Introduction
Breast cancer remains the leading cause of mortality
in females worldwide (1). Based
on the expression of estrogen and progesterone receptors, and
epidermal growth factor receptor 2 (Her2), breast cancer is
classified into 4 sub-types as follows: Luminal A, luminal B,
Her2-overexpressing and triple-negative breast cancer (TNBC)
(2). TNBC comprises a
heterogeneous group of cancer types (3). With no effective therapeutic target,
the current treatment of TNBC relies on combination therapy,
including chemotherapy (4).
Although TNBC usually responds well to chemotherapy, de novo
and acquired chemotherapy resistance remain to be overcome in order
to achieve an improved overall survival for patients with TNBC
(5). Notably, tumor metastasis is
an additional frequent obstacle when treating TNBC (6,7).
Cisplatin is a commonly used chemotherapeutic agent
administered to patients with TNBC (8). The antitumor properties of cisplatin
are primarily based on its ability to induce cell apoptosis by
causing DNA damage (9). However,
the efficacy of cisplatin is frequently compromised by the
insensitivity of malignant cells towards drug treatment and the
development of drug resistance (10,11). The underlying mechanism of
cisplatin resistance is complex. Previous studies on cancer cell
lines indicated that the activity of the p38 mitogen-activated
protein kinase signaling pathway was associated with cisplatin
sensitivity (12). An additional
study revealed that protein kinase B (Akt) was involved in
cisplatin-resistance by inhibiting cell apoptosis (13). Consequently, future studies on the
precise molecular mechanisms of cisplatin sensitivity are required
to meet current clinical requirements.
Islet 1 (ISL1) is a member of the LIM/homeodomain
family of transcription factors and was first cloned from
pancreatic insulin-producing cells of rats (14,15). Through binding the insulin gene
enhancer, ISL1 was identified to regulate insulin gene expression
(14). ISL1 is involved in the
development of numerous tissue types, including the nervous system,
pancreas and skeletal muscles (15). Previously, abnormal expression of
ISL1 has been demonstrated to be closely associated with cancer
development and progression (16). Immunohistochemical staining of
breast cancer samples revealed that the protein levels of ISL1 were
increased in tumor tissues from patients with TNBC compared with
those in other breast cancer sub-types (17). However, the role of ISL1 in TNBC
progression, and its underlying mechanism, remains unknown.
The present study aimed to explore the role of ISL1
in TNBC. The results of reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis revealed that ISL1
expression was significantly increased in TNBC tissues compared
with that in normal adjacent tissues. The present study also
demonstrated that ISL1 markedly promoted cell proliferation and
invasion in the TNBC MDA-MB-231 and MDA-MB-468 cell lines.
Additionally, overexpression of ILS1 markedly reversed
cisplatin-induced cell apoptosis in MDA-MB-231 and MDA-MB-468
cells. Furthermore, ILS1 inhibited cell apoptosis via upregulation
of the expression of the anti-apoptotic proteins,
phosphorylated-Akt (p-Akt) and B-cell lymphoma-2 (Bcl-2), and
downregulation of the expression of the pro-apoptotic protein,
Bcl-2-associated X protein (Bax). Taken together, these data
suggested that dysregulation of ILS1 participates in TNBC cell
progression and sensitivity to cisplatin, proposing ILS1 as a
promising therapeutic target in TNBC.
Materials and methods
Patients
Tumor tissues and their corresponding adjacent
(>5 cm) normal tissues were obtained from 35 patients with TNBC
who attended Tangshan People's Hospital (Tangshan, China) from
March, 2012 to September, 2015. In the present cohort, there were 9
patients <35 years old and 26 patients >35 years old (28
years old-65 years old). All tissues were stored at −80°C prior to
the extraction of nucleic acids. Written informed consent for use
of patient samples was obtained from all participants in the
present study prior to surgery. The experiments were performed
following approval from the Ethics Committee of Tangshan People's
Hospital.
Cell culture and reagents
The human TNBC MDA-MB-231 and MDA-MB-468 cell lines,
and 293 cell line were purchased from American Type Culture
Collection (Manassas, VA, USA). The 293, MDA-MB-231 and MDA-MB-468
cells were cultured in Dulbecco's modified Eagle's medium (DMEM;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences,
Logan, UT, USA), 100 U/ml penicillin and 100 μg/ml
streptomycin (Thermo Fisher Scientific, Inc.) in an incubator with
5% CO2 at 37°C.
Cisplatin was purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). For cisplatin treatment, MDA-MB-231 and
MDA-MB-468 cells (3×103 cells/well) were seeded into
96-well plates (Sarstedt, Nümbrecht, Germany) and incubated with 25
μM cisplatin in DMEM in an incubator with 5% CO2
at 37°C for 24 h, and then subjected to subsequent analyses.
ISL1 overexpression
The full-length sequence of ISL1 was amplified from
cDNA of 293 cells and cloned into the pcDNA3 plasmid (Addgene,
Inc., Cambridge, MA, USA). For ISL1 overexpression,
1×106 MDA-MB-231 and MDA-MB-468 cells were transfected
with 2 μg pcDNA3-ISL1 using Lipofectamine® 2000 (Thermo
Fisher Scientific, Inc.), while cells transfected with empty vector
served as controls. After 24 h, the cells were subjected to
following experiments.
RNA interference
MDA-MB-231 and MDA-MB-468 cells were transfected
with a scramble small interfering RNA (siRNA; 100 nM) used as
negative control or with ISL1 siRNA pool (containing three specific
siRNAs) (100 nM; cat. no. sc-37121, Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) using Lipofectamine RNAi MAX (Thermo Fisher
Scientific, Inc.), and incubated at 37°C for 72 h. Following this,
cells were subjected to subsequent experiments. Sequences of siRNAs
were listed as follow: Control siRNA: 5′-UUCUCCGAACGUGUCACGU-3′;
ISL1 siRNA pool: 5′-GGACCAGGCUCUAAUUCUATT-3′;
5′-AAAAGAAUGGAGGUGGAAG; GAGACAUGGUGGUUUA-3′.
Cell proliferation assay
Cell proliferation was assessed with a
Cell-Counting-Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), according to the manufacturer's protocols.
Briefly, MDA-MB-231 and MDA-MB-468 cells (3×103
cells/well) were seeded into 96-well plates. Following cell
attachment, cells were treated (100 nM, 37°C) with control siRNA,
ISL1 siRNA, pcDNA3 or pcDNA3-ISL1 for 72 h. To evaluate the effect
of ISL1 in cell proliferation, cells were treated (37°C) with
vehicle (dimethyl sulfoxide; Beijing Solarbio Science &
Technology, Co., Ltd., Beijing, China), 10 μM cisplatin, 10
μM cisplatin + pcDNA3-ISL1 or 10 μM cisplatin + ISL1
siRNA for 72 h. Next, CCK-8 solution (10 μl) was added into
each well and incubated (37°C) for 2 h. Subsequently, the
absorbance at 450 nm was recorded to determine cell
proliferation.
