Introduction
Degenerative intervertebral disc (IVD) disease and
associated chronic lower back pain constitute a major health
concern and have a significant socioeconomic burden (1,2).
The IVD consists of a gel-like nucleus pulposus (NP) surrounded by
the annulus fibrosus (AF) and thin hyaline cartilaginous end
plates, and provides a connection between the vertebral bodies.
During IVD degeneration, the disc undergoes a shift in balance from
anabolism to catabolism, including decreased production of aggrecan
and type II collagen, increased production of matrix
metalloproteinases and inflammatory factors, and increased cytokine
production (3–5). These changes are mediated by
disturbances in the function of cells residing in the disc
(6,7). It is well established that the NP
contains NP resident stem/progenitor cells (NPSCs), which serve a
major role in maintaining cellular homeostasis and regeneration
following aging and injury (8).
NPSCs have been found to maintain multipotent and self-renewal
potential when cultured in vitro (9,10),
and the resilience of the IVD cell population may be ensured if the
relevant stem cell populations give rise to differentiated progeny
over a person’s lifetime (11,12). Although the cellular identity of
NPSCs that reside in the degenerated IVD remains poorly defined,
numerous studies have reported that stem/progenitor cells
expressing mesenchymal stem cell (MSC) markers have been isolated
successfully from the degenerated IVD (13–15). However, the data on NPSCs
regarding phenotype signature and biological capacity during IVD
degeneration remain controversial (16,17).
Since the discovery and characterization of
multipotent MSCs in the bone marrow, identification of the NPSCs
has also principally depended on the MSC markers primarily
developed for bone marrow-derived MSCs (BMMSCs), despite their
tissue specificity.
The present study was undertaken to explore the
hypothesis that the regenerative potential of NPSCs isolated from
patients with degenerative IVD diseases declines with ageing and
IVD degeneration. The expression of MSC surface markers in NPSCs
from human degenerated discs was investigated in order to determine
their proliferation capacity and multi-lineage differentiation
potential, with the hope of elucidating the mechanism underlying
IVD degeneration and developing novel treatment strategies.
Materials and methods
Sample collection
A total of 10 NP tissue samples were obtained from
patients who underwent microendoscopic discectomy for degenerative
spine diseases. NP tissues were stored in PBS solution under
sterile conditions. The patient and sample details are summarized
in Table I. All the procedures
performed for the present study were approved by the medical ethics
committee of Jinan University (Guangzhou, China). Specific informed
consent was obtained in all cases.
 | Table ICharacteristics of the patients
enrolled in the study. |
Table I
Characteristics of the patients
enrolled in the study.
| Case no. | Age (years) | Sex | Symptoms | Diagnosis | Disc level | Pfirrmann
grade |
|---|
| 1 | 18 | M | BP-RP | Lumbar disc
herniation | L4/5 | II |
| 2 | 16 | F | BP-RP | Lumbar disc
herniation | L5/S1 | II |
| 3 | 34 | F | BP | Lumbar disc
herniation | L5/6 | IV |
| 4 | 25 | M | BP | Lumbar disc
herniation | L4/5 | III |
| 5 | 28 | F | BP | Lumbar disc
herniation | L5/S1 | III |
| 6 | 42 | M | BP-RP | Lumbar disc
herniation | L5/S1 | IV |
| 7 | 19 | F | BP-RP | Lumbar disc
herniation | L4/5 | III |
| 8 | 49 | F | BP | Lumbar disc
herniation | L4/5 | IV |
| 9 | 41 | M | BP | Lumbar disc
herniation | L5/S1 | III |
| 10 | 38 | F | BP | Lumbar disc
herniation | L5/S1 | III |
Isolation of MSC-like cells from NP
tissues
To distinguish gelatinous NP tissues from AF
regions, the collection of gelatinous NP tissue from surgically
removed human NP tissues was performed using a stereoscopic
microscope. An explant culture method was employed to isolate NPSCs
from NP tissue as previously described (18). In brief, NP tissues were cut into
1 mm3 pieces and incubated at 37°C in a 5%
CO2 incubator without culture medium for 2 h to allow
tissue attachment. Complete culture medium containing Dulbecco’s
Modified Eagle’s Medium (DMEM)/F12 supplemented with 20% fetal
bovine serum (FBS), 1% L-glutamine and 1% penicillin-streptomycin
(all from Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) was added to the tissue culture dishes and incubated for an
additional 17 days. The primary cells that had migrated out of the
NP tissues and attached to dishes were passaged by a 2-min
treatment with 0.25% trypsin and 0.02% EDTA at 37°C. The medium was
replaced every 2 days. Cells were further passaged when they
reached 80–90% confluence. All cells used in the experiments were
at passages 2–4.
Flow cytometry assay
Analysis of cell surface marker
expression
A flow cytometry assay was used to identify the
expression of specific surface markers in NPSCs. A total of
~1×106 cells were re-suspended in PBS (containing 5% FBS
and 1% BSA) to produce a single-cell suspension, then incubated
with fluorescein isothiocyanate-conjugated antibodies against human
CD29 (1:100 dilution, cat. no. ab150002, mouse monoclonal, Abcam,
Cambridge, MA, USA), CD44 (1:100 dilution, cat. no. ab46793, mouse
monoclonal, Abcam), CD73 (1:100 dilution, cat. no. ab106697, mouse
monoclonal, Abcam), CD90 (1:50 dilution, cat. no. ab134360, mouse
monoclonal, Abcam), CD105 (1:20 dilution, cat. no. ab53321, mouse
monoclonal, Abcam), CD11b (1:20 dilution, cat. no. ab28101, mouse
monoclonal, Abcam), CD14 (1:20 dilution, cat. no. ab91146, mouse
monoclonal, Abcam), CD24 (1:20 dilution, cat. no. ab30350, mouse
monoclonal, Abcam), CD34 (1:100 dilution, cat. no. ab187284, mouse
monoclonal, Abcam), CD45 (1:100 dilution, cat. no. ab157309, mouse
monoclonal, Abcam) and HLA-DR (1:50 dilution, cat. no. ab59476,
mouse monoclonal, Abcam) and appropriate isotype control-mouse
IgG2A-FITC (1:100; Miltenyi Biotech, Bergisch Gladbach, Germany) or
IgG1-PE (1:100; Molecular Probes, Life Technologies, Inc.; Thermo
Fisher Scientific, Waltham, MA, USA).
The samples were incubated in the dark at 4°C for 30
min. Finally, labelled cells were washed three times with PBS and
surface marker expression was detected using flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA).
Cell cycle analysis
Cells were harvested as previously described
(18), and washed twice with PBS
containing 2% FBS. The cells were then fixed in pre-chilled
absolute ethanol at 4°C for >1 h. An equal amount of PBS was
added twice for washing. A total of 100 µl RNaseA was added
at 37°C for 30 min, followed by the addition of propidium iodide
(PI) (Invitrogen; Thermo Fisher Scientific, Inc.) at 4°C in the
dark for 30 min. Cell cycle was analyzed by flow cytometry (BD
Biosciences), using BD FACSuite™ software.
Apoptosis analysis
Apoptosis and cell death were assessed by flow
cytometry using Annexin V-FITC (Invitrogen; Thermo Fisher
Scientific, Inc.) and PI. Cultured cells were detached, suspended
in PBS and stained with Annexin V-FITC and PI according to the
manufacturer’s protocol. Apoptotic cells were identified as an
Annexin V-positive/PI-negative population. Analysis was performed
using the CellQuest software Pro (BD Biosciences).
Cell proliferation and viability
analysis
Growth curves and population doubling
time
Cells at passage 3 were seeded at a density of
3×104 cells/well in a 24-well culture plate. The cells
were harvested by trypsinization from each well as previously
described (18), and a duplicate
using a hemocytometer to count the cells, every day for a total of
13 days. The growth curve was plotted with the cell culture time as
the horizontal axis and the cell number as the vertical axis. The
cell population doubling time was calculated as: DT=t (log 2)/(log
Nt−log N0) and the results were analyzed by
GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA,
USA).
