Introduction
Age-associated hearing loss, also referred to as
presbycusis, is a complex degenerative disease characterized by
hearing impairment (1).
Presbycusis is an increasingly important public health concern,
affecting 40% of individuals aged between 55 and 74 years old
(2). Aging-associated decline in
auditory sensitivity may be attributed to degeneration of the
central and/or peripheral auditory system (3). Central presbycusis refers to
age-associated degeneration in the auditory portion of the central
nervous system, which affects the ability to localize the temporal
and spatial origins of sounds and impairs speech understanding in
noisy environments (4).
Unfortunately, the mechanisms underlying these changes remain to be
fully elucidated.
The pathogenesis of presbycusis may be defined as
the sum of all conditions that lead to decreased hearing
sensitivity with advancing age. The cumulative effect of intrinsic
and extrinsic factors, including hereditary susceptibility,
inflammation, and oxidative stress, are reported to lead to hearing
loss (5-9). A heritability estimate indicates
that 35-55% of the variance in sensory presbycusis is attributed to
the effects of genes (10).
Certain candidate genes are well known to be associated with
oxidative stress, including manganese super-oxide dismutase (MnSOD)
(11). In addition, the increase
in reactive oxygen species (ROS) generation can lead to a state of
chronic inflammation (12). The
free radical theory of aging suggested by Denham Harman in 1956
suggested that endogenous reactive oxidants cause cumulative
oxidative damage to macromolecules, resulting in the aging
phenotype (13). Age-dependent
decreases in antioxidant mechanisms and profound accumulation of
ROS are causally linked to various health problems, including
cardiovascular disease, diabetes and neurodegenerative diseases.
NF-E2-related factor 2 (Nrf2) signaling is key in maintaining
antioxidant/oxidant homeostasis and in the defense against ROS
through modulation of a diverse set of cytoprotective enzymes,
including NAD(P) H quinone oxidoreductase 1 (NQO1), heme
oxygenase-1 (HO-1), and MnSOD (14,15), all of which have potent
antioxidant properties. Numerous studies have demonstrated that
oxidative stress serves a major role in the pathophysiology of
presbycusis (16,17). As an upstream regulator of Nrf2,
Wnt activation protects against oxidative stress-induced hair cell
damage (18). However, whether
the Nrf2 pathway is involved in central presbycusis remains to be
elucidated.
Mitochondria are important in the aging process
(19,20). Presbycusis, as one of the
age-associated diseases, has been associated with mitochondrial
oxidative damage (21). Although
mitochondrial (mt)DNA only encodes 13 mitochondrial proteins, it is
crucial for mitochondrial function (22). Due to the proximity of mtDNA to
the source of endogenous oxidants and the lack of any protective
histone covering, mtDNA is sensitive to oxidative stress (23). An accumulation of mtDNA mutations
and/or deletions may lead to mitochondrial dysfunction, further
increasing ROS generation and oxidative damage (24). Mutations of mtDNA include the
4,834-base pair (bp) deletion in rats and the 4,977-bp deletion in
humans, which are known as common deletions (CDs) and act as an
accurate biomarker for aging (25). In our previous studies, it was
found that mtDNA deletions may cause sensitivity to environmental
stress and aggravate hearing impairment (26). Previous studies have demonstrated
that Nrf2 may be involved in the cellular response to oxidative
stress and maintenance of mitochondrial function (27,28). However, the underlying association
between Nrf2 signaling and mtDNA damage in the process of aging
have not been thoroughly examined.
Natural aging can be experimentally modeled by the
chronic administration of D-galactose (D-gal). Animals treated with
D-gal exhibit increased oxidative stress, dysfunctional
mitochondria and neural damage; consequently, these animals exhibit
decreased cognitive function similar to the natural aging process
(29,30). Our previous studies demonstrated
that oxidative stress induced by D-gal may lead to central
presbycusis (31,32). The focus of the present study was
to investigate the role of Nrf2 signaling in the degeneration of
the auditory cortex using a mimetic aging model induced by D-gal.
Whether the Nrf2 signaling pathway is involved in mtDNA damage and
cell senescence in vitro was also investigated.
Materials and methods
Animals
A total of 162 male 4-week-old Sprague-Dawley rats,
weighing 89.25±13.60 g, were obtained from the Experimental Animal
Center of Tongji Medical College, Huazhong University of Science
and Technology (HUST; Wuhan, China). A previous study showed that
presbycusis is influenced by gender (33). Males demonstrate a higher
incidence of presbycusis with a rapid deterioration in the hearing
threshold and anatomical degeneration (33). Therefore, male rats were selected
for the experimental model, instead of female rats. All rats were
housed in an air-conditioned animal facility (22°C, 50-60% relative
humidity) with a 12:12-h light-dark cycle and allowed free access
to standard chow and tap water. Following acclimatization for 4
weeks, the 2-month-old rats were randomly allocated into two
groups: The normal saline (NS; n=81) and D-galactose (D-gal; n=81)
groups. The rats in the D-gal group were injected subcutaneously
with D-gal (500 mg/kg/day; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) for 8 weeks, whereas rats in the NS group received the
same volume of 0.9% normal saline (NS) on the same schedule,
injected once a day at a fixed time. Following the final injection,
the NS and D-gal groups were divided into three age subgroups:
4-month-old (immediately following the final injection),
10-month-old (6 months following the final injection) and
16-month-old (12 months following the final injection). All
procedures involving the care of animals were performed in
accordance with the guidelines of the Care and Use of Laboratory
Animals of the National Institutes of Health (34). The protocol was approved by the
Committee on the Ethics of Animal Experiments of HUST.
Cell culture and treatment
Well-differentiated rat pheochromocytoma (PC12)
cells induced by nerve growth factor were obtained from the
Shanghai Institutes for Biological Sciences of the Chinese Academy
of Cell Resource Center (Shanghai, China). The PC12 cells were
maintained and cultured in DMEM (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
100 IU/ml penicillin (Sigma-Aldrich; Merck KgaA) at 37°C in a
humidified atmosphere of 95% air and 5% CO2.
Target-specific Nrf2 small interfering (si)RNAs (GenePharma;
Shanghai, China) were designed to knock down the gene expression in
PC12 cells. An siRNA encoding a nonsense sequence was designed as
the negative control. The cells were transfected in 2 ml Opti-MEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 10 µl
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.),
with 200 nM Nrf2 siRNA or control siRNA according to the
manufacturer's protocol. The Opti-MEM medium was replaced 6 h later
with DMEM containing FBS. The following siRNA was used to knock
down the expression of Nrf2: siRNA-Nrf2, sense 5'-GCA AGA AGC CAG
AUA CAA ATT-3' and antisense 5'-UUU GUA UCU GGC UUC UUG CTT-3';
control, sense 5'-UUC UCC GAA CGU GUC ACG UTT-3' and antisense
5'-ACG UGA CAC GUU CGG AGA ATT-3'. The cells were incubated with
D-gal (Sigma-Aldrich; Merck KGaA) at 37°C in a humidified
atmosphere containing 5% CO2 for 48 h, with or without
oltipraz (Sigma-Aldrich; Merck KGaA) pretreatment for 1 h. Dimethyl
sulfoxide (DMSO; Sigma-Aldrich; Merck KgaA) was used for the
dissolution of oltipraz. The DMSO groups were pretreated with an
equal volume of DMSO (<0.2% final).
Measurements of malondialdehyde (MDA)
levels
The level of MDA in the auditory cortex of rats
(n=6/subgroup) was determined using a colorimetric kit (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) according to
the manufacturer's protocol.
Terminal deoxynucleotidyl
transferase-mediated deoxyuridine 5'-triphosphate nick-end labeling
(TUNEL) staining
The combination of ketamine and chlorpromazine can
be used for anesthesia in rats (35,36). In the present study, the rats
(n=6/subgroup) were anesthetized with a combination of ketamine
(100 mg/kg) and chlorpromazine (5 mg/kg) via intra-peritoneal
injection, according to our previous studies (37,38). Following deep anesthesia
determined by respiratory, palpebral reflex, pedal withdrawal
reflex, and cutaneous reflex (39,40), the animals were transcardially
perfused with 400 ml of saline followed by 4% paraformaldehyde
solution (pH 7.2-7.4). Following perfusion, the brain was dissected
from the skull, and the auditory cortex was separated and immersed
overnight in the same fixative. The right side was prepared for
TUNEL staining and the left side was prepared for
immunofluorescence. The following processes were performed, as
previously described (41).
Apoptosis in the auditory cortex of rats was detected using a TUNEL
assay (Roche Diagnostics GmbH, Mannheim, Germany) according to the
manufacturer's protocol. DAPI staining solution (1 µg/ml;
Beyotime Institute of Biotechnology, Haimen, China) was used to
counterstain the nuclei. The labeled cells were detected with a
laser scanning confocal microscope (Nikon Corporation, Tokyo,
Japan).
DNA extraction and quantification of the
mtDNA 4,834-bp deletion
Following deep anesthesia, the rats (n=6/subgroup)
were sacrificed. The brain was dissected from the skull and both
sides of the auditory cortex were obtained from each subgroup. All
removed tissue was frozen in a refrigerator (Siemens AG, Munich,
Germany) at −80°C. The right side was prepared for mtDNA analysis;
the left side was prepared for RNA analysis. Total DNA was
extracted from 20 mg of tissue in the auditory cortex or
107 cultured cells utilizing a Genomic DNA Purification
kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the
manufacturer's protocol. The percentages of CDs were determined by
TaqMan quantitative polymerase chain reaction (qPCR) assays. As the
D-Loop region is rarely deleted, it serves as the conservative
segment. The PCR primers and probes for the mtDNA CD (4,834-bp
deletion) and mtDNA D-loop were as previously described (42). The sequences used were as follows:
D-Loop, probe 5'-TTG GTT CAT CGT CCA TAC GTT CCC CTT A-3', forward
5'-GGT TCT TAC TTC AGG GCC ATC A-3' and reverse 5'-GAT TAG ACC CGT
TAC CAT CGA GAT-3'; Common deletion sequences, probe 5'-TCA CTT TAA
TCG CCA CAT CCA TAA CTG CTG T-3', forward 5'-GAT TAG ACC CGT TAC
CAT CGA GAT-3' and reverse 5'-CGA AGT AGA TGA TCC GTA TGC TGT A-3'.
PCR amplification was performed using an LC-480 real-time PCR
system (Roche Diagnostics GmbH) in a 20-µl reaction volume
consisting of 10 µl of a 2X TaqMan PCR mix (Takara Bio,
Inc., Dalian, China), 0.2 µl of each probe (10 mM), 0.4
µl of each forward and reverse primer (10 mM), 5 µl
of distilled water, and 4 µl of the sample DNA (10 ng/ml).
The amplification conditions were as follows: 30 sec at 95°C then
40 cycles of 10 sec at 95°C and 30 sec at 60°C. ∆Cq
(Cqdeletion-CqD-loop) was used to reflect the
abundance of the mtDNA 4,834-bp deletion. The relative expression
indicating the factorial difference in the deletions between the
experimental group and control group was calculated using the
2−∆∆Cq method (43),
where ∆∆Cq=∆CqmtDNA deletion in experimental group−∆Cq
mtDNA deletion in control group.
RNA extraction and reverse
transcription-qPCR (RT-qPCR) analysis
Total RNA was extracted from ~50 mg of tissue or
107 cultured cells with an RNA extraction kit (Omega
Bio-tek, Inc., Norcross, GA, USA) according to the manufacturer's
protocol. The cDNA was reverse transcribed using the PrimeScript RT
reagent kit (Takara Bio, Inc.). Fold changes in respective gene
expression were calculated by normalizing to the level of the
house-keeping gene GAPDH. The primer sequences designed for RT-qPCR
analysis are shown in Table I.
