Introduction
Prion disease, or transmissible spongiform
encephalopathy, refers to a group of fatal neurodegenerative
disorders reported in humans and animals (1). Prion diseases in humans include
Creutzfeldt-Jakob disease (CJD), fatal familial insomnia and
Gerstmann-Sträussler-Scheinker syndrome. The most common form of
human prion disease is sporadic CJD (sCJD), with a worldwide
incidence of ~1 case per million individuals annually (2,3).
Typically, sCJD presents with rapidly progressing ataxia, dementia
and myoclonus. The clinical duration of the disease is usually
<2 years, with the majority of patients succumbing to the
disease within 6 months.
A definitive diagnosis of sCJD requires
neuropathological or immunochemical detection of the prion protein
(PrPSc) (4).
PrPSc is partially protease-resistant and can induce its
normal cellular isoform (PrPC), to undergo a
conformational change. This occurs in a self-propagating manner
through a seeded aggregation process, resulting in accumulation of
PrPSc throughout the brain tissues, together with
accompanying spongiform alterations, neuronal loss and gliosis
(1,5,6).
Other than brain biopsy, there is currently no other
disease-specific pre-mortem diagnostic test for sCJD (7). Diagnosis of probable sCJD is based
on abnormal findings as determined by clinical examinations and
laboratory tests. Abnormal test results include periodic sharp wave
complexes on electroencephalogram, and altered signals on brain
magnetic resonance image and/or positive detection of 14-3-3
protein in the cerebrospinal fluid (CSF) (8). Postmortem examinations are rarely
performed in China due to cultural traditions (9). Therefore, methods that permit the
accurate diagnosis of sCJD are required.
Numerous studies have attempted to identify
biomarkers in CSF samples for the diagnosis of human prion
diseases. Proteins that have showed diagnostic values include
14-3-3, tau, S100 and neuron specific enolase (10-13). However, only a positive test
result for 14-3-3 protein in the CSF using western blotting is
included in the diagnostic criteria for sCJD (14,15). The detection of PrPSc
in the CSF of patients with sCJD and other types of human prion
diseases is difficult with routine testing methods, even using the
sensitive protein-misfolding cyclic amplification (PMCA)
technique.
Recently, a novel technique known as real-time
quaking-induced conversion (RT-QuIC) has been developed that is
based on amyloid fibril formation, a characteristic feature of
prion proteins (16-18). A number of studies have identified
that RT-QuIC has good sensitivity and specificity for the diagnosis
of sCJD using human CSF samples (16,17). In the present study, a number of
experimental variables that may influence RT-QuIC assay were
evaluated. Using the identified optimal conditions, the capacity of
the RT-QuIC assay to detect PrPSc in the brain
homogenates of 263K-infected hamsters and in the CSF samples of
probable sCJD patients was also evaluated.
Materials and methods
Ethics statement
The present study was approved by the Ethical
Committee of the National Institute for Viral Disease Control and
Prevention (Beijing, China) under the protocol 2009ZX10004-101,
including the use of brain samples of hamsters infected with the
scrapie strain 263K and CSF samples from probable sCJD patients and
non-CJD patients. Informed consent was obtained from all patients
prior to participation.
Expression and purification of the
hamster recombinant PrP (rPrP) protein
Hamster rPrPC protein (residues 23-231;
GenBank accession no. K02234) was prepared according to the method
described by Wilham et al (18) with a few modifications. Briefly,
the DNA sequence of hamster PrPC was ligated into the
pRSET vector (cat. no. V35120; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and then the recombinant plasmid was transformed
into BL21 (DE3)pLysS competent cells (cat. no. C1500; Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China). We
expressed rPrPC in 1 liter terrific broth medium (cat.
no. 71491; Novagen; Merck KGaA, Darmstadt, Germany). Next,
rPrPC protein was denatured with guanidine-HCL and
purified by chromatography using Ni-NTA Superflow resin (cat. no.
30430; Qiagen, Hilden, Germany) in an XK 16/40 column (GE
Healthcare Life Sciences, Little Chalfont, UK) at a flow rate of
1.9 ml/min. The concentration of rPrPC was adjusted to
~500 µg/ml as determined by the bicinchoninic acid reagent (cat.
no. 71285; EMD Millipore, Billerica, MA, USA). The purity of the
prepared rPrPC was evaluated by 15% SDS-PAGE and western
blot analysis. Briefly, proteins were transferred onto a
nitrocellulose filter membrane (cat. no. 10600001; GE Healthcare
Life Sciences), which was then blocked with 5% skim milk in
tris-buffered saline with 0.1% Tween-20 containing 0.1% Tween-20
for 1 h followed by incubation with the primary antibodies against
3F4 (cat. no. MAB1562-K; EMD Millipore) and β-actin (HX1827,
Beijing Huaxing Bochuang Gene Technology, Co., Ltd., Beijing,
China) overnight at 4°C. Subsequently, a horseradish
peroxidase-conjugated goat anti-mouse IgG secondary antibody (cat.
no. 31430; Thermo Fisher Scientific, Inc.) was incubated at 37°C
for 2 h. Both primary and secondary antibodies were usedat a
dilution of 1:5,000. The results were scanned with Bio-Rad
Molecular Imager (Bio-Rad Laboratories, Inc., Hercules, California,
USA) and analyzed by ImageJ software (National Institutes of
Health, Bethesda, MD, USA).
Preparation of brain homogenate (BH)
samples
A total of 10 3-week-old female Syrian golden
hamsters (45.8±1.3 g; cat. no. 501; Beijing Vital River Laboratory
Animal Technology Co., Ltd., Beijing, China) were intracerebrally
inoculated with 5 µl hamster-adapted scrapie agents 263K (19,20). Animals were housed withfree access
to food and water under a humidity between 40-70%, temperature
between 20-26°C and a 12 h light/dark cycle. The incubation time of
the 263K-infected hamsters was 70.5±4.93 days (21,22). Animals were sacrificed using ether
and exsanguinated, and then the brains were surgically removed from
each hamster. Subsequently, 10% BHs were prepared in lysis buffer
(100 mM NaCl, 10 mM EDTA, 0.5% Nonidet P-40, 0.5% sodium
deoxycholate, 10 mM Tris, pH 7.5) according to a previously
described protocol (21,22). In order to set up a
PrPSc panel for RT-QuIC, serially diluted (from
10−3 to 10−9) Sc-BH samples were prepared.
