Introduction
Malignant glioma is the most prevalent primary brain
tumour in the world, with >350,000 patients diagnosed with
glioma worldwide each year (1).
Despite the development in glioma therapy, the overall survival
rate following the diagnosis of patients remains poor (2,3).
In previous years, emerging reports have suggested that the
migration and self-renewal abilities of glioma cells contribute
substantially to tumour development, including tumour initiation,
metastasis, drug resistance and recurrence (4-8).
However, the influence of the tumour microenvironment on stemness
and the migration of glioma cells remains to be fully
elucidated.
Increasing evidence has indicated that the tumour
microenvironment is key in tumour development, including glioma
(9,10). The tumour microenvironment
comprises tumour cells, tumour stroma, blood vessels, infiltrating
inflammatory cells and various associated tissue cells.
Tumour-infiltrating inflammatory cells are mobilised and recruited
by tumour-derived factors, which contribute to the tumour
microenvironment. Macrophages are derived from monocytic precursors
and can undergo various differentiation or polarization processes
in tissues (11,12). Macrophage polarization is the
process of expressing different functional programmes in response
to microenvironmental signals (13). There are multiple polarization
statuses based on functional states that can acquire specific
phenotypes (14).
Tumour-associated macrophages (TAMs) can be divided into M1 and M2
subtypes, which have different roles in tumours, based on their
polarization status (15).
Studies have demonstrated that M1 and M2 subtype macrophages
exhibit tumour-suppressive and tumour-promoting functions,
respectively (16). M2 phenotype
TAMs (M2-TAMs) serve as the primary contributors to
tumour-infiltrating leukocytes and can be identified by several
surface markers, including CD163, CD206, Fizz1 and Arg1 (17,18). M2-TAMs directly facilitate tumour
initiation, progression and metastasis through the secretion of
proteolytic molecules to promote extracellular matrix remodelling
(19) or nonproteolytic proteins
to stimulate tumour cell proliferation, migration and invasion
(20,21). In addition, M2-TAMs interfere with
the antitumour functions of other immune cells (22). Consequently, investigating the
specific mechanism underlying the tumour-supportive role of M2-TAMs
in glioma progression is essential.
Furthermore, the high activity of the transforming
growth factor-β (TGF-β) pathway in human glioma is associated with
a poor prognosis (23). M2-TAMs
have been demonstrated to contribute to the accumulation of TGF-β1
in glioma tissues (24). The role
of TGF-β1 in tumour development, including cell proliferation,
invasion, immune suppression and microenvi-ronment modification,
has been well researched (24-28). In addition, reports have
demonstrated that TGF-β1 can promote the self-renewal ability of
glioma cells (29). However, how
the TGF-β pathway affects the biological properties of glioma
cells, including stemness and migration abilities, remains to be
fully elucidated.
The TGF-β pathway is induced by binding to pairs of
receptor serine/threonine kinases, known as type I and type II
receptors, and then activates and phosphorylates the intracellular
effectors mothers against decapentaplegic homolog (SMAD)2/SMAD3,
which form a complex with SMAD4 and enters the nucleus for target
gene recognition and transcriptional regulation (30,31). Sex-determining region Y-box (SOX)
factors are a family of transcriptional regulators that comprise 20
members. SOX2 is important in glioma progression (32). The downregulation of SOX2 via RNA
interference in glioma cells impairs their proliferation and tumour
formation ability in vivo (33), whereas the ectopic elevation of
SOX2 increases cell proliferation and self-renewal activity
(33,34). SOX2, mediated by other members of
the SOX family, including SOX4, which functions downstream of the
TGF-β pathway (26), is one of
the crucial factors for the maintenance cancer cell stemness
(29). The inhibition of TGF-β
has been demonstrated to suppress the expression of SOX4, leading
to a decrease in the level of SOX2 and impairment of glioma
tumourigenicity (26). However,
the effects of M2-TAMs on the expression of SOX family members to
mediate stemness and migration abilities in glioma cells remain to
be fully elucidated.
The present study aimed to elucidate the effects and
specific mechanisms of M2-TAMs on the stemness and migration of
glioma cells. It was demonstrated that M2-TAMs induced the stemness
and migration abilities of glioma cells via secreting TGF-β1,
leading to activation of the SMAD2/3 pathway and the upregulation
of SOX4 and SOX2, whereas the TGF-β pathway inhibitor SB431542 was
shown to eliminate their interaction. Furthermore, implanted
tumours in a mouse model, formed by glioma cells pre-treated with
TGF-β1 protein or co-cultured with M2-TAMs, exhibited an increase
in tumour size and growth rate compared with those formed by glioma
cells exposed to TGF-β inhibitor or no treatment. Taken together,
the results provided novel insights and strategies for the
treatment of gliomas.
Materials and methods
Cell culture and reagents
The U251 human glioma cell line and the THP-1 human
monocytic cell line were obtained from the American Type Culture
Collection (Manassas, VA, USA). The U251 cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) with 10% foetal bovine serum (FBS; HyClone,
GE Healthcare Life Sciences, Logan, UT, USA) and 1%
penicillin/streptomycin (Thermo Fisher Scientific, Inc.). The THP-1
cells were cultured in RPMI-1640 (Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS and 1% penicillin/streptomycin. The cells
were cultured at 37°C in a humidified incubator with 5%
CO2. Recombinant human TGF-β1 protein (cat. no. ab50036)
was purchased from Abcam (Cambridge, MA, USA), and the TGF-β
inhibitor SB431542 (cat. no. HY-10431) was purchased from Medchem
Express (Monmouth Junction, NJ, USA). Phorbol myristate acetate
(PMA; cat. no. P1585) was purchased from EMD Millipore (Billerica,
MA, USA). Interleukin (IL)-4 (cat. no. ab222347) and IL-13 (cat.
no. ab221410) were purchased from Abcam.
Preparation of M2 phenotype TAMs and
co-culture
The M2-polarised macrophages were generated as
previously described (35).
Briefly, the THP-1 cells (1×106 cells/ml) were seeded
into the upper insert of a six-well Transwell plate (Corning Inc.,
Corning, MA, USA) and were treated with 320 nM PMA for 6 h at 37°C,
followed by incubation with PMA and IL-4 (20 ng/ml) and IL-13 (20
ng/ml) for an addition 18 h at 37°C. The samples were then washed
with PBS to remove all PMA, and the M2-TAMs were co-cultured with
U251 cells (2×105 cells per well) without direct contact
for 48 h at 37°C. The co-cultured U251 cells were then washed and
harvested for subsequent experiments.
