Introduction
Arthrogryposis-renal dysfunction-cholestasis (ARC)
syndrome is a life-threatening autosomal recessive multisystem
disorder caused by germline mutations in VPS33B-interacting
protein, apical-basolateral polarity regulator (VIPAR) or
vacuolar protein sorting 33 homolog B (VPS33B) (1). The principle clinical manifestations
of ARC include renal tubular dysfunction, cholestasis, ichthyosis,
central nervous system malformation and congenital joint
contractures involving multiple organ systems (2,3).
It has been recognized that ARC syndrome exhibits notable clinical
variability, and the prognosis of this condition is particularly
poor, with the majority of patients not surviving beyond the first
year of life (4,5). Furthermore, there is currently no
specific treatment for this syndrome.
Mutations in VPS33B are detectable in 75-77%
of patients with a clinical diagnosis of ARC syndrome (3,6). A
better understanding of the molecular pathology of this disorder is
of vital importance for the development of an appropriate
therapeutic regimen. VPS33B encodes a 617-amino-acid
protein, which is a homolog of the class C yeast vacuolar protein
sorting, and the VPS33B protein contains a Sec-1 domain involved in
intracellular protein sorting and vesicular trafficking (7). It has also been reported that
VPS33B is a downstream target gene of the
hnf6/vhnf1 signaling pathway that is important for
zebrafish biliary development (8). In addition, the VPS33B protein can
interact with soluble N-ethylmaleimide-sensitive factor attachment
protein receptors (SNAREs), which are involved in vesicular
exocytosis and synaptic transmission to facilitate vesicle
targeting and fusion (9).
Therefore, the interaction between the mutant protein expressed in
VPS33B mutants and the SNAREs at the late endosomal stage
may be impeded, leading to abnormal secretion of lamellar granules,
and localization or accumulation of plasma proteins (2,10).
Abnormal protein trafficking and impairment in the maturation of
multi-vesicular bodies in megakaryocytes underlie the α-granule
deficiency in a mouse model of VPS33B deficiency and in
patients with ARC (11). The
VPS33B-VIPAR complex may regulate apical-basolateral polarity via
the Rab11a-dependent apical recycling pathway and the
transcriptional regulation of epithelial cadherin (1). This complex also regulates the
delivery of lysyl hydroxylase 3 into newly identified post-Golgi
collagen IV carriers, which are essential for the modification of
lysine residues in multiple collagen types (12).
In the study by Hanley et al (13), a murine model with a
liver-specific deletion of VPS33B (VPS33B
fl/fl-AlfpCre) was successfully established, as
indicated by the abnormalities identified in mice, which were
similar to those observed in children with ARC syndrome.
Furthermore, the analysis of gene expression profiles provided an
insight into the possible regulatory mechanisms responsible for ARC
syndrome. However, only the gene expression and pathway analysis of
the microarray data were performed in the aforementioned study. To
further elucidate the molecular basis of ARC, the gene expression
profiles deposited by Hanley et al (13) were downloaded in the present study
in order to conduct weighted gene co-expression network analysis
and to identify potential therapeutic drugs.
Materials and methods
Microarray data and preprocessing
The gene expression profile of GSE83192 (13), generated by the GPL16570 platform
(Affymetrix Mouse Gene 2.0 Array; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), was downloaded from Gene Expression Omnibus
database (http://www.ncbi.nlm.nih.gov/geo/). This dataset
contained six liver tissue samples from liver-specific
VPS33B knockout (VPS33Bfl/fl-AlfpCre) mice
and four liver tissue samples from control
(VPS33Bfl/fl) mice. The raw data preprocessing
was conducted using the oligo package in R software (www.r-project.org), including conversion of the data
format, filling-in of missing data with the median values (14), background correction using the
MicroArray Suite method (15) and
normalization of the sequencing data using the quantile method
(16).
Differential expression analysis and
hierarchical clustering
The Limma package (17) was used to perform differential
expression analysis for normalized values. In addition, P-values
were adjusted for the false discovery rate (FDR) via the method
described by Benjamini and Hochberg (18). The thresholds for differentially
expressed gene (DEG) screening were set at FDR<0.05 and
|log2 fold change|>1. The expression values of
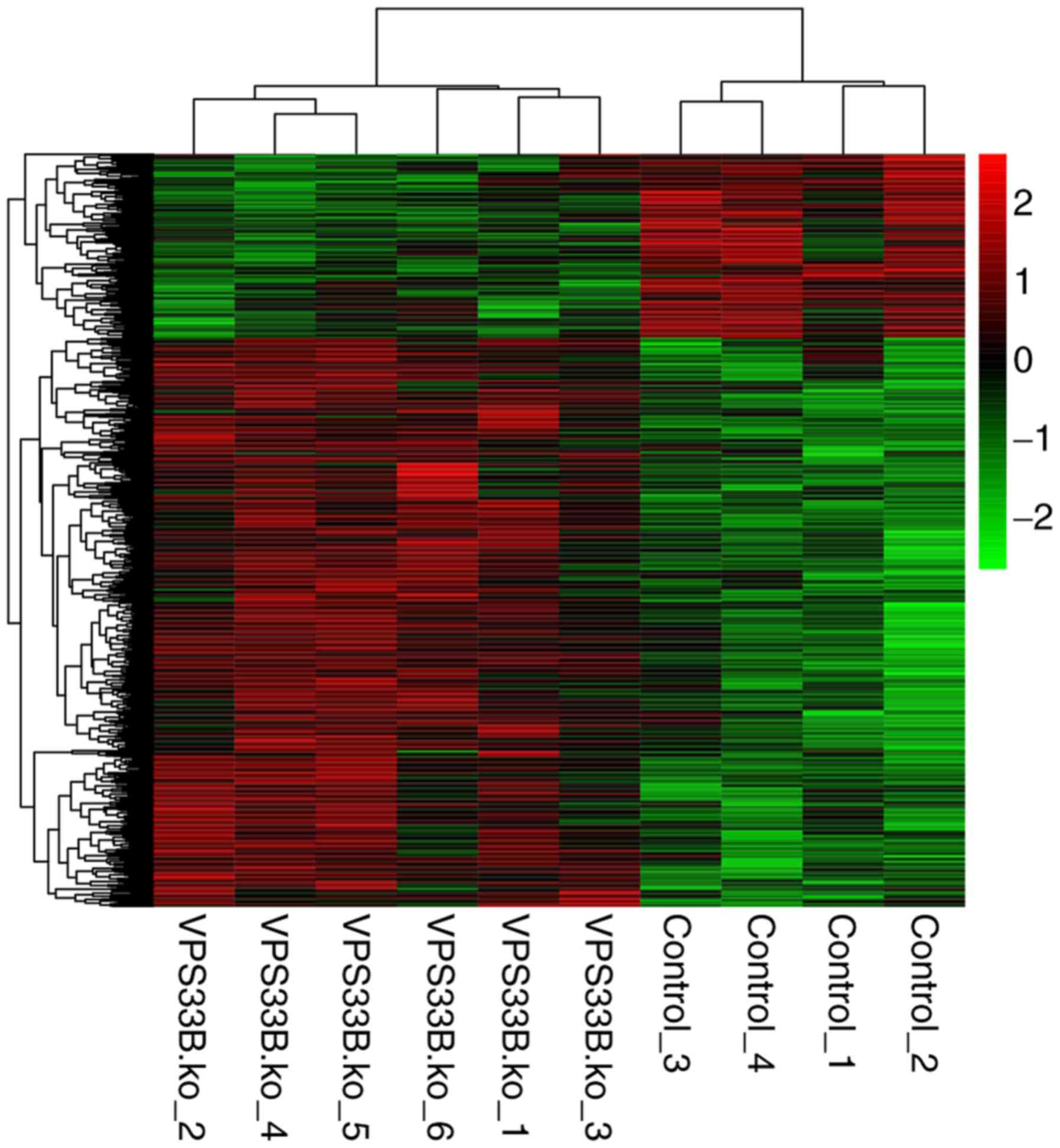
screened DEGs were hierarchically clustered by the pheatmap package
(19) in R to intuitively observe
the differences in gene expression levels.
