Introduction
Periodontitis is a chronic inflammation of
supporting tissues caused by specific bacteria adhering to and
growing on the surfaces of the teeth, which can even cause dental
pulp defects and numerous systemic diseases, including pneumonia
and cancer (1–3). Available treatments, such as
gingivectomy, orthodontic therapy and periodontal splint fixation,
are associated with high recurrence rate and pain (4,5).
Recently, stem cell regeneration appears to provide a new direction
in the treatment of periodontitis (6). Dental pulp stem cells (DPSCs) and
periodontal ligament stem cells (PLSCs) represent the basis for an
effective treatment due to their regenerative ability (7). Short treatment time, absence of pain
and no recurrence are the most marked advantages of using these
stem cells (8–10). However, local inflammation not
only blocks the normal physiological cell activities, but also
damages the cells (7). Therefore,
enhancing the treatment effect associated with the understanding of
the mechanism is of utmost importance, while improving the inflamed
microenvironment and/or promoting rapid cell proliferation and
differentiation may represent potential therapeutic strategies.
Periodontitis is characterized by neutrophils and
macrophages (MΦs) that infiltrate the tissues surrounding the teeth
and mediate inflammation, representing the main reason for the
worsening of the microenvironment, causing damage and bone
resorption (11). MΦs can be
divided into M1 and M2, depending on their metabolism (12). M1 secret pro-inflammatory
cytokines and chemokines, while M2 secret cytokines that decrease
inflammation, including interleukin (IL)-10 and transforming growth
factor (TGF)-β (12,13). Previous studies have reported that
certain inflammatory factors or a specific local microenvironment
promote stem cell proliferation (14,15). Therefore, promoting MΦ
transformation from M1 to M2 can reduce inflammation and achieve a
therapeutic effect.
High-fluence low-power laser irradiation (HF-LPLI)
serves an important role in regulating cell proliferation,
differentiation, apoptosis and other cell physiological activities
(16). It has been demonstrated
that HF-LPLI can also serve a role in regulating inflammation when
used for an appropriated period of time (17). HF-LPLI light sources include the
He-Ne laser and the GaAs semiconductor laser. In addition, this
technique has been reported to promote inflammation resolution,
which in turn is associated with myeloperoxidase activity, as well
as cyclooxygenase (COX)-1 and COX-2 gene expression (17).
Therefore, in the present study, it was hypothesized
that HF-LPLI may also promote inflammation resolution by inducing
neutrophil apoptosis and MΦ reprogramming subsequent to appropriate
inflammation activation of stem cells, as well as consequent
improvement of osteogenic differentiation, thereby enhancing the
efficacy of stem cell treatment to combat periodontitis. Following
in vitro validation of this hypothesis and the associated
mechanism of action by several techniques, an animal periodontitis
model was designed. Stem cell therapy was used, while HF-LPLI was
subsequently administered. Finally, the difference in cell
proliferation and osteogenic differentiation was evaluated between
the control and experimental groups.
Materials and methods
DPSC and PLSC isolation, identification
and culture
DPSCs were obtained from dental pulp tissue
explants. The third molars of adult patients (age, 16–25 years)
were obtained from the Department of Stomatology at the Liaocheng
People's Hospital (Liaocheng, China). All the patients included
were informed of the condition and agreed to participate in the
research. Teeth were first cleaned by normal saline containing 3%
antibiotic-antimycotic solution (Gibco; Thermo Fisher Scientific,
Inc.), and then sterilized dental fissure burs were used to expose
the pulp chamber. Subsequently, dental pulp tissues were dissected
into fragments (<0.5 mm), placed into a 6-cm dish containing
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 20% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and
incubated at 37°C with 5% CO2 for 2–3 weeks. DPSCs were
routinely passaged, and third passage cells were used for further
experiments. An inverted phase contrast microscope (Nikon
Corporation, Tokyo, Japan) was used to observe the cell morphology
(18,19).
PLSCs were obtained from teeth from the same
hospital. Initially, the tooth was washed by normal saline
containing 3% antibiotic-antimycotic solution (Gibco; Thermo Fisher
Scientific, Inc.). Pulp tissues were separated from the surface of
the tooth. In total, 1 g/l collagenase type I and 2.4 g/l dispase
(Gibco; Thermo Fisher Scientific, Inc.) were used to digest the
tissues for 1 h at 37°C. Samples were then centrifuged at 400 ×
g for 4 min at 4°C by TD5Z Multi-frame Centrifuge (Jintan
Changzhou Instrument Factory, Changzhou, China), and the pellet was
collected. Cells were resuspended in DMEM containing 20% FBS. Cells
at the sixth passage were used in subsequent experiments (20,21).
Flow cytometry
DPSCs and PLSCs were identified by flow cytometry.
The cell suspension was prepared using an icy buffer (PBS), the
cell concentration was adjusted to 5×105 cells/ml. Next,
samples were centrifuged at 1,500 × g for 3 min at 37°C and the
appropriate fluorescent-labeled antibodies were added to each
sample. Anti-STRO-1 antibody was used in DPSCs (cat. no. ab214086;
1 μg/ml), cluster of differentiation (CD)44 antibody was
used in PLSCs (cat. no. ab157107; 1 μg/ml), and CD133
antibody was used in both cell samples (cat. no. ab19898; 1
μg/ml; all purchased from Abcam (Cambridge, MA, USA).
Subsequent to antibody addition, the samples were centrifuged at
1,500 × g for 3 min at 37°C, followed by the addition of 0.5 ml
formaldehyde and incubated for 24 h in −4°C. Rabbit Anti-Rat IgG
H&L (horseradish peroxidase; cat. no. ab6734; Abcam) was added
as seconded antibody and incubated for 8 h in −4°C. Samples were
kept at 4°C until the end of the experimental analysis. Flow
cytometry (BD LSRFortessa™ cell analyzer; BD Biosciences, San Jose,
CA, USA) was finally used to identify the cells (18–21).
The two types of stem cells were cultured into
6-wells plates (5×104 cells/well). Cells were divided
into four groups, including the control, MΦ, MΦ+HF-LPLI, and the MΦ
followed by HF-LPLI groups. In these groups, the concentration of
MΦ was 1×104 cells/well. In the MΦ group, the cells were
co-cultured with MΦ for 6 h and without HF-LPLI treated. In the
MΦ+HF-LPLI group, stem cells were treated with HF-LPLI and
simultaneously co-cultured with MΦ. In the MΦ followed by HF-LPLI
group, stem cells were treated with HF-LPLI subsequent to
co-culturing with MΦ for 6 h.
Neutrophil and MΦ isolation and
culture
Male Sprague Dawley (SD) rats (age 6–8 weeks old,
250–280 g, n=10) were obtained from the Laboratory Animal Center of
Taishan Medical University (Taian, China). SD rats were cultured in
37°C, relative humidity 45% and 12-h light/dark environment with
low fat diet and free to water. The rats were then subjected to an
intraperitoneal injection of 1 ml 4% thiogly-collate (Guangzhou
SWAN Chemical Co., Ltd., Guangzhou, China; cat. no. 2365-48-2).
After 24 h, 10 ml cold Dulbecco's phosphate-buffered saline
containing 100 U/ml penicillin was injected into the peritoneum.
Peritoneal lavage fluids were mixed thoroughly and collected.
Neutrophils from the fluid were spun and re-suspended with
pre-warmed serum-free RPMI-1640 medium containing 0.05% bovine
serum albumin (WelGENE, Inc., Pohang, Korea). Cells were plated
into a 6-well plate (5×106 cells/well) (22), and divided into the control and
lipopolysaccharide (LPS) group for apoptosis detection by flow
cytometry. In the LPS group, cells were cultured in medium
containing 1 μg/ml LPS (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) which was used to create an inflammatory
culture condition.
Rat MΦs were purchased from the Shanghai Institutes
for Biological Sciences of the Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in RPMI-1640 medium
(WelGENE, Inc.) containing 10% FBS, 100 U/ml penicillin and 100
μg/ml streptomycin, followed by plating in a 6-well plate
(4×106 cells/well) and incubation at 37°C in 5%
CO2 for 48 h. MΦs were divided into three groups,
including the control, LPS and HF-LPLI groups, for
immunofluorescence detection. In the HF-LPLI group, HF-LPLI
treatment was applied following 1 μg/ml LPS incubation for
24 h.
HF-LPLI application
In the present study, a He-Ne laser with output
power at 632.8 nm and a 10.5-mW red laser were used for HF-LPLI
application. For in vitro experiments, samples were directly
treated under 20 J/cm2 HF-LPI for 1 h. Furthermore, oral
radiation was performed by optical fiber, 20 J/cm2 for 1
h to the first molars and their periodontal tissue with other teeth
covered by aluminum foil paper (16).
Apoptosis detection by flow
cytometry
Neutrophils and MΦs cultured in vitro were
divided into the control and LPS groups. The supernatant of cells
was collected into a 15 ml centrifuge tube. EGTA-free trypsin was
added to digest the neutrophils, and trypsin was added to the
corresponding centrifuge tube. Next, PBS was added to wash down the
cells attached on the tube. Samples were centrifuged at 1,000 × g
for 3 min, and then PBS was used to wash the cells twice, following
by further centrifugation at 1,000 × g for 3 min. Subsequently,
Annexin V was added according to the manufacturer's protocol
described in the FITC Annexin V Apoptosis Detection kit (BD
Biosciences). Flow cytometry (BD LSRFortessa™ cell analyzer; BD
Biosciences) was finally used to evaluate apoptosis (23–25).
