Introduction
Fluid shear stress (FSS) refers to friction that is
generated by blood flow, which acts on the vascular wall.
Endothelial cells (ECs) are directly exposed to FSS, and are able
to respond to differential stress induced by constantly varying
flow patterns and velocities by altering their shape, polarity and
patterns of gene activity (1).
Primary cilia are considered one of the major stress sensors in
vascular endothelium (2). The
primary cilium is a non-motile microtubule-based structure present
at the surface of almost every mammalian cell, which extends from
the basal body. The basal body functions as the template for
ciliogenesis, and regulates the entry and exit of proteins into and
out of the cilia that are required for cilia assembly (3). γ-tubulin, which is a basal body
marker, is associated with initiation of cilia assembly.
Intraflagellar transport 88 (IFT88) is a transport protein for
tubulin and is also a marker for ciliogenesis. In recent years, it
has been reported that primary cilia are key coordinators of
signaling pathways during development and tissue homeostasis;
therefore, cilia-associated disorders, known as ciliopathies, can
affect numerous organ systems, and include autosomal recessive
polycystic kidney disease and nephronophthisis (4). The mechanosensing function of
primary cilia depends on the following mechanoproteins: Polycystins
1 and 2 (5). An abrupt alteration
in FSS can be detected by these sensory proteins localized in the
cilia, and these alterations are transduced and translated via a
complex pathway of intracellular signaling. Notably, polycystin can
become functionally inactive following exposure to high FSS
(6). Previous studies have
suggested that alterations in FSS may also alter ciliary structure
by shortening the cilia or leading to depolymerization (7,8).
Furthermore, not all parts of the vasculature possess cilia.
Scanning electron microscopy indicated that primary cilia are
distributed in embryonic endocardium and participate in cardiac
differentiation (9), and are
found in the branches and bends of vessels, which is associated
with atherosclerosis (10). In
addition, the distribution pattern of monocilia is associated with
the pattern of shear stress, and primary cilia disassemble in ECs
under laminar FSS (7). In the
past few decades, it has been reported that shear flow is an
influencing factor in ciliogenesis (11). Although some studies have reported
that oscillatory fluid flow may stimulate the assembly of primary
cilia via an increase in the surrounding number of microtubules
(12), little is currently known
about the mechanism underlying flow-induced primary cilia assembly
and disassembly in ECs (11).
Recently, the Wnt signaling pathway has been
revealed to be associated with the primary cilium and FSS. The Wnt
proteins are a family of 19 highly conserved secreted glycoproteins
that act via frizzled (Frz) receptors, or via a complex that is
composed of Frz and low-density lipoprotein-receptor-related
proteins 5 and 6. At present, three distinct intracellular Wnt
signaling cascades are well established: The Wnt/β-catenin pathway,
which is the canonical Wnt signaling pathway; the Wnt/planar cell
polarity (PCP) signaling pathway; and the Wnt/Ca2+
pathway. The Wnt/β-catenin pathway is mediated by β-catenin, which
accumulates in the cytoplasm in the presence of Wnt, and can then
be translocated to the nucleus (12). The Wnt/PCP signaling pathway,
which was discovered in Drosophilia, serves a role in
establishing cell polarity during development of the organism
(13). In mammals, Wnt/PCP is
essential for neural tube closure, fur patterning, hair bundle
orientation in the inner ear and axonal guidance. The core PCP
genes include dishevelled segment polarity proteins 1–3
(Dvl1-3), VANGL planar cell polarity proteins 1-2
(Vangl1-2), Frz, cadherin, and Prickle-like proteins
1–4. Another group of PCP proteins comprises the ̔effector̓
molecules, including fuzzy planar cell polarity protein (Fuz),
inturned and Fritz (14). The Wnt
signaling pathway has been reported to be associated with the
mechanical force stimulation. In numerous tissue types,
particularly in the endothelium, PCP develops in response to shear
flow (15–17). To the best of our knowledge, the
mechanisms that differentially trigger and control these signaling
pathways remain to be fully understood. It has recently been
proposed that hemodynamic shear stress causes the polarization of
ECs in the direction of flow, and the noncanonical Wnt signaling
pathway may reduce endothelial shear sensitivity by regulating the
cell polarity (18). Notably, the
Wnt signaling pathway is essential for ciliogenesis. Numerous
proteins in the Wnt signaling pathway have been confirmed to
colocalize with the basal body, and mutations in these proteins
induce ciliary mislocalization or deficiency (19). Furthermore, the cilium functions
as a regulatory switch to control the balance between canonical and
noncanonical Wnt pathways (20).
However, the association between ciliogenesis, Wnt/β-catenin and
Wnt/PCP signaling pathways has yet to be elucidated. In addition,
very little is known about whether these two signaling pathways are
involved in primary ciliogenesis induced by FSS in ECs.
In the present study, to determine the role of
Wnt/β-catenin and Wnt/PCP signaling pathways in FSS-induced
ciliogenesis, the cells were subjected to differing velocities of
FSS for various durations using a shear stress device.
Subsequently, immunofluorescence and quantitative polymerase chain
reaction (qPCR) were used to assess activation of the Wnt/β-catenin
and Wnt//PCP signaling pathways, in order to determine the effects
of FSS on the two pathways and primary cilia in ECs. Furthermore,
the colocalization of Dvl2 and the basal body under low and laminar
FSS was analyzed. The results indicated that under low FSS for 12
h, ECs could induce the localization of Dvl2 to the basal body,
whereas laminar FSS led to the mislocalization of Dvl2, thus
suggesting that low FSS may induce primary ciliogenesis via the
Wnt/PCP signaling pathway.
Materials and methods
Cell culture
Primary human umbilical vein ECs (HUVECs) were
cultured in a humidified incubator at 37°C in vascular cell basal
medium in the presence of vascular endothelial growth factor
supplemented with 1% fetal bovine serum (FBS) and 1%
penicillin/streptomycin (all from American Type Culture Collection,
Manassas, VA, USA).
Cells grown on matrix-treated culture slides
(75×25×1.0 mm; Flexcell International Corporation, Burlington, NC,
USA) were subjected to FSS using a shear stress device
(Streamer®; Flexcell International Corporation) for 0,
6, 12 and 18 h at 37°C. As the inside diameter of the hose, the
length and width of the slide are fixed, the magnitude of FSS is
controlled by the flow rate of the liquid, which is controlled by
the injection pump, based on the following FSS formula:
FSS=6µQ/a2b, where µ refers to the apparent viscosity
of the media; a refers to height; b to width; and Q to flow rate.
Shear stress was regulated independently at 0, 1 and 15
dynes/cm2 using a computer-controlled peristaltic pump,
and cells that underwent FSS for 0 h were considered negative
control samples. Subsequently, cells were harvested for RNA and
protein extraction.
Immunofluorescence
Cells were fixed in 4% paraformaldehyde for 15 min
and permeabilized with 0.5% Triton X-100 for 3 min at 4°C in PBS.
Subsequently, the cells were blocked with 3% bovine serum albumin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) three times (5 min
per time) at room temperature in PBS. Protein expression was
detected by immunofluorescence using the following primary
antibodies at 4°C overnight: Mouse anti-Dvl2 (cat. no. sc-8026,
1:1,000) and mouse anti-β-catenin (cat. no. sc-59737, 1:2,000)
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA), goat anti-Vangl2
(cat. no. SAB2501092, 1:2,000), rabbit anti-γ-tubulin (cat. no.
T5192, 1:1,000), rabbit anti-Fuz (cat. no. HPA041779, 1:1,000) and
rabbit anti-IFT88 (cat. no. SAB1302866, 1:1,000) (Sigma-Aldrich;
Merck KGaA). Subsequently, cells were incubated with corresponding
secondary antibodies for 30 min at room temperature: Donkey Alexa
Fluor 488 or Alexa Fluor 594 anti-mouse, -rabbit or -goat (cat. no.
A-21202, 1:1,000; cat. no. A-21203, 1:1,000; cat. no. A-210207,
1:1,000; cat. no. A-11058, 1:1,000; Molecular Probes; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Nuclei were counter-stained
with DAPI (Invitrogen; Thermo Fisher Scientific, Inc.), and images
were captured using a fluorescence microscope (Olympus IX83;
Olympus Corporation, Tokyo, Japan) at room temperature.
