Introduction
Breast cancer has the highest incidence rate of all
female malignancies (1). Breast
cancer-related death ranks first among all cancer-related mortality
in females (2). The prevention
and treatment of breast cancer have received much attention in the
field of oncology for years. Recently, there have been
breakthroughs in the treatment of breast cancer, especially in
endocrine therapy and molecular-targeted therapy (3-6).
However, these therapies may only benefit certain patients; for
triple-negative breast cancer or patients with a heavy tumor
burden, the treatment choice is limited and the prognosis is still
poor (7-9). Novel agents are urgently needed to
enrich current treatment strategies. Following the success of
artemisinin and paclitaxel, Traditional Chinese Medicine and
natural plant extracts have received increasing attention in
medical research (10,11), as they have important roles in the
prevention and treatment of breast cancer (12).
Flavonoids are a class of small-molecule
polyphenolic compounds and secondary metabolites of plants.
Naturally occurring flavonoids are widely distributed in the roots,
stems, and leaves of plants (13,14). Flavonoids perform various
biological activities, including anti-inflammatory, antioxidant,
anti-allergic and antiviral activities (15,16). Furthermore, flavonoids have
effects in tumor prevention and treatment (17-19). Dietary intake of flavonoids has
been negatively correlated with the risk incidence of a number of
different types of cancer (20,21).
Fisetin, a natural flavonoid found in a variety of
edible and medicinal plants, has been suggested to possess
anti-tumor activity (13,22). Fisetin inhibits the proliferation,
metastasis and invasiveness of lung cancer cells (23,24). A similar anti-tumor effect of
fisetin has been observed in preclinical studies of colorectal
cancer, prostate cancer, pancreatic cancer and melanoma (25-28), and it has been indicated that
phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mechanistic
target of rapamycin (mTOR) may be the target signaling pathway of
fisetin (22). The PI3K/Akt/mTOR
pathway is also known to have a central role in various cellular
processes that contribute to the malignant phenotype of breast
cancer (29-31). Previous studies have reported that
fisetin and/or its nanoparticles induced cytotoxicity in MCF-7 and
MDA-MB-231 cells by apoptosis in vitro (32-37), and another study reported the
anti-tumor effect of fisetin in an MCF-7-bearing xenograft tumor
model in vivo (38).
However, the underlying mechanism of how fisetin induces apoptosis
of breast cancer cells remains to be elucidated. Considering the
role of fisetin in the prevention and treatment of other tumors,
the present study investigated the effect of fisetin on mammary
carcinoma cells proliferation, migration and invasion, and explored
the potential underlying molecular mechanisms.
Materials and methods
Cell culture
Mouse mammary carcinoma 4T1 cells were purchased
from the Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). Luciferase-labeled 4T1 cells
(4T1-luc2) were provided by Caliper Life Sciences; PerkinElmer,
Inc. (Waltham, MA, USA). Human breast cancer cells (MDA-MB-231 and
MCF-7) and HUV-EC-C human umbilical vein endothelial cells were
purchased from the Cell Resource Center of the Institute of Basic
Medical Sciences, Chinese Academy of Medical Science (Beijing,
China). RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin was used for culture of 4T1, 4T1-luc2 and
MDA-MB-231 cells. MCF-7 and HUV-EC-C cells were cultured in
Dulbecco's modified Eagle medium (Gibco; Thermo Fisher Scientific,
Inc.). All cells were maintained in incubators at 37°C in an
atmosphere of 5% CO2 and 95% humidity. Fisetin (>98%
purity), purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany), was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich;
Merck KGaA), and storage solutions were prepared at a concentration
of 80 mM. In all cell experiments, the final concentration of DMSO
was controlled and limited to <0.1% (v/v).
Examination of the effect of fisetin on
the viability of breast cancer cells
Exponentially growing cells (4T1, MCF-7 and
MDA-MB-231) were seeded into 96-well plates (1×103
cells/well) and were routinely cultured for 24 h. Subsequently, 100
µl fisetin-containing medium was added to each well; the
final concentrations of fisetin were adjusted to 0, 20, 40 and 80
µM. Negative groups consisting of fisetin+medium+MTT without
cells and medium+MTT without cells were used as controls, to
eliminate underestimation of the fisetin effect. Following
incubation for 24 and 48 h, viable cells were measured via MTT
assay (Sigma-Aldrich; Merck KGaA) using DMSO to dissolve the purple
formazan, and optical density was measured at 570 nm using a
microplate reader (Thermo Fisher Scientific, Inc.). The optical
density of fisetin+medium+MTT was subtracted from values obtained
when cells were present. Experiments were performed in
triplicate.
Examination of the effect of fisetin on
the proliferation, migration, and invasiveness of mammary carcinoma
cells via the electrical cell-substrate impedance sensing (ECIS)
method ECIS proliferation array
ECIS is a research platform that allows real-time,
quantitative, non-invasive monitoring of cell behavior. The ECIS
system is composed of an eight-well cell culture array with
gold-plated electrodes attached to the bottom of the wells and an
impedance detection system. Due to the physical properties of ECIS,
the number of attached cells is proportional to the change in
resistance. Therefore, cellular behavior can be monitored in real
time without interruption or labeling by recording cell-induced
resistance (39,40). The ECIS array 8WCP (Applied
Biophysics, Inc., Troy, NY, USA) was prepared, and electrodes were
stabilized according to the manufacturer's protocol (39,40). Basic resistance data of the array
were collected overnight in the Muti-Fre mode. After the curves
were stable, the array was removed. Logarithmically growing 4T1
cells were exposed to fisetin in the array. The concentration of
4T1 cells was adjusted to 6×104 cells/well. The final
concentrations of fisetin were adjusted to 0, 20, 40 and 80
µM. Cell proliferation was determined by measuring the cell
growth-induced resistance changes. Experiments were performed in
triplicate.
ECIS wound healing array
A total of 1×105 4T1 cells/ml was added
to each well of the 8W1E array (Applied Biophysics, Inc.). Once the
resistance had reached a plateau, the medium was replaced with
serum-free culture medium supplemented with fisetin (0, 2, 4 and 8
µM) for 6 h. Subsequently, 4T1 cells were subjected to
electronic wounding, which resulted in cell death in the active
electrode region and decreased cell impedance. Cells located
outside of the electrode area migrated to the electrode region,
which caused another rise in impedance. Wound-healing process was
recorded by continuous impedance measurements for 10 h in the
incubator at 37°C with 5% CO2. The metastatic capability
of the 4T1 cells was determined via an analysis of the cell
growth-induced changes in resistance. Experiments were performed in
triplicate.
ECIS invsion array
HUV-EC-C suspension (1×105 cells/well)
was added to each well of the 8W1E array. Once the resistance value
of HUV-EC-C reached a plateau, fisetin (0, 10, 20 and 40
µM)-treated 4T1 or fisetin (0, 20, 40 and 80
µM)-treated MDA-MB-231 cells were added to the array
(1×105 cells/well). HUV-EC-C cells were used to simulate
an artificial vascular endothelial layer. The progress of tumor
cell invasion with HUV-EC-C mimics tumor cell invasion of blood
vessels in humans. Penetration of the HUV-EC-C barrier by 4T1 cells
or MDA-MB-231 cells would lead to a decrease in resistance. The
invasive capability of the 4T1 cells and MDA-MB-231 cells was
determined by measuring the degree of decrease in the resistance
value. Experiments were performed in triplicate.
