Introduction
Ketamine (KTM) is an important drug for inducing
anesthesia that is used for sedation, as well as for relieving
pain, and is also applied to facilitate psychological treatments
due to its psychedelic nature (1–3).
KTM is often used to induce anesthesia in patients at high risk,
due to its hemodynamic stability, the absence of respiratory
depression, wide therapeutic range and short half-life (4,5).
In addition, KTM robustly elevates both cardiac output and blood
pressure (6). Studies by Mulvey
et al (7,8) have reported the safety and efficacy
of KTM, which serves as the first choice of anesthetic drug for
injury caused by natural disasters. However, patients recovering
from KTM anesthesia usually experience several undesirable
symptoms, including delirium, hallucination, confusion, and
sometimes a feeling of being 'outside of their body' or
'near-death.' Furthermore, KTM can cause excitotoxic damage and
neurotoxicity in the fragile neonatal brain (1,9,10),
and is also reported to induce apoptosis and necrosis in neuronal
cells in nature (9). Moderate or
high doses of KTM result in evident changes in the GABAergic
system, which increases the differentiation of hippocampal neurons
and causes severe neurotoxicity in the neonatal hippocampus
(11,12). The hippocampus is one of the most
vulnerable brain regions to numerous types of neurobiological
insults (13). Proliferation of
these vulnerable hippocampal cells serves an important role in
learning and memory processes (14). Moreover, the effects of KTM can be
mediated by the regulation of mitogen-activated protein kinase
(MAPK) signaling in the hippocampus (15). Excitotoxic and ischemic insults in
the hippocampus have been demonstrated to be affected by p38MAPK,
and the activation of p38MAPK may be part of a neuroprotective
response (16).
p38MAPK activates caspase-3 and regulates diverse
cellular functions, including senescence, apoptosis and cell cycle
arrest (17). The p38MAPK family
is usually stimulated by genotoxic agents, such as KTM and
cytokines, mediating stress responses and inflammation (16). KTM can decrease the activity of
the p38MAPK-mediated modulating pathway (18). In addition, a number of neurotoxic
agents have been demonstrated to induce apoptosis in a variety of
neuronal cell preparations mediated by the activation of p38
(19). Several studies have
suggested that p38MAPK gene silencing has protective functions
against apoptosis of hippocampal neuronal cells (13,18). However, further studies are
required, since the exact reasons for anesthesia-induced
hippocampal neurodegenerative disorders or memory loss remain
largely unknown (12).
Furthermore, the role of p38MAPK gene silencing in KTM-induced
apoptosis in hippocampal neurons has not been fully elucidated.
Therefore, the present study investigated the specific mechanisms
of p38MAPK gene silencing on KTM-induced apoptosis of rat
hippocampal neurons in order to provide a theoretical foundation
for the discovery of new therapeutic targets.
Materials and methods
Ethics statement
All animal experimentation conducted in the current
study was approved by the Animal Ethics Committee of Jining No. 1
People's Hospital (Jining, China) and abided by relevant protocols.
The environmental conditions of the facilities were in accordance
with the 'Laboratory Animal-Requirements of Environment and Housing
Facilities' guidelines (regulation no. GB14925-2001). All animal
breeding and experimental procedures were performed in accordance
with protocols issued by the 'Animal Management Regulations' and
the Association for Assessment and Accreditation of Laboratory
Animal Care International.
Cell extraction and culture
A total of 10 neonatal Sprague-Dawley (SD) rats
[3-days-old; 8–10 g; Shanghai SLAC Laboratory Animal Co., Ltd.,
Shanghai, China; production license no. SCXK (Hu) 2012-0002] were
sacrificed by cervical dislocation and then sterilized using 75%
ethanol. The skin and skull of the rats were dissected to expose
bilateral cerebral hemispheres. After the cerebral cortex was
separated using an ophthalmic tweezer, the bilateral hippocampal
tissues were extracted. The tissues were centrifuged at 130 × g at
4°C for 5 min, and the supernatant was discarded. Following
digestion with Accutase enzyme (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) three times the volume of the hippocampal
tissues, the hippocampal tissues were added to Dulbecco's modified
Eagle's medium (DMEM; Solarbio Science & Technology Co., Ltd.,
Shanghai, China) containing 10% fetal bovine serum (FBS; Sijiqing
Biological Engineering Materials Co., Ltd., Hangzhou, China), and
penicillin (100 U/ml) and streptomycin (100 µg/ml) (North
China Pharmaceutical Group Corp. Shijiazhuang, China), and
triturated into a cell suspension. The cell suspension was then
filtered by a 200-mesh copper sieve, and the filtered cell
suspension underwent a centrifugation (4°C; 200 × g) for 10 min
with the supernatant discarded and was subsequently added into
DMEM/F12 high-glucose medium containing 10% FBS, penicillin (100
U/ml) and streptomycin (100 µg/m) at 37°C with 5%
CO2. Hippocampal neurons in the logarithmic growth
phases were detached in 0.25% trypsin (Solarbio Science &
Technology Co., Ltd.) for preparation of the cell suspension.
Following cell counting using a blood cell counting chamber, the
cells were seeded in a 6-well plate (Corning Inc., Corning, New
York, USA) at a density of 1×105/well for subsequent
experiments.
Cell observation and identification
Neurons were seeded to a 6-well plate containing
coverslips precoated with lysine (Sigma-Aldrich; Merck KGaA). The
plate was then transferred to an incubator with 5% CO2
at 37°C. After 72 h of culture, a DMEM/F12 containing 10% FBS was
added to the plate in replace of the old medium, followed by the
addition of 10 mol/l arabinoside (Jun Rui Biotechnology, Shanghai,
China). At days 3, the medium was changed and the arabinoside was
removed. Cell morphology and growth were observed under an inverted
phase contrast microscope (Olympus Corp., Tokyo, Japan) at days 1,
3, 7 and 21. Neurons at 7 days of culture were collected with a
micropipette, washed with PBS three times (5 min each time), fixed
with 4% paraformaldehyde for 15 min at room temperature, and then
washed with PBS for a further three times. Neurons were cultured
with mouse anti-rat class III β-tubulin primary antibody (AT809,
1:250; Beyotime Institute of Biotechnology, Shanghai, China) in a
wet box (containing wet filter paper) at 4°C for 12 h, in order to
prevent the coverslips from being dry. Neurons were then washed
with 1% PBS containing Tween-20 (PBST) three times (5 min each
time), incubated with fluorescein isothiocyanate (FITC)-labeled
goat anti-rabbit secondary IgG antibody (1:400; Jackson Laboratory,
Bar Harbor, ME, USA) at 37°C for 1 h in the dark and washed with 1%
PBST three times (5 min each time). Neurons were incubated in 5
µg/ml Hoechst 33342 (Sigma-Aldrich; Merck KGaA) for 10 min
at room temperature. Neurons were treated with a quenching agent
subsequent to washing with PBST five times (5 min each time), and
then activated by ultraviolet rays at an excitation and an emission
wavelength of 346 and 448 nm, respectively. Images of the
fluorescence staining of neurons and nuclei were captured and
observed under a high-power microscope.
