Introduction
Breast cancer is a common cause of mortality among
types of cancer worldwide, and the majority of cases of breast
cancer-associated mortality (90%) are caused by invasion and
metastasis (1,2). Increasing data demonstrates the
importance of the epithelial-mesenchymal transition (EMT) in breast
cancer invasion and metastasis (1,3,4).
EMT is a complex process in which epithelial cancer cells gain a
mesenchymal phenotype and lose their epithelial features. In this
process, the epithelial cells gain increased migratory and invasive
properties to become mesenchymal cells through the EMT. In breast
cancer, a cascade of EMT processes can lead to cell detachment,
migration, invasion and colonization at secondary sites (1,3,4).
Cancer stem-like cells (CSCs) are a unique population of cancer
cells, which are associated with tumor initiation, progression,
migration, invasion, resistance to chemotherapy and radiation
therapy, and relapse (5). It has
been reported that EMT results in the increase of CSC-like
properties in various types of cancer, including breast cancer,
whereas inhibiting the process of EMT may repress CSC formation
(6–8). Therefore, targeted the suppression
of EMT and CSCs is emerging as an attractive method for the
curative treatment of several type of cancer.
The Notch signaling pathway is a highly conserved
cell signaling pathway, which is important in cell proliferation,
survival, apoptosis and differentiation, and in the modulation of
EMT and CSC maintenance (9). The
abnormal activation of Notch signaling has been found to be
associated with tumor development and progression in breast cancer
(10,11). In mammals, there are four
different Notch receptors and five known ligands, which have been
implicated in tumorigenesis (10). Increased expression levels of
Notch receptors and their ligands in breast cancer tissues have
been observed compared with levels in normal control tissues
(11). Notch receptors are
considered to be breast oncogenes partly due to the fact that the
overexpression of Notch1 or Notch 4 can lead to the formation of
spontaneous murine mammary tumors in vivo (12). Furthermore, the enhanced
expression of Notch1 and/or its ligand in human mammary tumors has
been correlated with poor patient survival rates (13), and Notch has been demonstrated to
be important for the survival of CSCs (14). Therefore, Notch may be a potent
therapeutic target in breast cancer. Crosstalk between the
phosphoinositide 3-kinase (PI3K)/Akt pathway and Notch family
members (Notch1 and 3) has been shown to be critical in cancer
progression (15). The
PI3K/Akt/mammalian target of rapamycin (mTOR) pathway has a crucial
function in cell survival, proliferation, migration, invasion and
apoptosis, and it commonly activated in mammary tumors. Enhanced
PI3K/Akt activity has been associated with poor patient prognosis,
and it is considered a key signaling pathway leading to resistance
to standard therapies in breast cancer (16,17). It has been noted that the PI3K/Akt
pathway can promote CSC activity (18), therefore, targeting this pathway
may offer a potential strategy for overcoming resistance to
conventional breast cancer therapies (19). Combined therapy, including the
combination of endocrine and PI3K/Akt pathway inhibitors, has shown
clinical benefit, and novel combination strategies are now being
examined clinically for their efficacy in the treatment of patients
with breast cancer.
Enhancer of zeste homolog 2 (EZH2), a histone-lysine
N-methyltransferase enzyme, is a polycomb group protein involved in
the regulation of cell proliferation, stem cell maintenance,
differentiation and neoplastic cell transformation (20). The overexpression of EZH2,
identified in aggressive and metastatic breast cancer, has been
correlated with poor prognosis, and can predict patient survival
rate as an independent biomarker (20,21). Therefore, an increased level of
EZH2 is considered to indicate propensity for metastasis and poor
outcome in patients with mammary tumors (21). EZH2 is of interest as a
therapeutic target in triple negative breast cancer (TNBC) due to
its overexpression in this form of the malignancy (22). EZH2-targeted therapy may be a
novel and promising treatment strategy for patients with breast
cancer.
Quercetin-3-methyl ether is a natural compound found
in various plants, including Allagopappus viscosissimus (23), Opuntia ficus-indica var. saboten
(24), Lychnophora staavioides
(25), Rhamnus species (26), Semecarpus anacardium (27) and Larrea divaricate (28). Previous studies have demonstrated
that querectin-3-methyl ether acts as an anticarcinogenic flavonoid
in a number of human cancer cell lines, including HL-60, A431,
SK-OV-3, HeLa, and HOS cells (23), and that this compound induces
apoptosis in lymphoma cells through nitrosative stress (28). Our previous study confirmed that
quercetin-3-methyl ether had a potent inhibitory effect on skin
carcinogenesis (29) and
significantly inhibited cell growth in human epidermal growth
factor receptor 2 (HER2)-positive SK-BR-3 lapatinib-sensitive and
-resistant breast cancer cells (30). However, whether this compound can
influence TNBC and hormone-sensitive breast cancer cells remains to
be fully elucidated. The present study showed that
quercetin-3-methyl ether significantly inhibited cell proliferation
through the induction of apoptosis and cell cycle arrest at the
G2-M phase, and suppresses cell invasion and migration
in human TNBC and hormone-sensitive breast cancer cells, including
the triple negative MDAMB-231 cell line and the estrogen receptor
(ER)-positive/progesterone receptor (PR)-positive/HER2-negative
MCF-7 and T47D cell lines. Quercetin-3-methyl ether was shown to
exert these effects by inhibiting the EMT process and CSC formation
through repression of the Notch1 and PI3K/Akt signaling
pathways.
Materials and methods
Reagents
Quercetin-3-methyl ether, human insulin-like growth
factor 1 (IGF-1) and DAPT were purchased from Sigma-Aldrich; Merck
Millipore (Darmstadt, Germany). Dulbecco's modified Eagle's medium
(DMEM) was obtained from Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Fetal bovine serum (FBS) was obtained from
Gibco; Thermo Fisher Scientific, Inc. Antibodies against Cyclin B1
(cat. no. 4138), Cyclin-dependent kinase 1 (CDK1; cat. no. 2546),
B-cell lymphoma (Bcl)-2 (cat. no. 2870), Bcl-extra large (Bcl-xl;
cat. no. 2764), PI3K (cat. no. 3358), phosphorylated PI3K (cat. no.
3821), total Akt (cat. no. 4691S), phosphorylated Akt (Ser473; cat.
no. 4060S), mTOR (cat. no. 2983), phosphorylated mTOR (cat. no.
5536), phosphorylated Glycogen synthase kinase β (GSK3β; cat. no.
5558), Notch1 (cat. no. 4380), EZH2 (cat. no. 5246S),
tri-methyl-histone H3 (Lys27; cat. no. 9733), E-cadherin (cat. no.
3195), Vimentin (cat. no. 5741), Matrix metalloproteinase 2 (MMP2;
cat. no. 4022), SRY-box 2 (SOX2; cat. no. 3579), Nanog (cat. no.
4903) and GAPDH (cat. no. 5174) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA).
Cell culture
Human breast cancer cells (MCF-7, T47D and
MDA-MB-231) and human breast non-tumorigenic MCF-10A epithelial
cells were obtained from the Cell Bank Type Culture Collection of
the Chinese Academy of Sciences (Shanghai, China). MCF-7 and
MDA-MB-231 cells originate from human invasive adenocarcinoma, and
T47D cells originate from human ductal carcinoma. MCF-7 and T47D
cells are known to express estrogen and progesterone receptors,
belong to the luminal A group, and have marginal metastatic
potential. MDA-MB-231 cells belong to the triple-negative
basal-like group and are highly metastatic (31). The MCF-7, T47D and MDA-MB-231
cells were cultured at 37°C in a 5% CO2 incubator in
DMEM containing 10% FBS and 1% penicillin/streptomycin. The MCF-10A
cells were cultured in high-glucose DMEM medium with 10% FBS and 1%
penicillin/streptomycin at 37°C in a 5% CO2
incubator.
