Introduction
The etiology of psoriasis remains unclear. Modern
research has demonstrated that it is related to various factors,
including heredity, infection, immunity, spirit, endocrine, trauma,
diet and metabolic disorder (1).
At present, it is suggested that immune mediation accounts for the
major pathogenesis (2). Its
clinical manifestation consists of reddish and scaly skin damage,
with an initial reddish rash (2).
The epidermis is covered by layers of silvery scales, with dry,
flaking and crusting skin (2). In
addition, several skin symptoms are contiguous. Some may be
itching, suppurant and blood-stained. Psoriasis can be classified
into Plaque, erythrodermic, pustular, and arthritic types (3). Blood-heat syndrome in traditional
Chinese medicine is dominated by papule and maculopapule, where new
rash occurs continuously, basal skin is bright red in color, and
punctuate hemorrhage can be observed after scraping the scales
(4). In addition, it is
accompanied with various degrees of itching, which equals to the
progressive psoriasis in western medicine. Prospective treatment
and future drug therapies have been proposed under the guidance of
gradually updated pathogenesis concept (5). Furthermore, more attention has been
paid to the complicated pathogenesis inducing the pathological
changes of the disease (4).
Treatment may not be limited to the traditional antiproliferative,
antimetabolic and anti-inflammatory measures based on the cellular
morphological changes (1).
MicroRNAs (miRNAs) are derived from the non-coding
region of the genome (6). They
are a group of evolutionarily conserved single-strand RNAs with
length of ~21–24 nucleotides (7).
A miRNA can target the mRNA of multiple genes. In addition, the
mRNA of a single gene can be simultaneously regulated by multiple
miRNAs, suggesting a great diversity and complexity of miRNAs
biological activities (7).
Research focusing on the regulation of miRNAs in immune responses,
as well as their regulatory mechanisms, are currently a hot topic
in immunology. The involvement of miRNAs in regulating
inflammation, natural immune responses and antiviral immune
responses has been reported in numerous studies (7,8).
Previous research has indicated that NLR family
pyrin domain containing 3 (NLRP3) can be stimulated by various
pathogenic microorganisms (9). In
addition, it can be stimulated by multiple pathogen-associated
molecular patterns (PAMPs), damage-associated molecular patterns
(DAMPS) and environmental factors (9). These mainly include ATP, hyaluronic
acid, β-amyloid protein, extracellular glucose, monosodium urate
crystal, shoe stone, asbestos, ultraviolet ray, picric acid and
dinitrofluorobenzene (10).
Additionally, NLRP3 expression has been demonstrated to be
increased in multiple diseases, including myocardial infarction,
Alzheimer disease, type 2 diabetes and its complications, gouty
arthritis, lung disease and dermatitis (11). Toll-like receptor 4 (TLR4)
signaling regulates miR-155 to promote liver fibrosis and
alcohol-induced steatohepatitis (12). In the present study, it was
hypothesized that the function of miR-155 may increase
psoriasis-induced inflammation and that its expression may be
dependent on inflammasome activation.
Materials and methods
Imiquimod-induced skin-inflammation
model
Female BALB/c mice (6 week, 20–22 g, n=12) were
purchased from the Center of Laboratory Animal Science of Guangdong
(Guangdong, China), and housed at 22–23°C, 55–60% humidity, 12 h
light/dark cycle, and freely access to food and water. The mice
(n=12) were randomly assigned into two groups: Normal (n=6) and
psoriasis model (n=6). In the psoriasis model group, mice were
treated on regions of shaved skin with 62.5 mg of 5% imiquimod
cream (Aldara; 3M Pharmaceuticals, Maplewood, MN, USA) at 8:00 pm
for 7 days. The present study was approved by the Medical Ethics
Committee of Guangzhou Institute of Dermatology (Guangzhou,
China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay and microarray data
analysis
Total RNA from skin samples and cells was isolated
using TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Total RNA was reverse transcribed using 200 ng total RNA with
the RevertAid Reverse Transcription kit (Thermo Fisher Scientific,
Inc.). qPCR was conducted using a Roche LightCycler 480 PCR machine
(Roche Diagnostics, Basel, Switzerland) with the SYBR-Green
miScript PCR kit (Qiagen, Inc., Valencia, CA, USA). The reaction
conditions were: 95°C for 10 min, followed by 40 cycles of 95°C for
30 sec and 60°C for 30 sec. Primer sequences were as follows:
miR-155, forward 5′-TTAATGCTAATCGTGTAGGGG-3′ and reverse
5′-CGAATTCTAGAGCTCGAGGCAGG-3′; U6, forward 5′-CTCGCTTCGGCACA-3′ and
reverse 5′-CGAATTCTAGAGCTCGAGGCAGG-3′. mRNA expression was
calculated using the formula 2−ΔΔCq (12).
A total of 500 ng total RNA was used for microarray
data analysis (8×15 K G4471A-021828 platform) Agilent Technologies,
Inc., Santa Clara, CA, USA) (13). Array data was acquired using the
Agilent Feature Extraction software (Agilent Technologies, Inc.)
according to the manufacturer′s protocols. The GeneSpring GX v12.1
software package (Agilent Technologies, Inc.) was used for quantile
normalization and subsequent data processing.
Cell culture and cell transfection
HaCaT cells were cultured at 37°C/5% CO2
in MEM (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) containing
10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.) and
antibiotics (Sigma-Aldrich; Merck KGaA). miR-155, forward
5′-GATCCCTGTTAATGCTAATCGTGATAGGGGTTTTTGCCTCCAACTGACTCCTACATATTAGCATTAACAGA-3′,
reverse
5′-AGCTTCTGTTAATGCTAATATGTAGGAGTCAGTTGGAGGCAAAAACCCCTATCACGATTAGCATTAACAGG-3′;
miR-155 inhibitor, forward
5′-ACCCCUAUCACGAUUAGCAUUAA-3′,reverse5′-ACGUGACACGUUCGGAGAATT-3′;
negative control, forward 5′-CCCCCCCCCCCCCCCCCCCC-3′ and reverse
5′-CCCCCCCCCCCCCCCCCCCC-3′. miR-155 mimic, miR-155 inhibitor and
negative control oligos were purchased from Sangon Biotech Co.,
Ltd. (Shanghai, China). miR-155 mimic (100 ng), miR-155 inhibitor
(100 ng) and negative control (100 ng) were transfected into cells
using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.).
Following transfection for 6 h, the medium was removed and fresh
medium was added, containing 0.1 ng/ml human interleukin (IL)-4
(Sigma-Aldrich; Merck KGaA), 1 ng/ml human tumor necrosis factor
(TNF)-α (Sigma-Aldrich; Merck KGaA) and 10 ng/ml human interferon
(IFN)-γ (Sigma-Aldrich; Merck KGaA) for 24 h. Cells were then
cultured as keratinocyte-induced HaCaT cells, an in vitro
model for psoriasis, as previously reported in the literature
(14). Cells were induced with
100 ng/ml LPS (Beyotime Institute of Biotechnology, Jiangsu, China)
for 24 h. Cells were treated with NLRP3 inhibitor, MCC950 (2 nM;
MedChemExpress, Shanghai, China) for 8 h.
ELISA
Following culture of the in vitro model of
psoriasis for 8 h, the culture supernatant was centrifuged at 2,000
× g for 10 min at 4°C and TNF-α (H052), IL-18 (H015), IL-6 (H007)
and IL-1β (H002) levels were quantified with a ELISA kits (Nanjing
Jiancheng Biology Engineering Institute, Nanjing, China).
Absorbency was detected at 450 nm using an AIA 600II Automated
Enzyme Immunoassay Analyzer (Tosoh Corporation, Tokyo, Japan).
