Introduction
The endoplasmic reticulum (ER) is one of the largest
and most important cellular organelles, serving a vital role in the
synthesis, folding and modification of proteins, and is the largest
Ca2+ pool, participating in intracellular signal
transduction. Various pathophysiological stimuli can contribute to
the accumulation and aggregation of unfolded or misfolded proteins
within the ER, resulting in ER stress (1). When cells are exposed to ER stress,
a series of mechanisms are triggered, known as the unfolded protein
response (UPR). The UPR signaling pathways are activated by three
ER membrane-associated proteins, namely the PERK, ATF6 and IRE1
proteins (2), which act to meet
the folding demand within the ER and promote cell survival
(1,3-5).
However, if cells ultimately fail to balance the folding capacity
and restore homeostasis to the ER, uncontrolled ER stress will,
through the UPR pathways, initiate apoptosis (6,7).
Several molecules, including C/EBP homologous protein (CHOP),
Caspase-12, IRE1, JNK, Bax and Bak, have been reported to be
involved in ER stress-induced apoptosis (1). However, it has been demonstrated
that numerous pro-apoptotic factors induced by CHOP, such as DR5,
BIM, TRB3 and GADD34, promote protein synthesis and exacerbate
oxidative stress in stressed cells (8-11).
Furthermore, the involvement of CHOP-mediated apoptosis has been
demonstrated in various diseases, including diabetes, brain and
renal injury, neurodegenerative abnormalities, and even certain
cardiovascular diseases (3,12-15). Notably, CHOP serves a crucial role
in ER stress and cell apoptosis, and evidence suggests that CHOP is
deleterious to cells when they are subjected to ER stress (16).
One of the most serious diseases of the exocrine
pancreas is acute pancreatitis (AP), which is characterized by
perivascular infiltration and inflammation, as well as tissue
edema, hemorrhage, and acinar and fat necrosis in the pancreas
(17,18). However, the understanding on the
complicated pathogenesis of this disease is currently limited.
Several potential factors have been proposed to explain pancreatic
injury, including pathological intraacinar trypsinogen activation,
intracellular calcium overload, inflammatory mediators, bacterial
infections, apoptosis, activation of nuclear factor (NF)-κB and
oxidative stress (19-22). All these factors interact with
each other and form a complex network control system to mediate
acinar cell injury and the inflammatory response, and certain of
these are also considered to be potential causes of ER stress
(2,23-25). Indeed, the pancreas is
particularly vulnerable to ER stress since its cells possess
particularly abundant ER to support the organ's prominent role in
the synthesis of digestive enzymes (1,26,27). Recently, evidence surfaced that ER
stress and UPR were activated in AP, and were indispensable in the
acceleration of the development of AP.
ER stress induces significant morphological changes
during AP pathogenesis (28,29). Kubisch et al (30) reported that ER stress-associated
receptors, including IRE1, PERK and ATF6, along with their
downstream signaling pathway-associated molecules, such as eIF2,
XBP1, CHOP and Caspase-12, were markedly activated in the early
stage of AP in a rat model induced by arginine, leading to the
formation of vesicle particles inside the ER. It was also reported
that PERK and phosphorylation of eIF2a were activated at an early
stage (within 4 h) in cerulein-induced pancreatitis (31). In pancreatitis, excessive IRE1a
activated the phosphorylation of JNK and other ‘warning genes’,
such as p38 and NF-κB, which strongly responded to ER stress during
AP injury, promoting transcription of various inflammatory genes
and pancreatic neutrophil infiltration (32-34).
The aforementioned studies demonstrated that the ER
stress response serves a major role in pancreatic acinar injury and
AP induction. However, earlier studies indicated that ER stress had
a protective role in AP; for instance, XBP1 or PERK deficiency was
reported to aggravate the exocrine pancreatic dysfunction (35,36). Furthermore, in alcohol-induced
pancreatitis, ER stress was attenuated by a robust UPR involving
XBP1 in acinar cells, which markedly weakened eIF2 phosphorylation
and the expression of PERK, ATF4 and CHOP, controlling the severity
of ER stress and alleviating the AP injury (37). Thus, ER stress-response appears to
regulate the injury of pancreatic acinar cells differently, by
either aggravating or attenuating it, possibly depending on the
balance between CHOP-mediated pro-apoptotic and pro-survival ER
stress responses (3). However,
the direct involvement of CHOP in the AP pathological process has
not been fully explored.
Melatonin, which is secreted by the pineal gland,
exerts several prominent cell-protective functions, including
anti-inflammatory, antioxidant and anti-apoptotic properties
(38). Studies have confirmed
that pretreatment with melatonin markedly reduced lipid
peroxidation, tissue edema and inflammation in cerulein-induced AP
in rats (39). However, whether
melatonin exhibits an anti-inflammatory effect via the
CHOP-mediated pathway remains unclear.
Thus, in the present study, a lentivirus-mediated
RNA interference (RNAi) approach was used to specifically knockdown
the expression of CHOP to investigate whether and how the
CHOP-mediated pathway contributes to the development of AP injury
in the pancreatic tissue of Sprague-Dawley (SD) rats. The potential
role of melatonin in the CHOP-mediated signaling pathway to
decrease inflammation and apoptosis was further demonstrated in the
rat AP model.
Materials and methods
Animals
A total of 40 clean-grade male SD rats, weighing
200-300 g (6 to 8-weeks-old), were purchased from the Shanghai
Laboratory Animal Center, Chinese Academy of Sciences (Shanghai,
China). The animals were maintained under tandard conditions in a
room with a 12-h light/dark cycle, and were provided free access to
food and water. All animals were adapted in the animal center for
at least 1 week and deprived of rat chow for 12 h before
experimentation, but allowed unlimited access to water throughout
the experimental period. All procedures were performed in
accordance with the Guidelines for Animal Experiments of Wenzhou
Medical University (Wenzhou, China). All the animal studies
complied with the current ethical considerations and were approved
by the Laboratory Animal Ethics Committee of Wenzhou Medical
University.
Animal groups and procedures
All SD rats were anesthetized by intraperitoneal
injection of 10% chloral hydrate (300 mg/kg), and randomly assigned
to the negative control (NC; n=8), sham-operated (SO; n=8), AP
(n=8), melatonin treatment (MT; n=8) or CHOP knockdown (CK; n=8)
groups. In the SO group, the rats underwent the same surgical
procedure as other experimental groups, but without infusion with
5% taurocholate. The surgical procedure performed involved
cannulating the biliopancreatic duct via penetration of the
duodenum with a 24-guage catheter. In the AP group, after clamping
the hepatic duct using a microclip, AP was induced by an infusion
of 5% taurocholate (1 ml/kg body weight; Sigma-Aldrich; Merck KGaA)
into the biliopancreatic duct via a microinjection pump at a rate
of 0.2 ml/min. In the MT group, melatonin (50 mg/kg body weight;
Sigma-Aldrich; Merck KGaA) was administered via intraperitoneal
injection 30 min before AP was induced. In the CK group, rats
underwent the same surgical procedure as rats in the AP group but
also underwent lentiviral transfection silencing CHOP expression
prior to AP induction. All surgical procedures were performed under
sterile conditions. After 9 h, the rats were sacrificed by
exsanguination under anesthesia with chloral hydrate (300 mg/kg),
and the pancreatic tissues were rapidly collected and divided into
two parts. Part of the tissue samples was fixed in 4%
paraformaldehyde and prepared for routine paraffin-embedding prior
to immunohistochemical and pathological examination analyses, while
the remaining tissue was stored at -80°C for the western blot and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) analyses.
Lentivirus-mediated stable RNA
interference of CHOP in pancreatic tissue
A lentivirus (vehicle:
hU6-MCS-Ubiquitin-EGFP-IRES-puromycin), which carried a short
hairpin (sh)RNA targeting the CHOP gene (GenBank no. NM_024134),
was purchased from GeneChem Co., Ltd. (Shanghai, China). This
lentivirus was used for CHOP silencing and termed LV-shCHOP.