Reverse transcription (RT)-qPCR
RNA was isolated using the RNeasy kit (Takara Bio,
Inc., Otsu, Japan) according to the manufacturer's protocol. cDNA
was synthesized using the Superscript III reverse transcriptase and
random hexamer primers according to the manufacturer's protocol
(Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR was performed
using TB Green™ Premix Ex Taq™ II (Tli RnaseH Plus) (Takara Bio,
Inc.) on samples from three independent experiments, according to
the manufacturer's protocol. The thermocycling conditions were as
follows: Predenaturation at 95°C for 3 min; followed by 40 cycles
of denaturation, at 95°C for 10 sec, and annealing and extension at
60°C for 30 sec. All experiments were conducted in duplicate, and
GADPH was used as the housekeeping gene for normalization
and quantification. The relative fold-change in expression was
calculated using the 2−ΔΔCq method (Cq values <30)
(21). Analysis was performed
according to the manufacturer's protocol. Primer sequences are
listed in Table I.
 | Table IPrimer sequences of the genes
detected by quantitative polymerase chain reaction. |
Table I
Primer sequences of the genes
detected by quantitative polymerase chain reaction.
| Gene | Primer sequences
(5′-3′) |
|---|
|
β-catenin | F:
5′-CTGGCCATATCCACCAGAGT-3′ |
| R:
5′-GAAACGGCTTTCAGTTGAGC-3′ |
| Dvl2 | F:
5′-TTCAACGGAAGGGTGGTATC-3′ |
| R:
5′-TGGCAAAGGAGGTAAAGGTG-3′ |
| Vangl2 | F:
5′-ATCGGACTCTCCGGAACTTT-3′ |
| R:
5′-TCAGCAGATACTGCCCTGTG-3′ |
| Fuz | F:
5′-ACTGAGGAACCAGGCACAG-3′ |
| R:
5′-TCAAAGAAGTGGGGTGAGG-3′ |
| IFT88 | F:
5′-GAGAGGCTCTGCATTTGACC-3′ |
| R:
5′-CCTGCATCTTTTGCCTTTTC-3′ |
|
γ-tubulin | F:
5′-AGAACGGCTGAATGACAGGT-3′ |
| R:
5′-TTGATCTGGGAGAAGGATGG-3′ |
| GAPDH | F:
5′-CAGGAGGCATTGCTGATGAT-3′ |
| R:
5′-GAAGGCTGGGGCTCATTT-3′ |
Western blot analysis
Cells were harvested from each group, and
radioimmunoprecipitation assay lysis and extraction buffer (Thermo
Fisher Scientific, Inc.) containing 1% phenylmethylsulfonyl
fluoride was used to obtain cell lysates for protein expression
detection by western blotting. Protein concentration was determined
using the bicinchoninic acid method; protein samples (25 µg)
were then subjected to electrophoresis (5% stacking gel and 8%
separation gel) Samples were transferred to polyvinylidene fluroide
membranes in electric transfer buffer (0.58% Tris base, 0.29%
glycine, 0.037% SDS, 1% methanol) at 4°C for 120 min. Subsequently,
the membranes were blocked in Tris-buffered saline-0.5% Tween with
1% skimmed milk at room temperature for 2 h, and were probed with
rabbit anti-human Dvl2 antibody (cat. no. SAB2100634, 1:1,000;
Sigma-Aldrich; Merck KGaA) and mouse anti-human GAPDH antibody
(cat. no. ab9484, 1:10,000; Abcam) at 4°C overnight, followed by
incubation with horseradish peroxidase-conjugated sheep anti-rabbit
and rabbit anti-mouse secondary antibodies (cat. nos. A16172 and
61-6520, 1:3,000; Thermo Fisher Scientific, Inc.) at room
temperature for 2 h, respectively. The immunoreactive bands were
visualized using enhanced chemiluminescence (ECL) reagent (Clarity™
Western ECL Substrate; Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and semi-quantified using Quantity One software 4.6.7 (Bio-Rad
Laboratories, Inc.). The ratio of the optical density of the target
protein to that of GAPDH was estimated as the relative expression
level of the target protein.
Statistical analysis
All experiments were repeated three times. All data
are presented as the means ± standard error of the mean.
Differences between means were determined using unpaired two-tailed
Student's t-tests. P<0.05 was considered to indicate a
statistically significant difference using SPSS 15.01 (SPSS, Inc.,
Chicago, IL, USA).
Results
mRNA expression levels of the core
proteins in two Wnt signaling pathways under low and laminar
FSS
To assess the effects of various types of FSS on the
expression of core proteins in the Wnt/β-catenin and Wnt/PCP
pathways, the mRNA expression levels were measured and quantified
using qPCR (Fig. 1). Cells loaded
with FSS at 0 h were considered the negative control group. The
results demonstrated that the expression levels of Dvl2 were
increased with time and maximal expression was detected at 18 h.
Furthermore, the expression levels of Dvl2 under low FSS (1
dynes/cm2) were significantly higher than those under
laminar FSS (15 dynes/cm2) (P<0.05); expression
exhibited a 1.9-fold increase in response to low FSS compared with
laminar FSS at 18 h (Fig. 1A).
Conversely, the expression levels of Fuz were decreased with
time, and the effects of low FSS were less than those of laminar
FSS (Fig. 1B). Notably, the mRNA
expression levels of the core protein of the PCP signaling pathway,
Vangl2, were very low prior to 18 h; however, the expression
increased sharply under laminar FSS for 18 h, ~100-fold (Fig. 1C). Although β-catenin,
which is the core protein of the Wnt/β-catenin pathway, was also
influenced by laminar FSS, no significant alterations were noted
with time under low FSS, which was mostly equivalent to that of the
control group. At the early stage of laminar FSS, the expression
levels of β-catenin were significantly increased; expression
was 2-fold that of the control group. However, the expression was
decreased to basal levels with increasing time (Fig. 1D). Therefore, this protein may be
considered an effector at the early stage under the influence of
laminar FSS. The expression levels of Dvl2 protein were detected,
and were significantly increased in response to low FSS, by ~2-fold
(P<0.05). Furthermore, Dvl2 protein expression was suppressed
under laminar FSS (P<0.05) as compared with cells at 0 h
(Fig. 1E and F).