Construction of the p38MAPK-short hairpin
RNA (shRNA) lentivirus vector
Both ends of positive and negative strand templates
in an shRNA targeting p38MAPK (SABioscience; Qiagen GmbH, Hilden,
Germany) were annealed following the addition of BamHI
(GGATCC) and EcoRI (GAATTC), respectively. The shRNA
template was then subcloned into the pLL3.7 vector, which had been
linearized by BamHI and EcoRI. Subsequent to
screening, enzyme digestion, identification and sequencing
performed by Sangon Biotech Co., Ltd. (Shanghai, China), the
p38MAPK-shRNA was obtained. The lentivirus shuttle plasmid and
auxiliary packaging vector plasmid DNA solution were prepared using
the following procedure: Interference plasmids (10 µg
pRsv-REV, 15 µg pMDlg-Prre, 7.5 µg pMD2G and 20
µg p38MAPK-shRNA) were mixed, and sterile water was added
until a constant volume of 1,800 µl was obtained. Next, 200
µl CaCl2 (2.5 mol/l) was added and mixed evenly,
followed by addition of 2,000 µl borate buffered saline
(2X). The sample was kept at room temperature for 20–30 min, and
then 293T cells (American Type Culture Collection, Manassas, VA,
USA) were transfected at 80–90% density. Subsequently, the culture
liquid containing monolayer cells was mixed uniformly with the
pRsv-REV, pMDlg-Prre, pMD2G and p38MAPK-shRNA plasmids and calcium
phosphate as the transfection reagent, and the medium was replaced
with a complete medium at 8 h after transfection. Following
transfection for 48 h, the supernatant with lentivirus particles
was collected, centrifuged at 500 × g at 4°C for 10 min to remove
shedding cells and large cell debris, and then filtered using a
0.45 m polyvinylidene fluoride membrane. The lentivirus supernatant
was added to an ultra-clear SW28 centrifugation tube (Beckman
Coulter Life Sciences, Brea, CA, USA), followed by centrifugation
at 4°C at 900 × g for 2 h. PBS was used to rinse the precipitates,
which were then dissolved at 4°C, followed by centrifugation at 500
× g for 1 min. Lastly, the lentivirus supernatant was separately
packaged and stored at a −80°C refrigerator for further use.
Cell transfection and grouping
Neurons in the logarithmic growth phase were
detached prior to transfection, collected and seeded into a 6-well
plate. Transfection was performed using lentivirus plasmid when the
neurons were at 80–90% confluence. Next, the neurons were randomly
grouped into the following groups: KTM group, treated with 2,500
µmol/l KTM; KTM + SB203580 group, treated with 20.0
µmol/l SB203580 (p38MAPK inhibitor, PHZ1253, Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 2,500
µmol/l KTM solution; KTM + negative control (NC) group,
transfected with empty lentivirus plasmid and 2,500 µmol/l
KTM solution; KTM + p38MAPK-shRNA group, transfected with
p38MAPK-shRNA lentivirus and 2,500 µmol/l KTM solution; and
the control group, treated with an equal volume of culture medium.
KTM (KH080401; Fujian Gutian Pharmaceutical Co., Ltd., Ningde,
China) used in these groups was dissolved in DMEM. After
transfection for 48 h, the expression of enhanced green fluorescent
protein (EGFP; BioVision, Inc., Milpitas, CA, USA) in the cells was
observed under an inverted microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following transfection for 48 h, the total RNA was
extracted using the TRIzol one-step method (Invitrogen; Thermo
Fisher Scientific, Inc.). The optical density (OD) of total RNA was
measured at 260 and 280 nm using an ultraviolet spectrophotometer
to obtain the concentration of RNA. The ratio of the OD at these
wavelengths was also calculated to determine the purity of RNA.
Total RNA (1 µg) was then reversely transcribed into cDNA
using a PrimeScript™ RT-PCR kit (Perfect Real-Time; RR047A; Takara
Bio, Inc., Kyoto, Japan). The reaction was conducted at 37°C for 60
min and at 85°C for 5 min. Primers for qPCR were designed by Primer
Premier 5.0 software (Premier Biosoft, Palo Alto, CA, USA) and
synthesized by Thermo Fisher Scientific, Inc. (Invitrogen), with
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serving as an
internal control (Table I). qPCR
was performed using a Bio-Rad instrument (cat. no. 10021337;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). The One-Step SYBR
PrimeScript PLUS RT-PCR Kit (Takara Bio, Inc.) was used to
determine gene expression and the reaction system constituted: 5.3
µl 2X Taq MasterMix, 1 µl forward primer (5
µM), 1 µl reverse primer (5 µM), 1 µl
cDNA and 11.7 µl RNase free H2O. The reaction
conditions were as follows: Pre-denaturation at 95°C for 3 min,
denaturation at 95°C for 10 sec, annealing at 60°C for 20 sec, and
extension at 72°C for 30 sec (40 cycles). Relative expression of
the target genes was calculated using the 2−ΔΔCq method
(20,21).
 | Table IPrimer sequences used in quantitative
polymerase chain reaction. |
Table I
Primer sequences used in quantitative
polymerase chain reaction.
| Gene | Primer
sequence |
|---|
| p38MAPK | F:
5′-ACATCGTGTGGCAGTGAAGAAG-3′ |
| R:
5′-CTTTTGGCGTGAATGATGGA-3′ |
| GAPDH | F:
5′-GCACAGTCAAGGCTGAGAATG-3′ |
| R:
5′-ATGGTGGTGAAGACGCCAGTA-3′ |
| Caspase-3 | F:
5′-GAGACAGACAGTGGAACTGACAATG-3′ |
| R:
5′-GGCGCAAAGTGACTGGATGA-3′ |
| Bax | F:
5′-AGACACCTGAGCTGACCTTGGAG-3′ |
| R:
5′-GTTGAAGTTGCCATCAGCAAACA-3′ |
| Bcl-2 | F:
5′-TGAACCGGCATCTGCACAC-3′ |
| R:
5′-CGTCTTCAGAGACAGCCAGGAG-3′ |
Western blot analysis
Following transfection for 48 h, the total protein
was extracted with the addition of radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology), and the
concentration of total protein was determined using the
bicinchoninic acid assay (KeyGen Biotech Co., Ltd., Nanjing,
China). Extracted proteins were mixed with the loading buffer and
boiled at 100°C for 5 min. Protein (10 µg) from each group
was then subjected to vertical sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (10% SDS-PAGE) and
transferred at 250 mA for 45 min to a polyvinylidene fluoride
membrane (EMD Millipore, Billerica, MA, USA). Next, the membrane
was blocked with 5% skimmed milk in PBST for 1 h at room
temperature and incubated overnight at 4°C with GAPDH (ab8245;
1:1,000), p38MAPK (ab31828; 1:1,000), phosphorylated (p)-p38MAPK
(ab47363; 1:1,000), caspase-3 (ab2171; 1:500), B-cell lymphoma 2
(Bcl-2; ab32124; 1:1,000) and Bcl-2-associated X protein (Bax;
ab32503; 1:1,000; all purchased from Abcam, Cambridge, MA, USA)
primary antibodies. The membrane was subsequently washed with TBST
four times (5 min each time) prior to the addition of horseradish
peroxidase-labeled goat anti-mouse IgG secondary antibody (ab6728;
1:2,000; Abcam) and shaken at 37°C for 1 h. After washing three
times with TBST, the membrane was exposed and developed using
enhanced chemiluminescence (Pierce; Thermo Fisher Scientific,
Inc.), and the film was scanned. A gel documentation system (UVP,
LLC, Phoenix, AZ, USA) was used to analyze the OD value of the
target bands, and GAPDH served as the internal control.
MTT assay
Following transfection for 48 h, neurons were washed
two times with PBS, detached by 0.25% trypsin and made into a
single cell suspension. Cells in the suspension were counted and
seeded into a 96-well plate at a density of 3–6 (×103)
cells per well in 200 µl cell-culture medium. Each culture
was set-up in triplicate and incubated in a cell culture incubator
for 12, 24 and 48 h, respectively. After the different times of
incubation, 20 µl of 5 mg/ml MTT (Sigma-Aldrich; Merck KGaA)
was added to each well, and then a further 4-h culture was
conducted at 37°C in 5% CO2. The supernatant was
carefully removed with a syringe, and a total of 150 µl of
dimethyl sulfoxide (Amresco, LLC, Solon, OH, USA) was added to each
well and oscillated in a horizontal shaker for 10 min to dissolve
the crystallization. The OD of each well was measured at 570 nm
using a microplate reader (Bio-Rad Laboratories, Inc.). The cell
survival rate was calculated according to (ODexperimental
group − ODblank group)/(ODcontrol group
− ODblank group) × 100%, and the cell survival rate of
each group was evaluated by comparing it with that of the control
group. The experiment was conducted five times.
