Introduction
Peripheral neuropathic pain is produced by multiple
etiological factors, and spared nerve injury (SNI) is an important
model for exploring the cellular and molecular mechanism in
peripheral neuropathic pain (1,2).
It has been demonstrated that the agonists of α2-adrenoceptors
(α2-AR) have anti-nociceptive effects (3,4).
Tizanidine, a derivative of clonidine, is a highly selective
agonist of α2-AR, and has the same effects of clonidine, but the
side effects such as low arterial blood pressure and bradycardia
are lower (5,6). The primary site of tizanidine effect
is the spinal cord (7). Through
stimulating the 2α presynaptic receptors, tizanidine could prevent
the release of aspartic and glutamic acids (8). As other α2-AR agonists, tizanidine
has also been demonstrated to exhibit analgesic effects (9). For instance, tizanidine could reduce
postoperative pain and the need for tranquilizers through
inhibition of central sensitization (10). However, whether tizanidine exerts
anti-nociceptive effects in spared nerve injury model of
neuropathic pain has not been previously reported.
Toll-like receptor 4 (TLR4), belongs to TLR family,
and is an important transmembrane protein acting as a signal
transduction molecule in inflammatory responses (11,12). Stimulation of TLR4 can further
activate the nuclear factor-κB (NF-κB) signaling (13,14). NF-κB, a key transcriptional
factor, is universally expressed in various cells, responsible for
regulating the transcription of inflammatory cytokines, including
tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6,
and thus acts as a key regulator of inflammatory responses
(15,16). Under normal conditions, inhibitory
NF-κB inhibitors (IκBs) bind NF-κB in the cytoplasm (17-19). The activation of NF-κB begins with
the phosphorylation of IκBα, which further causes IκBα
ubiquitinated and degraded eventually (17-19). Once IκBα is degraded, NF-κB
translocates to nucleus and promotes the transcription of its
target genes, such as IL-1β, IL-6 and TNF-α (17-19). However, the effects of tizanidine
on the TLR4/NF-κB-mediated inflammation have not bee previously
reported.
This study investigated whether intrathecal
administration of tizanidine exhibits anti-nociceptive effects in
SNI model of rats, and we also studied the underlying molecular
mechanism, involving the activity of TLR4/NF-κB signaling pathway
and the production of inflammatory cytokines.
Materials and methods
Animals
This study was approved by Animal Care and Use
Committee of People's Hospital of Hunan Province (Changsha, China).
Animal experiments were consistent with the National Institutes of
Health Guide for the Care and Use of Laboratory Animals. Adult male
Sprague Dawley rats (200-250 g) were purchased from Animal Center
of Central South University (Changsha, China) and housed in
light-controlled (12 h dark/12 h light cycle) and
temperature-controlled (22±2˚C) room with free access to clear
water and food.
Surgical procedure of SNI model
Under enflurane (3.0 v%) anesthesia, an incision was
made in the skin on the lateral surface of the thigh of rats. The
biceps femoris muscle was then cut across, exposing the sciatic
nerve, as well as the sural, common peroneal and tibial nerves. The
common peroneal and tibial nerves were tightly ligated with 5.0
silk and sectioned distal to the ligation, removing 2-4 mm of the
distal nerve stump. Muscle and skin were enclosed in two layers,
avoiding any contact with or stretching of the intact sural
nerve.
Behavioral assessments were conducted at the
following time-points: postoperative week 0 (POW0) (designated as
the pretest baseline data), POW1, POW2 and POW3. All rats were
sacrificed periodically at POW3. The L4-5 segments of spinal cord
were dissected and split into left and right halves from the
ventral midline, which were then cut into the dorsal and ventral
horn at the level of the central canal. The dorsal horn of rats was
used to conduct the following analysis.
Grouping
The rats were randomly divided into 7 groups (n=6
for each group): sham group, SNI group, saline group (intrathecal
injection with 20 µl of sterile saline for 3 consecutive
days post-SNI), PDTC group (intrathecal injection with 2 nmol/20
µl of PDTC for 3 consecutive days post-SNI), tizanidine
group (intrathecal injection with 2.5 µg/20 µl of
tizanidine for 3 consecutive days post-SNI), saline + tizanidine
group (pretreatment with 20 µl of sterile saline at 30 min
before intrathecal injection with 2.5 µg/20 µl of
tizanidine for 3 consecutive days post-SNI), and BRL44408 +
tizanidine group (pretreatment with 15 µg/20 µl of
BRL44408 at 30 min before intrathecal injection with 2.5
µg/20 µl of tizanidine for 3 consecutive days
post-SNI).
Drug administration
Tizanidine (2.5 µg/20 µl), PDTC (2
nmol/20 µl) and BRL44408 (15 µg/20 µl; Sigma,
St. Louis, MO, USA) were diluted in normal saline (NS, 0.9% NaCl)
and loaded into 12.7 mm 30 gauge needle. Under inhalation
anaesthesia using isoflurane (2% in oxygen), the rats were injected
with drug at the L5-6 interspace.
Mechanical allodynia test
Mechanical allodynia was assessed by measuring the
paw withdrawal mechanical threshold (PWMT) in response to a
calibrated series of Von Frey hairs (Stoelting, Wood Dale, IL,
USA). Each filament was applied five times, and each application
lasted for 2 sec with 30 sec interval between trials. A positive
response to a filament was indicated by the withdrawal of a hind
paw upon application of a particular hair for at least 3/5
consecutive applications. The PWMT was defined as the smallest
value of the hair force in grams that elicited positive
responses.
Thermal hyperalgesia test
Thermal hyperalgesia was studied by measuring the
paw withdrawal thermal latency (PWTL) in response to a radiant heat
source. Rats in each group were put on an elevated glass platform,
and a radiant heat source (Model 336; IITC Life Science, Woodland
Hill, CA, USA) was applied to the plantar surface of the hind paw
through the glass plate. We measured the time from onset of radiant
heat application to withdrawal of the rat's hind paw, and both hind
paws were tested independently with 10 min interval between
trials.
Western blot analysis
The dorsal horns were lysed with ice-cold lysis
buffer. Proteins in the supernatants were quantified by using a BCA
kit (Thermo Fisher Scientific, Rockford, IL, USA) and separated
with 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE). Proteins were transferred onto a polyvinylidene
difluoride membrane (Thermo Fisher Scientific), which was then
incubated with phosphate-buffered saline (PBS) containing 5% milk
overnight at 4°C. The membrane was incubated with primary
antibodies at room temperature for 3 h, respectively, and then with
secondary antibody (both from Abcam, Cambridge, MA, USA) at room
temperature for 40 min. Super Signal West Pico Chemiluminescent
Substrate kit (Thermo Fisher Scientific) was used to detect
signals, according to the manufacturer's instructions. The relative
protein expression was analyzed by Image-Pro Plus software 6.0,
represented as the density ratio versus glyceraldehyde 3-phosphate
dehydrogenase (GAPDH).
Enzyme-linked immunosorbent assay
(ELISA)
ELISA was conducted to examine the production of
inflammatory cytokines in dorsal horns of rats in each group. Rat
IL-1β ELISA kit, IL-6 ELISA kit, and TNF-α ELISA kit (all from
Biorbyt, Shanghai, China) were used to measure the levels of IL-1β,
IL-6 and TNF-α, according to the manufacturer's instructions. The
optical density at 450 nm was detected by using Synergy™ Mx
microplate absorbance reader (BioTek, Winooski, VT, USA).
Statistical analysis
Data were expressed as mean ± standard deviation
from three separate experiments. SPSS 19 was used to perform
statistical analysis. Student's t-test was conducted when comparing
two groups, and one-way ANOVA was conducted when comparing more
than two groups. P<0.05 were considered statistically
significant.
