Introduction
Malignant melanoma is known for its exceptionally
high mortality rate among all types of skin cancer. Malignant
melanoma occurs due to an intricate interaction between endogenous
and exogenous factors. In total, >65% of malignant melanoma
cases are influenced by sun exposure and ~12% of cases are caused
by genetic factors, such as mutations of critical genes (including
cyclin-dependent kinase inhibitor 2A, melanocortin 1 receptor and
DNA repair genes) (1). A great
number of melanoma patients notably acquired driver oncogenic
mutations in genes that encode proteins associated with growth
factor receptor signaling pathways, such as the mitogen-activated
protein kinase (MAPK)/extracellular signal-regulated kinase and
phosphoinositide 3-kinase/protein kinase B pathways (2,3).
Treatments for advanced melanoma have been investigated for the
last decade. Surgical removal is the primary treatment for melanoma
due to its noticeable appearance on the skin, and early removal of
melanoma increases the chance of preventing metastasis.
Chemotherapy, immunotherapy and molecular-targeted therapies are
also well known treatment methods for melanoma, which have
increased the survival rate of melanoma patients; however, similar
to other types of cancer, resistance to these treatments has been
rising (4).
Resveratrol, a trans-3,4′,5-trihydroxystilbene, is a
dietary phenol present in numerous plants and dietary supplements,
and is commonly found in grapes. Resveratrol has been reported to
have an impact on every stage of carcinogenesis. In addition, it
serves as a chemopreventive agent due to its potential to mediate
signaling pathways that manage cell division, cell growth,
apoptosis, angiogenesis, metastasis and inflammation (5,6).
There have been numerous studies on resveratrol as an ideal
anticancer molecule, as it provokes a cytotoxic effect on cancer
cells, while it does not affect nonmalignant cells (7–9).
The main resveratrol-mediated chemotherapeutic mechanism is
apoptosis associated with the activation of p53, a tumor
suppressor, and induced activation of death receptor Fas/CD85/APO-1
in diverse cancer cells (10).
Resveratrol is also know to exhibit a preventive role in heart
disease, since it prevents coagulation and platelet aggregation,
modifies eicosanoid synthesis and mediates lipoprotein metabolism
(11).
Endoplasmic reticulum (ER) is the primary site for
protein folding and transporting, and maintenance of cellular
functions. ER stress occurs when cell homeostasis collapses. The
unfolded protein response (UPR) is a response to ER stress that
involves several different stress signaling and inflammatory
pathways (12). Failure to
resolve ER stress leads to apoptosis. One ER stress-induced
apoptosis pathway involves the activation of C/EBP homologous
protein (CHOP), a transcription factor, by ER stress and the
promotion of apoptosis by downregulation of B-cell lymphoma 2
(Bcl-2) (13,14). ER stress also stimulates
intracellular reactive oxygen species (ROS) production, and these
species then reinforce ER stress-mediated apoptosis (15). Oxidative stress is caused by
excess production of ROS and leads to cell death. ROS consist of
cytotoxic molecules, including superoxide
(O2−), hydrogen peroxide
(H2O2), singlet oxygen (1/2 O2)
and the hydroxyl radical (•OH). These ROS can damage all
cellular components, including proteins, lipids and DNA, and
further cause disruptions in normal cellular signaling (16). Oxidative and antioxidative stress
also occurs when the balance between pro-oxidants and antioxidants
collapses (17,18). While excessive ROS generation
induces oxidative stress, excessive reduction in the oxidative
stress level by antioxidants induces antioxidative stress, which
also has harmful effects on human health.
In the present study, it was attempted to determine
whether resveratrol induces ER stress-mediated apoptosis to
suppress cell growth. In addition, its effect on the intracellular
ROS level in the A375SM cell line was examined.
Materials and methods
Reagents and chemicals
Resveratrol was purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany) and was dissolved in dimethyl sulfoxide
(DMSO; Junsei Chemical Co., Ltd., Tokyo, Japan).
Cell culture and media
A malignant melanoma cell line, A375SM, was obtained
from the Korean Cell Line Bank (Seoul, Korea) and cultured in
Dulbecco's modified Eagle's medium (DMEM; HyClone; GE Healthcare
Life Sciences, Logan, UT, USA) supplemented with 10% (v/v)
heat-inactivated fetal bovine serum (FBS; Rocky Mountain
Biologicals, Inc., Missoula, MT, USA), 10 U/ml penicillin and 100
µg/ml streptomycin (Cellgro Mediatech; Corning Incorporated,
Corning, NY, USA), and 10 mM HEPES (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C in 5% CO2
and 95% air in a humidified cell incubator. Melanoma cells were
trypsinized using 0.05% trypsin/0.02% EDTA (GE Healthcare Life
Sciences).
Cell viability assay
The cell viability test was conducted for 6 days. On
the first day, A375SM cells (3×103 cells/well) were
seeded in 96-well plates (SPL Life Science, Seoul, Korea) and
cultured in DMEM supplemented with FBS, 1% penicillin and
streptomycin, and 1% HEPES at 37°C in a humidified atmosphere with
5% CO2. The following day, A375SM cells were treated
with concentrations of resveratrol ranging between 10−2
and 10 µM in DMEM supplemented with 0.1% DMSO for 4 days. On
the third day of this treatment, the media were changed to a fresh
version of the same media. The day after the four days of
treatments were completed (day 6), an EZ-cytox Cell Viability Assay
kit (DoGen Bio Co., Ltd., Seoul, Korea) was used to verify the cell
viability in each well. An ELISA plate reader (VERSAmax; Molecular
Devices, LLC, Sunnyvale, CA, USA) was used to measure the
absorbance at 480 nm. Resveratrol (1 µM) was expected to
produce a sufficient effect on ER-stress and ROS production and was
applied for subsequent analysis; a dose-dependent effect was also
investigated by using 1 and 10 µM resveratrol as 10
µM resveratrol did not induce cell damage >45%.
Western blot assay
The A375SM cell line was incubated in medium
containing 0.1% DMSO and resveratrol (1 and 10 µM) for 48 h.
Next, radioimmunoprecipitation assay buffer containing 50 mM
Tris-HCl (pH 8.0), 150 mM NaCl, 1% Triton X-100 (Sigma-Aldrich;
Merck KGaA), 0.1% sodium dodecyl sulfate and 0.5% deoxycholic acid
(Sigma-Aldrich; Merck KGaA) mixed with protease and phosphatase
inhibitors was used for harvesting proteins for western blot
analysis. The extracted proteins were incubated overnight at 4°C
and then centrifuged at 18,430 × g for 1 h at 4°C. The selected
protein concentration was obtained using a mixture of bicinchoninic
acid and copper (II) sulfate (both from Sigma-Aldrich; Merck KGaA).
