Introduction
Respiratory diseases have been considered to
contribute to the global burden of disease in the last two decades.
Lung diseases that have attributed to global mortality rates
include lower respiratory infection, chronic obstructive pulmonary
disease (COPD), lung cancer, tuberculosis and pneumonia infection.
According to the World Health Organization, by 2030, morbidity
rates due to respiratory diseases may increase based on the rise of
air pollution, chronic respiratory problems and respiratory
infections associated with all health-related issues worldwide.
Despite an alarming increase in this issue worldwide, the
complexity of chronic airway or lung inflammation remains a serious
limitation in establishing a clear understanding of such diseases
(1).
Lung inflammation is usually caused by pathogens or
by exposure to toxins, pollutants, irritants, and allergens. Acute
lung inflammation includes pneumonia and acute respiratory distress
syndrome; whereas chronic inflammation includes asthma and COPD
(2). In laboratory animals, lung
inflammation is detected by the presence of inflammatory markers,
including immune cells and cytokines, in the bronchoalveolar lavage
fluid of sacrificed animals (3).
The respiratory system comes into direct contact with a low level
of lipopolysaccharide (LPS) present as a contaminant in airborne
particles. Exposure to LPS results in several characteristics of
lung inflammation, including the upregulation of myosin light chain
phosphorylation and airway epithelial barrier permeability with
increasing levels of albumin, myeloperoxidase activity and
infiltration of neutrophils (4),
and can cause acute lung injury due to bilateral alveolar
infiltration, lung edema and respiratory failure. Therefore, a
better understanding of the pathogens or stress associated with
lung inflammation and potential targets for treatment which can
maintain lung homeostasis, is always dependent on the
characteristics of cells or tissue involvement.
Fibroblasts, which are traditionally recognized as
quiescent cells responsible for extracellular matrix production,
are known to be actively involved the immune system (5). In chronic lung diseases, the number
and phenotypes of fibroblasts cells are altered as they are able to
modulate the immune response, however, any impairment in immune
regulation involves the disruption of controlling activity and
leads to inflammation and damage (6). Previous studies have implicated
fibroblasts as cells which contribute to disease persistence and
define anatomical location. Therefore, fibroblasts are an
attractive therapeutic target as they assist in orchestrating the
infiltration of inflammatory agents (7). Essential oils, including hinoki
cypress leaf oil, have long been used as commercialized products,
including fragrances (8), air
purifiers and human-safe insect repellent (9).
The physiological activities of phytoncide extract
include anti-oxidant, antimicrobial, insecticidal and antifungal
properties (10). It can also be
used as an alternative antibiotic in weaning pigs via dietary
supplementation (11). The forest
bathing of phytoncide volatile oil has shown to increase natural
killer (NK) cells (12), decrease
stress hormones (13) and even
induce physiological relaxation through olfactory stimulation in
humansin aromatherapy (8).
Monoterpenes of plant essential oils exert several antitumor
activities (14), whereas
monoterpene 1.8-cineole of Eucalyptus globulus oil (15) and tymol, carvacrol,
p-Cymene from Lippia sidoides leaves (16,17) have shown inflammatory activities
in vivo. Chamaecyparis obtusa (C. obtusa)
essential oil extract has shown to have an anti-inflammatory effect
against paw edema and peritonitis (18). However, cypress leaf essential
oil-derived terpenes have not been examined in vitro or
in vivo for their potential in attenuating inflammatory
mediators in lung inflammation.
Therefore, the present study investigated the
anti-inflammatory effect of hinoki cypress leaf extracted essential
oil in LPS-stimulated WI38 fibroblast cells by inhibiting the
nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB)
pathway and its ability to enhance the lung tissue capacity of
Sprague-Dawley rats.
Materials and methods
Plant material and preparation of leaf
essential oil
Shredded and frozen hinoki (C. obtusa) leaves
(<1 cm in length) were kindly provided by Napoli Co., Ltd.
(Tonyoung, South Korea) in May 2017. The leaves (~600 g) were
placed in a 2 l round-bottom flask with 1.2 l de-ionized water and
a Clevenger-type apparatus was then attached to collect the
essential oil. Following 11 h of steam distillation, the volume of
essential oil was measured, and the oil was then transferred to a
15 ml vial and weighed. The oil yield was calculated according to
the following equation: Essential oil (w/w%) = (weight of essential
oil/weight of hinoki leaves) x 100.
Phytoncide essential oil with 100% mixture and 100%
purity at a concentration of 1 gm/ml (1 ml) stock was obtained from
Professor Sung Phil Mun of Chonbuk National University (Jeonju,
South Korea).
Essential oil analysis using GC/MS
A GC/MS QP 2010 (Shimadzu Corporation, Kyoto, Japan)
equipped with a CBP5 fused silica capillary column (30 m x 0.25 mm
i.d., 0.25 µm film thickness; Shimadzu Corporation) was used
for the analysis of the leaf essential oil constituents. The oven
temperature was maintained at 40°C for 5 min, and then increased by
3°C/min to 220°C and increased again by 10°C/min to 280°C and held
for 5 min. The injector temperature was 300°C and the interface
temperature was 230°C with the EI mode at 70 eV. He gas was used as
the carrier gas at a flow rate of 1 ml/min and the split ratio was
1:30. The MS scan range was m/z 35-500. Identification of the oil
components was based on comparisons of relative retention indices
calculated from a mixture of aliphatic hydrocarbons ranging between
C7 and C30 and from the NIST11 database (https://www.nist.gov).
Chemical and reagents
LPS and 3-(4,5-dimethylthiazol-
2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). α-minimum essential
medium (MEM) was purchased from Lonza Group, Ltd. (Walkersville,
MD, USA). Fetal bovine serum (FBS) and penicillin/streptomycin
(P/S) antibiotics were obtained from Gibco; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). The materials and chemicals
used for electrophoresis were obtained from Bio-Rad Laboratories,
Inc. (Hercules, CA, USA). The primary antibodies against rabbit
inducible nitric oxide synthase (iNOS; cat. no. 13120), p65 (cat
no. 8242S), phosphorylated (p)-p65 (cat. no. 3033S), nuclear factor
NF-κB inhibitor (IκB)-α (cat. no. 4812S), and anti-rabbit Alexa
fluor 594 (cat. no. 8889S) conjugate were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA). Cyclooxygenase-2
(COX-2; cat. no. SC-1745) goat polyclonal and donkey anti-goat
(cat. no. SC-2354) IgG HRP conjugate secondary antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Anti-rabbit (cat. no. ADI-SAB-300) IgG horseradish conjugate
secondary antibody was obtained from Enzo Life Sciences, Inc.
(Minneapolis, MN, USA). Goat anti-mouse (cat. no. A90-116P) IgG HRP
conjugate secondary antibody was purchased from Bethyl
Laboratories, Inc. β-actin (cat. no. MAB1501) antibody was
purchased from EMD Millipore (Billerica, MA, USA). All chemicals
used were of the highest grade commercially available.
Experimental animals
Male Sprague-Dawley rats (6-week-old, 150 g;
Samtaco, Osan, Korea) were used for the in vivo experiments.
The experiment was performed following the standard animal science
guidelines reviewed and approved by the Ethics Committee of
Gyeongnam Biological Resource Research Center (Gyeongnam, Korea).
Prior to use, the rats were acclimatized for 3 days in a normal
room ambience (room temperature: 20-24°C; relative humidity:
40-70%; 12 h light/dark cycle), with free access to standard rodent
chow and softened tap water. Each group consisted of three rats and
comprised the control and phytoncide essential oil-inhaled groups.