ELISA
The supernatants of the THP-1 cells and polarised
M2-polarised macrophages were centrifuged at 1,000 × g for 5 min
under 4°C prior to ELISA. The levels of TGF-β1 (cat. no. DB-100B),
epidermal growth factor (EGF; cat. no. DEG00), and IL-10 (cat. no.
D1000B) were measured using commercial ELISA kits (R&D Systems,
Inc., Minneapolis, MN, USA) according to the manufacturer's
protocol. Each sample was evaluated in triplicate.
Sphere formation assay of U251 cells
The U251 cells with or without co-culture were
plated on ultralow attachment plates (Corning Inc.) at a density of
20,000 cells/ml in serum-free DMEM, supplemented with B27
(Invitrogen; Thermo Fisher Scientific, Inc.) and 10 ng/ml EGF (BD
Biosciences, San Jose, CA, USA). Images of the spheres were
captured using a light microscope (Eclipse; Nikon Corporation,
Tokyo, Japan) and the spheres were quantified following 10 days of
culture.
Transwell assay
A total of 5×105 U251 cells were
suspended in serum-free DMEM and plated into the upper insert of a
six-well Transwell plate (Corning Inc.), and serum-containing
medium was added to the lower chamber. The cells were incubated at
37°C for 8 h. The non-migratory cells in the upper insert were
gently removed using cotton swabs, and the migratory cells were
fixed with 4% paraformaldehyde in room temperature for 10 min,
followed by staining with crystal violet solution. Images were
captured using a light microscope (Eclipse; Nikon Corporation) and
quantified by counting cell numbers of five randomly picked fields
of view for each well.
Western blot analysis
Western blot analysis was performed as described
previously (35). Briefly, the
total proteins of U251 cells and M2-TAMs were extracted using RIPA
lysis buffer (Abcam) containing the protease inhibitor PMSF (EMD
Millipore). The concentration of proteins was determined by Pierce
BCA Protein Assay kit according to manufacture's protocol (Thermo
Fisher Scientific, Inc.). A total of 20 µg of proteins were
subjected to 10% SDS-PAGE and were transferred onto polyvinylidene
fluoride membranes (EMD Millipore). The membranes were blocked with
5% milk in room temperature for 1 h and then were incubated with
rabbit anti-CD133 (cat. no. 86781), rabbit anti-CD44 (cat. no.
37259, 1:2,000), rabbit anti-CD206 (cat. no. 91992, 1:500), rabbit
anti-CD163 (cat. no. 93498), rabbit anti-SOX2 (cat. no. 3579),
mouse anti-SMAD2 (cat. no. 3103), rabbit anti-SMAD3 (cat. no.
9523), rabbit anti-p-SMAD2 (cat. no. 18338, 1:500), rabbit
anti-p-SMAD3 (cat. no. 9520, 1:500) from Cell Signaling Technology,
Inc. (Danvers, MA, USA), and rabbit anti-SOX4 (cat. no. ab86809),
mouse anti-vimentin (cat. no. ab8978, 1:2,000), mouse
anti-N-cadherin (cat. no. ab98952), rabbit anti-E-cadherin (cat.
no. ab40772), rabbit anti-matrix metal-loproteinase (MMP)-2 (cat.
no. ab37150), mouse anti-MMP-9 (cat. no. ab58803, 1:3,000) from
Abcam, and mouse anti-GAPDH (cat. no. SC-32233; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) antibodies at a dilution of
1:1,000 unless indicated otherwise overnight at 4°C. The membranes
were then washed by PBS with 0.05% Tween-20 and incubated with the
appropriate HRP-conjugated secondary antibodies (cat. no. 7074 for
rabbit and 7076 for mouse) from Cell Signalling Technology, Inc.,
at a dilution of 1:5,000 for 2 h at room temperature. The proteins
were detected using the enhanced chemiluminescence detection
reagent (Bio-Rad, Laboratories, Inc., Hercules, CA, USA). GAPDH was
used as a normalised control. Relative protein levels were
quantified by the comparison of grey values by ImageJ 1.51
(National Institutes of Health, Bethesda, MD, USA).
Tumour implantation
All experimental procedures involving animals were
performed in accordance with the Guidelines for the Care and Use of
Laboratory Animals of Xiangya Hospital, Central South University
(Changsha, China). In total 40 female BALB/c nude mice of 7-8 weeks
old and 16-18 g body weight were purchased from SJA Laboratory
Animal Company (Changsha, China) and maintained under 27°C in a
HEPA-filtered environment with cages in a 12-h light/dark cycle
room, with food and water sterilized by autoclaving. To generate a
subcutaneous xenograft mouse model, the mice were injected
subcutaneously with 2×106 U251 cells into the right
flanks following the indicated treatment for 48 h (n=8 mice per
group). The tumour sizes were measured every 5 days. After 30 days,
the mice were sacrificed by cervical dislocation, and solid tumours
were harvested.
Statistical analysis
All experiments were performed at least three times.