Subsequently, 50 µg total protein was used for 10%
SDS-polyacrylamide gel electrophoresis, and then the separated
proteins were transferred to a polyvinylidene fluoride membrane
(Bio-Rad Laboratories, Inc., Hercules, CA USA). The membrane was
subsequently incubated overnight at 4°C with the following
monoclonal primary antibodies: mouse monoclonal anti-p21 (1:1,000;
Abcam, Cambridge, MA, USA; ab188224), anti-cyclin B (1:1,000;
Abcam; ab2949), anti-cyclin E (1:1,000; Abcam; ab98952), anti-p53
(1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA; sc-126),
anti-phosphorylated (p)-p38 MAPK (1:1,000; Abcam; ab4822),
anti-CHOP (1:1,000; Cell Signaling Technology, Inc., Danvers, MA,
USA; 2895), anti-Bcl-2-associated X protein (Bax; 1:1,000; Santa
Cruz Biotechnology, Inc.; sc-7480), anti-Bcl-2 (1:200; Cell
Signaling Technology, Inc.; 15071), anti-GAPDH (1:12,000; Abcam;
ab130099), rabbit monoclonal anti-nuclear factor erythroid
2-related factor 2 (Nrf2; 1:2,000; Abcam; ab62352), anti-p27
(1:1,000; Abcam; ab32034) and anti-p-eukaryotic initiation factor
2α (eIF2α; 1:1,000; Cell Signaling Technology, Inc.; 9721). The
primary antibody binding was observed by incubating for 1 h at 4°C
with a secondary antibody, including anti-mouse IgG (1:3,000;
Thermo Fisher Scientific, Inc.; 170-6516) or anti-rabbit IgG
(1:3,000; Thermo Fisher Scientific, Inc.; 170-6515). A West-Q
Chemiluminescent Substrate Plus kit (GenDEPOT, Inc., Barker, TX,
USA) was utilized for protein detection. The band densities on the
membrane were utilized to quantify each protein expression through
CS Analyzer 4 software ver. 2.1.2 (Atto Corporation, Tokyo, Japan).
Each experiment was conducted at least three times, and the
expression levels of the aforementioned proteins were normalized to
that of GADPH protein.
Measurement of ROS generation
An assay using 2′,7′-dichlorofluorescein diacetate
(DCF-DA) was conducted to measure the cellular levels of ROS in the
A375SM cell line. Briefly, A375SM cells were seeded at a density of
3×105 cells per well in a 6-well plate with the culture
medium. After 48 h of incubation, the culture medium was replaced
with medium containing either 0.1% DMSO or resveratrol (1 and 10
µM). As a positive control for ROS production, 2 ml of 3%
H2O2 solution was added to the A375SM cell
line for 15 min. A new medium containing DCF-DA solution in DMEM
was substituted for 30 min. Subsequently, each well was washed with
PBS, and the A375SM cell line was visualized using a fluorescence
microscope (IX-73 inverted microscope; Olympus Corporation, Tokyo,
Japan). The amount of ROS formed by the resveratrol treatment was
quantified using CellSens Dimension software ver. 1.13 (Olympus
Corporation).
TUNEL assay
Apoptotic melanoma cells were detected using a
DeadEnd™ fluorometric TUNEL assay kit (Promega Corporation,
Madison, WI, USA) as described in the manufacturer's protocol.
Briefly, melanoma cells were seeded at a density of
3×105 cells per well in a 6-well plate. After 48 h of
incubation with medium containing 0.1% DMSO and resveratrol (1 and
10 µM), cells were fixed with 3.7% formaldehyde for 25 min
and incubated with recombinant terminal deoxynucleotidyl
transferase incubation buffer for 1 h at 37°C. The cells were then
stained with DAPI (Invitrogen; Thermo Fisher Scientific, Inc.), and
both apoptotic and DAPI-stained cells were visualized using a
fluorescence microscope (IX-73 inverted microscope, Olympus
Corporation). ImageJ software ver. 1.49 (National Institutes of
Health, Bethesda, MD, USA) was used for merging the images of DAPI
and TUNEL staining. The number of apoptotic A375SM cells produced
by resveratrol treatment was quantified using the CellSens
Dimension software ver. 1.13 (Olympus Corporation).
Statistical analysis
All experiments were performed a minimum of three
times, and the resulting data were analyzed with the GraphPad Prism
software ver. 5 (GraphPad Software, Inc., San Diego, CA, USA). All
data are presented as the mean ± standard deviation, and were
analyzed using one-way analysis of variance, followed by Dunnett's
test. Differences with P-values of <0.05 were recognized as
statistically significant.
Results
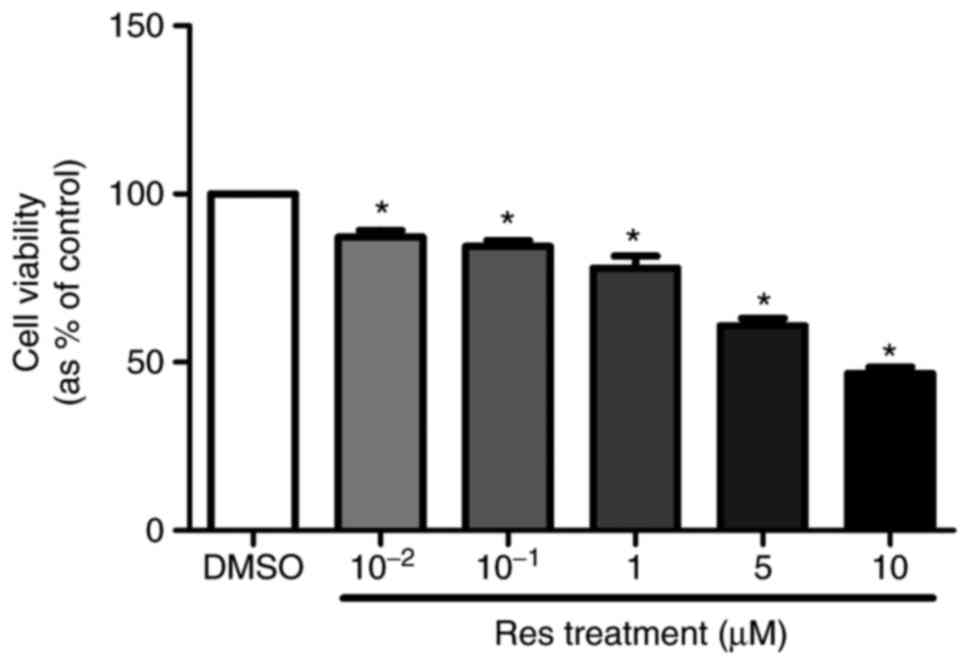
Cell proliferation of A375SM was
repressed by resveratrol
To evaluate the effects of resveratrol on A375SM
cell proliferation, the cells were treated with 0.1% DMSO (control)
or resveratrol (10−2, 10−1, 1, 5 and 10
µM) for 4 days. On day 6 of the treatment, EZ-cytox was
added to measure the cell viability. Resveratrol significantly
suppressed the cell viability of the melanoma cell line in a
dose-dependent manner (Fig. 1).
