Introduction
Systemic lupus erythematosis (SLE) is an autoimmune
disorder, which is linked to premature vascular disease (1,2).
The enhanced macrovascular risk has not been fully investigated
through exploring conventional risk factors or signals for vascular
disease (3). Lupus nephritis is a
major manifestation of SLE. Accordingly, SLE, as an autoimmune
disease, is also characterized by autoantibody generation, elevated
circulating inflammatory cytokines and injury to organs (4,5).
Furthermore, SLE is a disease with various causes, including
genetic and environmental factors (6). The pathogenesis of lupus nephritis
includes an abnormal T-cell response, accelerating the generation
of autoantibodies and immune complex deposits in renal or other
organs (7). It has been indicated
that T cells are essential for the pathogenesis of lupus nephritis,
which involves the regulation of T helper (Th) cells (8). Th cells are central regulators of
adaptive immune responses, which have an important role in the
pathogenesis of SLE by regulating the interactions between other
cells to regulate the generation of immunomodulatory cytokines
(9). Once activated by antigens,
the CD4+ T cells differentiate into various lineages of
Th cells, including Th1, -9 and -17, which are associated with
different types of cytokines (10). Th17 cells, which produce the
pro-inflammatory cytokine interleukin (IL)-17, are of particular
importance in the pathogenesis of SLE (11). According to previous studies,
patients with SLE had higher circulating IL-17 levels in comparison
with those in the controls (12).
Chemokine (C-X-C motif) ligand (CXCL)-1 is a murine homolog to
human CXCL-8 and a potent chemoattractant for neutrophils and
IL-17; it mediates pro-inflammatory responses primarily by
modulating the expression of other cytokines and chemokines,
including IL-6, IL-1β, tumor necrosis factor (TNF)-α and monocyte
chemoattractant protein (MCP)-1 (13,14).
3,7,3',4'-Tetrahydroxyflavone (fisetin), a naturally
occurring bioactive flavonol, has been reported to improve the
development of various diseases with its multiple bioactivities,
including the reduction of oxidative stress, inflammatory response
and apoptosis (15-17). In addition, fisetin was reported
to ameliorate immune cell infiltration to improve organ injury
(18). However, the crucial
effects of fisetin in human SLE progression have remained elusive.
The present study attempted to investigate whether fisetin has
potential value to ameliorate SLE in mice induced by
2,6,10,14-tetramethylpen-tadecane [pristane (PRI)]. Previously, PRI
has been suggested to induce nephritis in marine animals,
characterized by mesangial expansion, glomerular proliferation and
protein-uria, which are associated with elevated levels of
anti-double stranded (ds)DNA antibodies (19). Of note, the present study
indicated that fisetin reduces anti-autoimmune antibodies and
pro-inflammatory cytokines release, and regulates Th17-cell
differentiation through CXCL signaling. In addition, an in
vitro experiment indicated that fisetin had no cytotoxic
effects on treated cells. These results suggested that fisetin may
be used as a promising candidate to attenuate SLE in the
future.
Materials and methods
Animals and treatment
A total of 60 female C57BL/6 mice (weight, 18-20 g;
age, 10 weeks), were purchased from Nanjing Medical University
Animal Experiment Center (Nanjing, China). All experimental mice
were housed under specific pathogen-free conditions in static
microisolator cages with tap water ad libitum in a
temperature-controlled room (25±2°C, 50±5% humidity) with a 12-h
light/dark cycle and were fed a standard laboratory rodent diet
with water ad libitum. The mice experiments were performed
in a way to minimize animal suffering according to the Guide for
the Care and Use of Laboratory Animals by the National Institutes
of Health from 1996. The Institutional Animal Care and Use
Committee at Huai'an First People's Hospital, Nanjing Medical
University (Huai'an, China) approved the animal study protocols.
The animals were divided into 5 groups (n=10/group): Control (Con;
without any treatment); PRI group; PRI+25 mg/kg fisetin; PRI+50
mg/kg fisetin and PRI+100 mg/kg fisetin (20,21). Fisetin (purity, >98%) was from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and was administered
orally by gavage once a day. In order to induce lupus in mice, the
animals received an intraperitoneal (i.p.) injection of 0.5 ml PRI
(Sigma-Aldrich; Merck KGaA), as previously described (22). PBS was administered to the control
group. At 30 weeks following treatment with PRI or PBS, blood
samples were collected through retroorbital bleeding with capillary
tubes to obtain the serum. Next, the mice were sacrificed, and
renal and spleen tissue samples were immediately isolated and
stored at −80°C for the further experiments described below.
Proteinuria was measured through urine albumin-to-creatinine ratio
determined using the Albumin assay kit (A028-1; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) and Creatinine Assay kit
(C011-2; Nanjing Jiancheng Bioengineering Institute).
Cells and culture
The RAW264.7 murine macrophage cell line and the
293FT cell line were purchased from the American Type Culture
Collection (Manassas, VA, USA). The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM, Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% (v/v)
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
(v/v) penicillin/streptomycin in an incubator at 37°C in a
humidified atmosphere containing 5% CO2. For the
experiments, cells were pre-treated with 100 ng/ml
lipopolysaccharide (LPS; Sigma-Aldrich; Merck KGaA) for 2 h,
followed by incubation with fisetin (15 and 30 µM) for
another 24 h. Subsequently, all cells were harvested for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR),
western blot and flow cytometric analysis.
MTT assay
In order to measure the cytotoxicity of fisetin on
normal cells, 293FT and RAW264.7 cells were seeded into 96-well
plates at 1×103 cells/well in complete growth medium. On
the following day, the cells were treated with different
concentrations of fisetin ranging from 0-160 µM at 37°C for
24, 48 or 72 h. The cell viability was determined by an MTT assay
with the absorbance read at 570 nm (23).
Analysis of serum samples
The serum concentrations of anti-dsDNA antibodies
(5110; Alpha Diagnostic, San Antonio, TX, USA), anti-small nuclear
ribonucleoprotein antibody (AB-23240-A; anti-snRNP; Alpha
Diagnostic), IL-1β (MLB00C), IL-6 (M6000B), TNF-α (MTA00B),
interferon-γ (IFN-γ) (DY485), CXCL-1 (DY453), MCP-1/CCL-2 (MJE00),
chemokine (C-C motif) ligand 3 (CCL-3) (MMA00) and CXCL-2 (MM200;
all from R&D Systems, Minneapolis, MN, USA) were measured with
corresponding ELISA kits following the manufacturers'
protocols.
Histological analysis
To histologically evaluate the renal tissue samples
after various treatments, kidney tissue specimens were fixed in 10%
formalin at room temperature for 48 h and embedded in paraffin. A
series of 4-µm sections containing the hilum of the renal
tissue samples were stained with a periodic acid-Schiff (PAS) Stain
Kit (Abcam, Cambridge, MA, USA) following the manufacturer's
protocols. In brief, a semi-quantitative scoring system was applied
to calculate 10 various parameters, including mesangial deposits,
mesangial hypercellularity, mesangial sclerosis, endocapillary
sclerosis, endocapillary cellular infiltrate, endocapillary
organized crescents, endocapillary cellular crescents, tubular
atrophy, interstitial inflammation and interstitial fibrosis: 0, no
involvement; 0.5, minimal involvement (<10%); 1, mild
involvement (10-30%); 2, moderate involvement (31-60%); 3, severe
involvement (60%). An activity and chronicity index was determined
through compiling scores from groups of associated parameters (as
for the activity: Mesangial deposits, mesangial hypercellularity
and endocapillary cell infiltration; and as for the chronicity:
Tubular atrophy, endocapillary organized crescents, endocapillary
sclerosis and interstitial fibrosis). Spleen tissue samples were
soaked in 4% formalin overnight at room temperature, embedded in
paraffin and sectioned at 4 µm thickness. Sections were
stained with hematoxylin and eosin (H&E) and visualized by
light microscopy at a magnification of x400.
Flow cytometric analysis
The spleen tissue was disaggregated into single
cells as described previously (24) and stained for 30 min in dark at
room temperature for surface markers with the following antibodies:
CD3 (554832; 1:100), CD4 (553651; 1:100) and CD8 (557085; 1:100)
purchased from BD Biosciences (Franklin Lakes, NJ, USA). Spleen
cells were stimulated and then stained for intracellular cytokines.
RAW264.7 cells were treated as indicated above and then harvested
for CD3 conjugation. The following fluorochrome-conjugated
antibodies were used: CD4 (563933; 1:100 dilution, GK1.5), CD3
(561042; 1:100 dilution, 145-2C11), IL-17 (eBio17B7; 1:100
dilution) and RAR-related orphan receptor (ROR)γt (12-6988-82;
1:100 dilution, AFKJ-9), and all were purchased from BD Biosciences
or eBioscience (Thermo Fisher Scientific, Inc.). The antibodies
were stained in dark for 30 min Finally, the labeled cells were
quantified with a FACSCanto instrument (BD Biosciences).
T-cell differentiation in vitro
CD4+CD25− T cells were
isolated from spleens of 6-month-old mice as described previously
(25). Cells were purified using
a MACS magnetic column (Miltenyi Biotec GmbH, Bergisch Gladbach,
Germany) with a CD4+ T cell enrichment kit (11-0041-82;
eBioscience; Thermo Fisher Scientific, Inc.). The cells were then
stimulated with a plate-bound anti-CD3e antibody (5 µg/ml;
11-0038-42, eBioscience; Thermo Fisher Scientific, Inc.) and
anti-CD28 antibody (2 µg/ml; 16-0281-82, eBioscience; Thermo
Fisher Scientific, Inc.). For Th17-cell polarization, the following
exogenous cytokines and antibodies were added: Transforming growth
factor-β (5 ng/ml, R&D Systems); anti-IL-4 antibody
(46-7041-82; 10 µg/ml; eBioscience; Thermo Fisher
Scientific, Inc.); IL-6 (MAB406; 30 ng/ml; R&D Systems);
anti-IFN-γ antibody (MAB485; 10 µg/ml; eBioscience; Thermo
Fisher Scientific, Inc.) and IL-1β (AF-401-NA; 20 ng/ml; R&D
Systems). After 5 days of culture, the proportion of Th17 cells
among all cells was determined by flow cytometry.
