Introduction
Acute pulmonary thromboembolism (PTE), the
obstruction by a thrombus in the pulmonary artery or its branches,
is the most common form of pulmonary embolism (PE) (1). The common pathology of PTE comprises
hemodynamic instability, hypoxia, and pulmonary hypertension, which
may cause heart failure with hypotension (2,3).
PTE is reported to be a complex disease caused by a variety of
factors, including the vascular microenvironment and vascular cell
dysfunction (4,5). During an episode of PTE, a thrombus
trapped in the pulmonary blood vessels injures the vascular
endothelium, causing the unregulated release of pro‑inflammatory
mediators (2,6). Pulmonary vascular remodeling caused
by repeated pulmonary vascular injury may lead to secondary
pulmonary hypertension (7), a
major clinical consequence of PTE, which indicates that endothelial
injury induced by PTE may be key in the pathophysiological
consequences of PTE (8). PTE is a
common disease, which is a contributing factor to the global
non-communicable disease burden (8,9).
Previous studies have identified brain natriuretic
peptide (BNP) and N-terminal pro-BNP (NT-proBNP) in the blood as
biomarkers for predicting echocardiographic right ventricular
dysfunction in patients with acute PTE (10,11). Furthermore, it is reported that
troponin I, D-dimer, and plasma tenascin-C are positively
correlated with the occurrence of PTE, whereas the erythrocyte
sedimentation rate is downregulated in patients with PTE (12). Tang et al identified
interleukin 8 (IL‑8), tumor necrosis factor-α
(TNF‑α), and C‑X‑C motif chemokine ligand 5 as important
factors in PTE in rabbits (13).
In addition, it was revealed that these important genes are
associated with cardiovascular disease, pulmonary disease, immune
disease, and inflammation (13).
However, small chemical molecules targeting differently expressed
genes (DEGs) between the PTE group and the control group did not
screen out. The use of small chemical molecules that target
important disease-related genes has become an effective strategy
for disease treatment (14,15).
In the present study, the GSE84738 dataset
was obtained from the Gene Expression Omnibus (GEO) database.
Following data preprocessing, the DEGs between a PTE group and
control group were identified. Subsequently, the protein‑protein
interaction (PPI) network of these DEGs was visualized using
Cytoscape, and the most significantly clustered modules in the
network were analyzed. Subsequently, functional enrichment analysis
of the DEGs was performed. Furthermore, the chemical‑target
interaction networks were investigated using the Comparative
Toxicogenomics Database (CTD), visualized via Cytoscape. The
present study identified the important genes and their functional
enrichment in relation to the occurrence of PTE, and identified
small chemical molecules potentially useful for PTE treatment.
These findings may provide an important basis to detect mechanisms
underlying PTE and potential treatment methods for PTE.
Materials and methods
Data sources
The RNA expression profiling dataset of the European
rabbit (Oryctolagus cuniculus) was obtained from the GEO
database in the National Center for Biotechnology Information
(http://www.ncbi.nlm.nih.gov/geo/;
accession no. GSE84738) (13).
The dataset included eight samples (four control samples and four
PTE samples), collected from rabbit pulmonary artery tissue. All
samples were assessed, based on the GPL13288 Agilent‑020908
Oryctolagus cuniculus (rabbit) Oligo Microarray platform.
The use of animals was approved by the Animal Ethics Committee of
Affiliated Hospital of Nantong University (Nantong, China).
Screening of DEGs
The original file was downloaded and annotated. In
cases where one gene was detected by multiple probes, the mean
value of the probes was used to represent the expression level. The
gene expression matrix was separated into a control group and a PTE
group, and the DEGs between the two groups were screened according
to the Linear Models for Microarray package (version 3.30.3,
www.bioconductor.org/packages/release/bioc/html/limma.html)
in R software. The P‑values of these DEGs were calculated and
adjusted using an unpaired t-test and Benjamini-Hochberg method,
respectively, at a significance level of P<0.05. Subsequently,
the heatmap in R package, which is used for drawing heat-maps of
gene expression, was utilized to plot the heat map of DEGs
(16). Clustering between the
different samples and different genes was performed using Pearson’s
and Spearman’s correlation coefficients, respectively.
Analysis of the PPI network
The PPIs of the DEGs between the control group and
PTE group were extracted from the Search Tool for the Retrieval of
Interacting Genes/Proteins database (version 10.0, http://www.string‑db.org/) with default
parameters (species: Oryctolagus cuniculus) (17). Those PPIs meeting the combined
score requirement of >0.4 were used to construct the PPI
network, which was then visualized using Cytoscape (version 3.2.0,
http://cytoscape.org/) (18). In the PPI network, each node
represents a protein, and each edge between two nodes represents an
interaction between these two proteins. The degree is defined as
the number of proteins interacting with the node.
Furthermore, the degree centrality, a network
topology index, was used to analyze the scores of nodes in the
network. The nodes with higher degrees are considered to be
important in the PPI network, and may be the key nodes.
Subnet module analysis
Genes often have a regulatory role by interacting
with other cellular components. Proteins produced by genes in the
same module tend to have the same function or similar functions,
and where they act as a module they may have the same biological
role. The most significantly clustered modules in the network were
analyzed using the Multi Contrast Delayed Enhancement plugin of
Cytoscape (19).
GO and Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathway analysis of regulatory network modules
In order to examine the gene function and pathways
related to the DEGs, the Database for Annotation Visualization and
Integrated Discovery (DAVID) online tool (version 6.8, https://david‑d.ncifcrf.gov/) (20) was used to perform the Gene
Ontology (GO) functional and pathway enrichment analyses of DEGs
based on the GO database (20)
and KEGG pathway database (21).
Only function terms with an adjusted P‑value of <0.05 and count
≥2 were considered as significant.
Analysis of disease‑related chemistry
small molecule‑target gene interaction network
The CTD, a publicly available database (http://ctdbase.org/), provides manually curated
information on chemical-disease, gene-disease associations and
chemical‑gene/protein interactions. Based on PE‑related
chemical-gene interaction data provided by the CTD (22), the chemical‑gene interaction
association pairs were visualized via Cytoscape. Chemicals
interacting with a higher number of DEGs may be more important in
this disease.