Cell viability
Cell viability was measured with a Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) according to the manufacturer’s protocol. Briefly, cells
were seeded at a density of 3×104 cells/well in a
24-well cell culture plate. Proliferation rates were evaluated at
day 1, 3, 5, 7, 9, 11 and 13. The absorbance at 450 nm was measured
with a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). A colorimetric assay was used to create cell viability curves
using the mean results from three independent experiments.
Detection of the expression of marker
genes with reverse transcription-quantitative polymerase chain
reaction (RT-qPCR)
The gene expression of NP cell phenotypic markers,
NP progenitor cell-specific gene and pluripotent stem cell markers
was analyzed via RT-qPCR. mRNA was isolated from NPSCs using the
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) extraction
method. cDNA synthesis was performed using reverse transcriptase
SuperScript III according to the manufacturer’s protocol
(Invitrogen; Thermo Fisher Scientific, Inc.). All polymerase chain
reactions were conducted using ABI Prism 7500 (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and gene expression levels were
quantified using SYBR Green (Invitrogen; Thermo Fisher Scientific,
Inc.). The data were normalized to glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) values. Relative gene expression
levels were calculated by the comparative Cq method. The primers
used (synthesized by Invitrogen; Thermo Fisher Scientific, Inc.)
are listed in Table II.
 | Table IIPrimers used for this study. |
Table II
Primers used for this study.
| Gene name | Forward primer (5′
to 3′) | Reverse primer (5′
to 3′) |
|---|
| SOX9 |
AGCGAACGCACATCAAGAC |
CTGTAGGCGATCTGTTGGGG |
| ACAN |
TCGAGGACAGCGAGGCC |
TCGAGGGTGTAGCGTGTAGAGA |
| COL2A1 |
GGCAATAGCAGGTTCACGTACA |
CGATAACAGTCTTGCCCCACTT |
| Tie2 |
AATCACTATGAGGCTTGGCAACAT |
GCGTCTCACAGGTCCAGGAT |
| NANOG |
GATTTGTGGGCCTGAAGAAA |
CAGATCCATGGAGGAAGGAA |
| OCT4 |
GAGAAGGAGAAGCTGGAGCA |
AATAGAACCCCCAGGGTGAG |
| GAPDH |
CAGCGACACCCACTCCTC |
TGAGGTCCACCACCCTGT |
Multilineage differentiation
potential
Osteogenic and adipocytic differentiation of
subconfluent cells was induced by induction media for 21 days, as
previously described (18). For
chondrogenesis, a total of 3.5×105 cells were
centrifuged at 200 × g at room temperature for 5 min to form a
three-dimensional aggregate in a 15-ml conical tube, then incubated
with chondrogenic media containing DMEM/F12 supplemented with 10%
ITS, 10–7 M dexamethasone, 1 µM ascorbate-2-phosphate, 1%
sodium pyruvate and 10 ng/ml transforming growth factor-β1 (TGF-β1)
(Cyagen, Guangzhou, China) for 21 days. The induced medium was
replaced every 2–3 days.
For Alizarin Red S (ARS) staining, cells were fixed
in 4% paraformaldehyde (PFA) at room temperature for 10 min and
stained at room temperature for 5–10 min with 40 mM ARS solution
(Solarbio, Inc., Beijing, China). Oil red O solution (Solarbio,
Inc.) was used to stain intracellular lipid vacuoles at room
temperature for 5–10 min after fixation with PFA. To identify
chondrogenic differentiation, the cell pellets were stained with
Alcian blue (Solarbio, Inc.) at room temperature for 5–10 min,
fixed in PFA, as described previously, then frozen in OCT freezing
medium (Sakura Finetek USA, Inc., Torrance, CA, USA) and sectioned
into 5-µm slices.
Further quantitative analysis was performed using
ImageJ software (National Institutes of Health, Bethesda, MA,
USA).
Statistical analysis
The data are presented as mean ± standard deviation.
One-way analysis of variance (ANOVA) was conducted to analyze the
differences among different cell types. P<0.05 was considered to
indicate a statistically significant difference. If ANOVA indicated
a significant difference between the groups, the difference was
evaluated using the least significant difference test.
Results
Flow cytometry assay
Expression of cell surface
markers
The flow cytometry results demonstrated that NPSCs
from 2 young donors were highly positive for the expression markers
CD29, CD44, CD73, CD90 and CD105 at rates of >95% (Fig. 1A and C), and negative for CD11b,
CD14, CD34, CD45 and HLA-DR (<1%, Fig. 1B and D), which fulfilled the ISCT
requirements for MSC definition and were classified as the high
expression of MSC surface markers group (H-NPSCs). In NPSCs from
the remaining 8 donors that were aged >25 years, with one
exception aged <20 years, the expression of CD29 and CD105
exhibited interindividual variability; however, all rates were
<95%. The expression rates of CD73, CD44 and CD90 were >95%
and the expression of CD11b, CD14, CD34, CD45 and HLA-DR were
negative (<1%), thus classified as the low expression of MSC
surface markers group (L-NPSCs). Although H-NPSCs and L-NPSCs
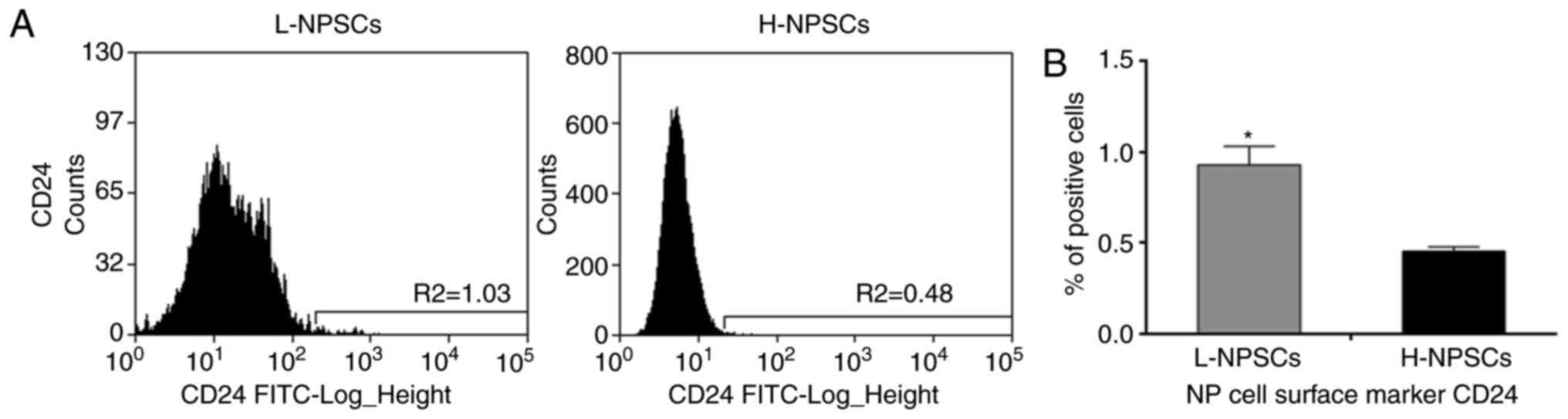
expressed the NP cell surface marker CD24 at low rates, the rate in
L-NPSCs was significantly higher compared with that in H-NPSCs
(P<0.05, Fig. 2). As the MSC
positive antigens were expressed at diverse levels for NPSCs
isolated from different degenerated disc samples, it was necessary
to explore further the possible variability in NPSC biological
characteristics with regards to the cell cycle, apoptosis,
proliferative capacity, differentiation potential and NP-specific
gene expression.