Caspase-3 activity analysis
The caspase-3 activity of neutrophils was detected
by a Caspase-3 Activity Assay kit (Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer's
protocol. Briefly, samples at 0, 2, 4 and 6 h following HF-LPLI
treatment were ground and then incubated in lysis buffer on ice for
15 min. The mixture was centrifuged at 16,000 × g for 10 min at
4°C, followed by the addition of 2 mmol Ac-DEVD-pNA (Sigma-Aldrich;
Merck KGaA) for 1 h. Absorbance was measured at 405 nm using a
microplate reader (Thermo Fisher Scientific, Inc.). The caspase-3
activity of each sample was calculated according to the standard
curve and normalized to the total protein concentration (26,27).
Immunofluorescence analysis
MΦs were seeded into 6-well plates at a density of
5×104 per well and incubated for 48 h, and then were
washed three times with PBS, fixed with 4% paraformaldehyde for 15
min and subsequently treated with 0.5% Triton X-100 for 20 min at
room temperature. Sufficient dilution of primary polyclonal
anti-rat CD86 (cat. no. MA110293; 1:1,000) or CD163 (cat. no.
AB-2716934; 1:1,000) antibodies against M1 and M2 (Thermo Fisher
Scientific, Inc.) was added to each slide, and incubated overnight
at 4°C. The slides were washed three times with PBS/Tween-20 for 3
min each time and then treated with secondary antibodies at 20–37°C
in the dark for 1 h. Next, samples were incubated with DAPI
(Beyotime Institute of Biotechnology) for 5 min. Slides were dried
using an absorbent paper, mounted with liquid sealing agents
containing anti-fluorescence quenching reagents and observed under
a fluorescent microscope for image acquisition (28,29).
NO concentration detection
DAF-FM DA (Beyotime Institute of Biotechnology) was
used to detect the NO concentration in adherent neutrophils. DAF-FM
DA was diluted to 1:1,000 using the dilution buffer provided in the
kit. Next, samples were incubated in an incubator for 20 min. Cells
were collected, treated with 5 μmol/l DAF-FM DA, incubated
at 37°C for 20 min and subjected to flow cytometry analysis
(30).
A total of 7 scalp needles were used to draw blood
from the caudal vein of the SD rats, then centrifuged 2,000 × g for
2 min at 37°C to obtain the rat serum. In order to detect the NO
concentration in the rat serum, a Nitric Oxide Assay kit
(Sigma-Aldrich; Merck KGaA) was used. The assay was conducted
according to the protocol provided in the kit, and NO was measured
using the Thermo Scientific™ SPECTRONIC™ 200 spectrophotometer
(Thermo Fisher Scientific, Inc.) (31).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
RT-qPCR was used to evaluate if DPSCs and PLSCs
maintained stem cell properties and osteogenesis differentiation
using the GoTaq® qPCR Master Mix, Promega A60021000
Reactions (Promega Corporation, Madison, WI, USA). Cells were
digested by TRIzol RNA separation reagent (Thermo Fisher
Scientific, Inc.). The RNA concentration was determined by
ultraviolet spectrophotometer. DEPC H2O was used to zero
instrument, it's also used to dilute RNA. The absorbance of RNA in
260 nm (A260) was tested. The analytical formula of RNA
concentration is A260X dilution multiple ×40 μg/ml. qPCR
reaction was then performed in a thin wall 0.5-ml reaction tube,
and the mixture consisted of 13.5 μl DEPC water, 2 μl
dNTP Mix, 1 μl oligo(dT) 12–18, 1 μl AMV/Tfl 5X
reaction buffer, 0.5 μl RNase inhibitor, 1 μl AMV-RT
reverse transcriptase. Firstly, samples were incubated at 42°C for
1 h, 95°C for 5 min, the cDNA templates were produced. Secondly, 10
μl cDNA tempaltes, 10 μl 2Xtap mix, 31.5 μl
DEPC water, 1 μl dNTP Mix, 2 μl specific upstream and
downstream primers of the target genes, including Sox2, Oct4, Klf4,
ALP, OCN and Runx2 (Table I), 5
μl 10X buffer and 0.5 μl DNA polymerases were add to
a new PCR tubes. The PCR thermal cycling conditions were: 95°C (5
min), 95°C (1 min), 55°C (90 sec), 72°C (90 sec) at 72°C and (10
min) at 4°C. The number of cycles (step 2-step 4) was 30. A total
of 10 μl PCR product was detected by 0.5% agarose gel
electrophoresis. Agarose gel electrophoresis was used to analyze
the PCR products observed under UV light. Analysis of relative gene
expression data using the 2−ΔΔCq method (32–34).
 | Table IPrimers used in quantitative
polymerase chain reaction. |
Table I
Primers used in quantitative
polymerase chain reaction.
| Target gene | Primer
sequence | Annealing
temperature (°C) | Cycles | Product size
(bp) |
|---|
| Oct4 | F:
5′-CAGTGCCCGAAACCCACAC-3′
R: 5′-GGAGACCCAGCAGCCTCAAA-3′ | 60 | 40 | 161 |
| Sox2 | F:
5′-ACACCAATCCCATCCACACT-3′
R: 5′-GCAAACTTCCTGCAAAGCTC-3′ | 60 | 40 | 224 |
| Klf4 | F:
5′-GAGCCCAAGCCAAAGAGG-3′
R: 5′-ATCCACAGCCGTCCCAGT C-3′ | 60 | 40 | 183 |
| ALP | F:
5′-CCACGTCTTCACATTTGGTG-3′
R: 5′-AGACTGCGCCTGGTAGTTGT-3′ | 60 | 40 | 196 |
| OCN | F:
5′-GGCAGCGAGGTAGTGAAGAG-3′
R: 5′-CTGGAGAGGAGCAGAACTGG-3′ | 60 | 40 | 230 |
| Runx2 | F:
5′-CCCGTGGCCTTCAAGGT-3′
R: 5′-CGTTACCCGCCATGACAGTA-3′ | 60 | 40 | 179 |
| GAPDH | F:
5′-GGGAAACTGTGGCGTGAT-3′
R: 5′-GAGTGGGTGTCGCTGTTGA-3′ | 60 | 40 | 299 |
EdU assay
In order to detect the proliferation and vitality of
cells, Click-iT™ EdU Flow Cytometry Assay kit (Alexa Fluor™ 488;
Thermo Fisher Scientific, Inc.) was used according to the
manufacturer's protocol. Briefly, cells were washed three times in
PBS containing 0.05% Triton X-100 prior to EdU-DNA detection. For
nuclear staining (blue), stem cell samples were treated with 1
μg/ml DAPI in PBS containing 0.1% Triton X-100. Next, the
samples were incubated for 1 h at 37°C in the dark. Newly
proliferating nucleotides were stained red by 2X EdU solution
according to the manufacturer's protocol. Then, the samples were
visualized under a fluorescence inverted microscopy (VMF400I,
Suzhou Jing Tong Instrument Co., Ltd. Suzhou, China) (35–37).
ELISA
The MΦ supernatant was collected from the three MΦ
groups cultured in vitro, including the control, LPS and
HF-LPLI cell groups. In addition, serum samples from the hearts of
rats in the control, periodontitis, and 1 or 3-week HF-LPLI
treatment groups were obtained by 2,000 × g for 15 min at 37°C.
Subsequently, 100 μl Standards and 100 μl samples
were added into the corresponding reaction plate well. Subsequent
steps were conducted according to the protocol provided by the
MitoBiogenesis™ In-Cell ELISA kit (Colorimetric; cat.
no. ab110217; Abcam). Finally, the optical density was measured at
450 nm (38,39).
Alizarin red S staining
For the evaluation of osteogenesis differentiation,
local tissues obtained from the animal model were washed twice with
PBS, fixed with 10% formaldehyde for 20 min and washed twice with
PBS. Cells were treated with 40 mM alizarin red S solution
(Sigma-Aldrich; Merck KGaA) for 20 min at room temperature.
Finally, samples were washed with distilled water for four times
for further evaluation under a microscope (40).
Animal experiment
Male SD rats (age 6–8 weeks old, 250–280 g) were
obtained from the Laboratory Animal Center in Taishan Medical
University (N=24 in total, n=8 for a group). SD rats were housed at
37°C, relative humidity 45% and dark environment with low fat diet
and free to water. All animal procedures were performed according
to the Guide for the Care and Use of Laboratory Animals (18), following the ARRIVE guidelines
(41), and were approved by the
Institutional Clinical Experiments Committee of the Liaocheng
People's Hospital (Liaocheng, China) and the Animal Care Committee.
