Introduction
Cervical cancer is the third most common tumor type
and the fourth leading cause of cancer-associated mortality in
women globally (1). It is
estimated that 530,000 new cases of cervical cancer and 270,000
cases of cervical cancer-associated mortality occur annually
worldwide (2). Radiotherapy (RT)
as the predominant therapeutic strategy or as an adjuvant treatment
can be applied to all stages of cervical cancer. Notably, v44% of
patients suffer from a relapse in cancer, among which, 35% of the
recurrent tumors are locoregional (3). The failure of RT is mainly
attributed to radioresistance, which is present in a subpopulation
of radioresistant cancer cells (4,5).
However, the underlying biological mechanisms that cause
radioresistance remain unclear. Therefore, there is an urgent
requirement to investigate the mechanisms governing radioresistance
in cervical cancer.
The development of human genome sequencing
technology has allowed long noncoding RNAs (lncRNAs) to be studied.
LncRNAs are a novel class of mRNA-like transcripts, which contain
>200 nucleotides, and are involved in numerous cellular events
(6). The identification of
lncRNAs has been considered a novel breakthrough to better
understand the initiation and progression of cancer (7). Notably, lncRNAs are also implicated
in RT and chemotherapy resistance (8).
Urothelial cancer associated 1 (UCA1), also known as
cancer-upregulated drug resistant, was initially discovered and
researched in bladder cancer (9).
Subsequently, the abnormal expression of UCA1 has been reported in
several other malignancies, where it functions as an oncogenic
lncRNA (10). Furthermore, UCA1
abundance is correlated with resistance to RT in prostate cancer
(11) and chemotherapy in various
types of cancer (12).
Therapeutic resistance is a complex, multifactorial
process. Previous studies have revealed that the increased
glycolysis of cancer cells is strongly correlated with
radioresistance (13,14). In cervical cancer, the interaction
between glucose metabolism and hypoxia has been proposed to be the
root of radioresistance (15).
Alteration in glucose metabolism is one of the main characteristics
of cancer; most tumor cells exhibit increased glycolysis and
decreased mitochondrial oxidative phosphorylation. This common
phenomenon is called ‘aerobic glycolysis’ or the ‘Warburg effect’
(16).
To the best of our knowledge, the biological role of
UCA1 in cervical cancer RT response has yet to be elucidated. The
present study tested the hypothesis that UCA1 was involved in the
radioresistance of cervical cancer cells via theglycolytic pathway.
The results demonstrated that the SiHa-irradiation-resistant (IRR)
and HeLa-IRR cell lines exhibited significantly increased UCA1
expression and glycolysis compared with in the parental cell lines.
Furthermore, UCA1 knockdown improved radiosensitivity, whereas UCA1
overexpression induced radioresistance. Inhibition of glycolysis
restored the sensitivity of IRR cells to irradiation. The present
data also suggested that UCA1 contributed to RT resistance through
the glycolytic pathway. By investigating the proteins associated
with glycolysis, it was revealed that hexokinase 2 (HK2) was the
crucial regulator in this process.
Materials and methods
Cell culture and drug treatment
Human cervical cancer cell lines HeLa and SiHa were
purchased from the Cell Resource Center of Shanghai Institute of
Life Sciences, Chinese Academy of Sciences (Shanghai, China). HeLa
and Siha cell lines were authenticated by short tandem repeat
profiling. The cervical cancer cells were cultured in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Biological Industries, Kibbutz Beit-Haemek, Israel) in a
humidified incubator containing 5% CO2 at 37°C. The
glycolysis inhibitor 2-deoxy-D-glucose (2-DG) was purchased from
Selleck Chemicals (Houston, TX, USA) and was dissolved in deionized
water at a concentration of 5 mM according to the manufacturer's
protocol; treatment of cells with deionized water only was
considered the control group. Cells were treated with 5 mM 2-DG for
24 h in an incubator containing 5% CO2 at 37°C to
inhibit glycolysis.
Establishment of IRR cervical cancer
cells
SiHa and HeLa cells (5×105) plated in 25
cm2 culture flasks were irradiated with 2 Gy X-ray
generated by 6 MeV β-rays from a Linear Accelerator (Siemens AG,
Berlin, German) with a 1.0-cm tissue compensation membrane.
Following irradiation, the cells with renewed culture medium were
immediately placed in an incubator containing 5% CO2 at
37°C. The cells were irradiated 5 days per week, and were then
allowed 7–10 days recovery prior to further irradiation. After
cells had been exposed to a total dose of 76 Gy irradiation, a
clonogenic assay was used to determine the level of resistance. The
parental cells, which underwent mock irradiation (exposed to 0 Gy
irradiation), were cultured under the same conditions. The
follow-up experiments were conducted 1 month after the last
irradiation.
Clonogenic survival assay
Cells (1,000, 2,000, 4,000, 6,000, 8,000 and 10,000)
were seeded into 6-well plates; a total of 24 h after being plated,
the cells were irradiated at doses of 0, 2, 4, 6, 8 and 10 Gy,
respectively. Subsequently, the cells were placed in an incubator
containing 5% CO2 at 37°C to allow colonies to form.
After 10–14 days, colonies were fixed with 4% paraformaldehyde for
15 min and stained with 0.1% crystal violet for 15 min at room
temperature. Colonies containing >50 cells were counted and
considered clonogenic survivors. Survival fraction = Number of
colonies/number of cells seeded × plating efficiency of the control
group. The control group plating efficiency was the ratio between
colonies formed and number of cells plated. The survival fraction
curve was plotted according to the single-hit multitarget formula:
S = 1 − (1−e−D/D0)N (17). The experiments were performed
three times.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using RNAiso
Plus (cat. no. 9108; Takara Biotechnology Co., Ltd., Dalian,
China). Using the RT-PCR kit (cat. no. RR047A; Takara Biotechnology
Co., Ltd.) RNA was reverse transcribed into cDNA, according to the
manufacturer's protocol. UCA1 expression was detected using
SYBR® Premix Ex Taq™ II (cat. no. RR820A; Takara
Biotechnology Co., Ltd.) according to the manufacturer's protocol,
and GAPDH expression was used as an internal control. Thermocycling
conditions for PCR were as follows: 95°C for 30 sec, followed by 40
cycles at 95°C for 5 sec and 60°C for 30 sec. The 2−ΔΔCq
method (18) was used to
calculate the results. Three independent experiments were
performed. Primers were synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China). The primer sequences are listed in Table I.
 | Table IPrimer and siRNA sequences. |
Table I
Primer and siRNA sequences.
| Primer or
siRNA | Sequence |
|---|
| UCA1 primer | F
5′-GACCCTACCCGGTCTTTATAG-3′ |
| R
5′-CTGATGGGCATGGCTTTATTC-3′ |
| GAPDH primer | F
5′-AGCCACATCGCTCAGACAC-3′ |
| R
5′-GCCCAATACGACCAAATCC-3′ |
| UCA1-siRNA1 | F
5′-GAGCCGAUCAGACAAACAATT-3′ |
| R
5′-UUGUUUGUCUGAUCGGCUCTT-3′ |
| UCA1-siRNA2 | F
5′-GGGCUUGGGACAUUUCACUTT-3′ |
| R
5′-AGUGAAAUGUCCCAAGCCCTT-3′ |
| UCA1-siRNA3 | F
5′-GGGAAUACUAUUCGUAUGATT-3′ |
| R
5′-UCAUACGAAUAGUAUUCCCTT-3′ |
| UCA1-NCsiRNA | F
5′-UUCUCCGAACGUGUCACGUTT-3′ |
| R
5′-ACGUGACACGUUCGGAGAATT-3′ |
Western blotting
Cells were lysed in cell lysis buffer (cat. no.
CW2333S; CWBIO, Beijing, China) supplemented with 1% protease
inhibitor (cat. no. CW2383; CWBIO). The cell lysate was centrifuged
at 15,000 × g for 15 min at 4°C. After centrifugation, the
supernatant was collected. Bicinchoninic acid assay was applied to
quantify protein concentration. Samples containing 20 µg
total protein were separated by 10% SDS-PAGE and then transferred
to a polyvinylidene fluoride membranes (Roche Diagnostics,
Shanghai, China). The membranes were then incubated in blocking
solution containing Tris-buffered saline containing 0.1% Tween
(TBST; pH 7.4) and 5% (mass-volume concentration) low-fat milk at
room temperature for 1 h. After being washed three times in TBST,
the membranes were incubated with rabbit polyclonal immunoglobulin
G anti-human antibodies: HK2 (1:1,000, cat. no. 22029-1-AP),
hypoxia-inducible factor 1α (HIF-1α; 1:400, cat. no. 20960-1-AP),
glucose transporter (GLUT)-1 (1:1,000, cat. no. 21829-1-AP), GLUT-4
(1:400, cat. no. 21048-1-AP), pyruvate kinase muscle isozyme M2
(PKM2; 1:1,000, cat. no. 15822-1-AP) and GAPDH (1:4,000, cat. no.