Western blot analysis
Following culture of the in vitro model of
psoriasis for 8 h, cells were lysed with RIPA buffer (Beyotime
Institute of Biotechnology) and protein concentration was
quantified using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology). A total of 50 μg proteins were
separated using 8–12% SDS-PAGE gels and blotted onto a
polyvinylidene difluoride membrane (Life Technologies, Grand
Island, NY, USA). The membrane was blocked with 5% non-fat dried
milk for 1 h at 37°C and then probed with antibodies against TLR4
(1:1,000; sc-10741; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), NF-κB (1:1,000; sc-109, Santa Cruz Biotechnology, Inc.),
NLRP3 (1:1,000; sc-66846, Santa Cruz Biotechnology), caspase-1
(1:1,000; sc-514, Santa Cruz Biotechnology, Inc.) and GAPDH
(1:5,000; sc-25778, Santa Cruz Biotechnology, Inc.) overnight at
4°C. Following three washes with PBST, the PVDF membrane was
incubated with goat anti-rabbit horseradish peroxidase-conjugated
secondary antibody (1:5,000; 7074, Cell Signaling Technology, Inc.,
Danvers, MA, USA) for 1 h at 37°C. Signals were developed using an
enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.) and
protein expression was quantified using Image-Pro Plus software
version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation
from three independent repeats. Statistical analyses were performed
by one-way analysis of variance following by a Tukey post-hoc test
using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
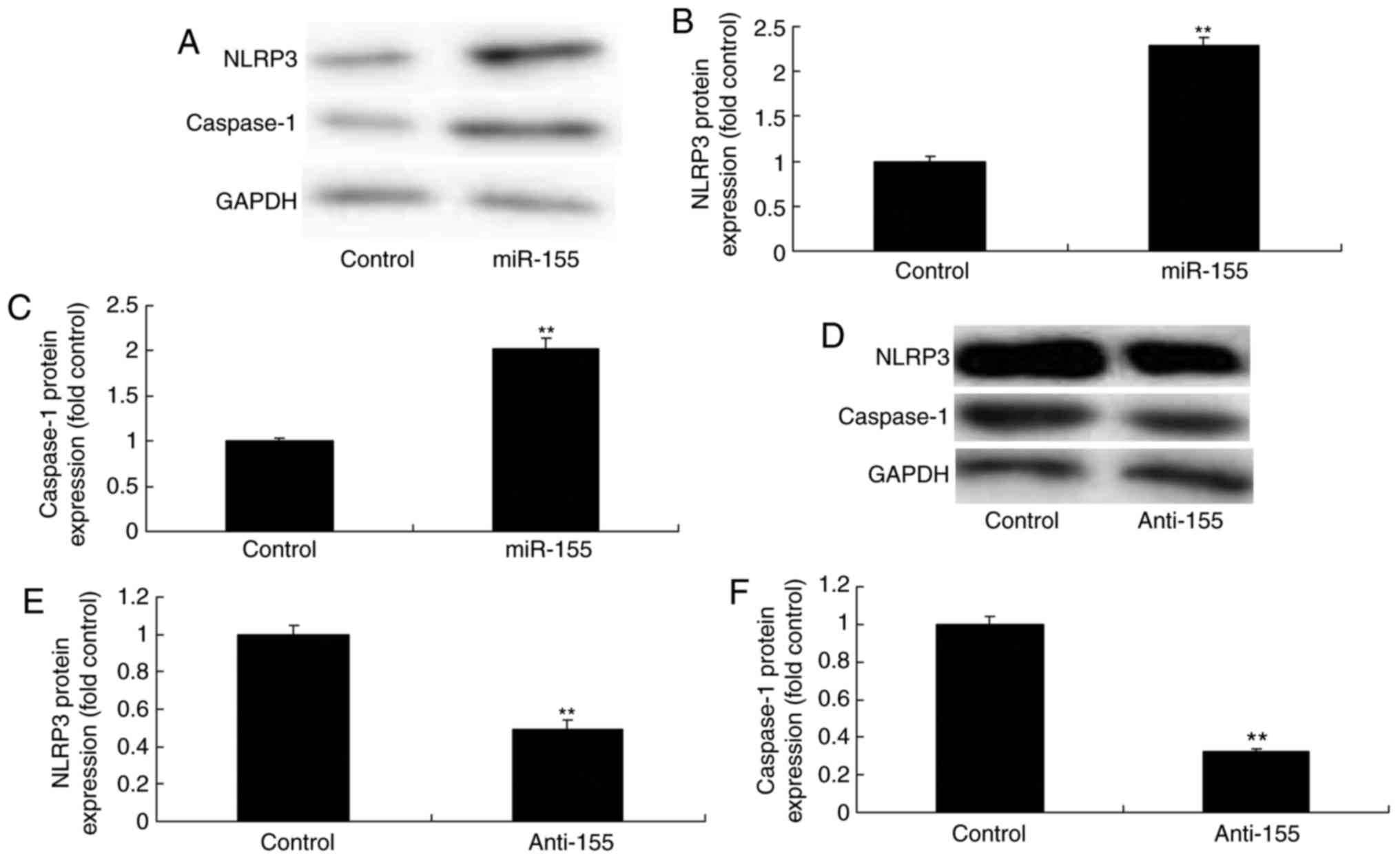
miR-155 expression in psoriasis
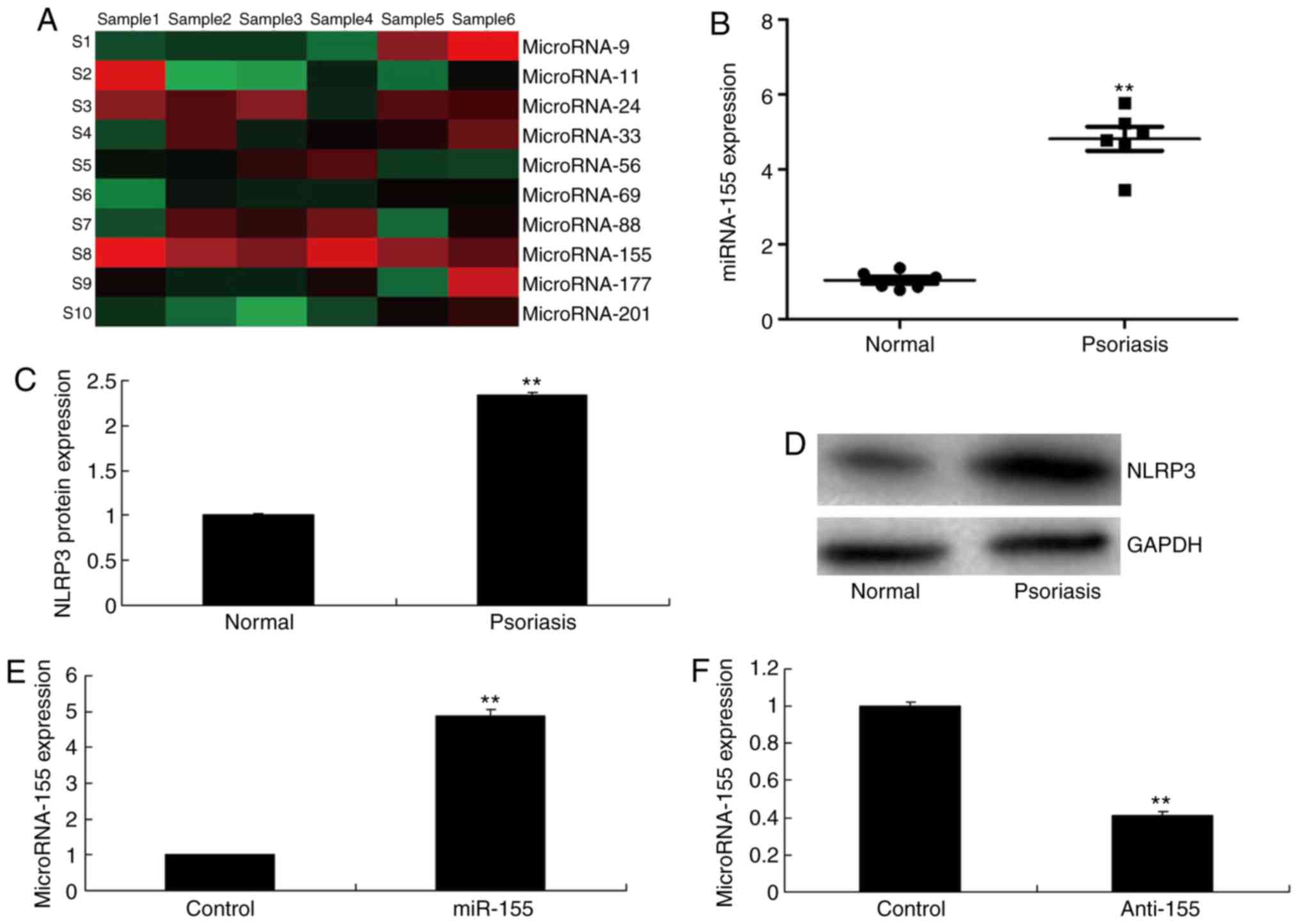
The expression of miR-155 in psoriasis was first
examined by using gene chip and qPCR analyses. In the in
vivo model of psoriasis, miR-155 expression levels were
significantly increased in psoriasis tissues compared with normal
tissues (Fig. 1A and B). Notably,
the protein expression levels of NLRP3 were also significantly
induced in the in vivo psoriasis model group, compared with
the control group (Fig. 1C and
D). Based on these initial experiments, it was hypothesized
that miR-155 may promote psoriasis by modifying gene expression.