Results
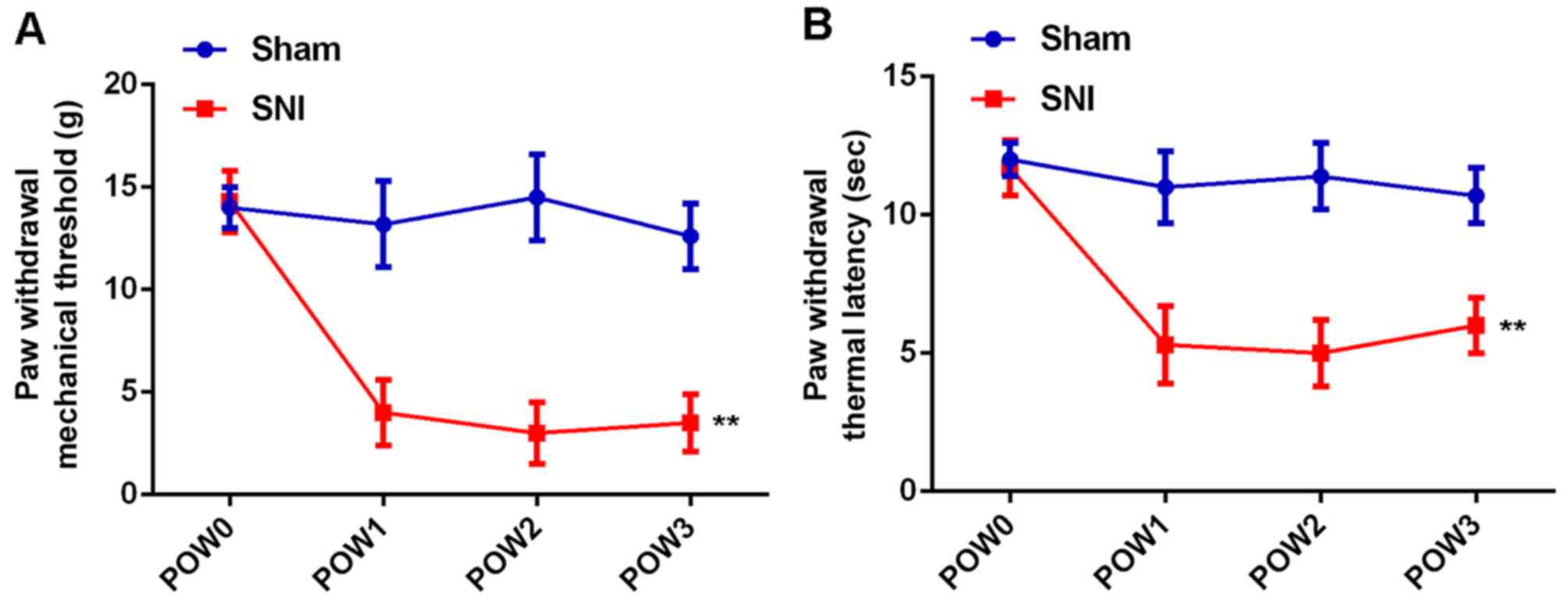
SNI induces mechanical and thermal
hyperalgesia and inflammatory responses in rats
In the study, we first conducted mechanical
allodynia test and thermal hyperalgesia test to determine the PWMT
and PWTL in SNI group, and the sham group was used as control. As
shown in Fig. 1, PWMT and PWTL
showed no difference at different time-points in the sham group.
However, the PWMT and PWTL were markedly reduced in SNI group,
which lasted for 3 weeks after surgery, indicating that rats in SNI
group showed significant mechanical and thermal hyperalgesia.
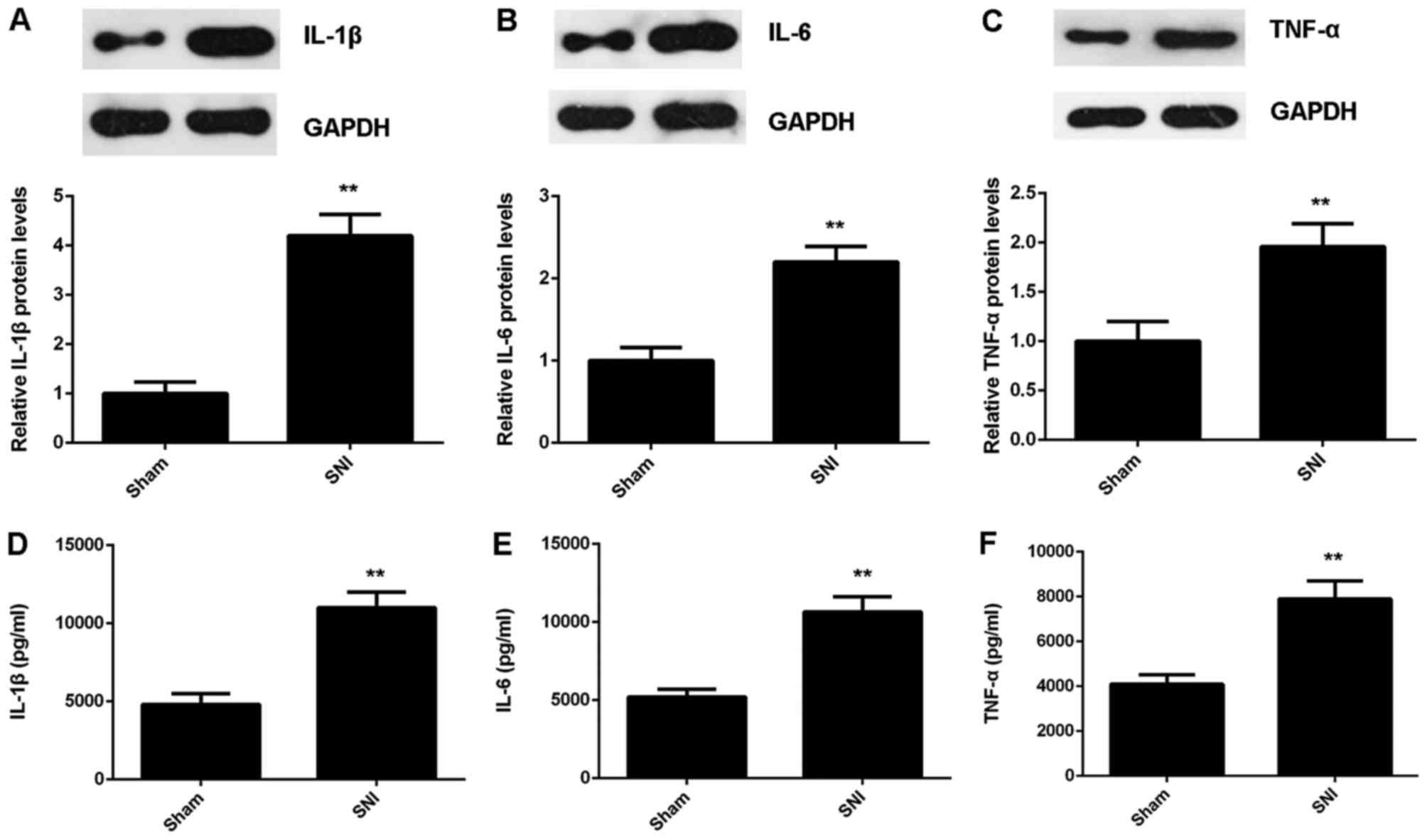
To further explore the underlying mechanism, we
examined the protein expression and secretion levels of
proinflammatory cytokines in the dorsal horns using western blot
analysis and ELISA, respectively. As shown in Fig. 2A-C, the protein levels of IL-1β,
IL-6 and TNF-α were significantly higher in the dorsal horns of
rats in the SNI group, when compared with those in the sham group.
Consistently, ELISA data showed that the production of these three
proinflammatory cytokines were also upregulated in the dorsal horns
of rats the SNI group (Fig.
2D-F).