Phytoncide essential oil (100 kg/cm3 maximum, as per the
recommendation of Chunbuk National University) was administered
through an oxygen channel into the cage for 4 weeks. After 4 weeks,
all mice were anesthetized with ether solution and sacrificed by
cervical dislocation.
Hematoxylin and eosin staining
The xenograft lung tissues were fixed with 4%
paraformaldehyde overnight. The tissues were then embedded with
paraffin. The embedded paraffin was removed from the samples with
100% xylazine and dehydrated with different concentrations of
ethanol (95, 90, 80, and 70%). The tissue samples were stained with
hematoxylin for 3 min and placed on 0.3% acid alcohol for
differentiation. The samples were rinsed with Scott’s tap water
prior to exposure to eosin solution for 3 min. Following staining
with hematoxylin and eosin, tissue samples were dried and protected
with a cover slide. The samples were then observed under a light
microscope.
Cell culture
The WI38 human embryonic fibroblast, lung
tissue-derived cell line was obtained from the Korean Cell Line
Bank (Seoul, Korea). The WI38 fibroblast cells were maintained in
α-MEM media supplemented with 20% heat-inactivated FBS and 1% P/S
at 37°C in a 5% CO2 incubator. The LPS was dissolved in
1X PBS.
Cell viability
To assess WI38 cell compatibility, the cells were
seeded at a density of 6×105 cells per well in 24-well
plates and treated with various concentrations of phytoncide
essential oil (1-50 µg/ml) and LPS (1-10 µg/ml)
followed by incubated at 37°C for 24 h. An MTT assay was performed
to evaluate cell viability. Following treatment, MTT solution (5
mg/ml in 1X PBS) was added followed by incubation for 3 h at 37°C
in the dark. The formazan crystals formed were solubilized by
incubating cells with 500 µl of DMSO. Cell absorbance was
read by an enzyme-linked immunosorbent assay (ELISA) plate reader
(BioTek Instruments, Inc., Winooski, VT, USA) at 540 nm. Cell
proliferation was quantified as a percentage compared with the
positive control and negative control group accordingly, which was
set at 100%.
Western blot analysis
Briefly, the WI38 cells, which had been pre-treated
with 1-10 µg/ml phytoncide essential oil for 1 h prior to 5
µg/ml of LPS for 24 h, were lysed overnight with RIPA lysis
buffer containing phosphatase inhibitor cocktail, protease
inhibitor and EDTA (Thermo Fisher Scientific, Inc.). The extracted
proteins were then centrifuged at 21,000 × g for 30 min at 4°C to
remove debris. The proteins were resolved using 10-12% SDS-PAGE and
subsequently transferred onto a poly-vinylidene difluoridemembrane
(Immunobilon-P, 0.45 mm; EMD Millipore) using the TE 77 Semi-Dry
transfer unit (GE Healthcare Life Sciences, Chalfont, UK). The
membranes were blocked with 5% non-fat milk in Tris-buffered saline
containing 1% Tween-20 (TBS-T, pH 7.4) or 1X phospho blocking
solution (TransLab Biosciences, Daejeon, Korea) at room temperature
for 1 h. The blots were probed with a 1: 1,000 dilutions for the
primary antibodies viz., iNOS, p65, p-p65, IκB-α and 1:250 for
COX-2 at 4°C for overnight. The membranes were washed five times
with TBS-T, and were then incubated with diluted enzyme-linked
secondary antibodies at 1:1,000 for anti-rabbit, 1:2,000 for
anti-goat and 1:3,000 for anti-mouse at room temperature for 3 h.
The membranes were then visualized using an enhanced
chemiluminescence kit and western blotting detection reagents (GE
Healthcare Life Sciences). ImageJ software v.1.51u (National
Institutes of Health, Bethesda, MD, USA) was used to quantify each
protein band, followed by densitometry reading performed following
normalization with the expression of β-actin.
Confocal imaging
Briefly, the cells were seeded on 15 mm microscope
cover glass (Paul Marienfeld GmbH & Co., KG, Lauda-Königshofen,
Germany) at a density of 5×106 cells/well in 12-well
plates and grown overnight, followed by pre-treatment with
essential oil and LPS stimulation for 24 h. For antibody labeling,
the cells were first fixed with 37% formaldehyde and 95% ethanol
(1:4) for 15 min at room temperature, followed by washing with 1X
PBS three times (5 min/wash) and blocking with 1% BSA (Bioshop,
Canada, Inc., Burlington, ON, Canada)/1X PBS for 1 h at room
temperature. The cells were probed overnight with a 1:100 ratio of
diluted antibody (p-p65) at 4°C. Following washing with 1X PBS four
times (7-10 min/wash), the cells were blocked with 1:250 diluted
anti-rabbit Alexa fluor 594 conjugate Red at room temperature for 1
h. The cells were then washed with 1X PBS and mounted with
4′,6-diamidino-2-phenylindole mounting solution on slides purchased
from Vector Laboratories, Inc. (Burlingame, CA, USA) and were
designated for confocal image analysis. All confocal images were
captured with a ×20 oil objective (numerical aperture 3.5) lenses
of Olympus Fluoview FV1000. FV10-ASW 3.1 viewer software (Olympus
Corporation, Tokyo, Japan) was used to extract the images.