Then, miR-155 mimics increased the expression of miR-155, and
anti-miR-155 mimics decreased the expression of miR-155 in
vitro model (Fig. 1E and
F).
Effects of miR-155 on the inflammatory
response of lipopolysaccharide (LPS)-induced HaCaT cells
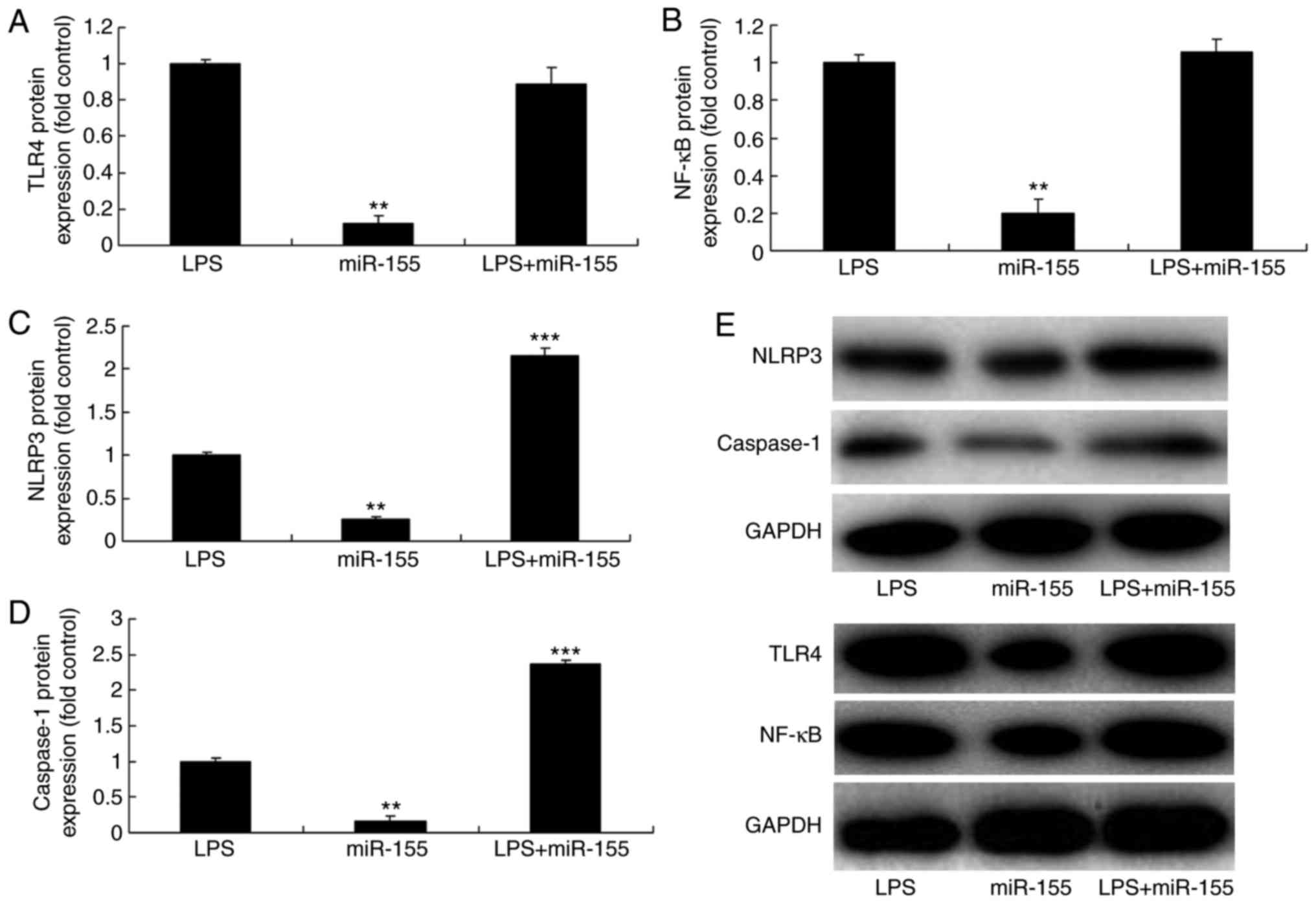
Next, LPS was used to induce HaCaT cells and a mimic
was used to overexpress miR-155. The results demonstrated that the
protein expression levels of TLR4, nuclear factor (NF)-κB, NLRP3
and caspase-1 were lower in the miR-155 overexpression alone group
compared with the LPS group (Fig.
2). The protein expression of TLR4 and NF-κB in the miR-155
overexpression and LPS groups was similar to those of the LPS alone
group (Fig. 2A, B, and E).
However, the protein expression of NLRP3 and caspase-1 in the
miR-155 overexpression and LPS group was higher compared with the
LPS alone group (Fig. 2C–E). In
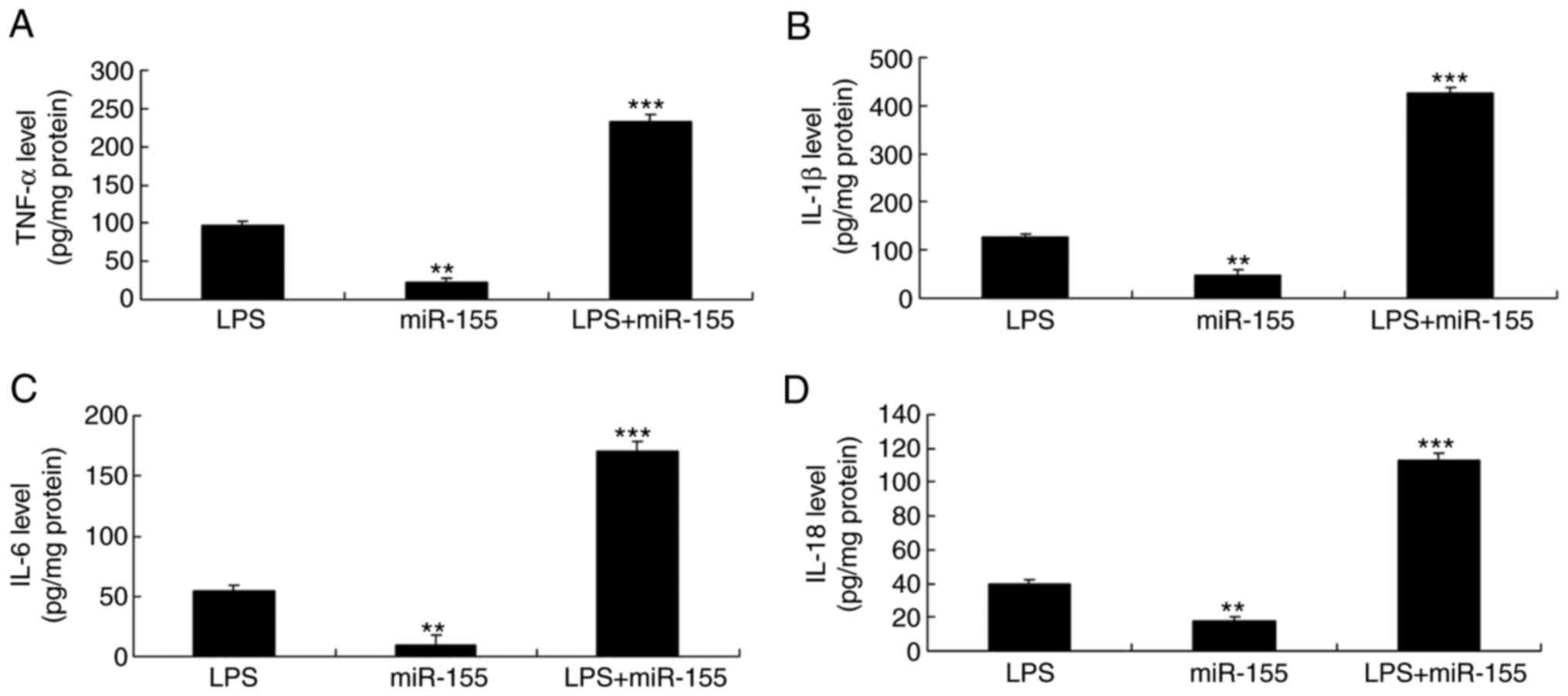
addition, the levels of secreted TNF-α, IL-18, IL-6 and IL-1β in
the culture supernatant of the miR-155 overexpression alone group
were lower compared with the LPS group (Fig. 3). However, the levels of secreted
TNF-α, IL-18, IL-6 and IL-1β levels in the miR-155 overexpression
and LPS group were higher than those of the LPS alone group
(Fig. 3). These results suggested
that miR-155 regulated the inflammasome NLRP3 activation, but did
not affect the TLR4/NF-κB signaling pathway.
