Introduction
Sepsis is a severe clinical syndrome resulting from
a systemic host response to infection. Septic-induced multiple
organ dysfunctions or shock are usually the main causes of death in
intensive care unit (ICU) patients (1,2).
Sepsis-induced cardiomyopathy (SICM) is attributed to various
mechanisms, including mitochondrial dysfunction (3), alteration of expression of nitric
oxide synthase (4), and
inflammatory cytokine overexpression-induced cardiomyocyte (CM)
apoptosis (5–7). Increasing evidence has demonstrated
that there are close links between inflammation and innate immune
response, which promote the progression of SICM (8–10).
Inflammation involving inflammasome activation, mediated by NLR
family pyrin domain containing 3 (NLRP3) and Toll-like receptor 4
(TLR4), has recently been established as an important mechanism
regulating cardiac dysfunction (11). TLR4 activation induces nuclear
factor (NF)-κB mediated proinflammatory cytokine expression
(12). Additionally, inflammatory
responses are amplified with the induction of NLPR3 in the heart
during SICM (13-15). The NLRP3 inflammasome, as a member
of the NOD-like receptor (NLR) family, is comprised of
apoptosis-associated speck-like protein (ASC), caspase-1 and NLRP3.
Previous studies have reported that the NLRP3 region, not only
controls pro-inflammatory cytokine production, but is also
associated with the pathogenesis of inflammatory diseases (16-18).
Apelin is a peptide hormone, which is widely
expressed in various tissues, and was recently identified as an
endogenous ligand for a G-protein-coupled receptor (19,20). Apelin has an important role in
regulating phosphoinositide 3-kinase/Akt signaling-mediated
angiectasis, inflammatory responses and cell proliferation
(21). A recent study has also
reported that anti-inflammatory effects were regulated by
apelin/NF-κB signaling (22).
In the present clinical study, apelin was
demonstrated to be upregulated in SICM and the myocardial function
was impaired with an enhanced inflammatory response following
sepsis induction. To elucidate the protective mechanism of apelin,
a cecal ligation and puncture (CLP)-induced sepsis-induced
myocardial injury model was established in rats. The TLR4 and NLRP3
signaling pathway-related protein levels were then measured by
western blot analysis, and the inflammatory factor levels were
analyzed by ELISA. The present study identified potential new
targets for the clinical treatment of sepsis.
Materials and methods
Subjects
A total of 73 subjects were recruited from Pudong
New Area Gongli Hospital (Shanghai, China) from April 2016 to June
2017. The normal control subjects (n=34) that were included in this
study, had no family history of sepsis or other chronic diseases,
were free of any major organ disease, and had a stable body weight
for at least 1 year. In the ICU, sepsis was defined as an acute
change in the total Sequential Organ Failure Assessment score ≥2
points as a result of the infection (n=39). The definition of
sepsis-induced cardiomyopathy (SICM) was based on an ejection
fraction (EF) <50% and a ≥10% decrease compared with the
baseline EF that recovered within 2 weeks (7). If the baseline was unknown, the
baseline was defined as an increase of >10% compared with the
initial EF assessed on admission. The EF improving to baseline
within 2 weeks was the definition of recovery. Inotropic agents,
such as dobutamine and epinephrine, were not used prior to
trans-thoracic echocardiography assessment. All clinical data were
gathered from the electronic medical records written by residents
or attending physicians of the emergency and critical care
departments. The clinical characteristics of the patients are
listed in Table I. To identify
the relative expression of apelin in sepsis, 2 ml plasma samples
from each sepsis patient or healthy volunteer were collected. An
informed consent was signed by each participant.
 | Table IComparison of the clinicopathological
characteristics in SICM patients and healthy controls. |
Table I
Comparison of the clinicopathological
characteristics in SICM patients and healthy controls.
| Clinical
parameter | SICM | Healthy
control | P-value |
|---|
| Sex | | | |
| Male | 26 (66.7%) | 20 (58.8%) | |
| Female | 13 (33.3%) | 14 (41.2%) | |
| Age | 71.6±10.7 | 51.6±15.9 | 0.0188 |
| HR (bpm) | 65.6±7.7 | 76.4±8.7 | 0.082 |
| EF (%) | 32.8±4.6 | 57.7±6.6 | 0.003 |
| FS (%) | 21.6±3.2 | 30.1±4.7 | 0.005 |
| LVESD (mm) | 29.3±2.8 | 33.2±7.0 | 0.067 |
| LVEDD (mm) | 37.2±4.4 | 46.7±5.7 | 0.023 |
| SV (µl) | 24.6±3.6 | 57.7±13.1 | 0.0032 |
| Long of ICU Stay
(days) | 23.0±22.9 | 0 | – |
| 28 d survival
(days) | 23 (59.0%) | – | – |
| PCT (ng/ml) | 3.5±3.1 | 78.6±58.6 | 0.0001 |
| WBC
(×109/l) | 15.5±10.2 | 6.5±1.1 | 0.0001 |
| CRP (mg/dl) | 128.1±54.4 | 8.7±11.2 | 0.0001 |
| Blood culture | | | |
| G (+) | 5 (7.7%) | 0 | – |
| G (−) | 23 (59.0%) | 0 | – |
| Fungus | 7 (17.9%) | | |
| Sources of
infection | | | |
| Lower respiratory
tract | 26 (66.7%) | 0 | – |
| Upper respiratory
tract | 0 | 0 | – |
| Urinary tract | 2 (5.1%) | 0 | – |
|
Intra-abdominal | 2 (5.1%) | 0 | – |
|
Musculoskeletal/soft tissue | 5 (12.8%) | 0 | – |
| Intravascular | 1 (2.6%) | 0 | – |
| Inflammatory
cytokines | | | |
| IL-6 (pg/ml) | 63.5±25.5 | 14.8±6.5 | 0.001 |
| IL-1β (pg/ml) | 124.6±84.1 | 22.5±11.6 | 0.001 |
| TNF-α (pg/ml) | 22.8±16.6 | 5.6±3.5 | 0.001 |
Sepsis model and treatment
Male Sprague Dawley rats (age, 7–8 weeks old;
weight, 200–250 g) were provided by Shanghai SLAC Laboratory Animal
Co. (Shanghai, China). Rats were provided with irradiated food,
free access to sterile acidified water, and were housed in
individual micro-isolators. For sepsis induction, animals were
anesthetized by intraperitoneal injection of 2% sodium
pentobarbital in saline (40 mg/kg; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and placed on a warming pad (37°C). CLP was
performed as described previously (23). Briefly, the rat abdomen was shaved
before a 2 cm long midline abdominal incision was made. In order to
prevent bowel obstruction, the cecum was carefully isolated and
~60% of the total cecum length was ligated below the ileocecal
valve. Two punctures were made on the opposite side of the
mesenteric with a sterile 18-gauge needle. The cecum was then
gently pressed to expel a small amount of stool from the puncture
site to ensure a full-thickness perforation. The cecum was returned
to the peritoneal cavity, and the abdominal incision was closed in
two layers. The cecums of sham-operated rats were not ligated or
punctured, but underwent the same procedure as aforementioned. All
rats were subcutaneously injected with normal saline (5 ml/100 g)
immediately following surgery in order to resuscitate. They were
then group-housed in a temperature-controlled room (22°C) in cages
with dry sawdust bedding. Fluid blocks and soft food were provided.