Based on the results of the cell viability assay, the resveratrol
concentrations of 1 and 10 µM were selected for further
experiments.
Resveratrol induced cell cycle arrest of
melanoma cell line
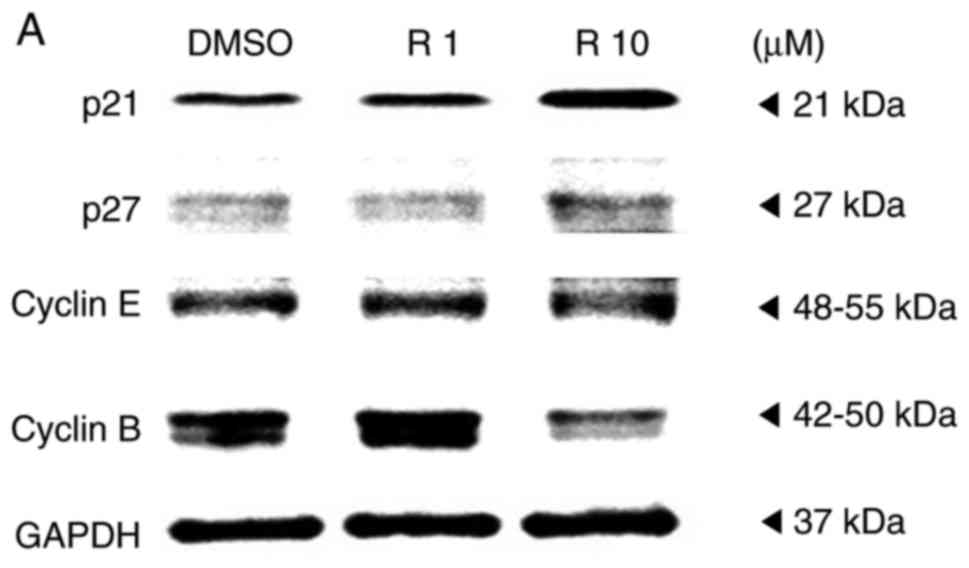
A western blot assay was performed on the A375SM
melanoma cell line to examine whether resveratrol influenced the
protein expression of genes controlling the cell cycle progression.
Melanoma cells were treated with the control or resveratrol (1 and
10 µM) for 48 h, and then the protein levels of cell
cycle-associated genes, including p21, p27, cyclin E and cyclin B,
were quantified. It was observed that the expression levels of
cyclin-dependent kinase inhibitors p21 and p27 were significantly
increased in a dose-dependent manner (Fig. 2). By contrast, the expression of
cyclin B was markedly decreased, whereas the expression of cyclin E
did not exhibit any significant difference among melanoma cells
that were treated with the control and resveratrol (1 and 10
µM), as shown Fig. 2B.
According to previous studies, upregulation of cyclin-dependent
kinase inhibitors, p21 and p27 arrests the cell cycle at the G2/M
phase by deregulating cyclin B1 levels, inducing G2 arrest to
hinder the replication of damaged DNA (19,20). These results indicate that
resveratrol may activate p21 and p27 to suppress the G2/M phase of
the cell cycle in A375SM melanoma cells.
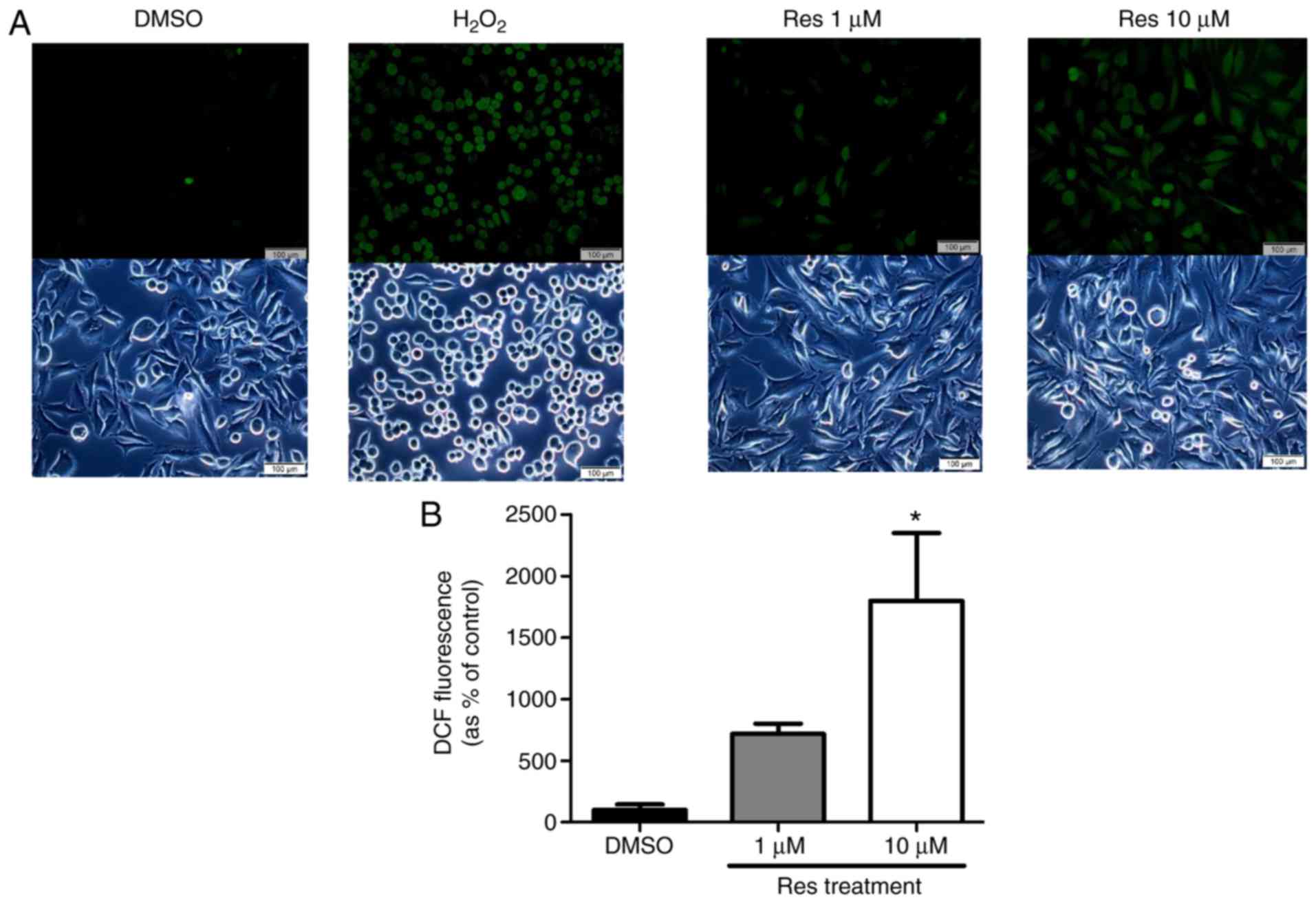
Resveratrol elevated ROS generation and
ER stress of melanoma cell line
The study further evaluated the ROS generation and
ER stress on the A375SM melanoma cell line when exposed to
resveratrol. Cellular ROS production in the A375SM cell line
exposed to 1 or 10 µM resveratrol for 48 h was measured
using a DCF-DA assay, using hydrogen peroxide as a positive
control. Fig. 3 displays the
induced ROS generation on the A375SM cell line, and the DCF
positive cells were significantly increased in a dose-dependent
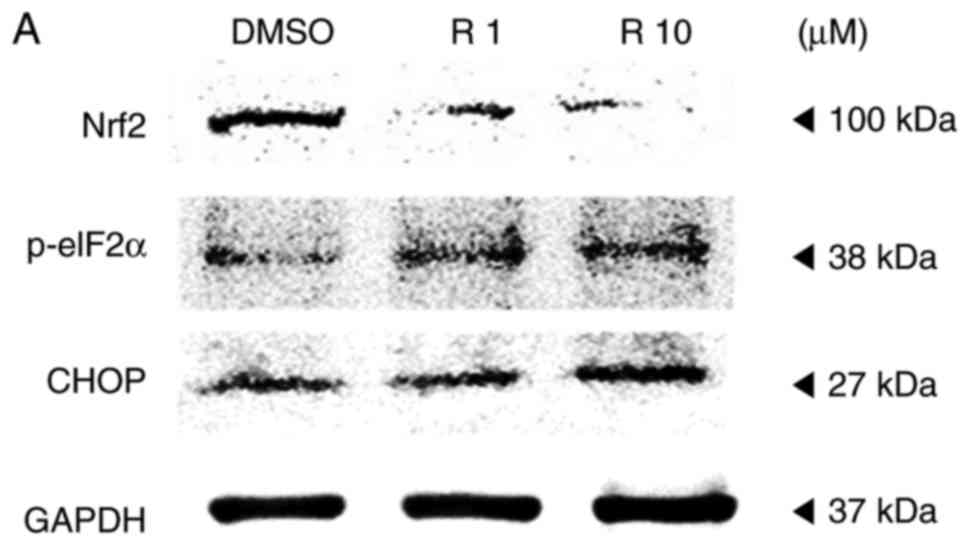
manner. Western blot analysis was also conducted to verify the
altered expression rates of the ROS and ER stress-associated
proteins Nrf2, p-eIF2α and CHOP as a result of resveratrol
treatment, compared with the control. The expression of the
