Introduction
The International Association for the Study of Pain
defines neuropathic pain (NPP) as pain that is a direct outcome of
trauma or diseases affecting the somatosensory nervous system
(1). The clinical manifestations
of NPP generally include spontaneous pain, hyperalgesia and
paresthesia. NPP has become a public health problem, which affects
a wide population globally (2).
Extensive research has been performed to elucidate the
neurobiological mechanisms of NPP, which is clinically managed by
drugs, including opioids and calcium channel blockers. However,
these therapies can be ineffective or can result in severe side
effects (3,4).
Evidence indicates that pain-related genes in
sensory neurons exert a pivotal effect in the development and
maintenance of NPP (5–7). Epigenetic mechanisms affect gene
expression without changing the original DNA sequence. Studies have
shown that epigenetic mechanisms are associated with synaptic
plasticity, learning and memory (8). DNA methylation, histone
modifications, including phosphorylation, acetylation,
ubiquitination and methylation, and microRNA expression are
involved in these epigenetic mechanisms. Histone modifications are
closely linked with cell development, aging, and several other
physiological and pathological processes. It has been shown that
histone methylation affects synaptic plasticity within the nervous
system, thus providing the basis for hyperalgesia (9).
Jumonji C domain (JMJD)-containing proteins form a
large family of histone demethylases. The JMJD family member JMJD6
can catalyze the demethylation of arginine 2 on histone H3 and
arginine 3 on histone H4 (10).
Co-bound JMJD6 and bromodomain-containing protein 4 anti-pause
enhancers may adjust the promoter proximal pause release of a
series of transcription units through long-range interactions
(11). In addition, JMJD6 can
regulate estrogen receptor-α methylation, thereby regulating
non-genomic estrogen signaling (12). As an epigenetic regulator, JMJD6
possesses a number of novel and unexpected nuclear functions, which
remain to be fully elucidated (11).
Nuclear factor-κB (NF-κB), a ubiquitous nuclear
transcription factor, is reported to regulate numerous genes
important for inflammation, central nervous system injury and NPP
(13–15). Peripheral nerve injury can
activate NF-κB, thus upregulating the expression of proinflammatory
cytokines, adhesion molecules and chemokines in the spinal cord,
subsequently leading to NPP. Evidence indicates that arginine
residues within NF-κB can be reversibly methylated by
histone-modifying enzymes, including arginine methyl transferase
and demethylase (16).
Furthermore, several downstream genes can be activated by NF-κB
methylation (16). It was found
that the p65 subunit can be methylated at arginine 30 by protein
arginine methyltransferase 5 in response to interleukin-1β (IL-1β),
which decreases the ability of NF-κB to interact with the inhibited
κB elements and enhances gene expression (17). A number of studies have indicated
that NF-κB can regulate the expression of tumor necrosis factor-α
(TNF-α), IL-1β and vascular endothelial growth factor (VEGF); these
mediators are also implicated in NPP (18,19).
In the present study, it was hypothesized that JMJD6
may exert its epigenetic effects in NPP in chronic constriction
injury (CCI) rats by regulating pain-associated mediators involved
in the NF-κB signaling pathway. The study evaluated the effects of
the intrathecal administration of a JMJD6-overexpressing lentiviral
vector (LV-JMJD6) on NPP through the mechanical withdrawal
threshold (MWT), performed by stimulating the plantar surface of
the left hind paw, and thermal withdrawal latency (TWL), performed
by stimulating the same position. The effects on the expression of
NF-κB and pain-associated factors, including TNF-α, IL-1β and VEGF,
were also examined in the CCI rats.
Materials and methods
Animals
Male Sprague-Dawley rats (220–250 g) were obtained
from Central South University (Changsha, China) and housed in clean
cages. The rats were allowed to adapt for 3 days and housed in
controlled conditions (22±0.5°C, 12:12 h dark/light cycle). All
rats were provided with free access to food and water. All
experiments were performed according to guidelines and protocols
approved by the Animal Care Committee of Central South University,
and were in accordance with the guidelines provided by the National
Institute of Health. During surgical procedures, humane care was
taken into consideration and animal suffering was minimized as much
as possible.
CCI
The rats were anesthetized by inhalation of
isoflurane (1–3%) prior to the initiation of surgical procedures.
The CCI model was implemented using the surgical procedures of
Bennet and Xie (20). The left
sciatic nerve was exposed using a blunt dissection technique. In
the CCI group, four snug ligatures (4-0 chromic gut) were placed
around the left sciatic nerve at an interval of 1.0 mm between
ligatures under a microscope. Care was taken to tie the ligatures,
so that the diameter of the sciatic nerve was gently constricted.
The desired degree of constriction did not arrest the circulation
thorough the superficial epineurial vasculature, and produced a
small and brief twitch in the muscle surrounding the exposure. For
the sham group, the sciatic nerve was exposed without ligation. To
avoid variation, all the sham and nerve ligations were performed by
the same individual, who was skilled in surgery. The incision was
closed in layers. All rats were housed in cages with 3–5 cm of soft
bedding following complete recovery from anesthesia.
Intrathecal catheter implantation
Under deep anesthesia, the rats were housed in a
prone position. A longitudinal skin incision was made at the L4–5
intervertebral space in the midline of the back. Subsequently, the
surrounding tissues were bluntly dissected and the intervertebral
space was exposed. A lumbar intrathecal catheter (PE-10; Smiths
Medical, Ashford, UK) was inserted into the subarachnoid space, as
previously reported (21).
Insertion of the catheter into the subarachnoid space was verified
by the appearance of side tail swing or hind leg twitch. The
success of catheter insertion was confirmed by definite outflow of
cerebrospinal fluid.
Construction of lentiviral vectors
Based on sense and anti-sense sequences, a BLAST
homology search was applied to guarantee that a single mRNA
sequence of JMJD6 (NM_001012143) was targeted. To obtain the target
gene fragment, polymerase chain reaction (PCR) primers were
designed as follows: JMJD6 (1,253 bp), sense
5′-GAGGATCCCCGGGTACCGGTCGCCACCATGAACCACAAGAGCAAGAAGC-3′ and
antisense 5′-TCCTTGTAGTCCATACCCCTGGAGGAACTGCGCTCTTTG-3′. PrimeSTAR
HS DNA polymerase (cat. no. R010B, Takara Biotechnology Co., Ltd.,
Dalian, China) and primers (GeneRay Biotech Co., Ltd., Shanghai,
China) were used for PCR amplification of JMJD6 cDNA. The PCR
thermocycling conditions were as follows: Initial denaturation at
98°C for 5 min; followed by 30 cycles at 98°C for 10 sec, 55°C for
10 sec, 72°C for 90 sec, 72°C for 8 min and then maintained at 4°C.
The GV358, pHelper 1.0 and pHelper 2.0 vectors (Shanghai GeneChem
Co., Ltd., Shanghai, China) were used as the lentivirus vectors.
Plasmid DNA was extracted using the Plasmid DNA Extract kit
(Qiagen, Inc., Valencia, CA, USA). Oligonucleotides were introduced
into the plasmid GV358-JMJD6-green fluorescent protein (GFP), and
the packaged plasmids were then cloned into the GV358-GFP
lentiviral vector (Shanghai GeneChem Co., Ltd.). The recombinant
vector and packaged plasmids were cotransduced into 293T cells
(American Type Culture Collection, Manassas, VA, USA). The final
titer of recombinant virus was 1×108 TU/ml.
Treatments
The rats were randomly divided into four groups:
Sham + normal saline (NS; 0.9% saline), CCI + NS, CCI + negative
control lentiviral vector (NC) and CCI + LV-JMJD6. The treatment
reagents (20 μl) containing lentiviral titers of
1×108 TU/ml were administered via the lumbar intrathecal
catheter on day 3 post-CCI. Following the administration of each
reagent, 15 μl of 0.9% saline was used to flush the catheter
to avoid reagent residue. All experiments complied with the
double-blind principle.
Assessment of pain behavior following
CCI
The rats were inspected carefully for changes,
including gait, posture of the affected hind paw, exercise ability
and whether autophagy was present (20). Behavioral assessments were used to
validate the success of CCI surgery. Only rats showing thermal
hyperalgesia and mechanical allodynia were used in the following
analysis. A 2390 Electronic von Frey anesthesiometer (IITC Life
Science, Woodland Hills, CA, USA) was used to measure mechanical
allodynia in all rats (22).