Flow cytometry with propidium iodide (PI)
staining
After transfection for 48 h, the cell suspension was
prepared by digestion with EDTA-free trypsin and centrifuged at 200
× g at 4°C for 5 min. The supernatant was discarded, and neurons
were washed three times with 5% cold PBS and re-suspended in 300
µl 5% PBS after the supernatant was aspirated. Next, the
samples were fixed for 12 h in 700 µl 75% absolute ethanol
and centrifuged for 5 min (4°C; 800 × g), followed by aspiration of
the supernatant. The neurons were then washed with PBS, stained
with 1% PI (Invitrogen; Thermo Fisher Scientific, Inc.) containing
RNase for 30 min and further washed with PBS, and the volume was
adjusted to 1 ml. The cell cycle progression was detected by
BD-Aria FACSCalibur flow cytometry (BD Biosciences, San Jose, CA,
USA). Each group had three samples and experiments were repeated
three times. The cell cycle distribution was analyzed by ModFit
software (Bio-Rad Laboratories, Inc.).
Enzyme-linked immunosorbent assay
(ELISA)
A telomerase PCR-ELISA kit (cat. no. 11854666910;
Roche Applied Science, Mannheim, German) was used to detect
telomerase activity. Neurons in the logarithmic growth phase were
seeded into 6-well plates with 2×105 cells/well.
Following incubation for 24 h, cells were rinsed twice with D-Hanks
buffer solution and detached by trypsin. Subsequent to
centrifugation at 3,000 × g for 10 min at 4°C, the telomerase
lysate was added into samples for lysis on ice for 30 min. Next,
cells were centrifuged at 16,000 × g for 20 min at 4°C, and the
cell supernatant was extracted for repeat sequence amplification
reaction as follows: Primer extension at 25°C for 25 min and
telomerase inactivation at 94°C for 5 min, followed by PCR
amplification with 30 cycles of at 94°C for 30 sec, 50°C for 30 sec
and 72°C for 30 sec, and finally, balance at 72°C for 10 min. The
PCR amplified products (5 µl) were mixed with denatured
reagents (20 µl), and left to stand at the room temperature
for 10 min. Subsequently, the hybrid mixture containing
digoxin-labeled probes which were complementary to telomeric repeat
sequences (225 µl) was added into samples. The product of
the aforementioned reaction (100 µl) was hybridized on the
microplates covered with biotin protein at 37°C avoiding light. The
anti-digoxin antibody of coupling peroxidase (100 µl/well)
was added to the samples at the room temperature for 30 min,
followed by addition of 3,5,3′,5′-tetramethylbenzidine with the
substrate of peroxidase for developing for 4 min. The reaction was
halted by adding the termination reagent. Subsequently, a
microplate reader (Thermo Fisher Scientific, Inc.) was employed in
order to detect the OD value at the wavelengths of 450 and 690
nm.
Flow cytometry with Annexin V-FITC/PI
staining
Subsequent to transfection for 48 h, neurons were
detached by EDTA-free trypsin, washed with PBS three times and then
centrifuged for 5 min (4°C, 800 × g). The supernatant was
aspirated, and the cell suspension (100 µl) suspended in
moderate binding buffer was selected in a 5-ml flow tube. Annexin
V-FITC/PI (1:2:50) was then added according to the protocol of the
Annexin V-FITC/PI apoptosis detection kit (M3021; Shanghai Meiji
Biotechnology Co., Ltd., Shanghai, China). Neurons
(1×106 cells) were resuspended in 100 µl staining
solution, mixed uniformly and stained for 15 min at room
temperature in the dark. FITC fluorescence was detected at 510-nm
and and PI fluorescence at 620-nm bandpass filters, respectively,
which were activated at an excitation wavelength of 488 nm using a
flow cytometer. Apoptosis in neurons was determined as follows:
Dead cells were represented in the upper left quadrant, negative
normal cells were in the lower left quadrant, late apoptotic cells
were in the upper right quadrant and early apoptotic cells were in
the lower right quadrant.
Statistical analysis
Statistical analyses were conducted using SPSS
version 21.0 software (IBM Corp., Armonk, NY, USA). Measurement
data are expressed as the mean ± standard deviation. Means between
groups were compared by t-test. Multiple groups were compared by
one-way analysis of variance after the homogeneity of variance
test. Pairwise comparisons among multiple groups were analyzed by
the least significant difference t-test. P<0.05 was considered
to be an indicator of a statistically significant difference.
Results
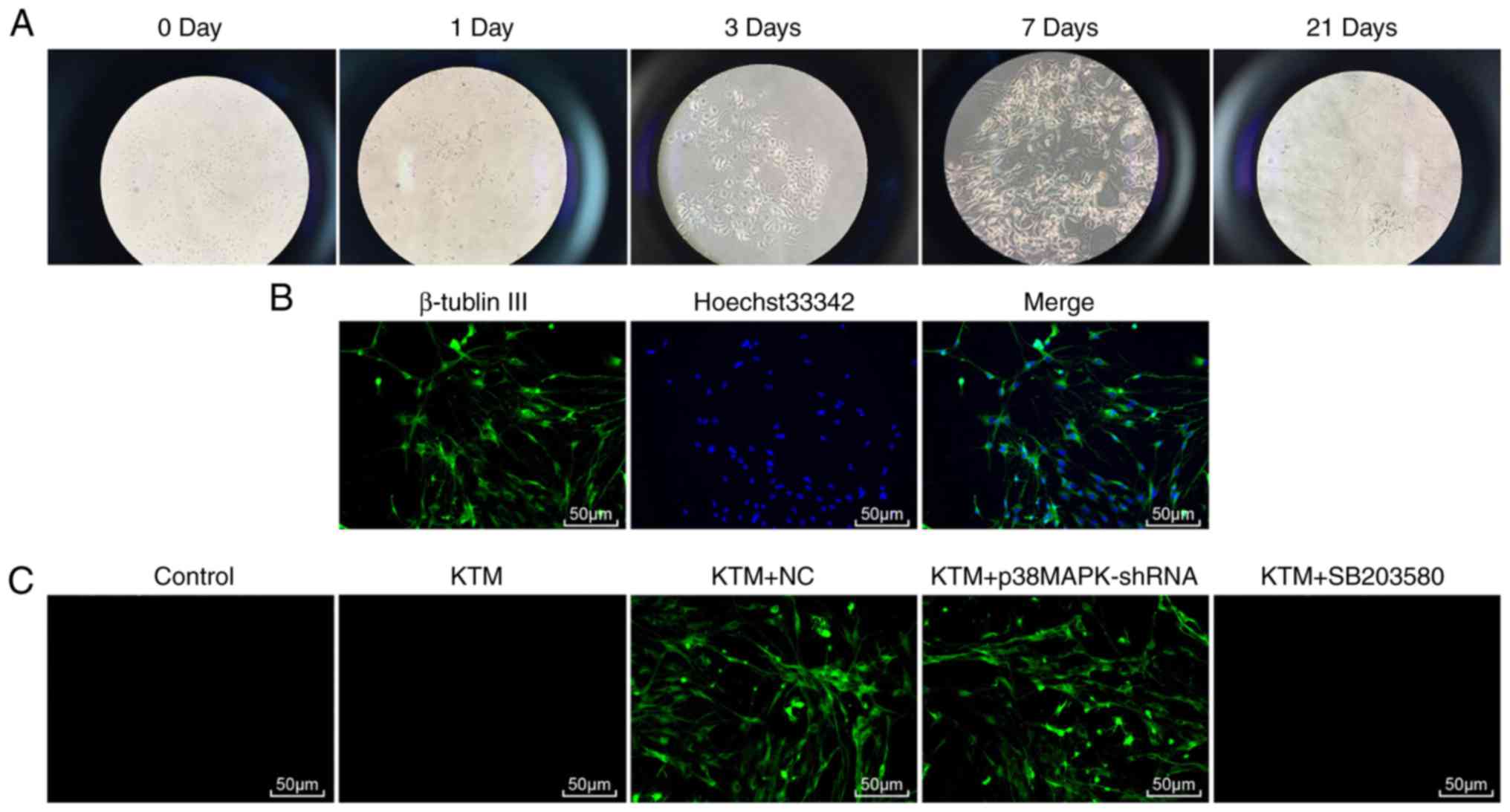
Morphological changes of hippocampal
neurons treated with KTM, and EGFP expression in the transfected
hippocampal neurons
The morphology and growth conditions of rat
hippocampal neurons were observed under a phase contrast inverted
microscope at days 0, 1, 3, 7 and 21 (Fig. 1A). After inoculation of
hippocampal neurons in neonatal rats (day 0), most cells adhered,
presented a round shape and were surrounded by halo; a few cells
also protruded. At day 1 after transfection, the neurons grew well
with different protrusion lengths and spindle morphology in the
majority of cells, whereas no clear connection was established
between neurons. After transfection for 3 days, cell protrusions
increased, cells were evidently elongated and thickened, and
connections between neurons began to form. The neurons were plump,
and there was a halo around them. After transfection for 7 days,
neurons were plumper, while the halo around the neurons was more
visible, and cell protrusions appeared more often and were longer.