Rats were divided into three groups of 8 rats each, including the
control, periodontitis and HF-LPLI groups. Subsequent to gingival
peeling and high-sugar feeding, all rats received azithromycin (10
mg/500 ml; Shanghai Yingrui Biopharma Co., Ltd., Shanghai, China)
for 4 days, followed by a 7-day antibiotic-free period. Next, rats
were anes-thetized with 10% chloral hydrate. A 0.2-mm wire was
placed in the dentogingival area of both mandibular first molars in
the periodontitis and HF-LPLI groups for 48 h, while the wire was
placed and immediately removed in the control group. All rats were
fed with the same food (at 8:00 a.m. and 8:00 p.m.). Rats in the
HF-LPLI groups underwent surgery, followed by a 1-week
20-J/cm2 HF-LPLI treatment for 1 h/day after local
injection of 1×105/ml stem cells in 100 μl,
followed by the in vivo bioluminescence imaging and H&E
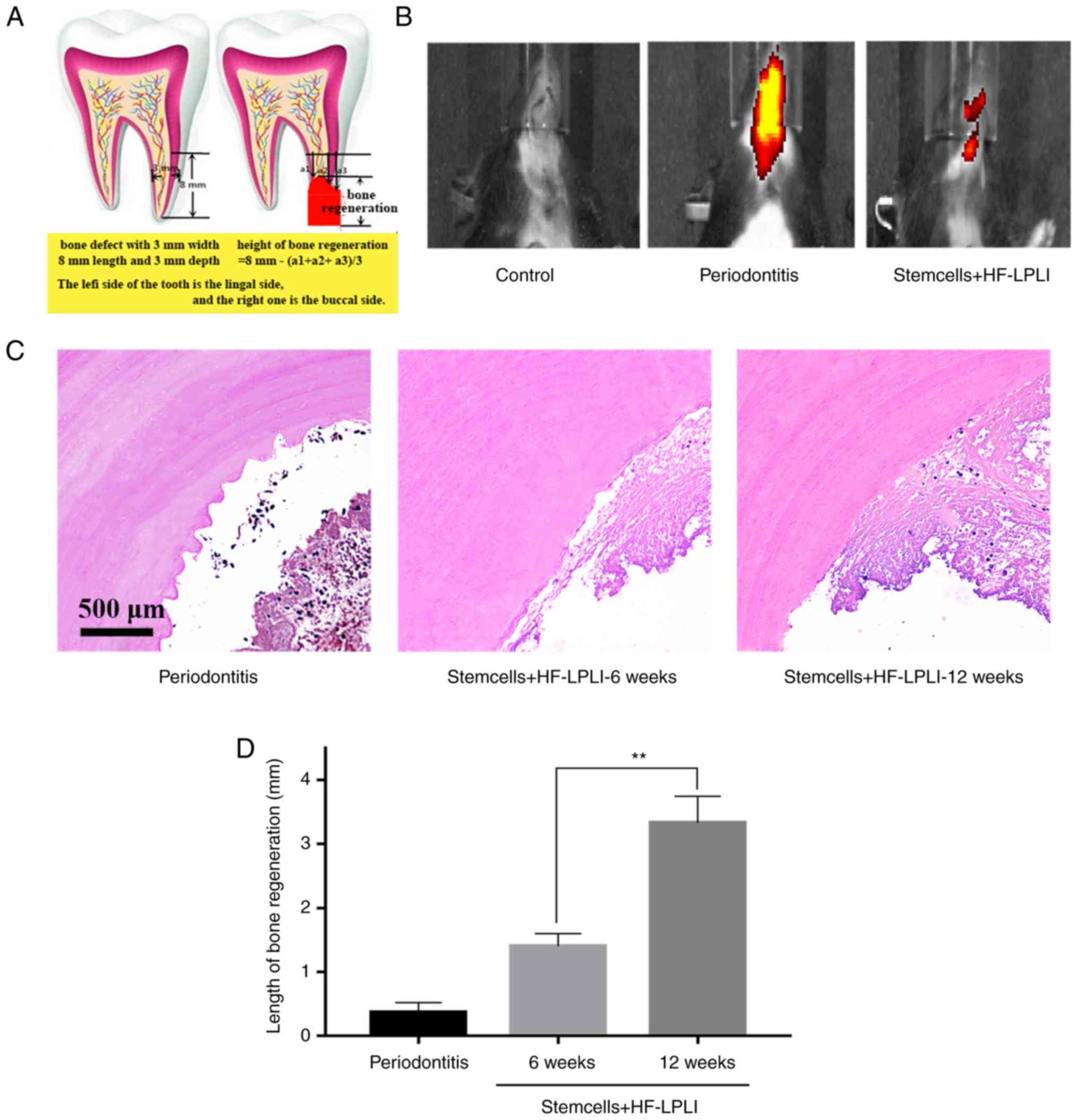
staining (42,43). The Height of bone regeneration was
calculated as 8 mm−(a1 + a2 + a3)/3. In the formula, a1, a2 and a3
were three random height measurements of residual defect height
following bone regeneration.
In vivo bioluminescence imaging
SD rats were divided into three groups, including
the control (6 weeks), HF-LPLI/6-week and HF-LPLI/12-week groups,
and were analyzed at the corresponding time point. Rats were
anesthetized with 10% chloral hydrate and treated with fluorescein
that was injected through the tail vein. At 60 min post-infection
images were obtained after intraperitoneal injection of D-luciferin
(150 mg/kg body weight; Thermo Fisher Scientific, Inc.), followed
by 50 μl D-luciferin (0.75 mg in PBS) through the oral
cavity. The IVIS Lumina image system (Xenogen Corporation, Alameda,
CA, EUA) was used to capture the images (44).
Hematoxylin and eosin (H&E)
staining
Periodontal tissue ~200×200 μm fragments from
the animal model were dewaxed by xylene, washed with an alcohol
gradient and then soaked in distilled water. Next, tissues were
stained with Harris hematoxylin solution for 5 min. Subsequent to
rinsing, sections underwent color separation in 0.5%
hydrochloric-alcohol solution for 5 sec, followed by washing two
times with 95% ethyl alcohol for 5 min each, two times with
absolute ethyl alcohol for 5 min each and xylene + ethyl alcohol
(1:1) for 5 min. Finally, samples were subjected to xylene cleaning
and neutral gum sealing, and followed by visualization under light
microscope (Nikon Corporation, Tokyo, Japan) (35–37).
Statistical analysis
Statistical analysis was performed using the SPSS
software package (version 13; SPSS, Inc., Chicago, IL, USA). The
results are expressed as the mean ± standard deviation, and the
analysis of variance test was used to evaluate the statistical
significance between two groups. P<0.05 was considered to
indicate a statistically significant when the.
Results
HF-LPLI induces MΦ transformation and
neutrophil apoptosis
The inflammatory reaction is one of the causes of
periodontal tissue damage. The results of the present study
revealed that HF-LPLI promoted inflammation resolution in
vitro by promoting neutrophil apoptosis and MΦ reprogramming.
Flow cytometry demonstrated that cells obtained and labeled by
STRO-1 and CD 44 were the DPSCs and PLSCs we wanted (data not
shown). Furthermore, it also demonstrated neutrophil apoptosis
increased subsequent to HF-LPLI treatment, ranging between 7.28 and
18.1% (Fig. 1A). In addition,
HF-LPLI induced a time-dependent increase of caspase-3 activation,
which was 2.8±0.17 times higher than the initial value at 6 h
(Fig. 1B). This further
demonstrated the role of HF-LPLI in promoting apoptosis. In fact,
the MΦ apoptosis was also tested following HF-LPLI treatment, and
the results indicated that HF-LPLI had no effect on MΦ apoptosis
(data not shown). In the pre-experiments, it was observed that 6 h
was the best time period for inflammation to promote the function
of stem cells (data not shown). In the tests conducted in the
present study, all the related experimental design selected
inflammation treatment to last 6 h (data not shown).
Immunofluorescence analysis detected no significant differences in
the morphology of different types of MΦs, while the reprogramming
of MΦs was confirmed. The LPS group exhibited an increase in the
number of CD86-M1 (red staining), while the HF-LPLI-treated group
mainly exhibited green staining, corresponding to CD163-M2
(Fig. 1C). Following HF-LPLI
treatment, the number of CD86-M1 decreased from 73.21±2.23% to
18.29±1.47% (P<0.05), while CD163-M2 increased from 9.26±1.07%
to 58.88±1.97% (Fig. 1D). In
addition, DAF-FM IL-6 and tumor necrosis factor α (TNF-α) levels
significantly decreased following HF-LPLI treatment, while the
levels of anti-inflammatory cytokines, including IL-10 and TGF-β,
secreted by M2 were increased (Fig.
1E and F).