Proteinase K-resistant PrP signals were detectable in Sc-BH samples
of 10−3 using routine western blotting.
CSF samples
A total of 70 CSF samples from probable sCJD
(63.3±8.8 years old, male/female ratio: 1.12) and 48 CSF samples
from non-CJD patients (55.1±14.5 years old, male/female ratio:
1.28) were included in the current study. Informed consent was
obtained from all patients prior to participation. All the samples
were collected at the CJD surveillance network of hospitals without
any additional treatments and sent to the CJD Surveillance Center
during Jan to Dec in 2015. The diagnosis of probable sCJD was
conducted according to the diagnostic criteria for CJD issued by
the World Health Organization and the surveillance document for CJD
issued by Chinese Center for Disease Control and Prevention
(Beijing, China) (9). Non-CJD
cases included patients whose clinical manifestations and
examinations did not fulfill the diagnostic criteria for human
prion disease even after follow-up, or patients who had other
diagnoses. The samples were collected from the CSF Bank in the
China CJD Surveillance Center attached to China CDC (Beijing,
China), and 200 µl from each CSF sample was obtained. All enrolled
CSF samples were free of blood contamination. Routine CSF
biochemistry assays of those specimens, including cell count,
glucose level and total protein, were all within the normal
ranges.
Evaluation of the effect of various main
elements on the reactivity of RT-QuIC
To identify any factors that affect the RT-QuIC
assay for detecting PrPSc, a set of variables were
independently evaluated. The basic elements in the working buffer
were 1X PBS, 85-340 mM NaCl, 0.5-2 mM EDTA, 10-80 µM thioflavin T
(ThT) and 5-20 µg rPrPC. These concentrations were
selected based on published data and our previous experiments
(16,23). In addition, 10−5
diluted BH from 263K-infected hamster at the terminal stage (Sc-BH)
and BH from age-matched normal hamsters (Nor-BH) were also prepared
as the positive and negative controls, respectively.
RT-QuIC assay
RT-QuIC was conducted in a black 96-well,
optical-bottomed plate (Nunc 265301; Thermo Fisher Scientific,
Inc.) on a BMG FLUOstar microplate reader (BMG LABTECH GmbH,
Ortenberg, Germany). Next, 1 µl diluted BH or 5-30 µl CSF sample
was mixed with 1X PBS, 170 mM NaCl, 1 mM EDTA, 0.01 mM ThT, 0.001%
SDS and 10 µg rPrPC in a final volume of 100 µl. Each
sample was assayed in triplicated. Each reaction contained the
following control groups: Blank (reaction buffer only), positive
(Sc-BH) and negative (Nor-BH). The working conditions were as
follow: Temperature, 50°C; vibration speed, 1.996 × g;
vibration/incubation time, 90/30 sec; total reaction time, 90 h.
ThT fluorescence (excitation wavelength, 450 nm; emission
wavelength, 480 nm) was automatically measured every 30 min and
expressed as relative fluorescence units (rfu). The cutoff value
was set as the mean value of the negative controls plus 3 times the
standard deviation. A sample was considered to be positive when ≥2
wells revealed positive reaction curves.
Results
Determination of appropriate
rPrPC concentration
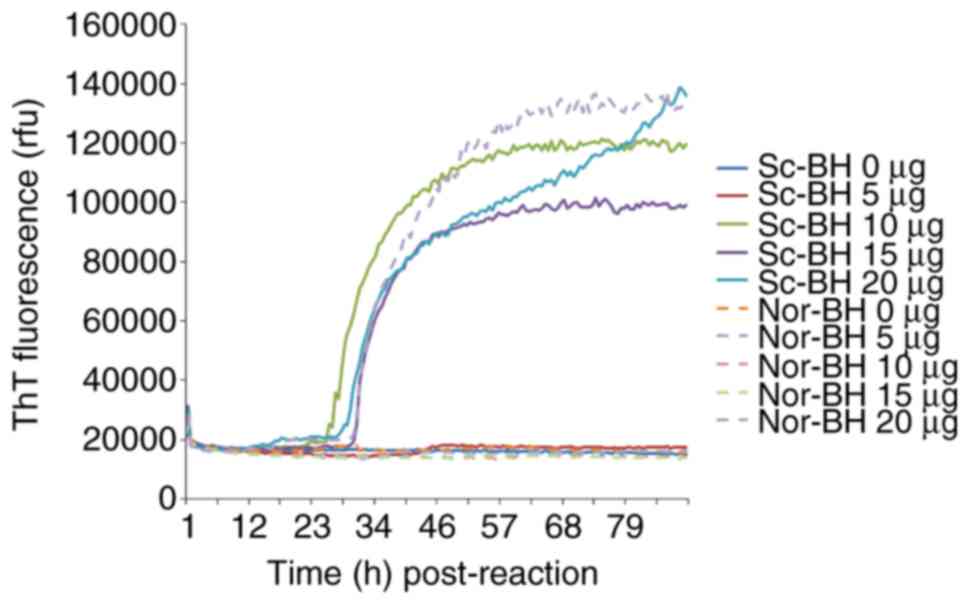
Different amounts (5, 10, 15 and 20 µg) of purified
rPrPC, serving as the substrate, were subjected to
RT-QuIC. Using Sc-BH as the seed, which induces the fibrillation of
rPrPC, increases in the ThT fluorescence curves were
observed in the reactions of 10, 15 and 20 µg rPrPC,
which began to increase at ~30 h post-reaction, while only a slight
elevation of the ThT value was detected in the 5 µg
rPrPC reaction (Fig.