Neurons moved closer to each other and began to form cell
populations, and cell protrusions formed a dense nerve fiber
network. After transfection for 21 days, the degeneration of
neurons, pyknosis and fragmentation of nuclei were evident. Cell
protrusions were reduced in size and severed, and the halo around
the cells faded.
Neurons were also observed under a laser scanning
confocal microscope (LSCM) using immunofluorescence staining
(Fig. 1B). The cytoplasm and
protuberance of positive neurons appeared green, and nuclei were
stained blue. A total of five visual fields were randomly selected.
The purity of neurons was identified as 92.1±2.9%. Subsequently,
the neuron transfection efficiency was detected. After transfection
for 48 h, the EGFP expression clearly increased, with >75% cells
exhibiting green fluorescence. Furthermore, the neuron transfection
efficiency in the KTM + NC and KTM + p38MAPK-shRNA groups was 76
and 79%, respectively (Fig.
1C).
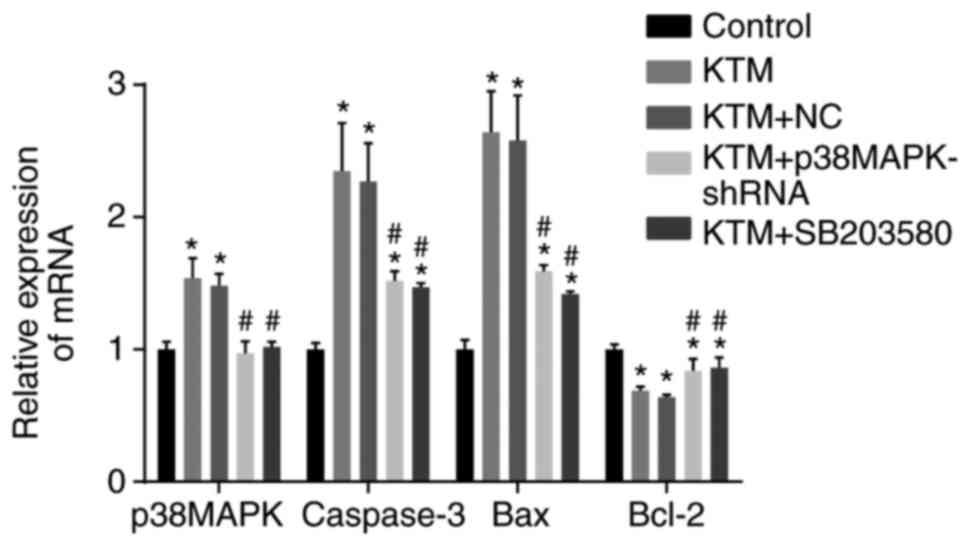
Silencing p38MAPK decreases the mRNA
expression levels of p38MAPK, caspase-3 and Bax, and increases
Bcl-2 mRNA expression
RT-qPCR was employed to measure the mRNA expression
levels of p38MAPK, caspase-3, Bax and Bcl-2 in rat hippocampal
neurons among the five groups. According to the results of RT-qPCR,
in the KTM and KTM + NC groups, the mRNA expression levels of
p38MAPK, caspase-3 and Bax were elevated, while Bcl-2 was reduced,
as compared with the control group (all P<0.05). In the KTM +
SB203580 and KTM + p38MAPK-shRNA groups, the mRNA expression of
p38MAPK exhibited no significant difference compared with the
control group, while the mRNA levels of caspase-3 and Bax were
markedly elevated and Bcl-2 significantly declined (all P<0.05).
In comparison with the KTM group, the KTM + p38MAPK-shRNA and KTM +
SB203580 groups exhibited a markedly downregulated p38MAPK,
caspase-3 and Bax expression, and an upregulated Bcl-2 expression
(all P<0.05; Fig. 2). These
findings revealed that silencing p38MAPK decreases the mRNA
expression levels of p38MAPK, caspase-3 and Bax, and increases
Bcl-2 mRNA expression in cells treated with KTM.
Silencing p38MAPK decreases the protein
levels of p38MAPK, caspase-3 and Bax, and increases Bcl-2 protein
expression
Western blot analysis was employed to measure the
protein expression levels of p38MAPK, caspase-3, Bax and Bcl-2, as
well as the extent of p38MAPK phosphorylation, in rat hippocampal
neurons among the five groups. As demonstrated by the results of
this analysis in Fig. 3, compared
with the control group, the KTM and KTM+NC groups exhibited
increased protein expression levels of p38MAPK, p-p38MAPK,
caspase-3 and Bax, and decreased expression of Bcl-2 (all
P<0.05). In the KTM + SB20358 and KTM + p38MAPK-shRNA groups,
the levels of p38MAPK and p-p38MAPK protein showed no significant
difference compared with the control group (P>0.05), while the
expression levels of caspase-3 and Bax were markedly increased and
Bcl-2 evidently decreased (all P<0.05). Compared with the KTM
group, the KTM + p38MAPK-shRNA and KTM + SB203580 groups displayed
significantly decreased protein levels of p38MAPK, p-p38MAPK,
caspase-3 and Bax, and increased Bcl-2 protein expression (all
P<0.05). These findings revealed that silencing p38MAPK
decreases the protein expression levels of p38MAPK, caspase-3 and
Bax, and increases Bcl-2 protein expression in cells treated with
KTM.
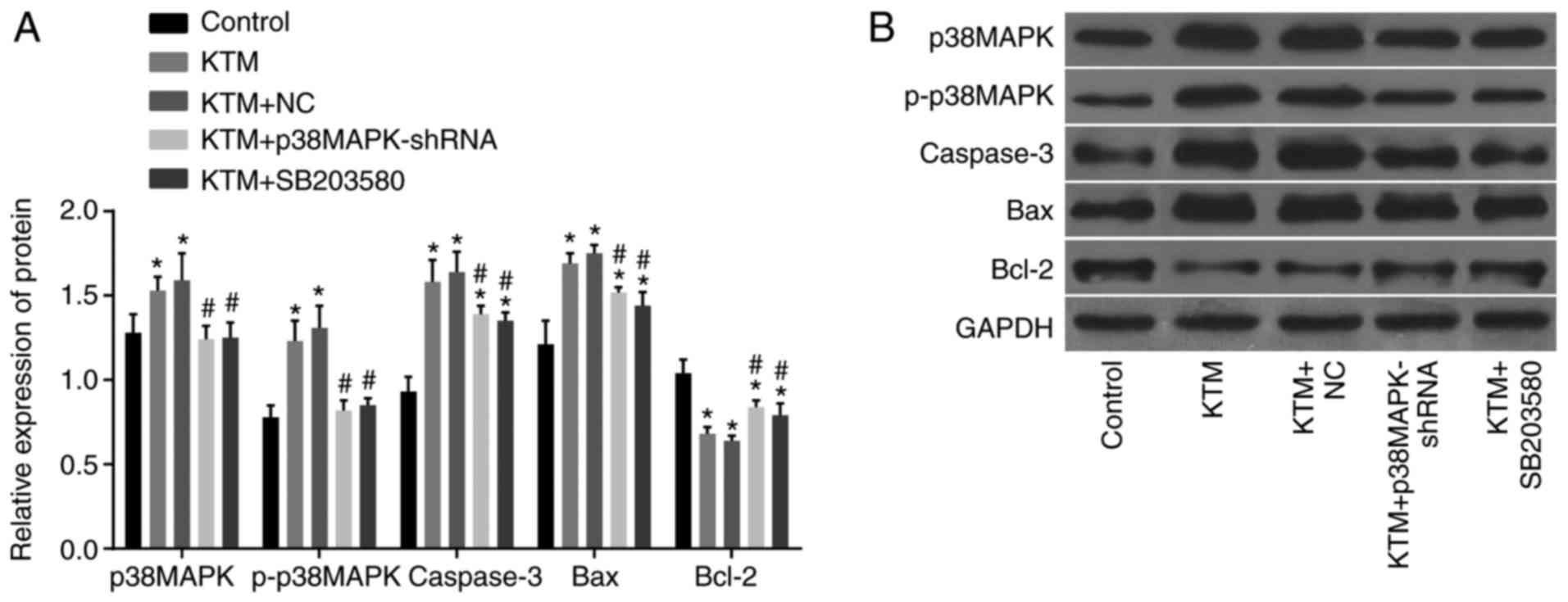 | Figure 3Western blot analysis demonstrates
that silencing p38MAPK decreases the protein expression levels of
p38MAPK, caspase-3 and Bax, and the extent of p38MAPK
phosphorylation, while it increases Bcl-2 protein expression. (A)
Relative protein expression in each group following transfection,
as detected by western blot analysis. (B) Gray value of associated
proteins in each group following transfection.