RT-qPCR
Total RNA extraction of cells and spleen tissue
samples was performed using TRIzol reagent (Life Technologies;
Thermo Fisher Scientific, Inc.). Total RNA (1 µg) was
reverse transcribed using the Moloney murine leukemiavirus RT
system (Promega Corp., Madison, WI, USA). A mixture of 2 µl
RT product with 7.3 µl nuclease-free H2O, 0.4
µl ROX I (50X), 10 µl TaqMan PCR Mixture (2X) and 0.3
µl TaqMan MiRNA Assay (20X) primer was prepared. The
reaction was performed at 42°C for 1 h and terminated by
deactivation of the enzyme at 70°C for 10 min. PCR was performed
using SYBR-Green (Bio-Rad Laboratories, Inc., Hercules, CA, USA) in
an ABI PRISM 7900HT detection systems (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Equal amounts of cDNA were diluted and
amplified through real-time PCR using All-in-One qPCR Mix
(GeneCopoeia, Maryland, USA) in a 20-µl reaction volume
containing 10 µl of 2X All-in-One qPCR Mix (GeneCopoeia,
Maryland, USA), 1 µl of 2 µM forward primer, 1
µl of 2 µM reverse primer, 1 µl of cDNA and 6
µl of nuclease-free water. All of the primers were from
Invitrogen (Thermo Fisher Scientific, Inc.). Amplification of
pre-denatured products was performed at 94°C for 60 sec followed by
45 cycles at 95°C for 30 sec, 58°C for 30 sec and 72°C for 30 sec;
this was followed by 95°C for 10 sec, 65°C for 45 sec and 40°C for
60 sec. Fold induction values were calculated according to the
2−ΔΔCq method, where ΔCt represents the differences in
cycle threshold number between the target gene and GAPDH, and ΔΔCt
represents the relative change in the differences between control
and treatment groups (26). The
following primers were used: IL-6 forward, 5'-CAA GCA ACA ATG GAC
TTT AGG-3' and reverse, 5'-GAG CCA TTA ACT ATG GAA CCA-3'; IL-1β
forward, 5'-AGA CTC CAC ATT ACA GGC AAT GCC-3' and reverse, 5'-AAT
CGC TAC GGA TCT CCA GA-3'; MCP-1 forward, 5'-AGA TCA GGC GAT GCA
TGT AGA CG-3' and reverse, 5'-CAA TAC CAG TGG ACA AAC GAC-3'; IFN-γ
forward, 5'-ACA AGC TCA AGT ACC CAA GGT ACA-3' and reverse, 5'-GTG
CTA CTG GTG TGA TCA-3'; TNF-α forward, 5'-ATG TCC GTC CTC ATC GGT
AGC-3' and reverse, 5'-CCG GAG GAC ACA GTC CAC C-3'; IL-17 forward,
5'-CCC ATG AGA TAC ACG GGT A-3' and reverse, 5'-CCG ATA CTG CAA GGT
GAA C-3'; GAPDH forward, 5'-TCA CAT GAA CCG GAC AGA GG-3' and
reverse, 5'-CCA ATC CTA CGA CAC CGA CTA AC-3'.
Western blot analysis
The cells and spleen tissue samples were homogenized
with hypotonic buffer [25 mM Tris-HCl (pH 8.0), 1 mM EDTA, 5
µg/ml leupeptin, 1 mM Pefabloc SC, 50 µg/ml
aprotinin, 5 µg/ml soybean trypsin inhibitor and 4 mM
benzamidine] at 10% (wt/vol) to yield a homogenate. The final
supernatants were the obtained by centrifugation at 12,000 x g for
20 min at 4°C. The protein concentration was determined with a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.) with bovine serum albumin as a standard. Subsequently, equal
amounts of total protein (20-40 µg) were subjected to 10%
SDS-PAGE and electrophoretically transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were then blocked with 5% skimmed milk Tris buffered
saline with 0.1% Tween 20 (TBST), washed, and then incubated with
the following primary antibodies at 1:1,000 dilution overnight at
4°C: Rabbit anti-IL-17 (13838), rabbit anti-CXCL-1 (PA1-29220),
rabbit anti-CD3 (4443), rabbit anti-CXCL-2 (701126), rabbit
anti-CCL-3 (MA5-24364), rabbit anti-CXCR-2 (PA5-38620) and rabbit
anti-GAPDH (2118; Cell Signaling Technology, Inc., Danvers, MA,
USA), followed by incubation with HRP-conjugated goat anti rabbit
secondary antibody (1:5,000; ab6721; Abcam) for 2 h at room
temperature. Western blot bands were observed using the GE
Healthcare ECL Western Blotting Analysis System (GE Healthcare,
Little Chalfont, UK) and exposed to X-ray film (Eastman Kodak,
Rochester, NY, USA). Protein expression levels were quantified by
determining the grey value, standardized to the housekeeping gene
(GAPDH) and expressed as a fold of the control.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Statistical analyses were performed using GraphPad PRISM
(version 6.0; GraphPad Inc., La Jolla, CA, USA). Analysis of
variance with Dunnet's least-significant difference post-hoc test
was performed for comparison between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Fisetin treatment reduces auto-antibodies
and nephritis in PRI-induced mice
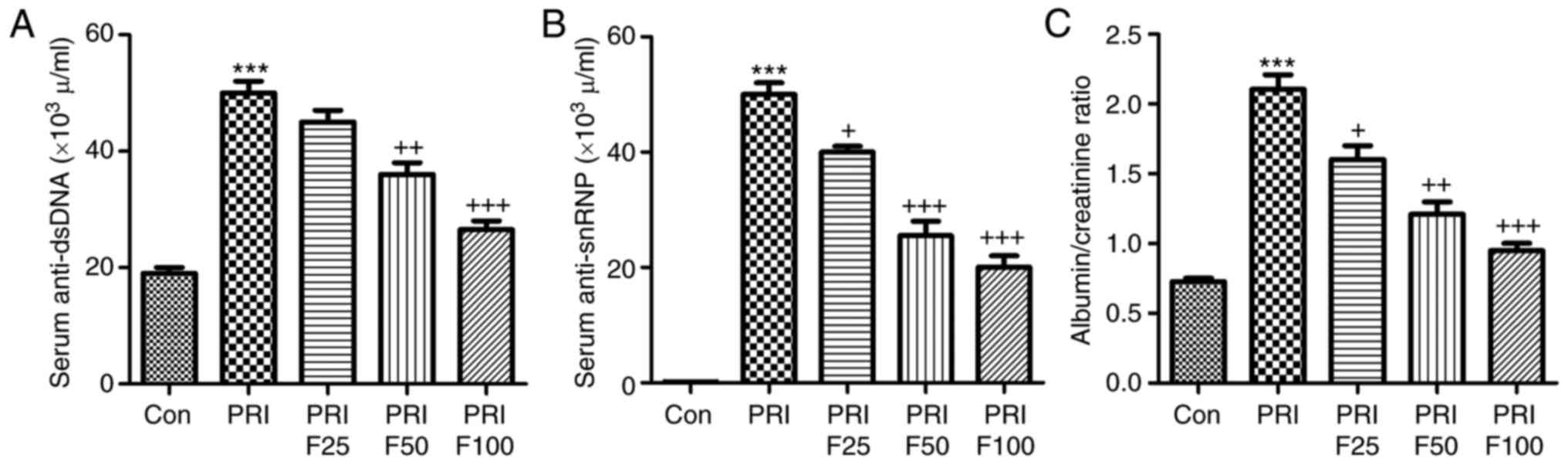
Compared with the Con group, anti-dsDNA antibodies
were significantly increased in PRI-induced mice (Fig. 1A). Of note, fisetin administration
significantly reduced PRI-induced anti-dsDNA antibody levels in a
dose-dependent manner. In addition, the levels of anti-snRNPs were
markedly increased in PRI-treated mice, but were reduced by fisetin
in a dose-dependent manner (Fig.
1B). Finally, the ratio of urine albumin to creatinine was
evaluated to assess lupus nephritis after PRI administration. As
presented in Fig. 1C, PRI
treatment significantly enhanced the ratio of albumin to
creatinine, suggesting that it caused injury in mice, which was
ameliorated by fisetin treatment. These results indicated that
fisetin may improve various autoimmune features of lupus in mice
induced by PRI.