The data are expressed as the mean ± standard deviation. The
statistical significance between the specific group and control was
analysed using SPSS 13.0 statistical software (IBM Corps., Armonk,
NY, USA). Statistical evaluation was performed using Student's
t-test (two-tailed) between two groups or one-way analysis of
variance followed by Tukey's post hoc test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
M2-polarised macrophage identification
and detection of TGF-β1 secretion
M2-polarised macrophages were obtained from THP-1
human monocyte cell line polarization by PMA, IL-4, and IL-13
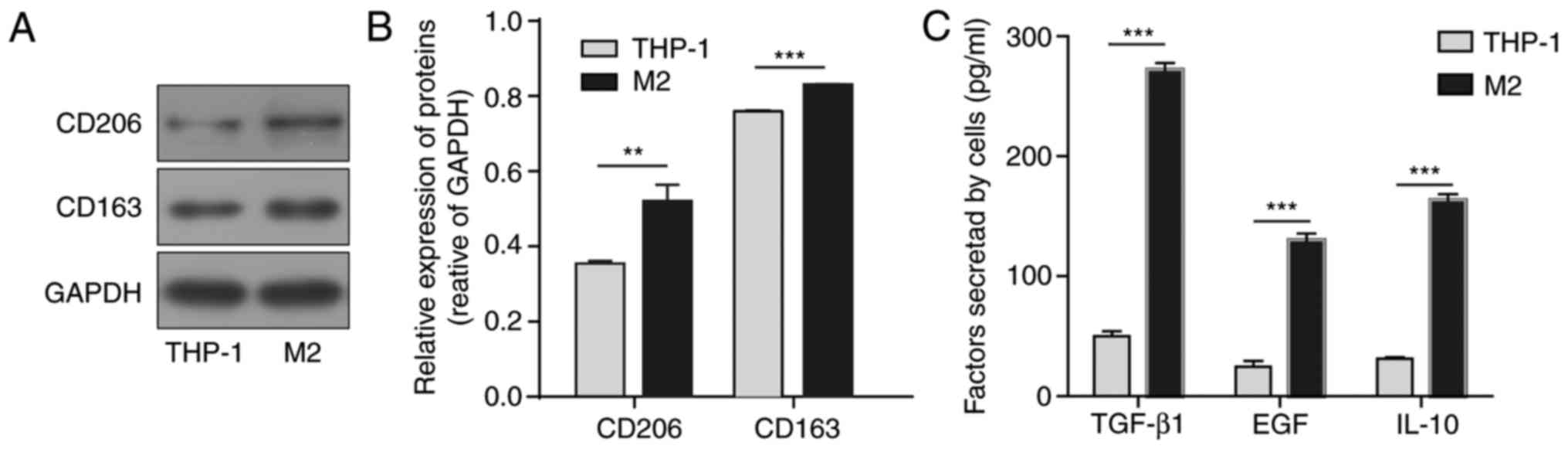
treatment. Markers for the M2 phenotype, CD206 and CD163, were
assessed by western blot analysis. As presented in Fig. 1A and B, the protein levels of
CD206 and CD163 were significantly increased following stimulation
compared with the levels in THP-1 cells. The relative growth
factors and cytokines secreted from the M2-polarised macrophages
were assessed by ELISA. Compared with the original THP-1 cells,
M2-polarised macrophages exhibited significant increases in the
concentrations of secreted TGF-β1, EGF and IL-10 (Fig. 1C). Due to the crucial role of
TGF-β1 in tumour development, TGF-β1 was selected for further
investigation.
M2-TAMs enhance the stemness and
migration abilities of glioma cells
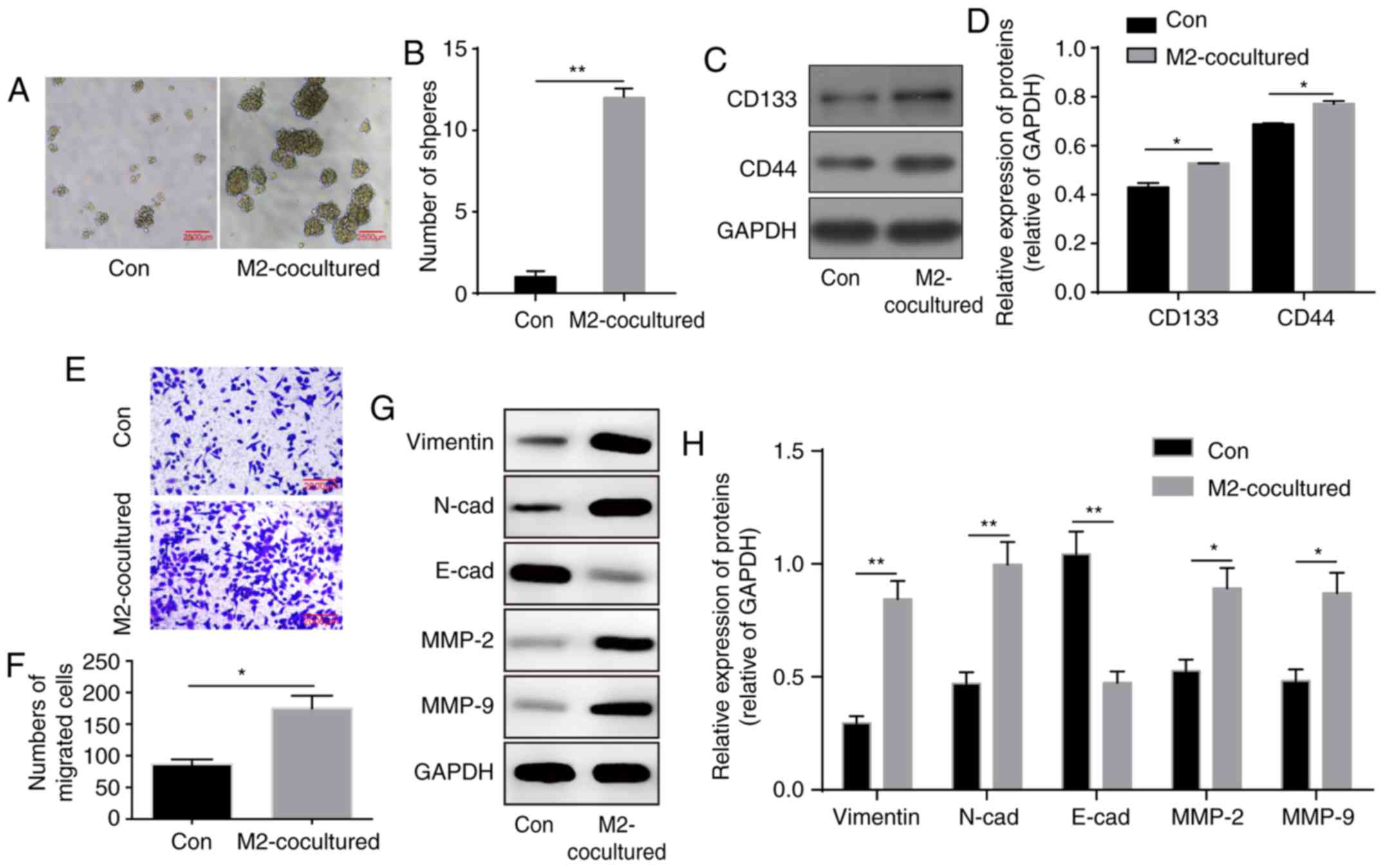
To demonstrate the influence of M2-TAMs on glioma
cells, the co-culture system was prepared. Following co-culture for
48 h, the U251 cells were harvested, and tumour formation capacity
was assessed by the sphere formation assay. Compared with the U251
cells co-cultured with THP-1 cells, the U251 cells co-cultured with
M2-TAMs exhibited a notable increase in sphere formation ability
(Fig. 2A and B). Similarly, the
expression levels of the stemness markers CD133 and CD44 were
significantly enhanced in the U251 cells (Fig. 2C and D), indicating a significant
increase in the stemness ability of glioma cells.