Cell invasion assay
Cell invasion ability was measured by a Transwell
assay. Briefly, 3×104 MDA-MB-231 and MDA-MB-468 cells
were plated onto the upper side of Matrigel-coated (30 min at 37°C)
Transwell chambers (Corning Incorporated, Corning, NY, USA) with
serum-free DMEM, while the lower chamber was filled with DMEM
containing 10% FBS. Following culture (37°C) for 24 h, the cells in
the upper chamber were removed by a cotton swab, while the cells
that had invaded into the reverse face of the membranes were fixed
(1 h at room temperature) with methanol (4%) and stained (1 h at
room temperature) with crystal violet (0.25%). Images were captured
from 3 randomly chosen fields and the number of cells was then
quantified using a light microscope (SZX16-3111; Olympus
Corporation, Tokyo, Japan). The experiment was repeated three
times.
Cell apoptosis assay
Cell apoptosis was detected with the Fluorescein
isothiocyanate-Annexin V/Apoptosis Detection kit (Thermo Fisher
Scientific, Inc.) with a BD FACSCalibur flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). Upon treatment of control
siRNA or ISL1 siRNA, and vehicle, 10 μM cisplatin, 10
μM cisplatin + pcDNA3-ISL1 or 10 μM cispl-atin + ISL1
siRNA, the MDA-MB-231 and MDA-MB-468 cells were harvested,
re-suspended in 500 μl pre-cooled 1X binding buffer from
Fluorescein isothiocyanate-Annexin V/Apoptosis Detection kit, and
mixed with 5 μl Annexin V and 2.5 μl propidium iodide
(PI). The scatter plots contained the following results: Quadrant 4
(Q4), healthy cells (Annexin V-/PI-); Q3,
apoptotic cells at an early stage (Annexin
V+/PI-); Q2, apoptotic cells at an advanced
stage (Annexin V+/PI+); and Q1, mechanically
injured cells (Annexin V-/PI+). Flow
cytometry results were analyzed using FlowJo 10.4.2 (FlowJo LLC,
Ashland, OR, USA). The apoptotic rate was calculated as the ratio
of apoptotic cells in Q3 + Q2 to the total number of cells.
Western blotting
Antibodies against ISL1 (cat. no. sc-390793;
1:1,000), Bcl-2 (cat. no. sc-7382; 1:1,000) and Bax (cat. no.
sc-7480; 1:1,000) were purchased from Santa Cruz Biotechnology,
Inc. The antibody against GAPDH was obtained from Sigma-Aldrich
(Merck KGaA). Secondary antibodies against mouse (cat. no.
SA00001-1; 1:10,000) and rabbit (cat. no. SA00001-1; 1:10,000) were
products of Proteintech Group, Inc., (Chicago, IL, USA). Western
blotting was performed following standard procedures: In brief,
protein lysates from MDA-MB-231 cells were prepared using
radioimmunoprecipi-tation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China), quantified by the method of BCA
(Beyotime Institute of Biotechnology, Shanghai, China), 20
μg protein per line were separated by 8% SDS-PAGE and then
transferred to polyvinylidene fluoride membranes. Subsequently, the
membranes were blocked (1 h at room temperature) with 5% non-fat
milk (5 g milk powder in 100 ml TBST with 0.1% Tween 20) and
incubated (1 h at room temperature) with the aforementioned primary
and secondary antibodies. The blots were developed with SuperSignal
West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific,
Inc.). GAPDH served as an internal control. Quantitative analysis
of protein expression was achieved by Image J version 1.8.0
(National Institutes of Health, Bethesda, MD, USA).
RT-qPCR
Total RNA was extracted from patient tissues with
TRIzol® reagent (Life Technologies; Thermo Fisher Scientific,
Inc.). The concentration and quality of the extracted RNA was
analyzed by NanoDrop 2000 (Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA). The RNA was reverse transcribed into cDNA
using the PrimeScript RT Reagent kit (Takara Bio, Inc., Otsu,
Japan). RT-qPCR was performed using the SYBR Green kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and an ABI Prism 7500
Sequence Detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). GAPDH served as an internal control.
Thermocycling conditions used in the RT-qPCR was as follows:
Denaturation: 95°C for 10 min, followed by annealing and
elongation: 40 cycles at 95°C for 15 sec, 60°C for 60 sec, and
final elongation: 1 cycle at 95°C for 15 sec. The qPCR primer
sequences were as follows: ISL1-forward, 5′-CTG CTT TTC AGC AAC TGG
TCA-3′; ISL1-reverse, 5′-TAGGACTGGCTACCATGCTGT-3′; GAPDH-forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′; and GAPDH-reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. The relative expression of gene was
analyzed using the 2−ΔΔCq method (18).
Immunohistochemistry
Tumor tissues and the adjacent normal tissues from
patients were first sliced into 4 μm-thick sections.
Afterwards, sections were fixed (24 h at 4°C) using 7.5% buffered
formalin and embedded with paraffin. Then depa-raffinization and
antigen retrieval [in (pH 6.0) citrate buffer at 98°C for 5 min, in
3% H2O2 for 15 min at 98°C in a dark place]
were performed prior to incubation with the primary ISL1 antibody
(cat. no. sc-390793; 1:50) overnight at 4°C. Subsequently, slides
were incubated with horseradish peroxidase-conjugated secondary
antibody (ZSGB-BIO; OriGene Technologies, Inc., Beijing, China) at
37°C for 30 min. The 3,3-diaminobenzidine detection kit (Wuhan
Boster Biological Technology, Ltd., Wuhan, China) acted as the
final chromogen and the nucleus was counterstained (3 min at room
temperature) with hematoxylin (0.5%). Images were captured from at
least 6 representative fields of view at magnification, ×400. The
immunostaining was assessed by two independent pathologists from
Tangshan People's Hospital with no knowledge of the groups.
Statistical analysis
All data were analyzed using GraphPad Prism 6.0
(GraphPad Software, Inc., La Jolla, CA, USA) and expressed as the
mean ± standard deviation. Comparisons between two groups and
multiple groups were performed by unpaired Student's t-test and
one-way analysis of variance followed by a Student-Newman-Keuls
test, respectively. The χ2 test was applied for the
analysis of the association between ISL1 and clinicopathological
characteristics of patients. P<0.05 was considered to indicate a
statistically significant difference. Each experiment was
replicated three times.