Following placement of each rat into the plexiglas chamber, the MWT
test was performed by stimulating the plantar surface of the left
hind paw. Each Von Frey filament was held for ~5 sec. A positive
response was a rapid withdrawal of the hind paw during stimulation.
When a positive response occurred, a filament with a lower force
was used. If a negative response occurred, a filament with a higher
force was used. A Hargreaves Tes7370 device (UgoBasile S.R.L,
Comerio, Italy) was also applied to measure thermal hyperalgesia.
The rats were placed on the surface of a glass plate, above which
was a plexiglass chamber. Heat stimulation was positioned at the
exposure site on the left hind paw. Each rat was assessed three
times at intervals of 5 min. The MWT and TWL of each rat were
assessed 1 day prior to CCI, and on days 3, 7, 10 and 14 following
CCI surgery. To avoid variance, all behavioral assessments were
performed by the same observer.
Total RNA isolation and reverse
transcription-quantitative (RT-q)PCR analysis
On day 14 post-CCI, the rats were sacrificed and the
lumbar spinal cord was removed. TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used to extract
total RNA. The OD260 of RNA was verified to calculate
quantities of RNA using a spectrophotometer (Nanodrop 2000; Thermo
Fisher Scientific, Inc.). Total RNA was reverse transcribed into
cDNA using a reverse transcriptase kit (Takara Biotechnology Co.,
Ltd.) following the manufacturer’s protocol and Oligo dT was used
for the reverse transcription. The RT-qPCR conditions were as
follows: 95°C for 5 min, followed by 40 cycles of 94°C for 20 sec,
56°C for 30 sec, and 72°C for 20 sec. All samples were run in
duplicate. RT-qPCR was performed using a Viia™ 7 Real-Time PCR
System (Thermo Fisher Scientific, Inc.) and Quantifast SYBR Green
PCR 2x Master Mix (Qiagen GmbH, Hilden, Germany). The following
primers were used for amplification: IL-1β,
5′-AGTCTGCACAGTTCCCCAAC-3′ (forward) and 5′-TTAGGAAGACACGGGTTCCA-3′
(reverse); β-actin, 5′-TCGTGCGTGACATTAAAGAG-3′ (forward) and
5′-ATTGCCGATAGTGATGACCT-3′ (reverse); TNF-α,
5′-AGAGCCCCCAATCTGTGTC-3′ (forward) and 5′-TTCAGCGTCTCGTGTGTTTC-3′
(reverse); VEGF, 5′-CACTGAGGAGTCCAACATCACC-3′ (forward) and
5′-CATCTCTCCTATGTGCTGGCCT-3′ (reverse). β-actin was used as a
control during PCR. SYBR Green assays were applied to measure mRNA
expression, and relative expression levels of target mRNA were
calculated using the 2−ΔΔCq method (23).
Western blot analysis
The L4–6 spinal cord segments were prepared for
extraction of total protein. Tissues were homogenized in
radioimmunoprecipitation assay lysis buffer (cat. no. P0013C;
Beyotime Institute of Biotechnology, Haimen, China) containing 1 mM
phenylmethylsulfonyl fluoride (cat. no. ST506; Beyotime Institute
of Biotechnology) and following centrifugation at 20,000 × g for 15
min at 4°C, the supernatant was collected. The protein
concentration was then determined using a BCA protein Assay Kit
(Pierce; Thermo Fisher Scientific, Inc.). A 10% SDS-PAGE gel was
used to separate the protein samples (50 μg of each sample),
which were then transferred onto PVDF membranes. Subsequently, 5%
non-fat dry milk blended with phosphate-buffered saline (PBS) and
0.05% Tween 20 was applied to block membranes for 1 h, followed by
incubation with mouse anti-JMJD6 (cat. no. SC-28348; 1:500; Santa
Cruz Biotechnology Inc., Dallas, TX, USA), rabbit anti-NF-κB p-p65
(cat. no. ab86299; 1:2,000; Abcam, Cambridge, UK), rabbit
anti-NF-κB-p65 (cat. no. 10745-1-AP; 1:1,500; ProteinTech Group,
Inc., Chicago, IL, USA), rabbit anti-TNF-α (cat. no. AB1837P;
1:3,000; Merck Millipore, Darmstadt, Germany), rabbit anti-IL-1β
(cat. no. AB1832P; 1:7,000; Merck Millipore), rabbit anti-VEGF
(cat. no. 5365-100; 1:2,000, BioVision, Inc., Milpitas, CA, USA) or
mouse anti-β-actin (cat. no. ab8226; 1:1,000; Abcam) primary
antibodies at 4°C overnight. The membranes were then incubated with
goat anti-rabbit horseradish peroxidase (HRP)-IgG (cat. no. ab6721;
1:2,000; Abcam) or goat anti-mouse HRP-conjugated IgG (cat. no.
ab205719; 1:2,000; Abcam) secondary antibodies for 1 h at room
temperature. An ECL kit (Pierce; Thermo Fisher Scientific, Inc.)
was used to visualize protein bands. Densitometric analysis was
performed using Quantity One software version 4.1 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Protein levels were
standardized to β-actin.
Double immunofluorescent labeling
Paraformaldehyde (4%) was used to post-fix the
spinal cord tissues of the CCI + LV-JMJD6 rats for 8 h, following
which the samples were embedded in paraffin and sections were cut
at a thickness of 5 μm. To retrieve antigens and remove
peroxidase activity, the deparaffinized sections were treated with
0.01 M citrate buffer at 96°C for 1 h, followed by 3%
H2O2 for 25 min. The sections were then
blocked with 10% donkey serum (cat. no. 017-000-121; Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 30 min
and incubated with mouse monoclonal anti-JMJD6 antibody (1:50;
Santa Cruz Biotechnology, Inc.) overnight at 4°C, followed by
incubation with red dihydroxyfluorene [Alexa Fluor 594-conjugated
goat anti-mouse lgG (H+L); cat. no. SA00006-3; ProteinTech Group,
Inc.] for 2 h. The sections were then incubated with rabbit
polyclonal anti-p65 antibody (1:100; ProteinTech Group, Inc.)
overnight at 4°C, and then with green dihydroxyfluorene [Alexa
Fluor 488-conjugated Affinipure Goat anti-rabbit IgG (H+L); cat.
no. SA00006-2; ProteinTech Group, Inc.] for 2 h. Images of the
double-labeled sections were captured with a Nikon microscope
(Eclipse Ti-SR; Nikon, Tokyo, Japan).
Co-immunoprecipitation analysis
Spinal cord segments were prepared for the
extraction of total protein. The samples were collected and
solubilized in immunoprecipitation lysis buffer containing 25 mM
Tris, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1% SDS, 0.5% Triton-X and
5% glycerol (pH 7.4). The homogenates were incubated on ice for 15
min followed by centrifugation to remove insoluble material (12,000
g, 4°C, 15 min). The lysates were incubated with a primary antibody
(2 μg IgG), vs. normal IgG (2 μg, as a control) of
the corresponding animal species overnight at 4°C. Subsequently, 20
μl of Protein A/G agarose beads (Santa Cruz Biotechnology,
Inc.) was added to the antigen-antibody complex suspension and
incubated for an additional 2 h at 4°C on a rocking platform. The
immunocomplexes were then obtained and separated from other unbound
proteins (3,000 g, 4°C, 3 min), and washed three times with IP
lysis buffer. 12% SDS-PAGE and PVDF membranes were used. The
membranes were blocked with 5% non-fat dry milk in PBS with 0.05%
Tween 20 for 1 h. Primary antibody incubation with anti-JMJD6 (cat.
no. SC-28348; 1:200; Santa Cruz Biotechnology) and anti-p65 (cat.
no. 10745-1-AP; 1:500; ProteinTech Group, Inc.) was performed at
room temperature on a shaker for 1 h and then at 4°C overnight,
followed by incubation with horseradish peroxidase-conjugated goat
anti-rabbit or goat anti-mouse secondary antibody (1:3,000; Santa
Cruz Biotechnology, Inc.) for 1 h at room temperature. Protein
brands were visualized by chemiluminescence using an ECL kit and
quantified using Quantity One software.