10494-1-AP) (ProteinTech Group, Inc., Chicago, IL, USA) overnight
at 4°C. Subsequently, the membranes were incubated with an
horseradish peroxidase-conjugated anti-rabbit secondary antibody
(1:2,000; cat. no. sc-2357; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) at room temperature for 1 h. After being treated with
enhanced chemiluminescence Plus solution (cat. no. WBKLS0010; EMD
Millipore, Billerica, MA, USA), the protein bands were visualized
through exposure to X-ray film. Densitometric analysis was
performed using ImageJ bundled with 64-bit Java 1.8.0_112 software
(National Institutes of Health, Bethesda, MD, USA) and expression
was normalized to GAPDH housekeeping protein expression.
Evaluation of glucose consumption and
lactate production
To evaluate glucose and lactate concentration,
glucose (cat. no. GOGA20; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and lactate assay kits (cat. no. K667-100; BioVision,
Inc., Milpitas, CA, USA) were used, according to the manufacturers'
protocols. Glucose and lactate concentrations were determined
according to a standard curve, and were normalized to cell number.
Glucose consumption was defined as the difference in glucose
concentrations between the original media and the media after 24 h
of cell culture.
Transfection of cervical cancer cell
lines
A total of 3×105 cells were seeded into
6-well plates. SiHa-IRR and HeLa-IRR cells were transfected with 5
mM plasmids and small interfering (si)RNAs using
Lipofectamine® 2000 (cat. no. 11668-019; Invitrogen;
Thermo Fisher Scientific, Inc.) at room temperature, according to
the manufacturer's protocol. Cells that were transfected with a
mock plasmid or negative control siRNA were considered control
cells. The full-length UCA1 sequence (Suzhou GenePharma Co., Ltd.)
was cloned into a pcDNA3.1 (+) vector (Suzhou GenePharma Co., Ltd.,
Suzhou, China) for overexpression of UCA1. For the knockdown of
UCA1, three siRNAs against UCA1 (Suzhou GenePharma Co., Ltd.) were
used (Table I). A total of 24 or
48 h post-transfection, cells were harvested and subjected to the
subsequent experiments. Cells were simultaneously transfected with
the UCA1 over-expression plasmid and treated with 2-DG; briefly, a
total of 24 h post-transfection with pcDNA3.1/UCA1 (pcDNA-U), the
parental cells were treated with 5 mM 2-DG for 24 h, and were then
irradiated at doses of 0, 2, 4, 6, 8 and 10 Gy.
Statistical analysis
All data were analyzed using SPSS standard version
13.0 software (SPSS, Inc., Chicago, IL, USA). Student's t-test and
one-way analysis of variance followed by the Bonferroni post hoc
test were used to evaluate comparisons between groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Establishment of IRR cell lines
After low-dose long-term irradiation to a total dose
of 76 Gy, IRR cells were separated from the parental cell
populations. A clonogenic assay was performed to validate the
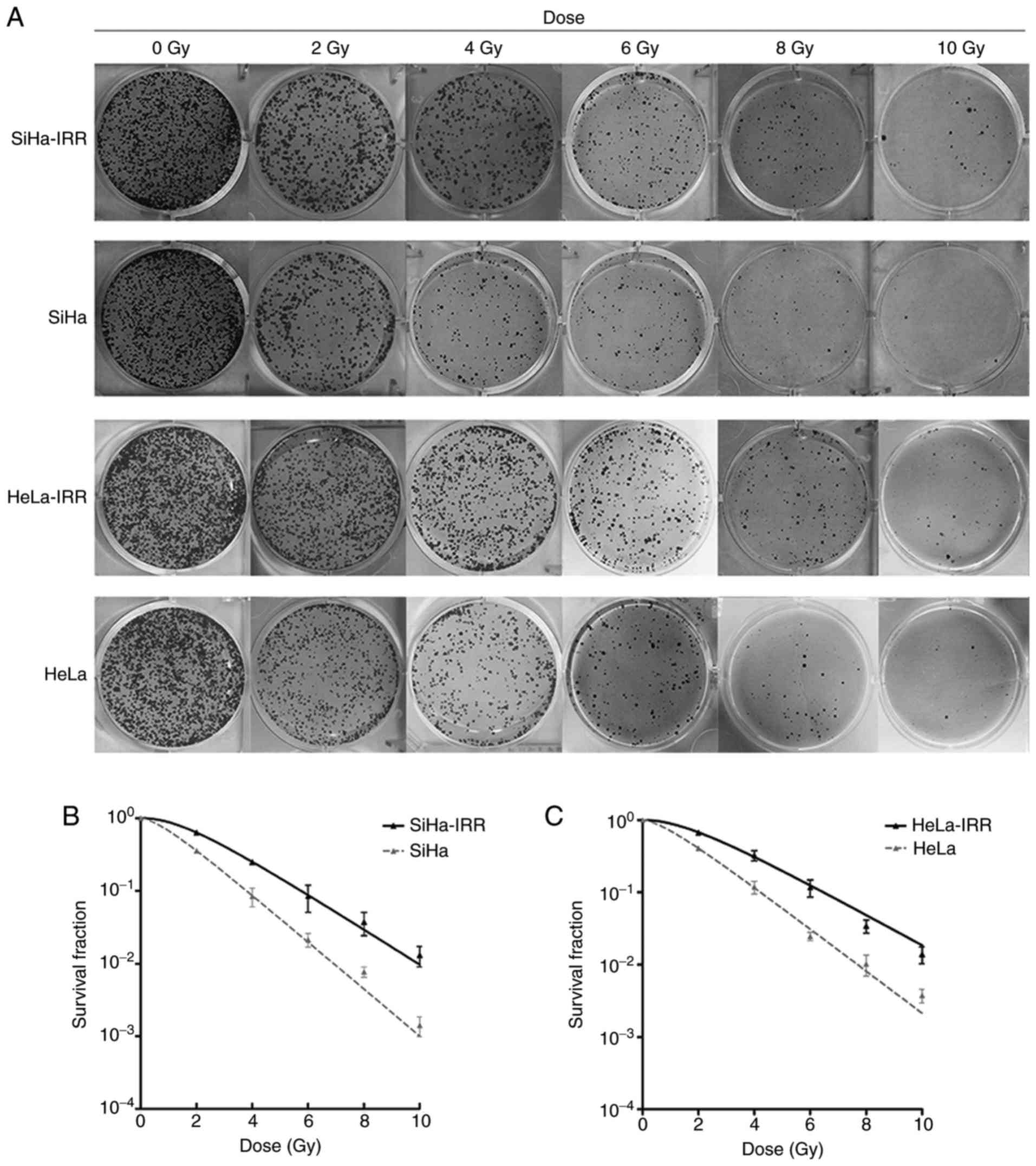
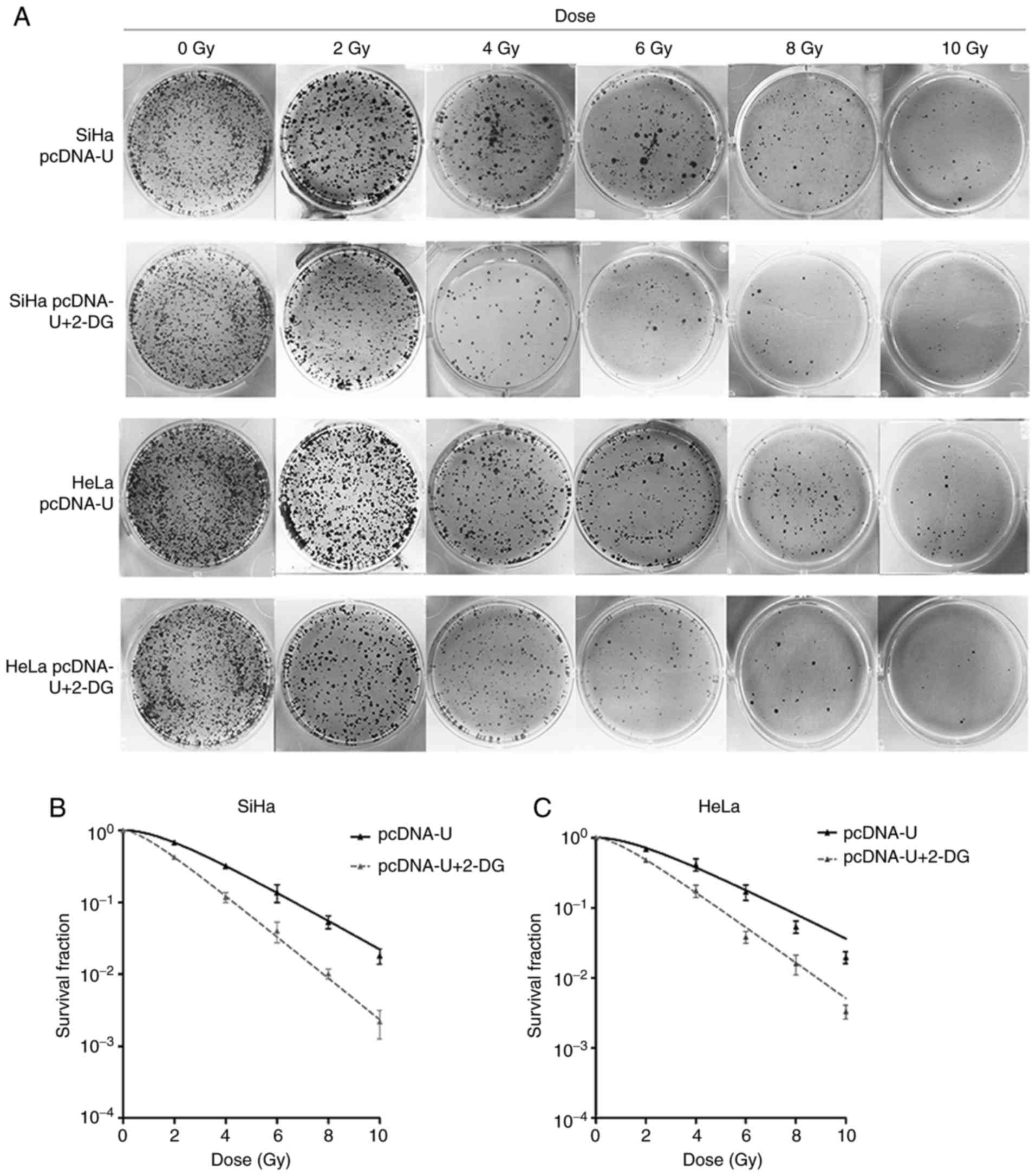
radioresistance of the subpopulation cells (Fig. 1). The IRR cells (SiHa-IRR and
HeLa-IRR) exhibited increases in survival fraction at 2, 4, 6, 8
and 10 Gy compared with the SiHa and HeLa parental cells (Fig. 1B and C). Furthermore,
radiobiological parameters of the SiHa-IRR and HeLa-IRR cells were
greater than those of the SiHa and HeLa cells, respectively
(Table II; P<0.05). These
data indicated that the SiHa-IRR and HeLa-IRR cells acquired a
stronger ability to form foci following exposure to irradiation
compared with the SiHa and HeLa cells. Therefore, it was confirmed
that the IRR cell lines were successfully established.