Flow cytometric analysis of
apoptosis
Apoptosis was assessed using an Annexin V/Propidium
Iodide (PI) Apoptosis Detection kit (cat. no. KGA 008; Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China). Following treatment with
fisetin (0, 20, 40 and 80 µM) for 24 h, 4T1 cells were
harvested and resuspended in 500 µl binding buffer on ice.
The cells were mixed thoroughly with 5 µl Annexin
V/fluorescein isothiocyanate and then with 5 µl PI.
Following incubation at room temperature for 15 min in the dark,
apoptotic cells were examined using a flow cytometer (Beckman
Coulter, Inc., Brea, CA, USA), and data was analyzed with EXPO32
Analysis Software (Version 1.1C; Beckman Coulter, Inc.).
Experiments were performed in triplicate.
Western blot analysis of
PI3K/Akt/mTOR-related proteins
Following treatment of the 4T1 cells with fisetin
(0, 20, 40 and 80 µM) for 24 h, proteins were extracted from
the cells by lysis in radioimmunoprecipitation assay buffer
(Applygen Technologies, Inc., Beijing, China) with protease
inhibitor cOmplete tablets (Roche Applied Science, Penzberg,
Germany) and PhosSTOP phosphatase inhibitor cocktail tablets (Roche
Applied Science). Western blotting was performed as previously
described (41,42). The primary antibodies, purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA), were
anti-mTOR (cat. no. 2983), anti-phosphorylated (p)-mTOR (cat. no.
5536), anti-Akt (cat. no. 9272), anti-p-Akt (cat. no. 9271),
anti-PI3K (cat. no. 4263), anti-p-PI3K (cat. no. 13857), anti-B
cell lymphoma (Bcl)-2 associated X protein (Bax; cat. no. 14796),
anti-Bcl-extra large (Bcl-xL; cat. no. 2764), anti-P70 (cat. no.
2708), and anti-p-p70 (cat. no. 9234). Reference protein (β-actin)
antibody (cat. no. E021020-01) was purchased from Beijing
GuanXingYun Sci & Tech Co., Ltd. (Beijing, China). The membrane
was incubated with above primary antibody in 5% BSA (Amresco, LLC,
Solon, OH, USA) at 1:1,000, and then incubated with the Dylight™
680- (cat. no. 072-06-15-06) or 800-labeled secondary antibody
(cat. no. 072-07-18-06; KPL, Inc., Gaithersburg, MD, USA) at
1:8,000. Odyssey infrared imaging system V3.0 (LICOR Biosciences
Company, USA) was used for membrane scanning. The signal intensity
of each protein was measured with Image-Pro Plus Version 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA).
Murine 4T1 mammary tumor model
All animal experiments were carried out in
accordance with the National Research Council (US) Committee for
the Update of the Guide for the Care and Use of Laboratory Animals
(43), and were approved by the
Animal Ethics Committee of Beijing Hospital of Traditional Chinese
affiliated with Medicine Capital Medical University (application
no. 2017020201). A total of 30 female BALB/c mice (age, 6-8 weeks;
weight, 18±2 g) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. (Beijing, China), and housed under
specific pathogen-free conditions at a constant temperature
(20±2°C) and 40-50% humidity in a 12-h light/dark cycle with free
access to food and water. The 4T1 orthotopic mammary tumor model
was established as previously described (41,44). Briefly, 1×104 4T1 cells
were injected into the left fourth mammary fat pad during
isoflurane gas anesthesia. At 12 days following inoculation, tumor
length (L) and width (W) were measured using electronic vernier
caliper, and tumor volume (V) was calculated (V=LxW2/2).
Mice were reordered and numbered according to tumor volume from
small to large, and randomly divided into the following three
groups (n=10/group) according to the block random sequence
generated by SPSS 19.0 (IBM Corp., Armonk, NY, USA): Vehicle group,
fisetin group and control group. The mice were administered an
intraperitoneal injection of 30 µl vehicle alone
(DMSO:polyethylene glycol 200, 1:4), fisetin (223 mg/kg), or normal
saline every day for 3 successive weeks, respectively. At 33 d
following inoculation, the number of photons emitted by the 4T1
orthotopic tumors was measured using an In Vivo Optical
Imaging Spectrum system (Caliper Life Sciences; PerkinElmer, Inc.)
as previously described followed the manufacturer's protocol
(41,44,45). At 34 days, mice were sacrificed,
and the tumors were collected and weighed.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
Apoptosis was analyzed using an In Situ Cell
Death Detection kit (Roche Applied Science). The 4T1 breast tumors,
fixed in 4% paraformaldehyde at 4°C for 24 h, were
paraffin-embedded and sectioned. Tissue sections were
deparaffinized and rehydrated according to standard protocols, and
then incubated for 15-30 min at room temperature with proteinase K
working solution. Subsequently, the TUNEL reaction mixture was
added to the tumor sections. Following incubation in a humidified
container for 2 h, the sections were mounted using anti-
fluorescence quenching agent (Beyotime Institute of Biotechnology,
Haimen, China) and observed in five fields under a fluorescence
microscope (BX-53; Olympus Corporation, Tokyo, Japan) at 200×
magnification.
Live and kidney function assay
A blood sample (~0.8 ml) was harvested from the
heart prior to sacrifice, serum was collected via centrifugation at
827 × g for 15 min at room temperature. Serum levels of alanine
amino transferase (ALT), aspartate amino transferase (AST), blood
urea nitrogen (BUN) and creatinine (CREA) were measured using assay
kits (cat. nos. C009, C010, C013 and C011, respectively; Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) according to
the manufacturer's protocols.
