Introduction
Gastric cancer (GC) is the second most common
malignant tumor worldwide, in terms of incidence as well as
mortality (1). There is a long
process before normal gastric mucosa becomes carcinomatous. During
this process, normal gastric mucosa initially develops precancerous
lesions, some of which may eventually become GC. Recent studies
have demonstrated that epithelial-to-mesenchymal transition (EMT)
is crucial role for the invasion and metastasis of all types of
epithelial malignancies (2–4).
EMT is a process whereby cells lose their epithelial traits of
cell-cell adhesion and acquire mesenchymal traits, such as motility
and invasiveness. In addition, it has been demonstrated that EMT is
associated with inflammation. Importantly, inflammatory cytokines,
particularly interleukin (IL)-1β, can cause normal epithelial and
cancer cells to undergo EMT (5,6).
However, the intrinsic mechanism has not been fully elucidated.
Melatonin is an important hormone that is
synthesized and secreted by the pineal gland. Its biological roles
include regulation of the circadian rhythm, as well as antioxidant,
anti-aging and immunoregulatory actions. Various studies have
reported that melatonin has significant antitumor activity against
several types of cancers, such as breast, lung and colorectal
cancer (7–9). Although the exact mechanism remains
unknown, the anticancer effects of melatonin may be mediated by
multiple pathways, including antagonism of growth factors,
induction of tumor cell apoptosis, enhancement of immunity,
adjustment of the psychological state, and synergy with other
treatments (10–14).
Nuclear factor kappa B (NF-κB) is a multifunctional
transcription factor, which not only regulates numerous
pathophysiological responses, but also plays an important role in
tumor development, particularly in the progression of tumor
invasion and metastasis (15–17). Numerous studies have indicated
that continued activation of NF-κB is crucial for tissue malignant
transformation (18,19). However, the complex mechanism
through which NF-κB regulates tumor metastasis is unclear.
It has been reported that NF-κB inhibits the
expression of E-cadherin and desmoplakin and induces the expression
of vimentin by promoting the expression of Snail (20), ZEB-1 and ZEB-2 (21), thereby initiating EMT. Other
studies have demonstrated that melatonin exerts an inhibitory
effect on NF-κB (22). However,
to the best of our knowledge, there is currently no study
investiagting these pathways in GC. The aim of the present study
was to validate the effects of IL-1β and melatonin on the
metastatic potential of gastric adenocarcinoma (GA) cell lines, and
examine whether melatonin inhibits EMT by decreasing the nuclear
translocation of NF-κB. Finally, the molecular mechanisms by which
melatonin inhibits EMT in GA cells by targeting IL-1β/NF-κB/matrix
metalloproteinase (MMP)2/MMP9 signaling were investigated, hoping
that this pathway may provide a novel therapeutic target for
GC.
Materials and methods
Cell culture
The MGC80-3 and SGC-7901 cell lines were purchased
from the Cell Bank of the Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). Cells were cultured in
RPMI-1640 medium (HyClone, Logan, UT, USA) with 10% fetal bovine
serum (HyClone) at 37°C in an incubator containing 5%
CO2.
Cell migration and invasion assays
For the invasion assay, MGC80-3 or SGC-7901 cells
were placed in 24-well Transwell chambers (Costar, Cambridge, MA,
USA) with 8-µm pore filters coated with 60 µl
ice-cold Matrigel (BD Biosciences, San Jose, CA, USA), and diluted
with serum-free RPMI-1640 medium. MGC80-3 or SGC-7901 cells
(5×104 per well) in 200 µl serum-free medium were
seeded into the upper chamber, and 600 µl medium containing
10% fetal bovine serum (as a chemoattractant) was added to the
lower chamber. After 24 h of incubation, the cells were fixed with
methanol and stained with crystal violet. Cotton swabs were used to
remove non-invading cells from the upper surface of the membrane.
Finally, cells were observed under a light microscope and
photographed using a digital microscopic imaging system (Olympus,
Tokyo, Japan). A total of 10 random fields were counted at a
magnification of ×200, and the results averaged for each condition.
The migration assay was performed following the same procedure as
described above, except that the wells were not coated with
Matrigel and the incubation time was 20 h.
Transfection with siRNA
siRNAs (GenePharma, Shanghai, China) were
transfected into cells using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc., Carlsbad, CA, USA) according to the
manufacturer’s instructions. Cells were exposed to 0, 0.1, 0.5 or
1.5 mM melatonin (Sigma-Aldrich; Merck KGaA, St. Louis, MO, USA)
dissolved in ethanol. To evaluate the effects of IL-1β (Prospec,
Rehovot, Israel), cells were treated with this agent for 30 min
after transfection. NF-κBp65 and control siRNA sequences are listed
in Table I.
 | Table IsiRNAs targeting specific genes. |
Table I
siRNAs targeting specific genes.
| Gene | Sequence
|
|---|
| Sense (5′-3′) | Antisense
(5′-3′) |
|---|
| NF-κBp65 siRNA |
GGAGUACCCUGAGGCUAUATT |
UAUAGCCUCAGGGUACUCCTT |
| Control siRNA |
UUCUCCGAACGUGUCACGUTT |
ACGUGACACGUUCGGAGAATT |
Western blot analysis
MGC80-3 or SGC-7901 cells were lysed on ice in
radioimmunoprecipitation assay lysis buffer containing 1%
phenylmethyl sulfonyl fluoride (Beyotime Institute of
Biotechnology, Nantong, China). Western blot analysis was used to
detect protein expression as described by Huang et al
(23). The primary antibody used
was rabbit polyclonal anti-human MMP2 (cat. no. 4022) or MMP9 (cat.
no. 3852) (1:1,000; Cell Signaling Technology, Danvers, MA, USA);
anti-β-actin (1:6,000; Cell Signaling Technology) was used as an
internal control.