Results
ISL1 is overexpressed in tumor tissues of
patients with TNBC
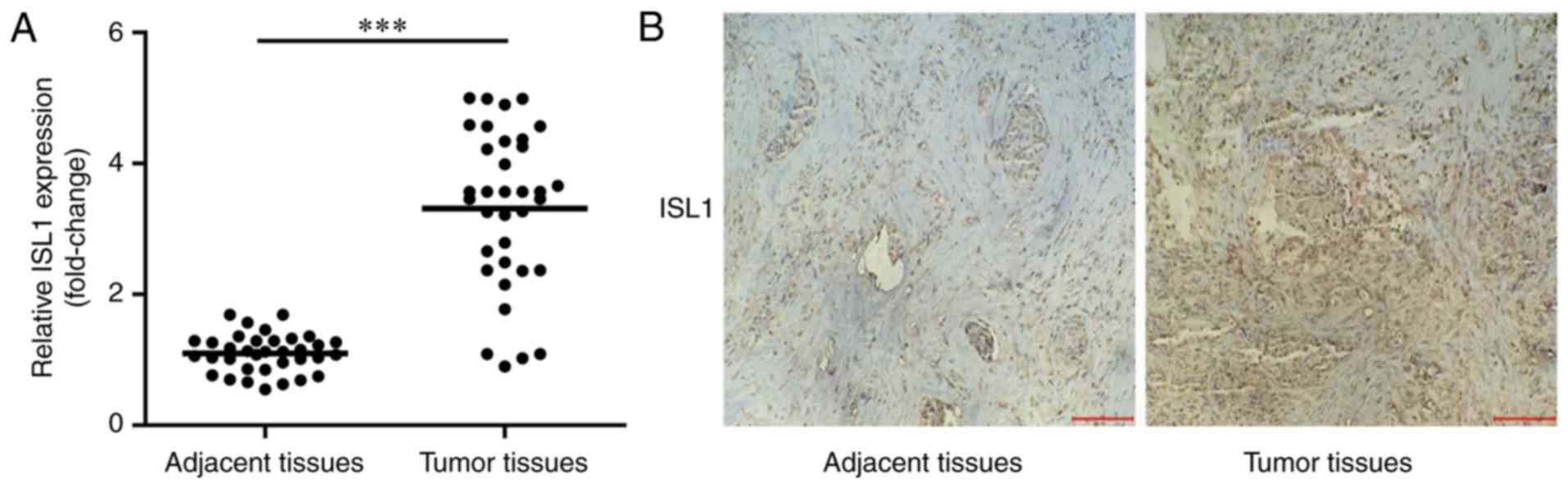
To identify whether ISL1 was increased in tumor
tissues, the present study detected mRNA expression levels of ISL1
in tumor tissues and normal adjacent tissues in 35 patients with
TNBC by RT-qPCR. As indicated in Fig.
1, ISL1 mRNA and protein levels were significantly increased in
tumor tissues compared with those in normal adjacent tissues.
In addition, the association between changes in ISL1
expression levels and the clinicopathological characteristics of
the patients are summarized in Table
I. There was no significant difference in ISL1 levels between
<35-year-old patients (3.15±1.35) and those aged ≥35-years
(3.37±1.21; P=0.64). There were 17 patients whose tumor-size was
<4 cm and 18 patients whose tumor-size was ≥4 cm, but no
significant difference in ISL1 levels was observed between these
two groups (3.39±1.25 vs. 3.24±1.97, respectively; P=0.71).
According to their histological grade by the Nottingham
modification of the Scarff-Bloom-Richardson (NSBR) grading scheme
(19), patients were grouped into
well/intermediate differentiation (n=14) and poor differentiation
groups (n=21), but no significant difference in ISL1 expression
levels was observed between the two groups (3.45±1.11 vs.
3.22±1.67, respectively; P=0.60). In total, 22 patients exhibited
Tis-T2 invasion depth, while 13 patients exhibited T3-T4 invasion
depth (19). The expression
levels of ISL1 in these patients were 3.17±1.37 and 3.56±1.77,
respectively, with no significant difference between the two groups
(P=0.36). By contrast, a significant difference in ISL1 levels was
observed for lymph node metastasis between N0 and N1-N3 (2.71±0.85
vs. 3.59±1.24; P=0.04). Similarly, a significant difference in ISL1
levels was observed for distant metastasis between M0 and M1
(3.13±1.25 vs. 4.22±1.49; P= 0.04).
 | Table IAssociation between ISL1 expression