Effects of miR-155 expression on
inflammation
Then, the in vitro psoriasis model of
keratinocyte-induced HaCaT cells was used to either upregulate or
silence miR-155 expression by transfecting a mimic or an inhibitor,
respectively (Fig. 1E, F).
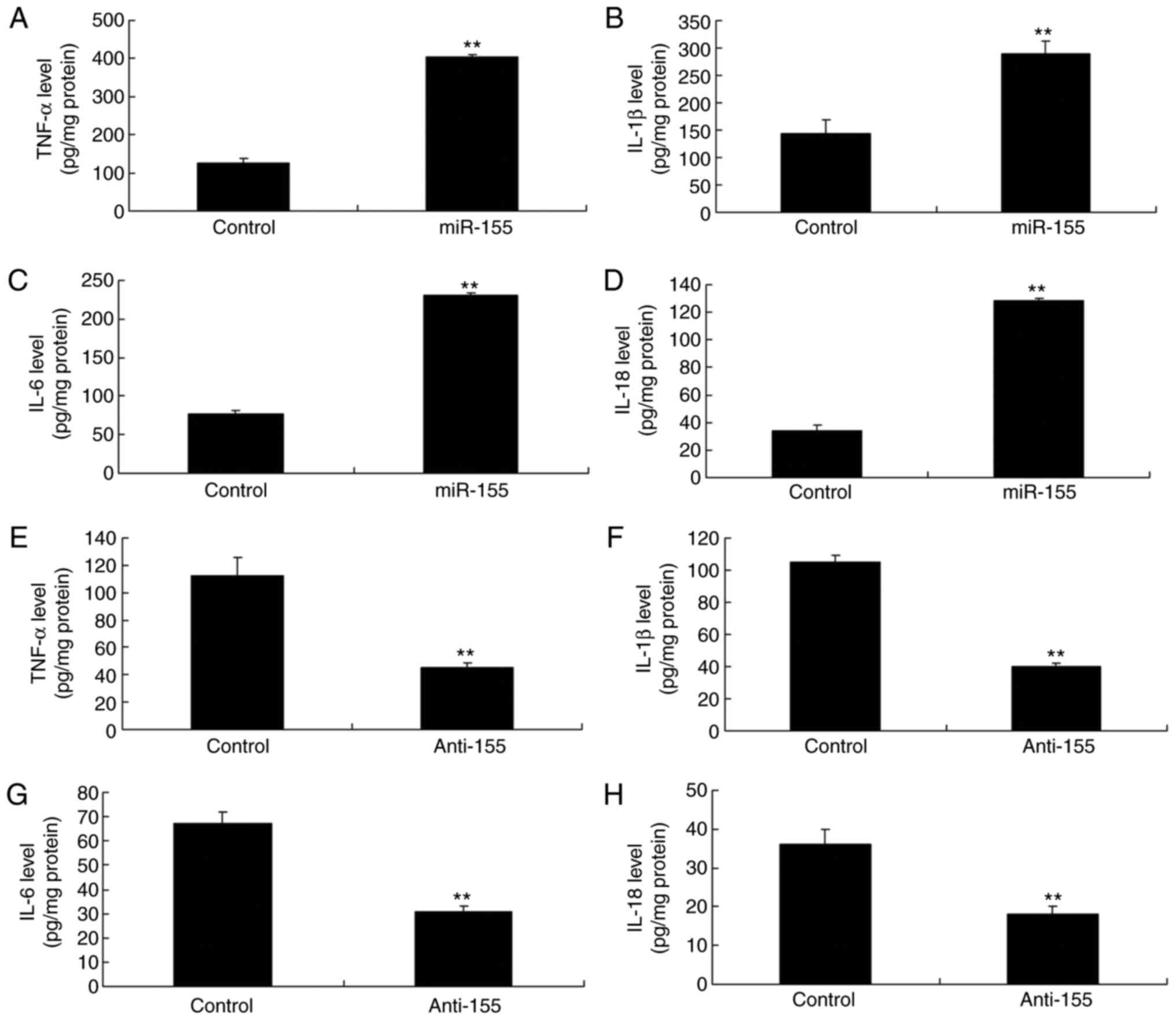
Overexpression of miR-155 significantly increased TNF-α, IL-18,
IL-6 and IL-1β levels (Fig.
4A–D). By contrast, silencing of miR-155 significantly
decreased TNF-α, IL-18, IL-6 and IL-1β levels in
keratinocyte-induced HaCaT cells (Fig. 4E–H).
miR-155 regulates the NLRP3/caspase-1
signaling pathway
To examine the potential mechanism by which miR-155
may mediate inflammation in psoriasis, the expression levels of
proteins in the NLRP3/caspase-1 pathway were measured. As
illustrated in Fig. 5,
overexpression of miR-155 significantly enhanced NLRP3 and
caspase-1 protein expression, while miR-155 silencing significantly
suppressed NLRP3 and caspase-1 protein expression in
keratinocyte-induced HaCaT cells.
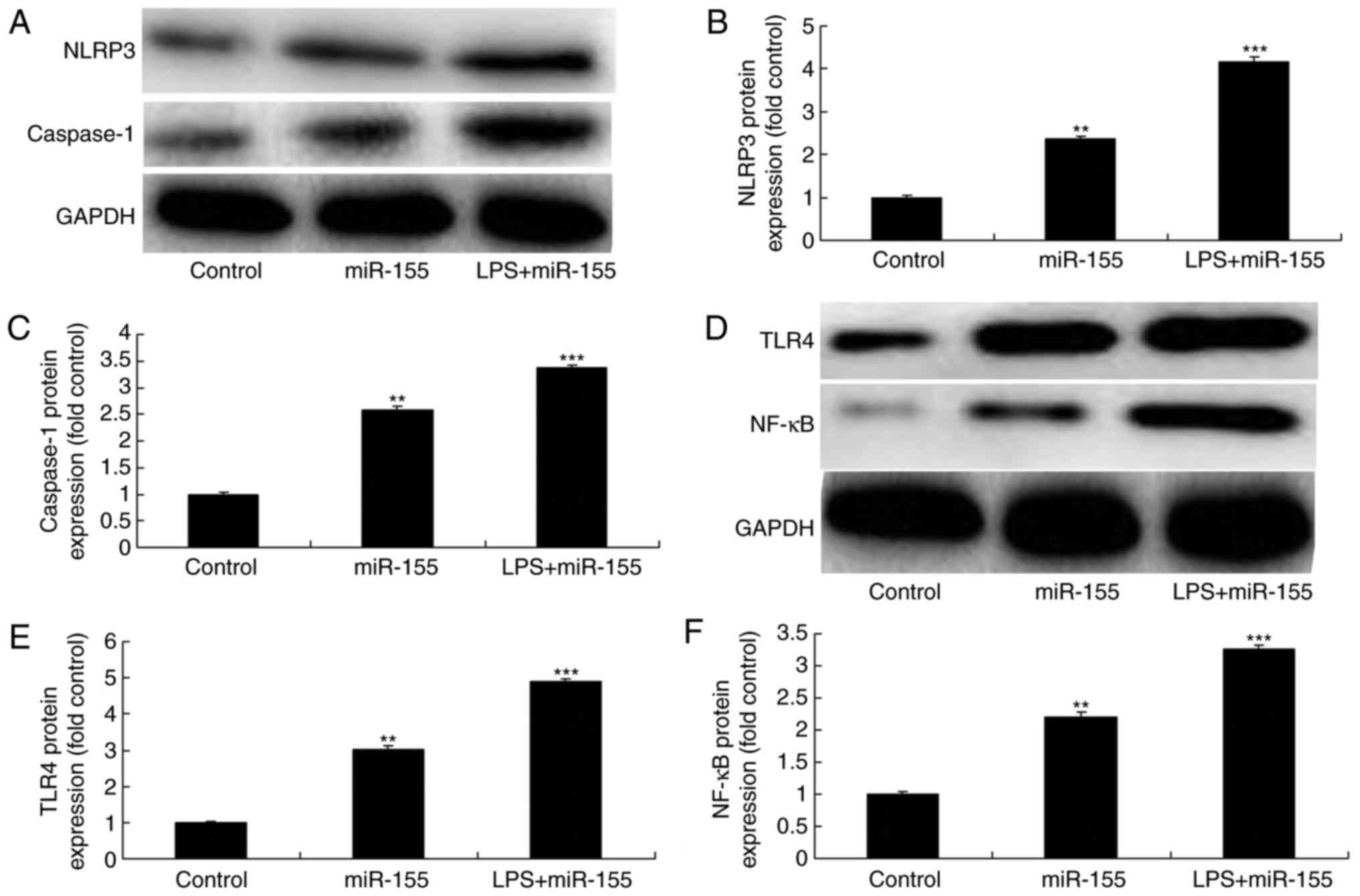
Activation of TLR4 by LPS stimulation
enhancess miR-155-mediated inflammation in keratinocyte-induced
HaCaT cells
Bala et al (15) have demonstrated that TLR4
signaling regulates miR-155 to promote liver fibrosis and
alcohol-induced steatohepatitis. To directly examine the upstream
pathways of miR-155 in psoriasis-related inflammation, the
TLR4/NF-κB signaling pathway was analyzed. NLRP3 and caspase-1
protein expression levels were significantly increased by LPS
stimulation and miR-155 overexpression, compared with the miR-155
overexpression alone group (Fig.