Identification of co-expression
modules
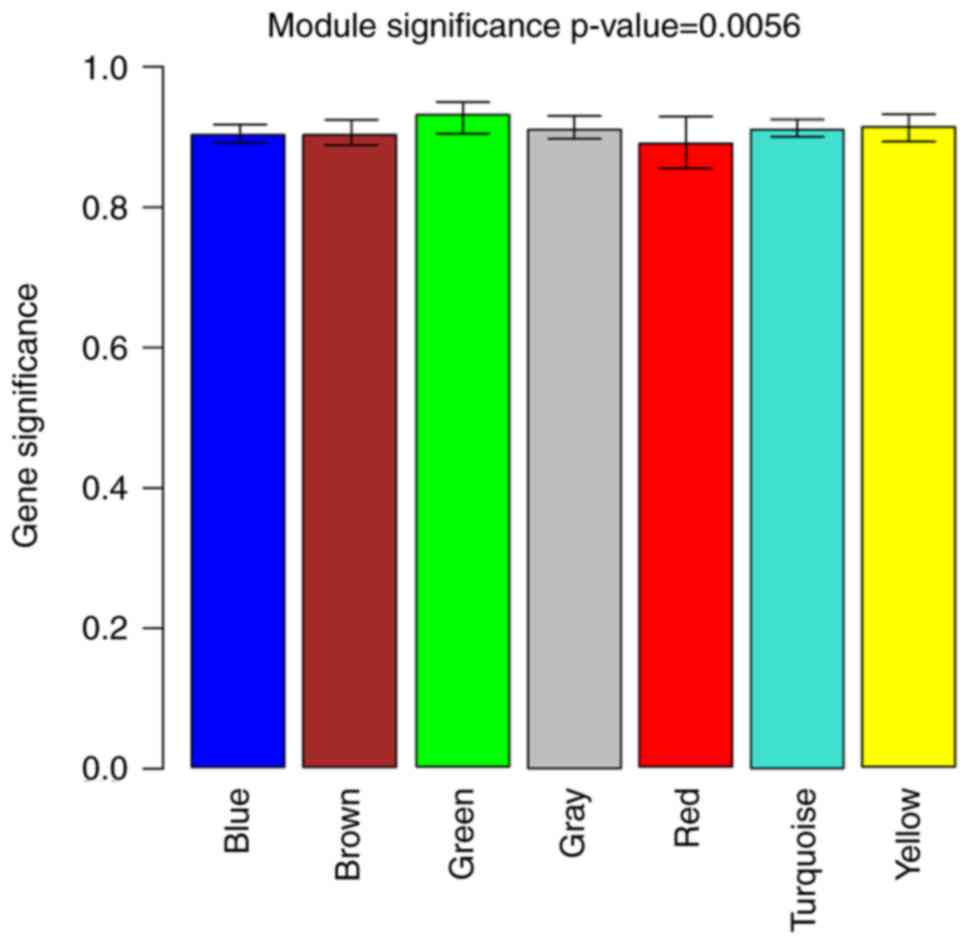
The gene significance (GS) values, defined as the
log of the P-value, indicated the difference in the mRNA expression
between VPS33B knockout and control mice. The module
significance (MS), defined as the mean value of GS for all genes in
a given module, was calculated for each module to identify its
connection with the disease status. Two representative
co-expression modules with the highest MS values were selected
since a higher MS value indicates a closer connection.
Construction and analysis of the
protein-protein interaction (PPI) network
The DEGs in the two selected representative
co-expression modules mentioned earlier were adopted for PPI
network construction. The database Search Tool for the Retrieval of
Interacting Genes/Proteins (22)
was employed to collect pairwise PPIs among the DEGs. Cytoscape
software (23) was applied for
visualization of the interaction associations, and the Molecular
Complex Detection (MCODE) plugin (24) was used to create the modules with
the following parameters: Degree cut-off=2, node score cut-off=0.2,
and K-core=2. BiNGO (25),
another plugin of Cytoscape, was used to annotate module function
with an adjusted P-value of <0.05.
Enrichment analysis and potential
therapeutic drug identification
The Gene Ontology (GO) annotations of the PPI
network were performed by GOstat (26) in three categories, namely
biological process (BP), cellular component (CC) and molecular
function (MF), with P<0.05. The Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathway enrichment analysis was conducted by the
KEGG Orthology-Based Annotation System server (27), and P<0.05 was used as the
cut-off criterion. Bioactive small molecules of putative relevance
to the ARC syndrome were searched for using the Connectivity Map
(CMAP) database, with the criteria set to |connectivity
score|>0.9 and P<0.05 (28). A connectivity score closer to −1
implied that this small molecule may have a stronger therapeutic
effect.
Results
DEG screening and hierarchical
clustering
Subsequent to data preprocessing, 1,016 DEGs,
including 768 upregulated and 248 downregulated genes, were
identified in the VPS33B knockout mice as compared with the
control mice. The expression values of screened DEGs were
hierarchically clustered by pheatmap package, and the color
contrast indicated that there were significant differences in gene
expression between the VPS33Bfl/fl-AlfpCre and
VPS33Bfl/fl mice (Fig. 1).