Gelatin zymography
The protease activities of MMP2 and MMP9 from the
supernatants of MGC80-3 or SGC-7901 cells were measured by gelatin
zymography. Briefly, 8% SDS-PAGE gel containing 1 mg/ml gelatin
(Xinfan Technologies Inc., Shanghai, China) was used to separate
the proteins with electrophoresis at a constant current of 40 mA.
Renaturing and developing the gels was conducted following the
manufacturer’s instructions. The gels were then stained with
Coomassie blue and destained with a solution containing 7% acetic
acid until clear bands of gelatin appeared on the dark background,
indicating gelatin degradation. The gels were digitized using a
scanner.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from MGC80-3 or SGC-7901
cells with TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). An M-MLV RT kit (Invitrogen; Thermo Fisher Scientific, Inc.)
was used for reverse transcription. The relative expression of
β-catenin, fibronectin, E-cadherin, vimentin, Snail, NF-κB, MMP2
and MMP9 was detected using SYBR Green qPCR SuperMix (Roche, Basel,
Switzerland) on a Two-step Real-Time PCR system (ABI7500, Thermo
Fisher Scientific, Singapore). The primer sequences are given in
Table II. Relative expression
levels were normalized to glyceraldehyde 3-phosphate dehydrogenase
and calculated using the 2−ΔΔCq method.
 | Table IIPrimers used for RT-qPCR. |
Table II
Primers used for RT-qPCR.
| Gene | Sequence
| Product size
(bp) |
|---|
| Sense (5′-3′) | Antisense
(5′-3′) |
|---|
| β-catenin |
TGCCAAGTGGGTGGTATAGAG |
CGCTGGGTATCCTGATGTGC | 331 |
| Fibronectin C |
GAGCTTCCCCAACTGGTAACCC |
GGTGGCACCTCTGGTGAGGC | 432 |
| E-cadherin |
TTGCTCACATTTCCCAACTCCTC |
CACCTTCAGCCATCCTGTTTCTC | 234 |
| Vimentin |
GCTGAATGACCGCTTCGCCAACT |
AGCTCCCGCATCTCCTCCTCGTA | 144 |
| Snail |
TCTAGGCCCTGGCTGCTACAA |
ACATCTGAGTGGGTCTGGAGGTG | 131 |
| NF-κB |
CTGCATCCACAGTTTCCAGAACC |
ACGCTGCTCTTCTATAGGAACTTGG | 295 |
| MMP2 CC |
TGATGTCCAGCGAGTG |
AGCAGCCTAGCCAGTCG | 103 |
| MMP9 |
CAGTCCACCCTTGTGCTCTTC |
TGCCACCCGAGTGTAACCAT | 124 |
| GAPDH |
AGAAGGCTGGGGCTCATTTG |
AGGGGCCATCCACAGTCTTC | 238 |
Electrophoretic mobility shift assay
(EMSA)
EMSA was performed to determine the association
between melatonin and NF-κB activation in MGC80-3 and SGC-7901
cells. Nuclear extracts were prepared and an EMSA was performed
according to the manufacturer’s instructions (Viagene, Changzhou,
China). Briefly, cell cultures were placed on ice (1×107
per 100-mm dish), then rinsed three times with pre-cooled PBS prior
to collection into a pre-cooled micro-tube. Subsequently, the
cytoplasmic protein lysis buffer was added and the precipitate
resuspended until no mass was identified. Then, the cell lysate was
centrifuged at 14,000 × g for 10 min and the supernatant discarded.
Solution buffer was added on the inner wall of the tube and
centrifugation was performed several times at 12,000 × g for 5 min.
Subsequently, the extract buffer was added and the tubes were
vortexed at the highest setting, then gently rocked on ice using a
shaking platform. After centrifugation, the supernatant was
transferred to new pre-cooled tubes. The nuclear protein extracts
were incubated with a biotin-labeled NF-κB consensus
oligonucleotide probe (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) in
binding buffer at room temperature for 20 min. Following
incubation, the samples were separated on a 5.5% polyacrylamide
gel, transferred onto a nylon membrane and fixed on the membrane
via ultraviolet crosslinking. The biotin-labeled probe was detected
with streptavidin-HRP by ChemiDoc Touch Imaging System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Statistical analyses were performed with SPSS
version 17.0 software (SPSS Inc., Chicago, IL, USA). All data are
expressed as means ± standard deviation and were analyzed by the
one-way analysis of variance, followed by Dunnett’s T3 post-hoc
test. P<0.05 indicated a statistically significant
difference.
Results
Melatonin decreases IL-1β-induced EMT in
GA cells
The effect of melatonin on IL-1β-induced EMT was
investigated. MGC80-3 and SGC-7901 cells were treated with or
without IL-1β (20 ng/ml) for 30 min followed by incubation with
0.1, 0.5 or 1.5 mM melatonin for 24 h. Both GA cell types exhibited
some morphological changes, including assuming a slender and
spindle-like shape after treatment with IL-1β (Fig. 1A). These changes were inhibited by
treatment with melatonin. Furthermore, IL-1β promoted cell invasion
and migration, and this effect was decreased by melatonin, albeit
not in a concentration-dependent manner (Fig. 1B and C).
