Introduction
Rosa genus (family Rosaceae) contains over 150
species that are widespread in Asia, Europe, Middle East and North
America. Historically, roses were cultivated and used for medicinal
purpose by the ancient Chinese 5,000 years ago (1,2).
Recently, roses were used extensively in perfume, cosmetic,
pharmaceutical and food industries as rose oil, rose water, rose
concrete, rose hydrosol and dried petals (3).
Rose has served as folk medicine and raw material of
perfume in China for thousands of years (4). There are many species of roses in
China, R. Setat x R. Rugosa Yu et Ku is known as
Kushui rose (KR), which is a hybrid plant and was large-scale
cultivated in Kushui, Yongdeng country of Gansu Province for over
200 years (5). There are more
than 100 kinds of active ingredients in KR, and the contents of
essential oil and total flavonoids (rutin) reach 0.04% and 0.48/100
g, respectively. Especially, the content of citronellol is >50%
in essential oil, which is the highest content among the rose
around the world (6–8). But up to now, KR is still directly
used as raw material for sauces and essential oil. Comprehensive
utilization of KR is relatively insufficient and more attentions
should to be paid to its biological activities and mechanisms
(9).
Ras proteins are mutationally activated in as many
as 30% of all human tumors (10).
The over-activated Ras is a decisive factor in the formation of
human malignant tumors (11).
Mutation of ra gene leads to persistently activating Ras
protein, increasing intracellular level of Ras-GTP, excessive
proliferating of cells and finally leading to the occurrence of
cancer (12). Thus, Ras protein
has become a universally accepted target for drug screening
(10). Although recent studies
found certain compounds can specifically target mutational Ras, the
clinical application of these compounds continues to be a long way
off (13).
Cancers usually contain elevated levels of reactive
oxygen species (ROS), and over-activated Ras is closely related to
the increased ROS accumulation as well (14–16). Furthermore, free radical theory
postulates that ROS is the main determinant for the promotion of
cancer (17). One approach for
developing anticancer drugs is to target ROS production and
accumulation (18). For this
reason, many anticancer drugs are screened mainly focusing on their
ROS scavenging capacity (19). So
it is reasonable to infer that antioxidants are potential drug
candidates for treating over-activated Ras related cancer. Although
numerous antioxidants have been synthesized, many of them suffered
from toxic and side effects when tested as drug candidates
(20–24). Thus there is an increasing
interest in testing natural antioxidants as novel therapeutic
agents (25–29). Only very few natural antioxidants
were shown effective to combat cancers related to over-activated
Ras, such as antroquinonol, which is a cytotoxicity agent from
Antrodia camphorata (30,31). Interestingly, KR has been shown to
be a potent antioxidant (26,32,33). Hence we hypothesize that KR could
suppress over-activated Ras due to its antioxidant activity.
In Caenorhabditis elegans (C.
elegans), let-6 is a homologous gene to ra in
mammals, and Ras/MAPK signaling pathway determines the development
of worm vulva (34). The
over-activated Ras/MAPK pathway produces an abnormal multivulva
(Muv) phenotype, which can be reversed by antitumor drug candidates
(35). C. elegan is
recognized as a powerful tool for screening antitumor drug
candidates to suppress over-activated Ras/MAPK pathway (36–38). In this study, we applied the model
organism C. elegan to determine whether KR can suppress
over-activated Ras/MAPK pathway.
Therefore, the aim of this study was to determine
the antioxidant activity of Kushui rose decoction (KRD), evaluating
the inhibition activity on over-activated Ras of KR extracts,
identifying new applications of KR and finally to improve the
availability of KR and the local economics. Our results provide
evidence to substantiate that KR can serve as a potential drug
candidate for combating over-activated Ras-related cancer.
Materials and methods
Preparation of KR extracts
The decoction of KR (KRD) was extracted with
distilled water. Fifty milliliters of decoction was obtained from 4
g dried rose buds, defined as RD (80 mg/ml). The concentration is
presented as the content of the crude drug in solution (w/v). The
KRD were diluted to 0.1 mg/ml (RDL), 0.2 mg/ml (RDM), 0.4 mg/ml
(RDH), 0.8 mg/ml (RDHH), 1.6 mg/ml (RDHHH), respectively. The KR
essential oil and hydrosol were obtained from Dongfang Tianrun
(Tianjin, China) company as a gift, and the gradient was diluted by
the solvent (0.1% DMSO and 2% PEG-400, final concentration)
50-fold.
Maintenance conditions
MT2124, let-60 (n1046sd, gf) IV was a gift
from Howard Hughes Medical Institute. TJ356, zIs356
[daf-16p::daf-16a/b::GFP + rol-6]; CF1553, muIs84 [(pAD76)
sod-3p::GFP + rol-6]; AM263, rmIs175 [unc-54p::Hsa-sod-1
(WT)::YFP]; TJ375, gpIs1[hsp-16-2::GFP]; CL2166, dvIs19
[(pAF15)gst-4p::GFP::NLS] III and Escherichia coli OP50 were
provided by the Caenorhabditis Genetics Center (CGC), which is
funded by the NIH National Center for Research Resources. Worms
were maintained at 20°C by standard methods (39). Escherichia coli OP50 were
used as standard food source.
Drug treatment
The drug treatments were performed in 96-well
plates. All drugs except the rose essential oil were diluted by S
buffer. Around 80–100 synchronized worms at L1 larvae cultured to
adult in 180 µl of S buffer containing different treatment
substances were transferred to 96-well plates, and 1 mg/ml freshly
grown OP50 were added as a standard food resource.
N-Acety-L-Cysteine (NAC) was purchased from TCI (Shanghai, China).
Paraquat (PQ) was from Sigma. 2.5 mM NAC and 0.5 mM PQ were used as
positive and negative control, respectively.
Quantification of the wild-type phenotype
of let-60(gf) mutants
let-60(gf) mutants were treated as
previously described and cultured to adults after 3–4 days, and the
percentage of wild-type phenotype of let-60(gf)
mutants were scored by an inverted microscope (SY-057). The percent
of wild-type phenotype worms were calculated according to the
formula: PW(%)=100% ×
NW/(NW+NM); where PW is
the percentage of wild-type phenotype of worms, NW is
the number of wild-type phenotype of worms, NM is the
number of Muv phenotype of worms (40,41).
2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid (ABTS) radical
scavenging activity of KRD
The capacity of KRD on scavenging ABTS radical in
vitr was measured by the Total Antioxidant Capacity assay kit
with the ABTS method (Beyotime, Shanghai, China) (42).
1,1-Diphenyl-2-picrylhydrazyl (DPPH)
radical scavenging activity of KRD
Anti-radical activities of KRD was examined by
comparing to the known antioxidant ascorbic acid by DPPH (43). Briefly, 20 µl of rose
decoction or different concentration of ascorbic acid (10, 5, 2.5,
1.25, 0.625, 0.3125 and 0.15625 mM) were mixed with 580 µl
methanolic solution of DPPH (50 µM). The mixture was shaken
vigorously and allowed to stand at room temperature for 30 min.
