Introduction
Benign prostatic hyperplasia (BPH) is the most
common prostate disease in elderly men and affects nearly one-half
of all men aged >50 years (1–3).
Previous studies have demonstrated that an imbalance between
androgens and estrogens serves an important role in the development
and progression of BPH (4,5).
The growth and enlargement of prostatic tissue are dependent on
androgenic stimulation by the potent androgenic receptor agonist
dihydrotestosterone (DHT), which is generated by the conversion of
testosterone by the 5α-reductase enzyme. Therefore, 5α-reductase
inhibitors (finasteride and dutasteride) or α1-adrenergic blockers
are typically used for clinical management of BPH (5,6).
However, these drugs incur side effects including impotence,
ejaculatory dysfunction, painful ejaculation, decreased libido,
dizziness, headache and fatigue (7–9).
Furthermore, post-marketing studies clearly suggested that certain
patients exhibited side effects including rashes, tachycardia and
chest pain (10).
Although there has been much effort during the
previous decade to gain insight into the etiology of BPH, a
detailed understanding of the pathophysiological processes is
lacking. DHT increases the mitochondrial activity in prostatic
cells, resulting in excessive free radical formation and oxidative
damage to the prostate (11).
This oxidative stress is caused by an imbalance between the
production and scavenging of free radicals in the system, resulting
in damage to cellular components. Data suggest that oxidative
stress is associated with BPH (12), and various studies have indicated
the role of oxidative stress in either the initiation or the
progression of BPH (13–15). Augmenting the function of
antioxidants and redox systems against oxidative stress in male
reproductive tissues has been previously described, and a number of
types of antioxidants may prevent BPH development induced by
oxidative stress (13). Vitamin
E, selenium, diallyl sulfide and black tea extract have been
demonstrated to significantly modulate testosterone-induced
oxidative stress due to their antioxidant potential (14–16). Therefore, the use of exogenous
antioxidant therapy may be protective in BPH.
GV1001 is a 16-amino acid hTERT peptide that was
selected based on computer algorithms predicting strong HLA class
II binding properties and multiple nested HLA class I binding
motifs (17,18). GV1001 was first developed as a
chemotherapeutic drug for the treatment of various types of cancer,
including advanced pancreatic cancer, melanoma, non-small cell lung
cancer, advanced hepatocellular carcinoma and prostate cancer
(19–22). Previous studies have suggested
that GV1001 also exerts a protective effect against
ischemia-reperfusion injury through antioxidant effects, by
reducing reactive oxygen species (ROS) and suppressing the
inflammatory cascade (23).
GV1001 exhibits neuroprotective effects in neural stem cells
following treatment with H2O2 that appear to
be mediated by the scavenging of free radicals, increased survival
signals and decreased death signals (24). GV1001 exhibited anti-inflammatory
activity in LPS-stimulated pulpitis without significantly affecting
cell viability (25). although
the pharmacological activities of GV1001 have been extensively
investigated with regard to its anti-inflammatory, antipyretic and
antioxidant activities (23,26), to the best of our knowledge there
have been no studies evaluating the effect of GV1001 in
testosterone-induced BPH. In particular, the mechanisms by which
GV1001 regulate steroid hormone synthesis have not yet been
elucidated.
The present study investigated whether GV1001
protected rats from testosterone-induced BPH and explored the
protective mechanism of GV1001 in a model of testosterone-induced
BPH in castrated rats. The results revealed that GV1001 injection
significantly inhibited a testosterone-mediated increase in
prostate weight and relative prostate weight. Therefore, the
results of the present study may be used to support future clinical
applications of GV1001 in BPH prevention or therapy.
Materials and methods
Materials
GV1001 was kindly provided by GemVax & KAEL
(Seongnam, South Korea). Finasteride, testosterone propionate (TP)
and all other reagents used in the present study were purchased
from Sigma Aldrich; Merck KGaA (Darmstadt, Germany). Assay kits for
glutathione (GSH), SOD, CAT and MDA were purchased from Abcam
(Cambridge, MA, USA). All other assay kits were purchased from
Cayman Chemical Company (Ann Arbor, MI, USA).
Castration procedures and experimental
animal model
Specific pathogen-free male Sprague-Dawley rats aged
10 weeks (n=42; 280±5 g) that were routinely screened serologically
for relevant respiratory pathogens were purchased from Central Lab.
Animal, Inc. (Seoul, Korea). The rats were maintained in an animal
facility under standard laboratory conditions for 1 week prior to
the experiments and subjected to a 12 h light-dark cycle in a room
with controlled temperature (22±1°C) and humidity (55±10%), as well
as free access to rodent chow and tap water. All experimental
procedures were performed in accordance with the Korea Food and
Drug Administration (FDA) Guidelines for the Care and Use of
Laboratory Animals, and animal handling followed the dictates of
the national animal Welfare law of Korea. after 1 week of
acclimation, the rats were randomly distributed into experimental
groups. All experimental procedures were performed in accordance
with guidelines of the Committee for the Purpose of Control and
Supervision of Experiments on Animals of Sungkyunkwan University.
The present study was reviewed and approved by the Sungkyunkwan
University Animal Ethics Committee.
To exclude the effect of intrinsic testosterone,
castration was performed in all rats by removing the testes and
epididymis through the scrotal sac. BPH was induced in rats by
subcutaneous (s.c.) injections of TP (3 mg/kg) for 4 weeks
following castration (27,28).
Castrated rats were divided into six groups (n=6 for each): Group
1, the castration group, which received corn oil s.c.; Group 2, the
BPH group, which received TP (3 mg/kg) s.c.; Groups 3-5, the GV1001
groups, which received GV1001 (0.01, 0.1 or 1 mg/kg) and TP (3
mg/kg) s.c.; group 6, the positive control group, which received
finasteride (10 mg/kg) orally and TP (3 mg/kg) s.c. for 4 weeks
following castration. Animals from all groups were examined every
other day for morphological changes, and body weight (BW) was
measured. The dosage of GV1001 was based on data from our previous
dose-escalation study in patients with pancreatic cancer without
toxicity (18). Subsequent to a
treatment period of 4 weeks and overnight fasting, the rats were
anesthetized with tribromoethanol (250 mg/kg BW, i.p.). Blood
samples were collected and centrifuged at 300 × g for 10 min at
4°C. Prostate and seminal vesicles from each animal were excised,
weighed and immediately washed with ice-cold saline. A part of the
ventral lobe of the prostate was stored in neutral buffered
formalin for the histological studies, and the remainder of the
prostate was used for the biochemical assays. A 2% tissue
homogenate was prepared in ice-cold phosphate buffer containing
0.15 M KCl for use as the enzyme source.
Prostatic index (PI) and percent
inhibition
The PI was determined as the ratio of prostate
weight (PW) to BW of each rat using the formula: PW/BW. After 4
weeks, each rat was weighed and then sacrificed by cervical
dislocation. The prostates were removed and weighed immediately.
