Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an
aggressive and devastating form of cancer (1), and it is expected to become the
second leading cause of cancer-associated mortality by 2030 in the
United States (2). Despite
advances in multidisciplinary treatments for PDAC, improving
clinical outcomes has proven difficult. The major reasons for this
lack in improvement are associated with the difficulties in
providing an early diagnosis, the high malignant potential in terms
of invasiveness and metastasis, and resistance against
chemoradiotherapy (3). Therefore,
the elucidation of the molecular mechanism involved in the
progression of PDAC is urgently required.
Annexins comprise a family of calcium-dependent
phospholipid-binding proteins with diverse cellular functions,
including membrane-cytoskeleton organization and regulation of ion
channel activity (4). Diaz
indicated that tissue plasminogen activator (t-PA), which is
overexpressed in PDAC cells, binds specifically to annexin II
(ANX2), a member of the annexin family, on the extracellular
membrane of PDAC cells, leading to the activation of tumor cell
invasion (5). It has also been
reported that ANX2 contributes to the epithelial to mesenchymal
transition (EMT) of PDAC cells and liver metastasis in mouse PDAC
models (6). In our previous
study, ANX2 was identified as a gemcitabine-resistant factor in
PDAC using a comprehensive proteomics technique (7), and it was revealed that the
Akt/mammalian target of rapamycin signaling pathway is involved in
the mechanisms of gemcitabine resistance induced by ANX2 in PDAC
cells (8). Therefore, ANX2 has
diverse functional roles and is likely a crucial factor in the
progression of PDAC.
A dense stromal desmoplastic reaction surrounding
cancer cells is a hallmark histological feature in PDAC (3). The stromal components consist of
pancreatic stellate cells, cancer-associated fibroblasts,
macrophages, infiltrating immune cells, and extracellular matrix
(ECM), which orchestrate with cancer cells and are crucial in
cancer progression (9). These
interactions between cancer cells and the stroma in PDAC
carcinogenesis, metastasis and therapeutic resistance have been
thoroughly investigated using several approaches. Tenascin C (TNC)
is one of the major ECM components that accelerates the invasion
and metastasis of cancer cells (10,11). It has been reported that TNC
directly binds to ANX2 in PDAC cells (12); however, the molecular functions
induced by the interaction between ANX2 and stromal TNC in PDAC
progression remain to be elucidated.
The present study investigated the functional roles
of the interaction of ANX2 with stromal TNC in the progression of
PDAC. The interaction of these molecules drives pre-invasive
pancreatic intraepithelial neoplasia (PanIN) cells to transition to
the mesenchymal phenotype, and accelerates their self-renewal
capacity and anoikis resistance to induce metastasis. The findings
of the present study provide an improved understanding of the
functional effects induced by the interaction of these two
molecules in the tumor microenvironment of PDAC, which may assist
in the development of a novel therapeutic target for PDAC.
Materials and methods
Patients and human tissue samples
PDAC tissues were obtained from 78 consecutive
patients who underwent R0 (no residual tumor) or R1 (microscopic
residual tumor) surgical resection in the Department of General
Surgery, Chiba University Hospital (Chiba, Japan) between January
2006 and December 2010. All patients were diagnosed with primary
PDAC histologically. The Ethics Committees of Chiba University,
Graduate School of Medicine (Chiba, Japan) approved the protocol of
the present study, and written informed consent was obtained from
each patient prior to surgery.
Murine and human pancreatic cell lines
and culture conditions
The murine PanIN cells (KC), invasive PDAC cells
(KPC1 and KPC2), and liver metastatic PDAC cells (KPCLiv) were
kindly provided by Dr Sunil Hingorani (University of Washington,
Seattle, WA, USA) (13). In
brief, each cell line was isolated from a murine PanIN lesion
(Pdx1-cre; LSL-KrasG12D/+) for KC, a
primary PDAC in KPC mice (Pdx1-cre;
LSL-KrasG12D/+; p53R172H/+) for
KPC1 and KPC2, and a liver metastatic lesion in KPC mice
(Pdx1-cre; LSL-KrasG12D/+;
p53R172H/+) for KPCLiv. The PANC-1 human PDAC
cell line was obtained from the American Type Culture Collection
(Manassas, VA, USA). All cell lines were cultured in Dulbecco's
modified Eagle's medium (DMEM; Sigma-Aldrich; EMD Millipore,
Billerica, MA, USA) with 10% fetal bovine serum (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and antibiotics (1% penicillin
and streptomycin).
Western blot analysis
The proteins were extracted from the above cultured
cells with RIPA buffer (Sigma-Aldrich; EMD Millipore). Each protein
sample (20-50 µg) was lysed in buffer (Laemmli Sample
Buffer; Bio-Rad Laboratories, Inc., Hercules, CA, USA) containing
5% 2-mercaptoethanol and incubated for 10 min at 97°C. Following
the measurement of the protein concentration of each sample using
Pierce™ BCA Protein Assay kit (Thermo Fisher Scientific, Inc.), 30
µg of proteins were then separated by electrophoresis on
5-12.5% XV PANTERA Gels (DRC, Tokyo, Japan) and transferred onto a
membrane (PerkinElmer, Inc., Waltham, MA, USA). The membranes were
blocked in 5% skim milk diluted with 0.1% Tris-buffed saline with
Tween-20 at room temperature for 60 min. The membranes were then
incubated with the following primary antibodies overnight at 4°C:
Anti-ANX2 polyclonal antibody (1:10,000 dilution; cat. no.
11256-1-AP; ProteinTech Group, Inc., Rosemont, IL, USA), anti-TNC
monoclonal antibody (1:1,000 dilution; cat. no. 12221; Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-E-cadherin
poly-clonal antibody (1:1,000 dilution; cat. no. sc-7870; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), anti-vimentin
polyclonal antibody (1:1,000 dilution; cat. no. 3932; Cell
Signaling Technology, Inc.), and anti-β-actin monoclonal antibody
(1:2,000 dilution; cat. no. 5125; Cell Signaling Technology, Inc.).
Subsequently, the membranes were incubated at room temperature for
60 min with anti-rabbit IgG horseradish peroxidase secondary
antibody (1:2,000 dilution; cat. no. sc-2301; Santa Cruz
Biotechnology, Inc.), in blocking buffer. The membranes were then
incubated with enhanced chemiluminescence detection reagent
(Amersham™ ECL™ Prime western blotting detection reagent; GE
Healthcare Life Sciences, Chalfont, UK) and developed with a
LAS-4000UV mini luminescent image analyzer (Fujifilm, Tokyo,
Japan). The intensity of each band was quantified by densitometric
analysis using ImageJ software version 1.51 (National Institutes of
Health, Bethesda, MD, USA) and used to calculate the relative
protein level normalized to β-actin.
Small interfering RNAs (siRNAs) and
reagents
siRNAs specifically targeting mouse ANX2 mRNA
(m-siANX2) and human ANX2 mRNA (h-siANX2) to inhibit the expression
of ANX2 were purchased, including double-stranded synthetic
siANX2s; m-siANX2-1 SASI_Mm01_00187632: Forward,
5′-GCAAGUCCCUGUACUACUATT-3′; Reverse, 5′-UAGUAGUACAGGGACUUGCTT-3′;
m-siANX2-2 SASI_ Mm01_00187634: Forward,
5′-GUAUGAUGCUUCGGAACUATT-3′; Reverse, 5′-UAGUUCCGAAGCAUCAUACTT-3′;
h-siANX2-1 SASI_Hs01_00246294: Forward,
5′-GUUACAGCCCUUAUGACAUTT-3′, Reverse, 5′-AUGUCAUAAGGGCUGUAACTT-3′;
h-siANX2-2 SASI_Hs01_00246296: Forward,
5′-GAACUUGCAUCAGCACUGATT-3′; Reverse, 5′-UCAGUGCUGAUGCAAGUUCTT-3′
(Sigma-Aldrich; EMD Millipore), and sicontrol All Stars negative
control siRNA (Qiagen, Inc., Valencia, CA, USA). The cells
(1×105) were cultured for 24 h, and were then
transfected with siRNAs (30 nmol/l final concentration) in Opti-MEM
I reduced serum medium (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) using Lipofectamine™ RNAiMAX transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). At 72 h post-siRNA
transfection, whether knockdown was sufficient was evaluated using
western blot analysis. The cells were used for the subsequent
assays at 24 h post-transfection.