Briefly, the rats were anesthetized through intraperitoneal
injection of 300 mg/kg chloral hydrate. Subsequently, LV-shCHOP (50
µl; 4x108 TU/ml; GeneChem Co., Ltd.) was injected
into their left gastric artery of the rats via a microinjection
pump at a rate of 0.2 ml/min, and the left gastric artery was then
ligated to avoid reflux of the virus. After 5 days, the AP model
was induced in rats. After 9 h of induction, the rats were
sacrificed by exsanguination as aforementioned, and the pancreatic
tissues were rapidly collected; some tissues were stored as frozen
sections (14 µm). Subsequently, the green fluorescent
protein (GFP)-positive cells in pancreatic tissue were observed
with a Nikon inverted fluorescence microscope (Nikon Corporation,
Tokyo, Japan). The effect of CHOP expression silencing in the
pancreatic tissues was then analyzed by western blot analysis and
RT-qPCR.
Western blot analysis
Total proteins from pancreatic tissues were
extracted and homogenized in ice-cold lysis buffer containing
radioimmunoprecipitation assay, phosphatase and phenylmethane
sulfonyl fluoride (ratio, 100:10:1) for 30 min on ice. Next, the
extracts were transferred to a microcentrifuge tube and centrifuged
at 1.2x104 x g for 15 min (4°C), and protein
concentrations were determined using a BCA assay kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Subsequently, 45 µg
total protein was separated through 10% SDS-PAGE and then
transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA) at 300 mA for 1 h. The membranes
were blocked with 5% skim milk for 2 h at 25°C and then incubated
with primary antibodies overnight at 4°C. The primary antibodies
used in this analysis included 78 kDa glucose-regulated protein
(GRP78; cat. no. ab108615; Abcam, Cambridge, UK), CHOP (cat. no.
ab179823; Abcam), tumor necrosis factor α (TNF-α; cat. no. ab6671;
Abcam), B-cell lymphoma 2 (Bcl-2; cat. no. ab32124; Abcam),
Bcl-2-associated X protein (Bax; cat. no. ab182733; Abcam),
caspase-3 (cat. no. ab32351; Abcam), phospho-NF-κB inhibitor α
(p-IκBα; Ser 32; cat. no. 2859; Cell Signaling Technology, Inc.,
Danvers, MA, USA), phospho-NF-κB p65 (p-p65; Ser 536; cat. no.
3033; Cell Signaling Technology, Inc.), and β-actin (Cell Signaling
Technology, Inc.) at a 1:1,000 dilution. β-actin was used as an
internal control. On the following day, membranes were washed three
times with Tris-buffered saline/Tween-20 (TBST) and incubated for 1
h at room temperature with a goat anti-rabbit IgG secondary
antibody conjugated to horseradish peroxidase (1:5,000; Bioworld
Technology, Inc., St. Louis Park, MN, USA), and then washed three
times with TBST. Finally, the protein bands were visualized using a
Western Bright ECL detection kit (Advansta, Menlo Park, CA, USA).
The density of specific bands was quantified using Image Lab
software with a ChemiDoc MP imaging densitometer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Each experiment was
performed in triplicate.
RT-qPCR analysis
Total RNA from pancreatic tissues was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Next, total RNA was reverse transcribed into cDNA using an RT kit
(Thermo Fisher Scientific, Inc) at 25°C for 5 min, 42°C for 60 min
and 70°C for 5 min; the concentration of RNA was determined with a
spectrophotometer by measuring the optical density at 260/280 nm.
The qPCR procedure was subsequently conducted using a Real-Time
qPCR system (Bio-Rad Laboratories, Inc.) and the Takara Power
SYBR-Green PCR Master mix (cat. no. DRR820A; Takara Bio, Inc.,
Otsu, Japan). The PCR primers were synthe-sized by Sangon Biotech
Co., Ltd. (Shanghai, China), and the sequences were as follows:
GRP78, 5′-CAA GAA CCA ACT CAC GTC CA-3′ (forward) and 5′-ACC ACC
TTG AAT GGC AAG AA-3′ (reverse); CHOP, 5′-CCA GGA AAC GAA GAG GAA
GA-3′ (forward) and 5′-CTT TGG GAG GTG CTT G TG A-3′ (reverse);
Bcl-2, 5′-AGG ATT GTG GCC TTC TTT GA-3′ (forward) and 5′-CAG ATG
CCG GTT CAG GTA CT-3′ (reverse); Bax, 5′-CAG GAT CGA GCA GAG AGG
AT-3′ (forward) and 5′-GTC CAG TTC ATC GCC AAT TC-3′ (reverse);
caspase-3, 5′-ACT GGA CTG TGG CAT TGA GA-3′ (forward) and 5′-AAT
TTC GCC AGG AAT AGT AAC C-3′ (reverse); TNF-α, 5′-TGA TCC GAG ATG
TGG AAC TG-3′ (forward) and 5′-CGA GCA GGA ATG AGA AGA GG-3′
(reverse); IL-6, 5′-TAC CCC AAC TTC CAA TGC TC-3′ (forward) and
5′-GGT TTG CCG AGT AGA CCT CA-3′ (reverse); β-actin, 5′-CGT GAA AAG
ATG ACC CAG AT-3′ (forward) and 5′-ACC CTC ATA GAT GGG CAC A-3′
(reverse). β-actin was used as an internal control. The cDNA was
amplified over the course of a 40-cycle program at 95°C for 30 sec,
95°C for 5 sec and 60°C for 30 sec. Finally, the quantified
relative gene expression levels of GRP78, CHOP, Bcl-2, Bax,
caspase-3, IL-6 and TNF-α mRNA were calculated using the
2-ΔΔCq method (40).
Each experiment was performed in triplicate.
Histological analysis and pathological
scores of pancreatic tissues
The pancreatic tissue samples were fixed in 4%
paraformaldehyde for 24 h, then embedded in paraffin wax and
stained with hematoxylineosin. Alterations in the pancreatic tissue
were assessed by an experienced pathologist who was blinded to the
experimental protocol. Eight randomly selected visual areas in each
pathological section were observed under a light microscope (Nikon
Corporation) and scored by an experienced histologist using
standards obtained from a study by Schmidt et al (41). Briefly, the pancreatic tissues
were scored for edema, inflammatory cell infiltration, acinar cell
degeneration and hemorrhage, each on a scale for 0-3. The maximum
score for each visual area was 12. Each experiment was performed in
triplicate.
Immunohistochemical staining
Pancreatic tissue samples were fixed in 4%
paraformaldehyde and embedded in paraffin. Sections (4 µm)
were deparaffinized with xylene and hydrated through a graded
ethanol series, followed by microwave heat repair using citrate
buffer (pH 6.0; OriGene Technologies, Inc., Beijing, China) for
15-20 min and cooling for 10 min in an ice-water bath. Tissue
sections were then treated with 3% hydrogen peroxide (OriGene
Technologies, Inc.) to suppress the activity of endogenous
peroxidase. Next, the tissue sections were blocked with 5% goat
serum (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) for 1 h at room temperature and then incubated with
CHOP and Bcl-2 antibodies in a 1:100 dilution overnight at 4°C.
Subsequently, the sections were incubated with a goat anti-rabbit
secondary IgG antibody conjugated to biotin (undiluted; cat. no.
DS-0005; OriGene Technologies, Inc.) for 1 h at room temperature,
followed by staining with DAB (OriGene Technologies, Inc.) and
hema-toxylin (Solarbio Science & Technology Co., Ltd.). Images
were captured using a light microscope (Nikon Corporation) and
quantified using the ImagePro-Plus software 6.0 (Media Cybernetics,
Inc., Rockville, MD, USA). Each experiment was performed in
triplicate.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. SPSS version 20.0 software (IBM Corp., Armonk, NY, USA)
was used for statistical analysis, while data comparisons were made
using the analysis of variance and Newman-Keuls multiple comparison
tests, as appropriate. P<0.05 was considered to denote a
statistically significant difference.
