Introduction
Thymosin β4 (Tβ4), a 4.9-kDa small peptide composed
of 43 amino acids, was first extracted from the thymus and is the
most abundant type of thymosin in mammals (1). Tβ4 participates in regulating cell
proliferation, differentiation and motility (2). Our previous study demonstrated that
Tβ4 expression increases MC3T3-E1 cell viability on titanium (Ti)
discs by increasing adhesion and proliferation (3). Tβ4 is expressed and involved in the
initiation, formation and differentiation of tooth germ during
molar development (4,5). Tβ4-overexpressing transgenic mice
exhibit abnormal tooth development resembling enamel hyperplasia
(6). Tβ4 knockdown with small
interfering RNA (siRNA) significantly decreases mRNA levels of
noncollagenous proteins and type I collagen (COL type I) during
MDPC-23 odontoblastic cell differentiation (7).
Dentinogenesis is regulated in odontoblasts by a
complex signaling cascade that promotes the expression of dentin
matrix-associated proteins (8).
Dentin is composed of the mineral hydroxyapatite and organic
materials including collagen and noncollagenous proteins. Among
noncollagenous proteins, bone sialoprotein (BSP) is one of the
major proteins in mineralized tissues including bone, dentin,
cementum and calcified cartilage (9). BSP is a phosphorylated or sulfurized
glycoprotein with high levels of a sialic acid, for example
osteopontin, and tends to bind with hydroxyapatite (10,11). BSP serves as the nucleator of
primary apatite crystals, and it regulates the direction of
ribbon-like apatite crystal growth on collagen during
mineralization (12). Protamine
increases BSP expression via mitogen-activated protein kinase
(MAPK) signaling in osteoblasts (13). Transforming growth factor β1
(TGF-β1) inhibits BSP expression and osteoblast formation from
human mesenchymal stem cells via mothers against decapentaplegic
homolog 3 (Smad3)/β-catenin signaling (14). Tβ4 increases MC3T3-E1 cell
viability by activating the paired box (Pax)/focal adhesion kinase
(FAK) and FAK/growth factor receptor-bound protein 2
(Grb2)/Ras/extracellular signal-regulated kinase (ERK) signaling
pathways and promoting focal adhesion and proliferation on Ti discs
(3). In addition, Tβ4 increases
gastric cancer cell migration by down-regulating E-cadherin through
the ERK/glycogen synthase kinase 3α/β-catenin signaling pathway
(15).
The authors' previous study identified that Tβ4
protein expression was highest in the secretory odontoblast layer
during the advanced bell stage of dentinogenesis (7). It was also identified that Tβ4
promotes differentiation and mineralization of the osteoblastic
MC3T3-E1 cell line on Ti discs (16). These previous studies indicated
that Tβ4 serves as a principle molecule during the formation of
mineralized tissues including bone and dentin. However,
Tβ4-associated signaling is not fully understood in odontoblasts,
and specifically, the association between Tβ4 signaling and BSP has
not been elucidated. Therefore, we hypothesized and investigated
whether Tβ4 signaling may regulate BSP expression in odontoblasts.
To the best of our knowledge, the present study identified for the
first time that Tβ4 upregulates BSP by activating ERK and Smad3
signaling in MDPC-23 odontoblastic cells, suggesting its role as a
principle regulatory signaling molecule in dentin matrix formation
during dentinogenesis.
Materials and methods
Tissue preparation
A total of 3 pregnant adult ICR outbred female mice,
10-weeks old, weighing 50–60 g (Samtako Bio Korea Osan,
Gyeonggi-do, Korea), were used. The temperature and humidity was
maintained at 23±2°C and 60±10%, respectively. The animals were
kept in a 12 h light-dark photoperiod and provided with pelleted
mouse chow and tap water ad libitum. The animal protocols
were approved by the Institutional Animal Care and Use Committees
at Chosun University (Gwangju, Korea), and animal care was
performed using specific-pathogen-free systems according to the
Guide for the Care and Use of Laboratory Animals (17). The heads of mice at postnatal day
1 (PN1), PN3, PN5, PN15, and PN21 were used in the present study.
The heads, dissected from PN1, PN3, and PN5 mice, were fixed in 4%
paraformaldehyde (PFA) in diethylpyrocarbonate-treated
phosphate-buffer saline (DEPC-PBS, pH 7.4) at 4°C for 24 h. The
PN15 and PN21 mice were fixed by the intracardiac perfusion of 4%
PFA. The heads of the PN15 and PN21 mice were dissected and fixed
an additional 18 h in fresh 4% PFA at 4°C. Tissues were decalcified
in a solution of 10% ethylenediaminetetraacetic acid (EDTA)
supplemented with 1% PFA at 27–28°C for 4 weeks. After
decalcification, the heads were dehydrated by sequential washes in
70, 80, 90, 100 I, 100 II and 100% III at 27–28°C for 1 h and
finally in 100% IV ethanol for 18 h. Paraffin-embedded tissues were
cut into 6-μm thick sections using a Histocut 820 (Leica
Microsystems, Wetzlar, Germany) and were subsequently placed onto
glass slides and dried on a 37°C slide warmer overnight.
Synthesis of Tβ4 complementary RNA (cRNA)
probes and peptides
Gene-specific probes for the 405-bp Tβ4 cDNA were
designed according to methods described previously (5). The pGEM-3Z vector (Promega
Corporation, Madison, WI, USA) containing a Tβ4 cDNA insert was
linearized by digestion with restriction enzymes (EcoRI or
HindIII) to synthesize Tβ4 sense (S) and antisense (AS) cRNA
probes using an in vitro transcription kit (Roche Applied
Science, Penzberg, Germany). The probes were labeled with
digoxigenin (DIG)-11-UTP using a SP6/T7 DIG RNA Labeling kit (Roche
Diagnostics, Basel, Switzerland). The full amino acid sequence of
the mouse Tβ4 peptide, Ac-SDK PDM AEI EKF DKS KLK KTE TQE KNP LPS
KET IEQ EKQ AGE S, was synthesized by solution phase peptide
synthesis (A&PEP, Cheongju-si, Chungcheongbuk-do, Korea).
Membrane hybridization
Unlabeled S probes were anchored to the nucleic acid
transfer membranes (GE Healthcare Life Sciences, Little Chalfont,
UK) using a ultraviolet crosslinker (Stratagene; Agilent
Technologies, Inc., Santa Clara, CA, USA). The membrane-anchored
unlabeled S probes were incubated with DIG-labeled AS probes mixed
with unlabeled S or unlabeled AS (1–10 ng/μl) in the
hybridization solution. The DIG-labeled AS probes were reacted with
alkaline phosphatase (AP)-conjugated DIG antibodies and then
detected using a DIG nucleic acid detection kit (Roche
Diagnostics).
In situ hybridization
Tissue sections were deparaffinized sequentially in
xylene I and II at 27–28°C for 5 min. For rehydration, the sections
were incubated sequentially in 100, 90, 80 and 70% ethanol at
27–28°C for 1 min. Following deparaffinization and hydration, the
tissue sections were incubated with proteinase K at 37°C for 12 min
and then 4% PFA at 27–28°C for 10 min. Subsequently, sections were
immersed in 0.1 M triethanolamine-HCl (TEA-HCl) and 0.25% acetic
anhydride (in 0.1 M TEA) to remove background signals.