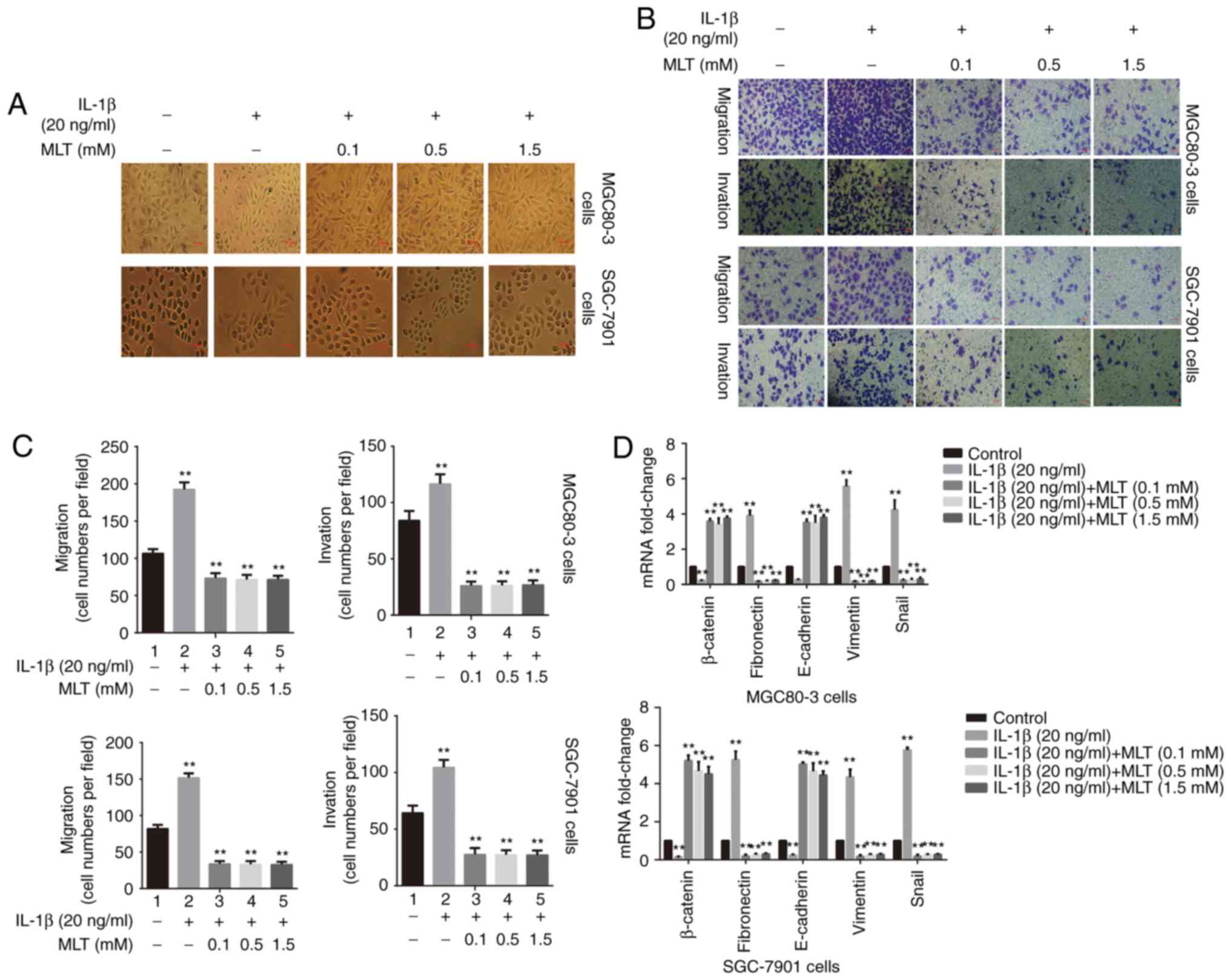 | Figure 1Melatonin suppresses the IL-1β-induced
EMT in GA cells. (A) Morphological differences between
IL-1β-treated MGC80-3 and SGC-7901 cells, and IL-1β-treated GA
cells with melatonin. GA cells were pretreated with 20 ng/ml IL-1β
for 30 min followed by incubation with melatonin (0.1, 0.5 and 1.5
mM) for 24 h. Representative images of cells treated with IL-1β
demonstrated that the morphological changes of epithelial cells
were reduced in the presence of melatonin (magnification, ×200). (B
and C) The effect of melatonin on IL-1β-induced migration and
invasion of GA cells was assessed using the Transwell assay.
Treatment of GA cells with IL-1β increased cell migration and
invasion, whereas these effects were inhibited by melatonin.
*P<0.05 and **P≤0.01 compared with the
control group. Bars indicate the mean ± standard deviation number
of cells per field of view (magnification, ×200) in the migration
and invasion assays. (D) Effect of IL-1β and melatonin on the
expression of EMT markers. The expression of EMT markers,
β-catenin, fibronectin, E-cadherin, vimentin and Snail were
evaluated by RT-qPCR. Relative expression was obtained using the
2−∆∆Cq method after normalization to GAPDH.
**P≤0.01 compared with the control group. IL-1β,
interleukin-1β; MLT, melatonin; GA, gastric adenocarcinoma; EMT,
epithelial-to-mesenchymal transition; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
To verify the effects of melatonin on EMT, the
expression of EMT markers was assessed by the RT-qPCR assay. The
results demonstrated that the mRNA expression of β-catenin and
E-cadherin was reduced, while the mRNA expression of fibronectin,
vimentin and Snail was increased, following addition of IL-1β (20
ng/ml). Melatonin significantly inhibited the changes in the
expression level of these genes (Fig.