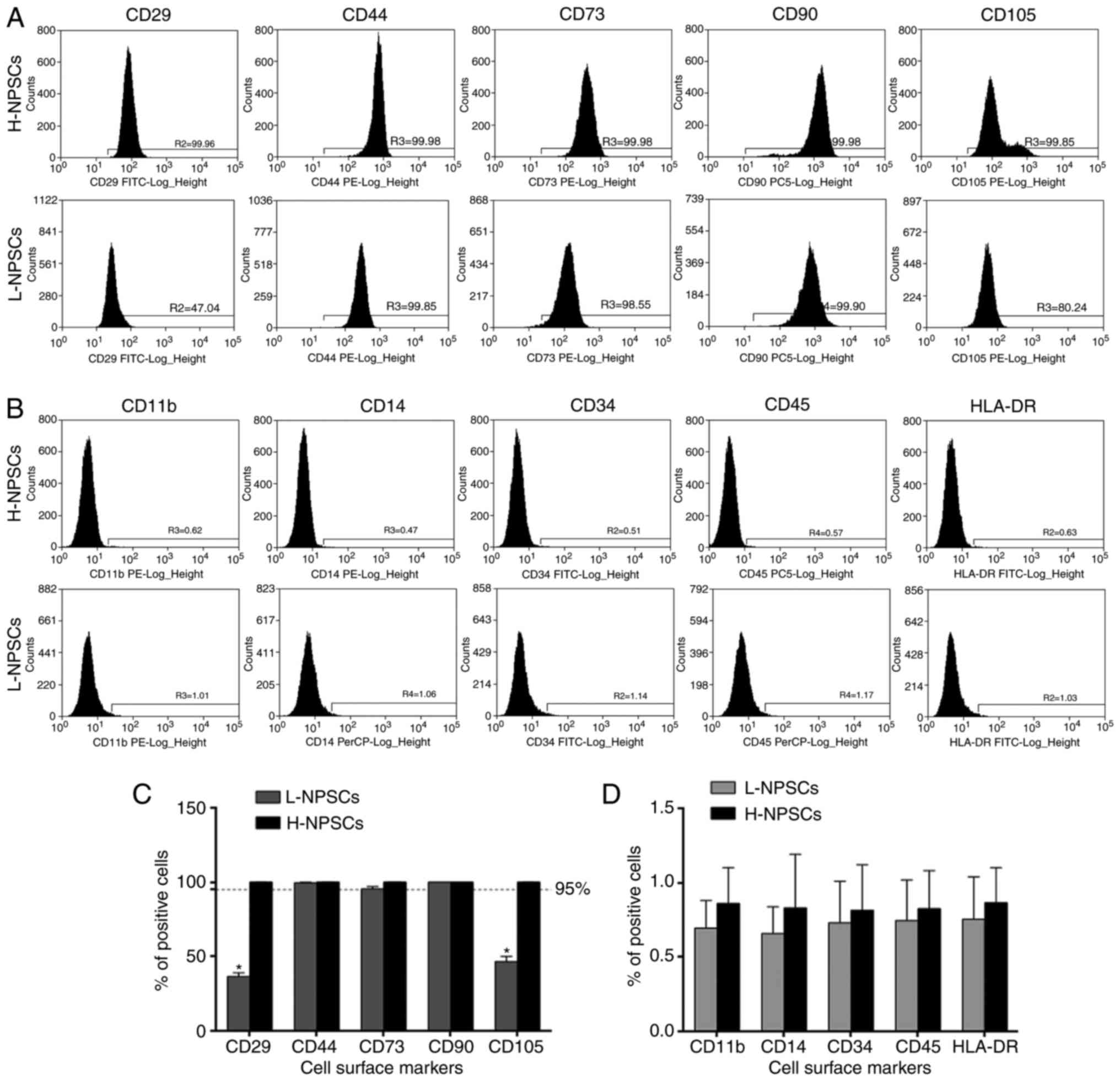 | Figure 1Flow cytometric analysis of the
expression of surface markers. (A) H-NPSCs and L-NPSCs were
positive for the MSC surface markers CD29, CD44, CD73, CD90 and
CD105. (B) H-NPSCs and L-NPSCs were negative (<1%) for the
hematopoietic stem cell surface markers CD11b, CD14, CD34, CD45 and
HLA-DR. (C) Comparative analysis of positivity for MSC surface
markers between L-NPSCs and H-NPSCs. (D) Comparative analysis of
positivity for hematopoietic stem cell surface markers between
L-NPSCs and H-NPSCs. The data are expressed as means ± standard
deviation, *P<0.05 vs. H-NPSCs. NP, nucleus pulposus;
MSC, mesenchymal stem cell; H-NPSC, high expression of MSC surface
markers group; L-NPSC, low expression of MSC surface markers
group. |
Cell cycle and apoptosis analysis
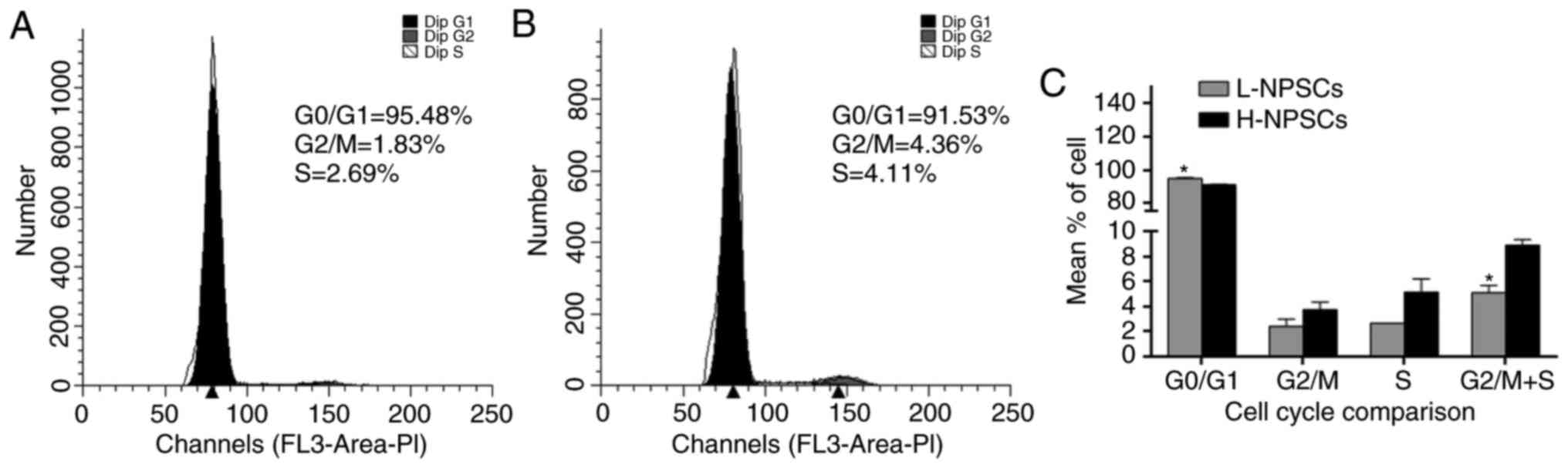
A significant difference was detected between
H-NPSCs and L-NPSCs in the G0/G1 phases of
the cell cycle (P<0.05). The percentage of H-NPSCs in the G2/M
and S phases (G2/M+S) was ~1.7-fold higher compared with
that of the L-NPSC group (P<0.05, Fig. 3). This result demonstrated a
reduced proliferative activity of L-NPSCs compared with H-NPSCs.