Cell growth assay
To examine the effect of quercetin-3-methyl ether on
breast cancer cell growth, the cells were seeded (3×103
cells/well) in 96-well plates with 10% FBS/DMEM and cultured at
37°C in a 5% CO2 incubator. After 24 h, the cells were
replenished with fresh medium and treated with quercetin-3-methyl
ether (0–20 µM) and/or DAPT (10 µM). Following
culturing for the indicated times (0–72 h), 10 µl of CCK-8
solution (Dojindo Molecular Technologies, Inc., Kumamoto, Japan)
was added into each well and the wells were incubated for 1 h at
37°C in a 5% CO2 incubator. Finally, the absorbance was
measured at 450 nm.
Cell cycle assay
The breast cancer cells were seeded
(2×105 cells/well) into six-well plates with 10%
FBS/DMEM and were incubated overnight at 37°C in a 5%
CO2 incubator. The cells were then starved in serum-free
medium for 24 h, treated with quercetin-3-methyl ether (0–20
µM) for 48 h, stained with propidium iodide and subjected to
cell cycle analysis according to a previously described method
(29,30).
Western blot analysis
The cancer cells (1×106) were cultured
overnight in a 10-cm dish, and starved in serum-free medium for 24
h. The starved cells were then treated with quercetin-3-methyl
ether (0–20 µM) or DAPT (10 µM) for 48 h in culture
medium containing 10% FBS. For the induction of phosphorlated PI3K
and Akt, the cells were pretreated with quercetin-3-methyl ether
for 2 h, and then exposed to 100 ng/ml IGF-1 for 20 min. The
harvested cells were lysed with lysis buffer (50 mM Tris-Cl pH 7.4,
150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) on ice
for 30 min. Following centrifugation at 12,000 × g for 15 min at
4°C, the supernatant was collected for further analysis. The total
protein concentration of the cell lysates was determined with a
dye-binding protein assay kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) according to the manufacturer's protocol. The
whole cell lysate proteins were subjected to western blot analysis
according to previous protocols (29,30). Following denaturation, the
proteins (50–100 µg the amount of total proteins varied
depending on different targeted proteins) were separated via 8–15%
SDS-PAGE and subsequently transferred to polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with blocking buffer (5% non fat milk) for 1 h at room
temperature and then incubated with dilutions of primary antibodies
(1:1,000) in blocking buffer overnight at 4°C Subsequently, the
membranes were incubated with secondary goat anti-rabbit or
goat-anti-mouse antibodies conjugated with horseradish peroxidase
(1:2,000) in blocking buffer for 1 h at room temperature. Protein
bands were visulized using an ECL detection kit (Bio-Rad
Laboratories, Inc). Quantity One 1-D Anylisis software (Bio-Rad
Laboratories, Inc.) was used for scanning and densitometric
analysis.
Apoptosis assay
The breast cancer cells were seeded
(2×105 cells/well) into six-well plates with 10%
FBS/DMEM and incubated overnight at 37°C in a 5% CO2
incubator. The cells were then starved in serum-free medium for 24
h, followed by treatment with quercetin-3-methyl ether (0–20
µM) for 48 h. Annexin V/propidium iodide staining was used
to analyze apoptotic cells according to the manufacturer's protocol
(AD10-10; Dojindo Molecular Technologies, Inc.). The fluorescence
intensity of the Annexin V/propidium iodide staining was detected
by flow cytometry.
Scratch wound-healing assay
A total of 4×105 cells were seeded into
12-well plates and cultured for 24 h, when an artificial wound was
made with a P20 pipette tip in each well. Following washing of the
cells three times with culture medium, fresh medium supplemented
with querectin-3-methyl ether (0–20 µM) was added. Images
were captured under an inverted microscope equipped with a camera
at different time points (24–72 h). The wound gap distance was
quantitatively determined with Image J software (National
Institutes of Health, Bethesda, MD, USA) and closure was determined
as follows: Closure (%)=migrated cell surface area/total surface
area ×100.
Migration and invasion assays
Migration and invasion assays were performed using a
Transwell chamber (8-µm pore size; Corning Incorporated,
Corning, NY, USA). For the migration assays, 5×104 cells
in serum-free medium were placed into the upper chamber. For the
invasion assays, the same number of cells in serum-free medium were
placed into the upper chamber with an insert coated in Matrigel (BD
Biosciences, San Jose, CA, USA). The lower compartments were filled
with 10% FBS-medium, and querectin-3-methyl ether (0–20 µM)
was added to the upper and lower compartments at the same
concentration. Following culture for 48 h, the cells in the upper
chamber were removed, and the cells in the lower chamber were
stained with crystal violet and counted in four views for each well
using a light microscope (TS100, Nikon Corporation, Tokyo,
Japan).
Mammosphere formation assay
Single-cell suspensions were cultured at a density
of 2,000 cells per well in six-well Ultra-Low Attachment Plates
(Corning Incorporated) with DMEM/F-12 medium containing 2% B27, 20
ng/ml EGF, 20 ng/ml bFGF, 10 ng/ml heparin, 0.4% bovine serum
albumin (EMD Millipore) and 1% penicillin/streptomycin. After 3
days, the resulting spheroids were treated with querectin-3-methyl
ether (0–20 µM) for another 7 days, following which the
mammospheres were counted under a light microscope (TS100, Nikon
Corporation).
Colony formation assay
The cells were seeded at a density of 500 cells per
well in 60-mm dishes with 10% FBS/DMEM and were incubated overnight
at 37°C in a 5% CO2 incubator. The cells were then
supplied with fresh medium and treated with vehicle control (DMSO),
quercetin-3-methyl ether (10 µM) and/or DAPT (10 µM).
The medium, with or without drug, was replaced every 3 days.
Following 8 days of incubation, the colonies were fixed with 10%
formaldehyde for 15 min and stained with 1.0% crystal violet
solution for 20 min. Finally, images were captured and colonies
were counted.