and clinicopathological parameters of patients with breast
cancer. |
Table I
Association between ISL1 expression
and clinicopathological parameters of patients with breast
cancer.
| Clinicopathological
parameters | N | ISL1
(fold-change) | P-value |
|---|
| Age, years | | | 0.64 |
| <35 | 9 | 3.15±1.35 | |
| ≥35 | 26 | 3.37±1.21 | |
| Tumor size, cm | | | 0.71 |
| <4 | 17 | 3.39±1.25 | |
| ≥4 | 18 | 3.24±1.97 | |
| Histological
grade | | | 0.60 |
| Well/intermediate
differentiation | 14 | 3.45±1.11 | |
| Poor
differentiation | 21 | 3.22±1.67 | |
| Invasion depth | | | 0.36 |
| Tis-T2 | 22 | 3.17±1.37 | |
| T3-T4 | 13 | 3.56±1.77 | |
| Lymph node
metastasis | | | 0.04a |
| N0 | 11 | 2.71±0.85 | |
| N1-N3 | 24 | 3.59±1.24 | |
| Distant
metastasis | | | 0.04a |
| M0 | 29 | 3.13±1.25 | |
| M1 | 6 | 4.22±1.49 | |
ISL1 promotes cell proliferation in TNBC
cells
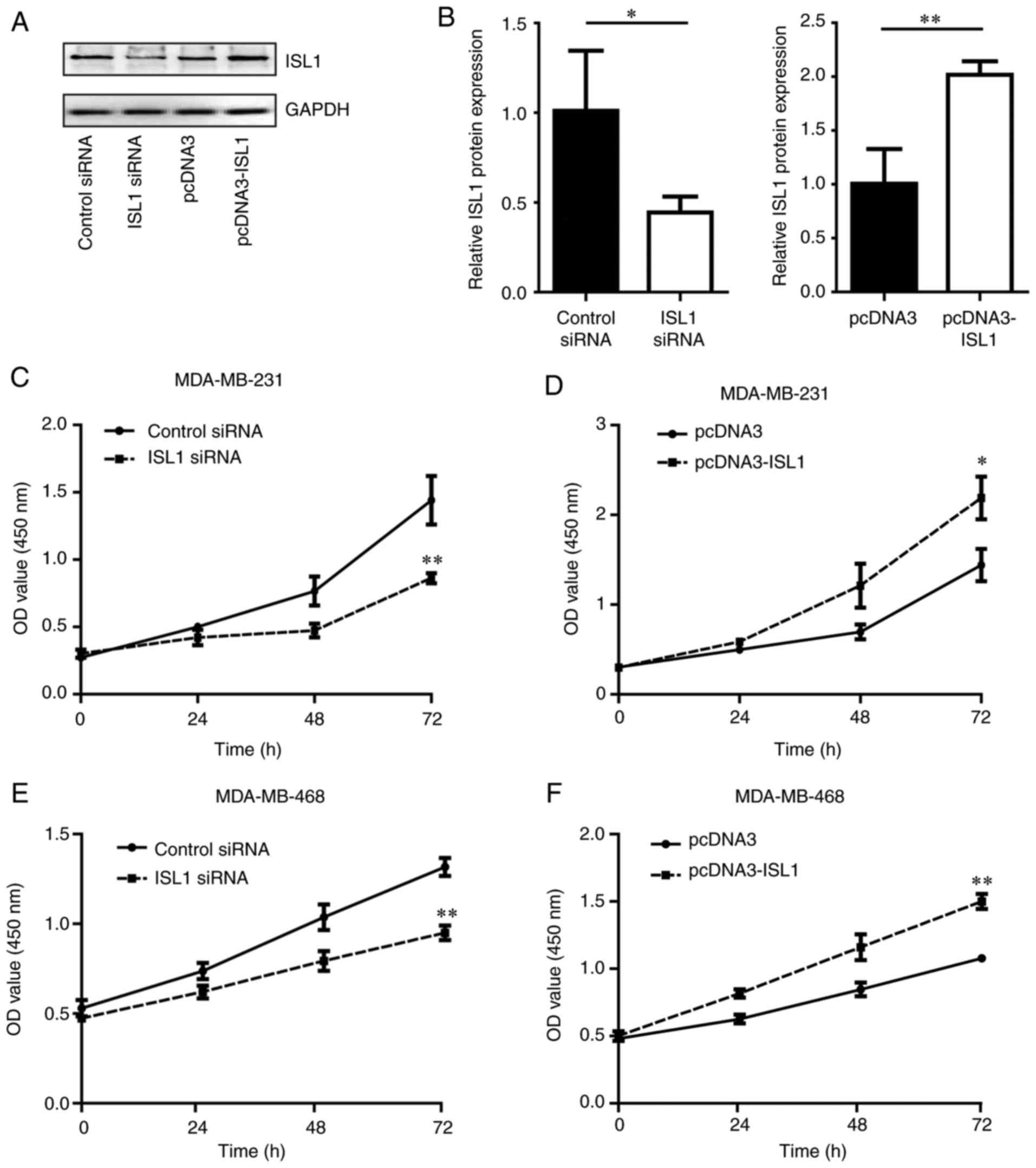
To additionally explore the function of ISL1 in TNBC
cells, the present study determined the proliferation of MDA-MB-231
and MDA-MB-468 cells in response to silencing and overexpressing of
ISL1. Western blotting confirmed that the ISL1 protein levels in
MDA-MB-231 cells were decreased upon silencing of ISL1 compared
with those in the control siRNA group. By contrast, the ISL1
protein levels were increased following overexpression of ISL1 in
MDA-MB-231 cells compared with those in the pcDNA3 group (Fig. 2A and B). In addition, silencing
ISL1 inhibited the proliferation of MDA-MB-231 and MDA-MB-468 cells
compared with that observed in the control siRNA groups, while
overexpression of ISL1 promoted cell proliferation in MDA-MB-231
and MDA-MB-468 cells in comparison with cells transfected with
pcDNA3 (Fig. 2C–F).
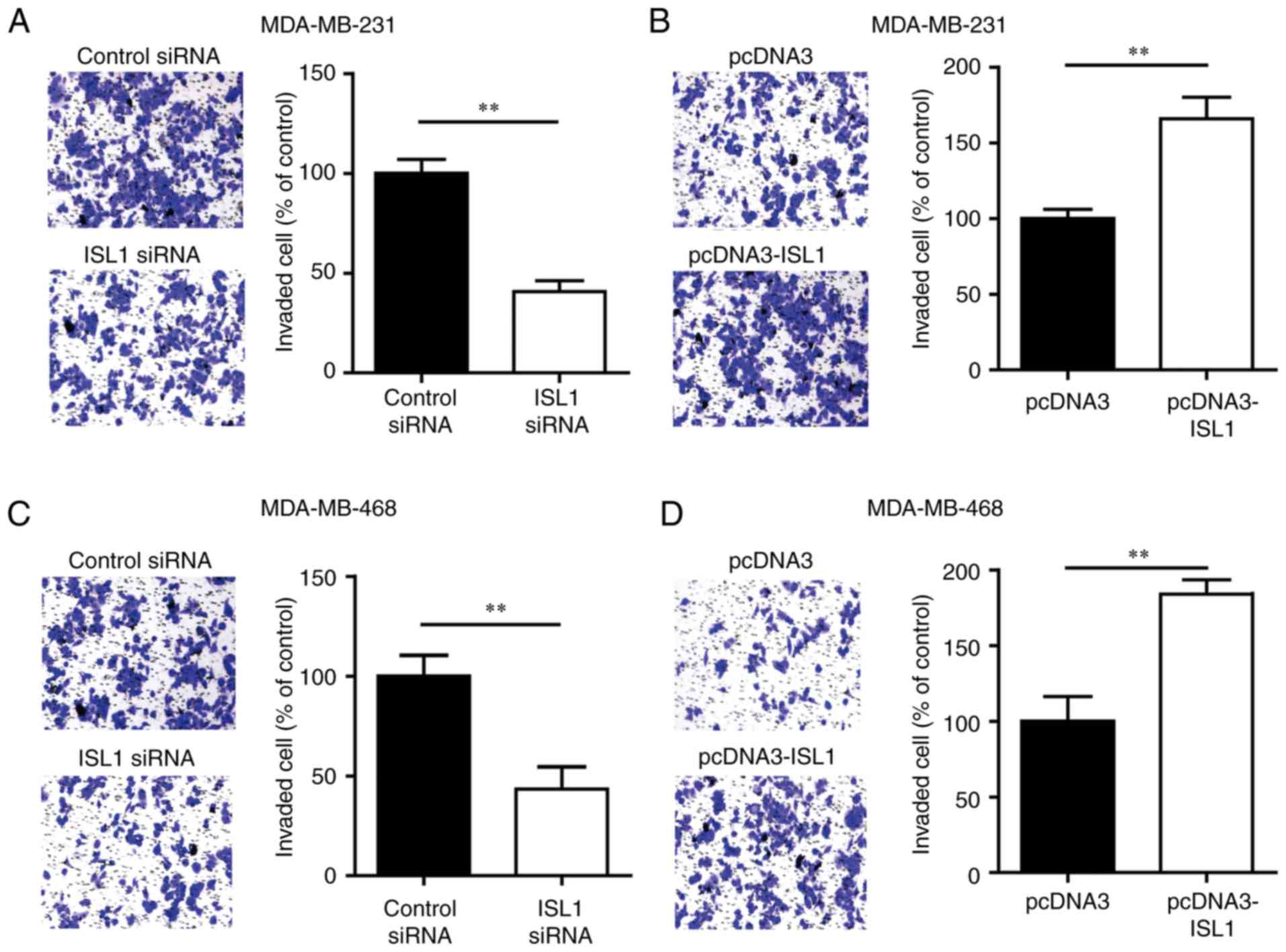
ISL1 improves the invasive ability of
TNBC cells
To explore the role of ISL1 in cell invasion, a
Transwell assay was used to evaluate the invasion ability of
MDA-MB-231 cells transfected with ISL1 siRNA or control siRNA.