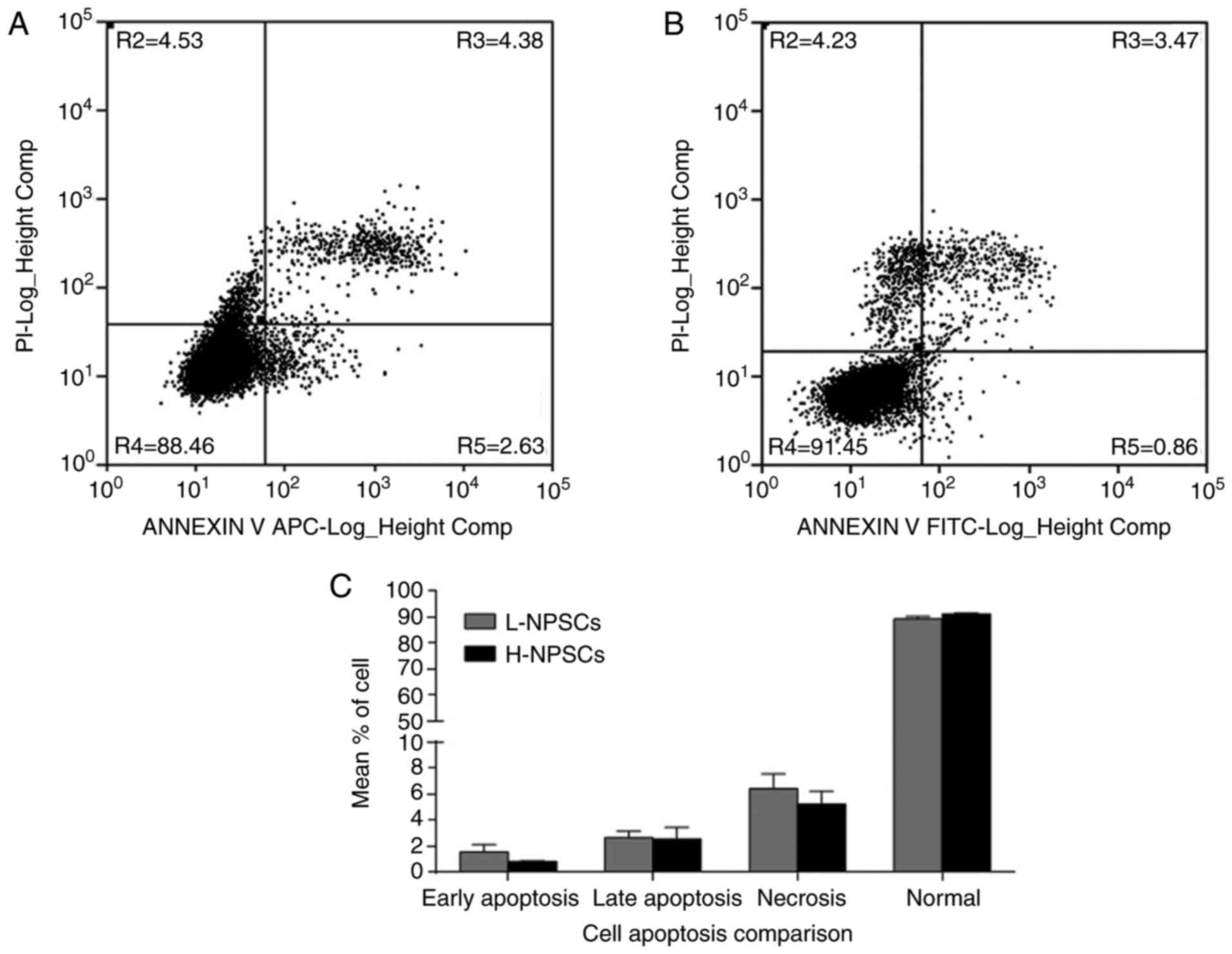
Similarly, the flow cytometry results demonstrated that the rates
of cell apoptosis and necrosis were higher in L-NPSCs when compared
with those in H-NPSCs (Fig.
4).
Morphology
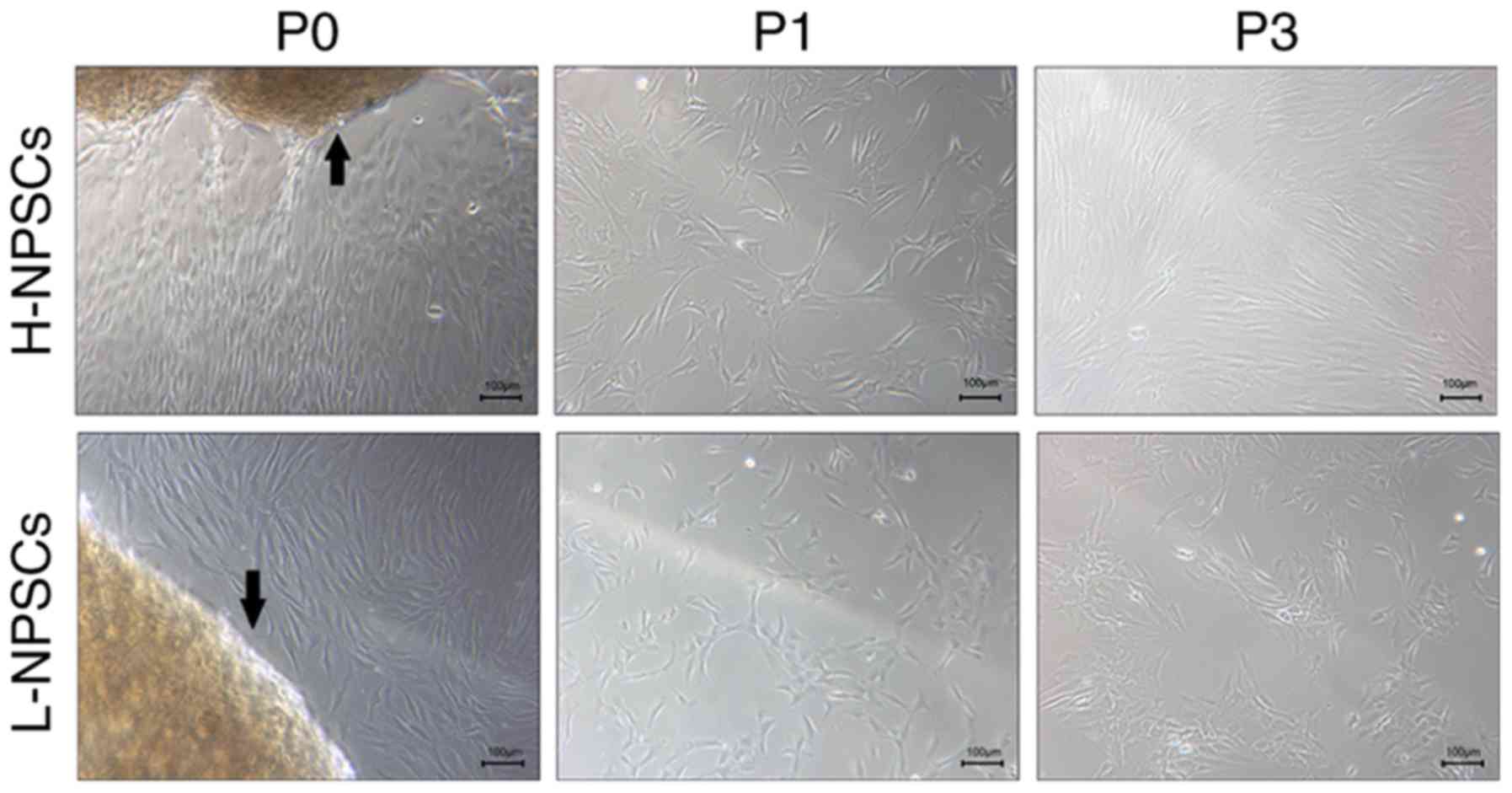
Cell morphology was assessed using phase-contrast
microscopy. At the primary passage (P0-P1), the majority of cells
among H-NPSCs and L-NPSCs exhibited a typical spindle shape, with a
more heterogeneous cell morphology observed in the L-NPSCs culture
(Fig. 5). Morphological changes
of cells were displayed during passaging; H-NPSCs maintained a
homogeneous population with typical MSC-shaped morphology; however,
L-NPSCs exhibited a heterogeneous morphology, with the appearance
of a varied proportion of polygonal or digital-shaped cells
(Fig. 5). Additionally, the
L-NPSC culture required a longer time to achieve confluence
compared with H-NPSCs.