PCR amplification was performed in an LC-480 real-time PCR system
(Roche Diagnostics GmbH) in a 20-µl reaction volume
consisting of 10 µl 2X SYBR Green II PCR Master Mix (Takara
Bio, Inc.), 1 µl each forward and reverse primer, 6
µl distilled water and 2 µl the sample cDNA. The
amplification conditions were as follows: 95°C for 5 min; 45 cycles
of 95°C for 10 sec, 60°C for 20 sec and 72°C for 20 sec; followed
by 95°C for 5 sec and 65°C for 60 sec. The relative mRNA expression
was calculated using the 2−∆∆Cq method.
 | Table IPrimer sequences for polymerase chain
reaction analysis. |
Table I
Primer sequences for polymerase chain
reaction analysis.
| Gene | Direction | Primer
sequence | Accession no. |
|---|
| NQO1 | Forward |
5'-GGGGACATGAACGTCATTCTCT-3' | NM_017000.3 |
| Reverse |
5'-AGTGGTGACTCCTCCCAGACAG-3' | |
| HO-1 | Forward |
5'-TGTCCCAGGATTTGTCCGAG-3' | NM_012580.2 |
| Reverse |
5'-ACTGGGTTCTGCTTGTTTCGCT-3' | |
| MnSOD | Forward |
5'-ATGTTGTGTCGGGCGGCGTGCAGC-3' | NM_017051.2 |
| Reverse |
5'-GCGCCTCGTGGTACTTCTCCTCGGT-3' | |
| GAPDH | Forward |
5'-GCAAGTTCAACGGCACAG-3' | NM_017008.4 |
| Reverse |
5'-GCCAGTAGACTCCACGACAT-3' | |
Transmission electron microscopy
(TEM)
Following anesthesia, the rats (n=3/subgroup) were
perfused transcardially with 0.9% oxygenated saline, followed by
2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2-7.4).
Following perfusion, the auditory cortex was isolated and immersed
overnight in the same fixative solution. In vitro, following
the indicated treatments, the cells were collected and immersed in
the fixative solution overnight. The following processes were
performed, as described in our previous study (41). The sections (60-100 nm) were
analyzed with a FEI TecnaiG220 TWIN microscope (Thermo
Fisher Scientific, Inc.) at ×1,700, ×3,500, and ×6,500
magnification.
Immunofluorescence
The processes of immunofluorescence were as
described in our previous study (37). The primary antibodies included
anti-kelch-like ECH-associated protein 1 (keap1; 1:100; cat. no.
ab150654; Abcam, Cambridge, MA, USA),
anti-8-hydroxy-2'-deoxyguanosine (8-OHdG; 1:200; cat. no.
sc-393871; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and
anti-Nrf2 (1:200; cat. no. ARG53382; Arigo Biolaboratories,
Hamburg, Germany). The secondary antibodies included FITC-labeled
donkey anti-rabbit IgG (H+L) (1:200; cat. no. ANT024; AntGene
Biotechnology, Inc., Wuhan, China) and FITC-labeled donkey
anti-mouse IgG (H+L) (1:200; cat. no. ANT023; AntGene
Biotechnology, Inc.). Fluorescent images were visualized with a
laser scanning confocal microscope (Nikon Corporation). The
quantification of immunofluorescence was performed using ImageJ
10.0 software (National Institutes of Health, Bethesda, MD,
USA).
Western blot analysis
Following deep anesthesia, six rats from each
subgroup were sacrificed. The tissue of the auditory cortex was
prepared for western blot analysis. The protein expression levels
of cleaved caspase-3 and cytochrome c in the auditory cortex
and PC12 cells were determined using western blot analysis.
Preparation of the cytosolic and mitochondrial fractions was
performed using a commercially available cytosol/mitochondria
fractionation kit (Beyotime Institute of Biotechnology) according
to the manufacturer's protocol. The proteins were extracted using
RIPA lysis buffer (Beyotime Institute of Biotechnology), which
contained a cocktail of phosphatase inhibitor and
phenylmethylsulfonyl fluoride, following the manufacturer's
protocol. Protein concentrations were determined using an Enhanced
BCA Protein Assay kit (Beyotime Institute of Biotechnology). Equal
quantities of protein (30 µg) were loaded onto 12%
SDS-polyacrylamide gels for electrophoresis, separated and
transferred to polyvinylidene difluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were incubated in blocking
solution [5% non-fat milk (BD Biosciences, Franklin Lakes, NJ, USA)
in Tris-buffered saline] for 1 h at room temperature. Subsequently,
the membranes were incubated overnight at 4°C with the following
primary antibodies: Anti-cleaved caspase-3 (1:200; cat. no. 9661;
Cell Signaling Technology, Inc., Danvers, MA, USA) and
anti-cytochrome c (1:500; cat. no. ab13575; Abcam). The
following processes were performed as previously described
(37). Quantification of the
western blot results was performed using ImageJ 10.0 software
(National Institutes of Health) to measure the intensities of the
bands.
Determination of mitochondrial membrane
potential (ΔΨm)
Cell ΔΨm was detected using a Mitochondrial Membrane
Potential Assay kit with JC-1 (Beyotime Institute of Biotechnology)
according to the manufacturer's protocol. Briefly, the cells were
seeded in 24-well plates at a density of 1×104 cells per
well. Following the indicated treatments, the cells were incubated
with JC-1 stain and incubated at 37°C for 20 min. The cells were
then washed three times with phosphate-buffered saline (PBS) and
immediately analyzed with a confocal laser-scanning microscope
(Nikon Corporation).
Cell viability test
Cell viability was assessed with the Cell Counting
Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) according to the manufacturer's protocol. Briefly,
the cells were seeded on a 96-well plate and left to attach
overnight. Following the indicated treatments, 10 µM of the
CCK-8 solution was dissolved in serum-free medium and added to each
well of the plates, and the plates were incubated for 30 min at
37°C. The absorbance at 450 nm was quantified on an automated
microplate reader (Bio-Tek Instruments, Inc., Winooski, VT,
USA).
Senescence-associated β-galactosidase
(SA-β-gal) staining assay
SA-β-gal is one of the best-characterized and
reliable methods for measuring senescence in vitro and in
vivo, by measuring the activity of β-Gal expressed by senescent
cells through immunohistochemistry (44). The number of senescent cells was
evaluated using a Senescence β-Galactosidase Staining kit (Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol. Briefly, following the indicated treatments, the cells
were washed with PBS at least three times and then fixed in
fixative solution for 15 min at room temperature. The fixed cells
were washed with PBS, stained by β-Gal staining solution and
incubated at 37°C overnight. Under a light microscope (Olympus
Corporation, Tokyo, Japan), the presence of blue granules in the
cytoplasm was considered as a positive result for β-Gal staining,
reflecting senescence of the examined cells.
Flow cytometry
Intracellular ROS generation was detected using
2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA; Beyotime
Institute of Biotechnology). Briefly, following treatment, the PC12
cells were incubated with 10 µM DCFH-DA for 30 min at 37°C.
The cells (1×106) were then suspended in PBS and
examined by flow cytometry (FACSCalibur; BD Biosciences). Apoptosis
in the cells was also evaluated using Annexin V/propidium iodide
(PI) staining (BD Biosciences) followed by flow cytometry,
according to the manufacturer's protocol.
Statistical analysis
All data are shown as the mean ± standard deviation.
Analyses were performed using GraphPad Prism 6 software (GraphPad
Software, Inc., San Diego, CA, USA). Two-tailed, unpaired Student's
t-tests were used to determine statistical significance when
comparing two groups, and one-way analysis of variance followed by
a Dunnett's multiple comparisons test was used when comparing more
than two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Neurodegeneration in the auditory
cortex
To investigate changes in the ultrastructure in the
auditory cortex, a TEM assay was used. In the NS groups, neurons of
the auditory cortex exhibited no notable ultrastructural changes in
the 4- and 10-month-old rats. In the D-gal groups, the auditory
cortex exhibited no notable ultrastructural changes in the
4-month-old rats (Fig. 1A-C).
Irregular nuclei, abundant lipofuscin, swollen mitochondria and
disrupted myelin were observed in the 16-month-old NS group rats.
However, the 10-month-old rats in the D-gal groups exhibited
similar ultrastructural changes to those observed in the
16-month-old NS group rats (Fig. 1D
and E). These ultrastructural changes were more prominent in
the 16-month-old D-gal group rats (Fig. 1F). These results revealed that
D-gal treatment accelerates neurodegeneration in the auditory
cortex of rats.
