Introduction
Lung cancer is one of the most common types of
malignant tumor and the leading cause of cancer-associated
mortality (1). The 5-year
survival of patients with lung cancer is only 16.6% (1,2).
Non-small cell lung cancer (NSCLC) represents ~85% of all incident
lung cancer cases, with adenocarcinoma, squamous cell carcinoma and
large cell carcinoma as the typical histopathological types
(3). Bone metastasis occurs in
~40% of NSCLC cases, resulting in serious morbidities and mortality
(4). Patients with NSCLC with
bone metastasis often experience severe pain, pathologic bone
fracture, spinal cord compression and hypercalcemia, which result
in a poor quality of life, decreased survival rates and increases
in medical expenditure (5).
Despite this clinical importance, the pathological mechanism of
bone metastasis in lung cancer remains poorly understood.
Sclerostin domain-containing protein 1 (SOSTDC1),
also known as WISE, USAG1 or ectodin, was previously studied in the
context of tooth development (6),
hair follicle formation (7), limb
morphogenesis (8) and trigeminal
ganglion formation (9). As a
regulator of cell differentiation and proliferation, SOSTDC1 was
identified to be associated with the development and progression of
multiple types of cancer, including breast, gastric, renal and
thyroid cancer (10–13). Zhou et al (14) indicated that SOSTDC1 inhibited
thyroid cancer metastasis by regulating epithelial-mesenchymal
transition (EMT). Previous studies confirmed SOSTDC1 as a
suppressor of Wnt and bone morphogenic protein (BMP) signaling
pathways (15,16). However, Zhou et al
(14) also suggested that SOSTDC1
may regulate phosphoinositide 3-kinase (PI3K)/protein kinase B
(Akt) and mitogen-activated protein kinase (MAPK)/extracellular
signal-regulated kinase (Erk) pathways in thyroid cancer,
indicating that SOSTDC1 may participate in complex carcinogenic
mechanisms. Liu et al (17) demonstrated that SOSTDC1 served as
a tumor suppressor through inhibiting proliferation in NSCLC cells.
However, the roles and mechanism of SOSTDC1 in NSCLC metastasis, in
particular bone metastasis, remain unclear.
The present study detected the expression of SOSTDC1
in primary and bone metastatic lung cancer tissues, and
demonstrated that SOSTDC1 expression was reduced in lung cancer
bone metastatic compared with primary NSCLC tissues. Furthermore,
through the overexpression or inhibition experiments on SOSTDC1,
SOSTDC1 was revealed to inhibit NSCLC cell proliferation,
migration, invasion, EMT and cancer cell-induced
osteoclastogenesis. Finally, RNA deep sequencing was performed to
predict the potential downstream targets of SOSTDC1 in NSCLC. These
results indicated that SOSTDC1 may serve key roles in NSCLC bone
metastasis.
Materials and methods
Clinical samples
A total of 141 paratumor lung tissues, 145 NSCLC
tissues and 49 lung cancer bone metastatic tissues were collected
from patients who underwent surgical resection at Changzheng
Hospital of the Second Military Medical University (Shanghai,
China) between January 2009 and December 2015. Clinical data of the
patients including age, sex, tumor size, the 7th American Joint
Committee on Cancer stage (18),
pathology grade (19) and the
expression level of SOSTDC1 are summarized in Table I. None of the patients received
neoadjuvant chemotherapy or radiotherapy prior to surgery. The
present study was approved by the Ethics Committee of Second
Military Medical University and written informed consent was
obtained from the surviving patients, or family members of those
who had succumbed.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Patient
characteristics | Disease phenotypes
|
|---|
| Primary NSCLC | NSCLC bone
metastasis |
|---|
| Age, years | 60.5±10.0 | 53.0±10.1 |
| Sex,
male/female | 104/41 | 25/24 |
| Types,
AD/SCC/unknown | 72/73/0 | 30/7/12 |
| Size, cm | 4.6±1.9 | 4.5±1.7 |
| Pathology grade,
I/II/III | 16/96/33 | – |
| T grade,
1/2/3/4 | 26/87/28/4 | – |
| N grade,
0/1/2/3 | 82/36/23/4 | – |
| AJCC grade,
1/2/3 | 55/54/36 | – |
| SOSTDC1,
high/low | 75/70 | 14/35 |
Immunohistochemistry (IHC)
Each tissue sample was fixed in 4% paraformaldehyde
at 4°C for 24 h, dehydrated through a graded series of ethanol (75,
85, 90 and 95%) for 2 h, and finally incubated with absolute
ethanol for 1 h at 4°C. Samples were then paraffin embedded, sliced
into 4-µm sections and stained for rabbit anti-human SOSTDC1
antibody (dilution, 1:1,000; cat. no. ab99340; Abcam, Cambridge,
MA, USA). The staining was performed using the Histostain-Plus
(DAB) kit (Shanghai Mingrui Biotech Co., Ltd., Shanghai, China)
following the manufacturer’s protocol. Briefly, sections were
heated for antigen retrieval, blocked by 1% bovine serum albumin
(BSA; Servicebio Inc., Wuhan, China), and incubated overnight at
4°C with the primary antibody. Subsequent to washing with PBS, the
slides were incubated with the goat anti-rabbit IgG horseradish
peroxidase (HRP)-conjugated secondary antibody (1:200; cat. no.
sc-2004; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at room
temperature for 1 h, and stained with DAB solution for 2–8 min at
room temperature under an electron optical microscope
(magnification, ×100). The staining intensity was scored as
follows: 0, negative; 1, weakly positive; 2, moderately positive;
and 3, strongly positive. The positivity was scored by percentage
according to four categories: 0, <5%; 1, 5–25%; 2, >25–50%;
3, >50–75%; and 4, >75%. The staining score was generated by
multiplying the staining intensity score with the percentage of
positivity, and was defined as either low expression (score ≤2) and
high expression (score ≥3).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA of clinical samples or cultured cells were
isolated using TRIzol® (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and reverse transcribed into cDNA by using Prime
Script™ RT Master Mix (Takara Bio, Inc., Otsu, Japan). RT was
performed at 37°C for 30 min followed by incubation for 5 sec at
85°C to inactivate the reverse transcriptase using the Prime Script
RT Master Mix (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer’s protocol. For qPCR, all reactions were performed
with a hot-start preincubation step of 5 min at 95°C, followed by
40 cycles of 25 sec at 95°C, 30 sec at 58°C and 20 sec at 72°C, and
a final 5 min step at 72°C using SYBR-Green qPCR Master Mix
(Bimake, Houston, TX, USA) on a 7900HT Fast Real-Time PCR system
(Thermo Fisher Scientific, Inc.),. Expression levels were
calculated using GAPDH as an internal control with the
2−ΔΔCq method (20).
All primers are summarized in Table
II.
 | Table IIPolymerase chain reaction primers
used in the present study. |
Table II
Polymerase chain reaction primers
used in the present study.