1). However, a positive increase of ThT value was also recorded
in part of duplication of 20 µg rPrPC using Nor-BH as
the seed, indicating a false positive reaction. Based on these
data, 10 µg rPrPC was suggested to be the optimal
working amount as the substrate in RT-QuIC.
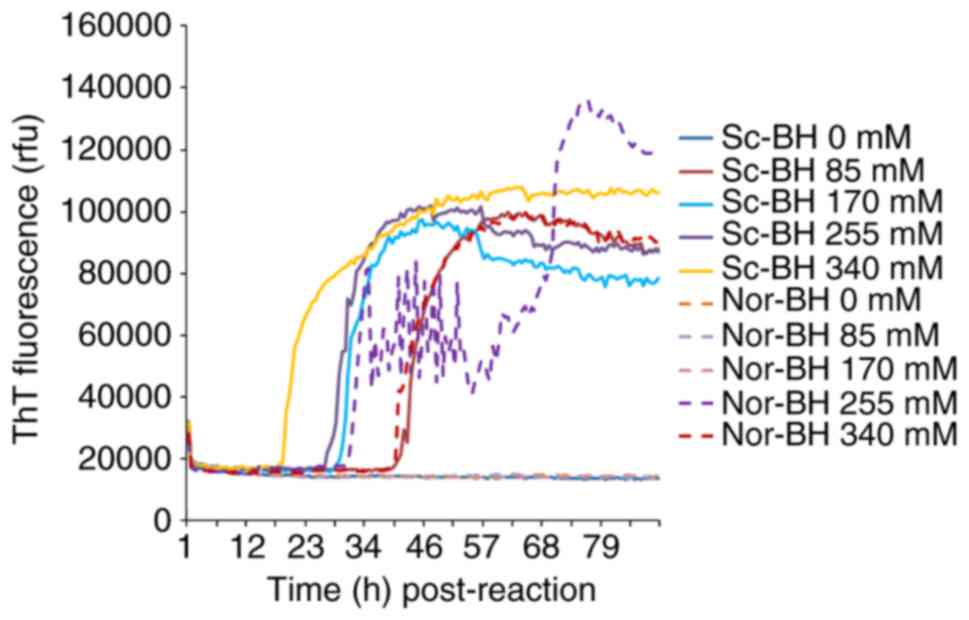
Determination of appropriate NaCl
concentration
Different concentrations of NaCl (0, 85, 170, 255
and 340 mM) were examined. In the condition of 10−5
dilutedSc-BH, four preparations containing NaCl demonstrated
positive reaction. The RT-QuIC reactivity was revealed to be NaCl
dose-dependent. Along with the increase in the amount of NaCl, the
positive conversion time post-reaction was decreased and the peak
values of ThT values increased. However, increased ThT curves were
also observed in the preparations of negative control containing
255 and 340 mM NaCl. This suggested that a high concentration of
NaCl was able to increase the reactivity of RT-QuIC, as well as
increase the possibility of false positive (Fig. 2). Thus, the NaCl concentration of
170 mM was selected for subsequent experiments.
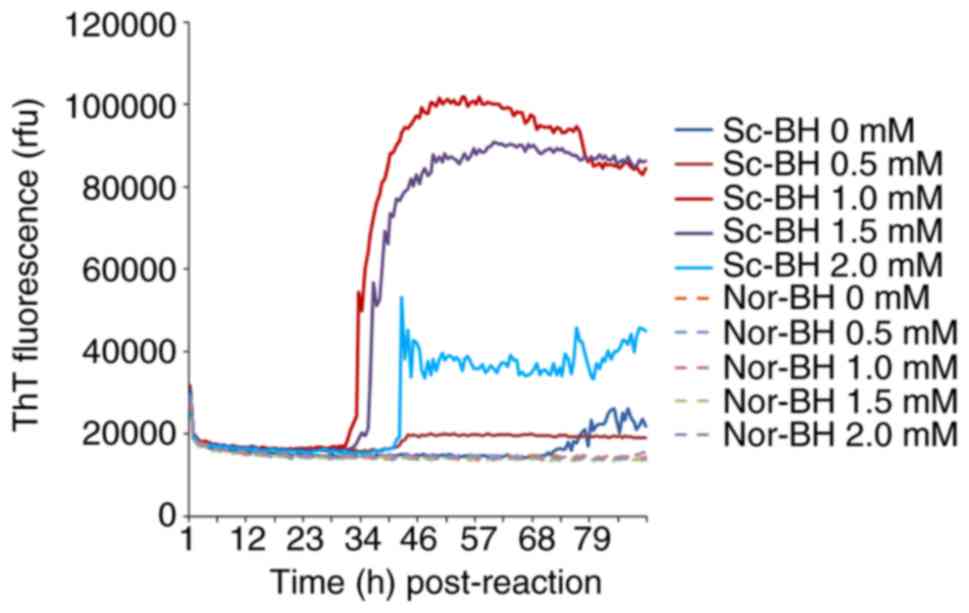
Determination of appropriate EDTA
concentration
Different concentration of EDTA (0, 0.5, 1, 1.5 and
2 mM) were also tested in the RT-QuIC assay containing
10−5 diluted Sc-BH. After 34 h, the reaction curve with
1 mM EDTA began to increase, followed by the curves of the 1.5 and
2 mM EDTA reactions. At a relatively late stage, the reactions with
0 and 0.5 mM EDTA exhibited weak positive results. These data
illustrated that certain concentrations of EDTA (particularly 1 mM)
help the reactivity of RT-QuIC assay, whereas high concentrations
of EDTA inhibit the assay (Fig.
3).
