Introduction
Platelet activation is an essential process to
repair injured blood vessels, restoring blood vascular integrity,
which is fundamental for the maintenance of vascular function.
However, aberrant platelet activation or hyperactivation of
platelets may give rise to thrombosis and result in thrombotic
vascular events, including atherosclerosis, arterial thrombosis and
myocardial infarction (1-5). Under physiological conditions,
platelets circulate through vessels with an intact and healthy
endothelium, remaining in an inactivated state. However, when the
endothelium is broken, the von Willebrand factor on the injured
vascular wall interacts with its receptor located on the surface of
platelets, which in turn induces platelet adhesion to extracellular
matrix (6). Platelet activation
results in the release of secondary mediators, including adenosine
diphosphate (ADP), thromboxane A2 (TXA2) and thrombin (7). These molecules promote further
adhesion, activation and aggregation of platelets, eventually
forming a platelet plug to stop bleeding and to repair injured
endothelium. The process of platelet activation is strongly
associated with multiple intracellular signaling pathways,
including the phosphoinositide 3-kinase (PI3K)/AKT serine/threonine
kinase (Akt) pathway. Following activation by extracellular
stimuli, PI3K is able to phosphorylate PI(4)P and PI(4,5)P2
to generate PI(3,4)P2 and PI(3,4,5)P3,
respectively (8). Akt is
recruited by PIP3 to the platelet plasma membrane with the
PIP3-binding domain, and phosphorylated/activated by
3-phosphoinositide dependent protein kinase 1 (PDPK1, additionally
termed PDK1) and mammalian target of rapamycin complex 2 (mTORC2)
(9-11). Activated Akt has critical roles in
platelet function by mediating various cellular responses,
including granule secretion, platelet aggregation and thrombus
formation (12-15). Therefore, inhibition of platelet
activation by suppressing the PI3K/Akt pathway may be a promising
strategy to treat thrombotic vascular diseases.
A variety of antiplatelet drugs (including aspirin
and clopidelgrel) demonstrate significant antiplatelet efficacy for
treatment of thrombotic vascular diseases. However, the
administration of most currently-used platelet activation
inhibitors frequently results in bleeding complications and drug
resistance (16,17). Recently, an increasing number of
studies are focusing on medicinal herbs, in order to discover
complementary and alternative compounds with relatively fewer
side-effects. Rhizoma Ligusticum Wallichii (RLW) is a
well-known medicinal herb, which has long been used in China to
clinically treat various cardiovascular disorders (18). Ligustrazine is one of the most
pharmacologically active compounds of RLW, which has been used for
anti-cardiovascular (19),
antiplatelet (20), ischemic
stroke (21), anti-Alzheimer’s
(22), neuroprotective (23) and anticancer (24) treatment. In cardiovascular and
cerebrovascular diseases, ligustrazine exhibits significant
therapeutic activity and may improve the microcirculation, by
expanding small arteries, removing blood stasis, and by
additionally having effects on antiplatelet aggregation,
antioxidation, calcium antagonists and antifibrosis (18,25-29). However, the molecular mechanism of
its mode of action has not been thoroughly elucidated. A
preliminary study from our group identified that treatment with
ligustrazine significantly inhibited ADP-induced platelet
aggregation and Akt phosphorylation (Li et al, unpublished
data). Therefore, it was hypothesized that ligustrazine
hydrochloride (LH; the clinical-grade form of ligustrazine) may
exhibit antiplatelet activities by suppressing the Akt signaling
pathway. To confirm this hypothesis, the present study used in
vitro and ex vivoplatelet activation models, established
by stimulating rat platelet-rich plasma (PRP) either with the
platelet activator ADP or with the specific Akt pathway activator
insulin-like growth factor-1 (IGF-1). The effects of LH on platelet
activation and the underlying molecular mechanisms were
investigated.
Materials and methods
Materials and reagents
Adrenaline hydrochloride was purchased from Fuyao
Group (Fuzhou, China). A thromboxane B2 (TXB2) enzyme immunoassay
kit was obtained from Enzo Life Sciences, Inc. (Farmingdale, NY,
USA). Antibodies against phosphorylated (p-)Akt, Akt, β-actin, and
horseradish peroxidase (HRP)-conjugated secondary antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
ADP, fluo-4-acetoxymethyl ester (Fluo-4 AM), IGF-1 and other
unstated chemicals were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany).
Preparation of LH
LH (>99.0%) was purchased from Beijing Putian
Tongchuang biotechnology Co., Ltd. (Beijing, China). LH was
dissolved in saline at a concentration of 20 mg/ml for ex
vivo experiments or 180 mM for in vitro experiments. The
chemical structures of ligustrazine and LH are presented in
Fig. 1.
Animals and treatment with LH
Male Sprague-Dawley (SD) rats of 6-8 weeks of age
(200-250 g) were purchased from Shanghai SLAC Laboratory Animal
Co., Ltd. (Shanghai, China). All animals were housed in a specific
pathogen-free environment with food and water supplied ad
libitumthroughout the experiment. The environment was
maintained at 22°C with a 12-h light/dark cycle and humidity of
55±5%. All the animal treatments were performed in compliance with
international ethical guidelines and the National Institutes of
Health Guidelines for the Care and Use of Laboratory Animals
(Bethesda, MD, USA). The experiments were approved by the
Institutional Animal Care and Use Committee of Fujian University of
Traditional Chinese Medicine (Fuzhou, China).