The tumour microenvironment is important in
facilitating tumour migration. In the Transwell migration assay,
co-culture with M2 phenotype macrophages significantly promoted the
migration ability of the glioma cells (Fig. 2E and F). Additionally, the protein
levels of epithelial-mesen-chymal transition (EMT)-associated
markers were altered, with increased levels of mesenchymal markers
vimentin, N-cadherin, MMP-2 and MMP-9, and decreased levels of the
epithelial marker E-cadherin (Fig. 2G
and H). In summary, these results indicated that M2-TAMs
facilitated the stemness and migration abilities of the glioma
cells.
TGF-β1 derived from M2-TAMs directly
increases the stemness and migration abilities of glioma cells
Due to the upregulation of TGF-β1 levels secreted
from M2-TAMs and its importance in tumour development, it was
hypothesised that M2-TAMs affecting the stemness and migration
abilities of glioma cells was achieved by TGF-β1 function. To
determine the contribution of TGF-β1 to glioma cells, the U251
cells were treated with human recombinant protein TGF-β1 (200 nM)
or TGF-β pathway inhibitor SB431542 (2 µM). Similar to co-culture
with M2-TAMs, TGF-β1 treatment increased U251 cell sphere formation
and the expression levels of stemness markers CD133 and CD44,
whereas the TGF-β inhibitor significantly reversed its effects
(Fig. 3A-D). Similarly, TGF-β1
treatment directly elevated the migration ability of the U251 cells
and promoted EMT, whereas its inhibitor led to inhibition of these
effects (Fig. 3E-H). These
results indicated that TGF-β1 derived from M2-TAMs is important in
the regulation of the stemness and migration abilities of glioma
cells.
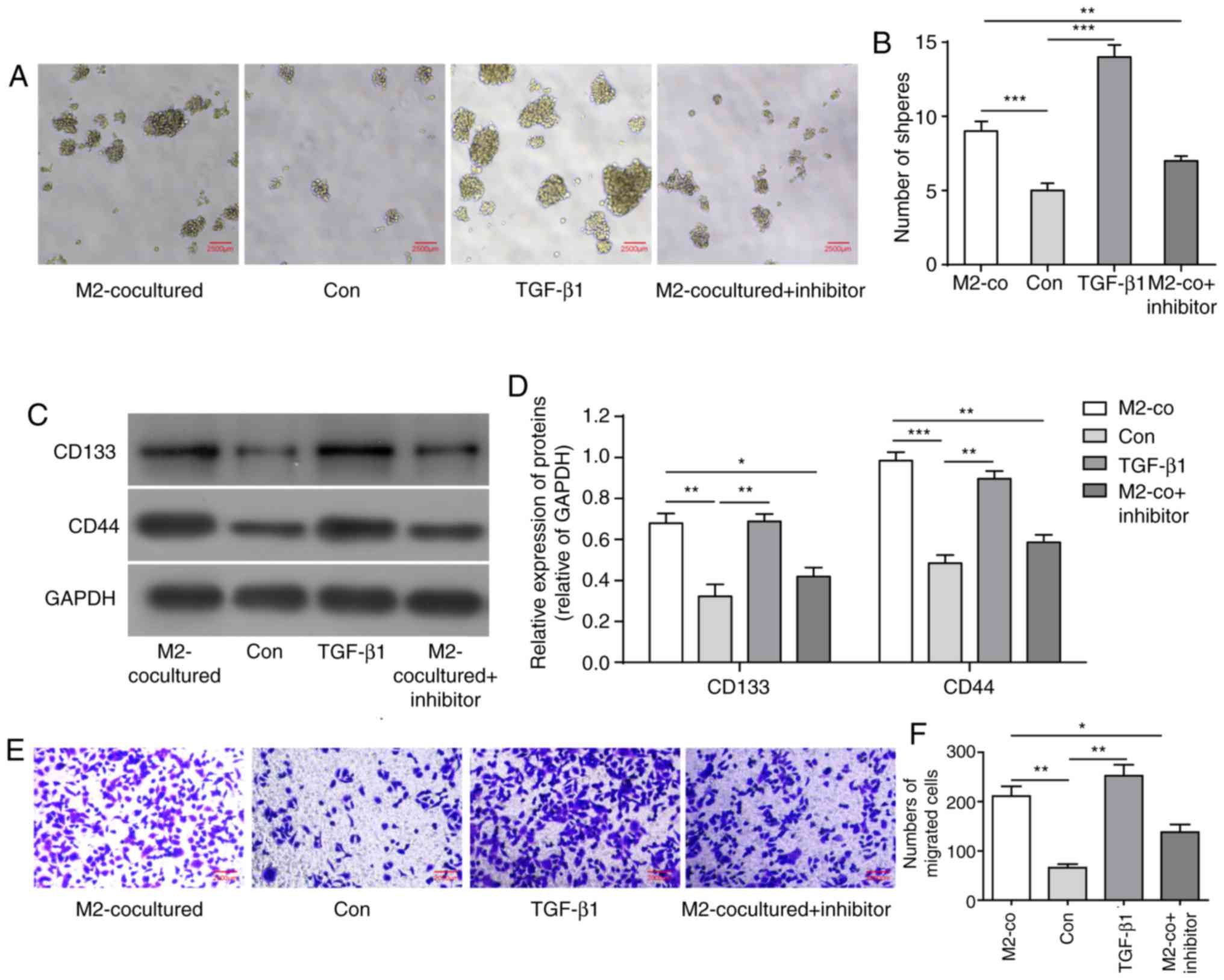 | Figure 3TGF-β1 derived from M2-TAMs directly
influence the stemness and migration of glioma cells. (A) Glioma
cell sphere formation ability and (B) results of statistical
analysis in the indicated groups. Scale bar=2,500 µm. (C)
Representative western blot images and (D) statistical results of
stemness markers CD133 and CD44 in the indicated groups. (E) Glioma
cell migration ability was measured using a Transwell assay in the
indicated groups. Scale bar=2,000 µm. (F) Statistical analysis of
migration ability in E. (G) Representative western blot images and
(H) results of statistical analysis of EMT-associated proteins of
glioma cells in the indicated groups. The results are
representative of three independent experiments. The error bars
represent the mean + standard deviation. P-values were determined
by one-way analysis of variance followed by Tukey's post hoc test.