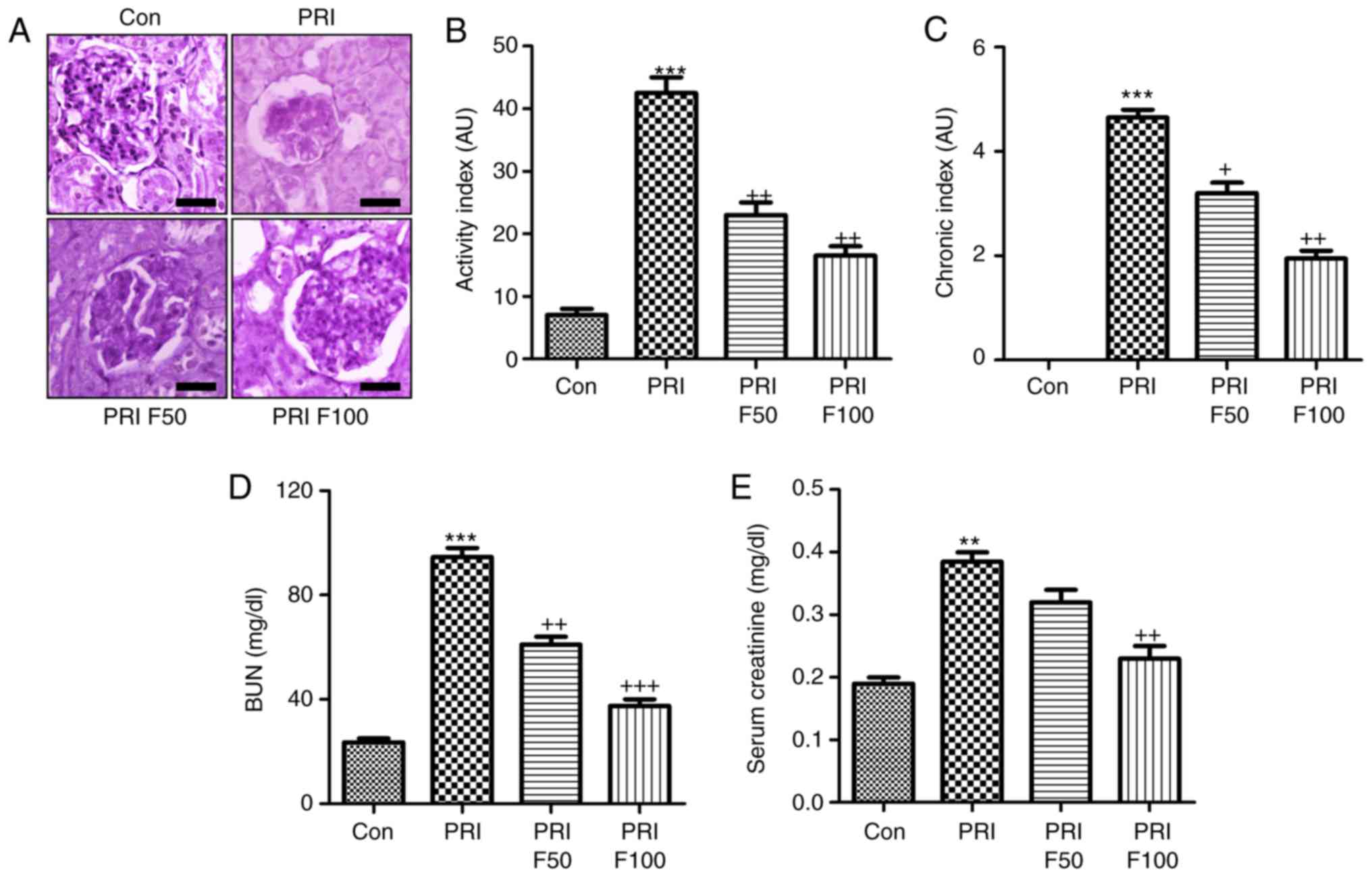
Fisetin reduces characteristics of
nephritis in a murine model of SLE
In order to determine the effect of fisetin on
PRI-induced nephritis in mice, the renal sections were
histologically examined by PAS staining, which was evaluated using
indexes of activity and chronicity. As presented in Fig. 2A and B, the activity index was
markedly enhanced by PRI induction, which was comparable to that in
the control group. Fisetin administration significantly reduced the
activity index, indicating that lupus nephritis was attenuated by
fisetin. Consistently, the PRI-induced elevation in the chronicity
index was also reduced by fisetin treatment (Fig. 2A and C). In addition, serum BUN
and creatinine levels were highly induced by PRI, which was
inhibited by fisetin, indicating renal injury induced by PRI and
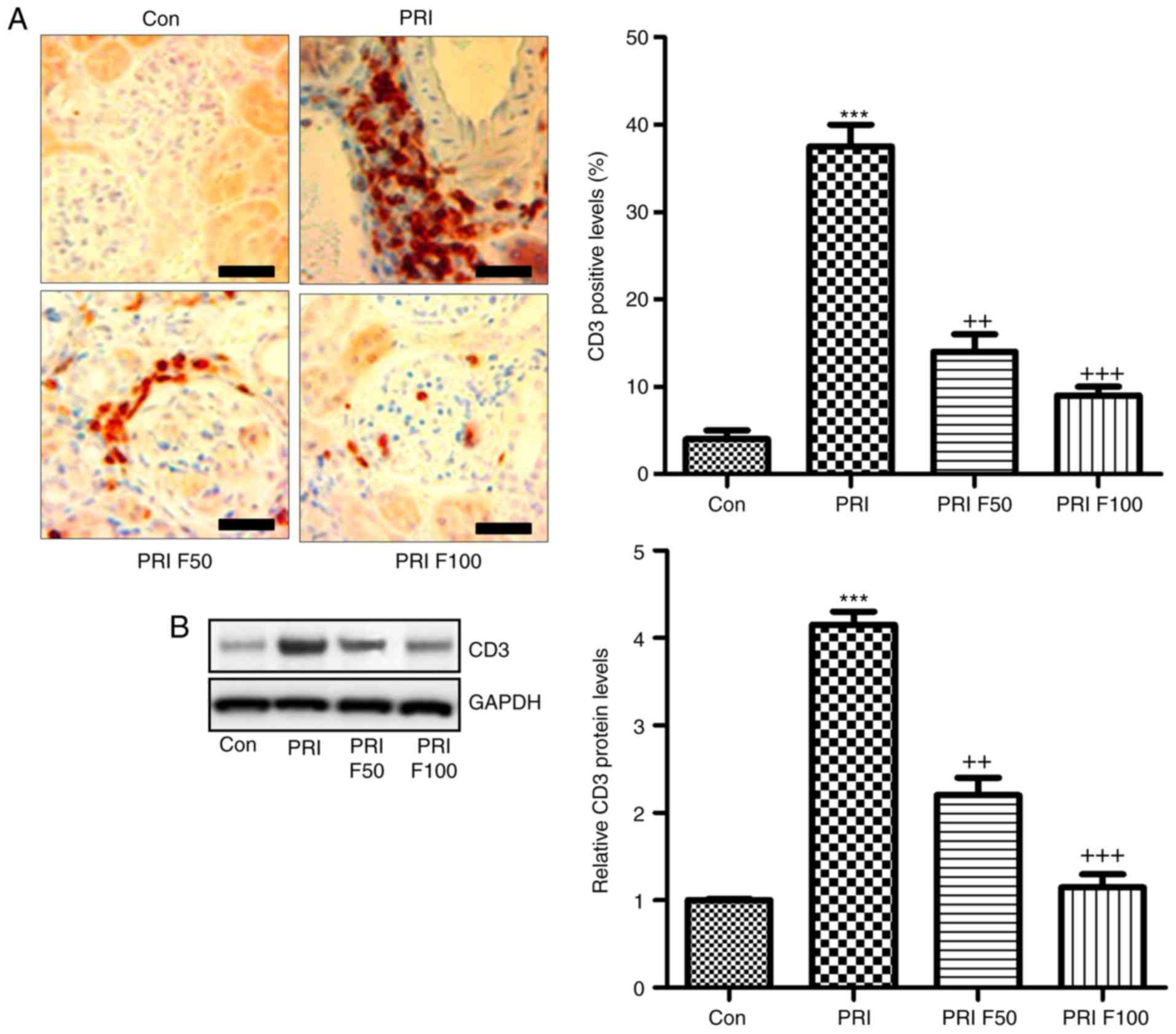
that fisetin had the capacity to attenuate nephritis (Fig. 2D and E). Furthermore, infiltration
of CD3+ cells was assessed to calculate the effects of
fisetin on SLE. The immunohistochemical analysis demonstrated that
PRI treatment led to increases of CD3+ cells throughout
the kidney tissue compared with the control group. Of note,
fisetin-treated mice exhibited significantly reduced infiltration
of CD3+ cells compared with that in the PRI group
(Fig. 3A). Western blot analysis
further indicated that PRI treatment increased CD3 levels, which
was inhibited by fisetin (Fig.
3B). Taken together, the above results demonstrated similar
results to those of other PRI-induced animal models of lupus, and
various features associated with lupus nephritis induced by PRI
administration were dose-dependently reduced by fisetin
treatment.
Fisetin reduces the circulating levels of
inflammatory signaling factors in mice with PRI-induced SLE
In order to assess the role of fisetin in
circulating inflammatory biomarkers associated with leukocyte
recruitment, the serum levels of CXCL-1, CXCL-2, CCL-3, CXCR-2,
IL-6, IFN-γ, TNF-α, IL-1β and MCP-1 were evaluated in PRI-induced
mice treated with or without fisetin. In the pathogenesis of lupus,
leukocyte extravasation has an important role (27). The results of the present study
indicated that serum CXCL-1 (Fig.
4A), CXCL-2 (Fig. 4B), CCL-3
(Fig. 4C) and CXCR-2 (Fig. 4D) were highly increased in
PRI-induced mice compared with those in the Con group. However,
after fisetin treatment, reduced levels of these signals were
observed. Next, the inflammatory cytokines IL-6, IFN-γ, TNF-α and
IL-1β, and the chemokines MCP-1 were assessed in the plasma of mice
after various treatments (Fig.
4E-I). The results indicated that PRI treatment enhanced the
levels of these factors, which were reduced by administration of
fisetin in a concentration-dependent manner. Thus, fisetin may
attenuate SLE due to reducing chemokines and cytokines in
PRI-induced mice.