For the ex vivo experiment, 24 SD rats were
randomly divided into the following three groups (n=8/group):
Control, Adrenaline and Adrenaline + LH. The Adrenaline + LH group
was administered LH (80 mg/kg; based on the clinical dosage and
transformation between human and rat) by intraperitoneal injection
daily for 7 days, while the other two groups received an equal
volume of saline. At the end of the treatment, the rats in the
Adrenaline + LH and Adrenaline groups were subcutaneously injected
with adrenaline hydrochloride at a dose of 1 mg/kg, whereas, the
rats in the Control group were subcutaneously injected with an
equal volume of saline. At 1 h following the injection, the
bleeding risk of SD rats from each group was evaluated and the
blood samples were collected for further ex vivo experiments
(including assays of platelet aggregation, analysis of
intracellular Ca2+ mobilization and TXB2 levels, in
addition to expression of associated proteins).
Blood collection and preparation of rat
platelets
Blood was collected from SD rats with or without LH
treatment. Rats were anesthetized with sodium pentobarbital (45
mg/kg) and blood was collected from the abdominal aorta and
anticoagulated with 3.8% sodium citrate solution (9:1, v/v). The
obtained blood samples (8-10 ml blood for each rat) were
centrifuged at room temperature for 15 min at 150 × g to obtain
PRP, which were centrifuged again at room temperature for 15 min at
150 × g to remove residual erythrocytes. The platelet-poor plasma
(PPP) was obtained by centrifugation of blood samples at room
temperature for 10 min at 1,000 × g. The final concentration of
platelets was adjusted to 3×108/ml with the PPP, which
was used as a reference solution for aggregation assays.
Assay of platelet aggregation
Platelet aggregation analysis was performed as
previously described (21). For
the in vitro experiment, subsequent to the preparation of
PRP as aforementioned, 330 μl rat PRP was incubated with 5.5
μM ADP or 300 μM IGF-1 for 5 min at 37°C, following
pretreatment with various concentrations of LH (0-3 mM) for 5 min
at 37°C. For the ex vivo experiment, an equal volume of PRP
was collected from rats in each group and incubated in 5.5
μM ADP for 5 min at 37°C. Following incubation, aggregation
of platelets in each group was monitored by measuring light
transmission via a platelet aggregometer (LBY-NJ4; Pulisheng
Instrument Co., Ltd., Beijing, China), and the % maximum platelet
aggregation was recorded.
Measurement of platelet intracellular
Ca2+ mobilization
For the in vitro experiment, 1 ml prepared
PRP (3×108 platelets/ml) was pretreated with LH (0, 1, 2
and 3 mM) in the presence of CaCl2 (1 mM) for 5 min at
37°C. For the ex vivo experiment, 1 ml prepared PRP
(3×108 platelets/ml) from the Control, Adrenaline and
Adrenaline + LH rat groups were suspended in CaCl2 (1
mM) for 5 min at 37°C. Subsequently, the platelets from in
vitro and ex vivo incubations were stimulated with ADP
(5.5 μM) or IGF-1 (300 μM) for 5 min at 37°C. The
platelets were incubated with Fluo-4/AM (20 μM) for 60 min
at 37°C in the dark and subsequently centrifuged at room
temperature for 15 min at 500 × g. The fluorescence intensity of
100,000 platelets/sample was examined using a flow cytometer (BD
Biosciences, San Jose, CA, USA) and analyzed via BD CellQuest™ Pro
(Version 6.0; BD Biosciences, San Jose, CA, USA).
Measurement of TXB2 expression
levels
For the in vitro experiment, 1 ml prepared
PRP (3×108 platelets/ml) was pretreated with LH (0, 1, 2
and 3 mM) for 5 min at 37°C. Subsequently, the platelets were
stimulated with ADP (5.5 μM) or IGF-1 (300 μM) for 5
min at 37°C. The PRP was centrifuged at 1,000 × g for 15 min at
4°C. The supernatant was collected and TXB2 levels were determined
using an ELISA kit (cat. no. ADI-900-002; Enzo Life Sciences, Inc.)
and expressed as pg/ml. For the ex vivo experiment, blood
was drawn from the Control, Adrenaline and Adrenaline + LH groups
at the end of the experiment. Plasma was prepared by centrifuging
for 15 min at 4°C at 1,600 × g, and the levels of TXB2 in plasma
were measured by ELISA, similarly to the in
vitroexperiment.
Tail bleeding assay
To evaluate the bleeding risk of LH, a modified tail
cutting method was used (30).
Following adrenaline hydrochloride or saline injection for 1 h, the
rats in each group were anesthetized using sodium pentobarbital (45
mg/kg). Subsequently, the tail was pre-warmed for 3 min in saline
solution at 37°C. Bleeding was induced by precise transection of
the mouse tail at 3 mm from the tip. The distal portion of the tail
(3 cm) was immersed vertically into saline solution at 37°C. The
time between the start of transection to bleeding cessation was
recorded as the bleeding time.
Western blot analysis
The expression levels of associated proteins in PRP
from in vitro and ex vivoexperiments were determined
by western blot analysis. PRP from each group was lysed with
mammalian cell radioimmunoprecipitation assay lysis buffer (cat.
no. P0013; Beyotime Institute of Biotechnology; Haimen, China)
containing protease and phosphatase inhibitor cocktails. Total
protein concentrations were determined by a bicinchoninic acid
assay. Equal amounts of total proteins (50 μg) were resolved
in 12% SDS-PAGE and electroblotted. The nitrocellulose membranes
were blocked with 5% skimmed milk at room temperature for 2 h and
incubated with primary antibodies targeting p-Akt (1:1,000; cat.
no. 4060), Akt (1:1,000; cat no. 4685) or β-actin (1:1,000; cat.
no. 4967) overnight at 4°C. Subsequently, the membranes were
incubated with the appropriate HRP-conjugated anti-rabbit antibody
(1:5,000; cat. no. 7074) at room temperature for 1 h, followed by
enhanced chemiluminescence detection (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). β-actin was used as a loading control.
Statistical analysis
All experiments were performed at least three times
and presented as the mean ± standard deviation. Statistical
analyses were performed using SPSS for Windows (version 18.0; SPSS,
Inc., Chicago, IL, USA). Analysis of three or more groups was
performed by one-way analysis of variance, followed by the
least-significant difference test. Values obtained in a number of
experiments were converted into % for comparison of controls with
treated samples. P<0.05 was considered to indicate a
statistically significant difference.