*P<0.05, **P<0.01 and
***P<0.001; M2-TAMs, M2 phenotype tumour-associated
macrophages; co, co-culture; TGF-β1, transforming growth factor-β1;
N-cad, N-cadherin; E-cad, E-cadherin; MMP, matrix
metalloproteinase; ns, not significant; Con, control. |
TGF-β1 derived from M2-TAMs induces the
stemness and migration abilities of glioma cells via the SMAD2/3
pathway
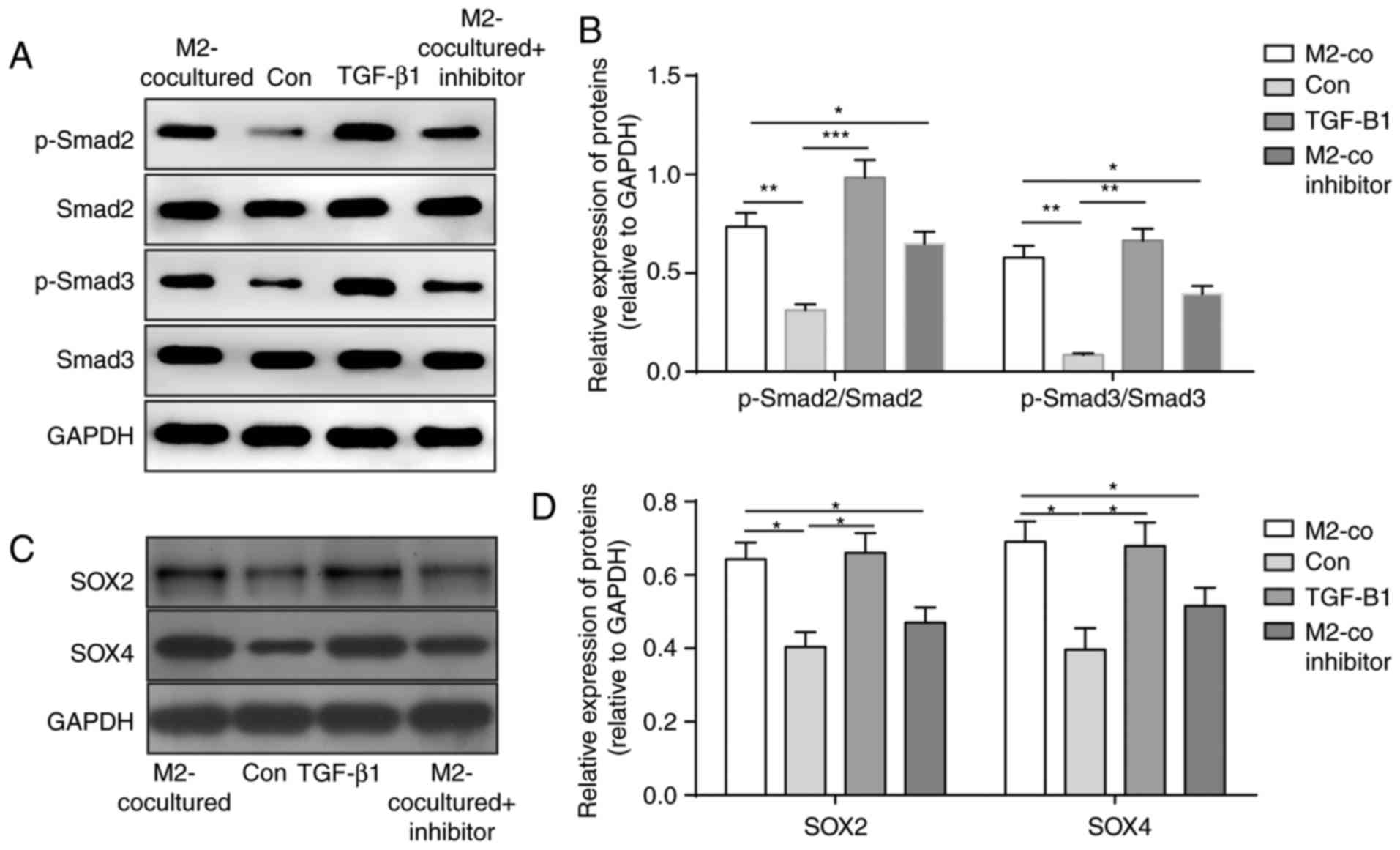
To determine the mechanism of the increased stemness
and migration abilities of glioma cells induced by M2-TAMs,
downstream signals of the TGF-β pathway in U251 cells were
detected. As shown in Fig. 4A and
B, the levels of p-SMAD2 and p-SMAD3 were significantly
upregulated following M2 coculture and TGF-β1 treatment, whereas
the TGF-β pathway inhibitor eliminated these effects (Fig. 4A and B). As the stemness of glioma
cells was increased, the levels of stemness-associated proteins
SOX2 and SOX4 were determined and demonstrated to be elevated in
the U251 cells in the co-culture system and those treated with
TGF-β1 (Fig. 4C and D). These
results suggested that TGF-β1 secreted from M2-TAMs activated the
TGF-β-SMAD2/3 pathway to induce the expression of SOX4 and SOX2 and
promote the stemness and migration abilities of glioma cells.