WGCNA analysis and PPI network
construction
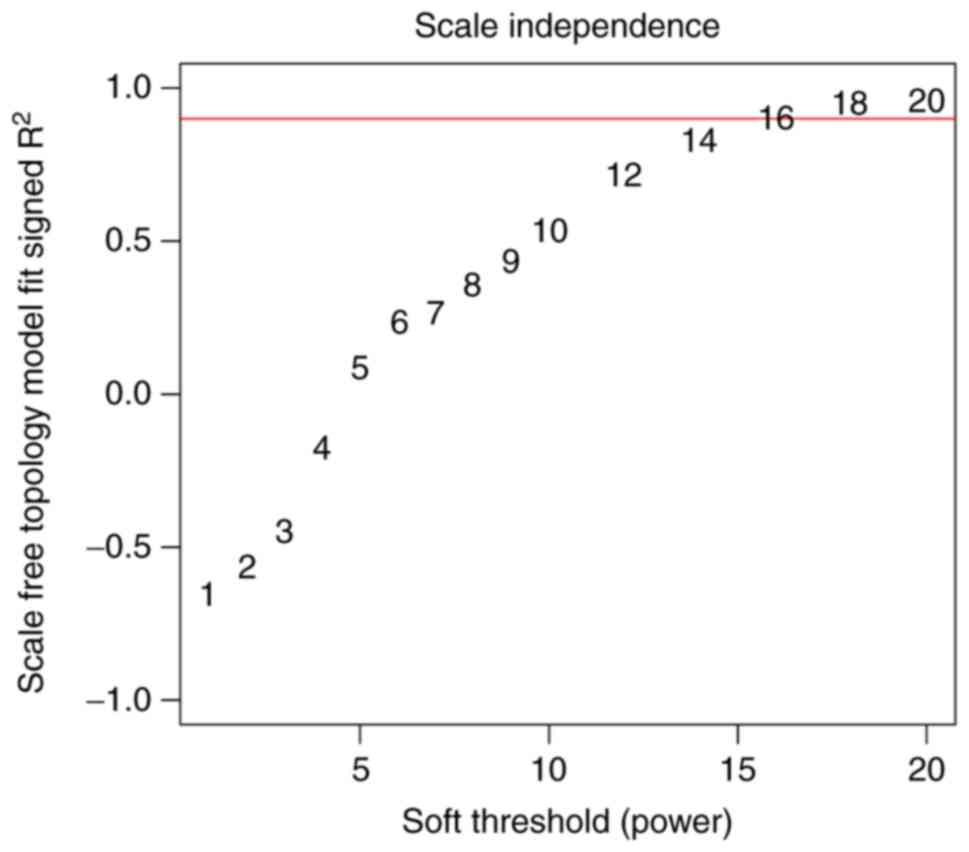
Based on the correlation coefficient between log (k)
and log P (k), the value of the adjacency matrix was set to 18 in
order to guarantee the scale-free topology of the co-expression
modules (Fig. 2). A gene
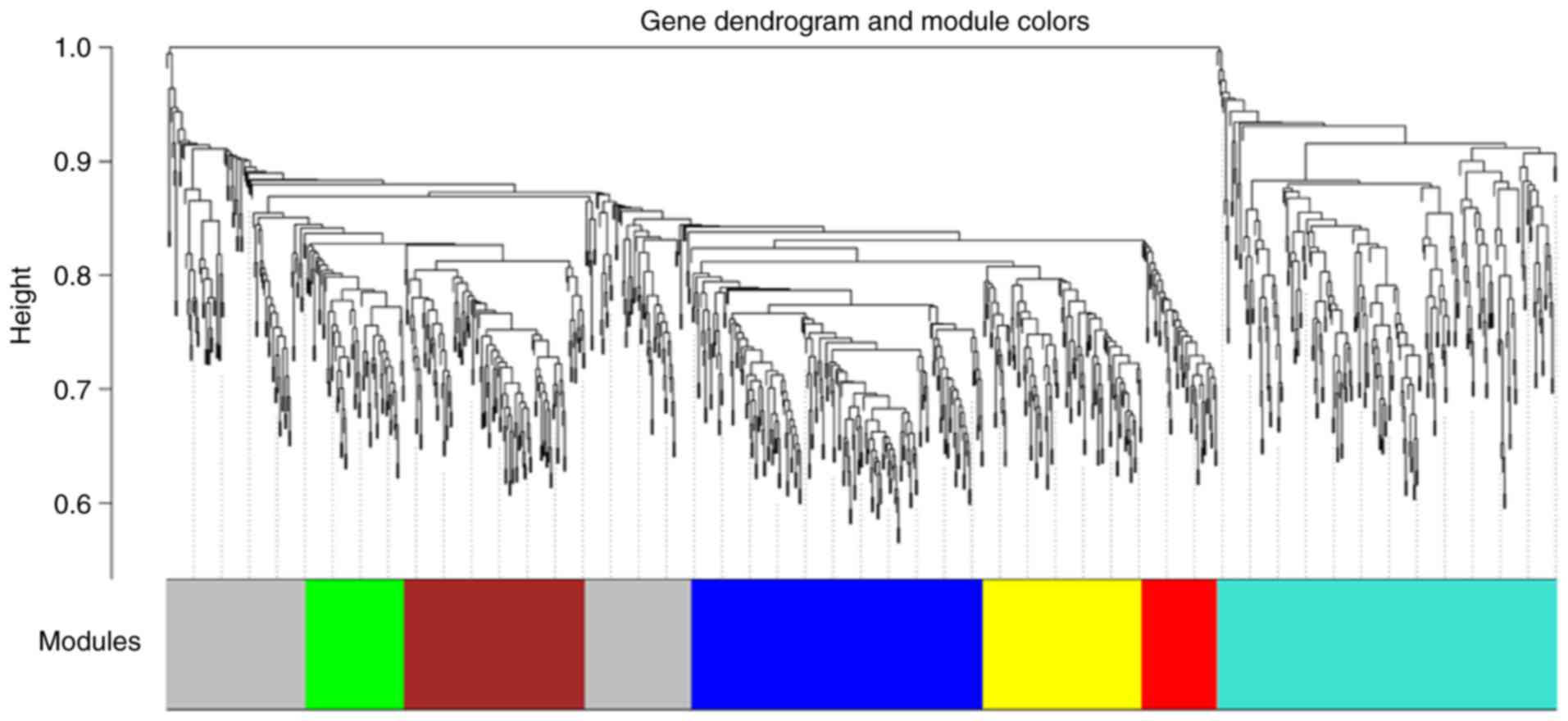
clustering tree with the cut-off height of 0.9 was then constructed
(Fig. 3), and the MS values of
all modules were >0.6 with a P<0.05 (Fig. 4). Two representative co-expression
modules, including the green module (72 DEGs) and the turquoise
module (247 DEGs), were selected. Subsequently, the PPI network was
constructed according to the PPIs of DEGs in these two
representative co-expression modules, and the constructed PPI
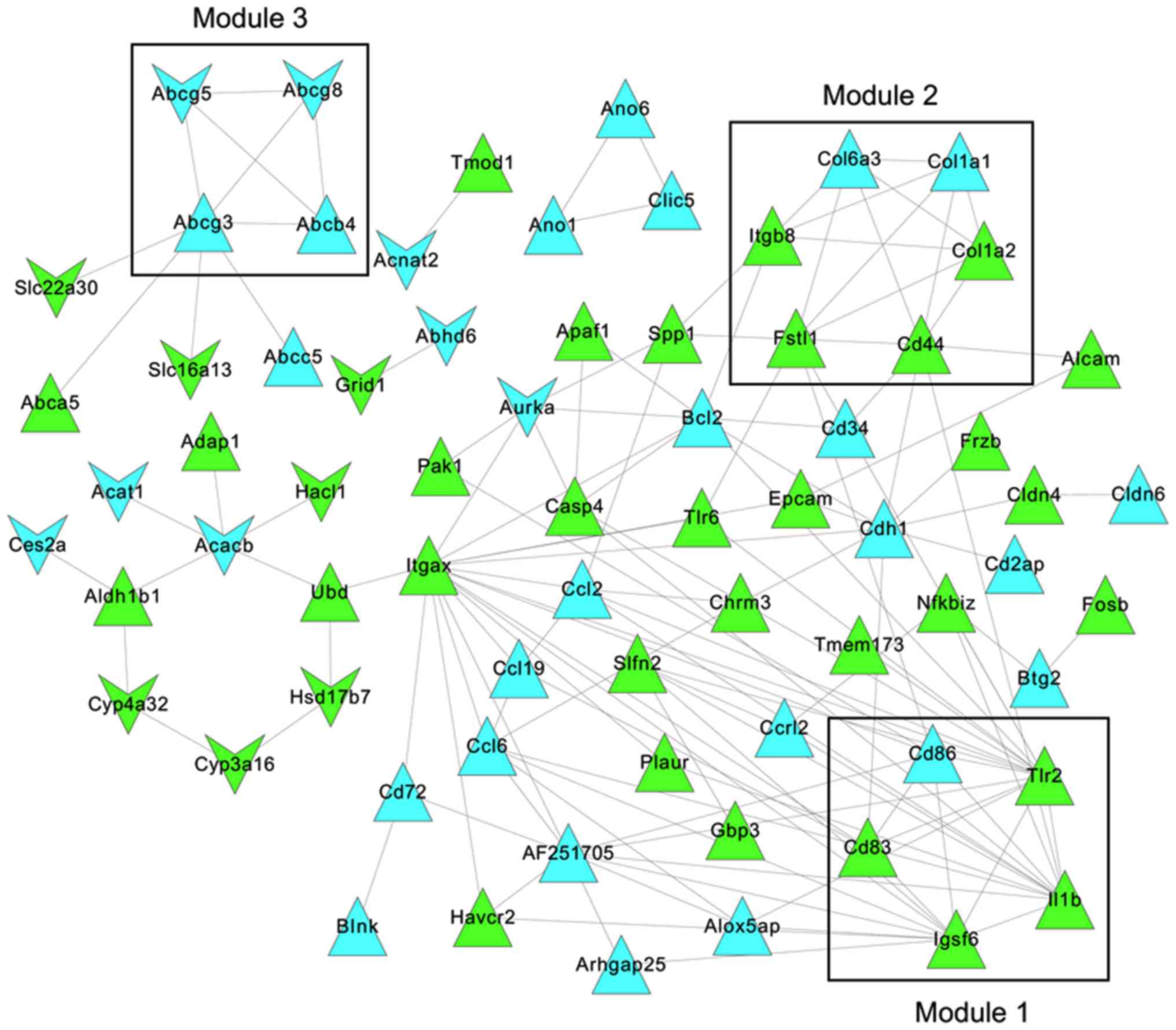
network contained 71 nodes and 135 PPIs (Fig. 5).
PPI network analysis
Three modules were identified from the constructed
PPI network by MCODE (Fig. 5).
The functional annotations revealed that the genes in module 1 were
mainly involved in the positive regulation of biological processes
(CD86, CD83, IL1B and TLR2; adjusted
P=1.32×10−3) and positive regulation of cytokine
production (CD83, IL1B and TLR2; adjusted
P=5.25×10−5; Table I).
The DEGs in module 2 were significantly enriched with respect to
protein heterotrimerization (COL1A1 and COL1A2;
adjusted P=2.85×10−6) and cellular component
organization (COL1A1, COL1A2 and CD44;
adjusted P=1.12×10−2). The four DEGs (ABCG8,
ABCG5, ABCB4 and ABCG3) in module 3 were
clearly associated with transport (adjusted P=1.49×10−3)
and the establishment of localization (adjusted
P=1.49×10−3). ABCG8 was observed to mainly
participate in intestinal cholesterol absorption, lipid digestion,
cholesterol efflux, intestinal absorption and sterol transport.
 | Table IEnriched GO terms for DEGs in the
three identified modules from the protein-protein interaction
network. |
Table I
Enriched GO terms for DEGs in the
three identified modules from the protein-protein interaction
network.