6A–C). In addition, TLR4 and NF-κB protein expression levels
were significantly induced by LPS stimulation and miR-155
overexpression, compared with the miR-155 overexpression alone
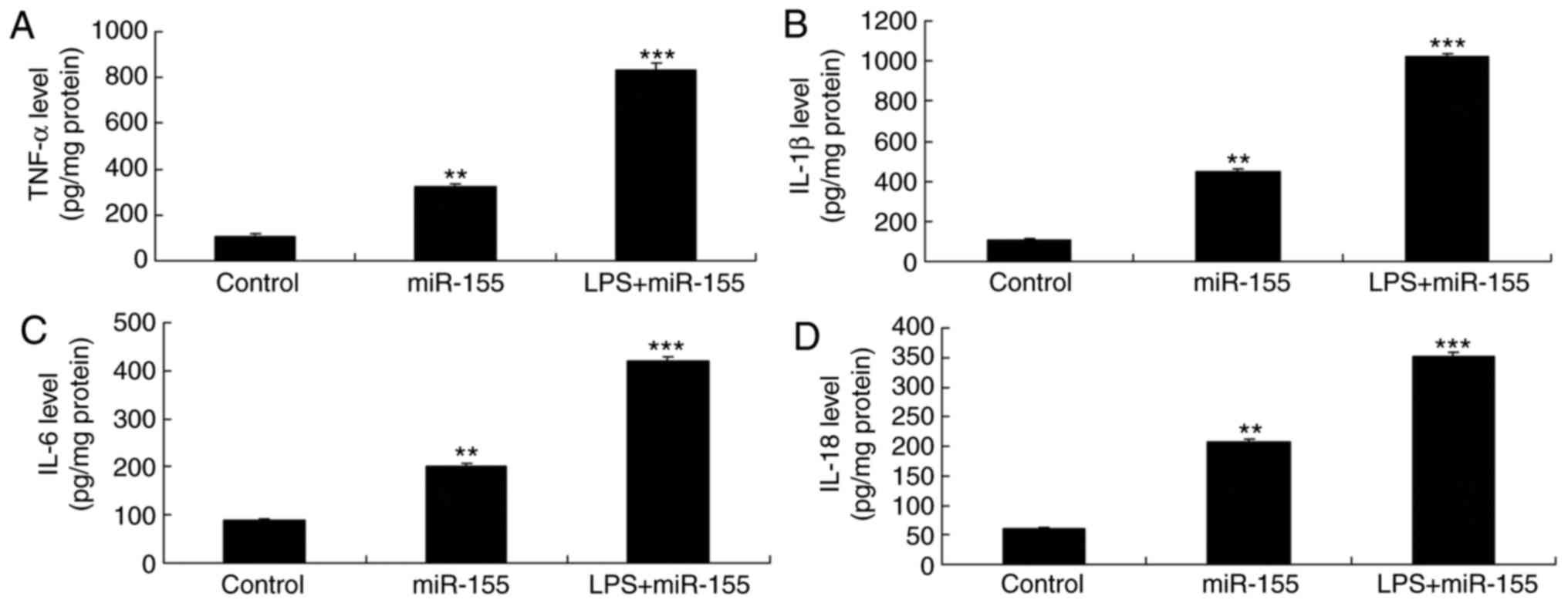
group (Fig. 6D–F) Furthermore,
LPS stimulation, and subsequently activation of TLR4, enhanced the
miR-155-mediated increase in the secreted levels of TNF-α, IL-18,
IL-6 and IL-1β in miR-155-overexpressing keratinocyte-induced HaCaT
cells, compared with the miR-155 overexpression alone group
(Fig. 7).
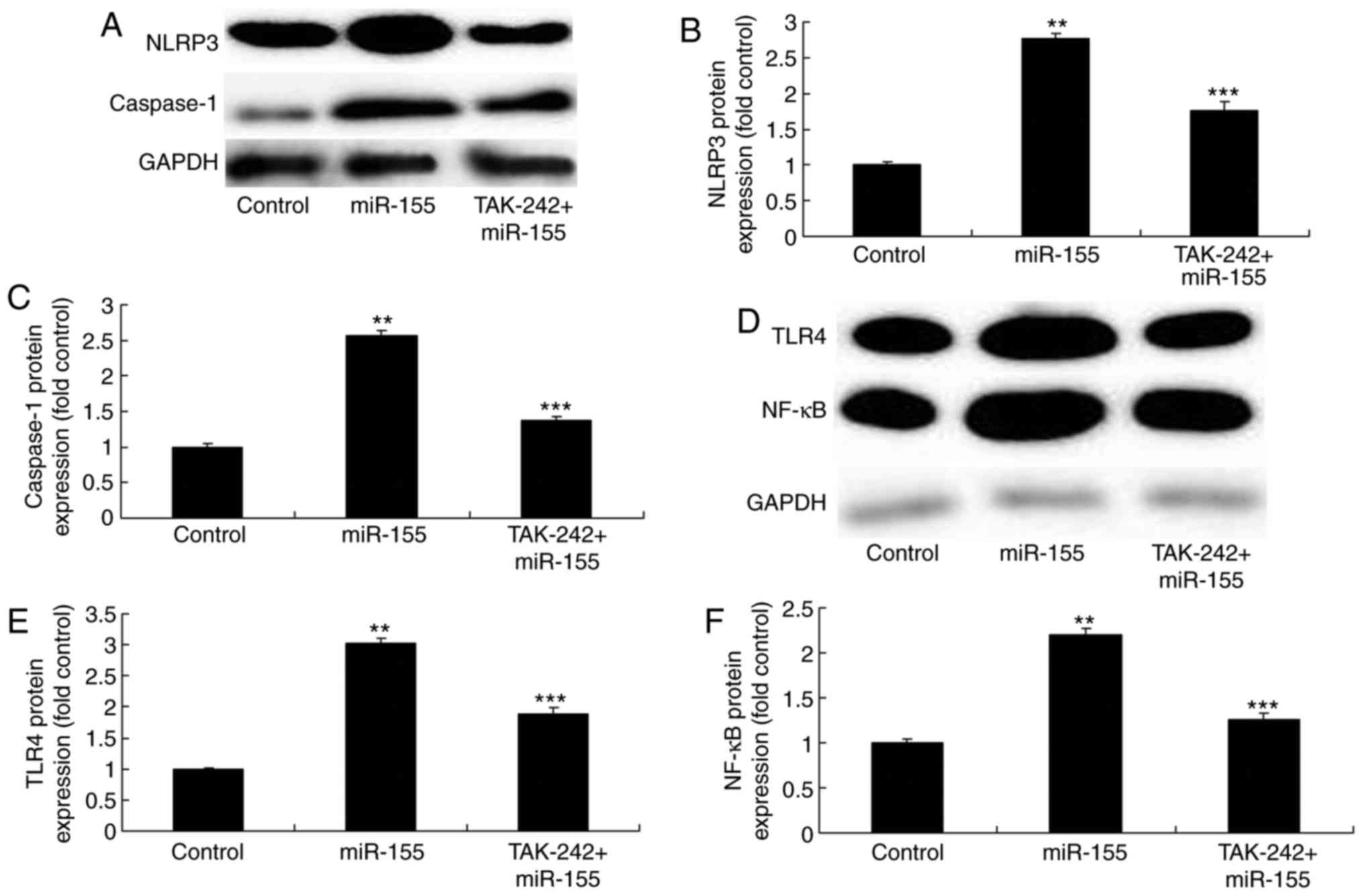
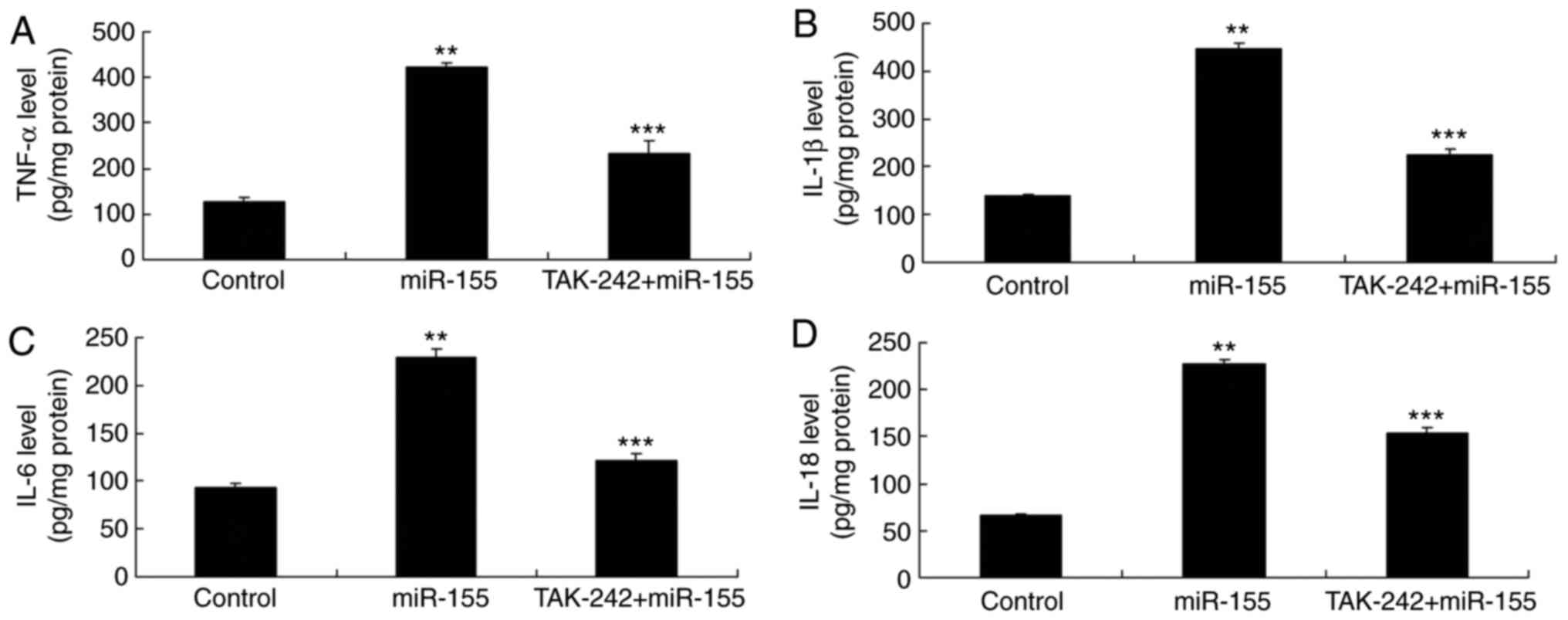
Inhibition of TLR4 reverses the
miR-155-mediated inflammation in keratinocyte-induced HaCaT
cells
The TLR4 inhibitor, TAK-242, was added in the cells
at 2 nM for 8 h to inhibit TLR4 activation. The results
demonstrated that TLR4 inhibition suppressed the NLRP3 and
caspase-1 protein expression levels in miR-155 overexpressing
keratinocyte-induced HaCaT cells, compared with the miR-155