Results
Identification of DEGs
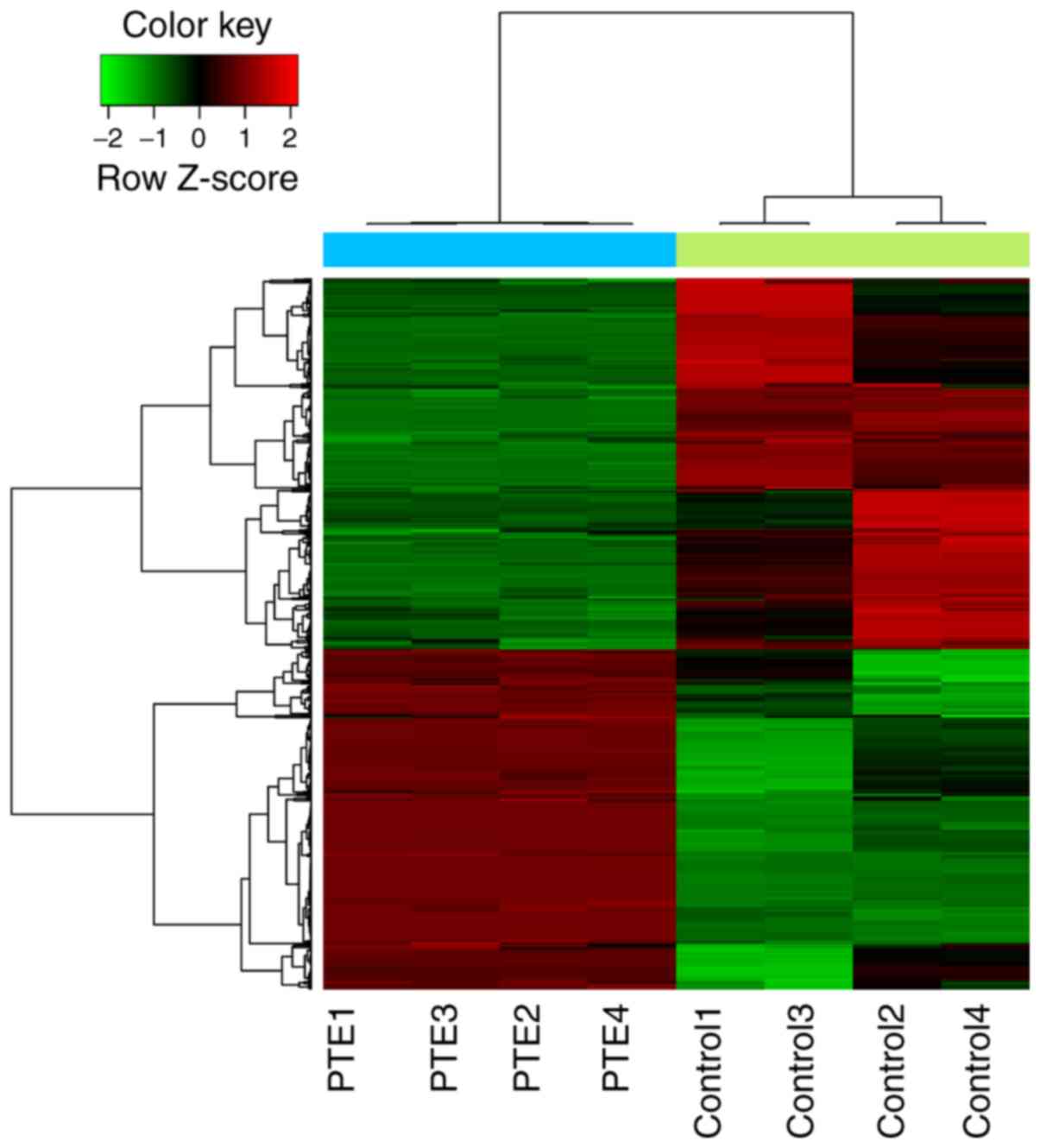
A total of 548 (262 upregulated and 286
downregulated DEGs) were identified in the PTE group, compared with
the control group. The results of the hierarchical clustering heat
map of DEGs showed that these DEGs were clearly distinguishable
between the different sample types (Fig. 1).
Functional enrichment analysis
The upregulated DEGs were significantly enriched in
27 KEGG pathways, 51 GO biological processes (BPs), 27 GO cellular
components (CCs) and 10 GO molecular functions (MFs). The
downregulated genes were significantly enriched in 11 KEGG
pathways, 15 GO BPs, 14 GO CCs, and 11 GO MFs. The enriched results
are presented in Table I. The
upregulated DEGs were largely enriched in the phagosome
(P=5.51E-09) and hematopoietic (P=4.39E-07, e.g. TNF) cell
lineages in the KEGG pathways, and the downregulated DEGs were
enriched in the arrhythmogenic right ventricular cardiomyopathy
(P=5.12E‑05) and calcium signaling (P=2.26E‑04) pathways in the
KEGG pathways (Table I).
Interleukin‑6 production (P=4.02E-07, e.g. TNF) in GO BP,
external side of plasma membrane (P=1.46E-05, e.g. TNF),
NADPH oxidase complex (P=2.08E‑05) in GO CC, and low‑density
lipoprotein particle binding (P=6.06E‑06) in MF were the
upregulated genes, and were enriched among the abovementioned GO
terms (Fig. 2A). The
downregulated genes were largely enriched in lens development in
camera-type eye (P=2.40E-05), sarcolemma (P=5.93E-05), and
structural constituent of eye lens (P=1.01E-08) (Fig. 2B).
