Introduction
Wound healing is a highly complex process,
associated with multiple biochemical pathways and a variety of
cellular activities, such as re-epithelialization (1) and angiogenesis (2). Following trauma, keratinocytes, the
predominant cell type in the epidermis, are activated to contribute
to the repair of cutaneous wounds (3). The activated keratinocytes
participate in re-epithelialization during wound healing via a
series of cellular activities, including cell migration and
proliferation (4). Therefore,
increasing the efficiency of migration and proliferation of
keratinocytes may accelerate the wound healing rate. However,
keratinocyte migration and proliferation remain not fully
elucidated, and therefore reliable and effective drugs that could
accelerate the above process have yet to be developed.
Icariin, a well-known traditional Chinese drug, is
extracted from Herba Epimedii. The pharmacological and
biological effects of icariin, including anti-inflammatory
(5), antitumor (6) and neuroprotective (7) effects, have been confirmed by
previous studies. Its effects on keratinocytes, however, remain
unclear. Previous studies have reported that icariin treatment
significantly induces the phosphorylation of AKT serine/threonine
kinase 1 (AKT) and extracellular signal-regulated kinase (ERK) in
dopaminergic EMS 23.5 cells (8).
Deng et al (9) have also
reported that icariin significantly upregulates the level of
phosphorylated (p)-AKT in rat nucleus pulposus cells. As the
phosphorylation of AKT and ERK are closely associated with the
migration and proliferation of keratinocytes (10), it is logical to hypothesize that
icariin could promote keratinocyte migration and proliferation,
thus exerting a beneficial effect on wound healing. Thus, the aim
of the present study was to examine the effects of icariin on
keratinocytes, and to further investigate the medicinal value of
icariin for the treatment of skin wounds.
In the present study, it was demonstrated that
icariin significantly accelerated the migration and proliferation
of keratinocytes. Furthermore, these processes were demonstrated to
be mediated through the activation of ERK and AKT signaling, and to
be accompanied by the upregulation of proliferation-associated
proteins. In addition, treatment with icariin accelerated wound
closure in vivo. These observations indicate that icariin
may be a suitable drug for the treatment of skin wounds.
Materials and methods
Cell culture and reagents
HaCaT cells were obtained from the Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China). Cells
were grown in Dulbecco's modified Eagle's medium (DMEM; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) containing 10%
heat-inactivated fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and 1% antibiotic-antimycotic
(100 μg/ml streptomycin and 100 U/ml penicillin) and
incubated at 37°C and 5% CO2. A Cell Counting Kit-8
(CCK-8) was purchased from MedChemExpress (Monmouth Junction, NJ,
USA). Antibodies against GAPDH (cat. no. ab37168), Cyclin D1 (cat.
no. ab134175) and Cyclin D3 (cat. no. ab63535) were purchased from
Abcam (Cambridge, UK). Antibodies against of AKT (cat. no. 4060),
p-Akt (cat. no. #4691), ERK (cat. no. 4695) and p-ERK (cat. no.
4370) were purchased from Cell Signaling Technology, Inc. (Danvers,
MA, USA). The second antibodies against HRP-Goat anti Rabbit (cat.
no. AS1107; 1:10,000) and HRP-Goat anti Mouse (cat. no. AS1106;
1:10,000) were purchased from Aspen Biotechnology Co., Ltd. (Wuhan,
China). An Akt inhibitor (MK-2206 2HCl) and an ERK inhibitor
(GDC-0994) were purchased from Selleck Chemicals (Houston, TX,
USA). ELISA kits (IL-6, IL-10, TNF-α; cat. nos. HM10205, HM10203
and HM10001 respectively) were purchased from Bio-Swamp Life
Science (Shanghai, China). Transwell chambers with 8 μm
pores were purchased from Corning, Inc. (Corning, NY, USA).
CCK-8 assay
The effect of icariin on HaCaT cell viability was
evaluated by CCK-8 assay. Briefly, 1×104 cells in 100
μl were seeded in each well of a 96-well plate and incubated
for 24 h at 37°C with 5% CO2. Various concentrations of
icariin as indicated (0–60 μM) were dissolved in fresh
medium and added to each well. After 24, 48 and 72 h of incubation,
10 μl of CCK-8 reagent was added to each well, and the cells
were further incubated for 2 h. The absorbance of each well was
measured using a microplate reader at 450 nm. The viability of
different groups was evaluated by comparing their optical density
(OD) values with that of the control group. The experiment was
conducted in triplicate.
Transwell assay
The effect of icariin on HaCaT cell migration was
assessed with a Transwell migration assay. After HaCaT cells were
starved in serum-free medium for 24 h, 1×105 cells in
200 μl serum-free growth medium were added to the upper
chambers of a 24-well Transwell plate. A total of 500 μl of
medium containing 10% FBS was added to each lower chamber, as a
chemoattractant. PBS (vehicle control) or 30 μM icariin were
added into the lower chamber. Following incubation for 24 h at
37°C, the migrated HaCaT cells were fixed with methanol, and
stained with 0.1% crystal violet. Images were obtained using a
light microscope (Olympus Corporation, Tokyo, Japan) at ×10
magnification. Cells were counted in five different optical fields
using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA). The experiment was conducted in
triplicate.
Western blot assay
For evaluation of the ERK and AKT pathways, cells
were treated with PBS or 30 μM icariin for 20 min. For
evaluation of the cyclin proteins, cells were treated with PBS, 30
μM icariin, 10 μM MK-2206 2HCl + 30 μM
icariin, or 10 μM GDC-0994 + 30 μM icariin for 24 h.
Following treatments as indicated, total protein was extracted with
RIPA lysis buffer (Wuhan Boster Biological Technology, Ltd., Wuhan,
China) and protease inhibitor cocktail (Roche Diagnostics, Basel,
Switzerland) on ice. The protein concentration was measured using a
BCA protein assay kit (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). A total of 40 μg of protein from each sample was
separated with 10% SDS-PAGE, and electrophoretically transferred to
a polyvinylidene fluoride membrane. Following transfer, the
membrane was blocked with 5% fat-free milk in PBST (PBS containing
0.05% Tween-20; Wuhan Boster Biological Technology, Ltd.) for 1 h
at room temperature and was incubated with the primary antibodies
targeting AKT (1:1,000), p-AKT (1:3,000), ERK (1:3,000), p-ERK
(1:1,000), Cyclin D1 (1:3,000), Cyclin D3 (1:1,000) and GAPDH
(1:10,000) at 4°C overnight. Subsequently, the membrane was
incubated with an HRP-conjugated secondary antibody for 1 h at room
temperature. Protein bands were visualized using an Odyssey
Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA).