overexpression alone group (Fig.
8A–C). Similarly, inhibition of TLR4 reduced the
miR-155-mediated upregulation of TLR4 and NF-κB, compared with the
miR-155 overexpression alone group (Fig. 8D–F). In addition, inhibition of
TLR4 reversed the miR-155-mediated increase in the secreted levels
of TNF-α, IL-18, IL-6 and IL-1β in keratinocyte-induced HaCaT
cells, compared with the miR-155 overexpression alone group
(Fig. 9).
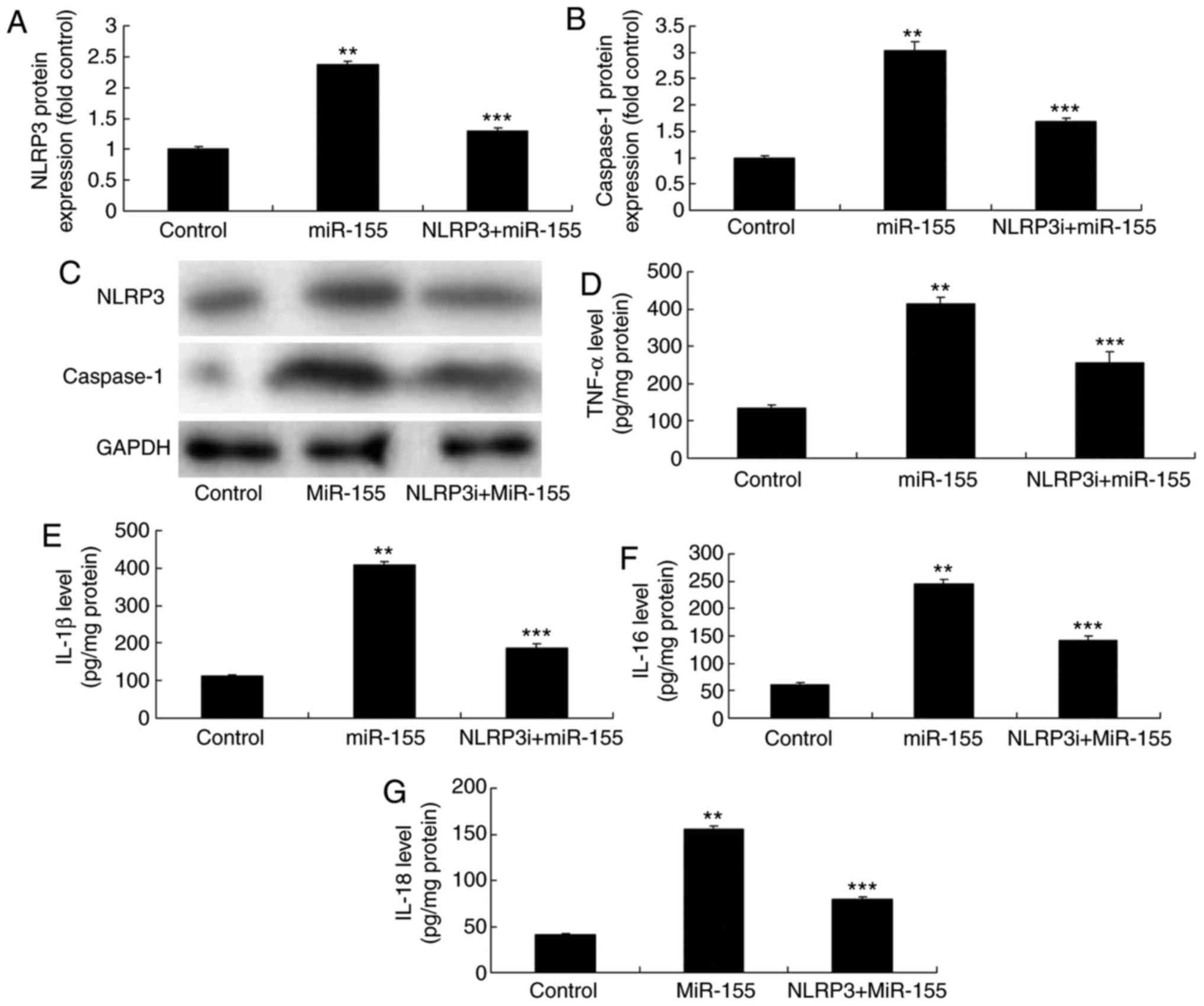
Inhibition of NLRP3 reverses the
miR-155-mediated inflammation in vitro
Finally, the hypothesis that miR-155-induced
regulation of inflammation-related genes may occur through NLRP3
was examined. The NLRP3 inhibitor, MCC950 (2 nM for 8 h), was added
to the cells, and protein expression was analyzed by western
blotting. Addition of the NLRP3 inhibitor suppressed NLRP3 and
caspase-1 protein expression levels in the in vitro
psoriasis model, compared with the miR-155 overexpression alone
group (Fig. 10A–C).