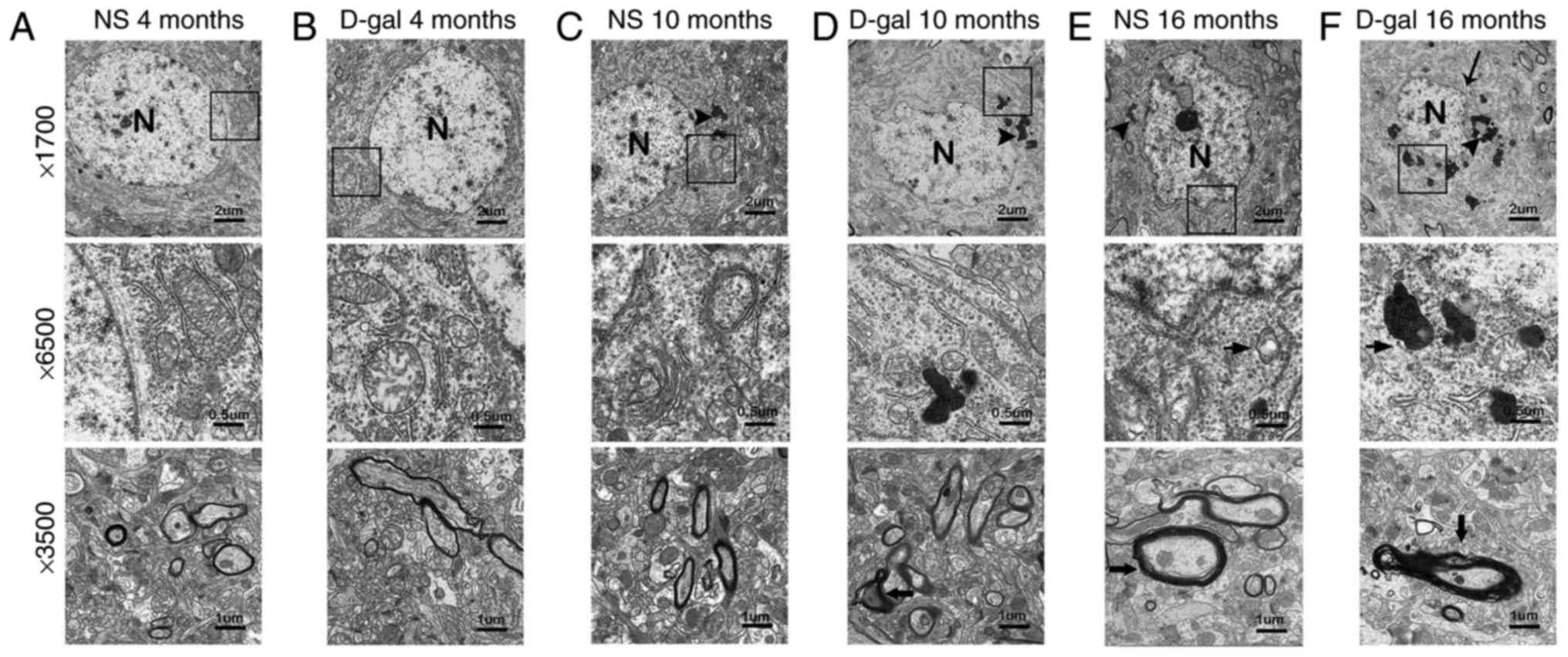 | Figure 1Ultrastructural morphology of the
auditory cortex. In the (A) 4-month-old NS group, (B) 4-month-old
D-gal group and (C) 10-month-old NS group, the nuclei were round,
the chromatin was uniform and the nuclear membrane was intact; the
mitochondria were normal and myelin in nerve fibers was intact. In
the (D) 10-month old D-gap group, (E) 16-month-old NS group and (F)
16-month-old D-gal group, irregular nuclei, abundant lipofuscin,
swollen mitochondria and disrupted myelin were observed. NS, normal
saline; D-gal, D-galactose. The arrowheads indicate the lipofuscin;
the bold arrows indicates the disrupted myelin; the normal arrows
indicate the nuclear membrane; N, nucleus. |
Cell apoptosis and MDA levels in the
auditory cortex
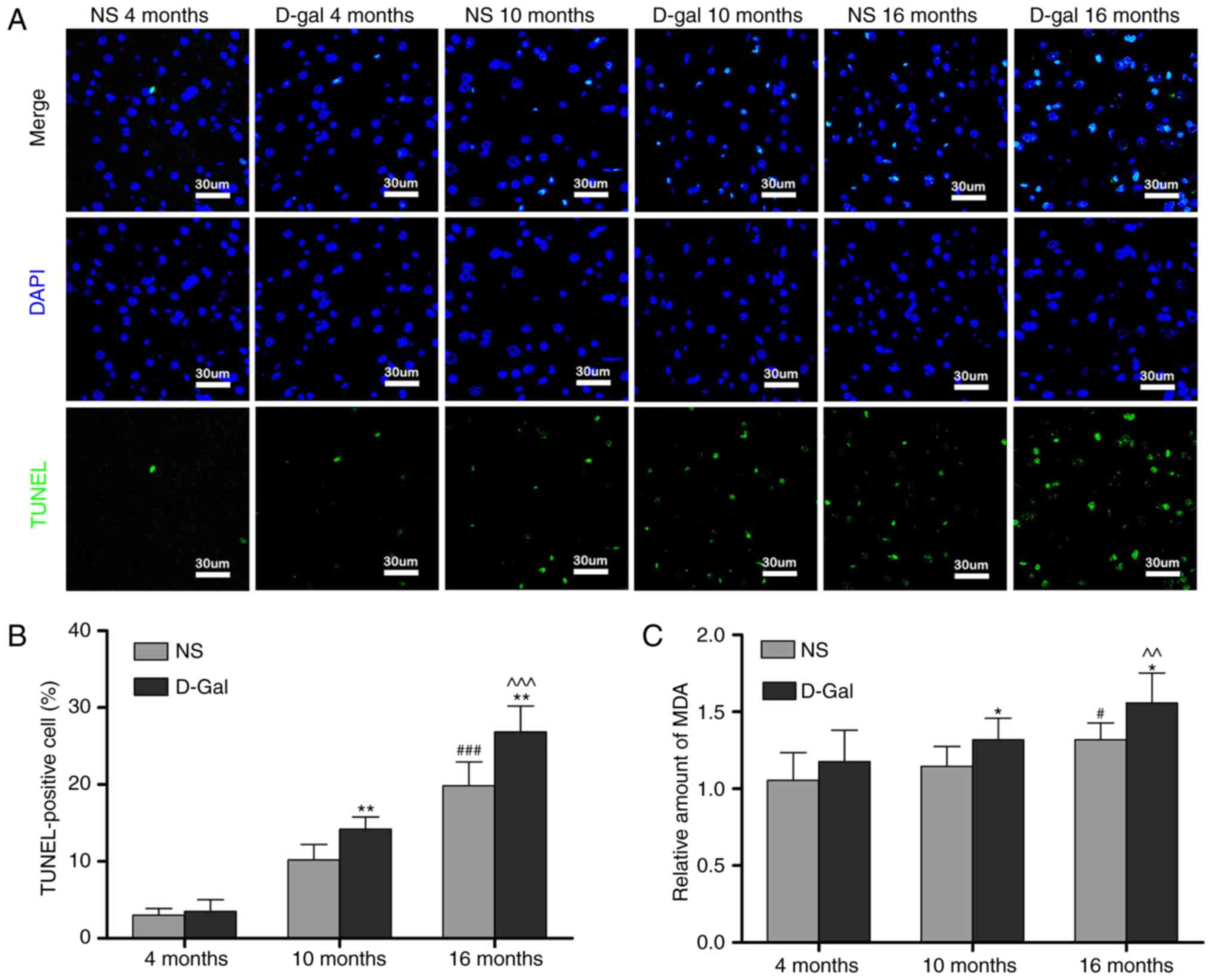
TUNEL staining was performed to investigate the
effects of D-gal and age on cell apoptosis in the auditory cortex.
Only low levels of TUNEL-positive cells were found in the
4-month-old NS and the 4-month-old D-gal groups. The number of
TUNEL-positive cells in the auditory cortex increased with age in
the NS groups. The number of TUNEL-positive cells was significantly
increased in the 10- and 16-month-old D-gal groups compared with
the age-matched NS groups (Fig. 2A
and B). MDA, a byproduct of lipid peroxidation induced by free
radicals, is widely used as a biomarker of oxidative stress
(45). Therefore, to determine
the level of oxidative stress, the levels of MDA in the auditory
cortex were measured. Compared with the 4-month-old NS group, the
MDA levels were significantly increased in the 16-month-old NS
group. In addition, compared with age-matched NS rats, MDA levels
in the D-gal-treated rats were significantly increased in the 10-
and 16-month-old groups (Fig.
2C). These data suggested that cell apoptosis and oxidative
stress levels in the auditory cortex were increased in the
D-gal-induced aging rats.
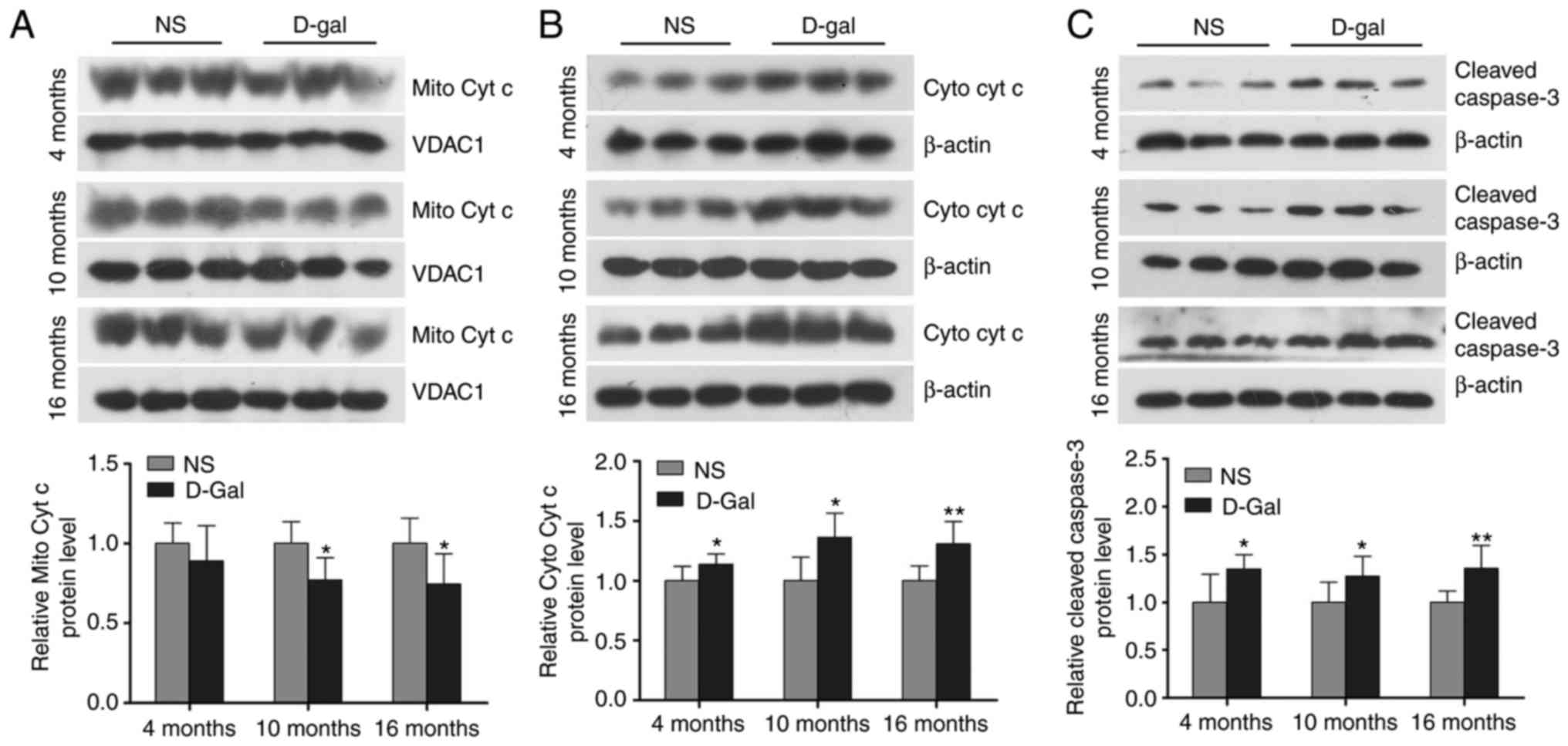
Increased mitochondrial cytochrome c
release and cleaved caspase-3 in D-gal-induced aging rats
Cytochrome c serves a critical role in the
mitochondrial-mediated apoptotic pathway and activation of caspase
in mammalian cells. Cleaved caspase-3 is a marker of the activation
of apoptosis. As shown in Fig.
3A-C, western blot analysis demonstrated that the release of
cytochrome c into the cytosolic fraction and cleaved
caspase-3 in the auditory cortex of the D-gal-induced mimetic aging
group were significantly increased compared with the age-matched
control groups. These data suggested that the release of
mitochondrial cytochrome c and the level of cleaved caspase-3 in
the auditory cortex was increased in the D-gal-induced aging
rats.