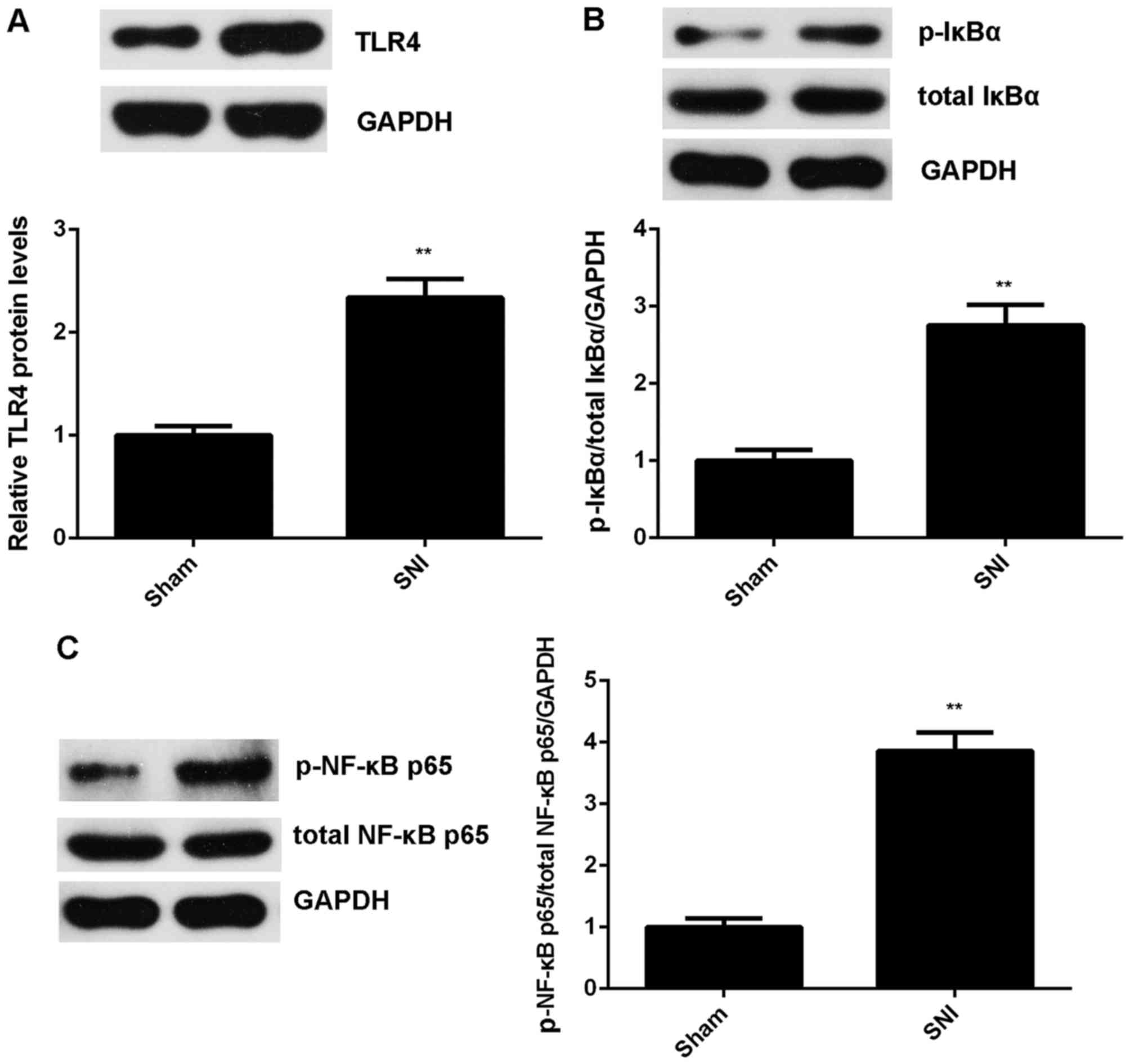
As TLR4/NF-κB signaling is a key regulator for
inflammatory responses (16), we
further examined the protein expression of TLR4 as well as the
activity of NF-κB. Western blot analysis showed that the protein
levels of TLR4 were significantly upregulated in the SNI group
compared with the sham group (Fig.
3A). In addition, the phosphorylation levels of IκBα and NF-κB
p65 proteins were also increased (Fig. 3B and C). These findings indicate
that the TLR4/NF-κB signaling was activated in the SNI group.
Accordingly, SNI induces mechanical and thermal hyperalgesia and
inflammatory responses in rats, probably through activation of
TLR4/NF-κB signaling.
Inhibition of TLR4/NF-κB signaling
attenuates SNI-induced mechanical and thermal hyperalgesia and
proinflammatory cytokine production in rats
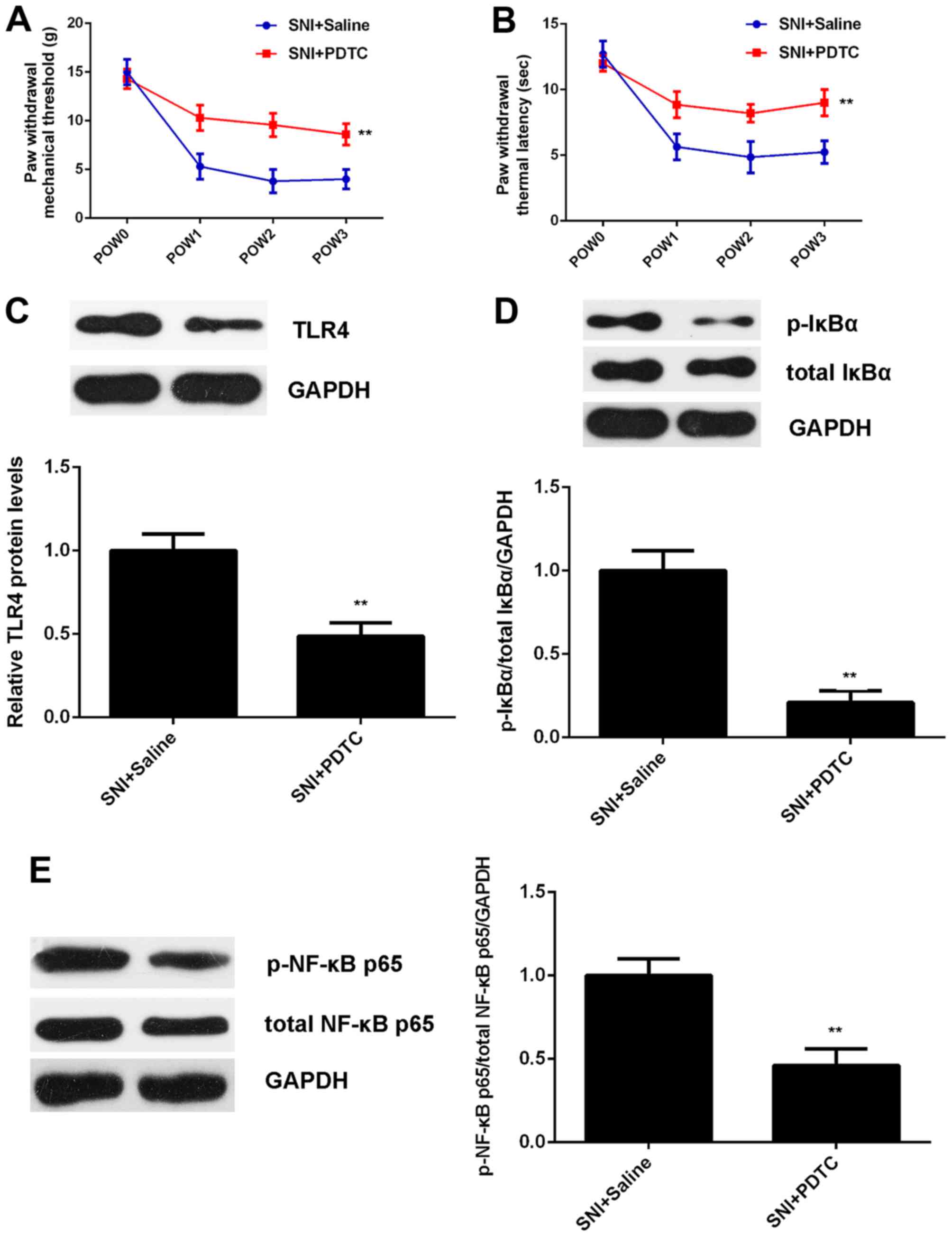
To further clarify whether TLR4/NF-κB signaling is
essential for the SNI-induced mechanical and thermal hyperalgesia,
PDTC, an inhibitor of TLR4/NF-κB signaling, was intrathecally
injected into rats for 3 consecutive days after SNI. Intrathecal
injection with the same amount of sterile saline was used as
control. The mechanical allodynia test and thermal hyperalgesia
test were performed at different time-points to determine the PWMT
and PWTL. As shown in Fig. 4A and
B, the PWMT and PWTL were significantly increased in the SNI +
PDTC group compared with the SNI + saline group, indicating that
treatment with PDTC attenuates SNI-induced mechanical and thermal
hyperalgesia.
To further confirm these findings, we examined the
activity of TLR4/NF-κB signaling. As shown in Fig. 4C-E, the protein levels of TLR4 as
well as the phosphorylation levels of IκBα and NF-κB p65 proteins
were remarkably reduced in the SNI + PDTC group compared with the
SNI + saline group, indicating that the treatment with PDTC indeed
inhibits the activation of TLR4/NF-κB signaling in SNI model of
rats.