Then the absorbance was measured at 517 nm against methanol as the
blank in a spectrophotometer. Lower absorbance of the reaction
mixture indicated higher free radical scavenging activity. The
percent of DPPH discolorations of the samples was calculated
according to the formula: I(%)=100% ×
(AB−AS)/AB; where AB is
the absorbance of the control reaction (containing all reagents
except the test sample), and AS is the absorbance of the
extracts/reference.
Superoxide anion, hydroxyl radicals and
hydrogen peroxide scavenging activity of KRD
The capacity of KRD on scavenging superoxide anion,
hydroxyl radicals and hydrogen peroxide in vitro,
respectively, by nitrotetrazolium blue chloride (NBT) method,
Fenton reaction and luminol-H2O2 method as
described (44).
Statistical analysis
The fluorescence signal intensity was quantified
using ImageJ software. The results are presented as the average of
three biological replicates. The data are analyzed by one-way ANOVA
and Tukey multiple comparison using SPSS 17.0. The significant
difference was set at a level of 0.05 among groups.
Results
KRD significant scavenging of the
radicals in vitro
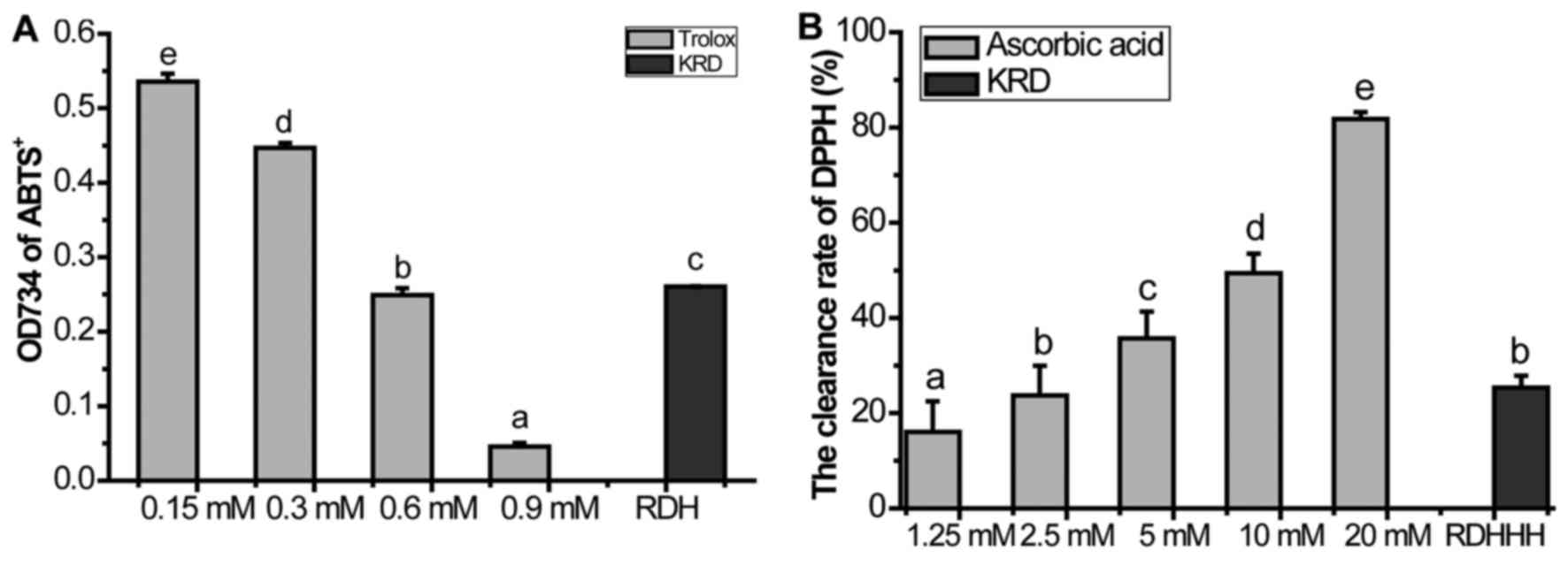
In ABTS radical scavenging activity test, Trolox was
used as a calibration reader with concentrations ranging from 0.15
to 0.9 mM and the obtained calibration was Y (scavenging rate) =
−0.6627 X (Trolox concentration, mM) + 0.6430 (r=0.9996). The
additive amounts of the samples were calculated based on the
obtained formula. Results were expressed in terms of mM Trolox per
1 mg/ml KRD. We found that 1 mg/ml KRD was equivalent to 1.1760 mM
Trolox (Fig. 1A).
In the DPPH radical scavenging activity test,
ascorbic acid was used as a calibration reader with concentrations
ranging from 0.625 to 20 mM and the obtained calibration was Y
(scavenging rate) = 0.0333 X (ascorbic acid concentration, mM) +
0.1532 (r=0.9964). The additive amounts of the samples were
calculated based on the obtained formula. Results were expressed in
terms of mM ascorbic acid per 1 mg/ml KRD. We found that 1 mg/ml
KRD was equivalent to 1.8844 mM ascorbic acids (Fig. 1B).
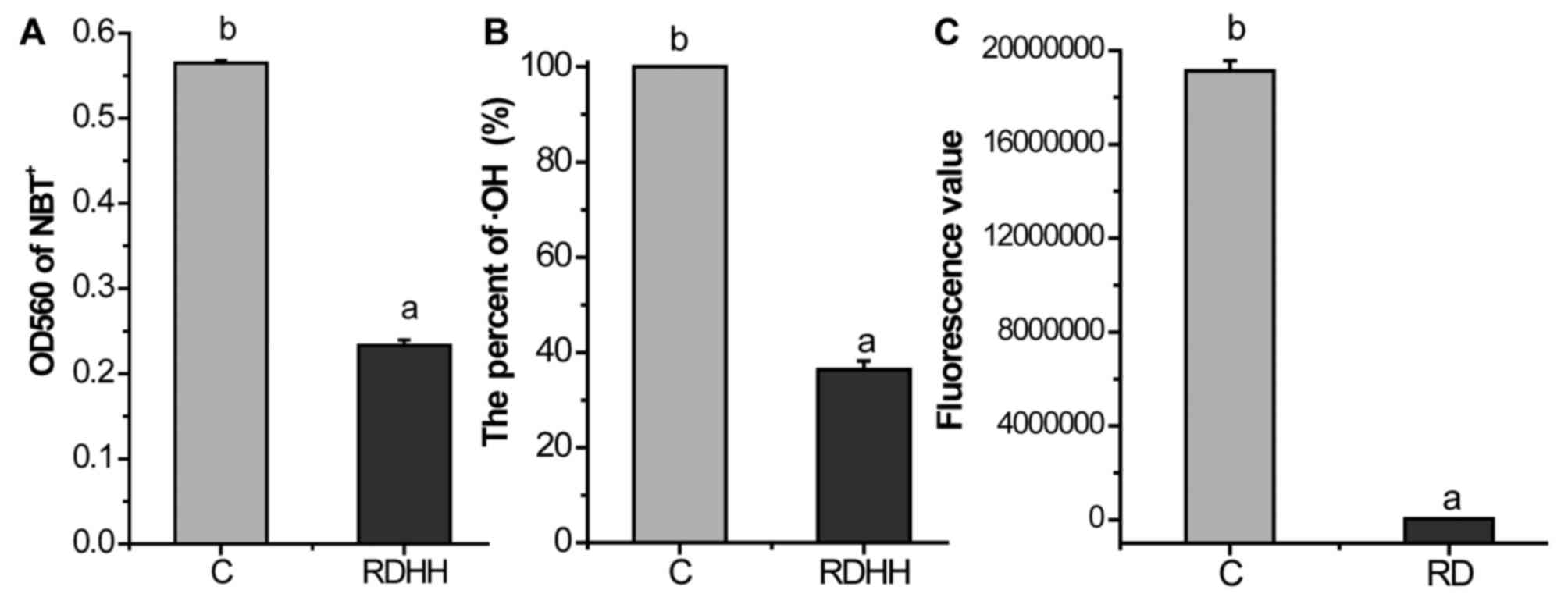
Additionally, we also tested the scavenging capacity
of KRD on hydroxyl radical, superoxide anion and hydrogen peroxide.