Percent inhibition was calculated as 100−[(treated group−negative
control)/(positive control−negative control) ×100].
Histopathological examination
All prostate specimens from each group were fixed
with 10% neutral-buffered formalin for 24 h at room temperature. To
assess morphological changes in the prostate, tissues were embedded
in paraffin, cut into 4-µm thick sections, and stained with
hematoxylin and eosin for 1 min at room temperature. Tissues were
subsequently mounted and cover-slipped using mounting medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and
then examined by light microscopy (Nikon Corporation, Tokyo,
Japan). The prostatic epithelial height (µm) was measured by
manually drawing a line through the acinar epithelia [30
measures/field (20 fields in total)]. The acinar luminar area
(µm2) was measured by drawing a line around the
luminar perimeter and calculating the acinar area.
Measurement of testosterone and DHT in
the prostate
For protein extraction, prostate tissues were washed
once with PBS and resuspended in lysis buffer (pH 8.0, 50 mM Tris,
150 mM NaCl, 5 mM EDTA, 1% NP-40, 0.1% SDS and 1 mM phenylmethane
sulfonyl fluoride). The homogenates were centrifuged at 12,000 × g
for 25 min at 4°C, and protein concentration in the supernatant
fractions was determined using a Bradford reagent (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Testosterone (cat. no.
582701; Cayman Chemical Company) and DHT (cat. no. 11-DHTHU-E01;
ALPCO, Salem, NH, USA) levels were measured using ELISA kits
according to their corresponding manufacturer's protocol. The
values were expressed per ng or pg per mg protein in the
prostate.
Assay for 5α-reductase enzyme
activity
The dorsolateral prostate (100 mg) was homogenized
in 1 ml of 40 mM sodium phosphate buffer (pH 6.5) containing
sucrose and DTT (1 mM) in 1X PBS. The homogenate was centrifuged at
5,000 × g for 10 min at 4°C. The supernatant was further
centrifuged for 1 h at 10,500 × g at 4°C. The resulting precipitate
was resuspended in 1 ml 40 mM sodium phosphate buffer (pH 7.4)
containing sucrose (0.3 M) and DTT (1 mM). This was used as the
enzyme source, as previously described (29). The protein content in the
supernatant fractions was determined using a Bio-Rad Protein Assay
kit (Bio-Rad Laboratories, Inc.). Each enzyme reaction was
conducted in duplicate. The activity of enzyme was evaluated with
SRD5α1 ELISA detection kit (cat. no. MBS700746) according to the
manufacturer's protocol (Biocompare, San Francisco, CA, USA).
Absorbance was measured at 450 nm with a microplate reader. The
concentration of SRD5α1 in the samples was subsequently determined
by comparing the optical density of the samples to the standard
curve.
Assay for antioxidant enzymes
Prostate tissue was homogenized (1/10 w/v) in tissue
lysis/extraction reagent containing protease inhibitors.
Homogenates were centrifuged at 12,000 × g for 25 min at 4°C. The
protein content in the supernatant fractions was determined using a
Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Inc.). The
activities of superoxide dismutase (SOD; cat. no. ab65353) and
catalase (CAT; cat. no. ab118184) and GSH (cat. no. ab65322) levels
were quantified using commercial kits according to the
manufacturer's protocols (Abcam), and the results were expressed as
U/mg protein, as described previously (30).
Lipid peroxidation assay
Concentration of malondialdehyde (MDA), an index of
lipid peroxidation, was determined based on thiobarbituric acid
reactive species production. MDA concentrations were calculated
using a TBARS Assay kit (cat. no. KGE013) according to the
manufacturer's protocol (R&D Systems, Inc., Minneapolis, MN,
USA) and normalized to protein levels. In brief, equal volumes (100
µl) sample and SDS were added to a 5 ml conical vial.
Following vortexing, samples were mixed with 0.4 ml of 1%
thiobarbituric acid in 50 mm NaOH and 0.2 ml (20%)
H3PO4. The mixture was heated to 100°C for 15
min. after 10 min incubation on ice, vials were centrifuged at
1,600 × g for 10 min at 4°C. The samples (100 µl) were
loaded onto 96-well assay plates, and the absorbance of each well
was measured at a wavelength of 540 nm using a microplate
reader.
Evaluation of apoptosis in prostate
tissues
Apoptosis in the prostate tissues was evaluated
using a terminal deoxynucleotidyl-transferase-mediated dUTP nick
end labelling (TUNEL) assay. Cell apoptosis was analyzed using an
in situ cell death-detection kit (Promega Corporation,
Madison, WI, USA) according to the manufacturer's protocol. Slides
were de-waxed with 100% xylene and rehydrated with pure ethanol.
The slides were fixed overnight at room temperature in 100 g/l
formaldehyde, treated with proteinase K and
H2O2 and labeled with DUTP in a humidified
chamber at 37°C for 1 h. Tunel-positive cell number was counted in
10 fields of view in each slide. Images were captured using a
confocal microscope (magnification, ×50; LSM510; Carl Zeiss AG,
Oberkochen, Germany).
Statistical analysis
Statistical analyses were performed with GraphPad
Prism software v5.03 (GraphPad Software, Inc., La Jolla, CA, USA).
All numerical data are presented as mean ± standard deviation.
P<0.05 and P<0.01 were considered to indicate a statistically
significant difference. Statistical analyses were performed using
Student's t-test or Mann-Whitney U test. one-way analysis of
variance with Tukey's post hoc test was used for comparison among
multiple groups.
Results
Effects of GV1001 on BW changes and
clinical signs
There were no significant differences in BW between
BPH rats treated with vehicle control or GV1001 (Table I).
 | Table Ieffect of GV1001 and finasteride on
BW, PW and PI of rats with benign prostate hyperplasia. |
Table I
effect of GV1001 and finasteride on
BW, PW and PI of rats with benign prostate hyperplasia.
| Treatment
groups | Dose, (mg/kg) | Body weight (g)
| PW, mg | PI (mg/g) | Inhibition % |
|---|
| Initial | Final |
|---|
| Vehicle
control | 0 | 287.9±3.97 | 351.4±8.81 | 14.5±4.62 | 0.04 | – |
| BPH model | 0 | 293.5±3.86 | 381.1±5.37 | 242.8±36.53 | 0.63 | – |
| GV1001 | 0.01 | 322.0±5.32 | 400.8±10.1 | 141.4±10.92a | 0.35a | 47.4a |
| GV1001 | 0.1 | 328.3±8.26 | 399.5±11.1 | 63.6±11.1b | 0.15b | 81.3b |
| GV1001 | 1 | 330.3±5.01 | 404.3±13.4 | 224.4±25.31 | 0.55 | 13.6 |
| Finasteride | 10 | 293.3±9.41 | 389.7±17.8 | 93.6±12.35b | 0.24b | 66.1b |
Effects of GV1001 on PW and PI
Animals treated with TP exhibited significant
increases in PW and PW:BW ratio compared with animals treated with
vehicle. Compared with the TP-induced BPH rats, rats treated with
0.01 and 0.1 mg/kg s.c. GV1001 exhibited significantly decreased
PW, by 41.73 and 73.7%, respectively. Similarly, 0.01 and 0.1 mg/kg
GV1001 treatment significantly decreased PI to 0.35 and 0.15,
respectively, compared with TP treatment (P<0.01). However,
these effects were not dose-dependent, as the high dose (1 mg/kg)
of GV1001 did not significantly decrease PW and PI, compared with
the BPH group (Table I). As a
positive control, finasteride (10 mg/kg, orally) markedly decreased
PW and PI to 61.4 and 66.1%, respectively, compared with vehicle
treatment (Table I).