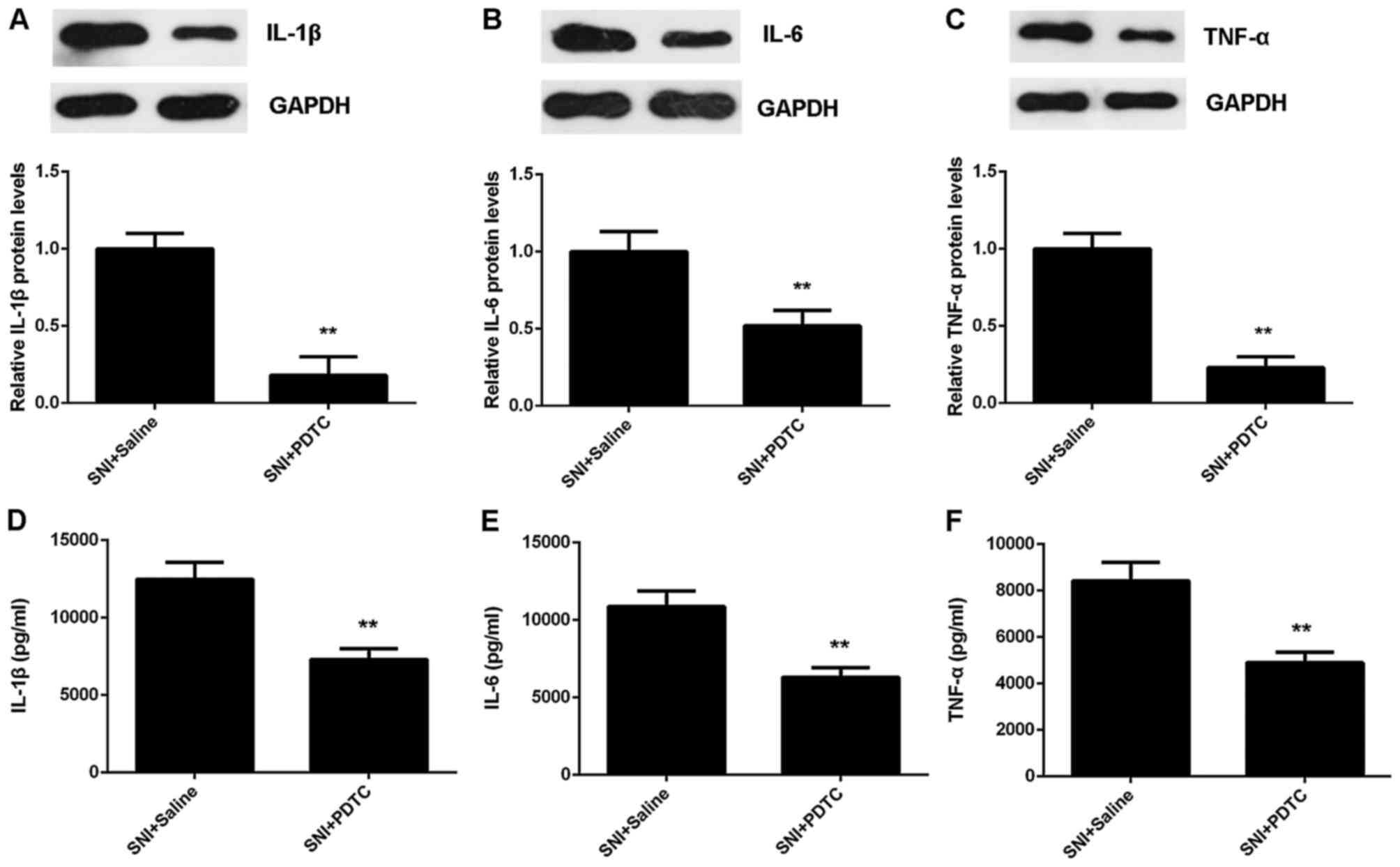
In addition, we found that the protein expression
and secretion of IL-1β, IL-6 and TNF-α were also down-regulated
after PDTC treatment, when compared with the SNI + saline group
(Fig. 5). Therefore, our data
suggest that the TLR4/NF-κB signaling is essential for the
SNI-induced mechanical and thermal hyperalgesia.
Treatment with tizanidine inhibits
SNI-induced mechanical and thermal hyperalgesia in rats
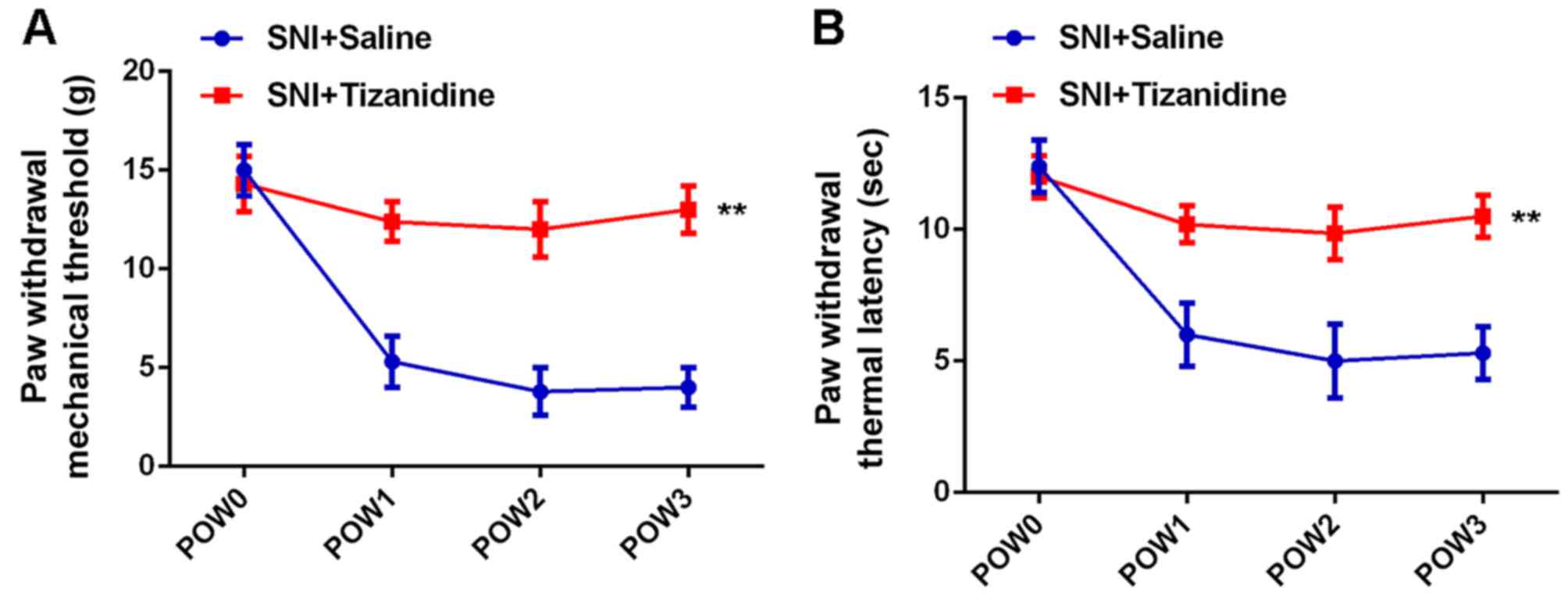
We studied the effects of tizanidine on SNI-induced
hyperalgesia in rats. tizanidine was intrathecally injected into
rats for 3 consecutive days after SNI. Intrathecal injection with
sterile saline was used as control. Results of mechanical allodynia
test and thermal hyperalgesia test showed that the PWMT and PWTL
were also increased in the SNI + tizanidine group compared with the
SNI + saline group, indicating that tizanidine could attenuate
SNI-induced mechanical and thermal hyperalgesia (Fig. 6).