Statistical analysis
As appropriate, data are expressed as the means ±
standard deviation, and significant differences were evaluated
using Student's t-test or one-way analysis of variance, with the
post hoc test following the latter being Tukey's test. GraphPad
Prism 7.0 software (GraphPad Software, Inc., La Jolla, CA, USA) was
used to process the data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Querectin-3-methyl ether inhibits cell
growth by inducing cell cycle arrest at the G2-M phase
and apoptosis in breast cancer cells
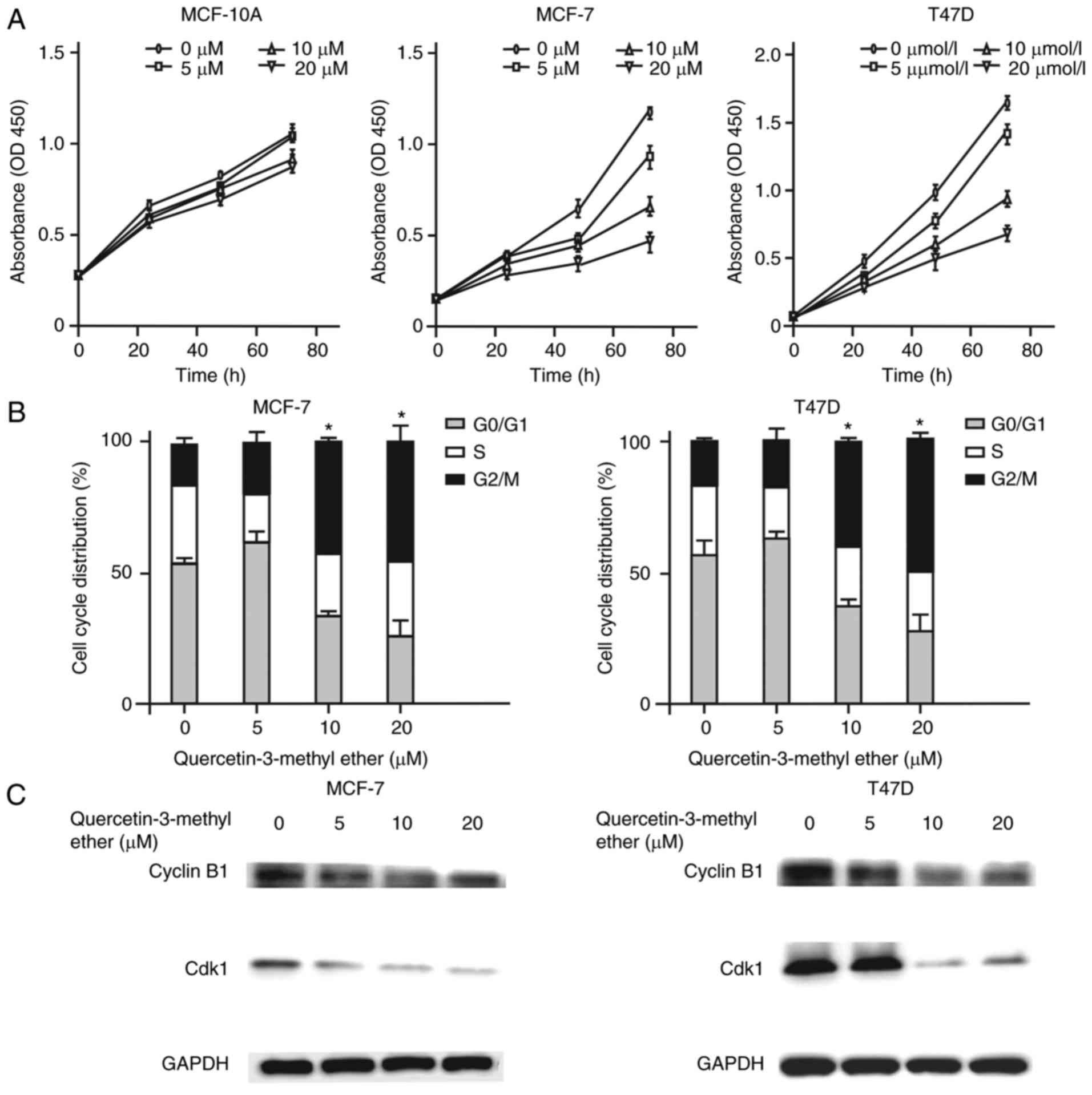
First, the effect of querectin-3-methyl ether on the
growth of MCF-10A, MCF-7 and T47D cells was examined. The results
revealed that treatment with this compound (0–20 µM) for 72
h had a marked inhibitory effect on the growth of breast cancer
cell lines, but had no significant effect on the growth of the
non-tumorigenic epithelial cells (MCF-10A; Fig. 1A). Subsequently, whether
quercetin-3-methyl ether inhibited cancer cell proliferation
through the regulation of cell cycle processes was determined. Flow
cytometric analysis of cell cycle distribution indicated that
treatment with 10 µM quercetin-3-methyl ether for 48 h
significantly increased the number of cancer cells at the
G2-M phase (P<0.05; Fig. 1B). As the G2-M phase is
regulated primarily by Cyclin B1/CDK1, the protein expression of
Cyclin B1 and CDK1 in quercetin-3-methyl ether-treated breast
cancer cells was subsequently determined. The results revealed a
marked decrease in the protein expression of these two genes in
MCF-7 and T47D cells following treatment with quercetin-3-methyl
ether for 48 h (Fig. 1C). To
examine whether quercetin-3-methyl ether inhibited cell growth via
the induction of cell death, the rate of apoptosis was detected in
the two hormone-sensitive breast cancer cell lines (MCF-7 and T47D)
treated with quercetin-3-methyl ether. The findings indicated that,
following treatment for 48 h, 10 µM quercetin-3-methyl ether
significantly induced apoptosis in the two cell lines (P<0.05;
Fig. 1D). The Bcl-2 family is
crucial in the regulation of apoptosis. Therefore, the effects of
quercetin-3-methyl ether on the protein expression of Bcl-2 family
members was also determined. The results demonstrated that the
levels of the key anti-apoptotic proteins Bcl-2 and Bcl-xl were
downregulated in the two cell lines following treatment for 48 h
(Fig. 1E). Collectively, these
data suggested that quercetin-3-methyl ether inhibited breast
cancer cell growth through inducing apoptosis and G2-M
phase cell cycle accumulation.
Querectin-3-methyl ether suppresses the
migration and invasion of breast cancer cells
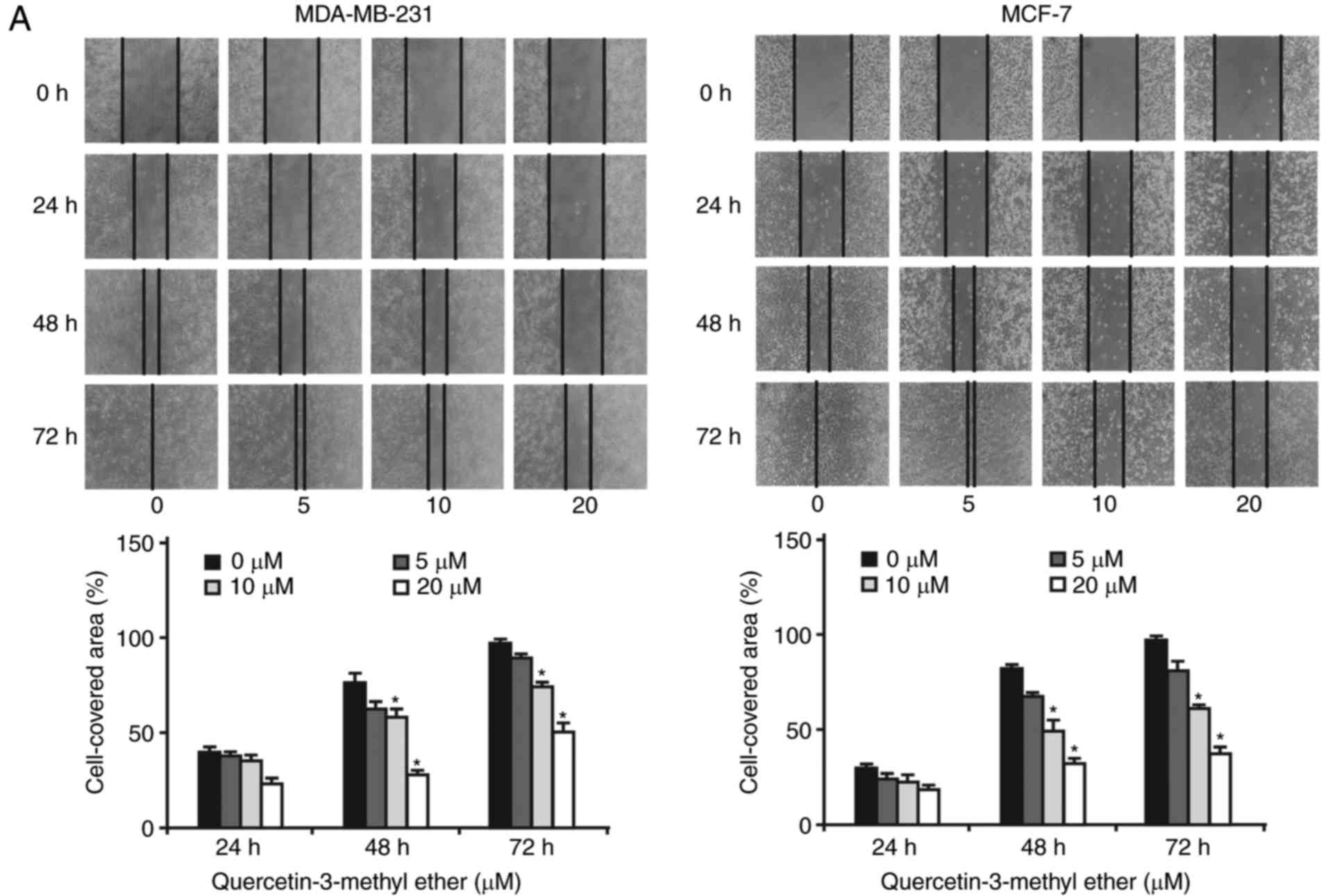
The effects of querectin-3-methyl ether on the
migration and invasion of breast cancer cells were subsequently
determined. A scratch wound-healing assay demonstrated that breast
cancer cells (MCF-7 and MDA-MB-231) had high mobile capacities;
whereas treatment with querectin-3-methyl ether (0-20 µM)
for 24-72 h significantly decreased the motility of breast cancer
cells into the wound gap in a dose- and time-dependent manner
(Fig. 2A). Accordingly, a
Transwell migration assay demonstrated that querectin-3-methyl
ether significantly inhibited breast cancer cell migration through
the Transwell insert membrane (Fig.