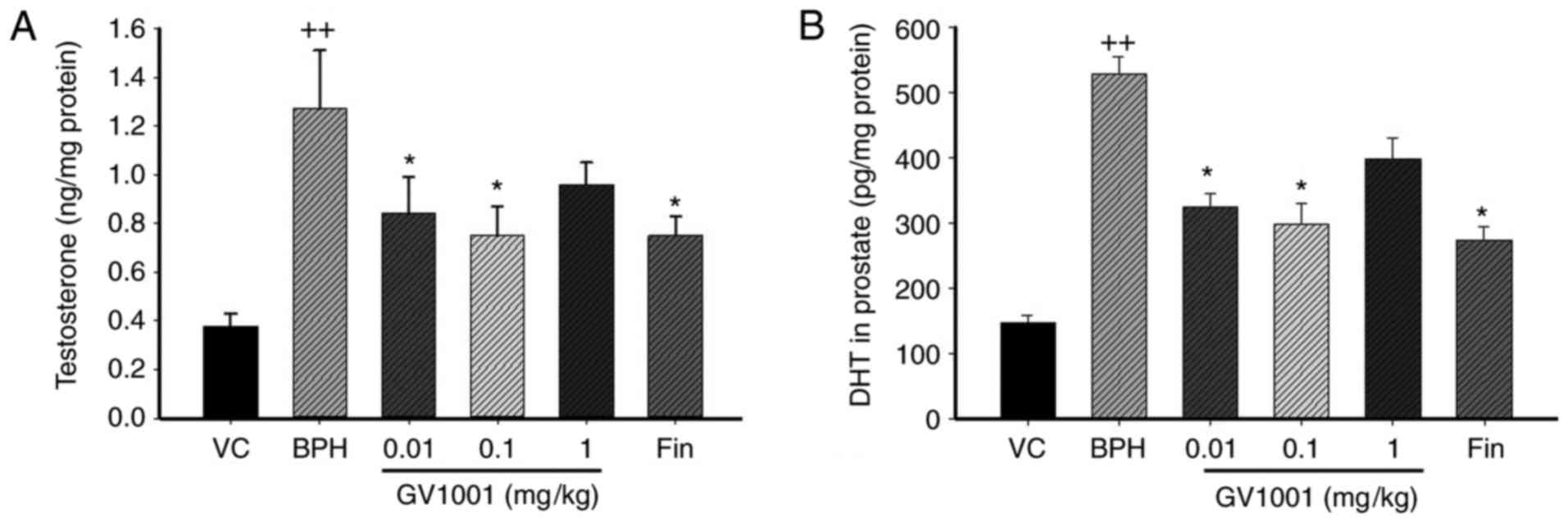
Effects of GV1001 on the levels of
testosterone and DHT in prostate
The major prostatic androgen is DHT, which is formed
from testosterone by the 5α-reductase enzyme (6,31).
Significantly increased levels of testosterone were identified in
rats of the TP-induced BPH group (1.36±0.07 ng/mg protein) compared
with the vehicle control group (0.38±0.17 ng/mg protein; P<0.01;
Fig. 1A). By contrast, rats in
the finasteride-treated group (0.78±0.14 ng/mg protein) showed
significantly decreased testosterone levels compared with those in
the BPH group (P<0.05). Similarly, rats in the GV1001 (0.1
mg/kg)-treated group (0.76±0.18 ng/mg protein) indicated a marked
decrease in testosterone levels compared with those of the BPH
group.
The DHT level in the prostate of BPH rats was
markedly increased compared with that in vehicle control rats
(524.24±78.69 vs. 149.09±12.60 pg/mg protein). However, the
prostatic DHT level in the finasteride-treated group (267.43±29.63
pg/mg protein) was significantly decreased compared with that of
the BPH-treated group. The level of prostatic DHT in the GV1001
(0.1 mg/kg)-treated group (297.17±43.05 pg/mg protein; P<0.05)
was also markedly decreased compared with that of BPH rats
(Fig. 1B).
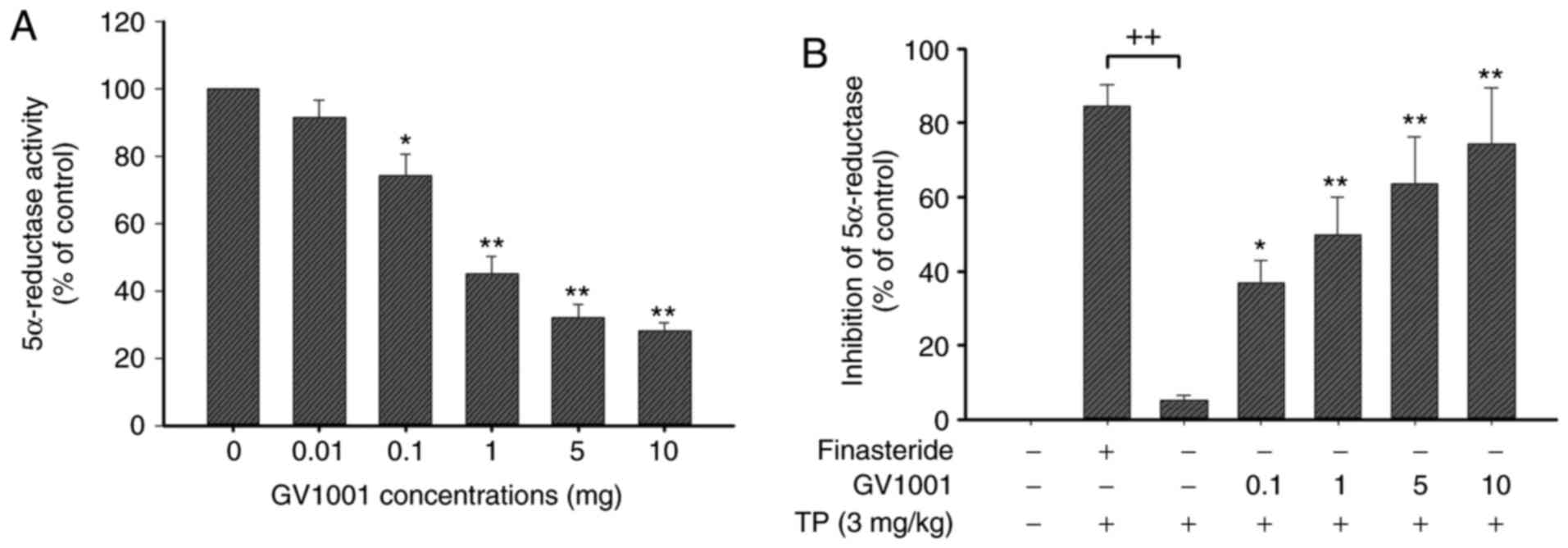
Effects of GV1001 on 5α-reductase
activity
To compare the inhibitory activity of GV1001, an
enzyme assay was performed using finasteride (steroidal positive
control) against an enzyme suspension of 5α-reductase prepared from
prostates of rats. This assay exhibited high sensitivity and
excellent specificity for the detection of human SRD5A2 (Fig. 2A). As demonstrated in Fig. 2B, GV1001 exhibited a
dose-dependent inhibitory activity (GV1001 1 mg, 40.5±5.2%
inhibition), whereas finasteride positive controls indicated potent
inhibition.