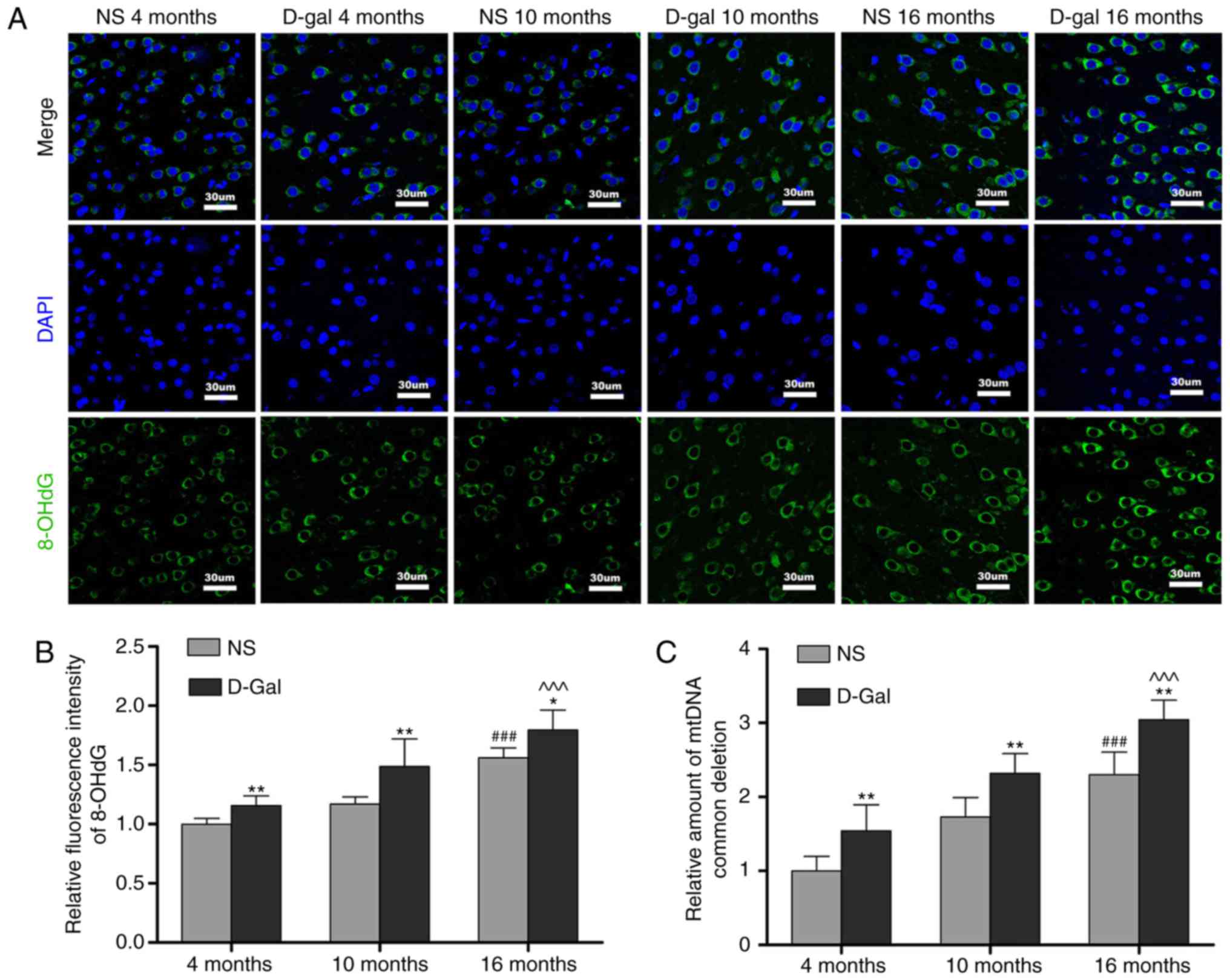
Formation of 8-OHdG and accumulation of
mtDNA CDs in the auditory cortex
The levels of oxidative damage of DNA are typically
assessed through biomarkers, including the formation of 8-OHdG
(46). The results of the
immunofluorescence assays (Fig. 4A
and B) revealed that the levels of 8-OHdG in the D-gal group
were significantly higher compared with those in the NS group at
different ages. Compared with the 4-month-old group, the 8-OHdG
levels in the 16-month-old group were markedly increased. In
addition, 8-OHdG fluorescence was observed predominantly in the
cytoplasm, suggesting that ROS induced mtDNA oxidative damage in
the auditory cortex. Mutations in mtDNA, including the 4,834-bp
deletion in rats and the 4,977-bp deletion in humans, referred to
as CDs, are closely associated with presbycusis (25). TaqMan qPCR analysis was performed
to evaluate the accumulation of CDs in the auditory cortex.
Consistent with the increase in 8-OHdG levels, CD levels increased
with age. Furthermore, CD levels were significantly increased in
the D-gal groups compared with those in the age-matched NS groups
(Fig. 4C). These results
indicated that oxidative damage to mtDNA was increased in the
auditory cortex of aging rats.
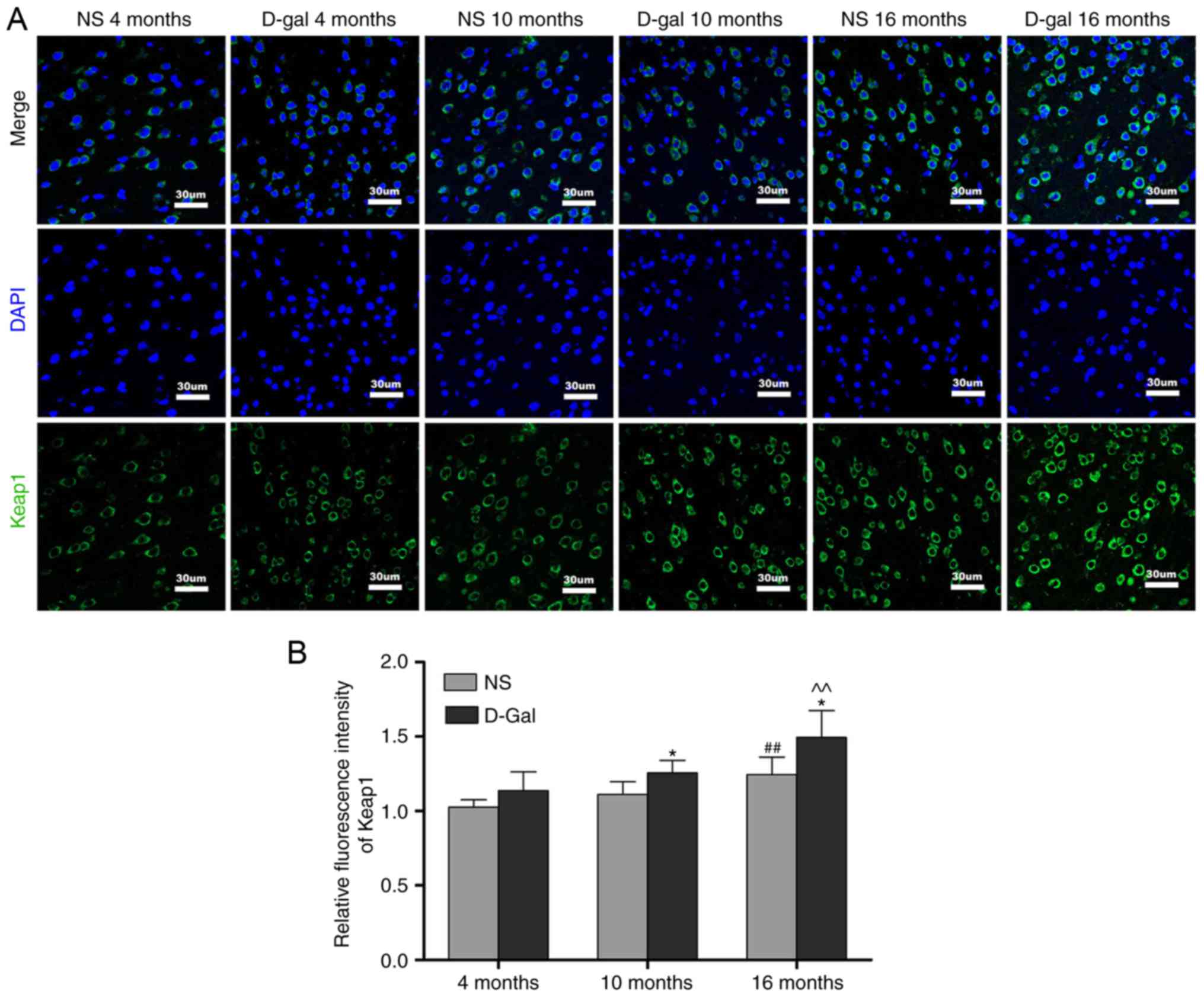
Age-associated changes of Nrf2 signaling
in the auditory cortex
Keap1 is a component of the E3 ubiquitin ligase
complex that targets Nrf2 for degradation (47). The results of the
immunofluorescence analysis demonstrated that the protein levels of
Keap1 were increased in the 16-month-old NS group, but that the
levels of Keap1 in the 16-month-old D-gal group exhibited a more
prominent increase. In addition, compared with the age-matched NS
groups, the level of Keap1 in the D-gal-treated groups were
significantly increased (Fig. 5A and
B). To further elucidate the role of the transcription factor
Nrf2 in the aging process, the levels of Nrf2 in the auditory
cortex were measured by immunofluorescence. Compared with the
4-month-old NS group, the level of Nrf2 was decreased in the
16-month-old NS group. Additionally, compared with the 4-month-old
D-gal group, the levels of Nrf2 in the 16-month-old D-gal group
exhibited a more marked decrease (Fig. 6A and B). In addition, the decline
in nuclear levels of Nrf2 coincided with a significant attenuation
in the expression of genes that are constitutively regulated by
Nrf2, including NQO1, HO-1 and MnSOD. The transcription of these
Nrf2 target genes was quantified by RT-qPCR analysis and gene
expression was normalized to GAPDH. The results demonstrated that
the mRNA levels of NQO1, HO-1 and MnSOD in the 16-month-old NS
group were significantly lower compared with those in the
4-month-old NS group (Fig. 6C-E).
Compared with the 4-month-old D-gal group, the mRNA levels of NQO1,
HO-1 and MnSOD were significantly decreased in the 16-month-old
D-gal group (Fig. 6C-E). Taken
together, these results demonstrated that Nrf2 signaling is
disrupted in D-gal-induced aging rats.
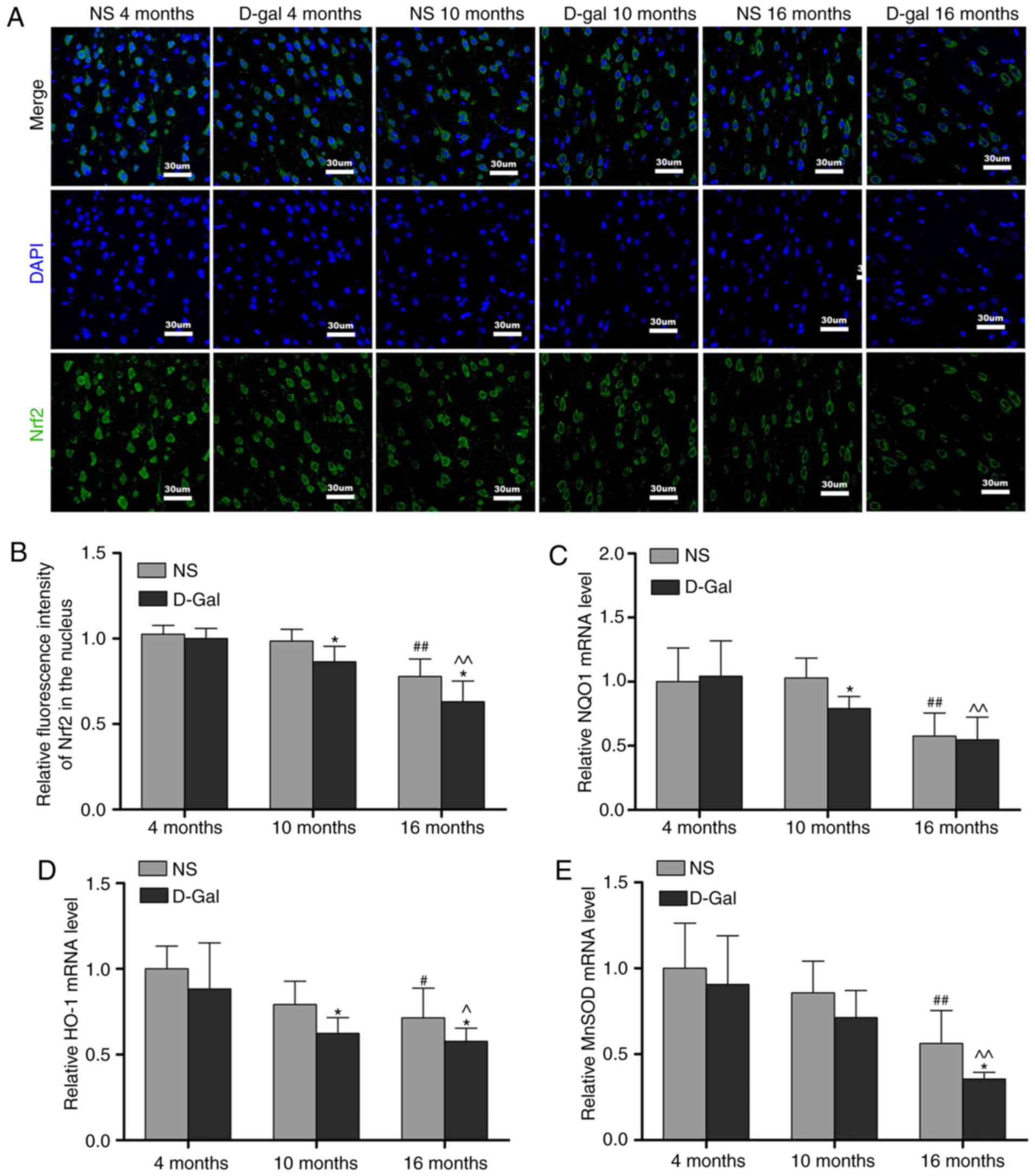 | Figure 6Age-associated decline of Nrf2
signaling in the auditory cortex. (A) Representative
immunofluorescence images stained with anti-Nrf2 antibody. (B)
Quantitative presentation of the fluorescence intensity of Nrf2 in
the nucleus. mRNA levels of (C) NQO1, (D) HO-1 and (E) MnSOD in the
auditory cortex. For all experiments, *P<0.05, vs.