*P<0.05 vs. the control group; #P<0.05
vs. the KTM group. KTM, ketamine; NC, negative control; p38MAPK,
p38 mitogen-activated protein kinase; p-, phosphorylated; shRNA,
short hairpin RNA; GAPDH, glyceraldehyde-3-phosphate dehydrogenase;
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein. |
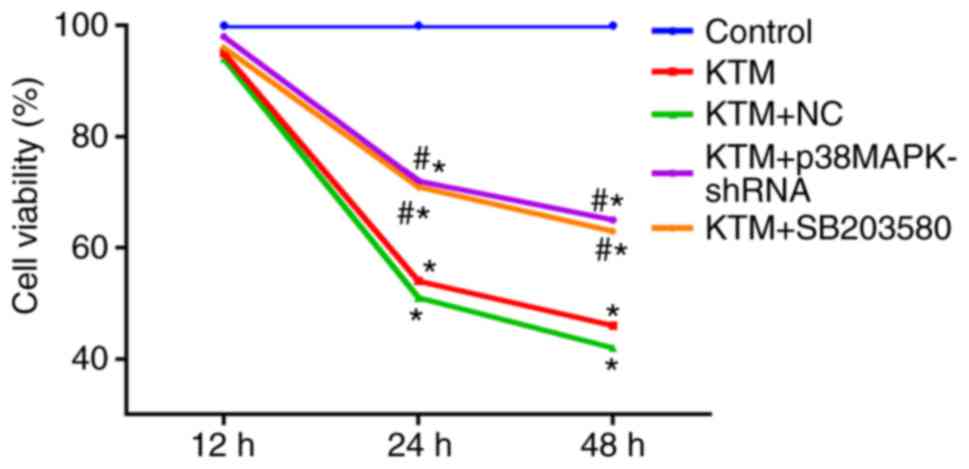
Silencing p38MAPK promotes hippocampal
neuron viability
An MTT assay was used to detect hippocampal neuron
viability. As shown in Fig. 4,
compared with the control group, the hippocampal neuron viability
was notably decreased after 24 h in the KTM, KTM+NC, KTM + SB203580
and KTM + p38MAPK-shRNA groups (all P<0.05). In the KTM +
SB203580 and KTM + p38MAPK-shRNA groups, the hippocampal neuron
viability significantly increased after 24 h compared with the KTM
group (all P<0.05). The results indicated that silencing p38MAPK
promotes the viability of hippocampal neurons treated with KTM.
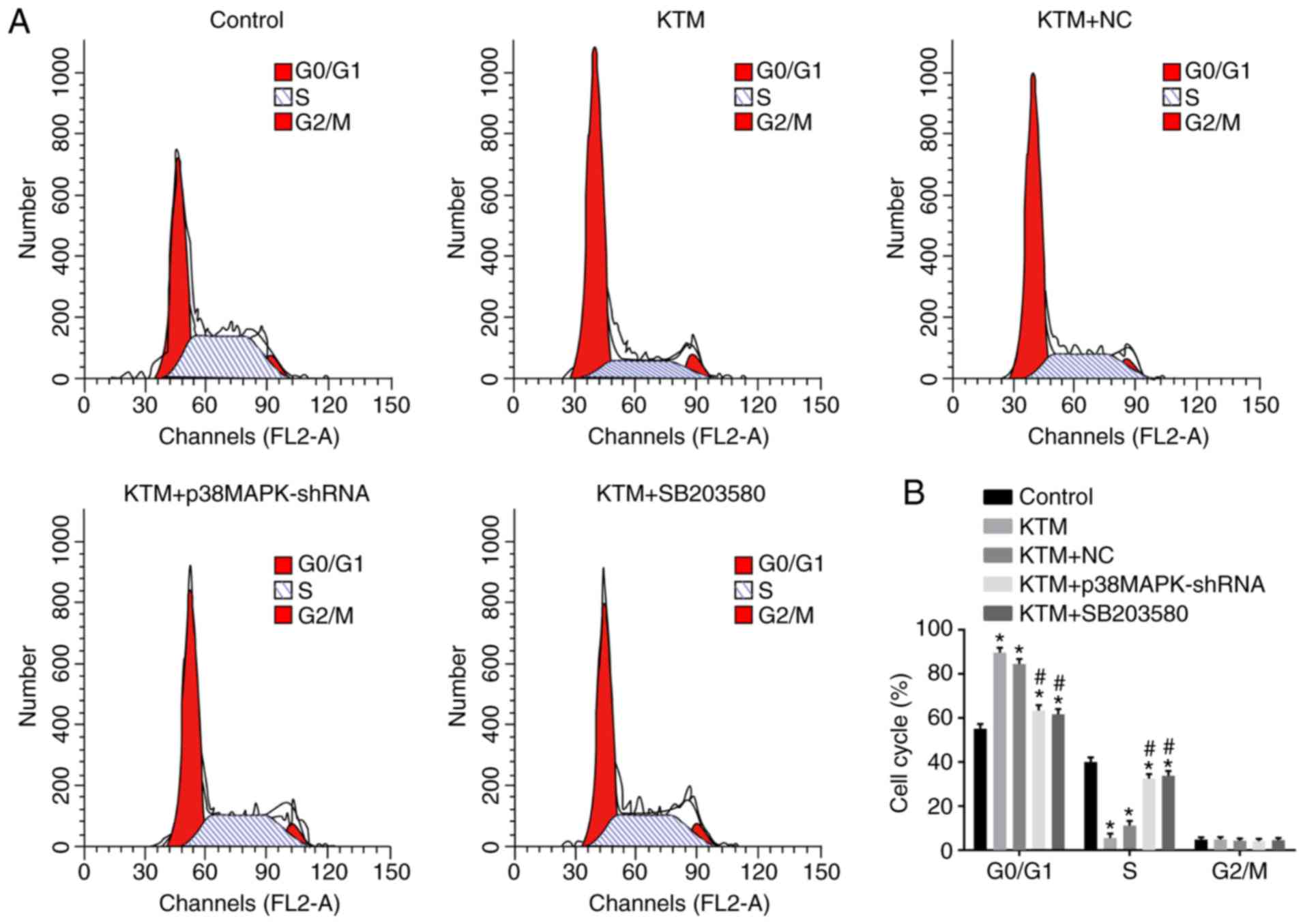
Silencing p38MAPK promotes cell cycle
progression of hippocampal neurons
Flow cytometry with PI staining was performed to
detect the cell cycle distribution of hippocampal neurons. As shown
in Fig. 5, compared with the
control group, neurons arrested in the G0/G1
phase and their percentage in S phase was evidently decreased in
the KTM, KTM+NC, KTM + SB203580 and KTM + p38MAPK-shRNA groups (all
P<0.05). In the KTM + p38MAPK-shRNA and KTM + SB203580 groups,
the proportion of hippocampal neurons arrested in
G0/G1 phase was decreased, while it was
increased in S phase, compared with the KTM group (all P<0.05).