Effect of GV1001 on prostate
histopathological examination
Sections from the control group demonstrated normal
histological architecture of the prostate (Fig. 3A). The tissues were tightly packed
with flattened cuboidal regular sized epithelium with a regular
acinar folding arrangement. In BPH rats, irregular acinar folding
with intraluminar projections, hemorrhage, cystic spaces and
nuclear conglomerates with marked hyperplasia, congestion and
vacuolation were observed as evidence of disruption of the
histoarchitecture (Fig. 3A–a).
The prostates from the TP-treated group also exhibited luminal
epithelial hyperplasia with intraluminal polyps and engorged blood
vessels (Fig. 3A–b). Co-treatment
with GV1001 (0.01 and 0.1 mg/kg) attenuated the pathological
alterations induced by testosterone (Fig. 3A-c and A-d). These effects were
similar to those observed in the finasteride-treatment group
(Fig. 3a-f). Treatment with
finasteride also resulted in regular unfolded acini. However, these
histopathological data did not reveal a dose-dependent restoration
of the histoarchitecture (Fig.
3A-e). Fig. 3B indicates the
representative prostate size following GV1001 treatment in BPH
rats. Injection of TP increased the epithelial height of the
prostate to 175×102 µm compared with that of
vehicle control rats. However, treatment with GV1001 at 0.01 mg/kg
significantly decreased epithelial height to 75.5×102
µm, compared with that of BPH rats (P<0.05). Finasteride
also significantly decreased the prostatic epithelial height to
61.5×102 µm compared with BPH (Fig. 3C).
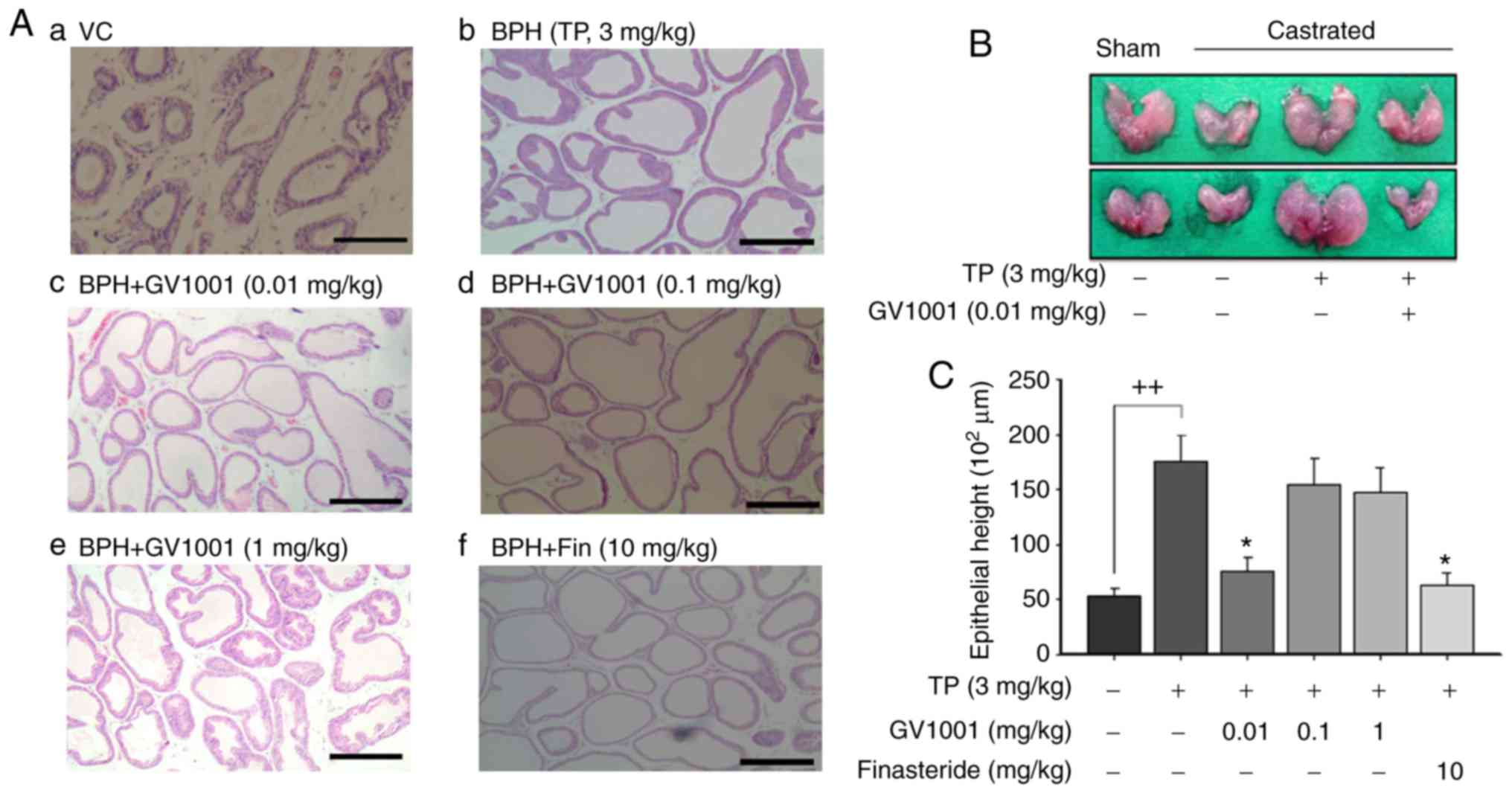 | Figure 3Effects of GV1001 on benign prostate
hyperplasia. (a) Histological examination of prostate tissue was
performed 24 h after the final TP injection. Prostate tissues were
fixed, sectioned at 4 µm thickness and stained with
hematoxylin & eosin solution. Scale bars=100 µm. (a)
Castration: Corn oil injection (s.c.) + oral administration of PBS;
(b) BPH: TP (3 mg/kg, s.c.) injection; (c) GV1001 injection (0.01
mg/kg, s.c.) of + TP (3 mg/kg) injection; (d) GV1001 injection (0.1
mg/kg, s.c.) of + TP (3 mg/kg) injection; (e) GV1001 injection (1
mg/kg, s.c.) of + TP (3 mg/kg) injection; (f) oral administration
of finasteride (10 mg kg/kg, Fin) + TP injection. GV1001 or
finasteride (Fin) treatment was performed 1 h prior to TP
injection. BPH, benign prostate hyperplasia. (B) Effects of GV1001
on prostate size. The rat model of BPH was generated by injection
of TP for 4 weeks after castration. Castration involved corn oil
injection (s.c.) + oral administration of PBS; BPH treatment
involved TP (s.c.) injection + oral administration of PBS; GV1001
treatment [injection of GV1001 (0.01 mg/kg) + TP (3 mg/kg, s.c.)