| GO ID | Adjusted
P-value | Description | DEGs |
|---|
| Module 1 | | | |
| GO:0048518 |
1.32×10−3 | Positive regulation
of biological process | CD86,
CD83, IL1B, TLR2 |
| GO:0050789 |
1.82×10−2 | Regulation of
biological process | CD86,
CD83, IL1B, TLR2 |
| GO:0065007 |
2.22×10−2 | Biological
regulation | CD86,
CD83, IL1B, TLR2 |
| GO:0001819 |
5.25×10−5 | Positive regulation
of cytokine production | CD83,
IL1B, TLR2 |
| GO:0031349 |
5.25×10−5 | Positive regulation
of defense response | CD86,
IL1B, TLR2 |
| GO:0031347 |
1.54×10−4 | Regulation of
defense response | CD86,
IL1B, TLR2 |
| GO:0001817 |
1.54×10−4 | Regulation of
cytokine production | CD83,
IL1B, TLR2 |
| GO:0048584 |
2.31×10−4 | Positive regulation
of response to stimulus | CD86,
IL1B, TLR2 |
| GO:0002684 |
2.31×10−4 | Positive regulation
of immune system process | CD86,
CD83, TLR2 |
| GO:0080134 |
2.31×10−4 | Regulation of
response to stress | CD86,
IL1B, TLR2 |
| GO:0051240 |
2.31×10−4 | Positive regulation
of multicellular | CD83,
IL1B, TLR2 |
| | organismal
process | |
| GO:0002682 |
4.86×10−4 | Regulation of
immune system process | CD86,
CD83, TLR2 |
| GO:0048583 |
6.88×10−4 | Regulation of
response to stimulus | CD86,
IL1B, TLR2 |
| GO:0010033 |
1.76×10−3 | Response to organic
substance | CD83,
IL1B, TLR2 |
| GO:0002376 |
1.80×10−3 | Immune system
process | CD86,
IL1B, TLR2 |
| GO:0051239 |
3.31×10−3 | Regulation of
multicellular organismal process | CD83,
IL1B, TLR2 |
| GO:0042221 |
3.31×10−3 | Response to
chemical stimulus | CD83,
IL1B, TLR2 |
| GO:0048519 |
5.00×10−3 | Negative regulation
of biological process | CD83,
IL1B, TLR2 |
| GO:0048522 |
5.02×10−3 | Positive regulation
of cellular process | CD83,
IL1B, TLR2 |
| GO:0050896 |
1.10×10−2 | Response to
stimulus | CD83,
IL1B, TLR2 |
| Module 2 | | | |
| GO:0070208 |
2.85×10−6 | Protein
heterotrimerization | COL1A1,
COL1A2 |
| GO:0070206 |
3.99×10−5 | Protein
trimerization | COL1A1,
COL1A2 |
| GO:0051291 |
2.91×10−3 | Protein
heterooligomerization | COL1A1,
COL1A2 |
| GO:0051259 |
6.07×10−3 | Protein
oligomerization | COL1A1,
COL1A2 |
| GO:0070271 |
9.81×10−3 | Protein complex
biogenesis | COL1A1,
COL1A2 |
| GO:0006461 |
9.81×10−3 | Protein complex
assembly | COL1A1,
COL1A2 |
| GO:0016043 |
1.12×10−2 | Cellular component
organization | COL1A1,
COL1A2, CD44 |
| GO:0065003 |
1.12×10−2 | Macromolecular
complex assembly | COL1A1,
COL1A2 |
| GO:0048646 |
1.12×10−2 | Anatomical
structure formation involved in morphogenesis | COL1A1,
CD44 |
| GO:0043933 |
1.12×10−2 | Macromolecular
complex subunit organization | COL1A1,
COL1A2 |
| Module 3 | | | |
| GO:0006810 |
1.49×10−3 | Transport | ABCG8,
ABCG5, ABCB4, ABCG3 |
| GO:0051234 |
1.49×10−3 | Establishment of
localization | ABCG8,
ABCG5, ABCB4, ABCG3 |
| GO:0051179 |
1.62×10−3 | Localization | ABCG8,
ABCG5, ABCB4, ABCG3 |
| GO:0030299 |
4.77×10−3 | Intestinal
cholesterol absorption | ABCG8 |
| GO:0044241 |
5.72×10−3 | Lipid
digestion | ABCG8 |
| GO:0033344 |
1.43×10−2 | Cholesterol
efflux | ABCG8 |
| GO:0050892 |
1.50×10−2 | Intestinal
absorption | ABCG8 |
| GO:0006855 |
1.67×10−2 | Drug transmembrane
transport | ABCB4 |
| GO:0015893 |
1.67×10−2 | Drug transport | ABCB4 |
| GO:0015918 |
1.67×10−2 | Sterol
transport | ABCG8 |
Functional and pathway enrichment
analysis of the PPI network
The functional enrichment analysis revealed that the
DEGs in the PPI network were significantly correlated with 10, 11
and 11 GO terms in the BP, CC and MF categories, respectively
(Table II). A total of 11 DEGs
(ALCAM, ITGAX, CLDN4, CD44,
ITGB8, CD34, CLDN6, BCL2, CDH1,
CD2AP and SPP1) were mainly involved in cell adhesion
(P=7.25×10−5). In addition, 7 DEGs (ALCAM,
CD83, CD86, ITGAX, CD44, CD34
and TLR2) were significantly associated with the external
side of the plasma membrane (P=4.44×10−4) and cell
surface (P=4.44×10−3). Meanwhile, the DEGs in the PPI
network were evidently associated with ATP-binding transport
activity (ABCG8, ABCG5, ABCG3, ABCC5,
ABCB4 and ABCA; P=4.71×10−3), cytokine
activity (CCL2, CCL19, IL1B, CCL6 and
SPP1; P=5.31×10−3), and cholesterol transporter
and sterol transporter activities (ABCG8 and ABCG5;
P=3.47×10−3). Furthermore, COL1A2 and
COL1A1 were associated with collagen type I
(P=9.57×10−3), fibrillar collagen
(P=3.31×10−3) and platelet-derived growth factor binding
(P=3.47×10−3).
 | Table IIFunctional annotations in the BP, CC
and MF categories for DEGs in the protein-protein interaction
network. |
Table II
Functional annotations in the BP, CC
and MF categories for DEGs in the protein-protein interaction
network.