 | Table IIRadiobiological parameters of the
radioresistant and parental cells. |
Table II
Radiobiological parameters of the
radioresistant and parental cells.
| Cells | SF2 | D0 | Dq |
|---|
| SiHa-IRR | 0.64±0.03a | 1.80±0.13b | 1.67±0.10b |
| SiHa | 0.36±0.02 | 1.33±0.19 | 0.76±0.11 |
| HeLa-IRR | 0.68±0.08b | 2.02±0.2c | 1.84±0.24c |
| HeLa | 0.41±0.03 | 1.47±0.22 | 0.85±0.13 |
UCA1 expression is significantly elevated
in SiHa-IRR and HeLa-IRR cells
To determine whether UCA1 is involved in the
radiation response of cervical cancer, the expression levels of
UCA1 were examined in the parental and IRR cell lines. As shown in
Fig. 2A, both parental and IRR
cell lines expressed UCA1. The expression levels of UCA1 were
significantly higher in the SiHa-IRR and HeLa-IRR cells compared
with in the parental cells (P<0.05). These findings indicated
that abundant UCA1 expression may contribute to the development of
radioresistance.
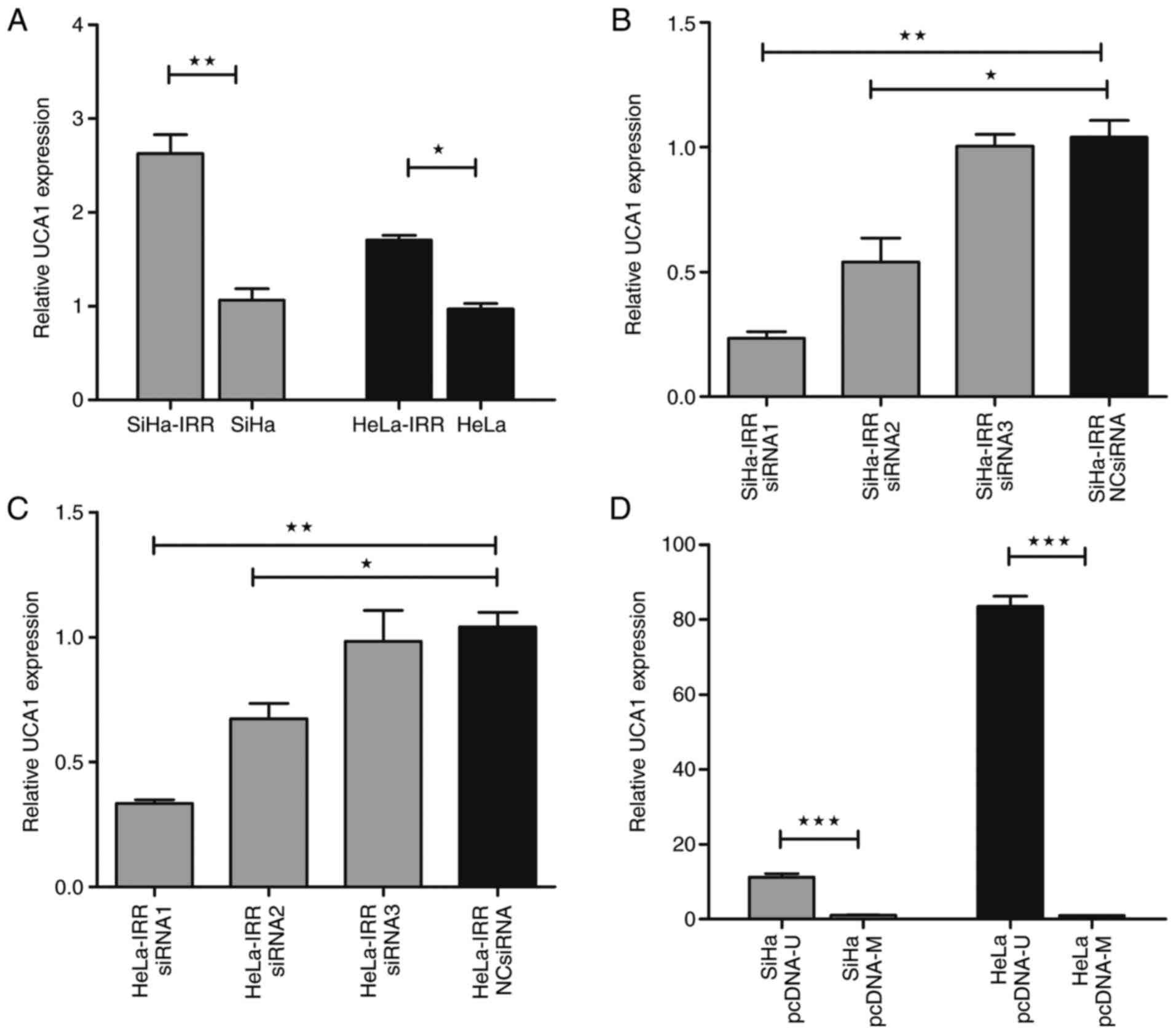 | Figure 2UCA1 expression is upregulated in
SiHa-IRR and HeLa-IRR cells. (A) RT-qPCR analysis of UCA1
expression in SiHa-IRR, SiHa, HeLa-IRR and HeLa cells. (B and C)
RT-qPCR analysis of UCA1 expression in SiHa-IRR and HeLa-IRR cells
transfected with UCA1-specific siRNAs or NC siRNA. (D) RT-qPCR
analysis of UCA1 expression in SiHa and HeLa cells transfected with
pcDNA-U or control pcDNA-M. Data are presented as the means ±
standard deviation from three separate experiments.
*P<0.05, **P<0.01,
***P<0.001. IRR, irradiation-resistant; NC, negative
control; pcDNA-M, pcDNA/Mock; pcDNA-U, pcDNA/UCA1; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; siRNA, small
interfering RNA; UCA1, urothelial cancer associated 1. |
Effects of UCA1 on cervical cancer
radioresistance
Since the SiHa-IRR and HeLa-IRR cells express higher
levels of UCA1, siRNA knockdown and plasmid overexpression assays
were conducted to explore the potential role of UCA1 in regulating
the response of cervical cancer cells to irradiation. After being
transfected with UCA1-specific siRNA1, siRNA2 or siRNA3, the
expression levels of UCA1 in SiHa-IRR and HeLa-IRR cells were
markedly decreased (Fig. 2B and
C). siRNA1 was used in subsequent experiments, since it was the
most efficient of the three siRNAs tested. In addition, the
expression levels of UCA1 in parental cells were markedly
upregulated post-transfection with pcDNA-U, particularly in HeLa
cells (Fig. 2D).