Silencing ISL1 markedly inhibited the invasion ability of
MDA-MB-231 cells compared with that of the control siRNA group
cells (Fig. 3A). Conversely,
overexpression of ISL1 increased the number of cells that invaded
into the lower chamber compared with that observed in the group
transfected with pcDNA3 (Fig.
3B). Similar results were observed in MDA-MB-468 cells
(Fig. 3C and D). These data
indicated that ISL1 may promote the metastasis of TNBC cells.
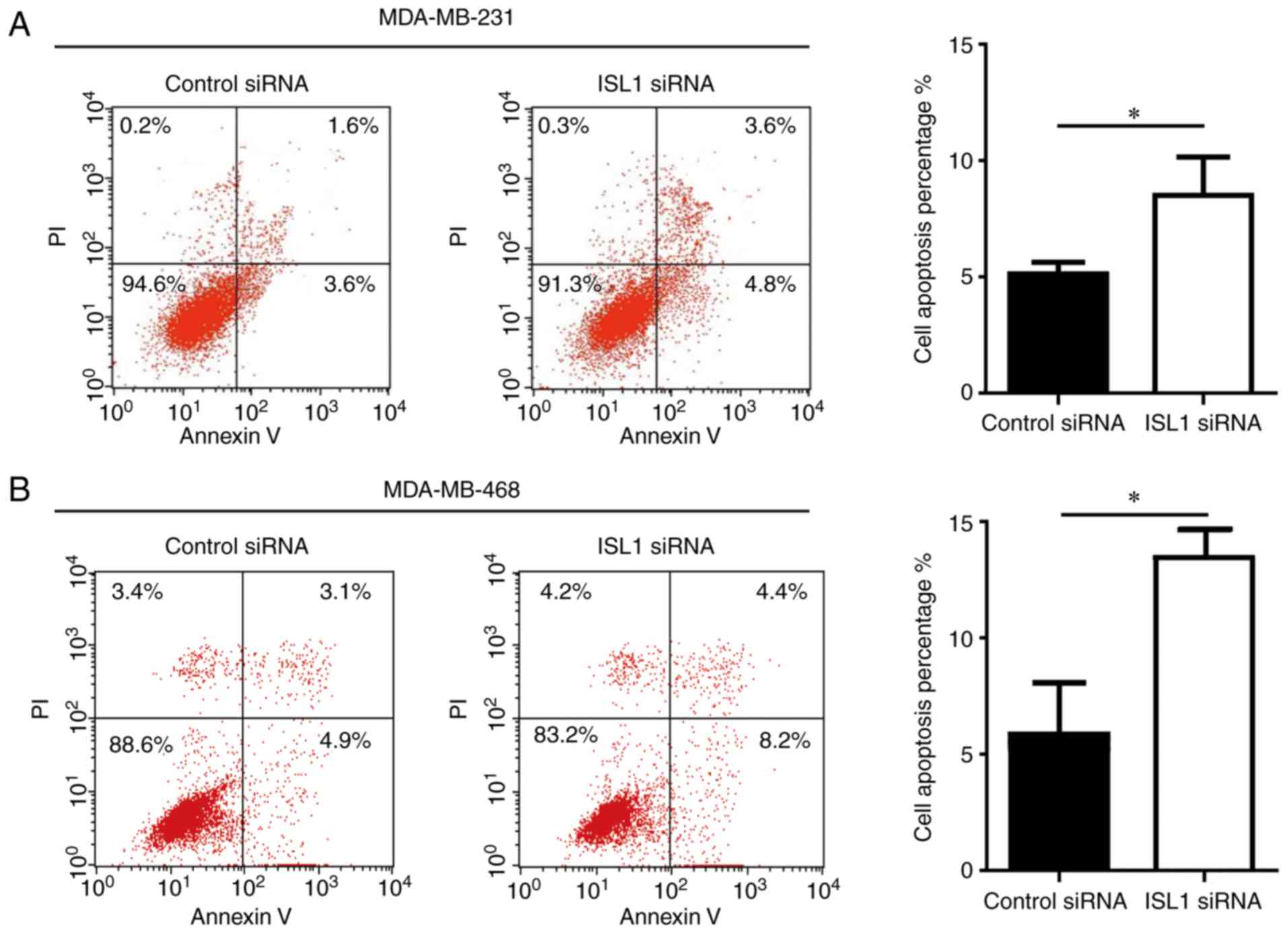
ISL1 exhibits anti-apoptotic ability in
TNBC cells
Next, the present study sought to examine the
function of ISL1 in TNBC cell apoptosis. Silencing ISL1 induced a
significant but relatively small increase in cell apoptosis in
MDA-MB-231 and MDA-MB-468 cells compared with that observed in the
control siRNA group (Fig. 4),
suggesting that ISL1 is involved in the regulation of cell
apoptosis in TNBC cells.
ISL1 inhibits cisplatin-induced cell
apoptosis in TNBC cells
As a modest anti-apoptotic ability was observed for
ISL1 in MDA-MB-231 cells, it was hypothesized that ISL1 may
antagonize the cell apoptosis induced by chemotherapy agents and
contribute to chemoresistance. The results revealed that cell
proliferation was markedly inhibited following cisplatin treatment
in the MDA-MB-231 and MDA-MB-468 cell lines, compared with that
observed in the vehicle group. Additionally, cisplatin-induced cell
proliferation was significantly decreased by overexpression of ISL1
and enhanced by ISL1 silencing (Fig.
5A and B). Flow cytometry analysis indicated that cisplatin
significantly increased apoptosis in TNBC cells compared with that
observed in control cells treated with vehicle. In addition,
cisplatin-induced cell apoptosis was reversed by ISL1
overexpression and increased by ISL1 silencing in TNBC cells
(Fig. 5C and D). These results
demonstrated that ISL1 may decrease the sensitivity of TNBC cells
towards cisplatin.
