Introduction
Many autoimmune diseases are caused by autoreactive
T helper (Th)1 clones, and dysregulated Th1/Th2 responses have long
been associated with the induction and regulation of autoimmunity
(1). However, the finding that a
lack of interferon γ makes otherwise experimental autoimmune
encephalomyelitis mice susceptible to disease (2) raised the question of whether another
T cell subset, other than Th1, may be required for the induction of
autoimmune diseases. Subsequently studies identified an independent
subset of interleukin (IL)‑17‑producing effector T helper (Th17)
cells, which are involved in the pathogenesis of numerous
experimental autoimmune diseases and human inflammatory states
(3). By contrast, regulatory T
cells (Tregs) are a subset of CD4+ T cells that express
forkhead box P3 (FOXP3) and are crucial in controlling inflammation
and maintaining self-tolerance (4).
Transforming growth factor (TGF)-β1 has been
recognized as a link between Treg and Th17 cell subsets. Th17 cells
are reciprocally related to Tregs given that TGF-β1 induces
differentiation of naive T cells into FOXP3+ Tregs,
whereas IL-6 inhibits the TGF-β1-induced expression of FOXP3 and
ultimately induces the generation of Th17 cells (3). By contrast, high concentrations of
TGF-β1 prevent the induction and effector functions of Th17 cells
by inducing FOXP3 (3). However,
numerous questions remain concerning the integration effects of
TGF-β signals and inflammatory stimuli on the plasticity between
Tregs and Th17 cells. Notably, BMP and activin membrane-bound
inhibitor (BAMBI) was identified as a TGF‑β1 rheostat that controls
Th17/Treg cell differentiation and the development of autoimmune
arthritis in mice (5). BAMBI is
structurally similar to TGF-β type I receptors (TGF-βRIs) but lacks
an intracellular kinase domain, causing it to antagonize TGF-β
family signalling (6). Although
BAMBI has been shown to serve roles in human lung inflammation
associated with chronic obstructive pulmonary disease (COPD) and
bacterial infection (7), the
precise expression and activity of BAMBI in human immune cells,
especially in the Th17/Treg cell paradigm, remains to be
determined.
Although smoking has been well established as a
central factor in many pathological conditions, such as neoplasms
and lung and cardiovascular diseases, little is known about the
processes and factors involved in changing immune arms in response
to cigarette smoke (8). Although
tobacco smoke was demonstrated to stimulate the production of
pro‑inflammatory cytokines (such as tumour necrosis factor α, IL-1
and IL-6) and to reduce anti‑inflammatory cytokine levels (such as
IL‑10), local homeostasis and cytokine networks, as well as genetic
predisposition, should be taken into account when analysing the
influence of cigarette smoke (8).
Our previous studies have demonstrated that cigarette smoking
disrupts the survival of CD4+CD8+ Tregs in
COPD, partially through muscarinic receptor-dependent mechanisms
(9,10). However, the pathological processes
and pathways underlying the Th17/Treg cell imbalance are still
unknown.
Our previous study also demonstrated that an
impaired TGF-β/BAMBI pathway promotes inflammation leading to
Th17/Treg imbalance, which is a new mechanism in smokers who
develop COPD (11). The present
study aimed to further characterize BAMBI expression in human
CD4+ cells and immune imbalance during activation and
cigarette smoke exposure. These data suggested that cigarette smoke
may disturb TGF-β-dependent BAMBI expression in human
TCR-stimulated CD4+ T cells, which, in turn, may cause
the dysfunction of Smad2/Smad3 phosphorylation following cigarette
smoke exposure. The results indicated that BAMBI was functionally
expressed in activated CD4+ T cells and may act as a
molecular switch to control TGF-β signalling and the Th17/Treg cell
balance. The TGF-β1/BAMBI pathway in T cells may serve a role in
immune dysfunction associated with cigarette smoke.
Materials and methods
Cell isolation
This study was conducted between January 2016 and
May 2017. Blood samples (20–30 ml) were obtained from 25 healthy
adults (age, 25–55 years; 15 male, 10 female) and immediately
placed on ice. Peripheral blood mononuclear cells (PBMCs) were
isolated from buffy coats of blood samples. All experiments were
performed in accordance with relevant manufacturer’s protocols.
Naive CD4+ T cells were isolated from PBMCs by MACS,
based on negative selection using the naive CD4+ T cell
isolation kit II (Miltenyi Biotec GmbH, Bergisch Gladbach,
Germany), according to the manufacturer’s instructions. The purity
of CD4+CD45RA+ T cells was >95%, as
measured by flow cytometry. This study was approved by the Ethics
Committee of Union Hospital, Tongji Medical School, Huazhong
University of Science and Technology (Wuhan, China); written
informed consent was received from each donor.
Cigarette smoke extract (CSE)
preparation
Aqueous CSE was prepared according to a modification
of our previously published method (10). Briefly, the smoke from one
cigarette (Huanghelou, Wuhan, China) was bubbled slowly into a tube
containing 2.5 ml of sterile RPMI-1640 medium (Beijing Suolaibao
Biotechnology Co., Ltd., Beijing, China). The resulting solution
was further filtered through 0.22 μm filters and was defined
as 100% CSE. The CSE was prepared fresh, within 30 min prior to
each experiment.
Cell viability assay
Purified naive CD4+ T cells
(1×105 cells/well) were cultured and incubated at 37°C
in 200 μl of complete medium containing 10% heat‑inactivated
(56°C for 30 min) foetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and 50 U/ml IL-2 (PeproTech,
Inc., Rocky Hill, NJ, USA) in 96-well plates and stimulated with
plate-bound anti-CD3 (monoclonal, OKT3; 5 μg/ml) and
anti‑CD28 (5 μg/ml) antibodies (both purchased from
eBioscience; Thermo Fisher Scientific, Inc.). Cells were stimulated
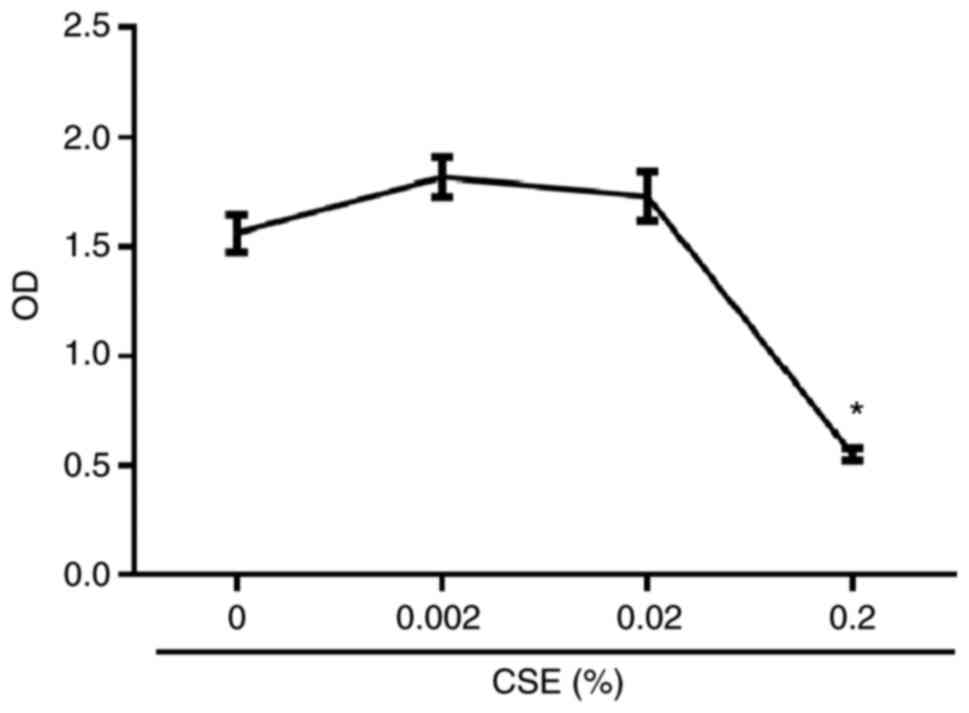
for 5 days in the presence of serial dilutions of CSE at 0, 0.002,
0.02 and 0.2%. Cell viability was tested by Cell Counting Kit-8
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan), according
to the manufacturer’s protocol. The safe concentrations of CSE at
0.002 and 0.02% used in subsequent experiments were chosen
following a cytotoxicity assay (Fig.