Statistical analysis
Data were statistically analyzed using SPSS 19.0
(IBM Corp., Armonk, NY, USA) and expressed as the mean + standard
deviation. Two-tailed Student's t-test was used to determine
statistical differences between two groups. Comparisons among
multiple groups were performed using one-way analysis of variance,
with post hoc Fisher's least significant difference test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Fisetin inhibits breast cancer cell
viability
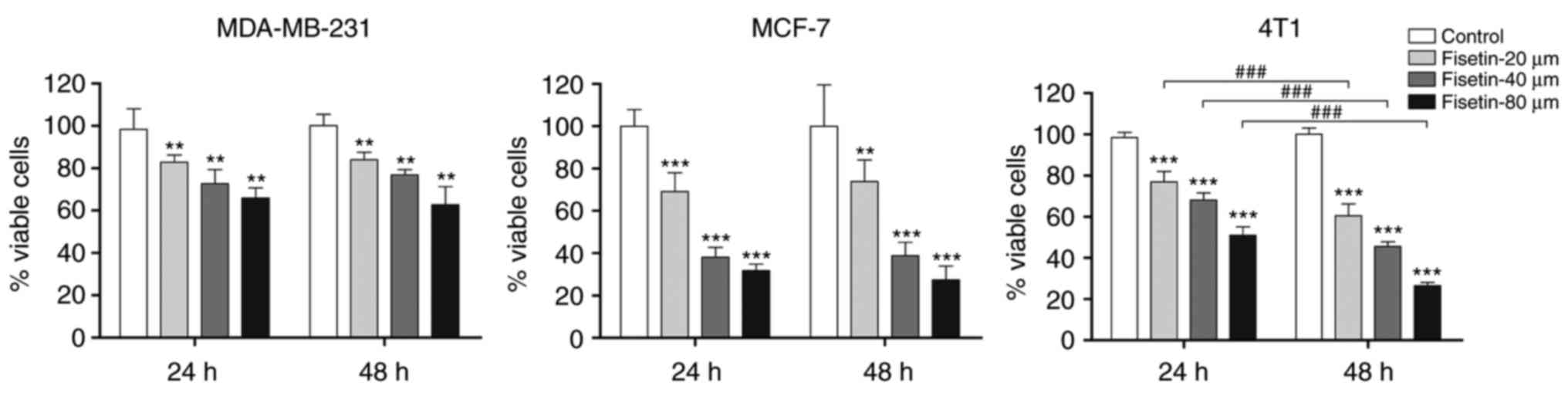
To explore the anti-tumor potency of fisetin against
breast cancer cells, the MTT assay was used to examine the effect
of fisetin on the viability of breast cancer cells (4T1, MCF-7 and
MDA-MB-231). The results demonstrated that fisetin significantly
reduced the number of viable 4T1, MCF-7 and MDA-MB-231 breast
carcinoma cells, compared with controls (Fig. 1). Furthermore, fisetin
significantly inhibited the proliferation of 4T1 cells in a
concentration- and time-dependent manner. Therefore, 4T1 cells were
selected for further study.
Fisetin inhibits the proliferation,
migration and invasiveness of mammary carcinoma cells - results
based on the ECIS platform
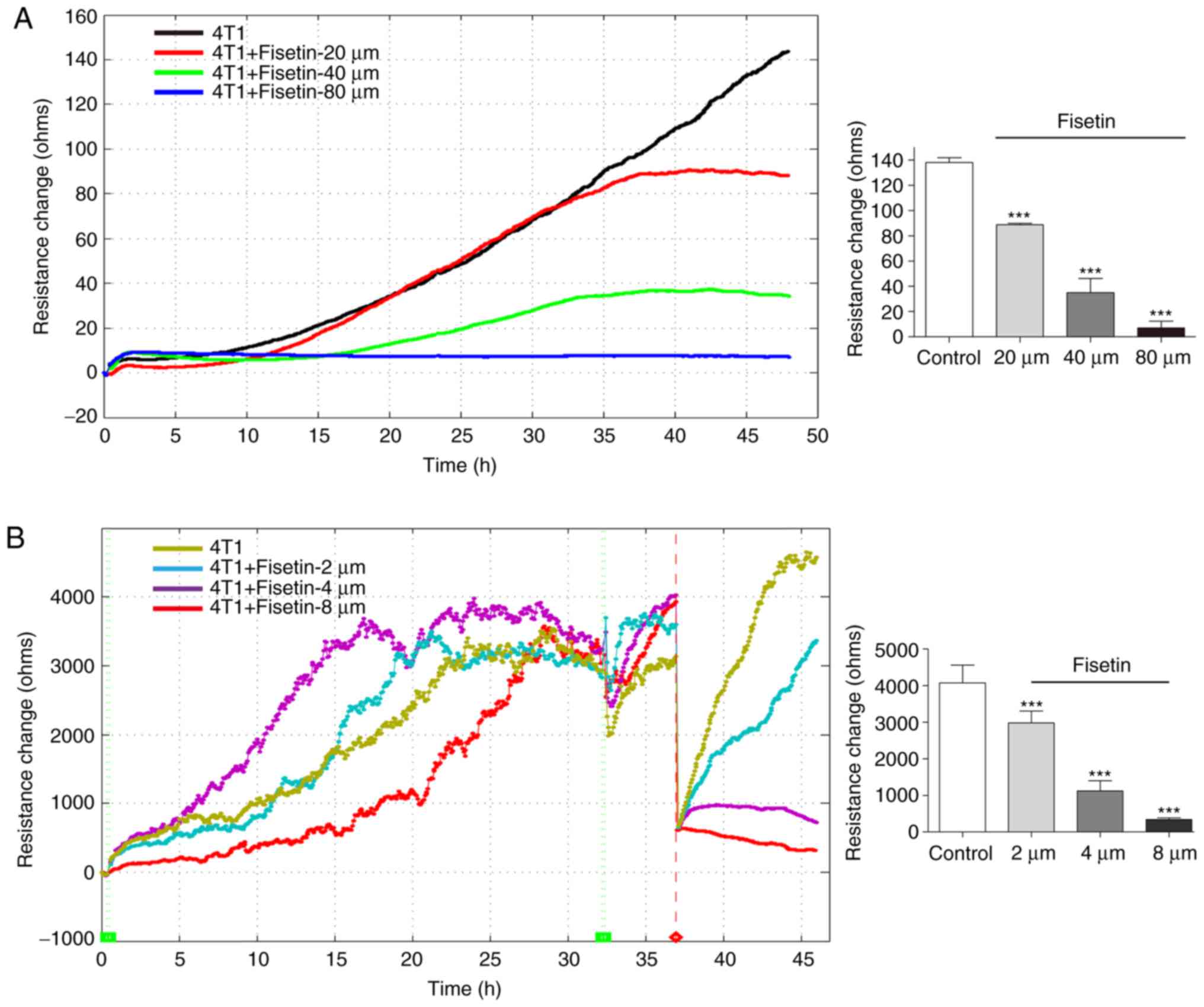
The inhibitory effect of fisetin was measured by
ECIS, cell proliferation was recorded by cell electric resistance
for 48 h. Consistent with the results of the MTT assay, ECIS
experiments demonstrated that fisetin significantly inhibited the
proliferation of 4T1 cells in a concentration-dependent manner
(Fig. 2A). The ECIS
platform-based wound-healing assay allows for the assessment of
metastatic capability. Following electrical wounding, the gradually
increasing value of resistance reflects the dynamic migration of
the healthy neighboring cells. The results demonstrated that the
untreated 4T1 cells rapidly recovered to the state observed prior
to electrical wounding. It was demonstrated that 4T1 cells that had
been treated with 2 µM fisetin also recovered to the state
prior to electrical wounding, but their recovery was less rapid
than the control group. Cells that had been treated with 4 or 8
µM fisetin were unable to completely recover (Fig. 2B). These results indicate that
fisetin inhibited the migration of 4T1 cells.
In the ECIS platform-based cell invasion method,
HUV-EC-C cells were used to simulate the in vivo vascular
endothelial layer. When tumor cells penetrated the HUV-EC-C
barrier, the resistance value decreased. As the growth of the
HUV-EC-C cells plateaued and a stable barrier layer was formed, the
layer was challenged with 4T1 and MDA-MB-231 cells. Fig. 2C indicated that 4T1 cells were
able to penetrate the HUV-EC-C barrier, which led to a decrease in
resistance. 4T1 cells that had been treated with 10 or 20 µM
fisetin influenced, but failed to breach, the HUV-EC-C junction.