The mortality and behavioral signs were monitored and recorded
every day following the CLP procedure.
To identify the effect of apelin on sepsis-induced
cardiomyopathy, apelin (2 mg/kg/day; American Peptide, Sunnyvale,
CA, USA) or an equal dose of normal saline were administered daily
intraperitoneally after the sepsis model was induced for 3 days.
Each group was comprised of 10 rats. Seven days post-surgery, the
heart and blood from the rats were collected for subsequent
experiments.
ELISA
Plasma inflammatory factors, interleukin (IL)-1β
(cat. no. RAB0273), IL-6 (cat. no. RAB0306), tumor necrosis factor
(TNF)-α (cat. no. RAB1089), and apelin, were measured with
commercially available ELISA kits (Sigma-Aldrich; Merck KGaA). All
procedures were performed according to the manufacturer's
instructions.
Immunohistochemical analysis
Myocardial tissue samples were fixed in 10% formalin
solution prior to embedding in paraffin. The 4 μm thickness
sections were stained with hematoxylin and eosin (H&E) or
terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling
(TUNEL) at room temperature. Nuclear were stain with DAPI. The
results were observed using an Axiophot light microscope (Zeiss AG,
Oberkochen, Germany) and photographed with a digital camera. A
total of 10 random fields in each group were selected for
statistical analysis.
Western blot analysis
Western blot analyses for TLR4 and NLRP3 signaling
pathway proteins in myocardial tissues were performed as previously
described (24). Briefly,
proteins in myocardial tissue lysates were separated by
electrophoresis and transferred to a polyvinylidene difluoride
membrane. The membranes were blocked and then incubated with
antibodies against GAPDH (cat. no. G5262; 1:200), NLRP3 (cat. no.
HPA012878; 1:200), ASC (cat. no. SAB4501315; 1:200), caspase-1
(cat. no. C5482; 1:200), TLR4 (cat. no. SAB1301541; 1:200), NF-κB
inhibitor α (IκBα; cat. no. SAB36552; 1:200), phosphorylated
(p)-IκBα (cat. no. SAB625455; 1:200), RELA proto-oncogene NF-κB
subunit (also known as P65) (cat. no. SAB251154; 1:200), and p-P65
(all from Sigma-Aldrich; Merck KGaA) (cat. no. SAB4536551; 1:200),
in TBST buffer overnight at 4°C. The membranes were then incubated
with secondary antibody linked to horseradish peroxidase
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Immunoreactive bands were developed using an enhanced
chemiluminescence reagent (Pierce; Thermo Fisher Scientific, Inc.).
Band densities were normalized to the density of GAPDH and
quantified with scanning densitometric analysis using ImageJ
software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation. Differences between treatment groups were analyzed using
one-way analysis of variance followed by Tukey's multiple
comparisons test, using GraphPad Prism 6.0 software (GraphPad
Software Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Inflammatory factors and cardiac
functional analyses in patients with SICM
In total, 73 participants with or without sepsis
were studied. Of these, 39 patients had sepsis and 34 subjects were
healthy controls. The sex distribution of each group was uniform.
In total, 26 patients with SICM were male (66.7%; Table I). There was a significant
difference in age between patients with SICM and those without SICM
(Table I). Among the patients
with SICM, the 28-day survival in-hospital was 59% and the average
ICU stay was 23 days. Among the patients with SICM, 26 (66.7%) had
respiratory infections, two (5.1%) had intra-abdominal infections,
two (5.1%) had urinary tract infections, five (12.8%) had
musculoskeletal or soft tissue infections, and one (2.6%) had an
intravascular infection (Table
I). The blood culture was positive in 35 patients with SICM;
five (7.7%) had Gram positive bacterial infections, 23 (59%) had
Gram negative bacterial infections, and 7 (17.9%) had fungal
infections (Table I).
Cardiac functional analyses revealed that, compared
with healthy controls, fractional shortening (FS), EF, stroke
volume (SV), left ventricular end-diastolic dimension (LVEDD) and
left ventricular end-systolic dimension (LVESD) were significantly
decreased in SICM patients, but the heart rate have no significant
difference between the two groups (Table I).