1).
Development of naive and differentiating
T cells in CSE‑conditioned medium
To investigate the effects of cigarette smoke on the
development of naive CD4+ T cells, purified cells
(5×105 cells/well) were incubated at 37°C in 1 ml
complete medium containing FBS and IL-2, as aforementioned, in
48-well plates with plate-bound anti-CD3/CD28, in the absence or
presence of CSE (0.002 and 0.02%). In parallel experiments, naive T
cells were cultured in vitro under Treg-polarizing
conditions (2 ng/ml TGF-β1) or Th17 cell-polarizing conditions (2
ng/ml TGF-β1 and 30 ng/ml IL-6; or 10 ng/ml IL-1β, 30 ng/ml IL-6
and 50 ng/ml IL-23), combined with or without CSE at the initiation
of culture. Recombinant human TGF-β1, IL-6, IL-1β and IL‑23 were
purchased from PeproTech, Inc. To confirm that the TGF-β1 produced
and activated in T cell receptor (TCR)-stimulated cells was indeed
responsible for BAMBI expression, a purified anti‑TGF‑β antibody
(500 ng/ml; clone 19D8; BioLegend, Inc., San Diego, CA, USA) that
is able to block human TGF-β1 activity was included in the culture.
The involvement of Smad3 was determined by treating cells with 1
μM of the Smad3‑specific inhibitor SIS3 (Sigma‑Aldrich;
Merck KGaA, Darmstadt, Germany), which caused potent and selective
inhibition of Smad3 function (12). After 5 days incubation, the
expression of T cell subsets and related molecules was determined
by flow cytometry.
Flow cytometry
The expression of T cells markers were analysed by
flow cytometry as described previously (10); surface or intracellular proteins
were stained with fluorescein isothiocy-anate-conjugated anti-CD4
(Clone RPA-T4; BD Biosciences, San Jose, CA, USA), phycoerythrin
(PE)-cyanine 7-conjugated anti-CD25 (Clone M-A251; BD Biosciences),
PE-conjugated anti-FOXP3 (Clone 236A/E7; eBioscience; Thermo Fisher
Scientific, Inc.), eFluor660-conjugated anti-IL-17A (Clone
eBio64DEC17; eBioscience; Thermo Fisher Scientific, Inc.), Alexa
Fluor (AF)-647-conjugated anti-BAMBI (Polyclonal; BIOSS, Beijing,
China) and AF647-conjugated anti-Smad2 (pS465/pS467)/Smad3
(pS423/pS425) (Clone O72-670; BD Biosciences) antibodies.
Intracellular staining for FOXP3, IL-17 and Smad2/Smad3 was
performed following fixation and permeabilization using the FOXP3
Staining Buffer Set (eBioscience; Thermo Fisher Scientific, Inc.),
as described previously (10).
Isotype controls were used to exclude non‑specific staining and to
identify the gates for negative/positive population selection. To
identify Th17 cells, 50 ng/ml phorbol 12-myristate 13-acetate
(Sigma-Aldrich; Merck KGaA), 1 μg/ml ionomycin
(Sigma-Aldrich; Merck KGaA) and 1 μl/ml GolgiStop (BD
Biosciences) were added to the culture medium on day 5 for 5 h. To
measure intracellular levels of phosphorylated (p)-Smad2/Smad3,
cells were treated with 2 ng/ml TGF-β1 for 30 min at 37°C on day 5
prior to harvesting. Flow cytometry was performed using the BD
LSRFortessa and analysed with FACSDiva Software v8 (BD Biosciences)
and FlowJo v10 software (Tree Star, Inc., Ashland, OR, USA).
Statistics
Data are expressed as the mean ± standard error of
the mean. Statistical analysis was performed by one-way ANOVA
followed by Dunnett’s multiple comparisons tests. Data analysis was
conducted with GraphPad Prism v7.0 (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference. No statistical method was
used to predetermine sample size.
Results
Treg differentiation upon CSE
exposure
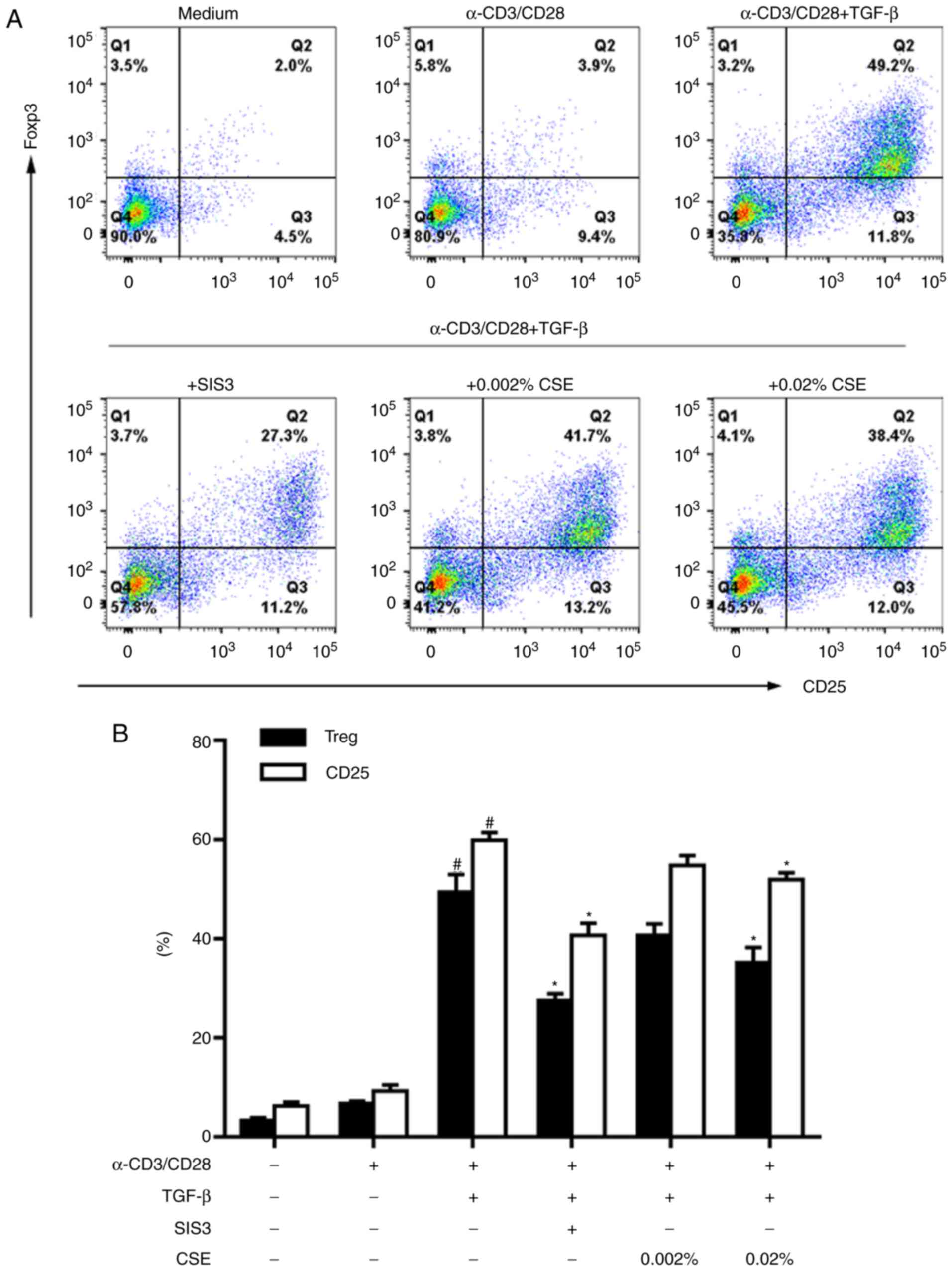
To better understand the induction of Tregs, the
expression levels of CD25 and FOXP3 were determined in naive
CD4+ T cells stimulated with anti-CD3/28 antibodies and
TGF-β1, in combination with or without the Smad3‑specific inhibitor
SIS3. According to classical in vitro Treg-polarizing
conditions (with anti-CD3/28 antibodies in the presence of TGF-β1)
(13,14), high levels of
CD25+FOXP3+ Tregs were induced successfully
during differentiation; whereas this induction was blocked by SIS3
treatment (Fig. 2).