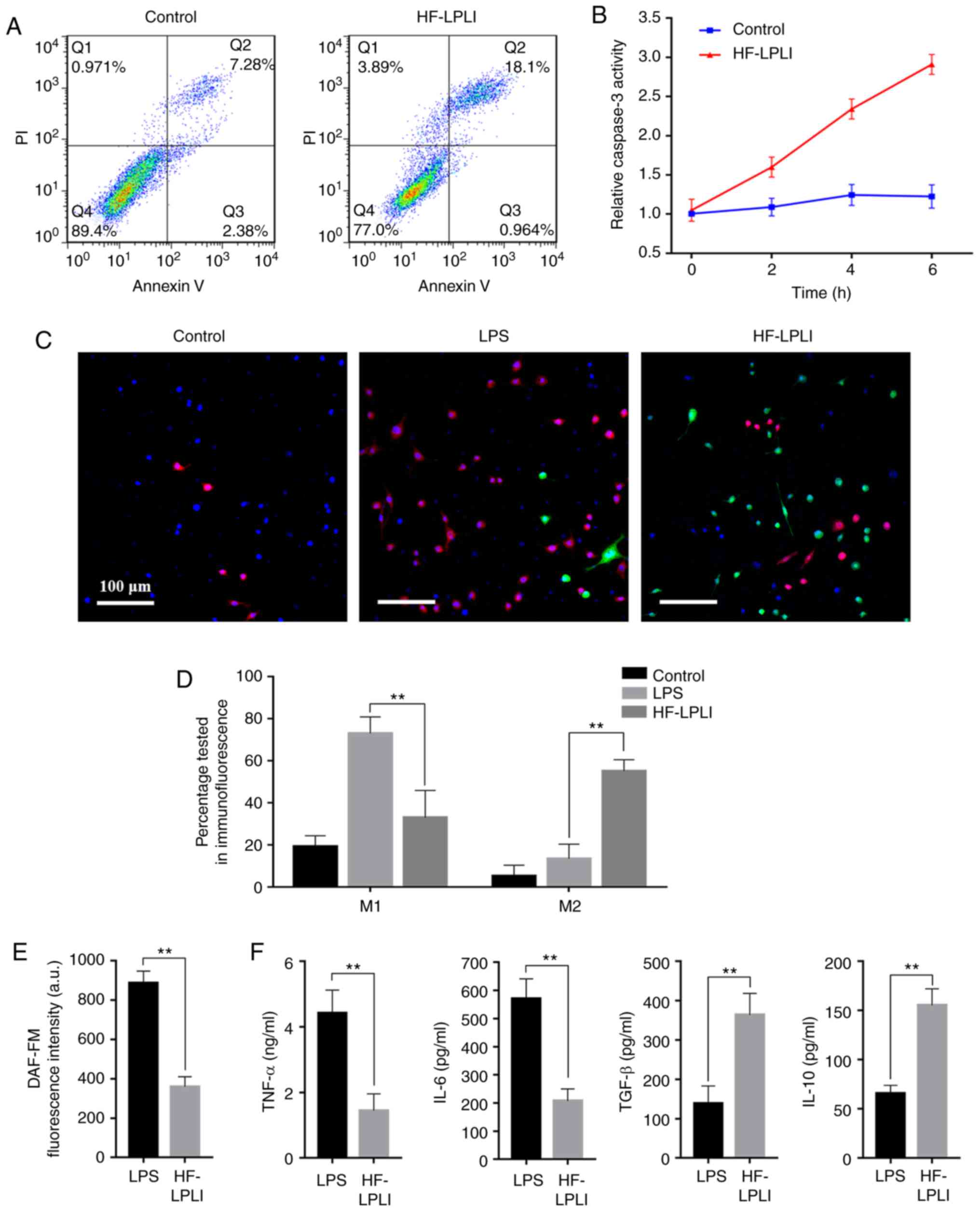 | Figure 1HF-LPLI promoted neutrophil apoptosis
and MΦ reprogramming, and inhibited the release of inflammatory
factors. (A) Apoptosis of HF-LPLI-treated neutrophils was detected
by flow cytometry. (B) Apoptosis activation in neutrophils,
detected by caspase-3 activity assay. (C) CD86 and CD163
immunofluo-rescent staining using red and green fluorophores,
respectively, and nuclei stained with DAPI (scale bar, 100
μm). (D) Bar graph of CD86- and CD163-positive MΦ percentage
by immunofluorescent staining. (E) NO concentration examined by
DAF-FM DA. (F) Concentration of inflammatory factors, including
TNF-α, IL-6, IL-10 and TGF-β, determined by ELISA. The results are
representative of 10 independent experiments, and are expressed as
the mean ± standard deviation. **P<0.01 vs. LPS group (n=10).
HF-LPLI, high-frequency low-power laser irradiation; MΦ,
macrophage; TNF-α, tumor necrosis factor α; IL, interleukin; TGF-β,
transforming growth factor-β; LPS, lipopolysaccharide. |
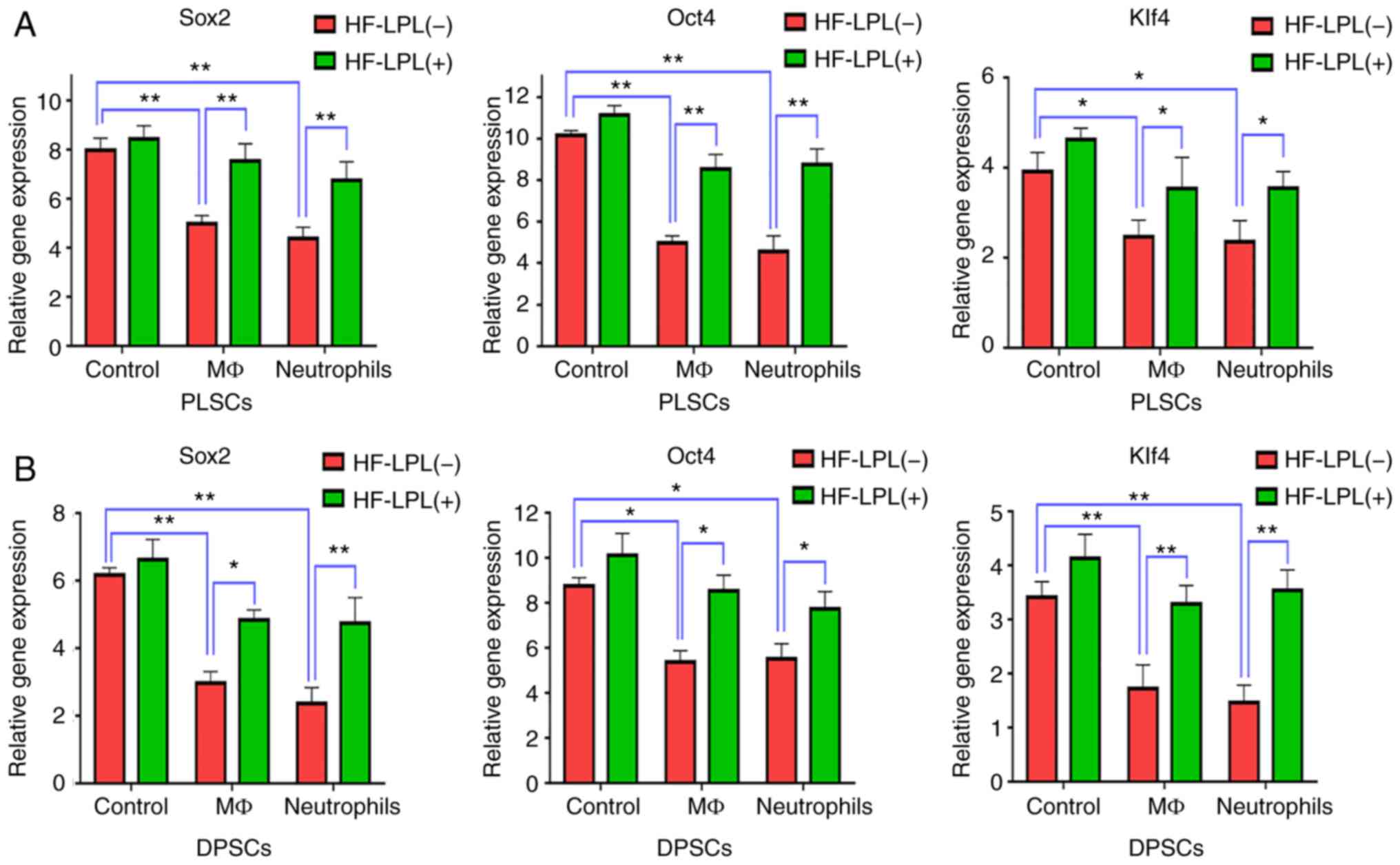
HF-LPLI maintains the stem cell
properties of DPSCs and PLSCs
RT-qPCR was used to detect the mRNA expression
levels of genes associated with the reprogramming of stem cells,
including Sox2, Oct4 and Klf4, prior to and following HF-LPLI
treatment. The results indicated that Sox2, Oct4 and Klf4
expression levels in DPSCs and PLSCs in the control group treated
with HF-LPLI slightly increased as compared with those in cells
without HF-LPLI treatment, although a significant difference was
not observed (P>0.05; Fig. 2A and
B). When stem cells were co-cultured with MΦs or neutrophils,
the expression of these three genes was significantly decreased
(P<0.05), while HF-LPLI treatment inhibited this effect and
increased the gene levels. More specifically, Oct4 expression in
PLSCs was decreased to a value of 4.3±0.2 and 4.2±0.2 when stem
cells were co-cultured with MΦs or neutrophils, respectively, while
this level was 10.2±0.3 in the culture of stem cells without
inflammatory cells, and HF-LPLI treatment was able to raise this
level to 6.0~8.0±0.4 to a value of <3 (Fig. 2A). In DPSCs, inflammatory cells
exhibited a marked effect on Sox2 expression, which was decreased
from 6.2±0.4 to a value of <3. However, HF-LPLI attenuated this
effect, increasing the Sox2 expression to ~5 (Fig. 2B).