Results
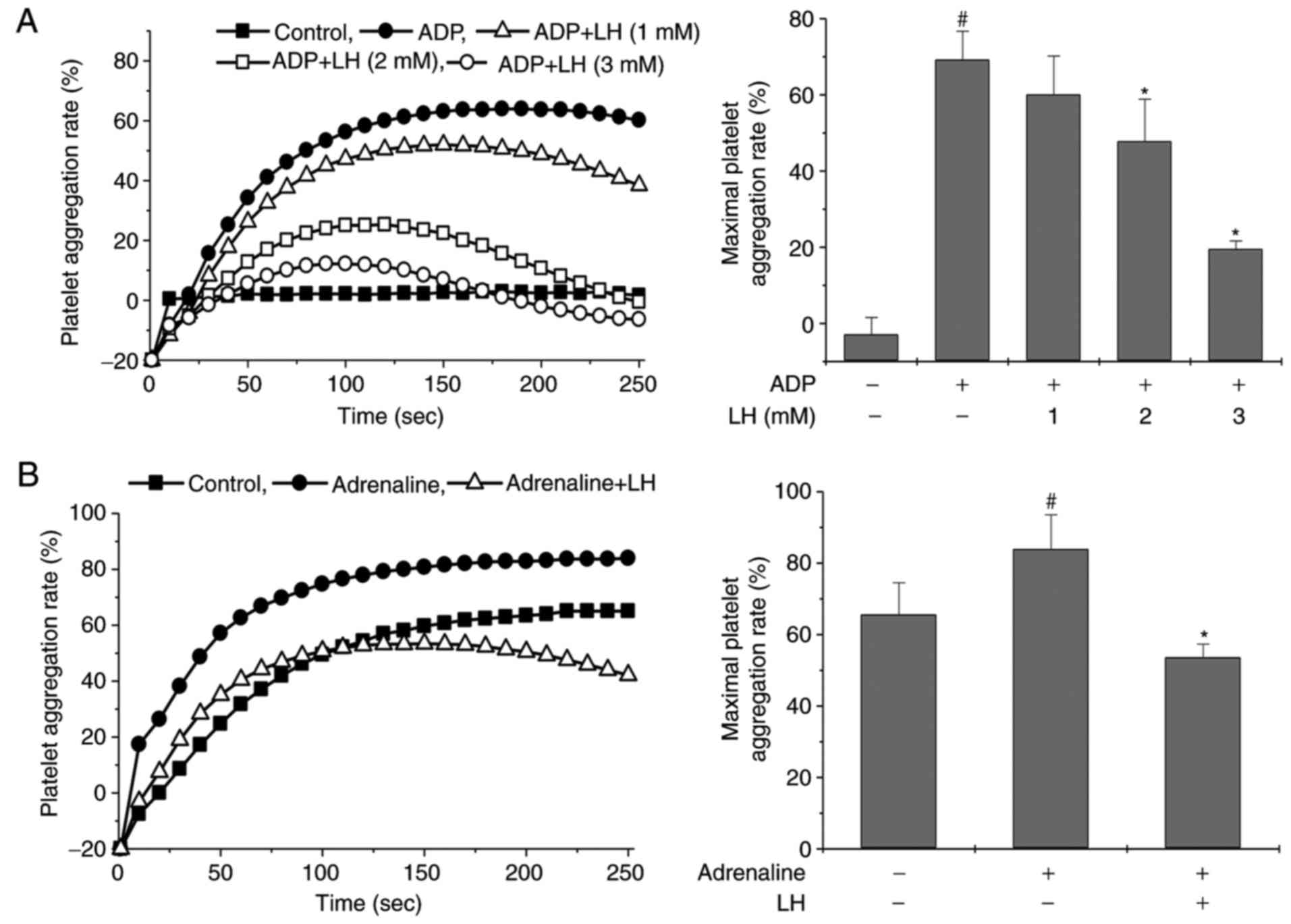
LH inhibits platelet aggregation in vitro
and ex vivo
To examine the potential therapeutic effects of
ligustrazine on platelet activation, rat PRP was incubated with LH,
followed by stimulation with ADP. As illustrated in Fig. 2A, ADP stimulation markedly induced
platelet aggregation in vitro, and this was significantly
inhibited by treatment with LH in a dose-dependent manner
(P<0.05 vs. untreated PRP; P<0.05 vs. ADP-stimulated PRP). To
verify the antiplatelet effect of LH, an ex vivo platelet
activation model was employed, where the rats were pretreated with
LH for 7 days followed by subcutaneous injection of adrenaline
hydrochloride for 1 h. Rat PRP from each group was collected and
stimulated with ADP (used as stimulation for detection of platelet
aggregation). As illustrated in Fig.
2B, treatment with LH significantly inhibited
adrenaline-induced platelet aggregation (P<0.05 vs. Control
group; P<0.05 vs. Adrenaline group). Taken together, these
results suggested that ligustrazine possesses potent properties of
suppressing platelet aggregation in vitro and ex
vivo.