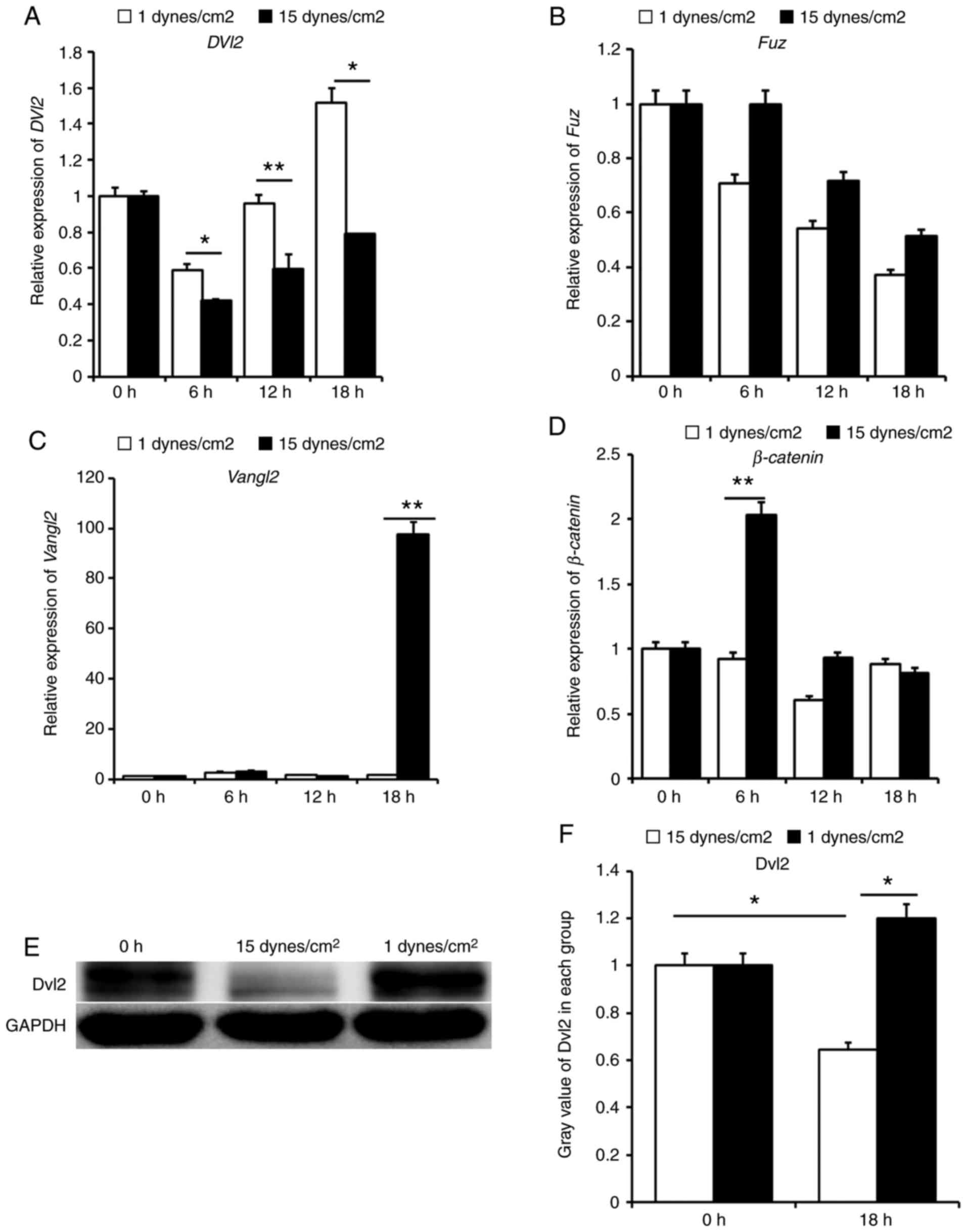 | Figure 1Relative expression of core proteins
in the Wnt signaling pathway and the protein expression levels of
Dvl2. (A) Low and laminar FSS promote the expression of Dvl2
with increasing time; the highest expression was observed after low
FSS loading for 18 h, which exhibited a 1.9-fold increase compared
with under laminar FSS. (B) Expression of Fuz was highest
during the early stage, and declined with increasing time; however,
no statistical difference was observed between each group. (C)
Expression levels of Vangl2 were low in almost all groups,
with the exception of the 18 h laminar FSS group. (D)
β-catenin exhibited a temporary increase under laminar FSS
at 6 h, ~2-fold of that under low FSS. (E) Expression levels of
Dvl2 were higher in the low FSS group compared with in the laminar
FSS group. (F) Gray value was estimated by Quantity One software;
relative expression in the low FSS group was ~2-fold that in the
laminar FSS group. Data are presented as the means ± standard error
of the mean, n=5. **P<0.01, *P<0.05 vs.
laminar FSS. FSS, fluid shear stress; Dvl2, dishevelled
segment polarity protein 2; Fuz, fuzzy planar cell polarity
protein; Vangl2, VANGL planar cell polarity protein 2. |
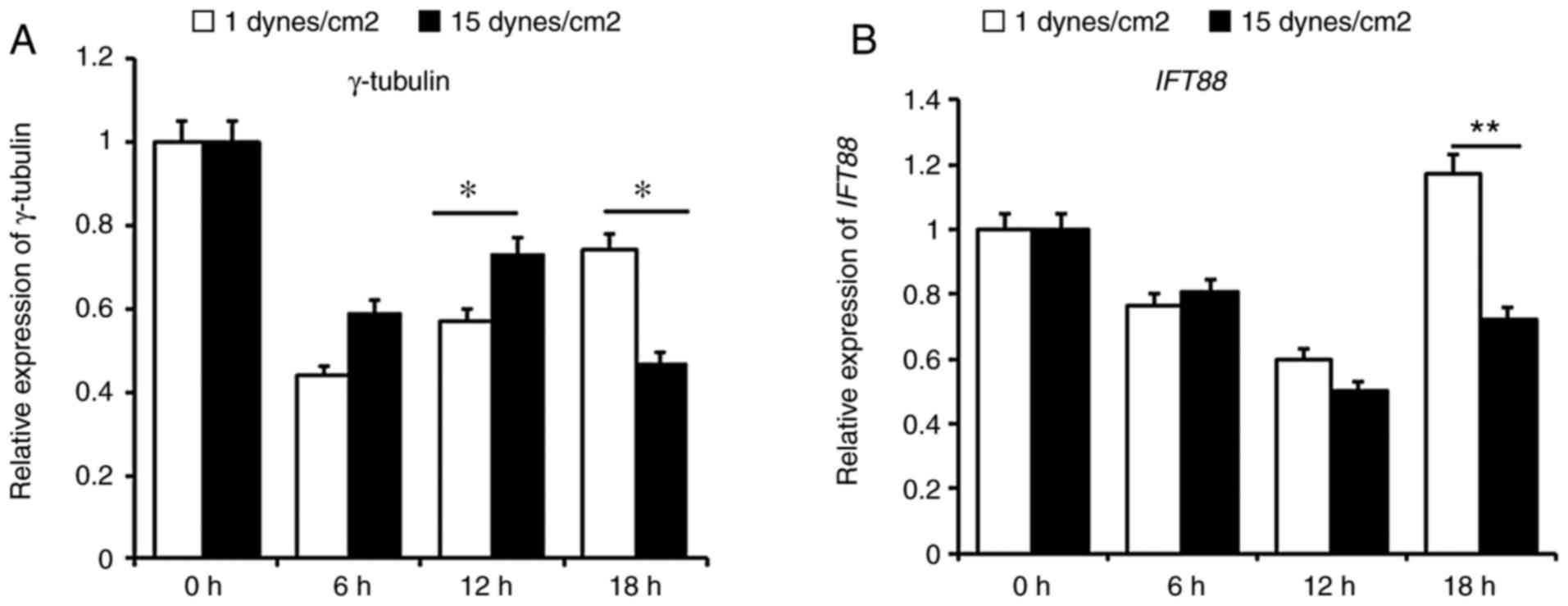
mRNA expression levels of the basal body
protein γ-tubulin and the primary cilia protein IFT88 under low and
laminar FSS
The mRNA expression levels of the basal body protein
γ-tubulin were assessed under low FSS (1
dynes/cm2) and laminar FSS (15 dynes/cm2) for
6, 12, and 18 h. Cells loaded with FSS at 0 h were considered a
negative control group. The results indicated that the expression
levels of γ-tubulin were higher under laminar FSS than under
low FSS before 12 h, with a considerable difference at 12 h
(P<0.05); however, this phenomenon was reversed after 18 h
(P<0.05) (Fig. 2A). Similar to
γ-tubulin, the mRNA expression levels of the primary cilia
protein, IFT88, were decreased before 12 h in each group,
and no significance was presented between these groups at 6 or 12
h. After 18 h, IFT88 expression was increased under low FSS
compared to that under laminar FSS (P<0.01) (Fig. 2B). These results indicated that
low FSS may promote membrane localization of the basal body of
primary cilia and cilia assembly.