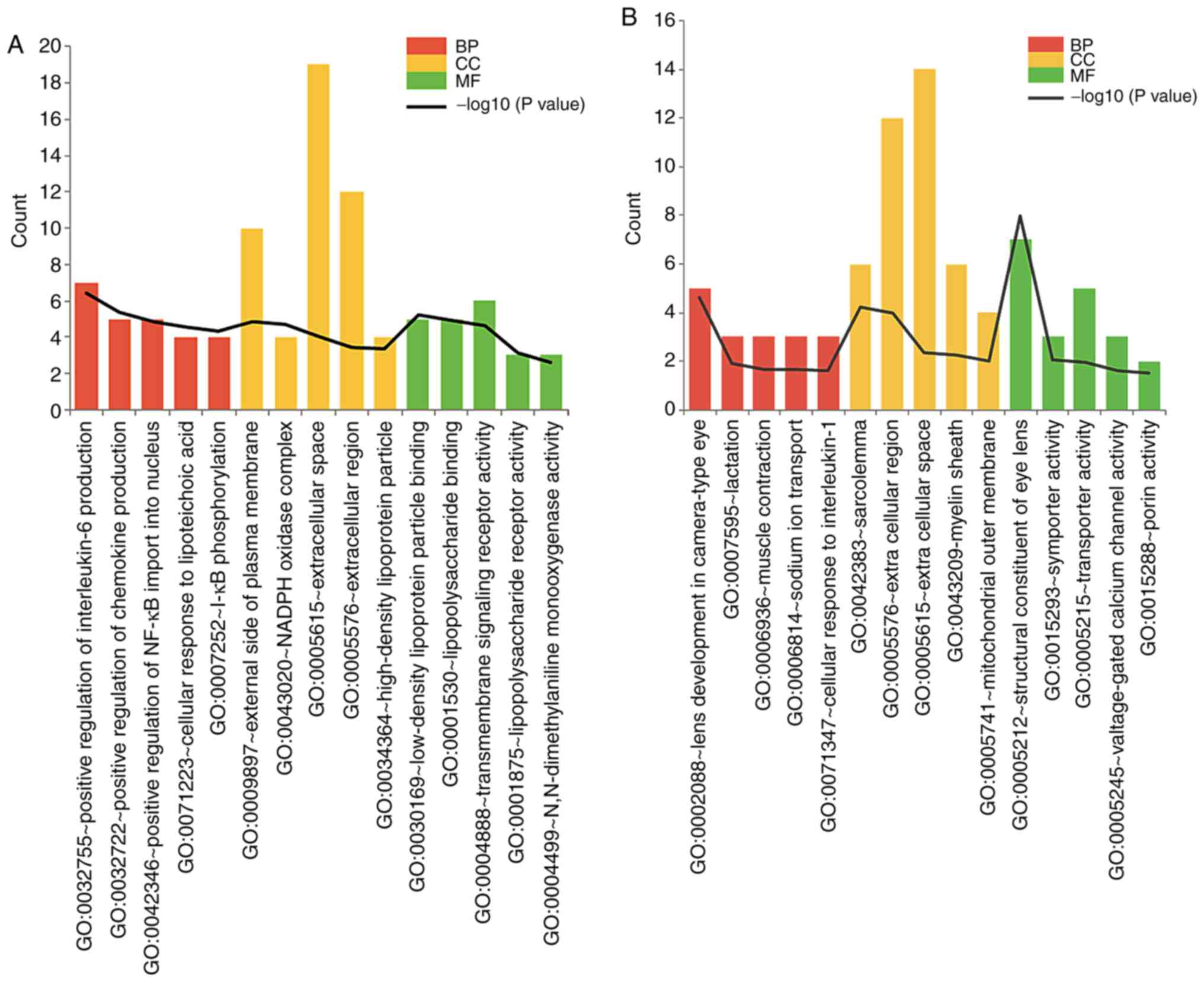 | Figure 2Top GO terms enriched in DEGs. Top 5
GO terms enriched in (A) upregulated and (B) downregulated DEGs.
Red, yellow, and green boxes represent GO BP, CC, and MF,
respectively. GO, gene ontology; DEGs, differentially expressed
genes; BP, biological process; CC, cellular component; MF,
molecular function. |
 | Table ITop 10 Kyoto Encyclopedia of Genes
and Genomes pathways enriched by differentially expressed
genes. |
Table I
Top 10 Kyoto Encyclopedia of Genes
and Genomes pathways enriched by differentially expressed
genes.
| Pathway ID | Pathway name | Count | P‑value |
|---|
| Upregulated | | | |
| ocu04145 | Phagosome | 15 | 5.51E-09 |
| ocu04640 | Hematopoietic cell
lineage | 10 | 4.39E-07 |
| ocu05323 | Rheumatoid
arthritis | 10 | 1.99E-06 |
| ocu05140 | Leishmaniasis | 9 | 4.03E-06 |
| ocu05162 | Measles | 9 | 1.80E-04 |
| ocu05142 | Chagas disease
(American trypanosomiasis) | 8 | 3.94E-04 |
| ocu04620 | Toll‑like receptor
signaling pathway | 7 | 8.54E‑04 |
| ocu05133 | Pertussis | 6 | 2.08E-03 |
| ocu05146 | Amoebiasis | 7 | 2.30E-03 |
| ocu05144 | Malaria | 5 | 3.61E-03 |
| Downregulated | | | |
| ocu05412 | Arrhythmogenic
right ventricular cardiomyopathy | 7 | 5.12E-05 |
| ocu04020 | Calcium signaling