Recombinant TNC (rTNC) treatment
The rTNC (TNC purified protein; EMD Millipore) was
added to the culture medium at room temperature at 2.5 µg/ml
for western blot analysis, 3D cell culture and the pancreatosphere
formation assay, and at 5.0 µg/ml for the invasion
assay.
Three-dimensional (3D) organotypic cell
culture system
3D organotypic pancreatic cell culture was performed
as previously described (14,15). Briefly, the cells transfected with
siANX2s or sicontrol were suspended in collagen solution with or
without rTNC (2.5 µg/ml final concentration). The
collagen-coated cells (1,250 cells/well) were poured into 4-well
chamber slides (Thermo Fischer Scientific, Inc.). The culture
medium containing rTNC was added to each well on a collagen layer,
followed by culture with replacement of the medium every 3 days.
After 7 days, images of the cells were captured using AxioVision
(version 4.3; Carl Zeiss AG, Oberkochen, Germany). The morphology
of cells was classified into three types: Spheroid cysts, irregular
cysts, and spindle-shaped cells; and their numbers were observed
and counted as previously described (16).
Cell invasion assay
Cell Biolabs CytoSelect™ 24-eell cell invasion assay
kits (Cell Biolabs, San Diego, CA, USA) utilizing basement
membrane-coated inserts were used according to the manufacturer's
protocol. In brief, cells transfected with siANX2s or sicontrol
were suspended in serum-free medium. Following overnight
starvation, the cells were seeded at 3.0×105 cells/well
in the upper chamber and incubated with medium containing serum
with/without 5.0 µg/ml rTNC in the lower chamber for 48 h.
The invasive cells passing through the basement membrane layer were
stained and quantified at an optical density (OD) of 595 nm in a
plate reader (iMark™ microplate reader; Bio-Rad Laboratories, Inc.)
following extraction.
Pancreatosphere formation assay
Pancreatosphere formation assays were performed as
described previously (16,17).
Briefly, the cells transfected with siANX2s or sicontrol in sphere
medium with or without 2.5 µg/ml rTNC were seeded at 15
cells/well in 96-well ultra-low attachment plates (Corning, Inc.,
Corning, NY, USA). Following 7 days of incubation at 37°C, the
cells were evaluated and the number of spheres with a diameter
>50 µm were counted using an inverted microscope (Axio
Observer Z1; Carl Zeiss AG, Oberkochen, Germany). The sphere
formation rate was assessed as the percent increase in the number
of spheres on day 7 with respect to the number of spheres observed
on day 1.
Anoikis assay
To assess anoikis resistance, which is the
resistance for apoptosis following loss of contact with the ECM, an
anoikis assay was performed as described previously (18). Briefly, the cells were
continuously rotated on tube rotator (HB-1000 Hybridizer
hybridization oven; Ultra-Violet Products, Inc., Upland, CA, USA)
for 24 h. Subsequently, 3,000 cells/well suspended in medium with
0.3% agar were seeded onto 24-well culture plates coated with a
lower layer of medium with 1% agar, and were cultured for 14 days
at 37°C. KPC cells (2,000/ml) transfected with siANX2s or sicontrol
in medium with or without 2.5 µg/ml rTNC were incubated in
medium without growth factor with rotation for 24 h at 37°C. The
colony formation assay was then performed (16). The number of colonies was
determined at 14 days following cell seeding. Tumor colonies were
visualized following staining with Giemsa stain solution (dilution
1:20, Waki Pure Chemical Industries, Ltd., Osaka, Japan).
Immunohistochemistry (IHC)
Formalin-fixed paraffin-embedded tissue samples were
cut into 4-µm-thick slices and deparaffinized. The antigens
were activated by autoclaving the tissue slides in citric acid
buffer (0.01 mol/l, pH 6.0) at 120°C for 10 min. The slides were
blocked with hydrogen peroxide (H2O2) diluted
to 3% with methanol for 15 min to inactivate endogenous peroxidase.
Immunohistochemical staining was performed using the
hyper-sensitive polymer method (Dako EnVision+ kit; Dako; Agilent
Technologies GmbH; Agilent Technologies GmbH, Waldbronn, Germany)
for ANX2 and using the labeled streptavidin-biotin-peroxidase
method (Dako; Agilent Technologies GmbH LSAB2 kit; Dako; Agilent
Technologies GmbH) for TNC, according to the manufacturer's
protocol. Following protein blocking, the slides were incubated
with the following primary antibodies: Anti-ANX2 polyclonal
antibody (1:200 dilution; cat. no. sc-9061; Santa Cruz
Biotechnology, Inc.) and monoclonal anti-human TNC antibody
(1:4,000 dilution; cat. no. T2551; Sigma-Aldrich; EMD Millipore)
overnight at 4°C. Counterstaining was performed with hematoxylin
prior to dehydration, penetration and mounting. Using an inverted
microscope (BX40; Olympus Corporation, Tokyo, Japan), the staining
intensities of ANX2 and TNC were evaluated independently by two
investigators with a pathologist. The ANX2 staining patterns were
scored as previously described (7,8):
Low expression, 0-30% of cancer cells with cell surface staining;
High expression, >30% of cancer cells with cell surface
staining. The TNC staining patterns were scored as follows: Low
expression, no expression in the stroma surrounding the cancer;
High expression, linear staining in the stroma surrounding the
cancer. The staining intensity of normal pancreatic ductal cells
was used as an internal positive control.
Statistical analysis
The correlations between ANX2/TNC staining
expression and the characteristics of patients with PDAC were
evaluated using the χ2 test. Survival rates were
calculated using Kaplan-Meier analysis and assessed using the
log-rank test. The in vitro experiments were performed at
least three times independently and data were analyzed using
Welch's t-test. P<0.05 was considered to indicate a
statistically significant difference. Values are expressed as the
mean ± standard error of the mean or standard deviation. The above
series of statistical analyses were performed using JMP®
PRO 13 software (SAS Institute Inc., Cary, NC, USA).