ImageJ software (National Institutes of Health, Bethesda, MD, USA)
was used for quantitative analysis of scanned densitometric values
of the proteins as ratios to GAPDH (used as a loading control).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cells were treated with PBS, 30 μM icariin,
10 μM MK-2206 2HCl + 30 μM icariin, or 10 μM
GDC-0994 + 30 μM icariin for 6 h. Following treatments,
total RNA was extracted from each group using RNA Iso Plus reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. RNA was reverse-transcribed into cDNA
using the PrimeScript RT Reagent kit (Takara Bio., Inc., Otsu,
Japan). qPCR was performed with SYBR Master Mix (Takara Bio., Inc.)
using a StepOne Real-Time PCR System (Life Technologies; Thermo
Fisher Scientific, Inc.). The thermocycling conditions were as
follows: Pre-denaturing at 95°C for 1 min, amplification for 40
cycles by denaturing at 95°C for 15 sec, annealing at 58°C for 20
sec and extension at 72°C for 45 sec, followed by a final
dissociation cycle of 95°C for 15 sec, 60°C for 1 min and 95°C for
15 sec. The relative mRNA quantity of each gene was normalized to
GAPDH and calculated using the 2−ΔΔCq method (11). The primer sequences were: Tumor
necrosis factor (TNF)-α, forward, 5′-CTCTTCTCCTTCCTGATCGTGG-3′ and
reverse, 5′-CTTGTCACTCGGGGTTCGAG-3′; interleukin (IL)-6, forward,
5′-TCAGCCCTGAGAAAGGAGACAT-3′ and reverse,
5′-GCTCTGGCTTGTTCCTCACTACT-3′; IL-10, forward,
5′-AACCTGCCTAACATGCTTCG-3′ and reverse, 5′-GAGTTCACATGCGCCTTGAT-3′;
and GAPDH, forward, 5′-CATCATCCCTGCCTCTACTGG-3′ and reverse,
5′-GTGGGTGTCGCTGTTGAAGTC-3′. The experiment was conducted in three
independent biological replicates.
ELISA
The levels of IL-6, IL-10 and TNF-α secreted by
HaCaT cells were measured using ELISA kits. The HaCaT cells were
seeded into a 24-well plate at a density of 2×104 cells
per well, incubated overnight, and then treated with PBS, 30
μM icariin, 10 μM MK-2206 2HCl + 30 μM
icariin, or 10 μM GDC-0994 + 30 μM icariin. Following
treatment for 24 h, supernatants were collected and the release of
IL-6, IL-10 and TNF-α into the media was quantified using the ELISA
kits, according to the manufacturer's instructions. The experiment
was conducted in triplicate.
Wound healing animal model
A total of ten male Sprague Dawley rats (age, 4
weeks) were purchased from the Center of Experimental Animals,
Tongji Medical College, Huazhong University of Science and
Technology (Wuhan, China). The rats were housed under standard
laboratory conditions with a 12-h light/dark cycle, with food and
water supplied. All animal studies were conducted under the review
and with the approval of the Ethics Committee of Tongji Medical
College (Wuhan, China). All experiments were performed in
accordance with the relevant guidelines and regulations of Tongji
Medical College.
The rats were randomly divided into two groups (n=5
per group): The control group and the icariin group. Rats were
anesthetized using 10% chloral hydrate (0.3 ml/100 g body weight).
The fur on the back of each rat was clipped, and a 1×1 cm
full-thickness wound was generated on each rat. A total of 1 ml
containing either 30 μM icariin (icariin group) or PBS
(control group) was injected into the surrounding tissue of the
wound each day for a total of 10 days. The wound area was monitored
by capturing digital photographs at 0, 4, 7 and 10 days post-injury
and quantified using Image-Pro Plus 6.0 software (Media
Cybernetics). To ensure that the wound areas could be compared
between the two groups, digital photos were captured using a ruler
for reference. Three authors collected the wound area data
independently and the mean value was used for statistical analysis.
The rate of wound closure was calculated using the following
formula: Wound closure (%)=[(original wound area-open area on at
time-point)/original wound area] ×100%.
Hematoxylin and eosin (H&E)
staining
Full thickness skin samples of all wounds were
collected for histological analysis after 10 days of PBS or icariin
was applied on the wound of animals. Tissue samples were fixed in
10% neutral-buffered formalin solution for 24 h, then transferred
and stored in 70% ethyl alcohol until paraffin embedded.
Paraffin-embedded samples were cut into 4 μm thick sections
for hematoxylin and eosin staining, under standard protocols.
Epidermal thickness assessment
The epidermal thickness was measured using the
H&E sections. Measurements and quantification were performed
with Image-Pro Plus 6.0 software (Media Cybernetics) under ×4
magnification. Epidermal thickness was evaluated in three random
fields of each sample. Three independent observers completed these
analyses. The mean value of each measurement was used for
statistical analysis.
Statistical analysis
Statistical analysis was performed with SPSS 12.0
software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 6.0
software (GraphPad Software, Inc., La Jolla, CA, USA). Data are
presented as the mean ± standard deviation. Differences between
samples were compared by t-test between two groups or one-way
analysis of variance (ANOVA) among multiple groups. The
Student-Newman-Keuls post hoc method was used to calculate the
P-value for pairwise comparisons following ANOVA. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cytotoxicity of icariin in
keratinocytes
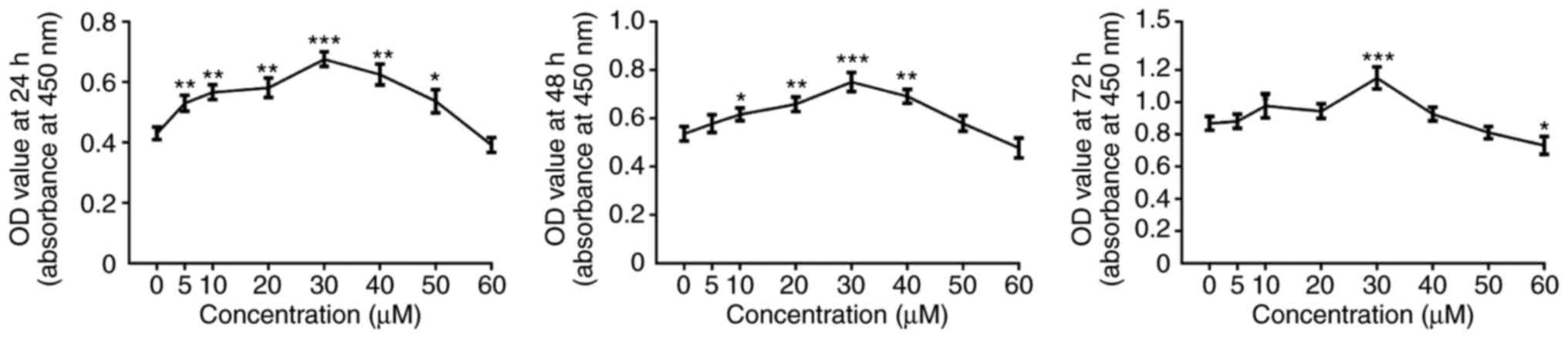
To evaluate the effect of icariin on keratinocyte
viability, a CCK-8 assay was used to test cell viability. At 24 h
following treatment with icariin, the cell viability in the 5–50
μM icariin treatment groups was higher compared with the
group treated with 0 μM icariin (Fig. 1). At 48 h, 10–40 μM icariin
significantly increased cell viability, whereas a concentration of
50 μM or 60 μM icariin did not affect viability
(Fig. 1). However, following 72 h
of treatment, 30 μM icariin significantly increased cell
viability, while 60 μM icariin significantly inhibited cell
viability, and 40 and 50 μM had no effect on cellular
viability (Fig. 1). Therefore,
the dose of 30 μM icariin was selected as optimal in the
subsequent experiments in order to prevent cytotoxicity.