injection] was performed 1 h prior to TP injection. (C) Effect of
GV1001 on proliferation of columnar epithelial cells. Values are
presented as mean ± standard deviation. ++P<0.01 vs.
vehicle control group and *P<0.05 vs. BPH group (TP,
3 mg/kg). BPH, benign prostate hyperplasia; Fin, finasteride; TP,
testosterone propionate; s.c., subcutaneous. |
Effect of GV1001 on prostatic antioxidant
enzyme activity
In BPH rats, the activities of SOD and CAT and the
level of GSH were significantly (P<0.01) decreased by 65.6, 68
and 85.95% of vehicle control-treated rats. By contrast, GV1001 0.1
mg/kg significantly restored SOD and CAT activity and GSH levels by
87.7, 75.8 and 79.3%, respectively, compared with vehicle control.
Finasteride also significantly restored SoD, CaT and GSH by 85.8,
79.01 and 84%, respectively, compared with the vehicle control
(Fig. 4).
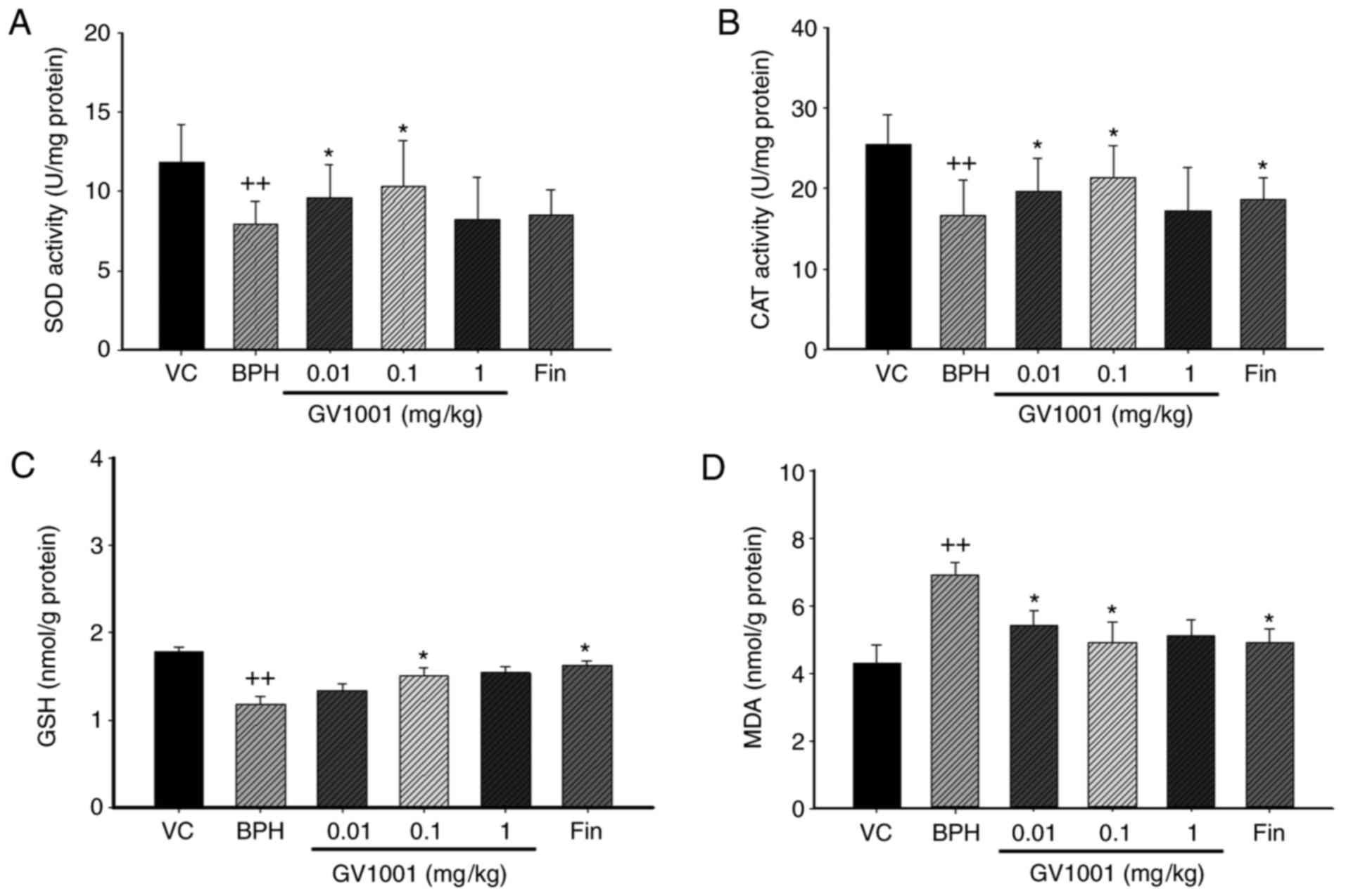 | Figure 4Effect of GV1001 on oxidative stress
in the prostates of BPH rats. (A) SOD, (B) CAT, (C) GSH and (D) MDA
concentrations were measured in prostate homogenates. Values are
presented as mean ± standard deviation (n=6).
++P<0.01 vs. the VC group, and *P<0.05
vs. the BPH group. VC, vehicle control; BPH, benign prostatic
hyperplasia; Fin, oral administration of finasteride and s.c.
injection of TP (3 mg/kg); GV1001, injection of GV1001 (0.01, 0.1,
and 1 mg/kg) and injection of TP (3 mg/kg). SOD, Superoxide
dismutase; CAT, catalase, GSH, glutathione; MDA, malondialdehyde;
TP, testosterone propionate. |
Effect of GV1001 on prostatic lipid
peroxides
To investigate the effect of GV1001 on oxidative
stress in BPH, the concentration of MDA, an indicator of lipid
peroxidation, in the prostate was measured. MDA concentration was
significantly increased in the TP-induced BPH group compared with
the vehicle control group, as previously described (32) (Fig.
4D). By contrast, MDA concentration was significantly decreased
in the 0.1 mg/kg-treated GV1001 group and the finasteride-treated
group compared with the TP-induced BPH group. These data suggest
that GV1001 injection prevented BPH by protecting the prostate from
oxidative stress.