Statistical analysis
Data are shown as the mean ± standard error of the
mean. For assessing mechanical and thermal hyperalgesia, two-way
analysis of variance was used to compare the thresholds. For
RT-qPCR and western blot analyses, one-way analysis of variance
followed by the Student-Newman-Keuls post hoc test was applied
using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
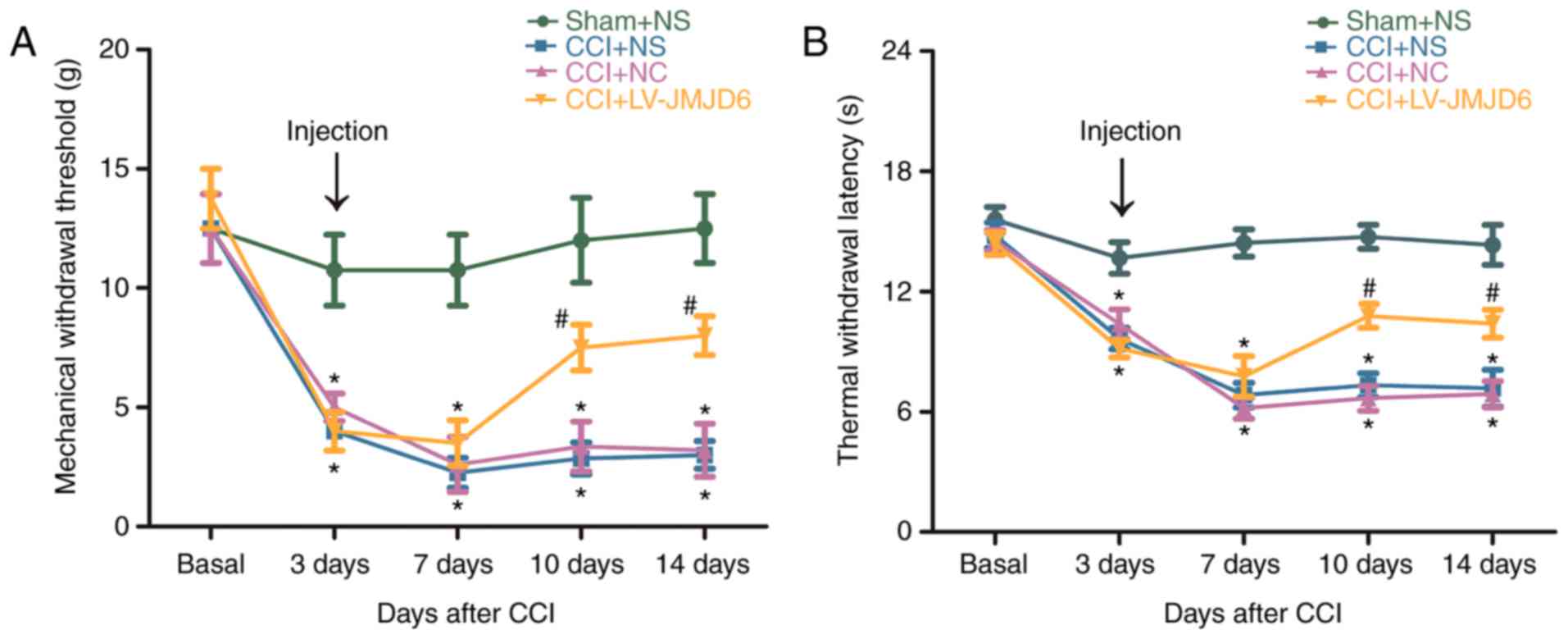
JMJD6 alleviates CCI-induced behavioral
changes
Following CCI surgery, the rats gradually showed
typical signs of hyperalgesia and allodynia, including foot
eversion, paw-licking and toe closing. The behaviors of the
sham-operated rats were not altered markedly. CCI-induced
mechanical allodynia and thermal hyperalgesia in the four groups
are shown in Fig. 1A and B. For
MWT and TWL prior to CCI, no significant difference was found
between any of the groups (P>0.05). Reductions in MWT and TWL in
the CCI rats were detectable at ~3 days post-CCI (P<0.05),
compared with the sham + NS-treated rats, indicating that the NPP
model was successfully established. In the CCI + NS and CCI + NC
rats, the MWT and TWL were reduced further on day 7 and continued
to decrease until day 14 post-CCI, compared with those in the sham
+ NS rats (P<0.05). In the CCI + LV-JMJD6 group, MWT and TWL
gradually recovered between day 10 and day 14, compared with that
in the CCI + NS and CCI + NC groups (P<0.05). JMJD6 alleviated
CCI-induced behavioral changes, suggesting a critical functional
connection between JMJD6 and NPP (Fig. 1).
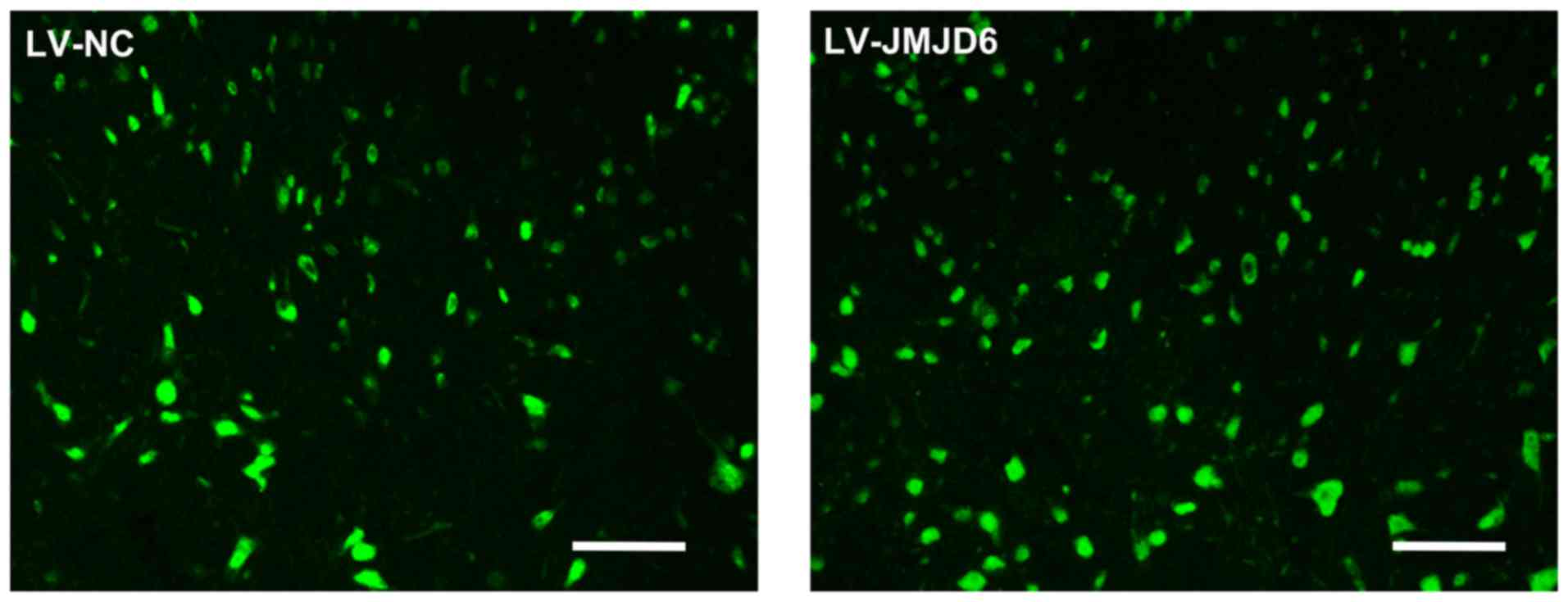
Effectiveness of lentiviral transfection
in spinal dorsal horn cells
The effectiveness of lentiviral transfection on
spinal cord tissue was detected on day 14 post-CCI. Lentiviral
vectors, which carried a plasmid expressing GFP, were delivered
into the subarachnoid space to transfect cells of the spinal cord
(Fig. 2). GFP-positive cells were
observed in the rats treated with LV-NC and LV-JMJD6, combined with
the protein expression of JMJD6 (Fig.
3A and B), which indicated the success of lentiviral
transfection.