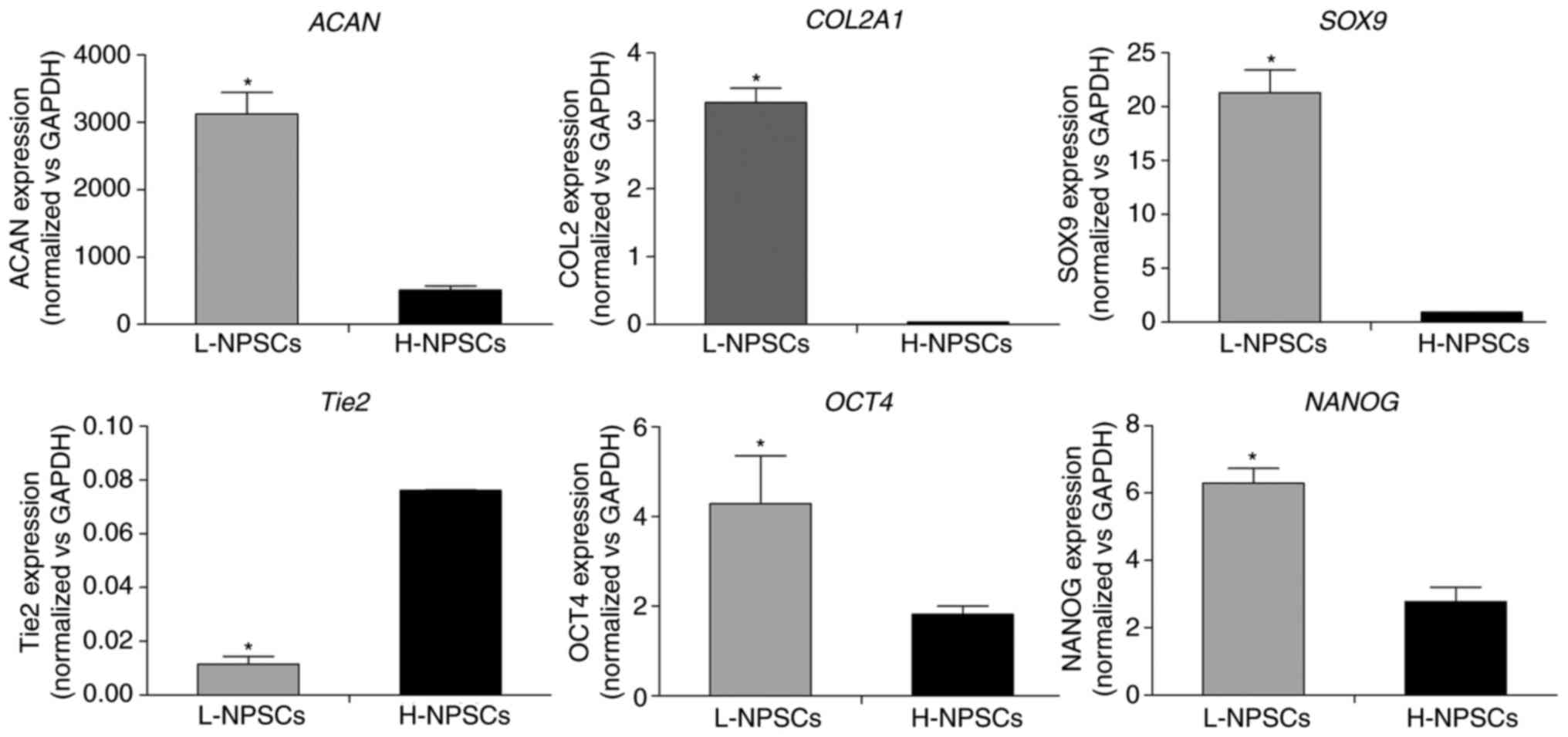
RT-qPCR analysis
The results in Fig.
6 demonstrated that the expression levels of SOX9,
COL2A1 and ACAN in L-NPSCs were 6-, 86- and 22-fold
higher, respectively, compared with those in H-NPSCs (P<0.05).
The opposite trend was observed for the NP progenitor cell-specific
marker Tie2, which demonstrated a significantly higher
expression levels in H-NPSCs compared with that in L-NPSCs
(Fig. 6, P<0.05). These
indices consistently demonstrated that part of cells in the L-NPSCs
group may have differentiated into NP cells.
Notably, the expression of OCT4 and
NANOG, as transcription factors mediating self-renewal and
an undifferentiated state (17),
were typically higher in L-NPSCs compared with H-NPSCs. These
findings suggested that the expression of pluripotency markers and
NP-specific markers were associated with the MSC immunophenotypic
pattern in NP-derived stem/progenitor cells.
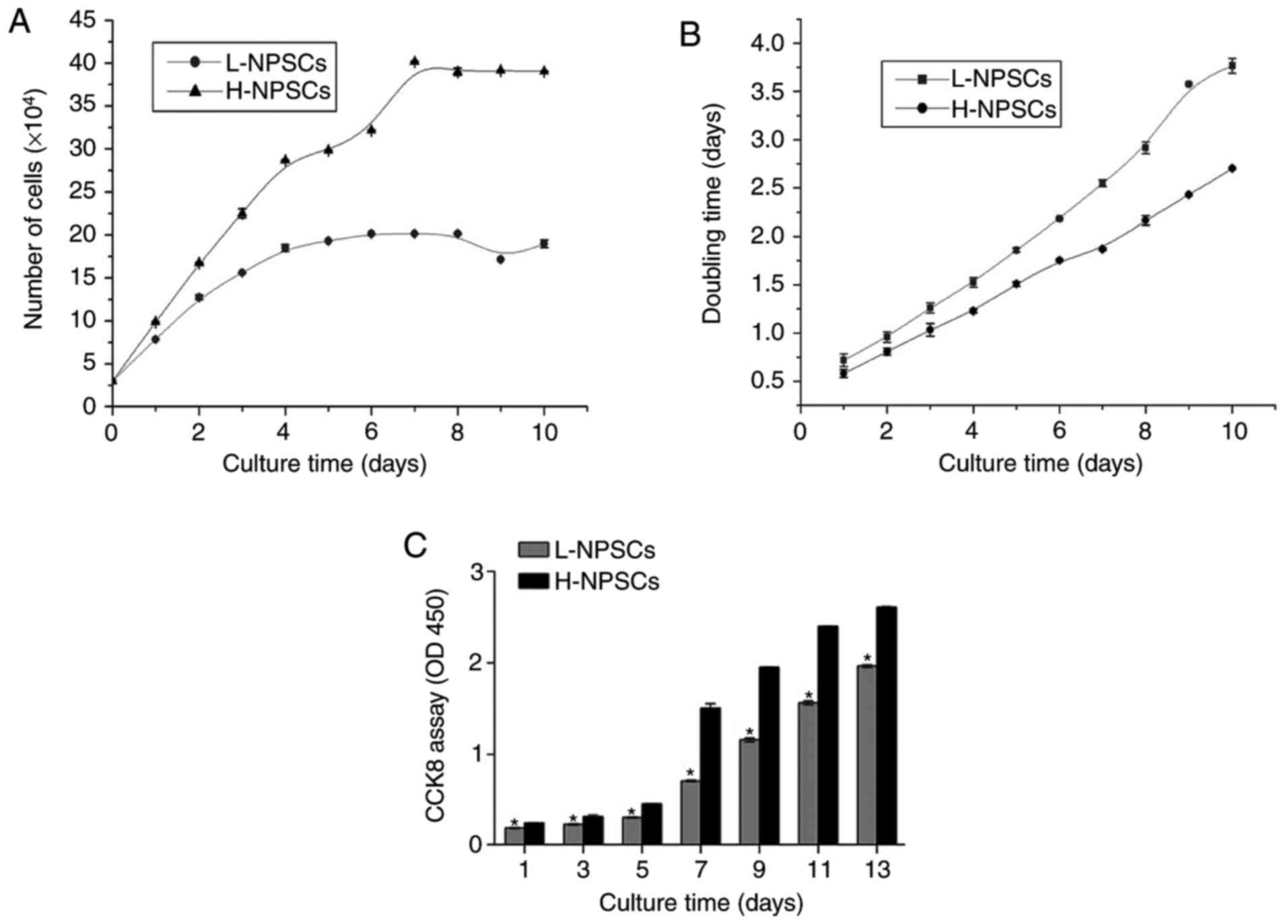
Cell proliferation and viability
analysis
In order to compare the proliferative capacity
between H-NPSCs and L-NPSCs, a growth curve was plotted and the
doubling time was calculated (Fig.