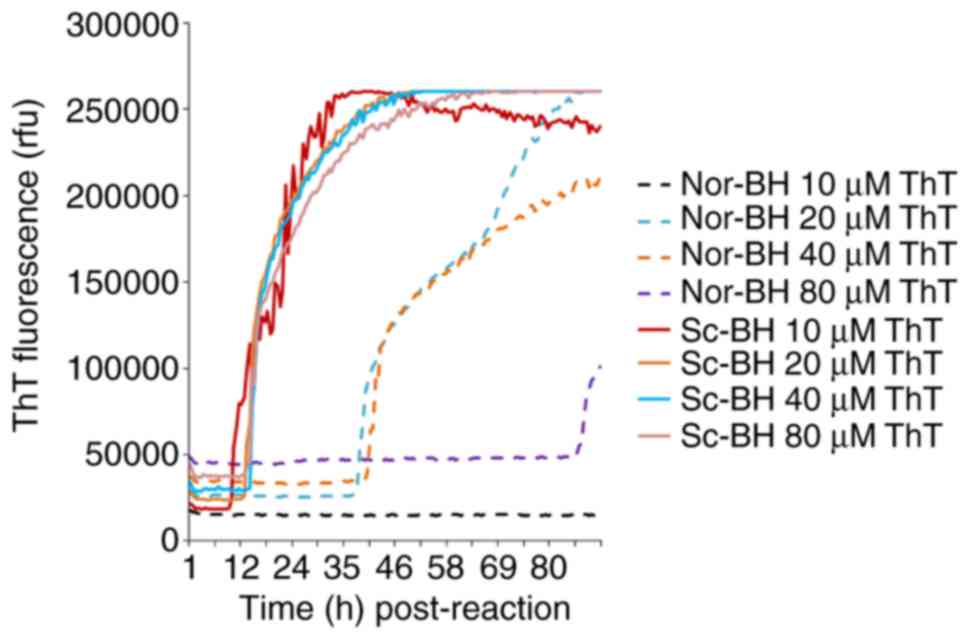
Determination of appropriate ThT final
concentration
Four different final concentrations of ThT (10, 20,
40 and 80 µM) were also examined in the RT-QuIC assay containing
10−5 diluted Sc-BH. All four preparations of
PrPSc exhibited positive curves at approximately 9-12 h
post-reaction, and reached similarly high ThT fluorescence values
after 30 h of reaction (Fig. 4).
However, increased curves were also observed in the negative
controls containing 20, 40 and 80 µM ThT, although these emerged
with relatively long lag phases (Fig.
4). These findings suggest that an increase in ThT
concentration does not enhance the sensitivity of RT-QuIC assay and
provides false positive results.
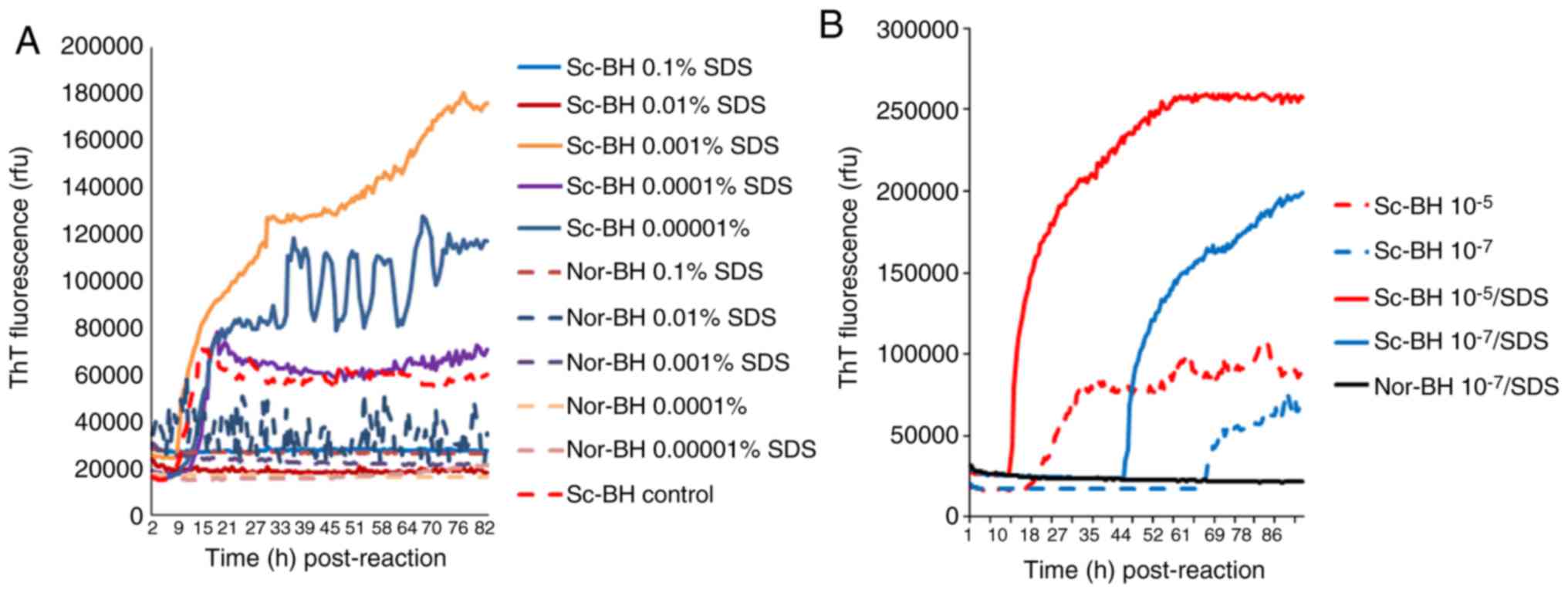
Determination of appropriate SDS
concentration
Different concentrations of SDS were added to
reactions containing 10−5 diluted Sc-BH, resulting in
final concentrations of SDS between 0.1 and 0.00001%. As shown in
Fig. 5A, positive reaction curves
were observed in the preparations of 0.001, 0.0001 and 0.00001%
SDS, among which the reaction using 0.001% SDS exhibited a markedly
shorter lag phase and higher ThT value. Positive reactivities were
markedly inhibited in the presences of 0.1 and 0.01% SDS, and a
false weak positive reaction was observed in the preparation of
Nor-BH containing 0.01% of SDS. Furthermore, the effect of 0.001%
SDS on the reactivity of different amounts of PrPSc was
evaluated. In the conditions of 263K BH diluted for 10−5
and 10−7 times, the presence of 0.001% SDS caused a
evidently quicker increase and higher ThT fluorescence values
(Fig. 5B).