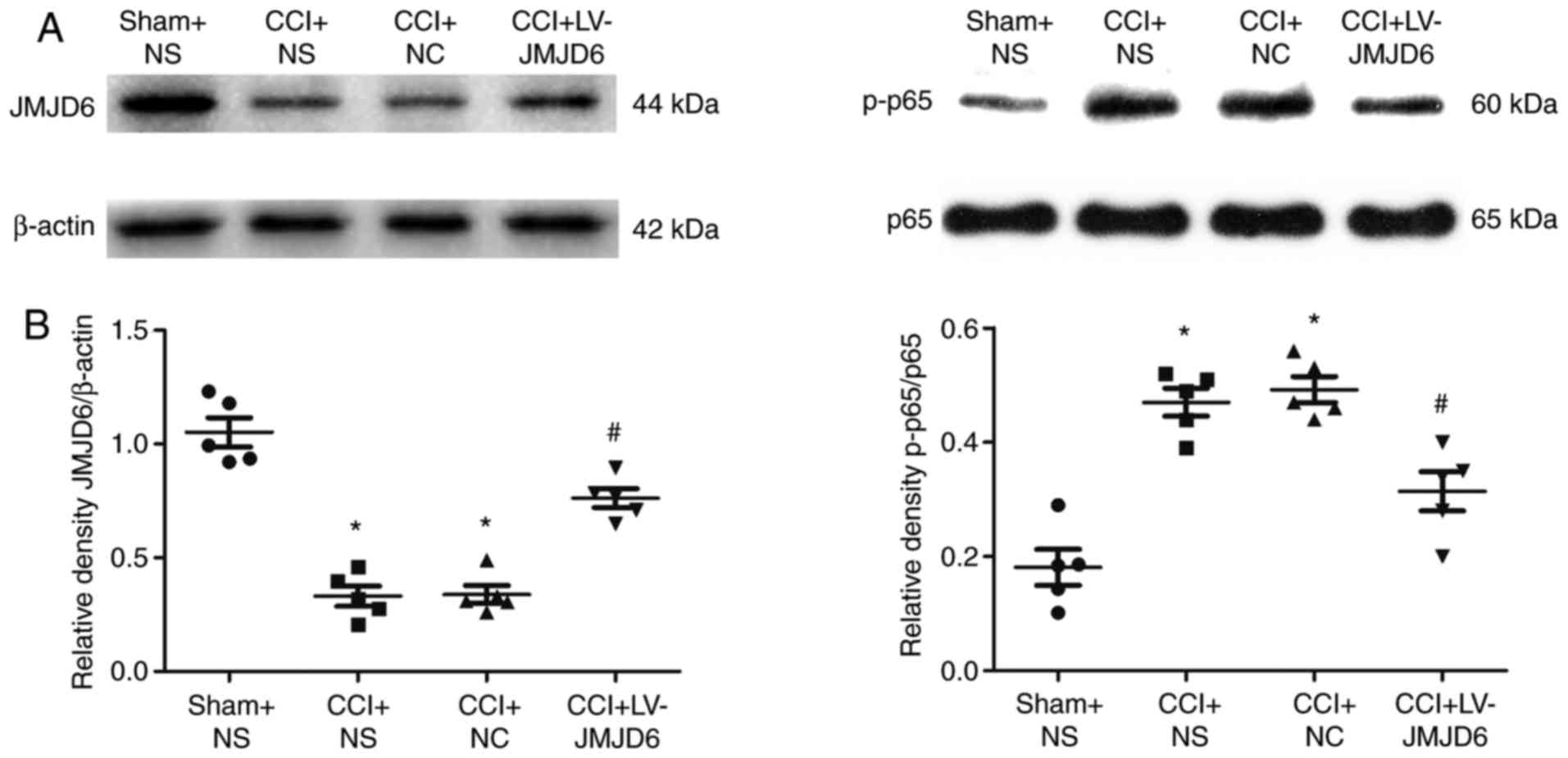
Lentiviral vector-mediated JMJD6
increases expression of JMJD6 in CCI rats
The expression of JMJD6 was confirmed by western
blot analysis (Fig. 3A and B).
Compared with the sham + NS group, the expression of JMJD6 in CCI
rats was significantly lower (P<0.05). Following CCI, the rats
were injected intrathecally with LV-JMJD6, the exogenous expression
of JMJD6 caused by the overexpressing lentiviral vector elevated
the level of JMJD6 and normalized the lower level of JMJD6 induced
by CCI surgery. The expression of JMJD6 was upregulated in the CCI
+ LV-JMJD6 group, compared with that in its negative control group
(CCI + NC group; P<0.05) and solvent control group (CCI + NS
group; P<0.05). These results also indicated that the lentiviral
vectors had transduced into the spinal cord successfully and the
exogenous expression of JMJD6 was attained. No difference in the
expression level of JMJD6 was observed between the CCI + NS and CCI
+ NC groups (P>0.05).
JMJD6 attenuates CCI-induced expression
of the NF-κB p-p65 subunit
The effects of JMJD6 on the expression of the NF-κB
p-p65 subunit were verified by western blot analysis. Compared with
the sham + NS group, the expression of the p-p65 subunit in the CCI
rats was increased significantly (P<0.05; Fig. 3A and B). However, intrathecal
injection with LV-JMJD6 markedly attenuated the expression of the
p-p65 subunit (P<0.05).
JMJD6 suppresses CCI-induced
proinflammatory cytokine activation in spinal segments
RT-qPCR and western blot analyses were used to
examine the mRNA and protein levels of proinflammatory cytokines,
respectively. Compared with the sham + NS group, the results
indicated that the expression levels of TNF-α and IL-1β were
markedly increased in the CCI + NS and CCI + NC groups (P<0.05;
Fig. 4). In the RT-qPCR (Fig. 4A) and western blot (Fig. 4B and C) analyses, JMJD6 attenuated
the CCI-induced changes in proinflammatory cytokine expression, as
demonstrated by reduced mRNA and protein levels of TNF-α and IL-1β
(P<0.05).
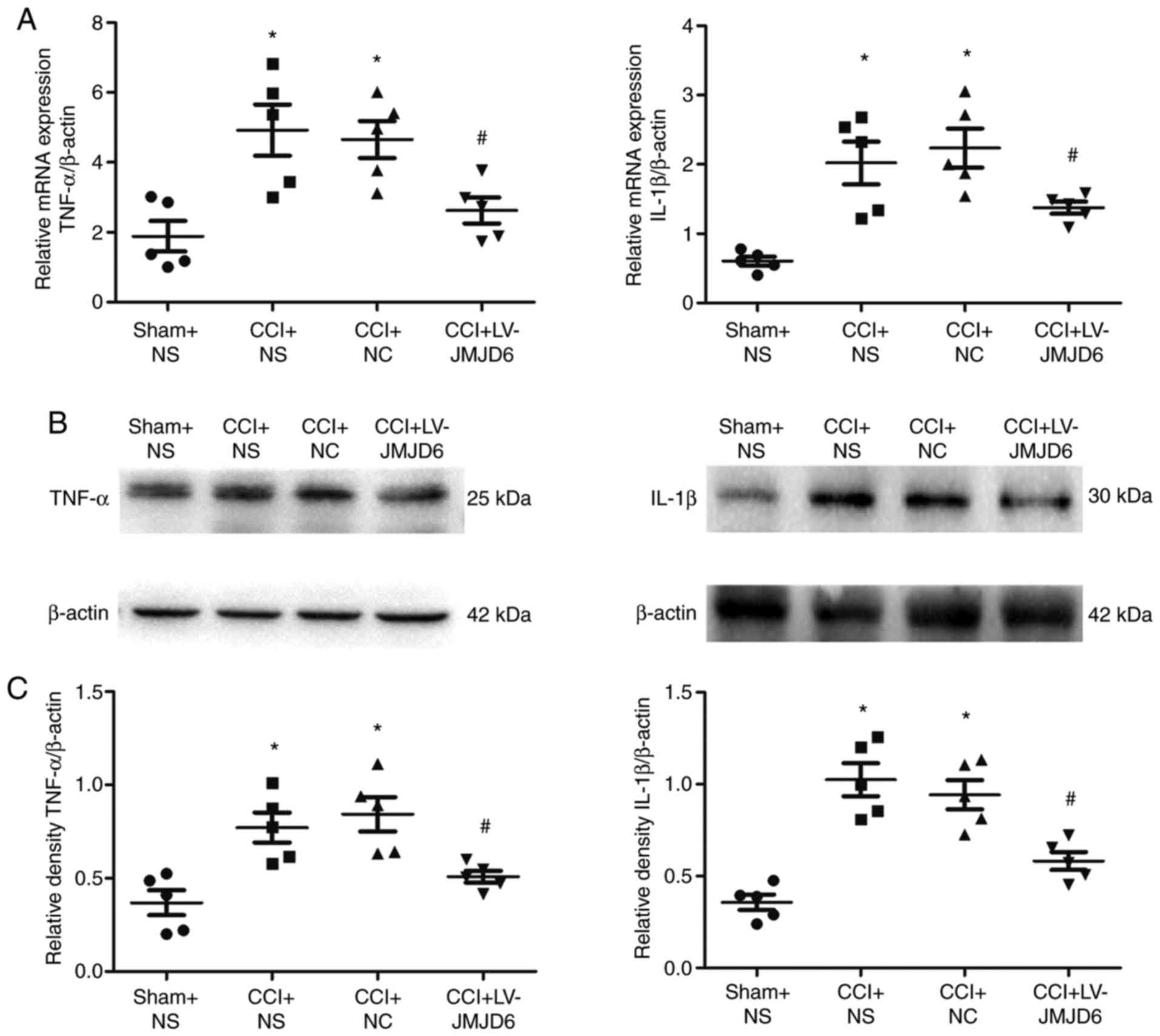 | Figure 4Effects of intrathecal injection with
LV-JMJD6 on chronic constriction injury (CCI)-induced
proinflammatory cytokine mRNA and protein expression. Animals were
treated with a JMJD6-overexpressing lentiviral vector (LV-JMJD6) 3
days following induction of NPP via CCI. Control animals received
sham surgery, NS, or NC. Spinal cord L4–6 samples were prepared for
reverse transcription-quantitative polymerase chain reaction or
western blot analyses 14 days following surgery. (A) Relative
quantification of mRNA levels of TNF-α and IL-1β (mean ± standard
error of the mean; n=5). β-actin was used as a control. (B)
Representative bands showing protein expression levels of TNF-α and
IL-1β. β-actin was used as a control. (C) Relative quantification
of protein levels of TNF-α/β-actin and IL-1β/β-actin (mean ±
standard error of the mean; n=5). One-way analysis of variance was
used as the statistical tests. *P<0.05, vs. sham + NS
group; #P<0.05, vs. CCI + NS and CCI + NC groups.