7). According to the growth curve, H-NPSCs accelerated rapidly
during days 3–7, and slowed down thereafter. L-NPSCs continued to
grow for 3–4 days, and reached a cell growth plateau at days 6–8
(Fig. 7A). The doubling time was
calculated based on the results illustrated in Fig. 7A, which demonstrated that L-NPSCs
had a much longer doubling time compared with H-NPSCs (Fig. 7B).
The viability of H-NPSCs and L-NPSCs was assessed
with the CCK-8 method (Fig. 7C).
The optical density (OD) values of H-NPSCs were significantly
higher compared with L-NPSCs, which were consistent with the
results of population doubling time and growth curves. According to
the abovementioned results, the growth ability of H-NPSCs was
markedly higher compared with that of L-NPSCs, indicating that
cells with higher intensity of MSC surface marker expression
exhibited a more prominent proliferative capacity.
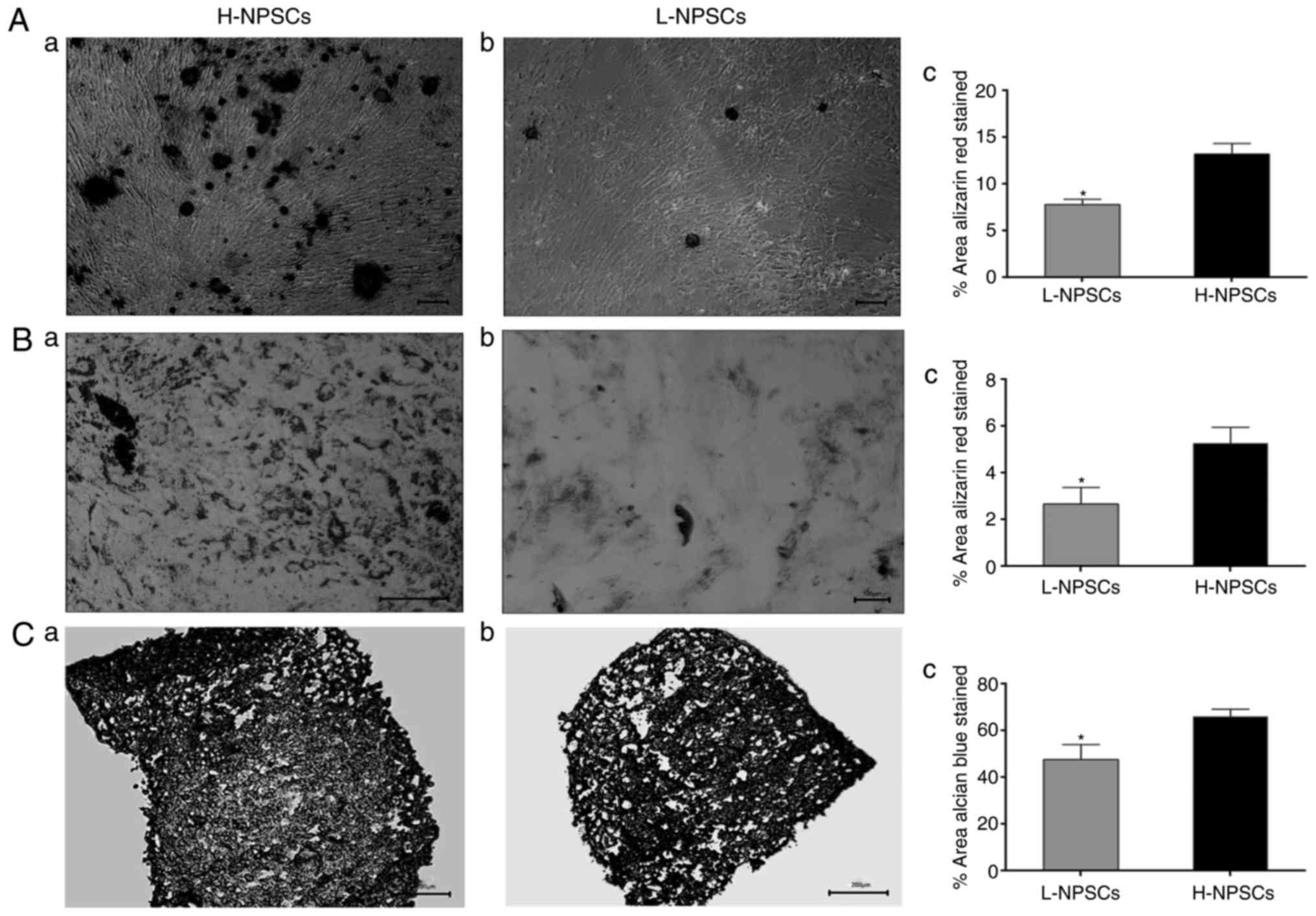
Multilineage differentiation
potential
Assays evaluating differentiation into osteogenic,
adipogenic and chondrogenic lineages were performed with specific
staining. After 3 weeks of osteogenic induction, H-NPSCs exhibited
an extensive mineralized matrix, as demonstrated by strong Alizarin
red staining. Conversely, L-NPSCs exhibited few calcium deposits
when stained by Alizarin red (Fig.
8A). Further quantitative analysis revealed that the percentage
of the positively stained area was significantly lower in L-NPSCs
compared with that in H-NPSCs. The examined NPSCs displayed
intracellular lipid vacuoles after 21 days of adipogenic induction,
which were visualized with Oil red O staining. However, the H-NPSCs
appeared to form an increased number of fat drops compared with the
L-NPSCs (Fig. 8B). Quantification
was performed by determining the percentage of the area that
contained Oil Red O-stained lipid vacuoles, and the percentage in
L-NPSCs was significantly lower compared with that in H-NPSCs.
Similarly, the amount of Alcian blue staining used to determine
chondrogenic differentiation was higher in H-NPSCs compared with
that in L-NPSCs (Fig. 8C).
Discussion
The identification of stem/progenitor cells within
human IVDs indicated the presence of a natural repair mechanism
within the disc, which may be activated for regeneration (19).
The characterization of the NPSCs was principally
based on the surface markers primarily developed for BMMSCs,
although there is a recent report on the existence of an NP
progenitor-specific marker (20).
A number of studies have reported that the markers that are
positive in BMMSCs, including CD29, CD44, CD73, CD90 and CD105, are
also positive in NPSCs from degenerated IVDs; and CD34 and CD45,
which are negative in BMMSCs, are also negative in NPSCs (13–17). However, in the present study, the
expression of CD29 and CD105 were significantly downregulated,
while CD44, CD73 and CD90 were expressed at rates >95% in
H-NPSCs compared with that in L-NPSCs, which expressed the examined
markers at rates <95% and failed to fulfill the ISCT requirement
for MSC definition.