Statistical analysis
The obtained results are expressed as the mean ±
standard deviation of a minimum three replicates in independent
experiments. The data were analyzed by one-way analysis of variance
followed by Tukey’s test for the comparison of multiple independent
variables using GraphPad Prism v.5.0 for Windows (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Quantification and characterization of
terpenes
Steam distillation of C. obtusa leaves
produced a light yellow-colored oil with a yield of 1.59% (w/w)
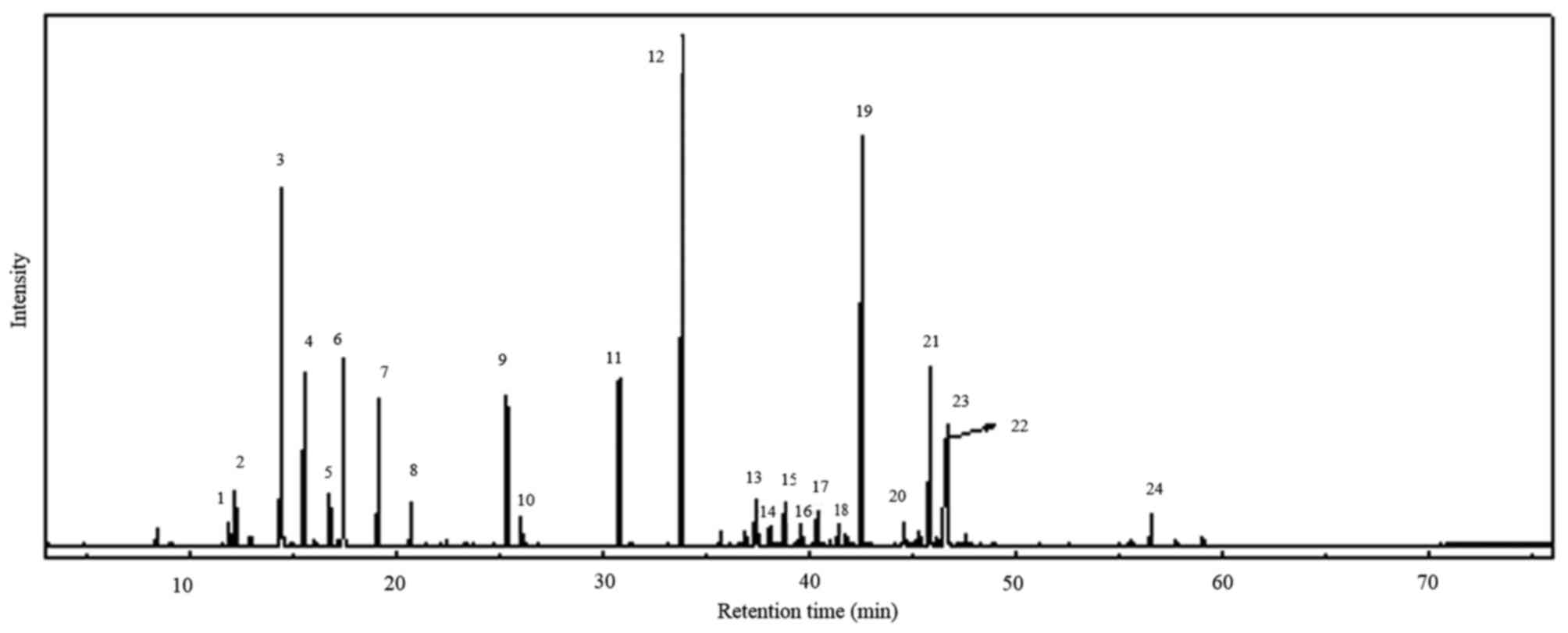
based on green leaf. The GC/MS analyzed peaks revealed >24
components in the total ion chromatogram, as shown in Fig. 1. A total of 23 compounds (Table I) were identified from the leaf
oil of C. obtusa. Among the leaf oil compounds, monoterpenes
and oxygenated monoterpenes were predominant (59.23%), followed by
sesquiterpenes and oxygenated sesquiterpenes (39.65%). Among the
monoterpenes and oxygenated monoterpenes, sabinene (10.42%) and
α-terpinyl acetate (18.89%) were the major compounds, with
oxygenated sesquiterpenes carrying elemol (17.45%) as the main
compound including sub major compounds listed in Table II. The compounds were identified
with reference to previous studies (19-22).
 | Table IChemical composition of the essential
oil obtained from Chamaecyparis obtusa leaf. |
Table I
Chemical composition of the essential
oil obtained from Chamaecyparis obtusa leaf.
| Peak no. | RT (min) | Compound | M+ | Formula | Area (%) | RIa | RIb | Type |
|---|
| 1 | 11.860 | Thujene | 136 |
C10H16 | 0.54 | 921 | 920 | M |
| 2 | 12.150 | α-Pinene | 136 |
C10H16 | 1.41 | 930 | 939 | M |
| 3 | 14.380 | Sabinene | 136 |
C10H16 | 10.42 | 971 | 976 | M |
| 4 | 15.490 | Myrcene | 136 |
C10H16 | 4.68 | 991 | 991 | M |
| 5 | 16.730 | δ-Carene | 136 |
C10H16 | 1.38 | 1,015 | 1,011 | M |
| 6 | 17.405 | Limonene | 136 |
C10H16 | 5.39 | 1,026 | 1,031 | M |
| 7 | 19.090 | γ-Terpinene | 136 |
C10H16 | 4.13 | 1,058 | 1,062 | M |
| 8 | 20.650 | Terpinolene | 136 |
C10H16 | 1.19 | 1,087 | 1,088 | M |
| 9 | 25.315 | Terpinen-4-ol | 154 |
C10H18O | 4.78 | 1,176 | 1,177 | M |
| 10 | 26.000 | α-Terpineol | 154 |
C10H18O | 0.90 | 1,189 | 1,195 | M |
| 11 | 30.750 | Bornyl acetate | 196 |
C12H20O2 | 5.52 | 1,286 | 1,289 | M |
| 12 | 33.820 | α-Terpinyl
acetate | 196 |
C12H20O2 | 18.89 | 1,351 | 1,352 | M |
| 13 | 37.360 | Thujopsene
(widdrene) | 204 |
C15H24 | 1.55 | 1,431 | 1,431 | S |
| 14 | 38.030 |
cis-Muurola-3,5-diene | 204 |
C15H24 | 0.73 | 1,447 | 1,446 | S |
| 15 | 38.770 | 1,2,3,5,6,7,8,8a-
Octahydro-1-methyl-6- methylene-4-(1- methylethyl) naphthalene | 204 |
C15H24 | 1.54 | 1,464 | 1,464 | S |
| 16 | 39.560 | Germacrene-D | 204 |
C15H24 | 0.78 | 1,482 | 1,480 | S |
| 17 | 40.330 | Unknown | 204 |
C15H24 | 1.19 | 1,500 | - | S |
| 18 | 41.370 | δ-Cadinene | 204 |
C15H24 | 0.67 | 1,507 | 1,524 | S |
| 19 | 42.525 | Elemol | 222 |
C15H26O | 17.45 | 1,552 | 1,550 | S |
| 20 | 44.550 C | edrol | 222 |
C15H26O | 0.81 | 1,603 | 1,596 | S |
| 21 | 45.805 | γ-Eudesmol | 222 |
C15H26O | 6.62 | 1,634 | 1,626 | S |
| 22 | 46.520 | β-Eudesmol | 222 |
C15H26O | 3.84 | 1,653 | 1,651 | S |
| 23 | 46.640 | α-Eudesmol | 222 |
C15H26O | 4.47 | 1,656 | - | S |
| 24 | 56.525 | Beyerene
(hibaene) | 272 |
C20H32 | 1.11 | 1,930 | 1,995 | D |
| Total | | | | | 99.99 | | | |
 | Table IIMain major compounds of essential oil
from hinoki cypress leaf analyzed by GC-MS. |
Table II
Main major compounds of essential oil
from hinoki cypress leaf analyzed by GC-MS.