2B). Following treatment with 10 µM of
querectin-3-methyl ether for 48 h, the rates of cell migration in
the MDA-MB-231 and MCF-7 cells were reduced to 38 and 26%,
respectively (P<0.05; Fig.
2B). Similarly, the Matrigel invasion assay revealed that
querectin-3-methyl ether treatment of the two cell lines
dose-dependently reduced the number of invasive cells that migrated
through the Matrigel base membrane from the upper to the lower
chamber (P<0.05; Fig. 2C).
Notably, the cell invasion rates were reduced to 55% (MDA-MB-231
cells) and 43% (MCF-7 cells) following treatment with 10 µM
of querectin-3-methyl ether for 48 h (P<0.001). These data
suggested that querectin-3-methyl ether had marked inhibitory
effects on the migratory and invasive capacities of breast cancer
cells. In addition, as shown in the images in Fig. 2C, the cell number in the MCF-7 0
µM group was higher than that in the MDA-MB-231 0 µM
group, however, a contrasting trend of cell number is shown in the
bar graph when comparing the two cell lines. The reason for this
was that cell number was counted in four views of each well using a
microscope (total of four views), whereas the images shown in
Fig. 2C were randomly selected
from one of the four views (single view). Although the images
suggest the cell number in the MCF-7 0 µM group was markedly
higher than that in the MDA-MB-231 0 µM group, the image
represents a single view and does not show the total number.
However, the cell counting results shown in the bar graph represent
the average values per well in three independent experiments. The
reason for the change in the cell lines was that suppression of the
migration and invasion in breast cancer cells was found. Therefore,
MDA-MB-231 cells were selected to examine the effects of
quercetin-3-methyl ether on cell invasion, metastasis and cancer
stem cell formation due to the fact that this cell line is a TNBC
line with high metastatic and malignant potential.
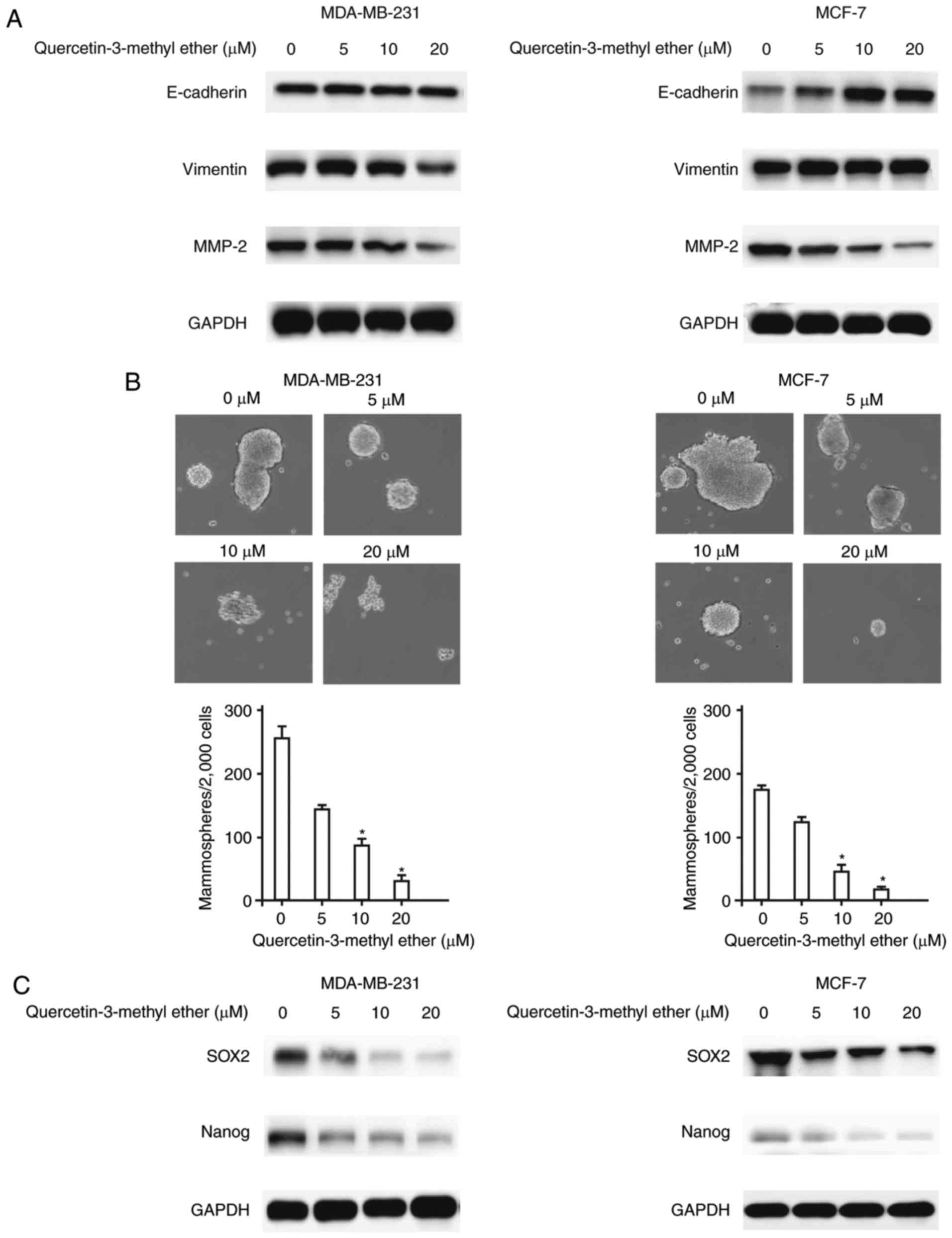
Querectin-3-methyl ether inhibits EMT and
CSC formation in breast cancer cells
EMT, an important program enabling epithelial cells
to acquire a mesenchymal phenotype, characterized by the decreased
expression of E-cadherin and the overexpression of Vimentin and
cellular proteases, including MMP-2, is closely linked with the
invasion and metastasis of breast cancer (1). The present study determined the
effect of querectin-3-methyl ether on the expression of EMT-related
genes in breast cancer cells. The data indicated that treatment
with querectin-3-methyl ether led to a reduction in the expression
of mesenchymal cell biomarkers, including Vimentin and MMP-2, and
concomitantly, the overexpression of epithelial cell biomarker
E-cadherin (Fig. 3A). As EMT may
contribute to the increase of CSC-like properties, and CSCs have
been implicated in cancer invasion and metastasis, the present
study examined whether querectin-3-methyl ether can suppress
mammosphere formation and the expression of stemness regulatory
genes, including SOX2 and Nanog, in breast cancer cells. The
results demonstrated that querectin-3-methyl ether effectively
reduced the number of mammospheres (P<0.05; Fig. 3B) and inhibited the protein
expression of SOX2 and Nanog in a dose-dependent manner (Fig. 3C). These findings suggested that
querectin-3-methyl ether inhibited breast cancer invasion and
metastasis, possibly by suppressing the intrinsic EMT process and
CSC formation.