CD105 is a receptor for transforming growth factor-β
(TGF-β) and is associated with cell adhesion and migration
(21). The decreased expression
of CD105 may signify declined capacities of homing and migration
(21). CD29 is an anchorage
protein involved in cell adhesion and migration, and its expression
indicates cell populations with a higher migratory capacity
(22). Although the low intensity
of CD105 expression was reported by Blanco et al (13), this result was different from
previous reports, which demonstrated that MSC-like cells from
degenerated NP tissues displayed a MSC marker profile that was
highly positive for CD73, CD90, CD105, CD44, CD56 and CD146, and
negative for CD45 (23,24). This may be attributed to a
different quality of the NP tissues obtained from surgical
specimens. In the present study, the specimens used were not any NP
tissues, but specifically the herniated NP tissues removed during
endoscopic discectomy. Hegewald et al (25) reported that cells harvested from
herniated NP tissue and grown in a 3D culture system possess
limited regenerative potential compared with cells from a
degenerated but contained NP compartment. Accordingly, we
hypothesized that the NP tissues L-NPSCs were isolated from may
have undergone more extensive degeneration compared with H-NPSCs
residing in NP tissues, and the activities of the resident NPSCs
would be altered accordingly.
The immunophenotypic data were supported by the
morphologic results: L-NPSCs exhibited a heterogeneous morphology
with the appearance of a varied proportion of polygonal or
digital-shaped cells, even at the 3rd passage; however, H-NPSCs
consistently exhibited a typical MSC morphology.
The expression of NP-specific progenitor marker and
NP cell marker Tie2, also referred to as CD202b, is a
cellular membrane receptor tyrosine kinase of the Tie family
(23). Tie2 has been
identified as a marker of NP precursor cells that were found to
exhibit multipotency and self-renewal capacity in animal and human
NP (23,26). The results of the present study
demonstrated that L-NPSCs and H-NPSCs expressed a low level of
Tie2; however, L-NPSCs displayed a significantly lower level
compared with H-NPSCs, suggesting that the number of progenitor
cells among L-NPSCs was decreased. This was also supported by the
immunophenotypic results, which demonstrated an upregulation of the
mature NP cell marker CD24 in L-NPSCs.
Native adult NP cells are conventionally described
as ‘chondrocyte-like’ and characterized through their rounded
morphology and expression of classic chondrogenic markers,
including SOX9, type II collagen (COL2A1) and
aggrecan (ACAN) (27).
SOX9 is the major regulator of the chondro-cytic phenotype
and serves as a potent promoter of COL2A1 gene expression,
which is almost exclusively produced in the chondrocyte. The
proteoglycan aggrecan is also a characteristic gene product of
chondrocytes (28). These markers
are characteristic of and relevant to healthy NP cells (29).
Gene expression analysis for the classic
chondrogenic marker genes ACAN, COL2A1 and SOX9 was
performed, in addition to OCT4 and NANOG, as
indicators of increased pluripotency and stemness. As expected,
significantly higher ACAN, COL2A1 and SOX9 gene
expression levels were identified in L-NPSCs in comparison with
H-NPSCs. These results verified that the frequency of
differentiated NP cells was increased in the cell population of
L-NPSCs. Of note, the levels of OCT4 and NANOG,
markers of multipotency, were found to be increased in L-NPSCs,
which is in agreement with the study of Brisby et al
(17). Taken together, these
results suggest that the higher expression level of OCT4 and
NANOG observed in L-NPSCs is due to an ongoing attempt to
counter degenerative processes in the degenerated IVD, since
L-NPSCs exhibited lower pluripotency.
Notably, the results of the present study
demonstrated that H-NPSCs have a higher proliferative capacity and
enhanced differentiation potential compared with L-NPSCs. The
reduced proliferation capacity of L-NPSCs was in agreement with the
results of cell cycle analysis indicating increased
G0/G1 and decreased G2/M phase
arrest and S phase entry. Furthermore, cell apoptosis analysis also
confirmed this result by revealing that the percentage of necrotic
and apoptotic cells among L-NPSCs was higher compared with H-NPSCs.
The lower proliferation rate of L-NPSCs may be explained by the
loss of positive MSC marker cells and the increase of CD24-positive
cells. It is well-established that the proliferation capability of
NP cells is weaker compared with that of MSC-like stem/progenitor
cells (20). Additionally, a
previous study also reported that the presence of CD24 was
associated with inferior proliferation and with low colony-forming
capability in NP tissues (30).
Multilineage potential was significantly higher in
H-NPSCs compared with that in L-NPSCs with regard to adipogenic,
chondrogenic and osteogenic differentiation potential. It was noted
that, although the expression of the classic chondrogenic markers
SOX-9, COL2A1 and ACAN was markedly
upregulated in L-NPSCs, L-NPSCs exhibited declined differentiation
potential to chondrocytes, suggesting the L-NPSCs group contained a
higher number of differentiated chondrocytes that bear little
differentiation potential.
The results of the present study predominantly
demonstrated that H-NPSCs with a higher intensity of MSC surface
marker expression, and with an improved proliferative capacity and
differentiation potential, were obtained from patients aged <20
years, while the majority of L-NPSC donors were aged >25 years,
with one exception. Sakai et al (20) reported that the frequency of
progenitor cells in NP tissues markedly decreased with age and
degeneration of the IVD. Furthermore, Yasen et al (31) demonstrated that the number of
endogenous progenitor cells and their proliferation capacity in
rabbit IVD decreased with age. Additionally, vertebral mesenchymal
stromal cells were demonstrated to decrease with age, which may be
responsible for the age-associated osteoporosis (32). It has been hypothesized that the
decline in the regenerative potential of NPSCs with age in IVD
plays a key role in disc aging and degeneration (20). These findings offered novel
insights into the biology of NPSCs during IVD degeneration. The
limitations of the present study were that H-NPSCs were harvested
from a relatively small sample size, and that H-NPSCs are
infrequently obtained from degenerated NP tissues. However, the
differences between L-NPSCs and H-NPSCs require further
investigation and identification, and more studies on human NPSCs
are required in order to provide an increased number of data
sets.
In conclusion, the present study demonstrated that
NPSCs with a higher expression intensity of MSC surface markers
exhibited a higher proliferative capacity and differentiation
potential, which may provide a novel strategy for evaluating the
regenerative potential of NPSCs. Additionally, the present study
may provide novel insights into the cellular mechanisms of IVD
degeneration considering that NP resident stem cells serve a key
role in maintaining the homeostasis of NP tissue.
Acknowledgments
Not applicable.
Funding
This study was supported by the Special Fund for
Scientific Research Fostering of the First Affiliated Hospital,
Jinan University, Guangzhou, Guangdong, China (grant no. 2017207),
by the Science and Technology Program of Guangzhou, China (grant
no. 201508020035, by the Special Fund of Science and Technology of
Guangzhou Province (grant no. 2017B030303001) and by the Science
and Technology Program of Guangzhou, China (grant no.
201704020162).
Availability of data and materials
Supporting data and materials associated with this
article can be accessed if required.
Authors’ contributions
This study was designed by JZ and HW; data were
collected by YS, JY, XZ and JL; data were analyzed and processed by
JY, HW, MT and LHC; the manuscript was written by YS and JY, and
revised by JZ. All the authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
All the procedures performed for the present study
were approved by the medical ethics committee of Jinan University
(Guangzhou, China). Specific informed consent was obtained in all
cases.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that there are no competing
interests.
References
|
1
|
Sakai D and Andersson GB: Stem cell
therapy for intervertebral disc regeneration: Obstacles and
solutions. Nat Rev Rheumatol. 11:243–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Breivik H, Eisenberg E and O’Brien T;
OPENMinds: The individual and societal burden of chronic pain in
Europe: The case for strategic prioritisation and action to improve
knowledge and availability of appropriate care. BMC Public Health.
13:12292013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Millward-Sadler SJ, Costello PW, Freemont
AJ and Hoyland JA: Regulation of catabolic gene expression in
normal and degenerate human intervertebral disc cells: Implications
for the pathogenesis of intervertebral disc degeneration. Arthritis
Res Ther. 11:R652009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Markova D, Anderson DG, Zheng Z,
Shapiro IM and Risbud MV: TNF-α and IL-1β promote a
disintegrin-like and metalloprotease with thrombospondin type I
Motif-5-mediated aggrecan degradation through syndecan-4 in
intervertebral disc. J Biol Chem. 286:39738–39749. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pattappa G, Li Z, Peroglio M, Wismer N,
Alini M and Grad S: Diversity of intervertebral disc cells:
Phenotype and function. J Anat. 221:480–496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao CQ, Wang LM, Jiang LS and Dai LY: The
cell biology of intervertebral disc aging and degeneration. Ageing
Res Rev. 6:247–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Risbud MV, Albert TJ, Guttapalli A,
Vresilovic EJ, Hillibrand AS, Vaccaro AR and Shapiro IM:
Differentiation of mesenchymal stem cells towards a nucleus
pulposus-like phenotype in vitro: Implications for cell-based
transplantation therapy. Spine (Phila Pa 1976). 29:2627–2632. 2004.