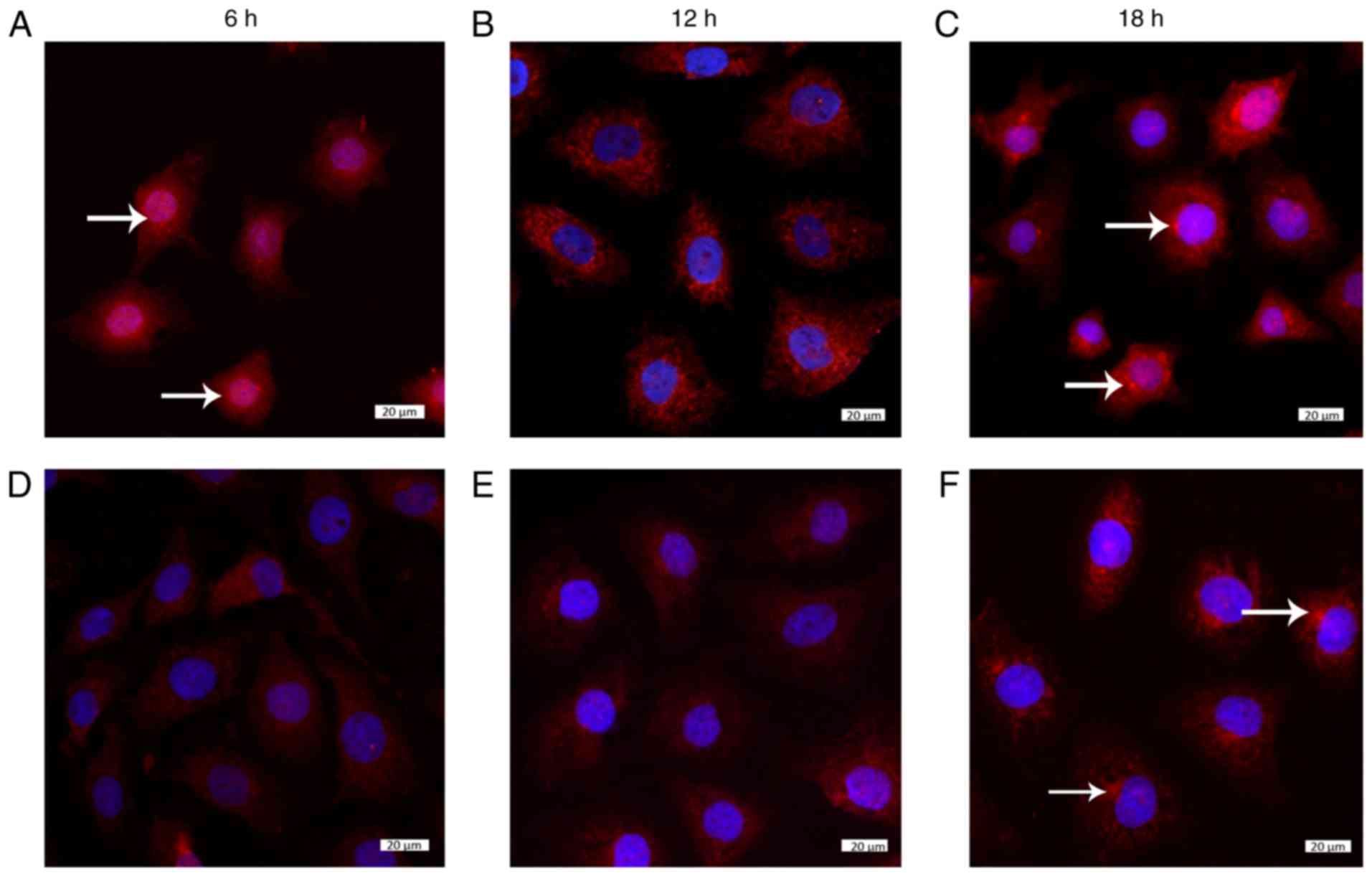
Localization of the core proteins in the
two Wnt signaling pathways under low and laminar FSS
In order to obtain the activation status of the
canonical and noncanonical Wnt signaling pathways under various
types of FSS, the localization of β-catenin (the core protein of
canonical Wnt signaling) and Dvl2, Vangl2 and Fuz (the core
proteins of Wnt/PCP signaling) was assessed by immunofluorescence
(Figs. 3Figure 4Figure 5Figure 6–7).
Under low FSS (1 dynes/cm2) for 6, 12 and
18 h, the localization of these proteins altered in a
time-dependent manner, with the exception of Fuz, which was
permanently localized at a specific point in the cytoplasm during
the whole process (Fig. 3A–C).
The levels of β-catenin were increased in the cytoplasm under low
FSS, and it entered the nucleus after 6 h, after which, it exited
and accumulated around the nucleus. Notably, after 18 h, β-catenin
was clustered in the cytoplasm (Fig.
4A–C). Similarly, the core protein of the PCP signaling
pathway, Vangl2, was scattered around the nucleus after 12 h under
low FSS, and some was aggregated in the cytoplasm at 18 h (Fig. 5A–C). In addition, under low FSS,
Dvl2 was mainly localized in the cytoplasm, with some aggregated
expression near the nucleus after 12 h (Figs. 6Bb–Db and 7Bb–Db).