HF-LPLI improves DPSC and PLSC
proliferation
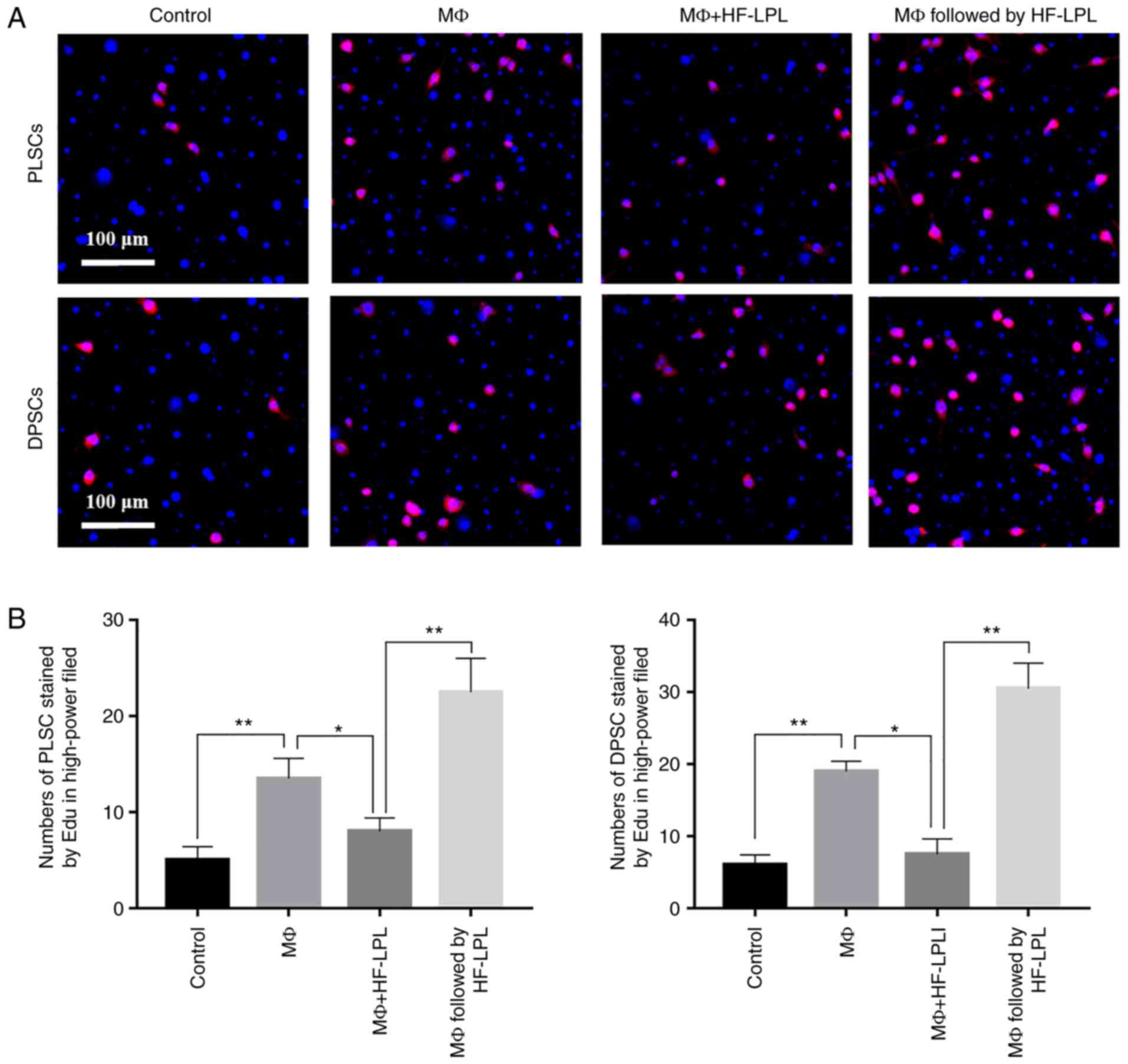
EdU assay results demonstrated that stem cell
proliferation was not evident when these cells were cultured alone,
while their proliferation was evident when co-cultured with MΦs.
When stem cells were co-cultured with MΦs and simultaneously
treated with HF-LPLI, the HF-LPLI treatment did not increase the
promotion of stem cell proliferation induced by inflammatory cells.
However, after co-culture for 6 h followed by treatment with
HF-LPLI, the stem cell proliferation was greatly improved, as shown
by the EdU results (Fig. 3A), and
the difference with the other experimental groups and the control
group was statistically significant (P<0.05). The number of
EdU-positive cells in the MΦ group was 13±1 and 19±1 for DPSCs and
PLSCs, respectively, while the number of cells was 5±1 and 6±1 in
the control group, respectively. When HF-LPLI treatment was
performed simultaneously with the MΦ co-culture in the stem cells,
the number of EdU-positive cells was 8±1 or 7±1 for DPSCs and
PLSCs, respectively. However, the number of proliferating cells was
21±2 or 29±2, respectively, when HF-LPLI treatment was performed at
6 h after MΦ co-culture (Fig.
3B).
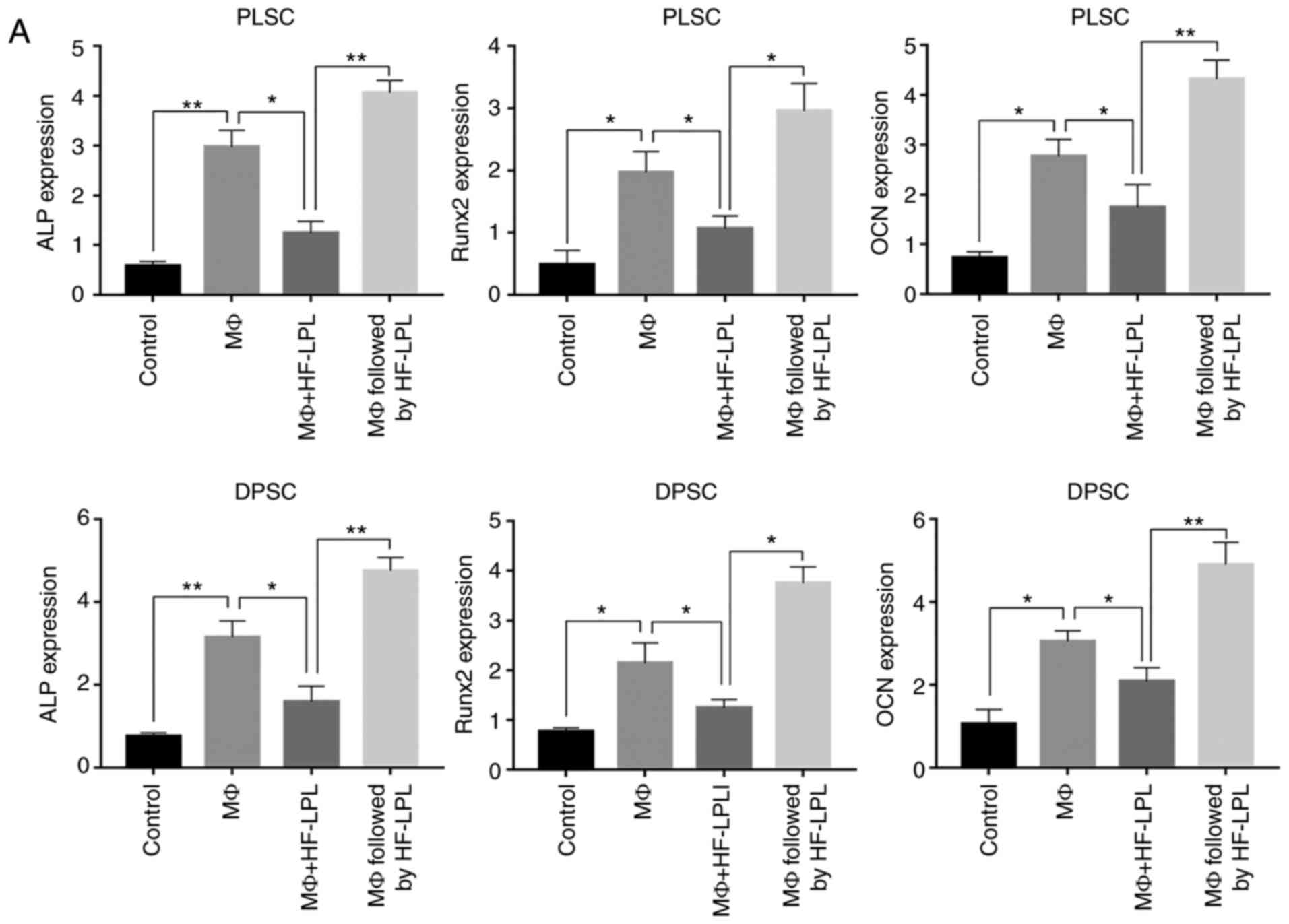
HF-LPLI promotes DPSC odontoblastic
differentiation
The present study next focused on the establishment
of the HF-LPLI ability to induce cell differentiation. ALP, OCN and
Runx2 gene expression levels were evaluated in cells obtained after
2 weeks of proliferation, as described earlier. The results
demonstrated that the relative expression levels of the three genes
in the MΦ group were significantly increased to ~2-3±0.2
(P<0.05; Fig. 4A). However,
gene expression was inhibited to nearly 1.3±0.2 when co-culture
with MΦs and HF-LPLI treatment were performed simultaneously, while
gene expression was promoted when HF-LPLI was performed after 6 h
of MΦ co-culture. In addition, OCN expression in DPSCs reached
4.7±0.8 (Fig. 4A).
Stem cells in the different MΦ and HF-LPLI groups
were then subjected to alizarin red S staining to identify the
calcium salt components in the cells. A significant increase in the
calcium ion composition was found in the MΦ followed by HF-LPLI
treatment group (Fig. 4B). For
alizarin red S staining, the absorbance at 570 nm was ~0.6 at 2
weeks and 0.9 at 4 weeks in the group with MΦ co-culture followed
by HF-LPLI treatment, while it was <0.5 in the control and MΦ
co-culture alone groups (Fig.
4C).