| Gene name | Forward | Reverse | Product length,
bp |
|---|
| Human SOSTDC1 |
AACAGCACGTTGAATCAAGCC |
GCCATCAGAGATGTATTTGGTGG | 123 |
| Mouse TRAP |
GCCCTTACTACCGTTTGC |
TCTCGTCCTGAAGATACTGC | 351 |
| Mouse NFATc1 |
CTCACCACAGGGCTCACTA |
GATGGCTCGCATGTTATTT | 284 |
| Mouse CTSK |
AGTAGCCACGCTTCCTAT |
CATCCACCTTGCTGTTAT | 182 |
| Human PAK6 |
ACCAATAGGCATGGAATGAAGG |
GCGGTCGGAAAGAGGAGTTG | 247 |
| Human CCL3 |
CCGTCACCTGCTCAGAATC |
CTGCTCGTCTCAAAGTAGTCA | 183 |
| Human BTC |
CCTGGGTCTAGTGATCCTTCA |
CTTTCCGCTTTGATTGTGTGG | 131 |
| Human WNT10A |
GGAGACTCGCAACAAGATCCC |
CGATGGCGTAGGCAAAAGC | 80 |
| Human CXCL10 |
CCAAGGGTACTAAGGAATC |
AGGTAGCCACTGAAAGAAT | 165 |
| Human CXCL2 |
GAGGCTGAGGAATCCAAGA |
CACAGAGGGAAACACTGCA | 253 |
| Human CXCL1 |
CCCCAAGAACATCCAAAGT |
GGAACAGCCACCAGTGAGC | 202 |
| Human CXCL8 |
TGGCAGCCTTCCTGATTT |
CTTCTCCACAACCCTCTG | 245 |
| Human GAPDH |
GGAGTCCACTGGCGTCTTCA |
GGGGTGCTAAGCAGTTGGTG | 191 |
| Mouse GAPDH |
TGTTTCCTCGTCCCGTAG |
CAATCTCCACTTTGCCACT | 108 |
| SOSTDC1-CDS |
AACTTAAGCTTGGTACATGCTTCCTCCTGCCATTCAT |
GCCACTGTGCTGGATCTAACTCATGCTGTGCTT | 652 |
| SOSTDC1-sh1 |
GTGGAAAGGACGCGGCGGGAACTGCGTTCCACCAAATATTCAAGACGTATTTGGTGGAACGCAGTTCCTTTTTTCCCGGGACGCGTTA |
TAACGCGTCCCGGGAAAAAAGGAACTGCGTTCCACCAAATACGTCTTGAATATTTGGTGGAACGCAGTTCCCGCCGCGTCCTTTCCAC | 88 |
| SOSTDC1-sh2 |
GTGGAAAGGACGCGGCGGCATTTCAGTAACACTGGACTTTCAAGACGAGTCCAGTGTTACTGAAATGCTTTTTTCCCGGGACGCGTTA |
TAACGCGTCCCGGGAAAAAAGCATTTCAGTAACACTGGACTCGTCTTGAAAGTCCAGTGTTACTGAAATGCCGCCGCGTCCTTTCCAC | 88 |
Construction of SOSTDC1 overexpression
and inhibition plasmids
For the SOSTDC1 overexpression plasmid, a vector
pcDNA3.1+ plasmid (Shanghai GeneChem Co., Ltd., Shanghai, China)
was enzyme digested by KpnI and EcoRV (Beijing
TransGen Biotech Co., Ltd., Beijing, China), and the code sequence
of SOSTDC1
(5′-ATGCTTCCTCCTGCCATTCATTTCTATCTCCTTCCCCTTGCATGCATCCTAATGAAAAGCTGTTTGGCTTTTAAAAATGATGCCACAGAAATCCTTTATTCACATGTGGTTAAACCTGTTCCAGCACACCCCAGCAGCAACAGCACGTTGAATCAAGCCAGAAATGGAGGCAGGCATTTCAGTAACACTGGACTGGATCGGAACACTCGGGTTCAAGTGGGTTGCCGGGAACTGCGTTCCACCAAATACATCTCTGATGGCCAGTGCACCAGCATCAGCCCTCTGAAGGAGCTGGTGTGTGCTGGCGAGTGCTTGCCCCTGCCAGTGCTCCCTAACTGGATTGGAGGAGGCTATGGAACAAAGTACTGGAGCAGGAGGAGCTCCCAGGAGTGGCGGTGTGTCAATGACAAAACCCGTACCCAGAGAATCCAGCTGCAGTGCCAAGATGGCAGCACACGCACCTACAAAATCACAGTAGTCACTGCCTGCAAGTGCAAGAGGTACACCCGGCAGCACAACGAGTCCAGTCACAACTTTGAGAGCATGTCACCTGCCAAGCCAGTCCAGCATCACAGAGAGCGGAAAAGAGCCAGCAAATCCAGCAAGCACAGCATGAGTTAG-3′)
was amplified by PCR (5 min at 94°C, followed by 35 cycles of 30
sec at 94°C, 30 sec at 60°C, 40 sec at 72°C and a final 5 min step
at 72°C) and inserted into pcDNA3.1+ using a Quick-Fusion Cloning
kit (Bimake) according to the manufacturer’s protocol. For the
SOSTDC1 short hairpin RNA (shRNA) plasmids, the vector pGenesil-1
plasmid (Shanghai GeneChem Co., Ltd.) was enzyme digested by
HindIII and BamH I (Beijing TransGen Biotech Co.,
Ltd.). The target sequences were synthesized (Genewiz Co., Ltd.,
Shuzhou, China) and inserted by using Quick-Fusion Cloning kit
(Bimake) according to the manufacturer’s protocol (40 ng plasmids
combined with 40 ng shRNA fragments). The PCR primers and shRNA
targets are summarized in Table
II.
Cell lines and culture
The NSCLC A549 and PC9 cell lines (Cell Bank of the
Type Culture Collection Committee of the Chinese Academy of
Science, Shanghai, China) were routinely maintained in DMEM (A549)
and RPMI-1640 (PC9) (both from Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), respectively. The RNA
samples of MRC-5, H1299, H522, H226, SK-MES-1 and H460 cells were
kindly provided by Institute of Biomedical Sciences and School of
Life Sciences, East China Normal University, China. Bone marrow
macrophages (BMMs) were isolated from C57/BL6 mice (n=4; Laboratory
Animal Center, Second Military Medical University; male; 6–7 weeks)
cultured in α-minimum essential medium (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS as described previously
(21). Mice were housed in a
specific pathogen-free laboratory animal center at a temperature of
20–26°C in a standard atmosphere, and were kept on a 12/12 h
light-dark cycle, and fed using standard rodent chow. Cells in the
logarithmic growth phase were transfected using DNA Transfection
Reagent (Bimake) once they reached 50–70% confluence, according to
the manufacturer’s protocol.
Western blot analysis
Cells were harvested with radioimmunoprecipitation
assay lysis buffer at 0°C for 30 min to obtain total proteins.
Proteins were quantified using a BCA Protein Assay kit (cat. no.
P0012S; Beyotime Institute of Biotechnology, Shanghai, China), then
20 µg protein/lane was separated on 10% SDS-PAGE gels and
transferred onto 0.22-mm nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA). The nitrocellulose membranes were blocked
using 1% BSA for 20 min at 37°C. Subsequent to washing with TBS for
10 min at room temperature three times, the membranes were
incubated overnight at 4°C with primary antibodies against SOSTDC1
(1:1,000; cat. no. ab99340; Abcam), cadherin 1 (1:1,000; CDH1; cat.
no. AF7718; Affinity Biosciences, Cincinnati, OH, USA), vimentin
(1:1,000; VIM; cat. no. AF7013; Affinity Biosciences), zinc finger
protein SNAI (1:1,000; SNAI1; cat. no. AF6032; Affinity
Biosciences) and β-actin (1:1,000; cat. no. AF7018; Affinity
Biosciences). The membranes were washed with TBS for 5 min at room
temperature three times. Proteins were detected through incubation
of the membranes with HRP-conjugated goat anti-rabbit IgG secondary
antibody (1:3,000; cat. no. sc-2004; Santa Cruz Biotechnology,
Inc.) at 37°C for 2 h.
Cell Counting Kit-8 (CCK-8) assay
A549 and PC9 cells were seeded in 96-well plates at
an initial density of 5×103 cells/well, cultured at 37°C
for 0, 12, 24, 48 and 72 h, and finally assessed using the CCK-8
(Bimake). The results were measured by absorbance at 450 nm using
an ELx800 microplate reader (BioTek Instruments Inc., USA).
Transwell migration and invasion
assay
Transwell chambers (8 µm pore size) without
Matrigel® (cat. no. 3422; Corning Incorporated, Corning,
NY, USA) or with Matrigel® (cat. no. 354480; Corning
Incorporated) were used for Transwell migration or invasion assays,
respectively. Cells were digested with Trypsin (Gibco; Thermo
Fisher Scientific, Inc.) and counted. A total of 1×105
cells in 100 µl medium without FBS were plated in the upper
chamber and 500 µl medium supplemented with 10% FBS was
placed in the bottom chambers as a chemoattractant. Non-migratory
cells on the upper membrane surface were carefully removed after 24
h incubation at 37°C. Cells on the bottom surface were fixed with
4% paraformaldehyde for 20 min at room temperature, stained at room
temperature with 0.1% crystal violet (1:1,000) for 30 min, then
counted by capturing images from five random fields under a light
microscope at magnification, ×400.
Osteoclast differentiation assay
BMMs with macrophage colony-stimulating factor
(M-CSF) stimulation (PeproTech, Inc., Rocky Hill, NJ, USA) as an
osteoclast differentiation model. BMMs were cultured at 37°C with
the conditional media from A549 or PC9 cells for 7 days. Then,
cells were fixed by 4% paraformaldehyde for 20 min at room
temperature and stained using a tartrate-resistant acid phosphatase
(TRAP) staining kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
according to the manufacturer’s protocol. TRAP-positive
multinucleated cells containing ≥3 nuclei were counted as mature
osteoclasts. The osteoclast cell numbers were counted by capturing
images from 5 random fields under a light microscope at
magnification, ×400.
RNA deep sequencing
PC9 cells were harvested following transfection with
OE-SOSTDC1 or OE-CTRL plasmids for 48 h using DNA Transfection
reagent (Bimake) according to the manufacturer’s protocol (1.6 ug
DNA/1 ml culture medium). Total RNA was extracted from the cells
using TRIzol reagent according the manufacturer’s protocol, and
subjected to RNA deep sequencing at Beijing Genomics Institute
(Shenzhen, China) on the BGISEQ-500 platform. The sequence results
were obtained as the fragment/kB of exons/million reads for each
transcript. The pathway analyses of the results of RNA deep
sequencing, including Gene Ontology (GO) analysis and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway analysis, were
further performed by Beijing Genomics Institute.