Results
ANX2 is expressed at high levels in
murine primary pancreatic neoplastic cells
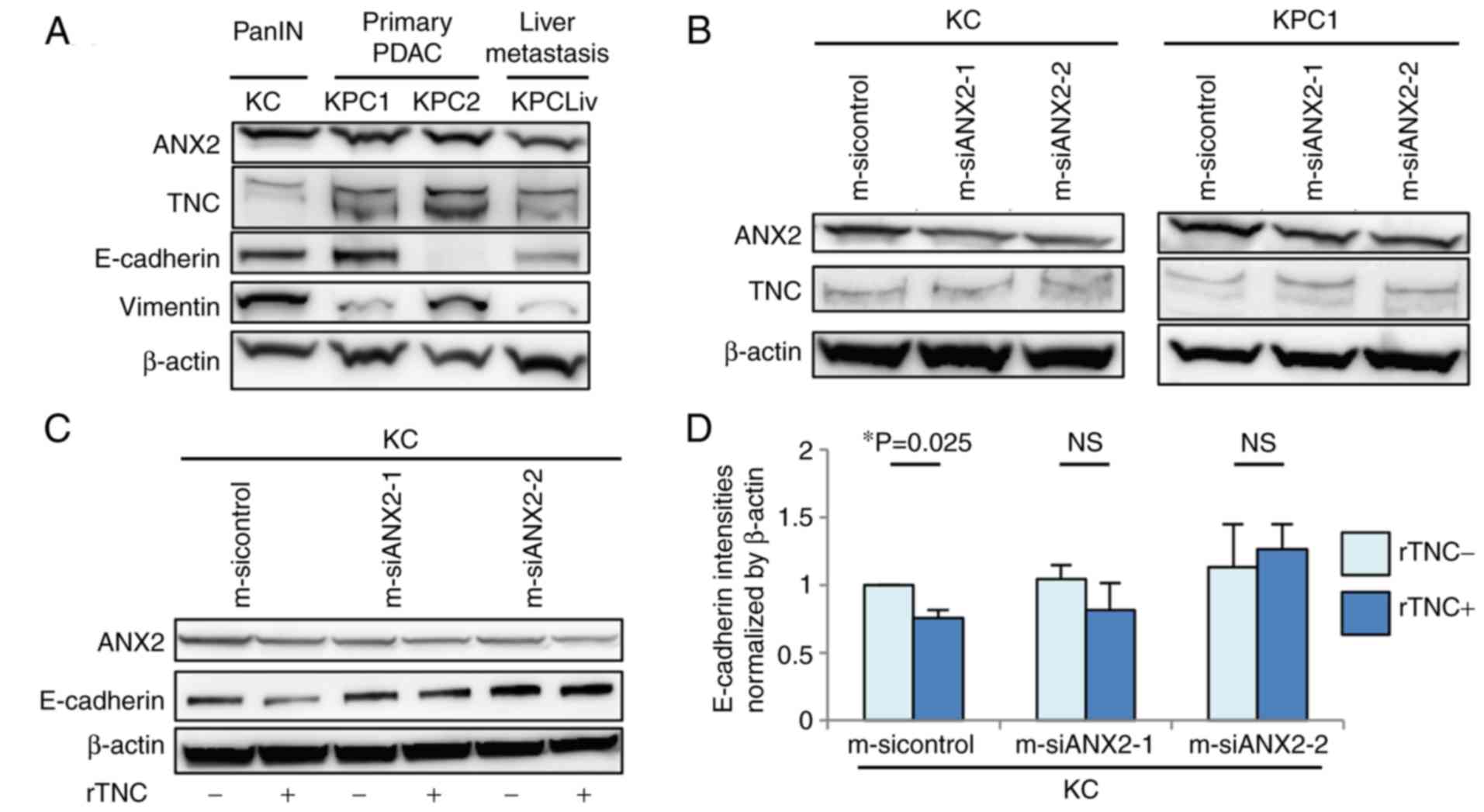
The present study examined the protein expression
levels of ANX2 and endogenous TNC among murine pancreatic
neoplastic cells isolated from the pancreas of KC mice or
pancreas/liver metastases of KPC mice, which are genetically
engineered mouse models. The expression of ANX2 was upregulated in
primary precancerous PanIN (KC) cells and invasive PDAC (KPC)
cells, but was relatively down-regulated in liver metastatic PDAC
(KPCLiv) cells, compared with that in primary KPC cells (Fig. 1A). Endogenous TNC in the invasive
KPC cells was expressed at a high level, compared with that in
precancerous and liver metastatic pancreatic cells. The present
study also assessed the correlation in protein expression between
ANX2 and representative epithelial/mesenchymal markers, E-cadherin
and vimentin, among these cell lines. There was no correlation in
expression between ANX2 and E-cadherin/vimentin according to the
western blot results.
Subsequently, the present study determined whether
the expression of ANX2 affects the expression of endogenous TNC.
ANX2 knockdown using ANX2-specific siRNAs did not affect the
expression of TNC in KC or KPC1 cells (Fig. 1B). Subsequently, whether ANX2 with
extrinsic TNC influences the expression of EMT markers in KC cells
was determined. The exposure to rTNC resulted in the significant
downregulation of E-cadherin in KC cells (P=0.025); however, there
was no effect on the expression of E-cadherin in the ANX2-knockdown
cells (Fig. 1C and D). Taken
together, these data indicated that ANX2 and extrinsic TNC may
induce pancreatic neoplastic cells to transition to the mesenchymal
phenotype during the progression of PDAC.
ANX2-TNC interaction regulates
mesenchymal morphology in pancreatic neoplastic cells
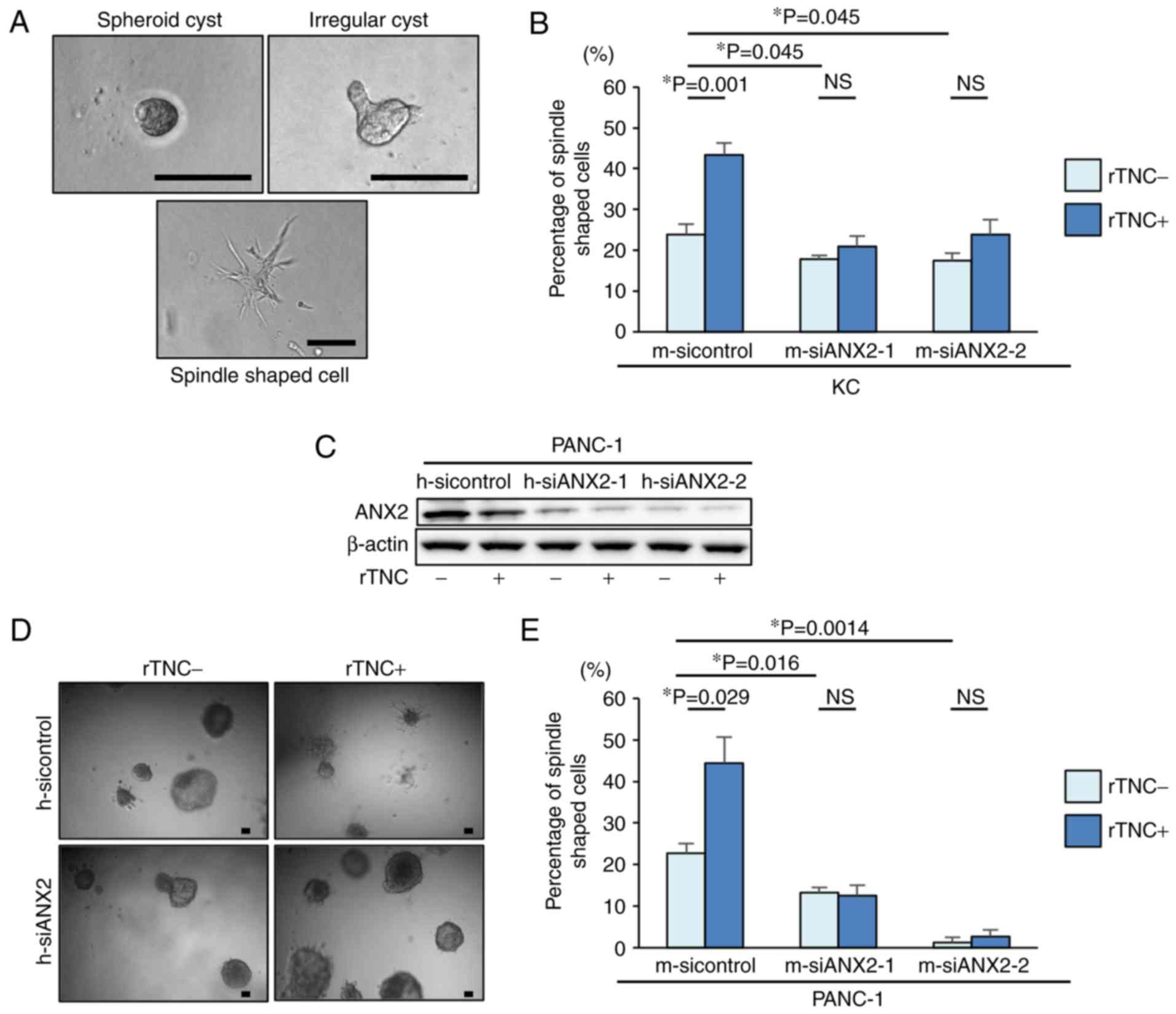
To examine the functional effects of ANX2 and TNC in
pancreatic neoplastic cells, the present study first used a
well-established 3D organotypic culture system. Based on the
cellular phenotype, the cultured KC cells exhibited representative
morphological characteristics, including spheroid cysts, irregular
cysts, and a spindle shape, in the 3D culture system (Fig. 2A). To determine whether ANX2, TNC,
or the ANX2-TNC interaction affects the morphological changes in
pancreatic neoplastic cells, ANX2 knockdown and rTNC treatment were
performed in KC cells. The ANX2-knockdown cells demonstrated
significantly altered morphology and a smaller population of
mesenchymal spindle-shaped cells, compared with the KC control
cells (Fig. 2B). Additionally, it
was observed that rTNC treatment resulted in a significant increase
spindle-shaped cells in the control cells (P=0.001) but not in the
ANX2-knockdown cells (Fig. 2B).