Both C-reactive and white blood cells, which are
biomarkers used in the diagnosis of sepsis and inflammation
response in SICM patients (25),
were significantly increased in SICM patients compared with healthy
controls (Table I), suggesting an
inflammatory response in SICM patients. ELISA analyses confirmed
that plasma levels of IL-6, TNF-α and IL-1β were significantly
increased following sepsis (Table
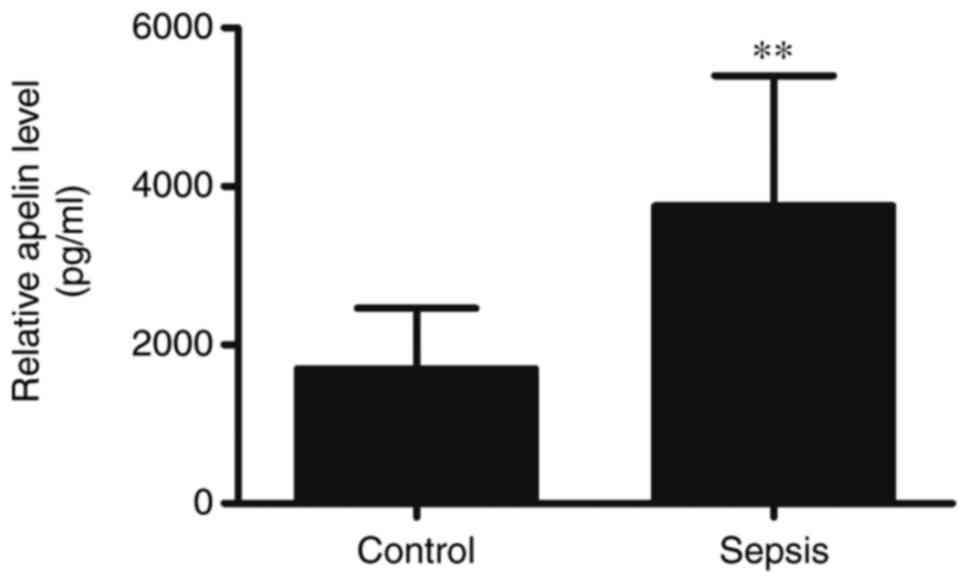
I), compared with healthy controls. Apelin, which has
anti-inflammatory effects, was also significantly increased in SICM
(Fig. 1), suggesting that the
expression of apelin might have had a protective effect against
SICM.
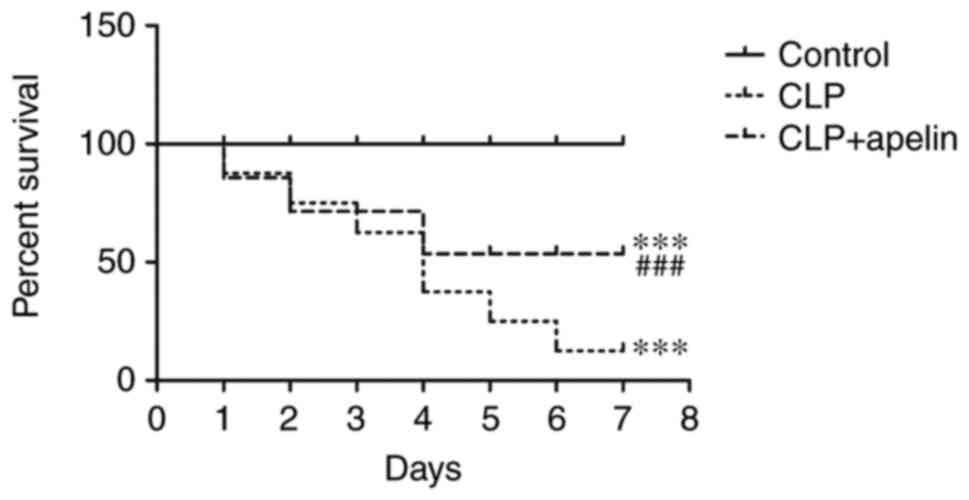
Apelin treatment improves survival and
attenuates CLP sepsis-induced myocardial injury
To determine the protective effects of apelin on
cardiac functions during sepsis, apelin (2 mg/kg/day) was
intraperitoneally administered for 3 consecutive days post-surgery,
and the mortality % was monitored every day for 7 days before the
rats were euthanized. The results demonstrated no deaths in the
control sham-operated group. Apelin treatment attenuated the
mortality of CLP-induced sepsis rats by 30–70%, demonstrating that
apelin treatment increased the survival of these animals (Fig. 2).