| Compound | Formula | Area (%) | RIa | RIb | Type |
|---|
| Sabinene |
C10H16 | 10.42 | 971 | 976 | Monoterpene |
| α-Terpinyl
acetate |
C12H20O2 | 18.89 | 1,351 | 1,352 | Oxygenated
monoterpene |
| Elemol |
C15H26O | 17.45 | 1,552 | 1,550 | Oxygenated
sesquiterpene |
| Myrcene |
C10H16 | 4.68 | 991 | 991 | Monoterpene |
| Limonene |
C10H16 | 5.39 | 1,026 | 1,031 | Monoterpene |
| γ-Terpinene |
C10H16 | 4.13 | 1,058 | 1,062 | Monoterpene |
| Terpinen-4-ol |
C10H18O | 4.78 | 1,176 | 1,177 | Oxygenated
monoterpene |
| Bornyl acetate |
C12H20O2 | 5.52 | 1,286 | 1,289 | Oxygenated
monoterpene |
| γ-Eudesmol |
C15H26O | 6.62 | 1,634 | 1,626 | Oxygenated
sesquiterpene |
| β-Eudesmol |
C15H26O | 3.84 | 1,653 | 1,651 | Oxygenated
sesquiterpene |
| α-Eudesmol
C15H26O | | 4.47 | 1,656 | – | Oxygenated
sesquiterpene |
| Total | | 86.19 | | | |
Phytoncide essential oil enhances lung
alveolar capacity
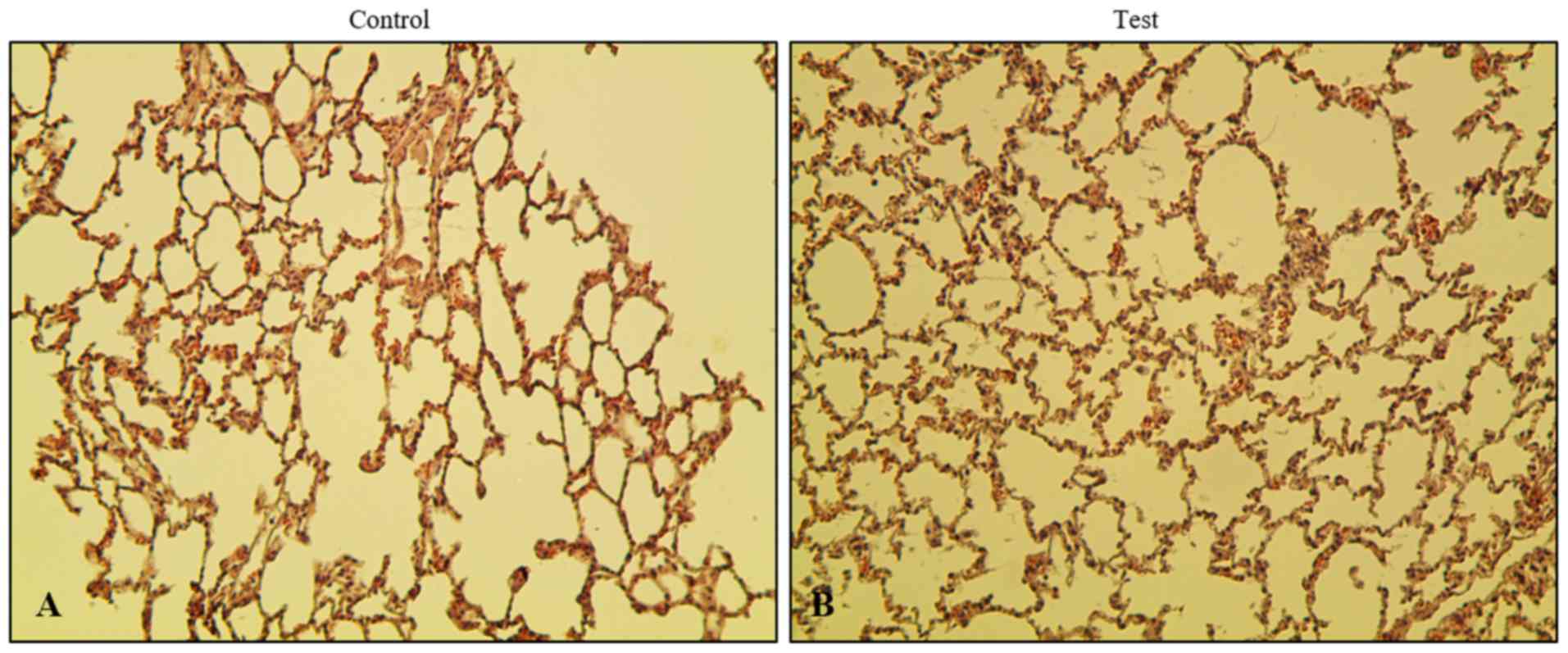
To evaluate the effect of phytoncide essential oil
on rats via olfactory stimulation, the lungs were removed followed
by subsequent fixing and staining. In order to investigate the
histopathological changes in the phytoncide essential
oil-administered or inhalation model, hematoxylin and eosin
staining was performed. It was observed that xenograft lung tissues
in the test group showed more alveoli in the lungs than the control
group (Fig. 2). No significant
difference between the control and test group was found when
comparing the thickness of the interstitial space layer, with the
exception ofin the nucleus. There was no exudate found inside the
alveolar layer. An increase of spontaneous volume and a decrease in
alveolar dead space were observed. In addition, no toxic
interstitial pneumonia or inflammation was observed in the test
group rats, confirming that there was no microbial infection in the
lungs. This observation indicates that the airway inhalation of
phytoncide essential oil by Sprague-Dawley rats did not cause any
microbial infection or inflammation over the course duration of
phytoncide administration. Instead, there was an enhancement in the
breathing capacity of the lungs.
Effect of phytoncide essential oil on
cell viability and LPS stimulation on WI38 fibroblast cells
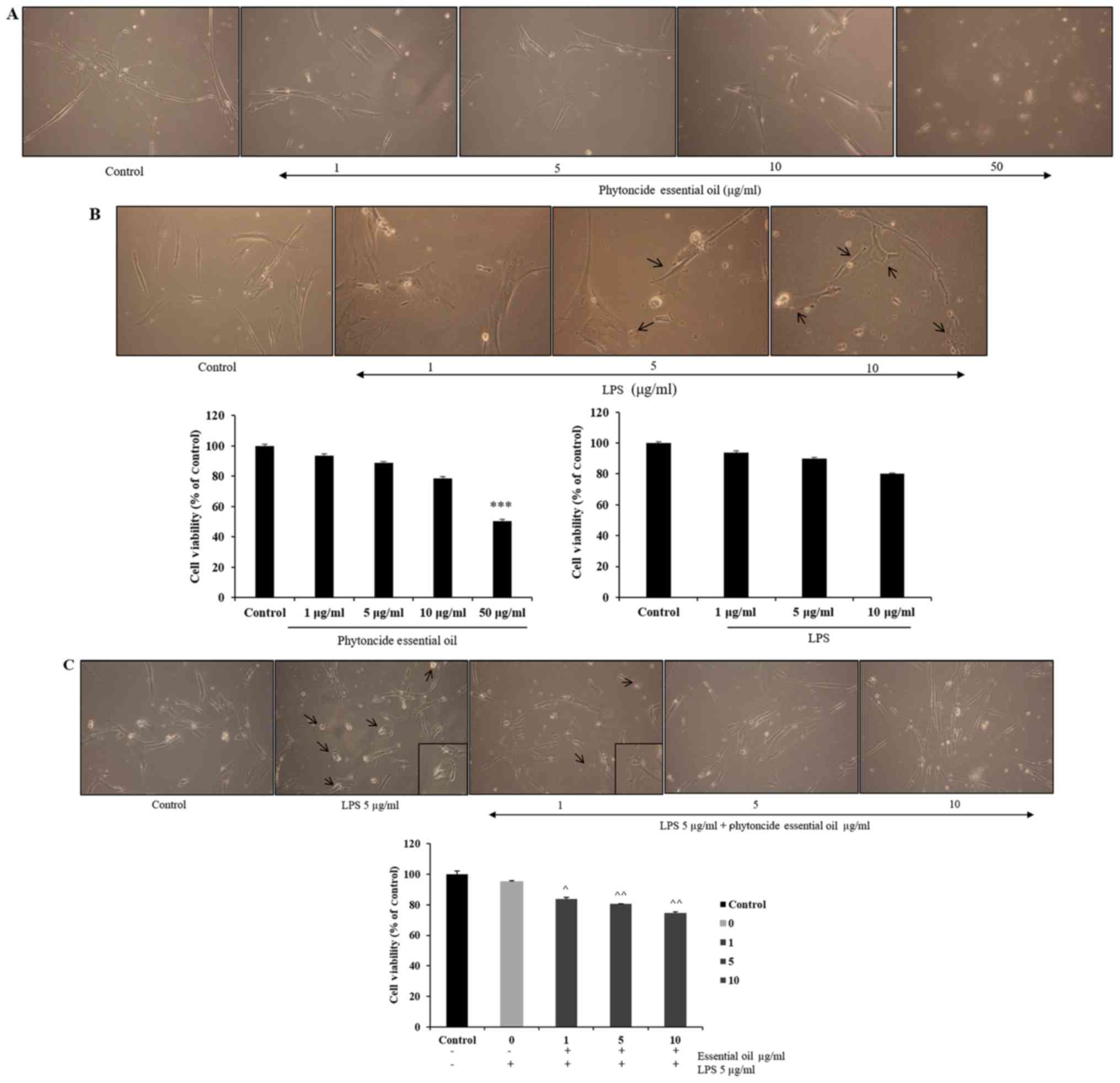
The effect of phytoncide from hinoki leaf extracted
essential oil on the viability of WI38 cells was measured at doses
ranging between 1 and 50 µg/ml and of LPS concentrations
ranging between 1 and 10 µg/ml using an MTT assay following
incubation of the cells for 24 h (Fig. 3A and B). It was observed that cell
viability was not affected until a 10 µg/ml dose of
phytoncide essential oil and significant cell growth inhibition
(47%) was observed at the 50 µg/ml dose. LPS stimulation led
to changes in cell morphology at 5 and 10 µg/ml
concentrations, compared with the subsequent control. The selected
concentrations of phytoncide essential oil were 1-10 µg/ml
based on the cell compatibility comparing with control; the LPS
stimulatory dose was selected as 5 µg/ml based on the
morphological changes. It was observed that the normal fibroblast
morphology of cells was altered, exhibiting swelling and
pseudopodia formation in the LPS-stimulated cells. Furthermore, it
was observed that pre-treatment with 1-10 µg/ml
concentrations of phytoncide essential oil significantly reduced
the stimulated swelling and pseudopodia formation induced by LPS in
a dose-dependent manner (Fig.