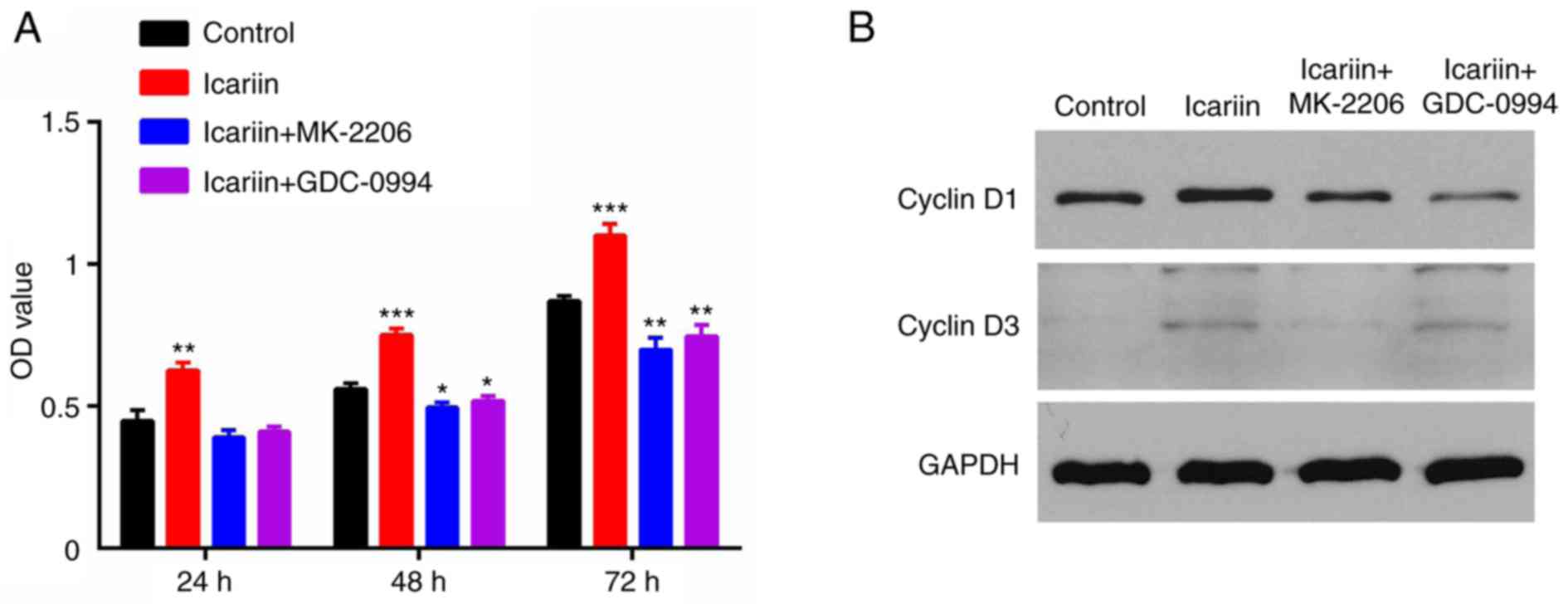
Icariin promotes the proliferation of
keratinocytes
A CCK-8 assay demonstrated that the proliferation of
HaCaT cells in the icariin group was higher compared with the
control group (Fig. 2A). To
further confirm the effect of icariin on cell proliferation, the
levels of Cyclin D1 and D3, two proteins that are closely
associated with cell proliferation, were quantified by western
blotting. The results demonstrated that the expression levels of
Cyclin D1 and D3 were markedly increased in the icariin treatment
group compared with the control group (Fig. 2B).
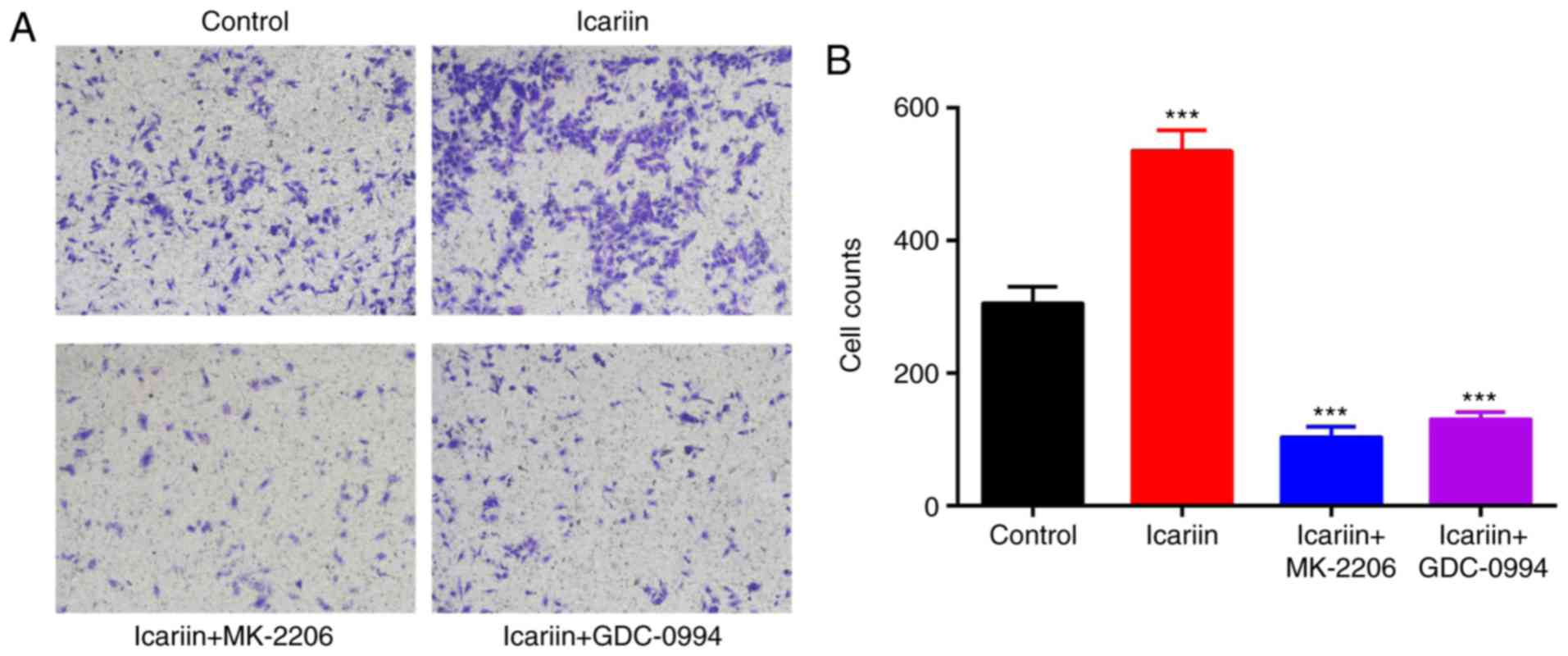
Icariin promotes the migration of
keratinocytes
To investigate the migration ability of the
keratinocytes treated with icariin, a Transwell migration assay was
performed. Representative images of the Transwell assay results at
24 h are presented in Fig. 3A.