3D organotypic cell culture was also performed using a
representative human PDAC cell line, PANC-1 cells. Western blot
analysis confirmed that the expression of ANX2 in the PANC-1 cells
was effectively suppressed by the specific siRNAs for human ANX2,
and rTNC treatment did not affect the expression of ANX2 in PANC-1
cells (Fig. 2C). Consistent with
the results in murine cells, the 3D cell culture experiments
revealed that rTNC treatment increased the percentage of
spindle-shaped cells in the control PANC-1 cells (P=0.029) but not
in the ANX2-knockdown PANC-1 cells (Fig. 2D and E). These results suggested
that ANX2 has a functional role in maintaining the mesenchymal
phenotype and that the ANX2-TNC interaction fosters this in
pancreatic neoplastic cells.
Interaction between ANX2 and TNC controls
cell invasion in pancreatic neoplastic cells
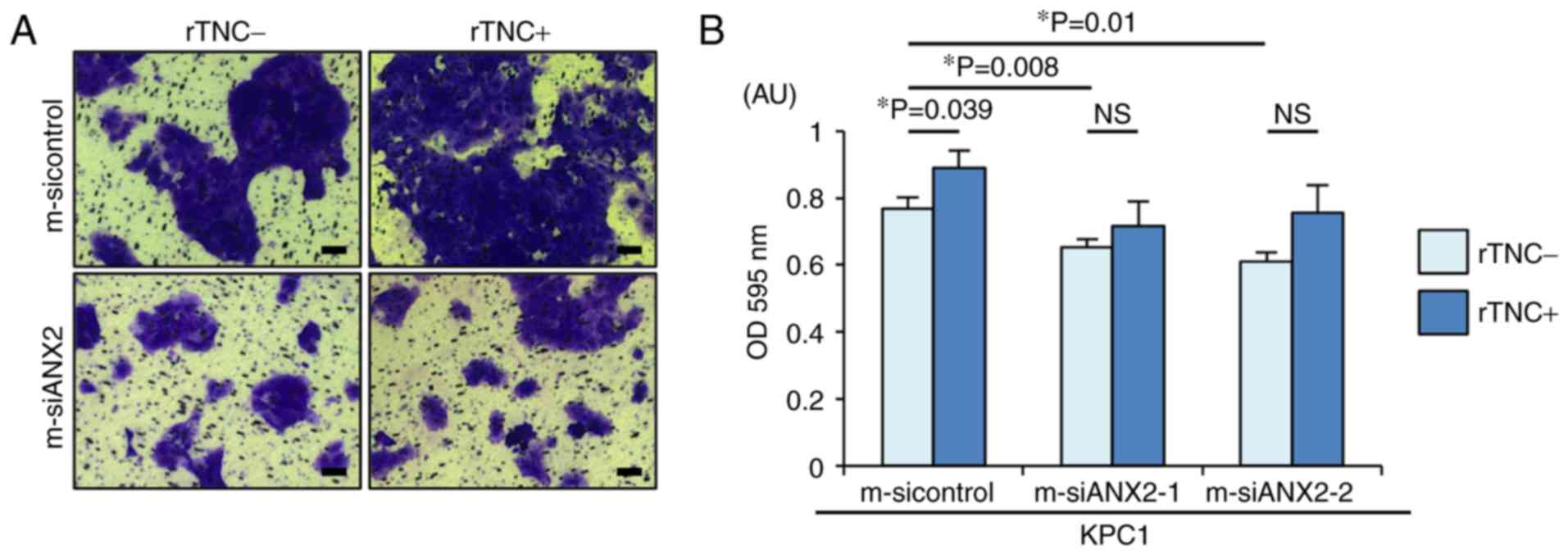
The present study aimed to determine whether the
ANX2-TNC interaction affects PDAC cell invasion, a representative
mesenchymal feature. As previously reported, ANX2 knockdown
resulted in significantly reduced invasion, compared with that
observed in the control cells (m-sicontrol, vs. m-siANX2-1;
P=0.008, m-sicontrol, vs. m-siANX2-2; P=0.01) (Fig. 3A and B). Notably, rTNC treatment
significantly facilitated cell invasion in the KPC1 control cells
but not in the ANX2-knockdown KPC1 cells (P=0.039). Taken together,
these results indicated that the ANX2-TNC interaction fosters cell
invasion in the invasive PDAC cells.