Querectin-3-methyl ether downregulates
Notch1, PI3K-AKT and EZH2 signals in breast cancer cells
EZH2, the catalytic subunit of polycomb repressive
complex 2, can downregulate gene transcription through histone H3
trimethylation on lysine 27 (H3K27me3). It has been reported that
EZH2 causes expansion of breast cancer stem cells through
activation of the Notch and PI3K/Akt signaling pathways (32). Therefore, the present study
determined changes in the levels of these proteins in the
hormone-sensitive and TNBC cells in response to querectin-3-methyl
ether treatment. The results indicated that querectin-3-methyl
ether markedly decreased the constitutive protein levels of
phosphorylated PI3K, phosphorylated AKT and phosphorylated mTOR in
MCF-7 and T47D cells (Fig. 4A).
Furthermore, the compound suppressed the levels of IGF-1-induced
phosphorylated PI3K, Akt and GSK3β in hormone-sensitive cells
(Fig. 4B). As quercetin-3-methyl
ether had a significant repressive effect on the expression of
Notch1 (Fig. 4C), the
combinational efficacy of this agent and the Notch signaling
inhibitor DAPT (a γ-secretase complex inhibitor) was examined. The
combination of these compounds exhibited an additive inhibitory
effect on the protein expression of Notch1, PI3K, Akt and mTOR in
MCF-7 and MDA-MB-231 cells (Fig.
4D). Furthermore, combining these two compounds had more marked
inhibitory effects on breast cancer cell proliferation and colony
formation (Fig. 4E and F). It was
also found that quercetin-3-methyl ether inhibited H3K27me3 signals
in a dose-dependent manner, but had no significant effect on the
expression of EZH2 (Fig. 4G).
These data suggested that quercetin-3-methyl ether suppressed EMT
and CSC formation in the hormone-sensitive breast cancer cells and
TNBC cells, possibly via the downregulation of H3K27me3 epigenetic
marks and the Notch1 and PI3K/Akt pathways.
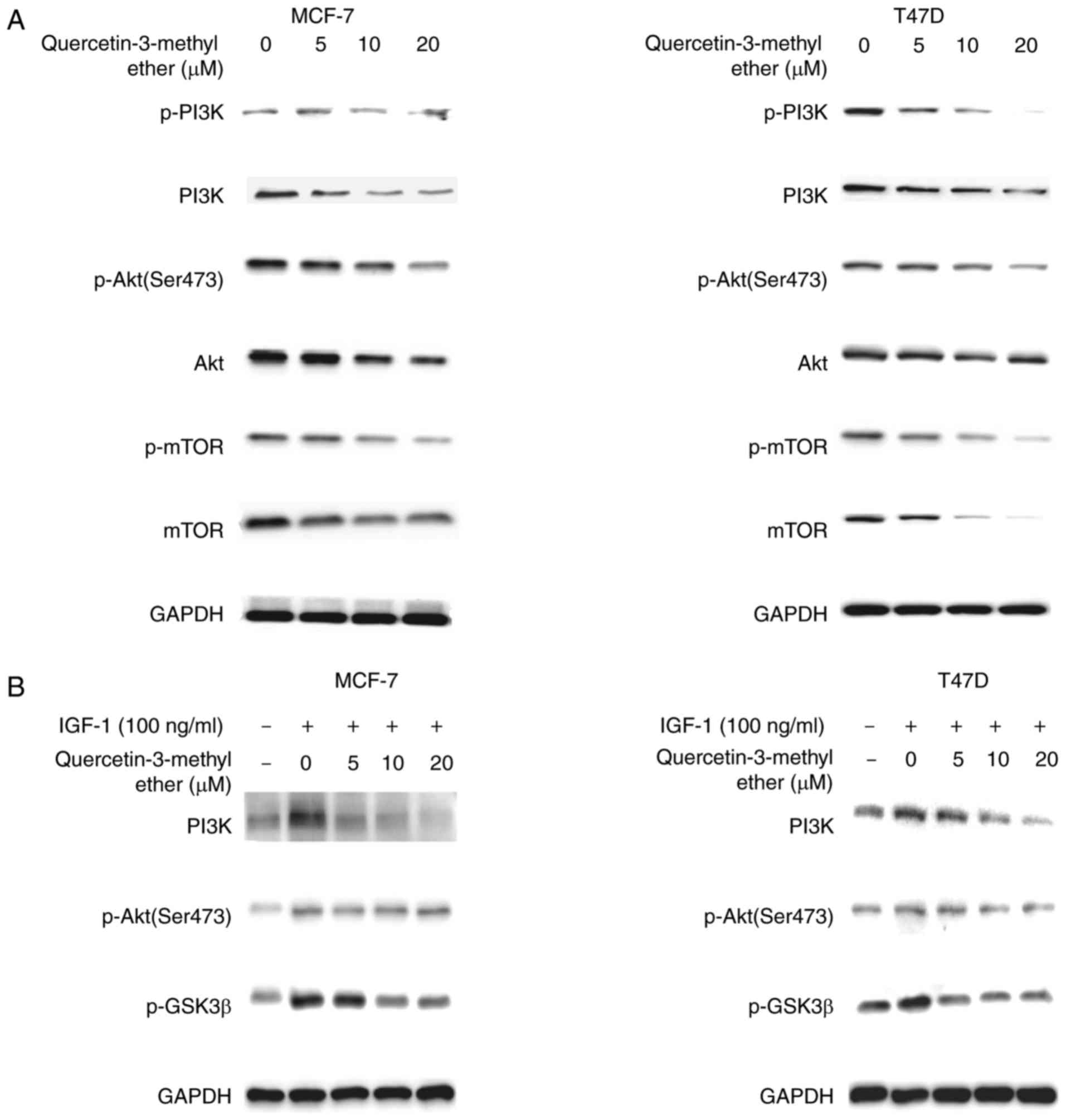 | Figure 4Querectin-3-methyl ether
downregulates Notch1, PI3K/Akt and EZH2 signals in breast cancer
cells. Cells were starved in serum-free medium for 24 h and then
treated with quercetin-3-methyl ether (0–20 µM) for 48 h.