| Term | Description | P-value | Count | DEGs |
|---|
| BP | | | | |
| GO:0007155 | Cell adhesion |
7.25×10−5 | 11 | ALCAM,
ITGAX, CLDN4, CD44, ITGB8, CD34,
CLDN6, BCL2, CDH1, CD2AP,
SPP1 |
| GO:0022610 | Biological
adhesion |
7.36×10−5 | 11 | ALCAM,
ITGAX, CLDN4, CD44, ITGB8, CD34,
CLDN6, BCL2, CDH1, CD2AP,
SPP1 |
| GO:0006952 | Defense
response |
7.02×10−5 | 10 | NFKBIZ,
TMEM173, CCL2, CD44, BCL2, TLR2,
CCL19, IL1B, APAF1, TLR6 |
| GO:0009611 | Response to
wounding |
7.13×10−5 | 9 | NFKBIZ,
CCL2, CD44, BCL2, TLR2, CCL19,
IL1B, TLR6, PLAUR |
| GO:0006955 | Immune
response |
5.71×10−4 | 9 | TMEM173,
CCL2, TLR2, CCL19, IL1B,
AF251705, TLR6, GBP3, CCL6 |
| GO:0006954 | Inflammatory
response |
2.84×10−4 | 7 | NFKBIZ,
CCL2, CD44, TLR2, CCL19, IL1B,
TLR6 CD83, CHRM3, BCL2, TLR2,
IL1B |
| GO:0051240 | Positive regulation
of multicellular organismal process |
4.23×10−3 | 5 | |
| GO:0006631 | Fatty acid
metabolic process |
6.48×10−3 | 5 | HACL1,
CYP4A32, ACNAT2, ALOX5AP, ACACB |
| GO:0016337 | Cell-cell
adhesion |
1.52×10−2 | 5 | CLDN4,
CLDN6, BCL2, CDH1, CD2AP |
| GO:0044093 | Positive regulation
of molecular function |
3.51×10−2 | 5 | CHRM3,
BCL2, TLR2, APAF1, TLR6 |
| CC | | | | |
| GO:0009897 | External side of
plasma membrane |
4.44×10−4 | 7 | ALCAM,
CD83, CD86, ITGAX, CD44, CD34,
TLR2 |
| GO:0045177 | Apical part of
cell |
4.78×10−4 | 6 | EPCAM,
ABCG8, ABCG5, CDH1, ABCB4,
SPP1 |
| GO:0009986 | Cell surface |
3.32×10−3 | 7 | ALCAM,
CD83, CD86, ITGAX, CD44, CD34,
TLR2 |
| GO:0005584 | Collagen type
I |
9.57×10−3 | 2 | COL1A2,
COL1A1 |
| GO:0016324 | Apical plasma
membrane |
1.20×10−2 | 4 | EPCAM,
ABCG8, ABCG5, ABCB4 |
| GO:0000267 | Cell fraction |
2.34×10−2 | 8 | ABCG5,
CYP3A16, CYP4A32, CLIC5, BCL2,
APAF1, ABCC5, ABCB4 |
| GO:0034707 | Chloride channel
complex |
2.39×10−2 | 3 | CLIC5,
ANO1, ANO6 |
| GO:0030054 | Cell junction |
2.47×10−2 | 7 | CHRM3,
CLDN4, CLDN6, CDH1, PAK1, ABCB4,
GRID1 |
| GO:0016323 | Basolateral plasma
membrane |
3.01×10−2 | 4 | EPCAM,
CD44, CDH1, PAK1 |
| GO:0005583 | Fibrillar
collagen | 3.31
×10−2 | 2 | COL1A2,
COL1A1 |
| MF | | | | |
| GO:0016887 | ATPase
activity |
4.71×10−3 | 6 | ABCG8,
ABCG5, ABCG3, ABCC5, ABCB4,
ABCA5 |
| GO:0005125 | Cytokine
activity |
0.53×10−3 | 5 | CCL2,
CCL19, IL1B, CCL6, SPP1 |
| GO:0008009 | Chemokine
activity |
9.65×10−3 | 3 | CCL2,
CCL19, CCL6 |
| GO:0042379 | Chemokine receptor
binding |
1.01×10−2 | 3 | CCL2,
CCL19, CCL6 |
| GO:0005254 | Chloride channel
activity |
2.59×10−2 | 3 | CLIC5,
ANO1, ANO6 |
| GO:0031404 | Chloride ion
binding |
2.90×10−2 | 3 | CLIC5,
ANO1, ANO6 |
| GO:0005253 | Anion channel
activity |
2.98×10−2 | 3 | CLIC5,
ANO1, ANO6 |
| GO:0043168g | Anion binding |
3.06×10−2 | 3 | CLIC5,
ANO1, ANO6 |
| GO:0017127 | Cholesterol
transporter activity |
3.47×10−2 | 2 | ABCG8,
ABCG5 |
| GO:0048407 | Platelet-derived
growth factor binding |
3.47×10−2 | 2 | COL1A2,
COL1A1 |
| GO:0015248 | Sterol transporter
activity |
3.47×10−2 | 2 | ABCG8,
ABCG5 |
In total, 8 KEGG pathways were identified for the
DEGs in the PPI network (Table
III). The members of the adenosine triphosphate-binding
cassette (ABC) family, such as ABCG8, ABCG5,
ABCG3, ABCC5, ABCB4 and ABCA5, were
significantly associated with the ABC transporters
(P=1.12×10−5). Certain other DEGs, including
ITGB8, COL1A2, COL1A1 and SPP1, were
evidently associated with extracellular matrix (ECM)-receptor
interactions (P=2.28×10−3) and focal adhesion
(P=1.03×10−2). Finally, the enriched Toll-like receptor
(TLR) signaling pathway was associated with CD86,
TLR2, IL1B, TLR6 and SPP1
(P=4.31×10−3).
 | Table IIISignificantly enriched pathways for
DEGs in the protein-protein interaction network. |
Table III
Significantly enriched pathways for
DEGs in the protein-protein interaction network.
| Term | Count | P-value | DEGs |
|---|
| mmu02010: ABC
transporters | 6 |
1.12×10−5 | ABCG8,
ABCG5, ABCG3, ABCC5, ABCB4,
ABCA5 |
| mmu04514: Cell
adhesion molecules | 7 |
5.31×10−4 | ALCAM,
CD86, CLDN4, ITGB8, CD34, CLDN6,
CDH1 |
| mmu04512:
ECM-receptor interaction | 5 |
2.28×10−3 | CD44,
ITGB8, COL1A2, COL1A1, SPP1 |
| mmu04620: Toll-like
receptor signaling pathway | 5 |
4.32×10−3 | CD86,
TLR2, IL1B, TLR6, SPP1 |
| mmu04510: Focal
adhesion | 6 |
1.03×10−2 | ITGB8,
BCL2, COL1A2, COL1A1, PAK1,
SPP1 |
| mmu00640:
Propanoate metabolism | 3 |
1.74×10−2 | ALDH1B1,
ACACB, ACAT1 |
| mmu00620: Pyruvate
metabolism | 3 |
3.12×10−2 | ALDH1B1,
ACACB, ACAT1 |
| mmu00071: Fatty
acid metabolism | 3 |
3.71×10−2 | CYP4A32,
ALDH1B1, ACAT1 |
Potentially therapeutic small
molecules
A total of six small molecules, including
mercaptopurine, ikarugamycin, camptothecin, quinostatin,
dexpanthenol and DL-thiorphan, were screened using the CMAP
database (Table IV). The score
for mercaptopurine was the lowest (connectivity score=−0.939),
indicating that this small molecule may be a potential drug for ARC
treatment.
 | Table IVIdentification of small molecules
with a potential therapeutic role in arthrogryposis-renal
dysfunction-cholestasis syndrome using the Connectivity Map
database. |
Table IV
Identification of small molecules
with a potential therapeutic role in arthrogryposis-renal
dysfunction-cholestasis syndrome using the Connectivity Map
database.
| Name | Connectivity
score | P-value |
|---|
| Mercaptopurine | −0.939 |
7.67×10−3 |
| Ikarugamycin | −0.906 |
1.62×10−3 |
| Camptothecin | 0.902 |
1.82×10−3 |
| Quinostatin | 0.904 |
1.88×10−2 |
| Dexpanthenol | 0.920 |
4.00×10−5 |
| DL-thiorphan | 0.975 |
9.70×10−4 |
Discussion
ARC, mainly caused by mutations in VPS33B, is
associated with abnormalities in polarized liver and kidney cells,
resulting in a multisystem disorder (1). In the present study, the microarray
data of liver tissue samples from liver-specific VPS33B
knockout mice and control mice were comprehensively analyzed. The
DEGs in two representative co-expression modules with the highest
MS values were selected for PPI network construction via WGCNA
analysis. Three further modules were identified from the PPI
network and annotated.