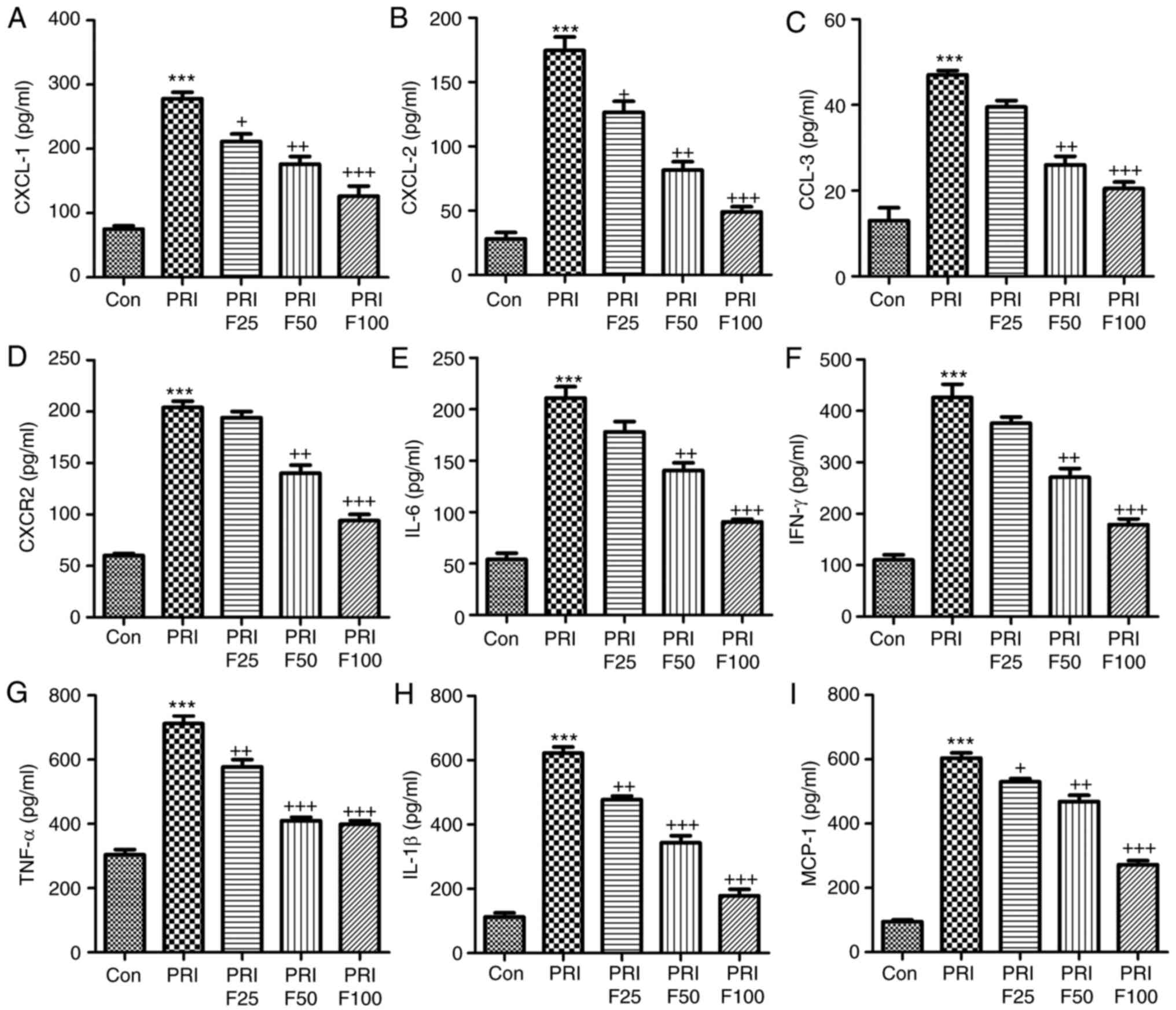 | Figure 4Fisetin reduces the levels of
inflammatory signaling proteins in mice with PRI-induced systemic
lupus erythematosus. The circulating levels of (A) CXCL-1, (B)
CXCL-2, (C) CCL-3, (D) CXCR-2, (E) IL-6, (F) IFN-γ, (G) TNF-α, (H)
IL-1β and (I) MCP-1 levels were determined by ELISA. Values are
expressed as the mean ± standard error of the mean (n=10 in each
group). ***P<0.001 vs. the Con group;
+P<0.05, ++P<0.01 and
+++P<0.001 vs. the PRI group. Con, control; PRI,
pristine; IL, interleukin; TNF, tumor necrosis factor; MCP,
monocyte chemoattractant protein; CXC, chemokine (C-X-C) motif;
CXCL, CXC ligand; CXCR, CXC receptor; CCL-3, chemokine (C-C motif)
ligand 3; F25/50/100, treatment with 25/50/100 mg/kg fisetin. |
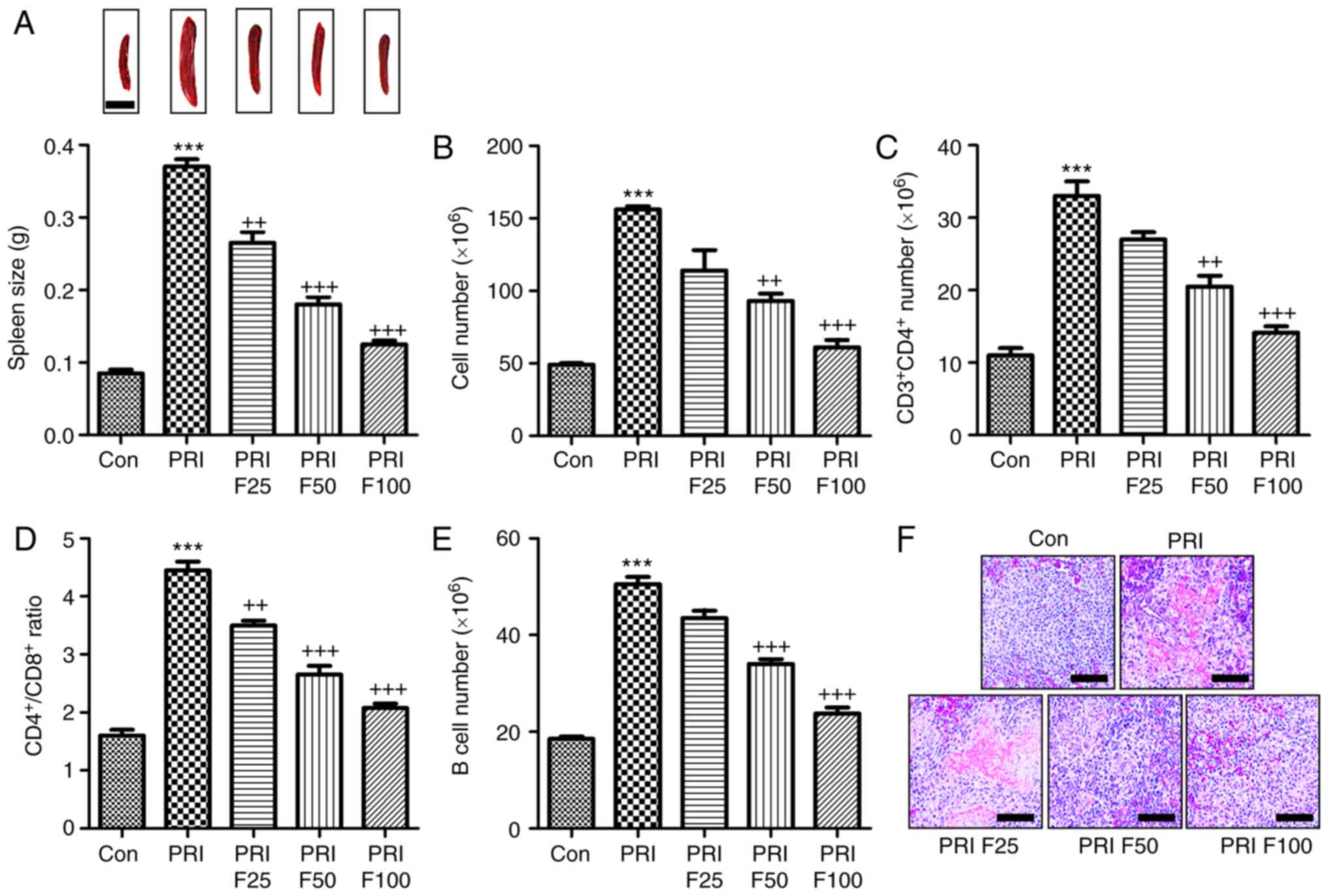
Fisetin inhibits PRI-induced changes the
composition of lymphocytes in the spleen of mice
According to previous studies, splenomegaly is a
characteristic of mice with SLE (27). As presented in Fig. 5A, the spleen size of mice induced
with PRI was larger than that of mice without any treatment. By
contrast, fisetin administration reduced the spleen size in
PRI-induced mice. In addition, the total number of lymphocytes in
the spleen tissue samples was markedly increased in PRI-treated
mice, which was reduced by fisetin treatment (Fig. 5B). Subsequently, the composition
of splenic lymphocytes was explored by flow cytometric analysis.
The results presented in Fig. 5C
indicated that the proportion of CD3+CD4+ T
cells was highly augmented in PRI-induced mice, which was inhibited
by fisetin administration. The ratio of CD4+ vs.
CD8+ T cells among the total splenic lymphocytes was
significantly increased in the PRI-treated mice, which was
attenuated by fisetin administration (Fig. 5D). Of note, a reduced
CD4+/CD8+ ratio was observed after fisetin
administration compared with that in mice treated with PRI only.
Furthermore, the B-cell number was also increased in the
PRI-induced mice compared with that in the control group (Fig. 5E). Finally, the H&E staining
indicated that PRI induced histological changes compared with
control group, which was attenuated by fisetin administration
(Fig. 5F). These results
demonstrated that PRI treatment caused an abundance of different
lymphocyte subsets in mice, which was reduced by fisetin
administration, indicating its role in improving SLE induced by PRI
in mice.