However, 4T1 cells that had been treated with 40 µM fisetin
failed to induce any change in the HUV-EC-C resistance (Fig. 2C). These results suggest that
fisetin reduced the invasive capability of 4T1 cells. This finding
was also confirmed in MDA-MB-231 cells (Fig. 2D).
Fisetin induces the apoptosis of mammary
carcinoma cells
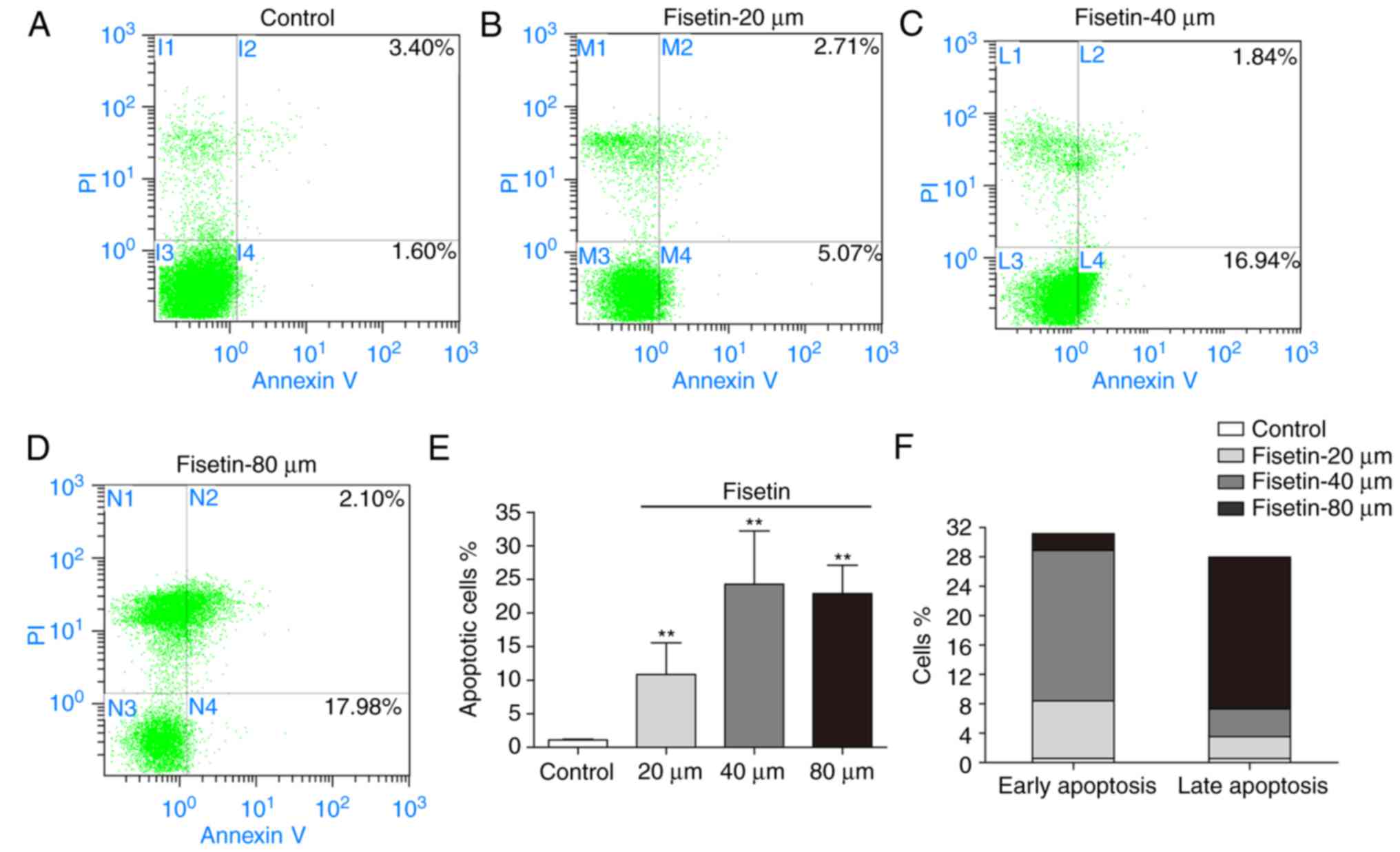
To examine the effect of fisetin on the apoptosis of
4T1 cells, cells were treated with fisetin for 24 h, followed by
Annexin V/PI double staining. The apoptotic rates of 4T1 cells
(early apoptosis + late apoptosis) were 10.82±4.73%, 24.28±7.92%,
and 22.89±4.21% in the 20, 40, and 80 µM fisetin groups,
respectively (Fig. 3A–E). Fisetin
at 40 µM primarily induced early apoptosis, whereas fisetin
at 80 µM primarily induced late apoptosis (apoptotic rate:
20.65±2.93%; Fig. 3F).
Fisetin regulates the PI3K/Akt/mTOR
pathway in 4T1 mammary carcinoma cells
Western blotting was performed to examine the
expression of PI3K, Akt, mTOR, P70, p-PI3K, p-Akt, p-mTOR, p-P70,
Bax, and Bcl-xL proteins in 4T1 cells, in an attempt to explore the
potential mechanism of fisetin intervention. The results
demonstrated that treatment of 4T1 cells with fisetin significantly
reduced the expression of Akt, P70, and mTOR. In addition, p-PI3K
and p-PI3K/PI3K, p-Akt and p-Akt/Akt, p-P70 and p-P70/P-70 and
p-mTOR were significantly decreased, Bax was significantly
upregulated, and Bcl-xL was significantly downregulated following
fisetin treatment in comparison with controls (Fig. 4). The very low expressions of
p-mTOR and mTOR in the fisetin-treated groups may have contributed
to the unexpected significant increase in the p-mTOR/mTOR ratio
(Fig. 4D).