CCI, chronic constriction injury; NPP, neuropathic pain; NS, normal
saline; NC, negative control lentivirus; TNF-α, tumor necrosis
factor-α; IL-1β, interleukin-1β. |
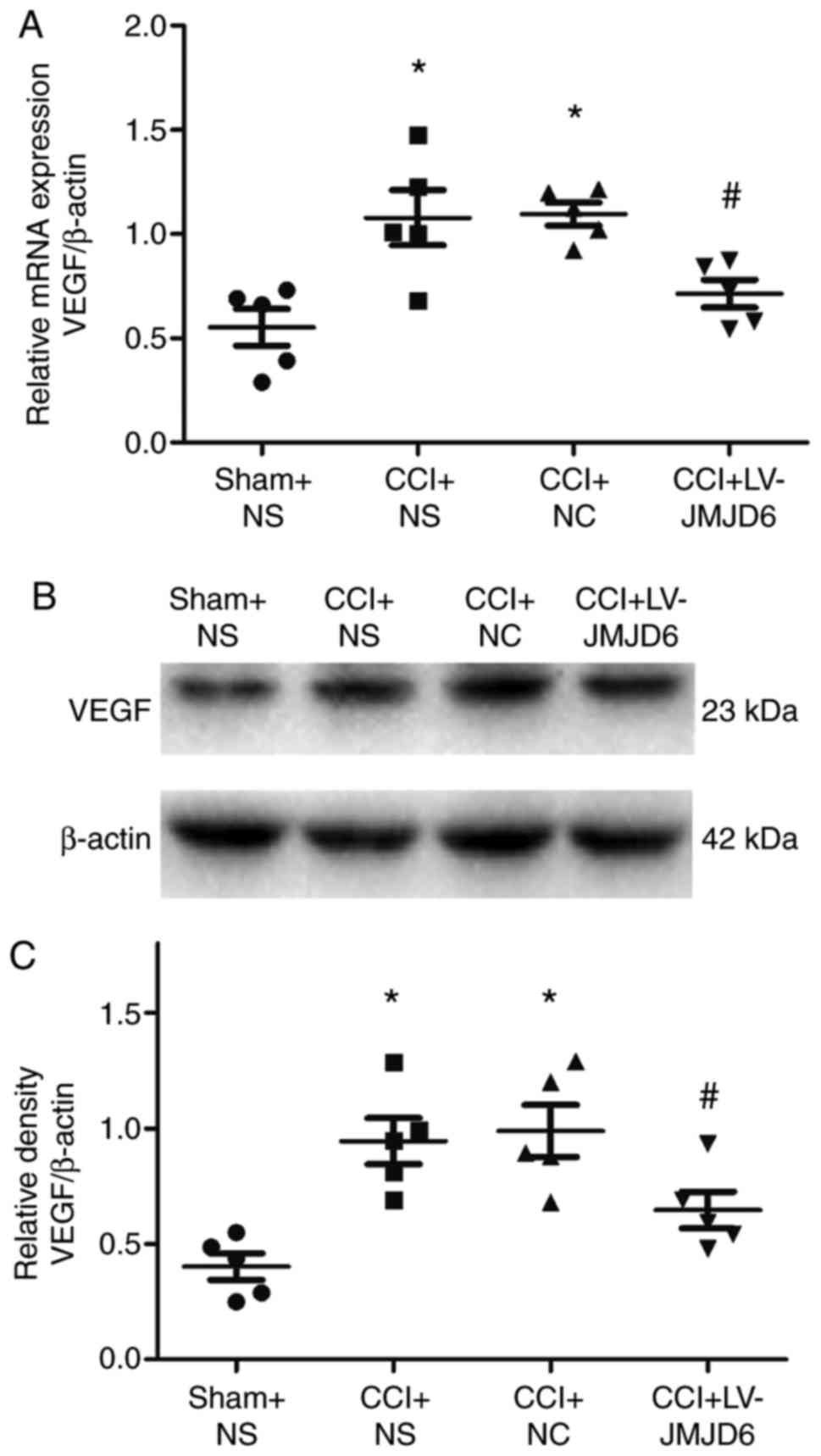
LV-JMJD6 injection decreases CCI-induced
expression of VEGF
The mRNA and protein expression levels of VEGF were
verified by RT-qPCR and western blot analyses, respectively
(Fig. 5A–C). Compared with the
sham + NS-treated group, the CCI rats exhibited significantly
higher expression levels of VEGF (P<0.05). However, the
expression of VEGF was lower following intrathecal injection of
LV-JMJD6 (P<0.05).