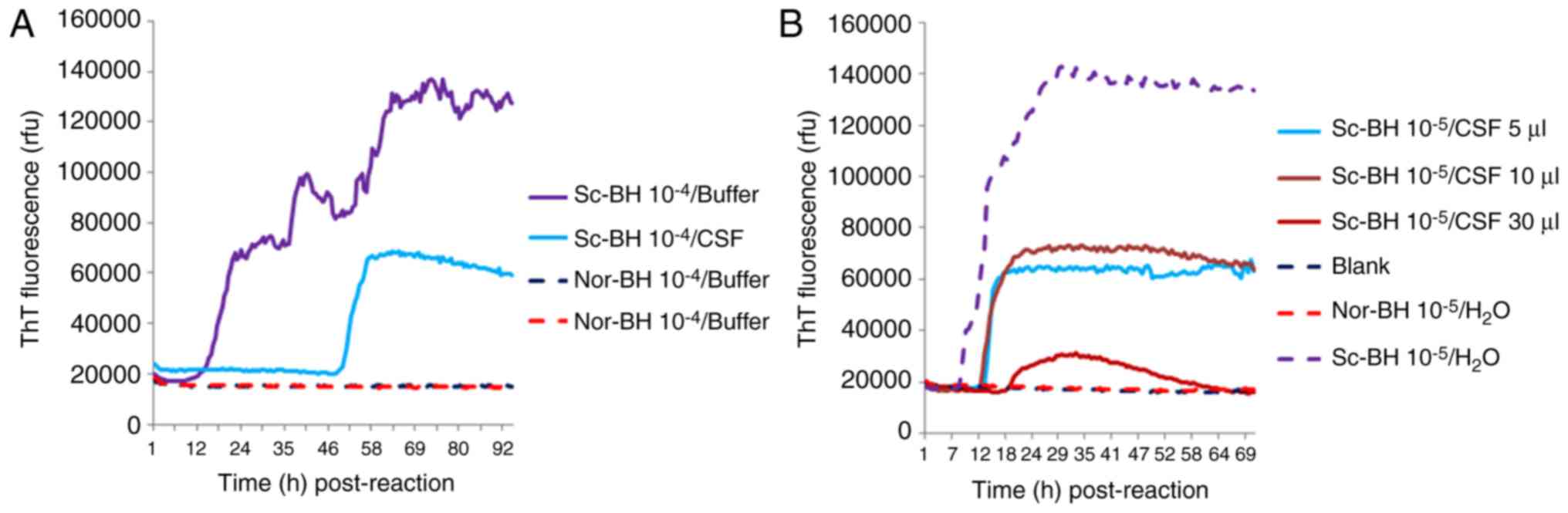
Determination of appropriate CSF
amount
To test the potential influence of human CSF on the
reactivity in RT-QuIC assays, 10−5 times diluted Sc-BH
was added into 30 µl CSF collected from a non-CJD patient with
major indexes in the CSF biochemistry within the normal range.
Compared with the same dilution of Sc-BH only, the lag time and
fluorescence peak in the reaction of Sc-BH in CSF were evidently
longer and lower, respectively (Fig.
6A). Furthermore, 10−5 times diluted Sc-BH was
separately mixed with 5, 10 and 30 µl CSF samples. The RT-QuIC
assay results demonstrated that the reactions with 5 and 10 µl CSF
displayed similar positive reactivities with a much shorter lag
phase and higher fluorescence peak in comparison with that of 30 µl
CSF (Fig. 6B). Thus, it appears
that certain unknown components of human CSF may hamper the RT-QuIC
assay; therefore, using relatively large amount of tested CSF in
RT-QuIC does not benefit the detecting ability for
PrPSc.
Final experimental conditions
Based on the aforementioned data, the experimental
conditions of RT-QuIC were set-up, and these involved the final
concentrations of 1X PBS, 170 mM NaCl, 1 mM EDTA, 0.01 mM ThT and
0.001% SDS with 10 µg rPrPC and 10 µl CSF in a total
reaction volume of 100 µl. The positive control was 10−5
diluted BH collected from 263K-infected hamsters and the negative
control was the same dilution of BH obtained from normal
hamsters.
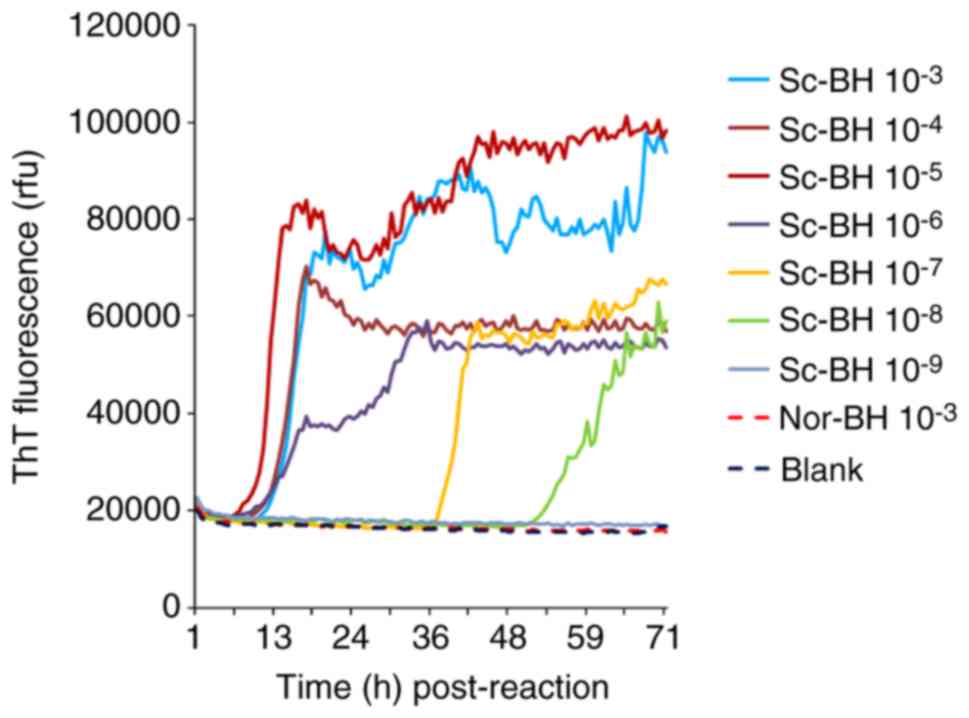
Evaluation of the capacity of the
optimized RT-QuIC assay in the detection of PrPSc in
brain tissues and CSF samples
The serially diluted Sc-BHs (10−3 to
10−9) were separately subjected to the optimized RT-QuIC
reactions. As shown in Fig. 7,
the ThT fluorescence values of the preparations of blank control
and Nor-BH were almost unchanged at 90 h post-reaction. By
contrast, the ThT values of the reactions containing relatively
high amounts of Sc-BH (between 10−3 and 10−6)
increased markedly after lag phases of approximately 9-12 h
post-reaction. The preparations of 10−7 and
10−8 diluted PrPSc BHs exhibited increased
ThT values at 37 and 52 h post-reaction, respectively. No positive
result was observed in the reaction of 10−9 diluted
PrPSc BH. Furthermore, the ThT fluorescence in the
preparations containing high amounts of PrPSc was
evidently higher in comparison with that of reactions containing
low amounts of PrPSc. The PrPSc detection
threshold of the RT-QuIC assay reported in the present study was
observed to be 10−8 diluted BH of 263K-infected
hamsters.