anti-oxidant factor Nrf2 was significantly decreased in a
dose-dependent manner in resveratrol-treated cells compared with
that in the control (Fig. 4),
which likely resulted from the increase in ROS production and ER
stress. However, treatment with 10 µM resveratrol
significantly increased the expression levels of p-eIF2α and CHOP,
which are ER stress-associated apoptosis markers, as demonstrated
in Fig. 4. These results
indicated that resveratrol may induce ROS generation and ER stress
to hinder the anti-oxidative effects of resveratrol and enhance the
apoptosis of melanoma cells.
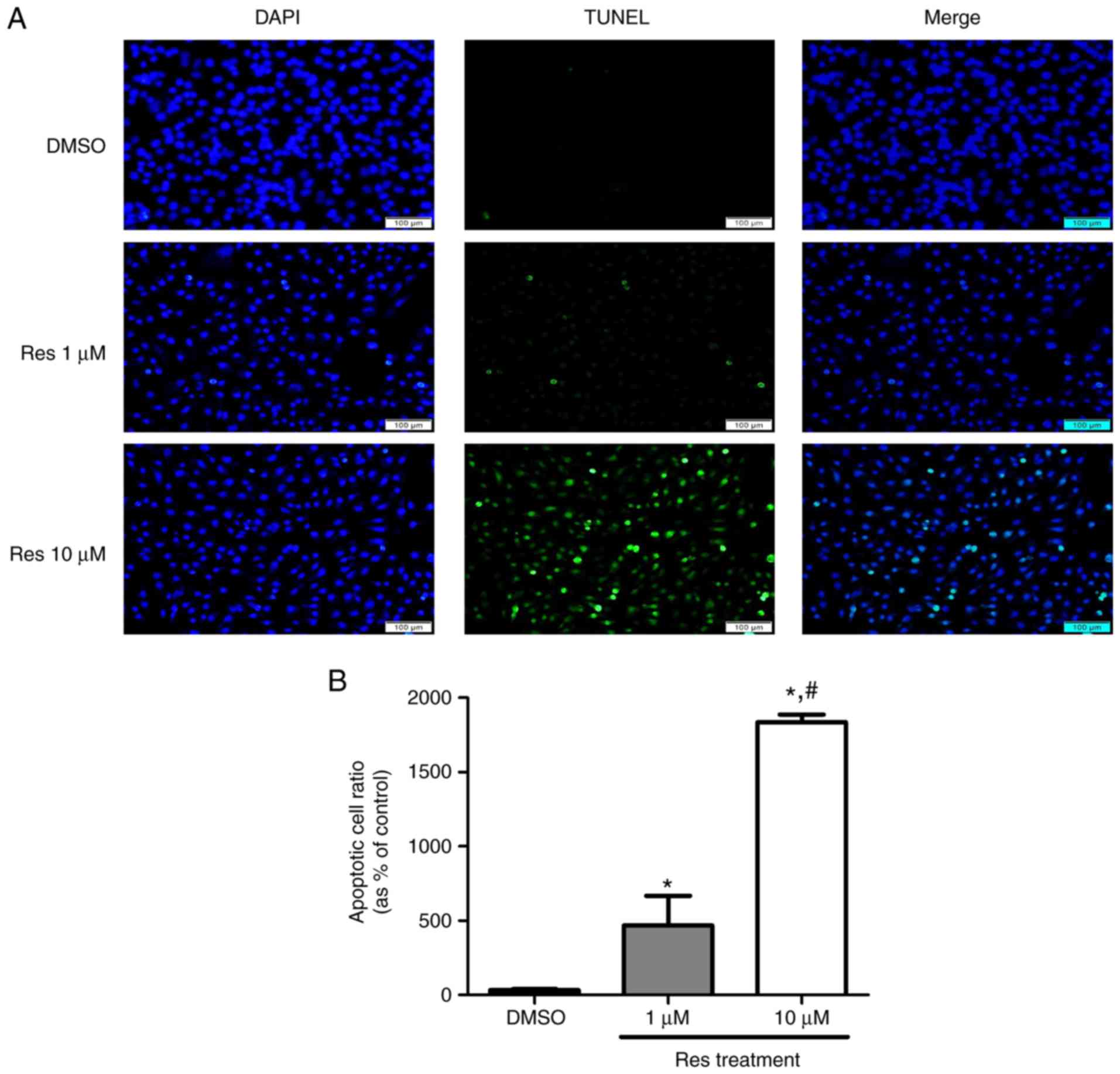
Resveratrol-induced apoptosis on melanoma
cell line
Melanoma cells that underwent apoptosis due to
resveratrol treatment were detected using a DeadEnd™ fluorometric
TUNEL assay kit, and the protein levels were verified using a
western blot assay. A375SM cells were treated with the control or
resveratrol (1 and 10 µM) for 48 h. Treatment with
resveratrol displayed increased cell death compared with that
observed in the control, as shown in Fig. 5. In addition, the higher
concentration of resveratrol was correlated with a marked increase
in melanoma cell death (Fig. 5)
compared with 1 µM resveratrol. The apoptosis-associated
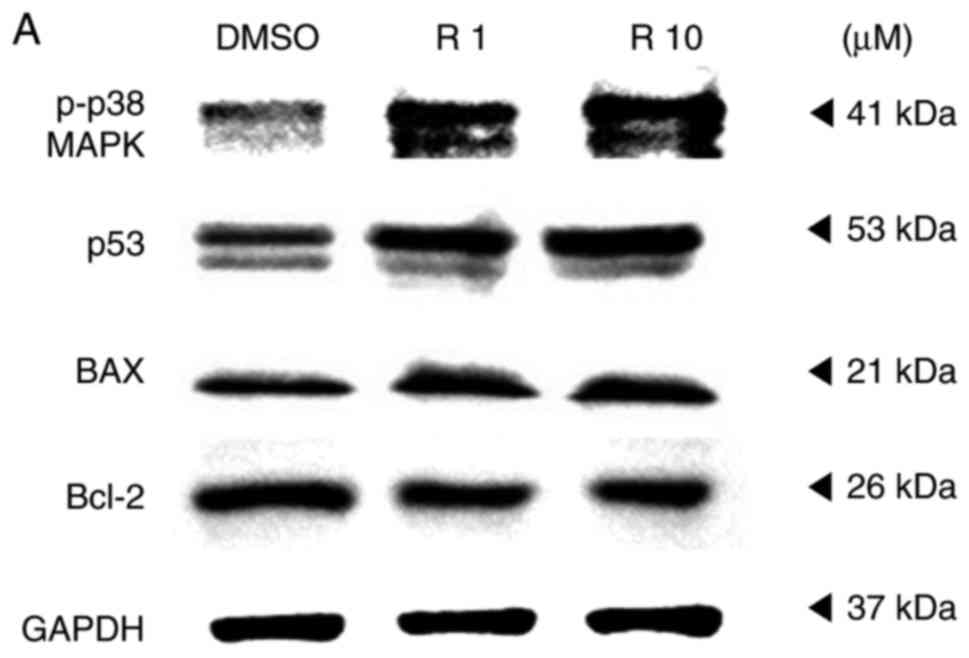
proteins p38, p53, Bax and Bcl-2 were also observed using a western
blot assay to support the TUNEL assay findings. The expression
levels of p53, a tumor suppressor and mediator of programmed cell
death, and of p-p38, which is upstream of and targets p53, were
increased in resveratrol-treated melanoma cells, as shown in
Fig. 6. When cells are under
stress, p53 interacts with the anti-apoptotic members Bcl-2 and
Bcl-xL and counterbalances their expression. This counterbalance
activates apoptosis through the induction of mitochondrial outer
membrane permeabilization factors, such as Bax, Bak and BH3-only
(21). In the present study, the
expression of the anti-apoptotic protein Bcl-2 was suppressed,
likely due to the activation of p53. By contrast, the expression of
the pro-apoptotic protein Bax was increased in a dose-dependent
manner (Fig. 6). These results
imply that the apoptosis that occurred in melanoma cells treated