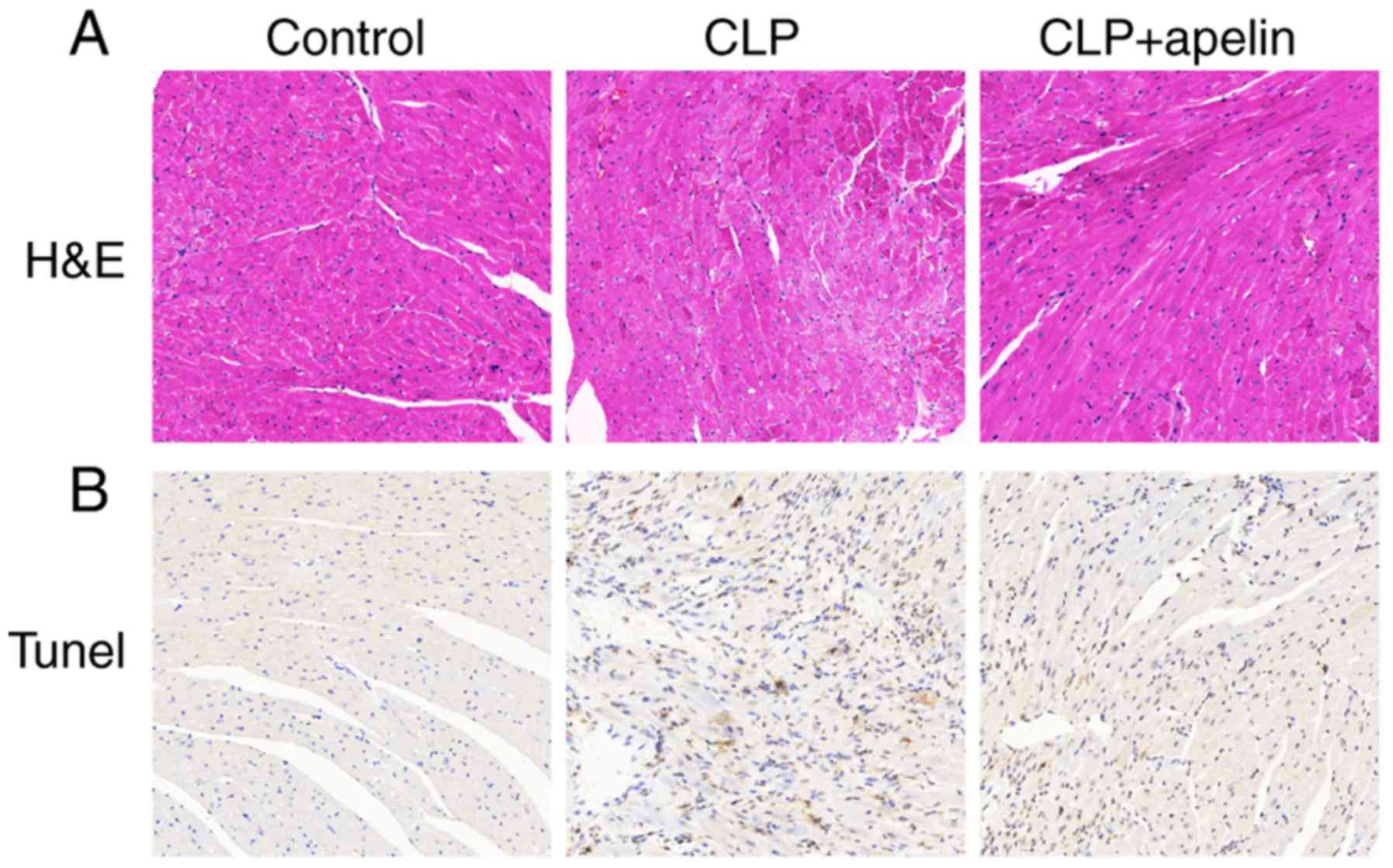
To identify pathological changes, myocardial tissue
was collected for H&E and TUNEL staining at 7 days
post-surgery. Fig. 3A illustrates
that the cell structures in the hearts of control sham-operated
rats were normal. However, the heart tissues of rats from the CLP
group, at seven days post-CLP, exhibited blurring of myocardial
stripes, myocardial fiber disarray, unclear cell boundary, small
necrosis focus, inflammatory cell infiltration and interstitial
edema (Fig. 3A). These
pathological changes were partially decreased following apelin
treatment (Fig. 3A). Previous
studies have reported that sepsis-induced cardiac dysfunction was
positively correlated with myocardial apoptosis (26,27). Thus, the TUNEL assay was used to
examine the rate of myocardial apoptosis in the experimental
groups. As illustrated in Fig.
3B, a marked increase in apoptotic cells was observed in the
heart tissues of the CLP rats compared with the control
sham-operated rats, confirming that CLP sepsis significantly
increased myocardial apoptosis. The numbers of apoptotic cells were
obviously decreased with apelin treatment (Fig. 3B), further confirming that apelin
had a protective effect on myocardial tissue.
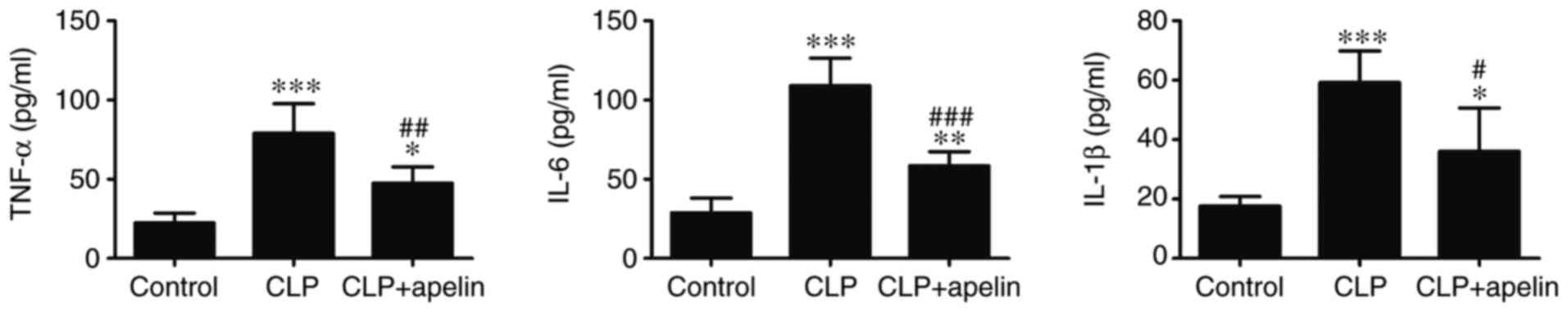
Apelin treatment attenuates the
sepsis-induced production of inflammatory cytokines by suppressing
TLR4 and NLRP3 signaling
Inflammatory cytokines, such as IL-1β, IL-6 and
TNFα, have been reported to suppress myocardial function (28). Thus, the effect of apelin on
CLP-induced and sepsis-mediated inflammatory cytokine production
was examined. Fig. 4 demonstrates
that the expression of inflammatory cytokines IL-1β, IL-6 and TNFα
were significantly increased following CLP-induced sepsis compared
with the sham controls. By contrast, apelin treatment significantly
reduced IL-1β, TNFα and IL-6 levels in plasma (Fig. 4).