Under laminar FSS (15 dynes/cm2) at 6 h,
no distinct localization of β-catenin was observed in the
cytoplasm; however, it clustered at 18 h (Fig. 4D–F). Conversely, under laminar
FSS, Vangl2 was always localized in the cytoplasm and dispersed
into the nucleus at 18 h (Fig.
5F). Under laminar FSS, Dvl2 was dispersed in the cytoplasm
before 18 h, and its expression was markedly reduced at 18 h
(Figs. 6Eb–Gb and 7Eb–Gb).
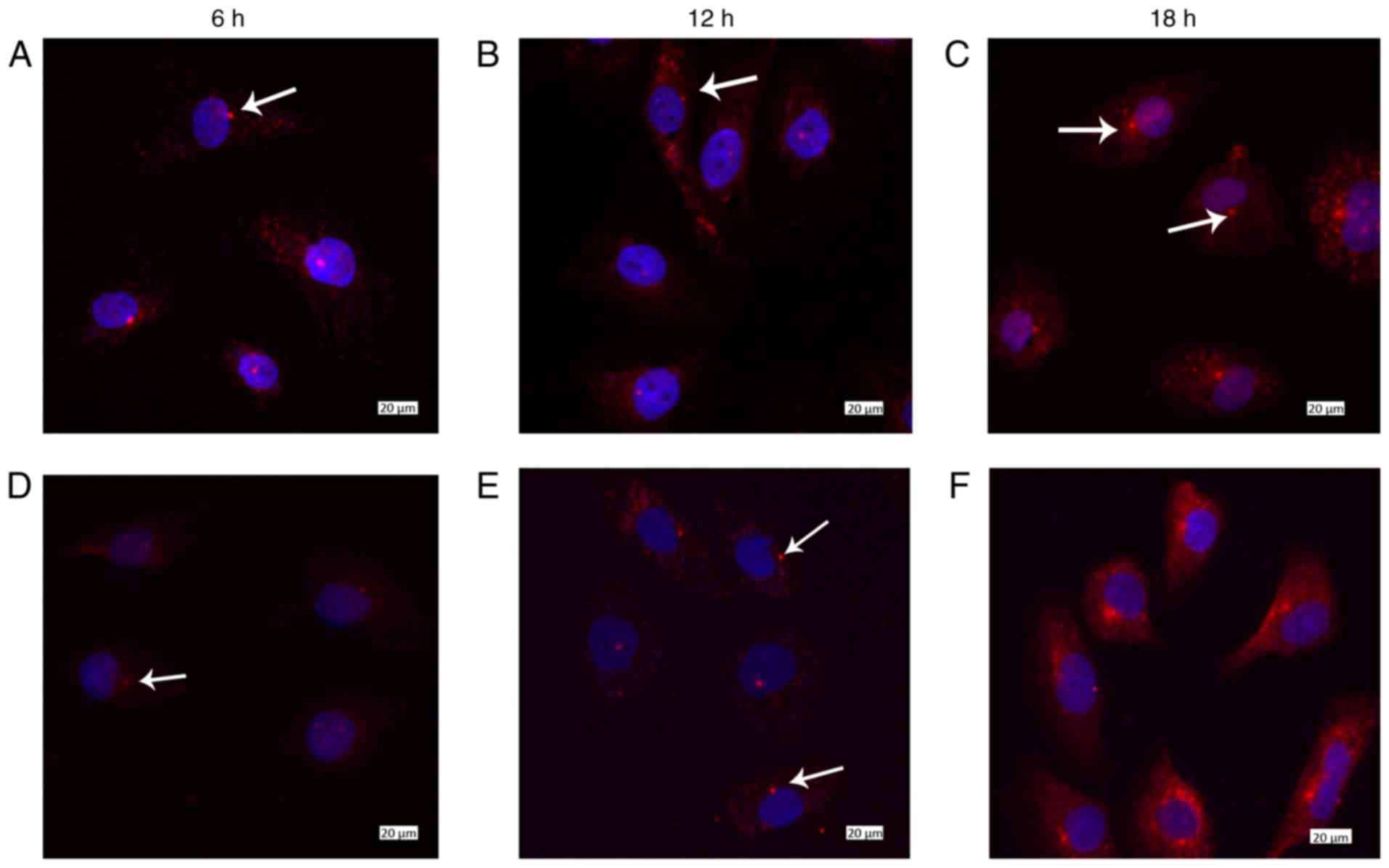
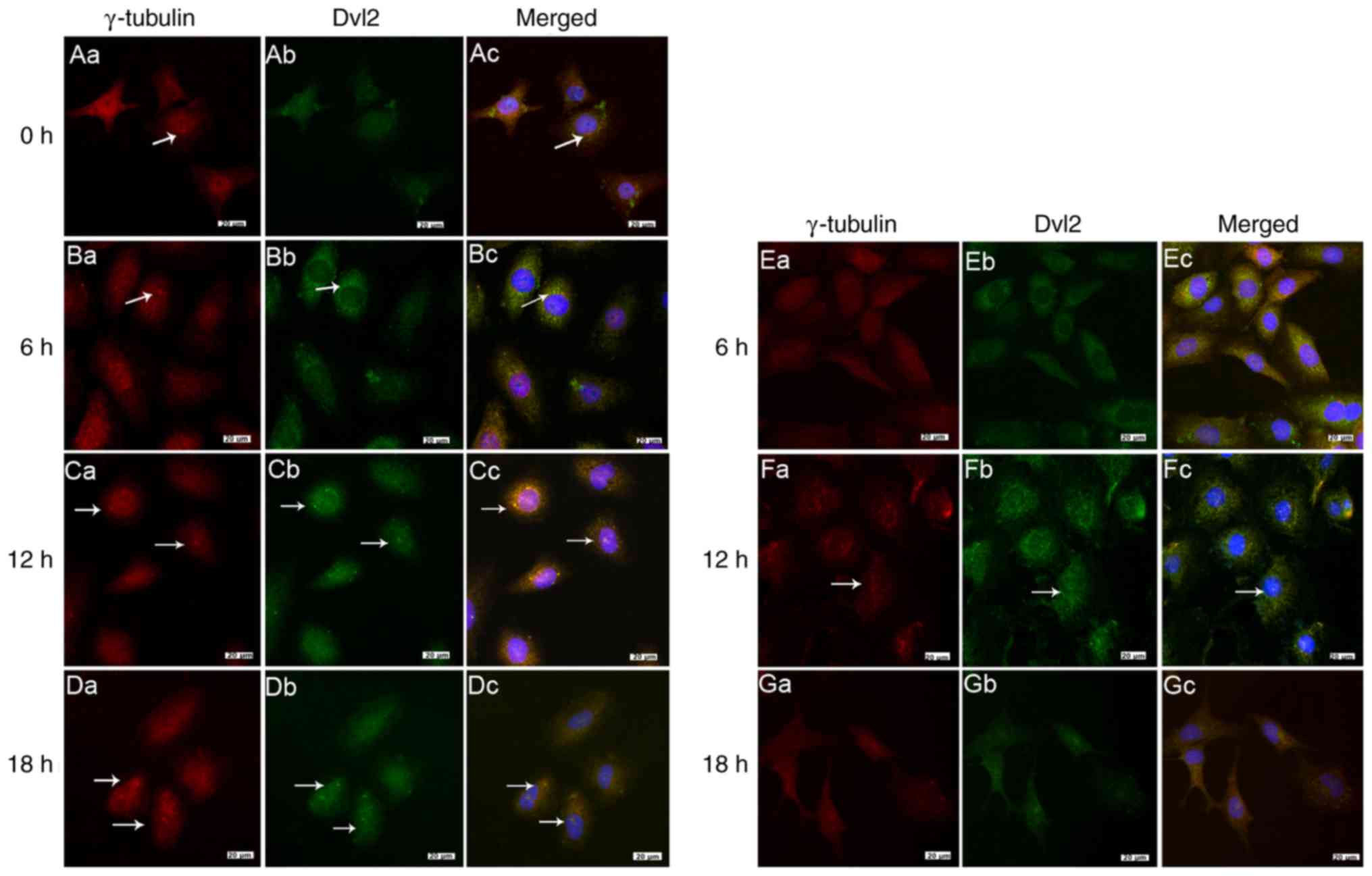
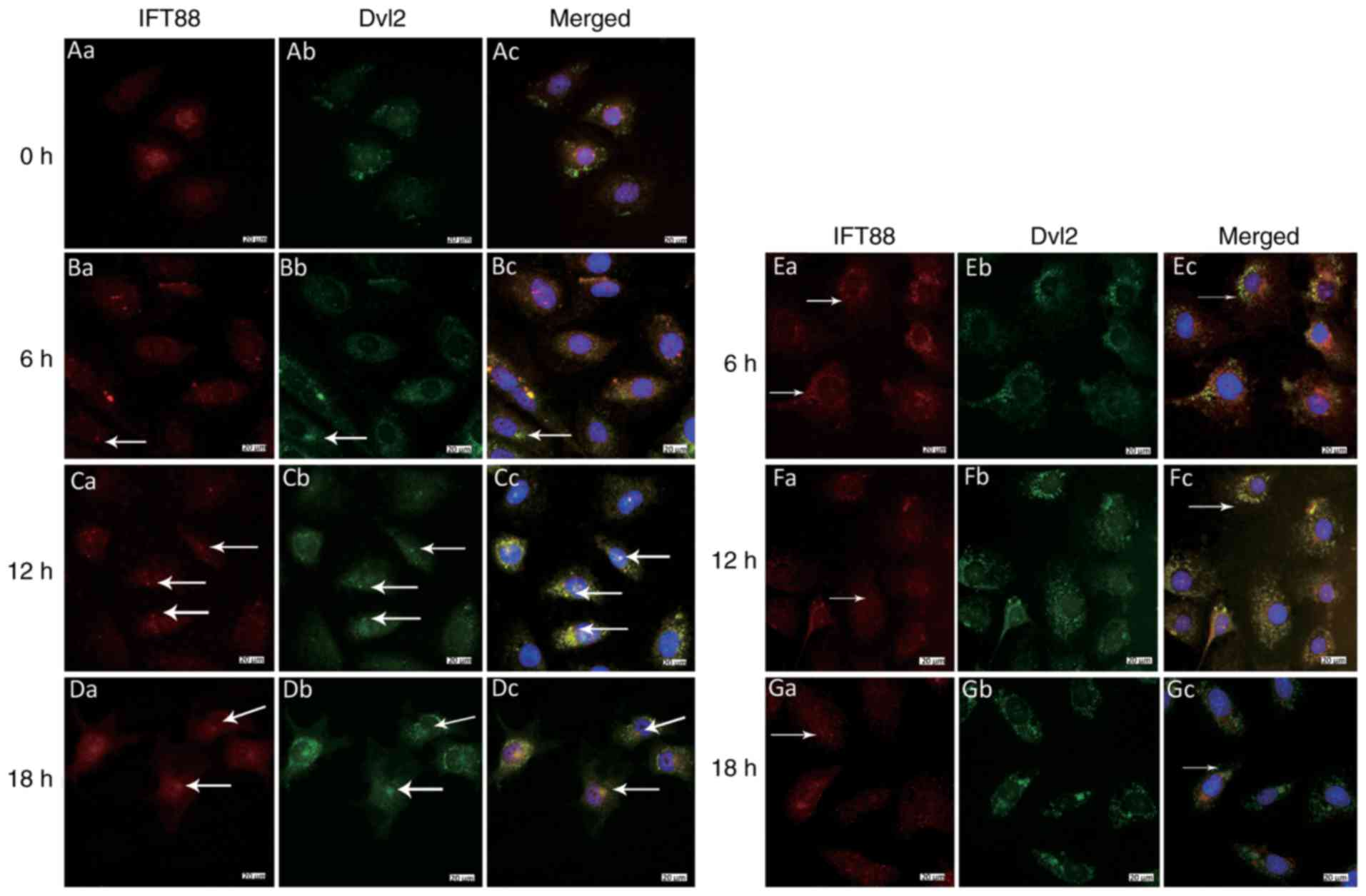
Localization of the basal body protein
γ-tubulin and the primary cilia protein IFT88
In most negative control cells loaded with FSS at 0
h, γ-tubulin was primarily localized in the nucleus, and only a
small number of cells exhibited cytoplasmic localization of this
protein (Fig. 6Aa). Notably,
localization in the cytoplasm was not fixed; therefore, some
protein could be localized in the proximity of the nucleus, whereas
some might be distally localized (Fig. 7Aa). When cells were loaded with
low FSS for 6 h, γ-tubulin was not expressed in all cells (Fig. 6Ba), whereas in some cells IFT88
began to localize to the edge of the nucleus (Fig. 7Ba). γ-tubulin was observed in the
majority of cells after 12 h (Fig.