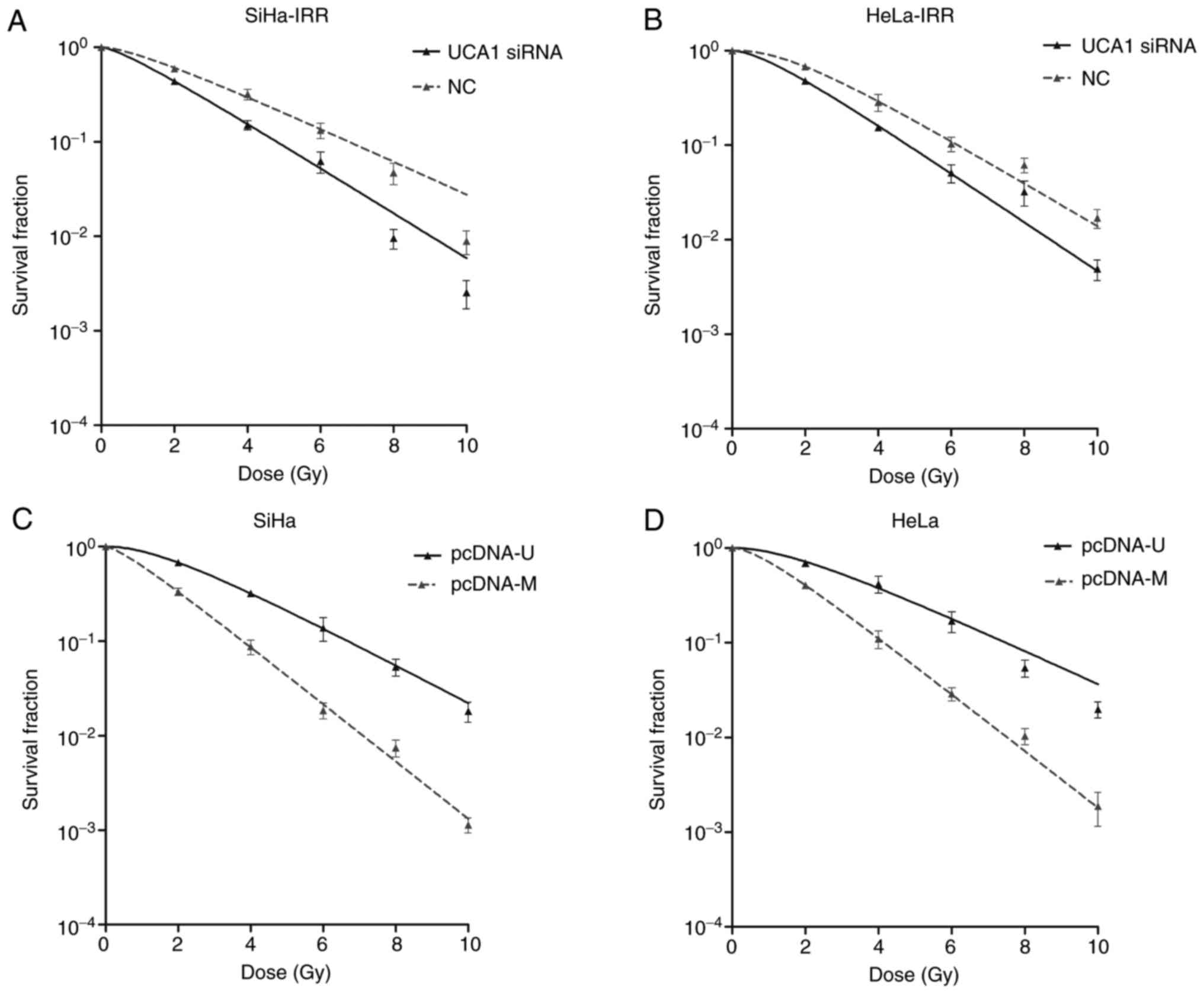
Post-transfection, the radioresistance of cervical cancer cells was
evaluated by clonogenic survival assays. Transfection with siRNA1
decreased the survival fraction compared with the control cells at
2, 4, 6, 8 and 10 Gy, thus suggesting that down-regulation of UCA1
expression may promote the sensitivity of SiHa-IRR and HeLa-IRR
cells to radiation (Fig. 3A and
B). Conversely, overexpression of UCA1 in the parental cell
lines, as induced by transfection with pcDNA-U, increased the
survival fraction at 2, 4, 6, 8 and 10 Gy, thus indicating that
augmentation of UCA1 expression may render SiHa and HeLa cells
resistant to radiation (Fig. 3C and
D). The relative radio-biological parameters are shown in
Table III (P<0.05). These
experiments further indicated that UCA1 may be considered an
important regulator of radioresistance in cervical cancer.
 | Table IIIRadiobiological parameters of the
radioresistant and parental cells exposed to various
treatments. |
Table III
Radiobiological parameters of the
radioresistant and parental cells exposed to various
treatments.
| Group | SF2 | D0 | Dq |
|---|
| SiHa-IRR +
2-DG | 0.36±0.02a | 1.37±0.05b | 0.71±0.05a |
| SiHa-IRR
control | 0.65±0.02 | 1.80±0.18 | 3.26±0.48 |
| HeLa-IRR +
2-DG | 0.43±0.03a | 1.57±0.08b | 0.82±0.14b |
| HeLa-IRR
control | 0.68±0.06 | 2.05±0.26 | 1.85±0.23 |
| SiHa-IRR UCA1
siRNA | 0.44±0.04a | 1.84±0.16a | 0.60±0.23b |
| SiHa-IRR NC
siRNA | 0.61±0.05 | 2.46±0.23 | 1.15±0.18 |
| HeLa-IRR UCA1
siRNA | 0.48±0.02a | 1.68±0.05b | 0.98±0.07a |
| HeLa-IRR NC
siRNA | 0.67±0.03 | 1.90±0.12 | 1.85±0.11 |
| SiHa pcDNA-U | 0.68±0.02a | 2.17±0.23c | 1.73±0.17a |
| SiHa pcDNA-M | 0.33±0.03 | 1.43±0.04 | 0.51±0.14 |
| SiHa pcDNA-U +
2-DG | 0.42±0.02d | 1.50±0.13e | 0.88±0.03f |
| HeLa pcDNA-U | 0.72±0.07a | 2.42±0.23a | 1.92±0.39b |
| HeLa pcDNA-M | 0.38±0.03 | 1.44±0.07 | 0.85±0.11 |
| HeLa pcDNA-U +
2-DG | 0.48±0.02f | 1.70±0.18f | 1.00±0.04e |
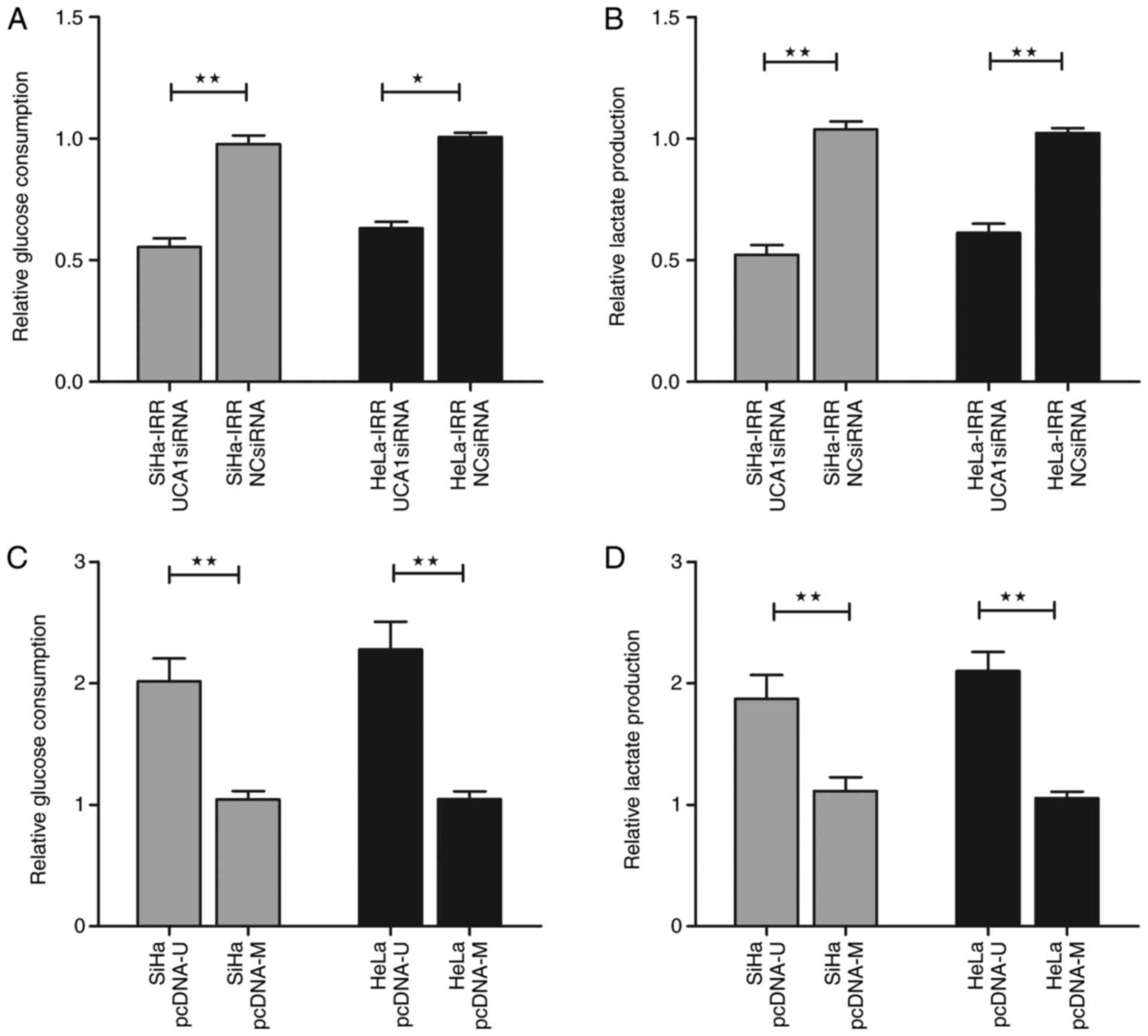
SiHa-IRR and HeLa-IRR cells exhibit
increased glycolysis
As aforementioned, dysregulated glycolysis
contributes to radioresistance in cancer. To investigate whether RT
alters the glucose metabolic profile in cervical cancer, the
present study aimed to measure glucose consumption and lactate
production in IRR and parental cells. As expected, the SiHa-IRR and
HeLa-IRR cell lines consumed more glucose and produced more lactate
compared with the SiHa and HeLa cells (Fig. 4A and B; P<0.05), thus
suggesting that abnormal activation of glycolysis may have a role
in cervical cancer radioresistance. To support the metabolic
results, the expression levels of proteins associated with glucose
metabolism were also measured. At the protein level, the expression
of limited enzymes HK2 and PKM, the regulator HIF-1α and the
glucose transporter GLUT-1 were increased in SiHa-IRR and HeLa-IRR
cells compared with in the parental cells (Fig. 4C; P<0.05); however, the
expression levels of the glucose transporter GLUT-4 were not
markedly altered (Fig. 4C). These
findings indicated that HK2, PKM, HIF-1α and GLUT-1, rather than
GLUT-4, may be involved in cervical cancer
radioresistance-associated glycolysis.