In order to determine the possible mechanism and
relationship between CLP sepsis-induced inflammatory responses and
apelin, TLR4/NF-kB pathway-related proteins were analyzed by
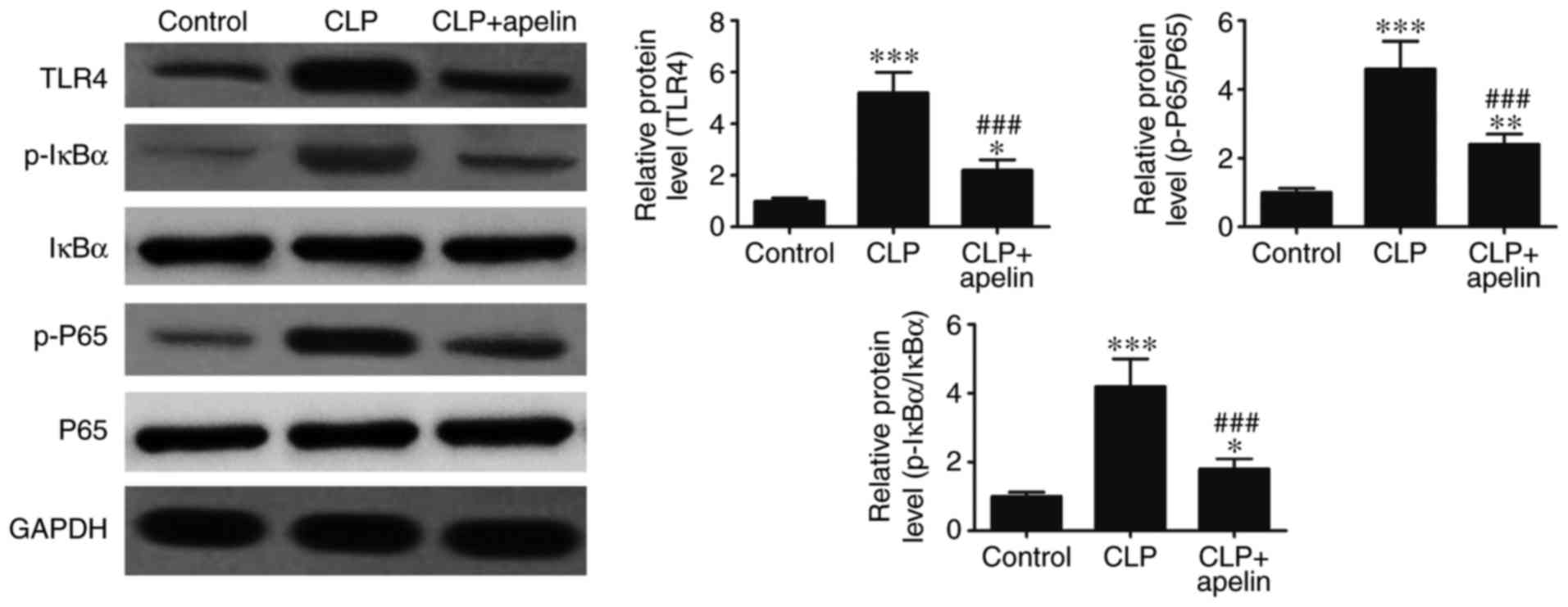
western blotting. Fig. 5
demonstrates that, compared with the CLP group, apelin treatment
significantly reversed the sepsis-induced upregulation of TLR4, and
the sepsis-induced phosphorylation of P65, and IκBα.
The present study demonstrated that apelin treatment
reduced the IL-1β, IL-6 and TNF-α expression in CLP-induced sepsis
rats. A previous study has reported that the inactive cytoplasmic
precursors pro-IL-1β, pro-IL-6 and pro-TNF-α can be cleaved into
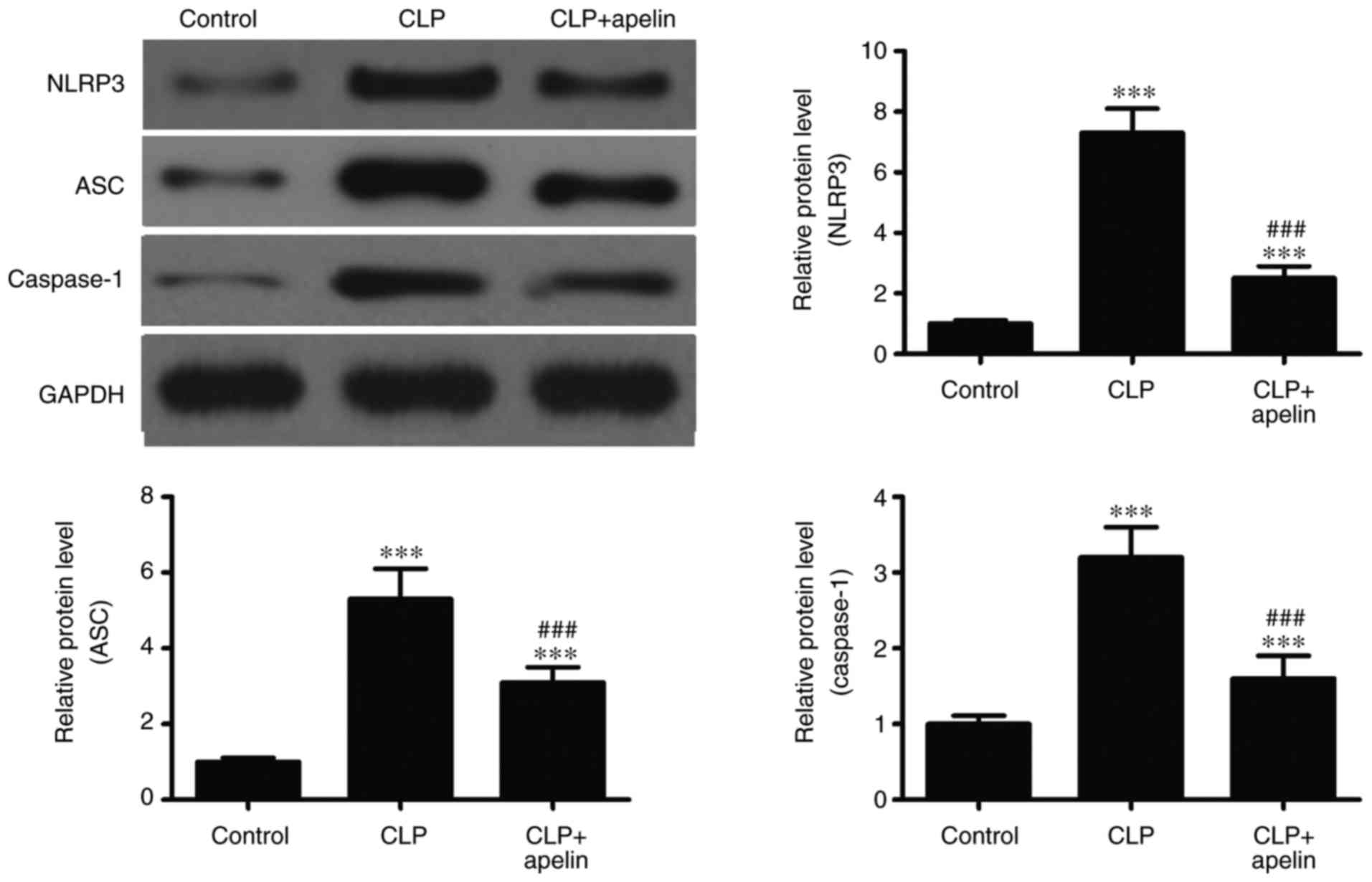
their active forms by caspase-1 (12). The NLRP3 inflammasome also serves
an important role in caspase-1 activation. Therefore, in order to
determine the role of the NLRP3 inflammasome in the inflammatory
responses of the myocardium, the levels of NLRP3
inflammasome-related proteins were analyzed by western blotting.
The results demonstrated that ASC, NLRP3 and caspase-1 protein
expression levels were significantly increased following sepsis,
but apelin treatment significantly reversed these effects (Fig. 6). Taken together, the present
results suggested that apelin inhibited the expression of
inflammatory cytokines by suppressing TLR4 and NLRP3 inflammasome
signaling.
Discussion
The present study revealed that expression of
inflammatory cytokines increased in SICM patients who were prone to
myocardial dysfunction. The results also revealed the increased
expression of plasma apelin in SICM patients, and in vivo
experiments demonstrated that apelin treatment reduced the heart
damage induced by CLP-mediated sepsis in a rat model. Apelin
treatment decreased the plasma levels of IL-6, TNF-α and IL-1β,
inhibited myocardial apoptosis, and protected against CLP
sepsis-induced myocardial dysfunction by inhibiting the TLR4 and
NLRP3 signaling pathways.
Inflammatory factors, including IL-6, TNF-α and
IL-1β, have been recognized to have critical roles in the
pathogenesis of CLP-induced sepsis (29,30). Several studies have reported that
IL-6, TNF-α and IL-1β are involved in myocardial dysfunction
(31,32). Furthermore, previous studies have
demonstrated that inhibition of the expression of these
inflammatory cytokines had a protective effect against SICM
(9,33,34). The effects of apelin on
inflammatory cytokine production were investigated in the present