To determine whether the stimulation of cigarette
smoke was associated with a change in Treg induction, CSE was added
to CD4+ T cell cultures at different non-cytotoxic
concentrations (0.002 and 0.02%; Fig.
1). Exposure to CSE alone did not induce naive CD4+
T cells to become CD25+FOXP3+ suppressor
cells (15). Under classical
Treg-polarizing conditions, however, CSE treatment notably reduced
the differentiation rate of Tregs (Fig. 2).
CD25 expression is one of the activation markers of
T cells. During Treg cell differentiation, a high induction of CD25
was also observed in CD4+ T cells following activation
with anti-CD3/28 antibodies in the presence of TGF-β1 (Fig. 2). Similar to the observed trend in
Treg generation, CD25 induction was inhibited by SIS3 and 0.02% CSE
treatment (Fig. 2).
CSE exposure in Th17 cell
differentiation
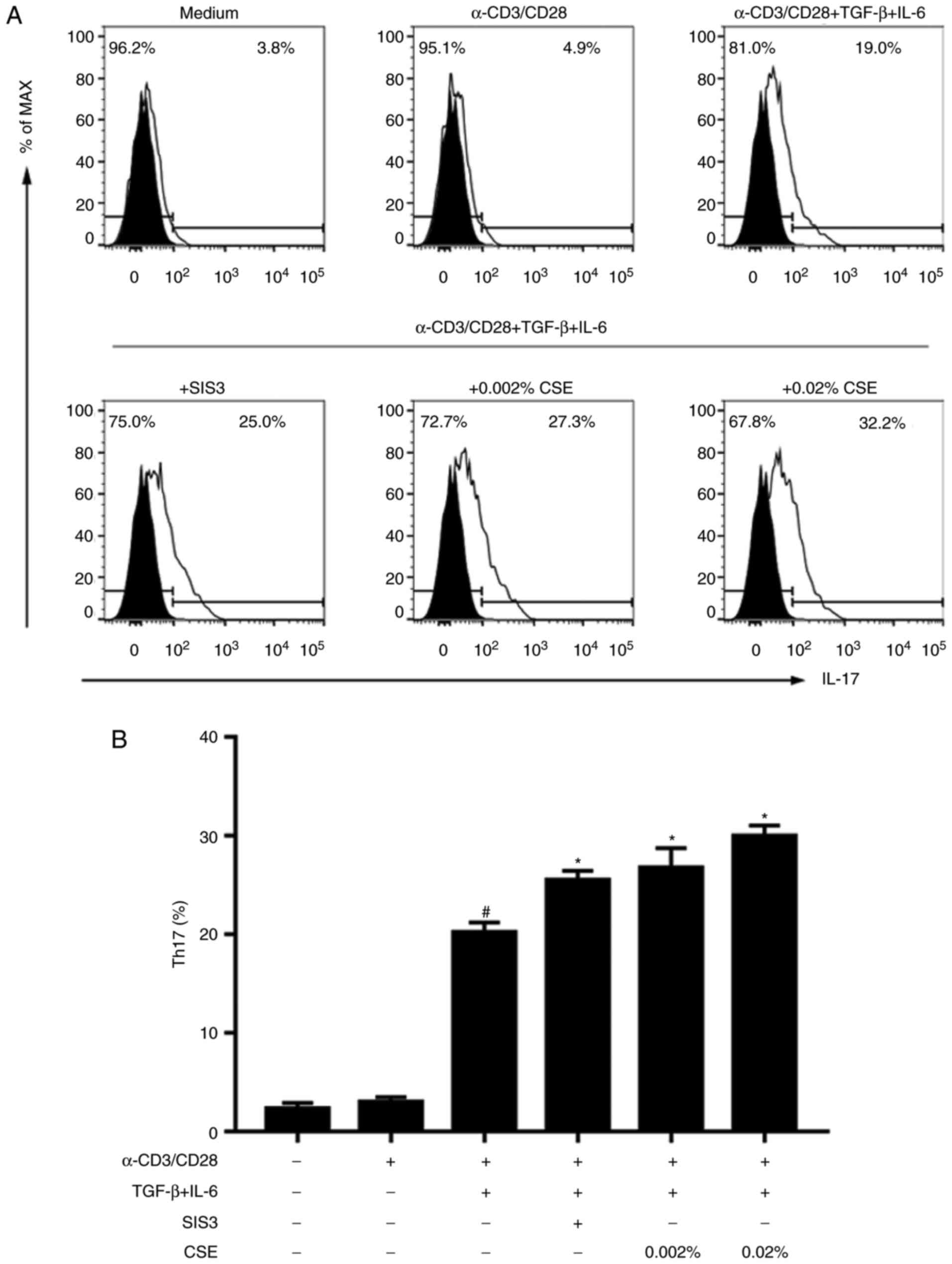
Classical differentiation of pro-inflammatory Th17
cells was also examined. In naive CD4+ T cells incubated
in the presence of TGF-β1 + IL-6 (the first protocol), Th17 cells
were successfully detected (Fig.
3). Notably, this induction was further enhanced in the
presence of SIS3, which indicated that weakened Smad3 signalling
may act as a regulator of Th17 cell skewing and Treg suppression.
Subsequently, the underlying effects of cigarette smoking on Th17
cell induction were further examined. A previous study reported
that the addition of CSE alone was unable to induce IL-17
expression in naive CD4+ T cells (15). Noatbly, under Th17 cell-polarizing
conditions (the first protocol), CSE induced the differentiation of
Th17 cells (Fig. 3).