age-matched NS group; #P<0.05 and
##P<0.01, vs. 4-month-old NS group;
^P<0.05 and ^^P<0.01, vs. 4-month-old
D-gal group. The data are presented as the mean ± standard
deviation. Nrf2, NF-E2-related factor 2; NQO1, NAD(P)H quinone
oxidoreductase 1; HO-1, heme oxygenase-1; MnSOD, manganese
superoxide dismutase; NS, normal saline; D-gal, D-galactose. |
Oltipraz activates the Nrf2 pathway and
increases cellular antioxidant activity in PC12 cells
The PC12 rat pheochromocytoma cell line, a
traditional cell line for neuroscience studies, is commonly used in
neurobiology and neuropharmacology in vitro (48,49). In addition, the PC12 cell line has
been widely used as a model to investigate oxidative stress-induced
cell injury and senescence (50-52). In the present study, a mimetic
aging model induced by D-gal was established in PC12 cells. The
cultured PC12 cells were treated with increasing concentrations of
D-gal (0-40 mg/ml) for 48 h and cell viability was then evaluated
using CCK-8. Cellular activity was altered by treatment with D-gal
in a concentration-dependent manner, exhibiting a decreasing
pattern that was statistically significant at 20 mg/ml. According
to these results (Fig. 7A), 15
mg/ml of D-gal was utilized to induce cell senescence in subsequent
experiments. As Nrf2 is an important regulator of redox genes, the
downregulation of NQO1, HO-1 and MnSOD genes was observed following
Nrf2 knockdown (Fig. 7B-D). The
results showed that Nrf2 can regulate the transcription of NQO1,
HO-1 and MnSOD genes. To investigate whether the Nrf2 signaling
pathway is involved in senescence and mtDNA damage, the PC12 cells
were pretreated with oltipraz, which has been found to activate
Nrf2, enhance glutathione biosynthesis and increase phase II
detoxification enzymes (53). To
select an optimal concentration of oltipraz, the PC12 cells were
pretreated with this agent at concentrations ranging between 0 and
100 µM for 1 h. The CCK-8 assay indicated that pretreatment
with oltipraz protected cells against injury induced by D-gal in a
concentration-dependent manner; the maximal cytoprotective effect
was observed at 50 µM oltipraz (Fig. 7E). To confirm activation of the
Nrf2 pathway by oltipraz, the transcription levels of antioxidant
genes NQO1, HO-1 and MnSOD were measured. Oltipraz (50 µM)
significantly increased the mRNA levels of NQO1, HO-1 and MnSOD
(Fig. 7F). To measure the
antioxidant activity of oltipraz, ROS generation in the PC12 cells
was assessed using DCFH-DA staining. The increased ROS levels under
D-gal treatment were found to be partially attenuated by the
addition of oltipraz (Fig. 7G and
H). These results demonstrated that activating the Nrf2 pathway
with oltipraz can increase cellular antioxidant activity.
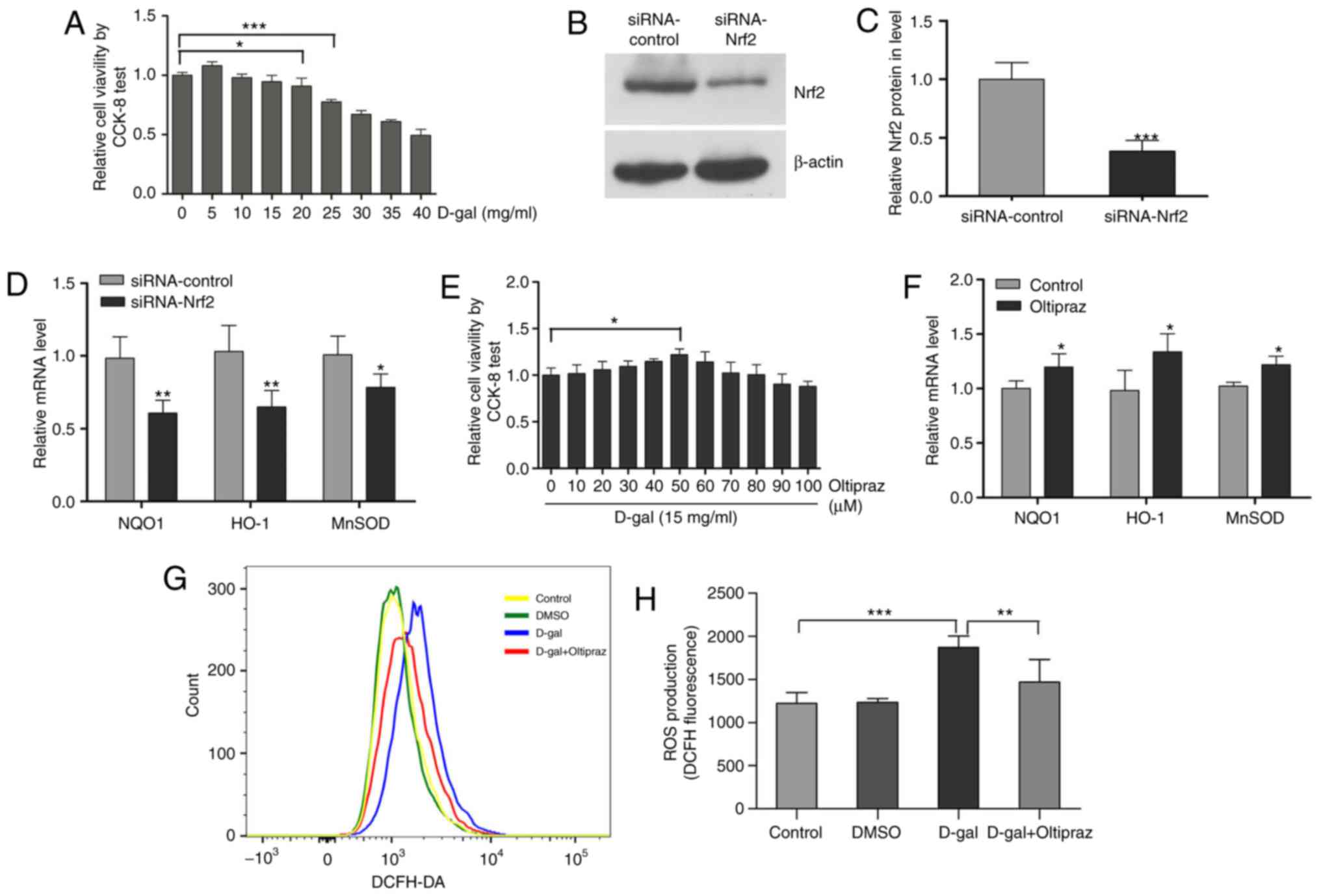 | Figure 7Oltipraz activates the Nrf2 pathway
and increases cellular antioxidant activity in PC12 cells. (A) An
optimal concentration of D-gal was selected using a CCK-8 test. (B)
Protein expression of Nrf2; (C) Nrf2 siRNA transfection decreased
the protein expression of Nrf2 in PC12 cells. (D) Nrf2 siRNA
transfection decreased the expression of Nrf2 target genes in PC12
cells. (E) A maximal cytoprotective concentration of oltipraz was
selected using the CCK-8 test. (F) mRNA levels of NQO1, HO-1 and
MnSOD in the control and oltipraz groups. (G) ROS levels were
measured in the treated cells using DCFH-DA staining and analyzed
by flow cytometry; (H) graph shows ROS levels. For all experiments,
*P<0.05, **P<0.01 and
***P<0.001. The data are presented as the mean ±
standard deviation. Nrf2, NF-E2-related factor 2; D-gal,
D-galactose; siRNA, small interfering RNA; CCK-8, Cell Counting
Kit-8; ROS, reactive oxygen species; DCFH-DA,
dichlorodihydrofluorescein diacetate; NQO1, NAD(P)H quinone
oxidoreductase 1; HO-1, heme oxygenase-1; MnSOD, manganese
superoxide dismutase; DMSO, dimethyl sulfoxide. |
Oltipraz protects PC12 cells against
D-gal-induced mtDNA oxidative damage, mtDNA CD and mitochondrial
dysfunction
It is well established that oxidative stress results
in nuclear DNA damage and mtDNA damage when the antioxidant/oxidant
equilibrium is disrupted (54).
The level of oxidatively damaged DNA is generally measured by the
formation of the biomarker 8-OHdG. Oltipraz pretreatment attenuated
the formation of 8-OHdG induced by D-gal (Fig. 8A and B). To determine
mitochondrial genome integrity, the levels of mtDNA CDs were
measured in PC12 cells by TaqMan qPCR and oltipraz was found to
decrease the incidence of mtDNA CDs induced by D-gal (Fig. 8C). To investigate whether the Nrf2
signaling pathway is involved in mitochondrial function, the ΔΨm
was examined using JC-1. The results demonstrated that oltipraz
attenuated the loss of ΔΨm induced by D-gal in PC12 cells (Fig. 8D and E). In addition, D-gal
treatment induced the release of cytochrome c into the
cytosolic fraction and the activation of caspase-3 in PC12 cells,
which were partially suppressed by oltipraz pretreatment (Fig. 8F-H). Taken together, these data
demonstrated that activating Nrf2 signaling with oltipraz
attenuates D-gal-induced mtDNA damage and mitochondrial
dysfunction.