1D). These findings indicated that melatonin inhibited
IL-1β-induced EMT in MGC80-3 and SGC-7901 cells.
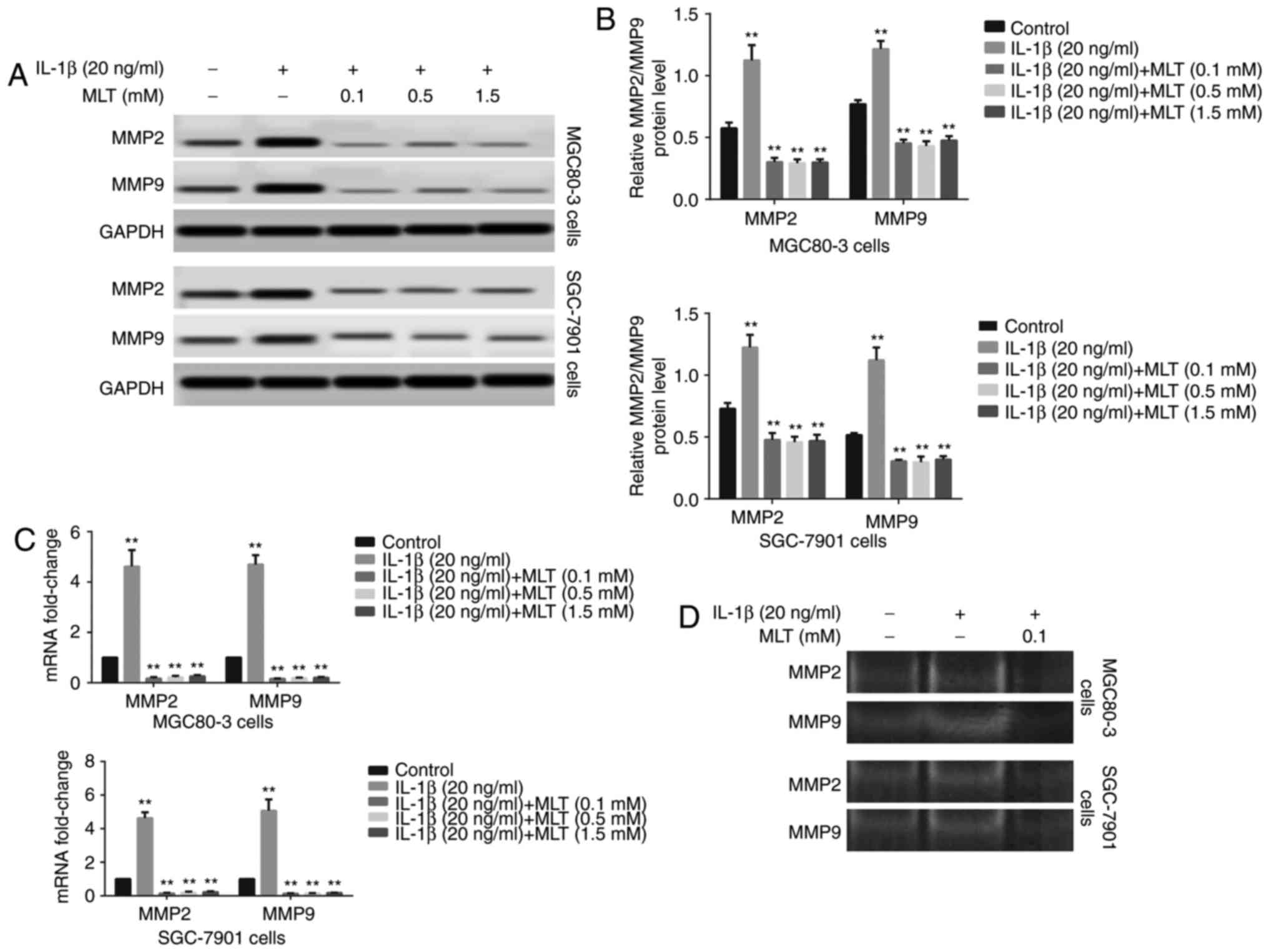
Melatonin inhibits the expression and
activity of MMP2 and MMP9, which are upregulated in IL-1β-induced
EMT
The expression of MMP2 and MMP9 is closely
associated with the invasion and metastasis of tumors (24). To investigate whether MMP2 and
MMP9 were involved in IL-1β-induced EMT in GA cells, western
blotting, RT-qPCR and gelatin zymography were applied to determine
their expression and activity in GA cells. As expected, the
expression and activity of MMP2 and MMP9 increased following
stimulation with IL-1β (Fig. 2).
Treatment with melatonin reduced these effects in MGC80-3 and
SGC-7901 cells (Fig. 2).