M2-TAMs facilitate solid tumour formation
in a mouse model
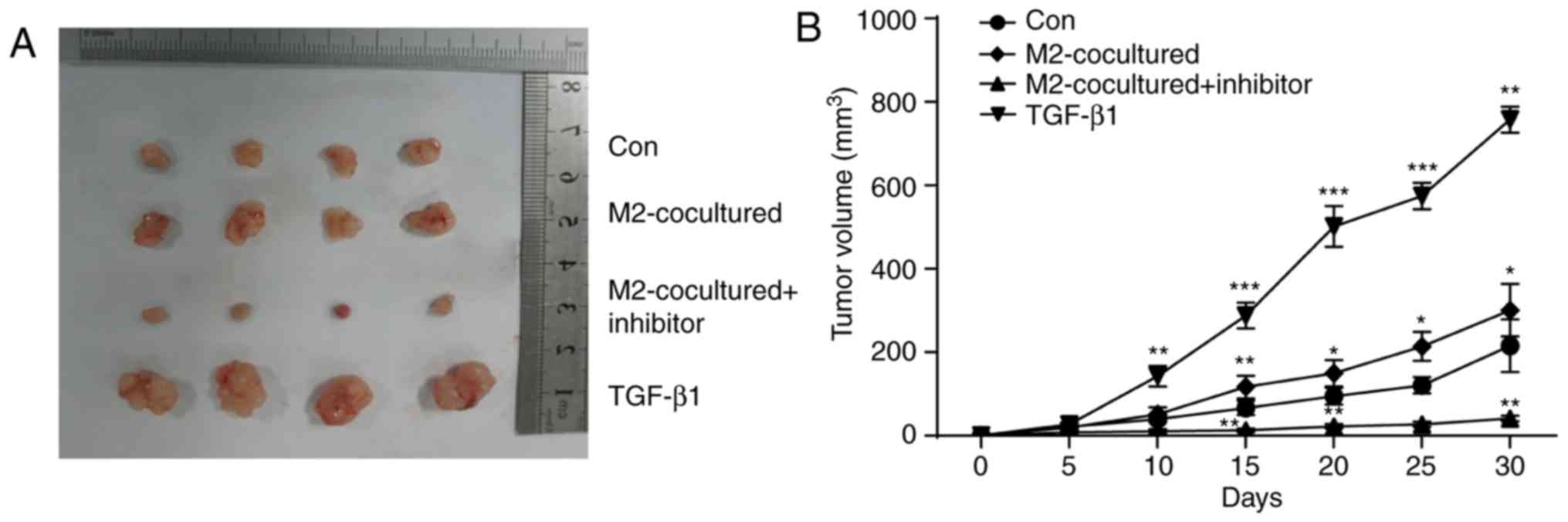
To investigate whether M2-TAMs can affect glioma
cell capacity for solid tumour formation in vivo, U251 cells
were subcutaneously implanted following different treatments. As
shown in Fig. 5A and B, the final
tumour sizes formed by cells co-cultured with M2-TAMs or those
treated with TGF-β1 were increased compared with those of the
control group. Additionally, TGF-β inhibitor treatment decreased
the size of the tumours, which were induced by co-culture with
M2-TAMs. The growth rate of tumours formed by U251 cells was also
accelerated by M2-TAM co-culture or TGF-β1 treatment, and the TGF-β
inhibitor reversed these effects. Therefore, these data
demonstrated that M2-TAMs facilitated solid tumour formation of
glioma cells in vivo through secreting TGF-β1 protein.
Discussion
The tumour-associated microenvironment provides cues
to cancer cells that regulate their self-renewal and metastatic
potential (36,37). The tumour microenvironment
features inflammation, which is the major contributing factor to
tumour formation and metastasis (38). Among tumour-infiltrating immune
cells, TAMs are the major constituents of the
inflammation-associated microenvironment in tumours (39). Previous studies have demonstrated
a positive correlation between the number of M2-TAMs and glioma
development, particularly glioma stem-like cells (21). However, the present study
investigated the function and mechanism of M2-TAM regarding the
stemness and migration abilities of glioma cells and the associated
specific pathways involved in this process. The results of the
present study demonstrated that M2-TAMs increased the stemness and
migration abilities of glioma cells via secreting TGF-β1, leading
to activation of the SMAD2/3 pathway and the upregulation of SOX4
and SOX2. These findings provide novel insights, strategies and
therapeutic targets for the treatment of glioma progression.
The initial results of the present study showed that
M2-TAMs derived from the THP-1 human monocyte cell line upregulated
M2 subtype surface markers CD206 and CD163 (Fig. 1A and B). Compared with THP-1
cells, M2-TAMs exhibited notably higher levels of TGF-β1, EGF and
IL-10, indicating the successful transition to M2 subtype
macrophages. Subsequently, to determine the cross-talk between
M2-TAMs and glioma cells, a co-culture system was established,
enabling communication through secreting factors from M2-TAMs.
M2-TAMs have been demonstrated to promote cell invasion in
pancreatic islets cancer (19)
and facilitate the stemness of cancer stem cells in breast cancer
(40). In the present study, the
glioma cells exhibited a higher tumour sphere formation capacity
and increased expression of stemness markers following co-cultured
with M2-TAMs, compared with those co-cultured with THP-1 cells,
indicating enhancement in their stemness and tumour development
ability induced by M2-TAMs. The M2-TAMs were also shown to promote
the migration ability of glioma cells and facilitate EMT
transition.
Previous reports have demonstrated that the level of
TGF-β receptor 2 (TGFBR2), as a specific receptor for TGF-β1, is
higher in glioma stem-like cells (21). Repression of the expression of
TGFBR2 in glioma notably decreased their invasion ability, even
when co-cultured with M2-TAMs, indicating that the TGF-β pathway
contributed the major function in the interaction between M2-TAMs
and glioma stem-like cells. The results demonstrated that direct
TGF-β1 protein treatment was able to achieve similar effects to
those induced by M2-TAMs in glioma cell performance, including
sphere formation capacity, self-renewal and migration abilities.
However, the TGF-β inhibitor significantly eliminated the effects
that were induced by M2-TAMs.
The TGF-β signalling cascade is initiated by binding
and activating its receptors (TGFBR1 and TGFBR2), leading to the
phosphorylation of intracellular effectors SMAD2/SMAD3 (30). To demonstrate the TGF-β pathway
activity in glioma cells, the protein levels of pSMAD2 and pSMAD3
were detected. The resulting data indicated that co-culture with
M2-TAMs or TGF-β1 protein stimulation significantly increased
SMAD2/3 phosphorylation activity, whereas the TGF-β pathway
inhibitor was shown to repress these effects (Fig. 4A and B). These results suggested
that the TGF-β1-SMAD2/3 pathway is key in the cross-talk between
M2-TAMs and glioma cells. Although Ye et al (21) also investigated M2-TAMs in glioma,
the study focused on the association between M2-TAMs and glioma
stem-like cells, and did not examine the specific associated
pathways downstream of TGF-β1. The present study demonstrated for
the first time, to the best of our knowledge, that M2-TAMs increase
the stemness and migration abilities of glioma cells via the
TGF-β1-SMAD2/3 pathway.