As showed in Fig. 2, KRD can mop
up 58.76% superoxide anion (Fig.
2A), 63.64% hydroxyl radical (Fig. 2B) and 99.80% hydrogen peroxide
(Fig. 2C), respectively. These
results showed that KRD possesses remarkable scavenging effect
against radicals in vitro.
KRD significantly suppresses
over-activated Ras/MAPK pathway, but not the essential oil and
hydrosol
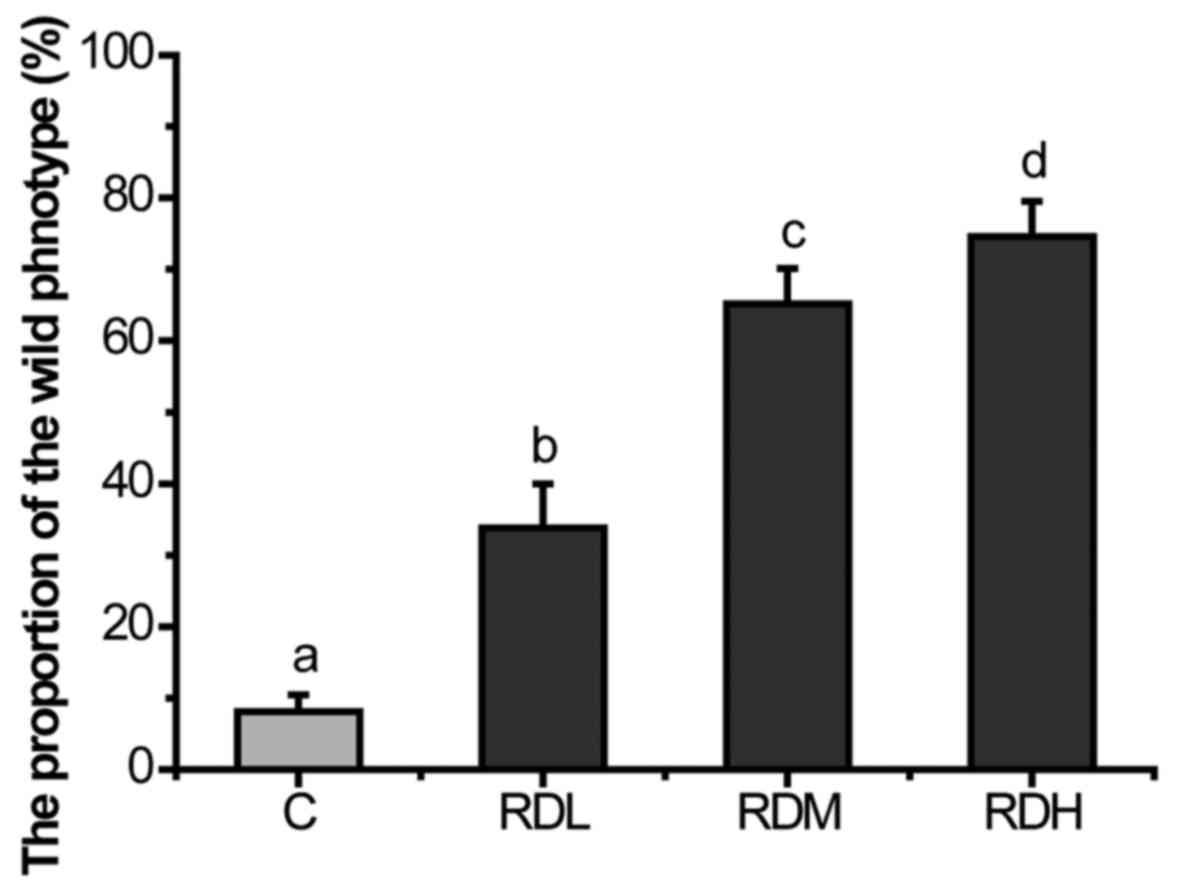
KRD inhibited the Muv phenotype of let-6
mutants strongly in a dose-dependent manner (Fig. 3). RDH increased the percent of
wild-type phenotype of let-60(gf) mutants to 74.58%.