There was no significant difference in the neuronal rate in
G2/M phase between the five groups (all P>0.05).
These findings indicated that silencing p38MAPK promotes cell cycle
progression of hippocampal neurons treated with KTM.
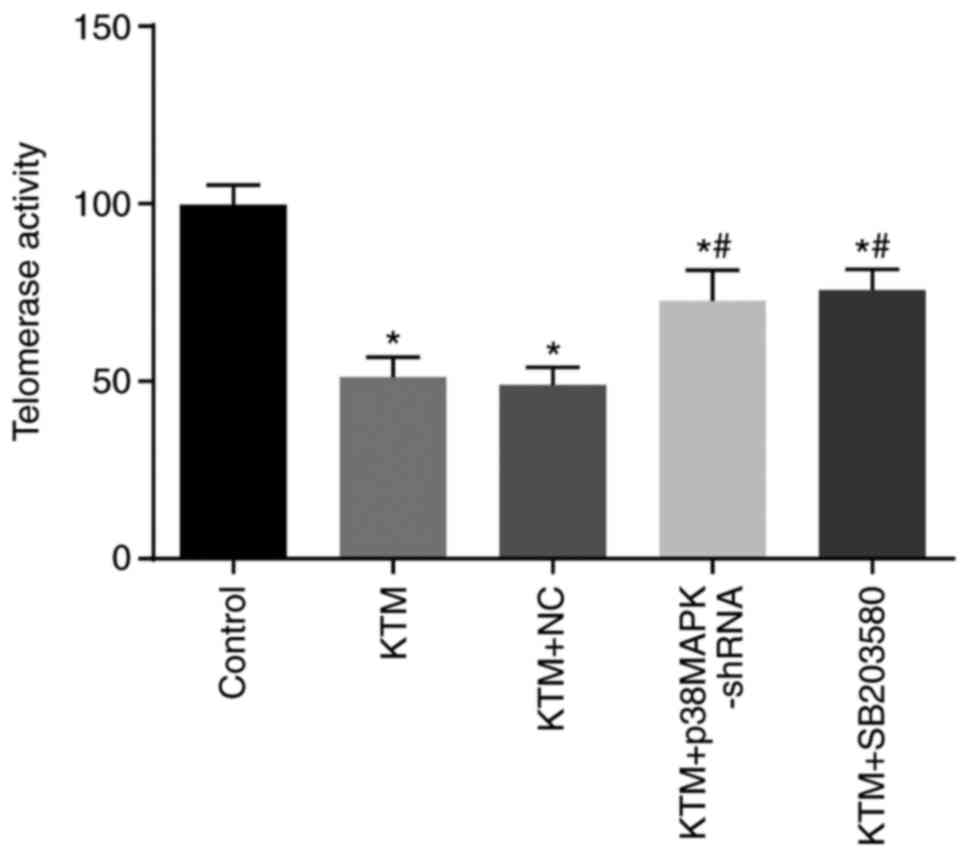
Silencing p38MAPK promotes telomerase
activity of hippocampal neurons
The telomerase activity of hippocampal neurons was
detected by ELISA. Compared with the control group, the telomerase
activity of hippocampal neurons significantly decreased in the
other four groups (all P<0.05). Compared with the KTM group, the
KTM + p38MAPK-shRNA and KTM + SB203580 groups displayed increased
telomerase activity of hippocampal neurons (both P<0.05), while
the KTM+NC group exhibited no significant difference (P>0.05;
Fig. 6). These findings implied
that silencing p38MAPK promotes the telomerase activity of
hippocampal neurons treated with KTM.
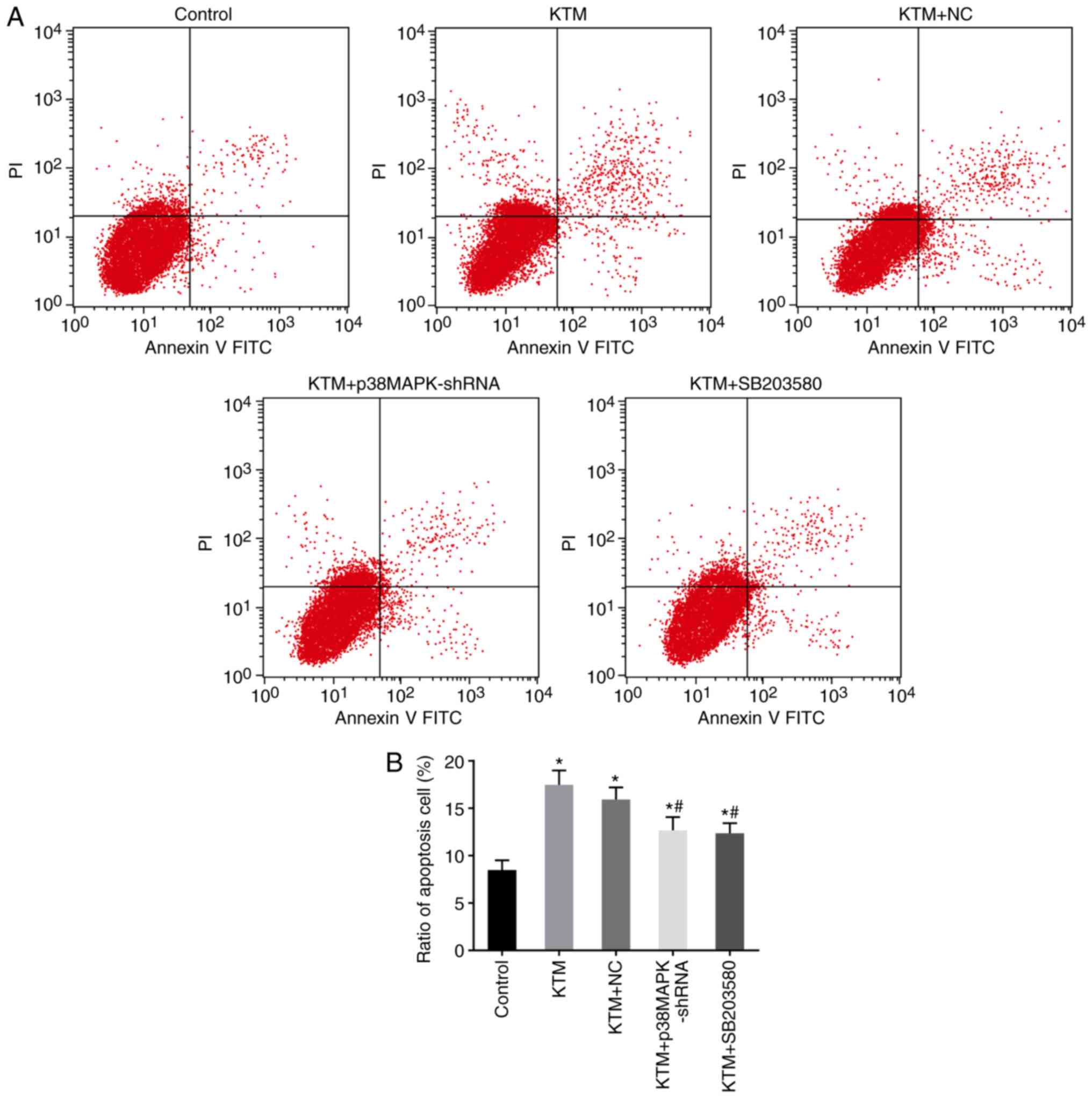
Silencing p38MAPK inhibits apoptosis of
hippocampal neurons
Flow cytometry with Annexin V-FITC/PI staining was
performed to detect the apoptosis of hippocampal neurons. As shown
in Fig. 7, following incubation
for 48 h, the apoptosis rate of rat hippocampal neurons increased
in the KTM, KTM+NC, KTM + SB203580 and KTM + p38MAPK-shRNA groups
compared with that in the control group (all P<0.05). In
comparison with the KTM group, the KTM + p38MAPK-shRNA and KTM +
SB203580 groups exhibited a decreased apoptosis rate of rat
hippocampal neurons (all P<0.05). These findings implied that
silencing p38MAPK suppresses the apoptosis of hippocampal neurons
treated with KTM.