Quantitative analysis of the numbers of migrated cells revealed
that the icariin-treated group exhibited a significant increase in
migration ability compared with the control group (P<0.001;
Fig. 3B).
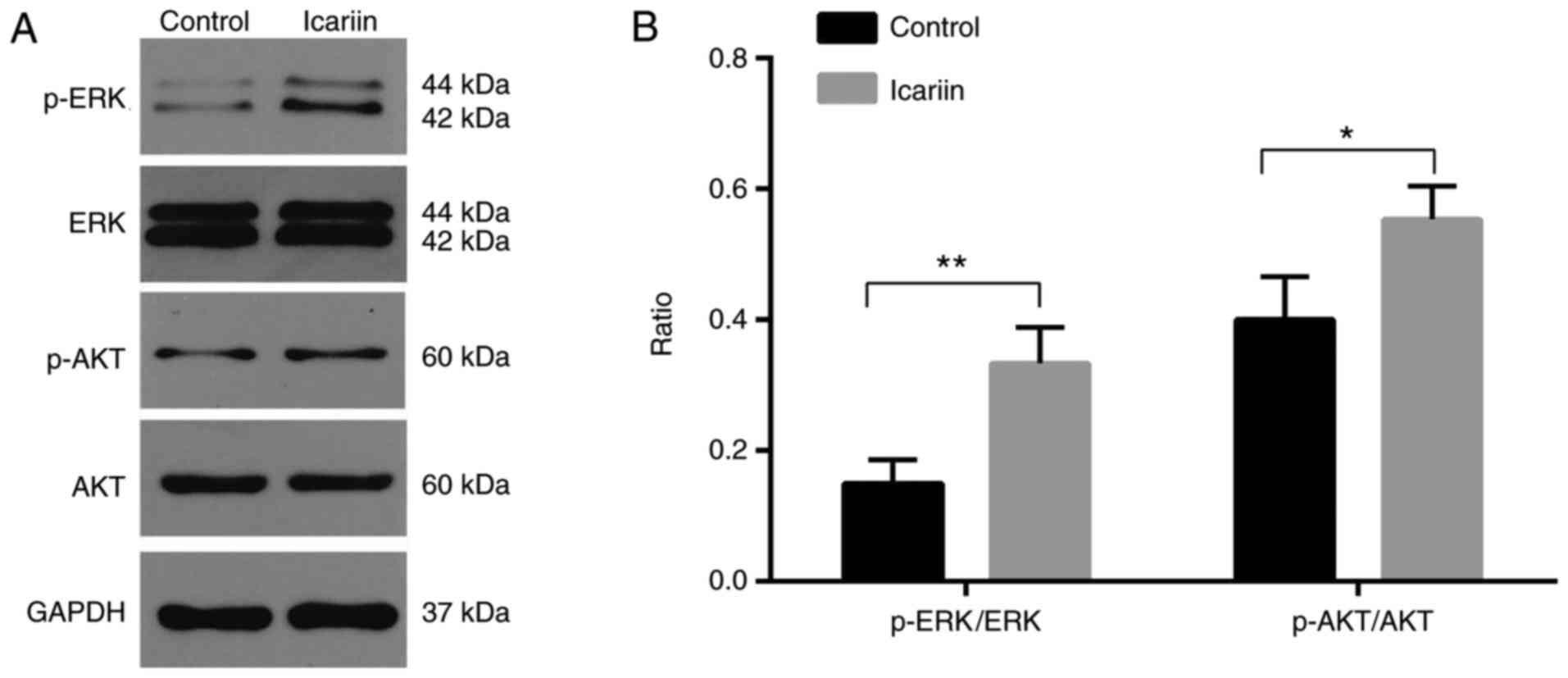
ERK and AKT are involved in the
icariin-mediated promotion of keratinocyte proliferation and
migration
To investigate which signaling pathways were
involved in the promotion of migration and proliferation by
icariin, the activation of the ERK and AKT signaling pathways was
assayed by western blotting. The results demonstrated that the
levels of p-ERK and p-AKT were significantly increased at 30 min
following treatment with icariin compared with the control group
(Fig. 4).
Subsequently, the effect of ERK and AKT inhibitors
was assessed on HaCaT cell migration and proliferation. HaCaT cells
were incubated with the MK-2206 2HCl AKT inhibitor (10 μM)
or the GDC-0994 ERK inhibitor (50 μM) for 2 h prior to
treatment with icariin. The icariin-mediated increase on cell
proliferation (Fig. 2) and
migration (Fig. 3) was
significantly blocked when cells were pretreated with the ERK or
the AKT inhibitor. These results indicated that phosphorylation and
activation of AKT and ERK were required for the effects of icariin
on keratinocytes.