Even at RDL as low as 0.05 mg/ml, the decoction still has efficacy
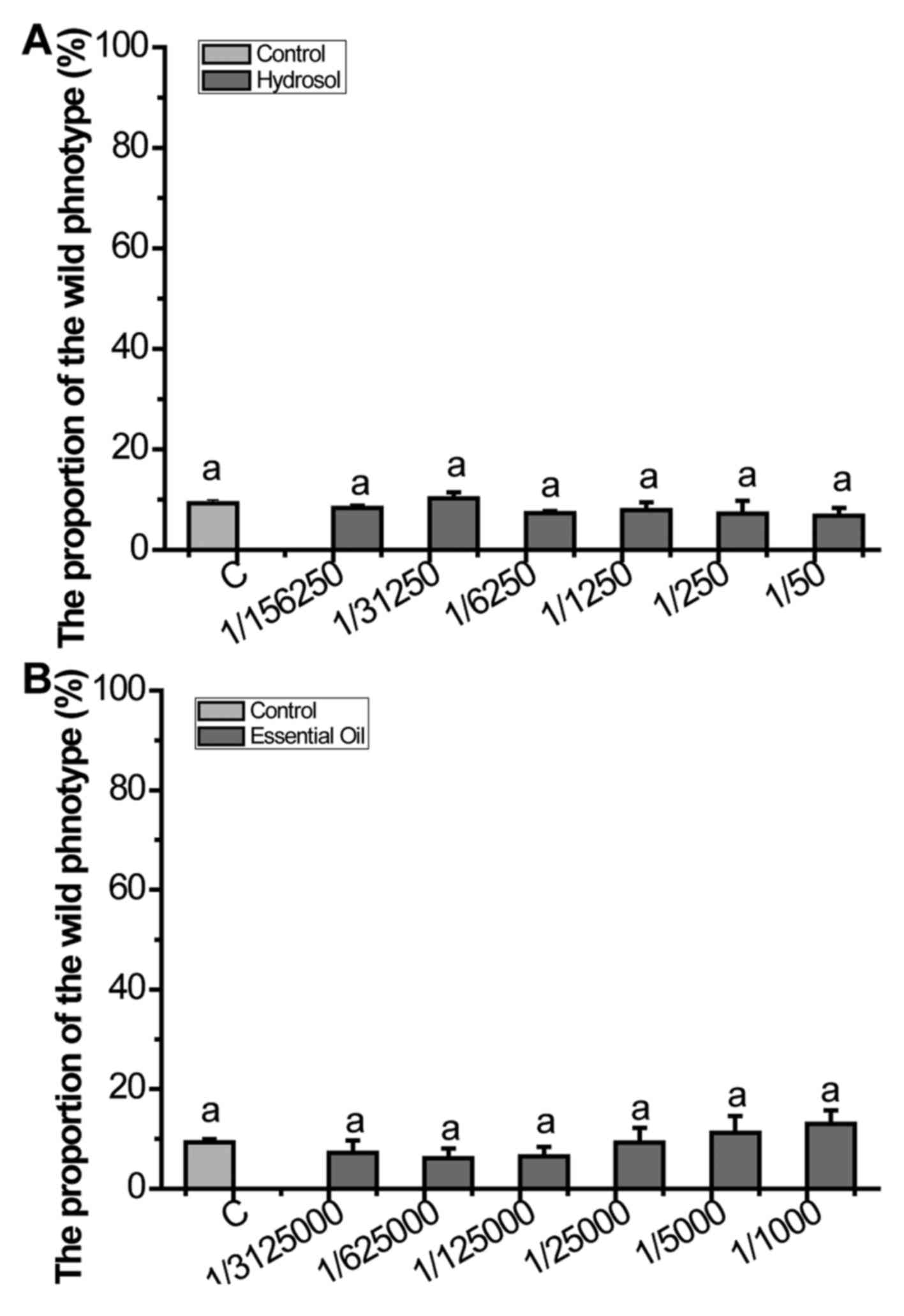
(33.83%). The essential oil is known to have high biological
activity, so we applied essential oil and hydrosol to assess
whether the essential oil can inhibit over-activated ras.
The results showed that neither of them can suppress over-activated
Ras (Fig. 4).
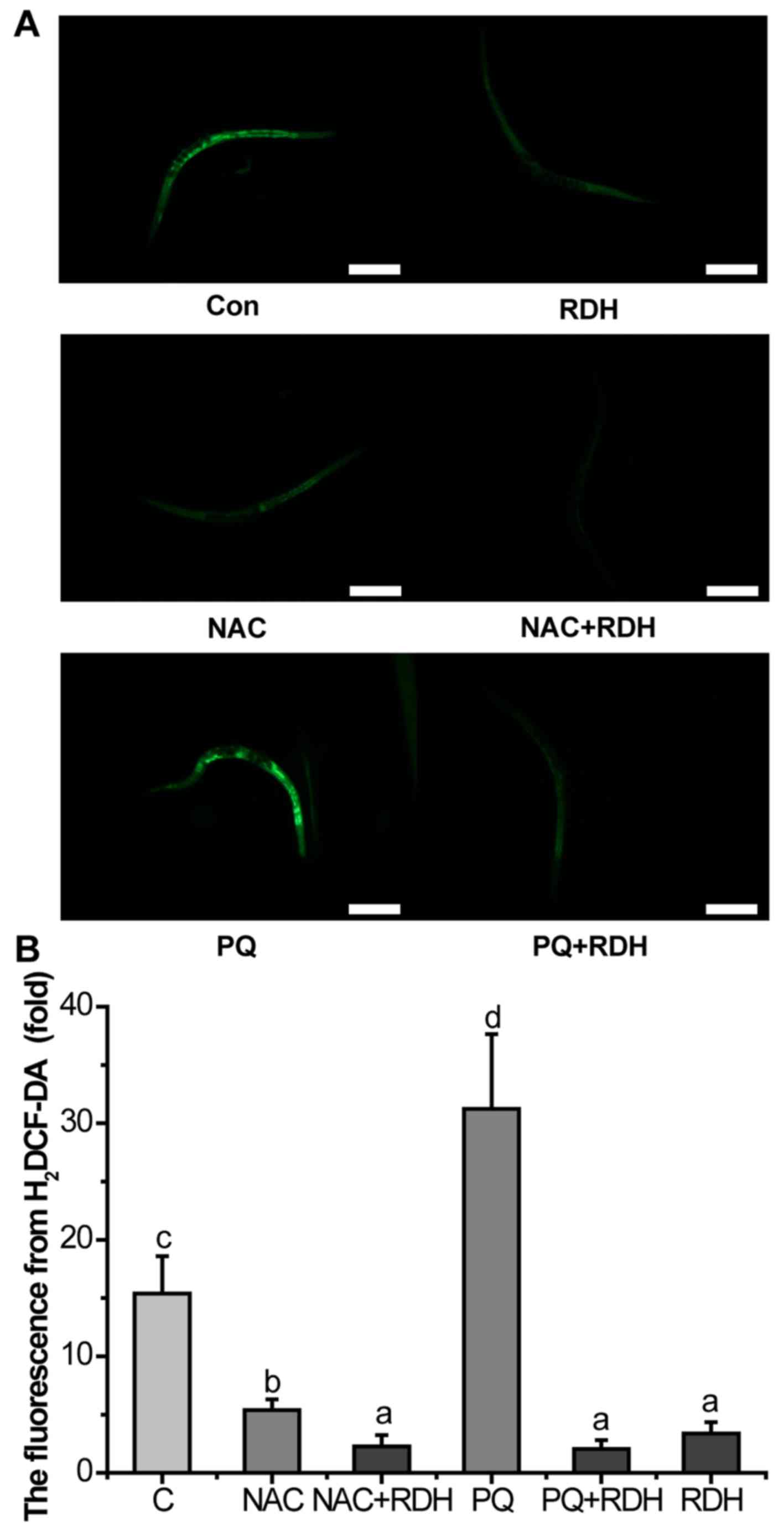
KRD significantly decreases the ROS level
in C. elegans
The level of ROS in let-60(gf) mutants
was measured by using the ROS probe H2DCF-DA. KRD can
strongly reduce ROS in vivo. NAC is an antioxidant that can
scavenge all kinds of ROS. NAC decreased the level of ROS of
let-60(gf) mutants, and KRD combined with NAC further
scavenged the ROS. PQ is a well-known pro-oxidant. PQ greatly
increased the accumulation of ROS, and KRD can almost completely
eliminate the ROS induced by PQ (Fig.
5).