Statistical analysis
SPSS 19.0 statistical software (IBM Corp., Armonk,
NY, USA) was used for statistical analysis. The Kaplan-Meier method
was used to establish survival curves, and log-rank test was
applied for comparative analysis of differences in patient
survival. All data are presented as mean ± standard error of the
mean (SEM). Factors measuring P≤0.1 from log-rank tests were
subjected to the Cox proportional hazard analysis and calculation
of the hazard ratio and 95% confidence interval. Statistics of the
mean value between groups were assessed using one-way analysis of
variance followed by the least significant difference method. All
experiments were repeated at least three times, and representative
experiments are presented. P<0.05 was considered to indicate a
statistically significant difference.
Results
SOSTDC1 is downregulated in NSCLC bone
metastatic tissues and associated with the survival outcomes of
patients with NSCLC
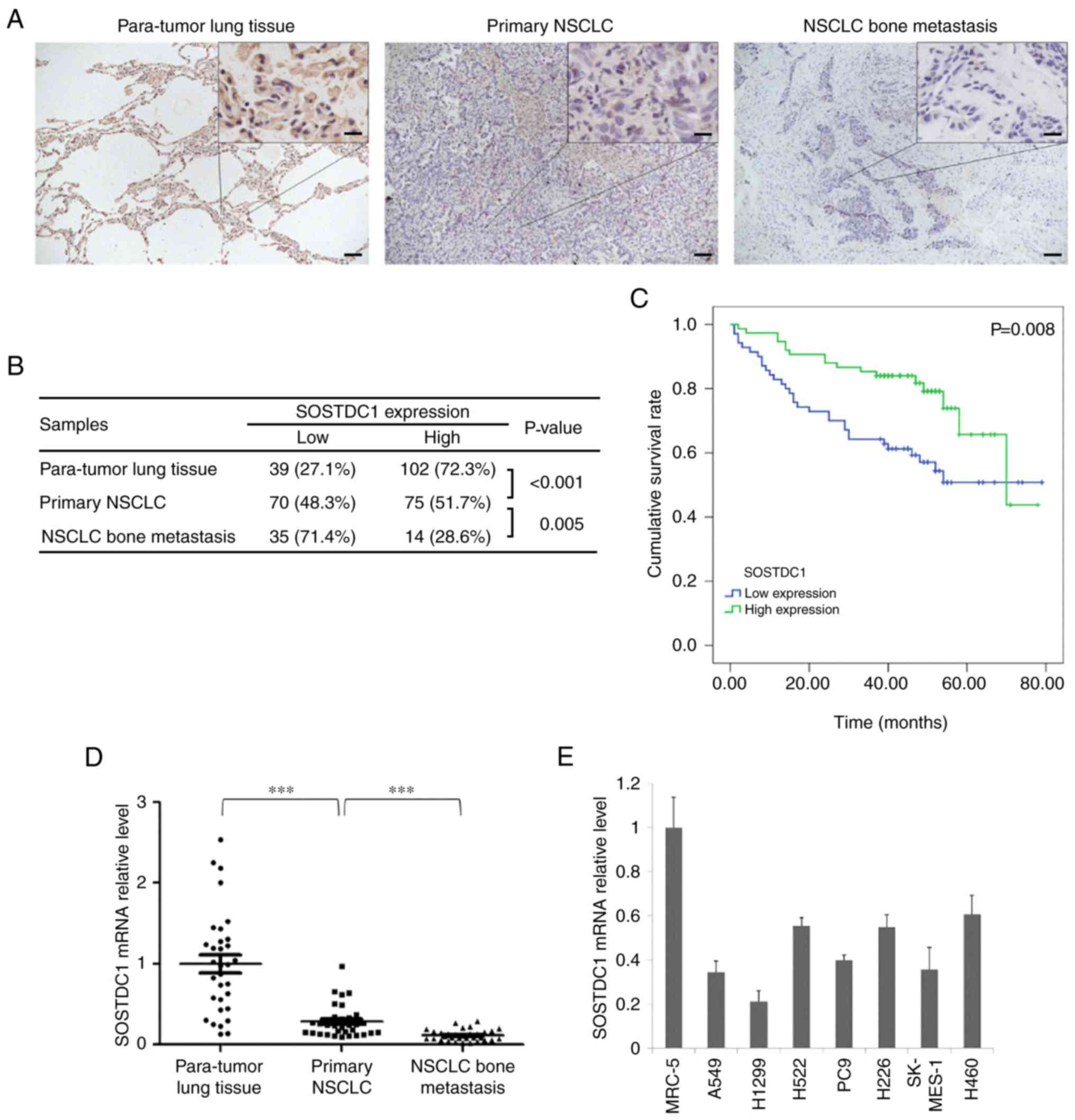
IHC staining was performed to detect the expression
of SOSTDC1 in 141 paratumor lung tissues, 145 primary NSCLC and 49
bone metastatic specimens. The results indicated that SOSTDC1
exhibited clear cytoplasmic expression, was significantly
downregulated in primary NSCLC tissues compared with that in
non-cancerous lung tissues, and additionally decreased in NSCLC
bone metastatic tissues (Fig. 1A and
B). In addition, Kaplan-Meier analysis demonstrated that
patients with primary NSCLC with high SOSTDC1 expression (n=75)
exhibited significantly improved overall survival compared with
those with low SOSTDC1 expression (n=70) (Fig. 1C). Multivariate COX analysis of
clinical factors additionally identified SOSTDC1 expression as an
independent prognostic factor for patients with NSCLC (Table III). Analysis of the association
between the clinical factors and SOSTDC1 expression suggested that
only T grade was significantly correlated with the SOSTDC1
expression level (Table IV). The
RT-qPCR assay also indicated that the mRNA level of SOSTDC1 was
markedly decreased in bone metastatic specimens (Fig. 1D). Then, differences in SOSTDC1
expression between lung cancer cell lines and normal lung cell line
(MRC-5) were compared. It was identified that SOSTDC1 was markedly
downregulated in NSCLC cancer cells compared with that in MRC-5
(Fig. 1E).
 | Table IIIUnivariate and multivariate analyses
of different prognostic factors for overall survival in 145
patients with non-small cell lung cancer. |
Table III
Univariate and multivariate analyses
of different prognostic factors for overall survival in 145
patients with non-small cell lung cancer.
| Prognostic
factors | Univariate analysis
| Multivariate
analysis
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (>60 vs.
≤60) | 1.519 | 0.845–2.731 | 0.162 | – | – | – |
| Sex (female vs.
male) | 0.690 | 0.357–1.333 | 0.269 | – | – | – |
| Types (AD vs.
SCC) | 1.277 | 0.709–2.299 | 0.416 | – | – | – |
| Size, cm (>5 vs.
≤5) | 1.838 | 1.030–3.281 | 0.040a | 1.778 | 0.918–3.441 | 0.088 |
| Pathology grade (II
vs. I) | 4.181 | 0.990–17.661 | 0.052 | 2.621 | 0.603–11.394 | 0.199 |
| Pathology grade
(III vs. I) | 4.439 | 0.975–20.202 | 0.054 | 3.072 | 0.669–14.113 | 0.149 |
| T grade (3–4 vs.
1–2) | 2.216 | 1.225–4.011 | 0.009a | 0.525 | 0.134–2.057 | 0.355 |
| N grade (1–3 vs.
0) | 1.912 | 1.075–3.402 | 0.027a | 1.063 | 0.520–2.175 | 0.866 |
| AJCC grade (2 vs.
1) | 0.939 | 0.421–2.097 | 0.879 | 0.644 | 0.263–1.573 | 0.334 |
| AJCC grade (3 vs.