study. The results demonstrated that apelin protected against CLP
sepsis-induced myocardial dysfunction by inhibiting inflammatory
cytokine production.
The TLR4 signaling pathway has an important role in
pathogenesis of sepsis, which subsequently induces NF-κB activation
and releases inflammatory cytokines. Previous studies have reported
that sepsis-induced cardiomyopathy is induced through the TLR4
signaling pathway (35,36). A variety of inflammatory genes are
regulated by transcriptional factor NF-κB (37), and a previous study has
demonstrated that the development of cardiomyopathy is closely
associated with NF-κB activation (9). In addition, the activation of NF-κB
was observed in a CLP-induced rat sepsis model (38,39). The present results suggested a
role of apelin in anti-inflammatory responses and revealed an
association between apelin and the TLR4 signaling pathway. The
present results demonstrated that CLP sepsis-induced TLR4
expression and NF-κB activation were inhibited following apelin
treatment. The present study also demonstrated that apelin
treatment significantly inhibited NLRP3 inflammasome-induced
myocardial dysfunction. NLRP3 is a multiprotein complex, which can
promote the secretion of IL-1β by activating caspase-1 (40). A previous study has demonstrated
that inhibition of NLRP3 inflammasome expression attenuated the
adverse factor-induced myocardial remodeling (41). In conclusion, the findings of the
present study provided evidence that apelin may have protective
effects against SICM, most likely through the inhibition of the
TLR4 and NLRP3 signaling pathways.
Funding
This work was funded by grants from the Shanghai
Pudong New Area Science and Technology Development Fund Innovative
Funding (Health) Program (grant no. PKJ2015-Y55) and the Shanghai
Pudong New Area Health System Key Specialty Construction Project
(grant no. PWZzk2017-05).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Author's contributions
QL, GL, GC and DG generated and analyzed the data.
LX, MH and MJ designed the experiments and drafted the manuscript.
All authors approved the final version of the manuscript.
Ethics approval and consent to
participate
All experiments involving human subjects or animals
were approved by the Human Ethics Committee of Pudong New Area
Gongli Hospital (Shanghai, China) and the Animal Ethics Committee
of Pudong New Area Gongli Hospital (Shanghai, China), respectively.