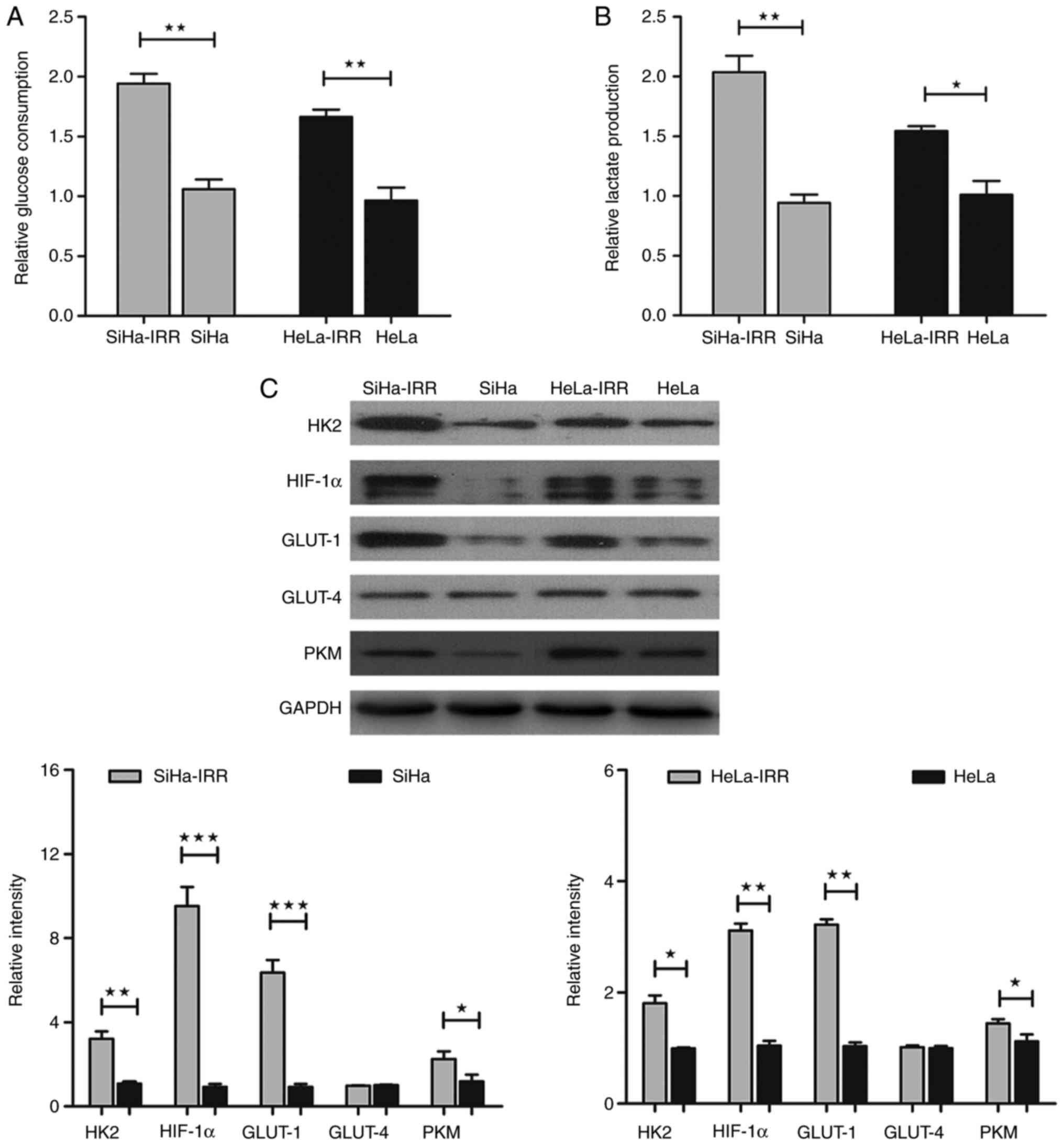 | Figure 4SiHa-IRR and HeLa-IRR cells exhibit
increased glycolysis. (A and B) Glucose consumption and lactate
production analyses of IRR and parental cells. (C) Western blot
analysis of the protein expression levels of HK2, HIF-1α, GLUT-1,
GLUT-4 and PKM in the IRR and parental cells. Data are presented as
the means ± standard deviation from three separate experiments.
*P<0.05, **P<0.01,
***P<0.001. GLUT, glucose transporter; HIF-1α,
hypoxia-inducible factor 1α; HK2, hexokinase 2; IRR,
irradiation-resistant; PKM, pyruvate kinase muscle isozyme M2. |
Inhibition of glycolysis restores the
radiosensitivity of SiHa-IRR and HeLa-IRR cells
In order to further address the function of
glycolysis in cervical cancer radioresistance, the present study
detected the radiosensitivity of SiHa-IRR and HeLa-IRR following
exposure to the glycolysis inhibitor 2-DG. Following treatment with
5 mM 2-DG for 24 h, IRR cells exhibited decreased glucose
consumption and lactate production (Fig. 5A and B). Since 2-DG inhibits
glycolysis mainly through competing with glucose for HK2, the
expression levels of HK2 were also evaluated. The results indicated
that the protein expression levels of HK2 were significantly
reduced by 2-DG, whereas the expression levels of the other
proteins were not significantly affected (Fig. 5C). Subsequently, the
radioresistance of IRR cells was assessed following treatment with
2-DG. SiHa-IRR and HeLa-IRR cells exhibited markedly decreased
survival fraction at 2, 4, 6, 8 and 10 Gy following treatment with
2-DG (Fig. 5D and E). The
relative parameters of radioresistant cells exposed to 2-DG were
also decreased compared with in the control cells without 2-DG
treatment (Table III;
P<0.05). Therefore, it may be suggested that inhibiting
glycolysis improves the sensitivity of cervical cancer cells to
irradiation.
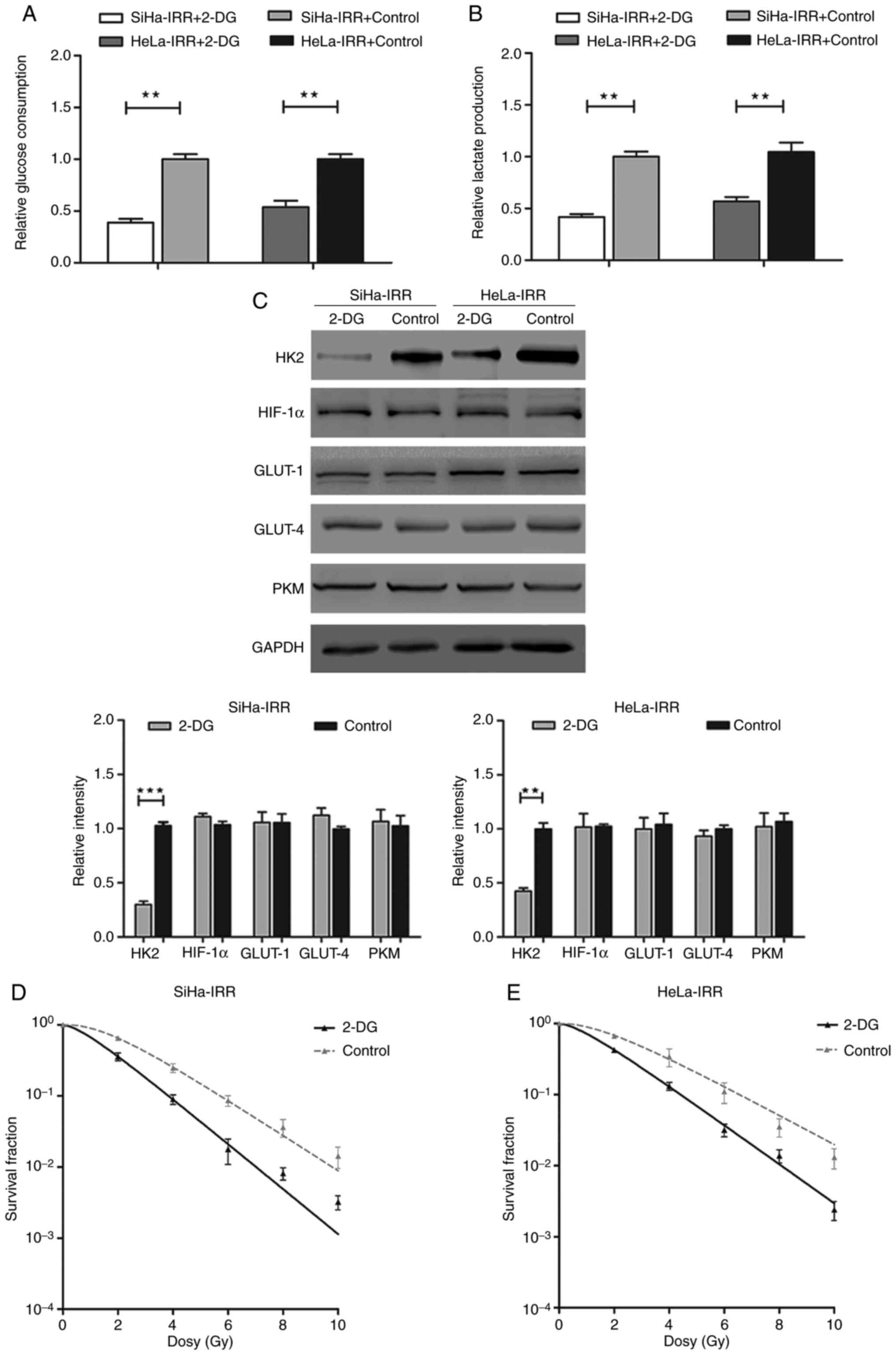 | Figure 5Inhibition of glycolysis by 2-DG
restores the radiosensitivity of SiHa-IRR and HeLa-IRR cells. (A
and B) Glucose consumption and lactate production analyses of IRR
cells treated with 2-DG. (C) Western blot analysis of the protein
expression levels of HK2, HIF-1α, GLUT-1, GLUT-4 and PKM in the IRR
cells treated with 2-DG. (D and E) 2-DG-treated IRR cell survival
fraction data were fitted to the single-hit multitarget model. Data
are presented as the means ± standard deviation from three separate
experiments. **P<0.01, ***P<0.001.