3C). Therefore, these data indicate that the morphological
observation of WI38 fibroblast cell inflammation by LPS can be
suppressed by the terpenes of essential oil from C. obtusa
leaf.
LPS-induced expression of iNOS and COX-2
is inhibited by phytoncide essential oil in WI38 fibroblast
cells
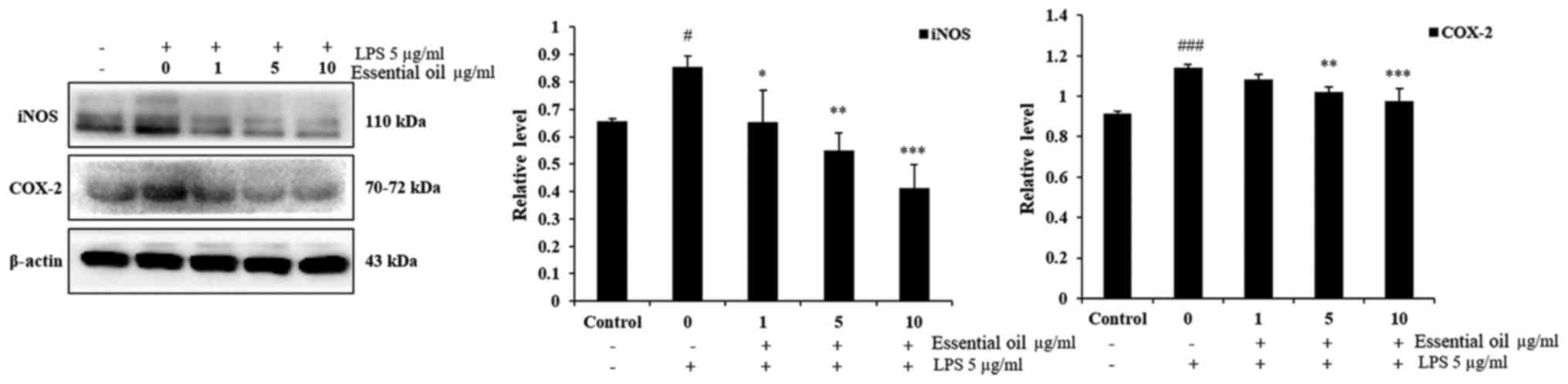
In order to investigate the anti-inflammatory effect
of phytoncide essential oil, western blot analysis was performed.
The experimental results of the western blot analysis determined
that 5 µg/ml LPS markedly increased the expression of iNOS
and COX-2 at 24 h duration in the WI38 fibroblast cells compared
with the untreated control group of WI38 cells. Pre-treatment of
the WI38 cells with 1-10 µg/ml phytoncide essential oil for
1 h led to a significant decrease in the expression of iNOS in a
dose-dependent manner compared with that in the LPS-stimulated
cells at 24 h. Furthermore, phytoncide essential oil significantly
suppressed the production of COX-2 in the LPS-stimulated WI38 cells
at 5 and 10 µg/ml doses. These data indicated that, the
terpenes of essential oil from C. obtusa leaf inhibits
LPS-stimulated protein secretion of iNOS and COX-2 in WI38
fibroblast cells (Fig. 4).
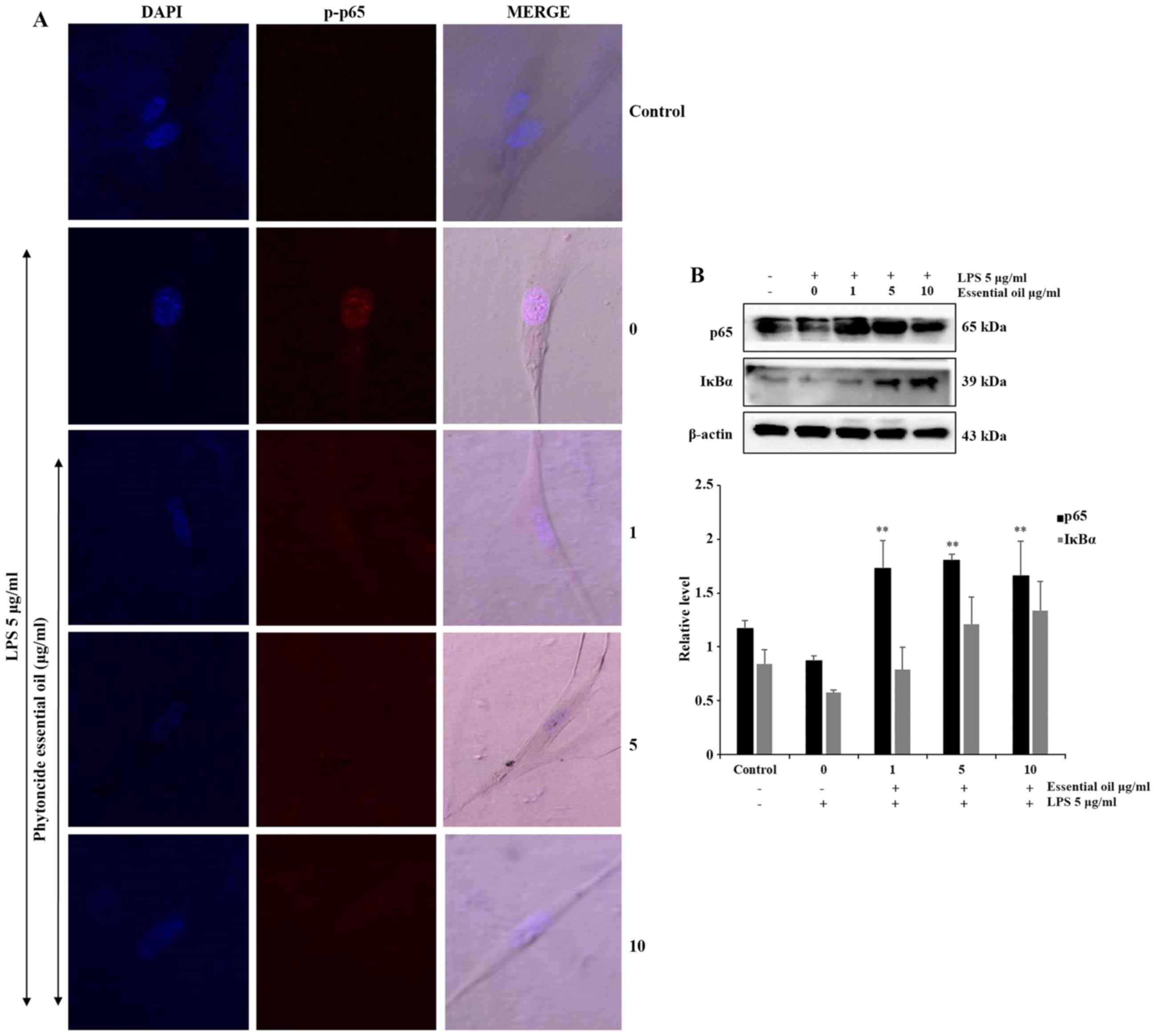
Phytoncide essential oil attenuates NF-κB
activation
NF-κB is activated at sites of inflammatory disease.