ANX2 and TNC maintain stem-like
characteristics in PDAC cells
To form metastatic colonies in a distant organ,
self-renewal properties are required for the disseminated cancer
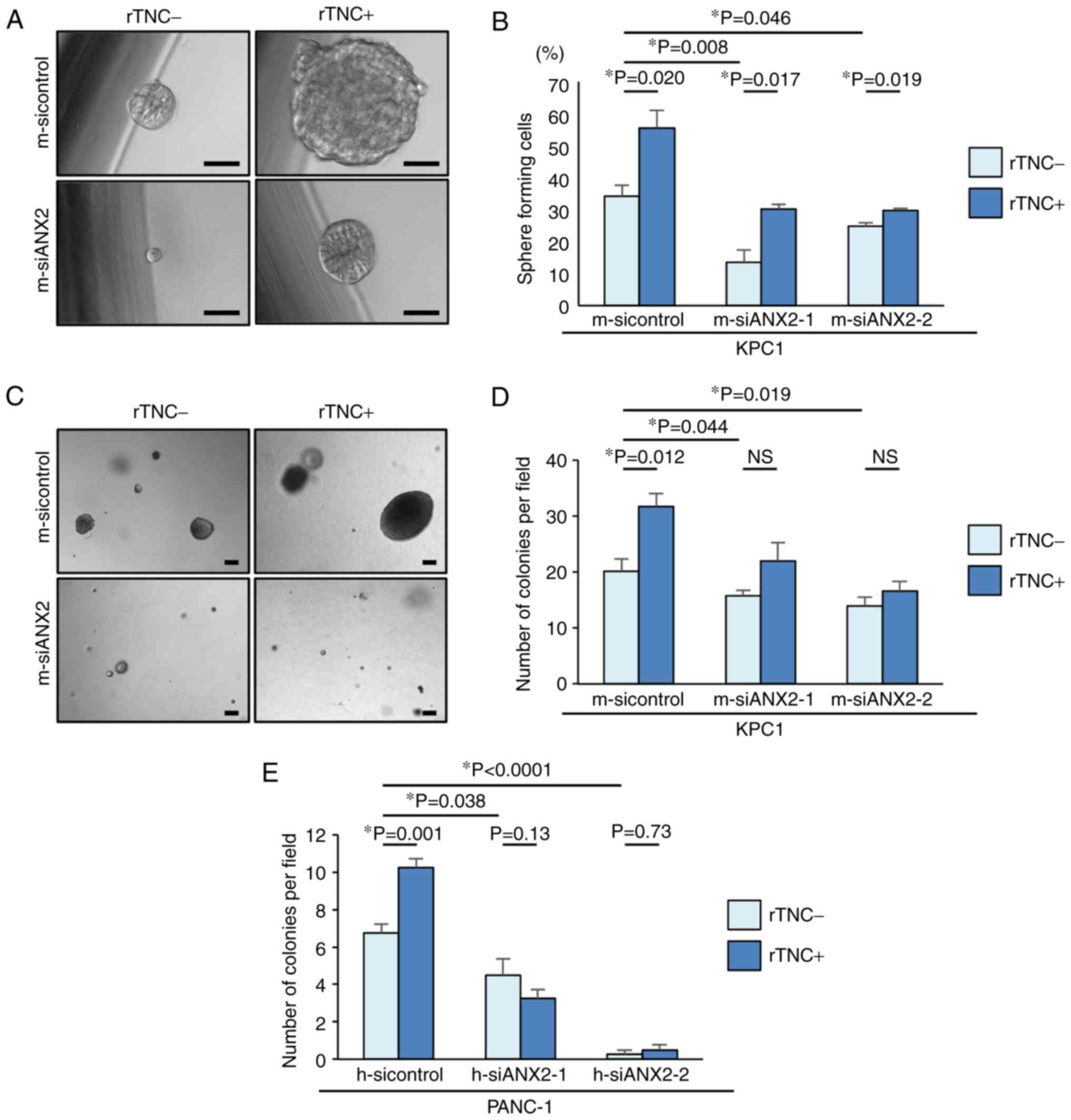
cells. Pancreatosphere formation assays were performed in the
present study to determine whether ANX2 confers the properties of
putative cancer stem cells (CSCs) to KPC1 cells. In the
pancreatosphere formation assays, ANX2 knockdown significantly
reduced the number of sphere-forming cells, compared with that in
the control KPC1 cells (m-sicontrol, vs. m-siANX2-1; P=0.008,
m-sicontrol, vs. m-siANX2-2; P=0.046) (Fig. 4A and B). Subsequently, whether
rTNC treatment also affected the self-renewal capacity of KPC1
cells was determined. Despite ANX2 knockdown, rTNC treatment
increased the number of sphere-forming cells in the KPC1 cells.
These results suggested that ANX2 and TNC have a functional role in
the stem-like properties of PDAC cells, independently.
ANX2-TNC interaction contributes to
anoikis resistance in PDAC cells
TNC is reported to accelerate the metastasis of
cancer cells (11). Based on the
association between the ANX2-TNC interaction and metastasis, it was
hypothesized that the interaction may prevent cancer cells from
anoikis following extravasation and subsequent colonization in
metastatic site. To clarify this hypothesis in vitro, ANX2
knockdown was performed using the specific ANX2 siRNAs. An anoikis
assay was performed to evaluate the potential role of the ANX2-TNC
interaction in resistance to apoptosis following loss of contact
from the ECM. Compared with that of the control KPC1 cells, the
resistance of the ANX2-knockdown KPC1 cells to anoikis was
significantly decreased (Fig. 4C and
D). rTNC treatment significantly fostered colony formation in
the KPC1 control cells but not in the ANX2-knockdown KPC1 cells
(P=0.012). These results were also confirmed in human PANC-1 cells
(Fig. 4E). These data suggested
that ANX2 knockdown led to functionally impaired
anchorage-independent growth and that the ANX2-TNC interaction
accelerated colony formation in vitro.
High expression of ANX2 and stromal TNC
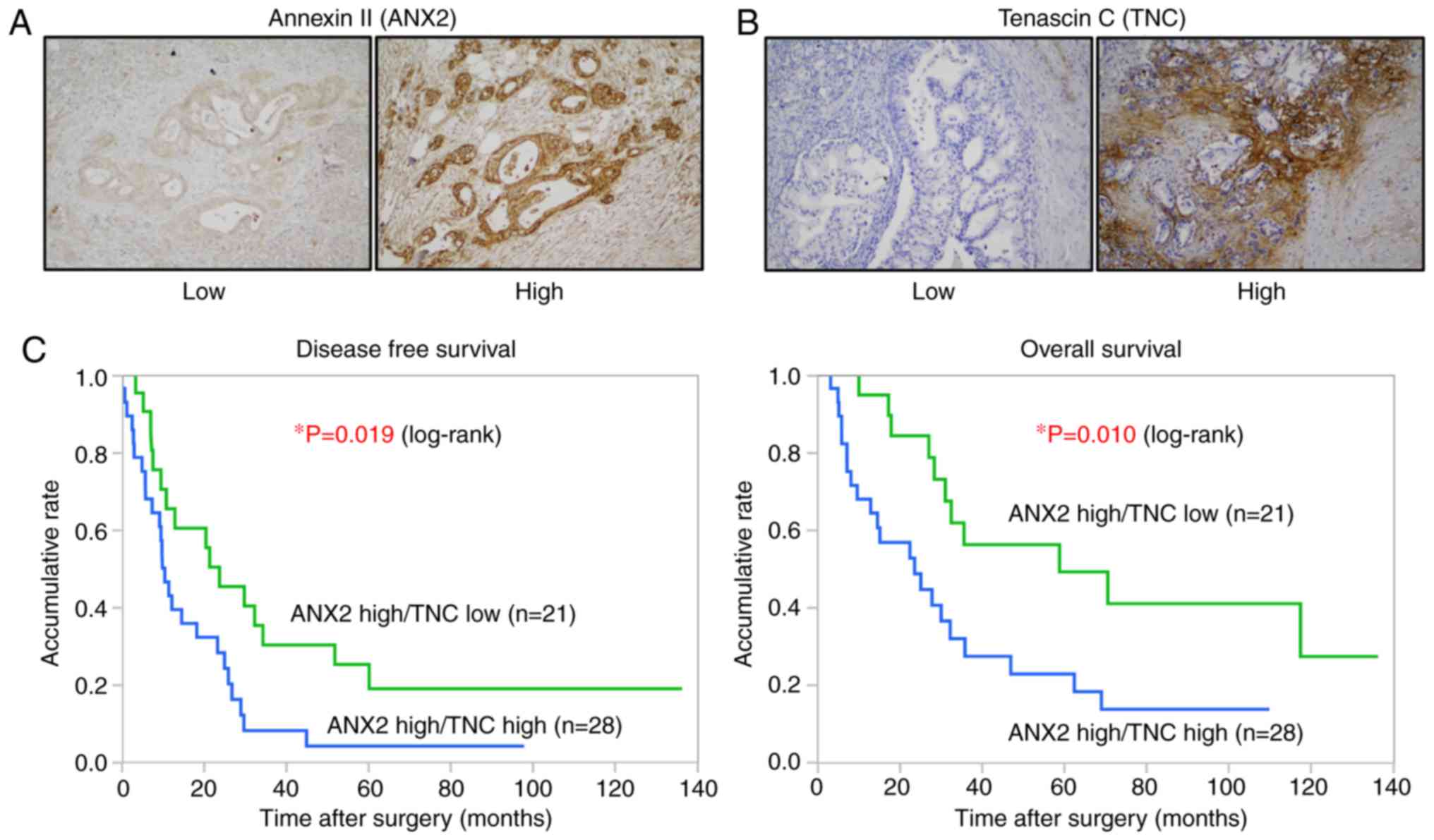
positively correlates with distant metastasis in human PDAC
The present study examined the correlation between
the expression of ANX2 and TNC and analyzed its clinical
significance in PDAC. The expression levels of ANX2 and TNC in 78
resected human PDAC tissues were assessed by IHC staining. As
previously shown, ANX2 was predominantly localized on the cell
surface of PDAC cells (7,8), whereas TNC was mainly expressed in
the stroma surrounding ductal carcinoma cells (Fig. 5A and B). Among the 78, 49 cases
(62.8%) were classified as ANX2 High expression, and 29 cases
(37.2%) were classified as ANX2 Low expression based on the
staining scores. The expression of TNC was also classified into the
TNC high group (43 cases, 55.1%) and TNC Low group (35 cases,
44.9%) based on the staining scores. To evaluate the interaction
between the expression of ANX2 and stromal TNC, all tissue samples
were categorized into four groups as follows: 28 cases (35.9%) in
the ANX2 High/TNC High group, 21 cases (26.9%) in the ANX2 High/TNC
Low group, 15 cases (19.2%) in the ANX2 Low/TNC High group, and 14
cases (17.9%) in the ANX2 High/TNC Low group. No direct correlation
between the expression of cancer cell surface ANX2 and stromal TNC
was found in the primary PDAC tissues (Table I).