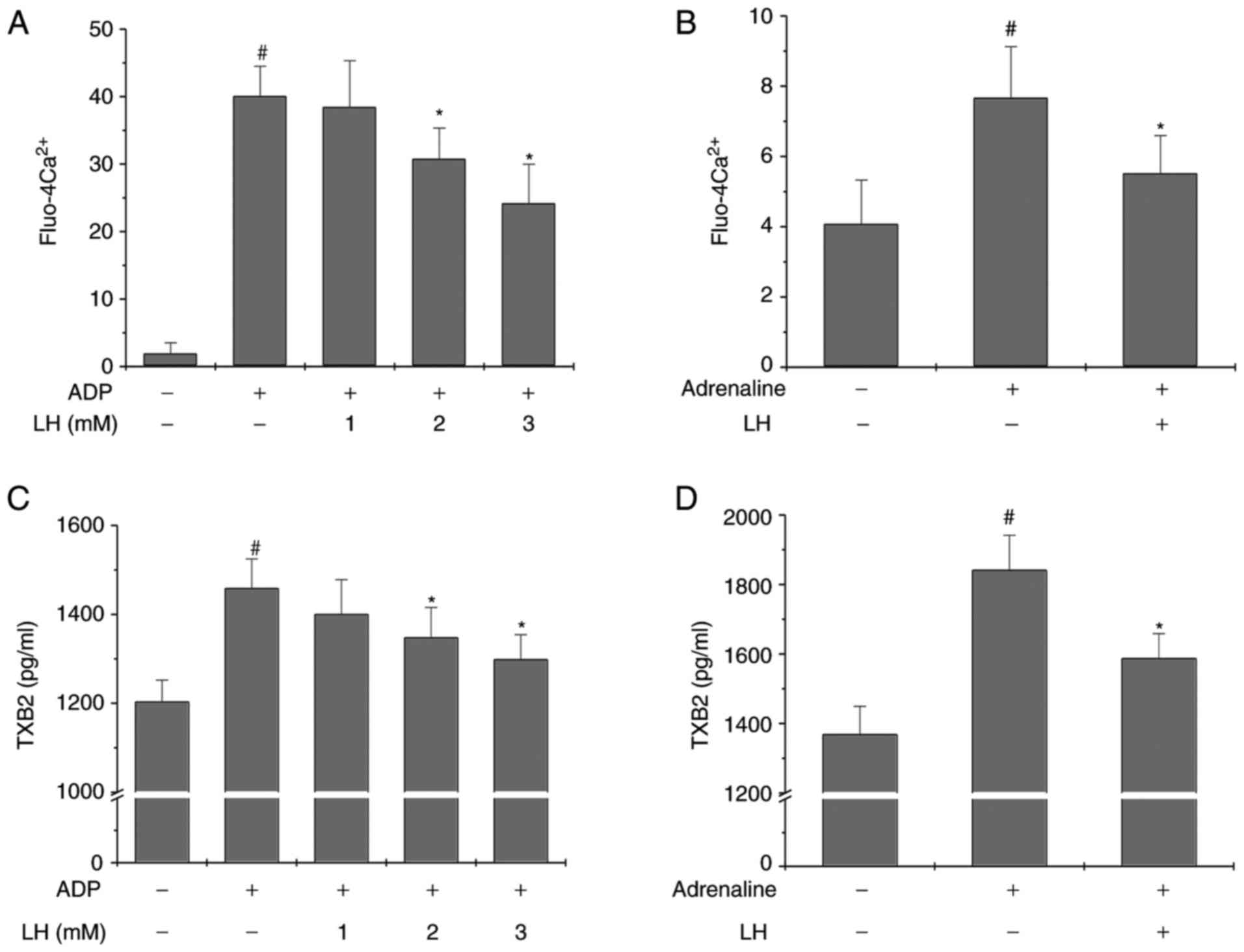
LH inhibits intracellular Ca2+
mobilization and TXA2 formation in vitro and ex vivo
To further determine the anti-platelet activity of
LH, its effect on Ca2+ mobilization and TXA2 secretion
was investigated. As presented in Fig. 3A and C, LH significantly and
dose-dependently inhibited ADP-induced Ca2+ mobilization
and TXB2 secretion in platelets in vitro (P<0.05 vs.
untreated PRP; P<0.05 vs. ADP-stimulated PRP). Furthermore,
similar results were observed in the ex vivoplatelet
activation model (Fig. 3B and D;
P<0.05 vs. Control group; P<0.05 vs. Adrenaline group),
suggesting that the anti-platelet activity of ligustrazine may be
mediated by the inhibition of Ca2+ mobilization and TXA2
formation.
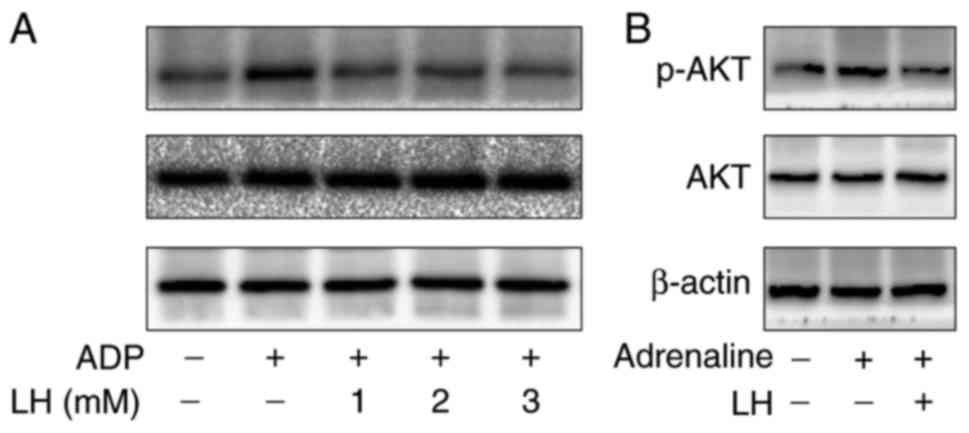
LH suppresses phosphorylation of Akt in
vitro and ex vivo
To examine the molecular mechanism of the
antiplatelet effects of LH, its effect on the