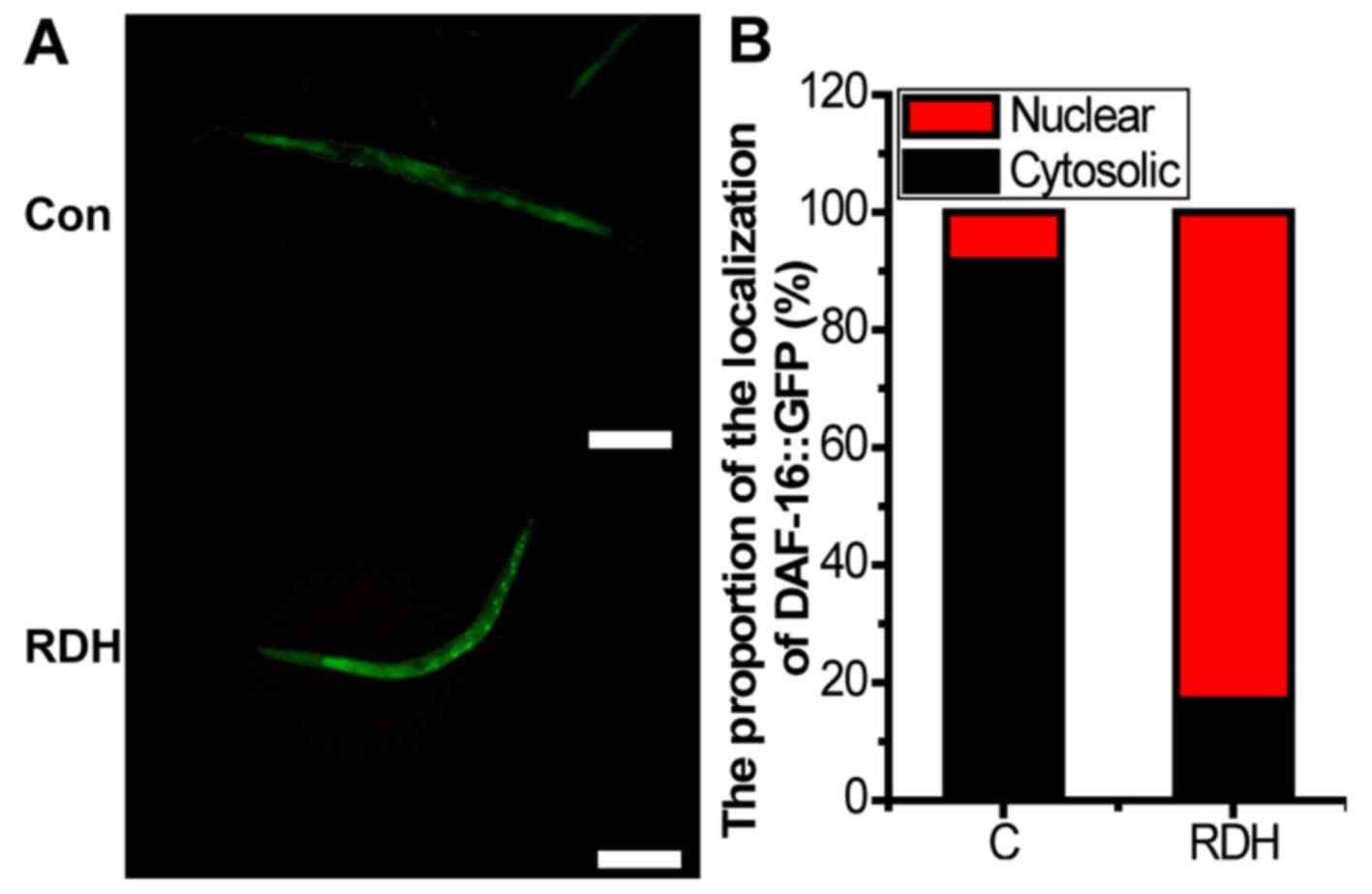
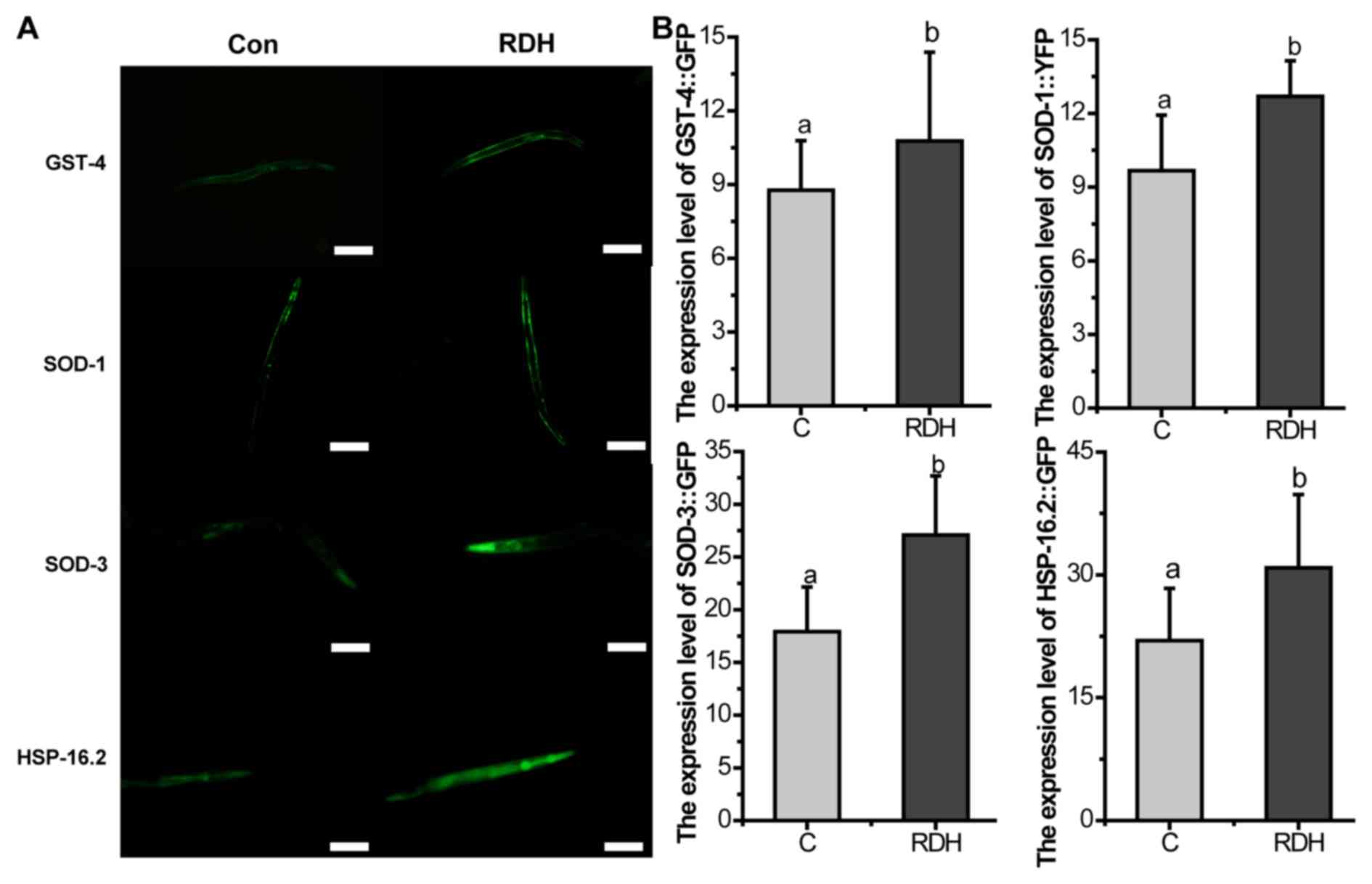
KRD significantly increases the oxidative
stress related proteins in C. elegans
DAF-16 is necessary for stress resistance (45). GSTs are main cellular
detoxification enzymes, and GST can protect the worms against
oxidative stress damage (46).