Hybridization was performed with the hybridization mixture
(hybridization solution, 1 ng/μl DIG-labeled S or
DIG-labeled AS). The sections were washed twice in 2X and 0.2X
saline-sodium citrate buffer containing 50% formamide, and then
incubated with a 1:500 dilution of AP-conjugated DIG antibodies at
27–28°C for 1 h. This DIG-labeled S or DIG-labeled AS probes
analysis was performed using a DIG nucleic acid detection kit (cat.
no. 11 175 041 910; Roche Diagnostics), and sections were
counterstained with 1% methyl green (10 mg/ml, Vector Laboratories,
Inc., Burlingame, CA, USA) at 27–28°C for 20 sec. The stained
tissue was observed by optical microscopy (Carl Zeiss AG,
Oberkochen, Germany) on bright field for Tβ4 mRNA detection. The
expression of Tβ4 mRNA in the tissue was analyzed using a bright
field microscope and Axiovision LE 4.6 software (Carl Zeiss
AG).
Cell culture
MDPC-23 cells (provided by Dr CT Hanks from
University of Michigan, Ann Arbor, MI, USA), an odonto-blastic cell
line derived from the dental papilla of fetal mouse molars, were
cultured in Dulbecco's modified Eagle medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Welgene, Inc., Gyeongsan-si, Korea) and 1%
antibiotic-antimycotic solution (Welgene, Inc.). Cells were
incubated in a humidified chamber maintained with 5% CO2
at 37°C.
Extraction of total RNA and reverse
transcription (RT) semi-quantitative polymerase chain reaction
(PCR)
Following serum starvation in serum free medium,
MDPC-23 cells were treated with 2 μg/ml Tβ4 (Tβ4/MDPC-23)
for 24 h (18). Total RNA was
extracted from the cells using TRIzol® reagent
(Molecular Research Center, Inc., Cincinnati, OH, USA) according to
the manufacturer's protocol, and PCR was performed using AmpONE
Taq premix (GeneAll Biotechnology, Co., Ltd., Seoul, Korea).
The following primers were synthesized by Bioneer, Co., Ltd.
(Daejeon, Korea) for RT-PCR analysis: BSP forward,
5′-ACCGGCCACGCTACTTTCTTTAT-3′; BSP reverse,
5′-TCCTCGTCGCTTTCCTTCACTTT-3′; GAPDH forward,
5′-CCATGGAGAAGGCTGGG-3′; GAPDH reverse, 5′-CAAAGTTGTCATGGATGACC-3′.
Each PCR reaction consisted of an initial denaturation at 95°C for
2 min followed by three-step cycling: Denaturation at 95°C for 20
sec, annealing at a temperature optimized for each primer pair for
10 sec (BSP, 60°C for 30 cycles; GAPDH, 56°C for 30 cycles), and
extension at 72°C for 30 sec. Single bands were observed at the
expected sizes of 358 bp for BSP (GenBank #L20232) and 199 bp for
GAPDH (GenBank #M33197) by agarose gel electrophoresis. The PCR
products were electrophoresed on 1.5% agarose gel buffered with
0.5X Tris-Borate-EDTA (TBE) buffer and stained with ethidium
bromide after amplification. The bands were visualized using a
Gel-Doc system (BioRad Laboratories, Inc., Hercules, CA, USA). Band
intensities were measured to determine differences in mRNA
expression using Science Lab Image Gauge software version 3.12
(Fujifilm Corporation, Tokyo, Japan).
Protein extraction and western blot
analysis
Following serum starvation in serum free medium,
MDPC-23 cells were treated with 2 μg/ml Tβ4 (Tβ4/MDPC-23) at
37°C for 6, 12 or 24 h. TGF-β1 (10 ng/ml; R&D Systems, Inc.,
Minneapolis, MN, USA) was also added to a group of MDPC-23 cells
(TGF-β1/MDPC-23) at 37°C for 30 min. Certain MDPC-23 cells were
treated with the mitogen activated protein kinase kinase inhibitor
PD98059 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or the
Smad3 phosphorylation inhibitor SIS3 (Sigma-Aldrich; Merck KGaA) at
5 μM (PD98059/MDPC-23 and SIS3/MDPC-23, respectively) at
37°C for 1 h to examine the effects of these inhibitors alone. In
addition, MDPC-23 cells were pretreated with PD98059 and SIS3 for 1
h prior to Tβ4 treatment (Tβ4/PD98059/MDPC-23 and Tβ4/SIS/MDPC-23,
respectively). Subsequent to the various treatments, proteins were
extracted from the cells using NP-40 lysis buffer [150 mM NaCl, 1%
NP-40, 50 mM Tris-HCl (pH 7.4), 2 mM Na3VO4,
2 mM Na4P2O, 50 mM NaF, 2 mM EDTA (pH 7.4),
0.1 μg/ml of leupeptin and 1 μg/ml of aprotinin], and
the proteins (30 μg per lane) were electro-phoresed on 7.5%
SDS-PAGE gels. Following electrophoresis, proteins were transferred
to PVDF membranes and blocked with either 5% non-fat dry milk or 5%
bovine serum albumin (BioShop Canada, Inc., Burlington, ON, Canada)
at 27–28°C for 1 h in TBS with 0.05% Tween-20 (TBS-T) buffer. The
membranes were blotted with specific antibodies at 4°C for 16 h
against phosphorylated (p)β-catenin (cat. no. 4176; 1:1,000),
β-catenin (cat. no. 8480; 1:1,000), pERK1/2 (cat. no. 9106;
1:2,500), ERK1/2 (cat. no. 9102; 1:2,500), pSmad2 (cat. no. 3108;
1:1,000), pSmad3 (cat. no. 9502; 1:1,000), or Smad2/3 (cat. no.
3102; 1:1,000) from Cell Signaling Technology, Inc., (Danvers, MA,
USA); BSP (cat. no. AB1854; 1:2,500) from Merck KGaA; and
runt-related transcription factor 2 (Runx2, cat. no. sc-10758;
dilution, 1:2,000) or β-actin (cat. no. sc-47778; 1:2,500) from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Membranes were
then blotted with horseradish peroxidase-conjugated goat
anti-rabbit (cat. no. sc-2004; 1:10,000) or mouse-IgG antibodies
(cat. no. sc-2005; 1:10,000) at 27–28°C for 1 h as appropriate
(Santa Cruz Biotechnology, Inc.). Immunoreactive bands were
detected using X-ray film (Fujifilm Corporation) following
treatment with enhanced chemiluminescent solution (Merck KGaA). The
intensity of expressed bands was measured by densitometry using
Science Lab Image Gauge software version 3.12 (Fujifilm
Corporation).
Immunofluorescence
MDPC-23 cells were serum starved in serum free
medium and then treated with 2 μg/ml Tβ4 and 5 μM
PD98059 or SIS3 for 24 h. Cells were fixed in 4% formaldehyde at
27–28°C for 15 min, treated with 0.1 M glycine, then permeabilized
with 0.2% Triton X-100 for 5 min, and blocked with 5% normal goat
serum (Vector Laboratories, Inc.) at 27–28°C for 1 h. Cells were
incubated with a 1:100 dilution of anti-rabbit BSP, pSmad3,
β-catenin, or Runx2 antibodies at 4°C overnight. A 1:1,000 dilution
of fluorescein isothiocyanate-conjugated goat-anti-rabbit IgG
(Santa Cruz Biotechnology, Inc.; cat. no. sc-2012) was used as a
secondary antibody. Cells were mounted with DAPI (Vector
Laboratories, Inc.) for nuclear staining and images were captured
at ×400, magnification using a fluorescence microscope (Carl Zeiss
AG). The fluorescence intensity of β-catenin, Runx2 and pSmad3
protein in the nucleus compared with the cytoplasm was measured by
ImageJ 1.8.0 software (National Institutes of Health, Bethesda, MD,
USA). At least 100 cells were counted in 3 different microscopic
fields. The measurement and quantification methods were performed
as described previously (19).