phosphorylation/activation of Akt was investigated. As presented in
Fig. 4, ADP markedly increased
the phosphorylation of Akt in vitro and ex vivo,
compared with the control group; this effect was however
significantly suppressed by treatment with LH. To further verify
the inhibitory effect of LH on Akt signaling, a specific Akt
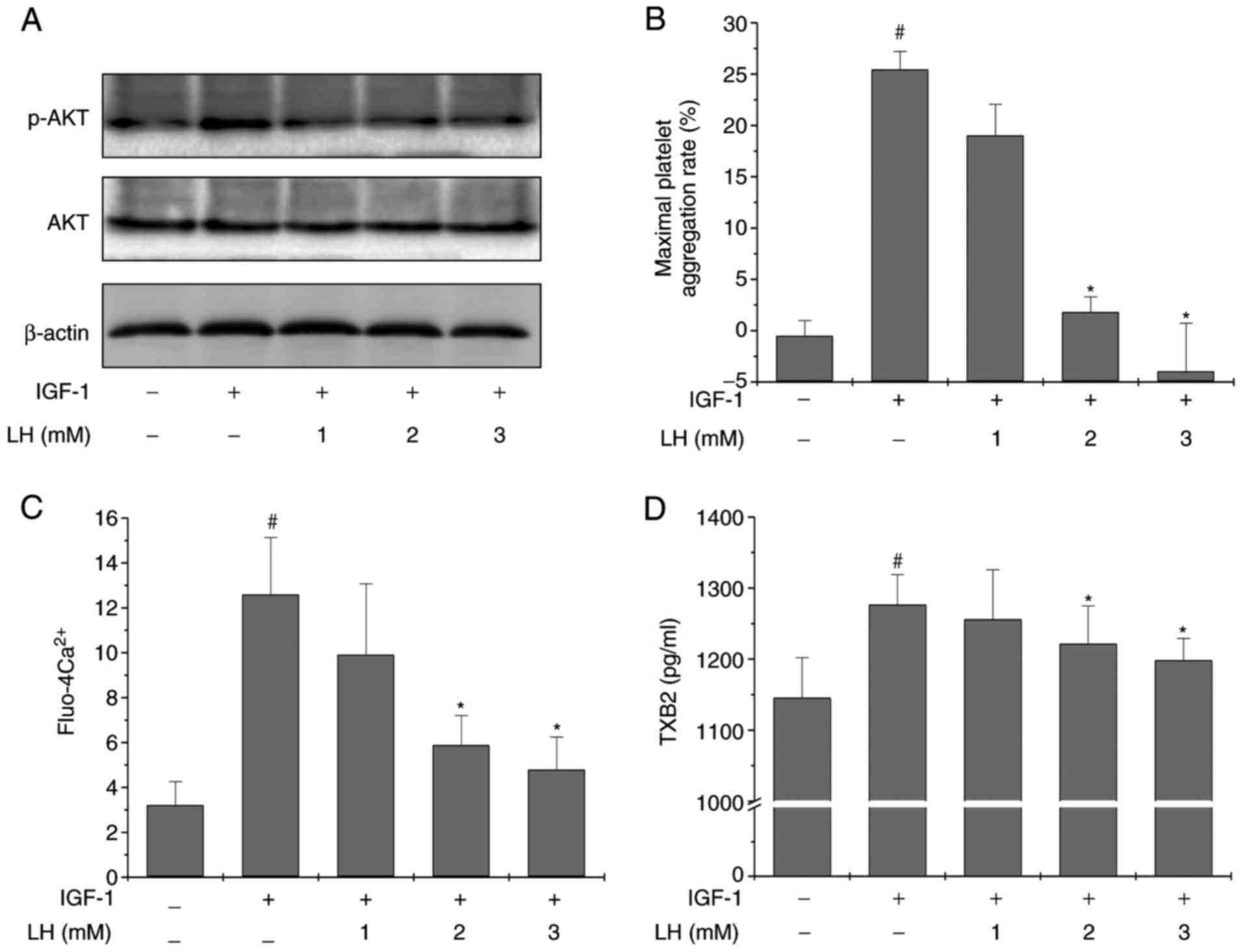
pathway activator, IGF-1, was employed. The results in Fig. 5A demonstrated that LH treatment
profoundly and dose-dependently suppressed IGF-1-induced Akt
phosphorylation. The levels of total Akt were unaltered during the
experiment. In addition, LH significantly inhibited IGF-1-induced
platelet aggregation, Ca2+ mobilization and TXB2
secretion in platelets (Fig.
5B–D; P<0.05 vs. untreated PRP; P<0.05 vs.
IGF-1-stimulated PRP). Collectively, these results suggested that
ligustrazine exerts its inhibitory effects on platelet activation
at least partly via suppression of the Akt signaling pathway.