SODs avoid the formation of highly aggressive ROS, such as
peroxynitrite or the hydroxyl radical (47). HSP-16.2 is also a stress-sensitive
reporter (48). The results of
the study showed that the proportion of DAF-16::GFP nuclear
translocation and the expression of GST-4, SOD-1, SOD-3 were all
significantly upregulated by KRD (Figs. 6 and 7).
Discussion
The free radical theory postulates that the
production of intracellular reactive oxygen species is the main
determinant and promotion of cancer (17). This is supported by findings from
a variety of studies, suggested that reduction of oxidative stress
is associated with the treatment of cancer (18). It is reasonable to conclude that
antioxidants are potential drug candidates for cancer therapy.
Further, over-activated Ras is closely related to the increased ROS
accumulation as well (14–16).
In C. elegans, let-6 is a homologous gene to
ra in mammals; let-60(gf) could cause the
multivulva phenotype. Thus, we employed C. elegan with
let-60(gf) mutant to determine whether KRD and its
extracts can suppress over-activated Ras/MAPK pathways thought
resistance to oxidative stress.
KRD had a very potent antioxidant activity in
vitro (Fig. 1). KRD (1 mg/ml)
was equivalent to 1.1760 mM Trolox or 1.8844 mM ascorbic acids,
respectively. As the results show in Fig. 2, KRD can mop up 58.76% superoxide
anion (Fig. 2A), 63.64% hydroxyl
radical (Fig. 2B) and 99.80%
hydrogen peroxide (Fig. 2C),
respectively. Thus, KRD can remarkable scavenge the radicals in
vitro.
In recent years, increased attention has been given
to rose, especially the essential oils (49) and rose essential oil is one of the
most expensive essential oils. The antioxidant activity of the rose
essential oil was previously studied, and high antioxidant capacity
was observed (26). Since KRD,
rose essential oil and its hydrosol all had potent antioxidant
capacity, the effect of KRD, rose essential oil and its hydrosol on
the over-activated Ras was further investigated. In this study, KRD
inhibited the Muv phenotype significantly in a dose-dependent
manner in let-6 mutants (Fig.
3). In Fig. 4, the results
show that neither rose essential oil nor hydrosol can suppress the
over-activated Ras, thus indicating that the active ingredient
which inhibit the over-activated Ras mainly exist in KRD.
Therefore, reminding us that soaking rose in hot water to prepare
'rose tea' is an effective way for health care.
Since KRD can significantly inhibit the
over-activated Ras (Fig. 3), and
have a potent antioxidant activity in vivo (Figs. 1 and 2), we attempted to illuminate whether
the mechanism of KRD is built on its antioxidant activity. The
results showed that KRD can strongly reduce ROS in vivo
(Fig. 5). NAC can decrease the
level of ROS of let-60(gf) mutants. KRD combined with
NAC can further scavenge ROS. PQ can substantially increase the
accumulation of ROS, and KRD can almost completely eliminate the
ROS induced by PQ. These phenomena indicated that KRD scavenged ROS
like NAC and resisted the oxidative stress induced by PQ in
let-60(gf) mutants (Fig. 5).
DAF-16/forkhead transcription factor, GSTs, SODs,
HSP-16.2 all can serve as stress-sensitive reporters. The
proportion of DAF-16::GFP nuclear translocation and the expression
of GST-4, SOD-1, SOD-3, HSP-16.2 were all increased by KRD
treatment (Figs. 6 and 7). These anti-oxidative stress related
factors were all upregulated by KRD, suggest that KRD inhibit
over-activated Ras via a mechanism that is based on its antioxidant
capacity and upregulating the anti-oxidative stress related
factors. So the antitumor effect of KRD in C. elegan is
shown by downregulation of Ras/MAPK pathway and resistance to
oxidative stress.
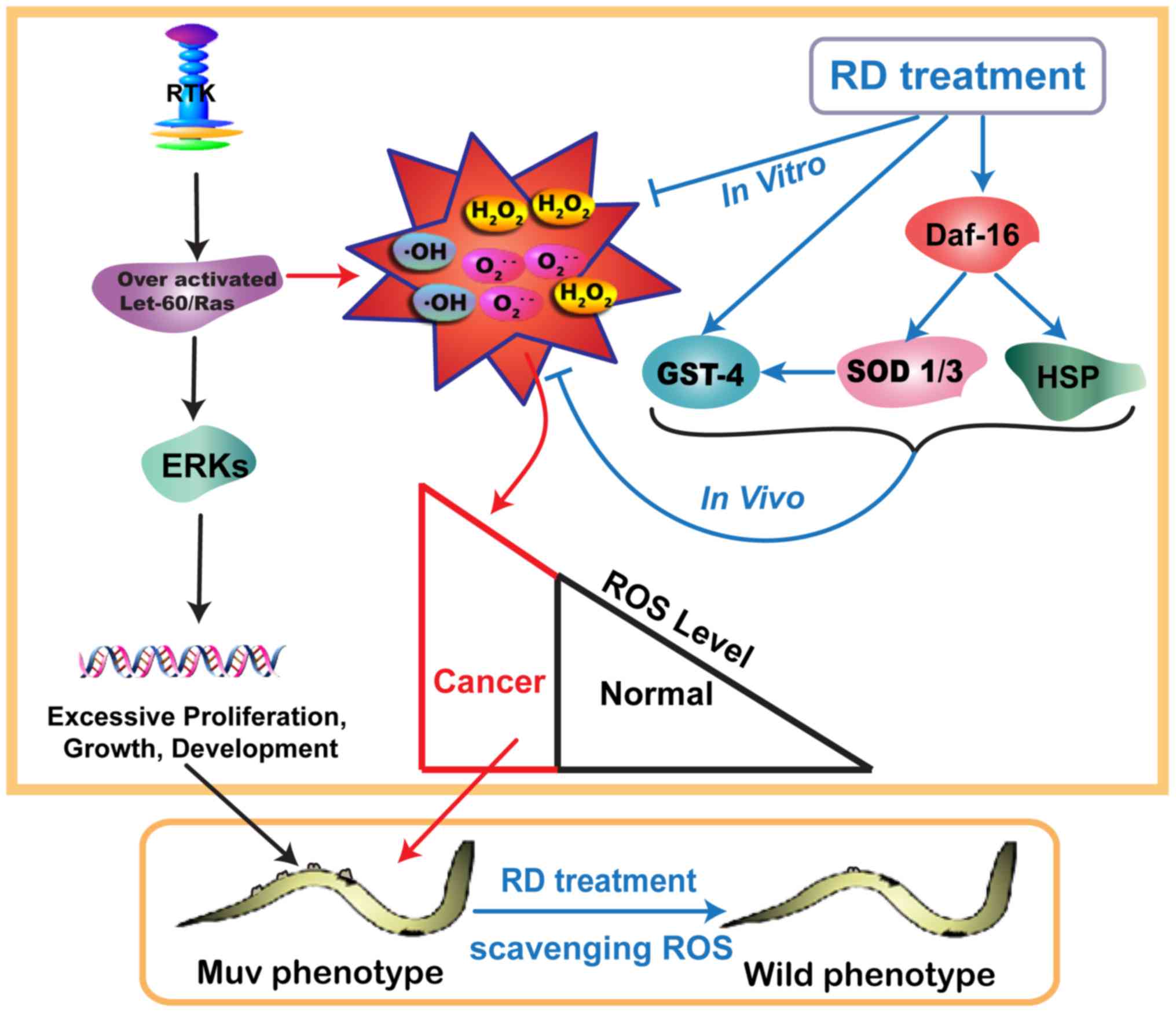
In conclusion, the mechanism of KRD that
significantly suppressed the over-activated Ras/MAPK pathway is
mainly dependent on the process as showed in Fig. 8. First of all, over-activated
Ras/MAPK pathway led to the excessive accumulation of ROS. Then
worms treated with KRD were able to scavenge ROS. Next, KRD
triggered the nuclear translocation of DAF-16 and the expression of
GST-4, SOD-1, SOD-3 and HSP-16.2 to protect the worms against
oxidative stress. Finally, the level of ROS returned to normal and
the Muv phenotype in let-60(gf) mutants was reverted
by KRD. The above indicates KRD can serve as a potential drug
candidate for combating over-activated Ras-related cancer.