Discussion
KTM serves as the first choice of anesthetic drug
for injury caused by natural disaster due to its action on the
elevation of cardiac output and blood pressure (6,8).
However, KTM has been reported to induce apoptosis of neural stem
cells (22) and to have adverse
impacts on the hippocampus, promoting neuronal apoptosis through a
mitochondrial pathway (15,23). Inhibition of the activation of
p38MAPK is a possible protective mechanism against neuronal injury
induced by cerebral ischemia-reperfusion (24). To reduce neuronal apoptosis, the
present study investigated the modulatory effect of p38MAPK on
KTM-induced apoptosis of rat hippocampal neurons. It was verified
that p38MAPK gene silencing is able to block KTM-induced apoptosis
of rat hippocampal neurons, which is of great significance for
alleviating the symptoms induced by KTM.
Initially, the results of the current study revealed
that apoptosis of neurons in KTM-treated groups increased and cell
activity decreased. KTM, as a channel blocker of
N-methyl-D-aspartate (NMDA) receptors, is widely used as a
pediatric anesthetic and is involved in increasing neuronal
toxicity in the developing brain (25,26). However, due to the involvement of
compensatory upregulated NMDA receptors, KTM at a high
concentration may lead to brain cell apoptosis (25). In addition, a study reported that
sublethal spinal KTM administration caused neuronal apoptosis of
rat pups (27). It has also been
demonstrated that KTM was able to prevent the activity of thalamic
neurons in rats (28). All these
findings are consistent with the observations of the present
study.
The current results also demonstrated that the
expression of p38MAPK and the extent of p38MAPK phosphorylation
increased in hippocampal neurons, and the downregulation of this
expression inhibited neuronal apoptosis. Accumulating evidence has
indicated the protective effects of silencing p38MAPK against
hippocampal apoptosis (29–31). Mammalian p38MAPK is activated by a
wide array of cell stressors and is responsive to inflammatory
cytokines (32). The p38MAPK is
also upregulated in certain diseases. For instance, sustained
activation of the p38MAPK signaling pathway in muscle stem cells as
a result of aging can cause damage to muscle regeneration (17). Certain subtypes of p38MAPK may
serve a pro-oncogenic role in cancer (32). Silencing p38MAPK was found to
promote the telomerase activity of hippocampal neurons in the
current study. It has previously been reported that inhibitors of
p38MAPK can be useful in the prevention and treatment of
hypoxia-induced neuronal injury (33), which is also consistent with the
present study.
Finally, the results of the current study revealed
that silencing p38MAPK in hippocampal neurons downregulated the
expression of the pro-apoptotic gene Bax and upregulated the
expression levels of caspase-3 and apoptosis-suppressing gene Bcl-2
subsequent to treatment with KTM. Liu et al (34) previously revealed a significant
elevation in the transcriptional activity of the caspase-3 gene
during neuronal apoptosis. Other studies have reported that p38MAPK
can regulate caspase-3 in several cell lines (35,36). Apart from these findings, p38MAPK
activity has been proven to serve a critical role in cell death
mediated by nitric oxide in neurons by stimulating Bax
translocation to the mitochondria and then activating the cell
death pathway (37). Bax is a
pro-apoptotic protein that regulates programmed cell death, and its
activation causes an increase in outer membrane permeabilization of
mitochondria, which finally results in apoptotic cascade events
(38). For instance, during the
process of sorbitol-induced apoptosis, sorbitol leads to increased
levels of Bax in response to p38MAPK signaling (39). By contrast, Bcl-2 rescues cells
from apoptosis (40). As an
anti-apoptotic factor, Bcl-2 can be phosphorylated by p38MAPK,
which regulates the anti-apoptotic role of Bcl-2 (41,42). Furthermore, activation of p38MAPK
under stress conditions is a key event in the early induction of
apoptosis (42). Thus, the ratio
of Bax and Bcl-2 contributes to cellular apoptosis (38). These findings further verify that
silencing p38MAPK suppresses the KTM-induced hippocampal neuron
apoptosis by decreasing Bax and caspase-3 expression levels, and
elevating the expression of Bcl-2.
In conclusion, the data of the present study
revealed that lentivirus-mediated p38MAPK gene silencing rescued
KTM-induced apoptosis of rat hippocampal neurons. Although further
experiments are necessary to confirm these results, the findings of
the current study are believed to have a significant implication
for identifying potential therapeutic strategies in the treatment
of apoptosis induced by KTM in hippocampal neurons.
Acknowledgments
The authors would like to express their sincere
appreciation to the reviewers for their critical comments on this
article.
Funding
Not applicable.
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
XQG, YLC, LZ and XZ made substantial contributions
to the design of the present study. YLC, ZRY and WMC collated the
data, and designed and developed the data in the manuscript YLC and
LZ performed data analyses and produced the initial draft of the
manuscript. All authors have read and approved the final submitted
manuscript.
Ethics approval and consent to
participate
All animal experimentation was approved by the
Animal Ethics Committee of Jining No. 1 People's Hospital (Jining,
China) and abided by relevant protocols.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morgan CJ and Curran HV: Independent
Scientific Committee on Drugs: Ketamine use: A review. Addiction.
107:27–38. 2012. View Article : Google Scholar
|
|
2
|
Marland S, Ellerton J, Andolfatto G,
Strapazzon G, Thomassen O, Brandner B, Weatherall A and Paal P:
Ketamine: Use in anesthesia. CNS Neurosci Ther. 19:381–389. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jansen KL: A review of the nonmedical use
of ketamine: Use, users and consequences. J Psychoactive Drugs.
32:419–433. 2000. View Article : Google Scholar
|
|
4
|
Bovill JG: Intravenous anesthesia for the
patient with left ventricular dysfunction. Semin Cardiothorac Vasc
Anesth. 10:43–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Missair A, Pretto EA, Visan A, Lobo L,
Paula F, Castillo-Pedraza C, Cooper L and Gebhard RE: A matter of
life or limb? A review of traumatic injury patterns and anesthesia
techniques for disaster relief after major earthquakes. Anesth
Analg. 117:934–941. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noh HJ, Bae YM, Park SH, Kim JG, Kim B,
Kim YS, Kim SH, Cho SI and Woo NS: The vasodilatory effect of
ketamine is independent of the N-methyl-D-aspartate receptor: Lack
of functional N-methyl-D-aspartate receptors in rat mesenteric
artery smooth muscle. Eur J Anaesthesiol. 26:676–682. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mulvey JM, Qadri AA and Maqsood MA:
Earthquake injuries and the use of ketamine for surgical
procedures: The Kashmir experience. Anaesth Intensive Care.
34:489–494. 2006.PubMed/NCBI
|
|
8
|
Mulvey JM, Awan SU, Qadri AA and Maqsood
MA: Profile of injuries arising from the 2005 Kashmir earthquake:
The first 72 h. Injury. 39:554–560. 2008. View Article : Google Scholar
|
|
9
|
Slikker W Jr, Zou X, Hotchkiss CE, Divine
RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA,
Hanig JP, et al: Ketamine-induced neuronal cell death in the
perinatal rhesus monkey. Toxicol Sci. 98:145–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scallet AC, Schmued LC, Slikker W Jr,
Grunberg N, Faustino PJ, Davis H, Lester D, Pine PS, Sistare F and
Hanig JP: Developmental neurotoxicity of ketamine: Morphometric
confirmation, exposure parameters, and multiple fluorescent
labeling of apoptotic neurons. Toxicol Sci. 81:364–370. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tozuka Y, Fukuda S, Namba T, Seki T and
Hisatsune T: GABAergic excitation promotes neuronal differentiation
in adult hippocampal progenitor cells. Neuron. 47:803–815. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang XL, Du BX, Chen J, Liu L, Shao WB
and Song J: MicroRNA-34a negatively regulates anesthesia-induced
hippocampal apoptosis and memory impairment through FGFR1. Int J
Clin Exp Pathol. 7:6760–6767. 2014.PubMed/NCBI
|
|
13
|
Liu B, Zhang H, Xu C, Yang G, Tao J, Huang
J, Wu J, Duan X, Cao Y and Dong J: Neuroprotective effects of
icariin on corticosterone-induced apoptosis in primary cultured rat
hippocampal neurons. Brain Res. 1375:59–67. 2011. View Article : Google Scholar
|
|
14
|
Ko IG, Shin MS, Kim BK, Kim SE, Sung YH,
Kim TS, Shin MC, Cho HJ, Kim SC, Kim SH, et al: Tadalafil improves
short-term memory by suppressing ischemia-induced apoptosis of
hippocampal neuronal cells in gerbils. Pharmacol Biochem Behav.