Results
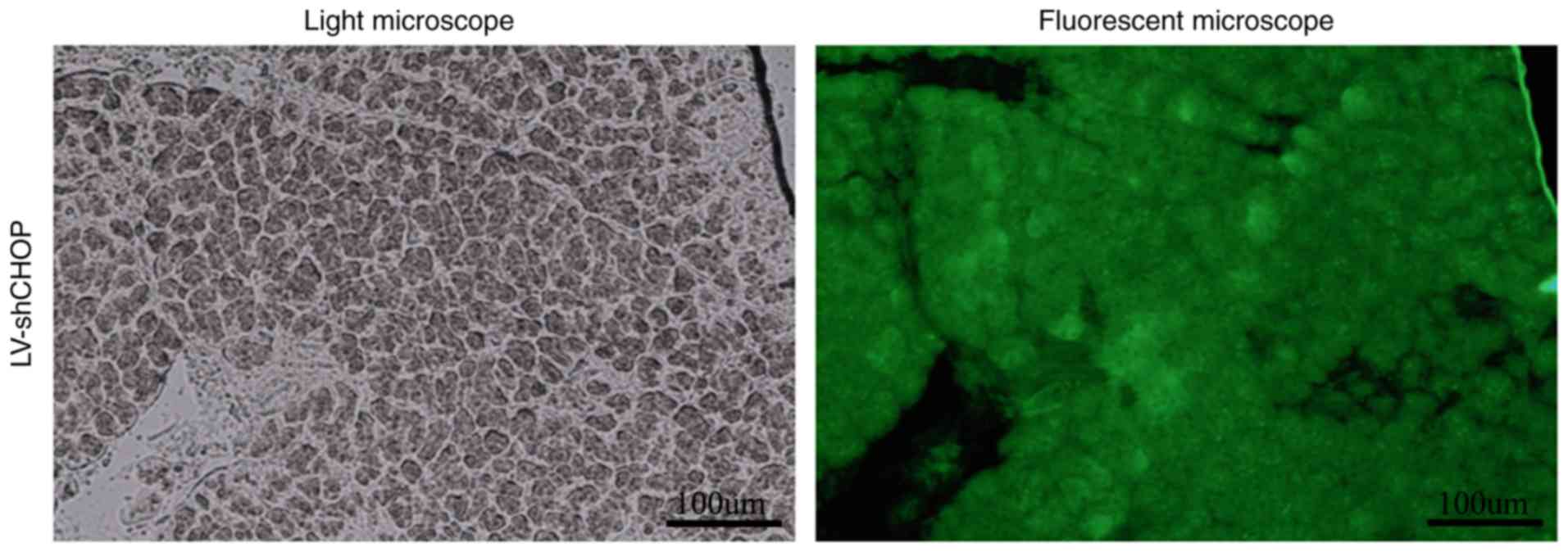
Transduction efficacy of
LV-shCHOP-mediated RNAi in pancreatic tissue
Highly efficient transduction (>85%) of LV-shCHOP
in pancreatic tissue was achieved after 5 days of infection
(Fig. 1). The GFP-positive cells
were also observed with a Nikon Inverted fluorescence microscope
(Fig. 1).
Effects of taurocholate and treatment
with melatonin on markers of ER stress in the pancreatic
tissue
As illustrated in Fig.
2A and B, GRP78 and CHOP levels were markedly elevated in the
AP group compared with the SO and NC groups. By contrast, the MT
and CK groups exhibited significantly reduced protein levels of
GRP78 and CHOP compared with the AP group. In the CK group,
silencing of CHOP expression in the pancreatic tissue was achieved,
which significantly attenuated the ER stress following the
induction of AP, while pre-treatment with melatonin also
downregulated the CHOP levels and restrained ER stress in AP.
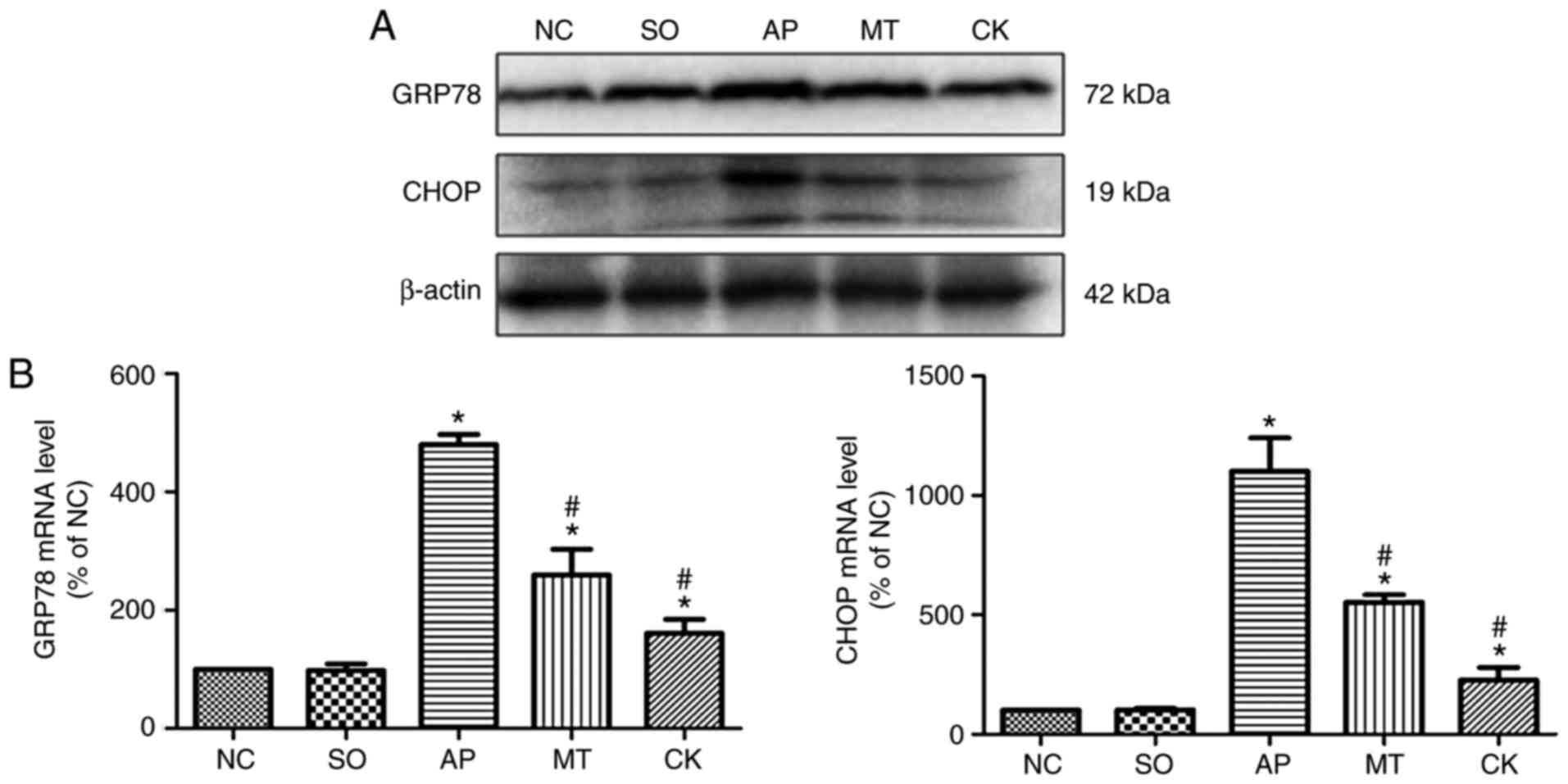 | Figure 2Expression of markers of ER stress in
pancreatic tissue obtained from rats after 9 h of treatment in the
NC, SO, AP, MT and CK groups. (A) GRP78 and CHOP protein expression
levels in the pancreatic tissue were examined by western blot
analysis, and β-actin was used as an internal control. (B) mRNA
levels of GRP78 and CHOP were quantified by reverse
transcription-quantitative polymerase chain reaction. Data (n=8 per
group) are expressed as the mean ± standard error of at least three
independent experiments. *P<0.05 vs. the SO and NC
groups; #P<0.05 vs. the AP group. CHOP, C/EBP
homologous protein; GRP78, 78 kDa glucose-regulated protein; NC,
negative control; SO, sham-operated; AP, acute pancreatitis; MT,
melatonin treatment; CK, CHOP knockdown. |
Effects of taurocholate and treatment
with melatonin on inflammation and apoptosis-associated molecules
in pancreatic tissue
TNF-α, p-p65 and p-IκBα levels were markedly
increased in the AP group compared with the SO and NC groups.