1) | 3.619 | 1.802–7.270 | <0.001a | 2.445 | 1.112–5.376 | 0.026a |
| SOSTDC1 (high vs.
low) | 0.449 | 0.248–0.812 | 0.008a | 0.505 | 0.276–0.922 | 0.026a |
 | Table IVAssociations between clinical factors
and SOSTDC1 expression. |
Table IV
Associations between clinical factors
and SOSTDC1 expression.
|
Characteristics | SOSTDC1 expression
| P-value |
|---|
| Low | High |
|---|
| Age, years | | | 0.096 |
| >60 | 42 | 34 | |
| ≤60 | 28 | 41 | |
| Sex | | | 0.581 |
| Male | 52 | 52 | |
| Female | 18 | 23 | |
| Types | | | 1.000 |
| AD | 35 | 37 | |
| SCC | 35 | 38 | |
| Size, cm | | | 0.856 |
| >5 | 20 | 23 | |
| ≤5 | 50 | 52 | |
| Pathology
grade | | | 0.133 |
| I | 4 | 12 | |
| II | 50 | 46 | |
| III | 16 | 17 | |
| T grade | | | 0.029a |
| 1–2 | 49 | 64 | |
| 3–4 | 21 | 11 | |
| N grade | | | 0.135 |
| 0 | 35 | 47 | |
| 1–3 | 35 | 28 | |
| AJCC grade | | | 0.185 |
| 1 | 23 | 32 | |
| 2 | 25 | 29 | |
| 3 | 22 | 14 | |
| Survival | | | 0.008a |
| Yes | 39 | 58 | |
| No | 31 | 17 | |
SOSTDC1 overexpression inhibits NSCLC
cell proliferation, migration, invasion and EMT
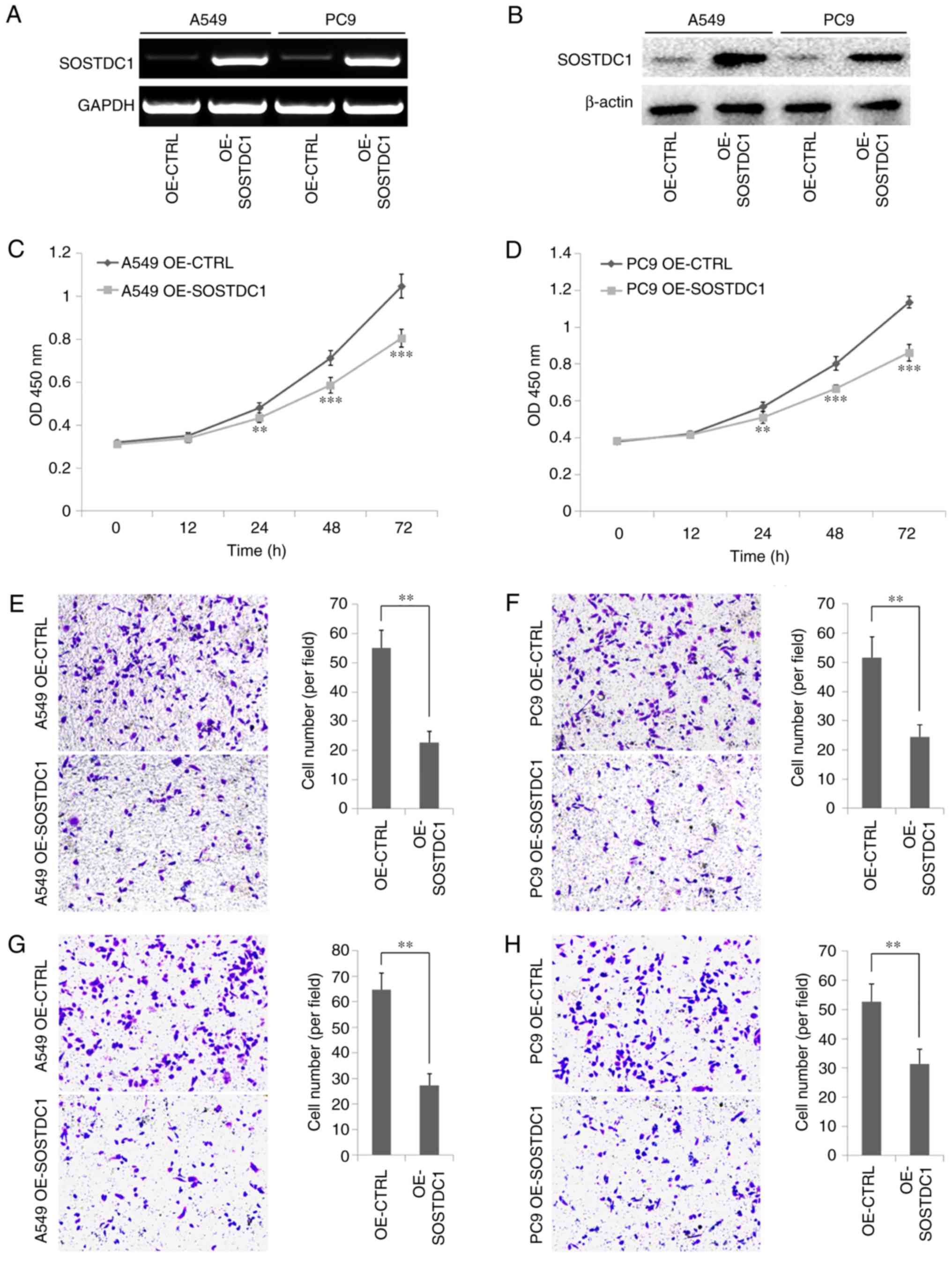
To detect the function of SOSTDC1 in NSCLC cells, a
SOSTDC1 overexpression plasmid was constructed and transfected into
A549 and PC9 cells. The efficiency of the overexpression plasmid
was confirmed by PCR and western blot analysis (Fig. 2A and B). The CCK-8 assay indicated
that SOSTDC1 overexpression inhibited A549 and PC9 cell
proliferation, and this inhibitory effect became more marked over
time (Fig. 2C and D). Cell
migratory abilities were detected by Transwell assay, and the
results demonstrated that the number of A549 and PC9 cells
migrating through the chamber membrane in OE-SOSTDC1 plasmid
transfection group was decreased significantly compared with that
in the control group (Fig. 2E and
F). The invasion capability associated with SOSTDC1 expression
was examined with Transwell chambers coated with Matrigel. As
expected, the number of cells migrating through the membrane was
markedly decreased when the expression of SOSTDC1 was upregulated
in A549 and PC9 cells (Fig. 3Gx and
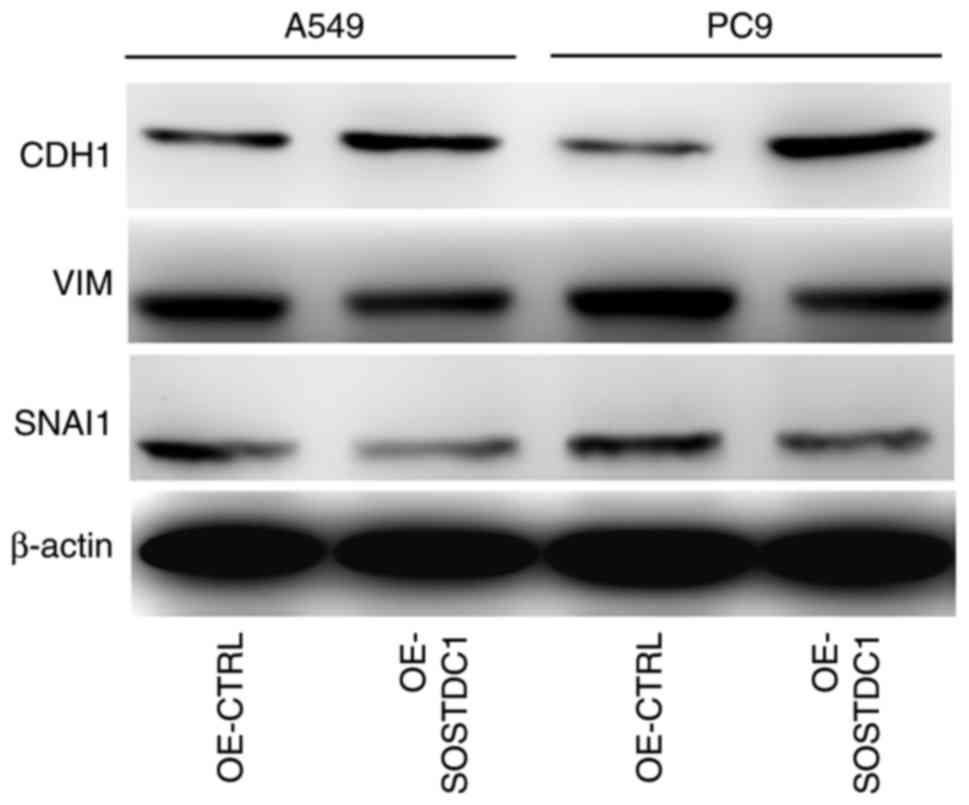
H). Knowing that the EMT process is an important factor
enhancing cell migration and invasion, the expression of epithelial
marker CDH1 and the mesenchymal markers VIM and SNAI1 following
overexpression of SOSTDC1 was detected in A549 and PC9 cells. The
results demonstrated that CDH1 was upregulated while VIM and SNAI1
were downregulated following SOSTDC1 overexpression in NSCLC cells
(Fig. 3), indicating that SOSTDC1
may suppress the process of EMT.