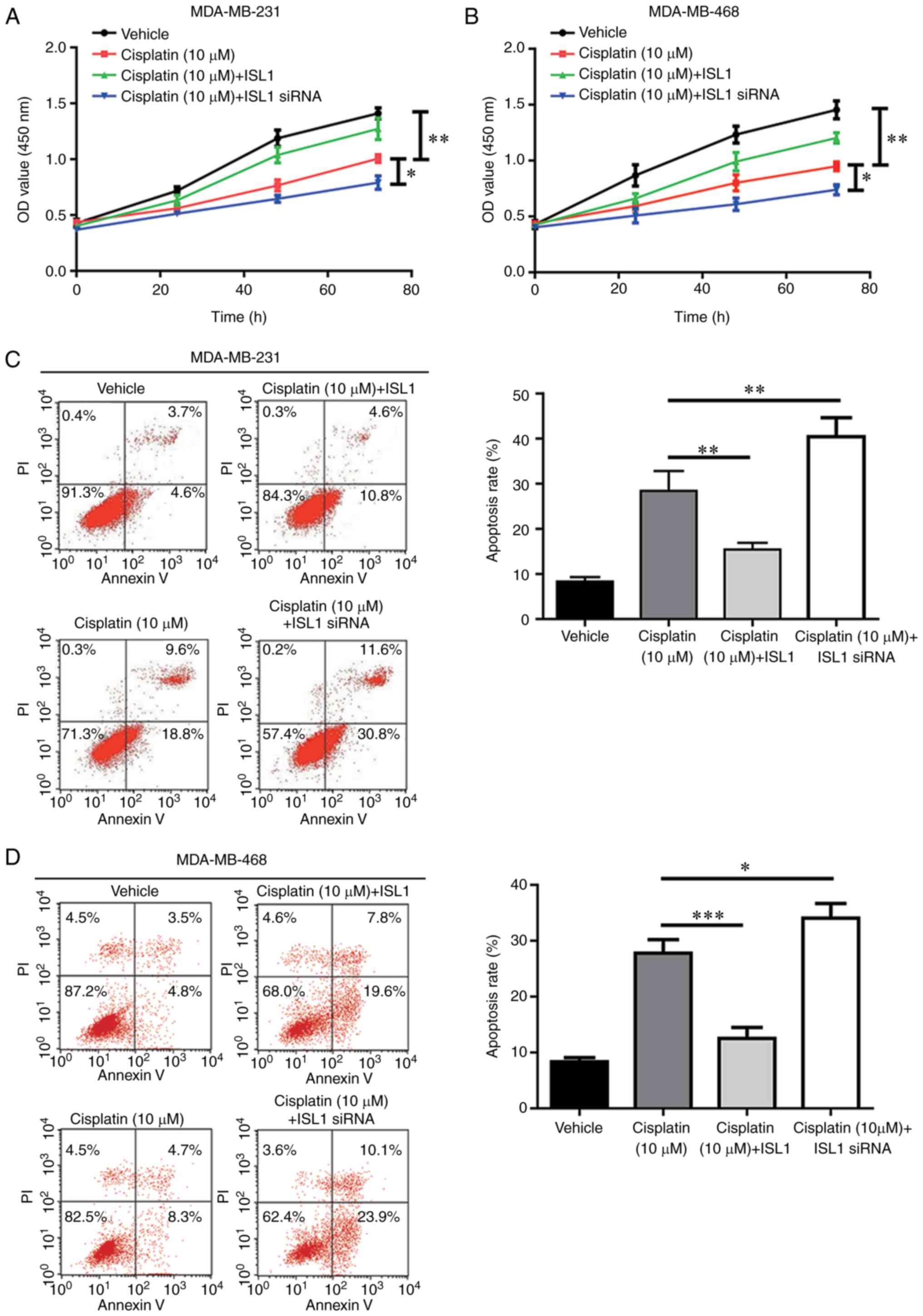 | Figure 5ISL1 decreases cisplatin sensitivity
of TNBC cells. (A) Compared with the vehicle group, treatment with
10 μM cisplatin induced a significant cell growth arrest,
which was partially reversed by ISL1 overexpression and augmented
by ISL1 silencing in MDA-MB-231 cells. (B) Compared with the
vehicle group, treatment with 10 μM cisplatin induced a
significant cell growth arrest, which was partially reversed by
ISL1 overexpression and augmented by ISL1 silencing in MDA-MB-468
cells. (C) Compared with the vehicle group, significant cell
apoptosis was induced by 10 μM cisplatin treatment, which
was reversed by ISL1 overexpression and augmented by ISL1 silencing
in MDA-MB-231 cells. (D) Compared with the vehicle group,
significant cell apoptosis was induced by 10 μM cisplatin
treatment, which was reversed by ISL1 overexpression and augmented
by ISL1 silencing in MDA-MB-468 cells. *P<0.05,
**P<0.01 and ***P<0.0001. siRNA, small
interfering RNA; ISL1, Islet 1; OD, optical density; PI, propidium
iodide. |
ISL1 regulates pro-apoptotic and
anti-apoptotic proteins
To explore the potential molecular mechanism of ISL1
in an anti-apoptosis pathway, the protein levels of pro-apoptotic
(Bax) and anti-apoptotic (Bcl-2 and p-Akt) proteins were detected
in MDA-MB-231 cells. Silencing ISL1 increased Bax protein levels,
and decreased Bcl-2 and p-Akt protein levels (Fig. 6A and B), while overexpression of
ISL1 decreased Bax protein levels, and increased Bcl-2 and p-Akt
protein levels (Fig. 6A and C).
These data indicated that ISL1 is involved in the modulation of
cell apoptosis through the regulation of anti-apoptotic and
pro-apoptotic proteins.
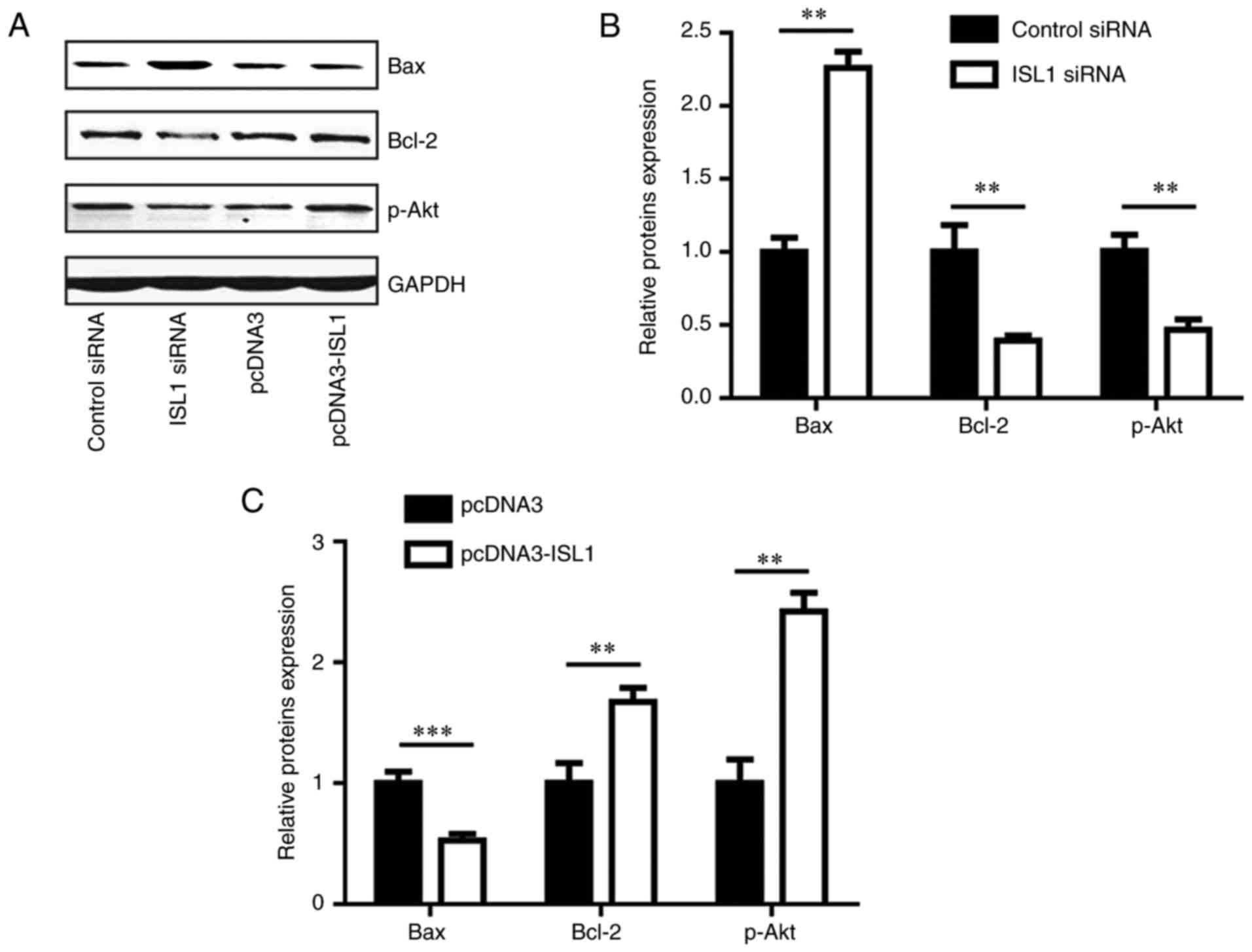 | Figure 6ISL1 regulated expression levels of
Bax, Bcl-2 and p-Akt in MDA-MB-231 cells. (A) Western blot analysis
indicated that ISL1 siRNA increased Bax protein level, and
decreased Bcl-2 and p-Akt protein levels in comparison with those
in the control siRNA group in MDA-MB-231 cells. Conversely,
pcDNA3-ISL1 decreased Bax protein level, and increased Bcl-2 and
p-Akt protein levels when compared with those of the pcDNA3 group.
(B) Densitometric analysis of the results from (A). (C)
Quantitative analysis of Bax, Bcl-2 and p-Akt protein level in A
following overexpression of ISL1, **P<0.01 and
***P<0.0001. Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; Akt, protein kinase B; p-Akt,
phosphorylated Akt; siRNA, small interfering RNA; ISL1, Islet
1. |
Discussion
Breast cancer remains a leading cause of mortality
in women worldwide (1). Compared
with other types of breast cancer, TNBC is characterized by
increased aggressive behavior and relatively poorer clinical
outcome (20). The present study
revealed that ISL1 was involved in the development of TNBC via
regulating cell proliferation, cell invasion and chemotherapy
sensitivity in TNBC cells.
Using Affymetrix, ISL1 was first observed to be
upregulated in biliary tract cancer tissues compared with normal
biliary epithelial scrapings (21). Subsequently, ISL1 was identified
as a marker for pancreatic endocrine tumors and metastasis, and was