Plasmid construction and
transfection
The 2.5 kb portion of the mouse BSP gene promoter
region from -2,472 to +41 was artificially synthesized (Bioneer,
Co., Ltd.; https://www.bioneer.co.kr) and cloned
into the Nhe I-Xho I site of the pGL3-basic vector (pGL3-BSP-Luc).
The pGL3-basic vector was supplied from Promega Corporation
(Madison, WI, USA). All construct identities were verified by DNA
sequencing and restriction enzyme digestion. MDPC-23 cells were
transfected with 2 μg/ml pGL3-basic vector (pGL3-Luc;
3.85×1011/ml) or 2 μg/ml pGL3-BSP-Luc
(2.53×1011/ml) for 48 h using WellFect-EX (Welgene,
Inc.) according to the manufacturer's protocol and transfected
cells were used subsequent experiment after serum starvation for 16
h.
Luciferase assay
The pGL3-BSP-Luc-transfected MDPC-23 cells were
treated with 2 μg/ml Tβ4 (pGL3-BSP-Luc/Tβ4), or 5 μM
PD98059 or SIS3 (pGL3-BSP-Luc/PD98059 or pGL3-BSP-Luc/SIS3,
respectively), for 24 h after serum starvation in serum free
medium. In addition, the pGL3-BSP-Luc/Tβ4 cells were pretreated
with PD98059 or SIS3 at 37°C for 1 h prior to Tβ4 treatment
(pGL3-BSP-Luc/PD98059 or SIS3/Tβ4) for 24 h. Protein extraction and
reaction with luciferase substrates were performed using a
luciferase assay kit (Promega Corporation; cat. no. E1500)
according to the manufacturer's protocol. Luciferase activity as
proxy for BSP expression was measured using a luminometer (Thermo
Fisher Scientific, Inc.). The luciferase activity of pGL3-basic
vector was used for normalization.
Statistical analysis
All experiments were performed at least in
triplicate. Values are expressed as the mean ± standard error of
the mean. Statistical analysis was performed using SPSS v25 (IBM
Corp., Armonk, NY, USA). Differences between samples among multiple
groups were compared by one-way analysis of variance (ANOVA). The
Tukey's post hoc method was used to perform pairwise comparisons
following ANOVA. P<0.01 was considered to indicate a
statistically significant difference.
Results
Tβ4 mRNA is expressed in odontoblasts
during postnatal dentinogenesis
To determine DIG-labeled AS binding activity, the
hybridization signal between the mixture of DIG-labeled AS and
unlabeled S was determined, and membrane-fixed unlabeled S was
detected upon the addition of 1 ng/μl of unlabeled S
mixture, but was not detected upon the addition of 5 or 10
ng/μl. In addition, the hybridization signal between the
mixture of DIG-labeled AS and unlabeled AS, and membrane-fixed
unlabeled S was most marked upon addition of the 1 ng/μl
unlabeled AS mixture, and was gradually decreased upon addition of
the 5 and 10 ng/μl solutions (Fig. 1A). Tβ4 mRNA was increased in
odontoblasts at the advanced bell stage (PN5), and was observed in
the odonto-blasts of the crown (PN15) and functional stages (PN21)
with a pattern similar to that observed in the early bell stage
(PN1). Dentin deposition was observed at PN1 during the early bell
stage and became thickened with the increase in enamel at PN3 and
PN5 during the advanced bell stage (Fig. 1B and C).
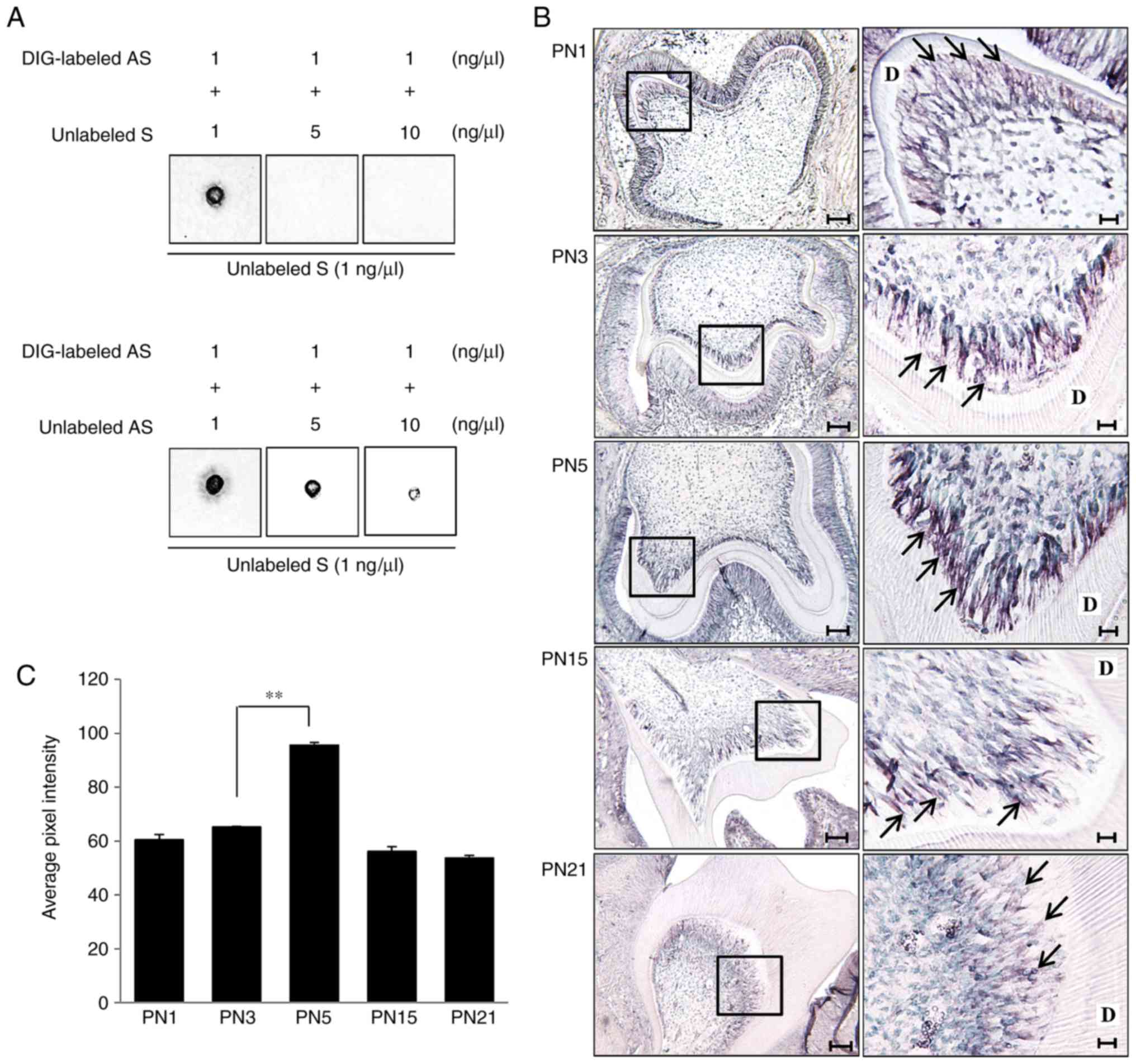 | Figure 1Specificity and binding activity
analysis of in situ probes through membrane hybridization
and Tβ4 mRNA expression in odontoblasts during dentinogenesis. (A)
The hybridization signal between DIG-labeled AS probes (1 ng/ml)
and membrane-dotted unlabeled S probes (1 ng/ml) was decreased by
increasing the concentration of unlabeled S (upper panel) or
unlabeled AS (lower panel) in the mixture. (B) Low (left,
magnification, ×100) and high (right, magnification, ×400)
magnification images of mouse molar tooth germ from PN1 to PN21.