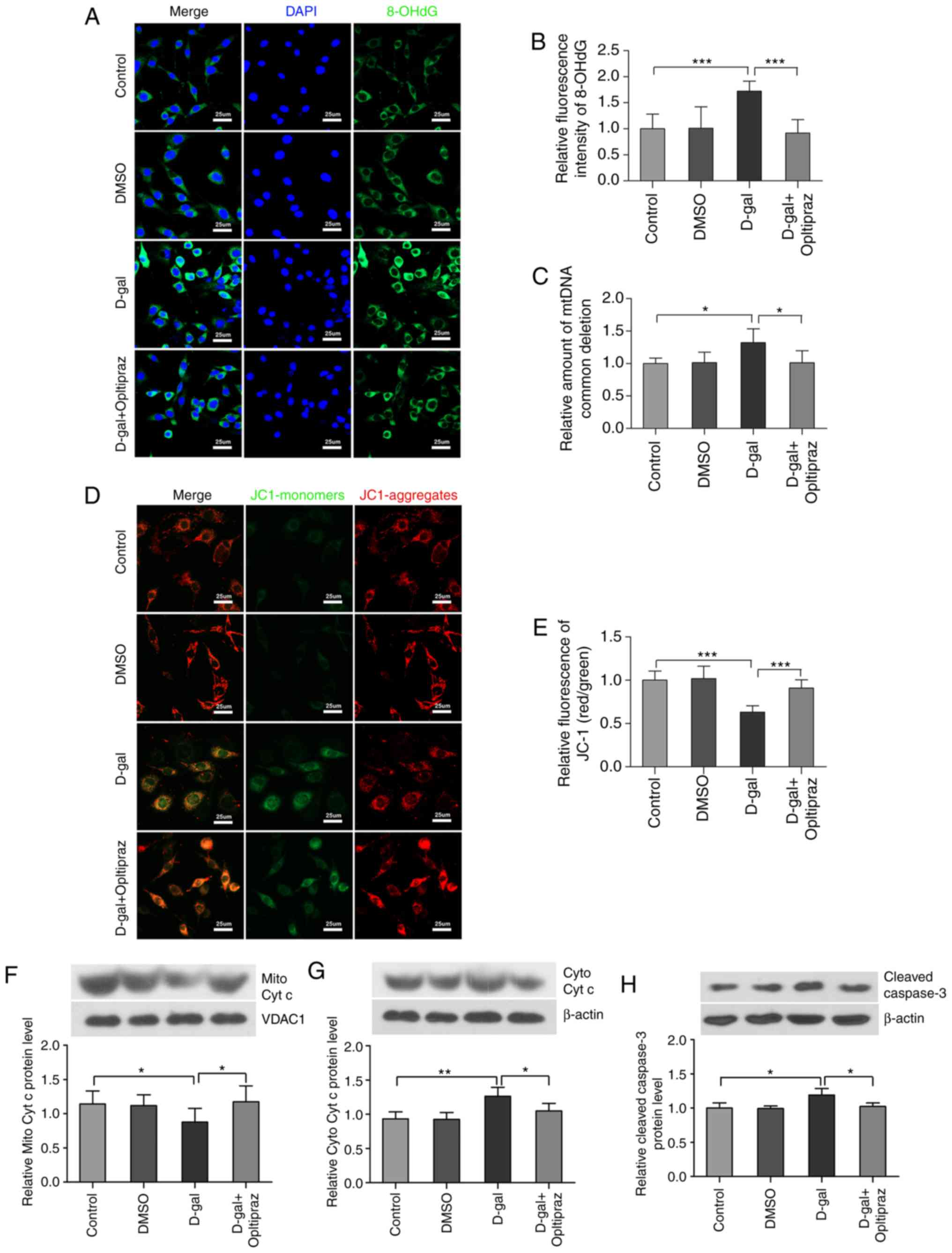 | Figure 8Oltipraz inhibits D-gal-induced
8-OHdG formation, mtDNA CDs and mitochondrial dysfunction. (A)
Representative confocal images of 8-OHdG. (B) Quantitative
assessment of 8-OHdG fluorescence in the treated cells. (C) Levels
of mtDNA CDs in the treated cells were analyzed by TaqMan
quantitative polymerase chain reaction. (D) Representative images
of JC-1. The red colour indicates the JC-1 aggregate fluorescence
from healthy mitochondria, the green colour indicates cytosolic
JC-1 monomers, merged images indicate the co-localization of JC-1
aggregates and monomers. (E) Quantitative assessment of JC-1
fluorescence. The results show the ratios of red to green JC-1 mean
fluorescence intensities in different groups. Western blot analysis
of (F) Mito Cyto c and (G) Cyto Cyt c levels. (H) Western blot
analysis of cleaved caspase-3 levels. For all experiments,
*P<0.05, **P<0.01 and
***P<0.001. The data are presented as the mean ±
standard deviation. D-gal, D-galactose; 8-OHdG,
8-hydroxy-2'-deoxyguanosine; Mito Cyt c, mitochondrial cytochrome
c; Cyto Cyt c, cytosolic cytochrome c; CD, common
deletion; DMSO, dimethyl sulfoxide. |
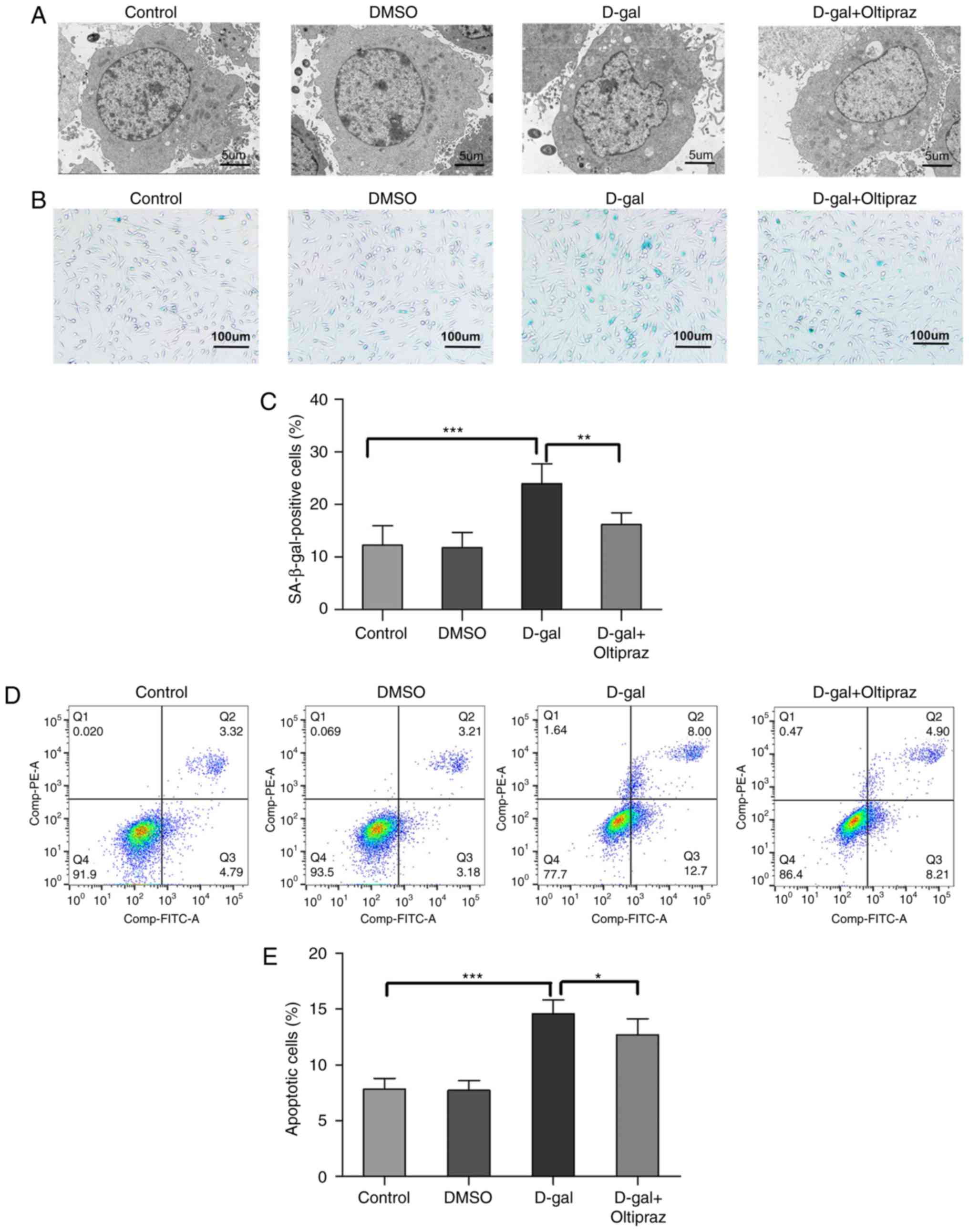
Oltipraz attenuates D-gal-induced
structural damage, apoptosis and senescence in PC12 cells
To further examine the cytoprotective effect of
oltipraz, the ultrastructure of the treated cells was observed by
TEM. Cells in the control and DMSO groups did not shown notable
ultrastructural changes; however, irregular nuclei and swollen
mitochondria were observed in the D-gal group. Notably, these
abnormal changes were alleviated in the D-gal group pretreated with
oltipraz (Fig. 9A). These data
indicated that oltipraz protected the cells from D-gal-induced
damage. SA-β-gal is the most widely known biomarker of cellular
senescence. To investigate whether the Nrf2 signaling pathway is
involved in cell senescence, SA-β-gal staining was performed in
PC12 cells and a marked increase in SA-β-gal-positive cells was
observed in the D-gal group, which was partially reversed by
oltipraz pretreatment (Fig. 9B and
C). Annexin V-FITC/PI staining and flow cytometry were used to
confirm the anti-apoptotic effect of oltipraz in the D-gal-treated
PC12 cells. The rate of apoptosis was increased by D-gal treatment,
but this effect was attenuated by pretreatment with oltipraz
(Fig. 9D and E). Taken together,
these findings demonstrated that oltipraz exerts a protective
effect against D-gal-induced cell damage, senescence and apoptosis
in PC12 cells.
Discussion
In the present study, apoptosis and abnormal
ultrastructural morphology were more prominent with aging in the
auditory cortex of the NS groups (Figs. 1 and 2A and B). Specifically, irregular
nuclei, abundant lipofuscin, swollen mitochondria and disrupted
myelin were observed in the 16-month-old NS group. The lifespan of
the majority of Sprague-Dawley rats is 2.5-3 years; thus, the age
of 16-months is equivalent to late adulthood in rats (55). The levels of apoptosis and changes
in ultrastructure morphology in the 10-month-old D-gal-treated rats
were similar to those in the 16-month-old NS rats, but were more
pronounced in the 16-month-old D-gal-treated group. Therefore, rats
in the 16-month-old D-gal-induced mimetic aging group were
classified as being the equivalent of old age. These data indicated
that the rat model of aging in the auditory cortex was successfully
established using D-gal.
In the present study, the data revealed that MDA
levels were significantly elevated in the auditory cortex of the
naturally aging and D-gal-induced aging rats (Fig. 2C). MDA is the final product of
lipid peroxidation (45), and its
accumulation suggests that oxidative stress in the auditory cortex
of the aging rat has become severe. Nrf2 signaling is critical in
the cellular response to oxidative stress (56). Previous studies in
Caenorhabditis elegans (C. elegans) demonstrated that the
knockdown of SKN-1, the homolog of Nrf2 in C. elegans,
shortens lifespan (57). A
previous study also demonstrated that repression of the
Nrf2-mediated antioxidant response is a key contributor to the
premature aging phenotype in Hutchinson-Gilford Progeria Syndrome
mesenchymal stem cells (58). In
the present study, it was observed that the levels of nuclear Nrf2
and Nrf2-regulated antioxidant genes were decreased in the auditory
cortex of the aging rats (Fig.
6). The mechanism underlying the downregulation of
Nrf2-mediated antioxidant genes in aging is likely multifaceted.
Keap1, a negative regulator of Nrf2, which acts as an adaptor
between Nrf2 and the ubiquitination ligase Cullin-3, promotes the
degradation of Nrf2 by the proteasome and inhibits the nuclear
translocation of Nrf2 (59). The
present study demonstrated that the expression of Keap1 was
increased in the auditory cortex of the aging rats (Fig. 5A and B). Increased levels of Keap1
and decreased levels of Nrf2 have also been observed in a chronic
renal failure rat model (60).
Decreased Nrf2 signaling may lead to failure of the cytoprotective
system and enhance the sensitivity of cells to oxidative stress.
Accordingly, the results of the present study demonstrated that
oxidative stress, apoptosis and degeneration in the auditory cortex
of aging rats may be associated with the dysregulation of Nrf2
signaling, which may be involved in the pathogenesis of central
presbycusis.