Fisetin inhibits SLE-associated increases
in splenic Th17 cells and pro-inflammatory cytokine expression
The present study attempted to evaluate the
proportion of Th17 cells among the splenic
CD3+CD4+ T cells through flow cytometric
analysis. As presented in Fig.
6A-C, the percentage of RORγ+ cells among
CD3+CD4+ T cells was significantly increased
in PRI-induced mice, as well as the percentage of IL-17-generating
CD3+CD4+ T cells, which were reduced by
fisetin treatment in a dose-dependent manner. In addition, the
pro-inflammatory cytokines in splenic cells were determined by
RT-qPCR analysis. The mRNA expression of pro-inflammatory cytokines
and MCP-1 was highly induced by PRI, which was in line with the
circulating levels. Of note, fisetin administration markedly
reduced the mRNA levels of these cytokines and MCP-1, indicating
its role in suppressing the inflammatory response in mice with SLE
(Fig. 6D). Finally, in order to
further confirm the capacity of fisetin to ameliorate SLE induced
by PRI in mice, the mRNA and protein levels of IL-17 were assessed
in the splenic cells through RT-qPCR, western blotting and ELISA.
As presented in Fig. 6E, PRI
induced IL-17, which was inhibited by fisetin.
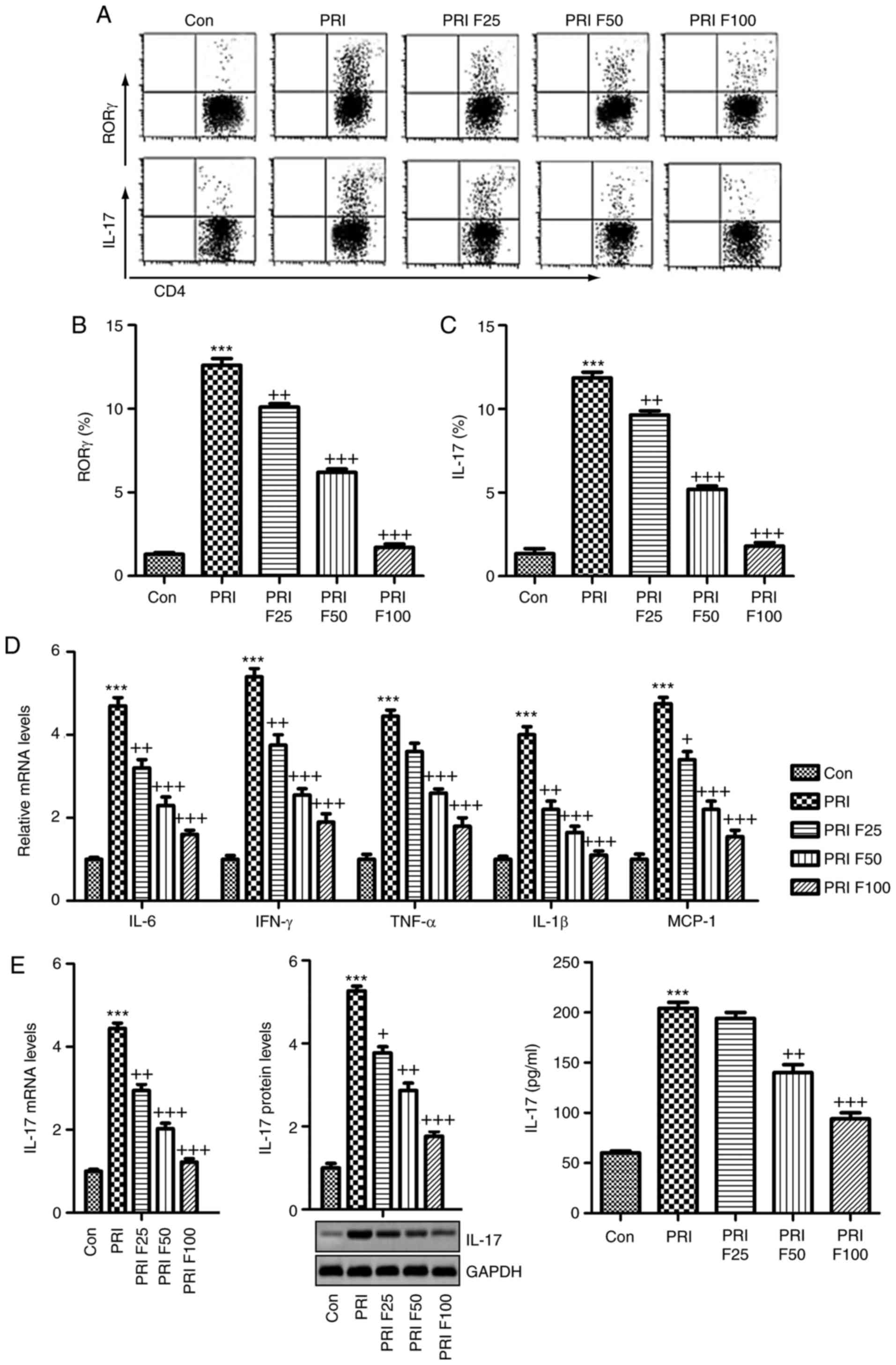 | Figure 6Splenic Th17 cells and
pro-inflammatory cytokine expression. The lymphocytes were isolated
from mice subjected to various treatments. (A) Flow cytometry was
used to evaluate RORγt+CD3+CD4+
Th17 cells among splenic CD4+ cells obtained from mice.
Quantification of (B) RORγ and (C) IL-17 from the flow cytometry
results. (D) The mRNA levels of the cytokines IL-6, IFN-γ, TNF-α,
IL-1β, and MCP-1β in the spleen tissue samples were assessed. (E)
IL-17 mRNA and protein levels were evaluated by reverse
transcription-quantitative polymerase chain reaction, western blot
and ELISA. Values are expressed as the mean ± standard error of the
mean (n=10 in each group). ***P<0.001 vs. the Con
group; +P<0.05, ++P<0.01 and
+++P<0.001 vs. the PRI group. Con, control; PRI,
pristine; F25/50/100, treatment with 25/50/100 mg/kg fisetin; Th17,
T-helper type 17; IL, interleukin; TNF, tumor necrosis factor; MCP,
monocyte chemoattractant protein; IFN, interferon; ROR, RAR-related
orphan receptor. |
Fisetin regulates CXCL signaling in
PRI-induced mice with SLE
The present study further investigated how CXCLs
signaling was altered in PRI-induced mice with or without fisetin
treatment. Western blot analysis indicated that the CXCL signaling
pathway was activated by PRI in mice with SLE, supported by an
elevation in CXCL-1, CXCL-2, CCL-3 and CXCR-2 protein expression
levels. Of note, fisetin administration significantly reduced the
abundance of CXCL-1, CXCL-2, CCL-3 and CXCR-2 protein, which may be
involved in the mechanisms by which fisetin attenuates SLE induced
by PRI treatment (Fig. 7A and
B).
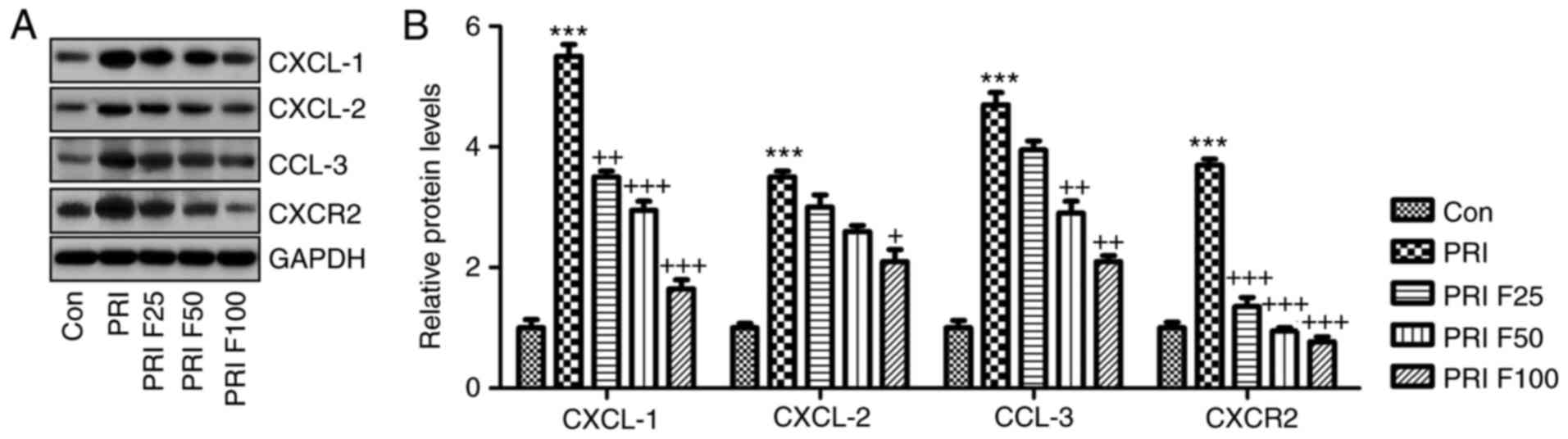 | Figure 7Role of fisetin in regulating the
CXCLs signaling pathway in mice with PRI-induced systemic lupus
erythematosus. (A) Western blot analysis was used to determine the
expression levels of CXCL-1, CXCL-2, CCL-3 and CXCR-2 in the spleen
tissue samples obtained from mice subjected to various treatments.
Representative images are provided. (B) Quantification of CXCL-1,
CXCL-2, CCL-3 and CXCR-2 protein levels from the western blots.