SOSTDC1 overexpression inhibits NSCLC
cell-induced osteoclast differentiation
To additionally investigate the role of SOSTDC1 in
NSCLC bone metastasis, BMMs were selected using M-CSF stimulation
for an osteoclast differentiation model. Conditional media from
A549 and PC9 cells transfected with OE-CTRL or OE-SOSTDC1 plasmids
were used as different stimuli during osteoclastogenesis. The TRAP
staining assay indicated that BMMs treated with the conditional
media from A549 and PC9 cells transfected with OS-SOSTDC1 plasmid
exhibited a decreased level of TRAP positive multinucleated
osteoclast formation as compared with the control (Fig. 4A and B). The mRNA levels of
nuclear factor of activated T cells, cytoplasmic 1 (NFATc1), TRAP
and cathepsin K (CTSK) were detected by RT-qPCR, as NFATc1, TRAP
and CTSK are all markers of osteoclasts (22). The results suggested that the mRNA
levels of NFATc1, TRAP and CTSK were all significantly decreased in
BMMs stimulated with the conditional media from SOSTDC1
overexpressing A549 and PC9 cells (Fig. 4C-E).
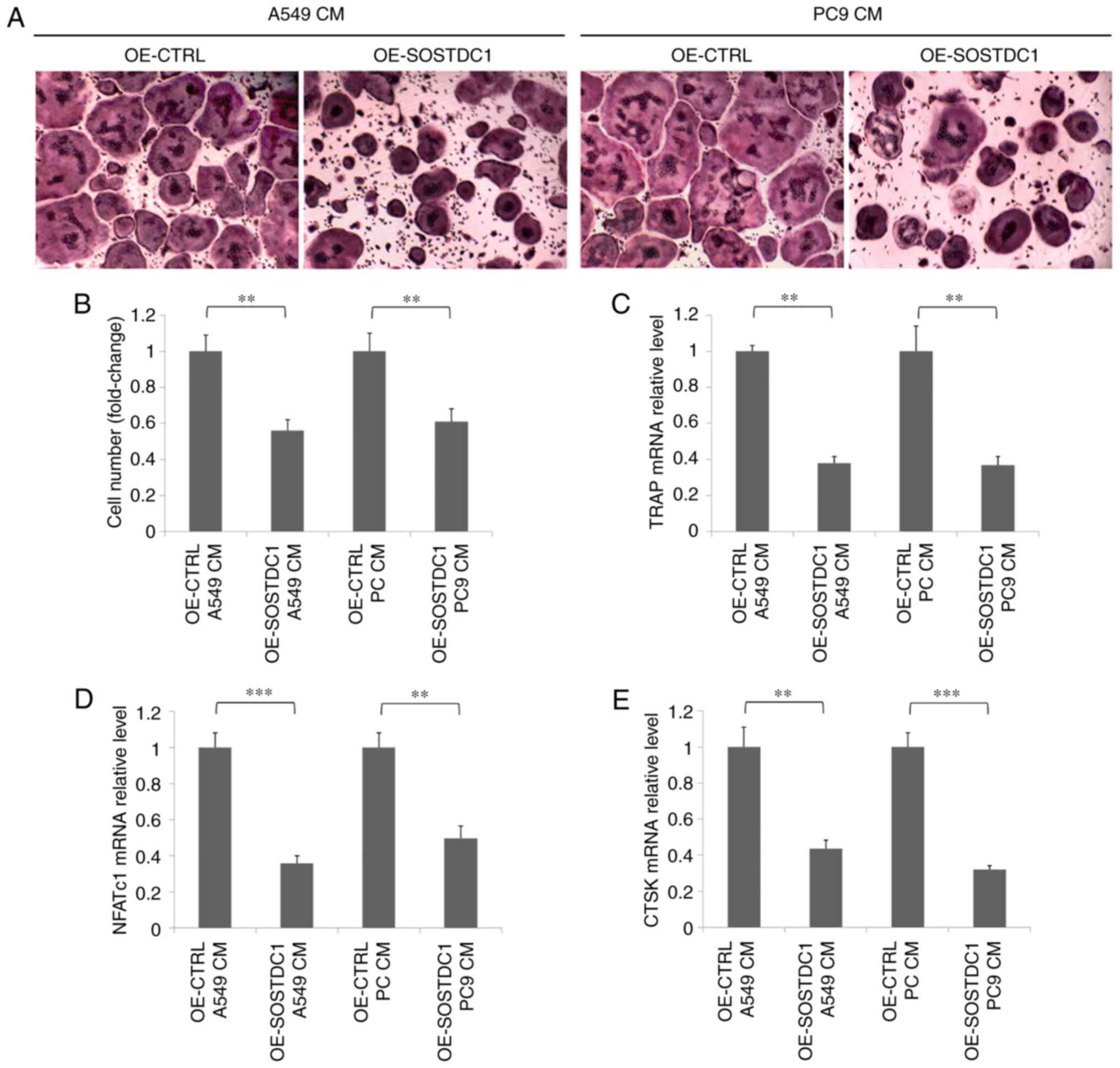 | Figure 4Overexpression of SOSTDC1 inhibits
NSCLC cell-induced osteoclast differentiation. (A) Mouse BMMs were
seeded and cultured with conditional medium containing macrophage
colony-stimulating factor (10 ng/ml) from A549 or PC9 cells
transfected with OE-CTRL or OE-SOSTDC1 plasmids for 7 days. Then,
TRAP staining was performed. (B) TRAP-positive osteoclasts were
counted. (C) RT-qPCR analysis of mRNA level of TRAP in BMMs. (D)
RT-qPCR analysis of NFATc1. (E) RT-qPCR analysis of CTSK.
**P<0.01 and ***P<0.001. SOSTDC1,
sclerostin domain-containing protein 1; OE, overexpression; CTRL,
control; TRAP, tartrate-resistant acid phosphatase; RT-qPCR,
reverse transcription quantitative polymerase chain reaction; BMMs,
bone marrow macrophages; NFATc1, nuclear factor of activated T
cells, cytoplasmic 1; CTSK, cathepsin K; CM, conditioned media. |
SOSTDC1 inhibition promotes NSCLC cell
proliferation, migration, invasion and cancer cell-induced
osteoclastogenesis
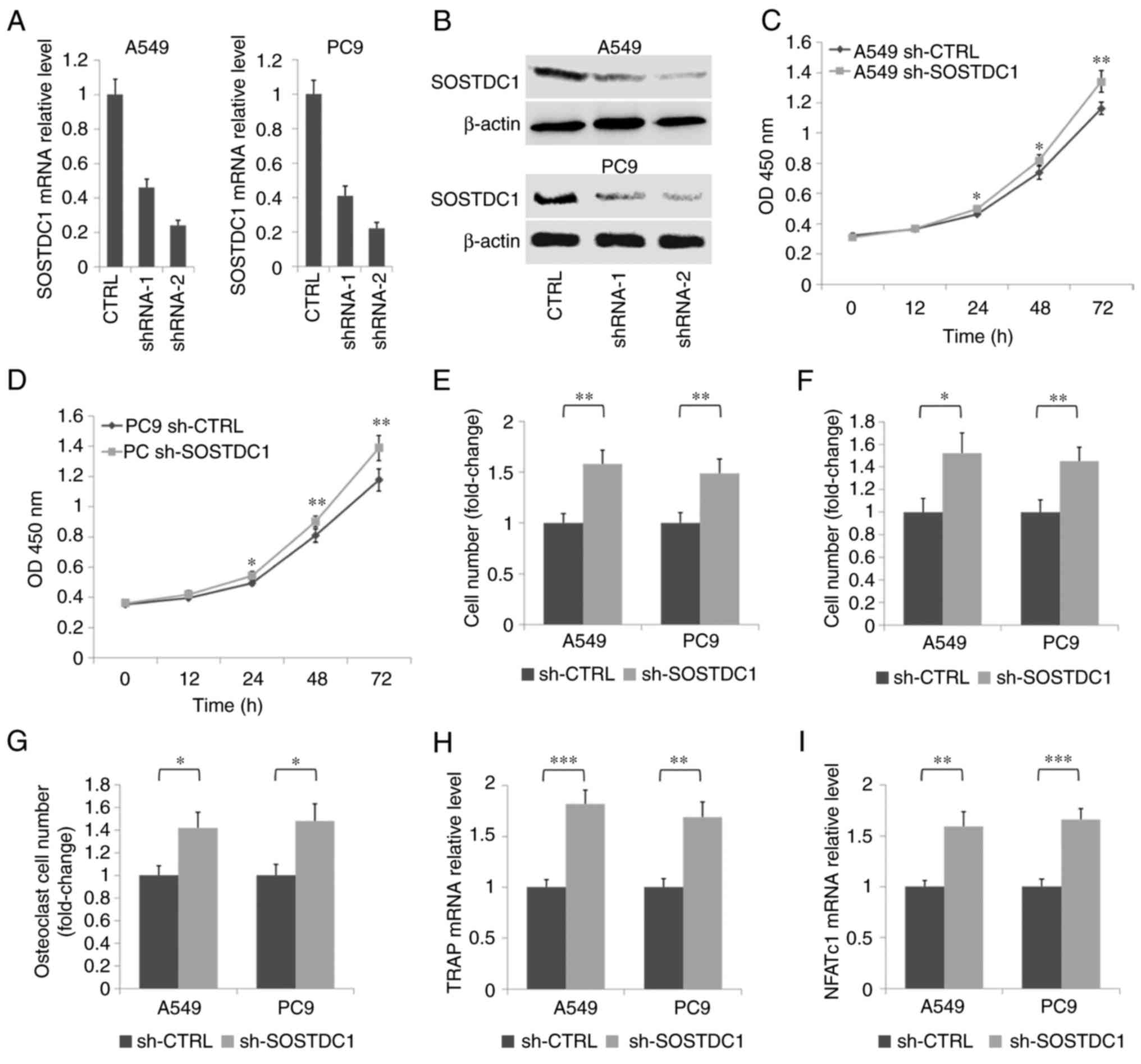
To additionally confirm the role of SOSTDC1 in
NSCLC, two shRNA plasmids for SOSTDC1 were constructed. RT-qPCR and