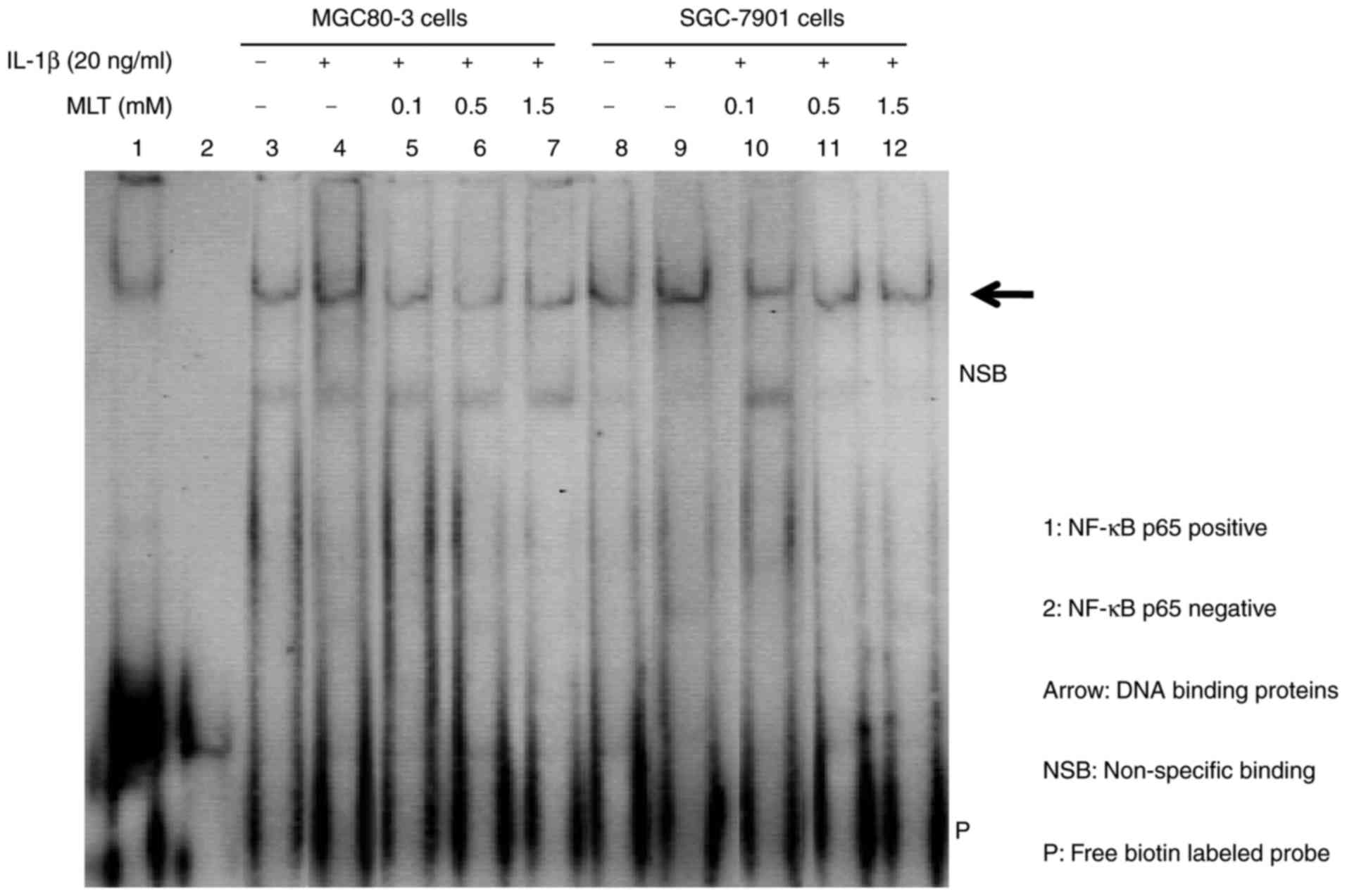
Melatonin suppresses the IL-1β-induced
activation of NF-κB in GA cells
Due to the key role of NF-κB in inflammation and
tumor cell invasion (25,26), it was hypothesized that the EMT
observed in GA cells under IL-1β induction was attributable to
increased NF-κB activity. MGC80-3 and SGC-7901 cells were
pretreated with or without IL-1β for 30 min. Melatonin was then
added for 24 h, and NF-κB activity was observed by EMSA analysis.
The results demonstrated that IL-1β induced NF-κB activation,
whereas melatonin suppressed this effect (Fig. 3).
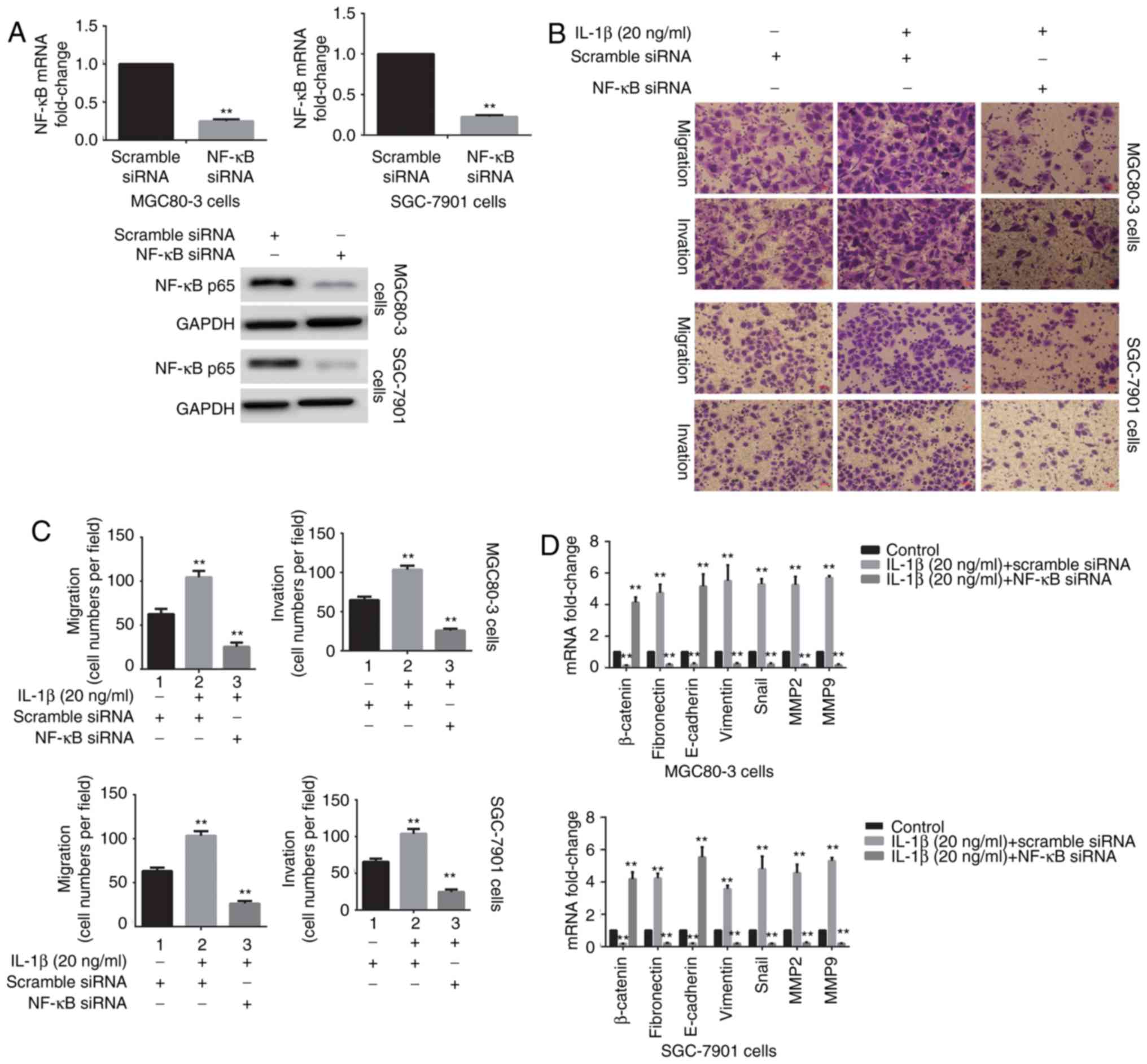
Inhibition of NF-κB activity reverses
IL-1β-induced EMT in GA cells
The potential role of NF-κB inhibition in preventing
the development of IL-1β-induced mesenchymal characteristics in GA
cells was investigated. A siRNA specific for NF-κB was applied to
these cells to block NF-κB activity. As shown in Fig. 4A, NF-κB siRNA markedly
downregulated the expression of NF-κB at both the mRNA and protein
levels. To verify that inhibiting NF-κB activity reversed the
IL-1β-induced EMT in GA cells, cell migration and invasion assays,
western blotting and RT-qPCR analysis were used. As expected,
downregulation of NF-κB not only significantly inhibited
IL-1β-induced cell migration and invasion, but also reduced the
expression of mesenchymal markers (Fig. 4B and F).
Discussion
A growing body of evidence indicates that there is a
close association between inflammation and tumor development.
Tumors are stimulated and maintained by inflammatory signals from
the tumor microenvironment, and this microenvironment plays a key
role in cancer development (27,28). EMT is a biological process whereby
epithelial cells assume the characteristics of mesenchymal cells.
During EMT, the ability of an epithelial cell to transform its
morphological characteristics into those of a mesenchymal cell is a
hallmark of tumor formation. Furthermore, numerous reports indicate
that EMT is implicated in tumor cell invasion and metastasis
(29). The inflammatory cytokine
IL-1β is closely associated with EMT in breast cancer (30).
Our results indicated that MGC80-3 and SGC-7901
cells displayed mesenchymal morphological characteristics, along