The expression levels of (A) p-PI3K, total PI3K, p-Akt, total Akt,
p-mTOR and total mTOR were detected by western blot analysis. (B)
Cells were starved in serum-free medium for 24 h and then treated
with quercetin-3-methyl ether (0–20 µM) for 2 h prior to
exposure to 100 ng/ml IGF-1 for 20 min. The expression levels of
p-PI3K, p-Akt and p-GSK3β were determined by western blot analysis.
(C) Cells were starved in serum-free medium for 24 h and then
treated with quercetin-3-methyl ether (0–20 µM) for 48 h.
The expression levels of Notch1, PI3K, Akt and mTOR were detected
by western blot analysis. (D) Cells were treated with 10 µM
quercetin-3-methyl ether and/or 10 µM DAPT for the indicated
times and expression levels of Notch1, PI3K, Akt and mTOR were
detected. (E) Cell growth was evaluated with an CCK-8 assay and (F)
colony formation was examined by a colony formation assay. The
combination of quercetin-3-methyl ether and DAPT exhibited more
marked inhibition of growth and colony formation capacity of MCF-7
and MDA-MB-231 cells. Data are presented as the mean ± standard
deviation. *P<0.05, drug-treated, vs. DMSO-treated.
(G) Expression of EZH2 and H3K27me3 were detected by western blot
analysis. PI3K, mTOR, mammalian target of rapamycin; p-,
phosphorylated; Q3ME, quercetin-3-methyl ether; IGF1, insulin-like
growth factor 1; EZH2, enhancer of zeste homolog 2. |
Discussion
The majority of cases of breast cancer-associated
mortality are caused by highly metastatic disease, the relatively
high rates of which have been attributed to the lack of effective
treatments. Breast cancer is a common and complicated malignant
disease characterized by a range of aberrations at the genomic and
molecular levels, which manifest in dysregulated signaling pathways
involved in the development, progression and metastasis of the
cancer. Therefore, the identification of novel agents that
specifically target the metastatic properties of cancer cells is
considered important for the effective clinical control of this
malignancy. There are three main subtypes of breast cancer based on
the primary biomarkers: Luminal tumors (ER- and PR-positive),
HER-2-positive tumors and TNBCs (negative for all three markers).
These cancer subtypes have distinct properties and prognoses. The
luminal type is well differentiated, whereas the HER-2-positive and
TNBC types are poorly differentiated. Our previous study
demonstrated that quercetin-3-methyl ether potently inhibited
anchorage-dependent or -independent growth of HER-2-positive human
breast cancer cells (SK-BR-3) sensitive or resistant to lapatinib
treatment (30). In the present
study, it was demonstrated that quercetin-3-methyl ether exhibited
inhibitory effects on the proliferation, migration and invasion of
cells, and simultaneously induced apoptosis in luminal tumor cells
(MCF-7 and T47D) and TNBC cells (MDA-MB-231).
EMT is frequently abnormally activated during cancer
invasion and metastasis. EMT is a reversible molecular process
involving the loss of epithelial markers, including E-cadherin, and
increased expression of mesenchymal markers, including Vimentin. It
has been reported that breast cancer cells typically exhibit an EMT
phenotype, characterized by the high expression of EMT-regulatory
transcription factors and mesenchymal markers and downregulation of
epithelial markers (33,34). Upon the downregulation of
E-cadherin, epithelial cells gain fibroblastic properties that
enable them to dissociate from the epithelium and exhibit enhanced
migratory capabilities. The findings of the present study indicated
that quercetin-3-methyl ether decreased the expression of Vimentin
and increased the expression of E-cadherin, suggesting an
inhibitory effect of EMT in breast cancer cells. In addition, EMT
is key in CSC formation. Previous clinical data have demonstrated
an association between the proportion of CSCs and poor prognosis in
patients with breast cancer. An improved understanding of CSCs is
expected to have important implications for cancer prevention and
therapy. In particular, inhibiting EMT and CSC formation is an
attractive strategy for cancer prevention and management. The
present study demonstrated that quercetin-3-methyl ether may
effectively inhibited EMT and CSC formation, and consequently,
reduced the migration and invasion capacities of breast cancer
cells. These results offer novel insight with potential clinical
applications into the anticancer mechanisms of flavonoids.
The PI3K/Akt/mTOR pathway has a key regulatory
function in cell survival, proliferation, migration, metabolism,
angiogenesis and apoptosis. It is the most frequently dysregulated
pathway and may be of importance as a possible therapeutic target
in breast cancer. Overactivation of this pathway has been linked
with increased cell growth and, clinically, poor prognosis in
various types of cancer (16,17,19). The results of the present study
demonstrated that quercetin-3-methyl ether significantly suppressed
the constitutive activation of the PI3K/Akt/mTOR signaling pathway
and the IGF-1-induced phosphorylation of PI3K, Akt and GSK-3β in
breast cancer cells. Increasing data indicates that crosstalk
between the PI3K/Akt pathway and Notch signaling is important in
cancer. Notably, Notch may regulate the Akt pathway in normal and
breast cancer cells. In previous studies, Notch signaling triggered
an autocrine signaling loop, which activated Akt, in breast
epithelial cells, and the suppression of Notch reduced the activity
of Akt in breast tumor cells (35,36). Additionally, the increased
expression of Notch1 in breast cancer has been significantly
correlated with metastasis, EMT and CSC formation. Previous studies
have indicated that inhibiting cell growth, migration and invasion,
and inducing apoptosis by the inhibition of Notch1 may be partly
achieved by inactivating AKT signaling (35). The present data demonstrated that
treatment with quercetin-3-methyl ether led to a decrease in the
expression of Notch1, PI3K, Akt and mTOR in TMBC and
hormone-sensitive breast cancer cell lines, and that the
combination of this compound with the Notch signaling inhibitor
DAPT had an additive inhibitory effect on the expression of these
proteins.
EZH2 protein is overexpressed in patients with
aggressive breast tumors and may be a significant biomarker of
recurrence and metastasis in breast cancer. Previous studies have
indicated that EZH2 serves a function in self-renewal of breast
CSCs, and is involved in EMT and cell invasion. Furthermore, it has
been reported that EZH2 contributes to the expansion of breast CSCs
via Notch signaling activation (32), and that overexpressed EZH2 protein
is associated with an increased level of phosphorylated Akt
(Ser473) in invasive breast cancer. The present study identified
that quercetin-3-methyl ether reduced the expression of H3K27me3,
Notch1, PI3K, Akt and mTOR, and inhibited the Notch1 and PI3K/Akt
signaling pathways in breast cancer cells. These data suggested
that quercetin-3-methyl ether repressed EMT and CSCs, possibly
through inhibition of the EZH2, Notch1 and PI3K/Akt signaling
pathways. Taken together, quercetin-3-methyl ether considerably
decreased H3K27 methylation, possibly by inhibiting EZH2
methyltransferase activity, which led to the repression of EMT
promotion, CSC expansion and cell cycle dysregulation (Fig. 5). This agent also inhibited Notch1
and PI3K/Akt signaling, which resulted in the downregulation of
protein markers associated with cell cycle, apoptosis, stem cell
pluripotency, and self-renewal, including CDK1, Cyclin B1, Bcl-xl,
Bcl-2, Sox2 and Nanog (Fig.