pathway | 9 | 2.26E‑04 |
| ocu05410 | Hypertrophic
cardiomyopathy | 6 | 1.24E-03 |
| ocu05414 | Dilated
cardiomyopathy | 6 | 1.66E-03 |
| ocu04080 | Neuroactive
ligand-receptor interaction | 9 | 2.90E-03 |
| ocu04022 | cGMP‑PKG signaling
pathway | 7 | 4.86E‑03 |
| ocu04261 | Adrenergic
signaling in cardiomyocytes | 6 | 1.11E-02 |
| ocu04066 | HIF‑1 signaling
pathway | 5 | 1.20E‑02 |
| ocu05416 | Viral
myocarditis | 4 | 2.74E-02 |
| ocu04151 | PI3K‑Akt signaling
pathway | 8 | 2.75E‑02 |
PPI network and module analysis
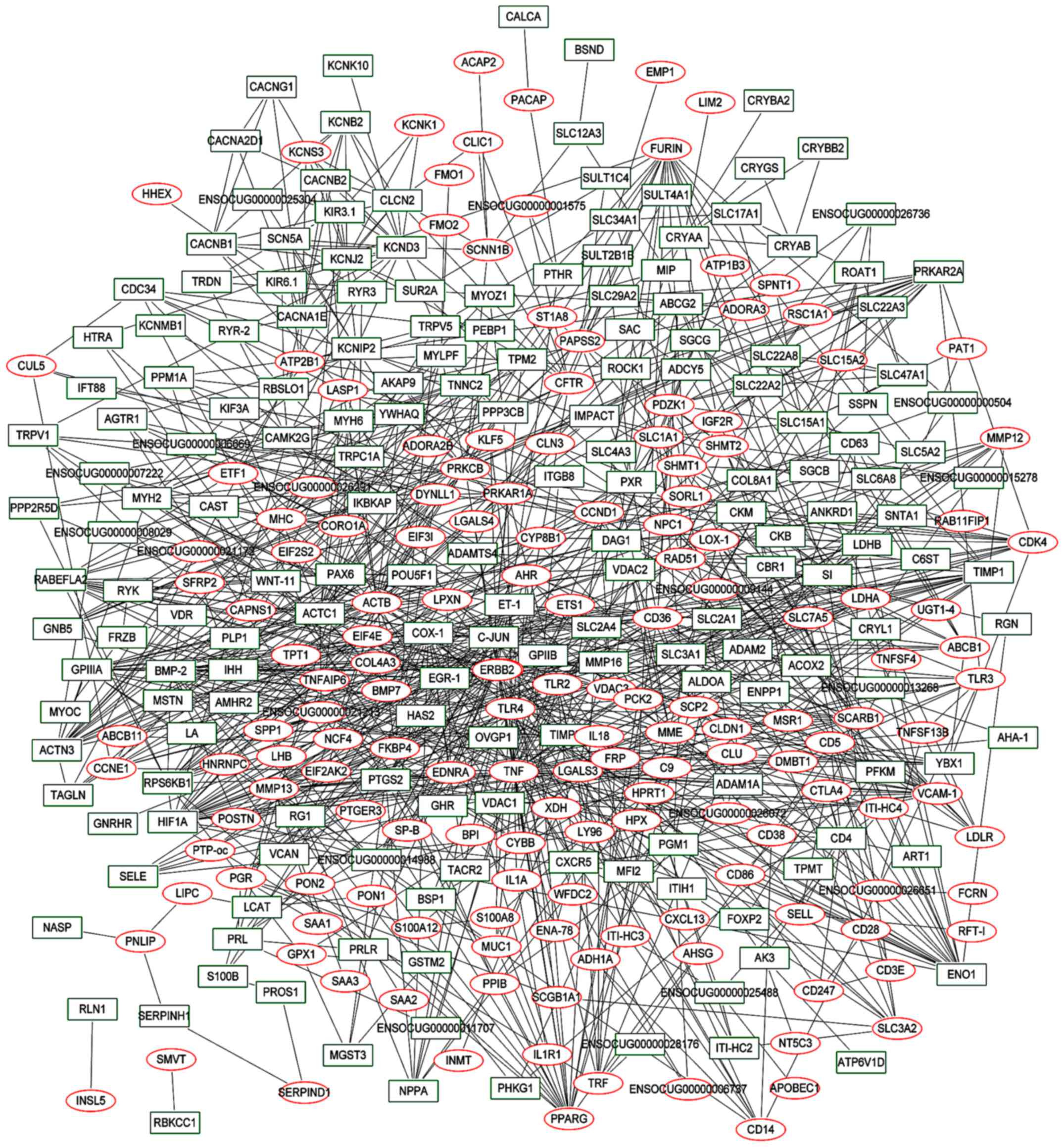
A total of 342 nodes and 1,128 interactional pairs
were included in the PPI network. Among them, 10 proteins had
>25 degrees (Fig. 3).
TNF and ERBB2 were the upregulated genes with the
highest degree in the PPI network, with 47 and 40 degrees
respectively. C‑JUN was the downregulated gene with the
highest degree of 46. Furthermore, C‑JUN was an interacting
protein of TNF and ERBB2.
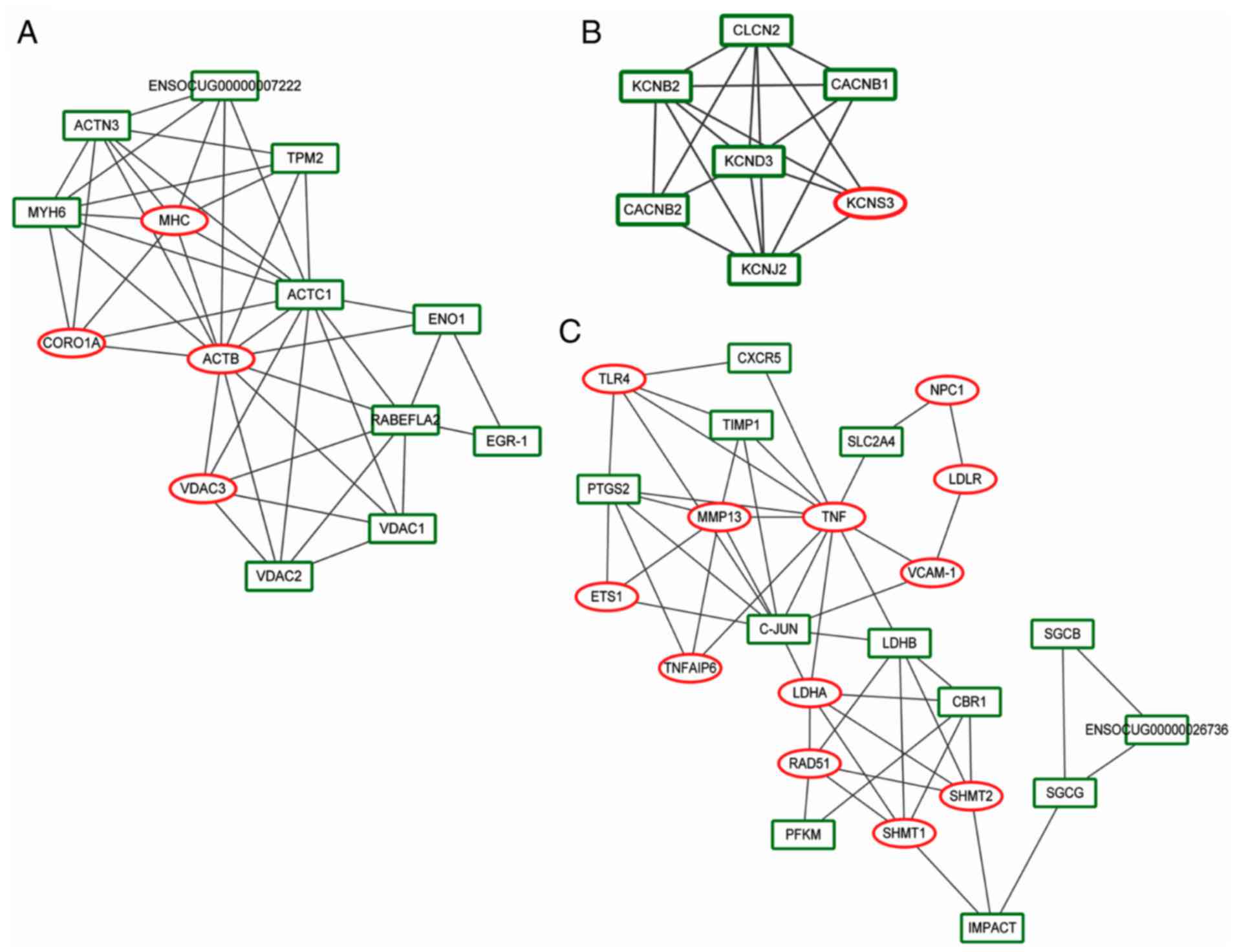
In total, three subnet modules (A‑C) were identified
with a score >4. Module A (score=6.769) included 14 nodes (e.g.
actin β) and 44 interactional pairs (Fig. 4A). Module B (score=6) had seven
nodes (e.g. potassium voltage-gated channel subfamily B member 2)
and 18 interactional pairs (Fig.