2-DG, 2-deoxy-D-glucose; GLUT, glucose transporter; HIF-1α,
hypoxia-inducible factor 1α; HK2, hexokinase 2; IRR,
irradiation-resistant; PKM, pyruvate kinase muscle isozyme M2. |
UCA1 regulates radioresistance through
the glycolytic pathway by modulating HK2 in cervical cancer
The present study indicated that UCA1 and glycolysis
may affect the radio-resistance of cervical cancer cells;
therefore, this study aimed to ascertain if UCA1 regulates the
radiation response through glycolysis (Figs. 6 and 7). In SiHa-IRR and HeLa-IRR cells, the
increases in glucose consumption and lactate production were
inhibited by UCA1 knockdown (Fig. 6A
and B; P<0.05). In addition, knockdown of UCA1 reduced the
protein expression levels of HK2 (P<0.05), but did not
significantly affect HIF-1α, GLUT-1, GLUT-4 and PKM expression
(Fig. 7A). Conversely,
overexpression of UCA1 in SiHa and HeLa cells augmented glucose
uptake and lactate production (Fig.
6C and D; P<0.05). Not unexpectedly, the protein expression
levels of HK2 were subsequently increased (P<0.05), whereas the
expression levels of other proteins were not markedly altered
(Fig. 7B). These results
indicated that UCA1, via the regulation of HK2, may function as a
mediator of radioresistance-associated glycolysis in cervical
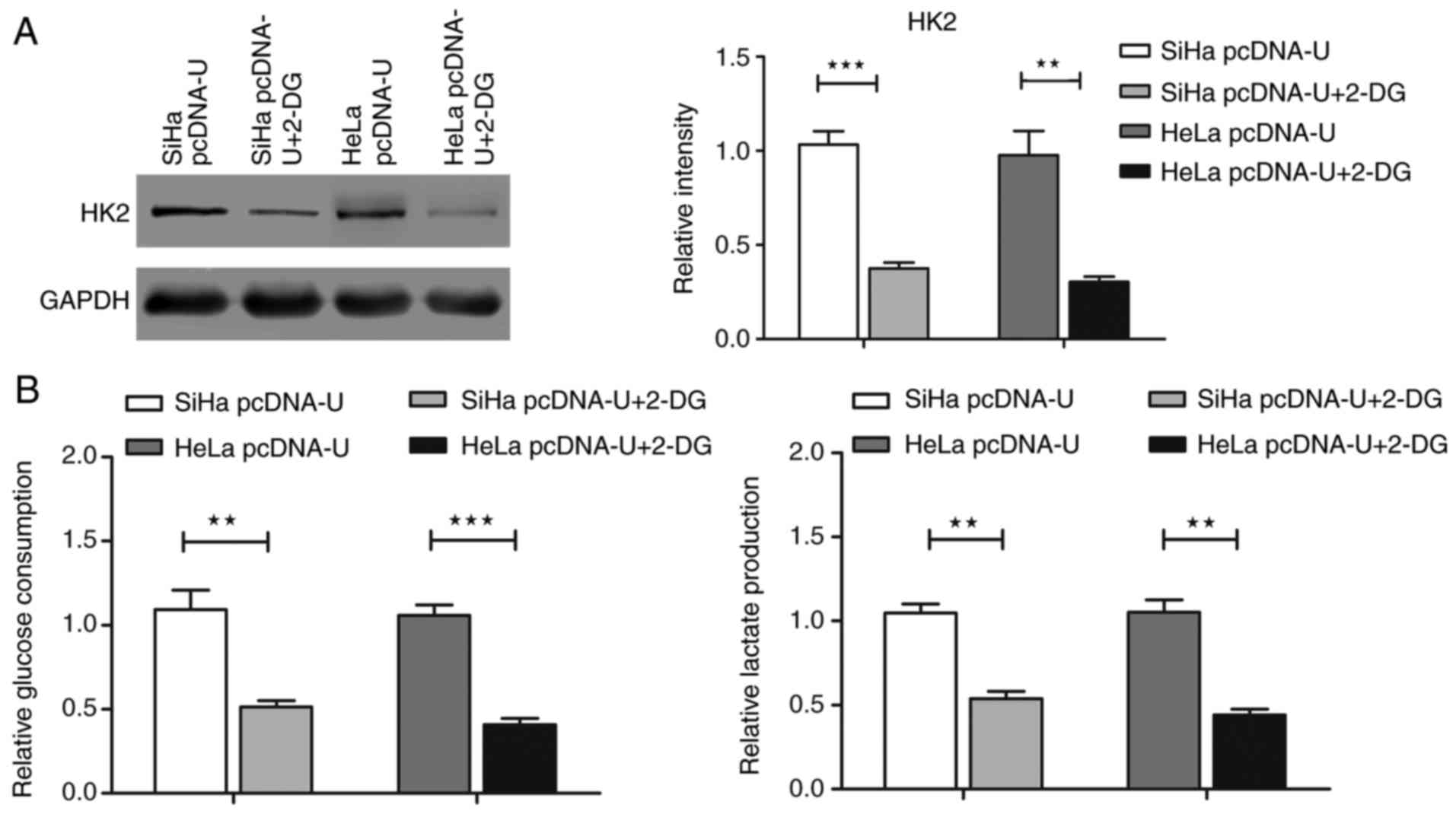
cancer cells. Finally, SiHa and HeLa cells were exposed to 2-DG
after being transfected with pcDNA-U plasmid. Compared with in the
cells transfected with pcDNA-U plasmid only, additional treatment
with 2-DG reversed the positive effects of UCA1 overexpression on
HK2 protein expression and glycolysis in SiHa and HeLa cells
(Fig. 8; P<0.05), and finally
reduced the radioresistance of cervical cancer cells (Fig. 9, Table III; P<0.05). These findings
suggested that the mediating effects of UCA1 on radioresistance of
cervical cancer cells might depend on the HK2/glycolytic
pathway.