also observed to be overexpressed in rhabdomyosar-coma (22,23). Furthermore, the results of
immunohistochemistry in 348 breast cancer tissues indicated that
ISL1 expression was notably increased in TNBC tissues compared with
that in tissues of other breast cancer sub-types (17). A recent study revealed that ISL1
promoted gastric cancer cell proliferation via the regulation of
cyclin-B1, cyclin-B2 and c-Myc (24). The present study revealed that
ISL1-knockdown inhibited cell proliferation, while ISL1
overexpression promoted cell proliferation in MDA-MB-231 and
MDA-MB-468 cells. In addition, ISL1-knockdown decreased the number
of MDA-MB-231 and MDA-MB-468 cells that invaded through Transwell
chambers, while ISL1 overexpression increased the invasion ability
of these cells. Together, these data suggest that ISL1 promotes the
progression of TNBC.
With no targeted therapy, the treatment of patients
with TNBC primarily relies on chemotherapy. However, de novo
and acquired chemoresistance usually result in poor drug response
and eventually lead to patient mortality (25). Numerous genes have been
demonstrated to contribute to chemotherapy resistance in TNBC
(26,27). The present study identified that
ISL1 knockdown induced a slight increase in cell apoptosis.
However, in the presence of cisplatin, ISL1 downregulation notably
increased the apoptosis of MDA-MB-231 and MDA-MB-468 cells.
Furthermore, ISL1 overexpression was able to reverse
cisplatin-induced apoptosis in MDA-MB-231 and MDA-MB-468 cells. The
ratio of Bcl-2/Bax, which is deregulated in cisplatin-induced cell
apoptosis, has been demonstrated to be critical for cell survival
(28,29). In addition, previous studies have
indicated that high expression levels of p-Akt contribute to
cisplatin resistance via inhibition of cell apoptosis in various
types of cancer (30,31). In the present study, ISL1
silencing markedly increased Bax protein levels, and decreased
Bcl-2 and p-Akt protein levels in MDA-MB-231 cells. Conversely, the
overexpression of ISL1 markedly decreased Bax levels, and increased
Bcl-2 and p-Akt levels. Collectively, the results of the present
study indicated that ISL1 may suppress cell apoptosis, particularly
cisplatin-induced cell apoptosis, in TNBC cells.
In conclusion, the present study identified ISL1 as
a potential oncogene in TNBC, as ISL1 promoted cell proliferation
and invasion and inhibited cell apoptosis in TNBC cells.
Furthermore, ISL1 overexpression reversed cisplatin-induced cell
apoptosis, indicating that ISL1 inhibited the sensitivity of TNBC
cells towards cisplatin. Therefore, ISL1 may be a promising
therapeutic target and a predictor of cisplatin sensitivity for
patients with TNBC.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Concept and design: HC. Acquisition of data: YZ, LW,
PG, ZS, NL, YL, JSh, JS, HD and YY. Analysis and interpretation of
data: HC, YZ, LW, PG and ZS. Writing and review of manuscript: HC,
YZ. Study supervision: HC
Ethics approval and consent to
participate
Written informed consent for use of patient samples
was obtained from all participants prior to surgery. The
experiments were performed following approval from the Ethics
Committee of Tangshan People's Hospital.