The five DEGs in module 1 included CD86,
CD83, IL1B, TLR2 and LGSF6. Pathway
enrichment analysis of the PPI network demonstrated that
CD86, TLR2, IL1B, TLR6 and SPP1
were significantly associated with the TLR signaling pathway
(P=0.004317). The TLRs are part of the naive immune system and
serve key roles in the elicitation of immune responses to microbes
(29). It has been suggested that
the VPS33B-VIPAR complex interacts with an active form of Rab11a
(1). In addition, Rab11a-positive
endosomes have been revealed to be important intermediates in the
transport of TLRs (TLR2 and TLR4) and TLR adaptor molecules to
phagosomes (30,31). In a study by Yu et al
(32), deletion of Rab11a
induced cytokine production and altered the intracellular
distribution of TLRs, indicating that Rab11a contributes to
intestinal host-microbial homeostasis through the sorting of TLRs.
The data of the present study revealed that CD83,
IL1B and TLR2 were significantly enriched with
respect to the positive regulation of cytokine production (adjusted
P=5.25×10−5). Thus, the identified DEGs, including
CD83, IL1B, TLR2 and TLR6, may
participate in the pathology of the ARC syndrome caused by
mutations in VPS33B via the TLR signaling pathway and
positive regulation of the cytokine production.
VPS33B serves a key role in the regulation of
vesicle-to-target SNARE complex formation and subsequent membrane
fusion (33). Furthermore,
inhibition of SNARE-mediated membrane traffic disrupted the
intracellular integrin trafficking that can provide a linkage
between the ECM and the cytoskeleton (34,35). In the present study, COL1A2
and COL1A1 in module 2 were significantly enriched with
respect to cellular component organization, ECM-receptor
interaction and focal adhesion. It has also been demonstrated that
loss of SNAP29 may cause alterations in the Rab11-expressing
domains of the endocytic recycling compartment and the structure of
focal adhesions, impairing endocytic recycling and cell motility
(36). Taken together, the
current study results provide evidence that mutations in
VPS33B may disturb cellular component organization,
ECM-receptor interactions and focal adhesion by regulating
COL1A2 and COL1A1.
It has been reported that vesicles containing ABC
transporters co-localize with Rab11a prior to their insertion into
the canalicular membrane (37).
In the present study, the four DEGs (ABCG8, ABCG5,
ABCB4 and ABCG3) in module 3 were significantly
involved in the ABC transporter pathway (P=1.12×10−5).
The ABC transporters are necessary for the energy-dependent biliary
secretion of bile acids, phospholipids, sterols (for instance,
ABCG8 and ABCG5 are sterol transporters) and non-bile acid organic
anions (38). Impaired bile acid
transport at the canalicular membrane, associated with reduced
amounts of ABC transporter proteins, may cause cholestasis (bile
secretory failure) (39). The
functional annotations for DEGs in the PPI network revealed that
ABCG8 and ABCG5 were evidently associated with
cholesterol transporter and sterol transporter activities. In
addition, ABCG8 mainly participated in intestinal
cholesterol absorption, lipid digestion, cholesterol efflux,
intestinal absorption and sterol transport, according to the module
annotations. Therefore, it may be speculated that mutations in
VPS33B influence sterol absorption and transport by
regulating ABCG8 and ABCG5.
In conclusion, the results of the present study
strongly indicate that the DEGs in the three identified modules
serve important roles in the pathogenesis of ARC caused by
mutations in VPS33B. Furthermore, CD83, IL1B,
TLR2 and TLR6 may participate in the pathology by
influencing the TLR signaling pathway and positive regulation of
cytokine production. The mutations in VPS33B may disturb the
cellular component organization, ECM-receptor interaction and focal
adhesion by dysregulation of COL1A2 and COL1A.
Finally, sterol absorption and transport may also be impeded by
mutations in VPS33B via the regulation of ABCG8 and
ABCG5 expression.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
XHan, MZ and LZ searched and downloaded gene
expression profile from the Gene Expression Omnibus database. MC,
LS, JB, XHao and BY made substantial contributions to analysis and
interpretation of microarray dataset. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cullinane AR, Straatman-Iwanowska A,
Zaucker A, Wakabayashi Y, Bruce CK, Luo G, Rahman F, Gürakan F,
Utine E, Ozkan TB, et al: Mutations in VIPAR cause an
arthrogryposis, renal dysfunction and cholestasis syndrome
phenotype with defects in epithelial polarization. Nat Genet.
42:303–312. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou Y and Zhang J: Arthrogryposis-renal
dysfunction-cholestasis (ARC) syndrome: From molecular genetics to
clinical features. Ital J Pediatr. 40:772014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gissen P, Tee L, Johnson CA, Genin E,
Caliebe A, Chitayat D, Clericuzio C, Denecke J, Di Rocco M,
Fischler B, et al: Clinical and molecular genetic features of ARC
syndrome. Hum Genet. 120:396–409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elmeery A, Lanka K and Cummings J: ARC
syndrome in preterm baby. J Perinatol. 33:821–822. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eastham KM, McKiernan PJ, Milford DV,
Ramani P, Wyllie J, van't Hoff W, Lynch SA and Morris AA: ARC
syndrome: An expanding range of phenotypes. Arch Dis Child.
85:415–420. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cullinane AR, Straatman-Iwanowska A, Seo
JK, Ko JS, Song KS, Gizewska M, Gruszfeld D, Gliwicz D, Tuysuz B,
Erdemir G, et al: Molecular investigations to improve diagnostic
accuracy in patients with ARC syndrome. Hum Mutat. 30:E330–E337.
2009. View Article : Google Scholar :
|
|
7
|
Carim L, Sumoy L, Andreu N, Estivill X and
Escarceller M: Cloning, mapping and expression analysis of VPS33B,
the human orthologue of rat Vps33b. Cytogenet Cell Genet. 89:92–95.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matthews RP, Plumb-Rudewiez N, Lorent K,
Gissen P, Johnson CA, Lemaigre F and Pack M: Zebrafish vps33b, an
ortholog of the gene responsible for human arthrogryposis-renal
dysfunction-cholestasis syndrome, regulates biliary development
downstream of the onecut transcription factor hnf6. Development.