View Article : Google Scholar
|
|
8
|
Weissman IL: Stem cells: Units of
development, units of regeneration, and units in evolution. Cell.
100:157–168. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu S, Liang H, Lee SM, Li Z, Zhang J and
Fei Q: Isolation and identification of stem cells from degenerated
human intervertebral discs and their migration characteristics.
Acta Biochim Biophys Sin (Shanghai). 49:101–109. 2017.
|
|
10
|
Henriksson HB, Svala E, Skioldebrand E,
Lindahl A and Brisby H: Support of concept that migrating
progenitor cells from stem cell niches contribute to normal
regeneration of the adult mammal intervertebral disc: A descriptive
study in the New Zealand white rabbit. Spine (Phila PA 1976).
37:722–732. 2012. View Article : Google Scholar
|
|
11
|
Chuah YJ, Lee WC, Wong HK, Kang Y and Hee
HT: Three-dimensional development of tensile pre-strained annulus
fibrosus cells for tissue regeneration: An in-vitro study. Exp Cell
Res. 331:176–182. 2015. View Article : Google Scholar
|
|
12
|
Gilbert HTJ, Hoyland JA and Richardson SM:
Stem cell regeneration of degenerated intervertebral discs: Current
status (Update). Curr Pain Headache Rep. 17:3772013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blanco JF, Graciani IF, Sanchez-Guijo FM,
Muntión S, Hernandez-Campo P, Santamaria C, Carrancio S, Barbado
MV, Cruz G, Gutierrez-Cosío S, et al: Isolation and
characterization of mesenchymal stromal cells from human
degenerated nucleus pulposus: Comparison with bone marrow
mesenchymal stromal cells from the same subjects. Spine (Phila PA
1976). 35:2259–2265. 2010. View Article : Google Scholar
|
|
14
|
Henriksson H, Thornemo M, Karlsson C, Hägg
O, Junevik K, Lindahl A and Brisby H: Identification of cell
proliferation zones, progenitor cells and a potential stem cell
niche in the intervertebral disc region: A study in four species.
Spine (Phila PA 1976). 34:2278–2287. 2009. View Article : Google Scholar
|
|
15
|
Risbud MV, Guttapalli A, Tsai TT, Lee JY,
Danielson KG, Vaccaro AR, Albert TJ, Gazit Z, Gazit D and Shapiro
IM: Evidence for skeletal progenitor cells in the degenerate human
intervertebral disc. Spine (Phila Pa 1976). 32:2537–2544. 2007.
View Article : Google Scholar
|
|
16
|
Mizrahi O, Sheyn D, Tawackoli W, Ben-David
S, Su S, Li N, Oh A, Bae H, Gazit D and Gazit Z: Nucleus pulposus
degeneration alters properties of resident progenitor cells. Spine
J. 13:803–814. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brisby H, Papadimitriou N, Brantsing C,
Bergh P, Lindahl A and Henriksson Barreto H: The presence of local
mesenchymal progenitor cells in human degenerated intervertebral
discs and possibilities to influence these in vitro: A descriptive
study in humans. Stem Cells Dev. 22:804–814. 2013. View Article : Google Scholar
|
|
18
|
Wu H, Zeng X, Yu J, Shang Y, Tu M, Cheang
LH and Zhang J: Comparison of nucleus pulposus stem/progenitor
cells isolated from degenerated intervertebral discs with umbilical
cord derived mesenchymal stem cells. Exp Cell Res. 361:324–332.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Deng G, Qiu Y, Huang X, Xi Y, Yu
J, Yang X and Ye X: Transplantation of allogenic nucleus pulposus
cells attenuates intervertebral disc degeneration by inhibiting
apoptosis and increasing migration. Int J Mol Med. 41:2553–2564.
2018.PubMed/NCBI
|
|
20
|
Sakai D, Nakamura Y, Nakai T, Mishima T,
Kato S, Grad S, Alini M, Risbud M, Chan D, Cheah K, et al:
Exhaustion of nucleus pulposus progenitor cells with ageing and
degeneration of the intervertebral disc. Nat Commun. 3:12642012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Conley BA, Koleva R, Smith JD, Kacer D,
Zhang D, Bernabéu C and Vary CP: Endoglin controls cell migration
and composition of focal adhesions: Function of the cytosolic
domain. J Biol Chem. 279:27440–27449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Molinos M, Almeida CR, Gonçalves RM and
Barbosa MA: Improvement of bovine nucleus pulposus cells isolation
leads to identification of three phenotypically distinct cell
subpopulations. Tissue Eng Part A. 21:2216–2227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia Z, Yang P, Wu Y, Tang Y, Zhao Y, Wu J,
Wang D, He Q and Ruan D: Comparison of biological characteristics
of nucleus pulposus mesenchymal stem cells derived from
non-degenerative and degenerative human nucleus pulposus. Exp Ther
Med. 13:3574–3580. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Turner S, Balain B, Caterson B, Morgan C
and Roberts S: Viability, growth kinetics and stem cell markers of
single and clustered cells in human intervertebral discs:
Implications for regenerative therapies. Eur Spine J. 23:2462–2472.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hegewald AA, Endres M, Abbushi A, Abraja
CM, Woiciechowsky C, Schmieder K, Kaps C and Thomé C: Adequacy of
herniated disc tissue as a cell source for nucleus pulposus
regeneration. J Neurosurg Spine. 14:273–280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tekari A, Chan SC, Sakai D, Grad S and
Gantenbein B: Angiopoietin-1 receptor Tie2 distinguishes
multipotent differentiation capability in bovine coccygeal nucleus
pulposus cells. Stem Cell Res Ther. 23:752016. View Article : Google Scholar
|
|
27
|
Tang X, Jing L and Chen J: Changes in the
molecular phenotype of nucleus pulposus cells with intervertebral
disc aging. PLos One. 7:e520202012. View Article : Google Scholar
|
|
28
|
Kim KW, Ha KY, Lee JS, Nam SW, Woo YK, Lim
TH and An HS: Notochordal cells stimulate migration of cartilage
end plate chondrocytes of the intervertebral disc in in vitro cell
migration assays. Spine J. 9:323–329. 2009. View Article : Google Scholar
|
|
29
|
Sive JI, Baird P, Jeziorsk M, Watkins A,
Hoyland JA and Freemont AJ: Expression of chondrocyte markers by
cells of normal and degenerate intervertebral discs. Mol Pathol.
55:91–97. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujita N, Miyamoto T, Imai J, Hosogane N,
Suzuki T, Yagi M, Morita K, Ninomiya K, Miyamoto K, Takaishi H, et
al: CD24 is expressed specifically in the nucleus pulposus of
intervertebral discs. Biochem Biophys Res Commun. 338:1890–1896.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yasen M, Fei Q, Hutton WC, Zhang J, Dong
J, Jiang X and Zhang F: Changes of number of cells expressing
proliferation and progenitor cell markers with age in rabbit
intervertebral discs. Acta Biochim Biophys Sin (Shanghai).
45:368–376. 2013. View Article : Google Scholar
|
|
32
|
D’Ippolito G, Schiller PC, Ricordi C, Roos
BA and Howard GA: Age-related osteogenic potential of mesenchymal
stromal stem cells from human vertebral bone marrow. J Bone Miner
Res. 14:1115–1122. 1999. View Article : Google Scholar
|