To access the efficacy of the optimized RT-QuIC
assay in human sCJD CSF samples, CSF samples from 70 patients that
fulfilled the diagnostic criteria for probable sCJD and 48 patients
that were classified as non-CJD were assayed. In total, 10 or 30 µl
CSF sample from each patient was separately tested. Using 30 µl
CSF, 11 (15.71%) samples in the probable sCJD group tested
positive, while all tested samples in the non-CJD group were
negative. Positive conversion times varied between 8 and 28 h
post-reaction, while the peak ThT value varied between 44,000 and
61,000 rfu. Using 10 µl CSF, 40 (57.14%) samples of probable sCJD
tested positive, whereas 11 samples of non-CJD also exhibited
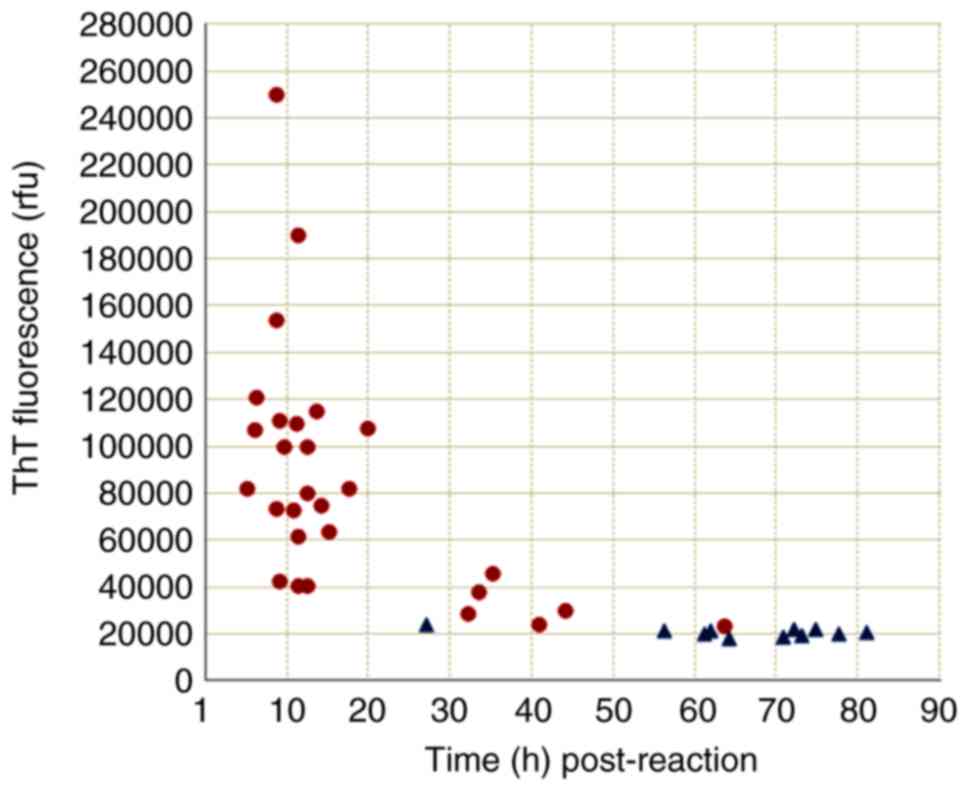
weakly positive results. Further analysis of the positive reaction
patterns in the RT-QuIC assay revealed notably different profiles
between the groups of probable sCJD (28 samples) and non-CJD (11
samples). As shown in Fig. 8, the
majority of the samples from the probable sCJD group tested
positive within 50 h post-reaction (27/28 samples), and exhibited
peak ThT values of >25,000 rfu (26/28 samples). By contrast,
only 1 case of non-CJD tested positive within 50 h post-reaction,
and the peak ThT values of all non-CJD case were <25,000 rfu.
These data indicate that applying the CSF RT-QuIC assay with the
aforementioned experimental conditions was able to detect
substantially more probable sCJD cases, with a significantly
shorter lag phase (<50 h post-reaction) and higher ThT
fluorescence values (>25,000 rfu).