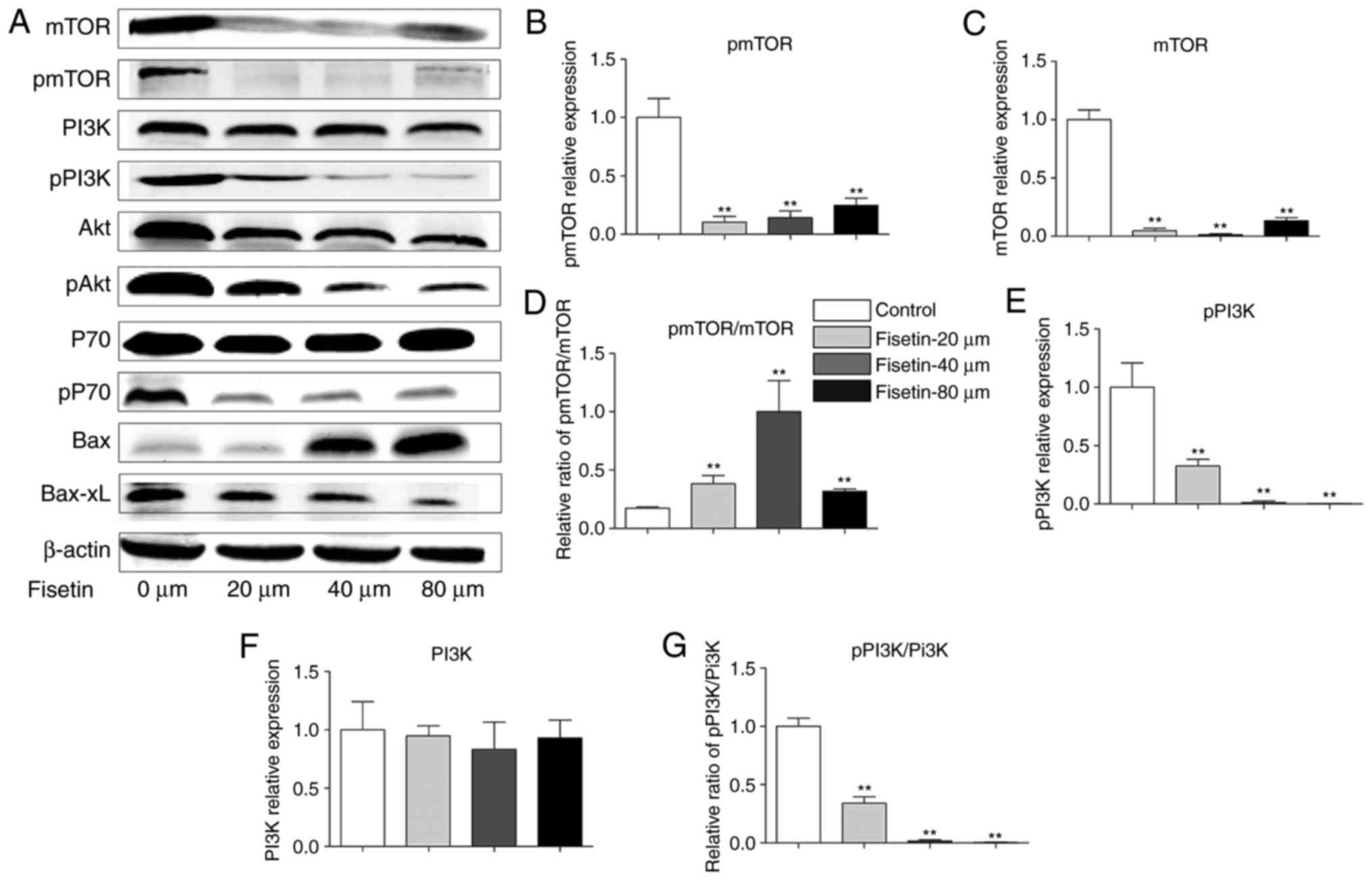 | Figure 4Fisetin regulates the PI3K/Akt/mTOR
pathway in 4T1 cells. (A) 4T1 cells were treated with fisetin (0,
20, 40 or 80 µM) for 24 h, and total protein was extracted
and subjected to western blot analysis. Mean + SD of band density
of (B) p-mTOR, (C) mTOR, (D) p-mTOR/mTOR, (E) p-PI3K, (F) PI3K, (G)
p-PI3K/PI3K, (H) p-Akt, (I) Akt, (J) p-Akt/Akt, (K) p-P70, (L) P70,
(M) p-P70/P70, (N) Bax and (O) Bcl-xL. Data are presented as the
mean + standard deviation (n=3) **P<0.01 vs. control.
PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; mTOR,
mechanistic target of rapamycin; p, phosphorylated; Bcl-xL, B cell
lymphoma-extra large; Bax, B cell lymphoma-2-associated X
protein. |
Fisetin inhibits the primary tumor growth
of 4T1 cells
To further determine the effect of fisetin on 4T1
cells in vivo, 4T1-luc2 cells were used to establish an
orthotopic-transplant model of mammary carcinoma. Significant
differences were detected in tumor volume and tumor weight between
the fisetin-treated and control groups, whereas vehicle
demonstrated no inhibitory effect (Fig. 5A and B). Although the difference
did not reach statistical significance, the number of photons
emitted from the tumor was markedly reduced in the fisetin-treated
group compared with the control group (Fig. 5C). The proportion of apoptotic
cells was markedly increased in the fisetin-treated group (Fig. 5D). The results of in vivo
experiments further demonstrated the inhibitory effect of fisetin
on mammary carcinoma. However, the results also demonstrated that
ALT and AST were elevated in the fisetin group (Fig. 5E and F), whereas no effect of
fisetin on BUN and CREA in tumor-bearing mice was indicated
(Fig. 5G and H).
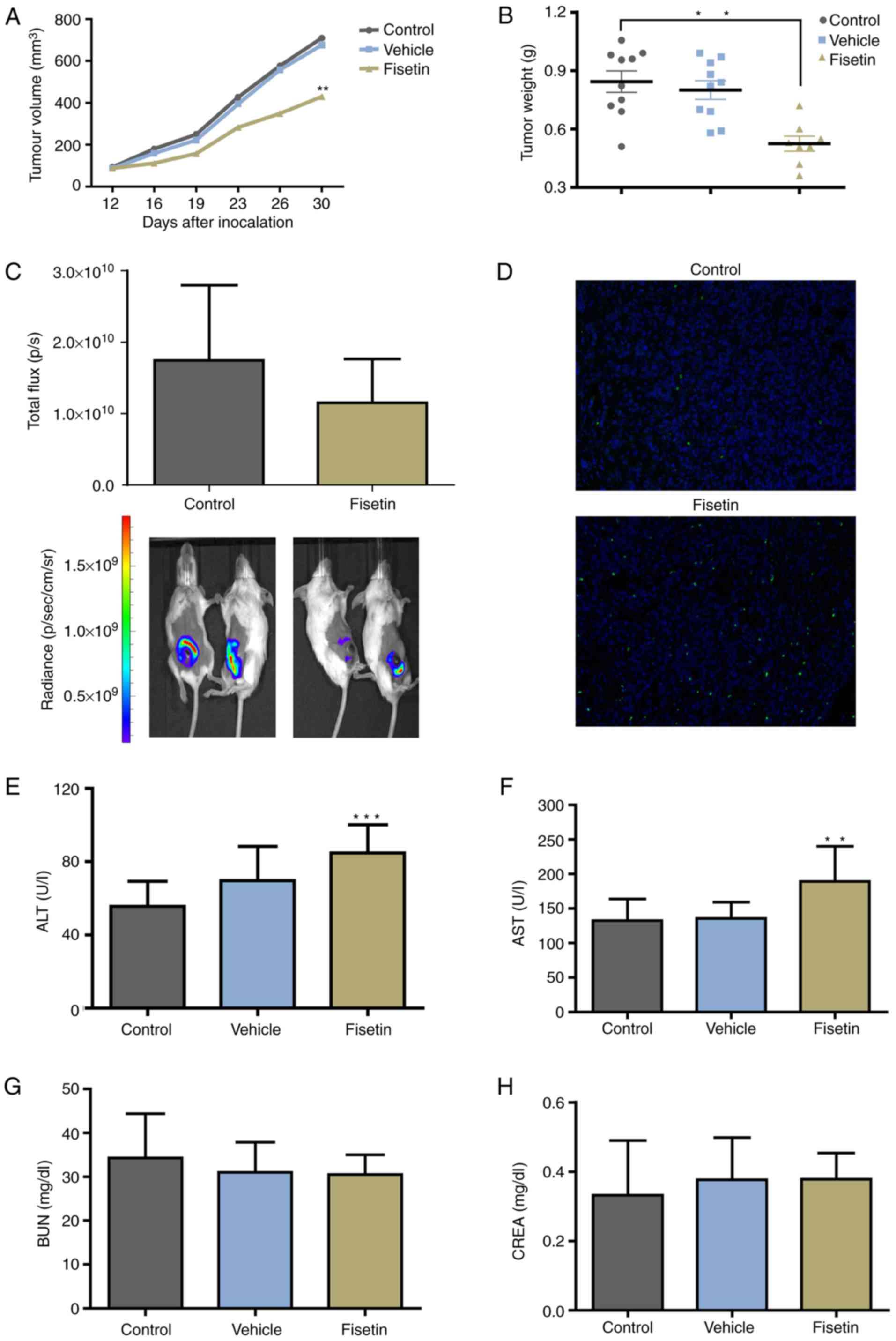 | Figure 5Fisetin inhibits tumor growth and
induces the apoptosis of 4T1 cells in vivo. In an
orthotopic-transplant model of mammary carcinoma, mice were treated
with fisetin, vehicle alone, or normal saline for 21 days. (A)
Tumor volume and (B) tumor weight were decreased in the
fisetin-treated group. (C) Bioluminescent signals of primary tumors
are expressed as total flux. Tumors were removed from the control
and fisetin groups, followed by the (D) terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling assay
to analyze apoptotic cells (magnification, ×200). (E and F) fisetin
may induce side effects on liver function. (G and H) No effect on
kidney function was observed. Data are expressed as the mean +
standard deviation. **P<0.01,
***P<0.001 vs. control. ALT, alanine amino
transferase; AST, aspartate amino transferase; BUN, blood urea
nitrogen; CREA, creatinine. |
Discussion
As a flavonoid compound that is widely present in
medicinal and edible plants, fisetin has become the focus of many
studies. Yang et al (36)
previously demonstrated that fisetin inhibited the proliferation of
Mcf-7 cells through the regulation of apoptosis and autophagy.