Values are expressed as the mean ± standard error of the mean (n=10
in each group). ***P<0.001 vs. the Con group;
+P<0.05, ++P<0.01 and
+++P<0.001 vs. the PRI group. Con, control; PRI,
pristine; CXC, chemokine (C-X-C) motif; CXCL, CXC ligand; CXCR, CXC
receptor; CCL-3, chemokine (C-C motif) ligand 3; F25/50/100,
treatment with 25/50/100 mg/kg fisetin. |
In order to further confirm the effects
of fisetin on SLE in vitro, RAW264.7 cells were included
According to the in vivo experiment,
induction of inflammation was a key molecular mechanism by which
SLE progressed. Hence, RAW264.7 cells were pre-treated with 100
ng/ml LPS for 2 h, followed by fisetin administration for another
24 h. As presented in Fig. 8A and
B, the LPS-induced increases in CD3 expression levels were
significantly reduced by fisetin. Furthermore, the mRNA levels of
the cytokines IL-6, IFN-γ, TNF-α and IL-1β, and the chemokine MCP-1
were all increased by LPS, which was inhibited by fisetin treatment
(Fig. 8C). Consistently, the
protein expression of CXCL-1, CXCL-2, CCL-3 and CXCR-2 was also
enhanced by LPS, which was markedly reduced by fisetin treatment
(Fig. 8D). In conclusion, the
above results indicated that in vitro, fisetin reduced
inflammation and CXCL pathway activation triggered by LPS as a
possible molecular mechanism by which it also attenuates SLE in
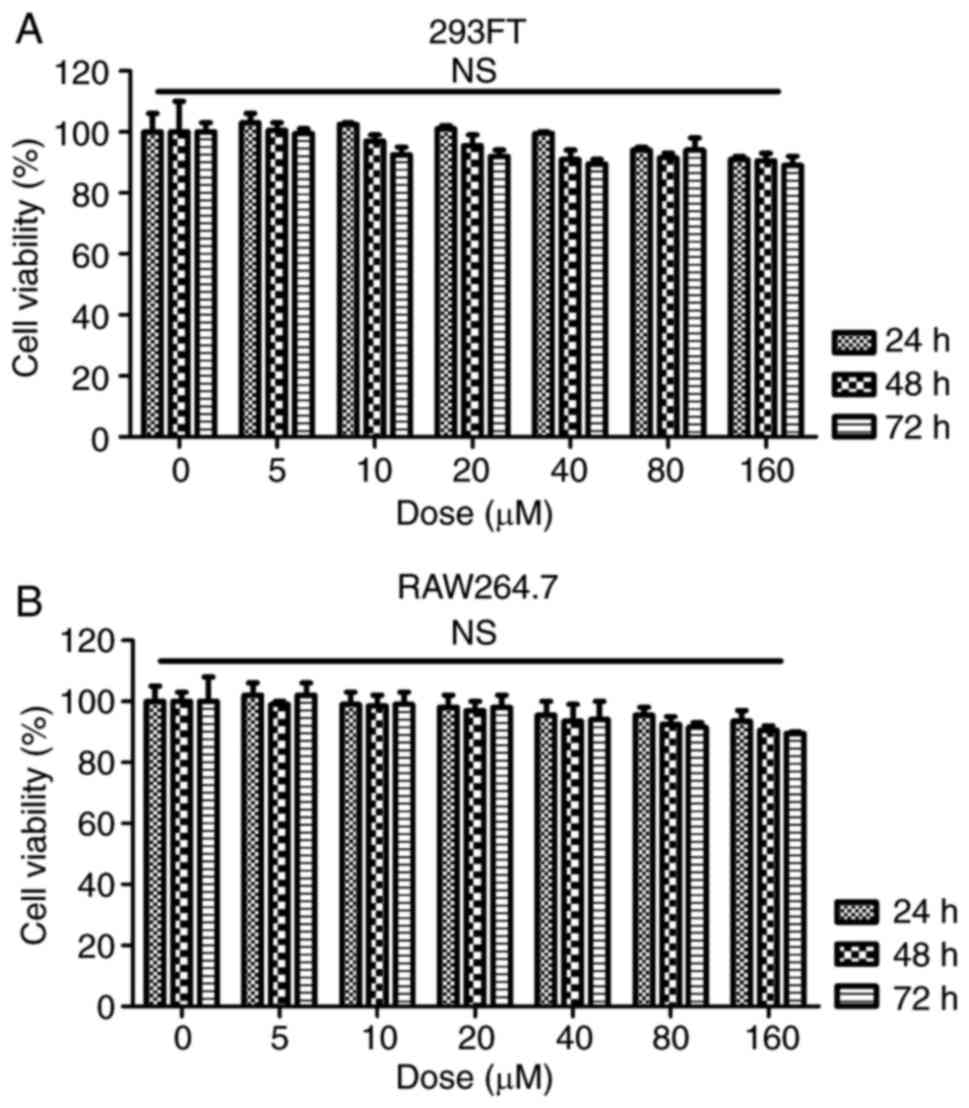
vivo. Finally, to assess whether fisetin had any possible
cytotoxic effects, the cell line 293FT and the RAW264.7 mouse
macrophage cell line of were treated with fisetin at 0, 5, 10, 20,
40, 80 or 160 µM for 24, 48 or 72 h. Subsequently an MTT
assay was used to measure the cell viability. The results presented
in Fig. 9A and B indicated no
significant difference in the number of viable 293FT and RAW264.7
cells after fisetin treatment for 24, 48 and even 72 h, which
suggested that fisetin was safe for SLE treatment under the above
conditions.
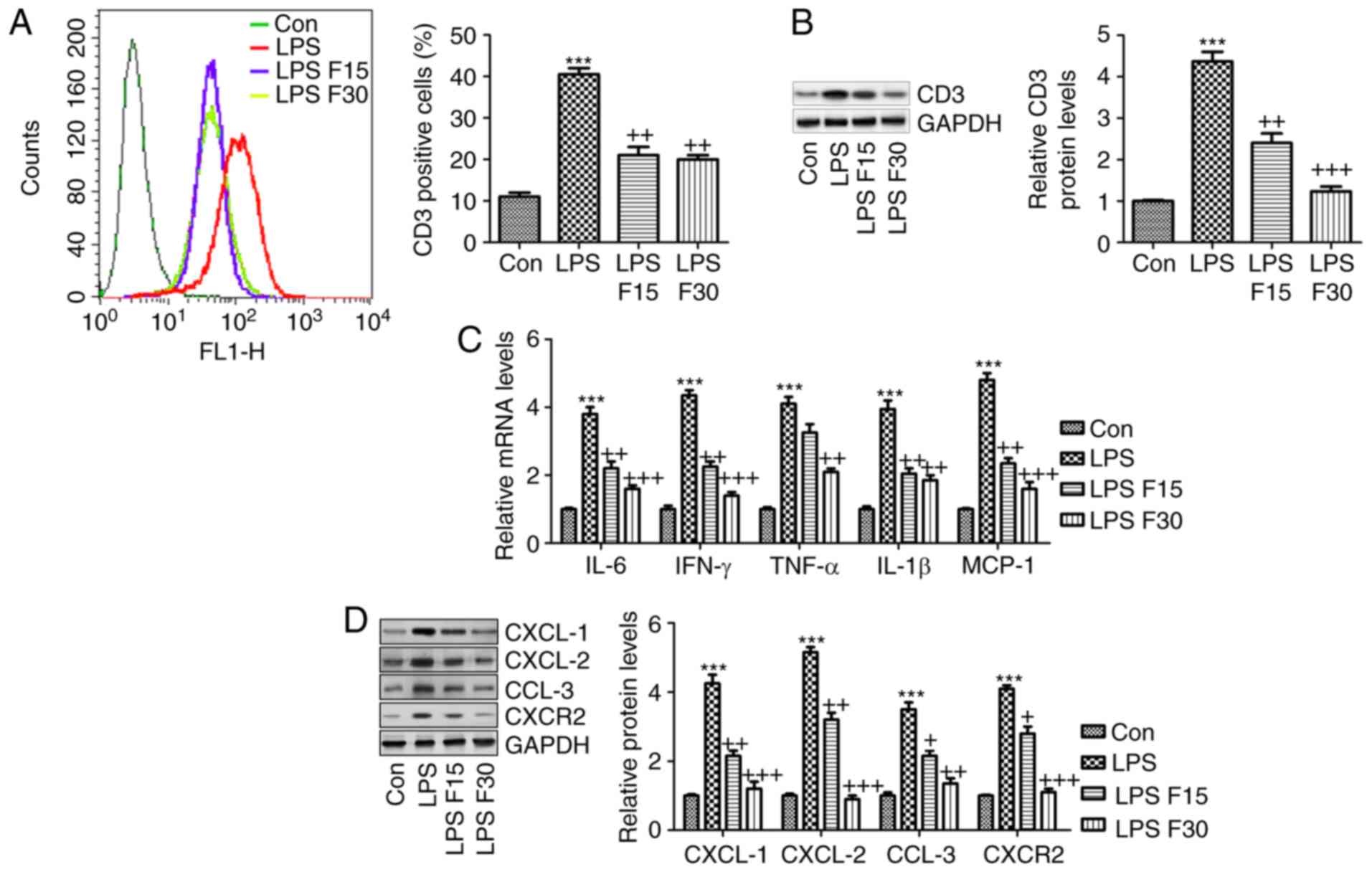 | Figure 8Fisetin reduces inflammation and
expression of CXCLs in LPS-induced RAW 264.7 cells. RAW 264.7 cells
were pre-treated with LPS (100 ng/ml) for 2 h, followed by
treatment with fisetin (15 or 30 µM) for another 24 h. (A)
Flow cytometric analysis was used to quantify CD3-expressing cells.