LH displays low risk of hemorrhage in
vivo
Multiple currently-used antithrombotic agents have
adverse effects, including impaired blood coagulation or prolonged
bleeding time. Therefore, the effect of LH on bleeding time was
investigated in vivo in rats using a cutting tail method
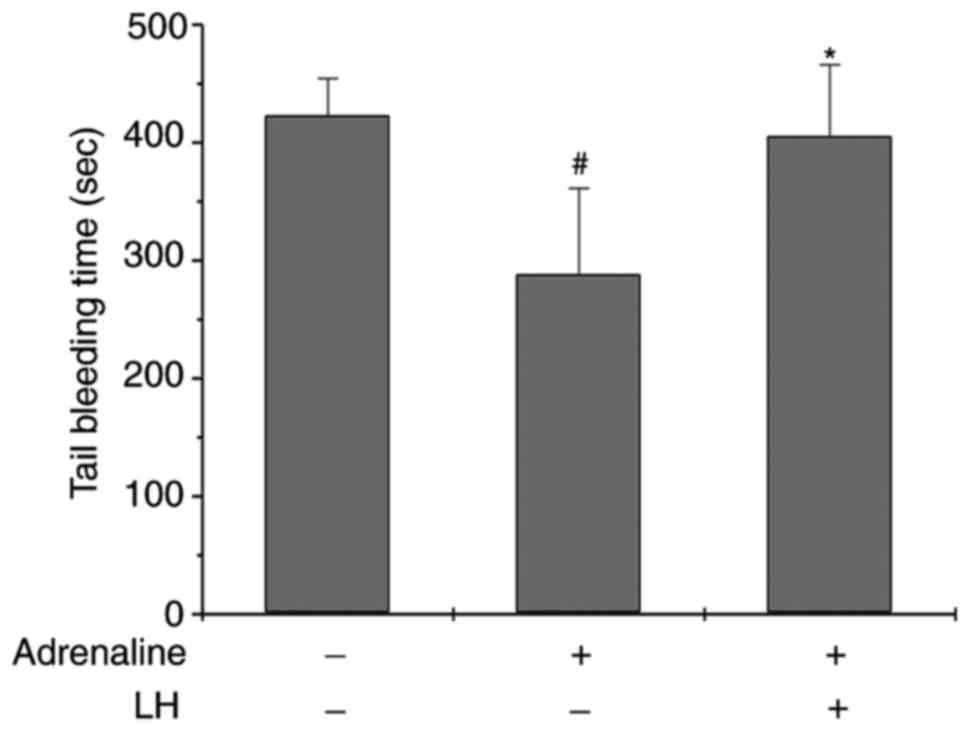
(30). As presented in Fig. 6, the bleeding time in the
Adrenaline-treated group was shorter compared with the control
group, while the bleeding time in the LH-pretreated group for 7
days was increased (P<0.05 vs. Control group; P<0.05 vs.
Adrenaline group). However, LH did not significantly prolong the
bleeding time compared with the control group, suggesting that
ligustrazine may have low bleeding risk.
Discussion
Due to the critical role of hyper-activation of
platelets in the development of thrombotic vascular diseases, the
process of platelet activation has become an attractive therapeutic
target. Numerous antiplatelet chemical drugs are used to prevent or
treat thrombosis. However, the currently available drugs have
specific clinical disadvantages, including gastrointestinal side-
effects and hemorrhage (16,17). Therefore, there is an urgent
requirement for safer and more effective antiplatelet agents
without these adverse effects. In recent years, novel therapeutic
agents have been derived from Chinese medicinal herbs and there is
growing interest in this area. Ligustrazine, one of the natural
alkaloids isolated from RLW that is commonly used to clinically
treat cardiac-cerebral diseases, has been demonstrated to possess
antiplatelet activity (19-22). However, the precise mechanism of
its potential therapeutic effects remains to be further elucidated.
Using in vitro and ex vivoplatelet activation models,
established by stimulating rat PRP with the platelet activator ADP,
the present study demonstrated that LH (the clinical-grade form of
ligustrazine) was able to significantly inhibit platelet
aggregation. In addition, LH was able to reverse adrenaline-induced
shortening of bleeding time, indicating the low bleeding risk of
ligustrazine. However, the rapid metabolism and short half-life of
ligustrazine severely limits its clinical application. Therefore,
novel chemical forms of ligustrazine with a longer half-life
require development in further studies. Additionally, a comparison
of LH with other antiplatelet medicines (including aspirin and
clopidelgrel) and their potential combinations require further
assessment.
Platelet activation may be induced by a variety of
agonists, including ADP and TXA2, which are released from the
granules of activated platelets. TXA2 exerts its function through
interaction with the thromboxane receptor, contributing to the
amplification of the initial platelet activation, which therefore
is a principal target for most currently-used antiplatelet agents
(31-33). Although different agonists induce
platelet activation through different mechanisms by respectively
binding to their specific receptors, they all result in an
elevation of intracellular Ca2+ concentration
[(Ca2+)i], an event termed Ca2+ mobilization
(34). Ca2+ is an
important second messenger that is critical for various cellular
processes, including platelet activation (35). Therefore, the present study
verified the antiplatelet activity of LH by examining
Ca2+ mobilization and TXA2 secretion, and the results
demonstrated that LH significantly and dose-dependently inhibited
ADP-induced Ca2+ mobilization and TXB2 secretion in
platelets in vitro and ex vivo. These results
suggested that the antiplatelet activity of ligustrazine may be
mediated by the inhibition of Ca2+ mobilization and TXA2
formation.
It is well documented that the process of platelet
activation is highly regulated by multiple pathways, including the
PI3K/Akt signaling pathway. Activation of the PI3K/Akt pathway
results in granule secretion and the second wave of platelet
aggregation (9,36-40). To examine the mechanism of the
antiplatelet activities of ligustrazine, its effect on the
phosphorylation/activation of Akt was determined. The present study
demonstrated that treatment with LH significantly suppressed
ADP-induced Akt phosphorylation in vitro and ex vivo.
Notably, using the specific Akt pathway activator IGF-1, the
present study further confirmed that LH significantly and
specifically suppressed the activation of Akt signaling.
Consequently, treatment with LH markedly inhibited IGF-1-induced
platelet aggregation, Ca2+ mobilization and TXB2
secretion in platelets. However, as phosphorylation of Akt is
regulated by upstream regulators (including PI3K, PH domain and
leucine rich repeat protein phosphatase 1, phosphatase and tensin
homolog and associated microRNAs) (41-43) and exerts its function by
regulating the expression of downstream molecules (including
mammalian target of rapamycin) (44), the effect of LH upstream and
downstream of Akt requires further assessment.
In conclusion, the present study proposed that
ligustrazine possesses a broad range of antiplatelet activities,
without apparent hemorrhagic side-effects. Suppression of Akt
signaling may be one of the mechanisms by which ligustrazine exerts
its antiplatelet function. The present results provide further
preliminary evidence to support that ligustrazine may be considered
a potent therapeutic agent against thrombotic vascular
diseases.
Acknowledgments
Not applicable.