with resveratrol was influenced by the phosphorylation of p38 and
activation of p53, which inhibited the expression of anti-apoptotic
factors and activated pro-apoptotic factors.
Discussion
Melanoma diagnosed at an early stage can be
effectively treated with surgical removal and radiation therapy
(4,22). Although a vast range of treatment
strategies are available for melanoma, ranging from chemotherapy to
molecular-targeted therapies, treatment resistance is unavoidable
in melanoma patients. Therefore, identifying novel methods for the
treatment or prevention of melanoma has become a focus point of
cancer research.
According to a review published in 2011, resveratrol
exhibited a chemopreventive role in various diseases, including
cancer (23). Therefore, the
current study examined how resveratrol, a compound found in various
types of food, influences melanoma at the cellular and protein
levels. It was observed that resveratrol inhibited melanoma cell
proliferation at a concentration of >10−2 µM.
The concentrations 1 and 10 µM were selected to further
examine the effect of resveratrol in a dose-dependent manner.
Resveratrol was demonstrated to activate the expression of p21 and
p27, which promoted cell cycle arrest in melanoma cells.
Furthermore, the effects of resveratrol on cyclin B and cyclin E
were assessed, both of which are highly expressed in melanoma that
exhibits metastatic tendencies (24). Resveratrol suppressed the
expression of cyclin B, but did not have a significant effect on
the expression of cyclin E.
In the current study, the generation of cellular ROS
in melanoma cells was observed using a DCF-DA assay. The density of
DCF-positive melanoma cells was increased in a dose-dependent
manner, which implies that A375SM cells cultured with resveratrol
generated a higher amount of ROS. Resveratrol increased the
cytosolic ROS generation by >5-fold (1 µM resveratrol)
and 15-fold (10 µM resveratrol) as compared with that in the
control. Although the glutathione/glutathione disulfide (GSH/GSSG)
ratio was not measured, it can easily be assumed that the increased
ROS generation by resveratrol reduced the GSH/GSSG ratio compared
with the control, and placed the melanoma cells under oxidative
stress.