91:629–635. 2009. View Article : Google Scholar
|
|
15
|
Reus GZ, Vieira FG, Abelaira HM, Michels
M, Tomaz DB, dos Santos MA, Carlessi AS, Neotti MV, Matias BI, Luz
JR, et al: MAPK signaling correlates with the antidepressant
effects of ketamine. J Psychiatr Res. 55:15–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rigon AP, Cordova FM, Oliveira CS, Posser
T, Costa AP, Silva IG, Santos DA, Rossi FM, Rocha JB and Leal RB:
Neurotoxicity of cadmium on immature hippocampus and a
neuroprotective role for p38 MAPK. Neurotoxicology. 29:727–734.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Segalés J, Perdiguero E and Muñoz-Cánoves
P: Regulation of muscle stem cell functions: A focus on the p38
MAPK signaling pathway. Front Cell Dev Biol. 4:912016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu XW, Ji EF, He P, Xing RX, Tian BX and
Li XD: Protective effects of the p38 MAPK inhibitor SB203580 on
NMDAinduced injury in primary cerebral cortical neurons. Mol Med
Rep. 10:1942–1948. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu HW, He GN, Ma H and Wang JK: Ketamine
reduces inducible superoxide generation in human neutrophils in
vitro by modulating the p38 mitogen-activated protein kinase
(MAPK)-mediated pathway. Clin Exp Immunol. 160:450–456. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tuo YL, Li XM and Luo J: Long noncoding
RNA UCA1 modulates breast cancer cell growth and apoptosis through
decreasing tumor suppressive miR-143. Eur Rev Med Pharmacol Sci.
19:3403–3411. 2015.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar
|
|
22
|
Mansouri S, Agartz I, Ögren SO, Patrone C
and Lundberg M: PACAP protects adult neural stem cells from the
neurotoxic effect of ketamine associated with decreased apoptosis,
ER stress and mTOR pathway activation. PLoS One. 12:e01704962017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bai X, Yan Y, Canfield S, Muravyeva MY,
Kikuchi C, Zaja I, Corbett JA and Bosnjak ZJ: Ketamine enhances
human neural stem cell proliferation and induces neuronal apoptosis
via reactive oxygen species-mediated mitochondrial pathway. Anesth
Analg. 116:869–880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng GY, Chen XC, Du J, Liu CY, Fang F,
Zhang J, Huang TW and Zeng YQ: Inhibitory action of propyl gallate
on the activation of SAPK/JNK and p38MAPK induced by cerebral
ischemia-reperfusion in rats. Yao Xue Xue Bao. 41:548–554. 2006.In
Chinese. PubMed/NCBI
|
|
25
|
Zou X, Patterson TA, Divine RL, Sadovova
N, Zhang X, Hanig JP, Paule MG, Slikker W Jr and Wang C: Prolonged
exposure to ketamine increases neurodegeneration in the developing
monkey brain. Int J Dev Neurosci. 27:727–731. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang C, Sadovova N, Fu X, Schmued L,
Scallet A, Hanig J and Slikker W: The role of the
N-methyl-D-aspartate receptor in ketamine-induced apoptosis in rat
forebrain culture. Neuroscience. 132:967–977. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Engelhardt T, Blaylock M and Weiss M:
Sublethal spinal ketamine produces neuronal apoptosis in rat pups.
Anesthesiology. 114:718–721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Foraster MA, Celada P, Jensen AA, Plath N,
Herrik KF and Artigas F: P2.010 Ketamine inhibits the activity of
thalamic neurons in anesthetized rats. Eur Neuropsychopharmacol.
26(Suppl 1): S31–S32. 2016. View Article : Google Scholar
|
|
29
|
Yang S, Zhou G, Liu H, Zhang B, Li J, Cui
R and Du Y: Protective effects of p38 MAPK inhibitor SB202190
against hippocampal apoptosis and spatial learning and memory
deficits in a rat model of vascular dementia. Biomed Res Int.
2013:2157982013. View Article : Google Scholar
|
|
30
|
Miskovic M, Lalic T, Radivojevic D,
Cirkovic S, Vlahovic G, Zamurovic D and Guc-Scekic M: Lower
incidence of deletions in the survival of motor neuron gene and the
neuronal apoptosis inhibitory protein gene in children with spinal
muscular atrophy from Serbia. Tohoku J Exp Med. 225:153–159. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cardaci S, Filomeni G, Rotilio G and
Ciriolo MR: p38MAPK/p53 signalling axis mediates
neuronal apoptosis in response to tetra-hydrobiopterin-induced
oxidative stress and glucose uptake inhibition: Implication for
neurodegeneration. Biochem J. 430:439–451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cuenda A and Rousseau S: p38 MAP-kinases
pathway regulation, function and role in human diseases. Biochim
Biophys Acta. 1773:1358–1375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lan A, Liao X, Mo L, Yang C, Yang Z, Wang
X, Hu F, Chen P, Feng J, Zheng D and Xiao L: Hydrogen sulfide
protects against chemical hypoxia-induced injury by inhibiting
ROS-activated ERK1/2 and p38MAPK signaling pathways in PC12 cells.
PLoS One. 6:e259212011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu W, Wang G and Yakovlev AG:
Identification and functional analysis of the rat caspase-3 gene
promoter. J Biol Chem. 277:8273–8278. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ohta T, Eguchi R, Suzuki A, Miyakaze S,
Ayuzawa R and Kaji K: Hypoxia-induced apoptosis and tube breakdown
are regulated by p38 MAPK but not by caspase cascade in an in vitro
capillary model composed of human endothelial cells. J Cell
Physiol. 211:673–681. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fan Y, Chen H, Qiao B, Luo L, Ma H, Li H,
Jiang J, Niu D and Yin Z: Opposing effects of ERK and p38 MAP
kinases on HeLa cell apoptosis induced by dipyrithione. Mol Cells.
23:30–38. 2007.PubMed/NCBI
|
|
37
|
Ghatan S, Larner S, Kinoshita Y, Hetman M,
Patel L, Xia Z, Youle RJ and Morrison RS: p38 MAP kinase mediates
bax translocation in nitric oxide-induced apoptosis in neurons. J
Cell Biol. 150:335–347. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Bi L, Ye Y and Chen J:
Formononetin induces apoptosis in PC-3 prostate cancer cells
through enhancing the Bax/Bcl-2 ratios and regulating the p38/Akt
pathway. Nutr Cancer. 66:656–661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu X, Li C, Wang YK, Jiang K and Gai XD:
Sorbitol induces apoptosis of human colorectal cancer cells via p38
MAPK signal transduction. Oncol Lett. 7:1992–1996. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang H, Chan H, Wang YY, Ouyang DY, Zheng
YT and Tam SC: Trichosanthin suppresses the elevation of p38 MAPK,
and Bcl-2 induced by HSV-1 infection in Vero cells. Life Sci.
79:1287–1292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Torcia M, De Chiara G, Nencioni L,
Ammendola S, Labardi D, Lucibello M, Rosini P, Marlier LN, Bonini
P, Dello Sbarba P, et al: Nerve growth factor inhibits apoptosis in
memory B lymphocytes via inactivation of p38 MAPK, prevention of
Bcl-2 phosphorylation, and cytochrome c release. J Biol Chem.
276:39027–39036. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
De Chiara G, Marcocci ME, Torcia M,
Lucibello M, Rosini P, Bonini P, Higashimoto Y, Damonte G,
Armirotti A, Amodei S, et al: Bcl-2 Phosphorylation by p38 MAPK:
Identification of target sites and biologic consequences. J Biol
Chem. 281:21353–21361. 2006. View Article : Google Scholar : PubMed/NCBI
|