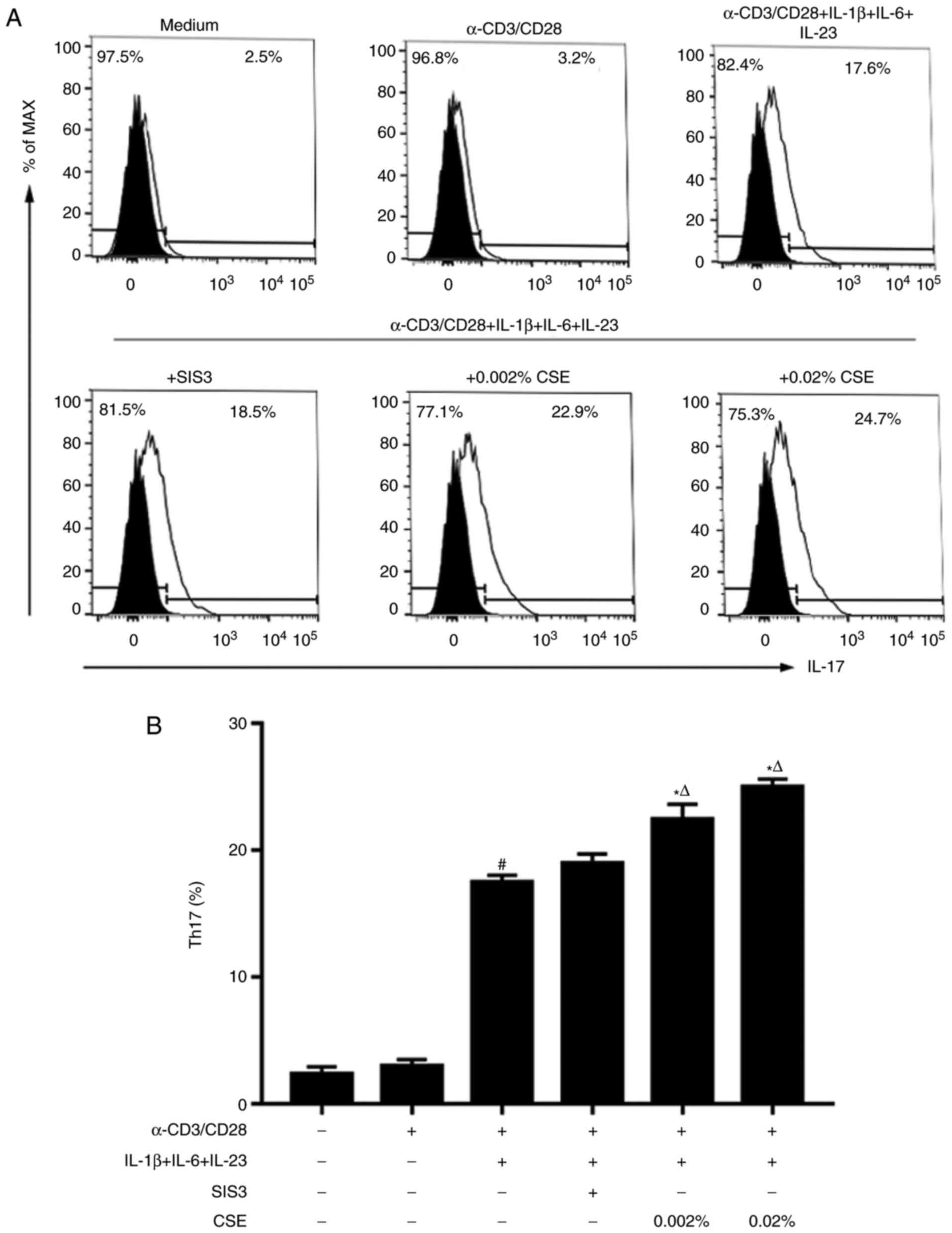
In addition, the combination of IL-1β, IL-6 and
IL-23 stimulation (the second protocol) was also able to initiate
in vitro Th17 cell differentiation (Fig. 4). The combination of IL-6, IL-1β
and IL-23 stimulation induced similar production levels of IL-17
between Th17 cells co-treated with or without SIS3 (Fig. 4). Notably, by the second protocol,
CSE induced statistically stronger expression of Th17 cells
compared with SIS3 treatment (Fig.
4).
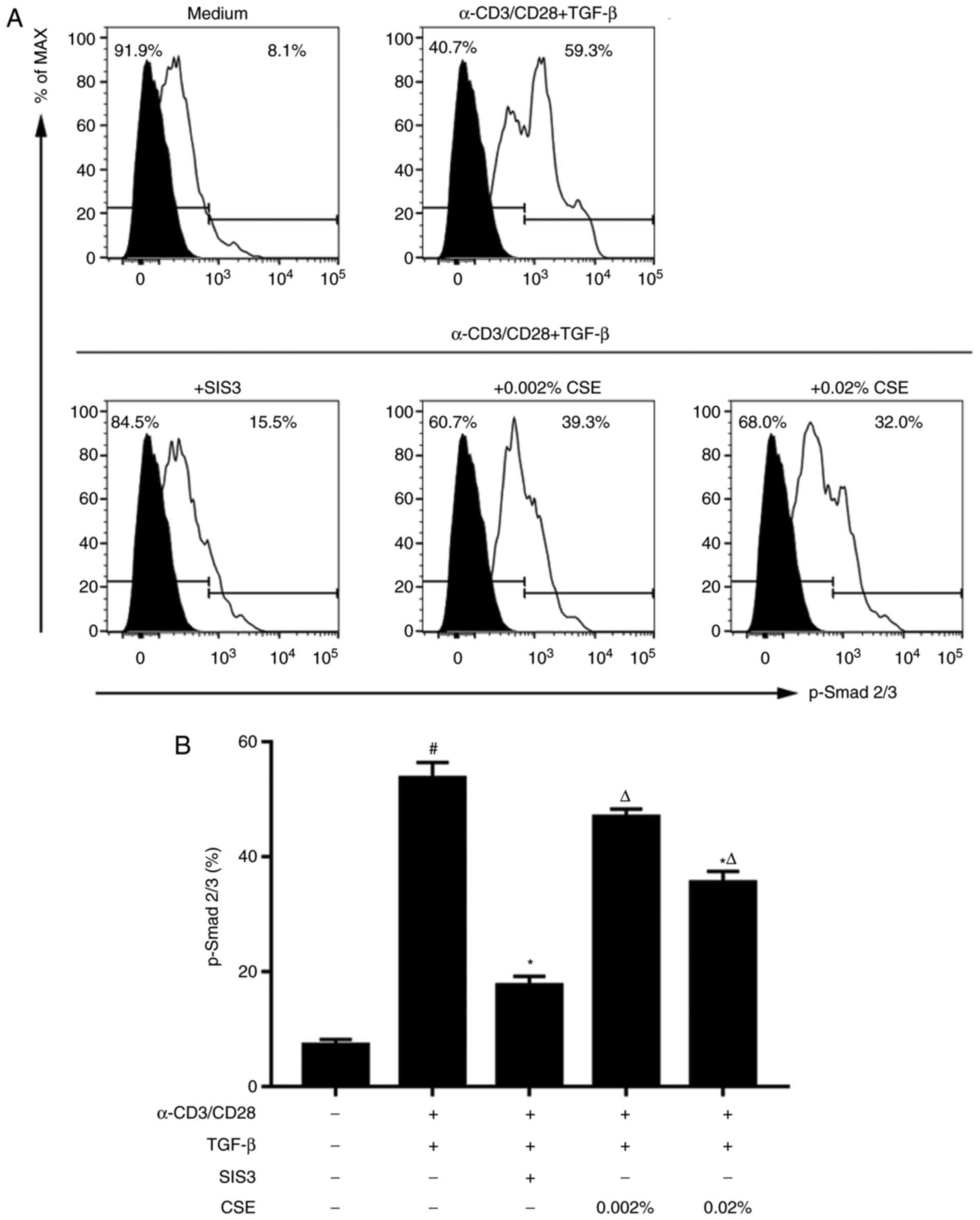
TGF‑β/Smad3 signalling is attenuated by
CSE exposure
As crucial downstream mediators in the TGF-β
signalling pathway, the potential role of Smad2/Smad3
phosphorylation was further evaluated in TCR-stimulated naive
cells. Results from flow cytometric analysis revealed a significant
increase in Smad2/Smad3 phosphorylation TGF-β1-treated cells
compared with expression in cells cultured in complete medium
without stimulation (Fig. 5).