Acknowledgments
The authors wish to thank Dr Kuang Yu Chen, Rutgers
University, and Dr Li Ming Hao, Harvard Medical School, for their
valuable suggestions and kind help in checking the English
presentation of this manuscript. MT2124 was kindly provided by
Howard Hughes Medical Institute.
Abbreviations:
|
ABTS
|
2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid
|
|
C. elegans
|
Caenorhabditis elegans
|
|
DAF-16
|
forkhead transcription factor
|
|
DPPH
|
1,1-diphenyl-2-picrylhydrazyl
|
|
GST-4
|
glutathione S-transferase-4
|
|
HSP-16.2
|
heat shock protein-16.2
|
|
KR
|
Kushui Rose (R. Setat x R.
Rugosa)
|
|
KRD
|
Kushui Rose decoction
|
|
NAC
|
N-acetyl cysteine
|
|
NBT
|
nitrotetrazolium blue chloride
|
|
PQ
|
paraquat
|
|
ROS
|
reactive oxygen
|
|
SOD
|
superoxide dismutase
|
Funding
This study was supported by the Ministry of Science
and Technology New Drug Project of MOST of China
(2015ZX09501-004-003-008); the National Natural Science Foundation
of China (31772147 and 31571989); and the Fundamental Research
Funds for the Central Universities (lzujbky-2018-40 and
lzujbky-2017-206).
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YLiu performed all the experiments and was a major
contributor to the writing of the manuscript. DF and ZZ prepared
the KR decoction. YLiu, DZ, XW and ZW analyzed the data. HL, YLi
and PC designed the experiments. XW, ZW, YLi and PC revised the
manuscript critically. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Slavov A, Kiyohara H and Yamada H:
Immunomodulating pectic polysaccharides from waste rose petals of
Rosa damascena Mill. Int J Biol Macromol. 59:192–200. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davis PH: Flora of Turkey and the East
Aegean Islands. Edinburgh University Press; Edinburgh: pp.
5671965
|
|
3
|
Kazaz S, Baydar H and Erbas S: Variations
in Chemical Compositions of Rosa damascena Mill. and Rosa canina L.
Fruits. Czech J Food Sci. 27:178–184. 2009.
|
|
4
|
Jin J: The comprehensive development of
rose. Chin Wild Plant Resour. 19:21–23. 2000.
|
|
5
|
Cai F, He Y and Li X: A Survey of Rosa
rugos germplasm resource. World Sci Technology-Modernization Tradit
Chin Med Materia Med. 3:75–80. 2007.In Chinese.
|
|
6
|
Gao Y, Mao XL, Wang WS, Chen Z and Huang
JY: Analysis of active components in kushui roses. Flavour
Fragrance Cosmetics. 22–26. 2014.
|
|
7
|
Zhai YL and Xie CJ: Kushui rose in
Lanzhou. Forestry of China. 44. 2010.
|
|
8
|
Zhou W, Zhou XP, Zhao GH, Liu HW, Ding L
and Chen LR: Studies of aroma components on essential oil of
Chinese kushui rose. Se Pu. 20:560–564. 2002.In Chinese.
|
|
9
|
Yu C, Zhao Y, Xu Q, Yu P and Zhang X: The
thinking of kushui rose (R. Setat x R. rugosa) industry
development. Gansu Sci Technol. 28:14–15. 2012.
|
|
10
|
Grunwald A, Gottfried I, Cox AD, Haklai R,
Kloog Y and Ashery U: Rasosomes originate from the Golgi to
dispense Ras signals. Cell Death Dis. 4:e4962013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Russo A, Ficili B, Candido S, Pezzino FM,
Guarneri C, Biondi A, Travali S, McCubrey JA, Spandidos DA and
Libra M: Emerging targeted therapies for melanoma treatment
(review). Int J Oncol. 45:516–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Friday BB and Adjei AA: K-ras as a target
for cancer therapy. Biochim Biophys Acta. 1756:127–144.
2005.PubMed/NCBI
|
|
13
|
Weinberg F and Chandel NS: Mitochondrial
metabolism and cancer. Ann NY Acad Sci. 1177:66–73. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Irani K, Xia Y, Zweier JL, Sollott SJ, Der
CJ, Fearon ER, Sundaresan M, Finkel T and Goldschmidt-Clermont PJ:
Mitogenic signaling mediated by oxidants in Ras-transformed
fibroblasts. Science. 275:1649–1652. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Lu B, Xu L, Yin LH, Wang XN, Peng
JY and Liu KX: The antioxidant activity and hypolipidemic activity
of the total flavonoids from the fruit of Rosa laevigata Michx. Nat
Sci. 2:175–183. 2010.
|
|
16
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bagchi D, Swaroop A, Preuss HG and Bagchi
M: Free radical scavenging, antioxidant and cancer chemoprevention
by grape seed proanthocyanidin: An overview. Mutat Res. 768:69–73.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bhandary B, Marahatta A, Kim HR and Chae
HJ: Mitochondria in relation to cancer metastasis. J Bioenerg
Biomembr. 44:623–627. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siddiqui IA, Adhami VM, Saleem M and
Mukhtar H: Beneficial effects of tea and its polyphenols against
prostate cancer. Mol Nutr Food Res. 50:130–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abdel-Hameed el-SS, Bazaid SA and Salman
MS: Characterization of the phytochemical constituents of Taif rose
and its antioxidant and anticancer activities. Biomed Res Int.