5).
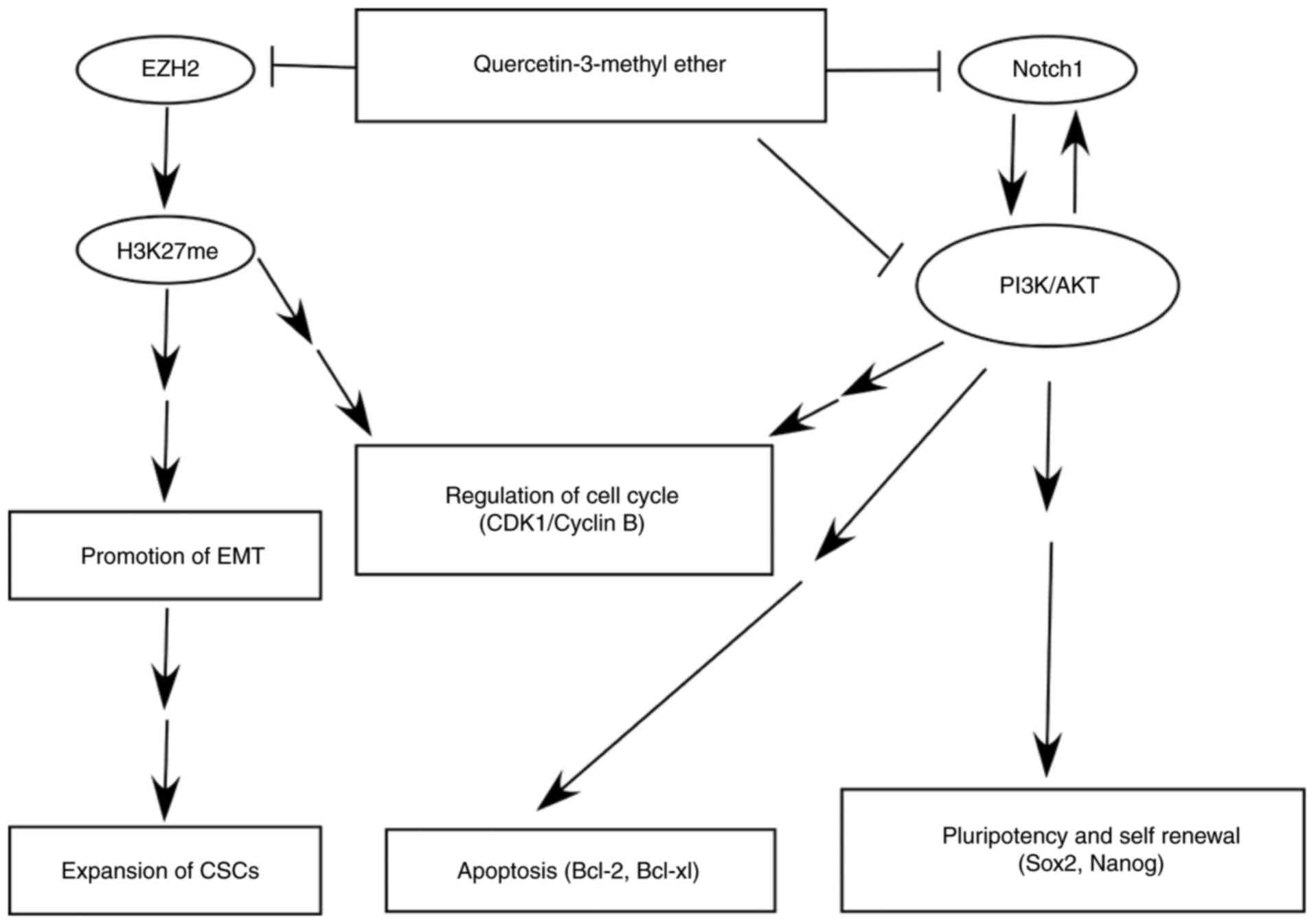 | Figure 5Simplified diagram of the proposed
anticancer mechanism of quercetin-3-methyl ether in breast cancer
based on the results of the present study. Quercetin-3-methyl ether
inhibits Notch1, PI3K/Akt and EZH2 signaling pathways, which
results in suppression of EMT and CSC formation, and induction of
cell cycle arrest and apoptosis. Ultimately, cancer cell
proliferation, migration and invasion are repressed by
quercetin-3-methyl ether. PI3K, EZH2, enhancer of zeste homolog 2;
CDK1, cyclin-dependent kinase 1; Bcl-2, B-cell lymphoma 2; Bcl-xl,
Bcl-extra large; Sox2, SRY-box 2; EMT, epithelial-mesenchymal
transition; CSCs, cancer stem-like cells. |
In conclusion, the present study demonstrated that
quercetin-3-methyl ether potently inhibited migration and invasion
in TNBC and hormone-sensitive breast cancer cells; this may have
occurred partly through the suppression of EMT and CSC induction,
an effect mediated via inhibition of the Notch1, PI3K/Akt and EZH2
signaling pathways. These findings suggest that quercetin-3-methyl
ether may be a potential chemopreventive and therapeutic drug for
the targeted eradication of CSCs in breast cancer.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81201710
and 81272434), the Guangdong Natural Science Foundation (grant no.
S2012010008259) and Guangdong Medical University (grant nos. XG1101
and STIF201105).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
Conception and design: LC, TL, KZ and JL;
Development of methodology: LC, BL, JZ, CL and JL; Acquisition of
data: LC, YY and ZY; Analysis and interpretation of data: LC, YY,
ZY and JL; Writing, review, and/or revision of the manuscript: LC
and JL; Administrative, technical, or material support: BL, JZ and
CL; Study supervision: KZ and JL.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Felipe Lima J, Nofech-Mozes S, Bayani J
and Bartlett JM: EMT in breast carcinoma-A review. J Clin Med.
5:E652016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu Y, Sarkissyan M and Vadgama JV:
Epithelial-mesenchymal transition and breast cancer. J Clin Med.
5:E132016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y and Zhou BP: Epithelial-mesenchymal
transition in breast cancer progression and metastasis. Chin J
Cancer. 30:603–611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asiedu MK, Beauchamp-Perez FD, Ingle JN,
Behrens MD, Radisky DC and Knutson KL: AXL induces
epithelial-to-mesenchymal transition and regulates the function of
breast cancer stem cells. Oncogene. 33:1316–1324. 2014. View Article : Google Scholar
|
|
6
|
Czerwinska P and Kaminska B: Regulation of
breast cancer stem cell features. Contemp Oncol. 19:A7–A15.
2015.
|
|
7
|
Zhao Z, Lu P, Zhang H, Xu H, Gao N, Li M
and Liu C: Nestin positively regulates the Wnt/β-catenin pathway
and the proliferation, survival and invasiveness of breast cancer
stem cells. Breast Cancer Res. 16:4082014. View Article : Google Scholar
|
|
8
|
Geng SQ, Alexandrou AT and Li JJ: Breast
cancer stem cells: Multiple capacities in tumor metastasis. Cancer
Lett. 349:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad
A, Banerjee S, Azmi AS, Miele L and Sarkar FH: Notch-1 induces
epithelial-mesenchymal transition consistent with cancer stem cell
phenotype in pancreatic cancer cells. Cancer Lett. 307:26–36. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clementz AG, Rogowski A, Pandya K, Miele L
and Osipo C: NOTCH-1 and NOTCH-4 are novel gene targets of PEA3 in
breast cancer: Novel therapeutic implications. Breast Cancer Res.