The right-hand column shows the area within the black box magnified
to ×400. Expression of Tβ4 mRNA was increased in odontoblasts
(arrows) at the advanced bell stage (PN5), and the expression at
the crown (PN15) and functional (PN21) stages were similar to
expression observed at the early bell stage (PN1). Scale bars=100
μm (left) and 20 μm (right). (C) The hybridization
signal intensity of Tβ4 mRNA was most marked in the PN5
odontoblasts. **P<0.01. Tβ4, thymosin β4; PN1,
postnatal day 1; PN3, postnatal day 3; PN5, postnatal day 5; PN15,
postnatal day 15; PN21, postnatal day 21; D, dentin; AS, antisense;
S, sense; DIG, digoxigenin. |
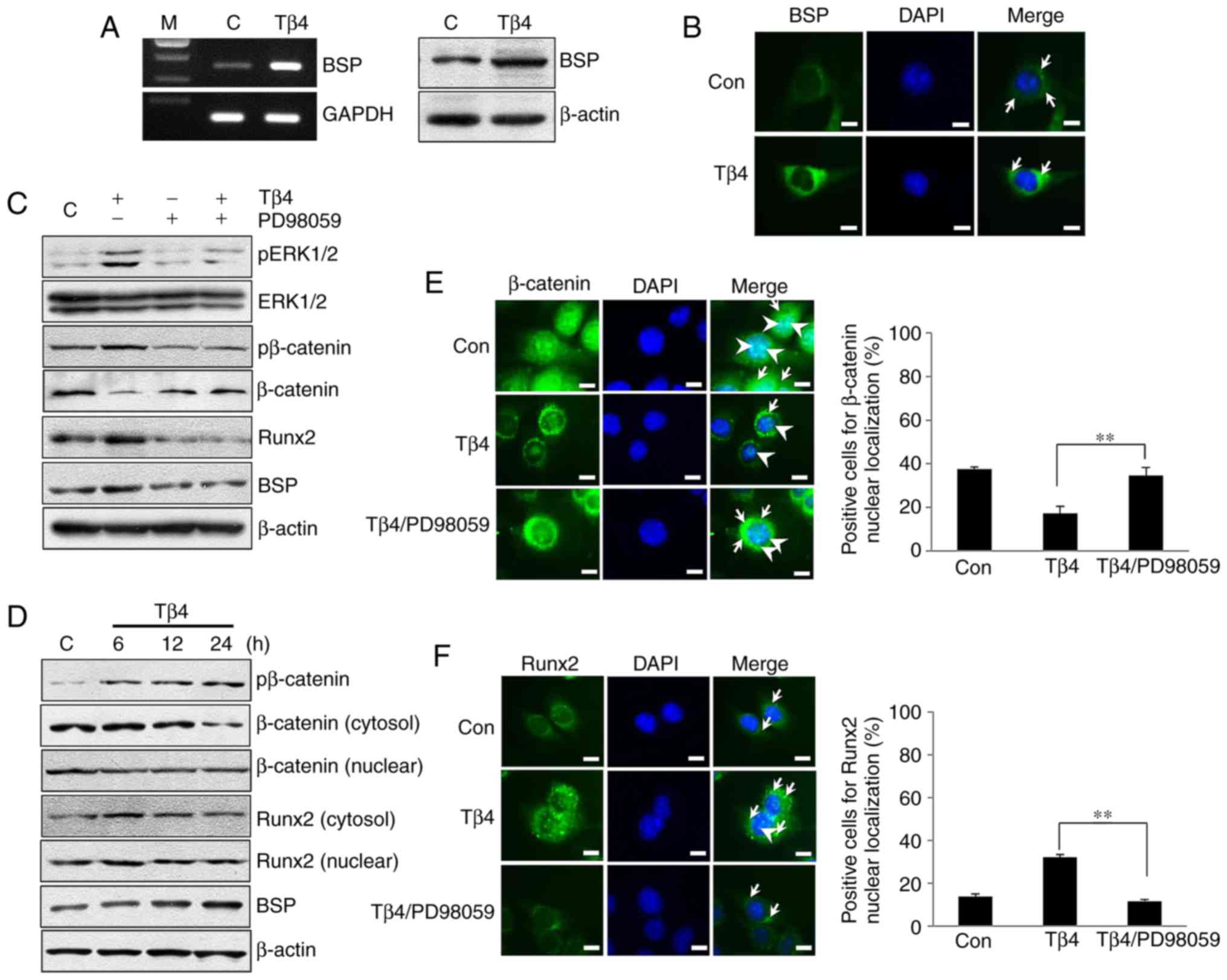
Tβ4 increases BSP expression by
activating ERK/Runx2 or ERK/β-catenin signaling in MDPC-23
cells
In MDPC-23 cells treated with Tβ4 for 24 h, the
levels of BSP mRNA and protein were increased compared with the
levels observed in untreated control cells (Fig. 2A). Furthermore, immunofluorescence
analysis indicated that Tβ4 treatment increased BSP protein levels
compared to the levels observed in untreated control cells
(Fig. 2B). To identify the effect
of Tβ4 on pERK1/2, pβ-catenin and β-catenin expression, MDPC-23
cells were treated with Tβ4 at different time intervals (5, 10, 15
and 30 min). The levels of pERK1/2 and pβ-catenin increased the
most, but β-catenin was decreased at 10 min in MDPC-23/Tβ4 cells
compared with that of the control (data not shown). pERK1/2 and
pβ-catenin levels were decreased in the PD98059/MDPC-23 and
Tβ4/PD98059/MDPC-23 groups compared with those in the Tβ4/MDPC-23
cells. β-catenin levels were increased in the PD98059/MDPC-23 group
compared with levels in the Tβ4/MDPC-23 cells. Expression levels of
Runx2 and BSP were increased in the Tβ4/MDPC-23 cells compared with
that in the untreated control cells, but levels of the 2 proteins
were decreased in the PD98059/MDPC-23 and Tβ4/PD98059/MDPC-23
groups compared with that in the Tβ4/MDPC-23 cells (Fig. 2C). Upon Tβ4 treatment for 6–24 h,
levels of pβ-catenin were increased but levels of cytosolic and
nuclear β-catenin protein were decreased in the Tβ4/MDPC-23 cells
compared with levels in the control cells. Cytosolic and nuclear
levels of Runx2 protein were increased in the Tβ4/MDPC-23 cells
after 6 h of treatment compared with that of the control cells. BSP
expression increased time-dependently in the Tβ4/MDPC-23 cells
compared with that in the control cells (Fig. 2D). Furthermore, levels of nuclear
β-catenin were increased in the Tβ4/PD98059/MDPC-23 cells compared
with levels in the Tβ4/MDPC-23 cells, and PD98059 inhibited the
Tβ4-induced nuclear localization of Runx2 (Fig. 2E and F).
Tβ4 increases BSP expression by
activating Smad3/Runx2 or Smad3/β-catenin signaling in MDPC-23
cells
pSmad2 levels were significantly increased in the
TGF-β1/MDPC-23 cells treated for 30 min compared with levels in the
control, but pSmad2 was not induced in the Tβ4/MDPC-23 cells.
pSmad3 levels were increased in the TGF-β1/MDPC-23 and Tβ4/MDPC-23
cells compared with levels in the control cells (Fig. 3A). Smad2 phosphorylation was not
induced in the Tβ4/MDPC-23 cells, but pSmad3 was significantly
increased in the Tβ4/MDPC-23 cells treated for 5 to 30 min compared
with that in the control (Fig.