Smad2/Smad3 phosphorylation was significantly inhibited in
TCR-stimulated cells co‑treated with SIS3, which corroborated the
specificity of the inhibitor.
To identify the potential for TGF-β/Smad signalling
as a possible mechanism for the differentiation of naive T cells by
CSE, the role of CSE in Smad2/Smad3 phosphorylation was evaluated.
Cells co-treated with 0.02% CSE exhibited a significant reduction
of Smad2/Smad3 phosphorylation compared with TGF-β-treated cells
(Fig. 5), but to a lesser extent
compared with SIS3 treatment. It is noteworthy that CSE treatment
did not completely inhibit p-Smad2/Smad3 expression to that level
of untreated cells.
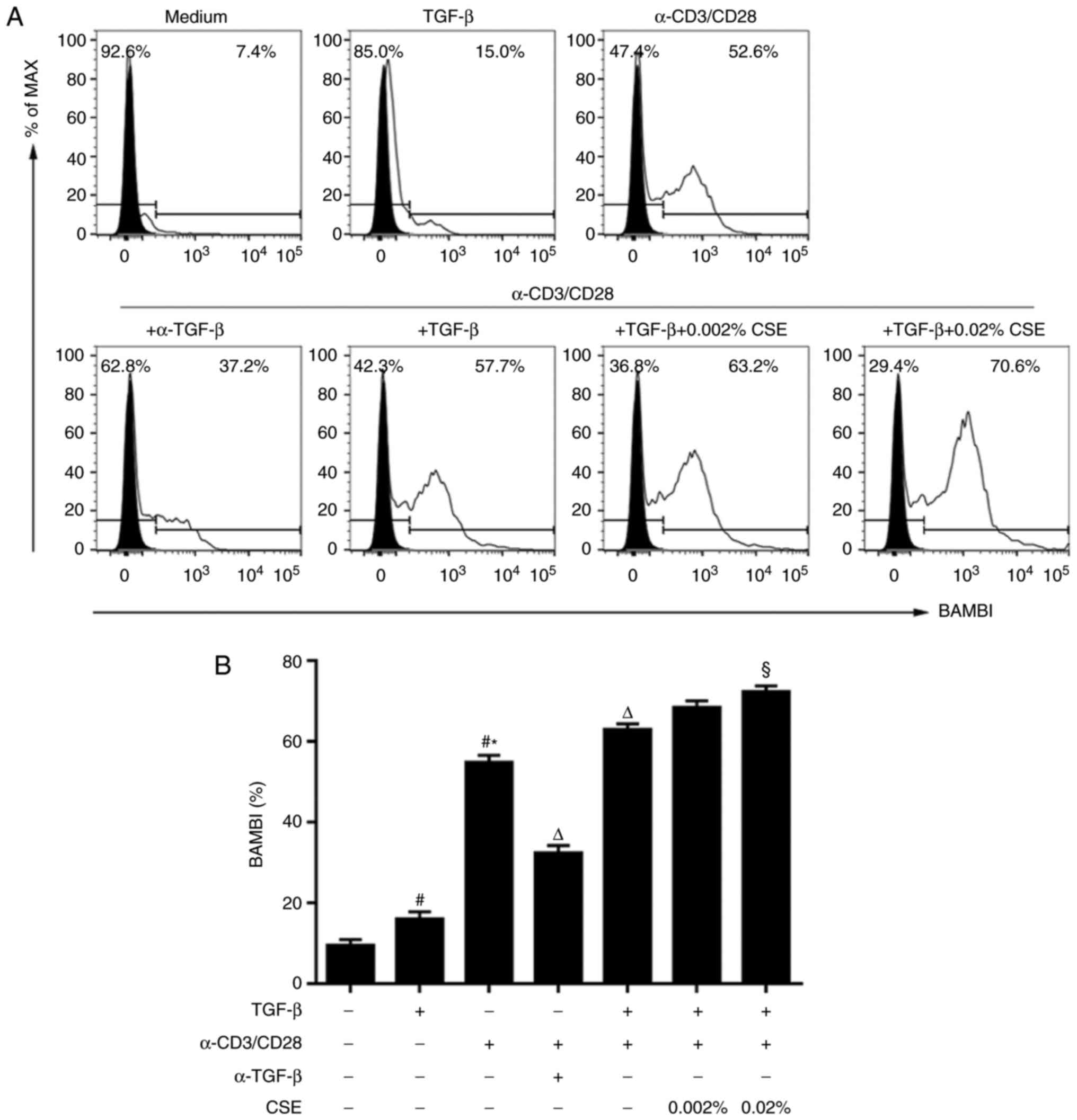
BAMBI expression is increased during
activation and CSE exposure
BAMBI acts as a TGF-β1 rheostat that regulates
Th17/Treg cell differentiation and the development of COPD and
autoimmune arthritis (5,9). Therefore, the potential expression
characteristics of BAMBI under the various activation levels and
the cytokine milieu conditions were investigated. Naive
CD4+ T cells from peripheral blood express low or
undetectable levels of BAMBI protein (5). Although treatment with TGF-β1 alone
was able to induce mild BAMBI expression compared with untreated
cells, anti-CD3/CD28 stimulation significantly increased BAMBI
expression compared with untreated and TGF-β-treated cells
(Fig. 6). In addition, the
endogenous active TGF-β produced by the TCR-activated cells was
necessary for sustained expression of BAMBI, since neutralization
of TGF-β1 with anti-TGF-β antibody significantly reduced BAMBI
induction in TCR‑stimulated cells (Fig. 6). Furthermore, it was observed
that the addition of exogenous TGF-β1 further enhanced TCR-induced
BAMBI expression compared with TCR-induction alone.
The potential effects of CSE on BAMBI expression
were also investigated. Under stimulation by anti-CD3/28 antibodies
in the presence of TGF-β1, CSE treatment induced a significant
increase of BAMBI expression at 0.02% concentration (Fig. 6).
Discussion
Although previous studies have demonstrated key
roles for tobacco smoke in immunity and inflammation, the cytokine
milieu as well as genetic susceptibility should also be taken into
account during analyses of effects of cigarette smoke (8). A previous study indicated that CSE
treatment alone does not induce differentiation of naive
CD4+ T cells (15).
Under specific differentiation conditions, however, CSE
substantially interfered with Treg development and concomitantly
promoted the differentiation of Th17 cells. Results from the
present study demonstrated that cigarette smoke-induced dysfunction
in pro/anti‑inflammation may be associated with Smad-mediated
canonical TGF-β signalling pathway and functionally expressed BAMBI
levels. BAMBI functions as a molecular switch to regulate the
balance between the development of Tregs and Th17 cells (5). These data may aid in guiding future
clinical strategies for maintaining immune tolerance and for the
treatment of smoking-induced immune disorders.
Although considerable progress has been made in
understanding the mechanisms by which tobacco smoking exerts
immunomodulatory functions in inflammation and autoimmunity
(8), these mechanisms are still
not fully understood. A balance of Tregs and Th17 cells is crucial
for the treatment of smoking-induced pathological conditions. To
determine the potential mechanisms for immune response following
cigarette smoke exposure, the present study examined the role of
CSE in freshly isolated naive CD4+ T cells from healthy
donors. In line with a previous study (15), in vitro CSE treatment alone
did not induce the development and differentiation of naive
CD4+ T cells, which indicated that the development of
Tregs and Th17 cells may be regulated by the cytokine milieu in
addition to cigarette smoke alone.