(B) CD3 expression levels were measured using western blot
analysis. (C) Reverse transcription-quantitative polymerase chain
reaction analysis of pro-inflammatory cytokines as indicated. (D)
CXCL-1, CXCL-2, CCL-3 and CXCR-2 expression levels were assessed
using western blot analysis. Values are expressed as the mean ±
standard error of the mean (n=8 in each group).
***P<0.001 vs. the Con group; +P<0.05,
++P<0.01 and +++P<0.001 vs. the LPS
group. Con, control; LPS, lipopolysaccharide; CXC, chemokine
(C-X-C) motif; CXCL, CXC ligand; CXCR, CXC receptor; CCL-3,
chemokine (C-C motif) ligand 3; F15/30, treatment with 15/30
µm fisetin. |
Discussion
SLE has been reported to be the most common
autoimmune disease among women aged 40-49 years (28,29). The course of SLE exhibits a great
variation among individuals, from mild to rapidly progressive
disease and eventually even a fatal outcome (30). Patients with SLE usually present
with high levels of auto-antibodies, pro-inflammatory cytokines and
nephritis (31). The underlying
pathogenesis of SLE is complex due to a variety of potential
disease mechanisms among individuals (32). Effective therapeutic strategies to
treat SLE are urgently required. Fisetin, which has been reported
to prevent pro-inflammatory cytokine expression and to have
immune-regulatory effects, was assessed in the present study as a
potential strategy to treat SLE induced by PRI in mice.
In the present study, pristane was used to induce
SLE in mice, which was characterized by elevated anti-dsDNA
antibodies, anti-snRNP antibodies and the ratio of albumin to
creatinine, indicating renal injury in mice. The renal architecture
was significantly preserved, as fisetin treatment prevented
PRI-induced histological alterations, indicating that nephritis was
caused in this model of lupus. Of note, fisetin administration
significantly reduced the levels of these antibodies, as well as
the activity and chronicity indexes determined from PAS staining
analysis.
Of note, in the SLE model, alterations in the
proportion of Th17 cells were identified among splenic
CD4+ cells and the expression of Th17-associated genes,
including IL-17. It is well known that Th17 cells are closely
associated with the pathogenesis of lupus nephritis via multiple
mechanisms (33). Th17 cells are
considered as a major type of pro-inflammatory T cell (34). In addition, a variety of cytokines
and transcription factors regulate the signaling pathways that
promote Th17 cell differentiation and enhance Th17 immune responses
(35). RORγt is an essential
transcription factor, which regulates the development and
progression of Th17 cells (36).
In the present study, PRI induced high expression of RORγ,
contributing to Th17-cell differentiation, which was reduced by
fisetin administration. Furthermore, IL-17, another important
cytokine, indicates the extent of Th17-cell differentiation, which
is associated with certain immune conditions (37,38). The present results indicated that
IL-17 was markedly upregulated at the gene and protein level in
PRI-induced mice in comparison to the control group, which was
inhibited by fisetin.
Cytokines are also considered to have a critical
role in SLE progression, which is associated with Th17-cell
differentiation (39).
Accordingly, IL-6, TNF-α, IL-1β, IFN-γ and MCP-1 drive the
differentiation of Th17 cells, and IL-17 promotes the expansion of
Th17 cells (40). Lupus-prone
mice universally display high IFN levels, thus providing excellent
animal models to investigate this matter (41). In the present study, the levels of
IL-6, TNF-α, IL-1β, IFN-γ and MCP-1 in the serum and in the splenic
lymphocytes were significantly increased in PRI-treated mice, which
may be another factor to enhance Th17 function and lupus nephritis
development. The CXCR-2-binding chemokines, CXCL-1 and -2, are
potential chemoattractants, which are associated with the immune
response (42). Inflammatory
molecules, including TNF-α, IL-1β, IFN-γ, MCP-1 and leukotrienes,
have been reported to be important for CXCL-1/2 recruitment
(43). According to previous
studies, pro-inflammatory cytokines, including TNF-α, may enhance
CXCL-1 and -2 expression in various diseases (44). Macrophages have an important role
in the progression of SLE (45,46). In addition, the two
pro-inflammatory cytokines CXCL-1 and -2 have been reported to be
linked to the progression of various diseases, including breast
cancer, lung injury and prostate cancer, via their upstream
signaling pathways, including nuclear factor-κB, which is
associated with the secretion of TNF-α and IL-1β (47). Similarly, in the present study,
CXCL-1, CXCL-2 and CXCR-2 were highly induced by PRI induction,
which was markedly reduced by fisetin administration. Furthermore,
CCL-3 was also enhanced in PRI-treated mice, and fisetin exerted an
inhibitory effect by regulating CCL-3 expression, which was in line
with CXCL-1/2 alterations in mice with SLE. Macrophages are the
central effector cells and regulatory cells in inflammation
(48,49). Therefore, RAW264.7 macrophages
were selected as an in vitro cell model in the present
study. Incubation with fisetin reduced the LPS-induced inflammatory
response and expression of CXCLs in RAW264.7 cells, contributing to
the attenuation of the immune response, which may be a potential
molecular mechanism by which SLE was alleviated.
In conclusion, the present study indicated that PRI
induced SLE in mice, which was inhibited by fisetin treatment in a
dose-dependent manner. Specifically, the elevated levels of Th17
cells were reduced by fisetin treatment, which was associated with
the suppression of pro-inflammatory cytokines and its downstream
signaling pathway of CXCLs, contributing to the disruption of Th17
cell differentiation in mice with SLE. Our present study suggested
that fisetin is a potential therapeutic strategy for SLE.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SX performed the experiments and YL supervised the
experiments and wrote the paper.
Ethics approval and consent to
participate
The animal study protocols were approved by the
Institutional Animal Care and Use Committee at Huai'an First
People's Hospital, Nanjing Medical University (Huai'an, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that no conflicts of interest
exist.
Acknowledgments
Not applicable.
References
|
1
|
Mikdashi JA, Wozniak M, Ashraf U and
Regenold W: Patterns of vascular brain injury in systemic lupus
e2rythematosus patients with ischemic strokes: Impact on
neuropsychological, neurobehavioral and physical function outcome.
Arthritis Rheumatol. 67:3568–3569. 2015.
|
|
2
|
Ajeganova S, Gustafsson T, Jogestrand T,
Frostegård J and Hafström I: Bone mineral density and carotid
atherosclerosis in systemic lupus erythematosus: A controlled
cross-sectional study. Arthritis Res Ther. 17:842015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fischer K, Sawicki M, Chamiak-Ciemińska K,
Stolarczyk J, Winikajtis-Burzyńska A, Milchert M, Ostanek L,
Bobrowska-Snarska D, Kapłon Ł, Przepiera-Będzak H, et al: A5.07 The
role of immunologic and inflammatory factors in the risk of
microvascular and macrovascular impairment development in systemic
lupus erythematosus-preliminary data. Ann Rheum Dis. 75:A44. 2016.
View Article : Google Scholar
|
|
4
|
Abou-Raya A, Abou-Raya S and Helmii M: The
effect of vitamin D supplementation on inflammatory and hemostatic
markers and disease activity in patients with systemic lupus
erythematosus: A randomized placebo-controlled trial. J Rheumatol.
40:265–272. 2013. View Article : Google Scholar
|
|
5
|
Leffler J, Martin M, Gullstrand B, Tydén
H, Lood C, Truedsson L, Bengtsson AA and Blom AM: Neutrophil
extracellular traps that are not degraded in systemic lupus
erythematosus activate complement exacerbating the disease. J
Immunol. 188:3522–3531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi J, Kim ST and Craft J: The
pathogenesis of systemic lupus erythematosusan update. Curr Opin
Immunol. 24:651–657
|
|
7
|
Madhok R: Systemic lupus erythematosus:
Lupus nephritis. BMJ Clin Evidence. 2015.1123:2015
|
|
8
|
Alunno A, Bartoloni E, Bistoni O,
Nocentini G, Ronchetti S, Caterbi S, Valentini V, Riccardi C and
Gerli R: Balance between regulatory T and Th17 cells in systemic
lupus erythematosus: The old and the new. Clin Dev Immunol.
2012.823085:2012.
|
|
9
|
Wahren-Herlenius M and Dörner T:
Immunopathogenic mechanisms of systemic autoimmune disease. Lancet.
382:819–831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shirota Y, Yarboro C, Fischer R, Pham TH,
Lipsky P and Illei GG: Impact of anti-interleukin-6 receptor
blockade on circulating T and B cell subsets in patients with
systemic lupus erythematosus. Ann Rheum Dis. 72:118–128. 2013.
View Article : Google Scholar
|
|
11
|
Hundorfean G, Neurath MF and Mudter J:
Functional relevance of T helper 17 (Th17) cells and the IL-17
cytokine family in inflammatory bowel disease. Inflamm Bowel Dis.
18:180–186. 2012. View Article : Google Scholar
|
|
12
|
Zhang W, Cai Y, Xu W, Yin Z, Gao X and
Xiong S: AIM2 facilitates the apoptotic DNA-induced systemic lupus
erythematosus via arbitrating macrophage functional maturation. J
Clin Immunol. 33:925–937. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun W, Jiao Y, Cui B, Gao X, Xia Y and
Zhao Y: Immune complexes activate human endothelium involving the
cell-signaling HMGB1-RAGE axis in the pathogenesis of lupus
vasculitis. Lab Invest. 93:626–638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adhami VM, Syed DN, Khan N and Mukhtar H:
Dietary flavonoid fisetin: A novel dual inhibitor of PI3K/Akt and
mTOR for prostate cancer management. Biochem Pharmacol.