 | Table ICorrelation between expression of
ANX2 and stromal TNC in pancreatic ductal adenocarcinoma
tissues. |
Table I
Correlation between expression of
ANX2 and stromal TNC in pancreatic ductal adenocarcinoma
tissues.
| Expression of
ANX2 | Stromal TNC
expression
| P-value |
|---|
| High (n=43) | Low (n=35) |
|---|
| High (n=49) | 28 | 21 | NS |
| Low (n=29) | 15 | 14 | |
High expression of ANX2 and TNC is
associated with hematogenous and peritoneal recurrence following
surgery and poor prognosis in human PDAC
Finally, to focus on the clinical significance of
these proteins in PDAC, the present study analyzed the
clinicopathological features of the stromal expression of TNC in
the ANX2 High group. As shown in Table II, the expression of TNC was
significantly associated with lymph node metastasis, distant
metastasis and advanced stage in the ANX2 High group. A significant
correlation was observed between hematogenous and peritoneal
recurrence following curative surgery in the ANX2 High/TNC High
group (P=0.017, χ2 test; Table III). Furthermore, the
Kaplan-Meier analyses showed that patients with a high expression
of TNC presented with significantly shorter disease-free survival
(DFS) and overall survival (OS) rates, compared with those with a
low expression of TNC in the ANX2 High group (DFS, P=0.019; OS,
P=0.010, log-rank test; Fig. 5C).
These clinical data suggested that the high expression of ANX2 and
TNC is associated with hematogenous and peritoneal recurrence and
poor prognosis in patients with PDAC.
 | Table IIClinicopathological features of
stromal expression of TNC in patients with pancreatic ductal
adenocarcinoma and high expression of ANX2. |
Table II
Clinicopathological features of
stromal expression of TNC in patients with pancreatic ductal
adenocarcinoma and high expression of ANX2.
| Clinicopathological
feature | Total (n=49) | Stromal expression
of TNC
| P-value |
|---|
| High (n=28) | Low (n=21) |
|---|
| Age (years) | 66.3±8.6 | 66.9±8.9 | 66.3±8.5 | NS |
| Gender
(male/female) | 24/25 | 14/14 | 10/11 | NS |
| Tumor size
(mm) | 28.4±8.4 | 29.6±7.8 | 26.6±9.1 | NS |
| N (0/1) | 11/37 | 3/24 | 8/13 | 0.026 |
| M (0/1) | 43/6 | 22/6 | 21/0 | 0.007 |
| UICC stage
(IIA≥/≥IIB) | 10/39 | 2/26 | 8/13 | 0.007 |
| Residual tumor
(R0/R1) | 35/14 | 19/9 | 16/5 | NS |
 | Table IIICorrelation between stromal
expression of TNC and recurrent form in the ANX2 High expression
group. |
Table III
Correlation between stromal
expression of TNC and recurrent form in the ANX2 High expression
group.
| Recurrent form | Total (n=49) | Stromal expression
of TNC
| P-value |
|---|
| High (n=28) | Low (n=21) |
|---|
| Local (−/+) | 26/23 | 13/15 | 13/8 | NS |
| Lymph node
(−/+) | 45/4 | 26/2 | 19/2 | NS |
| Hematogenous and
peritoneal (−/+) | 23/26 | 9/19 | 14/7 | 0.017 |
Discussion
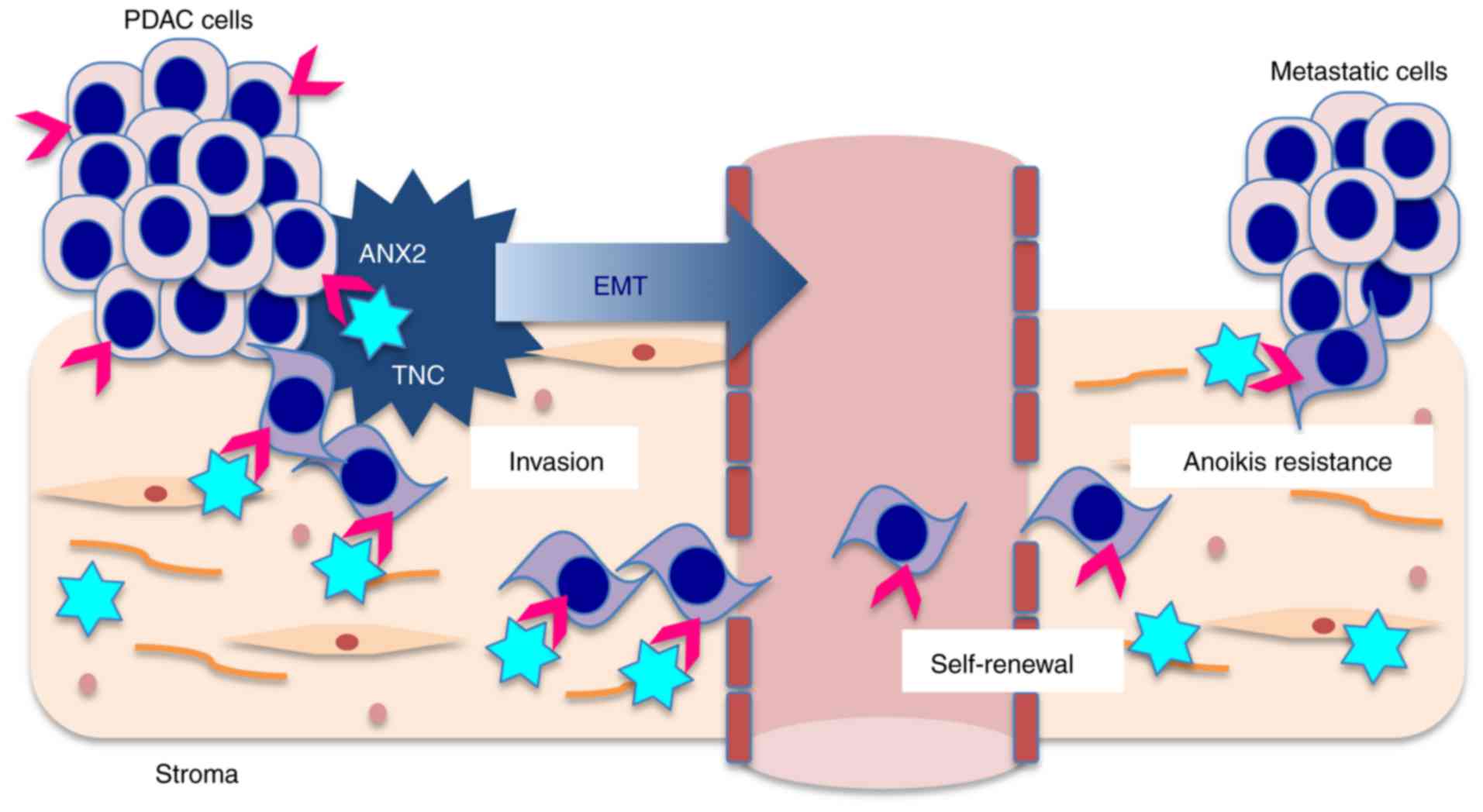
In the present study, it was demonstrated that ANX2
orchestrates with stromal TNC to induce EMT and promote distant
metastasis in PDAC cells (Fig.