Although resveratrol is known to have an antioxidant
effect, recent studies have demonstrated that resveratrol exhibits
both antioxidant and prooxidant properties, depending on its
concentration and the cell type (25,26). It has also been proposed that the
pro-oxidant action may be an important action mechanism of the
anticancer and apoptosis-inducing properties of resveratrol
(27). Correspondingly, the
present results observed that resveratrol displayed
apoptosis-inducing properties in the melanoma cell line, A375SM, by
acting as a pro-oxidant that promotes ROS formation.
Nrf2 is a mediator of cellular resistance to
oxidants and hyperactivation of the Nrf2 pathway establishes a
favorable environment for normal and malignant cells (28). In addition, Nrf2 has been
considered to protect the human body against cancer (29–31). However, certain tumor types
persistently express Nrf2, which allows the cancer to proliferate
and gain resistance to oxidants and anticancer drugs (32). Nrf2 is notable for its role as a
regulator of cellular defense mechanisms against oxidative stress;
however, recent studies revealed the dual nature of Nrf2 (29,33,34). Though it has a protective role
against cancer, constant expression of Nrf2 gave rise to strong
resistance of cancer to chemotherapeutic drugs (28). Since resveratrol-treated melanoma
cells demonstrated a decrease in Nrf2 expression in the present
study, it is hypothesized that chemotherapy-resistant melanoma
cells may regain sensitivity to chemotherapy with exposure to
resveratrol. Considering that Nrf2 is an anti-oxidant factor, its
reduced protein expression by resveratrol also supports the
occurrence of resveratrol-induced oxidative stress. In the context
of an existing melanoma, constant expression of Nrf2 gives rise to
resistance to chemotherapeutic drugs. Therefore, the decrease in
Nrf2 expression caused by resveratrol may prevent the development
of such resistance and thereby increase the sensitivity of melanoma
cells to chemotherapy. Similar to Nrf2, which has the dual effects
of protection against cancer and enhancement of chemoresistance,
resveratrol also has a dual nature, namely anti-oxidant and
pro-oxidant activities. Thereby, it is concluded that resveratrol
displayed an anti-melanoma effect through its pro-oxidant activity
and reduction of the chemoresistance assigned by Nrf2.
In addition to increasing intracellular ROS levels,
resveratrol also enhances ER stress (12). The UPR is modulated by three ER
membrane-associated proteins: PKR-like ER kinase (PERK),
inositol-requiring enzyme 1, and activating transcription factor-6.
Phosphorylation of eIF2α by the PERK kinase modulates its
translational response and promotes apoptotic cell death (35–37). In the present study, the
expression levels of p-eIF2α and CHOP were significantly increased
in A375SM cells treated with a high concentration of resveratrol,
thereby promoting programmed cell death.
Subsequent to confirming the impact of resveratrol
on the generation of ROS and ER stress, the present study further
determined that resveratrol induced the ROS-mediated p38-p53
pathway in melanoma cells and promoted apoptosis mediated by the
ROS-p38-p53 and ER stress pathway (38). The density of TUNEL-positive cells
was increased in a dose-dependent manner. It was further
demonstrated that resveratrol induced the mitochondrial apoptotic
pathway in melanoma cells through the ROS-p38-p53 pathway by
increasing the protein expression of p-p38 MAPK, and through the
p53 and ER stress pathway by increasing the protein expression of
p-eIF2α and CHOP. The enhanced ROS-p38-p53 and ER stress pathways
promoted apoptosis by downregulating Bcl-2 expression and
upregulating Bax expression (13).
In the present study, it was revealed that
resveratrol induced oxidative stress in melanoma cancer cells by
promoting ROS formation. The ROS-mediated oxidative stress induced
by resveratrol led to ER stress and mitochondrial dysfunction, both
of which induced the apoptosis of A375SM melanoma cells via
different pathways. Although it was not established which is the
main cause of the resveratrol-induced melanoma cell toxicity and
the resultant cell death, ER stress and mitochondrial dysfunction
can be considered as mechanisms of resveratrol in terms of the
induction of apoptosis in melanoma cells.
Taken together, these results revealed that
resveratrol generated intracellular ROS and ER stress in melanoma
cells. As canonical steps, eIF2α was phosphorylated, activating
CHOP, which induces an ER stress-mediated apoptosis pathway.
Furthermore, elevated ROS production led to the phosphorylation of
p38 MAPK and activation of p53. Activated p53 promoted cell cycle
arrest by activating p21 and p27, enhancing cell cycle arrest in
the G2/M phase by suppressing the expression of cyclin
B. The activated p53 and CHOP then accelerated apoptosis by
impeding Bcl-2 expression, upregulating the expression of Bax, as
shown in Fig. 7. In addition, the
decreased expression of Nrf2 caused by resveratrol should be
studied in order to determine whether it decreases melanoma
resistance to chemotherapeutic agents.