However, the CK and MT groups exhibited a significant reduction in
the levels of these molecules compared with the AP group (Fig. 3A and B). Furthermore, Fig. 4A and B demonstrates that the
levels of Bax and caspase-3 were significantly elevated, while
Bcl-2 level was reduced, in the AP group relative to the SO and NC
groups. By contrast, the CK and MT groups demonstrated
significantly enhanced protein levels of Bcl-2 and reduced protein
levels of Bax and caspase-3, compared with the AP group. These
levels indicated that knockdown of CHOP expression in pancreatic
tissue reduced the activation of the NF-κB pathway, inflammation
and apoptosis response following the induction of AP, while
pre-treatment with melatonin also relieved inflammation and
apoptosis in AP. Therefore, CHOP was observed to exacerbate AP
injury through inducing apoptosis, and activating the NF-κB pathway
and inflammation response. Besides, melatonin reduced apoptosis,
and alleviated AP severity and tissue injury through CHOP-mediated
pathways.
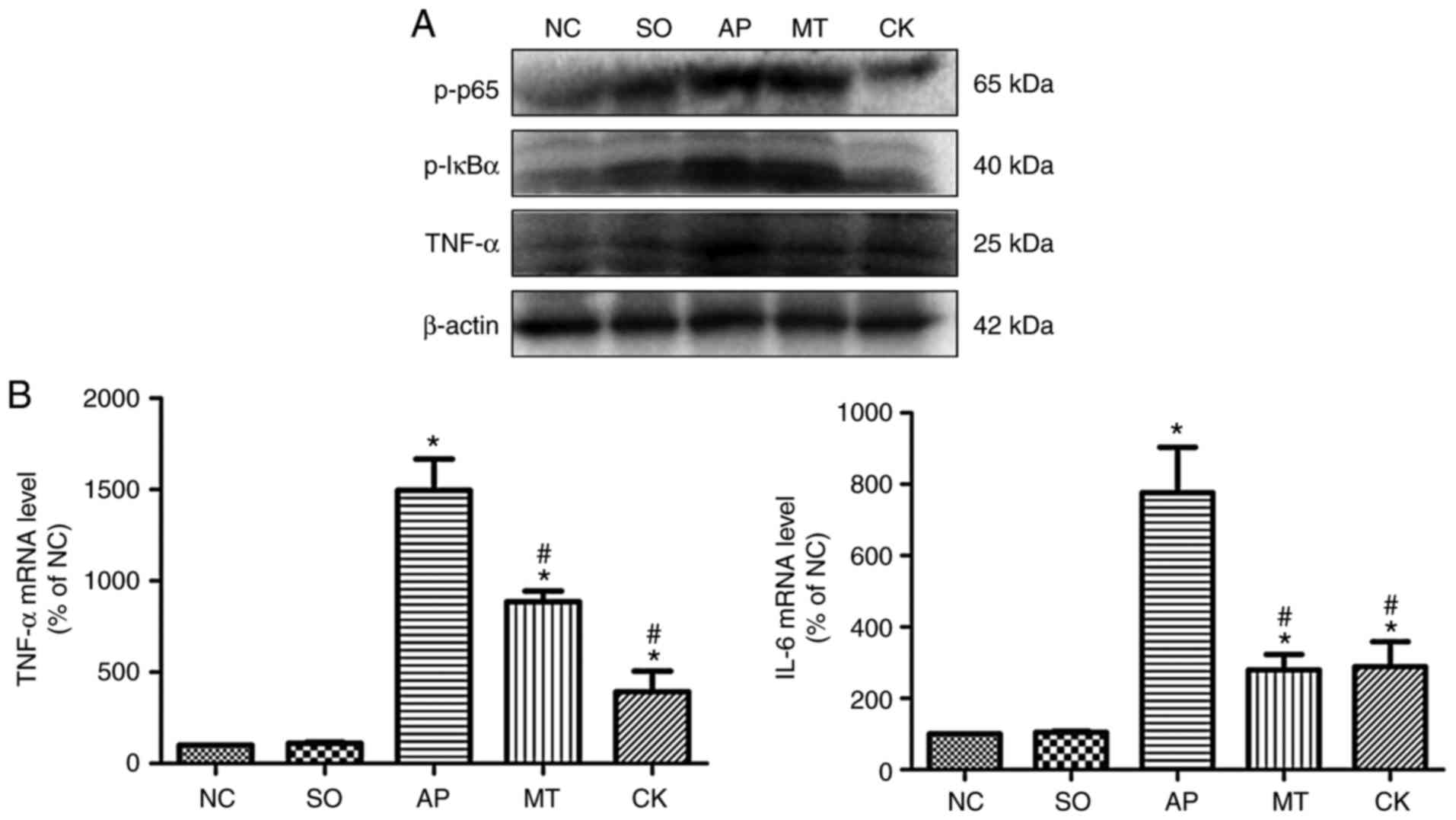 | Figure 3Expression levels of
inflammation-associated molecules in pancreatic tissue collected
from rats after 9 h of treatment in the different groups. (A)
p-p65, p-IκBα and TNF-α protein expression levels in the pancreatic
tissue, examined by western blot analysis. (B) mRNA levels of TNF-α
and IL-6 were quantified by reverse transcription-quantitative
polymerase chain reaction. Data (n=8 per group) are expressed as
the mean ± standard error of at least three independent
experiments. *P<0.05 vs. the SO and NC groups;
#P<0.05 vs. the AP group. IκBα, nuclear factor-κB
inhibitor α; TNF-α, tumor necrosis factor α; IL, interleukin; p-,
phosphorylated; NC, negative control; SO, sham-operated; AP, acute
pancreatitis; MT, melatonin treatment; CK, C/EBP homologous protein
knockdown. |
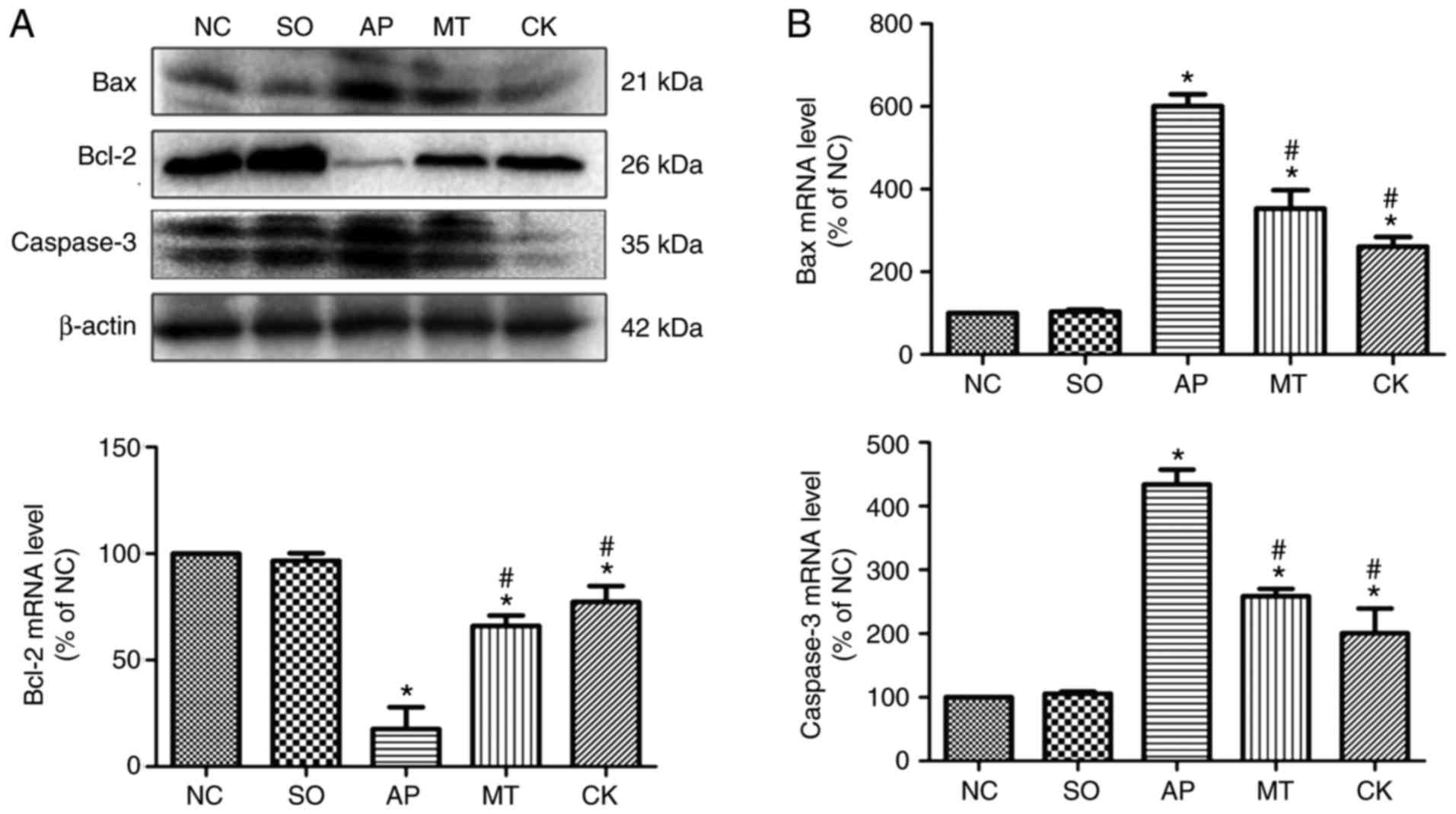 | Figure 4Expression levels of
apoptosis-associated molecules in pancreatic tissue samples
collected from rats after 9 h of treatment in the different groups.