Icariin treatment alters the production
of cytokines in keratinocytes
Following treatment of HaCaT cells with icariin (30
μM), the mRNA expression levels of cytokines were detected
by RT-qPCR (at 6 h), while the levels of secreted cytokines in the
cell supernatants were determined by ELISA kits (at 24 h). As
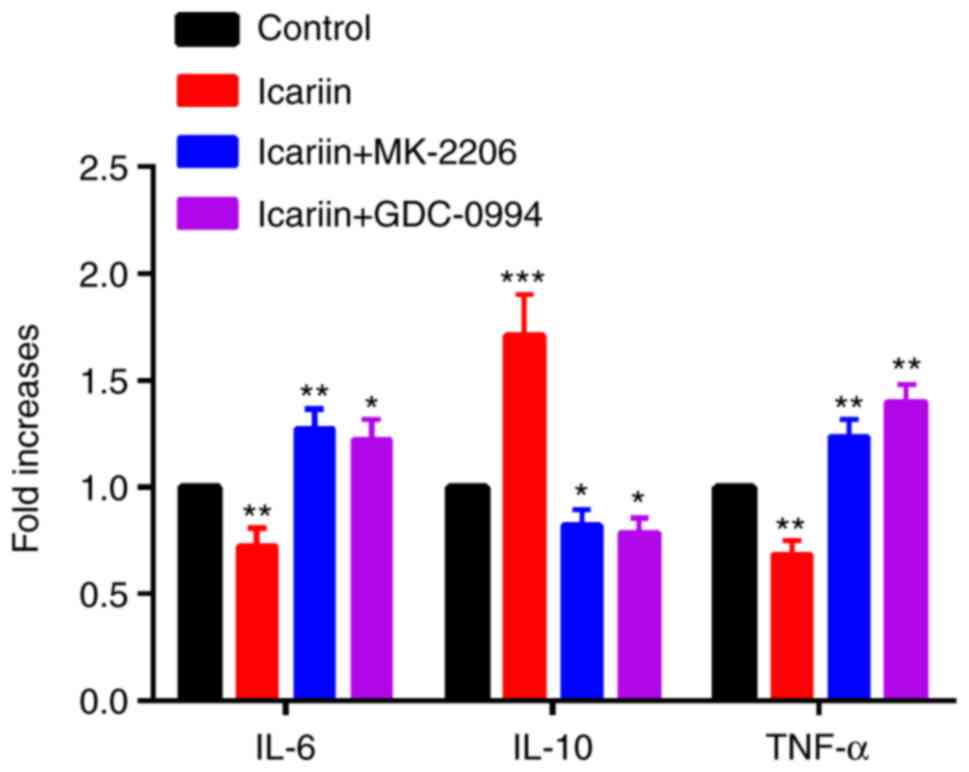
illustrated in Fig. 5, the IL-6
and TNF-α mRNA expression decreased and the IL-10 mRNA expression
increased in the icariin group compared with the control group. At
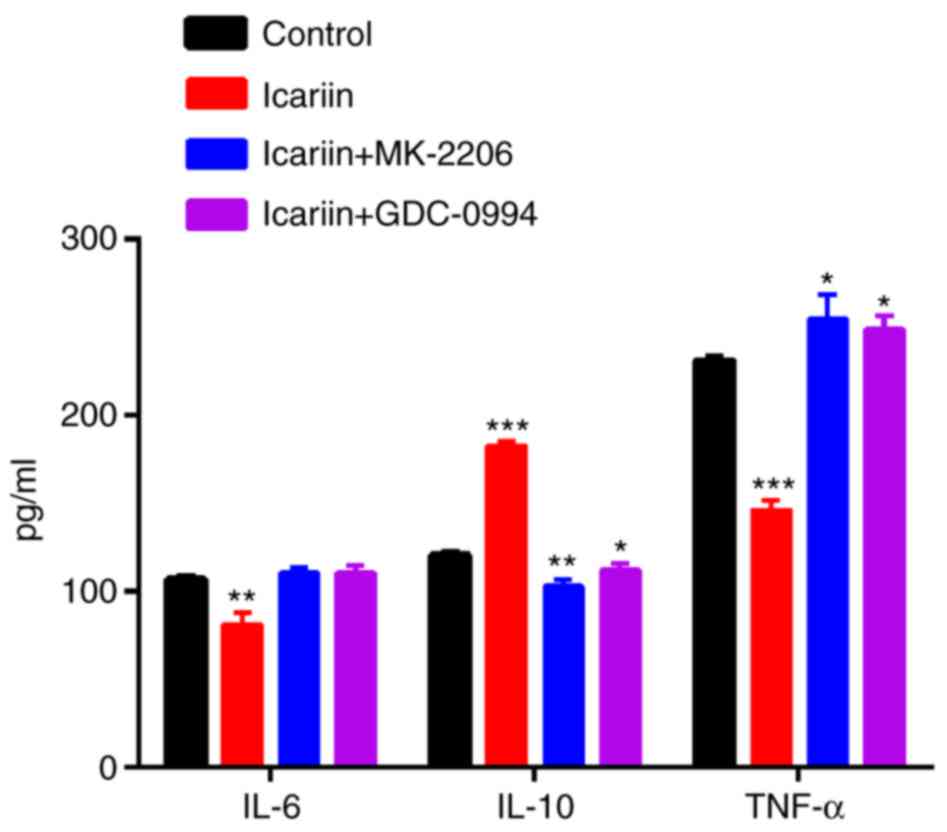
the protein level, icariin inhibited the secretion of IL-6 and
TNF-α, and induced the secretion of IL-10 (Fig. 6). Furthermore, we investigated if
the AKT and ERK signaling pathway was involved in this process.
Cells were pretreated for 2 h with 10 μM MK-2206 2HCl or 50
μM GDC-0994 prior to treatment with 30 μM icariin.
AKT and ERK inhibition resulted in a significant decrease of the
stimulatory effects of icariin to elicit the production of IL-10,
while the production of IL-6 and TNF-α were increased compared with
icariin treatment alone (Figs. 5
and 6).