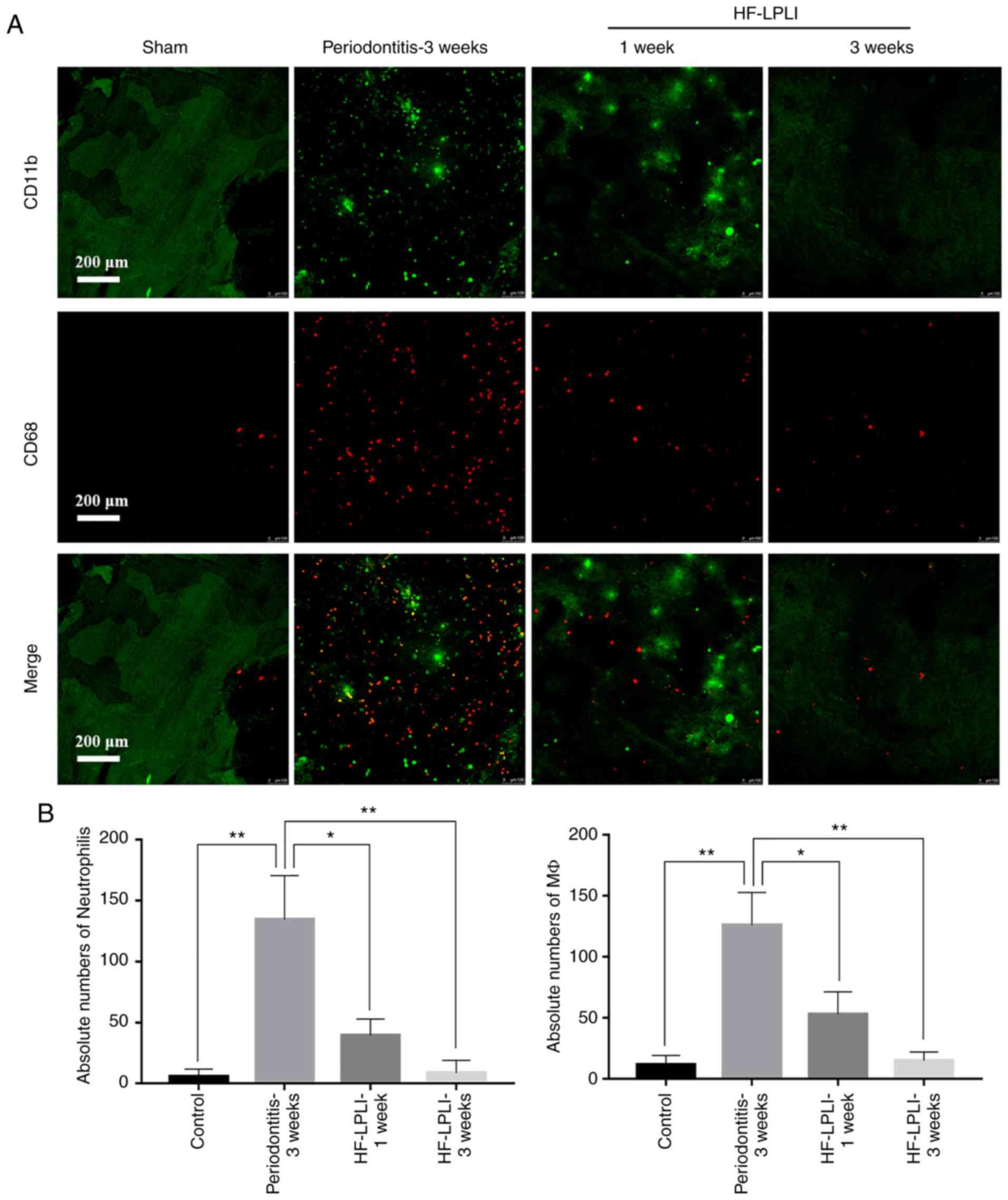
HF-LPLI reduces the infiltration of
inflammatory cells and release of inflammatory factors in
periodontitis rats
A large number of neutrophils and MΦs were detected
in the inflammatory sites in periodontitis rats during
immunofluorescence analysis. After 1 week of HF-LPLI treatment, the
number of neutrophils and MΦs in the surrounding tissues decreased
significantly from 131±6 and 119±5 to 44±3 and 52±4, respectively.
After 3 weeks of detection, further improvement in the number of
inflammatory cells was achieved, which were inhibited to 9±2 and
11±2, respectively. CD11b-labeled neutrophils and CD86-labeled MΦs
were abundant in the surrounding tissues of periodontitis rats,
while HF-LPLI application significantly decreased the staining for
the inflammatory cells. Furthermore, this effect was found to be
enhanced with time (Fig. 5A and
B).
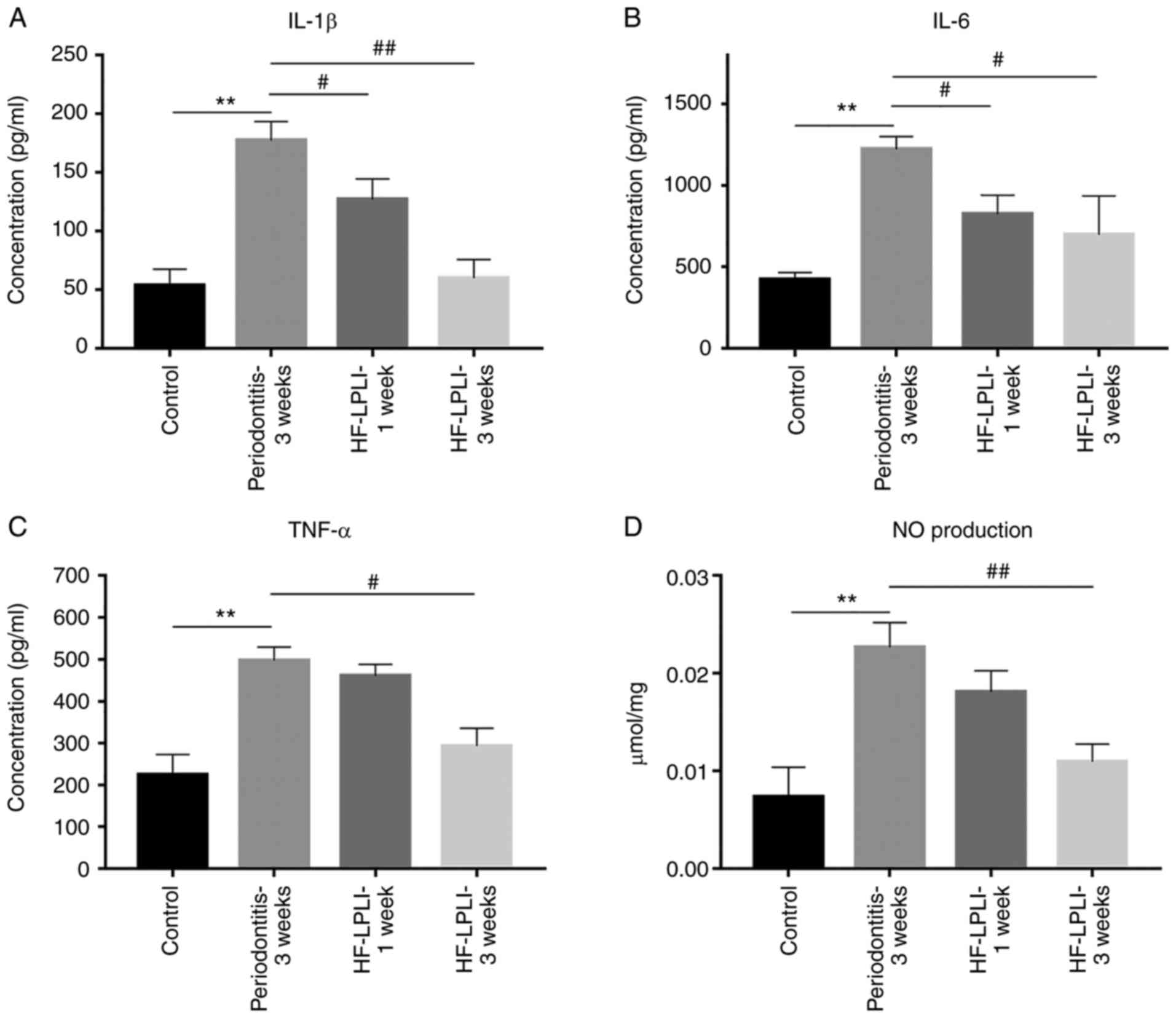
IL-1β, IL-6, TNF-α and NO levels in the blood of
rats belonging to different groups were subsequently examined, and
significantly increased plasma concentrations of pro-inflammatory
cytokines in rats with periodontitis were detected, as compared
with the control group. The levels observed in the periodontitis
rats were 173±12.6 pg/ml for IL-1β, 1,237±94.1 pg/ml for IL-6,
496±17.2 pg/ml for TNF-α and 0.02±0.004 μmol/ml for NO
(Fig. 6A–D). After 1-week HF-LPLI
treatment, these concentrations were significantly decreased, with
IL-6 reduction to a level of 733±62.3 pg/ml representing the best
result (Fig. 6B). After 3 weeks
of treatment, the concentration of pro-inflammatory cytokines
reached approximately the normal level. Taken together, these
results indicated that appropriate HF-LPLI treatment was able to
decrease the level of pro-inflammatory cytokines.
HF-LPLI induces dentin repair and
tertiary dentin in a rodent model
The results were further confirmed in the animal
model by evaluating the effectiveness of treating a dental defect
(3×3×8 mm) with stem cells (Fig.
7A). The results of in vivo imaging demonstrated the
presence of significant inflammation in the oral cavity of the
animal model, and the inflammation was reduced after 6 weeks of
treatment with stem cells followed by HF-LPLI (Fig. 7B). In addition, H&E staining
results revealed that the pulp defect was successfully
reconstructed in the rat model, and cell proliferation was clearly
time-dependent (Fig. 7C).