6). The in vitro data indicated that the interaction
between ANX2 and extrinsic TNC facilitated pre-invasive PanIN cells
to undergo EMT, and invasive PDAC cells to invade and metastasize.
In clinical samples, IHC analyses also revealed that the ANX2
High/TNC High expression group was significantly correlated with
distant metastasis, and was associated with hematogenous and
peritoneal recurrence and poor prognosis following curative
surgery.
The present study demonstrated that ANX2 was
upregulated in mouse primary invasive PDAC cells, compared with the
level in liver metastatic cells. To investigate the expression
patterns of ANX2, intrinsic TNC and EMT markers, two PDAC cell
lines (KPC1 and KPC2) were used, which originate from two different
mice with the same mutant strains of KPC (Pdx1-cre;
LSL-KrasG12D/+; p53R172H/+). However, the
expression pattern of EMT markers and morphology are different
between these two cell lines. These results may reflect that cancer
is heterogeneous in nature. Notably, ANX2 orchestrates with
extrinsic TNC to induce EMT, which is considered an important
process in the progression of PDAC from precancerous PanIN cells to
invasive PDAC cells. Using a 3D cell culture system, the present
study confirmed that ANX2 knockdown promoted the morphological
changes from the epithelial to the mesenchymal phenotype in
preinvasive KC cells. This finding indicated that ANX2 may be
required to induce the mesenchymal phenotype in PDAC cells.
Invasion is a typical property of mesenchymal cells
and a crucial step for metastasis (19,20). Previous studies have demonstrated
that ANX2 knockdown resulted in a significant reduction in cell
invasion in various types of cancer cells, including PDAC cells
(5,21,22). Consistent with this, the present
study demonstrated that ANX2 knockdown decreased PDAC cell
invasion. Certain previous studies have indicated that EMT is not
always essential for metastasis (23). However, cells undergoing EMT
significantly contribute to recurrent lung metastasis formation
following chemotherapy (24), and
the suppression of EMT leads to an enhanced sensitivity to
gemcitabine treatment (25).
Supporting these findings, our previous study demonstrated that the
expression of ANX2 was upregulated in gemcitabine-resistant PDAC
cells (7), and the Akt/mammalian
target of rapamycin signaling pathway was shown to be involved in
the mechanism of chemoresistance conferred by ANX2 in PDAC cells
(8).
TNC is a large glycoprotein located in the ECM,
which regulates the interactions between the parenchyma and
mesenchyme in conditions accompanying EMT and invasion (26,27). TNC is overexpressed in PanIN
lesions, and there is diffuse stromal expression in PDAC together
with localization of its cell receptor ANX2 on the tumor cells
(12). To determine whether the
ANX2-TNC interaction is crucially involved in the invasion and
metastasis of PDAC cells, the present study hypothesized that the
interaction of ANX2 with stromal TNC can orchestrate to have
functional roles and be a potential therapeutic target that
regulates the invasion, putative CSC properties and anoikis
resistance in the progression of PDAC. The present study observed
that extrinsic TNC induced a decrease in the expression of
E-cadherin, reflecting active EMT; furthermore, ANX2 knockdown
reduced the morphological mesenchymal properties of murine PanIN KC
cells. Therefore, these findings suggested that ANX2-TNC may
cooperate in precancerous KC cells to induce the cells to undergo
EMT during the progression of PDAC.
Oskarsson et al suggested that cancer
cell-derived TNC remains essential for metastasis outgrowth until
the tumor stroma takes over as a source of TNC in breast cancer
(11). In the present study, it
was also demonstrated that TNC enhanced the self-renewal capacity
of PDAC KPC cells. Notably, independent of rTNC treatment, the
expression of ANX2 also affected the self-renewal capacity of PDAC
cells. It has been demonstrated that EMT-inducing transcription
factors confer mesenchymal and stem cell properties (28). Using an anoikis assay, the present
study examined the final step of the metastatic cascade, namely
colony formation following extravasation from blood vessels, in
in vitro experiments. Supportively, the anoikis assays
clearly showed that the interaction of ANX2-TNC accelerated colony
formation of murine and human PDAC cells. Considering these
results, it may be possible that ANX2-TNC, in which ANX2 has
sequential effects on inducing invasiveness and self-renewal
capacity and TNC has effects in establishing a metastatic niche
with CSC properties and anoikis resistance, facilitates metastasis
during the metastatic cascade of PDAC.
In an analysis of clinical samples, a previous study
showed that the co-overexpression of ANX2 and TNC was an
independent factor of poor prognosis in patients with colorectal
carcinoma (29). In the present
study, the correlation between ANX2 and stromal TNC in human PDAC
tissues was examined. Unexpectedly, there was no positive
correlation between the expression of ANX2 and stromal TNC;
however, it was confirmed that a high expression of both ANX2 and
stromal TNC was significantly correlated with
hematogenous/peritoneal recurrence and poor prognosis of patients
with PDAC following curative surgery. Foley et al
demonstrated that ANX2 promoted PDAC metastasis in vivo by
knocking out ANX2 in KPC mice, a model that recapitulates the
progression of human PDAC (30).
Together with these data, the results of the present study
suggested that the high expression of ANX2-TNC leads to poorer
clinical outcomes for patients with PDAC.
In conclusion, the present study demonstrated that
the ANX2-TNC interaction sequentially regulated EMT, invasion and
metastatic colonization in the progression of PDAC. The ANX2-TNC
partnership accelerated the progression of PDAC from invasion to
metastasis. One of the limitations of the present study is that no
gain-of-function or rescue experiments were performed. Further
investigations are warranted to determine whether inhibition of the
ANX2-TNC interaction influences the progression of PDAC in
vivo and whether the ANX2-TNC axis may be a promising
therapeutic target for PDAC.
Acknowledgments
The authors would like to thank Dr Suzuki and Dr
Nishino (Department of General Surgery, Chiba University, Japan)
for their technical support in experiments.