6Ca–Da). Notably, γ-tubulin and IFT88 were localized to the
edge of the nucleus, at the same area, after 12–18 h under low FSS.
(Fig. 7Ca-Da).
At the early stage of laminar FSS, the aggregation
and localization of γ-tubulin near the nucleus was most marked at 6
h (Fig. 6Ea). However, when it
was loaded with laminar FSS for 12 h, only a few cells were
observed harboring γ-tubulin (Fig.
6Fa), and identification of cytoplasmic expression after 18 h
was difficult (Fig. 6Ga). In
addition, localization of IFT88 was rarely observed in the
cytoplasm during the whole process of laminar FSS loading (Fig. 7Ea-Ga).
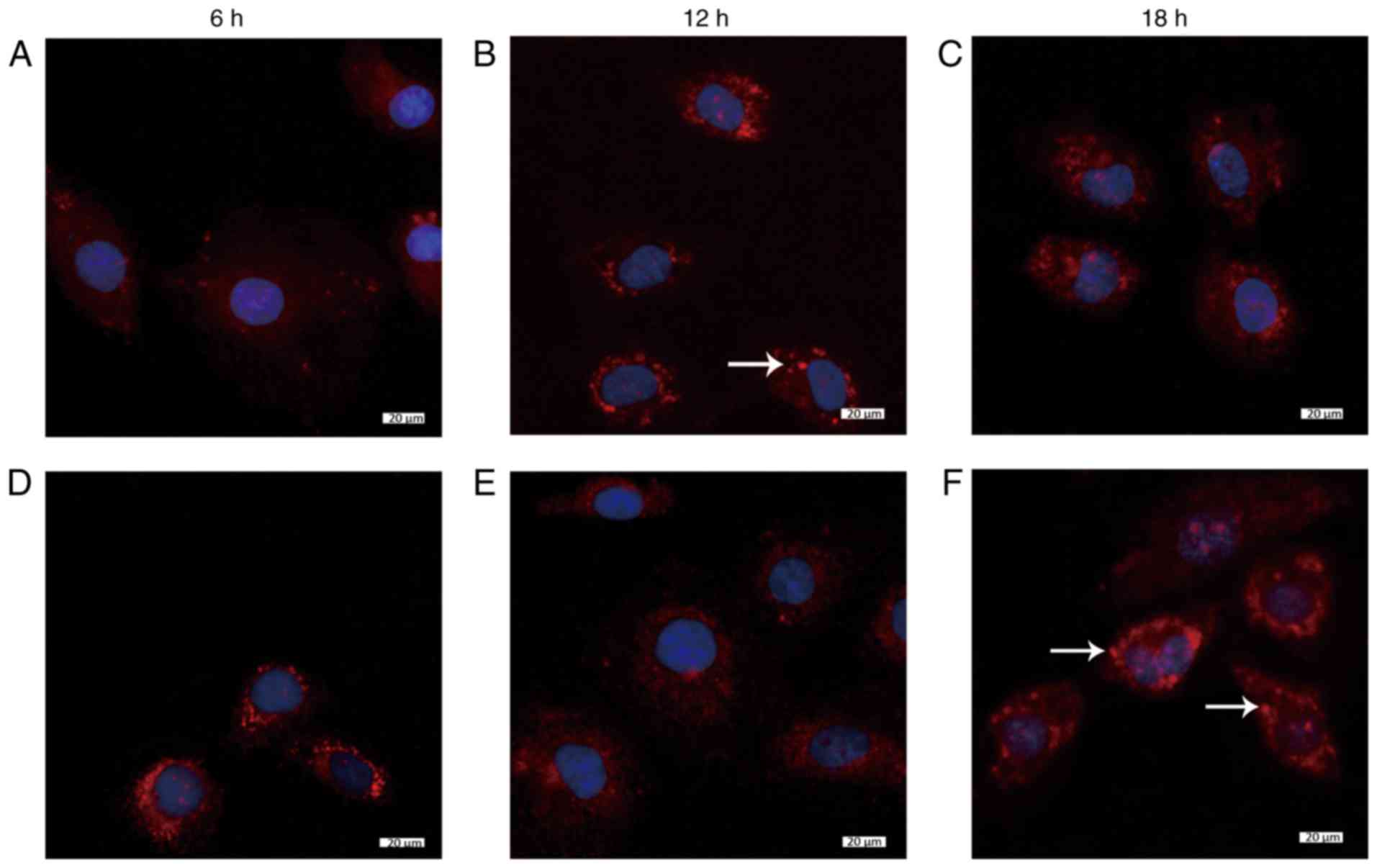
Colocalization of Dvl2 with γ-tubulin and
IFT88 under low and laminar FSS
The colocalization of Dvl2 with γ-tubulin and IFT88
was observed under low and laminar FSS. The results did not show
any localization of Dvl2 and IFT88 in the ciliated area in static
cells, which was considered as a negative control sample, and
γ-tubulin and IFT88 could only be observed in only some cells
(Figs. 6Ac and 7Ac). However, 12 h after loading with
low FSS (1 dynes/cm2), not only did the maximum number
of cells exhibit γ-tubulin localization, but they also exhibited
Dvl2 at the same position (Fig.
6Cc), and there colocalization of IFT88 and Dvl2 was detected
(Fig. 7Cc). IFT88 was mainly
localized at the primary cilia, whereas Dvl2 was detected at the
base, thus indicating that they were not on the same focal plane;
however, these were observed to be positioned in the same area.
Similarly, after 18 h, obvious colocalization of Dvl2 with
γ-tubulin and IFT88 was noted (Figs.
6Dc and 7Dc). Conversely, 12
h after loading with laminar FSS (15 dynes/cm2), Dvl2
colocalized with γ-tubulin in some cells (Fig. 6Fc); however, at 18 h, the
localization of γ-tubulin and IFT88 with Dvl2 was reduced. These
findings suggested that Dvl2 did not localize in a specific area
despite its abundant appearance in the cytoplasm, which indicates
that Dvl2 did not colocalize with γ-tubulin and IFT88 under laminar
FSS at 18 h (Figs. 6Gc and
7Gc).
Discussion
Under static conditions, little free β-catenin is
observed; however, it accumulates and translocates to the nucleus
when stimulated by specific signaling. Previous in vivo and
in vitro experiments indicated that activation of the
Wnt/β-catenin signaling pathway is the normal response to
mechanical stimulation (22).
Wnt/β-catenin increases the sensitivity of osteocytes to FSS
(23). A recent study
demonstrated that blood FSS increases the concentration of
β-catenin in the cytoplasm (24).
In the present study, the expression levels of β-catenin were
upregulated at the early stage of laminar FSS, and translocation to
the nucleus was observed at the early stage of low FSS, thus
suggesting that FSS could activate Wnt/β-catenin for a short
duration. Notably, although an upregulation of the protein was
observed under the action of laminar FSS, translocation to the
nucleus was not observed; therefore, further studies on the
activation of the downstream genes are required.
Dvl2 is a key scaffolding protein, and a branching
point at the Wnt/β-catenin and Wnt/PCP pathways, which has a
pivotal role in Wnt signaling. It acts as a molecular switch in the
activation of Wnt/PCP and the suppression of Wnt/β-catenin
pathways. In vascular ECs, Wnt signaling has been reported to
contribute towards the development of atherosclerosis; the risk
factor, hypercholesteremia, selectively activates canonical Wnt
signaling over the noncanonical pathway by specifically
facilitating membrane recruitment of Dvl and its interaction with
other proteins (25). However,
the effects of low FSS, another risk factor for atherosclerosis, on
Dvl2 are not yet fully understood. In osteoblast cells, the gene
expression levels of Dvl2 and β-catenin are affected
by mechanical stress (26,27).
In the present study, detection of the gene and protein expression
levels demonstrated the laminar FSS could inhibit the expression of
Dvl2 and its localization to the basal body, whereas low FSS led to
enhanced localization when loaded for 12–18 h. Therefore, in
vascular ECs, external FSS could influence the expression and
cellular localization of Dvl2.