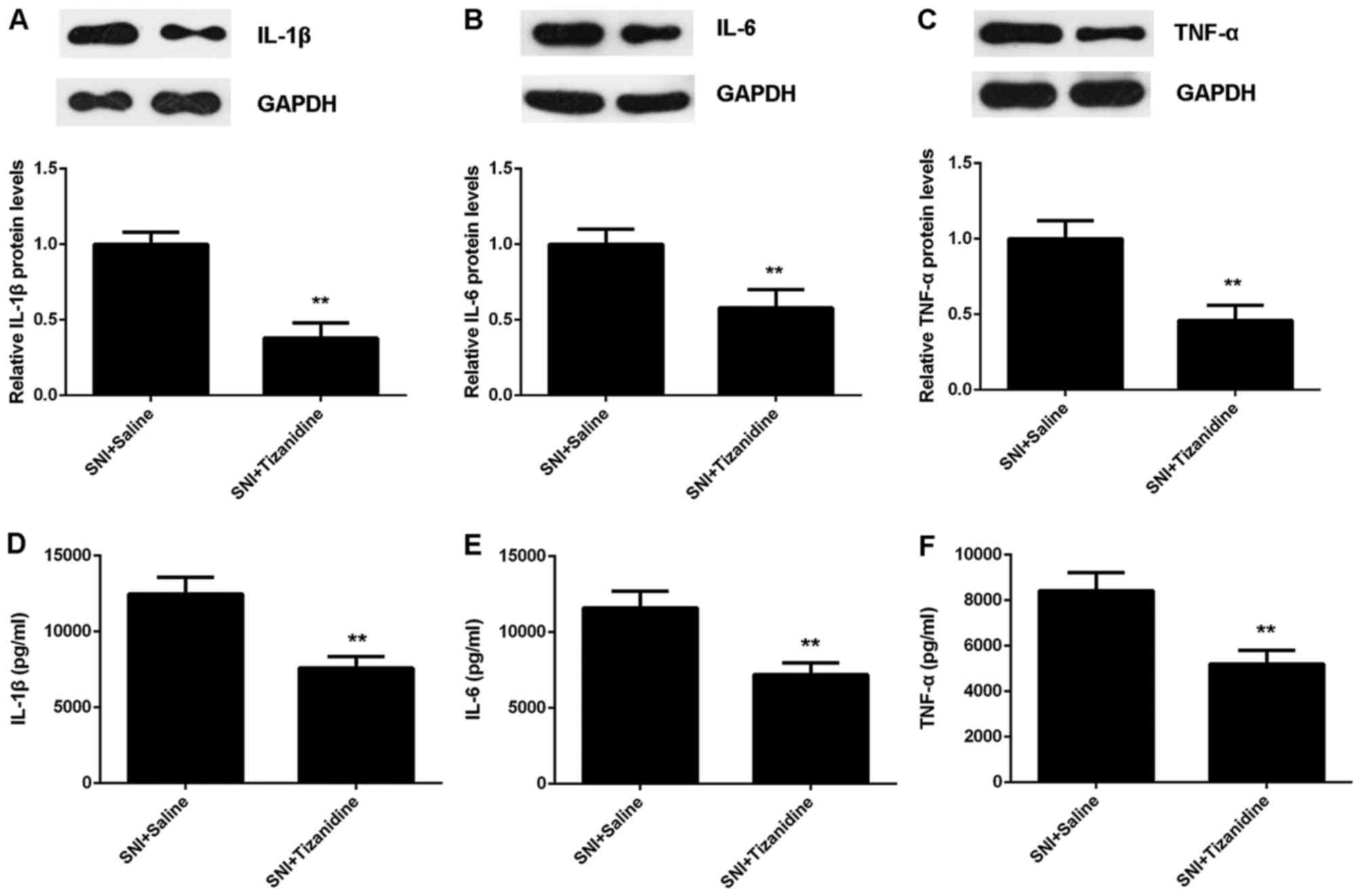
Moreover, western blot analysis and ELISA data
indicated that the protein expression and secretion of inflammatory
cytokines were also suppressed after tizanidine treatment in SNI
rats (Fig. 7). In addition, the
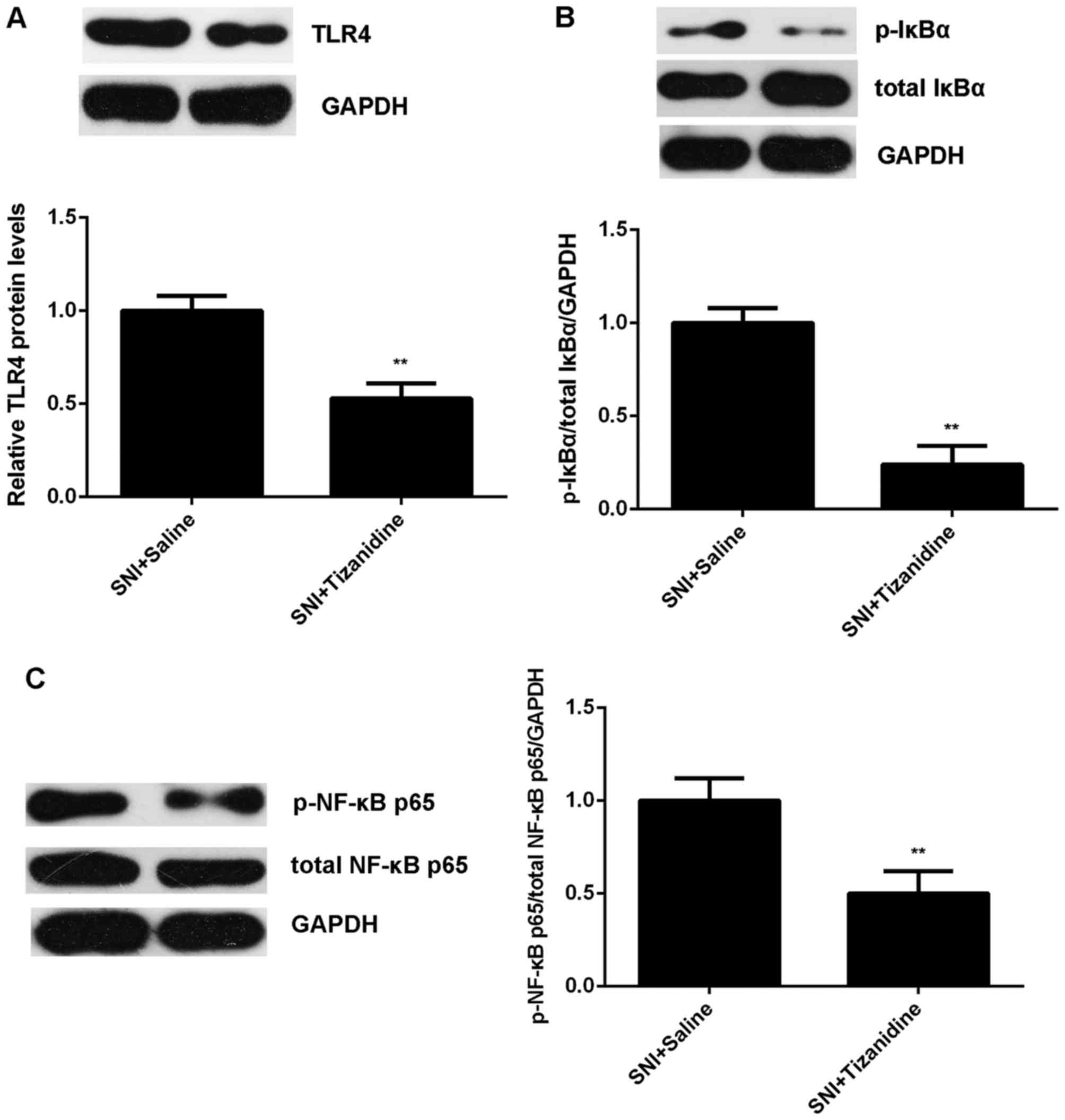
activity of TLR4/NF-κB signaling was also downregulated in the
tizanidine group compared with the saline group (Fig. 8). Taken together, we demonstrated
that treatment with tizanidine inhibits SNI-induced mechanical and
thermal hyperalgesia in rats through inhibition of TLR4/NF-κB
signaling-mediated inflammatory cytokines production in spinal
cord.
Pretreatment with α2-AR antagonist
reverses the inhibitory effects of tizanidine on SNI-induced
mechanical and thermal hyperalgesia in rats
As tizanidine is a highly selective α2-AR agonist
(5), we then performed
experiments to clarify whether the inhibitory effect of tizanidine
on SNI-induced hyperalgesia was through activation of α2-AR.