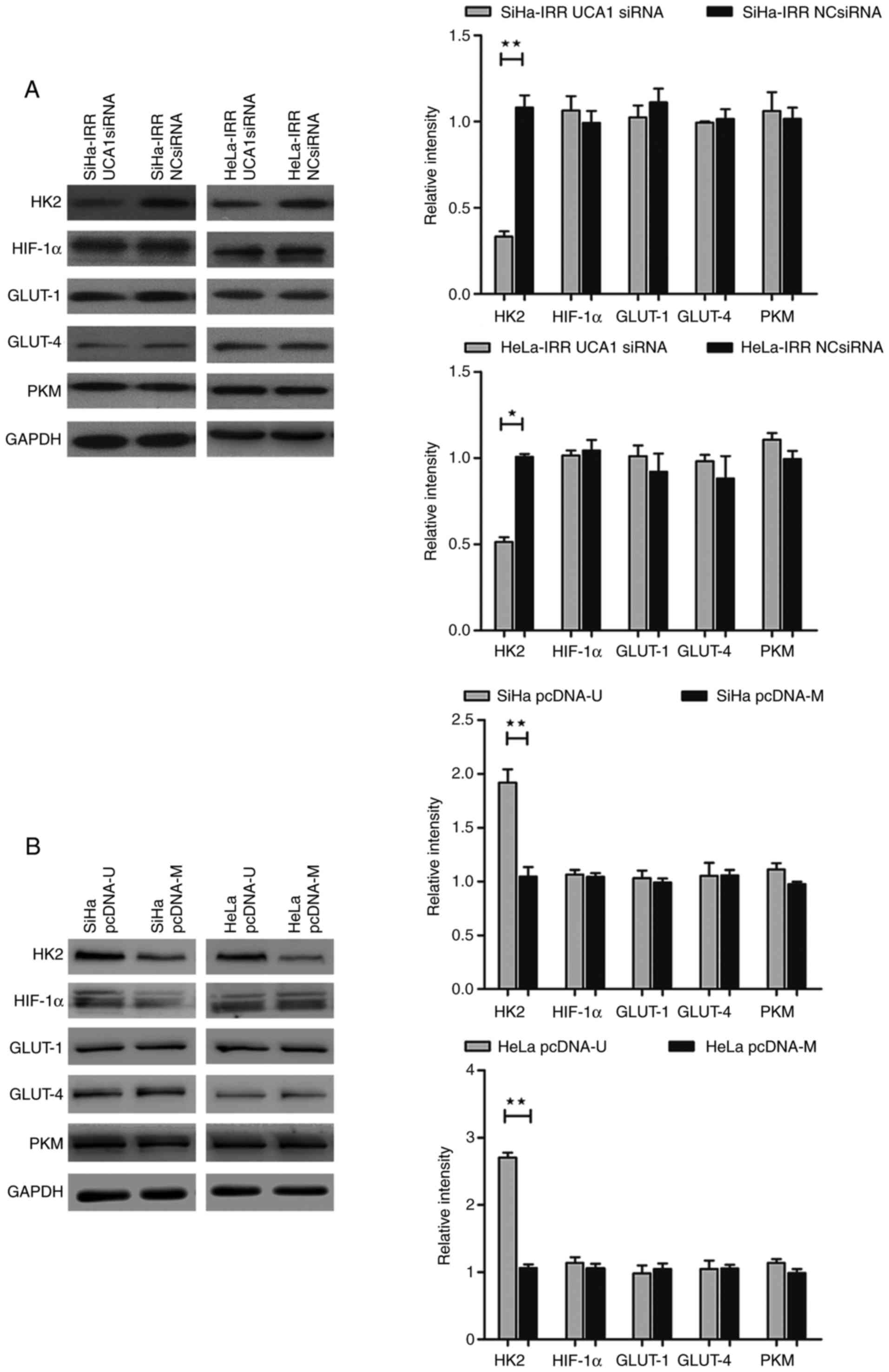 | Figure 7UCA1 regulates the glycolytic pathway
by targeting HK2 in cervical cancer cells. Western blot analysis of
protein expression levels of HK2, HIF-1α, GLUT-1, GLUT-4 and PKM in
(A) SiHa-IRR and HeLa-IRR cells transfected with UCA1 siRNA, and
(B) SiHa and HeLa cells transfected with pcDNA-U plasmid. Data are
presented as the means ± standard deviation from three separate
experiments. *P<0.05, **P<0.01. GLUT,
glucose transporter; HIF-1α, hypoxia-inducible factor 1α; HK2,
hexokinase 2; IRR, irradiation-resistant; NC, negative control;
pcDNA-M, pcDNA/Mock; pcDNA-U, pcDNA/UCA1; PKM, pyruvate kinase
muscle isozyme M2; siRNA, small interfering RNA; UCA1, urothelial
cancer associated 1. |
Discussion
Intrinsic or acquired radioresistance of cancer
cells remains a crucial obstacle in RT. The present study
established radio-resistant cervical cancer cell lines, which
exhibited a marked increase in clonogenic survival compared with in
the parental cell lines following irradiation. The roles of UCA1
and the glycolytic pathway in radioresistance were subsequently
investigated in SiHa-IRR and HeLa-IRR cells.
Dysregulation of lncRNAs induces numerous biological
effects (19). Recently, numerous
studies have proposed that lncRNAs mediate the irradiation response
through various signaling pathways and factors (20–22). The present results revealed that
UCA1 was markedly upregulated in SiHa-IRR and HeLa-IRR cells and
served an important role in the induction of radioresistance. In
cervical cancer, the lncRNA metastasis associated lung
adenocarcinoma transcript 1 (MALAT1) has been reported to modulate
radiosensitivity via microRNA-145 (23); however, to the best of our
knowledge, there are no studies regarding the effects of UCA1 on
cervical cancer radioresistance. Therefore, the present study is
the first to research the role of UCA1 in cervical cancer
radioresistance. Via transfection with siRNAs and plasmids, the
present study revealed that knockdown of UCA1 was able to reverse
the radioresistance of SiHa-IRR and HeLa-IRR cells. Conversely, in
SiHa and HeLa cells, overexpression of UCA1 had the opposite effect
on response to radiation. Based on these results, it may be
indicated that UCA1 expression is stimulated by irradiation and
functions as an important regulator of radioresistance in cervical
cancer. Furthermore, inconsistencies in SiHa and HeLa cell
radioresistance were detected. The HeLa cell line appeared to be
more resistant to RT than the SiHa cell line. This may also explain
the different responses of the two cell lines to UCA1
overexpression; increasing the UCA1 expression by >80-fold in
HeLa cells had almost the same effect on the radioresistance of
cells as increasing UCA1 expression by >20-fold in SiHa cells.
The involvement of aerobic glycolysis in cancer provides a good
advantage for cancer cell growth and leads to malignant progression
(24,25). Low-dose radiation promotes
metabolic transformation from oxidative phosphorylation to aerobic
glycolysis, leading to increased radioresistance in vitro
and in vivo (26). In the
present study, low-dose radiation (2 Gy) was used as the fraction
dose to establish IRR cells from parental cells. After exposure to
a total dose of 76 Gy radiation, SiHa-IRR and HeLa-IRR cells
exhibited increased glucose consumption and lactate production.
Furthermore, the protein expression levels of HK2, HIF-1α, GLUT-1
and PKM were markedly increased compared with in the parental
cells, thus suggesting that glycolysis was hyperactive in SiHa-IRR
and HeLa-IRR cells. In addition, treatment with the glycolysis
inhibitor 2-DG decreased the levels of glycolysis by reducing the
protein expression of HK2, thus improving the response of SiHa-IRR
and HeLa-IRR cells to RT. 2-DG is a glucose analog, which competes
with glucose for HK2, after which glucose cannot be converted to
glucose-6-phosphatese. In addition, HK2, combined with 2-DG, is
degraded in the mitochondria, thus resulting in a decrease in HK2
protein expression (27).
Facilitating the DNA repair process (28) and inducing cytotoxic stress
resistance (29,30) may be the underlying essential
mechanisms by which glycolysis regulates radioresistance.
Therefore, activation of glycolysis may facilitate cells to survive
following radiation, thus resulting in the generation of
radioresistance in cervical cancer.
H19 and downstream let-7, forming a double-negative
feedback loop, contribute to glucose metabolism in muscle cells
(31). HOX transcript antisense
RNA serves an important role in maintaining mitochondrial function
in cancer cells (32), and MALAT1
enhances arsenite-induced glycolysis in human hepatic L-02 cells
through HIF-1α stabilization (33). These previous studies indicated
that lncRNAs are crucial mediators of glucose metabolism. The
present study explored the connection between glycolysis and UCA1
in cervical cancer cells. Glucose consumption and lactate
production were decreased due to UCA1 knockdown in SiHa-IRR and
HeLa-IRR cells. Conversely, UCA1 overexpression caused an increase
in glycolysis in parental cells. These results suggested that UCA1
may regulate radioresistance-associated glycolysis in cervical
cancer, providing further evidence of lncRNAs modulating the
glycolytic pathway. LncRNAs usually affect glycolysis by targeting
associated enzymes and signaling pathways (34). The present study detected the
expression levels of glycolysis-associated proteins in IRR and
parental cells, only to find that the limited enzyme HK2 varied
along with alterations in UCA1 expression. This result is
consistent with the previous findings reported by Li et al
in 2014 (35). Li et al
revealed that UCA1 promotes glycolysis by upregulating HK2 via the
mammalian target of rapamycin-signal transducer and activator of
transcription 3/microRNA-143 pathway; to the best of our knowledge,
this previous study is the only study regarding the specific
mechanisms underlying the effects of UCA1 on HK2 regulation.
Therefore, there may be such regulatory pathways in cervical
cancer; this requires confirmation in future studies.
The present study also indicated that inhibiting
glycolysis could eliminate the enhancing effects of UCA1
overexpression on HK2 protein expression and cervical cancer
radioresistance, thus indicating that UCA1 may promote
radioresistance through the glycolytic pathway by targeting HK2 in
cervical cancer. UCA1 may represent a potential therapeutic target
for radioresistance, in order to improve the therapeutic effects of
RT and the prognosis of patients with cervical cancer.
In conclusion, the present study indicated that
radiation increases the expression of UCA1 and glycolysis is
enhanced through targeting the crucial enzyme HK2, which in turn
affects the sensitivity of cervical cancer cells to RT. Although
further research regarding the mechanism underlying the regulatory
effects of the UCA1/HK2/glycolytic pathway on radioresistance is
required, the present results improved current understanding of the
radioresistance regulatory network and identified potential novel
targets for the enhancement of RT success in cervical cancer.