GV1001 induces apoptosis in prostate
tissues
To determine whether the inhibitory effect of GV1001
on prostate growth was due to the apoptotic cell death pathway, the
effect of GV1001 on apoptosis in BPH rats was measured by
immunohistochemical staining using TUNEL. The number of
TUNEL-positive cells in the control group was 13.21±1.57, and that
in the prostatic hyperplasia-induced group was 1.25±0.54. The
numbers of positive cells in the 0.01, 0.1 and 1 mg/kg GV1001
groups were 25.67±2.36, 6.58±2.49 and 19.42±4.21, respectively.
Compared with the control and the BPH-induced groups, injection of
GV1001 resulted in a significantly higher number of apoptotic cells
(Fig. 5). GV1001 treatment
increased the proportion of TUNEL-positive cells compared with the
BPH-induced groups, demonstrating that the GV1001-mediated
inhibition of prostate growth is accompanied by induction of
apoptosis.
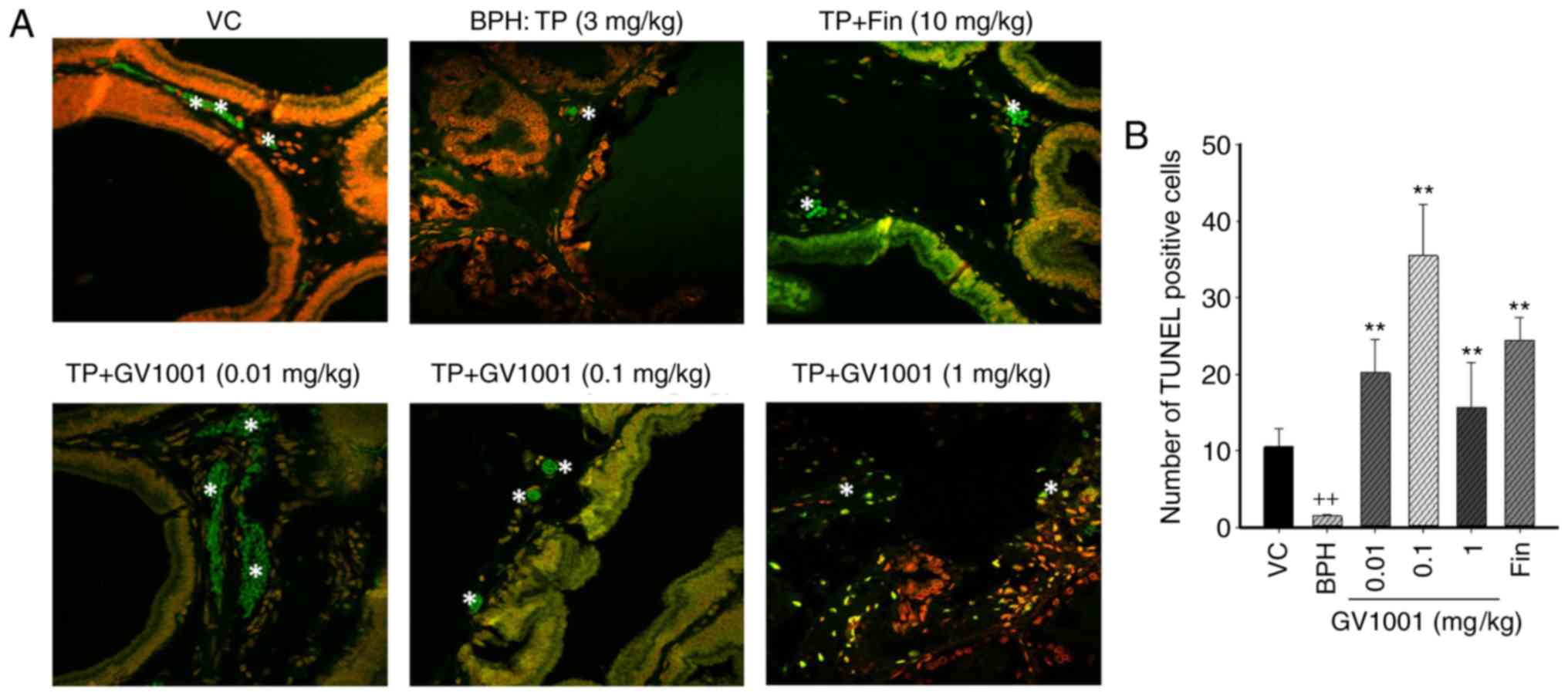 | Figure 5Immunohistochemical analysis of
apoptotic cell death in the prostate tissues. Cells undergoing
apoptosis were measured using the TUNEL assay. (a) Representative
sections are presented for the VC, BPH and GV1001+TP co-treatment
groups. Magnification, ×50 using Image Hub software. Tunel-positive
cells are indicated by white stars. (B) Mean numbers of positive
Tunel cells in the VC, BPH-induced, finasteride and GV1001-injected
groups. Data are presented as mean ± standard deviation.
++P<0.01 vs. the VC group, and **P<0.01
vs. the BPH (TP, 3 mg/kg) group. TUNEL, terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labelling; VC,
vehicle control; TP, testosterone propionate; BPH, benign prostatic
hyperplasia. |
Discussion
The progression of BPH is dependent on several
factors including growth factors, adrenergic stimulation and
chronic inflammation (32,33).
In particular, the cumulative production of ROS and reactive
nitrogen species serves a major role in the development of BPH,
which is considered a premalignant condition that evolves into
prostate diseases (12,30). In clinical studies, chronic
oxidative stress and an imbalance in antioxidant activity are more
prominent in patients with BPH compared with normal controls
(11,12). It has been hypothesized that BPH
is an inflammatory disease, and that inflammation may directly
contribute to prostate cell proliferation (34); however, an association between
prostatic inflammation and BPH has not been demonstrated
conclusively.
GV1001 is an anticancer drug and insulin sensitizer
that inhibits glycogenolysis and gluconeogenesis. Although the
anti-inflammatory, antipyretic and antioxidant activities of GV1001
have been extensively investigated, to the best of our knowledge
there have been no studies examining the effect of GV1001 in the
development of BPH. Therefore, the aim of the present study was to
investigate the protective effect of GV1001 in a
testosterone-induced BPH model, with an emphasis on its roles in
antioxidant balance and the inflammatory cascade.
Castration will lead to an imbalance of endogenous
hormones; however, injection of exogenous testosterone is an easier
method of inducing BPH in animals (35). As this method is also more similar
to the pathogenesis of clinical BPH, the protective effects of
GV1001 against testosterone-induced BPH in rats was evaluated.