Discussion
The results of RT-QuIC, which is considered to be a
sensitive assay, can be affected by a variety of factors. Under a
specific working temperature and vibration speed, the effect of
certain variables on the RT-QuIC detection capacity was evaluated
in the present study. Similarly to a previous study (12), a type of E. coli-expressed
full-length wild-type hamster rPrP was used as substrate in the
RT-QuIC assay reported in the current study. In a certain range of
rPrPC concentrations, the sensitivity of RT-QuIC
exhibits a positive association with the amount of the input
rPrPC, however, false positive results are also easily
inducible. PrP protein is capable of spontaneous fibrillation in
vitro when incubated at certain conditions, for it was reported
that agitation alone induces de novo conversion of
recombinant prion proteins to b-sheet rich fibrils (24). Using PMCA, a wild-type mouse rPrP
protein can be converted into a pathogenic isoform that has the
biochemical characteristics of PrPSc in vitro, as
well as the typical infectivity on experimental rodents (25). Therefore, careful consideration of
the rPrPC amount and of the normal negative control is
important, particularly when using different batches of purified
rPrPC protein.
Partially denatured rPrPC protein may
help to form fibrils in RT-QuIC assay. In addition, using specific
concentrations of PBS, NaCl, EDTA and SDS in the working buffer
benefits the induction of earlier and higher positive reactions in
the RT-QuIC assay. Meanwhile, excessive concentrations of salt and
cleaning surfactants lead to a false positive result or inhibit the
reactivity of RT-QuIC. ThT is a commonly used chemical for
diagnosis of the amyloid structure; however, it is not perfectly
specific for amyloid (26). The
spectroscopic change of ThT may differ largely, depending on the
particular protein and experimental conditions. In the present
study, it was also observed that a high concentration of ThT
induced evidently false positive reactions in the negative control
sample. Thus, a careful balance of the use of salt, cleaning
surfactants and ThT concentrations in the reaction buffer is
essential for ensuring the sensitivity and specificity of RT-QuIC
assay.
The current study also demonstrated that the amount
of human CSF used can inhibit the RT-QuIC assay. Human CSF samples
with normal biochemical profiles significantly reduced the ThT
fluorescence intensity and prolonged the lag time until a positive
reaction was detected. These results suggested that there were
certain unknown factors in human CSF that inhibited the
PrPSc amyloid formation in the RT-QuIC assay. An
improved RT-QuIC assay sensitivity was detected in reactions using
a relatively low amount of human CSF (5 and 10 µl) under the
experimental conditions of the current study. Further studies
identifying and removing any inhibitor(s) in human CSF would
improve the sensitivity of CSF RT-QuIC assay for sCJD
detection.
Using optimized experimental conditions in the
present study, samples containing 10−8 dilution of 263K
hamster BHwere successfully detected, which indicated a higher
PrPSc detection capacity in comparison with that of
routine western blot analysis (10−3) and that of PMCA
(10−5) using 10% BH of normal hamsters as a substrate
(27). A number of CSF samples
from probable CJD and non-CJD patients were also preliminarily
screened in the present study. Approximately 60% of the CSF samples
from the probable sCJD patient group tested positive. Notably, the
majority of these results occurred within 50 h post-reaction
(median, 11.85 h), and had high peak ThT fluorescence values
(median, 77,500 rfu). Certain CSF samples from the non-CJD patient
group also exhibited weakly positive reactions, however, these had
markedly longer lag phases (median, 70.70 h post-reaction) and
evidently reduced peak ThT values (median, 21,000 rfu). On the
basis of these data, it is proposed that <50 h post-reaction
and/or >25,000 rfu should be used as the cut-off values for
positive test results when applying the CSF RT-QuIC assay with the
experimental conditions reported in the current study. Certainly, a
larger number of samplesare required to further validate the
suitability of these criteria in the clinical diagnosis of
sCJD.
In conclusion, the present study evaluated the
factors which may affect the RT-QuIC assay, and confirmed that
RT-QuIC was capable of detecting traces of PrPSc.
Application of this assay using CSF samples in the present study
revealed the potential use as a pre-mortem tool for the diagnosis
of sCJD.
Funding
This work was supported by Chinese National Natural
Science Foundation Grants (grant nos. 81630062, 81301429 and
81572048), National Key Research and Development Plan (grant no.
2016YFC1202700) and SKLID Development Grant (grant nos.
2012SKLID102 and 2015SKLID503).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
KX designed the study, acquired the data and
prepared the manuscript; QS prepared the manuscript; JW, CG and BYZ
assisted in the RT-QuIC assay; CC performed statistical analysis;
WZ and QS prepared the samples; XPD, who was the corresponding
author, designed the study and revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of the National Institute for Viral Disease Control and
Prevention (Beijing, China; protocol 2009ZX10004-101). Informed
consent was obtained from all patients prior to participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
Acknowledgments
The authors would like to thank Dr Shelley Robison
for English language editing.
References
|
1
|
Prusiner SB: Prions Proc Natl Acad Sci
USA. 95:13363–13383. 1998. View Article : Google Scholar
|
|
2
|
Ladogana A, Puopolo M, Croes EA, Budka H,
Jarius C, Collins S, Klug GM, Sutcliffe T, Giulivi A, Alperovitch
A, et al: Mortality from Creutzfeldt-Jakob disease and related
disorders in Europe, Australia, and Canada. Neurology.
64:1586–1591. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen C and Dong XP: Epidemiological
characteristics of human prion diseases. Infect Dis Poverty.
5:472016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Budka H, Aguzzi A, Brown P, Brucher JM,
Bugiani O, Gullotta F, Haltia M, Hauw JJ, Ironside JW, Jellinger K,
et al: Neuropathological diagnostic criteria for Creutzfeldt-Jakob
disease (CJD) and other human spongiform encephalopathies (prion
diseases). Brain Pathol. 5:459–466. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borchelt DR, Scott M, Taraboulos A, Stahl
N and Prusiner SB: Scrapie and cellular prion proteins differ in
their kinetics of synthesis and topology in cultured cells. J Cell
Biol. 110:743–752. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prusiner SB: Novel properties and biology
of scrapie prions. Curr Top Microbiol Immunol. 172:233–257.