3B). In the Tβ4/MDPC-23 cells, the levels of pSmad3 and
pβ-catenin were increased compared with the control cells; however,
this phosphorylation was decreased in the SIS3/MDPC-23 and
Tβ4/SIS3/MDPC-23 groups. β-catenin levels were decreased in the
Tβ4/MDPC-23 cells compared with that in the control, but β-catenin
levels were similar to the control levels in the SIS3/MDPC-23 and
Tβ4/SIS3/MDPC-23 groups. The expression levels of Runx2 and BSP
were increased in the Tβ4/MDPC-23 cells compared with expression in
the control, but decreased levels were observed in the SIS3/MDPC-23
and Tβ4/SIS3/MDPC-23 groups compared with levels observed in the
Tβ4/MDPC-23 cells (Fig. 3C). The
levels of cytoplasmic and nuclear pSmad3 were increased from 6–24 h
in the Tβ4/MDPC-23 cells compared with that in the control. The
expression of BSP was increased in the Tβ4/MDPC-23 cells compared
with the expression in the control cells (Fig. 3D). Furthermore, nuclear pSmad3
levels were decreased in the Tβ4/SIS3/MDPC-23 cells compared with
that in the Tβ4/MDPC-23 cells, and the nuclear levels of β-catenin
were increased in the Tβ4/SIS3/MDPC-23 group (Fig. 3E and F).
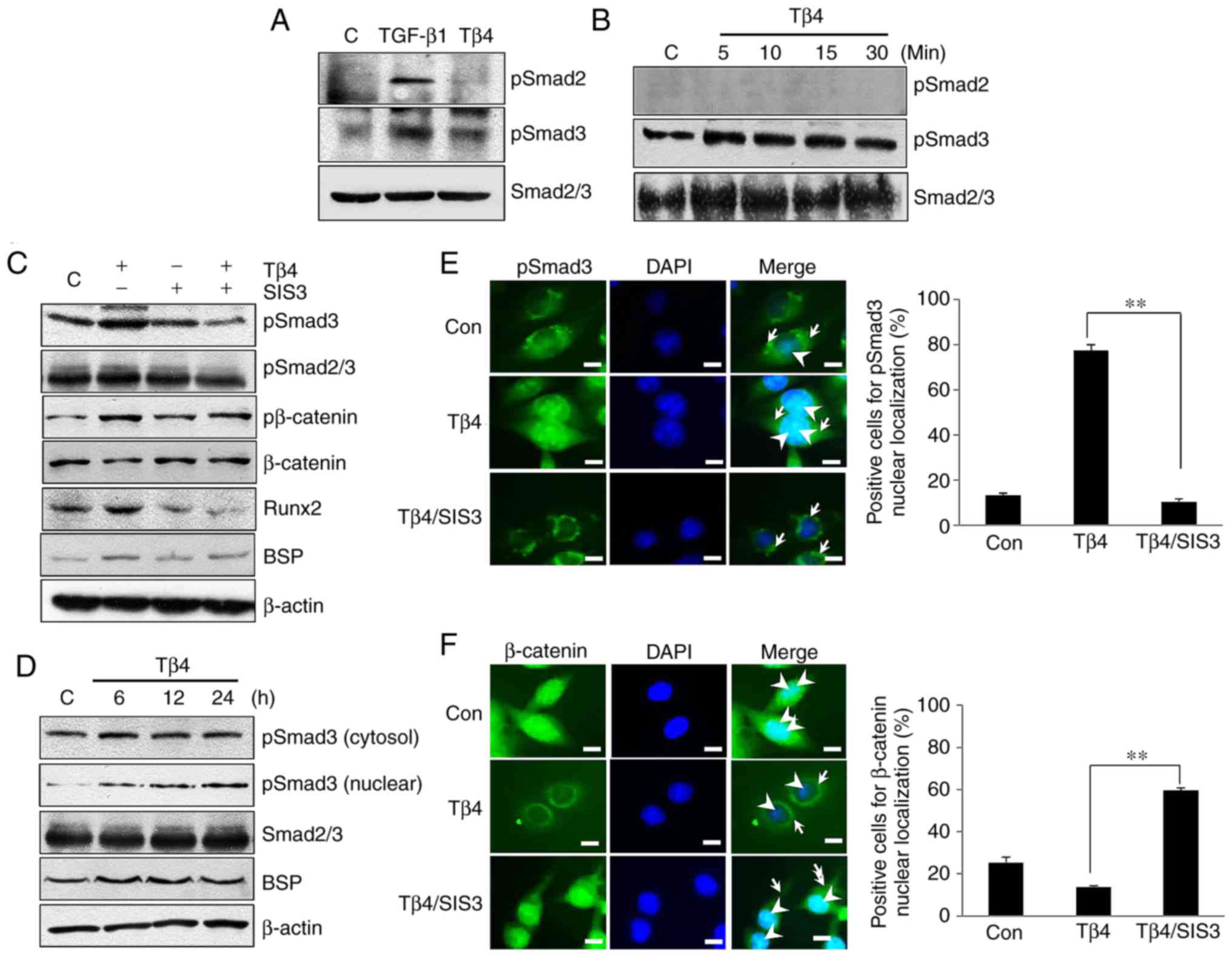 | Figure 3BSP expression through Smad3,
Smad3/Runx2 and Smad3/β-catenin signaling in Tβ4-treated MDPC-23
cells. (A) Levels of pSmad3, but not pSmad2, were increased in the
Tβ4/MDPC-23 cells compared with levels in the untreated control
cells. (B) Smad3 phosphorylation was increased by Tβ4 treatment at
all time points in MDPC-23 cells. (C) Levels of pSmad3 and
pβ-catenin were decreased in SIS3/MDPC-23 and Tβ4/SIS3/MDPC-23
cells compared with levels in the Tβ4/MDPC-23 cells. (D) Levels of
cytosolic and nuclear pSmad3 were increased in the Tβ4/MDPC-23
cells compared with levels in the untreated control cells. (E)
Immunofluorescence images indicating pSmad3 in the cytoplasm
(arrows) and nucleus (arrowheads). (F) Levels of β-catenin in the
cytoplasm (arrows) and nucleus (arrowheads) were decreased upon Tβ4
treatment but were increased in the SIS3/MDPC-23 cells compared
with that in the Tβ4/MDPC-23 cells. Scale bars=20 μm. The
histograms indicate the percentage of cells positive for pSmad3 and
β-catenin in the MDPC-23 group. **P<0.01. Tβ4,
Thymosin β4; BSP, bone sialoprotein; Con, control. Smad3, mothers
against decapentaplegic homolog 3; Smad2, mothers against
decapentaplegic homolog 2; p, phosphorylated; Runx2, runt-related
transcription factor 2. |
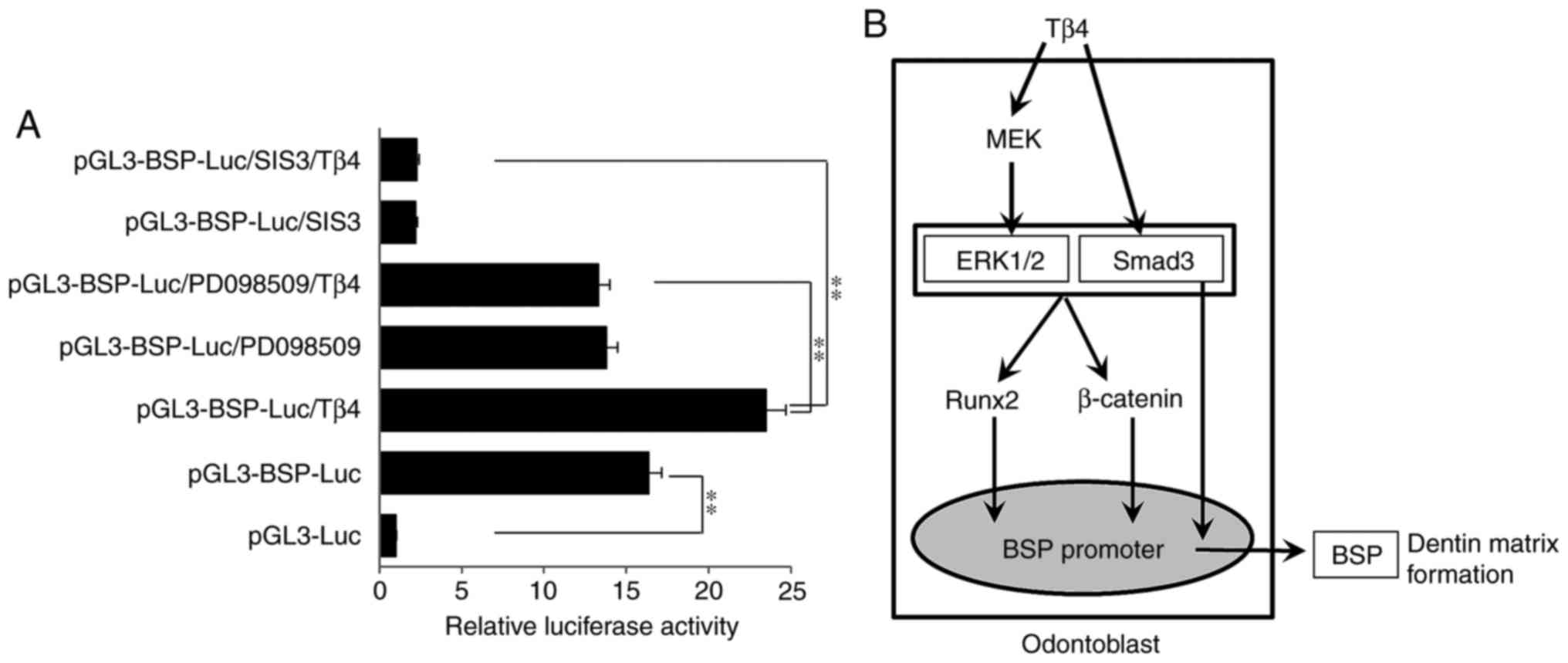
Tβ4 increases BSP promoter activity via
ERK and Smad3 signaling
Luciferase activity was significantly increased in
the pGL3-BSP-Luc cells compared with that in the pGL3-Luc cells,
and it was also increased in the pGL3-BSP-Luc/Tβ4 group compared
with that in the pGL3-BSP-Luc group (P<0.01). Compared with the
pGL-BSP-Luc/Tβ4 cells, the luciferase activity was significantly
decreased upon treatment with PD98059 or SIS3. Furthermore,
luciferase activity was decreased in the pGL-BSP-Luc/SIS3/Tβ4 cells
compared with in the pGL-BSP-Luc/PD98059/Tβ4 cells (Fig. 4A). Fig. 4B is a schematic diagram of the
experimental results demonstrating that Tβ4 induced BSP expression
via ERK or Smad3 signaling associated with Runx2 and β-catenin in
MDPC-23 cells.
Discussion
Odontoblast differentiation occurs actively at the
early bell stage, and secretory odontoblasts are present in the
cusp and cervical region of developing molars at advanced bell
stages (7,20,21). Secretory odontoblasts secrete
non-collagenous proteins including BSP, dentin sialoprotein,
osteocalcin (OCN) and osteonectin at the advanced bell stage
(22–24). Our previous study indicated that
Tβ4 protein levels are increased in odonto-blasts at the cusp and
cervical region of developing molars in the advanced bell stage,
compared with levels in early bell stages (7). Other studies suggested that Tβ4 is
associated with enamel development and tooth germ growth (6,25).
In the present study, Tβ4 mRNA was markedly expressed in
odontoblasts at the cusp region in the advanced bell stage