with increased cell migration and invasion, following treatment
with IL-1β. In addition, there were also changes in the expression
of EMT-related genes. Specifically, after adding IL-1β, the
expression of β-catenin and E-cadherin was reduced, while the
expression of fibronectin, vimentin and Snail was increased.
The expression of MMP2 and MMP9 is closely
associated with the invasion and metastasis of cancer (24). The results of the present study
are similar to those reported previously. The expression and
activity of MMP2 and MMP9 increased following stimulation with
IL-1β. To the best of our knowledge, this study is among the first
to demonstrate that IL-1β can promote EMT in GA cells; however, the
potential underlying molecular mechanisms remain largely
unknown.
Melatonin is a major hormone secreted mainly by the
pineal gland. Its anticancer mechanisms include acceleration of
tumor cell apoptosis, suppression of tumor cell proliferation,
immunostimulation and synergistic activity with other treatments.
Recent studies have demonstrated that melatonin notably decreases
the risk of death and treatment resistance of multiple
malignancies, particularly in breast cancer, colon cancer,
melanoma, lung cancer and malignant glioblastoma (7–9,31,32). A clinical study by Lissoni et
al has demonstrated that the regression rate in patients with
non-small-cell lung cancer or gastrointestinal cancer receiving
combination therapy with chemotherapeutic drugs and melatonin was
significantly higher compared with that in the group of patients
receiving chemotherapy alone. Moreover, the 2-year survival rate
was also higher in patients concomitantly treated with melatonin
(10).
In the present study, the effect of melatonin on
IL-1β-induced EMT in MGC80-3 and SGC-7901 cells was investigated.
Our findings suggested that melatonin inhibited the migration and
invasion on GA cells, albeit not in a concentration-dependent
manner. Furthermore, melatonin inhibited the IL-1β-induced
upregulation of MMP2 and MMP9 expression, and reversed the
increased expression of EMT markers. Thus, it was concluded that
melatonin decreased IL-1β-induced EMT in GA cells, although fully
elucidating the underlying mechanism requires further research.