Calcification observed under a microscope indicated the occurrence
of osteogenic differentiation. A statistically significant
difference in osteogenesis was observed among the periodontitis
animal model, the 6-week treatment and 12-week treatment groups
(P<0.05; Fig. 7D), suggesting
that HF-LPLI treatment was effective in vivo.
Discussion
DPSCs and PLSCs have previously been used in the
treatment of periodontal tissue for regeneration studies (45); however, the improvement of their
therapeutic effect and the underlying mechanism of their action
remain under debate. In order to identify a new strategy for the
application of DPSCs and PLSCs in the treatment of periodontitis,
HF-LPLI was used in the current study to induce cell orientation
and to identify the potential mechanism of promoting inflammation
reduction by enhancing neutrophil apoptosis and MΦ
reprogramming.
In the experiments of the present study, HF-LPLI was
found to promote neutrophil apoptosis and MΦ reprogramming, which
may via the inactivation of Akt/GSK3β signaling pathway (17). Neutrophil apoptosis reduced the
inflammatory response induced by periodontitis, while the
transformation of MΦs reduced the secretion of pro-inflammatory
cytokines and increased the release of anti-inflammatory cytokines,
such as IL-10 and TGF-β. M1 MΦs secrete ROS, RNS, TNF-α, IL-1,
IL-12, IL-23 and other chemokines, which are mainly involved in the
inflammatory response and host immune functions, and cause
inflammatory damage to normal tissues. Under the action of IL-4,
IL-13, IL-10 and TGF-β, the M1 MΦs are polarized to M2, which
secrete TGF-β, VEGF, EGF and other factors, particularly at the
late stage of inflammation, and promote the repair of trauma and
fibrosis (46). Thus, the
transformation of M1 to M2 can effectively promote the regression
of inflammation. Notably, the current study findings reported that
HF-LPLI promotes neutrophil apoptosis, but did not promote MΦ
apoptosis, and the mechanism involved in this effect warrants
further investigation.
This mechanism by which HF-LPLI promotes neutrophil
apoptosis and MΦ reprogramming led to the maintenance of stem cell
viability and increased the expression of stemness-associated
genes. The proliferation of stem cells was guaranteed at an early
stage of the treatment i.e., the stemness of the cells was well
maintained allowing for unhindered proliferation. These results
suggested a key role of HF-LPLI in the treatment of
periodontitis.
As shown by EdU proliferation assay, stem cell
proliferation was not significantly different from that in
MΦ+HF-LPLI simultaneously-treated groups when stem cells were
co-cultured with MΦs to create the inflammatory environment and
subjected to HF-LPLI simultaneous treatment. However, when HF-LPLI
treatment was applied 6 h after co-culture with MΦs, cell
proliferation was significantly enhanced. These results suggested
that inflammation obtained by HF-LPLI treatment for a certain
period of time served a role in starting and promoting the
proliferation of stem cells, while excessive inflammatory response
was reduced by HF-LPLI treatment at 6 h, improving the
micro-environment and further promoting stem cell proliferation.
Gene expression and alizarin red S staining demonstrated similar
results, further indicating that HF-LPLI promoted differentiation
following activation by appropriate inflammation. The results also
revealed that MΦ followed by HF-LPLI induced a better bone
differentiation at 4 weeks, particularly when DPSCs were used,
which achieved a better differentiation result compared with
PLSCs.
In the in vivo experiments, after induced
periodontitis was treated with stem cells, no treatment was
administered to the model to induce inflammation in 6 h. Proper
inflammation promoted the proliferation of stem cells and was
beneficial to subsequent differentiation. Subsequently, HF-LPLI was
used to reduce the number of inflammatory cells and inhibited the
release of inflammatory cytokines. The in vivo
bioluminescence imaging revealed that, after 6 weeks of HF-LPLI
treatment, the inflammation was reduced. As soon as inflammation
was inhibited, H&E staining indicated tissue regeneration due
to stem cell proliferation, and further osteogenic differentiation.
Therefore, stem cells appeared to successfully achieve
peri-odontitis treatment. At 12 weeks, the best treatment effect
was obtained, which was markedly greater than the effect at 6
weeks, suggesting a better osteogenic differentiation after an
additional 6 weeks.
In conclusion, HF-LPLI may represent a novel
strategy for improving the effect of stem cells treatment. However,
how to use HF-LPLI for achieving the best effect in the treatment
of periodontitis should be confirmed. Furthermore, the treatment
dose and the time required to maximize an anti-inflammatory effect,
as well as the effect of HF-LPLI on human periodontitis require
additional experiments.
Acknowledgments
Not applicable.
Funding
This study was supported by grants from the National
Key Research and Development Plan Young Scientists Program (no.
SQ2017ZY050258), the National Science Foundation of China (nos.
31200731 and 31170935), and the Joint Research Fund for Overseas
Chinese Young Scholars (no. 31028008).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
HY took part in all experiments and was the major
contributor in writing the manuscript. XW completed the cell
experiments and took part in data analysis. FK was responsible for
the cell culture and part of data analysis. ZC helped complete cell
experiments and other experiments in vivo. YM took part in
part animal experiments and manuscript writing. MD was in charge of
the overall planning and gave substantial advices for experiments
and manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal procedures were performed according to
the Guide for the Care and Use of Laboratory Animals, following the
ARRIVE guidelines, and were approved by the Institutional Clinical
Experiments Committee of the Liaocheng People's Hospital
(Liaocheng, China) and the Animal Care Committee. All the patients
included in the present study were informed of the condition and
agreed to participate in the research.
Patient consent for publication
All the patients included in the present study were
informed of the condition and agreed to participate in the
research.
Competing interests
The authors declare that there are no conflicts of
interest in connection with this article.
References
|
1
|
Cohen EE, LaMonte SJ, Erb NL, Beckman KL,
Sadeghi N, Hutcheson KA, Stubblefield MD, Abbott DM, Fisher PS,
Stein KD, et al: American cancer society head and neck cancer
survivorship care guideline. CA Cancer J Clin. 66:203–239. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katz J, Marc H, Porter S and Ruskin J:
Inflammation, peri-odontitis, and coronary heart disease. Lancet.
358:19982001. View Article : Google Scholar
|
|
3
|
Cotti E, Abramovitch K, Jensen J, Schirru
E, Rice DD, Oyoyo U and Torabinejad M: The influence of adalimumab
on the healing of apical periodontitis in ferrets. J Endod.
43:1841–1846. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garg K, Tripathi T, Rai P, Sharma N and
Kanase A: Prospective evaluation of psychosocial impact after one
year of orthodontic treatment using PIDAQ adapted for indian
population. J Clin Diagn Res. 11:ZC44–ZC48. 2017.PubMed/NCBI
|
|
5
|
Trupthi DV, Chowdhury S, Shah A and Singh
M: Treatment of mandibular fractures using intermaxillary fixation
and vacuum forming splints: A comparative study. J Maxillofac Oral
Surg. 13:519–524. 2014. View Article : Google Scholar
|
|
6
|
Kl V, Ryana H and Dalvi PJ: Autologous
periodontal stem cell assistance in periodontal regeneration
technique (SAI-PRT) in the treatment of periodontal intrabony
defects: A case report with one-year follow-up. J Dent Res Dent
Clin Dent Prospects. 11:123–126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu X, Zhang C, Huang GT, Cheung GS,
Dissanayaka WL and Zhu W: Transplantation of dental pulp stem cells
and platelet-rich plasma for pulp regeneration. J Endod.
38:1604–1609. 2012. View Article : Google Scholar
|
|
8
|
Ahmed NE, Murakami M, Kaneko S and
Nakashima M: The effects of hypoxia on the stemness properties of
human dental pulp stem cells (DPSCs). Sci Rep. 6:354762016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di Benedetto A, Carbone C and Mori G:
Dental pulp stem cells isolation and osteogenic differentiation a
good promise for tissue engineering. Methods Mol Biol.
1210:117–130. 2014. View Article : Google Scholar
|
|
10
|
Doan L, Kelley C, Luong H, English J,
Gomez H, Johnson E, Cody D and Duke PJ: Engineered cartilage heals
skull defects. Am J Orthod Dentofacial Orthop. 137:162 e1–9. 2010.
View Article : Google Scholar
|
|
11
|
Cardoso EM, Reis C and Manzanares-Cespedes
MC: Chronic periodontitis, inflammatory cytokines, and
interrelationship with other chronic diseases. Postgrad Med.
130:98–104. 2018. View Article : Google Scholar
|
|
12
|
Klar AS, Michalak-Micka K, Biedermann T,
Simmen-Meuli C, Reichmann E and Meuli M: Characterization of M1 and
M2 polarization of macrophages in vascularized human
dermo-epidermal skin substitutes in vivo. Pediatr Surg Int.
34:129–135. 2018. View Article : Google Scholar
|
|
13
|
Quero L, Hanser E, Manigold T, Tiaden AN
and Kyburz D: TLR2 stimulation impairs anti-inflammatory activity
of M2-like macrophages, generating a chimeric M1/M2 phenotype.
Arthritis Res Ther. 19:2452017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hingert D, Barreto Henriksson H and Brisby
H: Human mesenchymal stem cells pre-treated with IL-1β and
stimulated with BMP-3 enhance chondrogenesis. Tissue Eng Part A.