4B). Module C (score=4.435) included 24 nodes (e.g. TNF
and C‑JUN) and 51 interactional pairs (Fig. 4C). Genes in module A were most
enriched in cardiac muscle contraction (P=3.97E-03), hypertrophic
cardiomyopathy (P=4.79E-03) and dilated cardiomyopathy
(P=5.45E-03). Genes in module B were most enriched in the oxytocin
signaling pathway (P=1.33E‑03), whereas biosynthesis of antibiotics
in the KEGG pathways (P=1.61E‑03) was enriched in module C
genes.
PTE‑related chemical‑small
molecule‑target gene inter‑ action network
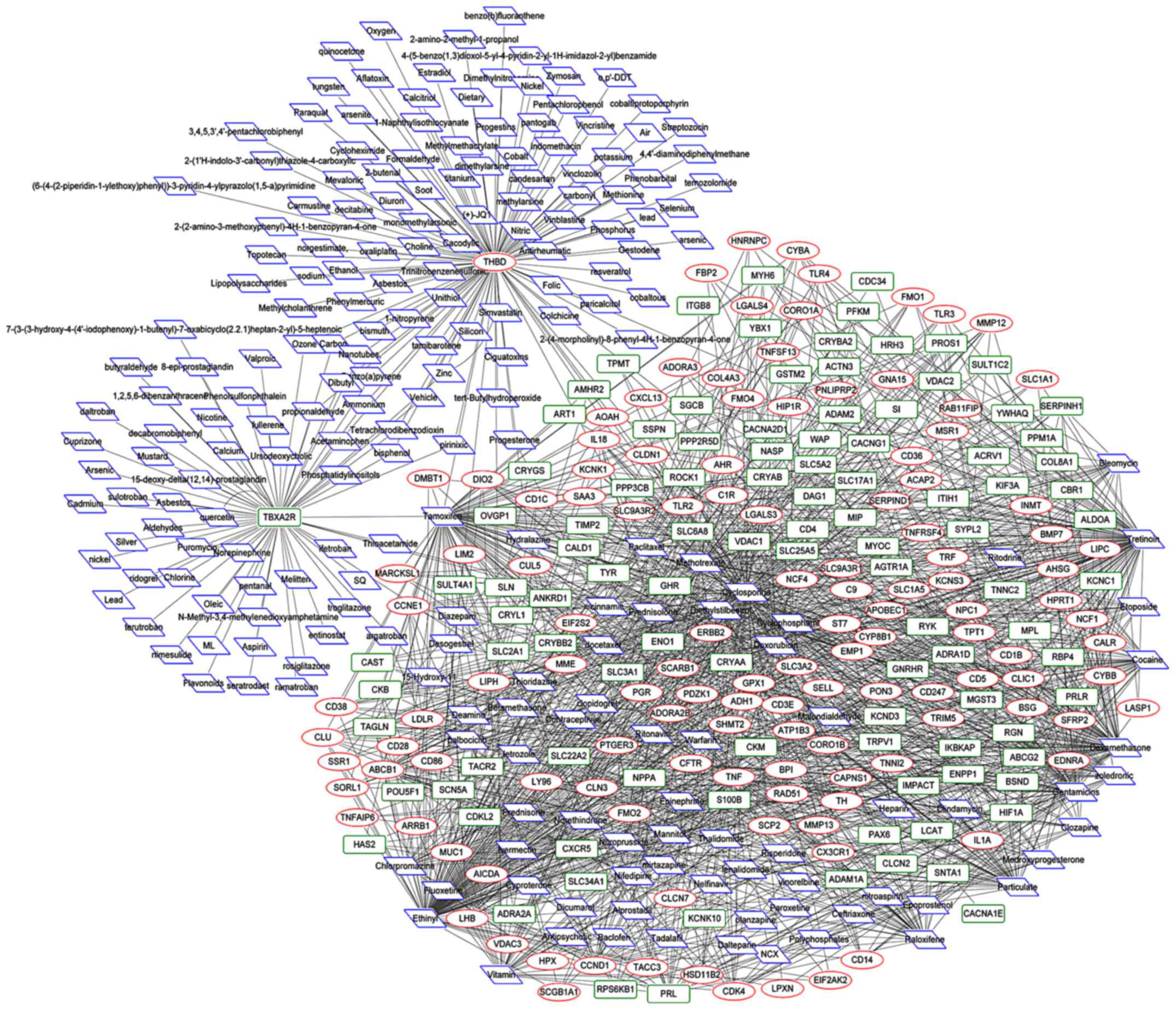
In the CTD database, 50,719 PE-related
chemical‑target interactions, including 1,537 DEG‑chemical
interactions, were detected (Fig.
5). A total of 1,537 interactional pairs, 127 upregulated DEGs,
105 downregulated DEGs, and 117 chemicals, including four with a
degree of >100 [ethinyl (135), cyclosporine (126),
thrombomodulin precursor (THBD) (113) and tretinoin (111)], were
found in the DEG‑chemical interaction network. Among them, ethinyl
targeted TNF, whereas ERBB2 were targeted by
cyclosporine. In addition to THBD being an upregulated gene,
it was an important chemical interacting with ethinyl, cyclosporine
and tretinoin. The chemical tretinoin targeted ERRB2
(Table II).
 | Table IITop five Kyoto Encyclopedia of Genes
and Genomes pathways enriched by differentially expressed genes in
the top 10 degrees and modules. |
Table II
Top five Kyoto Encyclopedia of Genes
and Genomes pathways enriched by differentially expressed genes in
the top 10 degrees and modules.