To better understand the induction process of Treg
development, the expression of CD25 and FOXP3 in CD4+ T
cells induced by specific-Treg differentiation conditions,
treatment with TGF-β1 were analysed. This induction was blocked by
a specific Smad3 inhibitor, SIS3. Notably, under Treg‑polarizing
conditions, the higher concentration of CSE remarkably inhibited
Treg differentiation. In keeping with the observed trend in Treg
generation, CD25 induction was also inhibited by SIS3 and by CSE
during Treg cell differentiation. In this regard, it has been
demonstrated previously that TGF-β1 collaborates with TCR
signalling to upregulate CD25 expression and that CD25 gene
regulatory region contains a Smad3 binding site that is required
for TGF-β1-mediated upregulation (16). Thus, the effects of CSE on Treg
differentiation depend not only on the CSE concentration, but also
on the differentiation milieu and activation status.
Th17 cells were also successfully induced using two
different in vitro Th17 cell differentiation protocols: One
used TGF-β1 and IL-6, and the other used a combination of IL-1β,
IL-6 and IL-23. Notably, low levels of endogenous TGF-β1 are still
indispensable for the second Th17 cell-polarizing condition
(17). Smad3 inhibition by SIS3
can promote Th17 cell differentiation in the presence of exogenous
TGF-β1 (the first protocol) but not endogenous TGF‑β1 (the second
protocol). However, CSE induced Th17 cell generation under both
Th17 cell-polarizing conditions, and induced stronger Th17
expression compared with SIS3 treatment in the second protocol,
which suggested that cigarette smoke may affect Th17 cells by
unknown mechanisms (such as by reactive oxygen species) in addition
to Smad signalling. Thus, although CSE itself does not induce the
development of naive T cells, under Th subtype‑specific stimulating
conditions CD4+ T cells had a decreased capacity to
differentiate into Tregs, and a corresponding increased capacity to
differentiate into Th17 cells upon CSE treatment.
A previous study reported that phosphorylation
status induces opposing roles of Smad2/Smad3 as cofactors of signal
transducer and activator of transcription 3 in Th17 differentiation
(18); however, the molecular
mechanisms by which context regulates phosphorylation status remain
unclear. Flow cytometric analysis suggested that Smad2/Smad3 is
phosphorylated following TCR stimulation with exogenous TGF-β1. The
results also demonstrated that higher concentrations of CSE were
able to partially inhibit TCR- and CD28-induced Smad2/Smad3
phosphorylation. However, CSE did not completely inhibit
p-Smad2/Smad3 to the level of non-activated cells, which may
partially be due to TCR-stimulated cells producing biologically
active TGF-β1 that was responsible for Smad2/Smad3 phosphorylation
(19). The effects of CSE on
p-Smad2/Smad3 in T cells were inconsistent with previous studies in
human bronchial epithelial cells (20) and foetal lung fibroblasts
(21), which reported that
exposure to CSE increased Smad3 phosphorylation. This discrepancy
may be due to differences of CSE doses and cell types.
Although a recent study defined the cytokine TGF-β1
to serve a crucial role in governing whether CD4+ T
cells differentiate into tolerogenic Tregs or pro‑inflammatory Th17
cells (22), the molecular
mechanisms underlying the mutually exclusive differentiation of the
two lineages remain incompletely understood. Using a BAMBI-knockout
mouse model of collagen‑induced arthritis, BAMBI was identified as
part of a rheostat-like mechanism for the control of TGF-β
availability and signalling strength (5). However, the response of human T
cells to polyclonal mitogens is more complicated compared with that
of mouse T cells (23). For
instance, human CD4+ cells activated without exogenous
TGF-β1 will still express FOXP3 through endogenous TGF-β1
activation by reactive oxygen species (19). In this regard, although previous
studies have demonstrated that BAMBI expression may be involved in
human lung inflammation associated with COPD and bacterial
infection (7), the present study
is the first, to the best of our knowledge, to report the in
vitro expression and role of BAMBI in human immune cells.
BAMBI is a 260-amino-acid-long transmembrane
glyco-protein that is structurally related to TGF-βRI, but lacking
the intracellular kinase domain (6). Although BAMBI expression may be
upregulated by TGF-β1 (24,25), bone morphogenetic protein (BMP)-4
(26) and Wnt/β-catenin (27) in various cell types, these effects
have not been observed in human preadipocytes (28), which indicated that the modulation
of BAMBI expression is cell type-dependent. Consistent with
previous reports using C57BL/6 mouse T cells (5), increased expression of BAMBI was
observed in activated CD4+ T cells from human peripheral
blood. Endogenous TGF-β1 produced by TCR-activated T cells may
serve a crucial role in BAMBI induction in human
CD4+CD25- T cells, and the addition of
exogenous TGF-β1 further enhanced this effect. Taken together,
these data demonstrated that endogenous and/or exogenous TGF-β1 may
serve as a link in BAMBI induction in naive human CD4+ T
cells following TCR stimulation. Additionally, we have provided the
first evidence that CSE needs to cooperate with TGF-β1 to provoke
induction of BAMBI expression. In summary, the effects of CSE on
BAMBI induction and expression depend not only on cell activation
status but also on local inflammatory stimuli, such as
endogenous/exogenous TGF-β1 and the concentration of CSE.
Although BAMBI has been reported to negatively
regulate TGF-β-family signalling mainly through Smad-dependent
pathways in various cells lines (29–31), it serves a positive role in
triggering Smad2/Smad3 phosphorylation in human preadipocytes
(28), which suggested cell
type‑specific differences. Given its high expression in activated
CD4+ T cells, the present study hypothesized that BAMBI
also serves a crucial haemostatic role by controlling TGF-β
signalling in human T cells. The results demonstrated that CSE
caused a enhancement of BAMBI expression under stimulation by
anti-CD3/28 antibodies and exogenous TGF-β1, which may result in a
concomitant inhibition of p-Smad2/Smad3. Additionally, BAMBI