Patient consent for publication
Written informed consent for use of patient samples
was obtained from all participants prior to surgery.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Patnaik JL, Byers T, DiGuiseppi C, Dabelea
D and Denberg TD: Cardiovascular disease competes with breast
cancer as the leading cause of death for older females diagnosed
with breast cancer: A retrospective cohort study. Breast Cancer
Res. 13:R642011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheang MC, Voduc D, Bajdik C, Leung S,
McKinney S, Chia SK, Perou CM and Nielsen TO: Basal-like breast
cancer defined by five biomarkers has superior prognostic value
than triple-negative phenotype. Clin Cancer Res. 14:1368–1376.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oualla K, El-Zawahry HM, Arun B, Reuben
JM, Woodward WA, Gamal El-Din H, Lim B, Mellas N, Ueno NT and Fouad
TM: Novel therapeutic strategies in the treatment of
triple-negative breast cancer. Ther Adv Med Oncol. 9:493–511. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brasó-Maristany F, Filosto S, Catchpole S,
Marlow R, Quist J, Francesch-Domenech E, Plumb DA, Zakka L,
Gazinska P, Liccardi G, et al: PIM1 kinase regulates cell death,
tumor growth and chemotherapy response in triple-negative breast
cancer. Nat Med. 22:1303–1313. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rakha EA, Reis-Filho JS and Ellis IO:
Basal-like breast cancer: A critical review. J Clin Oncol.
26:2568–2581. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu XC, Zhang J, Xu BH, Cai L, Ragaz J,
Wang ZH, Wang BY, Teng YE, Tong ZS, Pan YY, et al: Cisplatin plus
gemcitabine versus paclitaxel plus gemcitabine as first-line
therapy for metastatic triple-negative breast cancer (CBCSG006): A
randomised, open-label, multicentre, phase 3 trial. Lancet Oncol.
16:436–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pruefer FG, Lizarraga F, Maldonado V and
Melendez-Zajgla J: Participation of Omi Htra2 serine-protease
activity in the apoptosis induced by cisplatin on SW480 colon
cancer cells. J Chemother. 20:348–354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rottenberg S, Nygren AO, Pajic M, van
Leeuwen FW, van der Heijden I, van de Wetering K, Liu X, de Visser
KE, Gilhuijs KG, van Tellingen O, et al: Selective induction of
chemotherapy resistance of mammary tumors in a conditional mouse
model for hereditary breast cancer. Proc Natl Acad Sci USA.
104:12117–12122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar
|
|
12
|
Brozovic A and Osmak M: Activation of
mitogen-activated protein kinases by cisplatin and their role in
cisplatin-resistance. Cancer Lett. 251:1–16. 2007. View Article : Google Scholar
|
|
13
|
Zhou BP, Liao Y, Xia W, Spohn B, Lee MH
and Hung MC: Cytoplasmic localization of p21Cip1/WAF1 by
Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat
Cell Biol. 3:245–252. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karlsson O, Thor S, Norberg T, Ohlsson H
and Edlund T: Insulin gene enhancer binding protein Isl-1 is a
member of a novel class of proteins containing both a homeo- and a
Cys-His domain. Nature. 344:879–882. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hobert O and Westphal H: Functions of
LIM-homeobox genes. Trends Genet. 16:75–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo C, Wang W, Shi Q, Chen P and Zhou C:
An abnormally high expression of ISL-1 represents a potential
prognostic factor in gastric cancer. Hum Pathol. 46:1282–1289.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shin E, Lee Y and Koo JS: Differential
expression of the epigenetic methylation-related protein DNMT1 by
breast cancer molecular subtype and stromal histology. J Transl
Med. 14:872016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak and Schmittgen: Analysis of relative
gene expression data using real-time quantitative PCR and the
2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
19
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. The value of histologic grade
in breast cancer: Experience from a large study with long term
follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kong X, Liu W and Kong Y: Roles and
expression profiles of long non-coding RNAs in triple-negative
breast cancers. J Cell Mol Med. 22:390–394. 2018. View Article : Google Scholar
|
|
21
|
Hansel DE, Rahman A, Hidalgo M, Thuluvath
PJ, Lillemoe KD, Schulick R, Ku JL, Park JG, Miyazaki K, Ashfaq R,
et al: Identification of novel cellular targets in biliary tract
cancers using global gene expression technology. Am J Pathol.
163:217–229. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmitt AM, Riniker F, Anlauf M, Schmid S,
Soltermann A, Moch H, Heitz PU, Klöppel G, Komminoth P and Perren
A: Islet 1 (Isl1) expression is a reliable marker for pancreatic
endocrine tumors and their metastases. Am J Surg Pathol.
32:420–425. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Erlenbach-Wünsch K, Haller F, Taubert H,
Wurl P, Hartmann A and Agaimy A: Expression of the LIM homeobox
domain transcription factor ISL1 (Islet-1) is frequent in
rhabdomyosarcoma but very limited in other soft tissue sarcoma
types. Pathology. 46:289–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi Q, Wang W, Jia Z, Chen P, Ma K and
Zhou C: ISL1, a novel regulator of CCNB1, CCNB2 and c-MYC genes,
promotes gastric cancer cell proliferation and tumor growth.
Oncotarget. 7:36489–36500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schlotter CM, Tietze L, Vogt U, Heinsen CV
and Hahn A: Ki67 and lymphocytes in the pretherapeutic core biopsy
of primary invasive breast cancer: Positive markers of therapy
response prediction and superior survival. Horm Mol Biol Clin
Investig. Sep 22–2017, (Epub ahead of print). doi: https://doi.org/10.1515/hmbci-2017-0022.
View Article : Google Scholar
|
|
26
|
Wein L and Loi S: Mechanisms of resistance
of chemotherapy in early-stage triple negative breast cancer
(TNBC). Breast. 34(Suppl 1): S27–S30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gómez-Miragaya J, Palafox M, Paré L, Yoldi
G, Ferrer I, Vila S, Galván P, Pellegrini P, Pérez-Montoyo H, Igea
A, et al: Resistance to taxanes in triple-negative breast cancer
associates with the dynamics of a CD49f+ tumor-initiating
population. Stem Cell Reports. 8:1392–1407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saxena A, Viswanathan S, Moshynska O,
Tandon P, Sankaran K and Sheridan DP: Mcl-1 and Bcl-2/Bax ratio are
associated with treatment response but not with Rai stage in B-cell
chronic lymphocytic leukemia. Am J Hematol. 75:22–33. 2004.
View Article : Google Scholar
|
|
29
|
Chen L, Cui H, Fang J, Deng H, Kuang P,
Guo H, Wang X and Zhao L: Glutamine deprivation plus BPTES alters
etoposide- and cisplatin-induced apoptosis in triple negative
breast cancer cells. Oncotarget. 7:54691–54701. 2016.PubMed/NCBI
|
|
30
|
Liu S, Ren B, Gao H, Liao S, Zhai YX, Li
S, Su XJ, Jin P, Stroncek D, Xu Z, et al: Over-expression of BAG-1
in head and neck squamous cell carcinomas (HNSCC) is associated
with cisplatin-resistance. J Transl Med. 15:1892017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wen Q, Liu Y, Lyu H, Xu X, Wu Q, Liu N,
Yin Q, Li J and Sheng X: Long noncoding RNA GAS5, which acts as a
tumor suppressor via microRNA 21, regulates cisplatin resistance
expression in cervical cancer. Int J Gynecol Cancer. 27:1096–1108.
2017. View Article : Google Scholar : PubMed/NCBI
|