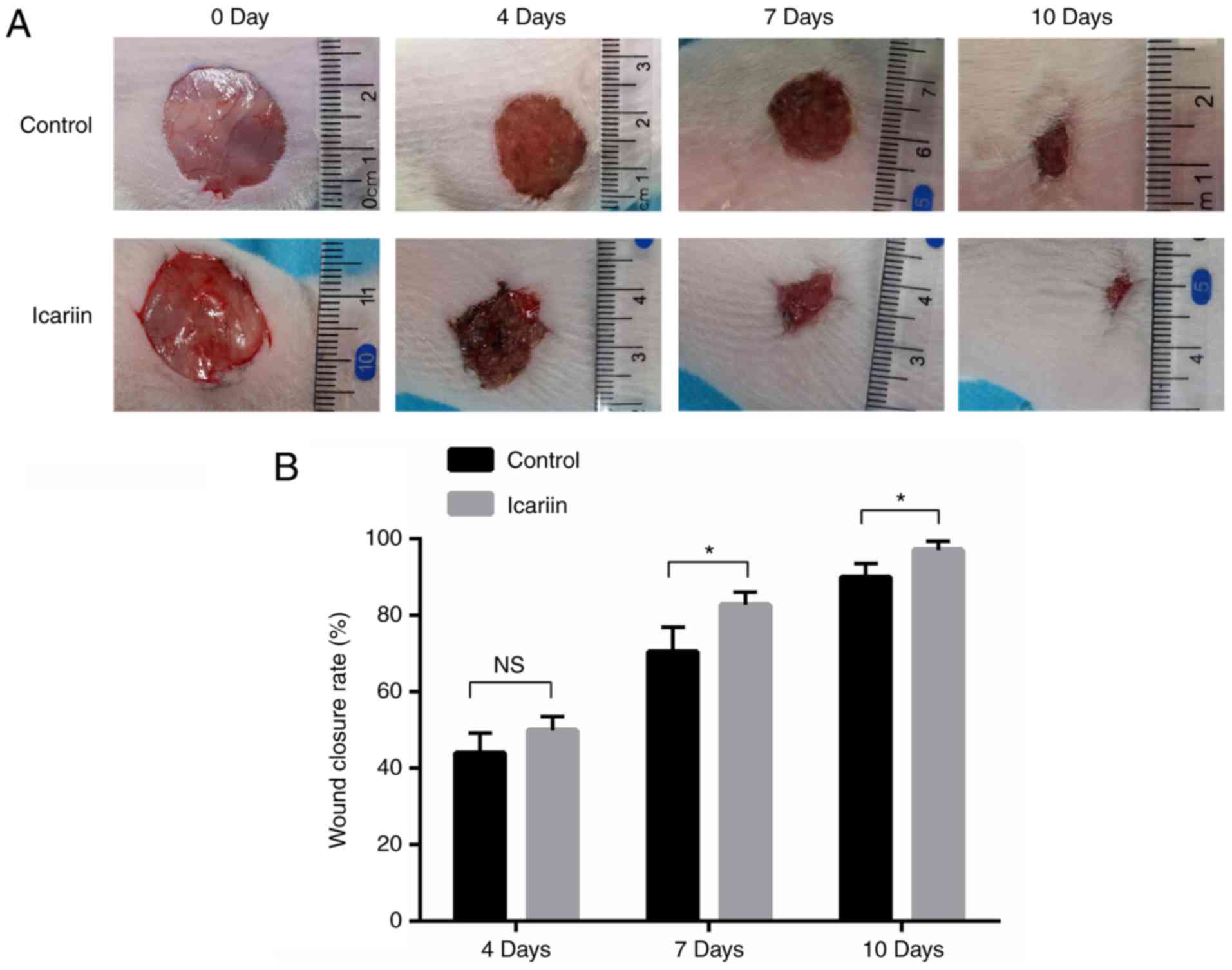
Icariin promotes wound healing in
vivo
To investigate whether icariin was beneficial for
wound closure in vivo, experimental rats were divided into
two groups, receiving either vehicle or icariin treatment following
the induction of skin wounds. The icariin group displayed a
significantly accelerated wound closure rate compared with the
control group (Fig. 7).
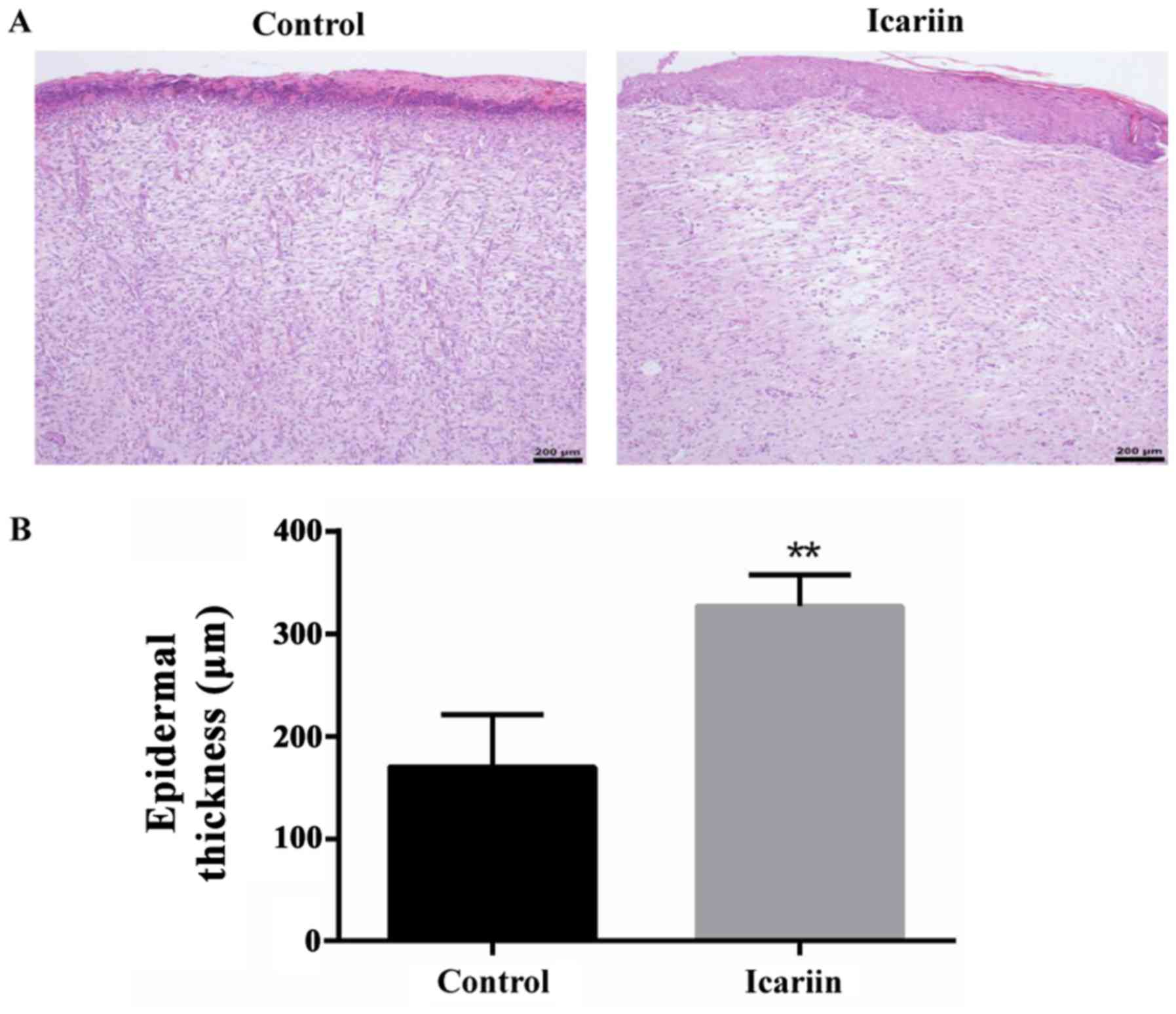
Furthermore, histopathological analysis and microscopic assessment
of epidermal thickness was performed on the final wound closure
sites. The epidermis was significantly thicker in the icariin group
compared with the control group (Fig.
8). These findings demonstrated that icariin was beneficial to
wound repair via stimulation of re-epithelialization and
keratinocyte proliferation.
Discussion
The cytotoxic effects of icariin on several cell
types, including SW 1353 chondrosarcoma (12) and human nucleus pulposus (5) cells, have been extensively explored.
In those studies, the dose of icariin was varied from 5 to 50
μM. In the present study, exposure to 60 μM icariin
for 72 h reduced cell proliferation, whereas concentrations of 50
μM or less had no growth inhibitory effect on HaCaT cells.