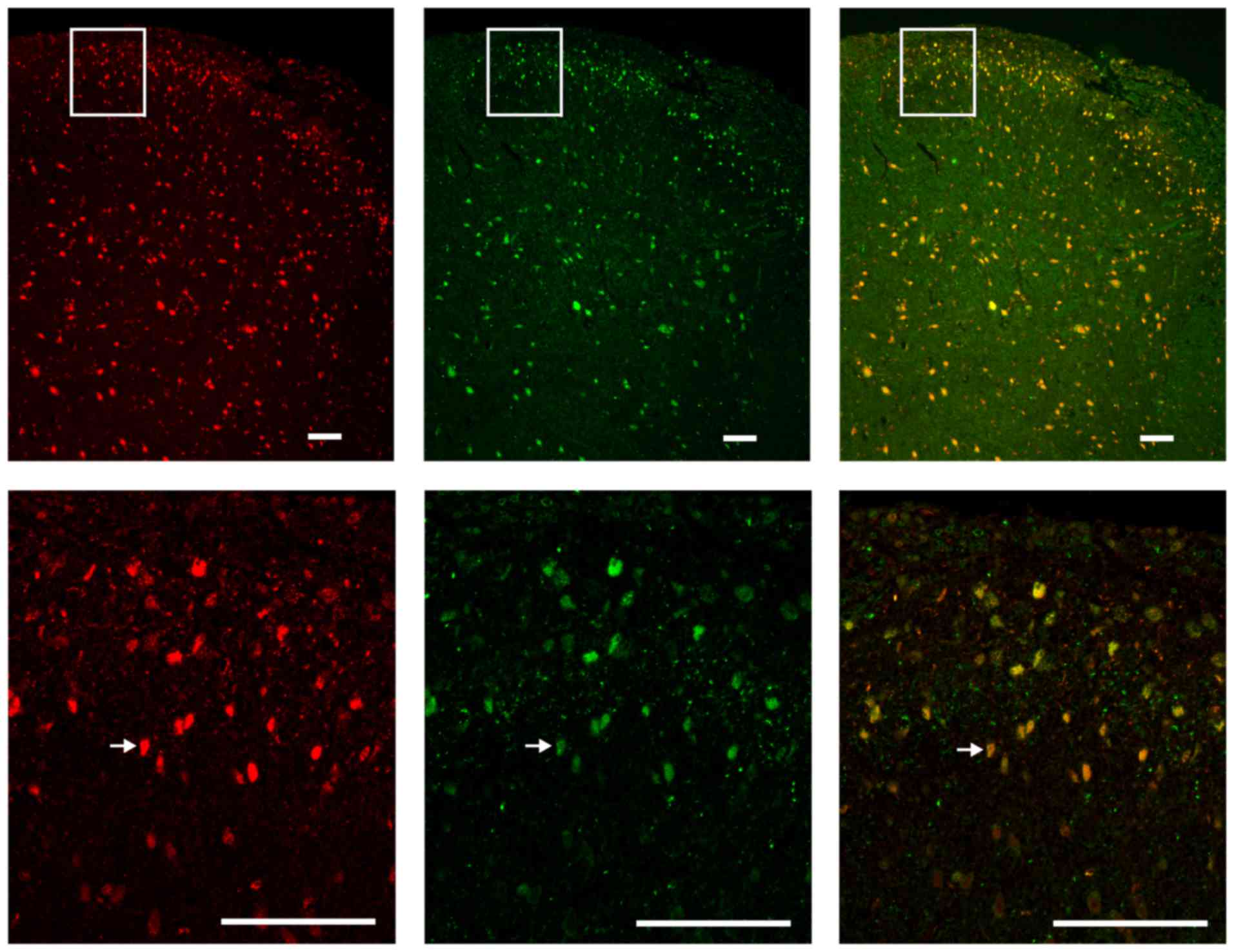
Colocalization of JMJD6 and NF-κB p65 in
the spinal cord
To assess the colocalization of JMJD6 and NF-κB p65
in the spinal cord of the CCI rats, double-labeling
immunofluorescence was performed. Red fluorescence was used to
label JMJD6-immunoreactive cells, green fluorescence was used to
label p65-immunoreactive cells. JMJD6 and p65 double-positive cells
(indicated in yellow in the merged image) in the spinal dorsal horn
nuclei indicated that JMJD6 may exert its function together with
p65 following CCI surgery (Fig.
6).
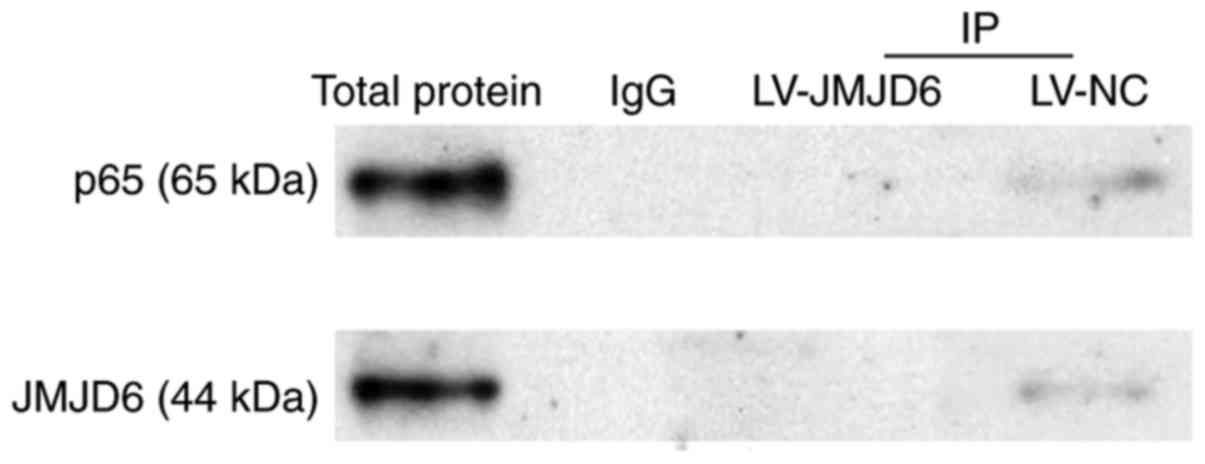
Absence of a direct interaction between
JMJD6 and p65
The earlier experiments in the present study
indicated the colocalization of JMJD6 and p65 in the spinal cord,
however, whether JMJD6 and p65 interact directly remained to be
elucidated. Therefore, co-immunoprecipitation analysis was used to
determine their potential interactions. Specimens from two groups
(CCI+LV-JMJD6, CCI+LV-NC) were detected. The results showed that
p65 protein was not observed in co-immunoprecipitation with JMJD6,
nor was JMJD6 protein observed in co-immunoprecipitation with p65
(Fig. 7), indicating that there
may be no direct interactions between JMJD6 and p65.
Discussion
The CCI model, which is a classic model in NPP
research, is achieved by placing loosely constrictive ligatures
around the common sciatic nerve. The postoperative behaviors of CCI
rats indicate that hyperalgesia, allodynia and, possibly,
spontaneous pain were produced, which consistently represent signs
in patients with NPP; CCI-induced hyperalgesia and allodynia
normally present on day 3 post-surgery (24–26), which indicate a successfully
established model. In the present study, treatments commenced on
day 3 following CCI, at which point MWT and TWL were markedly
decreased. In the LV-JMJD6-treated group, measurements in
behavioral assessments, including mechanical allodynia and thermal
hyperexcitability, recovered in a stepwise manner, compared with
those in the NC- and NS-treated CCI rats, and lasted until day 14,
indicating that JMJD6 exerted an effect.
JMJD6 is known to be a demethylase, and has been
implicated in an array of biological processes. Previous studies
have found that JMJD6 and the serine/arginine-rich protein U2AF65
co-regulate a large number of alternative splicing events, which
has important implications in development and disease processes
(27). However, the function of
JMJD6 in NPP remains to be elucidated. In the present study, the
protein expression of JMJD6 in the spinal cord was reduced in the
CCI rats. Intrathecal injection of the JMJD6-overexpessing
lentiviral vector attenuated CCI-induced NPP and upregulated the
protein expression of JMJD6, suggesting that JMJD6 may exert a
function in NPP.
The NF-κB pathway is pivotal in processes including
cell regulation, differentiation and stress responses (28). The activation of NF-κB enhances
gene expression by binding to target gene promoters (29). In several types of pain, NF-κB
affects inflammatory responses and immunity by regulating genes
encoding for chemokines, proinflammatory cytokines and adhesion
molecules in the spinal cord (30). The inhibition of NF-κB has been
used to attenuate chronic pain states. Intrathecal injection of the
NF-κB inhibitor pyrrolidine dithiocarbamate can alleviate
CCI-induced allodynia (31). In
the present study, expression of the NF-κB p-p65 subunit in the
spinal cord increased markedly following CCI, consistent with
previous findings that the activation of NF-κB is involved in or
required for the induction of allodynia and hyperalgesia (29). In addition, expression of the
NF-κB p-p65 subunit decreased following intrathecal injection of
LV-JMJD6, indicating that JMJD6 may suppress the activation of
NF-κB.
Gene expression is epigenetically regulated by
histone methyltransferases and demethylases via changing histone
methylation states in several cellular processes, including
differentiation and proliferation. The methylation states of
numerous non-histone proteins can also be changed by modifiers,
including effector proteins, which have key effects in cellular
signaling networks (32). It has
been noted that NF-κB signaling can be regulated by multiple
post-translational modifications (33). NF-κB can undergo arginine
methylation, and R30 of p65 can be demethylated by PRMT5, resulting
in the activation of NF-κB signaling (17). As a demethylase, JMJD6 may
regulate the effects of NF-κB in NPP. In the present study, the
expression of NF-κB p-p65 was examined following intrathecal
injection of JMJD6. The results showed a marked increase in the
expression of p-p65 following CCI; however, its activation was
inhibited in the LV-JMJD6-treated CCI rats, indicating that JMJD6
may effectively inhibit the activity of NF-κB and alleviate NPP
through epigenetic mechanisms.