132:5295–5306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peterson M and Emr SD: The class C Vps
complex functions at multiple stages of the vacuolar transport
pathway. Traffic. 2:476–486. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hershkovitz D, Mandel H, Ishida-Yamamoto
A, Chefetz I, Hino B, Luder A, Indelman M, Bergman R and Sprecher
E: Defective lamellar granule secretion in arthrogryposis, renal
dysfunction, and cholestasis syndrome caused by a mutation in
VPS33B. Arch Dermatol. 144:334–340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bem D, Smith H, Banushi B, Burden JJ,
White IJ, Hanley J, Jeremiah N, Rieux-Laucat F, Bettels R, Ariceta
G, et al: VPS33B regulates protein sorting into and maturation of
α-granule progenitor organelles in mouse megakaryocytes. Blood.
126:133–143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Banushi B, Forneris F, Straatman-Iwanowska
A, Strange A, Lyne AM, Rogerson C, Burden JJ, Heywood WE, Hanley J,
Doykov I, et al: Regulation of post-Golgi LH3 trafficking is
essential for collagen homeostasis. Nature Communications.
7:121112016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanley J, Dhar DK, Mazzacuva F, Fiadeiro
R, Burden JJ, Lyne AM, Smith H, Straatman-Iwanowska A, Banushi B,
Virasami A, et al: Vps33b is crucial for structural and functional
hepatocyte polarity. J Hepatol. 66:1001–1011. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Troyanskaya O, Cantor M, Sherlock G, Brown
P, Hastie T, Tibshirani R, Botstein D and Altman RB: Missing value
estimation methods for DNA microarrays. Bioinformatics. 17:520–525.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hubbell E, Liu WM and Mei R: Robust
estimators for expression analysis. Bioinformatics. 18:1585–1592.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rao Y, Lee Y, Jarjoura D, Ruppert AS, Liu
CG, Hsu JC and Hagan JP: A comparison of normalization techniques
for microRNA microarray data. Stat Appl Genet Mol Biol.
7:Article22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smyth GK: Limma: Linear models for
microarray data. Bioinformatics and computational biology solutions
using R and Bioconductor Springer. 397–420. 2005. View Article : Google Scholar
|
|
18
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate: A practical and powerful approach to
multiple testing. J R Statist Soc B. 57:289–300. 1995.
|
|
19
|
Wang L, Cao C, Ma Q, Zeng Q, Wang H, Cheng
Z, Zhu G, Qi J, Ma H, Nian H and Wang Y: RNA-seq analyses of
multiple meristems of soybean: Novel and alternative transcripts,
evolutionary and functional implications. BMC Plant Biol.
14:1692014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Langfelder P and Horvath S: WGCNA: An R
package for weighted correlation network analysis. BMC
Bioinformatics. 9:5592008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ravasz E, Somera AL, Mongru DA, Oltvai ZN
and Barabási AL: Hierarchical organization of modularity in
metabolic networks. Science. 297:1551–1555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Franceschini A, Szklarczyk D, Frankild S,
Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C
and Jensen LJ: STRING v9.1: Protein-protein interaction networks,
with increased coverage and integration. Nucleic Acids Res.
41(Database Issue): D808–D815. 2013. View Article : Google Scholar :
|
|
23
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bader GD and Hogue CW: An automated method
for finding molecular complexes in large protein interaction
networks. BMC Bioinformatics. 4:22003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maere S, Heymans K and Kuiper M: BiNGO: A
Cytoscape plugin to assess overrepresentation of gene ontology
categories in biological networks. Bioinformatics. 21:3448–3449.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beissbarth T and Speed TP: GOstat: Find
statistically over-represented Gene Ontologies within a group of
genes. Bioinformatics. 20:1464–1465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu J, Mao X, Cai T, Luo J and Wei L: KOBAS
server: A web-based platform for automated annotation and pathway
identification. Nucleic Acids Res. 34(Web Server Issue): W720–W724.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng J, Yang L, Kumar V and Agarwal P:
Systematic evaluation of connectivity map for disease indications.
Genome Med. 6:5402014. View Article : Google Scholar
|
|
29
|
Beutler B: Inferences, questions and
possibilities in Toll-like receptor signalling. Nature.
430:257–263. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Husebye H, Aune MH, Stenvik J, Samstad E,
Skjeldal F, Halaas O, Nilsen NJ, Stenmark H, Latz E, Lien E, et al:
The Rab11a GTPase controls Toll-like receptor 4-induced activation
of interferon regulatory factor-3 on phagosomes. Immunity.
33:583–596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sjoelund V, Smelkinson M and Nita-Lazar A:
Phosphoproteome profiling of the macrophage response to different
toll-like receptor ligands identifies differences in global
phosphorylation dynamics. J Proteome Res. 13:5185–5197. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu S, Nie Y, Knowles B, Sakamori R,
Stypulkowski E, Patel C, Das S, Douard V, Ferraris RP, Bonder EM,
et al: TLR sorting by Rab11 endosomes maintains intestinal
epithelial-microbial homeostasis. EMBO J. 33:1882–1895. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gissen P, Johnson CA, Morgan NV,
Stapelbroek JM, Forshew T, Cooper WN, McKiernan PJ, Klomp LW,
Morris AA, Wraith JE, et al: Mutations in VPS33B, encoding a
regulator of SNARE-dependent membrane fusion, cause
arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nat
Genet. 36:400–404. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Skalski M and Coppolino MG: SNARE-mediated
trafficking of alpha5beta1 integrin is required for spreading in
CHO cells. Biochem Biophys Res Commun. 335:1199–1210. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tayeb MA, Skalski M, Cha MC, Kean MJ,
Scaife M and Coppolino MG: Inhibition of SNARE-mediated membrane
traffic impairs cell migration. Exp Cell Res. 305:63–73. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rapaport D, Lugassy Y, Sprecher E and
Horowitz M: Loss of SNAP29 impairs endocytic recycling and cell
motility. PLoS One. 5:e97592010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wakabayashi Y, Dutt P, Lippincott-Schwartz
J and Arias IM: Rab11a and myosin Vb are required for bile
canalicular formation in WIF-B9 cells. Proc Natl Acad Sci USA.
102:15087–15092. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berge KE, Tian H, Graf GA, Yu L, Grishin
NV, Schultz J, Kwiterovich P, Shan B, Barnes R and Hobbs HH:
Accumulation of dietary cholesterol in sitosterolemia caused by
mutations in adjacent ABC transporters. Science. 290:1771–1775.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Strautnieks SS, Bull LN, Knisely AS,
Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V,
et al: A gene encoding a liver-specific ABC transporter is mutated
in progressive familial intrahepatic cholestasis. Nature genetics.
20:233–238. 1998. View
Article : Google Scholar : PubMed/NCBI
|