2013:3454652013. View Article : Google Scholar :
|
|
21
|
Bairati I, Meyer F, Gélinas M, Fortin A,
Nabid A, Brochet F, Mercier JP, Têtu B, Harel F, Mâsse B, et al: A
randomized trial of antioxidant vitamins to prevent second primary
cancers in head and neck cancer patients. J Natl Cancer Inst.
97:481–488. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bjelakovic G, Nikolova D, Gluud LL,
Simonetti RG and Gluud C: Antioxidant supplements for prevention of
mortality in healthy participants and patients with various
diseases. Cochrane Database Syst Rev. 3:CD0071762012.
|
|
23
|
Heinonen OP and Albanes D: The effect of
vitamin E and beta carotene on the incidence of lung cancer and
other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene
Cancer Prevention Study Group. N Engl J Med;330:1029–1035.
1994.
|
|
24
|
Omenn GS, Goodman GE, Thornquist MD,
Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL Jr, Valanis B,
Williams JH Jr, et al: Effects of a combination of beta carotene
and vitamin A on lung cancer and cardiovascular disease. N Engl J
Med. 334:1150–1155. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Velázquez E, Tournier HA, Mordujovich de
Buschiazzo P, Saavedra G and Schinella GR: Antioxidant activity of
Paraguayan plant extracts. Fitoterapia. 74:91–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baydar NG and Baydar H: Phenolic
compounds, antiradical activity and antioxidant capacity of
oil-bearing rose (Rosa damascena Mill.) extracts. Ind Crops Prod.
41:375–380. 2013. View Article : Google Scholar
|
|
27
|
Cociancich E and Pace R: Process for the
extraction of taxol and derivatives thereof from roots of plants of
the genus Taxus. US Patent US 5744333 A. Filed 28 April, 1998;
issued, 31 January, 1992.
|
|
28
|
Cragg GM and Newman DJ: Plants as a source
of anticancer agents. J Ethnopharmacol. 100:72–79. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sieber SM, Mead JA and Adamson RH:
Pharmacology of antitumor agents from higher plants. Cancer Treat
Rep. 60:1127–1139. 1976.PubMed/NCBI
|
|
30
|
Lee TH, Lee CK, Tsou WL, Liu SY, Kuo MT
and Wen WC: A new cytotoxic agent from solid-state fermented
mycelium of Antrodia camphorata. Planta Med. 73:1412–1415. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kumar VB, Yuan TC, Liou JW, Yang CJ, Sung
PJ and Weng CF: Antroquinonol inhibits NSCLC proliferation by
altering PI3K/mTOR proteins and miRNA expression profiles. Mutat
Res. 707:42–52. 2011. View Article : Google Scholar
|
|
32
|
Ng TB, He JS, Niu SM, Zhao L, Pi ZF, Shao
W and Liu F: A gallic acid derivative and polysaccharides with
antioxidative activity from rose (Rosa rugosa) flowers. J Pharm
Pharmacol. 56:537–545. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vinokur Y, Rodov V, Reznick N, Goldman G,
Horev B, Umiel N and Friedman H: Rose petal tea as an
antioxidant-rich beverage: Cultivar effects. J Food Sci.
71:S42–S47. 2006. View Article : Google Scholar
|
|
34
|
Mattiasson G: Analysis of mitochondrial
generation and release of reactive oxygen species. Cytometry A.
62:89–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu D, Zhi D, Zhou T, Yu Q, Wan F, Bai Y
and Li H: Realgar bioleaching solution is a less toxic arsenic
agent in suppressing the Ras/MAPK pathway in Caenorhabditis
elegans. Environ Toxicol Pharmacol. 35:292–299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dong Y, Guha S, Sun X, Cao M, Wang X and
Zou S: Nutraceutical interventions for promoting healthy aging in
invertebrate models. Oxid Med Cell Longev. 2012:7184912012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sternberg PW and Han M: Genetics of RAS
signaling in C. elegans. Trends Genet. 14:466–472. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhi J, Feng N, Liu DL, Hou RL, Wang MZ,
Ding XX and Li HY: Realgar bioleaching solution suppress ras
excessive activation by increasing ROS in Caenorhabditis elegans.
Arch Pharm Res. 37:390–398. 2014. View Article : Google Scholar
|
|
39
|
Brenner S: The genetics of Caenorhabditis
elegans. Genetics. 77:71–94. 1974.PubMed/NCBI
|
|
40
|
Hara M and Han M: Ras farnesyltransferase
inhibitors suppress the phenotype resulting from an activated ras
mutation in Caenorhabditis elegans. Proc Natl Acad Sci USA.
92:3333–3337. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reiner DJ, Gonzalez-Perez V, Der CJ and
Cox AD: Use of Caenorhabditis elegan to evaluate inhibitors of Ras
function in vivo. Methods Enzymol. 439:425–449. 2008. View Article : Google Scholar
|
|
42
|
Kampkötter A, Pielarski T, Rohrig R,
Timpel C, Chovolou Y, Wätjen W and Kahl R: The Ginkgo bilob extract
EGb761 reduces stress sensitivity, ROS accumulation and expression
of catalase and glutathione S-transferase 4 in Caenorhabditis
elegans. Pharmacol Res. 55:139–147. 2007. View Article : Google Scholar
|
|
43
|
Kaneria M, Kanani B and Chanda S:
Assessment of effect of hydroalcoholic and decoction methods on
extraction of antioxidants from selected Indian medicinal plants.
Asian Pac J Trop Biomed. 2:195–202. 2012. View Article : Google Scholar
|
|
44
|
Li XT, Zhang YK, Kuang HX, Jin FX, Liu DW,
Gao MB, Liu Z and Xin XJ: Mitochondrial protection and anti-aging
activity of Astragalus polysaccharides and their potential
mechanism. Int J Mol Sci. 13:1747–1761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang L, Jie G, Zhang J and Zhao B:
Significant longevity-extending effects of EGCG on Caenorhabditis
elegan under stress. Free Radic Biol Med. 46:414–421. 2009.
View Article : Google Scholar
|
|
46
|
Leiers B, Kampkötter A, Grevelding CG,
Link CD, Johnson TE and Henkle-Dührsen K: A stress-responsive
glutathione S-transferase confers resistance to oxidative stress in
Caenorhabditis elegans. Free Radic Biol Med. 34:1405–1415. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Afonso V, Champy R, Mitrovic D, Collin P
and Lomri A: Reactive oxygen species and superoxide dismutases:
role in joint diseases. Joint Bone Spine. 74:324–329. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu H, Zhao Y, Guo Y, Xu L and Zhao B:
Significant longevity-extending effects of a tetrapeptide from
maize on Caenorhabditis elegan under stress. Food Chem.
130:254–260. 2012. View Article : Google Scholar
|
|
49
|
Ge Q and Ma X: Composition and antioxidant
activity of antho-cyanins isolated from Yunnan edible rose (An
ning). Food Sci Hum Wellness. 2:68–74. 2013. View Article : Google Scholar
|