Among several key factors related to EMT, NF-κB is a
vital element involved in the inflammatory process (33), as well as controlling the
initiation and progression of several cancer types. NF-κB induces
the expression of Snail (20),
ZEB-1 and ZEB2 (21) to promote
EMT and cancer metastasis. It was also previously demonstrated that
melatonin potently inhibits the activity of NF-κB (22). In the present study, EMSA analysis
confirmed that IL-1β enhanced NF-κB transcriptional activity. Of
note, treatment with melatonin significantly suppressed
IL-1β-induced NF-κB transcriptional activity in MGC80-3 and
SGC-7901 cells. Further research demonstrated that NF-κB siRNA
markedly reduced the expression of MMP2, MMP9, fibronectin,
vimentin and Snail in IL-1β-treated GA cells, indicating that the
inhibition of NF-κB by a specific siRNA reversed the IL-1β-induced
EMT in GA cells. Furthermore, the cell migration and invasion assay
in GA cells treated with IL-1β and NF-κB siRNA corroborated the
decreased invasion and migration of these cells. Overall, our data
indicated that melatonin inhibited IL-1β-induced EMT by decreasing
the nuclear translocation of NF-κB.
In summary, the results of the present suggested
that melatonin suppresses IL-1β-induced EMT by reducing the
activity of NF-κB in GA cells. It was also determined that the
molecular mechanisms by which melatonin inhibits EMT in GA cells
involve targeting IL-1β/NF-κB/MMP2/MMP9 signaling.
Acknowledgments
Not applicable.
Funding
This research was supported by the Fujian Provincial
Natural Fund (2015J01472) for HH and Fujian Medical University
Nursery Fund (2015MP019) for XW.
Authors’ contributions
XW and HH designed the experiments. BW, JX, DH and
HZ conducted the research. XW, BW and HH worked on the manuscript.
All the authors have read and approved the final version of the
manuscript.
Availability of data and materials
The analyzed data are available from the
corresponding author upon reasonable request.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoshida T, Ozawa Y, Kimura T, Sato Y,
Kuznetsov G, Xu S, Uesugi M, Agoulnik S, Taylor N, Funahashi Y and
Matsui J: Eribulin mesilate suppresses experimental metastasis of
breast cancer cells by reversing phenotype from
epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial
transition (MET) states. Br J Cancer. 110:1497–1505. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tungsukruthai S, Sritularak B and
Chanvorachote P: Cycloartobiloxanthone inhibits migration and
invasion of lung cancer cells. Anticancer Res. 37:6311–6319.
2017.PubMed/NCBI
|
|
5
|
Jiménez-Garduño AM, Mendoza-Rodríguez MG,
Urrutia-Cabrera D, Domínguez-Robles MC, Perez-Yepez EA,
Ayala-Sumuano JT and Meza I: IL-1β induced methylation of the
estrogen receptor ERalpha gene correlates with EMT and
chemoresistance in breast cancer cells. Biochem Biophys Res Commun.
490:780–785. 2017. View Article : Google Scholar
|
|
6
|
Wang J, Bao L, Yu B, Liu Z, Han W, Deng C
and Guo C: Interleukin-1β promotes epithelial-derived alveolar
elastogenesis via αvβ6 integrin-dependent TGF-β activation. Cell
Physiol Biochem. 36:2198–216. 2015. View Article : Google Scholar
|
|
7
|
Kim W, Jeong JW and Kim JE: CCAR2
deficiency augments genotoxic stress-induced apoptosis in the
presence of melatonin in non-small cell lung cancer cells. Tumour
Biol. 35:10919–10929. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mao L, Yuan L, Xiang S, Zeringue SB,
Dauchy RT, Blask DE, Hauch A and Hill SM: Molecular deficiency
(ies) in MT1 melatonin signaling pathway underlies the
melatonin-unresponsive phenotype in MDA-MB-231 human breast cancer
cells. J Pineal Res. 56:246–253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pariente R, Bejarano I, Rodriguez AB,
Pariente JA and Espino J: Melatonin increases the effect of
5-fluorouracil-based chemotherapy in human colorectal
adenocarcinoma cells in vitro. Mol Cell Biochem. 440:43–51. 2018.
View Article : Google Scholar
|
|
10
|
Lissoni P: Biochemotherapy with standard
chemotherapies plus the pineal hormone melatonin in the treatment
of advanced solid neoplasms. Pathol Biol (Paris). 55:201–204. 2007.
View Article : Google Scholar
|
|
11
|
Martín-Renedo J, Mauriz JL, Jorquera F,
Ruiz-Andrés O, González P and González-Gallego J: Melatonin induces
cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line.
J Pineal Res. 45:532–540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Korkmaz A, Tamura H, Manchester LC, Ogden
GB, Tan DX and Reiter RJ: Combination of melatonin and a peroxisome
proliferator-activated receptor-gamma agonist induces apoptosis in
a breast cancer cell line. J Pineal Res. 46:115–116. 2009.
View Article : Google Scholar
|
|
13
|
Pizarro JG, Yeste-Velasco M, Esparza JL,
Verdaguer E, Pallàs M, Camins A and Folch J: The antiproliferative
activity of melatonin in B65 rat dopaminergic neuroblastoma cells
is related to the downregulation of cell cycle-related genes. J
Pineal Res. 45:8–16. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zupancic D, Jezernik K and Vidmar G:
Effect of melatonin on apoptosis, proliferation and differentiation
of urothelial cells after cyclophosphamide treatment. J Pineal Res.