Pre-intrathecal injection with BRL44408, an α2-AR antagonist, was
performed at 30 min before injection with tizanidine for 3
consecutive days after SNI. We then examined the PWMT and PWTL at
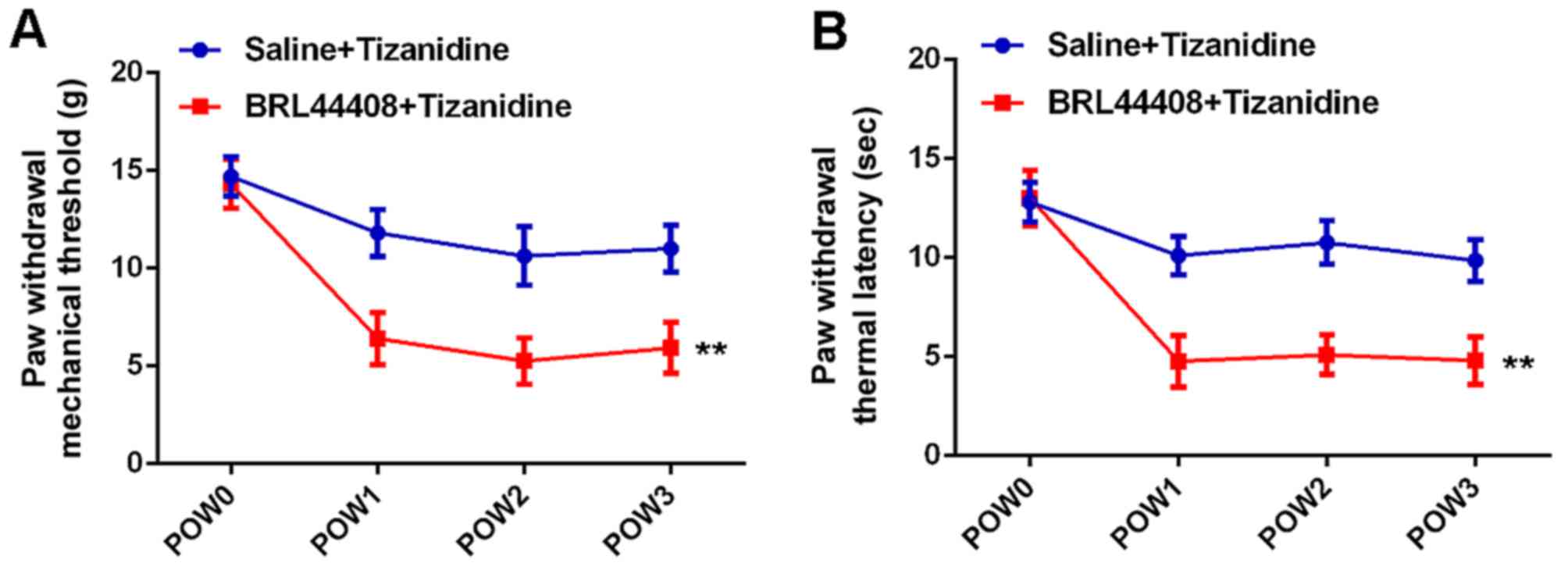
different time-points. As shown in Fig. 9, the PWMT and PWTL were
significantly reduced in the BRL44408 + tizanidine group compared
with the saline + tizanidine group, indicating that pretreatment
with BRL44408 reverses the inhibitory effects of tizanidine on
SNI-induced mechanical and thermal hyperalgesia.
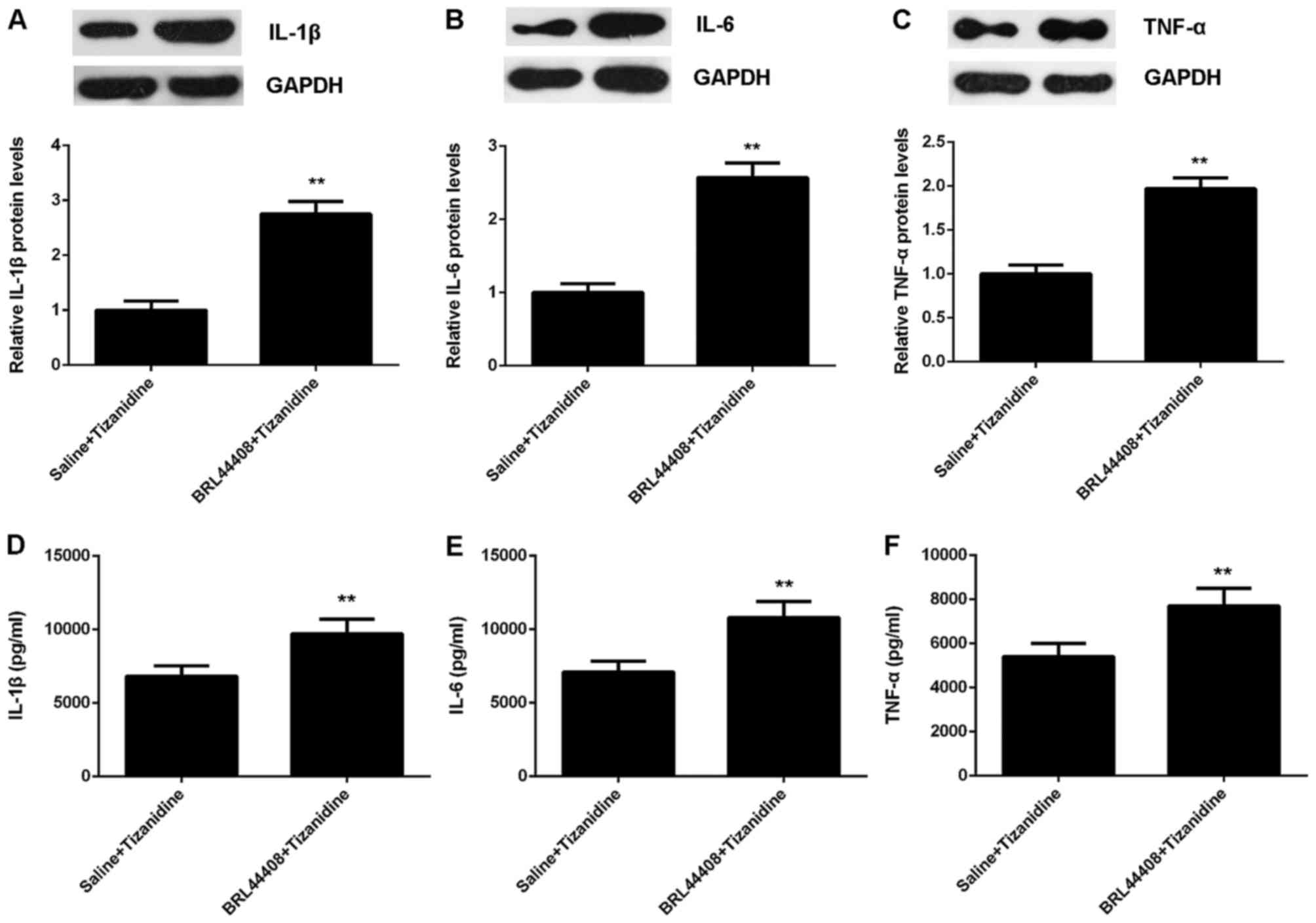
To further confirm these findings, we examined the
inflammatory responses in the spinal cord. Our data showed that the
protein expression and secretion of IL-1β, IL-6 and TNF-α were
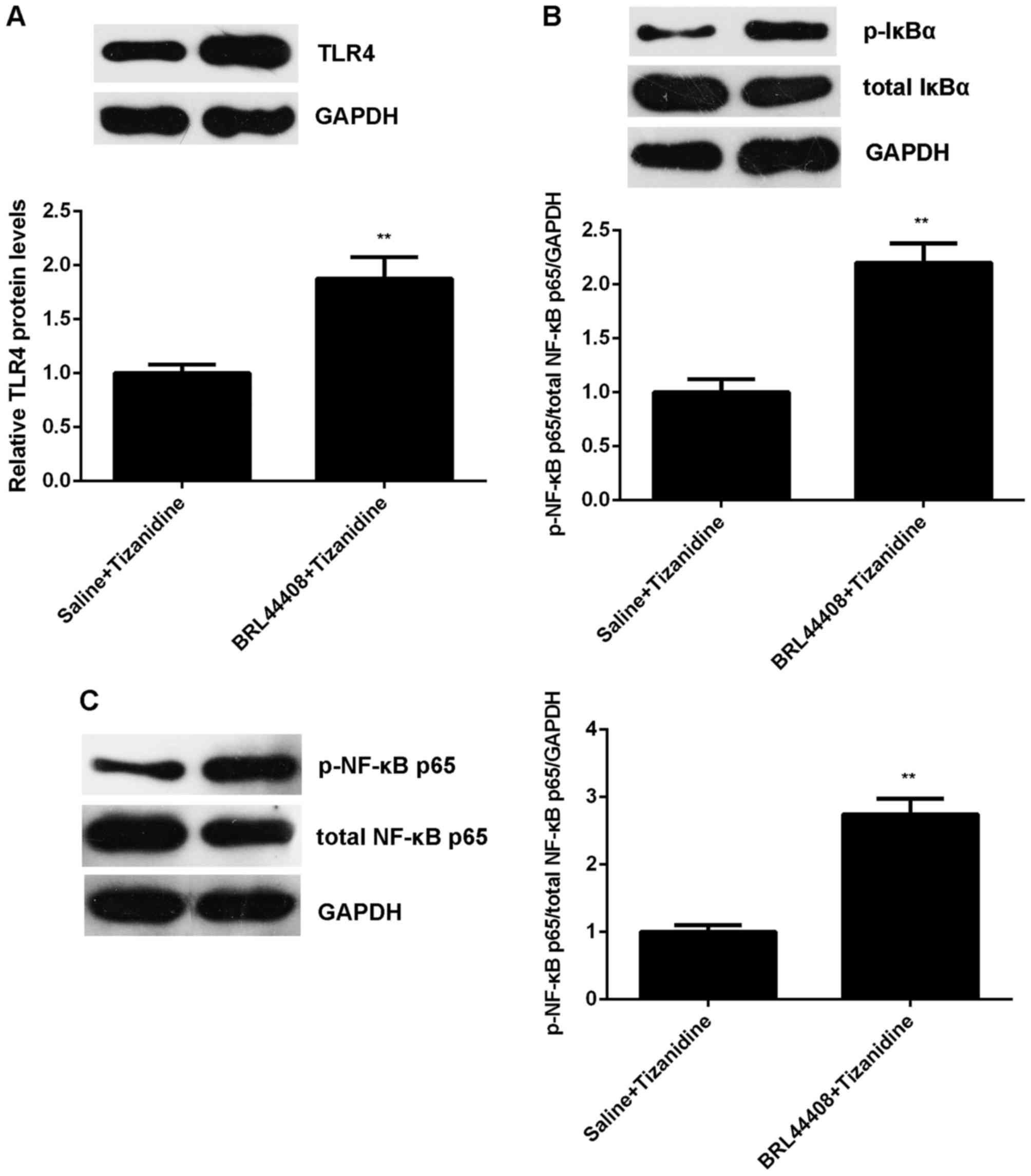
increased in pretreatment with BRL44408 (Fig. 10). Moreover, the protein levels
of TLR4 and the phosphorylation levels of IκBα and NF-κB p65
proteins were also upregulated in the BRL44408 + tizanidine group
compared with the saline + tizanidine group, indicating that the
TLR4/NF-κB signaling was activated when pretreated with BRL44408 in
SNI rats (Fig. 11). According to
these above findings, we demonstrated that the inhibitory effect of
tizanidine on SNI-induced mechanical and thermal hyperalgesia is
directly through activation of α2-AR in the spinal cord.