Animals in the BPH model group exhibited an increased PI compared
with the control animals, which confirmed that the model was
successfully established. In the present study, oral administration
of finasteride (10 mg/kg) as a positive control resulted in a
significant decrease in relative PW compared to BPH animals. The
PW, PI and hormone levels were measured in TP-induced BPH rats. BPH
rats exhibited an increased relative PW, elevated testosterone
level, prostatic epithelial hyperplasia, and decreased activities
of antioxidant enzymes in the prostate. Injection of GV1001 (0.01
and 0.1 mg/kg) effectively prevented the development of BPH, as
observed by decreased relative PW and PI values, and reduced serum
testosterone and DHT levels. Histological changes also indicated
that GV1001 (0.01 mg/kg) injection markedly decreased prostatic
epithelial hyperplasia.
Chronic inflammation leads to prostatic cell
proliferation by stimulating growth factors through oxidative
stress resulting from the endogenous production of ROS. Similarly,
an inverse correlation between the activities of antioxidant
enzymes and ROS generation, which activates the defense system of
the body against the damage caused by oxidative stress, has been
demonstrated previously (36,37). Therefore, potentiation of
antioxidant enzyme activities may prevent damage to target organs
by alleviating oxidative stress. GV1001 treatment significantly
increased the activities of the antioxidant enzymes responsible for
the decrease of testosterone-induced oxidative damage in prostatic
tissue. Therefore, GV1001 may effectively reverse the changes in
the activities of antioxidant enzymes during BPH, particularly at
doses >0.1 mg/kg. Previous studies have suggested that tumor
necrosis factor (TNF)-α increases levels of intracellular ROS,
which may exacerbate inflammatory processes (36,37). The suppressive effects of these
antioxidant compounds on the production of these inflammatory
mediators are due to their antioxidant activities. Therefore,
changes in the activities of prostatic antioxidant enzymes were
investigated in the present study.
The present study identified that the activities of
SOD and CAT were decreased in the BPH model group, whereas
treatment with GV1001 significantly increased the activities of
these enzymes. Furthermore, regions of prostatic inflammation will
produce free radicals, which attack the plasma membrane and result
in lipid peroxidation and MDA production (36). The level of MDA was elevated in
the model group compared with the sham group; however, treatment
with GV1001 attenuated the increase in MDA. Therefore, GV1001 may
successfully reverse the changes in antioxidant enzymes and lipid
peroxidation, suggesting that its anti-BPH effects were associated
with its antioxidant activities. Furthermore, the apoptotic index
measured by TUNEL assay demonstrated low levels of apoptotic cells
following induction of BPH by TP. Injection of 0.01, 0.1 and 1
mg/kg GV1001 restored normal apoptotic activity, as indicated by an
extensive degree of apoptotic body formation. In addition, the data
of the present study indicated a lower rate of apoptosis in the
rats that received the highest dose of GV1001 (1 mg/kg), compared
with 0.01 and 0.1 mg/kg GV1001. The increased number of apoptotic
bodies within the prostate tissues from rats treated with GV1001
may be considered to be the effect of the induction of apoptosis by
the antioxidant activity of GV1001, resulting in suppression of the
proliferation of prostate cells. Therefore, t the best of our
knowledge, the present study has demonstrated for the first time
that GV1001 inhibited prostate growth in vivo by promoting
apoptosis in prostatic cells. The results indicated that GV1001
prevented the development of BPH in the testosterone-induced BPH
model. This preventive effect is likely due to its potent
antioxidant capacity. Therefore, low dose GV1001 (0.1 mg/kg) may
represent a novel complementary therapy and may be used as a novel
therapeutic agent to prevent BPH in older men. However, additional
experimentation is required to conclusively determine that the
antioxidant reaction of GV1001 induced the apoptosis of prostate
cells.
Acknowledgments
We thank Professor Tae Chun Chung (Youngnam
University, Republic of Korea) and Professor Kyu-Bong Kim (Dankook
University, Republic of Korea) for technical help in the
histopathological evaluation of prostate.
Funding
This research was supported by National Research
Foundation of Korea (NRF) Grants funded by the Korean Government
(NRF-2015M3A9B6053068).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HSK and BML conceptualized and designed the study.
KSK and HYY acquired the data. SCC, KYL and YMK analyzed and
interpreted the data. KSK and HSK drafted the manuscript. KYL, BML
and HSK critically revised the manuscript for important
intellectual content. KSK and HYY performed the statistical
analysis. All the authors read and approved the final
manuscript
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Sungkyunkwan University Animal Ethics Committee. All experimental
procedures were performed in accordance with the Korea Food and
Drug Administration (FDA) Guidelines for the Care and Use of
Laboratory Animals, and animal handling followed the dictates of
the national animal Welfare Law of Korea. All experimental
procedures were performed in accordance with guidelines of the
Committee for the Purpose of Control and Supervision of Experiments
on Animals of Sungkyunkwan University. The present study was
reviewed and approved by the Sungkyunkwan University Animal Ethics
Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thorpe A and Neal D: Benign prostatic
hyperplasia. Lancet. 361:1359–1367. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee SWH, Chan EMC and Lai YK: The global
burden of lower urinary tract symptoms suggestive of benign
prostatic hyperplasia: A systematic review and meta-analysis. Sci
Rep. 7:79842017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee YJ, lee JW, Park J, Seo SI, Chung JI,
Yoo TK and Son H: Nationwide incidence and treatment pattern of
benign prostatic hyperplasia in Korea. Investig Clin Urol.
57:424–430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Henry G, Malewska A, Mauck R, Gahan J,
Hutchinson R, Torrealba J, Francis F, Roehrborn C and Strand D:
Molecular pathogenesis of human prostate basal cell hyperplasia.
Prostate. 77:1344–1355. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chughtai B, Forde JC, Thomas DD, Laor L,
Hossack T, Woo HH, Te AE and Kaplan SA: Benign prostatic
hyperplasia. Nat Rev Dis Primers. 2:160312016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carson C III and Rittmaster R: The role of
dihydrotestosterone in benign prostatic hyperplasia. Urology. 61(4
Suppl 1): S2–S7. 2003. View Article : Google Scholar
|
|
7
|
Gandhi J, Weissbart SJ, Smith NL, Kaplan
SA, Dagur G, Zumbo A, Joshi G and Khan SA: The impact and
management of sexual dysfunction secondary to pharmacological
therapy of benign prostatic hyperplasia. Transl Androl Urol.
6:295–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Traish AM, Melcangi RC, Bortolato M,
Garcia-Segura LM and Zitzmann M: Adverse effects of 5α-reductase
inhibitors: What do we know, don't know, and need to know? Rev
Endocr Metab Disord. 16:177–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
La Torre A, Giupponi G, Duffy D, Conca A,
Cai T and Scardigli A: Sexual dysfunction related to drugs: A
critical review. Part V: α-blocker and 5-ARI drugs.
Pharmacopsychiatry. 49:3–13. 2016.
|
|
10
|
Patel AK and Chappel CR: Benign prostatic
hyperplasia, treatment in primary health care. BMJ. 333:535–539.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Srivastava DS and Mittal RD: Free radical
injury and antioxidant status in patients with benign prostate
hyperplasia and prostate cancer. Indian J Clin Biochem. 20:162–165.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Minciullo PL, Inferrera A, Navarra M,
Calapai G, Magno C and Gangemi S: Oxidative stress in benign
prostatic hyperplasia: A systematic review. Urol Int. 94:249–254.