(A) Bax, Bcl-2 and caspase-3 protein expression levels in the
pancreatic tissue were examined by western blot analysis, and
β-actin was used as an internal control. (B) mRNA levels of Bax,
Bcl-2 and caspase-3 were quantified by reverse
transcription-quantitative polymerase chain reaction. Data (n=8 per
group) are expressed as the mean ± standard error of at least three
independent experiments. *P<0.05 vs. the SO and NC
groups; #P<0.05 vs. the AP group. Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X protein; NC, negative control;
SO, sham-operated; AP, acute pancreatitis; MT, melatonin treatment;
CK, C/EBP homologous protein knockdown. |
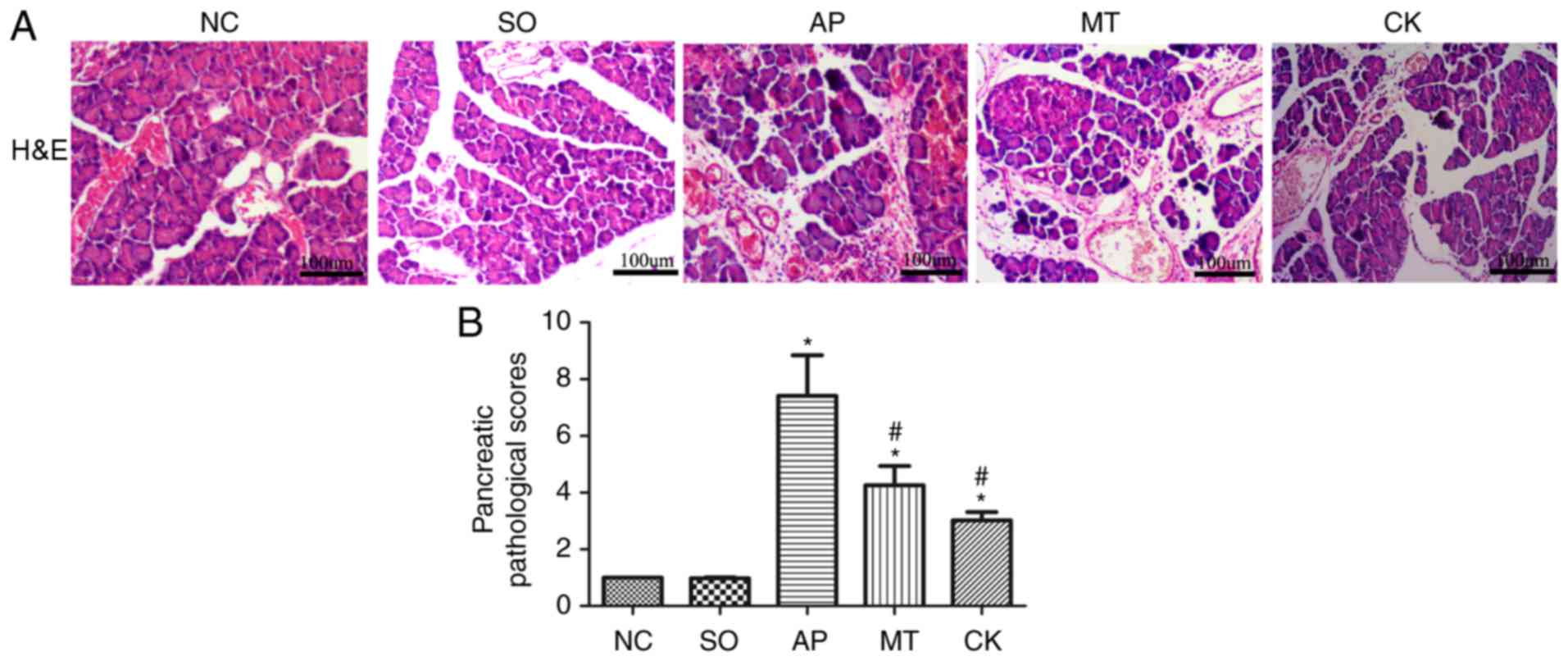
Pathological scoring and
histopathological examination of pancreatic tissues
Microscopic examination of pancreatic samples from
the SO group displayed a normal morphology at 9 h after surgery.
The AP group tissues exhibited evident edema, hemorrhage and
inflammatory cell infiltration. By contrast, the injury to
pancreatic tissues in the MT and CK groups was significantly
alleviated compared with the AP group (Fig. 5A). The mean pathological score of
pancreatic samples in the MT and CK groups was significantly lower
compared with the AP group, although it remained markedly higher
than that in the SO and NC groups (Fig. 5B).
Effect of taurocholate and treatment with
melatonin on CHOP and Bcl-2 expression in pancreatic tissue by
immunohistochemistry
The immunohistochemical analysis results further
revealed significantly higher expression of Bcl-2 and lower
expression of CHOP in the CK and MT groups, as compared with the
expression in the AP group (Fig. 6A
and B). This result further revealed that silencing of the CHOP
gene in pancreatic tissue significantly reduced the expression of
the CHOP protein; RT-qPCR and western blotting indicated that
silencing CHOP expression reduced the expression of pro-apoptotic
proteins, Bax and caspase-3, and increased that of anti-apoptotic
Bcl-2, which suggested a reduction in apoptosis during AP. In
addition, in the MT group, the expression of CHOP and apoptosis
were also significantly attenuated, indicating that melatonin
reduced apoptosis through CHOP-mediated pathways.