Informed consent was obtained from all individual participants
included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Papadopoulos P, Pistiki A,
Theodorakopoulou M, Christodoulopoulou T, Damoraki G, Goukos D,
Briassouli E, Dimopoulou I, Armaganidis A, Nanas S, et al:
Immunoparalysis: Clinical and immunological associations in SIRS
and severe sepsis patients. Cytokine. 92:83–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alam N, de Ven PM, Oskam E, Stassen P,
Kramer MH, Exter PV and Nanayakkara PW: Study protocol for a
multi-centre, investigator-initiated, randomized controlled trial
to compare the effects of prehospital antibiotic treatment for
sepsis patients with usual care after training emergency medical
services (EMS) personnel in early recognition (- The prehospital
antibiotics against sepsis (PHANTASi) trial. Acute Med. 15:176–184.
2016.
|
|
3
|
Li L, Hu BC, Chen CQ, Gong SJ, Yu YH, Dai
HW and Yan J: Role of mitochondrial damage during cardiac apoptosis
in septic rats. Chin Med J. 126:1860–1866. 2013.PubMed/NCBI
|
|
4
|
Ndongson-Dongmo B, Heller R, Hoyer D,
Brodhun M, Bauer M, Winning J, Hirsch E, Wetzker R, Schlattmann P
and Bauer R: Phosphoinositide 3-kinase gamma controls
inflammation-induced myocardial depression via sequential cAMP and
iNOS signalling. Cardiovasc Res. 108:243–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalbitz M, Fattahi F, Grailer JJ, Jajou L,
Malan EA, Zetoune FS, Huber-Lang M, Russell MW and Ward PA:
Complement-induced activation of the cardiac NLRP3 inflammasome in
sepsis. FASEB J. 30:3997–4006. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kakihana Y, Ito T, Nakahara M, Yamaguchi K
and Yasuda T: Sepsis-induced myocardial dysfunction:
Pathophysiology and management. J Intensive Care. 4:222016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sato R and Nasu M: A review of
sepsis-induced cardiomyopathy. J Intensive Care. 3:482015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hinkelbein J, Kalenka A, Schubert C,
Peterka A and Feldmann RE Jr: Proteome and metabolome alterations
in heart and liver indicate compromised energy production during
sepsis. Protein Pept Lett. 17:18–31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng G, Pan M, Jin W, Jin G and Huang Y:
MicroRNA-135a is up-regulated and aggravates myocardial depression
in sepsis via regulating p38 MAPK/NF-κB pathway. Int
Immunopharmacol. 45:6–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Turillazzi E, Fineschi V, Palmiere C and
Sergi C: Cardiovascular involvement in sepsis. Mediators Inflamm.
2016:85847932016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song E, Jahng JW, Chong LP, Sung HK, Han
M, Luo C, Wu D, Boo S, Hinz B, Cooper MA, et al: Lipocalin-2
induces NLRP3 inflammasome activation via HMGB1 induced TLR4
signaling in heart tissue of mice under pressure overload
challenge. Am J Transl Res. 9:2723–2735. 2017.PubMed/NCBI
|
|
12
|
Zhang X, Du Q, Yang Y, Wang J, Dou S, Liu
C and Duan J: The protective effect of Luteolin on myocardial
ischemia/reperfusion (I/R) injury through TLR4/NF-κB/NLRP3
inflammasome pathway. Biomed Pharmacother. 91:1042–1052. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hao H, Cao L, Jiang C, Che Y, Zhang S,
Takahashi S, Wang G and Gonzalez FJ: Farnesoid X receptor
regulation of the NLRP3 inflammasome underlies
cholestasis-associated sepsis. Cell Metab. 25:856–867. e8552017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang W, Tao A, Lan T, Cepinskas G, Kao R,
Martin CM and Rui T: Carbon monoxide releasing molecule-3 improves
myocardial function in mice with sepsis by inhibiting NLRP3
inflammasome activation in cardiac fibroblasts. Basic Res Cardiol.
112:162017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin L, Batra S and Jeyaseelan S: Deletion
of Nlrp3 augments survival during polymicrobial sepsis by
decreasing autophagy and enhancing phagocytosis. J Immunol.
198:1253–1262. 2017. View Article : Google Scholar
|
|
16
|
Kim MJ, Yoon JH and Ryu JH: Mitophagy: A
balance regulator of NLRP3 inflammasome activation. BMB Rep.
49:529–535. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shao QH, Zhang XL, Yang PF, Yuan YH and
Chen NH: Amyloidogenic proteins associated with neurodegenerative
diseases activate the NLRP3 inflammasome. Int Immunopharmacol.
49:155–160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Awad F, Assrawi E, Jumeau C,
Georgin-Lavialle S, Cobret L, Duquesnoy P, Piterboth W, Thomas L,
Stankovic-Stojanovic K, Louvrier C, et al: Impact of human monocyte
and macrophage polarization on NLR expression and NLRP3
inflammasome activation. PLoS One. 12:e01753362017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han S, Englander EW, Gomez GA and Greeley
GH Jr: Apelin regulates nuclear factor-κB's involvement in the
inflammatory response of pancreatitis. Pancreas. 46:64–70. 2017.