Inflammatory cytokines are involved in the
modulation of NPP. When tissues or nerves are damaged,
proinflammatory cytokines (TNF-α and IL-1β) are upregulated in the
spinal cord (18). These
cytokines are indicated to be downstream mediators of NF-κB, and it
has been reported that endoneural injection of an NF-κB inhibitor
at the nerve lesion can attenuate hyperalgesia and downregulate
cytokine mRNA levels at the site of injury (19). Therefore, in the present study,
the expression levels of TNF-α and IL-1β were assessed to examine
the effect of JMJD6 on NF-κB downstream mediators. The majority of
the findings suggested that the proinflammatory cytokines exerted
their effects mainly in the early stages of pain. However, studies
have also shown that central sensitization associated with NPP may
result from nerve injury-induced cytokine release, suggesting
long-term effects on nociceptive processing (34,35). For example, following peripheral
nerve injury, the expression of IL-1β continues to rise for at
least 35 days (34). Furthermore,
the neuronal release of cytokines can activate astrocytes, which
can release a large number of cytokines and amplify pain signaling
(18,36). These findings are consistent with
the results of the present study, which showed that the expression
levels of TNF-α and IL-1β were significantly increased on day 14
post-CCI (Fig. 4). In the present
study, the expression levels of TNF-α and IL-1β in the CCI rats
were attenuated by intrathecal injection of LV-JMJD6, indicating
that JMJD6 may affect the expression of downstream cytokines by
regulating NF-κB.
Changes in the expression of VEGF were also examined
in the present study. VEGF can regulate vascular function through
its effects on endothelial cells. Studies have shown that, in the
dorsal root ganglia of CCI rats, VEGF immunoreactivity is enhanced
(37). Treatment with an
anti-VEGF antibody via intrathecal injection in rats attenuates
reductions in MWT and TWL, which are induced by CCI surgery, and
reduces the expression of VEGF receptors (37). Several studies have shown that
NF-κB affects the expression of VEGF in various types of tumor. In
addition, Gao et al showed that the NF-κB inhibitor,
pyrrolidine dithiocarbamate hydrochloride, inhibited the expression
of VEGF in tumor tissues (38).
In the present study, the expression of VEGF was suppressed by
JMJD6, which may be regulated by NF-κB.
Based on the colocalization of JMJD6 and p65
observed in the present study, it was hypothesized that there may
be a functional connection between them. JMJD6 and p65 colocalize
within the nuclei in spinal dorsal horn, indicating that JMJD6 may
regulate NF-κB within the nucleus and downregulate the
transcription of mediators through a specific mechanism. However,
the co-immunoprecipitation results in the present study indicated
no direct interaction between JMJD6 and p65. According to the
results, JMJD6 effectively relieved NPP of CCI rats, and
downregulated the expression of NF-κB and its target genes,
suggesting an intermediate biomolecule or signaling element may
exist between JMJD6 and p65. It has been reported that TNF
receptor-associated factor 6 can regulate NF-κB and target genes
through demethylation by JMJD6 in innate immune responses (39), however, this remains unclear in
NPP. Therefore, future investigations are to focus on the
epigenetic mechanism of JMJD6 at the cellular and animal levels, by
assessing multiple time points in a long-term CCI model.
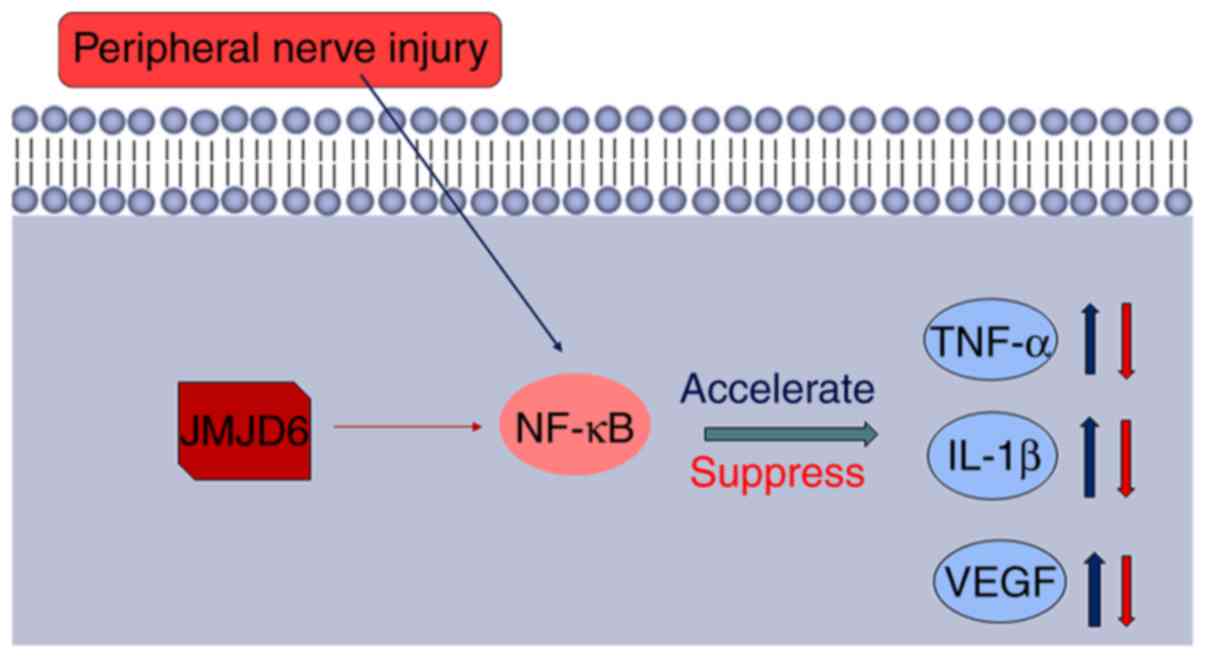
Based on the results of the present study, a novel
model for the regulation by JMJD6 of NF-κB in NPP was proposed
(Fig. 8). Peripheral nerve injury
leads to the activation of NF-κB. NF-κB then binds to specific
sites of target gene promoter regions to upregulate the expression
of TNF-α, IL-1β and VEGF, and other cytokines and adhesion
molecules. However, JMJD6 in the nucleus may remove the methyl
group from NF-κB and suppress the transcription of downstream
mediators, thereby downregulating TNF-α, IL-1β and VEGF, which may
be the reason for the alleviation of NPP.
In conclusion, the results of the present study
revealed that JMJD6 may exert key effects in NPP through regulating
NF-κB following CCI. Following CCI, the overexpression of JMJD6
functionally reversed pain behavior, upregulated the spinal cord
expression of JMJD6 and downregulated expression of the NF-κB p-p65
subunit and its downstream mediators, including TNF-α, IL-1β and
VEGF. In addition, JMJD6 and p65 colocalized within the nucleus,
although they did not interact directly, providing evidence for
their potential functional contact. The results of the present
study provide insights into the role of histone demethylation in
NPP, which warrants further investigation. JMJD6 is expected to be
a potential therapeutic target for NPP.
Glossary
Abbreviations
Abbreviations:
|
CCI
|
chronic constriction injury
|
|
GFP
|
green fluorescent protein
|
|
IL-1β
|
interleukin-1β
|
|
JMJD
|
Jumonji C domain
|
|
NF-κB
|
nuclear factor-κB
|
|
NPP
|
neuropathic pain
|
|
TNF-α
|
tumor necrosis factor-α
|
|
VEGF
|
vascular endothelial growth factor
|
Acknowledgments
The authors would like to thank Dr Melony Black,
Liwen Bianji and Edanz Group China (www.liwenbianji.cn/ac), for editing the English text
in a manuscript draft.
References
|
1
|
Gilron I, Baron R and Jensen T:
Neuropathic pain: Principles of diagnosis and treatment. Mayo Clin
Proc. 90:532–545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jensen TS and Finnerup NB: Allodynia and
hyperalgesia in neuropathic pain: Clinical manifestations and
mechanisms. Lancet Neurol. 13:924–935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Latremoliere A and Woolf CJ: Central
sensitization: A generator of pain hypersensitivity by central
neural plasticity. J Pain. 10:895–926. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kerstman E, Ahn S, Battu S, Tariq S and
Grabois M: Neuropathic pain. Handb Clin Neurol. 110:175–187. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benarroch EE: Central neuron-glia
interactions and neuropathic pain: Overview of recent concepts and
clinical implications. Neurology. 75:273–278. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu X, Li Q, Chang R, Yang D, Song Z, Guo
Q and Huang C: Curcumin alleviates neuropathic pain by inhibiting
p300/CBP histone acetyltransferase activity-regulated expression of
BDNF and cox-2 in a rat model. PLoS One. 9:e913032014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He Z, Guo Q, Xiao M, He C and Zou W:
Intrathecal lentivirus-mediated transfer of interleukin-10
attenuates chronic constriction injury-induced neuropathic pain
through modulation of spinal high-mobility group box 1 in rats.