Abbreviations:
|
ANX2
|
annexin II
|
|
TNC
|
tenascin C
|
|
PanIN
|
pancreatic intraepithelial
neoplasia
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
EMT
|
epithelial to mesenchymal
transition
|
|
CSC
|
cancer stem cell
|
|
IHC
|
immunohistochemistry
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
Funding
This study was supported by the Grant-in-Aid for
Scientific Research (KAKENHI): KIBAN B (grant no. 15H04925 to ST,
HY, SK and MM, and grant no. 17H04287 to MM, ST, HY, SK and MO);
KIBAN C (grant no. 16K08979 to HY, ST, SK and MM, grant no.
16K10589 to MO, ST and SK, and grant no. 17K10661 to SK and MO);
the Challenge Exploratory Research (grant no. 16K15607 to ST, HY,
SK and MM); and the Young Scientists B (grant no. 15K18439 to
SK).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NY contributed to the data acquisition, and drafting
and revision of the manuscript. ST contributed to the study
concept, experimental design, supervision and the data
interpretation, and drafting, revision and finalization of the
manuscript. YN and RS contributed to the data acquisition and
analysis. SK, KF, TT and SK contributed to the conception of the
study. HY, MM and MO contributed to the study concept and
supervision.
Ethics approval and consent to
participate
The Ethics Committees of Chiba University, Graduate
School of Medicine approved the protocol of the present study, and
written informed consent was obtained from each patient prior to
surgery.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gerke V, Creutz CE and Moss SE: Annexins:
Linking Ca2+ signalling to membrane dynamics. Nat Rev
Mol Cell Biol. 6:449–461. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Díaz VM, Hurtado M, Thomson TM, Reventós J
and Paciucci R: Specific interaction of tissue-type plasminogen
activator (t-PA) with annexin II on the membrane of pancreatic
cancer cells activates plasminogen and promotes invasion in vitro.
Gut. 53:993–1000. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng L, Foley K, Huang L, Leubner A, Mo
G, Olino K, Edil BH, Mizuma M, Sharma R, Le DT, et al: Tyrosine 23
phosphorylation-dependent cell-surface localization of annexin A2
is required for invasion and metastases of pancreatic cancer. PLoS
One. 6:e193902011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takano S, Togawa A, Yoshitomi H, Shida T,
Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Tomonaga T, et
al: Annexin II overexpression predicts rapid recurrence after
surgery in pancreatic cancer patients undergoing
gemcitabine-adjuvant chemotherapy. Ann Surg Oncol. 15:3157–3168.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kagawa S, Takano S, Yoshitomi H, Kimura F,
Satoh M, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Furukawa K, et
al: Akt/mTOR signaling pathway is crucial for gemcitabine
resistance induced by Annexin II in pancreatic cancer cells. J Surg
Res. 178:758–767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neesse A, Algül H, Tuveson DA and Gress
TM: Stromal biology and therapy in pancreatic cancer: A changing
paradigm. Gut. 64:1476–1484. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lowy CM and Oskarsson T: Tenascin C in
metastasis: A view from the invasive front. Cell Adh Migr.
9:112–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oskarsson T, Acharyya S, Zhang XH,
Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K,
Brogi E and Massagué J: Breast cancer cells produce tenascin C as a
metastatic niche component to colonize the lungs. Nat Med.
17:867–874. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Esposito I, Penzel R, Chaib-Harrireche M,
Barcena U, Bergmann F, Riedl S, Kayed H, Giese N, Kleeff J, Friess
H, et al: Tenascin C and annexin II expression in the process of
pancreatic carcinogenesis. J Pathol. 208:673–685. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hingorani SR, Wang L, Multani AS, Combs C,
Deramaudt TB, Hruban RH, Rustgi AK, Chang S and Tuveson DA:
Trp53R172H and KrasG12D cooperate to promote
chromosomal instability and widely metastatic pancreatic ductal
adenocarcinoma in mice. Cancer Cell. 7:469–483. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wescott MP, Rovira M, Reichert M, von
Burstin J, Means A, Leach SD and Rustgi AK: Pancreatic ductal
morphogenesis and the Pdx1 homeodomain transcription factor. Mol
Biol Cell. 20:4838–4844. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reichert M, Takano S, Heeg S, Bakir B,
Botta GP and Rustgi AK: Isolation, culture and genetic manipulation
of mouse pancreatic ductal cells. Nat Protoc. 8:1354–1365. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takano S, Reichert M, Bakir B, Das KK,
Nishida T, Miyazaki M, Heeg S, Collins MA, Marchand B, Hicks PD, et
al: Prrx1 isoform switching regulates pancreatic cancer invasion
and metastatic colonization. Genes Dev. 30:233–247. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rovira M, Scott SG, Liss AS, Jensen J,
Thayer SP and Leach SD: Isolation and characterization of
centroacinar/terminal ductal progenitor cells in adult mouse
pancreas. Proc Natl Acad Sci USA. 107:75–80. 2010. View Article : Google Scholar
|
|
18
|
Suzuki K, Takano S, Yoshitomi H, Nishino
H, Kagawa S, Shimizu H, Furukawa K, Miyazaki M and Ohtsuka M:
Metadherin promotes metastasis by supporting putative cancer stem
cell properties and epithelial plasticity in pancreatic cancer.
Oncotarget. 8:66098–66111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang F, Zhang L, Zhang B, Wei X, Yang Y,
Qi RZ, Ying G, Zhang N and Niu R: Anxa2 plays a critical role in
enhanced invasiveness of the multidrug resistant human breast
cancer cells. J Proteome Res. 8:5041–5047. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao P, Zhang W, Tang J, Ma XK, Dai JY, Li
Y, Jiang JL, Zhang SH and Chen ZN: Annexin II promotes invasion and
migration of human hepatocellular carcinoma cells in vitro via its
interaction with HAb18G/CD147. Cancer Sci. 101:387–395. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beerling E, Seinstra D, de Wit E, Kester
L, van der Velden D, Maynard C, Schäfer R, van Diest P, Voest E,
van Oudenaarden A, et al: Plasticity between epithelial and
mesenchymal states unlinks EMT from metastasis-enhancing stem cell
capacity. Cell Rep. 14:2281–2288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fischer KR, Durrans A, Lee S, Sheng J, Li
F, Wong S, Choi H, Rayes TE, Ryu S, Troeger J, et al:
Epithelial-to-mesenchymal transition is not required for lung
metastasis but contributes to chemoresistance. Nature. 527:472–476.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiquet-Ehrismann R and Chiquet M:
Tenascins: Regulation and putative functions during pathological
stress. J Pathol. 200:488–499. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cai J, Du S, Wang H, Xin B, Wang J, Shen
W, Wei W, Guo Z and Shen X: Tenascin-C induces migration and
invasion through JNK/c-Jun signalling in pancreatic cancer.
Oncotarget. 8:74406–74422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Emoto K, Yamada Y, Sawada H, Fujimoto H,
Ueno M, Takayama T, Kamada K, Naito A, Hirao S and Nakajima Y:
Annexin II overexpression correlates with stromal tenascin-C
overexpression: A prognostic marker in colorectal carcinoma.
Cancer. 92:1419–1426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Foley K, Rucki AA, Xiao Q, Zhou D, Leubner
A, Mo G, Kleponis J, Wu AA, Sharma R, Jiang Q, et al: Semaphorin 3D
autocrine signaling mediates the metastatic role of annexin A2 in
pancreatic cancer. Sci Signal. 8:ra772015. View Article : Google Scholar : PubMed/NCBI
|