84:1277–1281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ying TH, Yang SF, Tsai SJ, Hsieh SC, Huang
YC, Bau DT and Hsieh YH: Fisetin induces apoptosis in human
cervical cancer HeLa cells through ERK1/2-mediated activation of
caspase-8-/caspase-3-dependent pathway. Arch Toxicol. 86:263–273.
2012. View Article : Google Scholar
|
|
16
|
Currais A, Prior M, Dargusch R, Armando A,
Ehren J, Schubert D, Quehenberger O and Maher P: Modulation of p25
and inflammatory pathways by fisetin maintains cognitive function
in Alzheimer's disease transgenic mice. Aging Cell. 13:379–390.
2014. View Article : Google Scholar
|
|
17
|
Mitra S, Biswas S, Sinha A, Jana NR and
Banerjee EM: Therapeutic use of fisetin and fisetin loaded on
mesoporous carbon nanoparticle (MCN) in thioglycollate-induced
peritonitis. J Nanomed Nanotechnol. 6:3322015. View Article : Google Scholar
|
|
18
|
Rabb H, Noel S, Martina-Lingua MN, Racusen
LC, Hamad AR and Bandapalle S: Compositions and methods for the
study and treatment of acute kidney injury. US Patent 14/930,883.
Filed November 3, 2015 issued May 5, 2016.
|
|
19
|
Summers SA, Odobasic D, Khouri MB,
Steinmetz OM, Yang Y, Holdsworth SR and Kitching AR: Endogenous
interleukin (IL)-17A promotes pristane-induced systemic
autoimmunity and lupus nephritis induced by pristane. Clin Exp
Immunol. 176:341–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Léotoing L, Davicco MJ, Lebecque P,
Wittrant Y and Coxam V: The flavonoid fisetin promotes osteoblasts
differentiation through Runx2 transcriptional activity. Mol Nutr
Food Res. 58:1239–1248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JD, Huh JE, Jeon G, Yang HR, Woo HS,
Choi DY and Park DS: Flavonol-rich RVHxR from rhus verniciflua
stokes and its major compound fisetin inhibits inflammation-related
cytokines and angiogenic factor in rheumatoid arthritic
fibroblast-like synovial cells and in vivo models. Int
Immunopharmacol. 9:268–276. 2009. View Article : Google Scholar
|
|
22
|
Feng D, Yang L, Bi X, Stone RC, Patel P
and Barnes BJ: Irf5-deficient mice are protected from
pristane-induced lupus via increased Th2 cytokines and altered IgG
class switching. Eur J Immunol. 42:1477–1487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Angius F and Floris A: Liposomes and MTT
cell viability assay: An incompatible affair. Toxicology In Vitro.
29:314–319. 2015. View Article : Google Scholar
|
|
24
|
Donnenberg VS and Donnenberg A: Flow
cytometry on disaggregated solid tissues. Int Drug Discov. 6:14–18.
2011.
|
|
25
|
Yuan JS, Wang D and Stewart CN:
Statistical methods for efficiency adjusted real-time PCR
quantification. Biotechnol J. 3:112–123. 2008. View Article : Google Scholar
|
|
26
|
Ding Q, Zhao M, Yu B, Bai C and Huang Z:
Identification of tetraazacyclic compounds as novel potent
inhibitors antagonizing RORγt activity and suppressing Th17 cell
differentiation. PloS One. 10:e01377112015. View Article : Google Scholar
|
|
27
|
Janda J, Plattet P, Torsteinsdottir S,
Jonsdottir S, Zurbriggen A and Marti E: Generation of equine
TSLP-specific antibodies and their use for detection of TSLP
produced by equine keratinocytes and leukocytes. Vet Immunol
Immunopathol. 147:180–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Clowse MEB, Chakravarty E, Costenbader KH,
Chambers C and Michaud K: Effects of infertility, pregnancy loss,
and patient concerns on family size of women with rheumatoid
arthritis and systemic lupus erythematosus. Arthritis Care Res.
64:668–674. 2012. View Article : Google Scholar
|
|
29
|
Chung SA, Brown EE, Williams AH, Ramos PS,
Berthier CC, Bhangale T, Alarcon-Riquelme ME, Behrens TW, Criswell
LA, Graham DC, et al: Lupus nephritis susceptibility loci in women
with systemic lupus erythematosus. J Am Soc Nephrol. 25:2859–2870.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Askanase A, Shum K and Mitnick H: Systemic
lupus erythematosus: An overview. Soc Work Health Care. 51:576–586.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Llanos C, Mackern-Oberti JP, Vega F,
Jacobelli SH and Kalergis KM: Tolerogenic dendritic cells as a
therapy for treating lupus. Clin Immunol. 148:237–245. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen N, Liang D, Tang Y and Tak PP:
MicroRNAs-novel regulators of systemic lupus erythematosus
pathogenesis. Nat Rev Rheumatol. 8:701–709. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Talaat RM, Mohamed SF and Bassyouni IH:
Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus
erythematosus (SLE) patients: Correlation with disease activity.
Cytokine. 72:146–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xing Q, Wang B, Su H, Cui J and Li J:
Elevated Th17 cells are accompanied by FoxP3+ Treg cells decrease
in patients with lupus nephritis. Rheumatol Int. 32:949–958. 2012.
View Article : Google Scholar
|
|
35
|
Chatterjee M, Rauen T, Kis-Toth K,
Kyttaris VC, Hedrich CM, Terhorst C and Tsokos GC: Increased
expression of SLAM receptors SLAMF3 and SLAMF6 in systemic lupus
erythematosus T lymphocytes promotes Th17 differentiation. J
Immunol. 188:1206–1212. 2012. View Article : Google Scholar :
|
|
36
|
Yu Y, Liu Y, Shi FD, Zou H, Matarese G and
La Cava A: Cutting edge: Leptin-induced RORγt expression in
CD4+ T cells promotes Th17 responses in systemic lupus
erythematosus. J Immunol. 190:3054–3058. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reeves WH, Lee PY, Weinstein JS, Satoh M
and Lu L: Induction of autoimmunity by pristane and other naturally
occurring hydrocarbons. Trends Immunol. 30:455–464. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Persson EK, Uronen-Hansson H, Semmrich M,
Rivollier A, Hägerbrand K, Marsal J, Gudjonsson S, Håkansson U,
Reizis B, Kotarsky K, et al: IRF4 transcription-factor-dependent
CD103+CD11b+ dendritic cells drive mucosal T
helper 17 cell differentiation. Immunity. 38:958–969. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen DY, Chen YM, Wen MC, Hsieh TY, Hung
WT and Lan JL: The potential role of Th17 cells and Th17-related
cytokines in the pathogenesis of lupus nephritis. Lupus.
21:1385–1396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Barbado J, Martin D, Vega L, Almansa R,
Gonçalves L, Nocito M, Jimeno A, Ortiz de Lejarazu R and
Bermejo-Martin JF: MCP-1 in urine as biomarker of disease activity
in Systemic Lupus Erythematosus. Cytokine. 60:583–586. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Petri M, Wallace DJ, Spindler A,
Chindalore V, Kalunian K, Mysler E, Neuwelt CM, Robbie G, White WI,
Higgs BW, et al: Sifalimumab, a human anti-interferon-α monoclonal
antibody, in systemic lupus erythematosus: A phase I randomized,
controlled, dose-escalation study. Arthritis Rheum. 65:1011–1021.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lisi S, Sisto M, Lofrumento DD, D'Amore M,
De Lucro R and Ribatti D: A potential role of the GRO-α/CXCR2
system in Sjögren's syndrome: Regulatory effects of
pro-inflammatory cytokines. Histochem Cell Biol. 139:371–379. 2013.
View Article : Google Scholar
|
|
43
|
Zhao PW, Jiang WG, Wang L, Jiang ZY, Shan
YX and Jiang YF: Plasma levels of IL-37 and correlation with TNF-α,
IL-17A, and disease activity during DMARD treatment of rheumatoid
arthritis. PLoS One. 9:e953462014. View Article : Google Scholar
|
|
44
|
Moles A, Murphy L, Wilson CL, Chakraborty
JB, Fox C, Park EJ, Mann J, Oakley F, Howarth R, Brain J, et al: A
TLR2/S100A9/CXCL-2 signaling network is necessary for neutrophil
recruitment in acute and chronic liver injury in the mouse. J
Hepatol. 60:782–791. 2014. View Article : Google Scholar :
|
|
45
|
Tung YT, Chua MT, Wang SY and Chang ST:
Anti-inflammation activities of essential oil and its constituents
from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour
Technol. 99:3908–3913. 2008. View Article : Google Scholar
|
|
46
|
Zughaier S, Aneja R and Stephens DS:
Noscapine and analogs and methods related thereto. US Patent
8,841,317. Filed August 24, 2011 issued September 23, 2014.
|
|
47
|
Smiljanovic B, Grün JR, Biesen R,
Schulte-Wrede U, Baumgrass R, Stuhlmüller B, Maslinski W, Hiepe F,
Burmester GR, Radbruch A, et al: The multifaceted balance of TNF-α
and type I/II interferon responses in SLE and RA: How monocytes
manage the impact of cytokines. J Mol Med. 90:1295–1309. 2012.
View Article : Google Scholar
|
|
48
|
Schiffer L, Bethunaickan R, Ramanujam M,
Huang W, Schiffer M, Tao H, Madaio MP, Bottinger EP and Davidson A:
Activated renal macrophages are markers of disease onset and
disease remission in lupus nephritis. J Immunol. 180:1938–1947.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kuroiwa T and Lee EG: Cellular
interactions in the pathogenesis of lupus nephritis: The role of T
cells and macrophages in the amplification of the inflammatory
process in the kidney. Lupus. 7:597–603. 1998. View Article : Google Scholar
|