Acknowledgments
Not applicable.
Abbreviations:
|
RT
|
radiotherapy
|
|
lncRNAs
|
long non-coding RNAs
|
|
UCA1
|
urothelial cancer associated 1
|
|
IRR
|
irradiation-resistant
|
|
HK2
|
hexokinase 2
|
|
2-DG
|
2-deoxy-D-glucose
|
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81101979, 81572575
and 81602290), the Guangdong province Natural Scientific Grant
(grant nos. 2014A030313109 and 2016A020215059), the Guangdong
College Students' Innovation and Entrepreneurship Training Program
(grant no. 1055813194), the National College Students' Innovation,
Entrepreneurship Training Program (grant no. 201310558097) and the
Science and Technology Planning Project of Guangzhou (grant no.
201601020102).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LF participated in designing the project and
performed all experiments. CXH participated in the establishment of
IRR cells, and was a major contributor in writing the manuscript.
JL analyzed the data regarding the relationship between
UCA1/glycolytic pathway and cervical cancer cell radioresistance.
TG analyzed the data regarding the relationship between UCA1 and
the glycolytic pathway, and was a major contributor in writing the
manuscript. TTY and ZQL provided research ideas for the project and
were involved in study design.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Prevention CfDC a: Global Cancer
Statistics. Available from: http://www.cdc.gov/cancer/international/statistics.htm.
|
|
2
|
Organization WH: Human papillomavirus
(HPV) and cervical cancer [cited 2016 June 10]. Available from:
http://www.who.int/mediacentre/factsheets/fs380/en/.
|
|
3
|
Reducing uncertainties about the effects
of chemoradiotherapy for cervical cancer: Individual patient data
meta-analysis. The Cochrane database of systematic reviews.
2010.1:CD008285
|
|
4
|
Huang Z, Mayr NA, Yuh WT, Lo SS,
Montebello JF, Grecula JC, Lu L, Li K, Zhang H, Gupta N and Wang
JZ: Predicting outcomes in cervical cancer: A kinetic model of
tumor regression during radiation therapy. Cancer Res. 70:463–470.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang J, Yue JB, Liu J and Yu JM:
Repopulation of tumor cells during fractionated radiotherapy and
detection methods (Review). Oncol Lett. 7:1755–1760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kwok ZH and Tay Y: Long noncoding RNAs:
Lincs between human health and disease. Biochem Soc Trans.
45:805–812. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Chen W, Yang C, Wu W, Wu S, Qin X
and Li X: Long non-coding RNA UCA1a(CUDR) promotes proliferation
and tumorigenesis of bladder cancer. Int J Oncol. 41:276–284.
2012.PubMed/NCBI
|
|
10
|
Xue M, Chen W and Li X: Urothelial cancer
associated 1: A long noncoding RNA with a crucial role in cancer. J
Cancer Res Clin Oncol. 142:1407–1419. 2016. View Article : Google Scholar
|
|
11
|
Fotouhi Ghiam A, Taeb S, Huang X, Huang V,
Ray J, Scarcello S, Hoey C, Jahangiri S, Fokas E, Loblaw A, et al:
Long non-coding RNA urothelial carcinoma associated 1 (UCA1)
mediates radiation response in prostate cancer. Oncotarget.
8:4668–4689. 2017.
|
|
12
|
Wang H, Guan Z, He K, Qian J, Cao J and
Teng L: LncRNA UCA1 in anti-cancer drug resistance. Oncotarget.
8:64638–64650. 2017.PubMed/NCBI
|
|
13
|
Zhao H, Jiang H, Li Z, Zhuang Y, Liu Y,
Zhou S, Xiao Y, Xie C, Zhou F and Zhou Y: 2-Methoxyestradiol
enhances radiosen-sitivity in radioresistant melanoma MDA-MB-435R
cells by regulating glycolysis via HIF-1alpha/PDK1 axis. Int J
Oncol. 50:1531–1540. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimura T, Noma N, Sano Y, Ochiai Y,
Oikawa T, Fukumoto M and Kunugita N: AKT-mediated enhanced aerobic
glycolysis causes acquired radioresistance by human tumor cells.
Radiother Oncol. 112:302–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Noordhuis MG, Eijsink JJ, Roossink F, de
Graeff P, Pras E, Schuuring E, Wisman GB, de Bock GH and van der
Zee AG: Prognostic cell biological markers in cervical cancer
patients primarily treated with (chemo)radiation: A systematic
review. Int J Radiat Oncol Biol Phys. 79:325–334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jing Z, Gong L, Xie CY, Zhang L, Su HF,
Deng X and Wu SX: Reverse resistance to radiation in KYSE-150R
esophageal carcinoma cell after epidermal growth factor receptor
signal pathway inhibition by cetuximab. Radiother Oncol.
93:468–473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar
|
|
19
|
Fang Y and Fullwood MJ: Roles, functions,
and mechanisms of long non-coding RNAs in cancer. Genomics
Proteomics Bioinformatics. 14:42–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu X, Jiang H and Jiang X: Downregulation
of lncRNA ANRIL inhibits proliferation, induces apoptosis, and
enhances radiosensitivity in nasopharyngeal carcinoma cells through
regulating miR-125a. Cancer Biol Ther. 18:331–338. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang H, Hu X, Zhang H and Li W:
Down-regulation of LncRNA TUG1 enhances radiosensitivity in bladder
cancer via suppressing HMGB1 expression. Radiat Oncol. 12:652017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Z, Zhou Y, Tu B, Bu Y, Liu A and Kong
J: Long noncoding RNA MALAT1 affects the efficacy of radiotherapy
for esophageal squamous cell carcinoma by regulating Cks1
expression. J Oral Pathol Med. 46:583–590. 2017. View Article : Google Scholar
|
|
23
|
Lu H, He Y, Lin L, Qi Z, Ma L, Li L and Su
Y: Long non-coding RNA MALAT1 modulates radiosensitivity of HR-HPV+
cervical cancer via sponging miR-145. Tumour Biol. 37:1683–1691.
2016. View Article : Google Scholar
|
|
24
|
Kondoh H, Lleonart ME, Gil J, Wang J,
Degan P, Peters G, Martinez D, Carnero A and Beach D: Glycolytic
enzymes can modulate cellular life span. Cancer Res. 65:177–185.
2005.PubMed/NCBI
|
|
25
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lall R, Ganapathy S, Yang M, Xiao S, Xu T,
Su H, Shadfan M, Asara JM, Ha CS, Ben-Sahra I, et al: Low-dose
radiation exposure induces a HIF-1-mediated adaptive and protective
metabolic response. Cell Death Differ. 21:836–844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong JT and Zhou SH: Warburg effect,
hexokinase-II, and radioresistance of laryngeal carcinoma.
Oncotarget. 8:14133–14146. 2017.
|
|
28
|
Bhatt AN, Chauhan A, Khanna S, Rai Y,
Singh S, Soni R, Kalra N and Dwarakanath BS: Transient elevation of
glycolysis confers radio-resistance by facilitating DNA repair in
cells. BMC Cancer. 15:3352015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khodarev NN, Beckett M, Labay E, Darga T,
Roizman B and Weichselbaum RR: STAT1 is overexpressed in tumors
selected for radioresistance and confers protection from radiation
in transduced sensitive cells. Proc Natl Acad Sci USA.
101:1714–1719. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pitroda SP, Wakim BT, Sood RF, Beveridge
MG, Beckett MA, MacDermed DM, Weichselbaum RR and Khodarev NN:
STAT1-dependent expression of energy metabolic pathways links
tumour growth and radioresistance to the Warburg effect. BMC Med.
7:682009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao Y, Wu F, Zhou J, Yan L, Jurczak MJ,
Lee HY, Yang L, Mueller M, Zhou XB, Dandolo L, et al: The H19/let-7
double-negative feedback loop contributes to glucose metabolism in
muscle cells. Nucleic Acids Res. 42:13799–13811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng P, Xiong Q, Wu Y, Chen Y, Chen Z,
Fleming J, Gao D, Bi L and Ge F: Quantitative proteomics analysis
reveals novel insights into mechanisms of action of long noncoding
RNA Hox transcript antisense intergenic RNA (HOTAIR) in HeLa cells.
Mol Cell Proteomics. 14:1447–1463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luo F, Liu X, Ling M, Lu L, Shi L, Lu X,
Li J, Zhang A and Liu Q: The lncRNA MALAT1, acting through
HIF-1alpha stabilization, enhances arsenite-induced glycolysis in
human hepatic L-02 cells. Biochim Biophys Acta. 1862:1685–1695.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu C, Xue J, Zhu W, Jiao Y, Zhang S and
Cao J: Warburg meets non-coding RNAs: The emerging role of ncRNA in
regulating the glucose metabolism of cancer cells. Tumour Biol.
36:81–94. 2015. View Article : Google Scholar
|
|
35
|
Li Z, Li X, Wu S, Xue M and Chen W: Long
non-coding RNA UCA1 promotes glycolysis by upregulating hexokinase
2 through the mTOR-STAT3/microRNA143 pathway. Cancer Sci.
105:951–955. 2014. View Article : Google Scholar : PubMed/NCBI
|