To confirm the anti-inflammatory role of phytoncide essential oil
in WI38 cells, the expression of inflammatory proteins were
detected and evaluated. The phosphorylation of NF-κB or
translocation of NF-κB to the nucleus of the WI38 inflamed cells
were observed via immunofluorescence. The confocal imaging results
indicated that the LPS-treated cells exhibited a marked increase of
p-p65 in the nucleus, compared with the untreated or negative
control cells. The co-treated groups showed complete suppression of
the expression of p-p65 in the nucleus of WI38 cells pre-treated
with 5 and 10 µg/ml phytoncide essential oil (Fig. 5A). Furthermore, the protein
expression of the total form of p65 decreased in the LPS-stimulated
group was significantly increased in the phytoncide essential oil
co-treated groups, and NF-κB inhibitor or inactivated IκB-α protein
also decreased following LPS-stimulation and increased in the
phytoncide essential oil pre-treated group in the total cell lysate
(Fig. 5B). Therefore, these data
indicated that treatment with essential oil from C. obtusa
leaf containing terpenes inhibited the inflammation in WI38
fibroblast cells exposed to LPS stimulation by inhibiting the
translocation of NF-κB from the cytosol leading to nuclear
activation.
Discussion
Inflammation is a protective response to noxious
stimuli that occurs unavoidably at a cost to normal tissue
function, mainly depending on the type of cells and molecular
mediators involved, and are classified as acute vs. chronic and
local vs. systemic (23). The
characteristic features of several chronic inflammatory diseases
are their persistence and predilection for certain sites. The
prevalence of any disease is due to the lack of therapeutic targets
which have no side effects during treatment. The mechanism
underlying the development of inflammation and pathological pain in
disease is associated with an increase in pro-inflammatory
cytokines interleukin (IL)-6, IL-8, IL-β, tumor necrosis factor
(TNF)-α, chemo-kines, adhesion molecules, and matrix
metalloproteinases, followed by a decrease in anti-inflammatory
interleukin secretion, the production of COX-2 and iNOS and
activation of the NF-κB/Rel transcription family pathways (24).
Natural products and the therapeutic strategies
related to them are the foremost treatment methods in the
development for a potent medicine with no adverse effects. Several
reports on the anti-inflammatory effects of bioactive medicinal
herbs have explained these: Ailanthus altissima in ovalbumin
induced lung inflammation via the down-regulation of T helper 2
(Th2) cytokines and eotaxin transcripts in bone marrow-derived mast
cells (25); herb mixture PM014
attenuated lung inflammation in a murine model Balb/c mice against
COPD (26); pomegranate extract
attenuated cigarette smoking-induced inflammation in human alveolar
cells (27), and
curcumin-resveratrol combination led to anti-inflammatory and
proapoptotic effects in the MRC-5 human lung fibroblast cell line
(28).
Essential oils are complex volatile compounds, which
have potential therapeutic activity that depends mainly on their
types, including aldehydes, phenolics, terpenes, and other
antimicrobial compounds (29).
Essential oil mixed with mint, eucalyptus, spruce and frankincense
compounds have exhibited curative effects against acute airway
inflammation induced by air PM2.5 air pollutants in mice
(30). Phytoncide or wood
essential oil has been investigated for health beneficial
activities, including enhancing the activity of NK cells by the
induction of intracellular perforin, granzyme A, and granulysin in
NK-92MI human cells (31). Hinoki
cypress (C. obtusa) is a coniferous tree and predominant
cypress species in Asia, found in Japan and South Korea, which
contains volatile or essential oil in its leaves and twigs. The
direct inhalation of essential oil or contact with cypress wood has
been widely investigated for its physiological and psychological
relaxation properties without harmful effects on the body (8,12,13,32). In vitro studies have shown
that phytoncides decrease the capacity of LPS-induced inflammatory
responses in MAC-T mammary alveolar epithelial cells (33) and in RAW 264.7 macrophage cells
(18). Monoterpene β-thujaplicin
of C. obtusa have been shown to inhibit inflammatory
expression in RAW 264.7 cells and septic shock in mice (34); whereas α-phellandrene mono-terpene
in Schinus molle L. essential oil exhibits enhanced NK cell
activity in normal BALB/c mice (35). Similarly, in the present study,
exposure to terpenes of essential oil at different concentrations
led to the inhibition of LPS-induced inflammation in WI38 cells,
and inhalation of the essential oil enhanced the capacity of lung
alveoli in rats.
Inflammatory mediators, including eicosanoids, are
biosynthesized by COX and lipoxygenase enzymes, in which the
eicosanoid-generating enzyme, COX-2 has been found to be essential
for the production of prostaglandins in inflammatory diseases
(25,36). The up-regulation of COX-2 leads to
inflammatory cytokine release, which can further dictate the extent
and type of inflammatory response from immune and non-immune cells.
In chronic lung inflammation, pro-fibrotic and immunoregulatory Th2
cytokines are important in disease exaggeration (2). The role of iNOS protein in the
pathogenesis of allergen-induced airway inflammation and lung
fibrosis further explains the function of this protein in defining
the context of acute or chronic inflammatory response in disease
exaggeration (1). Several studies
have addressed the anti-inflammatory regulation by the inhibition
of iNOS and COX-2 in injured, allergy-evoked or damaged tissue and
cells, suggesting therapeutic targets for respiratory inflammatory
disease (1,25,37,38). Similarly, the present study showed
that terpenes in essential oil inhibited the LPS-stimulated
expression of iNOS and COX-2 inflammatory mediators in WI38
fibroblast cells.
