Cardiol Rev.
25:117–25. 2017. View Article : Google Scholar
|
|
15
|
Kukreja RC, Yin C and Salloum FN:
MicroRNAs: New players in cardiac injury and protection. Mol
Pharmacol. 80:558–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu S and Li G: MicroRNA expression and
function in cardiac ischemic injury. J Cardiovasc Transl Res.
3:241–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coskunpinar E, Cakmak HA, Kalkan AK,
Tiryakioglu NO, Erturk M and Ongen Z: Circulating miR-221-3p as a
novel marker for early prediction of acute myocardial infarction.
Gene. 591:90–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He XM, Zheng YQ, Liu SZ, Liu Y, He YZ and
Zhou XY: Altered plasma microRNAS as novel biomarkers for
arteriosclerosis obliterans. J Atheroscler Thromb. 23:196–206.
2016. View Article : Google Scholar
|
|
19
|
Timotin A, Pisarenko O, Sidorova M,
Studneva I, Shulzhenko V, Palkeeva M, Serebryakova L, Molokoedov A,
Veselova O, Cinato M, et al: Myocardial protection from
ischemia/reperfusion injury by exogenous galanin fragment.
Oncotarget. 8:21241–2152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
21
|
Wang K, Long B, Li N, Li L, Liu CY, Dong
YH, Gao JN, Zhou LY, Wang CQ and Li PF: MicroRNA-2861 regulates
programmed necrosis in cardiomyocyte by impairing adenine
nucleotide translocase 1 expression. Free Radic Biol Med. 91:58–67.
2016. View Article : Google Scholar
|
|
22
|
Burghardt TP, Sun X, Wang Y and Ajtai K:
Auxotonic to isometric contraction transitioning in a beating heart
causes myosin step-size to down shift. PLoS One. 12:e01746902017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tao L, Jia L, Li Y, Song C and Chen Z:
Recent advances of adapter proteins in the regulation of heart
diseases. Heart Fail Rev. 22:99–107. 2017. View Article : Google Scholar
|
|
24
|
Yang Q, He GW, Underwood MJ and Yu CM:
Cellular and molecular mechanisms of endothelial
ischemia/reperfusion injury: Perspectives and implications for
postischemic myocardial protection. Am J Transl Res. 8:765–777.
2016.PubMed/NCBI
|
|
25
|
Wang L, Niu X, Hu J, Xing H, Sun M, Wang
J, Jian Q and Yang H: After myocardial ischemia-reperfusion,
miR-29a, and Let7 could affect apoptosis through regulating IGF-1.
Biomed Res Int. 2015:2454122015. View Article : Google Scholar
|
|
26
|
Zhao Y, Ponnusamy M, Dong Y, Zhang L, Wang
K and Li P: Effects of miRNAs on myocardial apoptosis by modulating
mitochondria related proteins. Clin Exp Pharmacol Physiol.
44:431–440. 2017. View Article : Google Scholar
|
|
27
|
Makhdoumi P, Roohbakhsh A and Karimi G:
MicroRNAs regulate mitochondrial apoptotic pathway in myocardial
ischemia-reperfusion-injury. Biomed Pharmacother. 84:1635–1644.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu YF, Chu YY, Zhang XZ, Zhang M, Xie FG,
Zhou M, Wen HH and Shen HH: TGFβ1 protects myocardium from
apoptosis and oxidative damage after ischemia reperfusion. Eur Rev
Med Pharmacol Sci. 21:1551–1558. 2017.PubMed/NCBI
|
|
29
|
Fornari F, Gramantieri L, Ferracin M,
Veronese A, Sabbioni S, Calin GA, Grazi GL, Giovannini C, Croce CM,
Bolondi L and Negrini M: MiR-221 controls CDKN1C/p57 and CDKN1B/p27
expression in human hepatocellular carcinoma. Oncogene.
27:5651–5661. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rodríguez I, Coto E, Reguero JR, González
P, Andrés V, Lozano I, Martín M, Alvarez V and Morís C: Role of the
CDKN1A/p21, CDKN1C/p57, and CDKN2A/p16 genes in the risk of
atherosclerosis and myocardial infarction. Cell Cycle. 6:620–625.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haley SA, Zhao T, Zou L, Klysik JE,
Padbury JF and Kochilas LK: Forced expression of the cell cycle
inhibitor p57Kip2 in cardiomyocytes attenuates
ischemia-reperfusion injury in the mouse heart. BMC Physiol.
8:42008. View Article : Google Scholar
|