View Article : Google Scholar
|
|
20
|
Luo K, Long H, Xu B and Luo Y: Apelin
attenuates postburn sepsis via a phosphatidylinositol
3-kinase/protein kinase B dependent mechanism: A randomized animal
study. Int J Surg. 21:22–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang P, Yi LH, Meng GY, Zhang HY, Sun HH
and Cui LQ: Apelin-13 attenuates cisplatin-induced cardiotoxicity
through inhibition of ROS-mediated DNA damage and regulation of
MAPKs and AKT pathways. Free Radic Res. 51:449–459. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Hu W, Feng F, Xu J and Wu F:
Apelin-13 protects against myocardial infarction-induced myocardial
fibrosis. Mol Med Rep. 13:5262–5268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou Q, Pan X, Wang L, Wang X and Xiong D:
The protective role of neuregulin-1: A potential therapy for
sepsis-induced cardiomyopathy. Eur J Pharmacol. 788:234–240. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao M, Li L, Li C, Zhang P, Hu Q, Ma L
and Zhang H: Role of autophagy and apoptosis in wound tissue of
deep second-degree burn in rats. Acad Emerg Med. 21:383–391. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yao L, Liu Z, Zhu J, Li B, Chai C and Tian
Y: Clinical evaluation of circulating microRNA-25 level change in
sepsis and its potential relationship with oxidative stress. Int J
Clin Exp Pathol. 8:7675–7684. 2015.PubMed/NCBI
|
|
26
|
Zhang M, Wang X, Bai B, Zhang R, Li Y and
Wang Y: Oxymatrine protects against sepsis-induced myocardial
injury via inhibition of the TNF-α/p38-MAPK/caspase-3 signaling
pathway. Mol Med Rep. 14:551–559. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
An R, Zhao L, Xi C, Li H, Shen G, Liu H,
Zhang S and Sun L: Melatonin attenuates sepsis-induced cardiac
dysfunction via a PI3K/Akt-dependent mechanism. Basic Res Cardiol.
111:82016. View Article : Google Scholar
|
|
28
|
Zhao H, Zhang M, Zhou F, Cao W, Bi L, Xie
Y, Yang Q and Wang S: Cinnamaldehyde ameliorates LPS-induced
cardiac dysfunction via TLR4-NOX4 pathway: The regulation of
autophagy and ROS production. J Mol Cell Cardiol. 101:11–24. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee S, Nakahira K, Dalli J, Siempos II,
Norris PC, Colas RA, Moon JS, Shinohara M, Hisata S, Howrylak JA,
et al: NLRP3 inflammasome deficiency protects against microbial
sepsis via increased lipoxin B4 synthesis. Am J Respir
Crit Care Med. 196:713–726. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cho W, Koo JY, Park Y, Oh K, Lee S, Song
JS, Bae MA, Lim D, Lee DS and Park SB: Treatment of sepsis
pathogenesis with high mobility group box protein 1-regulating
anti-inflammatory agents. J Med Chem. 60:170–179. 2017. View Article : Google Scholar
|
|
31
|
Gao M, Ha T, Zhang X, Liu L, Wang X,
Kelley J, Singh K, Kao R, Gao X, Williams D and Li C: Toll-like
receptor 3 plays a central role in cardiac dysfunction during
polymicrobial sepsis. Crit Care Med. 40:2390–2399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ha T, Xia Y, Liu X, Lu C, Liu L, Kelley J,
Kalbfleisch J, Kao RL, Williams DL and Li C: Glucan phosphate
attenuates myocardial HMGB1 translocation in severe sepsis through
inhibiting NF-κB activation. Am J Physiol Heart Circ Physiol.
301:H848–H855. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma H, Wang X, Ha T, Gao M, Liu L, Wang R,
Yu K, Kalbfleisch JH, Kao RL, Williams DL and Li C: MicroRNA-125b
prevents cardiac dysfunction in polymicrobial sepsis by targeting
TRAF6-mediated nuclear factor κB activation and p53-mediated
apoptotic signaling. J Infect Dis. 214:1773–1783. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao F, Yang YZ, Feng XY, Fan TT, Jiang L,
Guo R and Liu Q: Interleukin-27 is elevated in sepsis-induced
myocardial dysfunction and mediates inflammation. Cytokine.
88:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vaez H, Rameshrad M, Najafi M, Barar J,
Barzegari A and Garjani A: Cardioprotective effect of metformin in
lipopoly-saccharide-induced sepsis via suppression of toll-like
receptor 4 (TLR4) in heart. Eur J Pharmacol. 772:115–123. 2016.
View Article : Google Scholar
|
|
36
|
Rameshrad M, Maleki-Dizaji N, Vaez H,
Soraya H, Nakhlband A and Garjani A: Lipopolysaccharide induced
activation of toll like receptor 4 in isolated rat heart suggests a
local immune response in myocardium. Iran J Immunol. 12:104–116.
2015.PubMed/NCBI
|
|
37
|
Afonina IS, Zhong Z, Karin M and Beyaert
R: Limiting inflammation-the negative regulation of NF-κB and the
NLRP3 inflammasome. Nat Immunol. 18:861–869. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang N, Mao L, Yang L, Zou J, Liu K, Liu
M, Zhang H, Xiao X and Wang K: Resveratrol protects against early
polymicrobial sepsis-induced acute kidney injury through inhibiting
endoplasmic reticulum stress-activated NF-κB pathway. Oncotarget.
8:36449–36461. 2017.PubMed/NCBI
|
|
39
|
Chen J, Kieswich JE, Chiazza F, Moyes AJ,
Gobbetti T, Purvis GS, Salvatori DC, Patel NS, Perretti M, Hobbs
AJ, et al: IκB kinase inhibitor attenuates sepsis-induced cardiac
dysfunction in CKD. J Am Soc Nephrol. 28:94–105. 2017. View Article : Google Scholar
|
|
40
|
Tschopp J and Schroder K: NLRP3
inflammasome activation: The convergence of multiple signalling
pathways on ROS production? Nat Rev Immunol. 10:210–215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li R, Lu K, Wang Y, Chen M, Zhang F, Shen
H, Yao D, Gong K and Zhang Z: Triptolide attenuates pressure
overload-induced myocardial remodeling in mice via the inhibition
of NLRP3 inflammasome expression. Biochem Biophys Res Commun.
485:69–75. 2017. View Article : Google Scholar : PubMed/NCBI
|