13:R632011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong Y, Shen S, Zhou Y, Mao F, Lin Y,
Guan J, Xu Y, Zhang S, Liu X and Sun Q: NOTCH1 is a poor prognostic
factor for breast cancer and is associated with breast cancer stem
cells. Onco Targets Ther. 9:6865–6871. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Callahan R and Raafat A: Notch signaling
in mammary gland tumorigenesis. J Mammary Gland Biol Neoplasi.
6:23–36. 2001. View Article : Google Scholar
|
|
13
|
Reedijk M, Odorcic S, Chang L, Zhang H,
Miller N, McCready DR, Lockwood G and Egan SE: High-level
coexpression of JAG1 and NOTCH1 is observed in human breast cancer
and is associated with poor overall survival. Cancer Res.
65:8530–8537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farnie G and Clarke RB: Mammary stem cells
and breast cancer-role of Notch signalling. Stem Cell Rev.
3:169–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palomero T, Dominguez M and Ferrando AA:
The role of the PTEN/AKT Pathway in NOTCH1-induced leukemia. Cell
Cycle. 7:965–70. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marty B, Maire V, Gravier E, Rigaill G,
Vincent-Salomon A, Kappler M, Lebigot I, Djelti F, Tourdès A,
Gestraud P, et al: Frequent PTEN genomic alterations and activated
phosphatidylinositol 3-kinase pathway in basal-like breast cancer
cells. Breast Cancer Res. 10:R1012008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baselga J: Targeting the
phosphoinositide-3 (PI3) kinase pathway in breast cancer.
Oncologist. 16(Suppl 1): S12–S19. 2011. View Article : Google Scholar
|
|
18
|
Singh JK, Farnie G, Bundred NJ, Simões BM,
Shergill A, Landberg G, Howell SJ and Clarke RB: Targeting CXCR1/2
significantly reduces breast cancer stem cell activity and
increases the efficacy of inhibiting HER2 via HER2-dependent and
-independent mechanisms. Clin Cancer Res. 19:643–656. 2013.
View Article : Google Scholar
|
|
19
|
Hernandez-Aya LF and Gonzalez-Angulo AM:
Targeting the phosphatidylinositol 3-kinase signaling pathway in
breast cancer. Oncologist. 16:404–414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kleer CG, Cao Q, Varambally S, Shen R, Ota
I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, et al: EZH2
is a marker of aggressive breast cancer and promotes neoplastic
transformation of breast epithelial cells. Proc Natl Acad Sci USA.
100:11606–11611. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inari H, Suganuma N, Kawachi K, Yoshida T,
Yamanaka T, Nakamura Y, Yoshihara M, Nakayama H, Yamanaka A, Masudo
K, et al: Expression of enhancer of zeste homolog 2 correlates with
survival outcome in patients with metastatic breast cancer:
Exploratory study using primary and paired metastatic lesions. BMC
Cancer. 17:1602017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pourakbar S, Pluard TJ, Accurso AD and
Farassati F: Ezh2, a novel target in detection and therapy of
breast cancer. Onco Targets Ther. 10:2685–2687. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rubio S, Quintana J, Eiroa JL, Triana J
and Estévez F: Acetyl derivative of quercetin 3-methyl
ether-induced cell death in human leukemia cells is amplified by
the inhibition of ERK. Carcinogenesis. 28:2105–2113. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee EH, Kim HJ, Song YS, Jin C, Lee KT,
Cho J and Lee YS: Constituents of the stems and fruits of Opuntia
ficus-indica var. saboten. Arch Pharm Res. 26:1018–1023. 2003.
View Article : Google Scholar
|
|
25
|
Takeara R, Albuquerque S, Lopes NP and
Lopes JL: Trypanocidal activity of Lychnophora staavioides Mart.
(Vernonieae, Asteraceae). Phytomedicine. 10:490–493. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei BL, Lu CM, Tsao LT, Wang JP and Lin
CN: In vitro anti-inflammatory effects of quercetin 3-O-methyl
ether and other constituents from Rhamnus species. Planta Med.
67:745–747. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar AD, Bevara GB, Kaja LK, Badana AK
and Malla RR: Protective effect of 3-O-methyl quercetin and
kaempferol from Semecarpus anacardium against
H2O2 induced cytotoxicity in lung and liver
cells. BMC Complement Altern Med. 16:3762016. View Article : Google Scholar
|
|
28
|
Martino R, Arcos ML, Alonso R, Sülsen V,
Cremaschi G and Anesini C: Polyphenol-rich fraction from Larrea
divaricata and its main flavonoid quercetin-3-methyl ether induce
apoptosis in lymphoma cells through nitrosative stress. Phytother
Res. 30:1128–1136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li J, Mottamal M, Li H, Liu K, Zhu F, Cho
YY, Sosa CP, Zhou K, Bowden GT, Bode AM, et al: Quercetin-3-methyl
ether suppresses proliferation of mouse epidermal JB6 P+ cells by
targeting ERKs. Carcinogenesis. 33:459–465. 2012. View Article : Google Scholar :
|
|
30
|
Li J, Zhu F, Lubet RA, De Luca A, Grubbs
C, Ericson ME, D'Alessio A, Normanno N, Dong Z and Bode AM:
Quercetin-3-methyl ether inhibits lapatinib-sensitive and
-resistant breast cancer cell growth by inducing G2/M
arrest and apoptosis. Mol Carcinog. 52:134–143. 2013. View Article : Google Scholar
|
|
31
|
Kim HY, Lee KM, Kim SH, Kwon YJ, Chun YJ
and Choi HK: Comparative metabolic and lipidomic profiling of human
breast cancer cells with different metastatic potentials.
Oncotarget. 7:67111–67128. 2016.PubMed/NCBI
|
|
32
|
Gonzalez ME, Moore HM, Li X, Toy KA, Huang
W, Sabel MS, Kidwell KM and Kleer CG: EZH2 expands breast stem
cells through activation of NOTCH1 signaling. Proc Natl Acad Sci
USA. 111:3098–3103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Armstrong AJ, Marengo MS, Oltean S, Kemeny
G, Bitting RL, Turnbull JD, Herold CI, Marcom PK, George DJ and
Garcia-Blanco MA: Circulating tumor cells from patients with
advanced prostate and breast cancer display both epithelial and
mesenchymal markers. Mol Cancer Res. 9:997–1007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lombaerts M, van Wezel T, Philippo K,
Dierssen JW, Zimmerman RM, Oosting J, van Eijk R, Eilers PH, van de
Water B, Cornelisse CJ, et al: E-cadherin transcriptional
downregulation by promoter methylation but not mutation is related
to epithelial-to-mesenchymal transition in breast cancer cell
lines. Br J Cancer. 94:661–671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu H, Bhaijee F, Ishaq N, Pepper DJ,
Backus K, Brown AS, Zhou X and Miele L: Correlation of Notch1, pAKT
and nuclear NF-κB expression in triple negative breast cancer. Am J
Cancer Res. 3:230–239. 2013.
|
|
36
|
Meurette O, Stylianou S, Rock R, Collu GM,
Gilmore AP and Brennan K: Notch activation induces Akt signaling
via an auto-crine loop to prevent apoptosis in breast epithelial
cells. Cancer Res. 69:5015–5022. 2009. View Article : Google Scholar : PubMed/NCBI
|