concordantly with the activation of dentin matrix synthesis during
dentinogenesis. Therefore, in accordance with previous studies, the
marked expression of Tβ4 mRNA in secretory odontoblasts indicated
that Tβ4 may regulate odontoblast differentiation or dentin
formation during tooth development. Our previous study demonstrated
that Tβ4 knockdown remarkably decreases mineralization and mRNA
expression of non-collagenous proteins including BSP during MDPC-23
cell differentiation (7). In bone
tissue, which is similar to dentin, transgenic BSP knockout mice
form undermineralized bones, and their cortical bones are thinner
compared with the cortical bones of normal mice (26). These studies suggest that Tβ4 may
regulate BSP expression during mineralized tissue development. In
the present study, Tβ4 treatment increased BSP mRNA and protein
levels in MDPC-23 cells compared with levels in untreated control
cells. This suggests that Tβ4 may regulate BSP expression in
odontoblasts through intracellular signal transduction during
dentinogenesis.
In a pulldown experiment to identify differential
interacting proteins, Tβ4 was demonstrated to increase
extracellular adenosine 5′-triphosphate (ATP) levels via ecto-ATP
synthase on the cell surface (27). Increased ATP was demonstrated to
activate P2X purinoreceptor 4 purinergic receptors to promote the
migration of endothelial cells. In a recent study, purinergic
receptor subtypes, including P2X4, were expressed in dental pulp
cells (DPCs) and ATP was revealed to promote the odon-toblastic
differentiation and mineralization of DPCs (28). Our previous studies demonstrated
autocrine and paracrine actions of Tβ4, similar to results of other
studies (27,29). Suppression of Tβ4 expression
significantly decreases the mRNA expression of
mineralization-associated factors, including BSP, and
mineralization in MDPC-23 cells (7). In addition, exogenous Tβ4 increases
osteoblast-like cell adhesion on Ti surfaces, but the suppression
of Tβ4 expression significantly inhibits this adhesion (3). Exogenous Tβ4 has also been indicated
to stimulate angiogenesis through increased endothelial cell
differentiation and migration, thereby allowing Tβ4 to function in
autocrine and paracrine manners (29). Therefore, based on these data from
our studies and additional previous studies, Tβ4 may regulate BSP
expression by autocrine or/and paracrine signaling and additional
studies are required for determining the receptor of Tβ4 in
odontoblasts during dentinogenesis.
Tβ4 is an actin-sequestering peptide that regulates
actin polymerization, and Tβ4 may also activate signaling pathways
involved with cell migration, adhesion and proliferation, including
ERK/β-catenin, Pax/FAK and FAK/Grb2/Ras/ERK in osteoblasts and
gastric cancer cells (3,15). Tβ4 is expressed in developing
mouse mandibles and is also involved in the initiation, formation
and differentiation of tooth germ during molar development
(4,5). In a previous study, Tβ4 promoted the
odontoblastic differentiation of dental pulp cells by activating
MAPKs [p38 MAPK, c-Jun N-terminal kinase (JNK) and ERK], and Smad
(Smad1/5/8 and Smad2/3) signaling pathways (30). ERK, p38 and JNK have critical
functions in signal transduction during embryo development, immune
response and neural canal development in vertebrates (31,32). In addition, MAPKs are the central
signal transducers that modulate osteogenesis and bone mass
(33,34). ERK1/2 signaling activates Runx2
and therefore OCN expression during osteoblast differentiation
(35). In the present study, it
was identified that Tβ4 increases pERK1/2 levels and Runx2
expression in MDPC-23 cells. Additionally, Tβ4 increased Runx2
nuclear translocation in MDPC-23 cells. In previous studies, Wnt3a
stimulation increased the nuclear translocation of β-catenin,
thereby decreasing the expression of Runx2, osterix, alkaline
phosphatase (ALP), BSP, and OCN in cementoblasts and dental
follicle cells (36,37). In the present study, Tβ4 reduced
cytoplasmic and nuclear β-catenin levels in MDPC-23 cells.