Discussion
The effect mechanism of tizanidine in neuropathic
pain remains largely unknown. Therefore, the present study
investigated the effects of tizanidine on neuropathic pain in
spared nerve injury (SNI) model of rats, as well as the underlying
molecular mechanism. We found that the rats in SNI group showed
significant mechanical and thermal hyperalgesia, accompanied by
increased production of proinflammatory cytokines in spinal dorsal
horn, and the activation of TLR4/NF-κB signaling. The inhibitor of
TLR4/NF-κB signaling PDTC significantly attenuated the SNI-induced
mechanical and thermal hyperalgesia and the production of
proinflammatory cytokines. Moreover, treatment with tizanidine also
attenuated the SNI-induced mechanical and thermal hyperalgesia,
suppressed production of the proinflammatory cytokines, and
inhibited the activation of TLR4/NF-κB signaling pathway, which
could be reversed by pretreatment with BRL44408, a selective α2-AR
antagonist.
Neuropathic pain induces allodynia and hyperalgesia
(20,21). SNI is a common model applied for
investigating the molecular mechanism underlying peripheral
neuropathic pain (22). Previous
studies have shown that SNI rats exhibited mechanical and thermal
hyperalgesia (23-25), consistent with our findings.
Moreover, inflammatory responses have been suggested to participate
in the SNI-induced peripheral neuropathic pain (22). Kobiela Ketz et al reported
that SNI could cause region-specific activation of macrophages and
microglia, and a pro-inflammatory microglial marker was expressed
in the spinal cord of SNI rats (22). Recently, Ding et al found
that IL-6 was important for the maintenance of SNI-induced
neuropathic pain (26). They
demonstrated that IL-6 and IL-6R in the red nucleus did not show
obvious change at 1 week and 2 weeks after SNI, but was
significantly upregulated at 3 weeks after injury (26). Moreover, injection of IL-6
antibody into the red nucleus contralateral to the nerve ligation
side at 3 weeks after injury dose-dependently increased the paw
withdrawal threshold of rats and alleviated SNI-induced mechanical
allodynia (26). Gui et al
demonstrated that IL-1β overproduction was a common cause for
neuropathic pain following peripheral nerve injury in rodents
(27). Besides, TNF-α has been
implicated in the development of neuropathic allodynia (28). In this study, we also found that
the expression and secretion levels of IL-1β, IL-6 and TNF-α were
significantly increased in the spine cord of SNI rats at 3 weeks
after injury. As TLR4/NF-κB signaling pathway has been demonstrated
to plays a pivotal role in inflammatory responses through the
regulation of the expression of proinflammatory cytokines,
chemokines and adhesion molecules (14), we further studied its activity.
Our data showed that the TLR4/NF-κB signaling was significantly
activated in the spine cord of rats at 3 weeks after SNI. Through
using the NF-κB inhibitor, we further confirmed that the TLR4/NF-κB
signaling is involved in the SNI-induced mechanical and thermal
hyperalgesia and inflammatory responses in spine cord of rats.
It has been reported that the main site where α2-AR
agonists function is the spinal cord (29,30). Tizanidine, a highly selective
α2-AR agonist, is widely used to treat the spasms, cramping, and
tightness of muscles caused by multiple sclerosis, spastic
diplegia, back pain, or some other injuries to the spine or the
central nervous system (31,32). Previous studies have shown that
tizanidine exerts analgesic potential in neuropathic pain (33,34). However, the underlying effect
mechanism of tizanidine in neuropathic pain remains largely
unknown. In the present study, we for the first time used SNI rat
model to investigate the anti-nociceptive effect of tizanidine on
SNI-induced neuropathic pain. Our data indicated that intrathecal
administration of tizanidine for 3 consecutive days after injury
significantly attenuated the SNI-induced mechanical and thermal
hyperalgesia. The following mechanism investigation showed that
tizanidine could inhibit the SNI-induced production of
proinflammatory cytokines as well as the activation of TLR4/NF-κB
signaling pathway in the spine cord. As the TLR4/NF-κB signaling
pathway participates in regulating the expression of these
proinflammatory cytokines, which are important for the maintenance
of SNI-induced neuropathic pain (14,26-28), we suggest that inhibition of
TLR4/NF-κB signaling activation is a potential mechanism through
which tizanidine exerts the anti-nociceptive effects in SNI rats.
Furthermore, as we found that pretreatment with α2-AR antagonist
significantly reversed the inhibitory effects of tizanidine on the
SNI-induced mechanical and thermal hyperalgesia, overproduction of
proinflammatory cytokines, and activation of TLR4/NF-κB signaling,
we suggest that the inhibitory effect of tizanidine on SNI-induced
neuropathic pain is directly through activation of α2-AR in spinal
cord.
In conclusion, to our knowledge, this study for the
first time demonstrates that tizanidine could attenuate the
mechanical and thermal hyperalgesia in SNI model of rats through
inhibiting the activation of TLR4/NF-κB p65 pathway, and thus
decreasing the production of inflammatory cytokines including
TNF-α, IL-1β and IL-6. These findings highlight the
anti-nociceptive effects of tizanidine neuropathic pain.
Funding
No funding was received.
Availability of data and material
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WP, YZ, LW and WW performed the experiments and
statistical analysis. WP wrote the manuscript. YZ and LL designed
the present study and revised the manuscript.
Ethics approval and consent to
participate
This study was approved by Animal Care and Use
Committee of People's Hospital of Hunan Province (Changsha, China).
Animal experiments were consistent with the National Institutes of
Health Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Yang YK, Lu XB, Wang YH, Yang MM and Jiang
DM: Identification crucial genes in peripheral neuropathic pain
induced by spared nerve injury. Eur Rev Med Pharmacol Sci.
18:2152–2159. 2014.PubMed/NCBI
|
|
2
|
Decosterd I and Woolf CJ: Spared nerve
injury: An animal model of persistent peripheral neuropathic pain.