western blot analysis indicated that the sh-SOSTDC1-2 plasmid
exhibited an improved inhibitory effect compared with sh-SOSTDC1-1
in A549 and PC9 cells (Fig. 5A and
B). Therefore, the sh-SOSTDC1-2 plasmid was used for subsequent
experiments. The CCK-8 assay demonstrated that the inhibition of
SOSTDC1 promoted the proliferation of A549 and PC9 cells (Fig. 5C and D). Transwell assays revealed
that the suppression of SOSTDC1 promoted the migration and invasion
of A549 and PC9 cells (Fig. 5E and
F). TRAP staining assay of BMMs stimulated with the conditional
media from A549 and PC9 cells demonstrated that the inhibition of
SOSTDC1 in NSCLC cells significantly decreased the osteoclast
formation induced by the cancer cells (Fig. 5G). Concurrently, RT-qPCR assays of
TRAP and NFATc1 confirmed the facilitation of SOSTDC1 inhibition in
osteoclastogenesis induced by A549 and PC9 cells (Fig. 5H and I). All these data suggest
that SOSTDC1 functioned as a tumor suppressor in NSCLC by
regulating cell proliferation, migration, invasion and
NSCLC-induced osteoclastogenesis.
Analysis of the potential downstream
targets of SOSTDC1 in NSCLC
To investigate the mechanism of SOSTDC1 in NSCLC
progression, RNA deep sequencing was performed to screen genes
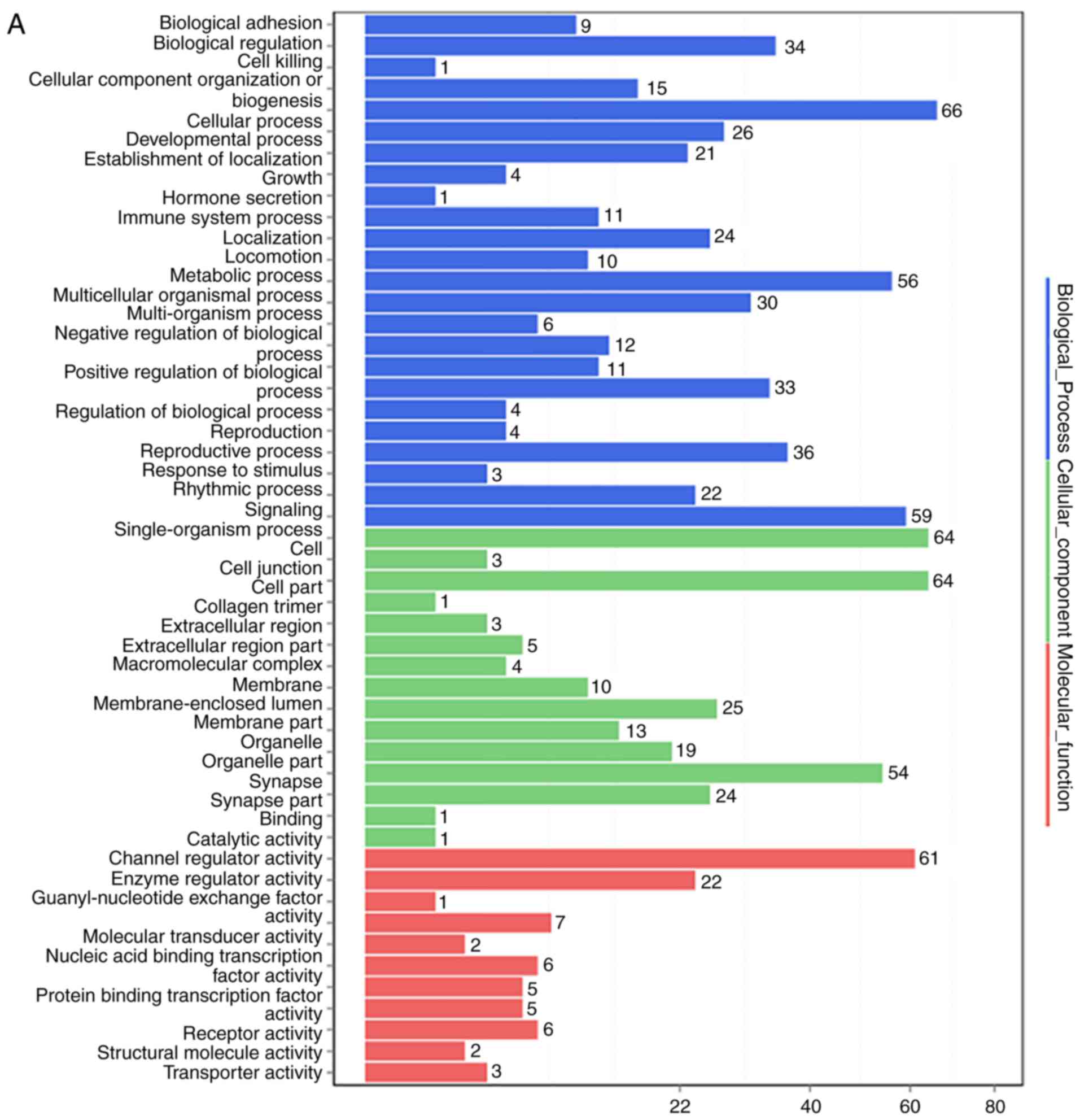
responsive to SOSTDC1 overexpression in PC9 cells (Fig. 6A). Pathway analysis indicated that
the changed genes were closely associated with cell growth and
death, cell motility, and cancer (Fig. 7). A selection of the altered genes
[statistical significance (P<0.05) with the changed rate
>40%] associated with cancer progression are exhibited in
Fig. 6B, and RT-qPCR assay was
used to validate the decrease in P21 (RAC1) activated kinase 6
(PAK6), C-C motif chemokine ligand 3 (CCL3), B-cell translocation
gene (BTG), Wnt family member 10A (WNT10A), C-X-C motif chemokine
(CXC) ligand 1 (CXCL1), CXC ligand 2 (CXCL2), CXC ligand 8 (CXCL8)
and CXC ligand 10 (CXCL10) mRNA expression following SOSTDC1
overexpression in A549 and PC9 cells (Fig. 6C-J). These results suggest that
the mechanisms of SOSTDC1 in NSCLC progression and bone metastasis
require additional investigation.