Abbreviations:
|
ADP
|
adenosine diphosphate
|
|
IGF-1
|
insulin-like growth factor-1
|
|
LH
|
ligustrazine hydrochloride
|
|
PRP
|
platelet-rich plasma
|
|
RLW
|
Rhizoma Ligusticum
Wallichii
|
|
TXB2
|
thromboxane B2
|
Funding
The present study was sponsored by the Natural
Science Foundation of Fujian Province (grant no. 2018J01229) and
the National Natural Science Foundation of China (grant no.
81774135).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors’ contributions
KC and JP conceived and designed the experiments.
LL, AS and QL conducted the animal experiments. HC and JC performed
FACS analysis. LL, YC and LYL performed the western blotting, ELISA
and data analysis. HC and AS determined platelet aggregation
analysis. LL, JP and KC wrote and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments involving animals were approved by
the Institutional Animal Care and Use Committee of Fujian
University of Traditional Chinese Medicine (Fuzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smith T, Dhunnoo G, Mohan I and
Charlton-Menys V: A pilot study showing an association between
platelet hyperactivity and the severity of peripheral arterial
disease. Platelets. 18:245–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Georgescu A, Alexandru N, Andrei E, Dragan
E, Cochior D and Dias S: Effects of transplanted circulating
endothelial progenitor cells and platelet microparticles in
atherosclerosis development. Biol Cell. 108:219–243. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alexandru N, Popov D and Georgescu A:
Platelet dysfunction in vascular pathologies and how can it be
treated. Thromb Res. 129:116–126. 2012. View Article : Google Scholar
|
|
4
|
Sharma G and Berger JS: Platelet activity
and cardiovascular risk in apparently healthy individuals: A review
of the data. J Thromb Thrombolysis. 32:201–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Angiolillo DJ: The evolution of
antiplatelet therapy in the treatment of acute coronary syndromes:
From aspirin to the present day. Drugs. 72:2087–2116. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spiel AO, Gilbert JC and Jilma B: von
Willebrand factor in cardiovascular disease: Focus on acute
coronary syndromes. Circulation. 117:1449–1459. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brass LF, Manning DR, Cichowski K and
Abrams CS: Signaling through G proteins in platelets: To the
integrins and beyond. Thromb Haemost. 78:581–589. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Franke TF, Kaplan DR, Cantley LC and Toker
A: Direct regulation of the Akt proto-oncogene product by
phosphati-dylinositol-3,4-bisphosphate. Science. 275:665–668. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guidetti GF, Canobbio I and Torti M:
PI3K/Akt in platelet integrin signaling and implications in
thrombosis. Adv Biol Regul. 59:36–52. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moore SF, van den Bosch MT, Hunter RW,
Sakamoto K, Poole AW and Hers I: Dual regulation of glycogen
synthase kinase 3 (GSK3)αIIb/β3 by protein
kinase C (PKC)α and Akt promotes thrombin-mediated integrin α β
activation and granule secretion in platelets. J Biol Chem.
288:3918–3928. 2013. View Article : Google Scholar
|
|
11
|
Chen X, Zhang Y, Wang Y, Li D, Zhang L,
Wang K, Luo X, Yang Z, Wu Y and Liu J: PDK1 regulates platelet
activation and arterial thrombosis. Blood. 121:3718–3726. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin H, Stojanovic A, Hay N and Du X: The
role of Akt in the signaling pathway of the glycoprotein Ib-IX
induced platelet activation. Blood. 111:658–665. 2008. View Article : Google Scholar
|
|
13
|
Kim S, Mangin P, Dangelmaier C, Lillian R,
Jackson SP, Daniel JL and Kunapuli SP: Role of phosphoinositide
3-kinase beta in glycoprotein VI-mediated Akt activation in
platelets. J Biol Chem. 284:33763–33772. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reséndiz JC, Kroll MH and Lassila R:
Protease-activated receptor-induced Akt activation-regulation and
possible function. J Thromb Haemost. 5:2484–2493. 2007. View Article : Google Scholar
|
|
15
|
Chen J, De S, Damron DS, Chen WS, Hay N
and Byzova TV: Impaired platelet responses to thrombin and collagen
in AKT-1-deficient mice. Blood. 104:1703–1710. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Minno MN, Lupoli R, Palmieri NM,
Russolillo A, Buonauro A and Di Minno G: Aspirin resistance,
platelet turnover, and diabetic angiopathy: A 2011 update. Thromb
Res. 129:341–344. 2012. View Article : Google Scholar
|
|
17
|
Uchiyama S: Clopidogrel resistance:
Identifying and overcoming a barrier to effective antiplatelet
treatment. Cardiovasc Ther. 29:e100–e111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beijing Institute of Pharmaceutical
Industry: Studies of active components of Ligusticum Wallichii
Franch. I. Extraction, isolation and structure identification of
tetramethylpyrazine. Chin Med J. 7:420–421. 1977.In Chinese.
|
|
19
|
Li Z, Li D, Huang J, Zhang W, Ding Y and
Wang S: Preparation of cardiovascular disease-related genes
microarray and its application in exploring ligustrazine-induced
changes in endothelial gene expression. Pol J Pharmacol.
56:427–433. 2004.PubMed/NCBI
|
|
20
|
Zhang F, Ni C, Kong D, Zhang X, Zhu X,
Chen L, Lu Y and Zheng S: Ligustrazine attenuates oxidative
stress-induced activation of hepatic stellate cells by interrupting
platelet-derived growth factor-β receptor-mediated ERK and p38
pathways. Toxicol Appl Pharmacol. 265:51–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li S, Chen H, Wang X, Wu J, Jiang J and
Wang Y: Pharmacokinetic study of a novel stroke therapeutic,
2-[[(1,1-dimethyl-ethyl)oxidoimino]methyl]-3,5,6-trimethylpyrazine,
by a simple HPLC-UV method in rats. Eur J Drug Metab Pharmacokinet.