Pain. 87:149–158. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoon SY, Kang SY, Kim HW, Kim HC and Roh
DH: Clonidine reduces nociceptive responses in mouse orofacial
formalin model: Potentiation by Sigma-1 receptor antagonist BD1047
without impaired motor coordination. Biol Pharm Bull. 38:1320–1327.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li SS, Zhang WS, Ji D, Zhou YL, Li H, Yang
JL, Xiong YC, Zhang YQ and Xu H: Involvement of spinal microglia
and interleukin-18 in the anti-nociceptive effect of
dexmedetomidine in rats subjected to CCI. Neurosci Lett. 560:21–25.
2014. View Article : Google Scholar
|
|
5
|
Mirbagheri MM, Chen D and Rymer WZ:
Quantification of the effects of an alpha-2 adrenergic agonist on
reflex properties in spinal cord injury using a system
identification technique. J Neuroeng Rehabil. 7:292010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawamata T, Omote K, Yamamoto H, Toriyabe
M, Wada K and Namiki A: Antihyperalgesic and side effects of
intrathecal clonidine and tizanidine in a rat model of neuropathic
pain. Anesthesiology. 98:1480–1483. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davies J and Johnston SE: Selective
antinociceptive effects of tizanidine (DS 103-282), a centrally
acting muscle relaxant, on dorsal horn neurones in the feline
spinal cord. Br J Pharmacol. 82:409–421. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koyuncuŏglu H, Ariciŏglu F, Uresin Y,
Dizdar Y and Esin Y: Effects of tizanidine on morphine physical
dependence: Attenuation and intensification. Pharmacol Biochem
Behav. 42:693–698. 1992. View Article : Google Scholar
|
|
9
|
Yazicioğlu D, Caparlar C, Akkaya T, Mercan
U and Kulaçoğlu H: tizanidine for the management of acute
postoperative pain after inguinal hernia repair: A
placebo-controlled double-blind trial. Eur J Anaesthesiol.
33:215–222. 2016. View Article : Google Scholar
|
|
10
|
Kabayel DD, Ozdemir F, Unlu E, Bilgili N
and Murat S: The effects of medical treatment and rehabilitation in
a patient with adult tethered cord syndrome in the late
postoperative period. Med Sci Monit. 13:CS141–CS144.
2007.PubMed/NCBI
|
|
11
|
Roy A, Srivastava M, Saqib U, Liu D,
Faisal SM, Sugathan S, Bishnoi S and Baig MS: Potential therapeutic
targets for inflammation in toll-like receptor 4 (TLR4)-mediated
signaling pathways. Int Immunopharmacol. 40:79–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rocha DM, Caldas AP, Oliveira LL, Bressan
J and Hermsdorff HH: Saturated fatty acids trigger TLR4-mediated
inflammatory response. Atherosclerosis. 244:211–215. 2016.
View Article : Google Scholar
|
|
13
|
Zhang J, Xia J, Zhang Y, Xiao F, Wang J,
Gao H, Liu Y, Rong S, Yao Y, Xu G, et al: HMGB1 TLR4 signaling
participates in renal ischemia reperfusion injury and could be
attenuated by dexamethasone-mediated inhibition of the ERK/NF-κB
pathway. Am J Transl Res. 8:4054–4067. 2016.
|
|
14
|
Liu DL, Zhao LX, Zhang S and Du JR:
Peroxiredoxin 1-mediated activation of TLR4/NF-κB pathway
contributes to neuroinflammatory injury in intracerebral
hemorrhage. Int Immunopharmacol. 41:82–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu S, Hu X, Tao Y, Ping Z, Wang L, Shi J,
Wu X, Zhang W, Yang H, Nie Z, et al: Strontium inhibits titanium
particle-induced osteoclast activation and chronic inflammation via
suppression of NF-κB pathway. Sci Rep. 6:362512016. View Article : Google Scholar
|
|
16
|
Huang Y, Chen R and Zhou J: E2F1 and
NF-κB: Key mediators of inflammation-associated cancers and
potential therapeutic targets. Curr Cancer Drug Targets.
16:765–772. 2016. View Article : Google Scholar
|
|
17
|
Fuentes E, Rojas A and Palomo I: NF-κB
signaling pathway as target for antiplatelet activity. Blood Rev.
30:309–315. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pujari R, Hunte R, Khan WN and Shembade N:
A20-mediated negative regulation of canonical NF-κB signaling
pathway. Immunol Res. 57:166–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schuster M, Annemann M, Plaza-Sirvent C
and Schmitz I: Atypical IκB proteins - nuclear modulators of NF-κB
signaling. Cell Commun Signal. 11:232013. View Article : Google Scholar
|
|
20
|
Dos Reis RC, Kopruszinski CM, Nones CF and
Chichorro JG: Nerve growth factor induces facial heat hyperalgesia
and plays a role in trigeminal neuropathic pain in rats. Behav
Pharmacol. 27:528–535. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gong SS, Li YX, Zhang MT, Du J, Ma PS, Yao
WX, Zhou R, Niu Y, Sun T and Yu JQ: Neuroprotective effect of
matrine in mouse model of vincristine-induced neuropathic pain.
Neurochem Res. 41:3147–3159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kobiela Ketz A, Byrnes KR, Grunberg NE,
Kasper CE, Osborne L, Pryor B, Tosini NL, Wu X and Anders JJ:
Characterization of macrophage/microglial activation and effect of
photobiomodulation in the spared nerve injury model of neuropathic
pain. Pain Med. 18:932–946. 2017.
|
|
23
|
Ko MH, Yang ML, Youn SC, Lan CT and Tseng
TJ: Intact subepidermal nerve fibers mediate mechanical
hypersensitivity via the activation of protein kinase C gamma in
spared nerve injury. Mol Pain. 12:pii: 17448069166561892016.
View Article : Google Scholar
|
|
24
|
Tilley DM, Cedeño DL, Kelley CA, Benyamin
R and Vallejo R: Spinal cord stimulation modulates gene expression
in the spinal cord of an animal model of peripheral nerve injury.
Reg Anesth Pain Med. 41:750–756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Curto-Reyes V, Kirschmann G, Pertin M,
Drexler SK, Decosterd I and Suter MR: Neuropathic pain phenotype
does not involve the NLRP3 inflammasome and its end product
interleukin-1β in the mice spared nerve injury model. PLoS One.
10:e01337072015. View Article : Google Scholar
|
|
26
|
Ding CP, Xue YS, Yu J, Guo YJ, Zeng XY and
Wang JY: The red nucleus interleukin-6 participates in the
maintenance of neuropathic pain induced by spared nerve injury.
Neurochem Res. 41:3042–3051. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gui WS, Wei X, Mai CL, Murugan M, Wu LJ,
Xin WJ, Zhou LJ and Liu XG: Interleukin-1β overproduction is a
common cause for neuropathic pain, memory deficit, and depression
following peripheral nerve injury in rodents. Mol Pain. 12:pii:
17448069166467842016. View Article : Google Scholar
|
|
28
|
Li X, Wang J, Wang Z, Dong C, Dong X, Jing
Y, Yuan Y and Fan G: Tumor necrosis factor-α of Red nucleus
involved in the development of neuropathic allodynia. Brain Res
Bull. 77:233–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu L, Ji F, Liang J, He H, Fu Y and Cao
M: Inhibition by dexmedetomidine of the activation of spinal dorsal
horn glias and the intracellular ERK signaling pathway induced by
nerve injury. Brain Res. 1427:1–9. 2012. View Article : Google Scholar
|
|
30
|
Buerkle H and Yaksh TL: Pharmacological
evidence for different alpha 2-adrenergic receptor sites mediating
analgesia and sedation in the rat. Br J Anaesth. 81:208–215. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Malanga G, Reiter RD and Garay E: Update
on tizanidine for muscle spasticity and emerging indications.
Expert Opin Pharmacother. 9:2209–2215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Henney HR III and Chez M: Pediatric safety
of tizanidine: Clinical adverse event database and retrospective
chart assessment. Paediatr Drugs. 11:397–406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ou-Yang HD, Zeng WA, Li Q, He WX, Wang PZ,
Lin LL, Zhang ZQ and Liu XG: Effects of intrathecal ouabain and
tizanidine injection for treatment of neuropathic pain in rats. Nan
Fang Yi Ke Da Xue Xue Bao. 28:1760–1763. 2008.In Chinese.
PubMed/NCBI
|
|
34
|
Leiphart JW, Dills CV and Levy RM:
Alpha2-adrenergic receptor subtype specificity of intrathecally
administered tizanidine used for analgesia for neuropathic pain. J
Neurosurg. 101:641–647. 2004. View Article : Google Scholar : PubMed/NCBI
|