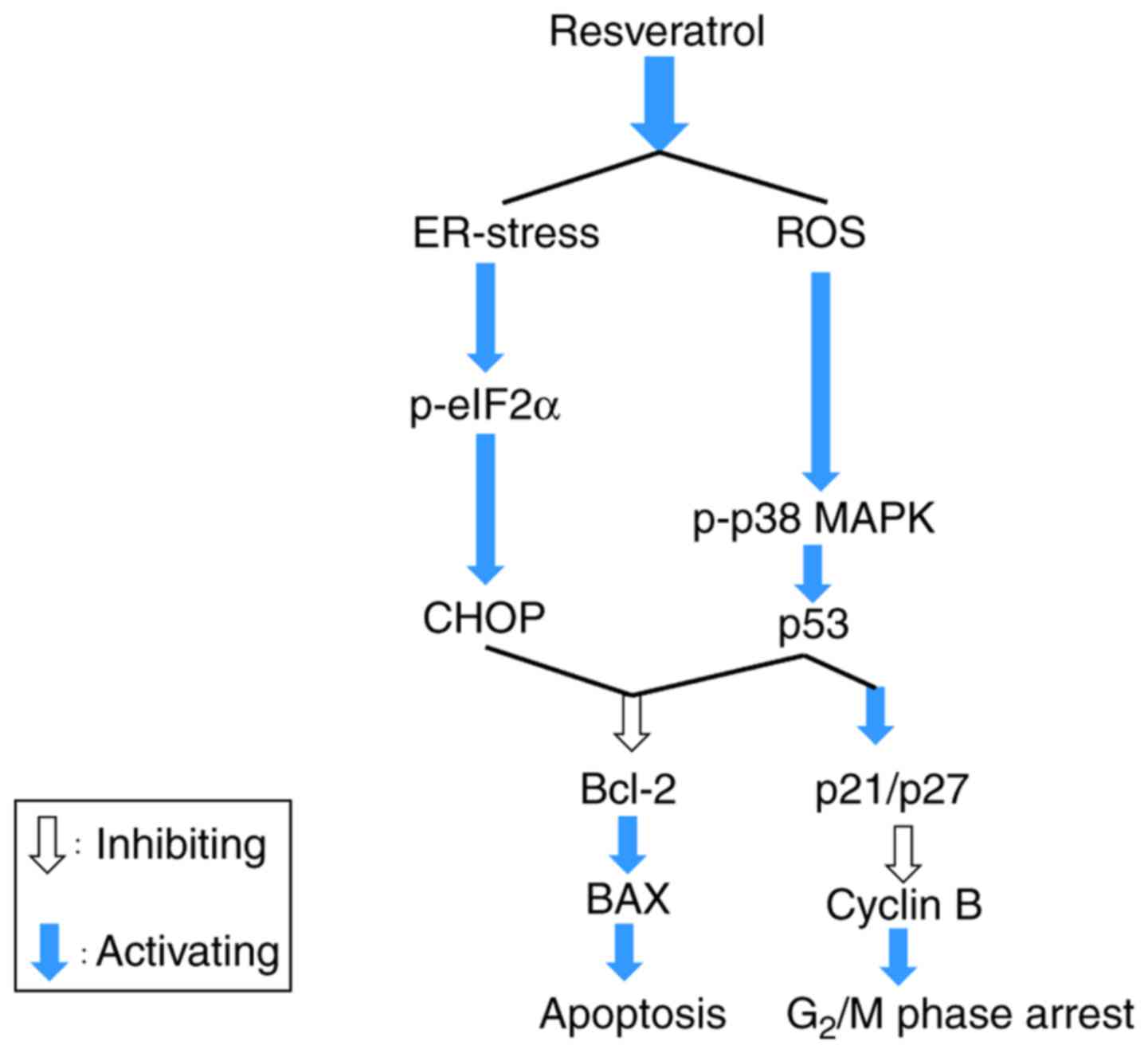 | Figure 7Summary of the role of resveratrol in
activating apoptosis and cell cycle arrest through enhancing ER
stress and ROS generation. Resveratrol impeded the growth of A375SM
cells via stimulating cell cycle arrest and apoptosis by elevating
the levels of p38, p53, and Bax, and decreasing the level of Bcl-2.
Resveratrol increased the intracellular ROS production and ER
stress-mediated apoptosis (p-eIF2α and CHOP) through deactivation
of the anti-oxidant factor Nrf2. Therefore, resveratrol accelerated
cell cycle arrest and apoptosis by boosting the ROS production and
ER stress. ROS, reactive oxygen species; ER, endoplasmic reticulum;
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; p-eIF2α,
phosphorylated eukaryotic initiation factor 2α; Nrf2, nuclear
factor erythroid 2-related factor 2; CHOP, C/EBP homologous
protein; MAPK, mitogen-activated protein kinase. |
Although these outcomes revealed novel insight that
may be helpful for melanoma treatment, there was a degree of
uncertainty in the present study. For instance, ROS involves a
diverse range of cell outcomes, including pyroptosis and apoptosis,
which are associated with DNA fragmentation that exhibits positive
results in a TUNEL assay. However, p53-mediated cell death usually
occurs by apoptosis rather than pyroptosis, as it was observed in
the current study. Although pyroptosis was not investigated herein,
the lack of a mechanism by which p53-mediated cell death leads to
pyroptosis results in the conclusion that resveratrol induced
A375SM melanoma cell death via ROS formation and p53-mediated
apoptosis. Increased cytosolic ROS generation by resveratrol was
also identified through the DCF-DA assay; however, this assay did
not demonstrate the exact site of ROS formation in the cell and the
types of ROS. For more specific representation of the role of
resveratrol in the melanoma microenvironment and its association
with ROS (the main cause of resveratrol-induced melanoma cell
toxicity and death), follow-up experiments examining pyroptosis,
associations with calcium ions and the ratio of GSH/GSSG will be
required, along with suitable in viv experiments, prior to
the application of resveratrol in clinical studies.
In conclusion, the present study demonstrated that
resveratrol impeded the viability of melanoma cells by activating
the expression of both p21 and p27, which suppressed the expression
of cyclin B and promoted cell cycle arrest. Furthermore,
resveratrol increased the generation of cellular ROS and
simultaneously induced the ER stress pathway in melanoma cells.
These results reveal a potential use for resveratrol in the
treatment for melanoma.
Acknowledgments
Not applicable.
Funding
This study was supported by the Global Research and
Development Center (GRDC) Program through the National Research
Foundation of Korea (NRF), funded by the Ministry of Education,
Science and Technology (MEST) of the Republic of Korea
(2017K1A4A3014959). In addition, this study was also supported by a
NRF grant funded by the MEST of the Republic of Korea
(2017R1D1A1A09000663).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JH, SK and KH made substantial contributions to
conception and design, and/or acquisition of data, and/or analysis
and interpretation of data. JH, JK and KC participated in drafting
the article or revising it critically for important intellectual
content. All authors gave final approval of the version to be
submitted and any revised version.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bandarchi B, Ma L, Navab R, Seth A and
Rasty G: From melanocyte to metastatic malignant melanoma. Dermatol
Res Pract. 2010:2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vogelstein B and Kinzler KW: The genetic
evolution of melanoma. N Engl J Med. 374:9962016.PubMed/NCBI
|
|
3
|
Wellbrock C and Arozarena I: The
complexity of the ERK/MAP-kinase pathway and the treatment of
melanoma skin cancer. Front Cell Dev Biol. 4:332016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heo JR, Kim NH, Cho J and Choi KC: Current
treatments for advanced melanoma and introduction of a promising
novel gene therapy for melanoma (Review). Oncol Rep. 36:1779–1786.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kraft TE, Parisotto D, Schempp C and
Efferth T: Fighting cancer with red wine? Molecular mechanisms of
resveratrol. Crit Rev Food Sci Nutr. 49:782–799. 2009. View Article : Google Scholar
|
|
6
|
Lee GA, Hwang KA and Choi KC: Roles of
dietary phytoestrogens on the regulation of epithelial-mesenchymal
transition in diverse cancer metastasis. Toxins (Basel). 8. pp.