SOX2 has been shown to be important in the
maintenance of stem cell activity, particularly for cancer stem
cells (32). SOX2 has been
identified as a frequently amplified gene in small cell lung cancer
(41). In breast cancer, SOX has
been reported to be upregulated in cancer stem cells (42). The downregulation of SOX2 in
glioma stem cells impairs their proliferation and tumour formation
ability (33). SOX4 acts
downstream of the TGF-β pathway and regulates the expression of
SOX2 (26). The inhibition of
TGF-β signalling is able to suppress the expression of SOX2 through
inhibiting SOX4 (26). SOX4 can
also regulate the TGF-β-induced EMT process (43). The results of the present study
showed that, when co-cultured with M2-TAMs or treated with TGF-β1
protein, the glioma cells exhibited increased expression of SOX4,
leading to elevated SOX2 at the same time. Therefore, these results
suggested that TGF-β1 activated the SMAD2/3 pathway to induce the
expression of SOX4 and SOX2, promoting the stemness and migration
abilities of the glioma cells. These results are the first, to the
best of our knowledge, to suggest that the SOX4/SOX2 axis is
involved in the regulation of M2-TAMs in the stemness and migration
abilities of glioma cells.
The in vivo tumour graft assay further
supported the above conclusion. The size and growth rate of tumours
formed by U251 cells were significantly increased by co-culture
with M2-TAMs and TGF-β1 protein treatment. Additionally, TGF-β
inhibitor treatment eliminated these effects induced by M2-TAMs.
From these results in vivo, it was concluded that M2-TAMs
accelerated the growth of solid tumours by the TGF-β1 pathway,
which may result from the increased stemness and migration
abilities of glioma cells that were observed in the in vitro
results.
In conclusion, the present study investigated the
contribution of M2-TAMs to the stemness and migration abilities of
glioma cells. It was demonstrated that TGF-β1 in the tumour
microenvironment secreted from M2-TAMs activated the SMAD2/3
pathway and then increased the expression levels of SOX4 and SOX2.
This resulted in elevation of the stemness and migration abilities
of the cells in vitro, by altering the gene expression
pattern associated with stemness and the EMT process, and increased
solid tumour sizes in vivo. The development of therapeutic
strategies against the communication among M2-TAMs and glioma cells
may be a potential approach to monitor glioma initiation and
progression.
Funding
The present study was supported by Beijing Medical
Health Public Welfare Foundation (grant no.
YWJKJJHKYJJ-B17468).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZL guarantees the integrity of the entire study and
was responsible for the design of the study and clarified
associated intellectual content. ZL also performed experimental
studies, data acquisition and data analysis and then edited the
whole manuscript and reviewed it. WK performed experimental studies
and manuscript editing. QZ performed data analysis and manuscript
preparation. YZ performed data acquisition and reviewed the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures involving animals were
performed in accordance with the Guidelines for the Care and Use of
Laboratory Animals of Xiangya Hospital, Central South
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Ostrom QT, Bauchet L, Davis FG, Deltour I,
Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh
KM, et al: The epidemiology of glioma in adults: A ‘state of the
science’ review. Neuro Oncol. 16:896–913. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zagzag D, Salnikow K, Chiriboga L, Yee H,
Lan L, Ali MA, Garcia R, Demaria S and Newcomb EW: Downregulation
of major histocompatibility complex antigens in invading glioma
cells: Stealth invasion of the brain. Lab Invest. 85:328–341. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J, Yuan J, Yuan X, Zhao J, Zhang Z,
Weng L and Liu J: MicroRNA-200b inhibits the growth and metastasis
of glioma cells via targeting ZEB2. Int J Oncol. 48:541–550. 2016.
View Article : Google Scholar
|
|
4
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:49–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sainz B Jr, Alcala S, Garcia E,
Sanchez-Ripoll Y, Azevedo MM, Cioffi M, Tatari M, Miranda-Lorenzo
I, Hidalgo M, Gomez-Lopez G, et al: Microenvironmental
hCAP-18/LL-37 promotes pancreatic ductal adenocarcinoma by
activating its cancer stem cell compartment. Gut. 64:1921–1935.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molina JR, Hayashi Y, Stephens C and
Georgescu MM: Invasive glioblastoma cells acquire stemness and
increased Akt activation. Neoplasia. 12:453–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hwang RF, Moore T, Arumugam T,
Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB and Logsdon CD:
Cancer-associated stromal fibroblasts promote pancreatic tumor
progression. Cancer Res. 68:918–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heusinkveld M and van der Burg SH:
Identification and manipulation of tumor associated macrophages in
human cancers. J Transl Med. 9:2162011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Steinman RM and Idoyaga J: Features of the
dendritic cell lineage. Immunol Rev. 234:5–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sica A, Larghi P, Mancino A, Rubino L,
Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P and Mantovani
A: Macrophage polarization in tumour progression. Semin Cancer
Biol. 18:349–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hamilton TA, Zhao C, Pavicic PG Jr and
Datta S: Myeloid colony-stimulating factors as regulators of
macrophage polarization. Front Immunol. 5:5542014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sielska M, Przanowski P, Wylot B,
Gabrusiewicz K, Maleszewska M, Kijewska M, Zawadzka M, Kucharska J,
Vinnakota K, Kettenmann H, et al: Distinct roles of CSF family
cytokines in macrophage infiltration and activation in glioma
progression and injury response. J Pathol. 230:310–321. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Staudt ND, Jo M, Hu J, Bristow JM, Pizzo
DP, Gaultier A, VandenBerg SR and Gonias SL: Myeloid cell receptor
LRP1/CD91 regulates monocyte recruitment and angiogenesis in
tumors. Cancer Res. 73:3902–3912. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ling EA and Wong WC: The origin and nature
of ramified and amoeboid microglia: A historical review and current
concepts. Glia. 7:9–18. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Komohara Y, Ohnishi K, Kuratsu J and
Takeya M: Possible involvement of the M2 anti-inflammatory
macrophage phenotype in growth of human gliomas. J Pathol.