Matthew et al (37)
recently demonstrated that fisetin inhibited the proliferation of
MDA-MB-468 and MDA-MB-231 cells through the induction of cell cycle
arrest and apoptosis. However, in vivo studies that have
investigated the effect of fisetin on breast cancer are scarce. The
present study demonstrated that fisetin inhibited the proliferation
of breast cancer cells. In addition, fisetin inhibited the
migration and invasiveness of 4T1 cells and induced their
apoptosis. In vivo experiments demonstrated that fisetin
inhibited the growth of 4T1 cell-derived orthotopically
transplanted tumors. The present study also demonstrated that
fisetin downregulated p-PI3K, p-Akt, and p-mTOR, and that Bax and
Bcl were associated with the fisetin-induced apoptosis of 4T1
cells.
PI3K is a phosphatidylinositol kinase that can
phosphorylate the third hydroxyl group of the inositol ring on the
cell membrane. PI3K activation leads to the production of
phosphatidylinositol (3,4,5)-trisphosphate, which is associated
with the phosphorylation and activation of Akt. Activated Akt is
translocated from the cell membrane to the cytoplasm and nucleus,
where it activates or inhibits downstream target proteins such as
Bcl-2, caspases, and mTOR through phosphorylation. This allows Akt
to regulate cell proliferation, differentiation, invasion,
apoptosis and energy metabolism (46-48). The PI3K/Akt/mTOR signaling pathway
is activated in approximately 70% of breast cancers, and its
activation is correlated with clinical characteristics and poor
prognosis (49,50). Inhibition of the PI3K/Akt pathway
suppresses the proliferation of tumor cells and enhances apoptosis
(51). Fisetin inhibits the
expression and activation of the PI3K/Akt/mTOR signaling pathway in
lung, prostate and colorectal cancer, and leukemia (22), which is consistent with the
results of the present study. The finding that fisetin targets and
inhibits the PI3K/Akt/mTOR signaling pathway provides rationale for
further investigation and the clinical application of fisetin.
Similar to other naturally occurring flavonoids,
fisetin has poor solubility. In previous in vivo
experiments, DMSO was used as the vehicle for the intraperitoneal
injection of fisetin (26). In
the in vivo experiments, mice were administered an
intraperitoneal injection of fisetin at a dose of 223 mg/kg, which
was determined based on the experimental dosage reported by Touil
et al (26). The toxicity
of fisetin observed in the present study may have been due to the
combined effects of the vehicle and fisetin. Fisetin significantly
increased ALT and AST levels, thus suggesting liver toxicity,
whereas the vehicle markedly increased ALT, which also indicated
potential liver toxicity. Apart from this potential role of the
vehicle, the high dose of fisetin is the present study may be the
key reason. The present research group previously attempted to
gavage and intraperitoneally inject fisetin at a dosage of 112
mg/kg, however, at this dose condition, neither inhibition nor
liver toxicity was observed (data not published). In the present
study, the results of ALT and AST assays suggested that the fisetin
dosage of 223 mg/kg may aggravate liver burden due to poor
bioavailability. Further studies regarding increasing the
bioavailability and reducing dose are required.
A number of potential limitations should be
considered in the present study. First, according to the MTT assay
performed in serum-containing medium and with fisetin-treatment of
24 or 48 h, fisetin may be cytotoxic to cells; therefore, the
effects on migration/invasion may be due to the effects on cell
viability. In spite of these inevitable disturbances, a number of
steps were taken to reduce the mixed effects. In the wound-healing
experiments, the medium was replaced with serum-free culture medium
supplemented with fisetin prior to electrical wounding, and the
fisetin-exposure time was reduced to 6 h. In the invasion
experiments, prior to exposure to the HUV-EC-C barrier, tumor cells
were treated with fisetin in the serum-free medium for 12 h.
Second, the in vitro experiments on invasiveness had a
potential flaw as HUV-EC-C cells are of human origin and 4T1 cells
are of mouse origin; hence the interactions between these cells
types may not reflect the in vivo situation when both
cell types are from the same species. On this issue, help was
sought from the ECIS manufacturer, who reported that the HUV-EC-C
barrier used in the ECIS model is the classic model developed by
their engineer, and that the stability of HUV-EC-C in the ECIS
model may not be achieved by other microvascular endothelial cells.
Therefore, the invasion-inhibition of the fisetin was confirmed
with MDA-MB-231 cells, which are from the same species of HUV-EC-C.
Furthermore, the invasion-inhibition reported in the fisetin
in vitro experiments is encouraging, and the impact
of fisetin on metastasis was explored in vivo. The
effect of fisetin on the 4T1 model was investigated. All animal
experiments culminated within 3 weeks, which was not long enough to
observe obvious lung metastasis in this model, according to
previous studies (41,44). During the 3-week treatment period,
in the fisetin-treated group, 2 mice succumbed and the mean body
weight decreased >10%, which was the humane endpoint established
by the ethical approval obtained. In this situation, it was
necessary to abandon the study, the effect of fisetin on lung
metastases was not examined in this model. The present research
group is working on fisetin-loaded material to ameliorate the poor
bioavailability, solubility, instability and permeability of
fisetin. Future studies regarding the effect on lung metastases
remain on the research schedule of the present authors.