Another core protein, Vangl2, is also affected by
FSS. Recently, Curtis-Whitchurch et al reported that laminar
flow (~15 dynes/cm2 for 2–48 h) was able to induce
phosphorylation of Vangl2 in cultured human ECs compared with under
static conditions; however, the disrupted flow led to uniform
distribution throughout the cells (28). In the current study, HUVECs were
exposed to laminar FSS (15 dynes/cm2) and low FSS (1
dynes/cm2) for 6, 12 and 18 h. qPCR demonstrated that
although Vangl2 was insensitive to low FSS, it could be
substantially upregulated by laminar flow. Similarly, fluorescence
labeling indicated that laminar FSS increased the expression of
this protein in the cytoplasm and it was also dispersed in the
nucleus at 18 h. Therefore, Vangl2 may not participate in cellular
activity induced by low FSS, which is essential for ECs under
physiological mechanical conditions.
Unlike Dvl2 and Vangl2, Fuz is an effector of PCP
signaling. However, studies regarding the effects of mechanical
forces on Fuz are absent. In the present study, it was demonstrated
that, although expression was decreased with increasing FSS time,
this was not statistically significant; therefore, it was suggested
that Fuz was not affected by FSS with increasing loading time.
These findings suggested that it may be a stable protein that is
not susceptible to external mechanical signals.
Ciliogenesis is considered to be associated with
cell-cycle dependent cellular progression. However, it has been
reported that it may also be influenced by external factors,
including osmotic stress (29).
Iomini et al reported that primary cilia of human ECs
disassemble under laminar FSS (8). Previous reports have also
demonstrated that ciliogenesis in ECs relies on the type of FSS
during the initial stages of cardiac development and
atherosclerosis (9,30). It was previously revealed that low
FSS could enhance ciliogenesis, whereas laminar FSS could suppress
ciliogenesis (31,32). However, the mechanism underlying
flow-induced ciliogenesis remains to be elucidated (31).
Both Wnt/β-catenin and Wnt/PCP signaling pathways
are associated with ciliogenesis. Several proteins, such as
inversin, Dvl, Vangl2 and inturned are enriched at the basal body
(20). Stabilization of Dvl2 at
the basal body is essential for the function of primary cilia. Dvl2
functions with inturned and Rho in the docking of basal bodies at
the apical plasma membrane (33).
Notably, it has been reported that Dvl2 may participate in the
disassembly of cilia. Lee et al reported that Dvl2
participates in primary cilia disassembly, which is regulated
throughout the cell cycle (33).
However, the possibility of tissue-, time- and
environment-dependent differences in ciliary association with Wnt
signaling cannot be excluded. Furthermore, whether this protein is
also required for the primary cilia assembly induced by FSS has not
yet been reported. In the present study, it was demonstrated that
the variation trends of the basal body protein γ-tubulin and the
cilia assembly protein IFT88 were the same as the core protein Dvl2
in the Wnt signaling pathway. In addition, Dvl2 was gradually
localized to the basal body during low FSS-induced ciliogenesis.
However, this phenomenon was not observed under laminar FSS.
Therefore, it may be hypothesized that in vascular ECs, Dvl2 is a
critical signal molecule for the assembly, instead of disassembly,
of primary cilia induced by mechanical stress.
Vangl2 is also known to be associated with basal
body orientation. In zebrafish embryos, although Vangl2 is not
required for ciliogenesis, it controls the posterior tilting of the
primary cilia and is required for asymmetric localization (34). It has previously been demonstrated
that Vangl2 participates in two PCP signals, while regulating the
apical docking and polarity of cilia in ependymal cells (14). Notably, the dock of ependymal
cilia basal bodies relies on the coupling between hydrodynamic
forces and the PCP protein, Vangl2, within a limited duration
(35). This feature indicates
that Vangl2 may have a role in ciliogenesis regulated by the
hydrodynamic force. In the present study, it was demonstrated that
the altered gene expression and cellular localization of Vangl2 was
in agreement with ciliogenesis under low FSS, as well as, with
cilia disassembly under laminar FSS. Furthermore, due to the
relationship of this protein with cilia disassembly (36), it was speculated that Vangl2 might
serve a dual role in ciliary fate influenced by FSS. Under low FSS,
although the expression of the protein did not change, its
cytoplasmic localization could guide basal body orientation. In
addition, under laminar FSS, the strong expression and cellular
accumulation of the protein might be associated with the primary
cilia disassembly via another pathway.
Fuz also localizes to the basal body. In
Xenopus and mice, the loss of Fuz protein disrupts
ciliogenesis, which in turn, might impair the formation of primary
cilia in the skin (37–39). Fuz controls cilia assembly and
signaling by recruiting Rab8 and Dvl to the primary cilium
(40). Furthermore, Fuz is
required for normal IFT dynamics in vertebrate cilia, and has a
specific role in trafficking of retrograde, but not anterograde,
IFT-B proteins (41). The present
study indicated that, although Fuz was not markedly influenced by
various types of FSS with regards to expression and localization,
it was located at the base of primary cilia in the cytoplasm during
the whole process. Considering that it appears to control the
subcellular localization of the core PCP protein Dvl2, it may be
inferred that, although Fuz was not regulated by an external
mechanical signal, its stationary cellular localization may
propitiously orient Dvl2 to pitch at the basal body.
Although there is no direct evidence indicating the
localization of β-catenin to the basal body, its translocation to
the nucleus has been associated with mutation-induced ciliopathies
(42), thereby suggesting an
indirect relationship between β-catenin and ciliogenesis.
Immunofluorescence demonstrated that both low and laminar FSS
loading for a prolonged period promoted the clustering of this
protein at a specific point in the cytoplasm, in the region of the
basal body, thus suggesting that mechanical stress is crucial in
stabilizing β-catenin in the cytoplasm. Although the alterations in
β-catenin cellular location are not synchronous with that of the
basal body protein γ-tubulin, and Vangl2 and Fuz in the PCP
signaling pathway under various types of FSS, its localization to
the ciliary region indicated its potential connection to primary
cilia. Since the trigger of these two signaling pathways is
diverse, and the correlation between cilia and Wnt/β-catenin is
currently unclear, an in-depth investigation into whether β-catenin
is essential for FSS-induced cilia assembly or disas-sembly is
required.
In conclusion, the results demonstrated that low FSS
promoted the expression of Dvl2 and its colocalization with
the basal body. Furthermore the expression of Vangl2 was
increased by laminar FSS, and β-catenin was translocated into the
nucleus at the early stage of low FSS. These findings suggested
that Dvl2 may participate in low FSS-induced ciliogenesis and
β-catenin may participate at the early stage, whereas Vangl2 may be
associated with laminar FSS-induced cilia disassembly. The results
have important clinical significance for exploring the relationship
between shear stress and the inflammatory response of endothelial
cells.
Acknowledgments
The authors would like to thank Ms. Honglin Lu
(Research Center for Medicine and Biology, Zunyi Medical
University, Zunyi, China) for providing support for instruments and
equipment, and Dr Ming Zhuo (Department of Surgery, University of
Texas Medical Branch, Galveston, TX, USA) for providing advice on
writing.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no.
NSFC-31360278) and the Guizhou Province's Collaborative Foundation
(grant no. LKZ [2013]28).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS and YS conceived and designed the study. YL, XL
and BS performed the experiments. DL processed data. XS and YS
wrote the paper. XS, YS, YL and XL reviewed and edited the
manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hahn C and Schwartz MA:
Mechanotransduction in vascular physiology and atherogenesis. Nat
Rev Mol Cell Biol. 10:53–62. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Egorova AD, van der Heiden K, Poelmann RE
and Hierck BP: Primary cilia as biomechanical sensors in regulating
endothelial function. Differentiation. 83(Suppl): S56–S61. 2012.
View Article : Google Scholar
|
|
3
|
Satir P, Pedersen LB and Christensen ST:
The primary cilium at a glance. J Cell Sci. 123:499–503. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shaheen R, Szymanska K, Basu B, Patel N,
Ewida N, Faqeih E, Al Hashem A, Derar N, Alsharif H, Aldahmesh MA,
et al: Characterizing the morbid genome of ciliopathies. Genome
Biol. 17:2422016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nauli SM, Alenghat FJ, Luo Y, Williams E,
Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, et al:
Polycystins 1 and 2 mediate mechanosensation in the primary cilium
of kidney cells. Nat Genet. 33:129–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nauli SM, Kawanabe Y, Kaminski JJ, Pearce
WJ, Ingber DE and Zhou J: Endothelial cilia are fluid shear sensors
that regulate calcium signaling and nitric oxide production through
polycystin-1. Circulation. 117:1161–1171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marshall WF: Cilia self-organize in
response to planar cell polarity and flow. Nat Cell Biol.