| Pathway ID | Pathway name | Count | P‑value | Genes |
|---|
| Degree top 10 | | | | |
| ocu05205 | Proteoglycans in
cancer | 5 | 7.67E-06 | ACTB, HIF1A, TNF,
ERBB2, TLR4 |
| ocu05140 | Leishmaniasis | 3 | 1.53E‑03 | TNF, PTGS2,
TLR4 |
| ocu04064 | NF-κB signaling
pathway | 3 | 1.69E‑03 | TNF, PTGS2,
TLR4 |
| ocu05410 | Hypertrophic
cardiomyopathy | 3 | 2.04E-03 | ACTB, ACTC1,
TNF |
| ocu05414 | Dilated
cardiomyopathy | 3 | 2.33E-03 | ACTB, ACTC1,
TNF |
| Module A | | | | |
| ocu04260 | Cardiac muscle
contraction | 3 | 3.97E-03 | ACTC1, MYH6,
TPM2 |
| ocu05410 | Hypertrophic
cardiomyopathy | 3 | 4.79E-03 | ACTB, ACTC1,
TPM2 |
| ocu05414 | Dilated
cardiomyopathy | 3 | 5.45E-03 | ACTB, ACTC1,
TPM2 |
| ocu04530 | Tight junction | 3 | 1.08E-02 | ACTB, MYH6,
ACTN3 |
| ocu04261 | Adrenergic
signaling in cardiomyocytes | 3 | 1.29E-02 | ACTC1, MYH6,
TPM2 |
| Module B | | | | |
| ocu04921 | Oxytocin signaling
pathway | 3 | 1.33E‑03 | CACNB1, CACNB2,
KCNJ2 |
| ocu05412 | Arrhythmogenic
right ventricular cardiomyopathy | 2 | 2.94E-02 | CACNB1, CACNB2 |
| ocu04260 | Cardiac muscle
contraction | 2 | 3.22E-02 | CACNB1, CACNB2 |
| ocu05410 | Hypertrophic
cardiomyopathy | 2 | 3.53E-02 | CACNB1, CACNB2 |
| ocu05414 | Dilated
cardiomyopathy | 2 | 3.77E-02 | CACNB1, CACNB2 |
| Module C | | | | |
| ocu01130 | Biosynthesis of
antibiotics | 5 | 1.61E‑03 | SHMT1, LDHB, LDHA,
SHMT2, PFKM |
| ocu00010 |
Glycolysis/gluconeogenesis | 3 | 1.07E‑02 | LDHB, LDHA,
PFKM |
| ocu01230 | Biosynthesis of
amino acids | 3 | 1.25E‑02 | SHMT1, SHMT2,
PFKM |
| ocu05140 | Leishmaniasis | 3 | 1.29E‑02 | TNF, PTGS2,
TLR4 |
| ocu04064 | NF-κB signaling
pathway | 3 | 1.42E‑02 | TNF, PTGS2,
TLR4 |
Discussion
The findings of the present study demonstrated that
TNF and ERBB2 were upregulated, and C‑JUN was
the main downregulated gene in the PTE group. Additionally,
ethinyl, cyclosporine, and tretinoin may have significant effects
in the treatment of PTE. Among them, TNF was targeted by
ethinyl and cyclosporine, ERBB2 was targeted by cyclosporine
and tretinoin, and C‑JUN was targeted by TNF and
ERBB2.
TNF is a multifunctional pro‑inflammatory
cytokine produced by macrophages. Previous studies have indicated
that inflammation is associated with the occurrence of PTE
(23). Due to stimulation caused
by hypoxia and trauma during PTE, inflammation may trigger
pulmonary platelet activation and endothelial dysfunction (24). Additionally, systemic inflammatory
and leukocytosis responses are prognostic factors for mortality
following 30 days of PTE (25).
In addition, pro‑inflammatory factors, including TNF and
IL‑6, are significantly correlated with PTE mortality rate
(26,27). Consistent with previous studies,
the results of the present study suggested that, compared with the
control group, TNF was upregulated in the PTE group, and
that it was mainly enriched in the hematopoietic cell lineage,
positive regulation of interleukin‑6 production, and external side
of the plasma membrane. Furthermore, it was targeted by small
chemical molecules, including ethinyl and cyclosporine. Ethinyl is
a contraceptive, although oral ethinyl increases the risk of venous
thromboembolisms including PTE (28,29). Cyclosporine is a calcineurin
inhibitor (CNI), which is beneficial for reducing the incidence of
complications during homologous transplantation, including vascular
toxicity (30). CNIs induce
proinflammatory cytokine production and endothelial activation in
the isolated mouse aorta and vascular smooth muscle cells (31). Cyclosporine also increases the
mRNA levels of proinflammatory cytokines, including TNF‑α,
IL‑6, C‑C motif chemokine ligand 5 (CCL5), and
CCL2, which are involved in vascular injury (31). Consistent with previous studies,
the results of the present study indicated that TNF was
targeted by cyclosporine. Cyclosporine promotes
inflammation‑induced monocyte adhesion to human intestinal
endothelial cells (32).
Therefore, ethinyl and cyclosporine may increase the incidence of
PTE via targeting TNF and promoting the onset of
inflammation.
Cyclosporine is also targeted by ERBB2, which
is a member of the epidermal growth factor receptor family.
ERBB2 is increased in patients with cancer, including breast
and ovarian cancer (33). In
breast cancer, ERBB2 is important in regulating oncogenic
microRNA expression (34).
ERBB2 is also a potent independent predictor of the
metastatic potential of breast cancer cells (35). However, few studies appear to have
investigated the role of ERBB2 in PTE. It is reported that
breast cancer is closely associated with the development of
vascular emboli (36). In the
present study, ERBB2 was the important upregulated gene in
the PTE group, as it had a higher degree in the PPI network, and it
was a target of the small chemical molecules, cyclosporine and
tretinoin. Tretinoin, a metabolite of retinol, can repair lung
tissue in pulmonary emphysema model rats (37). The role of tretinoin in promoting
alveolar regeneration involves the regulation of vascular
endothelial growth factor (VEGF), VEGF receptor 2, and
matrix metallo-proteinase 1 (38). Therefore, tretinoin may reduce the
degree of lung injury in PTE by downregulating ERBB2.
Cyclosporine may contribute to the occurrence of PTE via targeting
ERBB2 and TNF, thereby regulating the expression of
C‑JUN.
In humans, C-JUN is a protein encoded by JUN.
C-JUN in combination with C‑FOS, forms the early response
transcription factor, activator protein 1 (39). In addition, C-JUN cooperates with
nuclear factor‑κB to prevent apoptosis induced by TNF-α. C-JUN can
also protect hepatocytes from apoptosis, as hepatocytes lacking
C‑JUN show increased sensitivity to TNF-α‑induced apoptosis
(40). However, the role of C-JUN
in PTE has not been reported. As stated above, pro‑inflammatory
factors, including TNF and IL‑6, show a significant
correlation with PTE mortality rate. In addition, β4 integrin forms
a complex with ERBB2 and enhances activation of the transcription
factors signal transducer and activator of transcription 3 and
C-JUN (41). C-JUN is also
necessary for ERBB2-mediated hyperproliferation. Therefore,
C‑JUN may regulate TNF and ERBB2.