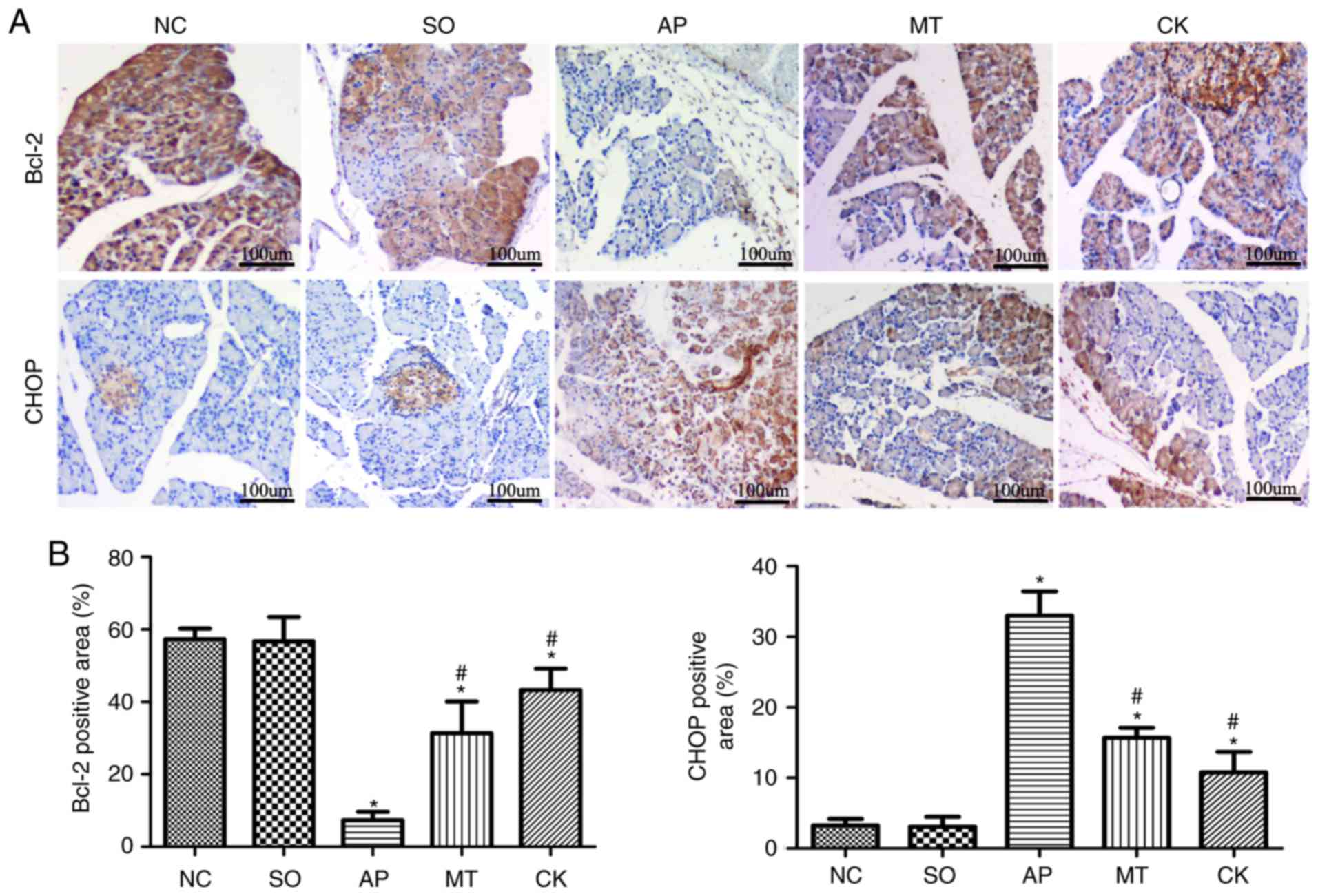 | Figure 6Expression levels of CHOP and Bcl-2
in pancreatic tissue samples from rats after 9 h of treatment in
the different groups. (A) Expression levels of CHOP and Bcl-2 in
the pancreatic tissue, as determined by immunohistochemistry
(original magnification, x40; bar, 100 µm). (B) Image
analysis of the area of CHOP and Bcl-2 staining. Data (n=8 per
group) are expressed as the mean ± standard error of at least three
independent experiments. *P<0.05 vs. the SO and NC
groups; #P<0.05 vs. the AP group. CHOP, C/EBP
homologous protein; Bcl-2, B-cell lymphoma 2; NC, negative control;
SO, sham-operated; AP, acute pancreatitis; MT, melatonin treatment;
CK, CHOP knockdown. |
Discussion
Although AP is one of the most lethal diseases of
the exocrine pancreas, the underlying mechanisms remain unclear.
Therefore, various cellular and animal models have been developed
in order to identify the early mechanisms of AP, which determine
the ultimate severity of the disease. In recent years, studies have
demonstrated that ER stress serves an indispensable role in the
development and progression of AP (42). The present study demonstrated that
the ER stress-induced CHOP-mediated pathway was activated in the
early stage of AP inflammation and served an important role in
exacerbating pancreatic injury by inducing apoptosis, NF-κB pathway
activation and the expression of proinflammatory cytokines.
Furthermore, the study also provided a new insight by demonstrating
that melatonin protects against pancreatitis inflammation via
inhibition of the CHOP-mediated pathway.
GRP78 is an important molecular chaperone (43), which is located in the ER and
widely used as an ER stress marker and a key regulator of the UPR.
Normally, it is expressed at a very low level, however, when
responding to ER stress, GRP78 expression significantly increases
and is separated from three ER transmembrane proteins (PERK, ATF6
and IRE1), thereby activating the UPR signaling pathway (1). In the current study, GRP78 was found
to be upregulated early in a rat AP model, suggesting that ER
stress was activated early in AP.
CHOP/GADD153 is an ER stress-specific transcription
factor that belongs to the C/EBP family of basic leucine zinc
finger structure transcription factors and is a signaling molecule
involved in ER stress-induced apoptosis (44). Previous studies have reported
that, under normal conditions, the expression of CHOP is low;
however, when responding to ER stress, IRE-1, PERK and ATF6 are
able to promote CHOP activation, with overexpression of CHOP
resulting in cell apoptosis (1,18,45), with the PERK signaling pathway
being the most important driver of this process. Ji et al
(46) identified through mice fed
with ethanol that hepatocellular apoptosis was significantly
reduced in CHOP-deficient mice compared with wild-type mice, while
other genes associated with apoptosis were also changed, such as
Jun D, Bcl-xl and Gadd45. ER stress is addressed via UPR-activated
CHOP protein, and thus far, several mechanisms have been proposed
for CHOP-mediated apoptosis. Such mechanisms include the increase
of the levels of pro-apoptosis proteins, such as Bax/Bak, the
decrease of the antiapoptotic protein Bcl-2 levels (47), the release of cytochrome c
from mitochondria, and the activation of apoptotic-associated
caspase cascade (48,49). All of these previous studies, as
well as the results of the current study, demonstrated that CHOP
was activated in the early stage of inflammation and also increased
over time, alongside increased Bax and caspase-3 levels and
decreased Bcl-2, supporting the idea that CHOP-mediated apoptosis
mainly occurs via a mitochondria-dependent pathway. Furthermore,
the present study western blot and RT-qPCR results indicated that
silencing of CHOP in pancreatic tissue suppressed apoptosis by
inhibiting the expression of Bax and caspase-3, and activating
Bcl-2 during AP. These results were also confirmed by
immunohistochemistry, which revealed a significantly higher
expression of Bcl-2 in the CK group as compared with that in the AP
group. All these results demonstrated that CHOP served a
potentially important role in AP-associated apoptosis. The results
of the present study were in agreement with previous findings
reporting that CHOP defi-ciency can protect cells from ER
stress-induced apoptosis (16).