36:95–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu W, Yu X, Luo XP, Yang SH and Zheng D:
Tetramethylpyrazine protects against scopolamine-induced memory
impairments in rats by reversing the cAMP/PKA/CREB pathway. Behav
Brain Res. 253:212–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng XR, Zhang L, Hu JJ, Sun L and Du GH:
Neuroprotective effects of tetramethylpyrazine on hydrogen
peroxide-induced apoptosis in PC12 cells. Cell Biol Int.
31:438–443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han J, Song J, Li X, Zhu M, Guo W, Xing W,
Zhao R, He X, Liu X, Wang S, et al: Ligustrazine suppresses the
growth of HRPC cells through the inhibition of cap-dependent
translation via both the mTOR and the MEK/ERK pathways. Anticancer
Agents Med Chem. 15:764–772. 2015. View Article : Google Scholar
|
|
25
|
Wang GJ: Changes of nail fold
microcirculation in patients with acute cerebral thrombosis treated
with ligustrazine. Chin J Neurol Psychiatry. 17:121–124. 1984.In
Chinese.
|
|
26
|
Sheu JR, Kan YC, Hung WC, Ko WC and Yen
MH: Mechanisms involved in the antiplatelet activity of
tetramethylpyrazine in human platelets. Thromb Res. 88:259–270.
1997. View Article : Google Scholar
|
|
27
|
Lin CI, Wu SL, Tao PL, Chen HM and Wei J:
The role of cyclic AMP and phosphodiesterase activity in the
mechanism of action of tetramethylpyrazine on human and dog cardiac
and dog coronary arterial tissues. J Pharm Pharmacol. 45:963–966.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu SF, Cai YN, Evans TW, McCormack DG,
Barer GR and Barnes PJ: Ligustrazine is a vasodilator of human
pulmonary and bronchial arteries. Eur J Pharmacol. 191:345–350.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng W, Hucks D, Priest RM, Kan YM and
Ward JP: Ligustrazine-induced endothelium-dependent relaxation in
pulmonary arteries via an NO-mediated and exogenous
L-arginine-dependent mechanism. Br J Pharmacol. 119:1063–1071.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alshbool FZ, Karim ZA, Vemana HP, Conlon
C, Lin OA and Khasawneh FT: The regulator of G-protein signaling 18
regulates platelet aggregation, hemostasis and thrombosis. Biochem
Biophys Res Commun. 462:378–382. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su XL, Su W, Wang Y, Wang YH, Ming X and
Kong Y: The pyrrolidinoindoline alkaloid Psm2 inhibits platelet
aggregation and thrombus formation by affecting PI3K/Akt signaling.
Acta Pharmacol Sin. 37:1208–1217. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sakariassen KS, Alberts P, Fontana P, Mann
J, Bounameaux H and Sorensen AS: Effect of pharmaceutical
interventions targeting thromboxane receptors and thromboxane
synthase in cardiovascular and renal diseases. Future Cardiol.
5:479–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fontana P, Zufferey A, Daali Y and Reny
JL: Antiplatelet therapy: Targeting the TxA2 pathway. J
Cardiovasc Transl Res. 7:29–38. 2014. View Article : Google Scholar
|
|
34
|
Varga-Szabo D, Braun A and Nieswandt B:
Calcium signaling in platelets. J Thromb Haemost. 7:1057–1066.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Berridge MJ, Bootman MD and Roderick HL:
Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev
Mol Cell Biol. 4:517–529. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Heraud JM, Racaud-Sultan C, Gironcel D,
Albigès-Rizo C, Giacomini T, Roques S, Martel V, Breton-Douillon M,
Perret B and Chap H: Lipid products of phosphoinositide 3-kinase
and phosphatidylinositol 4′,5′-bisphosphate are both required for
ADP-dependent platelet spreading. J Biol Chem. 273:17817–17823.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Canobbio I, Stefanini L, Cipolla L,
Ciraolo E, Gruppi C, Balduini C, Hirsch E and Torti M: Genetic
evidence for a predominant role of PI3Kbeta catalytic activity in
ITAM- and integrin-mediated signaling in platelets. Blood.
114:2193–2196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Woulfe DS: Akt signaling in platelets and
thrombosis. Expert Rev Hematol. 3:81–91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
O’Brien KA, Stojanovic-Terpo A, Hay N and
Du X: An important role for Akt3 in platelet activation and
thrombosis. Blood. 118:4215–4223. 2011. View Article : Google Scholar :
|
|
40
|
Stojanovic A, Marjanovic JA, Brovkovych
VM, Peng X, Hay N, Skidgel RA and Du X: A phosphoinositide
3-kinase-AKT-nitric oxide-cGMP signaling pathway in stimulating
platelet secretion and aggregation. J Biol Chem. 281:16333–16339.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Laurent PA, Severin S, Gratacap MP and
Payrastre B: Class I PI 3-kinases signaling in platelet activation
and thrombosis: PDK1/Akt/GSK3 axis and impact of PTEN and SHIP1.
Adv Biol Regul. 54:162–174. 2014. View Article : Google Scholar
|
|
42
|
Meuillet EJ: Novel inhibitors of AKT:
Assessment of a different approach targeting the pleckstrin
homology domain. Curr Med Chem. 18:2727–2742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ishibashi O, Akagi I, Ogawa Y and Inui T:
MiR-141-3p is upregulated in esophageal squamous cell carcinoma and
targets pleckstrin homology domain leucine-rich repeat protein
phosphatase-2, a negative regulator of the PI3K/AKT pathway.
Biochem Biophys Res Commun. 501:507–513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Razmara M, Heldin CH and Lennartsson J:
Platelet-derived growth factor-induced Akt phosphorylation requires
mTOR/Rictor and phospholipase C-γ1, whereas S6 phosphory-lation
depends on mTOR/Raptor and phospholipase D. Cell Commun Signal.
11:32013. View Article : Google Scholar
|