E1622016, View Article : Google Scholar
|
|
7
|
Marcsek ZL, Kocsis Z, Szende B and Tompa
A: Effect of formaldehyde and resveratrol on the viability of Vero,
HepG2 and MCF-7 cells. Cell Biol Int. 31:1214–1219. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reagan-Shaw S, Mukhtar H and Ahmad N:
Resveratrol imparts photoprotection of normal cells and enhances
the efficacy of radiation therapy in cancer cells. Photochem
Photobiol. 84:415–421. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baarine M, Thandapilly SJ, Louis XL, Mazué
F, Yu L, Delmas D, Netticadan T, Lizard G and Latruffe N:
Pro-apoptotic versus anti-apoptotic properties of dietary
resveratrol on tumoral and normal cardiac cells. Genes Nutr.
6:161–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Athar M, Back JH, Kopelovich L, Bickers DR
and Kim AL: Multiple molecular targets of resveratrol:
Anti-carcinogenic mechanisms. Arch Biochem Biophys. 486:95–102.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rojas C, Pan-Castillo B, Valls C, Pujadas
G, Garcia-Vallve S, Arola L and Mulero M: Resveratrol enhances
palmitate-induced ER stress and apoptosis in cancer cells. PLoS
One. 9:e1139292014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marciniak SJ, Yun CY, Oyadomari S, Novoa
I, Zhang Y, Jungreis R, Nagata K, Harding HP and Ron D: CHOP
induces death by promoting protein synthesis and oxidation in the
stressed endoplasmic reticulum. Genes Dev. 18:3066–3077. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rozpedek W, Pytel D, Mucha B, Leszczynska
H, Diehl JA and Majsterek I: The role of the PERK/eIF2α/ATF4/CHOP
signaling pathway in tumor progression during endoplasmic reticulum
stress. Curr Mol Med. 16:533–544. 2016. View Article : Google Scholar
|
|
15
|
Yen YP, Tsai KS, Chen YW, Huang CF, Yang
RS and Liu SH: Arsenic induces apoptosis in myoblasts through a
reactive oxygen species-induced endoplasmic reticulum stress and
mitochondrial dysfunction pathway. Arch Toxicol. 86:923–933. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Birben E, Sahiner UM, Sackesen C, Erzurum
S and Kalayci O: Oxidative stress and antioxidant defense. World
Allergy Organ J. 5:9–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Poljsak B, Šuput D and Milisav I:
Achieving the balance between ROS and antioxidants: When to use the
synthetic antioxidants. Oxid Med Cell Longev. 2013:9567922013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sies H: Oxidative stress: From basic
research to clinical application. Am J Med. 91:31S–38S. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Y, Wang K, Zhen S, Wang R and Luo W:
Carfilzomib induces G2/M cell cycle arrest in human endometrial
cancer cells via upregulation of p21(Waf1/Cip1) and p27Kip1. Taiwan
J Obstet Gynecol. 55:847–851. 2016. View Article : Google Scholar
|
|
20
|
Tyner AL: A new year, a new role for p21.
Cell Cycle. 8:1832009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vaseva AV and Moll UM: The mitochondrial
p53 pathway. Biochim Biophys Acta. 1787:414–420. 2009. View Article : Google Scholar
|
|
22
|
Maverakis E, Cornelius LA, Bowen GM, Phan
T, Patel FB, Fitzmaurice S, He Y, Burrall B, Duong C, Kloxin AM, et
al: Metastatic melanoma-a review of current and future treatment
options. Acta Derm Venereol. 95:516–524. 2015. View Article : Google Scholar
|
|
23
|
Shukla Y and Singh R: Resveratrol and
cellular mechanisms of cancer prevention. Ann NY Acad Sci.
1215:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Georgieva J, Sinha P and Schadendorf D:
Expression of cyclins and cyclin dependent kinases in human benign
and malignant melanocytic lesions. J Clin Pathol. 54:229–235. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heiss EH, Schilder YD and Dirsch VM:
Chronic treatment with resveratrol induces redox stress- and ataxia
telangiectasia-mutated (ATM)-dependent senescence in p53-positive
cancer cells. J Biol Chem. 282:26759–26766. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de la Lastra CA and Villegas I:
Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and
clinical implications. Biochem Soc Trans. 35:1156–1160. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh A, Sati S and Mishra R: Resveratrol:
Antioxidant-pro-oxidant. In J Tech Res Sci. 1:106–112. 2016.
|
|
28
|
Wang XJ, Sun Z, Villeneuve NF, Zhang S,
Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, et al: Nrf2
enhances resistance of cancer cells to chemotherapeutic drugs, the
dark side of Nrf2. Carcinogenesis. 29:1235–1243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Menegon S, Columbano A and Giordano S: The
dual roles of NRF2 in cancer. Trends Mol Med. 22:578–593. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hayes JD, McMahon M, Chowdhry S and
Dinkova-Kostova AT: Cancer chemoprevention mechanisms mediated
through the Keap1-Nrf2 pathway. Antioxid Redox Signal.
13:1713–1748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rachakonda G, Sekhar KR, Jowhar D, Samson
PC, Wikswo JP, Beauchamp RD, Datta PK and Freeman ML: Increased
cell migration and plasticity in Nrf2-deficient cancer cell lines.
Oncogene. 29:3703–3714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moon EJ and Giaccia A: Dual roles of NRF2
in tumor prevention and progression: Possible implications in
cancer treatment. Free Radic Biol Med. 79:292–299. 2015. View Article : Google Scholar
|
|
34
|
Lau A, Villeneuve NF, Sun Z, Wong PK and
Zhang DD: Dual roles of Nrf2 in cancer. Pharmacol Res. 58:262–270.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sood R, Porter AC, Ma K, Quilliam LA and
Wek RC: Pancreatic eukaryotic initiation factor-2alpha kinase (PEK)
homologues in humans, Drosophila melanogaster and Caenorhabditis
elegans that mediate translational control in response to
endoplasmic reticulum stress. Biochem J. 346:281–293. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: Cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi Y, Vattem KM, Sood R, An J, Liang J,
Stramm L and Wek RC: Identification and characterization of
pancreatic eukaryotic initiation factor 2 alpha-subunit kinase,
PEK, involved in translational control. Mol Cell Biol.
18:7499–7509. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu B, Cheng Y, Zhang B, Bian HJ and Bao
JK: Polygonatum cyrtonem lectin induces apoptosis and autophagy in
human melanoma A375 cells through a mitochondria-mediated
ROS-p38-p53 pathway. Cancer Lett. 275:54–60. 2009. View Article : Google Scholar
|