Additionally, the miR-155 overexpression-induced TNF-α, IL-18, IL-6
and IL-1β levels were significantly reduced in the in vitro
keratinocyte model following NLRP3 inhibition, compared with the
miR-155 overexpression alone group (Fig. 10D–G).
Discussion
Neurodermatitis, which is part of the cancer
category in traditional Chinese medicine, is referred to as
psoriasis. It is characterized by its refractoriness. Psoriasis has
gained names such as cowhide carcinoma and incised wound, according
to different skin lesion morphologies (5,16).
Psoriasis is the most common chronic, recurrent and inflammatory
dermatosis characterized by scaly erythema (2). It is the most common refractory
disease in skin, with a morbidity of ~0.1–3% (3). In China, the morbidity in the north
is higher compared with the south, in urban areas is higher
compared with rural areas, and in males is higher compared with
females. According to incomplete statistics, the morbidity of
psoriasis in China is over 2 million (16). However, the urban population in
China is surging in recent years, and China has witnessed an annual
new population of 20 million (5).
Epidemiological statistics suggest that ~0.1 million psoriasis
patients have increased in China every year. The present results
clearly demonstrate that miR-155 and NLRP3 expression were
significantly increased in psoriasis in vivo and in
vitro models compared with control groups.
miRNAs are extensively distributed in nematode,
fruit fly and multiple human tissues and cells (6). It is a type of single-strand
non-coding RNA with a length of ~21–25 nucleotides (6). miRNAs can bind at the
3′-untranslated region of mRNA, and negatively regulate the
expression of related proteins (6). Numerous previous studies have
indicated that miRNAs are closely related to inflammation (6,15).
They predominantly target important proteins in the NLRP3
inflammation-associated pathway, thus promoting or inhibiting the
inflammatory response (17). The
present study demonstrated that overexpression of miR-155
significantly increased TNF-α, IL-18, IL-6 and IL-1β levels in
keratinocyte-induced HaCaT cells, through the NLRP3 inflammasome.
Obora et al (18)
described that inflammation-induced miR-155 inhibits self-renewal
of neural stem cells. However, it remains to be elucidated how
miR-155 regulates NLRP3 in psoriasis.
The NLRP3 inflammasome is constituted by NLRP3, the
apoptosis-associated speck-like protein containing CARD (ASC), and
the cysteinyl aspartate specific proteinase-1, caspase-1 (19). NLRP3 binds to ASC once it is
activated (11), and then it can
recruit pro-caspase-1 and induce its self-cleavage to produce the
activated caspase-1 (19). The
activated caspase-1 can cleave the pro-IL-1β into active IL-1β,
thus activating the downstream inflammatory response (20). The present results demonstrated
that TLR4 induced the miR-155/NLRP3 inflammasome in
keratinocyte-induced HaCaT cells. Bala et al (15) reported that TLR4 signaling
regulates miR-155 to promote liver fibrosis and alcohol-induced
steatohepatitis. Taken together, these data suggest that TLR4
signaling regulates miR-155-induced inflammation through NLRP3
inflammasome in psoriasis.
The NLRP3 inflammasome-activated molecular mechanism
is extremely complicated, and it can be classified into three major
pathways (21). In the first
pathway, extracellular ATP stimulates the opening of the
P2X7-dependent ion channel (21).
This can promote K+ outflow and the formation of the
pannexin-1 membrane channel. Thus, the extracellular NLRP3 agonist
can enter the cell and directly promote the aggregation and
activation of the NLRP3 inflammasome (20). NLRP3 inflammasome enhances the
production of effector molecules, such as caspase-1 and IL-1p.
NF-κB in macrophages can upregulate NLRP3 expression (20). Intracellular inflammatory factors,
such as IL-1β, can also be released out of the cell, thus inducing
inflammatory response (22). In
the present study, addition of an NLRP3 inhibitor reversed the
miR-155 mediated increased inflammation in keratinocyte-induced
HaCaT cells. Artlett et al (23) reported that miR-155 required NLRP3
inflammasome-mediated collagen synthesis during fibrosis.
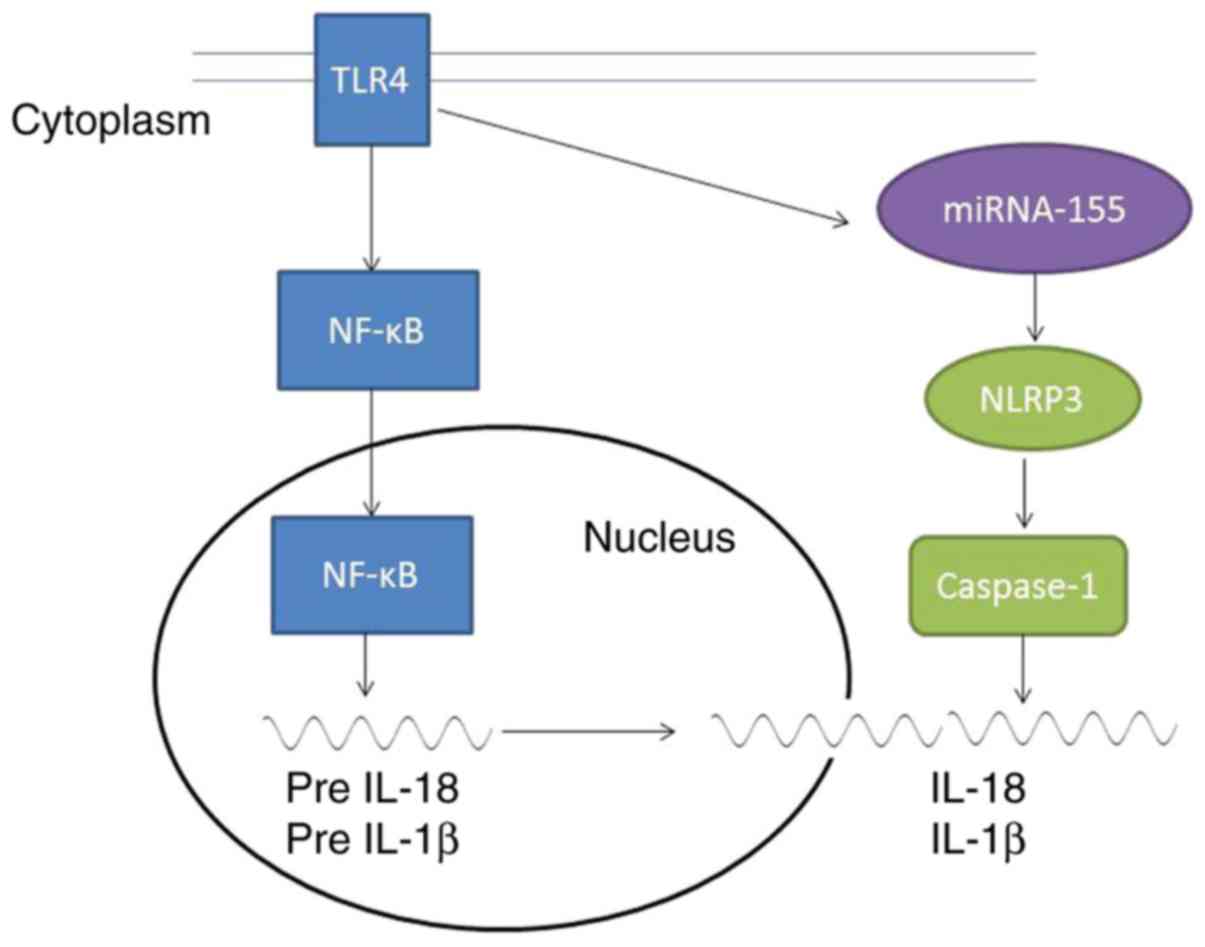
The present data imply that TRL4/miR-155 increased
inflammatory responses in psoriasis through inflammasome NLRP3
activation. TLR4/NF-κB signaling induced inflammation and
miR-155/NLRP3/caspase-1 promoted the production of IL-18 and IL-1β
in psoriasis (Fig. 11). Further
studies targeting miR-155 may provide new prospects in discovering
the causative factors involved in psoriasis and reveal novel
strategies for psoriasis treatment.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XZ designed the experiment; QL, JZ, WL, L L, XZ, XT,
WL, LZ performed the experiment; XZ and QL analyzed the data; XZ
wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Guangzhou Institute of Dermatology (Guangzhou,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reich K, Armstrong AW, Foley P, Song M,
Wasfi Y, Randazzo B, Li S, Shen YK and Gordon KB: Efficacy and
safety of gusel-kumab, an anti-interleukin-23 monoclonal antibody,
compared with adalimumab for the treatment of patients with
moderate to severe psoriasis with randomized withdrawal and
retreatment: Results from the phase III, double-blind, placebo- and
active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol.