24:775–7885. 2018. View Article : Google Scholar
|
|
15
|
Rubtsov Y, Goryunov K, Romanov A,
Suzdaltseva Y, Sharonov G and Tkachuk V: Molecular mechanisms of
immunomodulation properties of mesenchymal stromal cells: A new
insight into the role of ICAM-1. Stem Cells Int. 2017:65168542017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noda M, Aoki A, Mizutani K, Lin T, Komaki
M, Shibata S and Izumi Y: High-frequency pulsed low-level diode
laser therapy accelerates wound healing of tooth extraction socket:
An in vivo study. Lasers Surg Med. 48:955–964. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang L, Wu S and Xing D: High fluence
low-power laser irradiation induces apoptosis via inactivation of
Akt/GSK3β signaling pathway. J Cell Physiol. 226:588–601. 2011.
View Article : Google Scholar
|
|
18
|
Ferro F, Spelat R and Baheney CS: Dental
pulp stem cell (DPSC) isolation, characterization, and
differentiation. Methods Mol Biol. 1210:91–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eubanks EJ, Tarle SA and Kaigler D: Tooth
storage, dental pulp stem cell isolation, and clinical scale
expansion without animal serum. J Endod. 40:652–657. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Athanassiou-Papaefthymiou M, Papagerakis P
and Papagerakis S: Isolation and characterization of human adult
epithelial stem cells from the periodontal ligament. J Dent Res.
94:1591–1600. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tran HB, Doan VN, Le HT and Ngo LT:
Various methods for isolation of multipotent human periodontal
ligament cells for regenerative medicine. In Vitro Cell Dev Biol
Anim. 50:597–602. 2014. View Article : Google Scholar
|
|
22
|
Kawakami Y, Katayama T, Kishida M, Oda W
and Inoue Y: A case of streptobacillus moniliformis infection with
cutaneous leukocytoclastic vasculitis. Acta Med Okayama.
70:377–381. 2016.PubMed/NCBI
|
|
23
|
Nakahashi-Oda C, Udayanga KG, Nakamura Y,
Nakazawa Y, Totsuka N, Miki H, Iino S, Tahara-Hanaoka S, Honda S,
Shibuya K and Shibuya A: Apoptotic epithelial cells control the
abundance of Treg cells at barrier surfaces. Nat Immunol.
17:441–450. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lemos DR, Babaeijandaghi F, Low M, Chang
CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA and Rossi
FM: Nilotinib reduces muscle fibrosis in chronic muscle injury by
promoting TNF-mediated apoptosis of fibro/adipogenic progenitors.
Nat Med. 21:786–794. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu Q, Tian G, Tang Z, Gao J and Tan Y:
Adrenomedullin promotes the proliferation and inhibits apoptosis of
dental pulp stem cells involved in divergence pathways. J Endod.
42:1347–1354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jedicke N, Struever N, Aggrawal N, Welte
T, Manns MP, Malek NP, Zender L, Janciauskiene S and Wuestefeld T:
α-1-antitrypsin inhibits acute liver failure in mice. Hepatology.
59:2299–2308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su D, Hu X, Dong C and Ren J:
Determination of caspase-3 activity and its inhibition constant by
combination of fluorescence correlation spectroscopy with a
microwell chip. Anal Chem. 89:9788–9796. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Biczo G, Vegh ET, Shalbueva N, Mareninova
OA, Elperin J, Lotshaw E, Gretler S, Lugea A, Malla SR, Dawson D,
et al: Mitochondrial dysfunction, through impaired autophagy, leads
to endoplasmic reticulum stress, deregulated lipid metabolism, and
pancreatitis in animal models. Gastroenterology. 154:689–703. 2018.
View Article : Google Scholar
|
|
29
|
Wang Y, Woehrstein JB, Donoghue N, Dai M,
Avendano MS, Schackmann RCJ, Zoeller JJ, Wang SSH, Tillberg PW,
Park D, et al: Rapid sequential in situ multiplexing with DNA
exchange imaging in neuronal cells and tissues. Nano Lett.
17:6131–6139. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao Z, Duan X, Yao P, Cui W, Cheng D,
Zhang J, Jin Q, Chen J, Dai T and Shen W: Hydrogen gas is involved
in auxin-induced lateral root formation by modulating nitric oxide
synthesis. Int J Mol Sci. 18:E20842017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan Y, Du Y, Zheng H, Wang G, Li R, Chen J
and Li K: NS1 of H7N9 Influenza a virus induces NO-mediated
cellular senescence in neuro2a cells. Cell Physiol Biochem.
43:1369–1380. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dolezal E, Infantino S, Drepper F, Borsig
T, Singh A, Wossning T, Fiala GJ, Minguet S, Warscheid B, Tarlinton
DM, et al: The BTG2-PRMT1 module limits pre-B cell expansion by
regulating the CDK4-Cyclin-D3 complex. Nat Immunol. 18:911–920.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wolf Y, Boura-Halfon S, Cortese N, Haimon
Z, Sar SH, Kuperman Y, Kalchenko V, Brandis A, David E,
Segal-Hayoun Y, et al: Brown-adipose-tissue macrophages control
tissue innervation and homeostatic energy expenditure. Nat Immunol.
18:665–674. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jung JK, Gwon GJ, Neupane S, Sohn WJ, Kim
KR, Kim JY, An SY, Kwon TY, An CH, Lee Y, et al: Bortezomib
facilitates reparative dentin formation after pulp access cavity
preparation in mouse molar. J Endod. 43:2041–2047. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Owen KM, Campbell PM, Feng JQ, Dechow PC
and Buschang PH: Elevation of a full-thickness mucoperiosteal flap
alone accelerates orthodontic tooth movement. Am J Orthod
Dentofacial Orthop. 152:49–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pereira PD, Serra-Caetano A, Cabrita M,
Bekman E, Braga J, Rino J, Santus R, Filipe PL, Sousa AE and
Ferreira JA: Quantification of cell cycle kinetics by EdU
(5-ethynyl-2′-deox yuridine)-coupled-fluorescence-intensity
analysis. Oncotarget. 8:40514–40532. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu YC, Cheng WC, Chung MP, Su CC, Weng PW,
Cathy Tsai YW, Chiang HS, Yeh HW, Chung CH, Shieh YS and Huang RY:
Complicated root canal morphology of mandibular lateral incisors is
associated with the presence of distolingual root in mandibular
first molars: A cone-beam computed tomographic study in a taiwanese
population. J Endod. 44:73–79. 2018. View Article : Google Scholar
|
|
38
|
Sundaresan S, Meininger CA, Kang AJ,
Photenhauer AL, Hayes MM, Sahoo N, Grembecka J, Cierpicki T, Ding
L, Giordano TJ, et al: Gastrin induces nuclear export and
proteasome degradation of menin in enteric glial cells.
Gastroenterology. 153:1555–1567. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Loosen SH, Roderburg C, Kauertz KL,
Pombeiro I, Leyh C, Benz F, Vucur M, Longerich T, Koch A,
Braunschweig T, et al: Elevated levels of circulating osteopontin
are associated with a poor survival after resection of
cholangiocarcinoma. J Hepatol. 67:749–757. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsao YT, Huang YJ, Wu HH, Liu YA, Liu YS
and Lee OK: Osteocalcin mediates biomineralization during
osteogenic maturation in human mesenchymal stromal cells. Int J Mol
Sci. 18:E1592017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Knopp KL, Stenfors C, Baastrup C, Bannon
AW, Calvo M, Caspani O, Currie G, Finnerup NB, Huang W, Kennedy JD,
et al: Experimental design and reporting standards for improving
the internal validity of pre-clinical studies in the field of pain:
Consensus of the IMI-europain consortium. Scand J Pain. 7:58–70.
2017. View Article : Google Scholar
|
|
42
|
Yamaguchi H, Ishida Y, Hosomichi J, Suzuki
JI, Hatano K, Usumi-Fujita R, Shimizu Y, Kaneko S and Ono T:
Ultrasound microbubble-mediated transfection of NF-κB decoy
oligode-oxynucleotide into gingival tissues inhibits periodontitis
in rats in vivo. Plos One. 12:e01862642017. View Article : Google Scholar
|
|
43
|
Vargas-Sanchez PK, Moro MG, Santos F,
Anbinder AL, Kreich E, Moraes RM, Padilha L, Kusiak C, Scomparin DX
and Franco GCN: Agreement, correlation, and kinetics of the
alveolar bone-loss measurement methodologies in a ligature-induced
periodontitis animal model. J Appl Oral Sci. 25:490–497. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Silva-Dos-Santos D, Barreto-de-Albuquerque
J, Guerra B, Moreira OC, Berbert LR, Ramos MT, Mascarenhas BAS,
Britto C, Morrot A, Serra Villa-Verde DM, et al: Unraveling Chagas
disease transmission through the oral route: Gateways to
Trypanosoma cruzi infection and target tissues. PLoS Negl Trop Dis.
11:e00055072017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Aurrekoetxea M, Garcia-Gallastegui P,
Irastorza I, Luzuriaga J, Uribe-Etxebarria V, Unda F and Ibarretxe
G: Dental pulp stem cells as a multifaceted tool for bioengineering
and the regeneration of craniomaxillofacial tissues. Front Physiol.
6:2892015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Reales-Calderón JA, Aguilera-Montilla N,
Corbí ÁL, Molero G and Gil C: Proteomic characterization of human
proinflam-matory M1 and anti-inflammatory M2 macrophages and their
response to Candida albicans. Proteomics. 14:1503–1518. 2014.
View Article : Google Scholar
|