Mitochondria are considered to be key in the
progression of presbycusis (61).
Mammalian mtDNA is critical for mitochondrial function (24), and oxidative damage to mtDNA may
be attributed to an excess of ROS and/or inadequate antioxidant
defense. One of the major oxidative modifications of DNA is 8-OHdG,
which is an oxidative DNA mutagenic lesion that has been directly
correlated with the development of pathological processes (62,63). The present study demonstrated that
the levels of 8-OHdG in the auditory cortex increased with age
(Fig. 4A and B). mtDNA is
particularly susceptible to oxidative damage due to several
factors; it is in close proximity to the ROS-generating respiratory
chain, it is not covered by histones or other DNA-associated
proteins, and it lacks a robust repair system compared with that of
nuclear DNA (23). Consistent
with the other oxidative stress-related degenerative disorders,
including Alzheimer's disease, age-related macular degeneration,
and diabetic hearts (64-66), the present study showed that
8-OHdG fluorescence was observed predominantly in the cytoplasm of
cells, which suggests that mtDNA is the primary target of oxidative
damage. In accordance with the increased 8-OHdG levels, the
findings of the present study also demonstrated that the
accumulation of mtDNA CDs was increased in the aging rats (Fig. 4C). Furthermore, the release of
cytochrome c into the cytoplasm and cleaved caspase-3 in the
auditory cortex of the D-gal-induced mimetic aging group were
increased (Fig. 3). These results
demonstrated that oxidative damage to mtDNA and mitochondrial
stress-associated apoptosis are increased in the auditory cortex of
naturally aging and D-gal-induced aging rats. Nrf2 is a
transcription factor that regulates a number of antioxidant and
cytoprotective genes to protect against ROS-induced toxicity
(56). Nrf2-regulated antioxidant
enzymes, including NQO1, HO-1 and MnSOD, have potent antioxidant
properties. NQO1, an obligatory two-electron reductase, is a
ubiquitous cytosolic enzyme that catalyzes the reduction of quinone
substrates (67). As an important
Nrf2-dependent antioxidant response gene, NQO1 defects result in
increased susceptibility to DNA damage in the marrow cells of mice
(68). HO-1 catalyzes the
oxidative degradation of heme to biliverdin and carbon monoxide; a
deficiency in HO-1 may elevate the levels of ROS and superimpose
oxidative injury in endothelial cells (69). MnSOD is the main antioxidant
enzyme responsible for scavenging superoxide in the mitochondrial
matrix. It has been reported that decreased mitochondrial function
and increased mtDNA oxidative damage, including that by 8-OHdG, are
observed in the liver of MnSOD+/- mice (70). In the present study, it was
demonstrated that the expression levels of Nrf2-dependent
antioxidant genes (NQO1, HO-1 and MnSOD) were downregulated in the
auditory cortex of aging rats (Fig.
6C-E). It is likely that age-associated attenuation of the
antioxidant defense system in the auditory cortex, potentially due
to disruption of Nrf2 signaling, increases the sensitivity of mtDNA
to oxidative damage and leads to the development of a ‘vicious
cycle’, in which oxidative damage to mtDNA leads to further
mitochondrial dysfunction and oxidant generation. Taken together,
the findings of the present study, in addition to those of previous
studies, suggested that mtDNA damage in the auditory cortex may be
associated with Nrf2 signaling disruption during aging.
To further confirm the involvement of the Nrf2
pathway in mtDNA damage and aging, PC12 cells were pretreated with
oltipraz, which is a typical Nrf2 activator (53). The results demonstrated that
oltipraz activated the transcription of Nrf2-regulated antioxidant
genes (NQO1, HO-1 and MnSOD) and decreased the D-gal-induced
production of ROS in PC12 cells (Fig.
7F-H). Damage to mtDNA and mitochondrial dysfunction are
closely associated with aging (24,71). The present study demonstrated that
oltipraz attenuated D-gal-induced mtDNA damage and mitochondrial
dysfunction (Fig. 8). It is
possible that oltipraz upregulated the expression of stress
response genes (NQO1, HO-1 and MnSOD) and increased intracellular
antioxidant capacity, thus protecting mtDNA against ROS and
maintaining mitochondrial function. Onken and Driscoll reported
that the activation of SKN-1, the homolog of Nrf2 in C.
elegans, prolongs the lifespan of C. elegans (72). As markers of aging, cell apoptosis
and the number of SA-β-gal-positive cells were increased in the
D-gal-treated group in the present study, in accordance with our
previous studies (73,74). It was also observed that the
activation of Nrf2 signaling by oltipraz inhibited apoptosis and
delayed the senescence induced by D-gal (Fig. 9). Taken together, these results
indicate that the activation of Nrf2 signaling may be important in
maintaining mtDNA integrity and delaying aging.
In conclusion, Nrf2 signaling was found to be
decreased in the auditory cortex in a rat model of aging. The
activation of Nrf2 with oltipraz reduced mtDNA damage and delayed
cellular senescence. Therefore, decreased Nrf2-mediated antioxidant
responses may induce mtDNA damage, apoptosis and degeneration in
the auditory cortex, resulting in central presbycusis. The
restoration of Nrf2 signaling activity may represent a potential
therapeutic strategy for age-associated diseases, including central
presbycusis.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81230021),
the Major State Basic Research Development Program of China (973
Program; grant no. 2011CB504504) and the National Natural Science
Foundation of China (grant no. 81670929).
Availability of data and materials
The analyzed datasets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, WK and WJK conceived and designed the
experiments; YL, XZ, YH, HS, ZH, JY, HC, YS and XH performed the
experiments; YL, WK and WJK analyzed the data; YL, YH, WK and WJK
wrote the manuscript. All the authors have read and approved the
final version of this manuscript.
Ethics approval and consent to
participate
All procedures involving the care of animals were
performed in accordance with the guidelines of the Care and Use of
Laboratory Animals of the National Institutes of Health. The study
protocol was approved by the Committee on the Ethics of Animal
Experiments of HUST.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Fetoni AR, Picciotti PM, Paludetti G and
Troiani D: Pathogenesis of presbycusis in animal models: A review.
Exp Gerontol. 46:413–425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McCormack A and Fortnum H: Why do people
fitted with hearing aids not wear them. Int J Audiol. 52:360–368.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gates GA and Mills JH: Presbycusis Lancet.
366:1111–1120. 2005. View Article : Google Scholar
|
|
4
|
Walton JP, Frisina RD and O'Neill WE:
Age-related alteration in processing of temporal sound features in
the auditory midbrain of the CBA mouse. J Neurosci. 18:2764–2776.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao X, Tao Y, Lamas V, Huang M, Yeh WH,
Pan B, Hu YJ, Hu JH, Thompson DB, Shu Y, et al: Treatment of
autosomal dominant hearing loss by in vivo delivery of genome
editing agents. Nature. 553:217–221. 2018. View Article : Google Scholar :
|
|
6
|
Zhang Y, Tang W, Ahmad S, Sipp JA, Chen P
and Lin X: Gap junction-mediated intercellular biochemical coupling
in cochlear supporting cells is required for normal cochlear
functions. Proc Natl Acad Sci USA. 102:15201–15206. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun He, Waqas SZ, Zhang M, Qian X, Cheng
F, Zhang C, Zhang M, Wang S, Tang YM, et al: Reduced TRMU
expression increases the sensitivity of hair-cell-like HEI-OC-1
cells to neomycin damage in vitro. Sci Rep. 6:296212016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Menardo J, Tang Y, Ladrech S, Lenoir M,
Casas F, Michel C, Bourien J, Ruel J, Rebillard G, Maurice T, et
al: Oxidative stress, inflammation, and autophagic stress as the
key mechanisms of premature age-related hearing loss in SAMP8 Mouse
Cochlea. Antioxid Redox Signal. 16:263–274. 2012. View Article : Google Scholar
|
|
9
|
Uchida Y, Sugiura S, Sone M, Ueda H and
Nakashima T: Progress and prospects in human genetic research into
age-related hearing impairment. Biomed Res Int.
2014.390601:2014.
|
|
10
|
Gates GA, Couropmitree NN and Myers RH:
Genetic associations in age-related hearing thresholds. Arch
Otolaryngol Head Neck Surg. 125:654–659. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kinoshita M, Sakamoto T, Kashio A, Shimizu
T and Yamasoba T: Age-related hearing loss in Mn-SOD heterozygous
knockout mice. Oxid Med Cell Longev. 2013.325702:2013.
|
|
12
|
Hensley K, Mhatre M, Mou S, Pye QN,
Stewart C, West M and Williamson KS: On the relation of oxidative
stress to neuro-inflammation: Lessons learned from the G93A-SOD1
mouse model of amyotrophic lateral sclerosis. Antioxid Redox
Signal. 8:2075–2087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harman D: Aging: A theory based on free
radical and radiation chemistry. J Gerontol. 10:298–300. 1956.
View Article : Google Scholar
|
|
14
|
Venugopal R and Jaiswal AK: Nrf1 and Nrf2
positively and c-Fos and Fra1 negatively regulate the human
antioxidant response element-mediated expression of NAD(P)H:quinone
oxidoreductase(1) gene. Proc Natl Acad Sci USA. 93:14960–14965.
1996. View Article : Google Scholar
|
|
15
|
Scandalios JG: Oxidative stress: Molecular
perception and transduction of signals triggering antioxidant gene
defenses. Braz J Med Biol Res. 38:995–1014. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang H, Talaska AE, Schacht J and Sha SH:
Oxidative imbalance in the aging inner ear. Neurobiol Aging.
28:1605–1612. 2007. View Article : Google Scholar
|
|
17
|
Tavanai E and Mohammadkhani G: Role of
antioxidants in prevention of age-related hearing loss: A review of
literature. Eur Arch Otorhinolaryngol. 274:1821–1834. 2017.
View Article : Google Scholar
|
|
18
|
Liu L, Chen Y, Qi J, Zhang Y, He Y, Ni W,
Li W, Zhang S, Sun S, Taketo MM, et al: Wnt activation protects
against neomycin-induced hair cell damage in the mouse cochlea.
Cell Death Dis. 7:e21362016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bratic A and Larsson NG: The role of
mitochondria in aging. J Clin Invest. 123:951–957. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pinto M and Moraes CT: Mechanisms linking
mtDNA damage and aging. Free Radic Biol Med. 85:250–258. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Someya S and Prolla TA: Mitochondrial
oxidative damage and apoptosis in age-related hearing loss. Mech
Ageing Dev. 131:480–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park CB and Larsson NG: Mitochondrial DNA
mutations in disease and aging. J Cell Biol. 193:809–818. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang AL, Lukas TJ, Yuan M and Neufeld AH:
Increased mitochondrial DNA damage and down-regulation of DNA
repair enzymes in aged rodent retinal pigment epithelium and
choroid. Mol Vis. 14:644–651. 2008.PubMed/NCBI
|
|
24
|
Hebert SL, Lanza IR and Nair KS:
Mitochondrial DNA alterations and reduced mitochondrial function in
aging. Mech Ageing Dev. 131:451–462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Markaryan A, Nelson EG and Hinojosa R:
Quantification of the mitochondrial DNA common deletion in
presbycusis. Laryngoscope. 119:1184–1189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu J, Wang Y, Liu P, Li Q, Sun Y and Kong
W: Mitochondrial DNA common deletion increases susceptibility to
noise-induced hearing loss in a mimetic aging rat model. Biochem
Biophys Res Commun. 453:515–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dinkova-Kostova AT and Abramov AY: The
emerging role of Nrf2 in mitochondrial function. Free Radic Biol
Med. 88:179–188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Niture SK, Khatri R and Jaiswal AK:
Regulation of Nrf2-an update. Free Radic Biol Med. 66:36–44. 2014.