The redox-sensitive transcription factor, NF-κB, is
an important participant in a broad spectrum of inflammatory
networks that regulate cytokine activity in airway pathology
(39). Prominent transcription
factors in airway disease include NF-κB, activator protein-1
(AP-1), glucocorticoid receptors and nuclear factor of activated T
cells (40). Inflammatory target
proteins, including MMP-9, intercellular adhesion molecule-1,
vascular cell adhesion molecule-1, COX-2 and cytosolic
phospholipase A2, are associated with airway and lung inflammation
in response to various stimuli (41). Therapeutics that target the NF-κB
family can inhibit its translocation during inflammation in a
process termed trans-repression and can protect against the
condition of pulmonary and airway obstruction-associated
inflammation (23,39). Several studies have described the
therapeutic implications on lung diseases and its pathogenesis by
inhibiting the NF-κB pathway through microRNA-194 in infantile
pneumonia (42), and curcumin and
resveratrol combination in MRC-5 cells for idiopathic pulmonary
fibrosis (28). The suppression
of NF-κB in inflammatory diseases by natural compounds derived from
plants have been reported for inflammatory diseases by inhibiting
its downstream targets viz., and inflammatory mediators iNOS, COX-2
and PGE2 (43-47). The utilization of natural
compounds as a potential treatment method in inflammatory airway
diseases, for reversion to the healthy condition of cells or
tissues, is a prime target of investigation. Therefore, the present
study supports that terpenes in essential oil from the C.
obtusa leaf inhibits the translocation of NF-κB in WI38
cells.
The present study sheds light on the
anti-inflammatory mechanism of terpenes in phytoncide essential oil
from the Korean C. obtusa leaf by inhibiting the
inflammatory mediators iNOS and COX-2 and potent transcription
factor NF-κB in order to protect the lung from severe inflammatory
diseases (Fig 6). Therefore,
these findings suggest that terpene compounds in essential oil of
hinoki cypress can be used as a safe therapeutic agent for the
treatment of respiratory inflammation and improvement of
respiration capacity. However, further investigations are required
to provide further insight into the details of its molecular
mechanism.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Research
Foundation of Korea funded by MSIT (grant nos. 2012M3A9B8019303 and
2017R1A2B4003974).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
SR, SPM, SJL and GSK designed the study protocol. SR
performed the in vitro experiments, analyzed statistical
data, wrote and revised the manuscript. SR, SMK, HJL, VVGS, SEH and
EHK performed the in vivo experiment. SJL and JDH performed
the in vivo rat tissue experiment. SMK performed the
experimental analysis and manuscript revision. SPM performed the
GC/MS analysis and compound identification. GSK provided advise for
the experimental analysis.
Ethics approval and consent to
participate
The present study was performed following the
standard animal science guidelines reviewed and approved by the
Ethics Committee of Gyeongnam Biological Resource Research Center
(Gyeongnam, Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Naura AS, Zerfaoui M, Kim H, Abd Elmageed
ZY, Rodriguez PC, Hans CP, Ju J, Errami Y, Park J, Ochoa AC, et al:
Requirement for inducible nitric oxide synthase in chronic allergen
exposure-induced pulmonary fibrosis but not inflammation. J
Immunol. 185:3076–3085. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moldoveanu B, Otmishi P, Jani P, Walker J,
Sarmiento X, Guardiola J, Saad M and Yu J: Inflammatory mechanisms
in the lung. J Inflamm Res. 2:1–11. 2009.PubMed/NCBI
|
|
3
|
Stellari F, Bergamini G, Ruscitti F,
Sandri A, Ravanetti F, Donofrio G, Boschi F, Villetti G, Sorio C,
Assael BM, et al: In vivo monitoring of lung inflammation in
CFTR-deficient mice. J Transl Med. 14:2262016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eutamene H, Theodorou V, Schmidlin F,
Tondereau V, Garcia-Villar R, Salvador-Cartier C, Chovet M,
Bertrand C and Bueno L: LPS-induced lung inflammation is linked to
increased epithelial permeability: Role of MLCK. Eur Respir J.
25:789–796. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Linthout S, Miteva K and Tschöpe C:
Crosstalk between fibroblasts and inflammatory cells. Cardiovasc
Res. 102:258–269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vancheri C, Mastruzzo C, Tomaselli V,
Sortino MA, D’Amico L, Bellistrí G, Pistorio MP, Salinaro ET,
Palermo F, Mistretta A, et al: Normal human lung fibroblasts
differently modulate interleukin-10 and interleukin-12 production
by monocytes: Implications for an altered immune response in
pulmonary chronic inflammation. Am J Respir Cell Mol Biol.
25:592–599. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Flavell SJ, Hou TZ, Lax S, Filer AD,
Salmon M and Buckley CD: Fibroblasts as novel therapeutic targets
in chronic inflammation. Br J Pharmacol. 153(Suppl 1): S241–S246.
2008. View Article : Google Scholar
|
|
8
|
Ikei H, Song C and Miyazaki Y:
Physiological effect of olfactory stimulation by Hinoki cypress
(Chamaecyparis obtusa) leaf oil. J Physiol Anthropol. 34:442015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SH, Do HS and Min KJ: Effects of
essential oil from Hinoki cypressChamaecyparis obtusa, on
physiology and behavior of flies. PLoS One. 10:e01434502015.
View Article : Google Scholar
|
|
10
|
Abe T, Hisama M, Tanimoto S, Shibayama H,
Mihara Y and Nomura M: Antioxidant effects and antimicrobial
activites of phytoncide. Biocontrol Sci. 13:23–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang S, Jung JH, Kim HS, Kim BY and Kim
IH: Influences of phytoncide supplementation on growth performance,
nutrient digestibility, blood profiles, diarrhea scores and fecal
microflora shedding in weaning pigs. Asian-Australas J Anim Sci.
25:1309–1315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Q: Effect of forest bathing trips on
human immune function. Environ Health Prev Med. 15:9–17. 2010.
View Article : Google Scholar :
|
|
13
|
Li Q, Kobayashi M, Wakayama Y, Inagaki H,
Katsumata M, Hirata Y, Hirata K, Shimizu T, Kawada T, Park BJ, et
al: Effect of phytoncide from trees on human natural killer cell
function. Int J Immunopathol Pharmacol. 22:951–959. 2009.
View Article : Google Scholar
|
|
14
|
Sobral MV, Xavier AL, Lima TC and de Sousa
DP: Antitumor activity of monoterpenes found in essential oils.
Scientific World Journal. 2014.e9534512014.
|
|
15
|
Juergens UR: Anti-inflammatory properties
of the monoterpene 1.8-cineole: Current evidence for co-medication
in inflammatory airway diseases. Drug Res (Stuttg). 64:638–646.
2014. View Article : Google Scholar
|
|
16
|
Guerreiro M, Mernak M, Santana F, Pinheiro
N, Ramanholo BS, Capello T, Lolanda T, Martins M, Lago J and Prado
C: Essential oils reduced lung inflammation in a model of acute
lung injury. Eur Respir J. 44:39252014.
|
|
17
|
Games E, Guerreiro M, Santana FR, Pinheiro
NM, de Oliveira EA, Lopes FD, Olivo CR, Tibério IF, Martins MA,
Lago JH, et al: Structurally related monoterpenes p-Cymene,
carvacrol and thymol isolated from essential oil from leaves of
Lippia si Cham. (Verbenaceae) protect mice against elastase-induced
emphysemades. Molecules. 21:E13902016. View Article : Google Scholar
|
|
18
|
Park Y, Yoo SA, Kim WU, Cho CS, Woo JM and
Yoon CH: Anti-inflammatory effects of essential oils extracted from
Chamaecyparis obtusa on murine models of inflammation and RAW 264.7
cells. Mol Med Rep. 13:3335–3341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuiate JR, Bessière JM, Zollo PH and Kuate
SP: Chemical composition and antidermatophytic properties of
volatile fractions of hexanic extract from leaves of Cupressus
lusitanica Mill. from Cameroon. J Ethnopharmacol. 103:160–165.