1991.PubMed/NCBI
|
|
7
|
Manix M, Kalakoti P, Henry M, Thakur J,
Menger R, Guthikonda B and Nanda A: Creutzfeldt-Jakob disease:
Updated diagnostic criteria, treatment algorithm, and the utility
of brain biopsy. Neurosurg Focus. 39:E22015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zerr I, Kallenberg K, Summers DM, Romero
C, Taratuto A, Heinemann U, Breithaupt M, Varges D, Meissner B,
Ladogana A, et al: Updated clinical diagnostic criteria for
sporadic Creutzfeldt-Jakob disease. Brain. 132:2659–2668. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi Q, Zhou W, Chen C, Gao C, Xiao K, Wang
J, Zhang BY, Wang Y, Zhang F and Dong XP: Quality evaluation for
the surveillance system of human prion diseases in China based on
the data from 2010 to 2016. Prion. 10:484–491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsich G, Kenney K, Gibbs CJ, Lee KH and
Harrington MG: The 14-3-3 brain protein in cerebrospinal fluid as a
marker for transmissible spongiform encephalopathies. N Engl J Med.
335:924–930. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanchez-Juan P, Sanchez-Valle R, Green A,
Ladogana A, Cuadrado-Corrales N, Mitrová E, Stoeck K, Sklaviadis T,
Kulczycki J, Hess K, et al: Influence of timing on CSF tests value
for Creutzfeldt-Jakob disease diagnosis. J Neurol. 254:901–906.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Otto M, Wiltfang J, Cepek L, Neumann M,
Mollenhauer B, Steinacker P, Ciesielczyk B, Schulz-Schaeffer W,
Kretzschmar HA and Poser S: Tau protein and 14-3-3 protein in the
differential diagnosis of Creutzfeldt-Jakob disease. Neurology.
58:192–197. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beaudry P, Cohen P, Brandel JP,
Delasnerie-Lauprêtre N, Richard S, Launay JM and Laplanche JL:
14-3-3 protein, neuron-specific enolase, and S-100 protein in
cerebrospinal fluid of patients with Creutzfeldt-Jakob disease.
Dement Geriatr Cogn Disord. 10:40–46. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Collins S, Boyd A, Fletcher A, Gonzales M,
McLean CA, Byron K and Masters CL: Creutzfeldt-Jakob disease:
Diagnostic utility of 14-3-3 protein immunodetection in
cerebrospinal fluid. J Clin Neurosci. 7:203–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi Q, Gao C, Zhou W, Zhang BY, Chen JM,
Tian C, Jiang HY, Han J, Xiang NJ, Wang XF, et al: Surveillance for
Creutzfeldt-Jakob disease in China from 2006 to 2007. BMC Public
Health. 8:3602008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McGuire LI, Peden AH, Orru CD, Wilham JM,
Appleford NE, Mallinson G, Andrews M, Head MW, Caughey B, Will RG,
et al: Real time quaking-induced conversion analysis of
cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann
Neurol. 72:278–285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Atarashi R, Satoh K, Sano K, Fuse T,
Yamaguchi N, Ishibashi D, Matsubara T, Nakagaki T, Yamanaka H,
Shirabe S, et al: Ultrasensitive human prion detection in
cerebrospinal fluid by real-time quaking-induced conversion. Nat
Med. 17:175–178. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wilham JM, Orru CD, Bessen RA, Atarashi R,
Sano K, Race B, Meade-White KD, Taubner LM, Timmes A and Caughey B:
Rapid end-point quantitation of prion seeding activity with
sensitivity comparable to bioassays. PLoS Pathog. 6:e10012172010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang GR, Shi S, Gao C, Zhang BY, Tian C,
Dong CF, Zhou RM, Li XL, Chen C and Dong XP: Alternations of tau
protein and its phosphorylated profiles in the experimental
hamsters infected by scrapie agents 263K and 139A. Bing Du Xue Bao.
25:202–207. 2009.In Chinese. PubMed/NCBI
|
|
20
|
Zhang BY, Tian C, Han J, Gao C, Shi Q,
Chen JM, Jiang HY, Zhou W and Dong XP: Establishment of a stable
PrP(Sc) panel from brain tissues of experimental hamsters with
scrapie strain 263K. Biomed Environ Sci. 22:151–156. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Chen L, Zhang BY, Han J, Xiao XL,
Tian HY, Li BL, Gao C, Gao JM, Zhou XB, et al: Comparison study on
clinical and neuropathological characteristics of hamsters
inoculated with scrapie strain 263K in different challenging
pathways. Biomed Environ Sci. 17:65–78. 2004.PubMed/NCBI
|
|
22
|
Gao JM, Gao C, Han J, Zhou XB, Xiao XL,
Zhang J, Chen L, Zhang BY, Hong T and Dong XP: Dynamic analyses of
PrP and PrP(Sc) in brain tissues of golden hamsters infected with
scrapie strain 263K revealed various PrP forms. Biomed Environ Sci.
17:8–20. 2004.PubMed/NCBI
|
|
23
|
Zanusso G, Monaco S, Pocchiari M and
Caughey B: Advanced tests for early and accurate diagnosis of
Creutzfeldt-Jakob disease. Nat Rev Neurol. 12:325–333. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ladner-Keay CL, Griffith BJ and Wishart
DS: Shaking alone induces de novo conversion of recombinant prion
proteins to beta-sheet rich oligomers and fibrils. PLoS One.
9:e987532014. View Article : Google Scholar
|
|
25
|
Yuan Z, Yang L, Chen B, Zhu T, Hassan MF,
Yin X, Zhou X and Zhao D: Protein misfolding cyclic amplification
induces the conversion of recombinant prion protein to PrP
oligomers causing neuronal apoptosis. J Neurochem. 133:722–729.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biancalana M and Koide S: Molecular
mechanism of Thioflavin-T binding to amyloid fibrils. Biochim
Biophys Acta. 1804.1405–1412. 2010.
|
|
27
|
Orru CD, Wilham JM, Vascellari S, Hughson
AG and Caughey B: New generation QuIC assays for prion seeding
activity. Prion. 6:147–152. 2012. View Article : Google Scholar : PubMed/NCBI
|