The current observations indicate that UPR can
initiate an inflammatory response via various mechanisms (50). Besides its pro-apoptotic role. A
number of studies have also revealed a pro-inflammatory role for
the CHOP-mediated pathway (3,39,51,52), however, the mechanisms between
inflammation and CHOP have not been fully explored, particularly in
rat AP models. In the present study, it was proposed that the
pro-inflammatory mechanisms of the CHOP-mediated pathway may occur
via NF-κB activation. This was a possibility supported by the study
of Allagnat et al (2), who
reported that CHOP directly supported the activation of NF-κB
pathway and subsequent expression of pro-inflammatory cytokines,
which aggravated ER stress and promoted CHOP expression. In turn,
CHOP promoted the degradation of IκBα and amplified the activity of
the NF-κB pathway. NF-κB is a member of the transcription factor
family involved in the transcriptional regulation of a variety of
inflammatory genes and serves an important role in the inflammatory
response (2). Such a positive
feedback loop may exacerbate inflammatory responses and injury,
resulting in the development of AP. Consistently, the present study
proved this point, as silencing of CHOP was able to significantly
reduce NF-κB activation and inflammatory cytokine levels in an
in vivo AP model. Additionally, pathological examination and
immunohistochemistry analysis results revealed that CHOP silencing
significantly reduced pancreatic injury and inflammatory cell
infiltration. According to the experimental results, it was
concluded that the CHOP-mediated pathway effectively promoted the
inflammatory response in AP. Nevertheless, the potential molecular
mechanism associating the inflammatory response and the
CHOP-mediated pathway is of great importance and deserves further
investigation.
Previous results have indicated that melatonin
prevents or attenuates the severity of experimental severe acute
pancreatitis (SAP) (39).
However, the mechanisms of its anti-inflammatory effects in AP are
currently unclear. Recently, ER stress was reported to be involved
in the development and progression of AP, and several studies have
confirmed that melatonin suppressed ER stress in different models
of cell damage. For instance, treatment with melatonin resulting in
attenuation of cell apoptosis after brain ischemia was associated
with the reduction of ER stress (53), and similar effects were also
observed in lethal fulminant hepatitis (54). The main finding of the present
study was that melatonin significantly alleviated pancreatic
injury, inflammation and apoptosis in the AP rat model, accompanied
by downregulation of the expression of CHOP (a hallmark of
ER-associated apoptosis) and GRP78 (ER stress marker). The role of
CHOP in aggravating the inflammation and apoptosis in AP was
confirmed earlier in the present study; therefore, it is
hypothesized that melatonin can inhibit the CHOP-mediated pathway
to reduce injury in AP.
In conclusion, the current study indicates that the
ER stress-induced, CHOP-mediated pathway is activated soon after
the induction of AP. This pathway subsequently exacerbates AP
injury not only by inducing the apoptosis of pancreatic tissue, but
also by enhancing tissue inflammation. Therefore, the CHOP-mediated
pathway may represent a potential target for developing new
protection strategies against AP injury. Furthermore, the findings
of the present study provided further preclinical evidence of the
pancreas-protective effect of melatonin during AP via inhibition of
the CHOP-mediated pathway. Owing to its efficacy and low toxicity,
it is also suggested that melatonin urgently requires further
evaluation through clinical applications.
Acknowledgements
All the authors would like to thank Yina Chen at the
Department of Gastroenterology at Yuyao People's Hospital of
Zhejiang (Yuyao, China) for the technical support.
Funding
The present study was supported by the Public
Projects of Zhejiang Province (grant no. 2016C33215), the Zhejiang
Provincial Natural Science Foundation of China (grant no.
LQ17H030004) and the Science and Technology Bureau of Wenzhou,
Zhejiang Province, China (grant no. Y20150158).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
QZ and JH performed the majority of the experiments
and analyzed the data. HZ and JL performed the molecular
investigations. QZ and QC participated equally in the treatment of
animals. JW and YJ designed and coordinated the research. HY and YS
performed the statistical analyses. QZ wrote the manuscript.
Ethics approval and consent to
participate
All procedures were performed in accordance with the
Guidelines for Animal Experiments of Wenzhou Medical University
(Wenzhou, China). All the animal studies complied with the current
ethical considerations and were approved by the Laboratory Animal
Ethics Committee of Wenzhou Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kubisch CH and Logsdon CD: Endoplasmic
reticulum stress and the pancreatic acinar cell. Expert Rev
Gastroenterol Hepatol. 2:249–260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Allagnat F, Fukaya M, Nogueira TC,
Delaroche D, Welsh N, Marselli L, Marchetti P, Haefliger JA,
Eizirik D and Cardozo AK: C/EBP homologous protein contributes to
cytokine-induced pro-inflammatory responses and apoptosis in
β-cells. Cell Death Differ. 19:1836–1846. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miyazaki Y, Kaikita K, Endo M, Horio E,
Miura M, Tsujita K, Hokimoto S, Yamamuro M, Iwawaki T, Gotoh T, et
al: C/EBP homologous protein deficiency attenuates myocardial
reperfusion injury by inhibiting myocardial apoptosis and
inflammation. Arterioscler Thromb Vasc Biol. 31:1124–1132. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu JS, Li WM, Chen YN, Zhao Q and Chen QF:
Endoplasmic reticulum stress is activated in acute pancreatitis. J
Dig Dis. 17:295–303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nashine S, Liu Y, Kim BJ, Clark AF and
Pang IH: Role of C/EBP homologous protein in retinal ganglion cell
death after ischemia/reperfusion injury. Invest Ophthalmol Vis Sci.
56:221–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schröder M and Kaufman RJ: The mammalian
unfolded protein response. Annu Rev Biochem. 74:739–789. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohoka N, Yoshii S, Hattori T, Onozaki K
and Hayashi H: TRB3, a novel ER stress-inducible gene, is induced
via ATF4-CHOP pathway and is involved in cell death. EMBO J.
24:1243–1255. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamaguchi H and Wang HG: CHOP is involved
in endoplasmic reticulum stress-induced apoptosis by enhancing DR5
expression in human carcinoma cells. J Biol Chem. 279:45495–45502.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Puthalakath H, O'Reilly LA, Gunn P, Lee L,
Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin
J, Motoyama N, et al: ER stress triggers apoptosis by activating
BH3-only protein Bim. Cell. 129:1337–1349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song B, Scheuner D, Ron D, Pennathur S and
Kaufman RJ: Chop deletion reduces oxidative stress, improves beta
cell function, and promotes cell survival in multiple mouse models
of diabetes. J Clin Invest. 118:3378–3389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oyadomari S, Koizumi A, Takeda K, Gotoh T,
Akira S, Araki E and Mori M: Targeted disruption of the Chop gene
delays endoplasmic reticulum stressmediated diabetes. J Clin
Invest. 109:525–532. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Milhavet O, Martindale JL, Camandola S,
Chan SL, Gary DS, Cheng A, Holbrook NJ and Mattson MP: Involvement
of GADD153 in the pathogenic action of presenilin-1 mutations. J
Neurochem. 83:673–681. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paschen W, Gissel C, Linden T, Althausen S
and Doutheil J: Activation of GADD153 expression through transient
cerebral ischemia: Evidence that ischemia causes endoplasmic
reticulum dysfunction. Brain Res Mol Brain Res. 60:115–122. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prasanthi JR, Larson T, Schommer J and
Ghribi O: Silencing GADD153/CHOP gene expression protects against
Alzheimer's disease-like pathology induced by 27-hydroxycholesterol
in rabbit hippocampus. PLoS One. 6:E264202011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zinszner H, Kuroda M, Wang X, Batchvarova
N, Lightfoot RT, Remotti H, Stevens JL and Ron D: CHOP is
implicated in programmed cell death in response to impaired
function of the endoplasmic reticulum. Genes Dev. 12:982–995. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Esrefoglu M, Gul M, Ates B, Batcioglu K
and Selimoglu MA: Antioxidative effect of melatonin, ascorbic acid
and nacetylcysteine on caerulein induced pancreatitis and
associated liver injury in rats. World J Gastroenterol. 12:259–264.
2006. View Article : Google Scholar
|
|
18
|
Wang XZ, Lawson B, Brewer JW, Zinszner H,
Sanjay A, Mi LJ, Boorstein R, Kreibich G, Hendershot LM and Ron D:
Signals from the stressed endoplasmic reticulum induce
C/EBP-homologous protein (CHOP/GADD153). Mol Cell Biol.
16:4273–4280. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song JM, Liu HX, Li Y, Zeng YJ, Zhou ZG,
Liu HY, Xu B, Wang L, Zhou B and Wang R: Extracellular heat-shock
protein 70 aggravates cerulein-induced pancreatitis through
toll-like receptor-4 in mice. Chin Med J (Engl). 121:1420–1425.