View Article : Google Scholar
|
|
29
|
Cui X, Wang L, Zuo P, Han Z, Fang Z, Li W
and Liu J: D-galactose-caused life shortening in Drosophila
melanogaster and Musca domestica is associated with oxidative
stress. Biogerontology. 5:317–325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ho SC, Liu JH and Wu RY: Establishment of
the mimetic aging effect in mice caused by D-galactose.
Biogerontology. 4:15–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kong WJ, Wang Y, Wang Q, Hu YJ, Han YC and
Liu J: The relation between D-galactose injection and mitochondrial
DNA 4834 bp deletion mutation. Exp Gerontol. 41:628–634. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun HY, Hu YJ, Zhao XY, Zhong Y, Zeng LL,
Chen XB, Yuan J, Wu J, Sun Y, Kong W and Kong WJ: Age-related
changes in mitochondrial antioxidant enzyme Trx2 and
TXNIP-Trx2-ASK1 signal pathways in the auditory cortex of a mimetic
aging rat model: Changes to Trx2 in the auditory cortex. FEBS J.
282:2758–2774. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Willott JF: Effects of sex, gonadal
hormones, and augmented acoustic environments on sensorineural
hearing loss and the central auditory system: Insights from
research on C57BL/6J mice. Hear Res. 252:89–99. 2009. View Article : Google Scholar :
|
|
34
|
The National Academies Collection: Reports
funded by National Institutes of Health. National Research Council
Committee for the Update of the Guide for the Care and Use of
Laboratory. Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US), National Academy of
Sciences; Washington, DC: 2011
|
|
35
|
Ouahchi Y, Duclos C, Marie JP and Verin E:
Implication of the vagus nerve in breathing pattern during
sequential swallowing in rats. Physiol Behav. 179:434–441. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Louro TM, Matafome PN, Nunes EC, Xavier da
Cunha F and Seiça RM: Insulin and metformin may prevent renal
injury in young type 2 diabetic Goto-Kakizaki rats. Eur J
Pharmacol. 653:89–94. 2011. View Article : Google Scholar
|
|
37
|
Zeng L, Yang Y, Hu Y, Sun Y, Du Z, Xie Z,
Zhou T and Kong W: Age-related decrease in the mitochondrial
sirtuin deacetylase Sirt3 expression associated with ROS
accumulation in the auditory cortex of the mimetic aging rat model.
PLoS One. 9:e880192014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu X, Wang Y, Sun Y, Chen S, Zhang S, Shen
L, Huang X, Lin X and Kong W: Reduced expression of Connexin26 and
its DNA promoter hypermethylation in the inner ear of mimetic aging
rats induced by d-galactose. Biochem Biophys Res Commun.
452:340–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alves HN, da Silva AL, Olsson IA, Orden JM
and Antunes LM: Anesthesia with intraperitoneal propofol,
medetomidine, and fentanyl in rats. J Am Assoc Lab Anim Sci.
49:454–459. 2010.PubMed/NCBI
|
|
40
|
Ferrari L, Turrini G, Rostello C, Guidi A,
Casartelli A, Piaia A and Sartori M: Evaluation of two combinations
of Domitor, Zoletil 100, and Euthatal to obtain long-term
nonrecovery anesthesia in Sprague-Dawley rats. Comp Med.
55:256–264. 2005.PubMed/NCBI
|
|
41
|
Yuan J, Zhao X, Hu Y, Sun H, Gong G, Huang
X, Chen X, Xia M, Sun C, Huang Q, et al: Autophagy regulates the
degeneration of the auditory cortex through the AMPK-mTOR-ULK1
signaling pathway. Int J Mol Med. 41:2086–2098. 2018.PubMed/NCBI
|
|
42
|
Nicklas JA, Brooks EM, Hunter TC, Single R
and Branda RF: Development of a quantitative PCR (TaqMan) assay for
relative mitochondrial DNA copy number and the common mitochondrial
DNA deletion in the rat. Environ Mol Mutagen. 44:313–320. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
44
|
Itahana K, Campisi J and Dimri GP: Methods
to detect biomarkers of cellular senescence. Biological Aging:
Methods and Protocols. Tollefsbol TO: Humana Press; Totowa, NJ: pp.
21–31. 2007, View Article : Google Scholar
|
|
45
|
Ayala A, Muñoz MF and Argüelles S: Lipid
peroxidation: production, metabolism, and signaling mechanisms of
malondi-aldehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev.
2014.360438:2014.
|
|
46
|
Valavanidis A, Vlachogianni T and Fiotakis
C: 8-hydroxy-2'-de-oxyguanosine (8-OHdG): A critical biomarker of
oxidative stress and carcinogenesis. J Environ Sci Health C Environ
Carcinog Ecotoxicol Rev. 27:120–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Suzuki T and Yamamoto M: Molecular basis
of the Keap1-Nrf2 system. Free Radic Biol Med. 88:93–100. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee J, Song K, Huh E, Oh MS and Kim YS:
Neuroprotection against 6-OHDA toxicity in PC12 cells and mice
through the Nrf2 pathway by a sesquiterpenoid from Tussilago
farfara. Redox Biol. 18:6–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sáez-Orellana F, Fuentes-Fuentes MC, Godoy
PA, Silva-Grecchi T, Panes JD, Guzmán L, Yévenes GE, Gavilán J,
Egan TM, Aguayo LG and Fuentealba J: P2X receptor overexpression
induced by soluble oligomers of amyloid beta peptide potentiates
synaptic failure and neuronal dyshomeostasis in cellular models of
Alzheimer's disease. Neuropharmacology. 128:366–378. 2018.
View Article : Google Scholar
|
|
50
|
Park HJ, Zhao TT, Lee KS, Lee SH, Shin KS,
Park KH, Choi HS and Lee MK: Effects of (−)-sesamin on
6-hydroxydopamine-induced neurotoxicity in PC12 cells and
dopaminergic neuronal cells of Parkinson's disease rat models.
Neurochem Int. 83–84:19–27. 2015. View Article : Google Scholar
|
|
51
|
Zhang Y, Wang Z, Li X, Wang L, Yin M, Wang
L, Chen N, Fan C and Song H: Dietary iron oxide nanoparticles delay
aging and ameliorate neurodegeneration in drosophila. Adv Mater.
28:1387–1393. 2016. View Article : Google Scholar
|
|
52
|
Denisova NA, Cantuti-Castelvetri I, Hassan
WN, Paulson KE and Joseph JA: Role of membrane lipids in regulation
of vulnerability to oxidative stress in PC12 cells: Implication for
aging. Free Radic Biol Med. 30:671–678. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lee JS and Surh YJ: Nrf2 as a novel
molecular target for chemoprevention. Cancer Lett. 224:171–184.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Evans MD, Dizdaroglu M and Cooke MS:
Oxidative DNA damage and disease: Induction, repair and
significance. Mutat Res. 567:1–61. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Andreollo NA, Santos EFd, Araújo MR and
Lopes LR: Idade dos ratos versus idade humana Qual é a relação?
ABCD Arq Bras Cir Dig. 25:49–51. 2012. View Article : Google Scholar
|
|
56
|
Florczyk U, Łoboda A, Stachurska A,
Józkowicz A and Dulak J: Role of Nrf2 transcription factor in
cellular response to oxidative stress. Postepy Biochem. 56:147–155.
2010.In Polish.
|
|
57
|
Jasper H: SKNy worms and long life. Cell.
132:915–916. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kubben N, Zhang W, Wang L, Voss TC, Yang
J, Qu J, Liu GH and Misteli T: Repression of the antioxidant NRF2
pathway in premature aging. Cell. 165:1361–1374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kobayashi A, Kang MI, Watai Y, Tong KI,
Shibata T, Uchida K and Yamamoto M: Oxidative and electrophilic
stresses activate Nrf2 through inhibition of ubiquitination
activity of Keap1. Mol Cell Biol. 26:221–229. 2006. View Article : Google Scholar :
|
|
60
|
Kim HJ and Vaziri ND: Contribution of
impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in
chronic renal failure. Am J Physiol Renal Physiol. 298:F662–F671.
2010. View Article : Google Scholar
|
|
61
|
Chen H and Tang J: The role of
mitochondria in age-related hearing loss. Biogerontology. 15:13–19.
2014. View Article : Google Scholar
|
|
62
|
de Souza-Pinto NC, Hogue BA and Bohr VA:
DNA repair and aging in mouse liver: 8-oxodG glycosylase activity
increase in mitochondrial but not in nuclear extracts. Free Radic
Biol Med. 30:916–923. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Souza-Pinto NC, Croteau DL, Hudson EK,
Hansford RG and Bohr VA: Age-associated increase in
8-oxo-deoxyguanosine glycosylase/AP lyase activity in rat
mitochondria. Nucleic Acids Res. 27:1935–1942. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mecocci P, MacGarvey U and Beal MF:
Oxidative damage to mitochondrial DNA is increased in Alzheimer's
disease. Ann Neurol. 36:747–751. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang AL, Lukas TJ, Yuan M and Neufeld AH:
Age-related increase in mitochondrial DNA damage and loss of DNA
repair capacity in the neural retina. Neurobiol Aging.
31:2002–2010. 2010. View Article : Google Scholar
|
|
66
|
Cividini F, Scott BT, Dai A, Han W, Suarez
J, Diaz-Juarez J, Diemer T, Casteel DE and Dillmann WH:
O-GlcNAcylation of 8-Oxoguanine DNA glycosylase (Ogg1) impairs
oxidative mitochondrial DNA lesion repair in diabetic hearts. J
Biol Chem. 291:26515–26528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dinkova-Kostova AT and Talalay P:
NAD(P)H:Quinone acceptor oxidoreductase 1 (NQO1), a multifunctional
antioxidant enzyme and exceptionally versatile cytoprotector. Arch
Biochem Biophys. 501:116–123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bauer AK, Faiola B, Abernethy DJ, Marchan
R, Pluta LJ, Wong VA, Roberts K, Jaiswal AK, Gonzalez FJ,
Butterworth BE, et al: Genetic susceptibility to benzene-induced
toxicity: Role of NADPH: Quinone oxidoreductase-1. Cancer Res.
63:9292003.PubMed/NCBI
|
|
69
|
True AL, Olive M, Boehm M, San H, Westrick
RJ, Raghavachari N, Xu X, Lynn EG, Sack MN, Munson PJ, et al: Heme
oxygenase-1 deficiency accelerates formation of arterial thrombosis
through oxidative damage to the endothelium, which is rescued by
inhaled carbon monoxide. Circ Res. 101:893–901. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Williams MD, Van Remmen H, Conrad CC,
Huang TT, Epstein CJ and Richardson A: Increased oxidative damage
is correlated to altered mitochondrial function in heterozygous
manganese superoxide dismutase knockout mice. J Biol Chem.
273:28510–28515. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gaziev AI, Abdullaev S and Podlutsky A:
Mitochondrial function and mitochondrial DNA maintenance with
advancing age. Biogerontology. 15:417–438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Onken B and Driscoll M: Metformin induces
a dietary restriction-like state and the oxidative stress response
to extend C. elegans healthspan via AMPK, LKB1, and SKN-1. PLoS
One. 5:e87582010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen X, Zhao X, Cai H, Sun H, Hu Y, Huang
X and Kong W and Kong W: The role of sodium hydrosulfide in
attenuating the aging process via PI3K/AKT and CaMKKβ/AMPK
pathways. Redox Biol. 12:987–1003. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhao XY, Sun JL, Hu YJ, Yang Y, Zhang WJ,
Hu Y, Li J, Sun Y, Zhong Y, Peng W, et al: The effect of
overexpression of PGC-1α on the mtDNA4834 common deletion in a rat
cochlear marginal cell senescence model. Hear Res. 296:13–24. 2013.
View Article : Google Scholar
|