44:299–306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng Z, Geh E, Chen L, Meng Q, Fan Y,
Sartor M, Shertzer HG, Liu ZG, Puga A and Xia Y: Inhibitor of
kappaB kinase beta regulates redox homeostasis by controlling the
constitutive levels of glutathione. Mol Pharmacol. 77:784–792.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang H, Jang SW, Pak JH and Shim S:
Glaucine inhibits breast cancer cell migration and invasion by
inhibiting MMP-9 gene expression through the suppression of
NF-kappaB activation. Mol Cell Biochemist. 403:85–94. 2015.
View Article : Google Scholar
|
|
17
|
Yang SS, Li XM, Yang M, Ren XL, Hu JL, Zhu
XH, Wang FF, Zeng ZC, Li JY, Cheng ZQ, et al: FMNL2 destabilises
COMMD10 to activate NF-κB pathway in invasion and metastasis of
colorectal cancer. Br J Cancer. 117:1164–1175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krenn PW, Hofbauer SW, Pucher S, Hutterer
E, Hinterseer E, Denk U, Asslaber D, Ganghammer S, Sternberg C,
Neureiter D, et al: ILK induction in lymphoid organs by a
TNFα-NF-κB-regulated pathway promotes the development of chronic
lymphocytic leukemia. Cancer Res. 76:2186–2196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barham W, Chen L, Tikhomirov O, Onishko H,
Gleaves L, Stricker TP, Blackwell TS and Yull FE: Aberrant
activation of NF-κB signaling in mammary epithelium leads to
abnormal growth and ductal carcinoma in situ. BMC Cancer.
15:6472015. View Article : Google Scholar
|
|
20
|
Lu Z, Li Y, Wang J, Che Y, Sun S, Huang J,
Chen Z and He J: Long non-coding RNA NKILA inhibits migration and
invasion of non-small cell lung cancer via NF-κB/Snail pathway. J
Exp Clin Cancer Res. 36:542017. View Article : Google Scholar
|
|
21
|
Chua HL, Bhat-Nakshatri P, Clare SE,
Morimiya A, Badve S and Nakshatri H: NF-kappaB represses E-cadherin
expression and enhances epithelial to mesenchymal transition of
mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2.
Oncogene. 26:711–724. 2007. View Article : Google Scholar
|
|
22
|
Li W, Wu J, Li Z, Zhou Z, Zheng C, Lin L,
Tan B, Huang M and Fan M: Melatonin induces cell apoptosis in Mia
PaCa-2 cells via the suppression of nuclear factor-κB and
activation of ERK and JNK: A novel therapeutic implication for
pancreatic cancer. Oncol Rep. 36:2861–2867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Q, Lan F, Zheng Z, Xie F, Han J,
Dong L, Xie Y and Zheng F: Akt2 kinase suppresses
glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-mediated apoptosis
in ovarian cancer cells via phosphorylating GAPDH at threonine 237
and decreasing its nuclear translocation. J Biol Chem.
286:42211–42220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Tubergen EA, Banerjee R, Liu M, Vander
Broek R, Light E, Kuo S, Feinberg SE, Willis AL, Wolf G, Carey T,
et al: Inactivation or loss of TTP promotes invasion in head and
neck cancer via transcript stabilization and secretion of MMP9,
MMP2, and IL-6. Clin Cancer Res. 19:1169–1179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aggarwal BB: Nuclear factor-kappaB: The
enemy within. Cancer Cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aggarwal BB, Vijayalekshmi RV and Sung B:
Targeting inflammatory pathways for prevention and therapy of
cancer: Short-term friend, long-term foe. Clin Cancer Res.
15:425–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin LX: Inflammatory immune responses in
tumor microenvironment and metastasis of hepatocellular carcinoma.
Cancer Microenviron. 5:203–209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leonardi GC, Candido S, Cervello M,
Nicolosi D, Raiti F, Travali S, Spandidos DA and Libra M: The tumor
microenvironment in hepatocellular carcinoma (Review). Int J Oncol.
40:1733–1747. 2012.PubMed/NCBI
|
|
29
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soria G, Ofri-Shahak M, Haas I,
Yaal-Hahoshen N, Leider-Trejo L, Leibovich-Rivkin T, Weitzenfeld P,
Meshel T, Shabtai E, Gutman M and Ben-Baruch A: Inflammatory
mediators in breast cancer: Coordinated expression of TNFα &
IL-1β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal
transition. BMC Cancer. 11:1302011. View Article : Google Scholar
|
|
31
|
Martin V, Sanchez-Sanchez AM,
Puente-Moncada N, Gomez-Lobo M, Alvarez-Vega MA, Antolín I and
Rodriguez C: Involvement of autophagy in melatonin-induced
cytotoxicity in glioma-initiating cells. J Pineal Res. 57:308–316.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim TK, Lin Z, Tidwell WJ, Li W and
Slominski AT: Melatonin and its metabolites accumulate in the human
epidermis in vivo and inhibit proliferation and tyrosinase activity
in epidermal melanocytes in vitro. Mol Cell Endocrinol. 404:1–8.
2015. View Article : Google Scholar :
|
|
33
|
Pal S, Bhattacharjee A, Ali A, Mandal NC,
Mandal SC and Pal M: Chronic inflammation and cancer: Potential
chemoprevention through nuclear factor kappa B and p53 mutual
antagonism. J Inflamm (Lond). 11:232014. View Article : Google Scholar
|