Based on the CCK-8 assay in the present study, 30 μM icariin
was identified as the optimal concentration for promoting cell
proliferation at 72 h.
Once a skin wound occurs, keratinocytes migrate to
the wound site. The stimulatory role of icariin on keratinocyte
migration was demonstrated in the present study by a Transwell
assay. The present results suggested that icariin promoted the
migration of keratinocytes. Consistent with the current results,
migration is also promoted by icariin in other cell types; icariin
could induce human umbilical cord mesenchymal stem cell migration
in chronic liver injury in mice (13). The treatment of endothelial
progenitor cells with icariin also promoted cell migration
(14). The effect of icariin on
keratinocyte proliferation was demonstrated in the present study by
CCK-8 and western blot assays. In the icariin group, the rate of
keratinocyte proliferation was higher compared with the untreated
control group. The expression levels of Cyclin D1 and D3, proteins
with important roles in cell proliferation, were also upregulated
in the icariin group. Additionally, the present in vivo
study demonstrated that icariin could accelerate
re-epithelialization and wound healing rate. There are various
types of cells involved in the process of wound healing. Icariin
may also have effects on other cell types, such as fibroblasts and
mesenchymal stem cells. The effects of icariin on proliferation of
other cell types may also contribute to the accelerated wound
healing rate, and this will be further explored in future
studies.
In the present study, the mechanisms involved in the
effects of icariin on the migration and proliferation of
keratinocytes were investigated. Icariin has been reported to exert
effects on migration and proliferation in various types of cells
through multiple signaling pathways (15,16). AKT and ERK have important roles in
the regulation of cell growth, proliferation, differentiation,
death and other processes (17–19). Notably, previous studies have
reported that AKT and ERK are involved in the migration and
proliferation of keratinocytes (20,21), indicating that they may be
associated with the migration and proliferation of keratinocytes
induced by icariin. The present study identified that icariin
promoted the phosphorylation of ERK and AKT in keratinocytes. This
result was consistent with previous studies regarding the effect of
icariin on the phosphorylation of ERK and AKT in other cells types.
Furthermore, the present results demonstrated that pretreatment
with an ERK or AKT inhibitor significantly inhibited the
icariin-induced migration and proliferation of keratinocytes,
suggesting that ERK and AKT activation were critical for the
effects of icariin. In addition, Cyclin D1 and D3 were
significantly downregulated following pretreatment with the AKT or
ERK inhibitor.
A number of cytokines and chemokines act as
mediators of cell migration, proliferation and differentiation
during wound healing. A variety of cytokines are secreted by
activated keratinocytes during the proliferation and
re-epithelialization phase of wound healing, and act as
chemoattractants to activate neighboring keratinocytes (22). IL-10 is a regulatory cytokine
produced by various cells types that can inhibit the production of
proinflammatory cytokines, such as IL-6 and TNF-α (23). IL-6 derived from macrophages,
fibroblasts and keratinocytes, affects granulation tissue
formation, re-epithelialization and angiogenesis (24). TNF-α is produced by a variety of
cell types, including keratinocytes, macrophages and mast cells. It
serves dual roles in decreasing granulation tissue production and
collagen fiber arrangement (25,26). However, excessive production of
proinflammatory cytokines, such as IL-1β, TNF-α and IL-6, may
result in harmful inflammation and the risk of sepsis (27). As illustrated in Fig. 5, the mRNA expression of IL-6 and
TNF-α was significantly decreased in the icariin-treated group.
Conversely, the mRNA expression of IL-10 was increased in the
icariin-treated group. In accordance with this result, IL-6 and
TNF-α secreted levels were significantly inhibited in the culture
supernatants of icariin-treated HaCaT cells at 24 h, while the
icariin-treated group secreted more IL-10, compared with the
control group. These results indicated that icariin, not only
inhibited the secretion of proinflammatory cytokines, but also
induced keratinocytes to secrete anti-inflammatory cytokines. AKT
and ERK have been reported to regulate cytokine production and
release, and have been demonstrated to serve an important role in
wound repair (28,29). Specific inhibitors for AKT and ERK
blocked the downregulation of IL-6 and TNF-α and the upregulation
of IL-10 induced by icariin treatment, suggesting that these
kinases were involved in the process of icariin-induced cytokine
expression alterations.
Collectively, the present study demonstrated that 30
μM icariin was the optimal concentration for promoting
growth in HaCaT cells. Icariin treatment enhanced the migration and
proliferation of keratinocytes by activating the AKT and ERK
signaling pathways. In addition, icariin altered the expression of
cytokines through the AKT and ERK signaling pathways. Furthermore,
icariin accelerated wound healing in vivo. Therefore,
icariin may be considered a promising drug for the treatment of
skin wounds. Further studies are still required to confirm the
effect of icariin and its constituent in vivo models.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Science
Foundation of China (grant no. 81772345), the Science and
Technology Department of Hubei Province (grant no. 2016CFB424), and
the Development Center for Medical Science and Technology National
Health and Family Planning Commission of the People's Republic of
China (grant no. ZX-01-C2016024).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
BM and GL designed the study and prepare the
manuscript. YL, JL and LH performed the cell experiments. LH and WZ
performed the animal experiments. SY and YW analyzed the data. GL
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal studies were conducted under the review
and with the approval of the Ethics Committee of Tongji Medical
College (Wuhan, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gugerell A, Kober J, Schmid M, Buchberger
E, Kamolz LP and Keck M: Botulinum toxin A: Dose-dependent effect
on reepithelialization and angiogenesis. Plast Reconstr Surg Glob
Open. 4:e8372016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen X, Zhang M, Wang X, Chen Y, Yan Y and
Zhang L and Zhang L: Peptide-modified chitosan hydrogels promote
skin wound healing by enhancing wound angiogenesis and inhibiting
inflammation. Am J Transl Res. 9:2352–2362. 2017.PubMed/NCBI
|
|
3
|
Kim MH, Wu WH, Choi JH, Kim JH, Jun JH, Ko
Y and Lee JH: Galectin-1 from conditioned medium of
three-dimensional culture of adipose-derived stem cells accelerates
migration and proliferation of human keratinocyte and fibroblasts.
Wound Repair Regen. Aug 30–2017.(Epub ahead of print). View Article : Google Scholar
|
|
4
|
Gonzalez AC, Costa TF, Andrade ZA and
Medrado AR: Wound healing-A literature review. An Bras Dermatol.