The low solubility and low bioavailability of
fisetin limit its further investigation. Previous studies have
attempted to overcome these issues with the use of drug delivery
vectors and improved preparation techniques (35,52), and it is expected that future
studies will provide strategies for further clinical and
translational research on fisetin.
Acknowledgments
The authors would like to thank Professor Sa Liu
(Beijing Institute of Heart Lung and Blood Vessel Disease, Beijing,
China) for technical support with the cell apoptosis
experiments.
Funding
The present study was funded by the Beijing Natural
Science Foundation (grant nos. 7162084 and 7174312), National
Natural Science Foundation of China (grant nos. 81373815 and
81673924), The Beijing Municipal Education Commission (grant no.
KM201510025025), and The Specialized Research Fund for the Doctoral
Program of Higher Education of China (grant no.
20131107110014).
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
XS, GZ and XW conceived and designed the project and
prepared the manuscript. XS, XMM, QWL, YY and KXC conducted the
animal experiments. XS, XX, JS and MY conducted the cell
experiments. LY and GY analyzed the data. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Beijing Hospital of Traditional Chinese Medicine,
affiliated with Capital Medical University (Beijing, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gemignani ML and Hetzel DJ: Current
advances in endocrine therapy options for premenopausal women with
hormone receptor positive breast cancer. Gynecol Oncol.
147:153–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wander SA, Mayer EL and Burstein HJ:
Blocking the cycle: Cyclin-dependent kinase 4/6 inhibitors in
metastatic, hormone receptor-positive breast cancer. J Clin Oncol.
35:2866–2870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guerrero-Zotano AL and Arteaga CL:
Neoadjuvant trials in ER+ breast cancer: A tool for
acceleration of drug development and discovery. Cancer Discov.
7:561–574. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu X, Huang W and Fan M: Emerging
therapies for breast cancer. J Hematol Oncol. 10:982017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shachar SS, Jolly TA, Jones E and Muss HB:
Management of triple-negative breast cancer in older patients: How
is it different? Oncology (Williston Park). 32:58–63. 2018.
|
|
8
|
Lee A and Djamgoz MBA: Triple negative
breast cancer: Emerging therapeutic modalities and novel
combination therapies. Cancer Treat Rev. 62:110–122. 2018.
View Article : Google Scholar
|
|
9
|
Wang C, Kar S, Lai X, Cai W, Arfuso F,
Sethi G, Lobie PE, Goh BC, Lim LHK, Hartman M, et al: Triple
negative breast cancer in Asia: An insider's view. Cancer Treat
Rev. 62:29–38. 2018. View Article : Google Scholar
|
|
10
|
Chao J, Dai Y, Verpoorte R, Lam W, Cheng
YC, Pao LH, Zhang W and Chen S: Major achievements of
evidence-based traditional Chinese medicine in treating major
diseases. Biochem Pharmacol. 139:94–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goyal S, Gupta N, Chatterjee S and Nimesh
S: Natural plant extracts as potential therapeutic agents for the
treatment of cancer. Curr Top Med Chem. 17:96–106. 2017. View Article : Google Scholar
|
|
12
|
Sun X, Zhang X, Nian JY, Guo J, Yin Y,
Zhang GL, Yu MW, Zhang Y, Wang XM, Yang GW, et al: Chinese herbal
medicine as adjunctive therapy to chemotherapy for breast cancer: A
systematic review and meta-analysis. Evid Based Complement Alternat
Med. 2016:32819682016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khan N, Syed DN, Ahmad N and Mukhtar H:
Fisetin: A dietary antioxidant for health promotion. Antioxid Redox
Signal. 19:151–162. 2013. View Article : Google Scholar :
|
|
14
|
Hodek P, Trefil P and Stiborová M:
Flavonoids-potent and versatile biologically active compounds
interacting with cytochromes 450. Chem Biol Interact. 139:1–21.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jang HS, Kook SH, Son YO, Kim JG, Jeon YM,
Jang YS, Choi KC, Kim J, Han SK, Lee KY, et al: Flavonoids purified
from Rhus verniciflua Stokes actively inhibit cell growth and
induce apoptosis in human osteosarcoma cells. Biochimic Biophys
Acta. 1726:309–316. 2005. View Article : Google Scholar
|
|
16
|
Moon YJ, Wang X and Morris ME: Dietary
flavonoids: Effects on xenobiotic and carcinogen metabolism.
Toxicol In Vitro. 20:187–210. 2006. View Article : Google Scholar
|
|
17
|
Wang G, Wang JJ, Guan R, Du L, Gao J and
Fu XL: Strategies to target glucose metabolism in tumor
microenvironment on cancer by flavonoids. Nutr Cancer. 69:534–554.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
George VC, Dellaire G and Rupasinghe HPV:
Plant flavonoids in cancer chemoprevention: Role in genome
stability. J Nutr Biochem. 45:1–14. 2017. View Article : Google Scholar
|
|
19
|
Magne Nde CB, Zingue S, Winter E,
Creczynski-Pasa TB, Michel T, Fernandez X, Njamen D and Clyne C:
Flavonoids, breast cancer chemopreventive and/or chemotherapeutic
agents. Curr Med Chem. 22:3434–3446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zamora-Ros R, Agudo A, Lujan-Barroso L,
Luján-Barroso L, Romieu I, Ferrari P, Knaze V, Bueno-de-Mesquita
HB, Leenders M, Travis RC, et al: Dietary flavonoid and lignan
intake and gastric adenocarcinoma risk in the European Prospective
Investigation into Cancer and Nutrition (EPIC) study. Am J Clin
Nutr. 96:1398–1408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frankenfeld CL, Cerhan JR, Cozen W, Davis
S, Schenk M, Morton LM, Hartge P and Ward MH: Dietary flavonoid
intake and non-Hodgkin lymphoma risk. Am J Clin Nutr. 87:1439–1445.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Syed DN, Adhami VM, Khan MI and Mukhtar H:
Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin.
Anticancer Agents Med Chem. 13:995–1001. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khan N, Afaq F, Khusro FH, Mustafa Adhami
V, Suh Y and Mukhtar H: Dual inhibition of phosphatidylinositol
3-kinase/Akt and mammalian target of rapamycin signaling in human
nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int
J Cancer. 130:1695–1705. 2012. View Article : Google Scholar
|
|
24
|
Liao YC, Shih YW, Chao CH, Lee XY and
Chiang TA: Involvement of the ERK signaling pathway in fisetin
reduces invasion and migration in the human lung cancer cell line
A549. J Agric Food Chem. 57:8933–8941. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ravichandran N, Suresh G, Ramesh B and
Siva GV: Fisetin, a novel flavonol attenuates
benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice.
Food Chem Toxicol. 49:1141–1147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Touil YS, Seguin J, Scherman D and Chabot
GG: Improved antiangiogenic and antitumour activity of the
combination of the natural flavonoid fisetin and cyclophosphamide
in Lewis lung carcinoma-bearing mice. Cancer Chemother Pharmacol.