However, the results of the present study have not
been experimentally validated, which is a limiting factor that
requires resolution in the future. In addition, the regulation of
small chemical molecules by PTE-related gene expression was not
investigated. The sample size used in the present study was also
small (four control and four PTE pulmonary artery samples).
In conclusion, the findings of the present study
indicate that small chemical molecules, cyclosporine and ethinyl,
may trigger PTE by regulating the expression of TNF and
ERBB2. Furthermore, tretinoin may delay the progression of
PTE via targeting ERBB2. Taken together, cyclosporine,
ethinyl, and tretinoin may be promising potential targets for PTE
treatment.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Shanghai
Medical Key Subject Construction Project (grant no. ZK2015B15), the
Shanghai Weak Discipline Construction Plan of Shanghai Municipal
Commission of Health and Family Planning (grant no. 2016ZB0202),
the Minhang District Natural Science Research Project (grant no.
2017MHZ56) and the Shanghai Minhang District Center Hospital Grade
Project (grant no. 2016MHJC10).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
KS provided the conception and design of the study.
ZX and CQ acquired the data. JW, YL and ML analyzed and interpreted
the data and performed the statistical analysis. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The use of animals was approved by the Animal Ethics
Committee of Affiliated Hospital of Nantong University (Nantong,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
PTE
|
pulmonary thromboembolism
|
|
PE
|
pulmonary embolism
|
|
BNP
|
brain natriuretic peptide
|
|
NT‑proBNP
|
N‑terminal pro‑BNP
|
|
DEGs
|
differently expressed genes
|
|
GEO
|
Gene Expression Omnibus
|
|
PPI
|
protein‑protein interaction
|
|
CTD
|
Comparative Toxicogenomics
Database
|
|
GEO
|
Gene Expression Omnibus
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
BP
|
biological process
|
|
CC
|
cellular component
|
|
MF
|
molecular function
|
|
HCM
|
hypertrophic cardiomyopathy
|
|
CNIs
|
calcineurin inhibitors
|
References
|
1
|
Santhakumar R, Ramalingam PK, Gayathri K,
Manjunath BV, Karuppusamy N, Vetriveeran B, Selvamani S, Vishnuram
P and Natarajan K: Pulmonary thromboembolism presenting as multiple
pulmonary cavities. J Assoc Physicians India. 64:85–86.
2016.PubMed/NCBI
|
|
2
|
Wood KE: Major pulmonary embolism: Review
of a pathophysiologic approach to the golden hour of
hemodynamically significant pulmonary embolism. Chest.
121:8779052002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kostadima E and Zakynthinos E: Pulmonary
embolism: Pathophysiology, diagnosis, treatment. Hellenic J
Cardiol. 48:94–107. 2007.PubMed/NCBI
|
|
4
|
Evans CE, Humphries J, Mattock K, Waltham
M, Wadoodi A, Saha P, Modarai B, Maxwell PH and Smith A: Hypoxia
and upregulation of hypoxia-inducible factor 1{alpha} stimulate
venous thrombus recanalization. Arterioscler Thromb Vasc Biol.
30:2443–2451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yılmaz M: Evaluation of Tp‑e interval,
Tp‑e/qt ratio and Tp‑e/Qtc ratio in patients with acute pulmonary
thromboembolism. Am J Cardiol. 121:e192018. View Article : Google Scholar
|
|
6
|
Battistini B: Modulation and roles of the
endothelins in the pathophysiology of pulmonary embolism. Canad J
Physiol Pharmacol. 81:555–569. 2003. View
Article : Google Scholar
|
|
7
|
Kimura H, Okada O, Tanabe N, Tanaka Y,
Terai M, Takiguchi Y, Masuda M, Nakajima N, Hiroshima K, Inadera H,
et al: Plasma monocyte chemoattractant protein-1 and pulmonary
vascular resistance in chronic thromboembolic pulmonary
hypertension. Am J Respir Crit Care Med. 164:319–324. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wan J, Lu LJ, Miao R, Liu J, Xu XX, Yang
T, Hu QH, Wang J and Wang C: Alterations of bone marrow‑derived
endothelial progenitor cells following acute pulmonary embolism in
mice. Exp Biol Med (Maywood). 235:989–998. 2010. View Article : Google Scholar
|
|
9
|
Raskob GE, Angchaisuksiri P, Blanco AN,
Buller H, Gallus A, Hunt BJ, Hylek EM, Kakkar A, Konstantinides SV,
McCumber M, et al: Thrombosis: A major contributor to global
disease burden. Arterioscler Thromb Vasc Biol. 34:2363–2371. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pieralli F, Olivotto I, Vanni S, Conti A,
Camaiti A, Targioni G, Grifoni S and Berni G: Usefulness of bedside
testing for brain natriuretic peptide to identify right ventricular
dysfunction and outcome in normotensive patients with acute
pulmonary embolism. Am J Cardiol. 97:1386–1390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kostrubiec M, Pruszczyk P, Bochowicz A,
Pacho R, Szulc M, Kaczynska A, Styczynski G, Kuch‑Wocial A,
Abramczyk P, Bartoszewicz Z, et al: Biomarker‑based risk assessment
model in acute pulmonary embolism. Eur Heart J. 26:2166–2172. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Babaoglu E, Hasanoglu HC, Senturk A,
Karalezli A, Kilic H, Aykun G and Oztuna D: Importance of
biomarkers in risk stratification of pulmonary thromboembolism
patients. J Investig Med. 62:328–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang Z, Wang X, Huang J, Zhou X, Xie H,
Zhu Q, Huang M and Ni S: Gene expression profiling of pulmonary
artery in a rabbit model of pulmonary thromboembolism. PLoS One.
11:e01645302016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He X, Zhang Z, Zhang Q and Yuan G:
Selective recognition of G‑quadruplex in the vascular endothelial
growth factor gene with small‑molecule natural products by
electrospray ionization (ESI) mass spectrometry and circular
dichroism (CD) spectrometry. Canad J Chem. 90:55–59. 2012.