Considering all these results together, Tβ4 signal transduction
increases BSP expression through ERK/Runx2 or ERK/β-catenin in
MDPC-23 cells.
Smad proteins are additional important signal
transducers associated with tooth development. BMP-2 increases
Runx2 expression through the Smad1/5 pathway, which also increases
the expression of ALP and OCN through the β-catenin pathway in
dental papilla cells (38,39).
TGF-β1 inhibits the differentiation of mesenchymal stem cells into
osteoblasts by elevating β-catenin levels via Smad3, protein kinase
A (PKA) and phosphoinositide 3-kinase (PI3K) signaling, thereby
decreasing BSP expression (14).
Cytosolic Smad2 or Smad3 translocate into the nucleus by
interacting with Smad4, where they regulate the expression of genes
associated with bone matrix synthesis including Runx2, ALP, COL
type I and OCN in MC3T3-E1 cells (40,41). In a recent study, Tβ4 knockdown
decreased Runx2 expression via Smad1/5/8 and PI3K/protein kinase B
(Akt) signaling in dental epithelial cells (42). The results of the present study
indicated that Tβ4 increased Smad3 phosphorylation and expression
of Runx2, but did not increase the phosphorylation of Smad2 in
MDPC-23 cells. In addition, Tβ4 increased nuclear pSmad3 levels in
MDPC-23 cells. Tβ4 also decreased cytoplasmic and nuclear β-catenin
through Smad3 signaling, similarly to pERK1/2 signaling, in MDPC-23
cells. Therefore, the present results indicated that Tβ4 increased
BSP expression through Smad3/Runx2, Smad3/β-catenin and Smad3
signal transduction in MDPC-23 cells.
According to the results of the present study, Tβ4
induced BSP expression via ERK and Smad3 signaling in MDPC-23
cells. However, it is necessary to determine which pathway is more
important for Tβ4-mediated BSP expression in MDPC-23 cells. A
previous study suggested that protamine-induced BSP promoter
activation was significantly more suppressed by ERK1/2 inhibitor
treatment (U0126) compared with protein kinase C or PKA inhibitor
treatment (H7 or KT5720) in ROS 17/2.8 osteoblast-like cells
(13). SIS3 treatment inhibits
TGF-β1-induced β-catenin transcription and stabilization in
mesenchymal stem cells, but these phenomena are not affected by
PD98059 treatment (14).
Additionally, Tβ4 increases Runx2 expression via Smad1/5/8 and
PI3K/Akt signaling to a lesser degree compared with ERK/MAPK
signaling in dental epithelial cells (42). In the present study, Tβ4 increased
BSP promoter activity, which was decreased by PD98059 or SIS3
treatment in MDPC-23 cells. Notably, BSP promoter activity was
significantly more inhibited by SIS3 treatment compared with
PD98059 treatment. These results indicated that Tβ4 activated BSP
transcription via ERK and Smad3 signaling, and that Smad3 signaling
contributed to Tβ4-induced BSP transcription in MDPC-23 cells to a
greater extent compared with ERK signaling.
In conclusion, Tβ4 induced BSP in MDPC-23 cells
through ERK and Smad3 pathways, suggesting its role as a signaling
molecule for regulating BSP secretion in odontoblasts. These events
indicate that Tβ4 signaling may be associated with dentin matrix
formation in odontoblasts during dentinogenesis. Additional
examination of the receptor of Tβ4 in odontoblasts during
dentinogenesis is required.
Funding
The present study was supported by the National
Research Foundation of Korea funded by the Ministry of Science, ICT
& Future Planning (grant no. R13-2008-010-01001-0).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BDC, HJL and MJJ contributed to the design of the
study and performed the experiments. BDC, SYL, MHL, and KSK
analyzed the experimental data. BDC and MJJ drafted the manuscript.
DSL and SJJ were involved in revising manuscript critically for
important intellectual content. All the authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Animal studies were approved by the Institutional
Animal Care and Use Committees of Chosun University (Gwangju,
Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Low TL, Hu SK and Goldstein AL: Complete
amino acid sequence of bovine thymosin beta 4: A thymic hormone
that induces terminal deoxynucleotidyl transferase activity in
thymocyte populations. Proc Natl Acad Sci USA. 78:1162–1166. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldstein AL, Hannappel E and Kleinman HK:
Thymosin beta4: Actin-sequestering protein moonlights to repair
injured tissues. Trends Mol Med. 11:421–429. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi BD, Jeong SJ, Lee HY, Lim DS, Lee BH,
Bae CS and Jeong MJ: The effect of thymosin β4 for osteoblast
adhesion on titanium surface. J Nanosci Nanotechnol. 15:5663–5667.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamaza H, Matsuo K, Kiyoshima T, Shigemura
N, Kobayashi I, Wada H, Akamime A and Sakai H: Detection of
differentially expressed genes in the early developmental stage of
the mouse mandible. Int J Dev Biol. 45:675–680. 2001.PubMed/NCBI
|
|
5
|
Akhter M, Kobayashi I, Kiyoshima T, Matsuo
K, Yamaza H, Wada H, Honda JY and Sakai H: Possible functional
involvement of thymosin beta 4 in developing tooth germ of mouse
lower first molar. Histochem Cell Biol. 124:207–213. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cha HJ, Philp D, Lee SH, Moon HS, Kleinman
HK and Nakamura T: Over-expression of thymosin beta 4 promotes
abnormal tooth development and stimulation of hair growth. Int J
Dev Biol. 54:135–140. 2010. View Article : Google Scholar
|
|
7
|
Choi BD, Yun SH, Jeong SJ, Wang G, Kim HJ,
Lim DS and Jeong MJ: Expression of thymosin β4 in odontoblasts
during mouse tooth development. Int J Mol Med. 29:841–847. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matalová E, Lungová V and Sharpe P:
Development of tooth and associated structure. Stem cell biology
and tissue engineering in dental science. ScienceDirect: Elsevier
Inc; San Diego: pp. 335–346. 2015, View Article : Google Scholar
|
|
9
|
Macneil RL, Sheng N, Strayhorn C, Fisher
LW and Somerman MJ: Bone sialoprotein is localized to the root
surface during cemen-togenesis. J Bone Miner Res. 9:1597–1606.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oldberg A, Franzén A and Heinegård D: The
primary structure of a cell-binding bone sialoprotein. J Biol Chem.