68:445–455. 2011. View Article : Google Scholar
|
|
27
|
Syed DN, Afaq F, Maddodi N, Johnson JJ,
Sarfaraz S, Ahmad A, Setaluri V and Mukhtar H: Inhibition of human
melanoma cell growth by the dietary flavonoid fisetin is associated
with disruption of Wnt/β-catenin signaling and decreased Mitf
levels. J Invest Dermatol. 131:1291–1299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murtaza I, Adhami VM, Hafeez BB, Saleem M
and Mukhtar H: Fisetin, a natural flavonoid, targets chemoresistant
human pancreatic cancer AsPC-1 cells through DR3-mediated
inhibition of NF-kappaB. Int J Cancer. 125:2465–2473. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Costa RLB, Han HS and Gradishar WJ:
Targeting the PI3K/AKT/mTOR pathway in triple-negative breast
cancer: A review. Breast Cancer Res Treat. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schettini F, Buono G, Trivedi MV, De
Placido S, Arpino G and Giuliano M: PI3K/mTOR inhibitors in the
treatment of luminal breast cancer. Why, when and to whom? Breast
Care. 12:290–294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guerrero-Zotano A, Mayer IA and Arteaga
CL: PI3K/AKT/mTOR: Role in breast cancer progression, drug
resistance, and treatment. Cancer Metastasis Rev. 35:515–524. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arif H, Sohail A, Farhan M, Rehman AA,
Ahmad A and Hadi SM: Flavonoids-induced redox cycling of copper
ions leads to generation of reactive oxygen species: A potential
role in cancer chemoprevention. Int J Biol Macromol. 106:569–578.
2018. View Article : Google Scholar
|
|
33
|
Ghosh P, Singha Roy A, Chaudhury S, Jana
SK, Chaudhury K and Dasgupta S: Preparation of albumin based
nanoparticles for delivery of fisetin and evaluation of its
cytotoxic activity. Int J Biol Macromol. 86:408–417. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Noh EM, Park YJ, Kim JM, Kim MS, Kim HR,
Song HK, Hong OY, So HS, Yang SH, Kim JS, et al: Fisetin regulates
TPA-induced breast cell invasion by suppressing matrix
metalloproteinase-9 activation via the PKC/ROS/MAPK pathways. Eur J
Pharmacol. 764:79–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kadari A, Gudem S, Kulhari H, Bhandi MM,
Borkar RM, Kolapalli VR and Sistla R: Enhanced oral bioavailability
and anticancer efficacy of fisetin by encapsulating as inclusion
complex with HPβCD in polymeric nanoparticles. Drug Deliv.
24:224–232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang PM, Tseng HH, Peng CW, Chen WS and
Chiu SJ: Dietary flavonoid fisetin targets caspase-3-deficient
human breast cancer MCF-7 cells by induction of
caspase-7-associated apoptosis and inhibition of autophagy. Int J
Oncol. 40:469–478. 2012.
|
|
37
|
Smith ML, Murphy K, Doucette CD,
Greenshields AL and Hoskin DW: The dietary flavonoid fisetin causes
cell cycle arrest, caspase-dependent apoptosis, and enhanced
cytotoxicity of chemotherapeutic drugs in triple-negative breast
cancer cells. J Cell Biochem. 117:1913–1925. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang L, Zhang DZ and Wang YX: Bioflavonoid
fisetin loaded α-tocopherol-poly(lactic acid)-based polymeric
micelles for enhanced anticancer efficacy in breast cancers. Pharm
Res. 34:453–461. 2017. View Article : Google Scholar
|
|
39
|
Saxena NK, Taliaferro-Smith L, Knight BB,
Merlin D, Anania FA, O'Regan RM and Sharma D: Bidirectional
crosstalk between leptin and insulin-like growth factor-I signaling
promotes invasion and migration of breast cancer cells via
trans-activation of epidermal growth factor receptor. Cancer Res.
68:9712–9722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Saxena NK, Sharma D, Ding X, Lin S, Marra
F, Merlin D and Anania FA: Concomitant activation of the JAK/STAT,
PI3K/AKT, and ERK signaling is involved in leptin-mediated
promotion of invasion and migration of hepatocellular carcinoma
cells. Cancer Res. 67:2497–2507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Zhang GL, Sun X, Cao KX, Shang
YW, Gong MX, Ma C, Nan N, Li JP, Yu MW, et al: Gubenyiliu II
inhibits breast tumor growth and metastasis associated with
decreased hepa-ranase expression and phosphorylation of erk and akt
pathways. Molecules. 22:E7872017. View Article : Google Scholar
|
|
42
|
Sun JQ, Zhang GL, Zhang Y, Nan N, Sun X,
Yu MW, Wang H, Li JP and Wang XM: Spatholobus suberectus column
extract inhibits estrogen receptor positive breast cancer via
suppressing ER MAPK PI3K/AKT pathway. Evid Based Complement
Alternat Med. 2016:29343402016. View Article : Google Scholar
|
|
43
|
National Research Council (US) Committee:
Guide for the care and use of laboratory animals. 8th. Washington
(DC): 2011
|
|
44
|
Zhang Y, Zhang GL, Sun X, Cao KX, Ma C,
Nan N, Yang GW, Yu MW and Wang XM: Establishment of a murine breast
tumor model by subcutaneous or orthotopic implantation. Oncology
Lett. 15:6233–6240. 2018.
|
|
45
|
Jenkins DE, Oei Y, Hornig YS, Yu SF,
Dusich J, Purchio T and Contag PR: Bioluminescent imaging (BLI) to
improve and refine traditional murine models of tumor growth and
metastasis. Clin Exp Metastasis. 20:733–744. 2003. View Article : Google Scholar
|
|
46
|
Lien EC, Dibble CC and Toker A: PI3K
signaling in cancer: Beyond AKT. Curr Opin Cell Biol. 45:62–71.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rodgers SJ, Ferguson DT, Mitchell CA and
Ooms LM: Regulation of PI3K effector signalling in cancer by the
phosphoinositide phosphatases. Biosci Rep. 37:2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Carvalho S and Schmitt F: Potential role
of PI3K inhibitors in the treatment of breast cancer. Future Oncol.
6:1251–1263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Aleskandarany MA, Rakha EA, Ahmed MA, Powe
DG, Paish EC, Macmillan RD, Ellis IO and Green AR: PIK3C A
expression in invasive breast cancer: A biomarker of poor
prognosis. Breast Cancer Res Treat. 122:45–53. 2010. View Article : Google Scholar
|
|
50
|
López-Knowles E, O'Toole SA, McNeil CM,
Millar EK, Qiu MR, Crea P, Daly RJ, Musgrove EA and Sutherland RL:
PI3K pathway activation in breast cancer is associated with the
basal-like phenotype and cancer-specific mortality. Int J Cancer.
126:1121–1131. 2010. View Article : Google Scholar
|
|
51
|
Rychahou PG, Jackson LN, Silva SR,
Rajaraman S and Evers BM: Targeted molecular therapy of the PI3K
pathway: Therapeutic significance of PI3K subunit targeting in
colorectal carcinoma. Ann Surg. 243:833–842. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Seguin J, Brullé L, Boyer R, Lu YM, Ramos
Romano M, Touil YS, Scherman D, Bessodes M, Mignet N and Chabot GG:
Liposomal encapsulation of the natural flavonoid fisetin improves
bioavailability and antitumor efficacy. Int J Pharm. 444:146–154.
2013. View Article : Google Scholar : PubMed/NCBI
|