View Article : Google Scholar
|
|
15
|
An R, Hagiya Y, Tamura A, Li S, Saito H,
Tokushima D and Ishikawa T: Cellular phototoxicity evoked through
the inhibition of human ABC transporter ABCG2 by cyclin‑dependent
kinase inhibitors in vitro. Pharm Res. 26:449–458. 2009. View Article : Google Scholar
|
|
16
|
Wang L, Cao C, Ma Q, Zeng Q, Wang H, Cheng
Z, Zhu G, Qi J, Ma H, Nian H and Wang Y: RNA‑seq analyses of
multiple meristems of soybean: Novel and alternative transcripts,
evolutionary and functional implications. BMC Plant Biol.
14:1692014. View Article : Google Scholar
|
|
17
|
Szklarczyk D, Franceschini A, Kuhn M,
Simonovic M, Roth A, Minguez P, Doerks T, Stark M, Muller J, Bork
P, et al: The STRING database in 2011: Functional interaction
networks of proteins, globally integrated and scored. Nucleic Acids
Res. 39(Database Issue): D561–D568. 2011. View Article : Google Scholar :
|
|
18
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:249825042003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bader GD and Hogue CW: An automated method
for finding molecular complexes in large protein interaction
networks. BMC Bioinformatics. 4:22003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar
|
|
22
|
Davis AP, Grondin CJ, Lennon‑Hopkins K,
Saraceni‑Richards C, Sciaky D, King BL, Wiegers TC and Mattingly
CJ: The comparative toxicogenomics database’s 10th year
anniversary: Update 2015. Nucleic Acids Res. 43(Database Issue):
D914–D920. 2015. View Article : Google Scholar
|
|
23
|
Yan C, Wang X, Su H and Ying K: Recent
progress in research on the pathogenesis of pulmonary
thromboembolism: An old story with new perspectives. Biomed Res
Int. 2017.6516791:2017.
|
|
24
|
Wrobel JP, Thompson BR and Williams TJ:
Mechanisms of pulmonary hypertension in chronic obstructive
pulmonary disease: A pathophysiologic review. J Heart Lung
Transplant. 31:5572012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jo JY, Lee MY, Lee JW, Rho BH and Choi WI:
Leukocytes and systemic inflammatory response syndrome as
prognostic factors in pulmonary embolism patients. BMC Pulm Med.
13:742013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Halici B, Sarinc US, Günay E, Nural S, Sen
S, Akar O, Celik S and Unlu M: Assessment of inflammatory
biomarkers and oxidative stress in pulmonary thromboembolism:
Follow‑up results. Inflammation. 37:1186–1190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marchena Yglesias PJ, Nieto Rodríguez JA,
Serrano Martínez S, Belinchón Moya O, Cortés Carmona A, Díaz de
Tuesta A, Bruscas Alijarde MJ and Ruiz Ribó MD: Acute-phase
reactants and markers of inflammation in venous thromboembolic
disease: Correlation with clinical and evolution parameters. An Med
Interna. 23:105–110. 2006.In Spanish. PubMed/NCBI
|
|
28
|
Santosa F, Moerchel C, Berg C and Kröger
K: Disproportional increase of pulmonary embolism in young females
in Germany: Trends from 2005 to 2014. J Thromb Thrombolysis.
43:417–422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bird ST, Delaney JA, Etminan M, Brophy JM
and Hartzema AG: Drospirenone and non-fatal venous thromboembolism:
Is there a risk difference by dosage of ethinyl‑estradiol. J Thromb
Haemost. 11:1059–1068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang X, Sung YK, Tian W, Qian J, Semenza
GL and Nicolls MR: Graft microvascular disease in solid organ
transplantation. J Mol Med (Berl). 92:797–810. 2014. View Article : Google Scholar
|
|
31
|
Rodriguesdiez R, Gonzálezguerrero C,
Ocañasalceda C, Rodriguesdiez RR, Egido J, Ortiz A, Ruizortega M
and Ramos AM: Calcineurin inhibitors cyclosporine A and tacrolimus
induce vascular inflammation and endothelial activation through
TLR4 signaling. Sci Rep. 6:279152016. View Article : Google Scholar
|
|
32
|
Rafiee P, Johnson CP, Li MS, Ogawa H,
Heidemann J, Fisher PJ, Lamirand TH, Otterson MF, Wilson KT and
Binion DG: Cyclosporine a enhances leukocyte binding by human
intestinal microvascular endothelial cells through inhibition of
p38 MAPK and iNOS. Paradoxical proinflammatory effect on the
micro-vascular endothelium. J Biol Chem. 277:35605–35615. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chavez‑Blanco A, Perez‑Sanchez V,
Gonzalez‑Fierro A, Vela‑Chavez T, Candelaria M, Cetina L, Vidal S
and Dueñas‑Gonzalez A: ER2 expression in cervical cancer as a
potential therapeutic target. BMC Cancer. 4:592004. View Article : Google Scholar
|
|
34
|
Balwierz AK: ERBB2 as a driver of an
invasive phenotype of cells grown in 3D culture and an important
regulator of oncogenic miRNAs’ expression in breast cancer.
2017.
|
|
35
|
Ahmed AR: HER2 expression is a strong
independent predictor of nodal metastasis in breast cancer. J Egypt
Natl Cancer Inst. 28:219–227. 2016. View Article : Google Scholar
|
|
36
|
Zhou X, De Luise C, Shen R, Bate A and
Gatto N: Incidence of pulmonary embolism among postmenopausal (pm)
women with Er+/HER2− breast cancer.
Pharmacoepidemiol Drug Safety. 24:372–373. 2015.
|
|
37
|
Massraro GD and Massaro D: Retinoic acid
treatment abrogates elastase-induced pulmonary emphysema in rats.
Nat Med. 3:675–677. 1997. View Article : Google Scholar
|
|
38
|
Jian‑Lin XU, Fan XY, Guo YL, Jin MA and
Wang WM: Effects of all-trans-retinoic acid on emphysema model in
rats. Chin J New Drugs Clin Remed. 29:109–114. 2010.In Chinese.
|
|
39
|
Faubert BL and Kaminski NE: AP‑1 activity
is negatively regulated by cannabinol through inhibition of its
protein components, c‑fos and c‑jun. J Leukoc Biol. 67(259):
2011–266. 2000. View Article : Google Scholar
|
|
40
|
Eferl R, Ricci R, Kenner L, Zenz R, David
JP, Rath M and Wagner EF: Liver tumor development. c‑Jun
antagonizes the proapoptotic activity of p53. Cell. 112:181–192.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo W, Pylayeva Y, Pepe A, Yoshioka T,
Muller WJ, Inghirami G and Giancotti FG: Beta 4 integrin amplifies
ErbB2 signaling to promote mammary tumorigenesis. Cell.
126:489–502. 2006. View Article : Google Scholar : PubMed/NCBI
|