216:15–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gocheva V, Wang HW, Gadea BB, Shree T,
Hunter KE, Garfall AL, Berman T and Joyce JA: IL-4 induces
cathepsin protease activity in tumor-associated macrophages to
promote cancer growth and invasion. Genes Dev. 24:241–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li D, Wang X, Wu JL, Quan WQ, Ma L, Yang
F, Wu KY and Wan HY: Tumor-produced versican V1 enhances
hCAP18/LL-37 expression in macrophages through activation of TLR2
and vitamin D3 signaling to promote ovarian cancer progression in
vitro. PLoS One. 8:e566162013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen
L, Xiao HL, Wang B, Yi L, Wang QL, et al: Tumor-associated
microglia/macrophages enhance the invasion of glioma stem-like
cells via TGF-beta1 signaling pathway. J Immunol. 189:444–453.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morford LA, Dix AR, Brooks WH and Roszman
TL: Apoptotic elimination of peripheral T lymphocytes in patients
with primary intracranial tumors. J Neurosurg. 91:935–946. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bruna A, Darken RS, Rojo F, Ocaña A,
Peñuelas S, Arias A, Paris R, Tortosa A, Mora J, Baselga J and
Seoane J: High TGFbeta-Smad activity confers poor prognosis in
glioma patients and promotes cell proliferation depending on the
methylation of the PDGF-B gene. Cancer Cell. 11:147–160. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Massagué J: TGFbeta in cancer. Cell.
134:215–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bierie B and Moses HL: Transforming growth
factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth
Factor Rev. 21:49–59. 2010. View Article : Google Scholar
|
|
26
|
Jun F, Hong J, Liu Q, Guo Y, Liao Y, Huang
J, Wen S and Shen L: Epithelial membrane protein 3 regulates TGF-β
signaling activation in CD44-high glioblastoma. Oncotarget.
8:14343–14358. 2017. View Article : Google Scholar
|
|
27
|
Araki S, Eitel JA, Batuello CN,
Bijangi-Vishehsaraei K, Xie XJ, Danielpour D, Pollok KE, Boothman
DA and Mayo LD: TGF-beta1-induced expression of human Mdm2
correlates with late-stage metastatic breast cancer. J Clin Invest.
120:290–302. 2010. View
Article : Google Scholar
|
|
28
|
Donkor MK, Sarkar A, Savage PA, Franklin
RA, Johnson LK, Jungbluth AA, Allison JP and Li MO: T cell
surveillance of oncogene-induced prostate cancer is impeded by T
cell-derived TGF-β1 cytokine. Immunity. 35:123–134. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ikushima H, Todo T, Ino Y, Takahashi M,
Miyazawa K and Miyazono K: Autocrine TGF-beta signaling maintains
tumori-genicity of glioma-initiating cells through Sry-related
HMG-box factors. Cell Stem Cell. 5:504–514. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moustakas A and Heldin CH: The regulation
of TGFbeta signal transduction. Development. 136:3699–3714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng XH and Derynck R: Specificity and
versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev
Biol. 21:659–693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garros-Regulez L, Aldaz P, Arrizabalaga O,
Moncho-Amor V, Carrasco-Garcia E, Manterola L, Moreno-Cugnon L,
Barrena C, Villanua J, Ruiz I, et al: mTOR inhibition decreases
SOX2-SOX9 mediated glioma stem cell activity and temozolomide
resistance. Expert Opin Ther Targets. 20:393–405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alonso MM, Diez-Valle R, Manterola L,
Rubio A, Liu D, Cortes-Santiago N, Urquiza L, Jauregi P, Lopez de
Munain A, Sampron N, et al: Genetic and epigenetic modifications of
Sox2 contribute to the invasive phenotype of malignant gliomas.
PLoS One. 6:e267402011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garros-Regulez L, Garcia I,
Carrasco-Garcia E, Lantero A, Aldaz P, Moreno-Cugnon L,
Arrizabalaga O, Undabeitia J, Torres-Bayona S, Villanua J, et al:
Targeting SOX2 as a therapeutic strategy in glioblastoma. Front
Oncol. 6:2222016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang WC, Chan ML, Chen MJ, Tsai TH and
Chen YJ: Modulation of macrophage polarization and lung cancer cell
stemness by MUC1 and development of a related small-molecule
inhibitor pterostilbene. Oncotarget. 7:39363–39375. 2016.PubMed/NCBI
|
|
36
|
Lonardo E, Hermann PC, Mueller MT, Huber
S, Balic A, Miranda-Lorenzo I, Zagorac S, Alcala S,
Rodriguez-Arabaolaza I, Ramirez JC, et al: Nodal/Activin signaling
drives self-renewal and tumorigenicity of pancreatic cancer stem
cells and provides a target for combined drug therapy. Cell Stem
Cell. 9:433–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lonardo E, Frias-Aldeguer J, Hermann PC
and Heeschen C: Pancreatic stellate cells form a niche for cancer
stem cells and promote their self-renewal and invasiveness. Cell
Cycle. 11:1282–1290. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cabarcas SM, Mathews LA and Farrar WL: The
cancer stem cell niche-there goes the neighborhood. Int J Cancer.
129:2315–2327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Solinas G, Schiarea S, Liguori M, Fabbri
M, Pesce S, Zammataro L, Pasqualini F, Nebuloni M, Chiabrando C,
Mantovani A and Allavena P: Tumor-conditioned macrophages secrete
migration-stimulating factor: A new marker for M2-polarization,
influencing tumor cell motility. J Immunol. 185:642–652. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang J, Liao D, Chen C, Liu Y, Chuang TH,
Xiang R, Markowitz D, Reisfeld RA and Luo Y: Tumor-associated
macrophages regulate murine breast cancer stem cells through a
novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells.
31:248–258. 2013. View Article : Google Scholar
|
|
41
|
Rudin CM, Durinck S, Stawiski EW, Poirier
JT, Modrusan Z, Shames DS, Bergbower EA, Guan Y, Shin J, Guillory
J, et al: Comprehensive genomic analysis identifies SOX2 as a
frequently amplified gene in small-cell lung cancer. Nat Genet.
44:1111–1116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leis O, Eguiara A, Lopez-Arribillaga E,
Alberdi MJ, Hernandez-Garcia S, Elorriaga K, Pandiella A, Rezola R
and Martin AG: Sox2 expression in breast tumours and activation in
breast cancer stem cells. Oncogene. 31:1354–1365. 2012. View Article : Google Scholar
|
|
43
|
Vervoort SJ, Lourenço AR, van Boxtel R and
Coffer PJ: SOX4 mediates TGF-β-induced expression of mesenchymal
markers during mammary cell epithelial to mesenchymal transition.
PLoS One. 8:e532382013. View Article : Google Scholar
|