76:418–431. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balak DM, van Doorn MB, Arbeit RD,
Rijneveld R, Klaassen E, Sullivan T, Brevard J, Thio HB, Prens EP,
Burggraaf J and Rissmann R: IMO-8400, a toll-like receptor 7, 8,
and 9 antagonist, demonstrates clinical activity in a phase 2a,
randomized, placebo-controlled trial in patients with
moderate-to-severe plaque psoriasis. Clin Immunol. 174:63–72. 2017.
View Article : Google Scholar
|
|
3
|
Hayashi M, Umezawa Y, Fukuchi O, Ito T,
Saeki H and Nakagawa H: Efficacy and safety of ustekinumab
treatment in elderly patients with psoriasis. J Dermatol.
41:974–980. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao L, Chen X, Cai L, Zhang C, Wang Q,
Jing S, Chen G, Li J, Zhang J and Fang Y: Randomized, double-blind,
placebo-controlled, multiple-dose study of the safety, tolerability
and pharmacokinetics of benvitimod, a candidate drug for the
treatment of psoriasis. J Clin Pharm Ther. 39:418–423. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yao DN, Lu CJ, Wen ZH, Yan YH, Xuan ML, Li
XY, Li G, He ZH, Xie XL, Deng JW, et al: Oral PSORI-CM01, a Chinese
herbal formula, plus topical sequential therapy for
moderate-to-severe psoriasis vulgaris: Pilot study for a
double-blind, randomized, placebo-controlled trial. Trials.
17:1402016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Q, Wu DH, Han L, Deng JW, Zhou L, He
R, Lu CJ and Mi QS: Roles of microRNAs in psoriasis: Immunological
functions and potential biomarkers. Exp Dermatol. 26:359–367. 2017.
View Article : Google Scholar
|
|
7
|
Li Y, Su J, Li F, Chen X and Zhang G:
miR-150 regulates human keratinocyte proliferation in hypoxic
conditions through targeting HIF-1alpha and VEGFA: Implications for
psoriasis treatment. PLoS One. 12:e01754592017. View Article : Google Scholar
|
|
8
|
Yu X, An J, Hua Y, Li Z, Yan N, Fan W and
Su C: MicroRNA-194 regulates keratinocyte proliferation and
differentiation by targeting Grainyhead-like 2 in psoriasis. Pathol
Res Pract. 213:89–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dupaul-Chicoine J, Arabzadeh A, Dagenais
M, Douglas T, Champagne C, Morizot A, Rodrigue-Gervais IG, Breton
V, Colpitts SL, Beauchemin N and Saleh M: The Nlrp3 inflammasome
suppresses colorectal cancer metastatic growth in the liver by
promoting natural killer cell tumoricidal activity. Immunity.
43:751–763. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Orlowski GM, Sharma S, Colbert JD, Bogyo
M, Robertson SA, Kataoka H, Chan FK and Rock KL: Frontline science:
Multiple cathepsins promote inflammasome-independent,
particle-induced cell death during NLRP3-dependent IL-1β
activation. J Leukoc Biol. 102:7–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan C, Liu C, Wang T, He Y, Zhou Z, Dun
Y, Zhao H, Ren D, Wang J, Zhang C and Yuan D: Chikusetsu saponin
IVa ameliorates high fat diet-induced inflammation in adipose
tissue of mice through inhibition of NLRP3 inflammasome activation
and NF-kappaB signaling. Oncotarget. 8:31023–31040. 2017.PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
13
|
Maegdefessel L, Spin JM, Raaz U, Eken SM,
Toh R, Azuma J, Adam M, Nakagami F, Heymann HM, Chernogubova E, et
al: Erratum: miR-24 limits aortic vascular inflammation and murine
abdominal aneurysm development. Nat Commun. 6:65062015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang L, Cao J, Cao L, Gao L, Yang Y and
Xu L: Puerarin induces cell apoptosis in human chondrosarcoma cell
line SW1353 via inhibition of the PI3K/Akt signaling pathway. Oncol
Lett. 14:5585–5590. 2017.PubMed/NCBI
|
|
15
|
Bala S, Csak T, Saha B, Zatsiorsky J,
Kodys K, Catalano D, Satishchandran A and Szabo G: The
pro-inflammatory effects of miR-155 promote liver fibrosis and
alcohol-induced steatohepatitis. J Hepatol. 64:1378–1387. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gottlieb A, Sullivan J, van Doorn M,
Kubanov A, You R, Parneix A, Hugot S and Milutinovic M: Secukinumab
shows significant efficacy in palmoplantar psoriasis: Results from
GESTURE, a randomized controlled trial. J Am Acad Dermatol.
76:70–80. 2017. View Article : Google Scholar
|
|
17
|
Zhao M, Wang LT, Liang GP, Zhang P, Deng
XJ, Tang Q, Zhai HY, Chang CC, Su YW and Lu QJ: Up-regulation of
microRNA-210 induces immune dysfunction via targeting FOXP3 in
CD4(+) T cells of psoriasis vulgaris. Clin Immunol. 150:22–30.
2014. View Article : Google Scholar
|
|
18
|
Obora K, Onodera Y, Takehara T, Frampton
J, Hasei J, Ozaki T, Teramura T and Fukuda K: Inflammation-induced
miRNA-155 inhibits self-renewal of neural stem cells via
suppression of CCAAT/enhancer binding protein β (C/EBPβ)
expression. Sci Rep. 7:436042017. View Article : Google Scholar
|
|
19
|
Hoyt LR, Randall MJ, Ather JL, DePuccio
DP, Landry CC, Qian X, Janssen-Heininger YM, van der Vliet A, Dixon
AE, Amiel E and Poynter ME: Mitochondrial ROS induced by chronic
ethanol exposure promote hyper-activation of the NLRP3
inflammasome. Redox Biol. 12:883–896. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lv Z, Wei Z, Zhang Z, Li C, Shao Y, Zhang
W, Zhao X, Li Y, Duan X and Xiong J: Characterization of NLRP3-like
gene from Apostichopus japonicus provides new evidence on
inflammation response in invertebrates. Fish Shellfish Immunol.
68:114–123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carlström M, Ekman AK, Petersson S,
Söderkvist P and Enerbäck C: Genetic support for the role of the
NLRP3 inflammasome in psoriasis susceptibility. Exp Dermatol.
21:932–937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Irrera N, Vaccaro M, Bitto A, Pallio G,
Pizzino G, Lentini M, Arcoraci V, Minutoli L, Scuruchi M, Cutroneo
G, et al: BAY 11-7082 inhibits the NF-kappaB and NLRP3 inflammasome
pathways and protects against IMQ-induced psoriasis. Clin Sci
(Lond). 131:487–498. 2017. View Article : Google Scholar
|
|
23
|
Artlett CM, Sassi-Gaha S, Hope JL,
Feghali-Bostwick CA and Katsikis PD: mir-155 is overexpressed in
systemic sclerosis fibroblasts and is required for NLRP3
inflammasome-mediated collagen synthesis during fibrosis. Arthritis
Res Ther. 19:1442017. View Article : Google Scholar : PubMed/NCBI
|