upregulation by CSE may lead to the suppression of Treg
differentiation and the promotion of Th17 generation in response to
TGF-β1 stimulation, partially via Smad-dependent pathways. Thus, as
in mouse cells, BAMBI may function as a crucial regulator of
Th17/Treg plasticity through TGF-β/Smad-dependent pathways in human
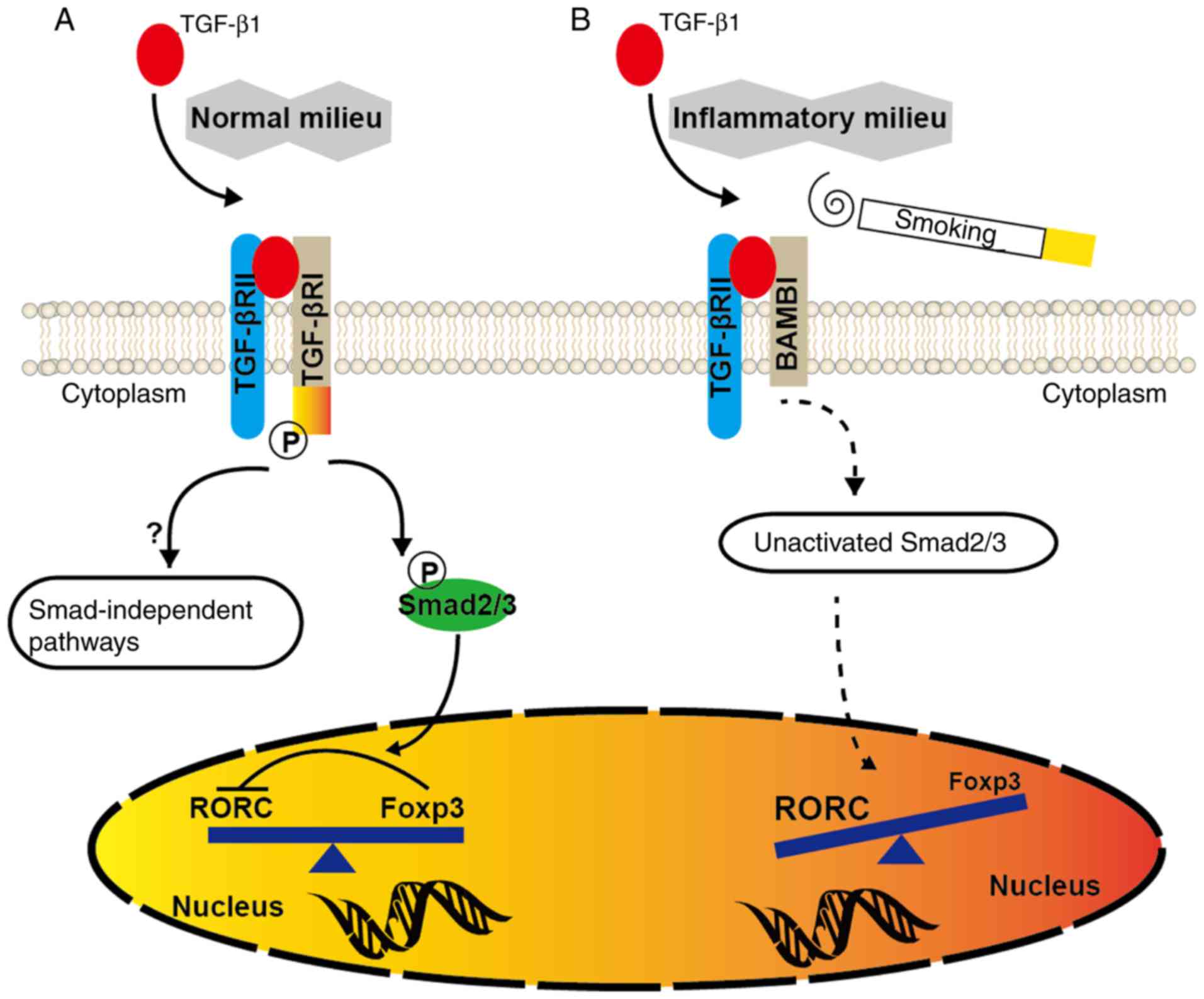
CD4+ T cells. It was proposed that at the steady state
or in the absence of any inflammatory insult, TGF‑β1 produced in
the immune system will facilitate the generation of Tregs, which
keep effector T cells in check (Fig.
7). However, upon inflammation or CSE exposure, BAMBI
overexpression by the activated immune system in susceptible
individuals may suppress the generation of TGF-β-induced Tregs and
evoke pro‑inflammatory responses dominated by Th17 cells.
BAMBI is an inhibitor or regulator of TGF-β
superfamily members, including BMP and activin, in addition to
TGF-β (6). The present study
focussed solely on TGF-β, as activin-A or BMP-2/4 seem to have only
synergistic effects on endogenous and/or exogenous TGF-β-induced
Treg generation (32,33). However, the present study has
several limitations that warrant mention; the focus was primarily
on TGF-superfamily signalling without considering the Wnt/β-catenin
pathway, although BAMBI also functions as a positive regulator of
Wnt signalling (28,34), which further negatively modulates
Treg function (35). In addition,
further work is needed to demonstrate the supplemental role of
non-Smad TGF-β signals by mitogen-activated protein kinases,
including c-Jun N-terminal kinase, p38 and, in particular,
extracellular signal-regulated kinase (ERK) (36), as previous studies have
demonstrated that BAMBI is able to facilitate ERK1/2
phosphorylation (37) and ERK
inactivation mediates the TGF-β1-induced expression of FOXP3
(38). Furthermore, functional
studies such as BAMBI knockdown and/or overexpression in human T
cells in vitro are warranted to clarify the mechanism by
which CSE exerts its effects.
In conclusion, results from the present study
confirmed that BAMBI may be able to regulate the balance between
protective Tregs and pathogenic Th17 cells through a rheostat-like
mechanism in the human immune system. CSE, together with TGF-β1,
was demonstrated to effect BAMBI expression and reduce Smad3
phosphorylation, which may act as a master regulator of Th17
induction and Treg suppression. These results may be beneficial in
improving our understanding of the reciprocal regulation of these
two cell lineages, and may aid in the development of better
approaches for the treatment of smoking‑associated inflammation or
other T cell‑mediated autoimmune diseases.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant nos. 81370146, 81570032
and 81500031). The funders had no role in study design, data
collection and analysis, decision to publish, or preparation of the
manuscript.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
HJL and GC contributed to data analysis and
interpretation. LC and MZ contributed to cell isolation and
culture. XZX contributed to the design of the study. XZX and XNT
were involved in volunteer recruitment. HJL, GC, ZJM and SWS wrote
the manuscript and critically revised the paper. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Union Hospital, Tongji Medical College, Huazhong University of
Science and Technology, and informed written consent was obtained
from each subject.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
BAMBI
|
BMP and activin membrane-bound
inhibitor
|
|
COPD
|
chronic obstructive pulmonary
disease
|
|
CSE
|
cigarette smoke extract
|
|
TGF-β
|
transforming growth factor β
|
|
Th
|
T helper cell
|
|
Treg
|
regulatory T cell
|
Acknowledgments
Not applicable.
References
|
1
|
Nicholson LB and Kuchroo VK: Manipulation
of the Th1/Th2 balance in autoimmune disease. Curr Opin Immunol.
8:837–842. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tran EH, Prince EN and Owens T: IFN-gamma
shapes immune invasion of the central nervous system via regulation
of chemokines. J Immunol. 164:2759–2768. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Korn T, Bettelli E, Oukka M and Kuchroo
VK: IL-17 and Th17 cells. Annu Rev Immunol. 27:485–517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Postigo J, Iglesias M, Alvarez P, Jesús
Augustin J, Buelta L, Merino J and Merino R: Bone morphogenetic
protein and activin membrane-bound inhibitor, a transforming growth
factor β rheostat that controls murine treg Cell/Th17 cell
differentiation and the development of autoimmune arthritis by
reducing interleukin-2 signaling. Arthritis Rheumatol.
68:1551–1562. 2016. View Article : Google Scholar
|
|
6
|
Onichtchouk D, Chen YG, Dosch R, Gawantka
V, Delius H, Massagué J and Niehrs C: Silencing of TGF-beta
signalling by the pseudoreceptor BAMBI. Nature. 401:480–485. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dromann D, Rupp J, Rohmann K, Osbahr S,
Ulmer AJ, Marwitz S, Röschmann K, Abdullah M, Schultz H, Vollmer E,
et al: The TGF-beta-pseudoreceptor BAMBI is strongly expressed in
COPD lungs and regulated by nontypeable haemophilus influenzae.
Respir Res. 11:672010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arnson Y, Shoenfeld Y and Amital H:
Effects of tobacco smoke on immunity, inflammation and
autoimmunity. J Autoimmun. 34:J258–J265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen G, Zhou M, Chen L, Meng ZJ, Xiong XZ,
Liu HJ, Xin JB and Zhang JC: Cigarette smoke disturbs the survival
of CD8+ Tc/Tregs partially through muscarinic
receptors-dependent mechanisms in chronic obstructive pulmonary
disease. PLoS One. 11:e1472322016.
|
|
10
|
Zhang MQ, Wan Y, Jin Y, Xin JB, Zhang JC,
Xiong XZ, Chen L and Chen G: Cigarette smoking promotes
inflammation in patients with COPD by affecting the polarization
and survival of Th/Tregs through Up-regulation of muscarinic
receptor 3 and 5 expression. PLoS One. 9:e1123502014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang JC, Chen G, Chen L, Meng ZJ, Xiong
XZ, Liu HJ, Jin Y, Tao XN, Wu JH and Sun SW: TGF-β/BAMBI pathway
dysfunction contributes to peripheral Th17/Treg imbalance in
chronic obstructive pulmonary disease. Sci Rep. 6:319112016.