2006. View Article : Google Scholar
|
|
20
|
Su YC and Ho CL: Composition and two
activities of the leaf essential oil of Litsea acuminata (Blume)
Kurata from Taiwan. Rec Nat Prod. 7:27–34. 2013.
|
|
21
|
Wang SY, Wang YS, Tseng YH, Lin CT and Liu
CP: Analysis of fragrance compositions of precious coniferous woods
grown in Taiwan. Holzforschung. 60:528–532. 2006. View Article : Google Scholar
|
|
22
|
Xu Brittany M, Baker George L, Sarnoski
Paul J and Goodrich-Schneide Renée M: A comparison of the volatile
components of cold pressed Hamlin and Valencia (Citrus sinensis
(L.) Osbeck) orange oils affected by Huanglongbing. J Food Qual.
2017.6793986:2017.
|
|
23
|
Okin D and Medzhitov R: Evolution of
inflammatory diseases. Curr Biol. 22:R733–R740. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tak PP, Firestein GS and NF-kappa B: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin MH, Yook J, Lee E, Lin CX, Quan Z, Son
KH, Bae KH, Kim HP, Kang SS and Chang HW: Anti-inflammatory
activity of Ailanthus altissima in ovalbumin-induced lung
inflammation. Biol Pharm Bull. 29:884–888. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee H, Kim Y, Kim HJ, Park S, Jang YP,
Jung S, Jung H and Bae H: Herbal formula, PM014, attenuates lung
inflammation in a murine model of chronic obstructive pulmonary
disease. Evid Based Complement Alternat Med. 2012.769830:2012.
|
|
27
|
Husari A, Hashem Y, Bitar H, Dbaibo G,
Zaatari G and El Sabban M: Antioxidant activity of pomegranate
juice reduces emphysematous changes and injury secondary to
cigarette smoke in an animal model and human alveolar cells. Int J
Chron Obstruct Pulmon Dis. 11:227–237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kloesch B, Dietersdorfer E, Loebsch S and
Steiner G: Anti-inflammatory and pro-apoptotic effects of curcumin
and resveratrol on the human lung fibroblast cell line MRC-5.
Altern Integr Med. 3:1742014.
|
|
29
|
Swamy MK, Akhtar MS and Sinniah UR:
Antimicrobial properties of plant essential oils against human
pathogens and their mode of action: An updated review. Evid Based
Complement Alternat Med. 2016.3012462:2016.
|
|
30
|
Wang H, Song L, Ju W, Wang X, Dong L,
Zhang Y, Ya P, Yang C and Li F: The acute airway inflammation
induced by PM2.5 exposure and the treatment of essential oils in
Balb/c mice. Sci Rep. 7:442562017. View Article : Google Scholar :
|
|
31
|
Li Q, Nakadai A, Matsushima H, Miyazaki Y,
Krensky AM, Kawada T and Morimoto K: Phytoncides (wood essential
oils) induce human natural killer cell activity. Immunopharmacol
Immunotoxicol. 28:319–333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ikei H, Song C and Miyazaki Y:
Physiological effects of touching hinoki cypress (Chamaecyparis
obtusa). J Wood Sci. 64:226–236. 2018. View Article : Google Scholar
|
|
33
|
Kang S, Lee JS, Lee HC, Petriello MC, Kim
BY, Do JT, Lim DS, Lee HG and Han SG: Phytoncide extracted from
pinecone decreases LPS-induced inflammatory responses in bovine
mammary epithelial cells. J Microbiol Biotechnol. 26:579–587. 2016.
View Article : Google Scholar
|
|
34
|
Shih MF, Chen LY, Tsai PJ and Cherng JY:
In vitro and in vivo therapeutics of β-thujaplicin on LPS-induced
inflammation in macrophages and septic shock in mice. Int J
Immunopathol Pharmacol. 25:39–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin JJ, Lin JH, Hsu SC, Weng SW, Huang YP,
Tang NY, Lin JG and Chung JG: Alpha-phellandrene promotes immune
responses in normal mice through enhancing macrophage phagocytosis
and natural killer cell activities. In Vivo. 27:809–814.
2013.PubMed/NCBI
|
|
36
|
Lanas A, Haggerty P and Hirschowitz BI: In
vitro studies of anti-inflammatory agents and prostaglandin e2
effects on stimulated normal human fibroblast cultures.
Inflammopharmacology. 2:377–387. 1994. View Article : Google Scholar
|
|
37
|
Seo HR, Choi MJ, Choi JM, Ko JC, Ko JY and
Cho EJ: Malvidin protects WI-38 human fibroblast cells against
stress-induced premature senescence. J Cancer Prev. 21:32–40. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thangavel J, Samanta S, Rajasingh S,
Barani B, Xuan YT, Dawn B and Rajasingh J: Epigenetic modifiers
reduce inflammation and modulate macrophage phenotype during
endotoxemia-induced acute lung injury. J Cell Sci. 128:3094–3105.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schuliga M: NF-kappaB signaling in chronic
inflammatory airway disease. Biomolecules. 5:1266–1283. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Barnes PJ: Transcription factors in airway
diseases. Lab Invest. 86:867–872. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee IT and Yang CM: Inflammatory
signalings involved in airway and pulmonary diseases. Mediators
Inflamm. 2013.791231:2013.
|
|
42
|
Xie F, Yang L, Han L and Yue B:
MicroRNA-194 regulates lipopolysaccharide-induced cell viability by
inactivation of nuclear factor-κ B pathway. Cell Physiol Biochem.
43:2470–2478. 2017. View Article : Google Scholar
|
|
43
|
de Cassia da Silveira E Sa R, Andrade LN,
Dos Reis Barreto, de Oliveira R and de Sousa DP: A review on
anti-inflammatory activity of phenylpropanoids found in essential
oils. Molecules. 19:1459–1480. 2014. View Article : Google Scholar
|
|
44
|
Kim KN, Ko YJ, Yang HM, Ham YM, Roh SW,
Jeon YJ, Ahn G, Kang MC, Yoon WJ, Kim D, et al: Anti-inflammatory
effect of essential oil and its constituents from fingered citron
(Citrus medica L. var. sarcodactylis) through blocking JNK, ERK and
NF-κB signaling pathways in LPS-activated RAW 264.7 cells. Food
Chem Toxicol. 57:126–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chiang YM, Lo CP, Chen YP, Wang SY, Yang
NS, Kuo YH and Shyur LF: Ethyl caffeate suppresses NF-kappaB
activation and its downstream inflammatory mediators, iNOS, COX-2,
and PGE2 in vitro or in mouse skin. Br J Pharmacol. 146:352–363.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wei J, Zhang X, Bi Y, Miao R, Zhang Z and
Su H: Anti-inflammatory effects of cumin essential oil by blocking
JNK, ERK, and NF-κB signaling pathways in LPS-stimulated RAW 264.7
cells. Evid Based Complement Alternat Med. 2015.474509:2015.
|
|
47
|
Raha S, Lee HJ, Yumnam S, Hong GE,
Venkatarame Gowda Saralamma V, Ha YL, Kim JO, Kim YS, Heo JD, Lee
SJ, et al: Vitamin D2 suppresses amyloid-β 25–35 induced microglial
activation in BV2 cells by blocking the NF-κB inflammatory
signaling pathway. Life Sci. 161:37–44. 2016. View Article : Google Scholar : PubMed/NCBI
|