263:19430–19432. 1988.PubMed/NCBI
|
|
11
|
Stubbs JT III, Mintz KP, Eanes ED, Torchia
DA and Fisher LW: Characterization of native and recombinant bone
sialoprotein: Delineation of the mineral-binding and cell adhesion
domains and structural analysis of the RGD domain. J Bone Miner
Res. 12:1210–1222. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hunter GK and Goldberg HA: Modulation of
crystal formation by bone phosphoproteins: Role of glutamic
acid-rich sequences in the nucleation of hydroxyapatite by bone
sialoprotein. Biochem J. 302:175–179. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou L, Matsumura H, Mezawa M, Takai H,
Nakayama Y, Mitarai M and Ogata Y: Protamine stimulates bone
sialoprotein gene expression. Gene. 516:228–237. 2013. View Article : Google Scholar
|
|
14
|
Zhou SH: TGF-β regulates β-catenin
signaling and osteoblast differentiation in human mesenchymal stem
cells. J Cell Biochem. 112:1651–1660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ryu YK, Lee YS, Lee GH, Song KS, Kim YS
and Moon EY: Regulation of glycogen synthase kinase-3 by thymosin
beta-4 is associated with gastric cancer cell migration. Int J
Cancer. 131:2067–2077. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeong SJ and Jeong MJ: Effect of thymosin
beta4 on the differentiation and mineralization of MC3T3-E1 cell on
a titanium surface. J Nanosci Nanotechnol. 16:1979–1983. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
No authors listed. Environment, housing,
and management. Guide for the Care and Use of Laboratory Animals.
8th edition. National Research Council of the National Academies:
National Academies Press; Washington, DC: pp. 41–88. 2011
|
|
18
|
Oh JM, Ryoo IJ, Yang Y, Kim HS, Yang KH
and Moon EY: Hypoxia-inducible transcription factor (HIF)-1 alpha
stabilization by actin-sequestering protein, thymosin beta-4 (TB4)
in Hela cervical tumor cells. Cancer Lett. 264:29–35. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang XH, Xie YT, Cai YP, Ren J and Ma T:
Effects of hepatitis C virus core protein and nonstructural protein
4B on the Wnt/β-catenin pathway. BMC Microbiol. 17:1242017.
View Article : Google Scholar
|
|
20
|
Lisi S, Peterkova R, Peterka M, Vonesch
JL, Ruch JV and Lesot H: Tooth morphogenesis and pattern of
odontoblast differentiation. Connect Tissue Res. 44(Suppl 1):
S167–S170. 2003. View Article : Google Scholar
|
|
21
|
Zhang J, Zhang ZG, Morris D, Li Y, Roberts
C, Elias SB and Chopp M: Neurological functional recovery after
thymosin beta4 treatment in mice with experimental auto
encephalomyelitis. Neuroscience. 164:1887–1893. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujisawa R and Kuboki Y: Changes in levels
of osteonectin in bovine dentine during tooth development. Arch
Oral Biol. 34:89–92. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D'Souza RN, Bronckers AL, Happonen RP,
Doga DA, Farach-Carson MC and Butler WT: Developmental expression
of a 53 KD dentin sialoprotein in rat tooth organs. J Histochem
Cytochem. 40:359–366. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bronckers AL, Engelse MA, Cavender A,
Gaikwad J and D'Souza RN: Cell-specific patterns of Cbfa1 mRNA and
protein expression in postnatal murine dental tissues. Mech Dev.
101:255–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ookuma YF, Kiyoshima T, Kobayashi I,
Nagata K, Wada H, Fujiwara H, Yamaza H, Nonaka K and Sakai H:
Multiple functional involvement of thymosin beta-4 in tooth germ
development. Histochem Cell Biol. 139:355–370. 2013. View Article : Google Scholar
|
|
26
|
Malaval L, Wade-Gueye NM, Boudiffa M, Fei
J, Zirngibl R, Chen F, Laroche N, Roux JP, Burt-Pichat B, Duboeuf
F, et al: Bone sialoprotein plays a functional role in bone
formation and osteoclastogenesis. J Exp Med. 205:1145–1153. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Freeman KW, Bowman BR and Zetter BR:
Regenerative protein thymosin beta-4 is a novel regulator of
purinergic signaling. FASEB J. 25:907–915. 2011. View Article : Google Scholar
|
|
28
|
Wang W, Yi X, Ren Y and Xie Q: Effects of
adenosine triphos-phate on proliferation and odontoblastic
differentiation of human dental pulp cells. J Endod. 42:1483–1489.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grant DS, Rose W, Yaen C, Goldstein A,
Martinez J and Kleinman H: Thymosin beta4 enhances endothelial cell
differentiation and angiogenesis. Angiogenesis. 3:125–135. 1999.
View Article : Google Scholar
|
|
30
|
Lee SI, Kim DS, Lee HJ, Cha HJ and Kim EC:
The role of thymosin beta 4 on odontogenic differentiation in human
dental pulp cells. PLoS One. 8:e619602013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuan CY, Yang DD, Samanta Roy DR, Davis
RJ, Rakic P and Flavell RA: The Jnk1 and Jnk2 protein kinases are
required for regional specific apoptosis during early brain
development. Neuron. 22:667–676. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong C, Davis RJ and Flavell RA: MAP
kinases in the immune response. Annu Rev Immunol. 20:55–72. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ge C, Xiao G, Jiang D and Franceschi RT:
Critical role of the extracellular signal-regulated kinase-MAPK
pathway in osteo-blast differentiation and skeletal development. J
Cell Biol. 176:709–718. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Greenblatt MB, Shim JH, Zou W, Sitara D,
Schweitzer M, Hu D, Lotinun S, Sano Y, Baron R, Park JM, et al: The
p38 MAPK pathway is essential for skeletogenesis and bone
homeostasis in mice. J Clin Invest. 120:2457–2473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiao G, Gopalakrishnan R, Jiang D, Reith
E, Benson MD and Franceschi RT: Bone morphogenetic proteins,
extracellular matrix, and mitogen-activated protein kinase
signaling pathways are required for osteoblast-specific gene
expression and differentiation in MC3T3-E1 cells. J Bone Miner Res.
17:101–110. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nemoto E, Koshikawa Y, Kanaya S, Tsuchiya
M, Tamura M, Somerman MJ and Shimauchi H: Wnt signaling inhibits
cement-oblast differentiation and promotes proliferation. Bone.
44:805–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Silvério KG, Davidson KC, James RG, Adams
AM, Foster BL, Nociti FH Jr, Somerman MJ and Moon RT: Wnt/β-catenin
pathway regulates bone morphogenetic protein (BMP2)-mediated
differentiation of dental follicle cells. J Periodontal Res.
47:309–319. 2012. View Article : Google Scholar
|
|
38
|
Cho YD, Yoon WJ, Woo KM, Baek JH, Park JC
and Ryoo HM: The canonical BMP signaling pathway plays a crucial
part in stimulation of dentin sialophosphoprotein expression by
BMP-2. J Biol Chem. 285:36369–36376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Koizumi Y, Kawashima N, Yamamoto M,
Takimoto K, Zhou M, Suzuki N, Saito M, Harada H and Suda H: Wnt11
expression in rat dental pulp and promotional effects of Wnt
signaling on odontoblast differentiation. Congenit Anom (Kyoto).
53:101–108. 2013. View Article : Google Scholar
|
|
40
|
Massagué J and Wotton D: Transcriptional
control by the TGF-beta/Smad signaling system. EMBO J.
19:1745–1754. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kaji H, Naito J, Sowa H, Sugimoto T and
Chihara K: Smad3 differently affects osteoblast differentiation
depending upon its differentiation stage. Horm Metab Res.
38:740–745. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Someya H, Fujiwara H, Nagata K, Wada H,
Hasegawa K, Mikami Y, Jinno A, Sakai H, Koyano K and Kiyoshimai T:
Thymosin beta 4 is associated with RUNX2 expression through the
Smad and Akt signaling pathways in mouse dental epithelial cells.
Int J Mol Med. 35:1169–1178. 2015. View Article : Google Scholar : PubMed/NCBI
|