View Article : Google Scholar
|
|
12
|
Jinnin M, Ihn H and Tamaki K:
Characterization of SIS3, a novel specific inhibitor of Smad3, and
its effect on transforming growth factor-beta1-induced
extracellular matrix expression. Mol Pharmacol. 69:597–607. 2006.
View Article : Google Scholar
|
|
13
|
Sekiya T and Yoshimura A: In vitro Th
differentiation protocol. Methods Mol Biol. 1344:183–191. 2016.
View Article : Google Scholar
|
|
14
|
Fantini MC, Dominitzki S, Rizzo A, Neurath
MF and Becker C: In vitro generation of CD4+
CD25+ regulatory cells from murine naive T cells. Nat
Protoc. 2:1789–1794. 2007. View Article : Google Scholar
|
|
15
|
Garn H, Turowska A, Weissmann N and
Baumgartl N: Effects of cigarette smoke extract-conditioned medium
on T helper cell development and differentiation in vitro. Am J
Respir Crit Care Med. 191:A27332015.
|
|
16
|
Kim HP, Kim BG, Letterio J and Leonard WJ:
Smad-dependent cooperative regulation of interleukin 2 receptor
alpha chain gene expression by T cell receptor and transforming
growth factor-beta. J Biol Chem. 280:34042–34047. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chung Y, Chang SH, Martinez GJ, Yang XO,
Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, et al:
Critical regulation of early Th17 cell differentiation by
interleukin-1 signaling. Immunity. 30:576–587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoon JH, Sudo K, Kuroda M, Kato M, Lee IK,
Han JS, Nakae S, Imamura T, Kim J, Ju JH, et al: Phosphorylation
status determines the opposing functions of Smad2/Smad3 as STAT3
cofactors in TH17 differentiation. Nat Commun. 6:76002015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amarnath S, Dong L, Li J, Wu Y and Chen W:
Endogenous TGF-beta activation by reactive oxygen species is key to
Foxp3 induction in TCR-stimulated and HIV-1-infected human
CD4+CD25- T cells. Retrovirology. 4:572007.
View Article : Google Scholar
|
|
20
|
Milara J, Peiro T, Serrano A and Cortijo
J: Epithelial to mesenchymal transition is increased in patients
with COPD and induced by cigarette smoke. Thorax. 68:410–420. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Farid M, Kanaji N, Nakanishi M, Gunji Y,
Michalski J, Iwasawa S, Ikari J, Wang X, Basma H, Nelson AJ, et al:
Smad3 mediates cigarette smoke extract (CSE) induction of VEGF
release by human fetal lung fibroblasts. Toxicol Lett.
220:1261342013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li MO and Flavell RA: TGF-beta: A master
of all T cell trades. Cell. 134:392–404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Horwitz DA, Zheng SG, Wang J and Gray JD:
Critical role of IL-2 and TGF-beta in generation, function and
stabilization of Foxp3+CD4+ Treg. Eur J
Immunol. 38:912–915. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sekiya T, Oda T, Matsuura K and Akiyama T:
Transcriptional regulation of the TGF-beta pseudoreceptor BAMBI by
TGF-beta signaling. Biochem Biophys Res Commun. 320:680–684. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xavier S, Gilbert V, Rastaldi MP, Krick S,
Kollins D, Reddy A, Bottinger E, Cohen CD and Schlondorff D: BAMBI
is expressed in endothelial cells and is regulated by
lysosomal/autolysosomal degradation. PLoS One. 5:e129952010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karaulanov E, Knochel W and Niehrs C:
Transcriptional regulation of BMP4 synexpression in transgeni
Xenopus. EMBO J. 23:844–856. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sekiya T, Adachi S, Kohu K, Yamada T,
Higuchi O, Furukawa Y, Nakamura Y, Nakamura T, Tashiro K, Kuhara S,
et al: Identification of BMP and activin membrane‑bound inhibitor
(BAMBI), an inhibitor of transforming growth factor-beta signaling,
as a target of the beta-catenin pathway in colorectal tumor cells.
J Biol Chem. 279:6840–6846. 2004. View Article : Google Scholar
|
|
28
|
Luo X, Hutley LJ, Webster JA, Kim YH, Liu
DF, Newell FS, Widberg CH, Bachmann A, Turner N, Schmitz-Peiffer C,
et al: Identification of BMP and activin membrane‑bound inhibitor
(BAMBI) as a potent negative regulator of adipogenesis and
modulator of autocrine/paracrine adipogenic factors. Diabetes.
61:124–136. 2012. View Article : Google Scholar
|
|
29
|
Shangguan L, Ti X, Krause U, Hai B, Zhao
Y, Yang Z and Liu F: Inhibition of TGF-/β/Smad signaling by BAMBI
blocks differentiation of human mesenchymal stem cells to
carcinoma‑associated fibroblasts and abolishes their protumor
effects. Stem Cells. 30:2810–2819. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan X, Lin Z, Chen F, Zhao X, Chen H, Ning
Y and Chen YG: Human BAMBI cooperates with Smad7 to inhibit
transforming growth factor-beta signaling. J Biol Chem.
284:30097–30104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Villar AV, García R, Llano M, Cobo M,
Merino D, Lantero A, Tramullas M, Hurlé JM, Hurlé MA and Nistal JF:
BAMBI (BMP and activin membrane-bound inhibitor) protects the
murine heart from pressure-overload biomechanical stress by
restraining TGF-β signaling. Biochim Biophys Acta. 1832.323–335.
2013.
|
|
32
|
Huber S, Stahl FR, Schrader J, Lüth S,
Presser K, Carambia A, Flavell RA, Werner S, Blessing M, Herkel J
and Schramm C: Activin a promotes the TGF-beta-induced conversion
of CD4+CD25- T cells into Foxp3+
induced regulatory T cells. J Immunol. 182:4633–4640. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu L, Ma J, Wang X, Wang J, Zhang F, Yu J,
He G, Xu B, Brand DD, Horwitz DA, et al: Synergistic effect of
TGF-beta superfamily members on the induction of Foxp3+
Treg. Eur J Immunol. 40:142–152. 2010. View Article : Google Scholar :
|
|
34
|
Lin Z, Gao C, Ning Y, He X, Wu W and Chen
YG: The pseudo-receptor BMP and activin membrane-bound inhibitor
positively modulates Wnt/beta-catenin signaling. J Biol Chem.
283:33053–33058. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Loosdregt J, Fleskens V, Tiemessen MM,
Mokry M, van Boxtel R, Meerding J, Pals CE, Kurek D, Baert MR,
Delemarre EM, et al: Canonical Wnt signaling negatively modulates
regulatory T cell function. Immunity. 39:298–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Derynck R and Zhang YE: Smad-dependent and
Smad- independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mai Y, Zhang Z, Dong P, Yang H, Yang G and
Sun S: BAMBI inhibits porcine preadipocyte differentiation by
facilitating ERK1/2 phosphorylation. Sheng Wu Gong Cheng Xue Bao.
30:1531–1540. 2014.In Chinese.
|
|
38
|
Luo X, Zhang Q, Liu V, Xia Z, Pothoven KL
and Lee C: Cutting edge: TGF-beta-induced expression of Foxp3 in T
cells is mediated through inactivation of ERK. J Immunol.
180:2757–2761. 2008. View Article : Google Scholar : PubMed/NCBI
|