91:614–620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hua W, Zhang Y, Wu X, Kang L, Tu J, Zhao
K, Li S, Wang K, Song Y, Luo R, et al: Icariin Attenuates
Interleukin-1β-induced inflammatory response in human nucleus
pulposus cells. Curr Pharm Des. 23:6071–6078. 2018. View Article : Google Scholar
|
|
6
|
Li X, Sun J, Hu S and Liu J: Icariin
induced B16 melanoma tumor cells apoptosis, suppressed tumor growth
and metastasis. Iran J Public Health. 43:847–848. 2014.
|
|
7
|
Jiang MC, Chen XH, Zhao X, Zhang XJ and
Chen WF: Involvement of IGF-1 receptor signaling pathway in the
neuro-protective effects of Icaritin against MPP(+)-induced
toxicity in MES23.5 cells. Eur J Pharmacol. 786:53–59. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen WF, Wu L, Du ZR, Chen L, Xu AL, Chen
XH, Teng JJ and Wong MS: Neuroprotective properties of icariin in
MPTP-induced mouse model of Parkinson's disease: Involvement of
PI3K/Akt and MEK/ERK signaling pathways. Phytomedicine. 25:93–99.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng X, Chen S, Zheng D, Shao Z, Liang H
and Hu H: Icariin prevents H2O2-induced
apoptosis via the PI3K/Akt pathway in rat nucleus pulposus
intervertebral disc cells. Evid Based Complement Alternat Med.
2017:26942612017.
|
|
10
|
Yoon SW, Lee KP, Kim DY, Hwang DI, Won KJ,
Lee DW and Lee HM: Effect of absolute from Hibiscus syriacus L.
Flower on wound healing in keratinocyte. Pharmacogn Mag. 13:85–89.
2017.PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
12
|
Zeng L, Wang W, Rong XF, Zhong Y, Jia P,
Zhou GQ and Li RH: Chondroprotective effects and multi-target
mechanisms of Icariin in IL-1 beta-induced human SW 1353
chondrosarcoma cells and a rat osteoarthritis model. Int
Immunopharmacol. 18:175–181. 2014. View Article : Google Scholar
|
|
13
|
Cui H, Liu Z, Wang L, Bian Y, Li W, Zhou
H, Chu X and Zhao Q: Icariin-treated human umbilical cord
mesenchymal stem cells decrease chronic liver injury in mice.
Cytotechnology. 69:19–29. 2017. View Article : Google Scholar :
|
|
14
|
Tang Y, Jacobi A, Vater C, Zou L, Zou X
and Stiehler M: Icariin promotes angiogenic differentiation and
prevents oxidative stress-induced autophagy in endothelial
progenitor cells. Stem Cells. 33:1863–1877. 2015. View Article : Google Scholar
|
|
15
|
Gu ZF, Zhang ZT, Wang JY and Xu BB:
Icariin exerts inhibitory effects on the growth and metastasis of
KYSE70 human esophageal carcinoma cells via PI3K/AKT and STAT3
pathways. Environ Toxicol Pharmacol. 54:7–13. 2017. View Article : Google Scholar
|
|
16
|
Liu Y, Huang L, Hao B, Li H, Zhu S, Wang
Q, Li R, Xu Y and Zhang X: Use of an osteoblast overload damage
model to probe the effect of icariin on the proliferation,
differentiation and mineralization of MC3T3-E1 cells through the
Wnt/β-Catenin signalling pathway. Cell Physiol Biochem.
41:1605–1615. 2017. View Article : Google Scholar
|
|
17
|
Sun P, Sun X, Zhao W, Ren M, Zhang C, Wang
Z and Xu W: Lemur tyrosine kinase-3 suppresses growth of prostate
cancer via the AKT and MAPK signaling pathways. Cell Physiol
Biochem. 42:2582–2592. 2017. View Article : Google Scholar
|
|
18
|
Jeong YJ, Hoe HS, Cho HJ, Park KK, Kim DD,
Kim CH, Magae J, Kang DW, Lee SR and Chang YC: Suppression of c-Myc
enhances 21WAF1/CIP1-mediated G1 cell cycle arrest
through the modulation of ERK phosphorylation by ascochlorin. J
Cell Biochem. 119:2036–2047. 2018. View Article : Google Scholar
|
|
19
|
Krajarng A, Chulasiri M and Watanapokasin
R: Etlingera elatior extract promotes cell death in B16 melanoma
cells via down-regulation of ERK and Akt signaling pathways. BMC
Complement Altern Med. 17:4152017. View Article : Google Scholar :
|
|
20
|
Zhou T, Yang Z, Chen Y, Chen Y, Huang Z,
You B, Peng Y and Chen J: Estrogen accelerates cutaneous wound
healing by promoting proliferation of epidermal keratinocytes via
Erk/Akt signaling pathway. Cell Physiol Biochem. 38:959–968. 2016.
View Article : Google Scholar
|
|
21
|
Zhao B, Liu JQ, Zheng Z, Zhang J, Wang SY,
Han SC, Zhou Q, Guan H, Li C, Su LL and Hu DH: Human amniotic
epithelial stem cells promote wound healing by facilitating
migration and proliferation of keratinocyte via ERK, JNK and AKT
signaling pathways. Cell Tissue Res. 365:85–99. 2016. View Article : Google Scholar
|
|
22
|
Freedberg IM, Tomic-Canic M, Komine M and
Blumenberg M: Keratins and the keratinocyte activation cycle. J
Invest Dermatol. 116:633–640. 2001. View Article : Google Scholar
|
|
23
|
Couper KN, Blount DG and Riley EM: IL-10:
The master regulator of immunity to infection. J Immunol.
180:5771–5777. 2008. View Article : Google Scholar
|
|
24
|
Feng Y, Sanders AJ, Morgan LD, Harding KG
and Jiang WG: Potential roles of suppressor of cytokine signaling
in wound healing. Regen Med. 11:193–209. 2016. View Article : Google Scholar
|
|
25
|
Gragnani A, Müller BR, Silva ID, Noronha
SM and Ferreira LM: Keratinocyte growth factor, tumor necrosis
factor-alpha and interleukin-1 beta gene expression in cultured
fibroblasts and keratinocytes from burned patients. Acta Cir Bras.
28:551–558. 2013. View Article : Google Scholar
|
|
26
|
Serra MB, Barroso WA, da Silva NN, Silva
SDN, Borges ACR, Abreu IC and Borges MODR: From inflammation to
current and alternative therapies involved in wound healing. Int J
Inflam. 2017:34062152017. View Article : Google Scholar
|
|
27
|
Mahlapuu M, Hakansson J, Ringstad L and
Björn C: Antimicrobial peptides: An emerging category of
therapeutic agents. Front Cell Infect Microbiol. 6:1942016.
View Article : Google Scholar
|
|
28
|
Sung YY, Kim YS and Kim HK: Illicium verum
extract inhibits TNF-α- and IFN-γ-induced expression of chemokines
and cytokines in human keratinocytes. J Ethnopharmacol.
144:182–189. 2012. View Article : Google Scholar
|
|
29
|
Yano S, Komine M, Fujimoto M, Okochi H and
Tamaki K: Interleukin 15 induces the signals of epidermal
proliferation through ERK and PI 3-kinase in a human epidermal
keratinocyte cell line, HaCaT. Biochem Biophys Res Commun.
301:841–847. 2003. View Article : Google Scholar
|