Pain Physician. 16:E615–E625. 2013.PubMed/NCBI
|
|
8
|
Descalzi G, Ikegami D, Ushijima T, Nestler
EJ, Zachariou V and Narita M: Epigenetic mechanisms of chronic
pain. Trends Neurosci. 38:237–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SY, Levenson JM, Korsmeyer S, Sweatt
JD and Schumacher A: Developmental regulation of Eed complex
composition governs a switch in global histone modification in
brain. J Biol Chem. 282:9962–9972. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang B, Chen Y, Zhao Y and Bruick RK:
JMJD6 is a histone arginine demethylase. Science. 318:444–447.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu W, Ma Q, Wong K, Li W, Ohgi K, Zhang
J, Aggarwal A and Rosenfeld MG: Brd4 and JMJD6-associated
anti-pause enhancers in regulation of transcriptional pause
release. Cell. 155:1581–1595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poulard C, Rambaud J, Hussein N, Corbo L
and Le Romancer M: JMJD6 regulates ERα methylation on arginine.
PLoS One. 9:e879822014. View Article : Google Scholar
|
|
13
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar
|
|
14
|
Gerondakis S, Fulford TS, Messina NL and
Grumont RJ: NF-κB control of T cell development. Nat Immunol.
15:15–25. 2014. View
Article : Google Scholar
|
|
15
|
Pan YD, Guo QL, Wang E, Ye Z, He ZH, Zou
WY, Cheng ZG and Wang YJ: Intrathecal infusion of pyrrolidine
dithiocarbamate for the prevention and reversal of neuropathic pain
in rats using a sciatic chronic constriction injury model. Reg
Anesth Pain Med. 35:231–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu T and Stark GR: NF-κB: Regulation by
methylation. Cancer Res. 75:3692–3695. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei H, Wang B, Miyagi M, She Y, Gopalan B,
Huang DB, Ghosh G, Stark GR and Lu T: PRMT5 dimethylates R30 of the
p65 subunit to activate NF-κB. Proc Natl Acad Sci USA.
110:13516–13521. 2013. View Article : Google Scholar
|
|
18
|
Ji RR, Berta T and Nedergaard M: Glia and
pain: Is chronic pain a gliopathy? Pain. 154(Suppl 1): S10–S28.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakaue G, Shimaoka M, Fukuoka T, Hiroi T,
Inoue T, Hashimoto N, Sakaguchi T, Sawa Y, Morishita R, Kiyono H,
et al: NF-kappaB decoy suppresses cytokine expression and thermal
hyperalgesia in a rat neuropathic pain model. Neuroreport.
12:2079–2084. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bennett GJ and Xie YK: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Milligan ED, Hinde JL, Mehmert KK, Maier
SF and Watkins LR: A method for increasing the viability of the
external portion of lumbar catheters placed in the spinal
subarachnoid space of rats. J Neurosci Methods. 90:81–86. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cunha TM, Verri WA Jr, Valério DA,
Guerrero AT, Nogueira LG, Vieira SM, Souza DG, Teixeira MM, Poole
S, Ferreira SH and Cunha FQ: Role of cytokines in mediating
mechanical hyper-nociception in a model of delayed-type
hypersensitivity in mice. Eur J Pain. 12:1059–1068. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Zhu XY, Huang CS, Li Q, Guo QL, Wang Y, He
X and Liao J: Temporal distribution of p300/CBP immunoreactivity in
the adult rat spinal dorsal horn following chronic constriction
injury (CCI). Cell Mol Neurobiol. 33:197–204. 2013. View Article : Google Scholar
|
|
25
|
Zhang YQ, Guo N, Peng G, Wang X, Han M,
Raincrow J, Chiu CH, Coolen LM, Wenthold RJ, Zhao ZQ, et al: Role
of SIP30 in the development and maintenance of peripheral nerve
injury-induced neuropathic pain. Pain. 146:130–140. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu XY, Huang CS, Li Q, Chang RM, Song ZB,
Zou WY and Guo Q: p300 exerts an epigenetic role in chronic
neuropathic pain through its acetyltransferase activity in rats
following chronic constriction injury (CCI). Mol Pain. 8:842012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yi J, Shen HF, Qiu JS, Huang MF, Zhang WJ,
Ding JC, Zhu XY, Zhou Y, Fu XD and Liu W: JMJD6 and U2AF65
co-regulate alternative splicing in both JMJD6 enzymatic activity
dependent and independent manner. Nucleic Acids Res. 45:3503–3518.
2017. View Article : Google Scholar :
|
|
28
|
Yao Z, Nie L, Zhao Y, Zhang Y, Liu Y, Li J
and Cheng L: Salubrinal suppresses IL-17-induced upregulation of
MMP-13 and extracellular matrix degradation through the NF-κB
pathway in human nucleus pulposus cells. Inflammation.
39:1997–2007. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo JG, Zhao XL, Xu WC, Zhao XJ, Wang JN,
Lin XW, Sun T and Fu ZJ: Activation of spinal NF-ΚB/p65 contributes
to peripheral inflammation and hyperalgesia in rat adjuvant-induced
arthritis. Arthritis Rheumatol. 66:896–906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu H, Lei X and Zhang Q: Moderate
activation of IKK2-NF-κB in unstressed adult mouse liver induces
cytoprotective genes and lipogenesis without apparent signs of
inflammation or fibrosis. BMC Gastroenterol. 15:942015. View Article : Google Scholar
|
|
31
|
Yin Q, Fan Q, Zhao Y, Cheng MY, Liu H, Li
J, Lu FF, Jia JT, Cheng W and Yan CD: Spinal NF-κB and chemokine
ligand 5 expression during spinal glial cell activation in a
neuropathic pain model. PLoS One. 10:e01151202015. View Article : Google Scholar
|
|
32
|
Alam H, Gu B and Lee MG: Histone
methylation modifiers in cellular signaling pathways. Cell Mol Life
Sci. 72:4577–4592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Perkins ND: Post-translational
modifications regulating the activity and function of the nuclear
factor kappa B pathway. Oncogene. 25:6717–6730. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gustafson-Vickers SL, Lu VB, Lai AY, Todd
KG, Ballanyi K and Smith PA: Long-term actions of interleukin-1beta
on delay and tonic firing neurons in rat superficial dorsal horn
and their relevance to central sensitization. Mol Pain. 4:632008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Echeverry S, Shi XQ and Zhang J:
Characterization of cell proliferation in rat spinal cord following
peripheral nerve injury and the relationship with neuropathic pain.
Pain. 135:37–47. 2008. View Article : Google Scholar
|
|
36
|
Toyokawa G, Cho HS, Masuda K, Yamane Y,
Yoshimatsu M, Hayami S, Takawa M, Iwai Y, Daigo Y, Tsuchiya E, et
al: Histone lysine methyltransferase Wolf-Hirschhorn syndrome
candidate 1 is involved in human carcinogenesis through regulation
of the Wnt pathway. Neoplasia. 13:887–898. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu S, Xu C, Li G, Liu H, Xie J, Tu G,
Peng H, Qiu S and Liang S: Vatalanib decrease the positive
interaction of VEGF receptor-2 and P2X2/3 receptor in chronic
constriction injury rats. Neurochem Int. 60:565–572. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao P, Gao YJ and Liang HL: Effect of
NF-κB inhibitor PDTC on VEGF and endostatin expression of mice with
Lewis lung cancer. Asian Pac J Trop Med. 8:220–224. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tikhanovich I, Kuravi S, Artigues A,
Villar MT, Dorko K, Nawabi A, Roberts B and Weinman SA: dynamic
arginine methylation of tumor necrosis factor (TNF)
receptor-associated factor 6 regulates toll-like receptor
signaling. J Biol Chem. 290:22236–22249. 2015. View Article : Google Scholar : PubMed/NCBI
|