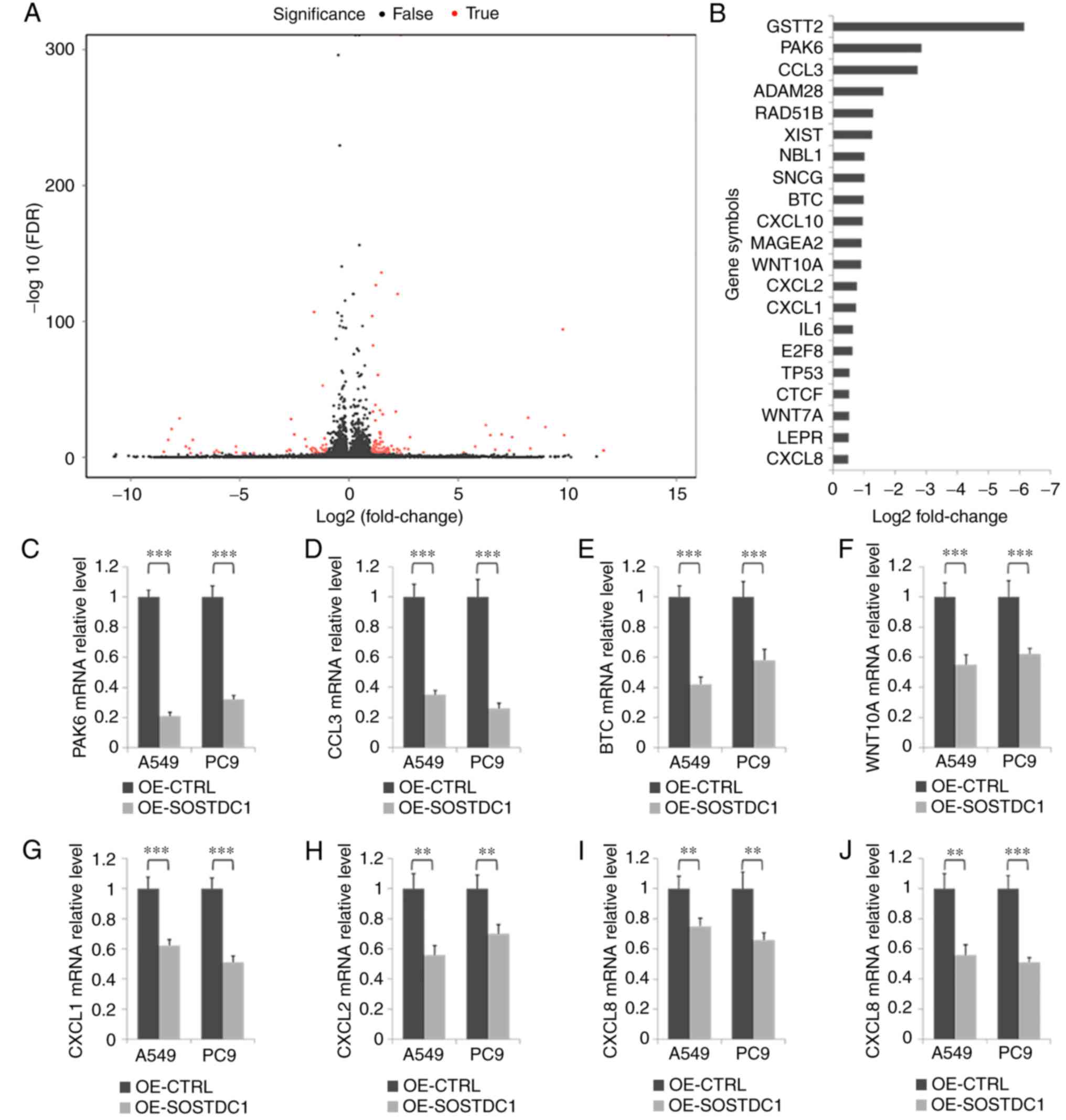 | Figure 6Analysis of potential relevant
downstream genes of SOSTDC1 in NSCLC cells. (A) RNA deep sequencing
of PC9 cells was performed following transfection with OE-CTRL or
OE-SOSTDC1 plasmids. (B) The fold-change of a selection of the
genes associated with tumor progression in RNA sequencing. (C-J)
RT-qPCR assay was performed to detect changes in mRNA expression of
several genes involved in cancer following transfection with
OE-CTRL or OE-SOSTDC1 plasmids in A549 and PC9 cells, including (C)
PAK6, (D) CCL3, (E) BTC, (F) WNT10A, (G) CXCL1, (H) CXCL2, (I)
CXCL8 and (J) CXCL10. **P<0.01 and
***P<0.001. OE, overexpression; CTRL, control;
SOSTDC1, sclerostin domain-containing protein 1; shRNA, CTRL,
control; PAK6; P21 (RAC1) activated kinase 6; CCL3, C-C motif
chemokine ligand 3; BTC, betacellulin; WNT10A, Wnt family member
10A; CXCL1, C-X-C motif chemokine (CXC) ligand 1; CXCL2 CXC ligand
2; CXCL8, CXC ligand 8; CXCL10, CXC ligand 10. |
Discussion
The present study firstly demonstrated the
downregulation of SOSTDC1 in NSCLC bone metastasis compared with
primary tumors, and additionally identified that low SOSTDC1
expression in NSCLC predicted poor prognosis of the patients.
Functionally, SOSTDC1 overexpression suppressed NSCLC cell
proliferation, migration, invasion, EMT and cancer cell-induced
osteoclastogenesis, while SOSTDC1 knockdown produced the opposite
effect. In addition, RNA deep sequencing and RT-qPCR assays
indicated that SOSTDC1 was associated with NSCLC progression and
bone metastasis through regulating PAK6, CCL3, BTG, WNT10A, CXCL1,
CXCL2, CXCL8 and CXCL10. All these results suggest that SOSTDC1
served an inhibitory role in NSCLC progression and bone
metastasis.
The formation of NSCLC bone metastasis requires
osteolysis and cancer growth induced by the interaction of cancer
cells and osteoclasts or its precursors. Previous studies have
suggested that SOSTDC1 was downregulated in various types of
cancer, while the decreased expression of SOSTDC1 accelerated cell
proliferation and predicted poor prognosis in gastric, thyroid and
breast cancer (10–12). Zhou et al (14) additionally demonstrated that
SOSTDC1 promoted thyroid cancer metastasis by activating EMT. In
lung cancer, SOSTDC1 was also confirmed as a tumor suppressor
through the regulation of cell proliferation (17). However, whether SOSTDC1 is
involved in NSCLC metastasis remains unclear. SOSTDC1 was first
examined in 2003 as the antagonist of BMPs (BMP 2, 4, 6 and 7)
(23), and later was confirmed to
serve key roles in bone remodeling by activating osteoblasts and
osteoclasts (24,25). Consistent with the role of BMPs in
bone, SOSTDC1 deficiency accelerated fracture healing by promoting
the expansion of periosteal mesenchymal stem cells (26). However, to the best of our
knowledge, the role of SOSTDC1 in bone metastasis has not yet been
described. Due to the potential effects of SOSTDC1 in the bone
micro-environment and cancer progression, we hypothesized that
SOSTDC1 was involved in the occurrence of bone metastasis. The
result of the present study indicated that SOSTDC1 was
downregulated in NSCLC bone metastatic lesions compared with that
in primary lesions, and may suppress bone metastasis through
inhibiting cell proliferation, migration, invasion, EMT and cancer
cell-induced osteoclast differentiation. Knowing that EMT and bone
resorption are two key processes during bone metastasis in NSCLC,
SOSTDC1 may prove to a potential prognostic biomarker for NSCLC
bone metastasis.
Previous studies identified SOSTDC1 as a suppressor
of BMP and Wnt signaling pathways: Recently, Togo et al
(27) demonstrated that SOSTDC1
antagonized RUNX2 during tooth development. Gopal et al
(12) revealed that SOSTDC1 was
involved in CpG methylation in gastric cancer. Zhou et al
(14) suggested that SOSTDC1
promoted thyroid cancer progression through the regulation of the
PI3K/Akt and MAPK/Erk pathways. Concurrently, SOSTDC1 was
demonstrated to be repressed by estrogen (28) and E4BP4 (11), and activated by FGF signaling
(29). These data indicate that
SOSTDC1 may participate in complex signaling pathways.
Nevertheless, the exact molecular mechanism of SOSTDC1 in NSCLC
require elucidation. Therefore, RNA deep sequencing was performed
in the present study to explore the potential downstream targets of
SOSTDC1 in NSCLC. Notably, it was identified that besides certain
components of the Wnt family, several members of the CXCL family
(CXCL1/2/8/10) were downregulated following SOSTDC1 overexpression.
These CXCLs were considered the downstream targets of the Erk
pathway (30) and certain other
pathways, including tumor necrosis factor and nuclear factor
kappa-light-chain enhancer of activated B cells signaling (31), and identified as the catalyst of
osteoclastogenesis and osteolysis (18,32,33). These results may assist in
understanding the mechanism of SOSTDC1 in NSCLC bone metastasis.
Altogether, the present study demonstrated that SOSTDC1 served a
potential role in inhibiting NSCLC bone metastasis, which may
provide useful insights for future studies on the treatment of
NSCLC bone metastasis. Limitations of the present study included
the absence of an in vivo experiment of SOSTDC1 in NSCLC
bone metastasis, and the incomplete clinical data of the samples,
in particular the absence of treatment choice, which may have
affected the prognosis of the patients. Additional studies are
required to confirm the clinical significance and the molecular
mechanism of SOSTDC1 in NSCLC and bone metastasis.