2015. View Article : Google Scholar
|
|
13
|
Jena AK, Vasisht K, Sharma N, Kaur R,
Dhingra MS and Karan M: Amelioration of testosterone induced benign
prostatic hyperplasia by Prunus species. J Ethnopharmacol.
190:33–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prasad S, Kalra N and Shukla Y: Modulatory
effects of diallyl sulfide against testosterone induced oxidative
stress in Swiss albino mice. Asian J Androl. 8:719–723. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siddiquia IA, Raisuddin S and Shukla A:
Protective effects of black tea extract on testosterone induced
oxidative damage in prostate. Cancer Lett. 227:125–132. 2005.
View Article : Google Scholar
|
|
16
|
Parekh MH, Lobel R, Oconnor LJ, Legget RE
and Levin RM: Protective effect of vitamin E on the response of the
rabbit bladder to partial outlet obstruction. J Urol. 166:341–346.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kyte JA: Cancer vaccination with
telomerase peptide GV1001. Expert Opin Investig Drugs. 18:687–694.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shaw VE, Naisbitt DJ, Costello E,
Greenhalf W, Park BK, Neoptolemos JP and Middleton GW: Current
status of GV1001 and other telomerase vaccination strategies in the
treatment of cancer. Expert Rev Vaccines. 9:1007–1016. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bernhardt SL, Gjertsen MK, Trachsel S,
Møller M, Eriksen JA, Meo M, Buanes TG and Gaudernack G: Telomerase
peptide vaccination of patients with non-resectable pancreatic
cancer: A dose escalating phase I/II study. Br J Cancer.
95:1474–1482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Middleton G, Ghaneh P, Costello E,
Greenhalf W and Neoptolemos JP: New treatment options for advanced
pancreatic cancer. Expert Rev Gastroenterol Hepatol. 2:673–696.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hunger RE, Kernland Lang K, Markowski CJ,
Trachsel S, Møller M, Eriksen JA, Rasmussen AM, Braathen LR and
Gaudernack G: Vaccination of patients with cutaneous melanoma with
telomerase-specific peptides. Cancer Immunol Immunother.
60:1553–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brunsvig PF, Kyte JA, Kersten C, Sundstrøm
S, Møller M, Nyakas M, Hansen GL, Gaudernack G and Aamdal S:
Telomerase peptide vaccination in NSCLC: A phase II trial in stage
III patients vaccinated after chemoradiotherapy and an 8-year
update on a phase I/II trial. Clin Cancer Res. 17:6847–6857. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee YK, Nata'atmaja BS, Kim BH, Pak CS and
Heo CY: Protective effect of telomerase-based 16-mer peptide
vaccine (gV1001) on inferior epigastric island skin flap
survivability in ischaemia-reperfusion injury rat model. J Plast
Surg Hand Surg. 51:210–216. 2017. View Article : Google Scholar
|
|
24
|
Park HH, Yu HJ, Kim S, Kim G, Choi NY, Lee
EH, Lee YJ, Yoon MY, Lee KY and Koh SH: Neural stem cells injured
by oxidative stress can be rejuvenated by GV1001, a novel peptide,
through scavenging free radicals and enhancing survival signals.
Neurotoxicology. 55:131–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ko YJ, Kwon KY, Kum KY, Lee WC, Baek SH,
Kang MK and Shon WJ: The anti-inflammatory effect of human
telomerase-derived peptide on P. gingivalis
lipopolysaccharide-induced inflammatory cytokine production and its
mechanism in human dental pulp cells. Mediators Inflamm.
2015:3851272015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi J, Kim H, Kim Y, Jang M, Jeon J,
Hwang YI, Shon WJ, Song YW, Kang JS, Lee WJ and Choi J: The
anti-inflammatory effect of GV1001 mediated by the downregulation
of eno1-induced pro-inflammatory cytokine production. Immune Netw.
15:291–303. 2015. View Article : Google Scholar
|
|
27
|
Lee MY, Shin IS, Seo CS, Lee NH, Ha HK,
Son JK and Shin HK: Effects of Melandrium firmum methanolic extract
on testosterone-induced benign prostatic hyperplasia in Wistar
rats. Asian J Androl. 14:320–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Steers WD: 5alpha-reductase activity in
the prostate. Urology. 58(6 Suppl 1): S17–S24. 2001. View Article : Google Scholar
|
|
29
|
Ali MI, Kondreddi HD and Veeresh B:
Protective effect of 2-hydroxy-4-methoxy benzoic acid on
testosterone induced benign prostatic hyperplasia in Wister rats.
Eur J Pharmacol. 698:397–403. 2013. View Article : Google Scholar
|
|
30
|
Aydin A, Arsova-Sarafinovska Z, Sayal A,
Eken A, Erdem O, Erten K, Ozgök Y and Dimovski A: Oxidative stress
and antioxidant status in non-metastatic prostate cancer and benign
prostatic hyperplasia. Clin Biochem. 39:176–179. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sultan C, Paris F, Terouanne B, Balaguer
P, Georget V, Poujol N, Jeandel C, Lumbroso S and Nicolas JC:
Disorders linked to insufficient androgen action in male children.
Hum Reprod Update. 7:314–322. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Culig Z, Hobisch A, Cronauer MV, Radmayr
C, Hittmair A, Zhang J, Thurnher M, Bartsch G and Klocker H:
Regulation of prostatic growth and function by peptide growth
factors. Prostate. 28:392–405. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jacobsen SJ, Girman CJ and Lieber MM:
Natural history of benign prostatic hyperplasia. Urology. 58(6
Suppl 1): S5–S16. 2001. View Article : Google Scholar
|
|
34
|
Bostanci Y, Kazzazi A, Momtahen S, Laze J
and Djavan B: Correlation between benign prostatic hyperplasia and
inflammation. Curr Opin Urol. 23:5–10. 2013. View Article : Google Scholar
|
|
35
|
Chung LW, Matsuura J and Runner MN: Tissue
interactions and prostatic growth. I Induction of adult mouse
prostatic hyperplasia by fetal urogenital sinus implants. Biol
Reprod. 31:155–163. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vral A, Magri V, Montanari E, Gazzano G,
Gourvas V, Marras E and Perletti G: Topographic and quantitative
relationship between prostate inflammation, proliferative
inflammatory atrophy and low-grade prostate intraepithelial
neoplasia: A biopsy study in chronic prostatitis patients. Int J
Oncol. 41:1950–1958. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao M, Ding H, Zhong G, Lu J, Wang H, Li Q
and Wang Z: The effects of transrectal radiofrequency hyperthermia
on patients with chronic prostatitis and the changes of MDA, NO,
SOD, and Zn levels in pretreatment and posttreatment. Urology.
79:391–396. 2012. View Article : Google Scholar
|