12:314–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iomini C, Tejada K, Mo W, Vaananen H and
Piperno G: Primary cilia of human endothelial cells disassemble
under laminar shear stress. J Cell Biol. 164:811–817. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van der Heiden K, Groenendijk BC, Hierck
BP, Hogers B, Koerten HK, Mommaas AM, Gittenberger-de Groot AC and
Poelmann RE: Monocilia on chicken embryonic endocardium in low
shear stress areas. Dev Dyn. 235:19–28. 2006. View Article : Google Scholar
|
|
10
|
Van der Heiden K, Hierck BP, Krams R, de
Crom R, Cheng C, Baiker M, Pourquie MJ, Alkemade FE, DeRuiter MC,
Gittenberger-de Groot AC and Poelmann RE: Endothelial primary cilia
in areas of disturbed flow are at the base of atherosclerosis.
Atherosclerosis. 196:542–550. 2008. View Article : Google Scholar
|
|
11
|
Espinha LC, Hoey DA, Fernandes PR,
Rodrigues HC and Jacobs CR: Oscillatory fluid flow influences
primary cilia and microtubule mechanics. Cytoskeleton (Hoboken).
71:435–445. 2014. View
Article : Google Scholar
|
|
12
|
Angers S and Moon RT: Proximal events in
Wnt signal transduction. Nat Rev Mol Cell Biol. 10:468–477. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maung SM and Jenny A: Planar cell polarity
in Drosophila. Organogenesis. 7:165–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boutin C, Labedan P, Dimidschstein J,
Richard F, Cremer H, André P, Yang Y, Montcouquiol M, Goffinet AM
and Tissir F: A dual role for planar cell polarity genes in
ciliated cells. Proc Natl Acad Sci USA. 111:E3129–E3138. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jia YY, Li F, Geng N, Gong P, Huang SJ,
Meng LX, Lan J and Ban Y: Fluid flow modulates the expression of
genes involved in the Wnt signaling pathway in osteoblasts in 3D
culture conditions. Int J Mol Med. 33:1282–1288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McCue S, Dajnowiec D, Xu F, Zhang M,
Jackson MR and Langille BL: Shear stress regulates forward and
reverse planar cell polarity of vascular endothelium in vivo and in
vitro. Circ Res. 98:939–946. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tatin F, Taddei A, Weston A, Fuchs E,
Devenport D, Tissir F and Makinen T: Planar cell polarity protein
Celsr1 regulates endothelial adherens junctions and directed cell
rearrangements during valve morphogenesis. Dev Cell. 26:31–44.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Franco CA, Jones ML, Bernabeu MO, Vion AC,
Barbacena P, Fan J, Mathivet T, Fonseca CG, Ragab A, Yamaguchi TP,
et al: Non-canonical Wnt signalling modulates the endothelial shear
stress flow sensor in vascular remodelling. Elife. 5:e077272016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
May-Simera HL and Kelley MW: Cilia, Wnt
signaling, and the cytoskeleton. Cilia. 1:72012. View Article : Google Scholar
|
|
20
|
Wallingford JB and Mitchell B: Strange as
it may seem: The many links between Wnt signaling, planar cell
polarity, and cilia. Genes Dev. 25:201–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Bonewald LF and Johnson ML: Osteocytes,
mechanosensing and Wnt signaling. Bone. 42:606–615. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lara-Castillo N, Kim-Weroha NA, Kamel MA,
Javaheri B, Ellies DL, Krumlauf RE, Thiagarajan G and Johnson ML:
In vivo mechanical loading rapidly activates beta-catenin signaling
in osteocytes through a prostaglandin mediated mechanism. Bone.
76:58–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lai JK and Stainier DY: Pushing yap into
the nucleus with shear force. Dev Cell. 40:517–518. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sheng R, Kim H, Lee H, Xin Y, Chen Y, Tian
W, Cui Y, Choi JC, Doh J, Han JK and Cho W: Cholesterol selectively
activates canonical Wnt signalling over non-canonical Wnt
signalling. Nat Commun. 5:43932014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen X, Guo J, Yuan Y, Sun Z, Chen B, Tong
X, Zhang L, Shen C and Zou J: Cyclic compression stimulates
osteoblast differentiation via activation of the Wnt/beta-catenin
signaling pathway. Mol Med Rep. 15:2890–2896. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Niu Q, Li F, Zhang L, Xu X, Liu Y, Gao J
and Feng X: Role of the Wnt/beta-catenin signaling pathway in the
response of chondrocytes to mechanical loading. Int J Mol Med.
37:755–762. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Curtis-Whitchurch L, Rekapally H and
Hoying J: Phosphorylation and redistribution of Vangl2, a planar
cell polarity protein, in endothelial cells in response to laminar
shear stress. Faseb J. 31:2017.
|
|
29
|
Solter KM and Gibor A: The relationship
between tonicity and flagellar length. Nature. 275:651–652. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Slough J, Cooney L and Brueckner M:
Monocilia in the embryonic mouse heart suggest a direct role for
cilia in cardiac morphogenesis. Dev Dyn. 237:2304–2314. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li XM, Sheng X, Guo PF, Chen CH and He L:
Changes on morphology and ciliogenesis of hUVECs loaded on
different flow shear stress. Life Sci Res. 19:13–18. 2015.
|
|
32
|
Park TJ, Mitchell BJ, Abitua PB, Kintner C
and Wallingford JB: Dishevelled controls apical docking and planar
polarization of basal bodies in ciliated epithelial cells. Nat
Genet. 40:871–879. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee KH, Johmura Y, Yu LR, Park JE, Gao Y,
Bang JK, Zhou M, Veenstra TD, Yeon Kim B and Lee KS: Identification
of a novel Wnt5a-CK1varepsilon-Dvl2-Plk1-mediated primary cilia
disas-sembly pathway. EMBO J. 31:3104–3117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Borovina A, Superina S, Voskas D and
Ciruna B: Vangl2 directs the posterior tilting and asymmetric
localization of motile primary cilia. Nat Cell Biol. 12:407–412.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guirao B, Meunier A, Mortaud S, Aguilar A,
Corsi JM, Strehl L, Hirota Y, Desoeuvre A, Boutin C, Han YG, et al:
Coupling between hydrodynamic forces and planar cell polarity
orients mammalian motile cilia. Nat Cell Biol. 12:341–350. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fliegauf M, Benzing T and Omran H: When
cilia go bad: Cilia defects and ciliopathies. Nat Rev Mol Cell
Biol. 8:880–893. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park TJ, Haigo SL and Wallingford JB:
Ciliogenesis defects in embryos lacking inturned or fuzzy function
are associated with failure of planar cell polarity and Hedgehog
signaling. Nat Genet. 38:303–311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gray RS, Abitua PB, Wlodarczyk BJ,
Szabo-Rogers HL, Blanchard O, Lee I, Weiss GS, Liu KJ, Marcotte EM,
Wallingford JB and Finnell RH: The planar cell polarity effector
Fuz is essential for targeted membrane trafficking, ciliogenesis
and mouse embryonic development. Nat Cell Biol. 11:1225–1232. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dai D, Zhu H, Wlodarczyk B, Zhang L, Li L,
Li AG, Finnell RH, Roop DR and Chen J: Fuz controls the
morphogenesis and differentiation of hair follicles through the
formation of primary cilia. J Invest Dermatol. 131:302–310. 2011.
View Article : Google Scholar
|
|
40
|
Zilber Y, Babayeva S, Seo JH, Liu JJ,
Mootin S and Torban E: The PCP effector Fuzzy controls cilial
assembly and signaling by recruiting Rab8 and Dishevelled to the
primary cilium. Mol Biol Cell. 24:555–565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brooks ER and Wallingford JB: Control of
vertebrate intraflagellar transport by the planar cell polarity
effector Fuz. J Cell Biol. 198:37–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lancaster MA, Schroth J and Gleeson JG:
Subcellular spatial regulation of canonical Wnt signalling at the
primary cilium. Nat Cell Biol. 13:700–707. 2011. View Article : Google Scholar : PubMed/NCBI
|