In conclusion, the present study demonstrated that
SOSTDC1 was downregulated in NSCLC bone metastasis compared with
that in primary tumors, and functioned as a potential tumor
suppressor by regulating cell proliferation, migration, invasion,
EMT and cancer cell-induced osteoclast differentiation. In
addition, a number of potential downstream target genes of SOSTDC1
were identified that may be associated with bone metastasis in
NSCLC cells. These data may assist in developing novel diagnostic
and treatment strategies for NSCLC bone metastasis.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81501927, 51573207
and 81572641).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
GC, TW and WZ conceived and designed the study; JW,
XY and GB analyzed and interpreted the patient data; HG, ZH, SH,
and GC performed the experiments; GC and TW wrote the manuscript;
JX and TL performed the statistical analysis and data presentation;
JX, TL and WZ reviewed the manuscript and agreed to be accountable
for all aspects of the work; All authors read and approved the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of our center and written informed consent was obtained
from the surviving patients, or family members of those who had
succumbed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Howlader N, Noone AM, Yu M and Cronin KA:
Use of imputed population-based cancer registry data as a method of
accounting for missing information: Application to estrogen
receptor status for breast cancer. Am J Epidemiol. 176:347–356.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoffman PC, Mauer AM and Vokes EE: Lung
cancer. Lancet. 355:479–485. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vicent S, Luis-Ravelo D, Antón I,
García-Tuñón I, Borrás-Cuesta F, Dotor J, De Las, Rivas J and
Lecanda F: A novel lung cancer signature mediates metastatic bone
colonization by a dual mechanism. Cancer Res. 68:2275–2285. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahn Y, Sims C, Murray MJ, Kuhlmann PK,
Fuentes-Antrás J, Weatherbee SD and Krumlauf R: Multiple modes of
Lrp4 function in modulation of Wnt/β-catenin signaling during tooth
development. Development. 144:2824–2836. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Närhi K, Tummers M, Ahtiainen L, Itoh N,
Thesleff I and Mikkola ML: Sostdc1 defines the size and number of
skin appendage placodes. Dev Biol. 364:149–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Collette NM, Yee CS, Murugesh D, Sebastian
A, Taher L, Gale NW, Economides AN, Harland RM and Loots GG: Sost
and its paralog Sostdc1 coordinate digit number in a Gli3-dependent
manner. Dev Biol. 383:90–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shigetani Y, Howard S, Guidato S,
Furushima K, Abe T and Itasaki N: Wise promotes coalescence of
cells of neural crest and placode origins in the trigeminal region
during head development. Dev Biol. 319:346–358. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang W, Guan H, He X, Ke W, Xu L, Liu L,
Xiao H and Li Y: Down-regulation of SOSTDC1 promotes thyroid cancer
cell proliferation via regulating cyclin A2 and cyclin E2.
Oncotarget. 6:31780–31791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rawat A, Gopisetty G and Thangarajan R:
E4BP4 is a repressor of epigenetically regulated SOSTDC1 expression
in breast cancer cells. Cell Oncol. 37:409–419. 2014. View Article : Google Scholar
|
|
12
|
Gopal G, Raja UM, Shirley S, Rajalekshmi
KR and Rajkumar T: SOSTDC1 down-regulation of expression involves
CpG methylation and is a potential prognostic marker in gastric
cancer. Cancer Genet. 206:174–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blish KR, Wang W, Willingham MC, Du W,
Birse CE, Krishnan SR, Brown JC, Hawkins GA, Garvin AJ, D’Agostino
RB Jr, et al: A human bone morphogenetic protein antagonist is
down-regulated in renal cancer. Mol Biol Cell. 19:457–464. 2008.
View Article : Google Scholar :
|
|
14
|
Zhou Q, Chen J, Feng J, Xu Y, Zheng W and
Wang J: SOSTDC1 inhibits follicular thyroid cancer cell
proliferation, migration, and EMT via suppressing PI3K/Akt and
MAPK/Erk signaling pathways. Mol Cell Biochem. 435:87–95. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Henley KD, Gooding KA, Economides AN and
Gannon M: Inactivation of the dual Bmp/Wnt inhibitor Sostdc1
enhances pancreatic islet function. Am J Physiol Endocrinol Metab.
303:E752–E761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Itasaki N, Jones CM, Mercurio S, Rowe A,
Domingos PM, Smith JC and Krumlauf R: Wise, a context-dependent
activator and inhibitor of Wnt signalling. Development.
130:4295–4305. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu L, Wu S, Yang Y, Cai J, Zhu X, Wu J,
Li M and Guan H: SOSTDC1 is down-regulated in non-small cell lung
cancer and contributes to cancer cell proliferation. Cell Biosci.
6:242016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koul R, Rathod S, Dubey A, Bashir B and
Chowdhury A: Comparison of 7th and 8th editions of the UICC/AJCC
TNM staging for nonsmall cell lung cancer in a non-metastatic North
American cohort undergoing primary radiation treatment. Lung
Cancer. 123:116–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Driver BR, Barrios R, Ge Y, Haque A, Tacha
D and Cagle PT: Folate receptor α expression level correlates with
histologic grade in lung adenocarcinoma. Arch Pathol Lab Med.
140:682–685. 2016. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔC T method. Methods. 25:402–408.
2001. View Article : Google Scholar
|
|
21
|
Wang T, Yin H, Wang J, Li Z, Wei H, Liu Z,
Wu Z, Yan W, Liu T, Song D, et al: MicroRNA-106b inhibits
osteoclastogenesis and osteolysis by targeting RANKL in giant cell
tumor of bone. Oncotarget. 6:18980–18996. 2015.PubMed/NCBI
|
|
22
|
Zhou W, Yin H, Wang T, Liu T, Li Z, Yan W,
Song D, Chen H, Chen J, Xu W, et al: MiR-126-5p regulates
osteolysis formation and stromal cell proliferation in giant cell
tumor through inhibition of PTHrP. Bone. 66:267–276. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Laurikkala J, Kassai Y, Pakkasjärvi L,
Thesleff I and Itoh N: Identification of a secreted BMP antagonist,
ectodin, integrating BMP, FGF, and SHH signals from the tooth
enamel knot. Dev Biol. 264:91–105. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kamiya N: The role of BMPs in bone
anabolism and their potential targets SOST and DKK1. Curr Mol
Pharmacol. 5:153–163. 2012. View Article : Google Scholar
|
|
25
|
Okamoto M, Murai J, Yoshikawa H and
Tsumaki N: Bone morphogenetic proteins in bone stimulate
osteoclasts and osteoblasts during bone development. J Bone Miner
Res. 21:1022–1033. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Collette NM, Yee CS, Hum NR, Murugesh DK,
Christiansen BA, Xie L, Economides AN, Manilay JO, Robling AG and
Loots GG: Sostdc1 deficiency accelerates fracture healing by
promoting the expansion of periosteal mesenchymal stem cells. Bone.
88:20–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Togo Y, Takahashi K, Saito K, Kiso H,
Tsukamoto H, Huang B, Yanagita M, Sugai M, Harada H, Komori T, et
al: Antagonistic functions of USAG-1 and RUNX2 during tooth
development. PLoS One. 11:e01610672016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fujita K, Roforth MM, Demaray S, McGregor
U, Kirmani S, McCready LK, Peterson JM, Drake MT, Monroe DG and
Khosla S: Effects of estrogen on bone mRNA levels of sclerostin and
other genes relevant to bone metabolism in postmenopausal women. J
Clin Endocrinol Metab. 99:E81–E88. 2014. View Article : Google Scholar :
|
|
29
|
Prochazkova M, Häkkinen TJ, Prochazka J,
Spoutil F, Jheon AH, Ahn Y, Krumlauf R, Jernvall J and Klein OD:
FGF signaling refines Wnt gradients to regulate the patterning of
taste papillae. Development. 144:2212–2221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JW, Wang P, Kattah MG, Youssef S,
Steinman L, DeFea K and Straus DS: Differential regulation of
chemokines by IL-17 in colonic epithelial cells. J Immunol.
181:6536–6545. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kanda N, Hau CS, Tada Y, Tatsuta A, Sato S
and Watanabe S: Visfatin enhances CXCL8, CXCL10, and CCL20
production in human keratinocytes. Endocrinology. 152:3155–3164.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hardaway AL, Herroon MK, Rajagurubandara E
and Podgorski I: Marrow adipocyte-derived CXCL1 and CXCL2
contribute to osteolysis in metastatic prostate cancer. Clin Exp
Metastasis. 32:353–368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu P, Lee S, Knoll J, Rauch A, Ostermay
S, Luther J, Malkusch N, Lerner UH, Zaiss MM, Neven M, et al: Loss
of menin in osteoblast lineage affects osteocyte-osteoclast
crosstalk causing osteoporosis. Cell Death Differ. 24:672–682.
2017. View Article : Google Scholar : PubMed/NCBI
|