2008.
|
|
20
|
van Minnen LP, Blom M, Timmerman HM,
Visser MR, Gooszen HG and Akkermans LM: The use of animal models to
study bacterial translocation during acute pancreatitis. J
Gastrointest Surg. 11:682–689. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu JH and Kim H: Oxidative stress and
inflammatory signaling in cerulein pancreatitis. World J
Gastroenterol. 20:17324–17329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tando Y, Algul H, Schneider G, Weber CK,
Weidenbach H, Adler G and Schmid RM: Induction of IkappaB-kinase by
chole-cystokinin is mediated by trypsinogen activation in rat
pancreatic lobules. Digestion. 66:237–245. 2002. View Article : Google Scholar
|
|
23
|
Xue X, Piao JH, Nakajima A, Sakon-Komazawa
S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H and Nakano H:
Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein
response (UPR) in a reactive oxygen species (ROS)-dependent
fashion, and the upr counteracts ros accumulation by TNFalpha. J
Biol Chem. 280:33917–33925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin W, Harding HP, Ron D and Popko B:
Endoplasmic reticulum stress modulates the response of myelinating
oligodendrocytes to the immune cytokine interferon-gamma. J Cell
Biol. 169:603–612. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malhotra JD and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress: A vicious cycle or a
double-edged sword? Antioxid Redox Signal. 9:2277–2293. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Case RM: Synthesis, intracellular
transport and discharge of exportable proteins in the pancreatic
acinar cell and other cells. Biol Rev Camb Philos Soc. 53:211–354.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Berridge MJ: The endoplasmic reticulum: A
multifunctional signaling organelle. Cell Calcium. 32:235–249.
2002. View Article : Google Scholar
|
|
28
|
Deng WH, Chen C, Wang WX, Yu J, Li JY and
Liu L: Effects of ORP150 on appearance and function of pancreatic
beta cells following acute necrotizing pancreatitis. Pathol Res
Pract. 207:370–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hartley T, Siva M, Lai E, Teodoro T, Zhang
L and Volchuk A: Endoplasmic reticulum stress response in an INS-1
pancreatic beta-cell line with inducible expression of a
folding-deficient proinsulin. BMC Cell Biol. 11:592010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kubisch CH, Sans MD, Arumugam T, Ernst SA,
Williams JA and Logsdon CD: Early activation of endoplasmic
reticulum stress is associated with arginine-induced acute
pancreatitis. Am J Physiol Gastrointest Liver Physiol.
291:G238–G245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sans MD, DiMagno MJ, D'Alecy LG and
Williams JA: Caerulein-induced acute pancreatitis inhibits protein
synthesis through effects on eIF2B and eIF4F. Am J Physiol
Gastrointest Liver Physiol. 285:G517–G528. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu P, Han Z, Couvillon AD, Kaufman RJ and
Exton JH: Autocrine tumor necrosis factor alpha links endoplasmic
reticulum stress to the membrane death receptor pathway through
IRE1alpha-mediated NF-kappaB activation and down-regulation of
TRAF2 expression. Mol Cell Biol. 26:3071–3084. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yun SW, Bae GS, Kim MS, Park KC, Koo BS,
Kim BJ, Kim TH, Seo SW, Shin YK, Lee SH, et al: Melittin inhibits
cerulein-induced acute pancreatitis via inhibition of the JNK
pathway. Int Immunopharmacol. 11:2062–2072. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murr MM, Yang J, Fier A, Gallagher SF,
Carter G, Gower WR and Norman JG: Regulation of Kupffer cell TNF
gene expression during experimental acute pancreatitis: The role of
p38-MAPK, ERK1/2, SAPK/JNK, and NF-kappaB. J Gastrointest Surg.
7:20–25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee AH, Chu GC, Iwakoshi NN and Glimcher
LH: Xbp-1 is required for biogenesis of cellular secretory
machinery of exocrine glands. EMBO J. 24:4368–4380. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Harding HP, Zeng H, Zhang Y, Jungries R,
Chung P, Plesken H, Sabatini DD and Ron D: Diabetes mellitus and
exocrine pancreatic dysfunction in Perk-/- mice reveals
a role for translational control in secretory cell survival. Mol
Cell. 7:1153–1163. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pandol SJ, Gorelick FS, Gerloff A and
Lugea A: Alcohol abuse, endoplasmic reticulum stress and
pancreatitis. Dig Dis. 28:776–782. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carloni S, Albertini MC, Galluzzi L,
Buonocore G, Proietti F and Balduini W: Melatonin reduces
endoplasmic reticulum stress and preserves sirtuin 1 expression in
neuronal cells of newborn rats after hypoxia-ischemia. J Pineal
Res. 57:192–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qi W, Tan DX, Reiter RJ, Kim SJ,
Manchester LC, Cabrera J, Sainz RM and Mayo JC: Melatonin reduces
lipid peroxidation and tissue edema in cerulein-induced acute
pancreatitis in rats. Dig Dis Sci. 44:2257–2262. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative pcr and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
41
|
Schmidt J, Rattner DW, Lewandrowski K,
Compton CC, Mandavilli U, Knoefel WT and Warshaw AL: A better model
of acute pancreatitis for evaluating therapy. Ann Surg. 215:44–56.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thrower EC, Gorelick FS and Husain SZ:
Molecular and cellular mechanisms of pancreatic injury. Curr Opin
Gastroenterol. 26:484–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hasnain SZ, Lourie R, Das I, Chen AC and
McGuckin MA: The interplay between endoplasmic reticulum stress and
inflammation. Immunol Cell Biol. 90:260–270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar
|
|
45
|
Matsumoto M, Minami M, Takeda K, Sakao Y
and Akira S: Ectopic expression of CHOP (GADD153) induces apoptosis
in M1 myeloblastic leukemia cells. FEBS Lett. 395:143–147. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ji C, Mehrian-Shai R, Chan C, Hsu YH and
Kaplowitz N: Role of CHOP in hepatic apoptosis in the murine model
of intra-gastric ethanol feeding. Alcohol Clin Exp Res.
29:1496–1503. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
McCullough KD, Martindale JL, Klotz LO, Aw
TY and Holbrook NJ: Gadd153 sensitizes cells to endoplasmic
reticulum stress by downregulating BCL2 and perturbing the cellular
redox state. Mol Cell Biol. 21:1249–1259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Martinou JC and Green DR: Breaking the
mitochondrial barrier. Nat Rev Mol Cell Biol. 2:63–67. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimer-izes in vivo with a conserved homolog, bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang K and Kaufman RJ: From
endoplasmic-reticulum stress to the inflammatory response. Nature.
454:455–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Suyama K, Ohmuraya M, Hirota M, Ozaki N,
Ida S, Endo M, Araki K, Gotoh T, Baba H and Yamamura K: C/EBP
homologous protein is crucial for the acceleration of experimental
pancreatitis. Biochem Biophys Res Commun. 367:176–182. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Namba T, Tanaka K, Ito Y, Ishihara T,
Hoshino T, Gotoh T, Endo M, Sato K and Mizushima T: Positive role
of CCAAT/enhancer-binding protein homologous protein, a
transcription factor involved in the endoplasmic reticulum stress
response in the development of colitis. Am J Pathol. 174:1786–1798.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sheng R, Liu XQ, Zhang LS, Gao B, Han R,
Wu YQ, Zhang XY and Qin ZH: Autophagy regulates endoplasmic
reticulum stress in ischemic preconditioning. Autophagy. 8:310–325.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tunon MJ, San-Miguel B, Crespo I, Laliena
A, Vallejo D, Alvarez M, Prieto J and Gonzalez-Gallego J: Melatonin
treatment reduces endoplasmic reticulum stress and modulates the
unfolded protein response in rabbits with lethal fulminant
hepatitis of viral origin. J Pineal Res. 55:221–228. 2013.
View Article : Google Scholar : PubMed/NCBI
|